Abstract
Motor functions improve during childhood and adolescence, but little is still known about the development of cortical motor circuits during early life. To elucidate the neurophysiological hallmarks of motor cortex development, we investigated the differences in motor cortical excitability and connectivity between healthy children, adolescents, and adults by means of navigated suprathreshold motor cortex transcranial magnetic stimulation (TMS) combined with high‐density electroencephalography (EEG). We demonstrated that with development, the excitability of the motor system increases, the TMS‐evoked EEG waveform increases in complexity, the magnitude of induced activation decreases, and signal spreading increases. Furthermore, the phase of the oscillatory response to TMS becomes less consistent with age. These changes parallel an improvement in manual dexterity and may reflect developmental changes in functional connectivity. Hum Brain Mapp 38:2599–2615, 2017. © 2017 Wiley Periodicals, Inc.
Keywords: transcranial magnetic stimulation, electroencephalography, motor cortex, child, adolescent, adult, gamma‐Amicobutyric Acid
INTRODUCTION
The first two decades of life represent a period of major developmental changes in sensory, motor, and cognitive abilities. There is a close relationship between motor and cognitive development [Mierau et al., 2016; Piek et al., 2008; Rigoli et al., 2012; Roebers et al., 2014]. Therefore, understanding of the normal development of cortical motor function could shed light on its deviations and thus on the origins of neurologic and psychiatric disorders that potentially emerge in childhood and adolescence.
Developmental magnetic resonance imaging (MRI) studies have demonstrated that gray matter volumes peak at the age of approximately 10–12 years [Giedd et al., 1999a] and thereafter decline throughout adolescence and adulthood [Sowell et al., 2001, 2003]. In contrast, the global white matter volume increases linearly between 4 and 22 years of age [Giedd et al., 1999a]. Age‐related decreases in gray matter are thought to reflect both synaptic pruning and myelination [Bartzokis et al., 2001; Giedd, 2004; Gogtay et al., 2004; Sowell et al., 2004], whereas age‐related increases in white matter are primarily thought to reflect progressive myelination. Within the isocortex, the primary cortical areas are among the first to mature [Gogtay et al., 2004; Sowell et al., 1999, 2001, 2003]. Recent evidence suggests that the gray matter of the motor and sensory cortices already reaches maturity by the age of 10 years, whereas the maturation of superficial white matter continues until the age of 18 years [Wu et al., 2014].
Consistent with earlier primary sensory and motor system ontogeny, resting state functional MRI results demonstrate that functional connectivity in the sensorimotor areas may exist as early as at the age of 2 weeks [Lin et al., 2008]. At 5–8 years, these networks supporting basic motor function and sensory processes already have a similar functional organization to that in mature adults [De Bie et al., 2012]. With age, throughout childhood until adulthood, the sensorimotor network evolves from a more random to a more efficient functional organization both locally and globally [Berchicci et al., 2015]. Studies using transcranial magnetic stimulation (TMS) suggest that the electrophysiological maturation of the fast corticospinal motor pathways controlling intrinsic hand muscles is complete before adolescence [Heinen et al., 1998; Nezu et al., 1997], but developmental changes in cortical excitability and inhibitory control continue until adulthood [Garvey et al., 2003; Schneider et al., 2016; Walther et al., 2009].
Transcranial magnetic stimulation combined with electroencephalography (TMS‐EEG) is a powerful technique for the direct investigation of cortical excitability and connectivity [Ilmoniemi et al., 1997]. Although the main impact of TMS on brain activity is localized at the site of stimulation, TMS also modulates neural circuits in remote brain regions [Bestmann et al., 2004; Denslow et al., 2005]. The temporal evolution of the distribution of TMS‐induced EEG potentials (TEPs) over the scalp probably reflects the spread of activation from the stimulated cortical site to ipsilateral and contralateral cortical areas via intra‐ and interhemispheric corticocortical fibers, and possibly also via subcortical structures, thus revealing the effective connectivity of the brain [Ilmoniemi et al., 1997; Komssi et al., 2002; Kähkonen et al., 2004; Paus et al., 2001]. In this context, TMS‐induced interhemispheric signal propagation has also been linked to the microstructural integrity of callosal fibers that connect homologous cortical regions, with either an inhibitory or facilitatory role [Voineskos et al., 2010]. In addition to analyzing TEPs in the time domain, further insights into the functioning of stimulated brain networks can be obtained through characterizing the oscillatory activity triggered or perturbed by TMS [Fuggetta et al., 2005; Rosanova et al., 2009; Thut et al., 2012].
In adults, single‐pulse TMS of the motor cortex elicits well‐characterized activity that lasts at least up to 300 ms and is composed at the vertex of a sequence of positive and negative deflections usually labeled after their latency and polarity, e.g. P30, N45, P60, N100, P180, and N280 [Bonato et al., 2006; Ferreri et al., 2011]. By combining high‐density TMS–EEG with pharmacology, early TEPs were recently demonstrated to be linked to gamma‐aminobutyric acid (GABA)A receptor‐mediated neurotransmission and later TEPs to GABAB receptor‐mediated neurotransmission [Premoli et al., 2014]. In addition to GABAB, later TEPs have also been proposed to be related to cholinergic neurotransmission [Ferreri et al., 2012; Rossini et al., 2015].
Studies on TEPs in children are scarce and have solely focused on the N100 component [Bender et al., 2005; D'Agati et al., 2014; Helfrich et al., 2012; Jarczok et al., 2016]. In their pioneering article, Bender et al. [2005] showed that TMS‐evoked N100 undergoes maturational decline and could serve as a test of cortical integrity and inhibitory function in children. Later studies have demonstrated that N100 modulation in the Go/NoGo task can differentiate between neurotypical children and children with attention deficit hyperactivity disorder [D'Agati et al., 2014] further supporting the role of N100 as a marker of inhibition. Moreover, according to a recent TMS‐EEG study, interhemispheric signal propagation increases as a function of age in healthy subjects and patients with autism spectrum disorder [Jarczok et al., 2016].
Although motor functions improve during childhood and adolescence, little is presently known about the development of cortical motor circuits. To clarify its physiological hallmarks, we investigated the differences in motor cortical excitability and connectivity between children, adolescents, and adults by means of navigated TMS‐EEG recordings. Since manual dexterity improves with age, we paralleled the results of TMS‐EEG targeted to primary motor cortex not only with age, but also with a test of manual dexterity to verify functional relevance of the neurophysiological results. We hypothesized that effective connectivity within the motor networks improves with age, and this could be revealed by a TMS‐elicited net activity with a progressive increase in TEP complexity and a decrease in amplitude. We also hypothesized that these changes would parallel the characteristics of the electrical oscillations evoked by stimulation of the motor cortex, which has never been studied in the developing brain.
SUBJECTS AND METHODS
Subjects
Ten children (mean age 126 months, SD 7.3 months), ten adolescents (mean age 188 months, SD 9.3 months), and ten adults (mean age 309 months, SD 48.5 months) participated in the study. There were 5 females and 5 males in each age group. All subjects were healthy and right‐handed according to a revised and reduced version of the Waterloo Handedness Questionnaire that included 20 items [Steenhuis et al., 1990]. The children were recruited from a population sample of children who participated in the Physical Activity and Nutrition in Children (PANIC) study at the Institute of Biomedicine, University of Eastern Finland [Eloranta et al., 2012]. As preterm birth has been shown to associate with reduced corticomotor excitability in childhood and early adolescence [Pitcher et al., 2012], data on gestational age (GA) of the children were retrospectively collected from the birth register provided by the National Institute for Health and Welfare. All, except for one girl (35 weeks GA) were born at term. The adolescents were recruited from a middle school near the hospital, and the adults were students from the University of Eastern Finland and personnel of the TMS laboratory. The exclusion criteria were common contraindications to MRI or TMS [Rossi et al., 2009, 2011].
All participants and the guardians of the children were informed about the nature of the study. After having received a detailed description of the procedure, the participants provided written informed consent. If the participant was under 15 years of age, a guardian also provided written informed consent. The Research Ethics Committee of the Hospital District of Northern Savo approved the study protocol (48/2010), and the study was carried out in accordance with the latest version of the Helsinki Declaration.
Study Protocol
The study protocol consisted of two separate visits, with MRI on the first visit and TMS‐EEG on the second visit. Gross manual dexterity was assessed with the Box and Block Test at the beginning of the second visit. The Box and Block Test is a timed test evaluating motor speed and skill. The participant moves as many blocks as possible within 60 seconds from one side of a box to the other [Mathiowetz et al., 1985]. Each hand was assessed separately.
Subjects were scanned with a 3.0 T scanner (Philips Achieva TX, Philips Healthcare, Eindhoven, The Netherlands). Structural three dimensional (3D) T1‐weighted MR images were acquired (TR 8.07 ms, TE 3.7 ms, flip angle 8°, 1 × 1 × 1 mm3 resolution) for neuronavigation in TMS. An experienced neuroradiologist screened all the structural MRIs before the TMS session.
TMS was performed with an eXimia stimulator (Nexstim Plc., Helsinki, Finland) and a biphasic figure‐of‐eight coil combined with the eXimia navigation system that enables continuous visualization of the stimulation site in relation to the individual cortical anatomic structure (3.2.2. research version).
