Abstract
The cortical and subcortical neural correlates underlying item and order information in verbal short‐term memory (STM) were investigated by means of digit span in 29 patients with direct electrical stimulation during awake surgery for removal of a neoplastic lesion. Stimulation of left Broca's area interfered with span, producing significantly more item than order errors, as compared to the stimulation of the supramarginal/angular gyrus, which also interfered with span but, conversely, produced more order than item errors. Similarly, stimulation of the third segment of the left superior longitudinal fasciculus (SLF‐III), also known as anterior segment of the arcuate fascicle (AF), produced more order than item errors. Therefore, we obtained two crucial results: first, we were able to distinguish between content and order information storage. Second, we demonstrated that the SLF‐III is involved in transferring order information from Geschwind's area to Broca's area. In a few patients, we demonstrated that also order information of nonverbal material was disrupted by left supramarginal gyrus stimulation. Order information is thus likely stored in the supramarginal gyrus, possibly independently from the nature of the material. Hum Brain Mapp 38:3011–3024, 2017. © 2017 Wiley Periodicals, Inc.
Keywords: memory short‐term, neurosurgery, direct electric stimulation, inferior parietal lobule, arcuate
INTRODUCTION
Verbal or phonological short‐term memory (STM) is the most extensively investigated component of working memory [Baddeley, 1996]. A popular model of phonological STM distinguishes between a phonological short‐term store, where memory traces fade after about 2 s, and an articulatory process of rehearsal, which is capable of refreshing the memory trace, preventing its decay [Baddeley, 1990].
Current knowledge of the neural correlates of phonological STM comes from traditional anatomoclinical studies in brain‐damaged patients (see Vallar and Papagno [2002] for a review), positron emission tomography [Awh et al., 1995; Paulesu et al., 1993; but see Poeppel, 1996], and fMRI studies [Henson et al., 2000], and from repetitive transcranial magnetic stimulation [Romero et al., 2006]. All of them support the hypothesis that the phonological short‐term store and the rehearsal process depend on the activity of two discrete regions in the left hemisphere: the inferior parietal lobule (more specifically, the supramarginal gyrus, Brodmann's area [BA] 40, but also the angular gyrus, BA 39, see for example Newhart et al. [2012], Vallar et al. [1997], Warrington et al. [1971]) and the inferior frontal operculum (BA 44 and BA 6, but also BA 45), respectively. More recently, a study performed on epileptic patients [Zamora et al., 2016] suggested an involvement of the lateral temporal cortex in active rehearsal. These results deserve confirmation, considering the type of population (intractable temporal lobe epilepsy) and the inclusion of left and right brain‐damaged patients. Recently, Baddeley [2007] has suggested a possible mapping of the arcuate fascicle (AF) onto the phonological loop, following Catani et al. [2005]'s model: the long segment connects Wernicke's area, where the phonological analysis occurs, directly to Broca's area, the neural correlate of the phonological output buffer; the third segment of the superior longitudinal segment (SLF‐III), also referred to as the anterior segment of the AF indirect pathway, conveys information from the supramarginal gyrus (phonological short‐term store) to Broca's area (phonological output buffer), while the posterior segment of the AF indirect pathway transfers the output of Wernicke's area (phonological analysis) to the supramarginal gyrus (phonological short‐term store) (Fig. 1). However, there is not yet direct evidence of such architecture.
Figure 1.
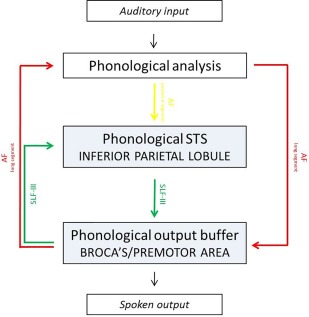
Suggested mapping of verbal short‐term memory onto the brain following Catani et al. [2005]. [Color figure can be viewed at http://wileyonlinelibrary.com]
In STM tasks, items are typically presented in an ordered sequence to be recalled in the same order. Hence, retention of order information is a critical component of STM. Experimental studies on verbal STM suggest that item and order information are stored separately [Bjork and Healy, 1974]. Indeed, phonological similarity improves item recall [Hulme et al., 1997; Saint‐Aubin and Poirier, 2000], but increases order errors (see Romani et al. [2008] for the role of the phonological representations in the retention of order). Moreover, item recall is more influenced by linguistic knowledge than order recall (see for example Saint‐Aubin and Poirier [1999]). More recent work suggests that serial order information can be processed using different codes such as item‐to‐item association, or start‐anchored or end‐anchored position (see for example Fischer‐Baum and McCloskey [2015]) or depending on their rhythm in spoken sequences [Hartley et al., 2016], and, finally, recall of temporal order information is more accurate with congruent spatial order information [Fischer‐Baum and Benjamin, 2014]. All these different codes for serial information could depend on different neural substrates, although this does not seem an economical solution.
As reported above, the left inferior parietal region is the most consistently activated region during verbal STM tasks (see also Majerus et al. [2006]), but its precise role is still controversial. It has been considered either a specific store for serial order information or a structure responsible for a more general function of attentional localization. As regard to the first hypothesis, listening to irrelevant speech during list presentation affects order information more than item information [Henson et al., 2003]. This disruptive effect occurs because auditory input has direct and obligatory access to the phonological short‐term store [Baddeley and Salamé, 1986; Papagno et al., 2008], whose neural correlates are represented in the left inferior parietal lobule, suggesting that the inferior parietal lobule is involved in maintaining serial order information. In contrast, Majerus et al. [2006] demonstrated, inside the parietal lobe, specific activation of the intraparietal sulcus (together with other different cortical areas, see below) in both order and item STM tasks; this finding led them to conclude that this structure acts as an attentional modulator of distant neural networks, which are specialized in processing order and language representations. In a following study, Majerus et al. [2009] investigated the neural specificity for verbal and visual STM tasks and found that this was restricted to sensory networks underlying the processing and storage of modality‐specific item information, while attentional and serial order processing were modality independent. However, as the authors acknowledge, verbal stimuli were presented visually, potentially enhancing similarity between order networks involved in verbal and visual STM conditions.
A direct test of the role of the inferior parietal region and of the AF (as suggested by Catani et al. [2005], see above) in verbal STM would be to assess STM errors during direct electrical stimulation (DES) in awake surgery with a spatiotemporal resolution unmatched by other methods [Desmurget et al., 2013]. This technique allows brain mapping with a spatial resolution about 1 cm2 [Ojemann et al., 1989]. During brain surgery for tumor resection, it is a common and recommended clinical practice to awaken patients to assess the functional role of selected brain regions, to maximize the extent of the resection while sparing the eloquent functions, generally with a particular attention to language and the motor‐sensory system [De Witt Hamer et al., 2012; Soffietti et al., 2010]. In this study, patients were asked to perform a digit span task while DES was temporarily applied to inactivate circumscribed regions around the tumor. By gathering the performances over the investigated areas and across participants, a map of the functional role of different brain regions can be built. The integrity of the phonological loop is crucial, for example, in sentence comprehension [Papagno et al., 2007; Romero Lauro et al., 2010] and foreign language learning [Baddeley et al., 1998, 1988; Papagno et al., 1991].
Therefore, by means of this technique, we aimed at directly verifying the involvement and the relative role in digit span performance of Broca's area, supramarginal gyrus, and AF. The working hypothesis was that stimulation of the supramarginal gyrus would induce significantly more order than item errors, consistently with the unattended speech disruptive effect on order information [Henson et al., 2003], due to the direct access of auditory input to the phonological short‐term store, whose neural correlates are located in the left inferior parietal lobule. In addition, following Baddeley [2007]'s model, stimulation of the long segment of the AF should produce phonological encoding errors, while stimulation of the SLF III could produce item and order errors.
We thus applied DES in 29 patients undergoing awake brain surgery for a tumor removal in the left dominant hemisphere. All patients performed a digit span during DES. The alternative predictions were as follows: (i) if the supramarginal gyrus is a verbal short‐term store, its disruption should impair digit span; (ii) if serial order information is stored there, then the supramarginal gyrus stimulation should produce significantly more order than item errors; (iii) finally, stimulation of the indirect segment of the AF should produce order errors when applied over the anterior tract and phonological errors when applied over the posterior tract.
