Abstract
The transition from voluntary to addictive behavior is characterized by a loss of regulatory control in favor of reward driven behavior. Animal models indicate that this process is neurally underpinned by a shift in ventral–dorsal striatal control of behavior; however, this shift has not been directly examined in humans. The present resting state functional magnetic resonance imaging (fMRI) study employed a two‐step approach to: (a) precisely map striatal alterations using a novel, data‐driven network classification strategy combining intrinsic connectivity contrast with multivoxel pattern analysis and, (b) to determine whether a ventral to dorsal striatal shift in connectivity with reward and regulatory control regions can be observed in abstinent (28 days) male cannabis‐dependent individuals (n = 24) relative to matched controls (n = 28). Network classification revealed that the groups can be reliably discriminated by global connectivity profiles of two striatal regions that mapped onto the ventral (nucleus accumbens) and dorsal striatum (caudate). Subsequent functional connectivity analysis demonstrated a relative shift between ventral and dorsal striatal communication with fronto‐limbic regions that have been consistently involved in reward processing (rostral anterior cingulate cortex [ACC]) and executive/regulatory functions (dorsomedial prefrontal cortex [PFC]). Specifically, in the cannabis‐dependent subjects, connectivity between the ventral striatum with the rostral ACC increased, whereas both striatal regions were uncoupled from the regulatory dorsomedial PFC. Together, these findings suggest a shift in the balance between dorsal and ventral striatal control in cannabis dependence. Similar changes have been observed in animal models and may promote the loss of control central to addictive behavior.
Keywords: anterior cingulate, addiction, cannabis, cognitive control, intrinsic connectivity contrast, data‐driven, functional connectivity, prefrontal cortex, reward, striatum
1. INTRODUCTION
Converging evidence from animal and human research indicates that maladaptations in fronto‐limbic‐striatal circuitries drive the loss of control that characterizes addiction (Fineberg et al., 2010; Morein‐Zamir & Robbins, 2015). The striatum lies at the core of this circuitry and critically contributes to both, acute drug reinforcement and the transition from voluntary to addictive use that is accompanied by a loss of control (Brand, Young, Laier, Wölfling, & Potenza, 2016; Everitt & Robbins, 2016; Fineberg et al., 2010).
The striatum contributes to several domains that undergo critical adaptations during the transition to addiction, including associative learning, behavioral control, incentive salience, and reward (Haber, 2016; Robbins, Gillan, Smith, de Wit, & Ersche, 2012). The functional heterogeneity of the striatum and its multifaceted contributions to addiction are mirrored in its complex organization into distinct subregions that communicate with the entire cortex via subregion‐specific fronto‐striatal loops (Haber, 2016). Based on their specific functions and projections, the striatum is divided into the ventral striatum (VS) that exhibits strong connections with ventral anterior cingulate and orbitofrontal regions regulating reward and reinforcement behavior, and the dorsal striatum (DS) that exhibits strong connections with dorsolateral and dorsomedial prefrontal regions engaged in regulatory control (Di Martino et al., 2008; Haber, 2016; Postuma & Dagher, 2006). This functional and network‐level seggregation has important implications for the transition to addiction, with overarching conceptualizations proposing that the loss of control is accompanied by a progressive shift from controlled toward reward‐driven impulsive behavior (Jentsch & Taylor, 1999; Noël, Brevers, & Bechara, 2013).
Partly driven by the ongoing discussion about cannabis legalization and concomitantly increasing demand for treatment of cannabis dependence (EMCDDA, 2008; Volkow et al., 2016), a number of studies have examined effects of cannabis use on the brain (overview see, e.g., Lorenzetti, Solowij, & Yücel, 2016; Weinstein, Livny, & Weizman, 2016). In line with the functions of the striato‐frontal circuitry and its proposed contribution to addiction task‐based neuroimaging studies demonstrated exaggerated striatal reactivity to cannabis cues (Filbey et al., 2016), reward anticipation (Jager, Block, Luijten, & Ramsey, 2013) and decreased frontal activity during executive control (Weinstein et al., 2016; Wrege et al., 2014; Yanes et al., 2018) in chronic users. This pattern may reflect exaggerated striatal reactivity in the context of deficient prefrontal control and mirror maladaptations that have been observed across addictive disorders (Brand et al., 2016; Goldstein & Volkow, 2011).
Despite the important contributions of these studies to characterize mechanisms of cannabis dependence, the task‐based approach does not allow direct evaluation of the ventral to dorsal striatal shift due to: (a) limitation of task‐based fMRI to regions engaged by the experimental paradigm (Weinstein et al., 2016) and (b) stimulus and context dependences of striatal alterations in cannabis dependence (Gilman, 2017; Zimmermann et al., 2017). Resting state fMRI functional connectivity (rsfMRI‐FC) approaches allow a more holistic assessment of functional alterations in the absence of task or contextual modulation. Moreover, these approaches have a high sensitivity for transient and lasting striatal alterations on the subregion‐specific network level (Di Martino et al., 2008; Di Martino et al., 2011). Two recent studies applied rsfMRI‐FC to determine lasting disruptions in the frontostriatal functional circuitries in cannabis dependence (Blanco‐Hinojo et al., 2017; Zimmermann, Yao, et al., 2017). However, findings were limited by the confirmatory nature of the rsfMRI‐FC analysis (Zimmermann, Yao, et al., 2017) or the use of literature‐based striatal seed regions (Blanco‐Hinojo et al., 2017) that have a high sensitivity to capture subregion specific functional networks in healthy subjects (Di Martino et al., 2008) but might not be sensitive to the specific alterations related to cannabis dependence. Despite supporting evidence for a striatal shift in response to masked drug cues in cannabis dependence (Wetherill et al., 2016), the ventral to dorsal shift has not been explicitly examined in the absence of external stimulation.
The present study therefore employed a two step approach to: (a) precisely map striatal alterations in cannabis‐dependent individuals using network classification and (b) determine whether a ventral to dorsal striatal shift in connectivity with reward and regulatory control regions can be observed. In an initial step, intrinsic striatal alterations were mapped using a novel fully data‐driven network classification approach that operates independently of a priori assumptions about striatal architecture and thus enables higher sensitivity to detect striatal functional changes (Martuzzi et al., 2011; Rubinov & Sporns, 2010). Next, altered functional communication of the determined striatal subregions was examined on the whole‐brain network level (for a similar approach see Walpola et al., 2017). To this end, rsfMRI data were acquired in n = 28 participants that fulfilled the diagnostic and statistical manual of mental disorders, fourth edition (DSM‐IV) criteria for cannabis dependence and n = 28 matched healthy controls. To control for subacute effects of cannabinoid metabolites (Vandevenne, Vandenbussche, & Verstraete, 2000), craving (Copersino et al., 2006), and rapid neural recovery of homeostatic receptor adaptations after cessation of chronic cannabis use (Hirvonen et al., 2012) participants underwent 28 days of cannabis abstinence before acquisition of the MRI data.
Based on current neurobiological frameworks suggesting that the transition to addiction is accompanied by regional‐specific neuroplastic changes in the ventral and dorsal striatum and accumulating evidence from task‐based neuroimaging studies suggesting that chronic cannabis use is associated with abberant reward and regulatory control processes (Everitt & Robbins, 2016; Weinstein et al., 2016; Wrege et al., 2014; Yanes et al., 2018) we expected to observe corresponding maladaptations on the level of the network level organization of the brain. Specifically, we hypothesized that: (a) global connectivity differences between the groups specifically map to the DS and VS, reflecting regional‐specific striatal adaptations and (b) a shift in the network communication of the striatal subregions reflecting increased communication within frontostriatal pathways related to reward processing and concomitantly decreased communication in pathways related to cognitive control.
2. METHODS
2.1. Participants
For the present study, data from n = 16 male cannabis‐dependent users and n = 16 healthy male controls from our previous publication on convergent task and resting functional connectivity alterations in cannabis users was included (Zimmermann, Yao, et al., 2017). To enhance statistical power for the present data‐driven approach, the sample size was increased to n = 28 male cannabis‐dependent subjects and n = 28 nonusing controls using identical inclusion and exclusion criteria. To control for potential confounding influences of menstrual cycle on striatal resting state activity as well as complex interactions between menstrual cycle and addiction‐related alterations in frontostriatal functioning (Franklin et al., 2015; Wetherill, Jagannathan, Hager, Maron, & Franklin, 2016; Wiers et al., 2016), only male subjects were included (for a similar approach see Zimmermann, Walz, et al., 2017).
