Abstract
In social interactions, humans are expected to regulate interpersonal distance in response to the emotion displayed by others. Yet, the neural mechanisms implicated in approach‐avoidance tendencies to distinct emotional expressions have not been fully described. Here, we investigated the neural systems implicated in regulating the distance to different emotions, and how they vary as a function of empathy. Twenty‐three healthy participants assessed for psychopathic traits underwent fMRI scanning while they viewed approaching and withdrawing angry, fearful, happy, sad and neutral faces. Participants were also asked to set the distance to those faces on a computer screen, and to adjust the physical distance from the experimenter outside the scanner. Participants kept the greatest distances from angry faces, and shortest from happy expressions. This was accompanied by increased activation in the dorsomedial prefrontal and orbitofrontal cortices, inferior frontal gyrus, and temporoparietal junction for angry and happy expressions relative to the other emotions. Irrespective of emotion, longer distances were kept from approaching faces, which was associated with increased activation in the amygdala and insula, as well as parietal and prefrontal regions. Amygdala activation was positively correlated with greater preferred distances to angry, fearful and sad expressions. Moreover, participants scoring higher on coldhearted psychopathic traits (lower empathy) showed reduced amygdala activation to sad expressions. These findings elucidate the neural mechanisms underlying social approach‐avoidance, and how they are related to variations in empathy. Hum Brain Mapp 38:1492–1506, 2017. © 2016 Wiley Periodicals, Inc.
Keywords: interpersonal distance, facial expressions, empathy, psychopathic traits, fMRI
Abbreviations
- FDR
False discovery rate
- IFG
Inferior frontal gyrus
- MNI
Montreal Neurological Institute
- OFC
Orbitofrontal cortex
- ROI
Region‐of‐interest
INTRODUCTION
Keeping an appropriate distance from the surrounding physical world is a defensive mechanism that serves a variety of functions including preventing injury [Graziano and Cooke, 2006]. Similarly, to successfully navigate the social world, humans are required to regulate interpersonal distance in order to preserve their own and others' personal space boundaries [Hayduk, 1983; Horowitz et al., 1964]. The violation of those boundaries typically generates discomfort and anxiety, and may trigger defensive responses in others, resulting in avoidant or aggressive behaviour [Ng et al., 2001; Regoeczi, 2008]. Despite being a key process in normal social functioning, few studies have directly examined the neurocognitive processes implicated in normal and abnormal interpersonal distance regulation. In particular, little attention has been given to how distance varies as a function of emotional social cues, such as facial expressions. Here, we used fMRI in combination with a computerized interpersonal distance task to investigate, for the first time, the influence of both emotional context and individual differences in empathy on the neural processes involved in distance regulation.
Facial expressions are among the most powerful communication cues in humans. They convey not only the emotional state, but also the behavioural intentions of an individual [Blair, 2003; Horstmann, 2003; Parkinson, 2005; Schmidt and Cohn, 2001], and are constantly used to guide social behaviour. The ability to adequately adjust interpersonal distance as a function of the emotions manifested by others is thus of utmost importance, especially in potentially threatening interactions (for example, withdrawing from someone expressing anger may protect against aggression). In the past, approach‐avoidance tendencies towards facial expressions have classically been examined using motoric tasks, wherein participants are instructed to either push or pull a joystick in response to different faces. The rationale behind such tasks was that pushing aversive stimuli (i.e., muscle extension) and pulling appetitive stimuli (i.e., muscle flexion) are automatic responses associated with shorter reaction times [Cacioppo et al., 1993; Marsh et al., 2005], although recent evidence suggests those responses may result from evaluative rather than purely automatic processes (see Laham et al., 2015 for a review). Studies using such paradigms have shown that angry faces generally motivate avoidance (faster pushing than pulling responses), whereas happy and fearful expressions elicit approach (faster pulling than pushing responses; Hammer and Marsh, 2015; Marsh et al., 2005; Seidel et al., 2010]. Yet, it is unclear how performance in these tasks is related to interpersonal distance in more naturalistic settings. Importantly, prior work does not elucidate what neurocognitive systems underlie approach‐avoidance tendencies to distinct emotions. Our first goal was therefore to examine the neural activation patterns to approaching and withdrawing faces displaying different emotional expressions, and to assess their association with interpersonal distance preferences.
At the neural level, the amygdala is one of the candidate regions thought to be implicated in social approach‐avoidance mechanisms [Bliss‐Moreau et al., 2011, 2013; Machado et al., 2009], and specifically in personal space regulation [Kennedy et al., 2009]. Existing neuroimaging studies have demonstrated an increase in amygdala activation in response to approaching relative to static faces, irrespective of emotion [Schienle et al., 2015], and to looming threatening stimuli [Mobbs et al., 2009; Mobbs et al., 2010]. Moreover, results obtained with both amygdala lesion patients and healthy participants suggested an association between amygdala function and interpersonal distance [Kennedy et al., 2009]. Based on these data, we hypothesized that activity within the amygdala would be enhanced in response to approaching compared to receding faces, and would be associated with greater interpersonal distance. A solid body of research has also supported a privileged role of the amygdala in processing fear‐related stimuli [Adolphs et al., 1994; Aube et al., 2015; Kryklywy et al., 2013; Phillips et al., 1998], particularly fearful facial expressions [Fusar‐Poli et al., 2009; Thomas et al., 2001; Whalen et al., 1998]. In light of this evidence, we also predicted the amygdala would be preferentially engaged in response to fearful faces relative to other expressions. In addition to the amygdala, animal and human studies have also suggested the involvement of a fronto‐parietal network in the maintenance of personal space, which includes the margins of the intraparietal sulcus, as well as somatosensory and premotor cortices [Brozzoli et al., 2013; Ferri et al., 2015; Graziano and Cooke, 2006; Holt et al., 2014]. Hence, we additionally expected these regions to be preferentially engaged in response to approaching faces. We were interested in assessing whether facial expressions would modulate activity within this network, although no predictions were formulated regarding potential emotion effects.
Interpersonal distance preferences are also likely influenced by personality. Importantly, emotional context and personality appear to be interacting factors, with some traits selectively affecting approach‐avoidance tendencies to specific emotions [Sambo and Iannetti, 2013; Schienle et al., 2015; Veenstra et al., 2016]. Personality traits associated with deficient emotional and empathic responses are characteristic of psychopathic personalities [Blair, 2015; Frick and White, 2008; Marsh, 2016], and may be particularly impactful in shaping interpersonal distance preferences in emotional contexts [Hammer and Marsh, 2015]. In clinical populations, there is evidence that some of the severe interpersonal problems associated with psychopathy (e.g., instrumental aggression) may be linked to atypical social approach‐avoidance mechanisms [von Borries et al., 2012]. Our second goal was to investigate whether psychopathic traits in a healthy community sample affects neural approach‐avoidance patterns and interpersonal distance to different emotional expressions, particularly those signaling distress. Recent work has demonstrated that coldhearted psychopathic traits, in particular, are associated with a preference for shorter interpersonal distances in healthy individuals [Vieira and Marsh, 2014]. These traits have also been linked to impairments in processing distress cues such as fearful and sad expressions in developmental populations [Blair et al., 2001; Dawel et al., 2012], and are associated with atypical amygdala function in both youth [Lozier et al., 2014; Viding et al., 2012] and healthy adult samples [Han et al., 2012]. In light of these findings and of the putative involvement of the amygdala in interpersonal distance regulation, we hypothesized that coldhearted psychopathic traits would be associated with a preference for shorter interpersonal distances to distress‐related emotions such as fear and sadness, which would be accompanied by reduced amygdala engagement. To our knowledge, this is the first study to investigate the neural processes implicated in interpersonal distance preferences in a healthy population, and to examine how those processes are simultaneously influenced by emotional context and individual differences in empathy.
MATERIALS AND METHODS
Participants
Twenty‐three participants (12 F, M = 20.96, SD = 2.48, range 18–29) were recruited through advertisements posted in the University of Western Ontario. All participants were right‐handed, had normal or corrected‐to‐normal vision, and reported having no history of psychiatric or neurological diagnoses, brain injuries or substance abuse. The study was approved by the Health Sciences Research Ethics Board at the University of Western Ontario (London, ON, Canada). Participants provided written informed consent and were compensated for their time.