MATERIALS AND METHODS
Participants
Patients were selected based on the site of their lesion, which should allow stimulation of the critical sites (namely Broca's region and the inferior parietal lobule). Moreover, we included only patients with a normal digit span (at least 4, following Italian norms) at the baseline neuropsychological examination. Twenty‐nine patients, 17 male and 12 female [mean age 41.86 (SD = 14.43, range = 15–74), mean education 12.55 years (SD = 4.17, range = 5–17)], were included in the study (see Table 1 for patients' demographic and clinical data). Nine of them (six male, mean age 38.11, SD 8.21, range 21–48, mean education 12.55, SD 4.5, range 8–17) underwent span assessment also during subcortical stimulation. Patients with low‐grade glioma and high‐grade glioma differed in age [t(27) = −3.1, P = 0.004], being the latter older (M = 49.92, SD 15.71) than low‐grade glioma patients (M = 35.31, SD 9.45), but not in educational level [t(27) = −0.33, P = 0.74, LGG = 12.31, SD 4.17; HGG = 12.85, SD 4.29].
Table 1.
Demographical and clinical data of the 29 patients submitted to direct electrical stimulation during awake surgery
| Sex | Age | Education | Symptom | Lesion site (left) | Volume (cm3) | Residual | WHO | Histology | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 30 | 8 | G | F2 | 1.92 | 0 | LGG | Oligodendroglioma II |
| 2 | F | 37 | 13 | G | T anterior–inferior | 14.99 | 0 | LGG | Oligodendroglioma II |
| 3 | F | 48 | 8 | P | Parietal parasagittal | 3.776 | 0 | LGG | Oligodendroglioma II |
| 4 | F | 15 | 8 | P | F mesial | 1.159 | 0 | LGG | Oligodendroglioma II |
| 5# | M | 40 | 17 | P+G | T pole, T inferior | 41.82 | 0 | LGG | Oligodendroglioma II |
| 6# | F | 40 | 8 | G | T superior, insula | 22.04 | 0 | LGG | Oligodendroglioma II |
| 7# | M | 42 | 8 | G | T mesial, lingula | 35.61 | 0 | LGG | Oligodendroglioma II |
| 8# | F | 43 | 17 | P | Insula medioposterior | 9.11 | 0 | LGG | Oligodendroglioma II |
| 9# | M | 48 | 17 | G | F anterior | 39.95 | 0 | LGG | Oligodendroglioma II |
| 10# | M | 34 | 8 | P | SMG | 1.45 | 0 | LGG | Hemangioma |
| 11# | M | 31 | 17 | G | SMG | 6.269 | 0 | LGG | Cavernous angioma |
| 12 | M | 31 | 17 | P+G | SMA | 7.3 | 0 | LGG | DNET I |
| 13* | F | 25 | 17 | headache | Angular gyrus | 4.3 | 0 | LGG | Pilocitic astrocitoma I |
| 14 | M | 36 | 13 | P+G | Insula, T1,2, orbitoF lateral | 104 | 6.24 | LGG | Astrocitoma (fibrillary) II |
| 15# | F | 44 | 8 | P | Lingual gyrus, precuneus inferior | 0.94 | 0 | LGG | Amarthoma |
| 16# | M | 21 | 13 | P+G | T mesial | 1.55 | 0 | LGG | DNT |
| 17 | F | 25 | 16 | G | F3, precentral | 3.5 | 0 | ELGG | Anaplastic ependimoma III |
| 18 | M | 43 | 17 | G | Orbitofrontal | 20.12 | 0 | ELGG | Anaplastic oligoastrocitoma III |
| 19* | M | 37 | 12 | P | T1 anterior, F orbitalis, insula | 95.07 | 0 | ELGG | Anaplastic astrocitoma III |
| 20 | M | 21 | 16 | G | Precentral | 3.05 | 0 | HGG | Anaplastic astrocitoma III |
| 21 | M | 42 | 8 | P | SMG, insula posterior | 2.62 | 0 | HGG | Oligoastrocitoma III |
| 22 | M | 52 | 8 | P | T1, insula | 18.43 | 3.87 | HGG | Glioblastoma IV |
| 23 | F | 57 | 17 | P | F3, precentral | 17.27 | 0 | HGG | Glioblastoma IV |
| 24 | M | 65 | 13 | G | Angular gyrus | 5.97 | 0 | HGG | Glioblastoma IV |
| 25 | F | 64 | 8 | G | F1, 2, 3 anterior | 29.4 | 0 | HGG | Glioblastoma IV |
| 26 | M | 74 | 5 | LD | T1,2,3 anterolateral | 24.02 | 0 | HGG | Glioblastoma IV |
| 27 | F | 58 | 17 | LD | T–O ventral | 14.28 | 0 | HGG | Glioblastoma IV |
| 28 | F | 60 | 17 | G | F3, insula | 12.49 | 0 | HGG | Metastasis |
| 29* | M | 51 | 13 | G | T2, 3 posterior | 3.01 | 0 | HGG | Metastasis |
The asterisk indicates patients submitted also to the symbol task. The symbol # indicates patients submitted to subcortical stimulation. Legend: M = male; F = female; G = generalized seizure; LD = language deficits; P = partial seizure; F1,F2, F3 = superior, middle, and inferior frontal gyrus, respectively; T = temporal; T1,2,3 = superior, middle, and inferior temporal gyrus, respectively; O = occipital; SMG = supramarginal gyrus; LGG = low‐grade glioma; ELGG = evolution of low‐grade glioma; HGG = high‐grade glioma
The protocol was approved by the local ethical committee (IBR: n.0011333/10) and the study was run in accordance with the Declaration of Helsinki.
Methods
An extensive neuropsychological evaluation (Supporting Information), an fMRI, and diffusion tensor imaging (DTI) tractography study were performed before surgery to allow the preoperative planning. fMRI also allowed assessing language dominance by having the patient performing an object naming task. The neuropsychological evaluation, performed in the week before surgery, was also apt to individually tailor the intraoperative testing. We did not use fMRI data other than for assessing language dominance and for further refinement of surgical planning, as a previous review has shown a lack of correspondence between sites of electrical stimulation and activation sites on fMRI [Giussani et al., 2010].
Patients underwent a follow‐up neuropsychological evaluation 3–7 days after surgery.
Tumor volume was calculated on fluid‐attenuated‐inversion‐recovery (FLAIR) scans for low‐grade glioma and on postcontrast T1‐weighted MRI scans for high‐grade glioma [Bello et al., 2014] via a computerized system (Brainlab iPlan Cranial Software 3.0, Feldkirchen, Germany). Histology was defined according to the World Health Organization (WHO) brain tumor classification.
We used a conservative criterion to choose the digit span length for each participant. Digit span is the traditionally employed task for clinical use to assess phonological STM capacity and the one for which Italian normative data are available (see Vallar and Papagno [2002] and Shallice and Cooper [2011]). As reported before, digit span is highly sensitive to either item processing abilities or serial order maintenance capacity [Majerus, 2013; Majerus et al., 2008]. Recent studies on the sensitivity and specificity of the digit span [Babikian et al., 2006; Woods et al., 2011] confirmed that this task is potentially sensitive to both order and item STM abilities. In particular, the assessment of the pattern and variability of order and item errors in the participants' performance on a computerized version of this task was crucial in the identification of subjects who were malingering [Woods et al., 2011]. That is, both aspects seem to critically contribute to the inherent specificity of the digit span.
We selected the length at which each participant was able to correctly repeat three sequences out of three; we then presented 20 sequences at this length. If accuracy was lower than 80%, we used a shorter sequence (digit span −1) for intraoperative assessment.
Therefore, each patient was submitted to a specific, ad hoc intraoperative protocol, designed according to his/her presurgical performance; each patient also served as a control for his/her own performance. In the case of digit span, stimuli were recorded by a male voice at a rate of 1 digit per second and played by means of a computer. We computed item and order errors separately. Substitutions of an item with a different one, omissions, and intrusions were considered item errors. As the span varied among participants, item errors for each sequence were calculated as a proportion of the total number of item errors out of the span length, averaged by participants for each stimulation site (e.g., Broca vs SMG‐AG). Order errors were those in which a correct item appeared in a wrong position. For order errors, we adopted the methodology suggested by Saint‐Aubin and Poirier [1999] to compensate for the fact that, if more items are recalled, the probability of an order error increases. Namely, we divided the total number of order errors by the total number of items recalled.