All cannabis users were diagnosed with a current cannabis dependence according to DSM‐IV during the 18 months before the examination. Diagnostic criteria were assessed by the structured clinical interview (SCID for DSM‐IV) conducted by a trained psychologist. To facilitate the determination of long‐term alterations associated with cannabis dependence that persist beyond subacute cannabinoid metabolites (Vandevenne et al., 2000), craving (Copersino et al., 2006), and rapid neural recovery (Hirvonen et al., 2012) participants were required to abstain from cannabis for 28 days prior to the assessment. Comparable abstinence periods have been employed in previous studies to differentiate enduring (long term) effects of cannabis use from transient (subacute) alterations and rapid neural recovery (overview in Crean, Crane, and Mason (2011); Ganzer, Bröning, Kraft, Sack, and Thomasius (2016); Schreiner and Dunn (2012)).
At the time point of recruitment, most cannabis‐dependent subjects were active cannabis users or in early phases of abstinence. Inclusion criteria for all participants were: (a) age between 18 and 35 years, (b) right‐handedness, (c) negative urine toxicology for cannabis and other illicit drugs (immunoassay, substance/cut‐off per milliliter: THC/50 ng, amphetamines/500 ng, cocaine/300 ng, methamphetamine/500 ng, MDMA/300 ng, opiates/300 ng, methadone/300 ng), and (d) self‐reported abstinence of >28 from cannabis validated by negative urine screens on the day of the fMRI assessment. One user reported having used cannabis on one occasion 14 days before the experiment, but was included due to negative urine toxicology. Exclusion criteria for all participants were: (a) profound DSM‐IV axis I or axis II disorders, such as psychotic or bipolar symptoms, (b) Beck Depression Inventory score > 20 indicating a moderate depression, (c) medical disorder, (d) current/regular medication, and (e) use of other illicit substances >75 lifetime occasions. Controls were included if their cumulative lifetime use of cannabis was <15 g.
Anxiety, mood and concentration were assessed on the day of the examination using validated scales including the social interaction anxiety scale (SIAS) (Mattick & Clarke, 1998), the state–trait anxiety inventory (STAI) (Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, 1983), positive and negative affect schedule (Crawford & Henry, 2004), and the d2 test of attention (Brickenkamp & Zillmer, 1998). These measures allowed control for potential confounding effects of common withdrawal symptoms. Cannabis use patterns and the use of other drugs were assessed using corresponding modules of a structured interview that has been used in previous studies of our group (e.g., Becker et al., 2015; Becker, Wagner, Gouzoulis‐Mayfrank, Spuentrup, & Daumann, 2010; Zimmermann, Yao, et al., 2017). Differences in potential confounders were examined by independent sample t test; in case of a nonnormal distribution of the underlying data nonparametric analyses were used. As a trade‐off between effects of acute nicotine and nicotine craving on neural activity all participants underwent a supervised abstinence from nicotine 1.5 hr before the fMRI assessment. Cannabis‐dependent subjects were recruited in cooperation with the LVR Clinics Bonn, Germany, Addiction Department that offers a specialized treatment program for cannabis dependence. Potential participants were contacted and invited for an initial screening session to further validate study participation criteria. Participants who agreed to stay abstinent for at least 28 days were scheduled for the fMRI assessment on a separate day following the abstinence period. Controls were recruited by advertisements and underwent identical study procedures. Written informed consent was obtained and study protocols were approved by the local ethics committee (Medical Faculty, University of Bonn).
Three cannabis users were excluded due to a history of exceeding couse of other illicit drugs and one was excluded due to excessive head movement during fMRI, leading to a final sample of n = 24 cannabis users and n = 28 healthy controls.
2.2. MRI data acquisition
Functional and structural MRI was acquired using a Siemens TRIO 3‐Tesla system with a 32‐channel head coil. rsfMRI data were acquired before any tasks using a T2*‐weighted echo‐planar imaging (EPI) pulse sequence (repetition time = 2,580 ms, echo time = 30 ms, number of slices = 47, slice thickness = 3.5 mm, no gap, field‐of‐view = 224 × 224 mm2, resolution = 64 × 64, flip angle = 80°, number of volumes = 180). To improve spatial normalization and exclude participants with apparent brain pathologies, a high‐resolution whole‐brain volume T1‐weighted images was acquired in addition using a 3D spoiled gradient echo pulse sequence (repetition time = 1,660 ms, echo time = 2.54 ms, flip angle = 9°, field‐of‐view = 256 × 256 mm2, acquisition matrix = 256 × 256, thickness = 0.8 mm, number of slices = 208).
Participants were instructed to lie still and relax with their eyes closed while thinking of nothing in particular, yet not to fall asleep during the resting state acquisition.
2.3. Preprocessing of the functional resting state data
rsfMRI data were preprocessed using standard statistical parametric mapping (SPM), analysis of functional neuroImages (AFNI) and FMRIB software library (FSL) routines in combination with advanced independent component analysis (ICA‐AROMA, Pruim et al., 2015). The first five volumes were discarded to allow MRI T1 equilibration. The remaining volumes were slice‐time corrected, spatially realigned to the first volume, and unwarped to correct for nonlinear distortions possibly related to head motion or magnetic field inhomogeneity using SPM12 (http://www.fil.ion.ucl.ac.uk/spm/software/spm12). The functional time series were further processed using FSL (http://www.fmrib.ox.ac.uk/fsl), including nonbrain removal using BET (Smith, 2002), spatial smoothing with a Gaussian kernel of 6 mm full width at half maximum, and grand‐mean scaling. Subsequently, the data were submitted to an ICA for automatic removal of motion artifacts (ICA‐AROMA, Pruim, Mennes, van Rooij, et al., 2015)—a procedure that has been shown to minimize the impact of motion on functional connectivity metrics and decrease the loss in temporal degrees of freedom compared to spike regression and scrubbing (Pruim, Mennes, Buitelaar, & Beckmann, 2015). Next, mean signals from white matter (WM) and cerebrospinal fluid (CSF), linear and quadratic drift across time, together with a series of sine and cosine functions removing all frequencies outside the range (0.01–0.08 Hz), were regressed out in a single regression step using AFNI's 3dTproject. Similar to the preprocessing protocol used by Pruim, Mennes, van Rooij, et al. (2015), WM and CSF time series were derived by determining the mean time series over voxels within predefined subject‐specific WM and CSF masks. To obtain these masks, we first thresholded the MNI152 average CSF and WM priors maps (95% of the robust range) and subsequently registered to native EPI space. Likewise, we applied FSL FAST (Zhang, Brady, & Smith, 2001) to the individual T1 images to derive a CSF and WM probability map and thresholded at 95% followed by registration to native EPI space. Multiplication of both masks resulted in the respective conservative CSF and WM masks. Finally, functional MRI data were registered to T1 and standard MNI152 space using Boundary‐Based Registration as implemented in FLIRT (Jenkinson, Bannister, Brady, & Smith, 2002; Jenkinson & Smith, 2001) and FNIRT nonlinear registration (Andersson, Jenkinson, & Smith, 2010) with 6 and 12 df, respectively, and interpolated to 2 × 2 × 2 mm.
2.4. Quality control for motion artifacts
One participant (cannabis user) was excluded due to excessive head movement (>2.5 mm or 2.5° absolute motion over the whole scan). Mean‐frame‐wise displacement (FD, Power, Barnes, Snyder, Schlaggar, & Petersen, 2012) and spike control was employed for data quality assurance and motion control. FD was calculated with the raw functional data for the remaining participants. Importantly, the two groups (controls and cannabis users) did not differ in mean FD (controls: mean ± SD = 0.087 ± 0.032, range from 0.044 to 0.145; cannabis users: mean ± SD = 0.083 ± 0.024, range from 0.042 to 0.142, p = .568, Cohen's d = 0.160). We also defined a spike in head movements as FD > 0.3 mm. Less than 7% scans were regarded as spikes in all of the participants and the two groups did not differ in the number of spikes (control: mean ± SD = 2.427 ± 3.501, range from 0 to 11; user: mean ± SD = 1.750 ± 2.723, range from 0 to 11. p = .445, Cohen's d = 0.214).
2.5. Determining altered striatal connectivity in cannabis dependence using the intrinsic connectivity contrast
To promote an unbiased determination of striatal subregions exhibiting altered functional connectivity related to cannabis dependence, a data‐driven network level approach was implemented (Walpola et al., 2017). To this end, whole‐brain voxel‐to‐voxel connectivity was computed for each striatal voxel using the intrinsic connectivity contrast (ICC), an index similar to “degree” in graph theory but without the need for a correlation threshold, which reflected the average r 2 of a given voxel with all other brain voxels (Martuzzi et al., 2011; Rubinov & Sporns, 2010). The corresponding ICC analysis was implemented by weighting the connections of a given voxel with every other voxel in the brain (restricted to the SPM gray matter mask >0.3) by their r 2 value (Martuzzi et al., 2011), thus both negative and positive correlations will be captured in positive ICC values.