Psychopathic Traits Assessment
Psychopathic personality traits were assessed using the Psychopathic Personality Inventory – Revised [Lilienfeld and Widows, 2005], a self‐report instrument designed to measure psychopathic traits in a dimensional manner. This is consistent with the idea that psychopathy is a set of traits continuously distributed in the general population rather than a clinical taxon and, like other personality disorders, it can be more reliably assessed using dimensional models of personality [Drislane et al., 2014; Marcus et al., 2004; Miller et al., 2001; Skeem et al., 2011]. The PPI‐R contains 154 items organized in seven subscales (social influence, fearlessness, stress immunity, Machiavellian egocentricity, rebellious non‐conformity, blame externalization, and carefree nonplanfulness) that load onto two higher‐order factors (PPI‐I or fearless dominance, and PPI‐II or Self‐centered impulsivity) and an eighth subscale, Coldheartedness (C), that is believed to be largely independent of both these factors, and is therefore regarded simultaneously as a subscale and a higher‐order dimension [Skeem et al., 2011]. Fearless dominance scores index interpersonal dominance and low anxiety (e.g., “When I'm in a frightening situation, I can “turn off” my fear almost at will”), whereas Self‐centered impulsivity scores are related to disinhibition and impulsive behavior (e.g., “I like to act first and think later;” Lilienfeld and Widows, 2005].
Coldheartedness scores index callousness and lack of sympathy for others (e.g., “When someone is hurt by something I say or do, that's their problem;” [Lilienfeld and Widows, 2005]. It is the component that best taps onto the low empathic concern dimension of psychopathy, considered the precursor and core feature of the adult psychopathic phenotype [Frick, 2016]. Importantly, Coldheartedness has been previously associated with amygdala dysfunction [Han et al., 2012], abnormalities in fearful face perception [Oliver et al., 2015], preferences for shorter interpersonal distances [Vieira and Marsh, 2014], and increased approach to angry faces [Hammer and Marsh, 2015] in community populations. Because we were particularly interested in investigating how emotional contexts shapes interpersonal distance preferences, we hypothesized coldhearted psychopathic traits would have the largest influence. Therefore, our analyses were focused on this component. Coldheartedness raw scores were converted into T scores (range = 33–67; M = 45.83, SD = 9.39) based on sex and age group norms [Lilienfeld and Widows, 2005] and used in the analyses.
Computerized Interpersonal Distance Task
In this task, we assessed participants' preferred distance to faces displaying five different emotions. Participants were presented a series of facial expressions and asked to adjust their size on the screen, in order to manipulate the perceived distance from each face (Fig. 1B). Forty faces from eight Caucasian actors (four male, four female) displaying angry, fearful, happy, sad and neutral expressions were selected from the NimStim Set of Facial Expressions [Tottenham et al., 2009]. Images were converted to grayscale, cropped to remove extraneous features around the face, and the edges smoothed. Participants were instructed to use as reference the distance they would normally keep “when having a conversation with a stranger.” The task comprised 40 approach (faces were initially small, simulating a greater distance) and 40 withdrawal trials (faces were bigger, simulating closer distance), presented randomly. In each trial, participants pressed “2” in a response box to bring the face closer (size increased by a factor of 1.13), and “3” to push it back (size decreased by a factor of 1.13). They were given a 5‐second interval to press the buttons as many times as necessary to achieve the desired distance, upon which they pressed “1” to lock their response and move on to the next trial. The task was programmed and delivered using E‐prime 2.0 (Psychology Software Tools Inc.).
Figure 1.
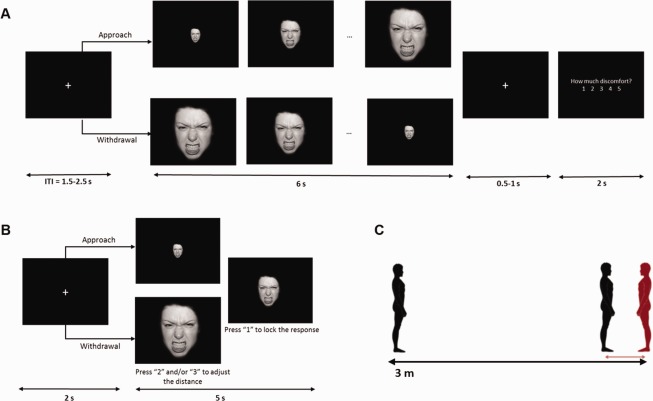
Schematic representation of the experimental tasks: imaging task (A), computerized interpersonal distance task (B) and “Stop‐distance” task (C). [Color figure can be viewed at http://wileyonlinelibrary.com]
fMRI Task
To investigate BOLD responses to approaching and withdrawing emotional faces that were not contaminated by motoric responses, participants underwent fMRI scanning while they passively viewed facial expressions (same stimuli as in the computerized interpersonal distance task) that either increased (Approach) or decreased (Withdrawal) in size, appearing to move towards or away from or them, respectively. Images increased or decreased by a factor of 1.13, resulting in 20 frames per trial. In each trial, participants were instructed to stay focused on the face (6 s), and then rate their level of discomfort (2 s) using a 5‐point scale (1—min, 5—max; Fig. 1A). Participants completed eight functional runs of 30 trials each (≈ 5 m). Each run included randomly presented faces from three actors displaying the five expressions (anger, fear, happiness, sadness, neutral) once in each direction (approach and withdrawal). Thus, in total, participants performed 240 trials of the task, 24 in each condition. The order of runs was randomized across participants. The task was programmed and delivered using E‐prime 2.0 (Psychology Software Tools Inc.).
“Stop‐Distance” Task
We were interested in confirming whether performance in our computerized task predicted interpersonal distance preferences in real interaction settings. We therefore also asked participants to complete a “Stop‐distance task” akin to that used in prior studies [Kennedy et al., 2009; Vieira and Marsh, 2014], which provided a more ecologically valid measure of preferred distance (Fig. 1C). In this task, performed outside the scanner, participants adjusted the distance between themselves and the experimenter across a series of trials. The task comprised an approach and a withdrawal block (4 trials each), counterbalanced across participants. In approach trials, the experimenter stood 3 m away and walked towards the participant at a natural gait (approximately 1m/s). In withdrawal trials, the experimenter started standing with her toes at about 3 cm from the participant's, and walked backwards. In both, participants were instructed to tell the experimenter to stop at their preferred distance (i.e., the distance at which they felt “the most comfortable”). Chin‐to‐chin distance was recorded in each trial using a digital laser tape measure (Bosch GLM 15).
Procedures
Functional neuroimaging took place in one session at the Centre for Functional and Metabolic Mapping of the Robarts Research Institute (University of Western Ontario). After providing written consent, participants were given a practice version of the experimental tasks on a laptop. They completed 8 practice trials of the scanning task and 16 trials of the computerized distance task, both featuring only neutral expressions of two actors not used in the experimental versions. For the “Stop‐distance task,” all participants were tested by the same experimenter in a room next to the scanner, either before or after the scan (counterbalanced). Following the scan, participants were administered the PPI‐R; [Lilienfeld and Widows, 2005]. Additionally, in line with prior suggestions that interpersonal distance preferences in higher coldheartedness individuals could be associated with aggressive tendencies, we also administered a self‐report measure of aggression (Reactive‐Proactive Aggression Questionnaire, RPQ; [Raine et al., 2006].
fMRI Acquisition, Preprocessing, and Analysis
Subjects were scanned in a single session using a 3T Siemens Scanner with a 32‐channel head coil. Whole‐brain functional images were taken with an echo‐planar T2*‐weighted imaging sequence while participants performed the scanning task (TR = 1250 ms, TE = 30 ms, FoV = 192 mm, flip angle = 40°, 57 interleaved slices of 2.00 mm isovoxels, 267 volumes per run). Scan parameters were chosen to optimize the signal‐to‐noise ratio for the amygdala based on recent recommendations [Morawetz et al., 2008; Robinson et al., 2004]. After 4 functional runs, a high resolution T1‐weighted anatomical scan was obtained (TR = 2300 ms, TE = 2.98 ms; FoV = 256 mm, flip angle = 9°, 192 axial slices of 1 mm isovoxels), during which participants performed the computerized distance task. Four more functional runs of the scanning task were collected after the anatomical scan.