During TMS, muscle activity was monitored online and recorded by stimulus‐locked electromyography (EMG) (Nexstim Plc., Helsinki, Finland). TMS‐induced motor evoked potentials (MEPs) were recorded using disposable Ag/AgCl surface electrodes placed on the right abductor pollicis brevis (APB) muscle using the belly‐tendon montage. During the TMS session, the participants sat in an adjustable chair with a headrest that ensured a stable head position. The reconstructed 3D brain surface at a depth where the gyral structure was distinct was used for navigation of the stimulation sites. First, the subject's motor representation area of the right APB muscle was mapped around the anatomical “hand knob” [Yousry et al., 1997] by keeping the stimulating current perpendicular to the central sulcus. The mapping was started with 50% of maximal stimulator output intensity, but was adjusted to obtain MEPs with amplitude of 300–500 µV. The precentral gyrus and the sulci surrounding it were stimulated to find the optimal site where MEPs of maximal amplitude were repeatedly recorded in the right APB muscle. At this optimal site, the coil was turned within ±90° from the original angle of stimulation in tangential plane to find the optimal current direction for stimulations. The optimal point with the optimal current direction was set as the stimulation target. At this site, the individual resting motor threshold (MT) for APB was determined using the TMS Motor Threshold Assessment Tool 2.0 with an amplitude limit of ≥50 µV [Awiszus, 2003, 2012]. For additional details, refer to [Julkunen, 2014; Säisänen et al., 2008].
TMS was focused on the above‐mentioned stimulation target with a stimulation intensity of 110% of the MT. Each participant underwent 150 TMS trials with interstimulus intervals randomized between 4.0 and 5.5 s. The TMS system delivered trigger pulses that synchronized the TMS and EEG systems. EEG was recorded with a 60‐channel TMS‐compatible amplifier (Nexstim Plc., Helsinki, Finland) continuously throughout the experiments from TMS‐compatible Ag/AgCl‐coated electrodes positioned according to the 10‐10 International System. The ground and reference electrodes were positioned on the midline forehead. Skin/electrode impedance was kept below 5 kOhm. Horizontal and vertical eye movements were detected by recording the electro‐oculogram with two electrodes located to the left and right of the external canthi.
In the EEG system, a sample‐and‐hold circuit was applied together with blocking of the amplifier input for 2 ms from the stimulus to avoid amplifier saturation. The data were recorded with a 1,450 Hz sampling frequency and 16‐bit precision.
During the TMS‐EEG recording, the subjects were instructed to keep their eyes open and to look at a fixation point on a screen in front of them. To mask coil‐generated clicks, white noise (obtained from the waveform of the TMS click), digitized and processed to produce a continuous audio signal with specific time‐varying frequencies [Massimini et al., 2005], was continuously delivered through earphones. The masking volume was adjusted until the subject reported that the TMS click was no longer audible.
Data Analysis
Scalp‐to‐cortex distance (SCD)
The scalp‐to‐cortex distance was measured using Nexstim navigation software. The optimal cortical representation site of the right APB muscle was identified on the reconstructed 3D brain surface at a depth where the gyri structure was distinct. Thereafter, the peeling distance was adjusted until the outmost part of the precentral gyrus was determined. This peeling depth was assessed as the distance between the scalp and the motor cortex (surface of gray matter).
EEG
Offline data analysis was conducted with custom‐made scripts using MATLAB (version 2008b, MathWorks, Natick, Mass., USA) and the public license toolbox EEGLAB [Delorme and Makeig, 2004]. EEG data were divided into segments of 600 ms, including a 100‐ms pre‐stimulus baseline. EEG signals were bandpass filtered between 1 and 80 Hz, down sampled from 1,450 to 725 Hz, and baseline corrected by using the 100‐ms pre‐stimulus time as the baseline. All epochs showing TMS‐EEG evoked activity contaminated by extreme values (the difference between the maximum and the minimum amplitude was either greater than 150 µV or smaller than 5 µV) were automatically rejected. After the automatic analysis, rejection results were visually inspected trial by trial and manually confirmed by an expert in TMS‐EEG data analysis. Furthermore, trials containing muscle activity, eye movements or blinks were rejected based on visual inspection. Following this procedure, all trials included were averaged for each channel and for each subject. The 20‐ms interval immediately following the TMS pulse was excluded from analyses to avoid the artifact caused by the currents induced by the magnetic field and the eventual TMS‐evoked muscular scalp responses. Recording reference was used in TEP analysis and in frequency domain analysis, while EEG derived from the average reference was used in source localization.
To examine responses in the time domain, the total EEG activity was first assessed using the global mean field power (GMFP) calculated as the root mean‐squared value of the EEG signal across all electrodes [Lehmann and Skrandies, 1980].
For the analysis of evoked responses, averaged TEPs over all the included trials for each electrode and each subject were used, and semi‐automatic amplitude/latency measurements of each component of the TEPs were performed. Within these analyses, the minimum and the maximum latency of the TEP components (i.e., range from which the automatic analyses were done) were manually given to a MATLAB script which then automatically detected the maximal/minimal baseline‐to‐peak amplitude within these time limits and calculated the latency of this peak. The latency ranges for different components were defined based on GMFP.
Following our a priori hypothesis of age‐related differences in inter‐ and intrahemispheric transmission, the area under the rectified curve was obtained between 50 and 150 ms post‐stimulus for the ipsilateral motor cortex (M1left) (average of C5, C3, CP5, and CP3), as well as for the ipsilateral frontal cortex (frontalleft) (average of FP1, AF1, F7, and F5), and between 60 and 160 ms for the contralateral motor cortex (M1right) (average of C6, C4, CP6, and CP4) [Jarczok et al., 2016; Voineskos et al., 2010]. These time intervals were chosen because 150 ms represents the mean duration of the probable GABAB‐receptor‐mediated inhibitory neurotransmission [Fitzgerald et al., 2009] and the average interhemispheric transfer time of 10 ms was chosen to account for the time it takes for the signal to propagate from the site of stimulation to the opposite hemisphere in adults [Ferbert et al., 1992]. Interhemispheric signal propagation time of 10 ms has also been applied to pediatric populations [Jarczok et al., 2016]. Interhemispheric propagation between motor cortices was assessed by computing the ratio between M1left and M1right [(M1right/M1left) * 100]. Ipsilateral (intra‐hemispheric) signal propagation was indexed in a similar fashion [(frontalleft/M1left) * 100].
Dipole localization was conducted in order to approximate the number and site of sources accounting for TEP components. The average TEPs were converted to common average reference TEPs for dipole localization (Curry 6.0.2., 2007 Compumedics Neuroscan, Charlotte, USA). The time windows for different components in separate groups were defined based on GMFP (Supporting Information Table). The number of dipoles was evaluated with principal component analysis (PCA) of the selected time range and components with a signal‐to‐noise ratio (SNR) higher than 1 were selected. Dipole fits were calculated with the fixed MUSIC algorithm using a realistic head model (BEM model) from one individual from each age group.
To examine responses in the frequency domain, event‐related spectral perturbation (ERSP) and inter‐trial coherence (ITC) were calculated. ERSP measures changes in the amplitude dynamics of the EEG spectrum relative to an experimental event (in this case, TMS). ERSP values are calculated for different frequency bands and are independent of the phase of the EEG‐evoked activity, and are considered to reflect event‐related desynchronization or synchronization. ITC values range from 0 (no phase locking) to 1 (maximal synchronization across trials) and provide a measure of synchronization of the EEG across different trials, independent of the signal amplitude. The ERSP and ITC were computed for each channel between 1 and 50 Hz using the fast Fourier transform and Hanning window tapering. The analyses were performed for the post‐stimulus period between 0 and 500 ms by using the pre‐stimulus time between −100 and −20 ms as a baseline. Furthermore, all the channel‐specific ERSP and ITC values were averaged to form global values including all channels.
Statistical Analyses
Statistical analyses were computed with MATLAB (version 2008b, MathWorks, Natick, Mass., USA) and SPSS for Windows, Version 22 (IBM Corporation, Somers, NY, USA). Differences between groups were defined as statistically significant if P < 0.05, and Bonferroni correction for multiple comparisons was applied when necessary for the number of age groups (P < 0.05/3). The following assessments were performed:
Developmental differences between age (in months), manual dexterity, SCD, MT, and GMFP area were assessed using two‐tailed Pearson's correlation coefficients. Similarly, two‐tailed Pearson's correlation coefficients between manual dexterity (right hand), MT and GMFP area were computed.
Differences in GMFP between groups were assessed by performing a t‐test at each time point in the 500‐ms post‐stimulus time interval. A GMFP area (20–500 ms post stimulus) comparison was made with one‐way ANOVA, and an independent samples t‐test was used for post‐hoc analyses.
Propagation of TMS‐evoked activity was tested with two‐tailed Pearson's correlation coefficients between age (in months), and intrahemispheric and interhemispheric transmission.
-
In statistical analysis of TEPs, the linear mixed‐effects model was used. The channels were divided into 15 regions (see Fig. 1 for further details). For analysis, we created variables to determine the regions: three in the anteroposterior (AP) direction (1 = frontal, 2 = central and 3 = parieto‐occipital) and five in the mediolateral (ML) direction (1 = left lateral, 2 = left mediolateral, 3 = medial, 4 = right mediolateral, 5 = right lateral). The baseline‐to‐peak amplitude of peaks of interest was used as a dependent variable, the participant was determined as a random effect, and the group, AP, and ML as fixed effects. TEPs (P50, N100, and for children and adolescents, also P300) were tested independently, and the results were Bonferroni corrected according to the number of peaks. Post hoc analyses were run to determine the source of statistically significant interactions. Before the post hoc analysis, we combined the variables group (children/adolescents/adults) with AP and ML (15 regions). Thereafter, a linear mixed model with variable AP_ML_GROUP as fixed effect and the peak of interest as the dependent variable was run. For TEP analyses, only results indicating statistically significant differences between the groups were reported.
Figure 1.