Next, resulting ICC maps were used for discriminating between cannabis‐dependent subjects and healthy controls using a multivoxel pattern analysis (MVPA) with a linear kernel support vector classifier as implemented in LIBSVM (http://www.csie.ntu.edu.tw/~cjlin/libsvm). A searchlight procedure with a three‐voxel radius was used to provide measures of classification accuracy in the neighborhood of each voxel in the striatum mask which was obtained from the Harvard‐Oxford Subcortical Structural Atlases (subcortical HO atlas, Harvard Center for Morphometric Analysis) by combining the accumbens, caudate, putamen, and pallidum masks (thresholded at 0% population probability) that were further masked by the gray matter mask to remove voxels with low gray matter signal. Classification was evaluated by a 10‐fold cross validation during which all participants were randomly assigned to 10 subsamples of five or six participants using MATLAB's cvpartition function. In each iteration, the optimal hyperplane was computed based on the multivariate pattern of ICC values of 47 or 46 participants and evaluated by the excluded five or six participants. The default cost parameter (C = 1) was automatically corrected for unbalanced groups by adjusting weights inversely proportional to group frequencies in the training data. The iteration was repeated 10 times with each group being the testing set once and then the average classification accuracy of each sphere was assigned to the center voxel in the sphere. The training set was scaled to [−1, 1], and the testing set was scaled using the same scaling factors before applying SVM (Hsu, Chang, & Lin, 2003). To avoid a potential bias of train‐test splits, the cross‐validation procedure was repeated five times by producing different splits in each repetition and the resultant maps were averaged to produce a convergent estimation. To test whether the resultant measures exceeded chance level, we used permutation tests to simulate the probability distribution of the classification. Briefly, we randomly shuffled the group labels and recomputed these measures (repeated 5,000 times) to build empirical distributions. The resultant maps were then converted to p values and familywise error (FWE) corrected for multiple comparisons.
2.6. Follow‐up seed‐to‐voxel functional connectivity
Given that ICC is predominantly an exploratory strategy that allows a data‐driven precise mapping of striatal regions characterized by their functional connectivity profile differences between the groups functional connectivity analysis was used to determine the associated networks that contribute to these differences (for a similar approach see Martuzzi et al., 2011; Walpola et al., 2017). Importantly, the rationale for the present analyses was based on addiction animal models that indicate a ventral‐to‐dorsal shift in striatal processing underlying the development of addiction (Everitt & Robbins, 2016). In line with our expectations, the MVPA on the ICC revealed group differences in the global connectivity profiles of ventral and dorsal striatal regions (details see Section 3). Consequently, the major aim of the follow‐up functional connectivity analysis was to determine regions that shift communication with ventral vs dorsal striatal nodes in drug dependence. To this end, two 6‐mm‐radius spheres (masked by the striatum mask and the gray matter mask) centered at the peak voxels derived from the MVPA results (DS and VS) were used as seed regions, and Fisher z‐transformed image maps were then created by calculating the correlation coefficient of each voxel in the brain with the mean time‐series of the seed regions. To determine the shift in ventral vs dorsal striatal networks associated with dependence, the subject‐level seed‐specific Z‐maps were submitted to a two‐way mixed ANOVA with seed (VS and DS) entered as a within‐subject factor and group (controls and cannabis users) entered as a between‐subject factor employed in the FSL's Randomise Tool. Corrections for multiple comparisons were conducted using permutation‐based inferences (10,000 permutations) with Threshold‐Free Clustering Enhancement (TFCE) which provides a strict control while improving replicability (Chen, Lu, & Yan, 2018; Smith & Nichols, 2009). For regions showing significant interaction effects (F test), the mean Fisher z‐transformed correlation coefficients were extracted from the underlying anatomical regions for further post hoc analysis within SPSS.
2.7. Validation using literature‐based VS and DS seeds
In addition, to validate the seed‐to‐voxel functional connectivity results, functional connectivity analyses were then rerun using predefined VS and DS masks. An anatomical VS mask was defined as nucleus accumbens (subcortical HO atlas) with the probability threshold set to 30% (228 voxels in total). Following Tinnermann, Geuter, Sprenger, Finsterbusch, and Büchel (2017), coordinates from different studies (Daw, O'doherty, Dayan, Seymour, & Dolan, 2006; Fitzgerald et al., 2011; Luijten, Schellekens, Kühn, Machielse, & Sescousse, 2017; Schönberg, Daw, Joel, & O'Doherty, 2007; Sweitzer et al., 2016) were averaged to create the mean coordinates of reported DS peak voxels. For those lateralized coordinates we multiplied x coordinates by −1 to cover both hemispheres. The DS mask was then created by drawing two 6‐mm‐radius spheres centered at ±13, 11, 14 (for bilateral DS) and masked by the striatum mask (223 voxels in total). To control for nongray matter signal, including WM and CSF, both VS and DS masks were masked by the gray matter mask.
2.8. Functional characterization of the determined networks
To support the functional characterization of the networks that exhibit a dependence‐associated shift in VS versus DS communication, the NeuroSynth decoder function (http://neurosynth.org/decode) was used to employ a large‐scale automated meta‐analysis map. The top 25 terms (excluding terms for brain regions) ranked by the correlation strengths between the regions exhibiting dependence‐associated alterations and the meta‐analytic map were visualized using word cloud with the size of the font scaled by correlation strength.
3. RESULTS
3.1. Group characteristics
The groups were comparable with respect to important potential confounders including socio‐demographics, attention and the use of licit drugs (Table 1). The lack of significant group differences in attention, mood, and state anxiety (Table 1) argues against strong confounding effects of cannabis withdrawal. As expected cannabis users reported greater lifetime experiences with other illicit drugs (Table 1) than controls. Cannabis use parameters are reported in Table 2. In post‐MRI interviews, none of the subjects reported having fallen asleep during the scan.
Table 1.
Group characteristics and drug use parameters
| Measure | Cannabis users mean (SD) | Controls mean (SD) | p |
|---|---|---|---|
| Age (years) | 24.00 (3.46) | 23.39 (2.86) | .49 |
| Education (years) | 14.50 (11.00–22.00)a | 14.25 (12.00–19.00)a | .75b |
| d2 concentration performance | 196.46 (39.61) | 205.43 (50.57) | .49 |
| STAI‐state | 34.17 (7.70) | 31.07 (7.71) | .16 |
| STAI‐trait | 35.87 (8.40) | 32.64 (7.22) | .14 |
| SIAS | 16.50 (8.00–46.00)a | 16.00 (5.00–33.00)a | .24b |
| Number of smokers | N = 22 | N = 23 | |
| Years of nicotine use | 8.23 (4.49) | 6.24 (3.72) | .11 |
| Cigarettes per day | 8.00 (2–20.00)a | 8.00 (1–30.00)a | .96b |
| Pack‐year | 4.16 (3.59) | 3.42 (3.84) | .50 |
| Alcohol occasions per week | 1.00 (0–4.00)a | 1.00 (0–4.00)a | .74b |
| Alcohol units per week | 5.00 (0–24.00)a | 4.90 (0–18.00)a | .92b |
| PANAS‐positive | 33.00 (16.00–42.00)a | 32.50 (22.00–48.00) | .67b |
| PANAS‐negative | 11.00 (10.00–25.00)a | 10.00 (10.00–14.00)a | .10b |
|
Past ecstasy use Lifetime occasions ecstasy |
N = 15 | N = 2 | – |
| 10.00 (1–75)a | (1, 8) | ||
|
Past cocaine use Lifetime occasions cocaine |
N = 11 | N = 0 | – |
| 6.00 (1–70)a | – | ||
|
Past amphetamine use Lifetime occasions amphetamine |
N = 15 | N = 1 | – |
| 20.00 (1–75)a | 30.00 | ||
|
Past hallucinogen use Lifetime amount hallucinogen |
N = 9 | N = 0 | – |
| 6.00 (1–50)a | – | ||
|
Past opiate use Lifetime occasions opiate |
N = 3 | N = 1 | – |
| (1, 1, 4) | 30.00c | ||
|
Past cannabis use % lifetime cannabis dependence |
N = 24 | N = 22 | – |
| 100% | 0% |
Abbreviations: PANAS = positive and negative affect schedule; SIAS = social interaction anxiety scale; STAI = state–trait anxiety inventory.
Median (range).
Mann–Whitney U test.
Prescription medicinal use.
Table 2.