Preprocessing of fMRI data was done using SPM12 (Wellcome Trust Centre for Neuroimaging, http://www.fil.ion.ucl.ac.uk). After slice timing correction, images were realigned to the volume acquired immediately before the anatomical scan, using 6 parameter rigid‐body transformations. They were then coregistered with the structural data, normalized to standard space using the Montreal Neurological Institute (MNI) template with a voxel size of 2 × 2 × 2 mm, and smoothed using a Gaussian kernel with an isotropic full‐width‐half‐maximum of 4 mm. The acquisition parameters (namely slice thickness and TE) and spatial smoothing kernel were based on recommendations by Morawetz and colleagues (2008] and Robinson and colleagues (2004] to optimize signal‐to‐noise ratio in the amygdala: the use of 2 mm slices has been associated with fewer susceptibility artifacts, and moderate smoothing has been recommended to balance sensitivity and specificity. Similar parameters have been employed in prior fMRI studies investigating the involvement of the amygdala in socio‐affective tasks in comparable populations [Han et al., 2012]. Additionally, a high‐pass filter cutoff of 128 seconds was applied to remove slow signal drifts.
First‐level analysis was based on the general linear model. Time‐series of each voxel were normalized by dividing the signal intensity of a given voxel at each point by the mean signal intensity of that voxel for each run and multiplying it by 100. Resulting regression coefficients thus represent a percent signal change from the mean. Regressors were created by convolving the train of stimulus events with a canonical hemodynamic response function. Ten events of interest were modelled, corresponding to each of the five facial expressions (Anger, Fear, Happiness, Sadness and Neutral) in each direction (Approach, Withdrawal) (6 s). In addition, 10 more regressors were created to model the same conditions during the response slides (discomfort ratings; 2 s). The six motion parameters estimated during realignment were also included in the model as regressors of no interest.
Statistical Analysis
Behavioural data
Our behavioural measures were the distance set in the computerized task, which were operationalized as [100 – percentage of face maximum size], and the discomfort ratings. We investigated the effects of Emotion and Direction on these measures through two 5 (Anger, Fear, Happiness, Sadness and Neutral) by 2 (Approach, Withdrawal) repeated‐measures ANOVAs. In addition, for the “Stop‐distance task,” we averaged all trials and performed a correlation analysis to test whether overall preferred distance outside the scanner predicted the overall distance set in the computerized task.
Finally, we used correlations to test the hypothesized association between Coldheartedness and distance to fearful and sad expressions, following evidence suggesting callous psychopathic traits are associated with impairments in processing distress cues. To isolate emotion‐related effects, we computed difference scores between each emotion and Neutral for each direction, and then averaged across approach and withdrawal scores. We performed bivariate correlations between the two resulting average difference scores and Coldheartedness. The threshold for statistical significance was set at P < 0.05 two‐tailed for all behavioural analyses. Greenhouse‐Geisser procedure was used to correct departures from sphericity, when necessary, and Sidak correction was used to adjust the significance level when multiple correlations were performed. Analyses were performed in SPSS Version 23 (IBM Corp).
fMRI data
To investigate the neural activation patterns to approaching and withdrawing emotional faces (6‐second interval), we performed a whole‐brain 5 (Anger, Fear, Happiness, Sadness and Neutral) by 2 (Approach, Withdrawal) ANOVA, with emotion and direction as within‐subject factors. To minimize Type I errors, only results found with a false discovery rate (FDR)‐corrected threshold of P < 0.05 are reported. To test hypothesized associations between brain activation and behaviour, we extracted the percent signal change of significant clusters and performed correlational analyses with both behavioural difference scores and Coldheartedness using SPSS. Sidak correction was used to adjust the significance level of multiple correlations.
To further investigate the association between Coldheartedness and amygdala activation to distress cues we performed region‐of‐interest (ROI) analyses using anatomically defined masks of the left and right amygdala. ROIs were created using the Automated Anatomical Labeling atlas (aal, + 1 dilation; Tzourio‐Mazoyer et al., 2002] within the WFU PickAtlas toolbox [Maldjian et al., 2004; Maldjian et al., 2003]. For each ROI, we performed regression analyses on the Approach‐Withdrawal average image for Fear and for Sadness, using Coldheartedness T scores as predictor. Significant clusters at a corrected threshold of P < 0.05 are reported.
RESULTS
Behavioural Results
In the computerized task, we found main effects of direction [F(1,22)=95.82, P < 0.001] and emotion [F(4,88)= 31.52, P < 0.001], but no significant interaction. The main effect of direction was characterized by greater preferred distances in the Approach than the Withdrawal condition. For the main effect of emotion, with the exception of neutral and sadness, all other emotions differed significantly from each other (all ps < 0.05), with greatest preferred distances for anger, followed by fear, sadness, neutral and happiness (Fig. 2A).
Figure 2.
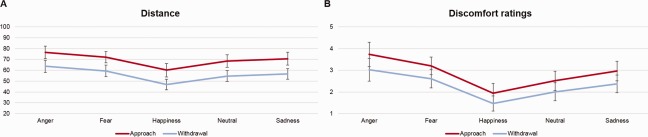
Mean distances set to each emotional expression in the computerized task (A), and mean discomfort ratings during the scanning task (B). [Color figure can be viewed at http://wileyonlinelibrary.com]
Finally, for ratings of discomfort, there were main effects of direction [F(1,22) = 9.98, P = 0.005], emotion [F(4,88)=35.06, P < 0.001], and a significant interaction [F(4,88) = 3.41, P = 0.016]. Greater discomfort was reported during Approach trials, and all emotions differed significantly from each other (all ps < 0.05), with highest discomfort ratings for angry expressions, followed by fear, sadness, neutral and happiness. To explore the interaction, we compared the approach—withdrawal discomfort difference scores per emotion in a repeated measures ANOVA, and found that the difference score for anger was greater than for happiness (P = 0.020); no other difference scores differed significantly (Fig. 2B).
To verify whether distances in the computerized task predicted interpersonal distance preferences, we examined the correlation between those distances and that chosen in the Stop‐distance task. We found that overall Stop distance was correlated at trend level with overall computerized distance (r = 0.396, P = 0.06).
Coldheartedness and distance
Correlation analysis revealed a trend for shorter distance to fearful faces in participants scoring higher in Coldheartedness (r = −0.403, P = 0.057). No correlation was found for sadness.
fMRI Results
Effects of direction and emotion on whole‐brain activation
As hypothesized, activation in the right amygdala (xyz = 24, 0, −14) was increased for approaching versus receding facial expressions, irrespective of emotion. The same pattern was found in the margins of the intraparietal sulcus, including the left superior (SPL; −28, −58, 44) and right inferior (IPL; 40, −56, 46) parietal lobules. Other regions with increased activation for approaching versus withdrawing faces included the bilateral insula (L: −28, 26, −2; R: 44, 20, −2), bilateral DLPFC (L: −50, 32, 30; R: 50, 34, 32), and a large cluster (8, −98, 18) that encompassed occipital, temporal and parietal visual areas (Table 1, 2, Fig. 3).
Table 1.
Descriptive statistics for age and PPI‐R factor T scores
| M(SD) | Range | |
|---|---|---|
| Age | 21 (2.5) | 18–29 |
| PPI‐R total T | 47.9 (9.2) | 40–68 |
| Fearless dominance T | 47.7 (11.1) | 27–68 |
| Self‐centered impulsivity T | 46.7 (7.1) | 36–61 |
| Coldheartedness T | 45.8 (9.4) | 33–67 |
Table 2.