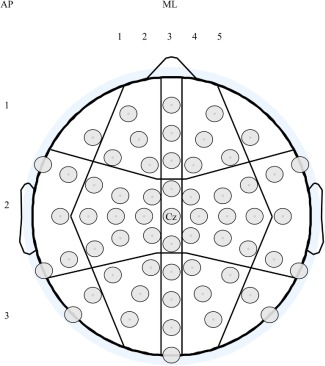 60 EEG channels were used in the analyses when exploring the topography of the TEP peaks. The channels were divided into fifteen regions according to their anteroposterior (AP; 1 = frontal, 2 = central and 3 = parieto‐occipital) and mediolateral (ML; 1 = left lateral, 2 = left mediolateral, 3 = medial, 4 = right mediolateral, 5 = right lateral) location.
60 EEG channels were used in the analyses when exploring the topography of the TEP peaks. The channels were divided into fifteen regions according to their anteroposterior (AP; 1 = frontal, 2 = central and 3 = parieto‐occipital) and mediolateral (ML; 1 = left lateral, 2 = left mediolateral, 3 = medial, 4 = right mediolateral, 5 = right lateral) location. The TEP latencies were analyzed as the mean latency in the region where the response was largest in amplitude. One‐way ANOVA and independent samples t‐tests were used for between‐group comparisons.
Statistical topographical analysis of ERSP and ITC differences was conducted for the alpha (8–12 Hz) and beta (15–25 Hz) frequency bands, which are known to be closely related to motor cortex function [Neuper and Pfurtscheller, 2001; Veniero et al., 2011]. Two time ranges (20–200 ms and 200–500 ms post‐stimulus) were analyzed. First, the data were false discovery rate (FDR) corrected for the number of channels, and thereafter Bonferroni corrected for the number of groups and time ranges. The time ranges were based on visual inspection of the global values obtained by averaging all channels.
RESULTS
General Indices
The examinations were well tolerated and no side effects were observed. No abnormalities were found in MRI. Manual dexterity improved significantly as a function of age (r = 0.644, P < 0.001 for left hand; r = 0.570, P = 0.001 for right hand). The scalp‐to‐cortex distance correlated significantly with age (r = 0.570, P = 0.001) being shortest in children and longest in adults.
MT decreased significantly with age (r = −0.497, P = 0.005). Furthermore, to exclude the effects of SCD behind the age‐related differences in MT, we ran a partial correlation between MT and age and the result remained significant (partial correlation, controlled for SCD, r = −0.498, P = 0.013). Manual dexterity for the right hand correlated with MT (r = −0.474, P = 0.008) (Fig. 2).
Figure 2.
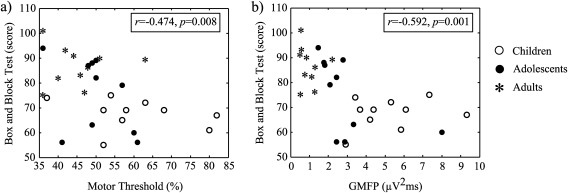
Correlation between the Box and Block test and MT (a) and Box and Block test and GMFP area (b). Better Box and Block test score is associated with lower MT and smaller GMFP. The difference between the age groups is also clearly seen.
The ratio of frontalleft/M1left (intrahemispheric transmission) correlated with age (r = 0.622, P < 0.001). Also the M1right/M1left ratio (interhemispheric transmission) yielded similar results (r = 0.586, P = 0.001). See also Table 1.
Table 1.
Measured or computed parameters shown as mean (S.D.)
| Parameter | Children | Adolescents | Adults |
|---|---|---|---|
| MT (%) | 60 (14) | 50 (8) | 45 (8) |
| Box and Block Test right (score) | 68 (6) | 75 (15) | 87 (8) |
| Box and Block Test left (score) | 65 (6) | 72 (12) | 83 (7) |
| GMFP area (µV2ms) | 5.2 (2.0) | 2.9 (1.9) | 1.0 (0.5) |
| Interhemispheric propagation (%) | 36 | 45 | 65 |
| Intrahemispheric propagation (%) | 29 | 26 | 57 |
| Scalp‐to‐cortex‐distance (mm) | 8.3 (1.2) | 11.2 (2.0) | 13.5 (2.8) |
Note: The mean MT of the children born at term was 61 (14), and the MT for the child born preterm (35 weeks GA) was 57.
TMS‐EEG Results
GMFP
The mean area of GMFP showed a significant decline with age (r = −0.690, P < 0.001). Manual dexterity for the right hand correlated with GMFP area (r = −0.592, P = 0.001) (Fig. 2). Group‐wise differences as a function of time are presented in Figure 3.
Figure 3.
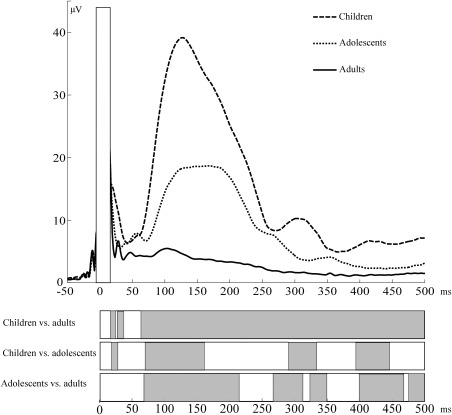
TMS‐evoked electrical activity presented as GMFP in children, adolescents, and adults. Statistically significant differences between groups are presented in the lower panel in grey.
TEPS, topography and sources
Upon visual inspection of grand average waveform, in children and adolescents, the TEP waveform was dominated by a large deflection peaking at around 130 ms, preceded by a positive deflection at around 50 ms and followed by positivity at around 300 ms (Fig. 4a). On the other hand, in adults, TEPs were composed of a sequence of deflections similar to what has been already described [Bonato et al., 2006; Ferreri et al., 2011; Kähkonen et al., 2004; Lioumis et al., 2009; Paus et al., 2001; Premoli et al., 2014] and reviewed [Ferreri and Rossini, 2013], with peaks at 34, 43, 51, 117, 190, and 257 ms (Fig. 4b). The group comparisons were only conducted for the peaks at around 50 and 100 ms, which appeared in all age groups, and separately for the positive peak around 300 ms between children and adolescents. For the purpose of comparison, we also measured the negative peak that was present in adults at around 300 ms, but statistical analyses between negative adult N280 and positive P300 in children and adolescents were not conducted. Summary of results from linear mixed model analyses are presented in Table 2.
Figure 4.
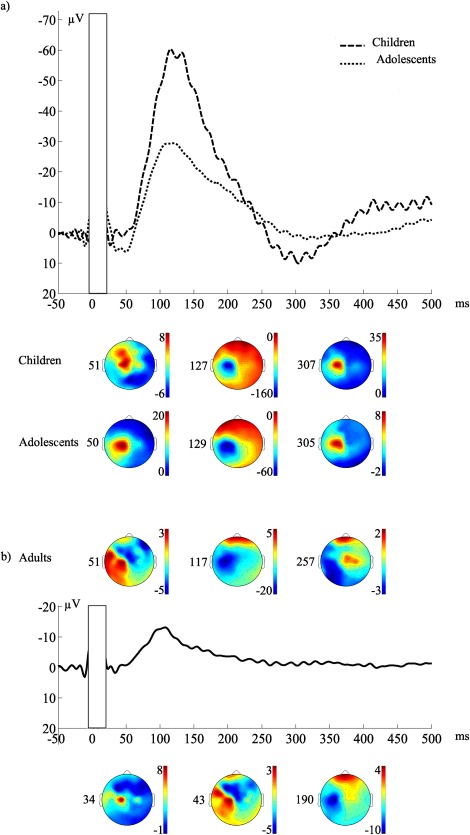
Grand average of the EEG responses and the topography of the TEP peaks presented as scalp distribution maps in children, adolescents, and adults using recording (forehead) reference. Zero corresponds to the TMS pulse. (a) Topography of the TEP peaks in children 51, 127, and 307, and in adolescents 50, 129, and 305 ms after 110%MT motor cortex stimulation. (b) Topography of the TEP peaks in adults 34, 43, 51, 117, 190, and 257 ms after 110%MT motor cortex stimulation.
Table 2.
The main effects and interactions from linear mixed model analysis
| P50 | N100 | P300 | ||||
|---|---|---|---|---|---|---|
| Main effect/interaction | F (df) | P | F (df) | P | F (df) | P |
| Group | 9.25 (2,27.18) | <0.01 | 8.81 (1,18.31) | <0.05 | ||
| AP × Group | 17.39 (4,1728) | <0.001 | 32.41 (4,1728) | <0.001 | 14.35 (2,1152) | <0.001 |
| ML × Group | 6.33 (8,1728) | <0.001 | 28.86 (8,1728) | <0.001 | 4.67 (4,1152) | <0.01 |
| AP × ML × Group | 3.09 (16,1728) | <0.001 | 7.56 (16,1728) | <0.001 | 3.15 (8,1152) | <0.01 |
P50 (maximal positivity between 40 and 70 ms)
The topography of P50 differed significantly between the groups (Table 2). In children and adolescents, the P50 response was largest in the central medial region (10.6 µV in children and 14.3 µV in adolescents), whereas in adults, it was largest in the central region at left lateral electrodes (4.3 µV). Post hoc analyses for the interaction between AP, ML, and group showed that in adolescents, P50 was larger than that of adults in the central medial region (P < 0.05). P50 latency did not differ significantly between the groups, as evaluated by the mean latency in the region where the response was largest (children 51 ms, adolescents 50 ms, adults 51 ms, P > 0.05 in all comparisons).
In children, one cortical dipole pointing radially out between electrodes C1 and Cz accounted for the P50. In adolescents, P50 was explained with two dipoles in the left hemisphere: one cortical dipole pointing radially out and located near electrode C1, and the other pointing tangentially left and located medially in the lingual gyrus. P50 in adults was also explained by two tangential cortical dipoles in the left hemisphere: one superficial dipole pointing laterally and another dipole just below it pointing in the opposite direction. Both dipoles were located in the vicinity of electrode C1. According to the literature and upon visual inspection, adults have two distinct peaks within the 30–60 ms time window. The additional N45 in adults was explained by two dipole components. The first dipole was tangential to the cortex, pointing from right to left, located under electrodes C1 and Cz. The second one was tangential to the cortex, pointing from left to right and located in the vicinity of electrodes Cz and CPz. See also Figure 5 and Supporting Information Table.