Cannabis use parameters
| Cannabis use parameter | Cannabis users mean ± SD (range) | Controls mean ± SD (range) |
|---|---|---|
| N | 24 | 22 |
| Age of first cannabis use | 15.17 ± 1.23 (13–17) | 16.36 ± 1.58 (13–19) |
| Days since last cannabis use | 30.00a (14–500) | 670.00a (120–3,000) |
| Maximum frequency of cannabis use (days/month) | 28.08 ± 4.51 (14–30) | – |
| Duration of regular cannabis use (months) | 78.38 ± 36.40 (19–144) | – |
| Lifetime amount of cannabis (g) | 1,616.5a (62–5,786) | 2a (0.1–12) |
Median.
3.2. Intrinsic connectivity contrast
The searchlight MVPA identified brain regions within the striatum that exhibit altered global connectivity patterns in cannabis users compared to controls. Specifically, the groups could be reliably discriminated in two regions that mapped onto the VS (peak voxel coordinates, 10, 12, −10 mapping onto the right nucleus accumbens, accuracy = 84.62%, voxel‐wise FWE‐corrected p = .040) and the DS (peak voxel coordinates, −12, 14, 16 mapping onto the left caudate nucleus, accuracy = 85.38%, voxel‐wise FWE‐corrected p = .026) (Figure 1).
Figure 1.
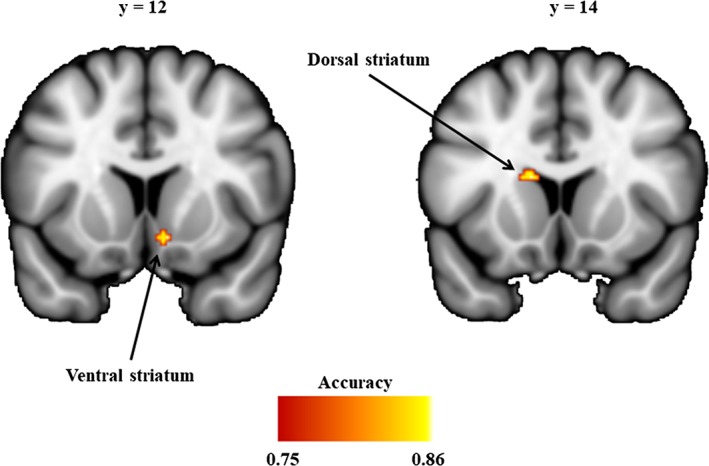
Striatum regions discriminating cannabis‐dependent subjects and nonusing controls. Images are thresholded at accuracy > = 0.75 (approximate p < .001, uncorrected) and cluster size >5 for display purpose
3.3. Follow‐up analyses—seed‐to‐voxel functional connectivity
The main effects of seed reflecting the differential connectivity patterns of the VS versus DS seeds further confirmed the mapping of the MVPA to the VS and DS: (a) the VS seed showed stronger connectivity with ventral regions of the frontal cortex including rostral anterior cingulate cortex (rACC) and orbitofrontal regions, whereas the DS seed exhibited stronger connectivity with the dorsal frontal regions including dorsolateral prefrontal cortex (dmPFC), a pattern resembling previous human and animal research (Di Martino et al., 2008; Haber, 2016). (b) There was a strong overlap between the networks identified for the VS and DS regions determined in the present study and the literature‐based seeds from the validation analysis (Figure 2).
Figure 2.
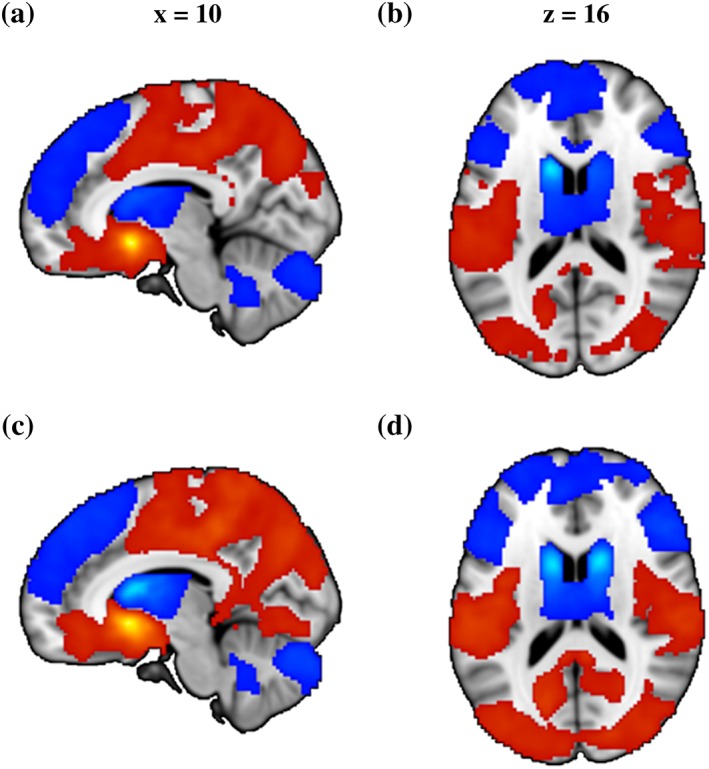
Brain regions showing significant main effect of seed on functional connectivity. (a) and (b) Results from data‐driven seeds. (c) and (d) Results from validation seeds. Red color indicated stronger functional connectivity with VS than DS and blue color indicated stronger functional connectivity with DS than VS. All images were thresholded at p < .05, TFCE corrected
Examining differences in seed‐based functional connectivity between the groups revealed no regions showing a significant main effect of group, arguing against unspecific striatal network changes in cannabis dependence. However, significant group × seed interaction effects were found mirroring a shift in network level communication of the striatal subregions in cannabis dependence. The interaction effects were observed in connectivity with the dmPFC and rACC across both analyses (Table 3 and Figure 3).
Table 3.
Brain regions showing significant group (control vs. user) by seed (VS vs. dorsal striatum) interaction
| x | y | z | (sub)Peak voxel F value | p value (TFCE corrected) | k | BA | Laterality | Label |
|---|---|---|---|---|---|---|---|---|
| Data‐driven seeds | ||||||||
| 2 | 52 | 42 | 24.22 | 0.020 | 376 | 9/32/24 | L/R | dmPFC |
| 10 | 36 | 2 | 23.40 | 0.022 | rACC | |||
| 10 | 30 | 48 | 21.42 | 0.026 | 163 | 8 | R | dmPFC |
| 6 | 44 | 54 | 16.48 | 0.039 | dmPFC | |||
| Validation seeds | ||||||||
| −6 | 44 | 20 | 21.86 | 0.004 | 2,147 | 8/9/10/24/32 | L/R | rACC |
| 12 | 38 | 2 | 27.39 | 0.005 | rACC | |||
| 2 | 52 | 42 | 26.29 | 0.007 | dmPFC | |||
x, y, z = MNI‐coordinates; k = cluster size; BA = Brodmann area; dmPFC = dorso‐medial prefrontal cortex; DS = dorsal striatum; rACC = rostral anterior cingulate cortex; VS = ventral striatum.
Figure 3.
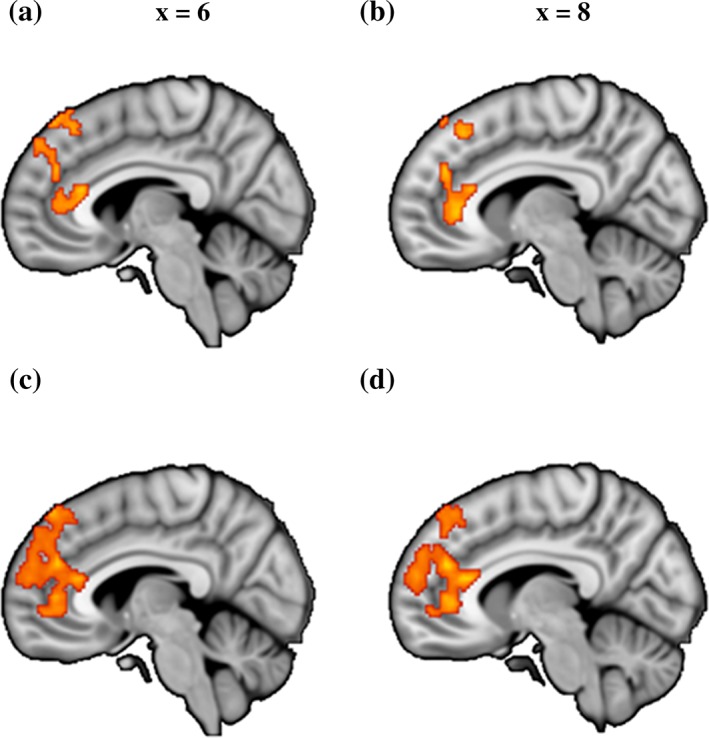
Brain regions showing significant group (control vs user) by seed (ventral vs dorsal striatum) functional connectivity interaction. (a) and (b) Results from data‐driven seeds. (c) and (d) Results from validation seeds. All images are thresholded at p < .05, TFCE corrected
3.4. Post hoc analyses
To further disentangle the direction of the changes using post hoc comparisons, mean Fisher z‐transformed correlation coefficients were extracted from anatomical masks referring to the dmPFC and rACC. Masks of the respective regions were defined using structurally defined regions. To obtain the rACC mask, we first thresholded the anterior cingulate gyrus (HO atlas) at 30% to remove voxels with low probability and the posterior boundary was then delineated as one slice anterior to the coronal plane where the connection of the corpus callosum in each hemisphere was no longer connected (Asami et al., 2008; McCormick et al., 2006). Similarly, to obtain the DMPFC mask, we thresholded the superior frontal gyrus (HO atlas) at 30% probability, excluding voxels greater than 10 mm from the midline of the brain (de la Vega, Chang, Banich, Wager, & Yarkoni, 2016) or posterior to the anterior boundary of supplementary motor area. As displayed in Figure 4a,c, post hoc pairwise comparisons revealed attenuated negative correlation (anticorrelation) between dmPFC and VS (t [50] = 3.23, p = .002, Cohen's d = 0.901), as well as decreased positive connectivity between dmPFC and DS (t [50] = −2.33, p = .024, Cohen's d = 0.649) in cannabis users relative to controls. Post hoc comparisons on the rACC demonstrated increased positive functional connectivity with the VS (t [50] = 2.32, p = .025, Cohen's d = 0.644) and concomitantly decreased positive functional connectivity with the DS (t [50] = −2.69, p = .009, Cohen's d = 0.751) in cannabis users compared to controls.
