Abstract
Global brain connectivity (GBC) identifies regions of the brain, termed “hubs,” which are densely connected and metabolically costly, and have a wide influence on brain function. Since obesity is associated with central and peripheral metabolic dysfunction we sought to determine if GBC is altered in obesity. Two independent fMRI data sets were subjected to GBC analyses. The first data set was acquired while participants (n = 15 healthy weight and 15 obese) tasted milkshake and the second with participants at rest (n = 33 healthy weight and 28 obese). In the resting state and during milkshake consumption GBC is consistently decreased in the ventromedial and ventrolateral prefrontal cortex, insula and caudate nucleus, and increased in brain regions belonging to the dorsal attention network including premotor areas, superior parietal lobule, and visual cortex. During milkshake consumption, but not at rest, additional decreases in GBC are observed in feeding‐related circuitry including the insula, amygdala, anterior hippocampus, hypothalamus, midbrain, brainstem and somatomotor cortex. Additionally, GBC differences were not accounted for by age. These results demonstrate that obesity is associated with decreased GBC in prefrontal and feeding circuits and increased GBC in the dorsal attention network. We therefore conclude that global brain organization is altered in obesity to favor networks important for external orientation over those monitoring homeostatic state and guiding feeding decisions. Furthermore, since prefrontal decreases are also observed at rest in obese individuals future work should evaluate whether these changes are associated with neurocognitive impairments frequently observed in obesity and diabetes. Hum Brain Mapp 38:1403–1420, 2017. © 2016 Wiley Periodicals, Inc.
Keywords: obesity, connectivity, feeding, resting state, reward, attention, internal milieu
Abbreviations
- BA
Brodmann Area
- BMI
Body mass index;
- DEBQ
Dutch Eating Behavior Questionnaire
- DMN
Default mode network
- FEAT
FMRIB Expert Analysis Tool
- FSL
FMRIB Software Library
- GBC
Global brain connectivity
- MNI
Montreal Neurological Institute
- SMA
Supplementary motor area
- SVD
Singular value decomposition
- TFCE
Threshold‐free cluster enhancement
- TFEQ
Three Factor Eating Questionnaire
INTRODUCTION
There is a growing literature documenting a variety of brain changes associated with obesity [Babiloni et al., 2011; Garcia‐Garcia et al., 2012; Garcia‐Garcia et al., 2013; Kullmann et al., 2011; Kullmann et al., 2013; Le et al., 2007; Marques‐Iturria et al., 2015; McFadden et al., 2013; Raji et al., 2010; Stanek et al., 2011; Stoeckel et al., 2009; Tregellas et al., 2011; Verstynen et al., 2012; Xu et al., 2013], some of which are linked to metabolic disturbances in central and peripheral insulin signaling and glucose metabolism [Gonzales et al., 2010; Kroemer et al., 2013; Page et al., 2011; Zhang et al., 2015]. Many studies also demonstrate widespread shrinkage in brain gray matter [Karlsson et al., 2013; Marques‐Iturria et al., 2015; Pannacciulli et al., 2006], decreased white matter integrity [Verstynen et al., 2012; Xu et al., 2013], altered cortical synchronization [Babiloni et al., 2011; Olde Dubbelink et al., 2008; Wijngaarden et al., 2015] and regional decrease in cerebral blood flow [Willeumier et al., 2011] in obese compared to healthy weight individuals. Accordingly, deficits in cognitive function are consistently observed [Coppin et al., 2014; Francis and Stevenson, 2013; Gunstad et al., 2007; Stoeckel et al., 2016].