Figure 5.
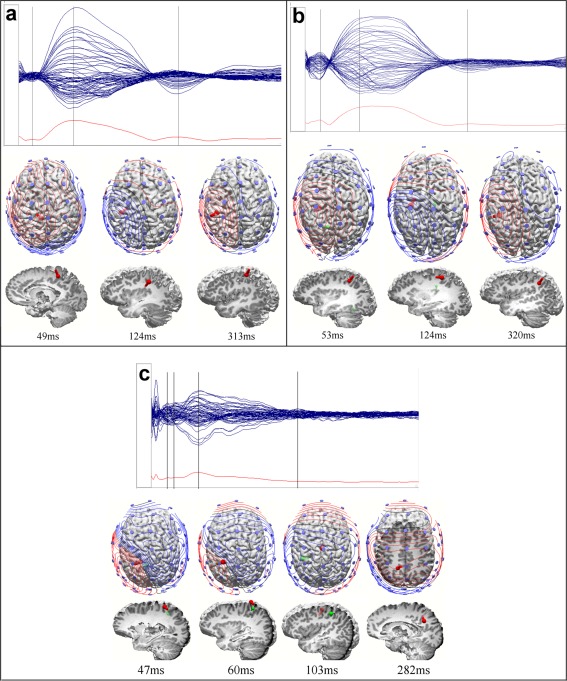
Dipole localization using average reference was conducted in order to approximate the number and site of sources accounting for TEP components. In upper panel, grand average of the EEG responses presented as butterfly plots and GMFP in time window 0–500 ms. Zero corresponds to the TMS pulse. Lower panel visualizes dipoles from top and left view of the brain. Also potential maps and electrode location are shown in top view. All voltage scales are optimized for each group and component. (a) TEP peaks in children 49 (P50), 124 (N100), and 313 (P300) ms after 110%MT motor cortex stimulation. (b) TEP peaks in adolescents 53 (P50), 124 (N100), and 320 (P300) ms after 110%MT motor cortex stimulation. (c) TEP peaks in adults 47 (N45), 60 (P50), 103 (N100), and 282 (N280) ms after 110%MT motor cortex stimulation
N100 (maximal negativity between 70 and 200 ms)
The N100 amplitude differed significantly between the groups (Table 2). In children, the N100 was significantly larger than in adults (−60.1 µV vs. −15.4 µV, P = 0.001), and there was a tendency for a larger response in children than in adolescents (−60.1 µV vs. −34.7 µV, P = 0.066), whereas the response did not differ between adults and adolescents. The N100 amplitude was maximal centrally at the left medio‐lateral channels in all age groups (−131.4 µV in children, −62.4 µV in adolescents and −24.0 µV in adults).
Also, significant differences in topography of N100 were found between the groups (Table 2). Post hoc analyses for group, AP, and ML revealed that the response in children was significantly higher than that of adults centrally in the left lateral and left mediolateral regions (P < 0.001 in both cases), and parieto‐occipitally in the left lateral and mediolateral regions (P = 0.024 and P < 0.001, respectively). Compared with adolescents, the response of children was higher in the central region in the left mediolateral electrode group. No differences between adolescents and adults were recorded.
The N100 latency did not differ significantly between the groups as evaluated by the mean latency in the left mediolateral region (127 ms in children, 129 ms in adolescents, and 117 ms in adults; P > 0.05 in all comparisons).
In children, the N100 was explained by two radial dipoles: one between C3 and C1 pointing in and the other, a weak dipole (SNR = 1.5) pointing out in the middle between Cz and C2. N100 in adolescents was accounted for by two dipoles: a cortical dipole tangential in a posterior‐to‐anterior direction between FC1 and C1 and a deep radial dipole parasagittally between FCz and Cz, pointing in. N100 in adults was explained by two dipoles: a parasagittal dipole pointing in radially close to the FCz electrode and a cortical dipole pointing tangentially to the right in the vicinity of C1. See also Figure 5 and Supporting Information Table.
P300 (maximal positivity between 200 and 400 ms)
The P300 was significantly larger in children (16.5 µV) than in adolescents (9.0 µV) (Table 2). Significant differences in topography of P300 were also found between these groups (Table 2). In the post hoc analysis of the group, AP, and ML interaction, the difference was only significant in the left central mediolateral region (P = 0.001), where the response was also maximal in both groups (33.2 µV in children and 16.0 µV in adolescents). The P300 latency did not differ between children and adolescents (307 and 305 ms, respectively).
In adults, the negative peak that was present within 200–400 ms time frame was maximal in the left central mediolateral region (peak amplitude −9.7 µV, mean latency 257 ms).
P300 was explained by one radial cortical dipole pointing out between C1 and Cz in both children and adolescents. N280 was explained by one deep radial dipole pointing out to Cz. See also Figure 5 and Supporting Information Table.
ERSP/ITC
The results of frequency analysis using ERSP and ITC are presented in Figure 6a and b. For ITC, a significant increment was found in both alpha and beta bands between children and adults, and the increment covered both of the analyzed time periods. Within the first 200 ms, it peaked at channels localized mainly in the left centro‐parietal regions, whereas in the later time range, differences were also seen in the contralateral hemisphere. Adolescents also showed increased ITC compared with adults in both alpha and beta ranges. This difference was not significant within the first 200 ms, but peaked in the 200–500 time range in left central and to a lesser extent in right central and frontal regions. There were no statistically significant differences between children and adolescents. In topographical analyses, ERSP values for alpha and beta showed no significant difference for either of the time ranges analyzed (data not shown).
Figure 6.
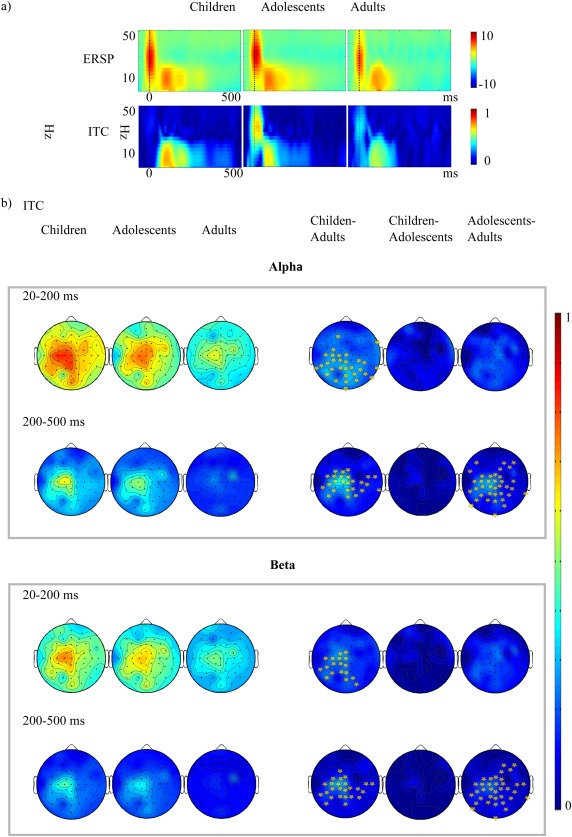
(a) The group‐wise ERSP (above) and ITC (below) values averaged from all channels. Zero corresponds to the TMS pulse. (b) ITC topography in alpha (8–15 Hz) and (beta 15–25) Hz range. In the right column the difference between the ITC distributions is shown (children minus adults, children minus adolescents and adolescents minus adults). The light dots represent the scalp positions where ITC is significantly increased in children and adolescents compared with adults.
DISCUSSION
Main Findings
The results of this study describe, for the first time, brain development in multiple aspects of TEPs to suprathreshold M1 stimulation. We demonstrated that typical development from childhood to adulthood is accompanied by changes in TEP morphology and amplitude, as well as a modification of cortical intrahemispheric and interhemispheric connectivity. Moreover, we revealed clear changes in the phase‐locking properties of alpha and beta rhythms. Finally, we showed that developmental changes in TMS‐related general neurophysiological indices (i.e., MT and GMFP) occur in parallel with improvement in manual dexterity.
Motor Network Developmental Changes in the Time Domain
Motor network excitability can be measured with various parameters. The MT is used to describe the pathway excitability. In accordance with the existing literature [Garvey et al., 2003; Moll et al., 1999; Nezu et al., 1997; Pitcher et al., 2015] we found a significant decrement in MT with age. Furthermore, decreased excitability in children was associated with poorer manual dexterity, which is also in line with previous reports [Pitcher et al., 2012]. MT reflects excitability in intracortical interneurons, upper motor neurons, lower motoneurons, and muscle fibers. At the cellular level, MT expresses membrane excitability [Ziemann et al., 1996]. Thus, MT reflects the developmental stage of myelination in the corticospinal tracts, cortical motor afferents, and synaptic efficacy within the motor cortex and spinal cord. Recent observations have suggested that most of the variance in MTs can be explained by the SCD and white matter fiber orientation in cortico‐pyramidal tracts [Herbsman et al., 2009]. It is noteworthy that our results do not support the view that developmental differences in MT would stem from the SCD, which was significantly shorter in children compared with adults.