Results of the 5 (emotion) by 2 (direction) ANOVA (FDR corrected P < 0.05; MNI coordinates are reported)—effects of direction and emotion
| Location | R/L | k | x | y | z | BA | Nature of effect |
|---|---|---|---|---|---|---|---|
| Main effect of emotion | |||||||
| Cerebellum | L | 55 | −10 | −88 | −28 | – | HA > FE, NE, SA |
| Inferior parietal lobule | R | 158 | 50 | −52 | 50 | 40 | AN = HA > FE, NE, SA |
| Angular gyrus | R | 47 | 60 | −50 | 32 | 40 | AN > FE, NE |
| Inferior parietal lobule | L | 51 | −42 | −30 | 44 | 40 | AN = HA > SA |
| Precentral gyrus | R | 78 | 28 | −22 | 76 | 6 | HA > FE, SA |
| Postcentral gyrus | L | 49 | −46 | −18 | 48 | 4 | HA > AN, FE, NE |
| Premotor cortex | R | 215 | 38 | 6 | 54 | 6, 8 | AN > SA; HA > FE, NE, SA |
| Ventrolateral prefrontal cortex | R | 62 | 56 | 20 | 18 | 45 | HA > NE, SA |
| Inferior frontal gyrus | L | 54 | −50 | 36 | −8 | 47 | AN > FE, HA, NE |
| Inferior frontal gyrus | R | 114 | 44 | 38 | −8 | 47 | AN > FE, NE; HA > NE; SA > NE |
| Dorsolateral prefrontal cortex | L | 111 | −20 | 50 | 24 | 10 | AN > FE, NE; HA > FE |
| Orbitofrontal cortex | R | 289 | 28 | 62 | 16 | 10 | AN > FE, NE, SA; HA > NE, SA |
| Dorsomedial prefrontal cortex | L/R | 415 | 0 | 64 | 28 | 9, 10 | AN > FE, SA; HA > FE |
| Main effect of direction | |||||||
| Middle occipital gyrus | R/L | 18624 | 8 | −98 | 18 | 17, 18, 19, 30, 31, 37 | A > W |
| Cerebellum | L | 53 | −12 | −76 | −44 | – | A > W |
| Intraparietal sulcus | L | 87 | −28 | −58 | 44 | 7 | A > W |
| Inferior parietal lobule | R | 506 | 40 | −56 | 46 | 40 | A > W |
| Amygdala | R | 48 | 24 | 0 | −14 | – | A > W |
| Premotor cortex | L | 49 | −48 | 6 | 38 | 9 | A > W |
| Supplementary motor area | L | 287 | −6 | 8 | 50 | 32 | A > W |
| Inferior frontal gyrus | L | 394 | −44 | 16 | −4 | 47 | A > W |
| Anterior insula/IFG | R | 638 | 44 | 20 | −2 | 46, 9 | A > W |
| Anterior insula | L | 43 | −28 | 26 | −2 | 47, 13 | A > W |
| Dorsolateral prefrontal cortex | L | 173 | −50 | 32 | 30 | 9, 46 | A > W |
| Dorsolateral prefrontal cortex | R | 1407 | 50 | 34 | 32 | 46 | A > W |
| Direction × Emotion | |||||||
| Precuneus | R | 47 | 14 | −66 | 32 | 31 | |
| Rolandic operculum/mid‐insula | L | 57 | −40 | 2 | 18 | 44 | |
| Anterior insula | R | 44 | 42 | 6 | −6 | 13 | |
| Inferior frontal gyrus | L | 65 | −40 | 22 | −12 | 47 | |
| Ventrolateral prefrontal cortex | R | 272 | 36 | 44 | 4 | 10 | |
| Ventrolateral prefrontal cortex | L | 123 | −34 | 52 | 24 | 10 |
Figure 3.
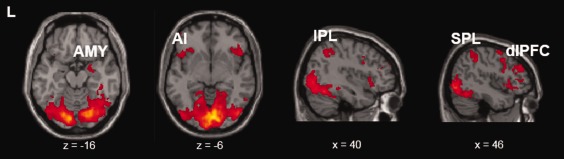
Clusters showing a main effect of Direction in the 5 × 2 ANOVA, including the bilateral visual cortex, fusiform gyrus, right inferior (IPL) and superior parietal lobules (SPL), right amygdala, bilateral anterior insula (AI) and bilateral dorsolateral prefrontal cortex (dlPFC; FDR‐corrected P < 0.05). [Color figure can be viewed at http://wileyonlinelibrary.com]
Contrary to our predictions, whole brain analysis found no main effect of emotion in the amygdala. Instead, results showed emotion affected activation in a prefrontal and parietal network that included the dorsomedial prefrontal cortex (dmPFC; 0, 64, 28), bilateral inferior frontal gyrus (IFG; L: −50, 36, −8; R: 44, 38, −8), right orbitofrontal cortex (OFC; 28, 62, 16), bilateral inferior parietal lobule (IPL; L: −42, −30, 44; R: 50, −52, 50), and angular/supramarginal gyrus (60, −50, 32) (Table 2, Fig. 4A). Percent signal change was extracted from significant clusters to delineate the nature of these effects. The largest frontal clusters (located in the dmPFC and OFC) showed increased activation to faces of anger and happiness relative to other expressions (all ps < .05), with parietal clusters located in the temporoparietal junction (IPL and angular/supramarginal gyrus) exhibiting a similar activation pattern (Fig. 4B).
Figure 4.
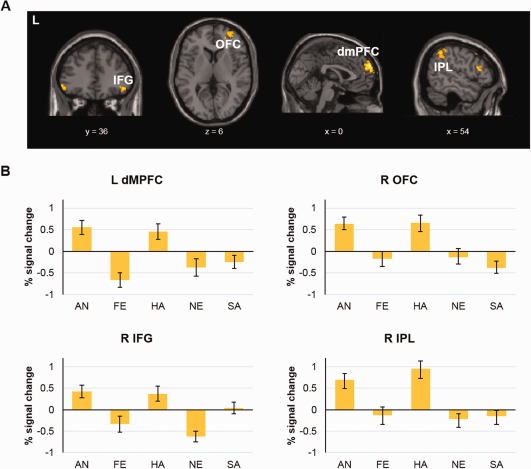
(A) Clusters showing a main effect of emotion in the 5 × 2 ANOVA, including the bilateral IFG right OFC, left IPL and left dorsomedial prefrontal cortex (dmPFC; FDR‐corrected P < 0.05). (B) Graphs depicting the percent signal change per facial expression in clusters showing a main effect of emotion (left dmPFC, right OFC, right IFG and right IPL). [Color figure can be viewed at http://wileyonlinelibrary.com]
Results also revealed clusters with a significant Emotion × Direction interaction. Of note, these regions included the right anterior insula (42, 6, −6) and left mid insula (−40, 2, 18), bilateral ventrolateral PFC (vlPFC; L: −34, 52, 24; R: 36, 44, 4), and left IFG (−40, 22, −12) (Table 2, Fig. 5). Percent signal change was extracted from each cluster and paired t‐tests were performed to compare activation in approach versus withdrawal trials, per emotion. In the insula (bilaterally), results showed increased activation to approach versus withdrawal for happy and angry faces, and an opposite pattern for sadness. Results also showed opposite activation patterns to approaching versus withdrawing faces for happy and sad expressions in the vlPFC (bilaterally), such that activation was greater for withdrawing versus approaching sad faces, and for approaching versus withdrawing happy faces. Finally, in the left IFG, results showed a more pronounced activation increase for approach versus withdrawal for angry and happy faces (all ps < 0.05; see S1 for a more detailed description of the interaction).
Figure 5.