Figure 4.
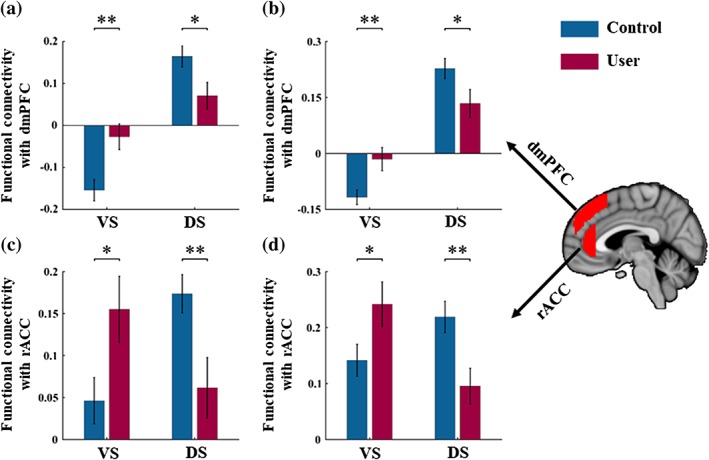
Patterns of functional connectivity associated with a significant group (control vs user) by seed (VS vs. DS) interaction. (a) and (c) Results from data‐driven seeds and (b) and (d) Results from validation seeds. Error bars reflect the SEM. *p < .05; **p < .01; VS: Ventral striatum; DS: Dorsal striatum; dmPFC: Dorsomedial prefrontal cortex; rACC: Rostral anterior cingulate cortex
3.5. Control analyses
Importantly, none of the cannabis users fulfilled a dependence for other illicit drugs, and cannabis was their main drug of abuse. However, in line with an increased prevalence of illicit drug use in addicted populations, the cannabis group had higher experience with recreational use of other drugs, particularly amphetamine (~60%). Given that previous studies reported altered striatal functioning in occasional amphetamine users (Schrantee et al., 2016), we additionally compared the altered neural indices between users with a history of amphetamine use versus no history. This additional control analysis did not reveal significant between group differences (all ps > 0.2), arguing against recreational amphetamine use as driver of the observed difference with the control group. Moreover, the control analysis with the literature‐derived validation seeds revealed comparable results as the data‐driven seeds, further confirming the robustness of the findings (Figure 4b,d).
3.6. Functional characterization of the altered networks
NeuroSynth decoding revealed that terms showing highest correlations with the dmPFC were predominantly referring to control‐related or reasoning‐related processes (Figure 5a), while a predominance of reward‐related or default network processes (Figure 5b) was found for the rACC.
Figure 5.
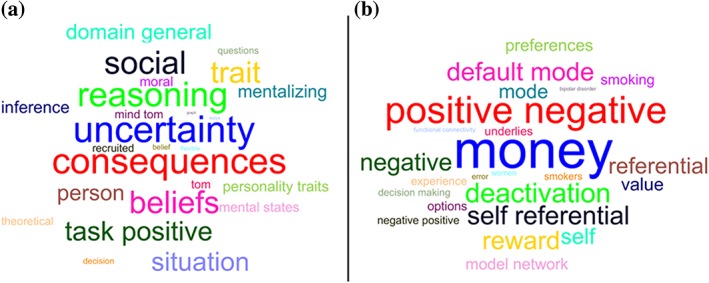
Word cloud showing the correlation strength between the ROIs and the meta‐analytic map. (a) The top 25 terms associated with dmPFC based on Neurosynth decoding and font size represents relative correlation strength of that term to dmPFC. (b) The same information for rACC
3.7. Association between cannabis use parameters and neural alterations
Exploring associations between neural alterations and the age of onset of cannabis use, cumulative exposure, and duration of abstinence (days since last use) did not reveal significant associations (all ps > 0.1). Corresponding exploratory analyses with nicotine use parameters did not reveal significant associations (all ps > 0.2).
4. DISCUSSION
The present study combined an innovative network level approach with resting state fMRI to determine regional‐specific striatal alterations and associated changes in functional communication in abstinent cannabis‐dependent subjects. In line with animal and human data that emphasize the specific contributions of the VS and DS, subregion‐specific differences between cannabis users and controls mapped to the nucleus accumbens and the caudate nucleus. Cannabis users displayed a relative shift between VS and DS communication with frontolimbic regions that have been consistently involved in reward processing (rACC) and executive/regulatory functions (dmPFC). Aberrant frontostriatal functional connectivity in the abstinent cannabis users was observed in the absence of a significant main effect in the comparison with the control group. This observation is partly in line with a previous study reporting that altered frontostriatal connectivity in chronic cannabis users normalizes during 28 days of abstinence (Blanco‐Hinojo et al., 2017). However, comparing the shift between the ventral and dorsal striatal functional networks in the present study revealed evidence for a persisting imbalance in the subregion specific frontostriatal circuits. Together, these findings argue against unspecific long‐term striatal changes in cannabis dependence but rather emphasize the importance of both, specific adaptations in dorsal and ventral subregions and a relative shift between the regions.
Moreover, employing a fully data‐driven network approach that combines the ICC with a pattern classification demonstrated that the general connectivity patterns of the VS and DS can reliably discriminate between cannabis‐dependent individuals and controls. As such, the present data are in line with previous studies reporting altered VS and DS activity in chronic cannabis users during drug cue reactivity, reward processing (Weinstein et al., 2016; Wrege et al., 2014; Yanes et al., 2018) and social decision making (Gilman, 2017).
Furthermore, our data emphasize the important contribution of striatal maladaptations in addiction which might reflect a common pathological pathway across addictive disorders. In accordance with a plethora of previous animal and human research suggesting a functional differentiation of the striatum along a dorsal to ventral gradient (Delgado, 2007) and a specific contribution of the striatal subregions to the transition to addiction (Everitt & Robbins, 2016), differences in the intrinsic function of both subregions were demonstrated. Specifically, the nucleus accumbens, a core reward processing node (Delgado, 2007), and the caudate nucleus, strongly engaged in cognitive processes and executive control (Haber, 2016), differed between both groups.
The mapping of the identified regions and their proposed functional contributions was further confirmed by the stronger connectivity of the VS with reward‐related limbic and (orbito‐)frontal regions, whereas the DS showed a stronger functional interaction with frontal regions such as the dorsolateral prefrontal cortex that are critically involved in cognitive and regulatory processes. Examining differences in the connectivity profiles of the identified VS and DS regions revealed a relative shift in the connectivity with the dmPFC, a region that has been implicated in regulatory downstream control over the striatum (Kragel et al., 2018; Robbins et al., 2012) and the rACC, a region engaged in reward processing via up‐stream signaling of the VS (Haber & Knutson, 2010). As such, the present findings are in accordance with recent quantitative and qualitative reviews suggesting alterations in networks engaged in cognitive control and reward processing in chronic cannabis users (Weinstein et al., 2016; Wrege et al., 2014; Yanes et al., 2018).