Another relevant parameter is the GMFP, which is an indicator of global cerebral activation in response to an external perturbation, also giving information on the functional connectivity of the stimulated area. When using a suprathreshold stimulus intensity, TMS elicited a notably enhanced GMFP response in children compared with adults, with the adolescents' responses lying between those of children and adults. This age‐dependent increment also associated with developmental changes in manual dexterity. Previous developmental TMS‐EEG studies have focused on TEP in the N100 latency range and shown that N100 is increased in children compared with adults [Bender et al., 2005; D'Agati et al., 2014; Helfrich et al., 2012]. Importantly, it has been demonstrated that the decline in N100 amplitude with development is not attributed only to age‐related differences in MT but represents true maturational effects [Bender et al., 2005] and might be associated with gray matter loss in the pre‐ and peripubescent period [Jarczok et al., 2016; Whitford et al., 2007]. Adding to the literature, we found an increment in GMFP amplitude that starts well before and goes well beyond the N100 latency range.
In adults, suprathreshold stimulation of M1 elicited the well‐known TEP waveform [Bonato et al., 2006; Ferreri et al., 2011; Kähkonen et al., 2004; Lioumis et al., 2009; Paus et al., 2001; Premoli et al., 2014]. The younger age groups, on the contrary, demonstrated a completely different waveform. Within the N45–P60 latency window, no distinction between N45 and P60 peaks was seen, but positivity at around 50 ms was observed instead. Topographically, this component was located more posteriorly compared with the adult N45, and more anteriorly and centrally compared with the typical P60 in adults. Furthermore, at around 300 ms, the centrally maximal TEP was negative in adults but positive in the younger age groups.
Our source analysis showed two dipoles for the N45 component in the motor region in adults. This is in accordance with previous adult studies, in which the origin of N45 has been localized to the ipsilateral central sulcus [Litvak et al., 2007; Paus et al., 2001]. The sources for the following components, P50 and N100, have similar behavior: both of these components in children were explained with one dipole, whereas adolescents and adults have two explaining sources. For P50, the sources we found were in the vicinity of sensorimotor areas, except for the second source for the P50 component in adolescents, which was located in the lingual gyrus. The localized source in the sensorimotor area is in concordance with an earlier study suggesting that the P50 peak could be a hallmark of sensorimotor interaction [Ferreri et al., 2012].
The N100 has been related to the activity of the cortico–striato–thalamo–cortical loops and/or long‐range corticocortical connections of the callosal fibers [Ferrarelli et al., 2010; Premoli et al., 2014]. In all age groups, this TEP was maximal in the left centroparietal region, with the dipoles suggesting a source in the motor cortex. In children, the N100 amplitude was significantly increased compared with older age groups. Of interest is that much of the amplitude augmentation was located under the stimulated area and in its vicinity.
The TEP component around 300 ms is explained with one dipole in all groups. The dipole of children and adolescents is more lateralized and in the vicinity of the primary motor area of the hand, pointing out radially, whereas the dipole in adults is closer to the midline in the parietal area and pointing radially to Cz. The source structure and neuropharmacology of the late TEP components is not known, but a link to GABAB‐related activity has been suggested [Ferreri and Rossini, 2013].
Consistent with the above‐mentioned finding of a local increase in TEP responses in the N100 latency range, we found that signal spreading within the hemisphere as well as the spreading of interhemispheric activity increased with development. As activation of M1 in one hemisphere sends an excitatory signal transcallosally that excites inhibitory interneurons in the contralateral M1, reducing the net excitatory output [Daskalakis et al., 2002], spreading of the interhemispheric signal has been related to the microstructure of the corpus callosum [Voineskos et al., 2010], and the facilitation of interhemispheric transfer as a function of development was interpreted to reflect the maturation of the corpus callosum [Jarczok et al., 2016]. Thus, one likely contributor to increased efficiency of signal propagation is the elaboration of the myelin sheet, which may support the functional integration of distant regions [Fair et al., 2007; Luna and Sweeney, 2004], as childhood and adolescent development is accompanied by the refinement of white matter connectivity between brain regions [Barnea‐Goraly et al., 2004; Battino et al., 1995; Chen et al., 2016; Eluvathingal et al., 2007; Giedd et al., 1999a; Keshavan et al., 2002; Lebel et al., 2008; Rajapakse et al., 1996; Snook et al., 2005]. It is generally agreed that maturation of the corpus callosum continues through adolescence into young adulthood [Barnea‐Goraly et al., 2004; Chen et al., 2016; Giedd et al., 1999b; Keshavan et al., 2002; Rajapakse et al., 1996; Snook et al., 2005], although some recent studies suggest that the growth of callosal regions containing motor fibers, that is, the body and isthmus, may be complete before the age of 10 years [Cancelliere et al., 2013; Kwon et al., 2014].
In addition to structural factors, the excitation–inhibition (glutamate/GABA) balance and GABA levels are strictly correlated with the strength of functional short‐range and long‐range connectivity [Ferreri and Rossini, 2013; Ghisleni et al., 2015; Sampaio‐Baptista et al., 2015; Stagg et al., 2014]. Accordingly, evidence strongly suggests that GABAB receptors are involved in interhemispheric connectivity [Daskalakis et al., 2002; Palmer et al., 2012]. In the hippocampus, the GABAAergic system attains mature properties before the onset of puberty. Neocortical GABAAergic projections may undergo massive modifications until the end of adolescence [Caballero et al., 2014; Chugani et al., 2001; Hashimoto et al., 2009; Kilb, 2012; Silveri et al., 2013; Wang and Kriegstein, 2009] although a recent study suggests that GABAAergic inhibitory motor circuits are largely mature by 10 years of age [Schneider et al., 2016]. Only a few (rodent) studies have investigated the role of GABAB function in development, providing evidence of the importance of GABAB receptor‐mediated signaling in normal brain maturation [Bolton et al., 2015; Prosser et al., 2001; Queva et al., 2003; Schuler et al., 2001]. However, there are no data on how GABAB functioning changes during different developmental periods. The concentration of glutamate, the major excitatory neurotransmitter in the central nervous system, remains relatively stable in both cerebral white and gray matter after early infancy [Bluml et al., 2013].
Given that there is evidence showing that functional connectivity without direct structural connections strengthens with development [Betzel et al., 2014], and that cortico‐cortical effective connectivity may depend on non‐structural factors such as medication and state of consciousness [Ferrarelli et al., 2010; Massimini et al., 2005; Premoli et al., 2014], different GABAergic neurotransmission in local inhibitory networks could in part underlie enhanced cortical activation and restricted propagation of TEPs from the stimulated area in the younger age groups in our study. In the future, a developmental paired‐pulse TMS‐EEG study assessing motor cortical short‐interval and long‐interval intracortical inhibition [Ferreri et al., 2011; Fitzgerald et al., 2008] could confirm the aforementioned association between developmental differences in GABA‐mediated neurotransmission and TEPs.
Motor Network Developmental Changes in the Frequency Domain
In addition to studying TEPs in the time domain, we used two complementary indices in the frequency domain: ITC and ERSP. The TMS‐evoked oscillations most likely result from the resetting of ongoing oscillatory activity, and are not activated by TMS itself [Fuggetta et al., 2005; Paus et al., 2001; Rosanova et al., 2009]. In our study, TMS of the motor cortex produced a robust and highly significant increase in phase locking in children and adolescents compared with adults, while the ERSP measures showed no statistically significant developmental differences. This reflects the similar amplitude dynamics of TMS‐evoked activity between the groups, but the consistency of the instantaneous phase was greater in children and adolescents than in adults. The ITC increment of children was not frequency specific and covered a large time range. This suggests that in childhood and adolescence, there may be a generic increase in the ability of motor circuits to engage in synchronous activity. Topographical analyses demonstrated that within the first 200 ms, differences peaked at channels mainly localized in the left centro‐parietal regions, whereas in the later time range, differences were also seen in the contralateral hemisphere. The difference between adolescents and adults did not reach statistical difference within the first 200 ms, but peaked in the 200–500 time range in left central and to a lesser extent in right central and frontal regions. Previous studies have proposed that evoked potentials in EEG result from the combination of power enhancement and phase synchrony in specific frequency bands [Cebolla et al., 2009; Cheron et al., 2007]. In the present study, no between‐group differences were observed in the power level of any of the evaluated frequency bands, whereas a significant increase in phase synchrony was detected in children and adolescences in comparison to adults. The greater phase synchrony in children and adolescences may thus explain the larger TEPs observed in these groups in comparison to those of adults. The electrical rhythms triggered by TMS probably reflect overall circuit properties at the level of whole cortical areas and connected thalamic, subcortical nuclei [Rosanova et al., 2009]. Thus, various factors, including differences in (GABAergic) inhibitory modulation of the evoked oscillations [Cho et al., 2015; Ferrarelli et al., 2008; Fukui et al., 2010; Julkunen et al., 2013], in signal variability, or in neural network connectivity [Haider et al., 2010; Lippe et al., 2009; Miyauchi et al., 2016; Moldakarimov et al., 2015; Vakorin et al., 2011], may explain the age‐related differences in ITC in our study. The exact interaction of the intracellular and network properties underlying enhanced ITC in children and adolescents merits further investigation.
Strengths and Limitations of the Study
Since the number of participants was low, the results of this study have to be considered as preliminary. In order to maximize the accuracy and reliability of our results, we used cutting edge technologies like HdEEG and neuronavigation. Furthermore, the number of trials averaged per subject was relatively high, thus minimizing the effect of coincidental errors on the TEP waveform. Despite the small group sizes, we found statistically significant changes in cortical excitability and connectivity between different age groups. These changes should be studied in the future in a larger population.
The possible influence of volume conduction, the transmission of electric or magnetic fields from an electric primary current source through biological tissue toward measurement sensors, is another potential limitation in TMS‐EEG studies. Jarczok et al. [2016] addressed this question by comparing TMS‐evoked interhemispheric transmission between different age groups, and found signal propagation to be higher in older subjects who can be assumed to have larger heads and therefore a longer distance between the two cortical areas (i.e., reduced effects of volume conduction). Our results showing reduced intra‐ and interhemispheric signal propagation in children compared with adults are in line with this, giving further support to the notion that age‐related differences in signal propagation are related to differences in connectivity and not an artifact caused by signal degradation.