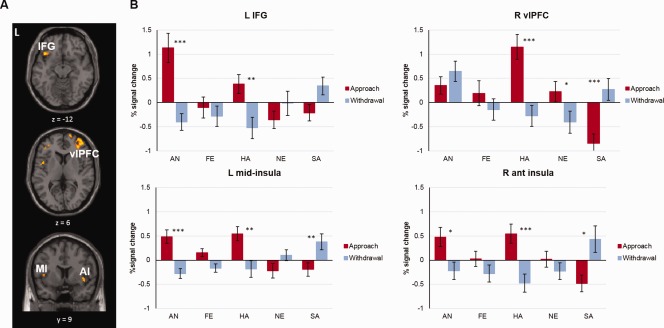
(A) Clusters showing a direction × emotion interaction in the 5 × 2 ANOVA, including the left IFG, right ventrolateral prefrontal cortex (vlPFC), left mid‐insula (MI) and right anterior insula (AI; FDR‐corrected P < 0.05). (B) Graphs depicting the percent signal change per facial expression and direction in clusters showing a significant interaction (left IFG, right vlPFC left MI and right AI) (*P < 0.05, ** P < 0.01, *** P < 0.001). [Color figure can be viewed at http://wileyonlinelibrary.com]
Amygdala activation and distance
We extracted the percent signal change from the right amygdala cluster identified in the ANOVA (main effect of direction) and tested the association between activation within this region and distance in the computerized task. As hypothesized, right amygdala activation was significantly associated with greater distances to angry (r = 0.61, P = 0.002), sad (r = 0.527, P = 0.010) and, at trend level, fearful (r = 0.504, P = 0.014) expressions (Fig. 6). We also performed exploratory correlations between amygdala percent signal change and discomfort ratings. Results showed trends for increased amygdala activation and higher discomfort to angry (r = 0.421, P = 0.045), sad (r = 0.462, P = 0.027) and fearful faces (r = 0.375, P = 0.078). No associations were found between amygdala activation and preferred distance (r = 0.006, P = 0.98) or discomfort ratings (r = 0.192, P = 0.381) to happy expressions.
Figure 6.
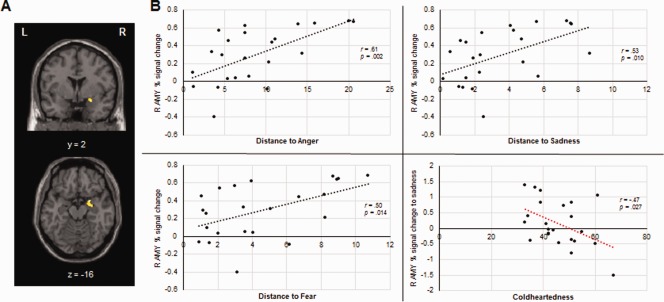
Scatter plots depicting the association between right amygdala activation (24, 0, −14) and distance set to angry (top left), sad (top right) and fearful expressions (bottom left); scatter plot depicting the association between right amygdala activation to sad faces and Coldheartedness scores (bottom right). [Color figure can be viewed at http://wileyonlinelibrary.com]
Amygdala activation and coldheartedness
Whole‐brain analysis showed enhanced activation in the right amygdala to approaching versus receding faces. Following evidence that callous psychopathic traits are associated with abnormal amygdala activity to distress cues [Han et al., 2012; Lozier et al., 2014], we hypothesized activation within this cluster in response to fearful and sad faces would be modulated by Coldheartedness. To test this hypothesis, we performed correlations between percent signal change in the right amygdala in response to approaching sad and fearful faces and Coldhearteness scores. Results showed a significant negative association between activation to sad faces and Coldheartedness (r = −0.47, P = 0.027) (Fig. 6B). No association was found for fear.
To confirm the relation between Coldheartedness and amygdala activation, we created bilateral anatomical masks of this region and performed regression analyses within SPM using Coldheartedness scores as predictors. The ROI analysis confirmed a cluster within the right amygdala (14 voxels; 22, −10, −12) wherein activation in response to sad faces was negatively associated with Coldheartedness (Fig. 7).
Figure 7.
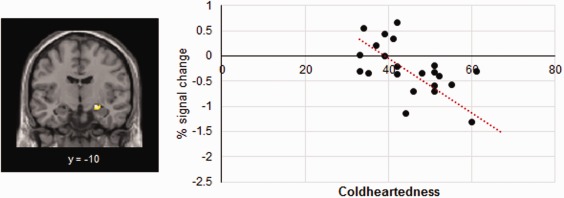
Scatter plot depicting the association between activation to sad faces in the right amygdala ROI and Coldheartedness (FWE‐corrected P < 0.05). [Color figure can be viewed at http://wileyonlinelibrary.com]
DISCUSSION
Successful social interactions rely on our ability to adequately adjust the distance from others based on relevant emotional cues. In this study, we used fMRI to investigate the neural systems implicated in regulating interpersonal distance to different emotional expressions. Our findings revealed that a network of regions, including the amygdala, insula, dlPFC and IPS, was preferentially activated to approaching versus receding faces. Also, prefrontal and parietal regions, such as dmPFC and TPJ, were modulated by emotion, showing increased activity in response to facial expressions of anger and happiness. Finally, our results suggested that characteristics of the perceiver may moderate these effects, with coldhearted psychopathic traits being associated with differential limbic responses to approaching sad expressions.
The Amygdala and Interpersonal Distance
As hypothesized, we found greater right amygdala activity in response to approaching relative to withdrawing faces. These findings are in agreement with prior work demonstrating the amygdala responds preferentially to looming versus receding threatening stimuli (Coker‐Appiah et al., 2013; Mobbs et al., 2010], and suggest this extends to salient social information, such as emotional faces. However, contrary to our predictions, amygdala effects were not modulated by emotional expression. This result may be surprising in light of evidence that the amygdala is more reliably activated in response to fearful faces than to other expressions [Fusar‐Poli et al., 2009], but it is consistent with previous studies showing greater amygdala activation to approaching faces irrespective of emotion [Schienle et al., 2015]. Existing accounts implicate the amygdala—particularly its basolateral nucleus—in encoding salient, ambiguous or unpredictable information [Davis et al., 2016; Whalen, 1998], and in evaluating and representing the intensity of emotional stimuli [Kryklywy et al., 2013]. Both these processes are likely brought to bear in our paradigm; when approached by other individuals, it is likely advantageous to both identify and assess the intensity of their emotional states, in order to infer their behavioural intentions and adjust our behavior accordingly. Our results suggest that, in the context of our task, spatial proximity was perhaps a more salient cue than emotion, as any approaching facial expression is potentially relevant. Furthermore, participants were required to rate their discomfort as facial stimuli approached their personal space. Judgements of discomfort may have caused generalized engagement of the amygdala, irrespective of the facial expression. Our correlational findings appear to support this interpretation; as predicted, we found a significant association between amygdala activity and interpersonal distance, in line with previous suggestions that this region is involved in personal space maintenance [Kennedy et al., 2009]. Specifically, our results showed increased amygdala activation to approaching faces predicted greater discomfort (at trend level) and distance to angry, fearful and sad faces. This is consistent with previous suggestions that the amygdala contributes to interpersonal distance regulation by generating feelings of discomfort when personal space is breached [Kennedy et al., 2009]. Taken together, our data implicate the amygdala in interpreting the behavioural significance of facial expressions as a function of their proximity, potentially as a means of preserving a safety margin around the individual while appropriate behavioural responses are selected and initiated.
Impact of Emotion and Direction on Parietal and Prefrontal Responses to Faces
Besides the amygdala, approaching faces elicited increased widespread activation that was independent of emotional expression across occipital, parietal, temporal and prefrontal regions. This included brain regions involved in defensive responses, such as the anterior insula [Mobbs et al., 2007; Mobbs et al., 2010], and regions previously implicated in personal space maintenance, such as the margins of the intraparietal sulcus (Holt et al., 2014].
Additionally, we examined whole‐brain approach‐avoidance patterns to different emotions. Main effects of emotion emerged in the prefrontal (namely, dmPFC, OFC, IFG) and parietal (TPJ) cortices, with activation within these regions being increased in response to faces of anger and happiness relative to other expressions. Interestingly, these two emotions corresponded to the greatest and shortest behavioural distances, and the highest and lowest discomfort ratings, respectively. In spite of conveying opposite social signals, both anger and happiness are approach‐oriented (i.e., both reflect a disposition to approach on behalf of the actor, Adams and Kleck, 2005] and dominant [Hess et al., 2000] emotions. It is plausible that personal space intrusions by individuals displaying dominant versus submissive expressions are interpreted differently by an observer. Hence, unlike fearful or sad faces expressing greater vulnerability [Hess et al., 2000], observers may interpret approaching angry and happy expressions as more likely to result in behavioural action upon themselves. It is possible that, in our data, the greater recruitment of mentalizing regions (e.g., dmPFC, IFG and TPJ) in response to angry and happy faces reflects privileged processing of facial expressions with more probable behavioural consequences for the observer, and therefore demands to predict their intentions. However, more research is needed to formally test this hypothesis.