MR Imaging Acquisition and Tractography Procedure
Diffusion MRI data were acquired before surgery on a 3 T MRI scanner (Siemens Verio, Erlangen Germany) with the following MR parameters: TR/TE = 15,000/96 ms, 8 b = 0 volumes and 64 diffusion gradient directions (b = 2,000 s/mm2); 64 axial slices were acquired with a FOV of 256 × 256 mm2 and an isotropic resolution of 2.0 × 2.0 × 2.0 mm3. We performed whole‐brain DTI tractography with the interpolated streamline algorithm in Diffusion Toolkit [Wang et al., 2007], applying the following thresholds: angle < 35° and FA > 0.10. Deterministic tractography with a two‐ROI approach was performed using Trackvis [http://www.trackvis.org] by a neuroradiologist with 12 years of experience in MR tractography. After delineation of inclusive and exclusive ROIs on fractional anisotropy (FA) maps, virtual dissections of the left AF, SLF‐III, and the AF‐p were performed connecting the ROIs in the dorsolateral prefrontal, inferior parietal lobule, and posterior temporal perisylvian regions as described by Catani et al [2005]. Virtual dissections of the left UF, IFOF, and ILF were performed connecting the ROIs delineated in the temporal stem, temporal pole, and occipital lobe, respectively, as described by Catani and Thiebaut de Schotten [2008].
Surgical Procedure
Volumetric FLAIR and postgadolinum T1‐weighted imaging were co‐registered and loaded into the Neuronavigation (Brainlab, Feldkirchen Germany) along with DTI reconstruction of the inferior fronto‐occipital fasciculus, uncinate fasciculus, superior longitudinal fasciculus, arcuate fasciculus, and corticospinal tract to allow cortical and subcortical mapping procedures (Bello et al. 2008). The surgery was aimed at the maximal feasible resection with the preservation of the patient's functional status. The number of stimulated sites varied between 30 and 40 for each subject. The number of stimulations specifically used to assess digit span varied between 10 and 27. DES was performed by using a bipolar stimulator (60 Hz biphasic pulse, 1 ms width, current intensity ranging 2–8 mA, trains lasting 1–4 s). Stimulation was repeated 3 times at the same site in case of an evoked disturbance, to acknowledge the relationship between DES and an induced response as bona fide, in accordance with the usual procedure adopted in brain mapping during awake surgery. In the case of digit span, stimulation was applied before the presentation of the second digit and lasted until the end of the sequence presentation. Therefore, the patient was stimulation free when he had to repeat the sequence. Throughout the cortical and subcortical language mapping, the electrocorticography was continuously monitored to detect after‐discharge spikes, both to reduce the probability of evoking a seizure and to avoid the probability that errors were caused by the effects of the current spread. The brain mapping procedure was video‐ and audio‐recorded and reviewed postoperatively by a neurosurgeon and a neuropsychologist. Each stimulation point was stereotactically recorded onto the navigation system (3D‐FLAIR, postgadolinium T1‐weighted MRI and direction‐encoded color DTI maps) and checked offline along video‐ and audio‐recording to ensure reliability of the anatomical assignment of each point, which was given intraoperatively upon cortical surface survey, enhanced by intraoperative ultrasound (Aloka Pro Sound Alpha7, convex probe 10‐3.5 MHz, Hitachi Medical) acquisition paired to the neuronavigation.
To limit the effects of brain shift during surgical procedure, first we identified functional boundaries at the beginning of the resection, after performing a small corticectomy; second, intraoperative ultrasound was used to monitor the occurrence of brain shift and, eventually, account for that through neuronavigation; the neuronavigation data set was enriched with preoperative tractography [Chang et al., 2011]. Concerning the possible effects of backward propagation, we used a bipolar probe (tip distance 4 mm) with 60 Hz biphasic pulse. All studies in this field, exploring cortical and subcortical functions, adopted this technique to investigate the central nervous system, due to its spatial and temporal resolution which is still unparalleled by other methods [Desmurget et al., 2013]. The current propagation in the white matter of the bipolar pulse technique is known to be millimetric on average [Riva et al., 2016].
Statistical Analyses
Descriptive analyses were performed to investigate the number of correct responses for the different stimulation sites. Number of correct responses (included as categorical variable: accurate vs nonaccurate) was submitted to a mixed‐effects logit model including areas of stimulation as independent variable (three levels: Broca's region, SMG‐AG, and nontarget areas). Additionally, a more contingent analysis was conducted to unveil the specific contribution of the stimulation over a functional site of Broca's region and SMG‐AG (for Broca's region: pars opercularis, triangularis, and orbitalis; for SMG‐AG: supramarginal and angular gyrus). Random effects for participants and items were further included. These and all the further models (except for ANOVAs) were implemented in R.
Then, we performed a Poisson regression [Cameron and Trivedi, 1998] including the number of correct responses (i.e., number of sequences that were accurately repeated) and the number of errors (i.e., number of incorrect sequences) as dependent variables, and areas of stimulation as independent variable. Importantly, we collapsed the two target regions (Broca's region and the SMG‐AG) and contrasted them with the nontarget regions (see below).
Finally, we estimated the rates of item and order errors elicited by stimulation over either Broca's region or SMG‐AG. These errors were analyzed by means of a repeated measures analysis of variance (ANOVA), the dependent variable being the proportions of item error and the proportions of order error and the independent variable the target areas (two levels: Broca's region vs SMG‐AG).
The data based on intraoperative cortical (27 patients, as two patients underwent only subcortical mapping for the digit span) and subcortical (9 patients) stimulation mapping results were analyzed separately.
RESULTS
At the preoperative evaluation, all patients but two (n. 16 and 23) performed in the normal range at language examination and, crucially, none showed an impaired digit span (see Table 2 for language and digit span examination). Two patients obtained a pathological score on verbal fluency on phonemic cue, but they did not show any impairment during spontaneous speech, in particular they did not show anomia or other aphasic deficits, such as agrammatism. Low‐grade and high‐grade glioma patients did not differ either in digit span or in language tasks or tumor volume (see Appendix). Therefore, we considered them as a single group.
Table 2.
Presurgery language examination and digit span
| Naming | Verbal fluency | Comprehension | Repetition | Digit span (n.v. ≥3.75) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Object (n.v. ≥41.5) | Action (n.v. ≥36.8) | Phonemic (n.v. ≥17) | Semantic (n.v. ≥25) | Sentence (n.v. ≥70.5) | Token test (n.v. ≥29) | Word (n.v. ≥34) | Nonword (n.v. ≥33) | Sentence (n.v. ≥18) | ||
| 1 | 48 | 45.5 | 33 | 51 | 98.60 | 31.75 | 36 | 35 | 20 | 6.75 |
| 2 | 46.18 | 43.7 | 28 | 43 | 96.90 | 31.50 | 36 | 35 | 20 | 6.50 |
| 3 | 48 | 49.80 | 50 | 66 | N.a. | 34 | 36 | 35 | 20 | 6 |
| 4 | 42.60 | 44.77 | 24 | 41 | 94.60 | 33.25 | 36 | 35 | 20 | 5.75 |
| 5# | 46.48 | 46.40 | 31 | 33 | 93.10 | 31.75 | 36 | 35 | 20 | 7.25 |
| 6# | 48 | 43.02 | 43 | 47 | 94.10 | 33.75 | 36 | 35 | 20 | 5 |
| 7# | 46.59 | 47.50 | 27 | 34 | 100 | 33.75 | 36 | 35 | 20 | 8 |
| 8# | 46.28 | 38.70 | 32 | 42 | 97.10 | 36 | 36 | 35 | 20 | 5.25 |
| 9# | 46.79 | 50 | 33 | 55 | 100 | 32 | 36 | 35 | 20 | 5.25 |
| 10# | 46.18 | 50 | 27 | 48 | 79.40 | 36 | 36 | 35 | 20 | 6 |
| 11# | 48 | 46.10 | 37 | 47 | 96 | 31.75 | 36 | 35 | 20 | 6.25 |
| 12 | 48 | 50 | 39 | 49 | 92 | 36 | 36 | 35 | 20 | 5.25 |
| 13* | 48 | 43.60 | 20 | 37 | N.a. | 36 | 36 | 35 | 20 | 6 |
| 14 | 48 | 45.70 | 23 | 47 | 96.60 | 32.50 | 36 | 35 | 20 | 6.50 |
| 15# | 48 | 46.50 | 29 | 37 | 100 | 30.75 | 36 | 35 | 20 | 5 |
| 16# | 40.77 | 37.60 | 21 | 18 | 100 | 32 | 36 | 35 | 20 | 4 |
| 17 | 44.77 | 42.7 | 14 | 40 | 85.30 | 31.50 | 36 | 35 | 20 | 3.75 |
| 18 | 46.79 | 49.8 | 29 | 39 | N.a. | 32.25 | 36 | 35 | 20 | 5.25 |
| 19* | 44.59 | 44.70 | 20 | 34 | 96.60 | 32 | 36 | 35 | 20 | 6.50 |
| 20 | 48 | 44.7 | 44 | 48 | N.a. | 36 | 36 | 35 | 20 | 5.50 |
| 21 | 48 | 46 | 28 | 50 | 94.10 | 36 | 36 | 35 | 20 | 5.50 |
| 22 | 44.79 | 42.1 | 28 | 38 | N.a. | 31.50 | 36 | 30 | 20 | 4 |
| 23 | 44.69 | 35.8 | 3 | 30 | 83.90 | 24.25 | 36 | 31 | 20 | 5.50 |
| 24 | 45.41 | 48.4 | 26 | 42 | 77.30 | 29.50 | 36 | 35 | 20 | 3.75 |
| 25 | 48 | 45.4 | 18 | 39 | N.a. | 33.25 | 36 | 35 | 20 | 6.25 |
| 26 | 47.82 | 42.5 | 34 | 55 | 87.40 | 32 | 36 | 34 | 20 | 6 |
| 27 | 48 | 47.8 | 36 | 34 | N.a. | 31.50 | 36 | 35 | 20 | 6.50 |
| 28 | 47.21 | 47.21 | 7 | 45 | 96.8 | 29.5 | 36 | 35 | 20 | 5.50 |
| 29* | 48 | 46.60 | 30 | 37 | 96.80 | 32.75 | 36 | 35 | 20 | 4.75 |
Pathological scores are reported in bold.