More specifically, both striatal regions demonstrated an uncoupling with dmPFC regulatory regions which may reflect deficient inhibitory control which is consistently observed in cannabis users (Wrege et al., 2014). The altered caudate‐dmPFC pathway suggests specific deficits in stop‐signal inhibition (Robbins et al., 2012), an inhibitory domain that has been shown to be deficient across addictive disorders (Acikalin, Gorgolewski, & Poldrack, 2017; Morein‐Zamir, Jones, Bullmore, Robbins, & Ersche, 2013).
By contrast, in cannabis‐dependent subjects the rACC exhibited increased connectivity with the VS and concomitantly decreased connectivity with the DS. Increased connectivity in the nucleus accumbens—ACC pathway in response to drug cues has previously been identified as a specific marker for dependent versus nondependent cannabis users (Filbey & Dunlop, 2014). Activation in the rACC and the adjacent ventromedial PFC has been found to reliably reflect subjective value and reward representations (Acikalin et al., 2017).
The pathway with the VS has been associated with the degree of delayed discounting‐associated inhibitory control (Li et al., 2013; see also circuit model by Robbins et al. (2012)) and increased reward‐seeking impulsivity in addictive disorders (Fineberg et al., 2010). Consistent with the present results on decreased DS‐rACC connectivity, a previous study reported attenuated connectivity in this pathway in nicotine‐dependent individuals (Sweitzer et al., 2016). This has been interpreted as disengagement of habitual behavior in favor of conscious perception of craving that promotes drug‐seeking behavior.
Together, the pattern of increased connectivity in the VS‐rACC pathway engaged in reward‐seeking impulsivity and concomitantly decreased striatal coupling with dmPFC regulatory regions may reflect a hyperactive striatal “impulsive” system and a hypoactive prefrontal inhibitory (“reflective”) system as proposed by dual‐process models of addiction (Jentsch & Taylor, 1999; Noël et al., 2013). The present findings indicate that the imbalalance between the systems extends to the task‐free state, suggesting that changes in the intrinsic organization of the striato‐frontal circuitries may promote loss of behavioral control in interaction with external stimuli such as drug‐associated cues.
Previous animal and human research indicate that alterations in striato‐frontal circuits (Becker et al., 2015; Ersche et al., 2012; George & Koob, 2010) and associated functional domains such as deficient regulatory control and increased impulsivity (Dalley, Everitt, & Robbins, 2011; Ersche, Turton, Pradhan, Bullmore, & Robbins, 2010) precede the escalation of drug use and represent predisposing risk factors for addiction. The retrospective design of the present study does not allow to disentangle predisposing alterations from neuroplastic changes that accompany the transition to cannabis dependence. As such, the observed intrinsic striatal alterations may represent predisposing factors that render individuals vulnerable for the development of cannabis dependence.
The present findings need to be considered in the context of limitations. (a) To facilitate the determination of elementary frontostriatal alterations in cannabis dependence the study focused on male participants. The rationale was based on an increasing number of studies reporting modulatory effects of menstrual cycle phase on frontostriatal processing and associated functions (e.g.,Diekhof & Ratnayake, 2016; Dreher et al., 2007) as well as recent studies suggesting that addiction‐related alterations in these circuits vary as a function of menstrual cycle (Franklin et al., 2015; Wetherill, Jagannathan, et al., 2016; for cannabis see Wiers et al., 2016). The focus on a male sample allowed to control for these potential confounding variables, however, comes at a cost of a limited generalization of the present findings to cannabis‐dependent women. In the context of emerging evidence for a higher sensitivity of women to the adverse effects of chronic cannabis use on the brain, including blunted dopaminergic reactivity in the striatum and impaired frontal activity (Paola Castelli et al., 2014; Wiers et al., 2016), future studies need to consider to explicitly examine sex differences, (b) due to the high prevalence of tobacco couse in cannabis addiction both samples included a high proportion of tobacco smokers, although the groups were matched with respect to tobacco‐use parameters complex interactions between cannabis and tobacco use may have contributed to the present findings, (c) cannabis users that were interested in participation but did not remain abstinent during the 28‐day period were not included in the study, the sample thus may represent a subgroup with relative low degrees of cannabis dependence, and (d) the cannabis sample size was relatively small which limited ability to assess within‐group clinical correlations.
Together, the present findings suggest a shift in the balance between DS and VS control of behavior in cannabis dependence. These alterations may reflect predisposing risk factors for the development of cannabis dependence or neuroplasticity changes that accompany the transition to dependence. Similar alterations have been previously validated in comprehensive animal models of addiction and may promote the loss of control central to addictive behavior.
CONFLICT OF INTERESTS
The authors declare no potential conflict of interests.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (NSFC, 91632117; 31530032), the Fundamental Research Funds for Central Universities (ZYGX2015Z002); the Science, Innovation and Technology Department of the Sichuan Province (2018JY0001) and the German Research Foundation (DFG, grant: BE5465/2‐1, HU1302/4‐1).
Zhou F, Zimmermann K, Xin F, et al. Shifted balance of dorsal versus ventral striatal communication with frontal reward and regulatory regions in cannabis‐dependent males. Hum Brain Mapp. 2018;39:5062–5073. 10.1002/hbm.24345
Funding information National Natural Science Foundation of China, Grant/Award Number: 91632117, 31530032; Deutsche Forschungsgemeinschaft, Grant/Award Number: BE5465/2‐1, HU1302/4‐1; Fundamental Research Funds for Central Universities , Grant/Award Number: ZYGX2015Z002; Science, Innovation and Technology Department of the Sichuan Province, Grant/Award Number: 2018JY0001
Feng Zhou and Kaeli Zimmermann contributed equally to this work.
REFERENCES
- Acikalin, M. Y. , Gorgolewski, K. J. , & Poldrack, R. A. (2017). A coordinate‐based meta‐analysis of overlaps in regional specialization and functional connectivity across subjective value and default mode networks. Frontiers in Neuroscience, 11, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson, J. L. , Jenkinson, M. , & Smith, S. (2010). Non‐linear registration, aka spatial normalisation. FMRIB technical report TR07JA2
- Asami, T. , Hayano, F. , Nakamura, M. , Yamasue, H. , Uehara, K. , Otsuka, T. , … Hirayasu, Y. (2008). Anterior cingulate cortex volume reduction in patients with panic disorder. Psychiatry and Clinical Neurosciences, 62(3), 322–330. [DOI] [PubMed] [Google Scholar]
- Becker, B. , Wagner, D. , Gouzoulis‐Mayfrank, E. , Spuentrup, E. , & Daumann, J. (2010). The impact of early‐onset cannabis use on functional brain correlates of working memory. Progress in Neuro‐Psychopharmacology and Biological Psychiatry, 34(6), 837–845. [DOI] [PubMed] [Google Scholar]
- Becker, B. , Wagner, D. , Koester, P. , Tittgemeyer, M. , Mercer‐Chalmers‐Bender, K. , Hurlemann, R. , … Daumann, J. (2015). Smaller amygdala and medial prefrontal cortex predict escalating stimulant use. Brain, 138(7), 2074–2086. [DOI] [PubMed] [Google Scholar]
- Blanco‐Hinojo, L. , Pujol, J. , Harrison, B. J. , Macià, D. , Batalla, A. , Nogué, S. , … Martín‐Santos, R. (2017). Attenuated frontal and sensory inputs to the basal ganglia in cannabis users. Addiction Biology, 22(4), 1036–1047. [DOI] [PubMed] [Google Scholar]
- Brand, M. , Young, K. S. , Laier, C. , Wölfling, K. , & Potenza, M. N. (2016). Integrating psychological and neurobiological considerations regarding the development and maintenance of specific internet‐use disorders: An interaction of person‐affect‐cognition‐execution (I‐PACE) model. Neuroscience & Biobehavioral Reviews, 71, 252–266. [DOI] [PubMed] [Google Scholar]
- Brickenkamp, R. , & Zillmer, E. (1998). The d2 test of attention. Hogrefe & Huber Seattle, WA. [Google Scholar]
- Chen, X. , Lu, B. , & Yan, C.‐G. (2018). Reproducibility of R‐fMRI metrics on the impact of different strategies for multiple comparison correction and sample sizes. Human Brain Mapping, 39(1), 300–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copersino, M. L. , Boyd, S. J. , Tashkin, D. P. , Huestis, M. A. , Heishman, S. J. , Dermand, J. C. , … Gorelick, D. A. (2006). Cannabis withdrawal among non‐treatment‐seeking adult cannabis users. The American Journal on Addictions, 15(1), 8–14. [DOI] [PubMed] [Google Scholar]
- Crawford, J. R. , & Henry, J. D. (2004). The positive and negative affect schedule (PANAS): Construct validity, measurement properties and normative data in a large non‐clinical sample. British Journal of Clinical Psychology, 43(3), 245–265. [DOI] [PubMed] [Google Scholar]
- Crean, R. D. , Crane, N. A. , & Mason, B. J. (2011). An evidence based review of acute and long‐term effects of cannabis use on executive cognitive functions. Journal of Addiction Medicine, 5(1), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley, J. W. , Everitt, B. J. , & Robbins, T. W. (2011). Impulsivity, compulsivity, and top‐down cognitive control. Neuron, 69(4), 680–694. [DOI] [PubMed] [Google Scholar]
- Daw, N. D. , O'doherty, J. P. , Dayan, P. , Seymour, B. , & Dolan, R. J. (2006). Cortical substrates for exploratory decisions in humans. Nature, 441(7095), 876–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Vega, A. , Chang, L. J. , Banich, M. T. , Wager, T. D. , & Yarkoni, T. (2016). Large‐scale meta‐analysis of human medial frontal cortex reveals tripartite functional organization. Journal of Neuroscience, 36(24), 6553–6562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado, M. R. (2007). Reward‐related responses in the human striatum. Annals of the New York Academy of Sciences, 1104(1), 70–88. [DOI] [PubMed] [Google Scholar]
- Di Martino, A. , Kelly, C. , Grzadzinski, R. , Zuo, X.‐N. , Mennes, M. , Mairena, M. A. , … Milham, M. P. (2011). Aberrant striatal functional connectivity in children with autism. Biological Psychiatry, 69(9), 847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino, A. , Scheres, A. , Margulies, D. S. , Kelly, A. , Uddin, L. Q. , Shehzad, Z. , … Milham, M. P. (2008). Functional connectivity of human striatum: A resting state FMRI study. Cerebral Cortex, 18(12), 2735–2747. [DOI] [PubMed] [Google Scholar]
- Diekhof, E. K. , & Ratnayake, M. (2016). Menstrual cycle phase modulates reward sensitivity and performance monitoring in young women: Preliminary fMRI evidence. Neuropsychologia, 84, 70–80. [DOI] [PubMed] [Google Scholar]
- Dreher, J.‐C. , Schmidt, P. J. , Kohn, P. , Furman, D. , Rubinow, D. , & Berman, K. F. (2007). Menstrual cycle phase modulates reward‐related neural function in women. Proceedings of the National Academy of Sciences, 104(7), 2465–2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Monitoring Centre for Drugs and Drug Addiction . (2008). Annual report 2008: the state of the drugs problem in Europe. Office for Official Publications of the European Communities, Luxembourg.