CONCLUSIONS
According to the results of our study, children have a different pattern of activation in response to motor cortex TMS than adults. They have a less complex waveform configuration, particularly within the first 100 ms post‐stimulus, a more consistent phase of the response, higher induced activation and more restricted signal spreading compared with adults. These results are well in accordance with the findings of developmental studies on intrinsic functional connectivity [Supekar et al., 2009], the evolution of brain connectivity patterns [Yap et al., 2011], and the development of neural systems underlying cognition [Fair et al., 2009]. Previous observations [Fair et al., 2009; Vakorin et al., 2011] and the results of the current study together show that the brain of children develops during maturation from strong local connectivity toward a more distributed, predominantly functional based connectivity pattern characterized by stronger integration.
Supporting information
Supporting Information Table 1.
ACKNOWLEDGMENTS
We thank Meri Julkunen and Sirpa Heikkinen‐Knuutila for their help with the measurements. Professor Petro Julkunen is acknowledged for providing invaluable help with MATLAB scripts. We also thank all the volunteers for participating in the study.
The authors declare that they have no conflicts of interest.
REFERENCES
- Awiszus F (2003): TMS and threshold hunting. Clin Neurophysiol Suppl 56:13–23. [DOI] [PubMed] [Google Scholar]
- Awiszus F (2012): On relative frequency estimation of transcranial magnetic stimulation motor threshold. Clin Neurophysiol 123:2319–2320. [DOI] [PubMed] [Google Scholar]
- Barnea‐Goraly N, Kwon H, Menon V, Eliez S, Lotspeich L, Reiss AL (2004): White matter structure in autism: Preliminary evidence from diffusion tensor imaging. Biol Psychiatry 55:323–326. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Beckson M, Lu PH, Nuechterlein KH, Edwards N, Mintz J (2001): Age‐related changes in frontal and temporal lobe volumes in men: A magnetic resonance imaging study. Arch Gen Psychiatry 58:461–465. [DOI] [PubMed] [Google Scholar]
- Battino D, Estienne M, Avanzini G (1995): Clinical pharmacokinetics of antiepileptic drugs in paediatric patients. Part II. Phenytoin, carbamazepine, sulthiame, lamotrigine, vigabatrin, oxcarbazepine and felbamate. Clin Pharmacokinet 29:341–369. [DOI] [PubMed] [Google Scholar]
- Bender S, Basseler K, Sebastian I, Resch F, Kammer T, Oelkers‐Ax R, Weisbrod M (2005): Electroencephalographic response to transcranial magnetic stimulation in children: Evidence for giant inhibitory potentials. Ann Neurol 58:58–67. [DOI] [PubMed] [Google Scholar]
- Berchicci M, Tamburro G, Comani S (2015): The intrahemispheric functional properties of the developing sensorimotor cortex are influenced by maturation. Front Hum Neurosci 9:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestmann S, Baudewig J, Siebner HR, Rothwell JC, Frahm J (2004): Functional MRI of the immediate impact of transcranial magnetic stimulation on cortical and subcortical motor circuits. Eur J Neurosci 19:1950–1962. [DOI] [PubMed] [Google Scholar]
- Betzel RF, Byrge L, He Y, Goni J, Zuo XN, Sporns O (2014): Changes in structural and functional connectivity among resting‐state networks across the human lifespan. Neuroimage 102 Pt 2:345–357. [DOI] [PubMed] [Google Scholar]
- Bluml S, Wisnowski JL, Nelson MD, Jr. , Paquette L, Gilles FH, Kinney HC, Panigrahy A (2013): Metabolic maturation of the human brain from birth through adolescence: Insights from in vivo magnetic resonance spectroscopy. Cereb Cortex 23:2944–2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton MM, Heaney CF, Murtishaw AS, Sabbagh JJ, Magcalas CM, Kinney JW (2015): Postnatal alterations in GABAB receptor tone produce sensorimotor gating deficits and protein level differences in adulthood. Int J Dev Neurosci 41:17–27. [DOI] [PubMed] [Google Scholar]
- Bonato C, Miniussi C, Rossini PM (2006): Transcranial magnetic stimulation and cortical evoked potentials: A TMS/EEG co‐registration study. Clin Neurophysiol 117:1699–1707. [DOI] [PubMed] [Google Scholar]
- Caballero A, Thomases DR, Flores‐Barrera E, Cass DK, Tseng KY (2014): Emergence of GABAergic‐dependent regulation of input‐specific plasticity in the adult rat prefrontal cortex during adolescence. Psychopharmacology 231:1789–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancelliere A, Mangano FT, Air EL, Jones BV, Altaye M, Rajagopal A, Holland SK, Hertzler DA, 2nd , Yuan W (2013): DTI values in key white matter tracts from infancy through adolescence. AJNR Am J Neuroradiol 34:1443–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebolla AM, De Saedeleer C, Bengoetxea A, Leurs F, Balestra C, d'Alcantara P, Palmero‐Soler E, Dan B, Cheron G (2009): Movement gating of beta/gamma oscillations involved in the N30 somatosensory evoked potential. Hum Brain Mapp 30:1568–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Zhang H, Yushkevich PA, Liu M, Beaulieu C (2016): Maturation along white matter tracts in human brain using a diffusion tensor surface model tract‐specific analysis. Front Neuroanat 10:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheron G, Cebolla AM, De Saedeleer C, Bengoetxea A, Leurs F, Leroy A, Dan B (2007): Pure phase‐locking of beta/gamma oscillation contributes to the N30 frontal component of somatosensory evoked potentials. BMC Neurosci 8:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho RY, Walker CP, Polizzotto NR, Wozny TA, Fissell C, Chen CM, Lewis DA (2015): Development of sensory gamma oscillations and cross‐frequency coupling from childhood to early adulthood. Cereb Cortex 25:1509–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugani DC, Muzik O, Juhasz C, Janisse JJ, Ager J, Chugani HT (2001): Postnatal maturation of human GABAA receptors measured with positron emission tomography. Ann Neurol 49:618–626. [PubMed] [Google Scholar]
- D'Agati E, Hoegl T, Dippel G, Curatolo P, Bender S, Kratz O, Moll GH, Heinrich H (2014): Motor cortical inhibition in ADHD: Modulation of the transcranial magnetic stimulation‐evoked N100 in a response control task. J Neural Transm (Vienna) 121:315–325. [DOI] [PubMed] [Google Scholar]
- Daskalakis ZJ, Christensen BK, Fitzgerald PB, Roshan L, Chen R (2002): The mechanisms of interhemispheric inhibition in the human motor cortex. J Physiol 543:317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bie HM, Boersma M, Adriaanse S, Veltman DJ, Wink AM, Roosendaal SD, Barkhof F, Stam CJ, Oostrom KJ, Delemarre‐van de Waal HA, Sanz‐Arigita EJ (2012): Resting‐state networks in awake five‐ to eight‐year old children. Hum Brain Mapp 33:1189–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A, Makeig S (2004): EEGLAB: An open source toolbox for analysis of single‐trial EEG dynamics including independent component analysis. J Neurosci Methods 134:9–21. [DOI] [PubMed] [Google Scholar]
- Denslow S, Lomarev M, George MS, Bohning DE (2005): Cortical and subcortical brain effects of transcranial magnetic stimulation (TMS)‐induced movement: An interleaved TMS/functional magnetic resonance imaging study. Biol Psychiatry 57:752–760. [DOI] [PubMed] [Google Scholar]
- Eloranta AM, Lindi V, Schwab U, Tompuri T, Kiiskinen S, Lakka HM, Laitinen T, Lakka TA (2012): Dietary factors associated with overweight and body adiposity in Finnish children aged 6‐8 years: The PANIC Study. Int J Obes 36:950–955. [DOI] [PubMed] [Google Scholar]
- Eluvathingal TJ, Hasan KM, Kramer L, Fletcher JM, Ewing‐Cobbs L (2007): Quantitative diffusion tensor tractography of association and projection fibers in normally developing children and adolescents. Cereb Cortex 17:2760–2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Dosenbach NU, Church JA, Cohen AL, Brahmbhatt S, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL (2007): Development of distinct control networks through segregation and integration. Proc Natl Acad Sci U S A 104:13507–13512. 17679691 [Google Scholar]
- Fair DA, Cohen AL, Power JD, Dosenbach NU, Church JA, Miezin FM, Schlaggar BL, Petersen SE (2009): Functional brain networks develop from a “local to distributed” organization. PLoS Comput Biol 5:e1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD (1992): Interhemispheric inhibition of the human motor cortex. J Physiol 453:525–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrarelli F, Massimini M, Peterson MJ, Riedner BA, Lazar M, Murphy MJ, Huber R, Rosanova M, Alexander AL, Kalin N, Tononi G (2008): Reduced evoked gamma oscillations in the frontal cortex in schizophrenia patients: A TMS/EEG study. Am J Psychiatry 165:996–1005. [DOI] [PubMed] [Google Scholar]
- Ferrarelli F, Massimini M, Sarasso S, Casali A, Riedner BA, Angelini G, Tononi G, Pearce RA (2010): Breakdown in cortical effective connectivity during midazolam‐induced loss of consciousness. Proc Natl Acad Sci 107:2681–2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreri F, Rossini PM (2013): TMS and TMS‐EEG techniques in the study of the excitability, connectivity, and plasticity of the human motor cortex. Rev Neurosci 24:431–442. [DOI] [PubMed] [Google Scholar]
- Ferreri F, Pasqualetti P, Määttä S, Ponzo D, Ferrarelli F, Tononi G, Mervaala E, Miniussi C, Rossini PM (2011): Human brain connectivity during single and paired pulse transcranial magnetic stimulation. Neuroimage 54:90–102. [DOI] [PubMed] [Google Scholar]