In picture naming of objects [Catricalà et al., 2013], half of the stimuli were colored pictures of living and half of nonliving items. Living (fruits, vegetables, and animals) and nonliving (tools, clothes, and furniture) stimuli were balanced for all relevant variables. In picture naming of actions, stimuli were black‐and‐white drawings.
In verbal fluency [Novelli et al., 1986] on phonological cue, the patient is asked to generate as many words as possible beginning with a given letter, namely F, P, and L. For each letter, the time allowed is 1 min. In verbal fluency by semantic cue, the procedure is the same, but categories such as makes of cars, fruits, and animals are used instead of letters.
Sentence comprehension [Cecchetto et al., 2012] included 100 items, 10 for sentence type: active, passive, dative, coordination of noun phrase, coordination of verb phrases, coordination of sentences, central embedded subject relatives, central embedded object relatives, subject relatives in right peripheral position, and object relatives in right peripheral position.
The 36‐item Token Test was used [De Renzi and Faglioni, 1978].
Intraoperative Digit Span Assessment
To collect a sufficient number of stimulation per area, we considered as target areas, Broca's region (including pars opercularis, triangularis, and orbitalis) and the SMG‐AG, and we gathered all the remaining parietal, temporal, and frontal sites as “control” sites. The location of Broca's area is variously described as being in the pars triangularis of the inferior frontal gyrus, in the pars opercularis, or more commonly in the “posterior inferior frontal gyrus.” A further functional definition was done intraoperatively, as previously described [Riva et al., 2016; Quinones‐Hinojosa et al., 2003]. As patients differed in their span (Table 2 and Supporting Information), ad‐hoc, “personalized” sequences were used for each patient.
Cortical stimulation
The analysis was conducted on the data collected on 27 patients during intraoperative cortical stimulation. We first observed the number of correct sequences. Concerning language, which was assessed before digit span, when stimulating SMG in one case we observed a sequence error in counting but no other types of error, while DES over Broca's area produced in three occasions phonemic paraphasias, in three a latency, and in one case articulatory difficulties. A proportion of 0.046 correct sequences (SD = 0.21) was observed during SMG‐AG stimulation, whereas the stimulation of Broca's region yielded a proportion of 0.17 correct sequences (SD = 0.38). In contrast, the average proportion of correct sequences when non‐target, control areas were stimulated was 0.84 (SD = 0.36). A series of logistic regressions showed that stimulation of control sites yielded significantly more correct sequences as compared to target sites (b = 4.061, SE= 0.402, Wald Z = 10.08, P < 0.001). The stimulation of control sites elicited more correct sequences also when compared separately to Broca's region (b = 3.24, SE = 0.34, Wald Z = 9.55, P < 0.001) or SMG‐AG (b = 4.71, SE = 0.56, Wald Z = 8.41, P < 0.001). Because the SMG/AG is much larger than BA, the chance to have a stimulation trial within the functional site is much higher for BA than for SMG/AG, producing a greater “artificial” percentage of errors in BA than in SMG/AG. In contrast, the comparison between SMG‐AG and Broca's region indicated globally a lower performance when stimulation was delivered over SMG‐AG (b = −1.46, SE = 0.57, Wald Z = −2.57, P < 0.01). We further investigated whether the stimulation over a specific site in Broca's region was more likely to elicit correct responses as compared to SMG‐AG. Results indicated that the stimulation over the pars orbitalis (b =5.56, SE = 1.74, Wald Z = 3.20, P < 0.00137) and triangularis (b = 5.56, SE = 1.74, Wald Z = 3.20, P < 0.054) caused a better performance in comparison with the stimulation over SMG‐AG. In contrast, the stimulation over the pars opercularis did not elicit more accurate responses with respect to SMG‐AG (b =1.06, SE = 0.79, Wald Z = 1.342, P = 0.179). Finally, we checked whether stimulation applied over the supramarginal gyrus caused a different pattern of results with respect to stimulation delivered over the angular gyrus. The logistic analysis confirmed that there was no significant difference (b =14.57, SE = 2284.10, Wald Z = 0.006, P = 0.995). Sites at which stimulation produced disruption either in order or item (or both) are depicted on Figure 2.
Figure 2.
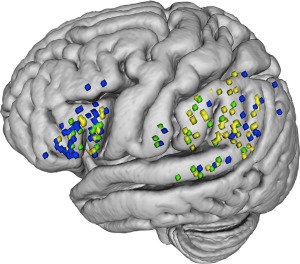
Sites where direct electrical stimulation disrupted digit span performance. Order errors sites are reported in yellow, item errors are reported in blue; sites where both types of error occurred are depicted in green. The stimulation points were recorded through both intra‐operative picture and stereotactic 3‐dimensional (3D) coordinates with the neuronavigation system. Upon off‐line visual verification of correspondence between the intra‐operative picture and the stereotactic coordinates, these points were then plotted on a 3D common Montreal Neurological Institute (MNI) brain for display purpose and to compare spatial location of error sites across all patients. [Color figure can be viewed at http://wileyonlinelibrary.com]
Single errors were then computed. A series of Poisson regressions showed that stimulation of target areas yielded more errors (M = 6.96; SD = 3.50) compared to control sites (M = 1.04, SD = 1.70), this difference being significant (b = −1.89, SE = 0.22, Wald Z = −8.54, P < 0.001).
Accordingly, participants were less likely to be accurate, and produced less correct responses (correct item in the right position), when target areas (M = 0.92; SD = 1.70) were stimulated as compared to control sites (M = 5.54; SD = 2.61). The difference was significant (b = 1.79, SE = 0.23, Wald Z = 7.90, P < 0.001).