Finally, we have uncovered a direction by emotion interaction in the insula, vlPFC and IFG, driven by opposite approach‐withdrawal patterns to sadness compared to angry and happy expressions. Specifically, across those regions, sad faces elicited greater activation in the withdrawal condition, whereas angry and happy faces were associated with enhanced activation during approach. Contrary to anger and happiness, sadness has been viewed as an avoidance‐oriented emotion [Adams and Kleck, 2003; Adams and Kleck, 2005] that signals vulnerability, which could help explain the opposite activation patterns to sad versus angry and happy expressions, particularly in regions previously implicated in processing emotion [insula; Jerram et al., 2014] and social dominance [IFG/vlPFC; Marsh et al., 2009]. However, questions remain as to the motivational value of a receding sad expression. It is noteworthy that prior behavioural studies have reported more complex approach‐avoidance patterns for sadness relative to other emotions. For example, Seidel and colleagues (2010] reported participants generally showed implicit approach to sad expressions (joystick task), but when asked to estimate how close/far they would get from a sad expression, their responses predominantly indicated avoidance. Taken together, these findings highlight remaining questions regarding the social value of sad expressions and call for addition research to be clarified.
Coldhearted Psychopathic Traits and Interpersonal Distance
A second goal of this study was to examine whether empathic abilities influenced interpersonal distance to emotional expressions signaling distress and its neural underpinnings. Our hypothesis was partially confirmed, with high Coldheartedness being associated with reduced right amygdala activation to sad expressions. To our knowledge, this is the first study reporting an association between amygdala hypoactivation to sad expressions and subclinical variation in coldhearted traits. The lack of additional empirical evidence for a link between coldhearted psychopathic traits and reduced amygdala sensitivity to sad faces could be due to the fact that most imaging studies on face processing, with either clinical or nonclinical populations, did not include sad expressions in their paradigms [Contreras‐Rodriguez et al., 2014; Han et al., 2012; Jones et al., 2009; Mier et al., 2014], or did not perform contrasts with sadness [Gordon et al., 2004]. Nonetheless, psychopathic and callous‐unemotional traits have been previously associated with atypical behavioural responses to sadness in youth [Blair et al., 2001; Woodworth and Waschbusch, 2008] and adult forensic samples [Dolan and Fullam, 2006; Hastings et al., 2008]. Additionally, there is evidence supporting the role of the amygdala in processing sad expressions [Fine and Blair, 2000]. Our findings thus lend support to the hypothesis that psychopathic personality traits related to low empathy are associated with diminished sensitivity to distress cues in others, possibly as a result of an amygdala dysfunction [Blair, 2013].
It should be noted that, at the behavioral level, we only found a trend level association between coldheartedness and distance to fearful expressions. This however, could have been due to reduced statistical power, especially given that our effect size (r = −0.4) was comparable to that of prior behavioral studies with larger sample sizes [r = −0.3; Vieira and Marsh, 2014]. Additional research with a larger sample and a greater range of Coldheartedness scores is needed to test the association between variation in these traits and behavioural responses to sad and fearful expressions. Overall, our results suggest dysfunctional social approach‐avoidance patterns in individuals with low trait empathy may be associated with atypical amygdala responses to social cues signaling distress.
Caveats and Future Directions
Prior work suggests neural and behavioural response patterns to emotional stimuli may also reflect variation in core affective dimensions such as valence and arousal [Bonnet et al., 2015; Weymar and Schwabe, 2016; Wilson‐Mendenhall et al., 2013]. A number of studies have provided a characterization of how different emotions map onto a valence/arousal space [Fontaine et al., 2007; Gerber et al., 2008; Mehu and Scherer, 2015; Yik et al., 2011]. These studies suggest that the dimensional profile associated with each emotion is relatively stable across prototypical expressions of that emotion. Hence, some of the emotion‐related effects in our data may reflect the inherent valence/arousal configuration of each emotion, as well as variations in other dimensions, such as dominance. Because dimensional ratings are not available for the experimental stimuli used in our study, we are unable to test the relative contribution of valence, arousal or dominance for the reported effects. Further research is needed to directly test the extent to which variation along different affective dimensions can account for approach/avoid preferences to distinct emotions. Regarding potential confounds, we have minimized the influence of gender and age on Coldheartedness by using sex and age‐normalized T scores instead of raw scores. Also, it is unlikely that level of education has played a role in the reported effects, given that all participants were recruited from a relatively homogenous undergraduate population. However, it has been suggested that gender may also influence the recognition of facial expressions [Forni‐Santos and Osorio, 2015] and empathic abilities [Christov‐Moore et al., 2014], processes which may relate to interpersonal distance preferences [Perry et al., 2015]. Recent work has also reported differential neural activation patterns to personal space intrusions by men and women [Wabnegger et al., 2016]. In the present data, exploratory analyses revealed that females preferred greater distances than males in the approach condition only, irrespective of emotional expression. Given that we were primarily interested in emotion‐specific effects on interpersonal distance and its neural substrates, no further gender differences were explored. Future research using larger mixed samples should assess how personal space preferences are affected by the gender.
One important limitation of this study is the relatively limited sample size for exploring individual differences, and consequently reduced statistical power, in comparison to prior work investigating neural correlates of socio‐affective processes as a function of psychopathic traits [Han et al., 2012].We have attempted to overcome this limitation by relying on correlational methods rather than group analysis to explore the effects of Coldheartedness in our data. Nevertheless, future work involving larger sample sizes would be valuable for further exploring these effects.
Conclusions
To our knowledge, this was the first study to explore the neural processes involved in regulating interpersonal distance in emotional contexts, and how those processes varied with individual differences in empathy. It differed from previous fMRI studies that examined the neural signatures of static emotional expressions [Fusar‐Poli et al., 2009], or only contrasted approach and static stimuli [Schienle et al., 2015]. Present findings implicate the amygdala in approach‐avoidance behaviour in social settings and, particularly, in interpersonal distance preferences to anger, sadness and fear displays. Moreover, we have demonstrated that individual differences in empathy may selectively affect approach‐avoidance tendencies to distress cues. Our hypotheses for this study were specific for low trait empathy as described and assessed for psychopathic personalities, given prior evidence of an association between those features and both interpersonal distance and amygdala dysfunction. Our findings concern community samples of individuals scoring high on coldhearted traits, and may not generalize to all psychopathic populations. However, in light of suggestions that the core deficits in psychopathy include reduced responsiveness to emotional cues in others [i.e., low empathy; Blair, 2015], the present findings may shed light on the potential neurobiological mechanisms disrupted in psychopathic individuals. Overall, the present work contributes to a better understanding of how people use emotional cues to guide social behaviour, and how individual differences in empathy influence this process.