- Ferreri F, Ponzo D, Hukkanen T, Mervaala E, Könönen M, Pasqualetti P, Vecchio F, Rossini PM, Määttä S (2012): Human brain cortical correlates of short‐latency afferent inhibition: A combined EEG‐TMS study. J Neurophysiol 108:314–323. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Daskalakis ZJ, Hoy K, Farzan F, Upton DJ, Cooper NR, Maller JJ (2008): Cortical inhibition in motor and non‐motor regions: A combined TMS‐EEG study. Clin EEG Neurosci 39:112–117. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Maller JJ, Hoy K, Farzan F, Daskalakis ZJ (2009): GABA and cortical inhibition in motor and non‐motor regions using combined TMS‐EEG: A time analysis. Clin Neurophysiol 120:1706–1710. [DOI] [PubMed] [Google Scholar]
- Fuggetta G, Fiaschi A, Manganotti P (2005): Modulation of cortical oscillatory activities induced by varying single‐pulse transcranial magnetic stimulation intensity over the left primary motor area: A combined EEG and TMS study. Neuroimage 27:896–908. [DOI] [PubMed] [Google Scholar]
- Fukui I, Burger RM, Ohmori H, Rubel EW (2010): GABAergic inhibition sharpens the frequency tuning and enhances phase locking in chicken nucleus magnocellularis neurons. J Neurosci 30:12075–12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvey MA, Ziemann U, Bartko JJ, Denckla MB, Barker CA, Wassermann EM (2003): Cortical correlates of neuromotor development in healthy children. Clin Neurophysiol 114:1662–1670. [DOI] [PubMed] [Google Scholar]
- Ghisleni C, Bollmann S, Poil S, Brandeis D, Martin E, Michels L, O'Gorman RL, Klaver P (2015): Subcortical glutamate mediates the reduction of short‐range functional connectivity with age in a developmental cohort. J Neurosci 35:8433–8441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN (2004): Structural magnetic resonance imaging of the adolescent brain. Ann N Y Acad Sci 1021:77–85. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL (1999a): Brain development during childhood and adolescence: A longitudinal MRI study. Nat Neurosci 2:861–863. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Rajapakse JC, Vaituzis AC, Liu H, Berry YC, Tobin M, Nelson J, Castellanos FX (1999b): Development of the human corpus callosum during childhood and adolescence: A longitudinal MRI study. Prog Neuropsychopharmacol Biol Psychiatry 23:571–588. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3rd , Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM (2004): Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci 101:8174–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider B, Krause MR, Duque A, Yu Y, Touryan J, Mazer JA, McCormick DA (2010): Synaptic and network mechanisms of sparse and reliable visual cortical activity during nonclassical receptive field stimulation. Neuron 65:107–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Nguyen QL, Rotaru D, Keenan T, Arion D, Beneyto M, Gonzalez‐Burgos G, Lewis DA (2009): Protracted developmental trajectories of GABAA receptor alpha1 and alpha2 subunit expression in primate prefrontal cortex. Biol Psychiatry 65:1015–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinen F, Fietzek UM, Berweck S, Hufschmidt A, Deuschl G, Korinthenberg R (1998): Fast corticospinal system and motor performance in children: Conduction proceeds skill. Pediatr Neurol 19:217–221. [DOI] [PubMed] [Google Scholar]
- Helfrich C, Pierau SS, Freitag CM, Roeper J, Ziemann U, Bender S (2012): Monitoring cortical excitability during repetitive transcranial magnetic stimulation in children with ADHD: A single‐blind, sham‐controlled TMS‐EEG study. PloS One 7:e50073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbsman T, Forster L, Molnar C, Dougherty R, Christie D, Koola J, Ramsey D, Morgan PS, Bohning DE, George MS, Nahas Z (2009): Motor threshold in transcranial magnetic stimulation: The impact of white matter fiber orientation and skull‐to‐cortex distance. Hum Brain Mapp 30:2044–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilmoniemi RJ, Virtanen J, Ruohonen J, Karhu J, Aronen HJ, Näätänen R, Katila T (1997): Neuronal responses to magnetic stimulation reveal cortical reactivity and connectivity. Neuroreport 8:3537–3540. [DOI] [PubMed] [Google Scholar]
- Jarczok TA, Fritsch M, Kroger A, Schneider AL, Althen H, Siniatchkin M, Freitag CM, Bender S (2016): Maturation of interhemispheric signal propagation in autism spectrum disorder and typically developing controls: A TMS‐EEG study. J Neural Transm (Vienna) 123:925–935. [DOI] [PubMed] [Google Scholar]
- Julkunen P (2014): Methods for estimating cortical motor representation size and location in navigated transcranial magnetic stimulation. J Neurosci Methods 232:125–133. [DOI] [PubMed] [Google Scholar]
- Julkunen P, Säisänen L, Könönen M, Vanninen R, Kälviäinen R, Mervaala E (2013): TMS‐EEG reveals impaired intracortical interactions and coherence in Unverricht‐Lundborg type progressive myoclonus epilepsy (EPM1). Epilepsy Res 106:103–112. [DOI] [PubMed] [Google Scholar]
- Kähkonen S, Wilenius J, Komssi S, Ilmoniemi RJ (2004): Distinct differences in cortical reactivity of motor and prefrontal cortices to magnetic stimulation. Clin Neurophysiol 115:583–588. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Diwadkar VA, DeBellis M, Dick E, Kotwal R, Rosenberg DR, Sweeney JA, Minshew N, Pettegrew JW (2002): Development of the corpus callosum in childhood, adolescence and early adulthood. Life Sci 70:1909–1922. [DOI] [PubMed] [Google Scholar]
- Kilb W (2012): Development of the GABAergic system from birth to adolescence. Neuroscientist 18:613–630. [DOI] [PubMed] [Google Scholar]
- Komssi S, Aronen HJ, Huttunen J, Kesäniemi M, Soinne L, Nikouline VV, Ollikainen M, Roine RO, Karhu J, Savolainen S, Ilmoniemi RJ (2002): Ipsi‐ and contralateral EEG reactions to transcranial magnetic stimulation. Clin Neurophysiol 113:175–184. [DOI] [PubMed] [Google Scholar]
- Kwon HG, Son SM, Jang SH (2014): Development of the transcallosal motor fiber from the corticospinal tract in the human brain: Diffusion tensor imaging study. Front Human Neurosci 8:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C (2008): Microstructural maturation of the human brain from childhood to adulthood. Neuroimage 40:1044–1055. [DOI] [PubMed] [Google Scholar]
- Lehmann D, Skrandies W (1980): Reference‐free identification of components of checkerboard‐evoked multichannel potential fields. Electroencephalogr Clin Neurophysiol 48:609–621. [DOI] [PubMed] [Google Scholar]
- Lin W, Zhu Q, Gao W, Chen Y, Toh CH, Styner M, Gerig G, Smith JK, Biswal B, Gilmore JH (2008): Functional connectivity MR imaging reveals cortical functional connectivity in the developing brain. AJNR Am J Neuroradiol 29:1883–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lioumis P, Kicic D, Savolainen P, Mäkelä JP, Kähkönen S (2009): Reproducibility of TMS‐Evoked EEG responses. Hum Brain Mapp 30:1387–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippe S, Kovacevic N, McIntosh AR (2009): Differential maturation of brain signal complexity in the human auditory and visual system. Front Human Neurosci 3:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvak V, Komssi S, Scherg M, Hoechstetter K, Classen J, Zaaroor M, Pratt H, Kahkonen S (2007): Artifact correction and source analysis of early electroencephalographic responses evoked by transcranial magnetic stimulation over primary motor cortex. Neuroimage 37:56–70. [DOI] [PubMed] [Google Scholar]
- Luna B, Sweeney JA (2004): The emergence of collaborative brain function: FMRI studies of the development of response inhibition. Ann N Y Acad Sci 1021:296–309. [DOI] [PubMed] [Google Scholar]
- Massimini M, Ferrarelli F, Huber R, Esser SK, Singh H, Tononi G (2005): Breakdown of cortical effective connectivity during sleep. Science 309:2228–2232. [DOI] [PubMed] [Google Scholar]
- Mathiowetz V, Volland G, Kashman N, Weber K (1985): Adult norms for the Box and Block Test of manual dexterity. Am J Occup Ther 39:386–391. [DOI] [PubMed] [Google Scholar]
- Mierau A, Felsch M, Hulsdunker T, Mierau J, Bullermann P, Weiss B, Struder HK (2016): The interrelation between sensorimotor abilities, cognitive performance and individual EEG alpha peak frequency in young children. Clin Neurophysiol 127:270–276. [DOI] [PubMed] [Google Scholar]
- Miyauchi E, Kitajo K, Kawasaki M (2016): TMS‐induced theta phase synchrony reveals a bottom‐up network in working memory. Neurosci Lett 622:10–14. [DOI] [PubMed] [Google Scholar]
- Moldakarimov S, Bazhenov M, Sejnowski TJ (2015): Feedback stabilizes propagation of synchronous spiking in cortical neural networks. Proc Natl Acad Sci 112:2545–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll GH, Heinrich H, Wischer S, Tergau F, Paulus W, Rothenberger A (1999): Motor system excitability in healthy children: Developmental aspects from transcranial magnetic stimulation. Electroencephalogr Clin Neurophysiol Suppl 51:243–249. [PubMed] [Google Scholar]
- Neuper C, Pfurtscheller G (2001): Evidence for distinct beta resonance frequencies in human EEG related to specific sensorimotor cortical areas. Clin Neurophysiol 112:2084–2097. [DOI] [PubMed] [Google Scholar]
- Nezu A, Kimura S, Uehara S, Kobayashi T, Tanaka M, Saito K (1997): Magnetic stimulation of motor cortex in children: Maturity of corticospinal pathway and problem of clinical application. Brain Dev 19:176–180. [DOI] [PubMed] [Google Scholar]