Finally, we compared item and order errors. First, we conducted a within subjects ANOVA including only 7 participants who received a stimulation over both control sites. The stimulation delivered over Broca's area caused a higher number of item errors (M = 0.25, SD = 0.12) as compared to the SMG‐AG (M = 0.08, SD = 0.07) and the difference was significant [F(1,6) = 24.53, P < 0.003]. Regarding order error, the opposite pattern of results was observed: a stimulation over the SMG‐AG elicited a higher number of order errors (M = 0.59, SD = 0.10) in comparison with Broca's area (M = 0.37, SD = 0.24); however, this difference was only marginally significant [F(1,6) = 4.59, P < 0.07]. Second, we conducted another analysis including all the patients who received stimulation over at least one target region and who did not show language deficits after the stimulation was delivered over the target sites. Stimulation produced, on average, a higher number of item errors when delivered over Broca's area (M = 0.28, SD = 0.11) than over the SMG‐AG (M = 0.10, SD = 0.09); this difference was significant [F(1, 28) = 22.93, P < 0.001]. On the other hand, the number of order errors was significantly higher [F(1, 28) = 7.67, P = 0.01] when stimulation was applied over SMG‐AG (M = 0.59, SD = 0.102) than over Broca's area (M = 0.41, SD = 0.225). Additionally, we run a logistic regression model based on the individual error pattern of each patient. We tested whether the likelihood to make more item than order errors (i.e., coded as 1) was modulated by the site of stimulation (Broca's area vs SMG‐AG), included as independent variable. The model revealed that the likelihood to make more item errors than order errors was significantly higher when DES was applied over Broca's area, whereas the opposite pattern was observed (b = −2.48, SE = 0.94, Wald Z = −2.631, P < 0.008) when DES was applied over SMG‐AG (Fig. 3).
Figure 3.
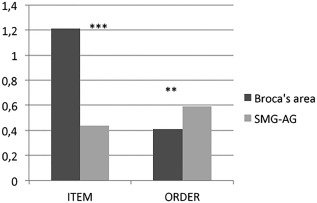
Proportion of item and order errors in digit span after stimulation of Broca's region, and supramarginal/angular gyrus (SMG/AG). The asterisks indicate a significantly higher proportion of item errors after stimulation of Broca's region with respect to the SMG/AG and a significantly higher number of order errors after stimulation of the SMG/AG with respect to Broca's area.
Subcortical stimulation
The data collected on 9 patients who underwent intraoperative subcortical stimulation are reported herein. The average proportion of correct sequences during stimulation of the white matter corresponding to the long segment of the AF was M = 0.83 (SD = 0.38) (Fig. 4), whereas the stimulation of the white matter corresponding to the SLF‐III yielded a proportion of M = 0.21 correct sequences (SD = 0.41) (Fig. 5). This difference was significant (Wald Z = −3.05, P < 0.002). Then we compared the proportion of item (M = 0.30, SD = 0.12) and order errors (M = 0.62, SD = 0.17) during stimulation of the SLF‐III. This difference was significant (t = 2.88, P = 0.045).
Figure 4.
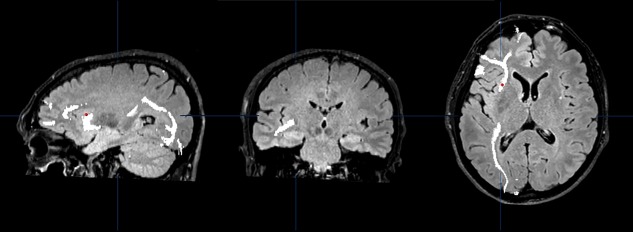
Oligodendroglioma in the left frontal lobe (patient N. 9). Sagittal, coronal and axial view of the trajectories of the left inferior fronto‐occipital fascicle (IFOF, in white) in the DTI study acquired before resection. DES applied over the IFOF did not interfere with digit span. The site of stimulation is marked with a red dot registered through the neuronavigation system. [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 5.
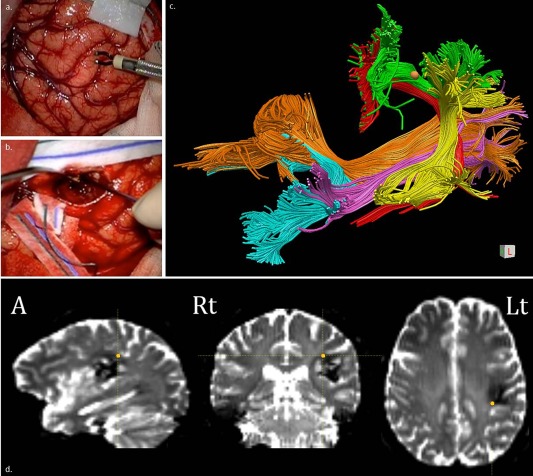
Cavernous angioma in the left supramarginal gyrus (patient N. 11). Direct Electrical Stimulation (DES) applied both cortically (left supramarginal gyrus) (a) and subcortically (direct pathway of the arcuate) (b) produced order errors. MR diffusion tractography (c and d). Coronal view of the trajectories of the long segment of the arcuate fascicle (AF, in red), posterior segment of the AF (AF‐p, in yellow), and superior longitudinal fascicle III (SLF‐III, in green), inferior longitudinal fascicle (ILF, in light orange), inferior fronto‐occipital fascicle (IFOF, in cyan) and cortico‐spinal tract (CST, in pink/violet) in the DTI study acquired before the resection. DES interfered with digit span when applied over the SLF‐III: the site of stimulation registered through neuronavigation system (c) and as 3‐dimensional coordinates as the orange spot (d) is highlighted as an orange dot. [Color figure can be viewed at http://wileyonlinelibrary.com]
Postsurgery Assessment
All patients but two underwent total removal of the tumor, with sparing of the long segment of the AF. In the two patients with partial removal, n 14 and n 22, the resection was 94% and 79%, respectively. At the postsurgery evaluation, only five patients (four cases with SMG or AG removal) showed an impaired digit span. However, the performance significantly decreased on average, as it is always the case in the week after surgery in the dominant hemisphere (see e.g., Papagno et al. [2012]) [mean digit span: presurgery 5.63 (SD 1.02), postsurgery 4.45, SD = 1.52, t(24) = 3.5, P = 0.0016)].
DISCUSSION
We assessed digit span in 29 patients during awake‐surgery for the removal of a left frontal or temporal glioma. The aim was to distinguish between regions involved in maintaining item and order information and to investigate the role of the AF in verbal STM. More specifically, we aimed at assessing whether the inferior parietal region is selectively involved in the retention of order information, as suggested by Henson et al. [2000].
First of all, we found a selective disruption of digit span when two regions were stimulated, namely, Broca's region and SMG‐AG, confirming previous results obtained with different methodologies. However, this is by far the most direct evidence, the first of this kind obtained with a large series of patients. Crucially, we also found stimulation to produce significantly more item errors when applied over Broca's area than over SMG‐AG, while the opposite pattern was evident for order errors. This result suggests that Broca's area is mainly involved in storing item (verbal) information while SMG‐AG maintains order information. Stimulation of the white matter underlying the SMG‐AG produced more order than item errors. It is not easy to certainly attribute stimulation to a specific fascicle, as all tracts run very close to each other at the stimulation site (Fig. 5c). Indeed, when the long segment of the AF and SLF‐III turn laterally to project to the inferior frontal gyrus, they share the same trajectory and are not distinguishable [Catani and Thiebaut de Schotten, 2012; Makris et al., 2005]. However, the critical site in our study was near the cortical ending in the inferior parietal lobule and not anteriorly where the two streamlines are not distinguishable. This “posterior” stimulation as well as the use of a bipolar probe with an intertips distance of 4 mm in our study, which allowed a focal stimulation of this large fascicle, can account for the absence of speech disturbances that, in contrast, were observed by van Geemen et al. [2014], who stimulated the ventral premotor cortex. We did not observe motor responses with this stimulation, either clinically or evidenced by continuous EMG recordings of selected orofacial muscles.
Disruption of the digit span was recorded in patients with DES of the SLF‐III (Fig. 5). The presence of a significant higher number of order errors similar to what happened after stimulation of SMG suggests that the SLF‐III transfers order information from the phonological store in the inferior parietal lobule to Broca's area, while (we could speculate) single‐item information reaches Broca's area (phonological output buffer) from Wernicke's area by means of the long segment of the AF. This distinction can account for the occurrence of the two different forms of conduction aphasia: a reproduction and a repetition one [Shallice and Warrington, 1977]. The former will depend from a lesion of the direct AF pathway, causing production deficits, and corresponding to conduction aphasia: indeed, information from Wernicke's area is transferred to the phonological output buffer for production; in this case, order information (stored in the phonological input buffer) is irrelevant (since single‐item reproduction is required). The latter form, which is the selective deficit of verbal STM, will depend on the involvement of the SLF‐III (indirect pathway) which transfers order information from the inferior parietal lobule to the phonological output buffer; in this case, serial order errors are produced. This can also explain while we did not record language deficits when stimulating SLF‐III.