- Ersche, K. D. , Jones, P. S. , Williams, G. B. , Turton, A. J. , Robbins, T. W. , & Bullmore, E. T. (2012). Abnormal brain structure implicated in stimulant drug addiction. Science, 335(6068), 601–604. [DOI] [PubMed] [Google Scholar]
- Ersche, K. D. , Turton, A. J. , Pradhan, S. , Bullmore, E. T. , & Robbins, T. W. (2010). Drug addiction endophenotypes: Impulsive versus sensation‐seeking personality traits. Biological Psychiatry, 68(8), 770–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt, B. J. , & Robbins, T. W. (2016). Drug addiction: Updating actions to habits to compulsions ten years on. Annual Review of Psychology, 67, 23–50. [DOI] [PubMed] [Google Scholar]
- Filbey, F. M. , & Dunlop, J. (2014). Differential reward network functional connectivity in cannabis dependent and non‐dependent users. Drug and Alcohol Dependence, 140, 101–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey, F. M. , Dunlop, J. , Ketcherside, A. , Baine, J. , Rhinehardt, T. , Kuhn, B. , … Alvi, T. (2016). fMRI study of neural sensitization to hedonic stimuli in long‐term, daily cannabis users. Human Brain Mapping, 37(10), 3431–3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fineberg, N. A. , Potenza, M. N. , Chamberlain, S. R. , Berlin, H. A. , Menzies, L. , Bechara, A. , … Hollander, E. (2010). Probing compulsive and impulsive behaviors, from animal models to endophenotypes: A narrative review. Neuropsychopharmacology, 35(3), 591–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald, K. D. , Welsh, R. C. , Stern, E. R. , Angstadt, M. , Hanna, G. L. , Abelson, J. L. , & Taylor, S. F. (2011). Developmental alterations of frontal‐striatal‐thalamic connectivity in obsessive‐compulsive disorder. Journal of the American Academy of Child & Adolescent Psychiatry , 50(9), 938‐948. e933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin, T. R. , Jagannathan, K. , Wetherill, R. R. , Johnson, B. , Kelly, S. , Langguth, J. , … Childress, A. R. (2015). Influence of menstrual cycle phase on neural and craving responses to appetitive smoking cues in naturally cycling females. Nicotine & Tobacco Research, 17(4), 390–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganzer, F. , Bröning, S. , Kraft, S. , Sack, P.‐M. , & Thomasius, R. (2016). Weighing the evidence: A systematic review on long‐term neurocognitive effects of cannabis use in abstinent adolescents and adults. Neuropsychology Review, 26(2), 186–222. [DOI] [PubMed] [Google Scholar]
- George, O. , & Koob, G. F. (2010). Individual differences in prefrontal cortex function and the transition from drug use to drug dependence. Neuroscience & Biobehavioral Reviews, 35(2), 232–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman, J. M. (2017). Neural correlates of social influence among cannabis users. Current Addiction Reports, 4(2), 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein, R. Z. , & Volkow, N. D. (2011). Dysfunction of the prefrontal cortex in addiction: Neuroimaging findings and clinical implications. Nature Reviews Neuroscience, 12(11), 652–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber, S. N. (2016). Corticostriatal circuitry. Dialogues in Clinical Neuroscience, 1, 7–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber, S. N. , & Knutson, B. (2010). The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology, 35(1), 4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirvonen, J. , Goodwin, R. , Li, C.‐T. , Terry, G. , Zoghbi, S. , Morse, C. , … Innis, R. (2012). Reversible and regionally selective downregulation of brain cannabinoid CB 1 receptors in chronic daily cannabis smokers. Molecular Psychiatry, 17(6), 642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu, C.‐W. , Chang, C.‐C. , & Lin, C.‐J. (2003). A practical guide to support vector classification. [Google Scholar]
- Jager, G. , Block, R. I. , Luijten, M. , & Ramsey, N. F. (2013). Tentative evidence for striatal hyperactivity in adolescent cannabis‐using boys: A cross‐sectional multicenter fMRI study. Journal of Psychoactive Drugs, 45(2), 156–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson, M. , Bannister, P. , Brady, M. , & Smith, S. (2002). Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage, 17(2), 825–841. [DOI] [PubMed] [Google Scholar]
- Jenkinson, M. , & Smith, S. (2001). A global optimisation method for robust affine registration of brain images. Medical Image Analysis, 5(2), 143–156. [DOI] [PubMed] [Google Scholar]
- Jentsch, J. D. , & Taylor, J. R. (1999). Impulsivity resulting from frontostriatal dysfunction in drug abuse: Implications for the control of behavior by reward‐related stimuli. Psychopharmacology, 146(4), 373–390. [DOI] [PubMed] [Google Scholar]
- Kragel, P. A. , Kano, M. , Van Oudenhove, L. , Ly, H. G. , Dupont, P. , Rubio, A. , … Gianaros, P. J. (2018). Generalizable representations of pain, cognitive control, and negative emotion in medial frontal cortex. Nature Neuroscience, 21(2), 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, N. , Ma, N. , Liu, Y. , He, X.‐S. , Sun, D.‐L. , Fu, X.‐M. , … Zhang, D.‐R. (2013). Resting‐state functional connectivity predicts impulsivity in economic decision‐making. Journal of Neuroscience, 33(11), 4886–4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzetti, V. , Solowij, N. , & Yücel, M. (2016). The role of cannabinoids in neuroanatomic alterations in cannabis users. Biological Psychiatry, 79(7), e17–e31. [DOI] [PubMed] [Google Scholar]
- Luijten, M. , Schellekens, A. F. , Kühn, S. , Machielse, M. W. , & Sescousse, G. (2017). Disruption of reward processing in addiction: An image‐based meta‐analysis of functional magnetic resonance imaging studies. JAMA Psychiatry, 74(4), 387–398. [DOI] [PubMed] [Google Scholar]
- Martuzzi, R. , Ramani, R. , Qiu, M. , Shen, X. , Papademetris, X. , & Constable, R. T. (2011). A whole‐brain voxel based measure of intrinsic connectivity contrast reveals local changes in tissue connectivity with anesthetic without a priori assumptions on thresholds or regions of interest. NeuroImage, 58(4), 1044–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattick, R. P. , & Clarke, J. C. (1998). Development and validation of measures of social phobia scrutiny fear and social interaction anxiety. Behaviour Research and Therapy, 36(4), 455–470. [DOI] [PubMed] [Google Scholar]
- McCormick, L. M. , Ziebell, S. , Nopoulos, P. , Cassell, M. , Andreasen, N. C. , & Brumm, M. (2006). Anterior cingulate cortex: An MRI‐based parcellation method. NeuroImage, 32(3), 1167–1175. [DOI] [PubMed] [Google Scholar]
- Morein‐Zamir, S. , Jones, P. S. , Bullmore, E. T. , Robbins, T. W. , & Ersche, K. D. (2013). Prefrontal hypoactivity associated with impaired inhibition in stimulant‐dependent individuals but evidence for hyperactivation in their unaffected siblings. Neuropsychopharmacology, 38(10), 1945–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morein‐Zamir, S. , & Robbins, T. W. (2015). Fronto‐striatal circuits in response‐inhibition: Relevance to addiction. Brain Research, 1628, 117–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noël, X. , Brevers, D. , & Bechara, A. (2013). A neurocognitive approach to understanding the neurobiology of addiction. Current Opinion in Neurobiology, 23(4), 632–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paola Castelli, M. , Fadda, P. , Casu, A. , Sabrina Spano, M. , Casti, A. , Fratta, W. , & Fattore, L. (2014). Male and female rats differ in brain cannabinoid CB1 receptor density and function and in behavioural traits predisposing to drug addiction: Effect of ovarian hormones. Current Pharmaceutical Design, 20(13), 2100–2113. [DOI] [PubMed] [Google Scholar]