- Palmer LM, Schulz JM, Murphy SC, Ledergerber D, Murayama M, Larkum ME (2012): The cellular basis of GABA(B)‐mediated interhemispheric inhibition. Science 335:989–993. [DOI] [PubMed] [Google Scholar]
- Paus T, Sipila PK, Strafella AP (2001): Synchronization of neuronal activity in the human primary motor cortex by transcranial magnetic stimulation: An EEG study. J Neurophysiol 86:1983–1990. [DOI] [PubMed] [Google Scholar]
- Piek JP, Dawson L, Smith LM, Gasson N (2008): The role of early fine and gross motor development on later motor and cognitive ability. Hum Mov Sci 27:668–681. [DOI] [PubMed] [Google Scholar]
- Pitcher JB, Schneider LA, Burns NR, Drysdale JL, Higgins RD, Ridding MC, Nettelbeck TJ, Haslam RR, Robinson JS (2012): Reduced corticomotor excitability and motor skills development in children born preterm. J Physiol 590:5827–5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher JB, Doeltgen SH, Goldsworthy MR, Schneider LA, Vallence AM, Smith AE, Semmler JG, McDonnell MN, Ridding MC (2015): A comparison of two methods for estimating 50% of the maximal motor evoked potential. Clin Neurophysiol 126:2337–2341. [DOI] [PubMed] [Google Scholar]
- Premoli I, Castellanos N, Rivolta D, Belardinelli P, Bajo R, Zipser C, Espenhahn S, Heidegger T, Muller‐Dahlhaus F, Ziemann U (2014): TMS‐EEG signatures of GABAergic neurotransmission in the human cortex. J Neurosci 34:5603–5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser HM, Gill CH, Hirst WD, Grau E, Robbins M, Calver A, Soffin EM, Farmer CE, Lanneau C, Gray J, Schenck E, Warmerdam BS, Clapham C, Reavill C, Rogers DC, Stean T, Upton N, Humphreys K, Randall A, Geppert M, Davies CH, Pangalos MN (2001): Epileptogenesis and enhanced prepulse inhibition in GABA(B1)‐deficient mice. Mol Cell Neurosci 17:1059–1070. [DOI] [PubMed] [Google Scholar]
- Queva C, Bremner‐Danielsen M, Edlund A, Ekstrand AJ, Elg S, Erickson S, Johansson T, Lehmann A, Mattsson JP (2003): Effects of GABA agonists on body temperature regulation in GABA(B(1))‐/‐ mice. Br J Pharmacol 140:315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajapakse JC, Giedd JN, Rumsey JM, Vaituzis AC, Hamburger SD, Rapoport JL (1996): Regional MRI measurements of the corpus callosum: A methodological and developmental study. Brain Dev 18:379–388. [DOI] [PubMed] [Google Scholar]
- Rigoli D, Piek JP, Kane R, Oosterlaan J (2012): An examination of the relationship between motor coordination and executive functions in adolescents. Dev Med Child Neurol 54:1025–1031. [DOI] [PubMed] [Google Scholar]
- Roebers CM, Rothlisberger M, Neuenschwander R, Cimeli P, Michel E, Jager K (2014): The relation between cognitive and motor performance and their relevance for children's transition to school: A latent variable approach. Hum Mov Sci 33:284–297. [DOI] [PubMed] [Google Scholar]
- Rosanova M, Casali A, Bellina V, Resta F, Mariotti M, Massimini M (2009): Natural frequencies of human corticothalamic circuits. J Neurosci 29:7679–7685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual‐Leone A (2009): Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol 120:2008–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual‐Leone A (2011): Screening questionnaire before TMS: An update. Clin Neurophysiol 122:1686. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Burke D, Chen R, Cohen LG, Daskalakis Z, Di Iorio R, Di Lazzaro V, Ferreri F, Fitzgerald PB, George MS, Hallett M, Lefaucheur JP, Langguth B, Matsumoto H, Miniussi C, Nitsche MA, Pascual‐Leone A, Paulus W, Rossi S, Rothwell JC, Siebner HR, Ugawa Y, Walsh V, Ziemann U (2015): Non‐invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin Neurophysiol 126:1071–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Säisänen L, Julkunen P, Niskanen E, Danner N, Hukkanen T, Lohioja T, Nurkkala J, Mervaala E, Karhu J, Kononen M (2008): Motor potentials evoked by navigated transcranial magnetic stimulation in healthy subjects. J Clin Neurophysiol 25:367–372. [DOI] [PubMed] [Google Scholar]
- Sampaio‐Baptista C, Filippini N, Stagg CJ, Near J, Scholz J, Johansen‐Berg H (2015): Changes in functional connectivity and GABA levels with long‐term motor learning. Neuroimage 106:15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider LA, Goldsworthy MR, Cole JP, Ridding MC, Pitcher JB (2016): The influence of short‐interval intracortical facilitation when assessing developmental changes in short‐interval intracortical inhibition. Neuroscience 312:19–25. [DOI] [PubMed] [Google Scholar]
- Schuler V, Luscher C, Blanchet C, Klix N, Sansig G, Klebs K, Schmutz M, Heid J, Gentry C, Urban L, Fox A, Spooren W, Jaton AL, Vigouret J, Pozza M, Kelly PH, Mosbacher J, Froestl W, Kaslin E, Korn R, Bischoff S, Kaupmann K, van der Putten H, Bettler B (2001): Epilepsy, hyperalgesia, impaired memory, and loss of pre‐ and postsynaptic GABA(B) responses in mice lacking GABA(B(1)). Neuron 31:47–58. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Sneider JT, Crowley DJ, Covell MJ, Acharya D, Rosso IM, Jensen JE (2013): Frontal lobe gamma‐aminobutyric acid levels during adolescence: Associations with impulsivity and response inhibition. Biol Psychiatry 74:296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snook L, Paulson LA, Roy D, Phillips L, Beaulieu C (2005): Diffusion tensor imaging of neurodevelopment in children and young adults. Neuroimage 26:1164–1173. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW (1999): In vivo evidence for post‐adolescent brain maturation in frontal and striatal regions. Nat Neurosci 2:859–861. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Delis D, Stiles J, Jernigan TL (2001): Improved memory functioning and frontal lobe maturation between childhood and adolescence: A structural MRI study. J Int Neuropsychol Soc 7:312–322. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW (2003): Mapping cortical change across the human life span. Nat Neurosci 6:309–315. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Toga AW (2004): Mapping changes in the human cortex throughout the span of life. Neuroscientist 10:372–392. [DOI] [PubMed] [Google Scholar]
- Stagg CJ, Bachtiar V, Amadi U, Gudberg CA, Ilie AS, Sampaio‐Baptista C, O'Shea J, Woolrich M, Smith SM, Filippini N, Near J, Johansen‐Berg H (2014): Local GABA concentration is related to network‐level resting functional connectivity. eLife 3:e01465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenhuis RE, Bryden MP, Schwartz M, Lawson S (1990): Reliability of hand preference items and factors. J Clin Exp Neuropsychol 12:921–930. [DOI] [PubMed] [Google Scholar]
- Supekar K, Musen M, Menon V (2009): Development of large‐scale functional brain networks in children. PLoS Biol 7:e1000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thut G, Miniussi C, Gross J (2012): The functional importance of rhythmic activity in the brain. Curr Biol 22:R658–6R663. [DOI] [PubMed] [Google Scholar]
- Vakorin VA, Lippe S, McIntosh AR (2011): Variability of brain signals processed locally transforms into higher connectivity with brain development. J Neurosci 31:6405–6413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veniero D, Brignani D, Thut G, Miniussi C (2011): Alpha‐generation as basic response‐signature to transcranial magnetic stimulation (TMS) targeting the human resting motor cortex: A TMS/EEG co‐registration study. Psychophysiology 48:1381–1389. [DOI] [PubMed] [Google Scholar]
- Voineskos AN, Farzan F, Barr MS, Lobaugh NJ, Mulsant BH, Chen R, Fitzgerald PB, Daskalakis ZJ (2010): The role of the corpus callosum in transcranial magnetic stimulation induced interhemispheric signal propagation. Biol Psychiatry 68:825–831. [DOI] [PubMed] [Google Scholar]
- Walther M, Berweck S, Schessl J, Linder‐Lucht M, Fietzek UM, Glocker FX, Heinen F, Mall V (2009): Maturation of inhibitory and excitatory motor cortex pathways in children. Brain Dev 31:562–567. [DOI] [PubMed] [Google Scholar]
- Wang DD, Kriegstein AR (2009): Defining the role of GABA in cortical development. J Physiol 587:1873–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitford TJ, Rennie CJ, Grieve SM, Clark CR, Gordon E, Williams LM (2007): Brain maturation in adolescence: Concurrent changes in neuroanatomy and neurophysiology. Hum Brain Mapp 28:228–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Lu LH, Lowes A, Yang S, Passarotti AM, Zhou XJ, Pavuluri MN (2014): Development of superficial white matter and its structural interplay with cortical gray matter in children and adolescents. Hum Brain Mapp 35:2806–2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap PT, Fan Y, Chen Y, Gilmore JH, Lin W, Shen D (2011): Development trends of white matter connectivity in the first years of life. PloS One 6:e24678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousry TA, Schmid UD, Alkadhi H, Schmidt D, Peraud A, Buettner A, Winkler P (1997): Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain 120 (Pt 1): 141–157. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Lonnecker S, Steinhoff BJ, Paulus W (1996): Effects of antiepileptic drugs on motor cortex excitability in humans: A transcranial magnetic stimulation study. Ann Neurol 40:367–378. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Table 1.