Direct stimulation of SMG produced a different effect on item and order, as reported above. This is in line with the hypothesis that item information is directly coded in the language network (see Burgess and Hitch [1999]), while order information is stored in a specific STM system. Accordingly, we also found errors of sequence during counting when stimulating this area. Our results confirm what has been found in two patients (the lesion is not specified) who recovered from aphasia [Attout et al., 2011] and presented with distinct pattern of impairment, one with residual language deficits and specific item impairment and the other with no residual language deficits and selective impairment for order in STM. This dissociation was confirmed in a series of aphasic patients [Majerus et al., 2015], and support to a neural model of order representation in the human brain comes from an fMRI study [Kalm and Norris, 2014].
Nonetheless, order errors were present, even though at a lesser extent, also when stimulating Broca's region. Possibly, the premotor cortex could be involved in grouping processes for item sequences, a process which is more likely to intervene during maintenance, as proposed by Marshuetz [2005].
Serial order is crucial for a wide range of verbal and nonverbal activities, including language, where sequences of sounds within words and words within sentences must be maintained (conduction aphasia errors are a typical example), and skilled motor performance such as imitating sequences of gestures (ideomotor apraxia is an example). As Baddeley [2012] suggests, it is more likely that “evolution has applied the same solution to a problem, maintaining serial order, that crops up in a range of different domains (see also Hurlstone et al., 2014).” To verify whether the SMG‐AG is involved in maintaining serial order in general, three patients of our sample (marked with an asterisk in Table 1) were also submitted to a visual symbol recognition task, using modified Chinese and Bengali characters (ignored by participants). For ethical reasons, strictly experimental tests could be performed when time and patient's clinical conditions allowed maintaining the awake period for two additional minutes (the time required for this additional task) without interfering with the surgical procedure. We presented two consecutive sequences of nonverbalizable symbols that could be identical or different in order (two inverted items) or in a single item. Also in this case, the length of sequences was assessed before surgery, with the same procedure described above. We found that the proportions of correct responses for identical versus order‐ or item‐different sequences were 0.92 (SD = 0.27), 0.54 (SD = 0.51), 0.81 (SD = 0.40), respectively. Owing to the limited number of observations, data of symbol digits were submitted to a set of Generalized Estimating Equations [Liang and Zeger, 1986]. There were significantly more correct responses when sequences were identical than when they differed in order (b = 2.27, SE = 0.89, Wald Z = 2.53, P < 0.01). As for the sequences that differed in items, participants produced the same number of correct responses as with identical sequences (b = 1.002, SE = 1.17, Wald Z = 0.87, P = 0.38), and more correct responses as compared to sequences that differed in order (b = −1.25, SE = 0.35, Wald Z = −3.51, P < 0.001). Importantly, we run a further analysis to demonstrate that sequences differing in order were not simply more difficult than sequences differing in item. The analysis confirmed that, without stimulation, the proportion of correct responses for sequences that differed in order (M = 1, SD = 0) or item (M = 0.93, SD = 0.27) was the same (b = 19.001, SE= 1821.075, Wald Z = 0.001, P = 0.99). Therefore, our data point in the direction of Baddeley's suggestion, with the left inferior parietal lobule as a strong candidate for maintaining interitem relations, although we are aware that three patients represent a very limited sample to draw conclusions (however, see Thiebaut de Schotten et al. [2005], who discuss DES results on line bisection on three patients). Using an n‐back task, Ravizza et al. [2004] found that the dorsal part of the inferior parietal cortex was active with high memory load regardless of information type (verbal vs nonverbal). However, the n‐back task is not a pure measure of verbal STM and, consequently, this study does not provide information about the localization of the phonological short‐term store (for a discussion, see Shallice and Cooper [2011]). Future research can follow this direction, using less invasive methodologies.
A limitation of our study is that patients with different histological lesions were included. However, this is the case for the majority of clinical studies of this type and we excluded extra‐axial lesions. Furthermore, as reported, HGG and LGG patients did not differ in severity of cognitive deficits. Another limitation is that only specific sites could be stimulated due to surgical constraints. Additional sites—such as right and left BA7, BA6, left and right intraparietal sulcus—have been found involved in order maintenance in neuroimaging studies, and we ourselves have detected order errors in Broca's area, although less frequently. Our results are partly in line with Marshuetz et al. [2000] and Marshuetz et al. [2006], who found peak activation foci for order versus control condition and order versus item condition in BA7/40 and item versus control and order versus control in Broca's area. Marshuetz et al. also found significant activation foci in the right parietal cortex, that we could not test, and in left BA 6, which has found to be involved in recoding/rehearsal (see, e.g., Henson et al. [2000]), coherently with the fact that the items were visually presented, while we used auditory verbal stimuli. Henson et al. [2000] reported different sites in an fMRI study on six subjects using five different visually presented tasks and participants used the index or middle finger of their right hand to make a “yes” or “no” response. Activation of left supramarginal and inferior frontal gyri in a Letter Probe (judgment for the presence or absence of that letter in the prior sequence) relative to a Letter Match task (same‐different judgment) was attributed to storage and retrieval of phonological information. The left lateral premotor cortex activation in a Sequence Probe task (match of the serial order) relative to Letter Probe task was attributed to subvocal rehearsal of serial order. The deactivation of left dorsolateral premotor cortex in a Grouped Probe (presentation of the sequence was grouped temporally into two groups) relative to Sequence Probe task, was attributed to the exploitation of temporal grouping in maintaining serial order. Finally, Majerus et al. [2006] in a larger series of participants tested different verbal STM conditions that probed recognition for word identity or word order. During order STM, the left IPS was functionally connected to serial/temporal order processing areas in the right IPS, premotor and cerebellar cortices, while during item STM, the left IPS was connected to phonological and orthographic processing areas in the superior temporal and fusiform gyri. However, the modalities of testing in all fMRI studies are very different from ours and have been run, in general, on a limited number of subjects, preventing any clear comparison. Moreover, in direct stimulation studies, it is possible to determine a causal role, while fMRI studies are correlation studies.
CONCLUSION
Although this study suffers the limitations typical of clinical studies, this is the first direct assessment of the relative role of Broca's area, SMG‐AG and, crucially, of the AF in verbal STM. A previous neuropsychological study assessing the role of the inferior parietal and inferior frontal cortex supported the distinct roles of these two structures in STM processing, but only as far as storage and rehearsal were concerned [Baldo and Dronkers, 2006]. Parietal patients had a grossly reduced span.
In this study, the results are clear‐cut: (i) item and order storage rely on different anatomical correlates, namely, Broca's area and the parietal inferior lobule, respectively; (ii) subcortical stimulation of the SLF‐III (anterior segment of the indirect AF pathway) interferes with digit span, producing significantly more order than item errors. Order information per se is stored in the supramarginal gyrus, probably independently from the material type.
We propose, therefore, a possible variation of the functioning of the phonological loop: auditory verbal information reaches Wernicke's area and the perceived items are transferred to Broca's area. Order information is stored in the supramarginal gyrus and then transferred to Broca's area where it is implemented to item information through rehearsal.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
The authors are grateful to Giulia Mattavelli and Leonor Romero Lauro for a preliminary discussion about target areas and how to collapse control sites. They are also grateful to Marcello Gallucci for suggestions about the statistical analyses.
The authors acknowledge that they have no conflicts of interest.
Mann–Whitney U test between low‐ and high‐grade glioma for language tasks, digit span, and tumor volume
Object naming: U = 93.5, z = 0.46, P = 0.64
Action naming: U = 88.5, z = 0.67, P = 0.49
Phonemic fluency: U = 64, z = 0.35, P = 0.72
Semantic fluency: U = 83.5, z = 0.89, P = 0.36
Token test: U = 68, z = 1.58, P = 0.11
Digit span: U = 73, z = 1.36, P = 0.17
Tumor volume: U = 89, z = −0.65, P = 0.51
REFERENCES
- Attout L, Van Der Kaa MA, George M, Majerus S (2011): The importance of distinguishing item and order memory for understanding short‐term memory deficits in brain‐damaged patients. Poster presented at the 49th Academy of Aphasia, Montreal October 16–18.