- Postuma, R. B. , & Dagher, A. (2006). Basal ganglia functional connectivity based on a meta‐analysis of 126 positron emission tomography and functional magnetic resonance imaging publications. Cerebral Cortex, 16(10), 1508–1521. [DOI] [PubMed] [Google Scholar]
- Power, J. D. , Barnes, K. A. , Snyder, A. Z. , Schlaggar, B. L. , & Petersen, S. E. (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage, 59(3), 2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruim, R. H. , Mennes, M. , Buitelaar, J. K. , & Beckmann, C. F. (2015). Evaluation of ICA‐AROMA and alternative strategies for motion artifact removal in resting state fMRI. NeuroImage, 112, 278–287. [DOI] [PubMed] [Google Scholar]
- Pruim, R. H. , Mennes, M. , van Rooij, D. , Llera, A. , Buitelaar, J. K. , & Beckmann, C. F. (2015). ICA‐AROMA: A robust ICA‐based strategy for removing motion artifacts from fMRI data. NeuroImage, 112, 267–277. [DOI] [PubMed] [Google Scholar]
- Robbins, T. W. , Gillan, C. M. , Smith, D. G. , de Wit, S. , & Ersche, K. D. (2012). Neurocognitive endophenotypes of impulsivity and compulsivity: Towards dimensional psychiatry. Trends in Cognitive Sciences, 16(1), 81–91. [DOI] [PubMed] [Google Scholar]
- Rubinov, M. , & Sporns, O. (2010). Complex network measures of brain connectivity: Uses and interpretations. NeuroImage, 52(3), 1059–1069. [DOI] [PubMed] [Google Scholar]
- Schönberg, T. , Daw, N. D. , Joel, D. , & O'Doherty, J. P. (2007). Reinforcement learning signals in the human striatum distinguish learners from nonlearners during reward‐based decision making. Journal of Neuroscience, 27(47), 12860–12867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrantee, A. , Ferguson, B. , Stoffers, D. , Booij, J. , Rombouts, S. , & Reneman, L. (2016). Effects of dexamphetamine‐induced dopamine release on resting‐state network connectivity in recreational amphetamine users and healthy controls. Brain Imaging and Behavior, 10(2), 548–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner, A. M. , & Dunn, M. E. (2012). Residual effects of cannabis use on neurocognitive performance after prolonged abstinence: A meta‐analysis. Experimental and Clinical Psychopharmacology, 20(5), 420–429. [DOI] [PubMed] [Google Scholar]
- Smith, S. M. (2002). Fast robust automated brain extraction. Human Brain Mapping, 17(3), 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, S. M. , & Nichols, T. E. (2009). Threshold‐free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage, 44(1), 83–98. [DOI] [PubMed] [Google Scholar]
- Spielberger, C. D. , Gorsuch, R. L. , Lushene, R. , Vagg, P. R. , & Jacobs, G. A. (1983). Manual for the State‐Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- Sweitzer, M. M. , Geier, C. F. , Addicott, M. A. , Denlinger, R. , Raiff, B. R. , Dallery, J. , … Donny, E. C. (2016). Smoking abstinence‐induced changes in resting state functional connectivity with ventral striatum predict lapse during a quit attempt. Neuropsychopharmacology, 41(10), 2521–2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinnermann, A. , Geuter, S. , Sprenger, C. , Finsterbusch, J. , & Büchel, C. (2017). Interactions between brain and spinal cord mediate value effects in nocebo hyperalgesia. Science, 358(6359), 105–108. [DOI] [PubMed] [Google Scholar]
- Vandevenne, M. , Vandenbussche, H. , & Verstraete, A. (2000). Detection time of drugs of abuse in urine. Acta Clinica Belgica, 55(6), 323–333. [DOI] [PubMed] [Google Scholar]
- Volkow, N. D. , Swanson, J. M. , Evins, A. E. , DeLisi, L. E. , Meier, M. H. , Gonzalez, R. , … Baler, R. (2016). Effects of cannabis use on human behavior, including cognition, motivation, and psychosis: A review. JAMA Psychiatry, 73(3), 292–297. [DOI] [PubMed] [Google Scholar]
- Walpola, I. C. , Nest, T. , Roseman, L. , Erritzoe, D. , Feilding, A. , Nutt, D. J. , & Carhart‐Harris, R. L. (2017). Altered insula connectivity under MDMA. Neuropsychopharmacology, 42(11), 2152–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein, A. , Livny, A. , & Weizman, A. (2016). Brain imaging studies on the cognitive, pharmacological and neurobiological effects of cannabis in humans: Evidence from studies of adult users. Current Pharmaceutical Design, 22(42), 6366–6379. [DOI] [PubMed] [Google Scholar]
- Wetherill, R. R. , Hager, N. , Jagannathan, K. , Mashhoon, Y. , Pater, H. , Childress, A. R. , & Franklin, T. R. (2016). Early versus late onset of cannabis use: Differences in striatal response to cannabis cues. Cannabis and Cannabinoid Research, 1(1), 229–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill, R. R. , Jagannathan, K. , Hager, N. , Maron, M. , & Franklin, T. R. (2016). Influence of menstrual cycle phase on resting‐state functional connectivity in naturally cycling, cigarette‐dependent women. Biology of Sex Differences, 7(1), 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiers, C. E. , Shokri‐Kojori, E. , Wong, C. T. , Abi‐Dargham, A. , Demiral, Ş. B. , Tomasi, D. , … Volkow, N. D. (2016). Cannabis abusers show hypofrontality and blunted brain responses to a stimulant challenge in females but not in males. Neuropsychopharmacology, 41(10), 2596–2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrege, J. , Schmidt, A. , Walter, A. , Smieskova, R. , Bendfeldt, K. , Radue, E.‐W. , … Borgwardt, S. (2014). Effects of cannabis on impulsivity: A systematic review of neuroimaging findings. Current Pharmaceutical Design, 20(13), 2126–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanes, J. A. , Riedel, M. C. , Ray, K. L. , Kirkland, A. E. , Bird, R. T. , Boeving, E. R. , . . . Laird, A. R. (2018). Neuroimaging meta‐analysis of cannabis use studies reveals convergent functional alterations in brain regions supporting cognitive control and reward processing. Journal of Psychopharmacology, 32(3), 283–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. , Brady, M. , & Smith, S. (2001). Segmentation of brain MR images through a hidden Markov random field model and the expectation‐maximization algorithm. IEEE Transactions on Medical Imaging, 20(1), 45–57. [DOI] [PubMed] [Google Scholar]
- Zimmermann, K. , Walz, C. , Derckx, R. T. , Kendrick, K. M. , Weber, B. , Dore, B. , … Becker, B. (2017). Emotion regulation deficits in regular marijuana users. Human Brain Mapping, 38(8), 4270–4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann, K. , Yao, S. , Heinz, M. , Zhou, F. , Dau, W. , Banger, M. , … Becker, B. (2017). Altered orbitofrontal activity and dorsal striatal connectivity during emotion processing in dependent marijuana users after 28 days of abstinence. Psychopharmacology, 235(3), 849–859. [DOI] [PubMed] [Google Scholar]