REFERENCES
- Adams RB Jr, Kleck RE (2003): Perceived gaze direction and the processing of facial displays of emotion. Psychol Sci 14:644–647. [DOI] [PubMed] [Google Scholar]
- Adams RB Jr, Kleck RE (2005): Effects of direct and averted gaze on the perception of facially communicated emotion. Emotion 5:3–11. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio H, Damasio A (1994): Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature 372:669–672. [DOI] [PubMed] [Google Scholar]
- Aube W, Angulo‐Perkins A, Peretz I, Concha L, Armony JL (2015): Fear across the senses: Brain responses to music, vocalizations and facial expressions. Soc Cogn Affect Neurosci 10:399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJ (2003): Facial expressions, their communicatory functions and neuro‐cognitive substrates. Philos Trans R Soc Lond B Biol Sci 358:561–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJ (2013): The neurobiology of psychopathic traits in youths. Nat Rev Neurosci 14:786–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJ (2015): Psychopathic traits from an RDoC perspective. Curr Opin Neurobiol 30:79–84. [DOI] [PubMed] [Google Scholar]
- Blair RJ, Colledge E, Murray L, Mitchell DG (2001): A selective impairment in the processing of sad and fearful expressions in children with psychopathic tendencies. J Abnorm Child Psychol 29:491–498. [DOI] [PubMed] [Google Scholar]
- Bliss‐Moreau E, Toscano JE, Bauman MD, Mason WA, Amaral DG (2011): Neonatal amygdala lesions alter responsiveness to objects in juvenile macaques. Neuroscience 178:123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss‐Moreau E, Moadab G, Bauman MD, Amaral DG (2013): The impact of early amygdala damage on juvenile rhesus macaque social behavior. J Cogn Neurosci 25:2124–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet L, Comte A, Tatu L, Millot JL, Moulin T, Medeiros de Bustos E (2015): The role of the amygdala in the perception of positive emotions: An “intensity detector”. Front Behav Neurosci 9:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozzoli C, Gentile G, Bergouignan L, Ehrsson HH (2013): A shared representation of the space near oneself and others in the human premotor cortex. Curr Biol 23:1764–1768. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Priester JR, Berntson GG (1993): Rudimentary determinants of attitudes. II: Arm flexion and extension have differential effects on attitudes. J Pers Soc Psychol 65:5–17. [DOI] [PubMed] [Google Scholar]
- Christov‐Moore L, Simpson EA, Coude G, Grigaityte K, Iacoboni M, Ferrari PF (2014): Empathy: Gender effects in brain and behavior. Neurosci Biobehav Rev 46 Pt 4:604–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coker‐Appiah DS, White SF, Clanton R, Yang J, Martin A, Blair RJ (2013): Looming animate and inanimate threats: The response of the amygdala and periaqueductal gray. Soc Neurosci 8:621–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras‐Rodriguez O, Pujol J, Batalla I, Harrison BJ, Bosque J, Ibern‐Regas I, Hernandez‐Ribas R, Soriano‐Mas C, Deus J, Lopez‐Sola M, Pifarre J, Menchon JM, Cardoner N (2014): Disrupted neural processing of emotional faces in psychopathy. Soc Cogn Affect Neurosci 9:505–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis FC, Neta M, Kim MJ, Moran JM, Whalen PJ (2016): Interpreting ambiguous social cues in unpredictable contexts. Soc Cogn Affect Neurosci 11:775–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawel A, O'Kearney R, McKone E, Palermo R (2012): Not just fear and sadness: Meta‐analytic evidence of pervasive emotion recognition deficits for facial and vocal expressions in psychopathy. Neurosci Biobehav Rev 36:2288–2304. [DOI] [PubMed] [Google Scholar]
- Dolan M, Fullam R (2006): Face affect recognition deficits in personality‐disordered offenders: Association with psychopathy. Psychol Med 36:1563–1569. [DOI] [PubMed] [Google Scholar]
- Drislane LE, Patrick CJ, Sourander A, Sillanmaki L, Aggen SH, Elonheimo H, Parkkola K, Multimaki P, Kendler KS (2014): Distinct variants of extreme psychopathic individuals in society at large: Evidence from a population‐based sample. Person Disord 5:154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri F, Costantini M, Huang Z, Perrucci MG, Ferretti A, Romani GL, Northoff G (2015): Intertrial variability in the premotor cortex accounts for individual differences in peripersonal space. J Neurosci 35:16328–16339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine C, Blair RJR (2000): Mini review: The cognitive and emotional effects of amygdala damage. Neurocase 6:435–450. [Google Scholar]
- Fontaine JR, Scherer KR, Roesch EB, Ellsworth PC (2007): The world of emotions is not two‐dimensional. Psychol Sci 18:1050–1057. [DOI] [PubMed] [Google Scholar]
- Forni‐Santos L, Osorio FL (2015): Influence of gender in the recognition of basic facial expressions: A critical literature review. World J Psychiatry 5:342–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick PJ (2016): Early identification and treatment of antisocial behavior. Pediatr Clin North Am 63:861–871. [DOI] [PubMed] [Google Scholar]
- Frick PJ, White SF (2008): Research review: The importance of callous‐unemotional traits for developmental models of aggressive and antisocial behavior. J Child Psychol Psychiatry 49:359–375. [DOI] [PubMed] [Google Scholar]
- Fusar‐Poli P, Placentino A, Carletti F, Landi P, Allen P, Surguladze S, Benedetti F, Abbamonte M, Gasparotti R, Barale F, Perez J, McGuire P, Politi P (2009): Functional atlas of emotional faces processing: A voxel‐based meta‐analysis of 105 functional magnetic resonance imaging studies. J Psychiatry Neurosci 34:418–432. [PMC free article] [PubMed] [Google Scholar]
- Gerber AJ, Posner J, Gorman D, Colibazzi T, Yu S, Wang Z, Kangarlu A, Zhu H, Russell J, Peterson BS (2008): An affective circumplex model of neural systems subserving valence, arousal, and cognitive overlay during the appraisal of emotional faces. Neuropsychologia 46:2129–2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon HL, Baird AA, End A (2004): Functional differences among those high and low on a trait measure of psychopathy. Biol Psychiatry 56:516–521. [DOI] [PubMed] [Google Scholar]
- Graziano MS, Cooke DF (2006): Parieto‐frontal interactions, personal space, and defensive behavior. Neuropsychologia 44:2621–2635. [DOI] [PubMed] [Google Scholar]
- Hammer JL, Marsh AA (2015): Why do fearful facial expressions elicit behavioral approach? Evidence from a combined approach‐avoidance implicit association test. Emotion 15:223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han T, Alders GL, Greening SG, Neufeld RW, Mitchell DG (2012): Do fearful eyes activate empathy‐related brain regions in individuals with callous traits? Soc Cogn Affect Neurosci 7:958–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings ME, Tangney JP, Stuewig J (2008): Psychopathy and identification of facial expressions of emotion. Person Individ Dif 44:1474–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayduk LA (1983): Personal space: Where we now stand. Psychol Bull 94:293–335. [Google Scholar]
- Hess U, Blairy S, Kleck RE (2000): The influence of facial emotion displays. gender, and ethnicity on judgements of dominance and affiliation. J Nonverb Behav 24:265–283. [Google Scholar]
- Holt DJ, Cassidy BS, Yue X, Rauch SL, Boeke EA, Nasr S, Tootell RB, Coombs G 3rd (2014): Neural correlates of personal space intrusion. J Neurosci 34:4123–4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz MJ, Duff DF, Stratton LO (1964): Body‐buffer zone; Exploration of personal space. Arch Gen Psychiatry 11:651–656. [DOI] [PubMed] [Google Scholar]
- Horstmann G (2003): What do facial expressions convey: Feeling states, behavioral intentions, or action requests? Emotion 3:150–166. [DOI] [PubMed] [Google Scholar]
- Jerram M, Lee A, Negreira A, Gansler D (2014): The neural correlates of the dominance dimension of emotion. Psychiatry Res 221:135–141. [DOI] [PubMed] [Google Scholar]
- Jones AP, Laurens KR, Herba CM, Barker GJ, Viding E (2009): Amygdala hypoactivity to fearful faces in boys with conduct problems and callous‐unemotional traits. Am J Psychiatry 166:95–102. [DOI] [PubMed] [Google Scholar]
- Kennedy DP, Glascher J, Tyszka JM, Adolphs R (2009): Personal space regulation by the human amygdala. Nat Neurosci 12:1226–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryklywy JH, Nantes SG, Mitchell DG (2013): The amygdala encodes level of perceived fear but not emotional ambiguity in visual scenes. Behav Brain Res 252:396–404. [DOI] [PubMed] [Google Scholar]
- Laham SM, Kashima Y, Dix J, Wheeler M (2015): A meta‐analysis of the facilitation of arm flexion and extension movements as a function of stimulus valence. Cogn Emot 29:1069–1090. [DOI] [PubMed] [Google Scholar]