- Awh E, Smith EE, Jonides J (1995): Human rehearsal processes and the frontal lobes: PET evidence. Ann NY Acad Sci 769:97–117. [DOI] [PubMed] [Google Scholar]
- Babikian T, Boone KB, Lu P, Arnold G (2006): Sensitivity and specificity of various digit span scores in the detection of suspect effort. Clin Neuropsychol 20:145–159. [DOI] [PubMed] [Google Scholar]
- Baddeley AD (1990): The development of the concept of working memory: Implications and contributions of neuropsychology In: Vallar G, Shallice T, editors. Neuropsychological Impairments of Short‐Term Memory. Cambridge: Cambridge University Press; pp 54–73. [Google Scholar]
- Baddeley AD (1996): Exploring the central executive. Q J Exp Psychol A Hum Exp Psychol 49:5–28. [Google Scholar]
- Baddeley AD (2007): Working Memory, Thought, and Action. Oxford: Oxford University Press; p 215. [Google Scholar]
- Baddeley AD (2012): Working memory: Theories, models, and controversies. Ann Rev Psychol 63:1–29. [DOI] [PubMed] [Google Scholar]
- Baddeley AD, Gathercole S, Papagno C (1998): The Phonological loop as a language learning device. Psychol Rev 105:158–173. [DOI] [PubMed] [Google Scholar]
- Baddeley AD, Papagno C, Vallar G (1988): When long term learning depends on short‐term storage. J Mem Lang 27:586–595. [Google Scholar]
- Baddeley AD, Salamé P (1986): The unattended speech effect: perception or memory? J Exp Psychol Learn Mem Cogn 12:525–529. [DOI] [PubMed] [Google Scholar]
- Baldo JV, Dronkers NF (2006): The role of inferior parietal and inferior frontal cortex in working memory. Neuropsychology 20:529–538. [DOI] [PubMed] [Google Scholar]
- Bello L, Gambini A, Castellano A, Carrabba G, Acerbi F, Fava E, Giussani C, Cadioli M, Blasi V, Casarotti A, Papagno C, Gupta AK, Gaini S, Scotti G, Falini A (2008): Motor and language DTI fiber tracking combined with intraoperative subcortical mapping for surgical removal of gliomas. Neuroimage 39:369–382. [DOI] [PubMed] [Google Scholar]
- Bello L, Riva M, Fava E, Ferpozzi V, Castellano A, Raneri F, Pessina F, Bizzi A, Falini A, Cerri G (2014): Tailoring neurophysiological strategies with clinical context enhances resection and safety and expands indications in gliomas involving the motor pathways. Neuro‐Oncol 16:1110–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork EL, Healy AF (1974): Short‐term order and item retention. J Verb Learn Verb Behav 13:80–97. [Google Scholar]
- Burgess N, Hitch J (1999): Memory for serial order: a network model of the phonological loop and its timing. Psychol Rev 106:551–581. [Google Scholar]
- Cameron AC, Trivedi PK (1998). Regression Analysis of Count Data. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Catani M, Thiebaut de Schotten M (2008): A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex 44:1105–1132. [DOI] [PubMed] [Google Scholar]
- Catani M, Thiebaut de Schotten M (2012). Atlas of Human Connections. Oxford: Oxford University Press. [Google Scholar]
- Catani M, Jones DK, ffichte DH (2005): Perisylvian language networks of the human brain. Ann Neurol 57:8–16. [DOI] [PubMed] [Google Scholar]
- Catricalá E, Della Rosa PA, Ginex V, Mussetti Z, Plebani V, Cappa SF (2013). An Italian battery for the assessment of semantic memory disorders. Neurol Sci 34:985–993. [DOI] [PubMed] [Google Scholar]
- Cecchetto C, Di Domenico A, Garraffa M, Papagno C (2012). Comprendo. Batteria per la comprensione di frasi negli adulti. Milano: Raffaello Cortina Editore. [Google Scholar]
- Chang EF, Clark A, Smith JS, Polley MY, Chang SM, Barbaro NM, Parsa AT, McDermott MW, Berger MS (2011): Functional mapping‐guided resection of low‐grade gliomas in eloquent areas of the brain: improvement of long‐term survival. Clinical article. J Neurosurg 114:566–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmurget M, Song Z, Mottolese C, Sirigu A (2013): Re‐establishing the merits of electrical brain stimulation. Trends Cogn Sci 17:442–449. [DOI] [PubMed] [Google Scholar]
- De Renzi E, Faglioni P (1978): Normative data and screening power of a shortened version of the token test. Cortex 14:41–49. [DOI] [PubMed] [Google Scholar]
- De Witt Hamer PC, Robles SG, Zwinderman A, Duffau H, Berger MS (2012): Impact of intraoperative stimulation brain mapping on glioma surgery outcome: A meta‐analysis. J Clin Oncol 30:2559–2565. [DOI] [PubMed] [Google Scholar]
- Fischer‐Baum S, Benjamin AS (2014): Time, space, and memory for order. Psychon Bull Rev 21:1263–1271. [DOI] [PubMed] [Google Scholar]
- Fischer‐Baum S, McCloskey M (2015): Representation of item position in immediate serial recall: Evidence from intrusion errors. J Exp Psychol Lang Mem Cogn 41:1426–1446. [DOI] [PubMed] [Google Scholar]
- Giussani C, Roux F‐E, Ojemann J, Sganzerla EP, Pirillo D, Papagno C (2010): Is preoperative functional magnetic resonance imaging reliable for language areas mapping in brain tumor surgery? Review of language functional magnetic resonance imaging and direct cortical stimulation correlation studies. Neurosurgery 66:1–8. [DOI] [PubMed] [Google Scholar]
- Hartley T, Hurlstone MJ, Hitch GJ (2016): Effects of rhythm on memory for spoken sequences: A model and tests of its stimulus‐driven mechanism. Cogn Psychol 87:135–178. [DOI] [PubMed] [Google Scholar]
- Henson RNA, Burgess N, Frith CD (2000): Recoding, storage, rehearsal, and grouping in verbal short‐term memory: an fMRI study. Neuropsychologia 38:426–440. [DOI] [PubMed] [Google Scholar]
- Henson R, Hartley T, Burgess N, Hitch G, Flude B (2003): Selective interference with verbal short‐term memory for serial order information: a new paradigm and tests of a timing‐signal hypothesis. Q J Exp Psychol 56:1307–1334. [DOI] [PubMed] [Google Scholar]
- Hulme C, Roodenrys S, Schweickert R, Brown G, Martin S, Stuart G (1997): Word‐frequency effects on short‐term memory tasks: Evidence for a redintegration process in immediate serial recall. J Exp Psychol Learn Mem Cogn (1997): 23:1217–1232. [DOI] [PubMed] [Google Scholar]
- Hurlstone MJ, Hitch GJ, Baddeley AD (2014): Memory for serial order across domains: An overview of the literature and directions for future research. Psychol Bull 140:339–373. [DOI] [PubMed] [Google Scholar]
- Kalm K, Norris D (2014): The representation of order information in auditory‐verbal short‐term memory. J Neurosci 34:6879–6886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang K‐Y, Zeger SL (1986): Longitudinal data analysis using generalized linear models. Biometrika 73:13–22. [Google Scholar]
- Majerus S (2013): Language repetition and short‐term memory: an integrative framework. Front Hum Neurosci 7:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majerus S, Attout L, Artielle M‐A, Van der Kaa M‐A (2015): The heterogeneity of verbal short‐term memory impairment in aphasia. Neuropsychologia 77:165–176. [DOI] [PubMed] [Google Scholar]
- Majerus S, D'Argembeau A, Martinez Perez T, Belayachi S, Van der Linden M, Collette F, Salmon E, Seurinck R, Fias W, Maquet P. (2009): The commonality of neural networks for verbal and visual‐short‐term memory. J Cogn Neurosci 22:2570–2593. [DOI] [PubMed] [Google Scholar]
- Majerus S, Poncelet M, Van der Linden M, Albouy G, Salmon E, Sterpenich V, Vandewalle G, Collette F, Maquet P (2006): The left intraparietal sulcus and verbal short‐term memory: Focus of attention or serial order? Neuroimage 32:880–891. [DOI] [PubMed] [Google Scholar]
- Majerus S, Poncelet M, Van der Linden M, Weekes B (2008): Lexical learning in bilingual adults: the relative importance of short‐term memory for serial order and phonological knowledge. Cognition 107:395–419. [DOI] [PubMed] [Google Scholar]
- Makris N, Kennedy DN, McInerney S, Sorensen AG, Wang R, Caviness VS, Pandya DN (2005): Segmentation of subcomponents within the superior longitudinal fascicle in humans: A quantitative, in vivo, DT‐MRI study. Cereb Cortex 15:854–869. [DOI] [PubMed] [Google Scholar]
- Marshuetz C (2005): Order information in working memory: An integrative review of evidence from brain and behaviour. Psychol Bull 131:323–339. [DOI] [PubMed] [Google Scholar]
- Marshuetz C, Reuter‐Lorenz PA, Smith EE, Jonides J, Noll DC (2006): Working memory for order and the parietal cortex: an event‐related functional magnetic resonance imaging study. Neuroscience 139:311–316. [DOI] [PubMed] [Google Scholar]
- Marshuetz C, Smith EE, Jonides J, DeGutis J, Chenevert TL (2000): Order information in working memory: fMRI evidence for parietal and prefrontal mechanisms. J Cogn Neurosci 12:130–144. [DOI] [PubMed] [Google Scholar]
- Miceli G, Capasso R, Laudanna A, Burani C (1994): Batteria per l'analisi dei deficit afasici. CEPSAG, Roma.
- Newhart M, Trupe LA, Gomez Y, Cloutman L, Molitoris JJ, Davis C, Leigh R, Gottesman RF, Race D, Hillis AE (2012): Asyntactic comprehension, working memory, and acute ischemia in Broca's area versus angular gyrus. Cortex 48:1288–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novelli G, Papagno C, Capitani E, Laiacona M, Vallar G, Cappa SF (1986): Tre test clinici di ricerca e produzione lessicale: taratura su soggetti normali. Archivio di Neurologia, Psicologia e Psichiatria 47:477–506. [Google Scholar]
- Ojemann G, Ojemann J, Lettich E, Berger M (1989): Cortical language localization in left, dominant hemisphere: an electrical stimulation mapping investigation in 117 patients. Neurosurgery 71:316–326. [DOI] [PubMed] [Google Scholar]
- Orsini A, Grossi D, Capitani E, Laiacona M, Papagno C, Vallar G (1987): Verbal and spatial immediate memory span: normative data from 1355 adults and 1112 children. Ital J Neurol Sci 8:539–548. [DOI] [PubMed] [Google Scholar]
- Papagno C, Casarotti A, Comi A, Gallucci M, Riva M, Bello L (2012): Measuring clinical outcomes in neuro‐oncology. A battery to evaluate low‐grade gliomas (LGG). J Neuro‐Oncol 108:269–275. [DOI] [PubMed] [Google Scholar]
- Papagno C, Cecchetto C, Reati F, Bello L (2007): Processing of syntactically complex sentences relies on verbal short‐term memory. Evidence from a STM patient. Cogn Neuropsychol 24:292–311. [DOI] [PubMed] [Google Scholar]
- Papagno C, Lucchelli F, Vallar G (2008): Phonological recoding, visual short‐term store and the effect of unattended speech: Evidence from a case of slowly progressive anarthria. Cortex 44:312–324. [DOI] [PubMed] [Google Scholar]
- Papagno C, Valentine T, Baddeley AD (1991): Phonological short‐term memory and foreign‐language vocabulary learning. J Mem Lang 30:331–347. [Google Scholar]
- Paulesu E, Frith CD, Frackowiak RS (1993): The neural correlates of the verbal component of working memory. Nature 362:342–345. [DOI] [PubMed] [Google Scholar]
- Poeppel D (1996): A critical review of PET studies of phonological processing. Brain Lang 55:317–351. [DOI] [PubMed] [Google Scholar]
- Quinones‐Hinojosa A, Ojemnn SG, Sanai N,MD, Dillon WP, Berger MS (2003): Preoperative correlation of intraoperative cortical mapping with magnetic resonance imaging landmarks to predict localization of the Broca area. J Neurosurg 99:311–318. [DOI] [PubMed] [Google Scholar]
- Ravizza SM, Delgado MR, Chein JM, Becker JT, Fiez JA (2004): Functional dissociations within the inferior parietal cortex in verbal working memory. Neuroimage 22:562–573. [DOI] [PubMed] [Google Scholar]
- Riva M, Fava E, Gallucci M, Comi A, Casarotti A, Alfiero T, Raneri FA, Pessina F, Bello L (2016): Monopolar high‐frequency language mapping: can it help in the surgical management of gliomas? A comparative clinical study. J Neurosurg 124:1479–1489. [DOI] [PubMed] [Google Scholar]
- Romani C, McAlpine S, Martin RC (2008): Concreteness effects in differential tasks: Implications for models of short‐term memory. Q J Exp Psychol 61:292–323. [DOI] [PubMed] [Google Scholar]
- Romero L, Walsh V, Papagno C (2006): The neural correlates of phonological short‐term memory: a repetitive transcranial magnetic stimulation (rTMS) study. J Cogn Neurosci 18:1147–1155. [DOI] [PubMed] [Google Scholar]
- Romero Lauro L, Reis J, Cohen L, Cecchetto C, Papagno C (2010): A case for the involvement of phonological loop in sentence comprehension. Neuropsychologia 48:4003–4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint‐Aubin J, Poirier M (1999): Semantic similarity and immediate serial recall: Is there a detrimental effect on order information? Q J Exp Psychol 52:A: 367–394. [DOI] [PubMed] [Google Scholar]
- Saint‐Aubin J, Poirier M (2000): Immediate serial recall of words and nonwords: Tests of the retrieval‐based hypothesis. Psychon Bull Rev 7:332–340. [DOI] [PubMed] [Google Scholar]
- Shallice T, Cooper RP (2011). The Organization of Mind. Oxford: Oxford University Press. [Google Scholar]
- Shallice T, Warrington EK (1977): Auditory‐verbal short‐term memory impairment and conduction aphasia. Brain Lang 4:479–491. [DOI] [PubMed] [Google Scholar]
- Soffietti R, Baumert BG, Bello L, von Deimling A, Duffau H, Frénay M, Grisold W, Grant R, Graus F, Hoang‐Xuan K, Klein M (2010): Guidelines on management of low‐grade gliomas: report of an EFNS–EANO* Task Force. Eur J Neurol 17:1124–1133. [DOI] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Urbanski M, Duffau H, Volle E, Levy R, Dubois B, Bartolomeo P (2005): Direct evidence for a parietal‐frontal pathway subserving spatial awareness in humans. Science 309:2226–2228. [DOI] [PubMed] [Google Scholar]
- Vallar G, Di Betta AM, Silveri MC (1997): The phonological short‐term store‐rehearsal system: Patterns of impairment and neural correlates. Neuropsychologia 35:795–812. [DOI] [PubMed] [Google Scholar]
- Vallar G, Papagno C (2002): Neuropsychological impairments of short‐term memory In: Baddeley AD, Kopelman MD, Wilson BA, editors. Handbook of Memory Disorders. Chichester: Wiley; pp 249–270. [Google Scholar]
- van Geemen K, Herbet G, Moritz‐Gasser S, Duffau H (2014): Limited plastic potential of the left ventral premotor cortex in speech articulation: evidence from intraoperative awake mapping in glioma patients. Hum Brain Mapp 35:1587–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Benner T, Sorensen AG, Wedeen VJ (2007): Diffusion toolkit: A software package for diffusion imaging data processing and tractography. Proc Intl Soc Mag Reson Med 15:3720. [Google Scholar]
- Warrington EK, Logue V, Pratt RTC (1971): The anatomical localisation of selective impairment of auditory verbal short‐term memory. Neuropsychologia 9:377–387. [DOI] [PubMed] [Google Scholar]
- Woods DL, Kishiyama MM, Yund EW, Herron TJ, Hink RF, Reed B (2011): Computerized analysis of error patterns in digit span recall. J Clin Exp Neuropsychol 33:721–734. [DOI] [PubMed] [Google Scholar]
- Zamora L, Corina D, Ojemann G (2016): Human temporal cortical single neuron activity during working memory maintenance. Neuropsychologia 86:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