- Lilienfeld, S.O. , Widows, M.R. (2005) PPI‐R: Psychopathic Personality Inventory—Revised. Lutz, FL: PsychologicalAssessmentResources. [Google Scholar]
- Lozier LM, Cardinale EM, VanMeter JW, Marsh AA (2014): Mediation of the relationship between callous‐unemotional traits and proactive aggression by amygdala response to fear among children with conduct problems. JAMA Psychiatry 71:627–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado CJ, Kazama AM, Bachevalier J (2009): Impact of amygdala, orbital frontal, or hippocampal lesions on threat avoidance and emotional reactivity in nonhuman primates. Emotion 9:147–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH (2003): An automated method for neuroanatomic and cytoarchitectonic atlas‐based interrogation of fMRI data sets. Neuroimage 19:1233–1239. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Burdette JH (2004): Precentral gyrus discrepancy in electronic versions of the Talairach atlas. Neuroimage 21:450–455. [DOI] [PubMed] [Google Scholar]
- Marcus DK, John SL, Edens JF (2004): A taxometric analysis of psychopathic personality. J Abnorm Psychol 113:626–635. [DOI] [PubMed] [Google Scholar]
- Marsh AA (2016): Understanding amygdala responsiveness to fearful expressions through the lens of psychopathy and altruism. J Neurosci Res 94:513–525. [DOI] [PubMed] [Google Scholar]
- Marsh AA, Ambady N, Kleck RE (2005): The effects of fear and anger facial expressions on approach‐ and avoidance‐related behaviors. Emotion 5:119–124. [DOI] [PubMed] [Google Scholar]
- Marsh AA, Blair KS, Jones MM, Soliman N, Blair RJ (2009): Dominance and submission: The ventrolateral prefrontal cortex and responses to status cues. J Cogn Neurosci 21:713–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehu M, Scherer KR (2015): Emotion categories and dimensions in the facial communication of affect: An integrated approach. Emotion 15:798–811. [DOI] [PubMed] [Google Scholar]
- Mier D, Haddad L, Diers K, Dressing H, Meyer‐Lindenberg A, Kirsch P (2014): Reduced embodied simulation in psychopathy. World J Biol Psychiatry 15:479–487. [DOI] [PubMed] [Google Scholar]
- Miller JD, Lynam DR, Widiger TA, Leukefeld C (2001): Personality disorders as extreme variants of common personality dimensions: Can the five‐factor model adequately represent psychopathy? J Pers 69:253–276. [DOI] [PubMed] [Google Scholar]
- Mobbs D, Marchant JL, Hassabis D, Seymour B, Tan G, Gray M, Petrovic P, Dolan RJ, Frith CD (2009): From threat to fear: The neural organization of defensive fear systems in humans. J Neurosci 29:12236–12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs D, Petrovic P, Marchant JL, Hassabis D, Weiskopf N, Seymour B, Dolan RJ, Frith CD (2007): When fear is near: Threat imminence elicits prefrontal‐periaqueductal gray shifts in humans. Science 317:1079–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs D, Yu R, Rowe JB, Eich H, FeldmanHall O, Dalgleish T (2010): Neural activity associated with monitoring the oscillating threat value of a tarantula. Proc Natl Acad Sci U S A 107:20582–20586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawetz C, Holz P, Lange C, Baudewig J, Weniger G, Irle E, Dechent P (2008): Improved functional mapping of the human amygdala using a standard functional magnetic resonance imaging sequence with simple modifications. Magn Reson Imaging 26:45–53. [DOI] [PubMed] [Google Scholar]
- Ng B, Kumar S, Ranclaud M, Robinson E (2001): Ward crowding and incidents of violence on an acute psychiatric inpatient unit. Psychiatr Serv 52:521–525. [DOI] [PubMed] [Google Scholar]
- Oliver LD, Mao A, Mitchell DG (2015): “Blindsight” and subjective awareness of fearful faces: Inversion reverses the deficits in fear perception associated with core psychopathic traits. Cogn Emot 29:1256–1277. [DOI] [PubMed] [Google Scholar]
- Parkinson B (2005): Do facial movements express emotions or communicate motives? Pers Soc Psychol Rev 9:278–311. [DOI] [PubMed] [Google Scholar]
- Perry A, Mankuta D, Shamay‐Tsoory SG (2015): OT promotes closer interpersonal distance among highly empathic individuals. Soc Cogn Affect Neurosci 10:3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Young AW, Scott SK, Calder AJ, Andrew C, Giampietro V, Williams SC, Bullmore ET, Brammer M, Gray JA (1998): Neural responses to facial and vocal expressions of fear and disgust. Proc Biol Sci 265:1809–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine A, Dodge K, Loeber R, Gatzke‐Kopp L, Lynam D, Reynolds C, Stouthamer‐Loeber M, Liu J (2006): The Reactive‐proactive aggression questionnaire: Differential correlates of reactive and proactive aggression in adolescent boys. Aggress Behav 32:159–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regoeczi WC (2008): Crowding in context: An examination of the differential responses of men and women to high‐density living environments. J Health Soc Behav 49:254–268. [DOI] [PubMed] [Google Scholar]
- Robinson S, Windischberger C, Rauscher A, Moser E (2004): Optimized 3 T EPI of the amygdalae. Neuroimage 22:203–210. [DOI] [PubMed] [Google Scholar]
- Sambo CF, Iannetti GD (2013): Better safe than sorry? The safety margin surrounding the body is increased by anxiety. J Neurosci 33:14225–14230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schienle A, Wabnegger A, Schongassner F, Leutgeb V (2015): Effects of personal space intrusion in affective contexts: An fMRI investigation with women suffering from borderline personality disorder. Soc Cogn Affect Neurosci 10:1424–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt KL, Cohn JF (2001): Human facial expressions as adaptations: Evolutionary questions in facial expression research. Am J Phys Anthropol Suppl 33:3–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel EM, Habel U, Kirschner M, Gur RC, Derntl B (2010): The impact of facial emotional expressions on behavioral tendencies in women and men. J Exp Psychol Hum Percept Perform 36:500–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skeem JL, Polaschek DL, Patrick CJ, Lilienfeld SO (2011): Psychopathic personality: Bridging the gap between scientific evidence and public policy. Psychol Sci Public Interest 12:95–162. [DOI] [PubMed] [Google Scholar]
- Thomas KM, Drevets WC, Whalen PJ, Eccard CH, Dahl RE, Ryan ND, Casey BJ (2001): Amygdala response to facial expressions in children and adults. Biol Psychiatry 49:309–316. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Marcus DJ, Westerlund A, Casey BJ, Nelson C (2009): The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Res 168:242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. Neuroimage 15:273–289. [DOI] [PubMed] [Google Scholar]
- Veenstra L, Schneider IK, Bushman BJ, Koole SL (2016): Drawn to danger: Trait anger predicts automatic approach behaviour to angry faces. Cogn Emot 1–7. [DOI] [PubMed] [Google Scholar]
- Viding E, Sebastian CL, Dadds MR, Lockwood PL, Cecil CA, De Brito SA, McCrory EJ (2012): Amygdala response to preattentive masked fear in children with conduct problems: The role of callous‐unemotional traits. Am J Psychiatry 169:1109–1116. [DOI] [PubMed] [Google Scholar]
- Vieira JB, Marsh AA (2014): Don't stand so close to me: Psychopathy and the regulation of interpersonal distance. Front Hum Neurosci 7:907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Borries LA, Volman I, de Bruijn ER, Bulten BH, Verkes RJ, Roelofs K (2012): Psychopaths lack the automatic avoidance of social threat: Relation to instrumental aggression. Psychiatry Res 200:761–766. [DOI] [PubMed] [Google Scholar]
- Wabnegger A, Leutgeb V, Schienle A (2016): Differential amygdala activation during simulated personal space intrusion by men and women. Neuroscience 330:12–16. [DOI] [PubMed] [Google Scholar]
- Weymar M, Schwabe L (2016): Amygdala and emotion: The bright side of it. Front Neurosci 10:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen PJ (1998): Fear, vigilance, and ambiguity: Initial neuroimaging studies of the human amygdala. Curr Direct Psychol Sci 7:177–188. [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA (1998): Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J Neurosci 18:411–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson‐Mendenhall CD, Barrett LF, Barsalou LW (2013): Neural evidence that human emotions share core affective properties. Psychol Sci 24:947–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodworth M, Waschbusch D (2008): Emotional processing in children with conduct problems and callous/unemotional traits. Child Care Health Dev 34:234–244. [DOI] [PubMed] [Google Scholar]
- Yik M, Russell JA, Steiger JH (2011): A 12‐point circumplex structure of core affect. Emotion 11:705–731. [DOI] [PubMed] [Google Scholar]