Global brain connectivity (GBC) derived from functional magnetic resonance imaging (fMRI) data, provides a sensitive measure of brain network integrity by quantifying the number of connections each node (i.e., the basic unit of a network, here a voxel) has with all other nodes within a network [Cole et al., 2010; Eguiluz et al., 2005; Rubinov and Sporns, 2010]. Clusters of densely connected nodes, also called “hubs” [Buckner et al., 2009; Cole et al., 2010; Eguiluz et al., 2005], are metabolically costly [Fulcher and Fornito, 2016; Liang et al., 2013; Tomasi et al., 2013] and are thought to mediate integrative information processing [Crossley et al., 2013; Harriger et al., 2012; van den Heuvel and Sporns, 2013]. Hubs have been found to be stable across studies [Tomasi and Volkow, 2010], and brain states, (i.e., resting state and task based fMRI data) [Buckner et al., 2009], but are consistently vulnerable to disruption in aging [Cao et al., 2014; Hampson et al., 2012] and disease, such as schizophrenia or dementia [Rubinov and Bullmore, 2013a; Rubinov and Bullmore, 2013b; Zhou et al., 2012]. They are also correlated with cognitive function [Cole et al., 2012]. To date GBC has not been examined in obesity. This is an important gap in the literature because obesity is characterized by peripheral and central metabolic alterations, such as insulin resistance [Grundy, 2016; Heni et al., 2015], insensitivity to a variety of circulating hormones, peptides and nutrients important for brain function and homeostasis [Carter et al., 2013; le Roux et al., 2006; Page et al., 2011; Zigman et al., 2016], as well as cognitive dysfunction [Coppin et al., 2014; Stoeckel et al., 2016]. In addition, obesity is highly comorbid with neurological and psychiatric disorders [Barnes and Yaffe, 2011; Egan et al., 2013; Shiri et al., 2010; Shiri et al., 2008; Tiihonen et al., 2009] where GBC is often used as a marker of pathology [Anticevic et al., 2013; Anticevic et al., 2014]. Taken together, these observations highlight the importance of clarifying the GBC signature unique to obesity.
We therefore used fMRI to examine the relationship between GBC [Buckner et al., 2009; Cole et al., 2010; Eguiluz et al., 2005; Scheinost et al., 2012] and body mass index (BMI) while participants engaged in ingestive behavior and while they were at rest. We hypothesized that obese relative to healthy‐weight subjects would have large‐scale altered GBC reflecting the deleterious influence of adiposity, diet, and metabolic dysfunction on brain circuits. The prefrontal cortex and hippocampus were of particular interest because structural and functional abnormalities are consistently observed in these regions [Erion et al., 2014; Gupta et al., 2015; Mueller et al., 2012; Willeumier et al., 2011]. We further reasoned that, areas with loss of GBC, reflecting reduced influence of widespread brain networks, may instead be preferentially connected to a restricted set of regions involved in appetitive and habitual behavior mirroring the increased response of these circuits to food cues in obesity [Dagher, 2012]. The following questions were addressed: (1) what and where are the effects of weight status (i.e., healthy weight vs. obese) on GBC? (2) Might changes in GBC be accompanied by altered seed based connectivity in feeding circuits? (3) Do these effects differ if subjects are at rest compared to when they are consuming a palatable and energy dense milkshake? (4) Does BMI account for more or similar variance in GBC compared to age?
EXPERIMENTAL PROCEDURES FOR EXPERIMENT 1
Participants
Data for experiment 1 were pooled from 52 individuals who participated in two different studies previously published by our group [Geha et al., 2013; Veldhuizen et al., 2013]. From this sample, 15 healthy weight individuals (BMI ≤ 25), and 15 obese (BMI ≥ 30) that were matched for age and gender were selected (Table 1). Participants were not taking daily medications; had no history of loss of consciousness; chronic medical illness, such as arthritis or heart disease; psychiatric disorders, such as mood, psychotic, anxiety or eating disorder; chemosensory impairment; or food allergies. All participants filled out the Three Factor Eating Questionnaire (TFEQ) [Stunkard and Messick, 1985] and the Dutch Eating Behavior Questionnaire (DEBQ) [Van et al., 1986]. Subjects were between 18 and 45 years old. The studies were approved by the Yale University Human Investigation Committee and subjects provided informed consent. (see Online Supporting Information).
Table 1.
Demographic characteristics and internal state ratings of subjects in experiment 1
| Healthy weight | Obese | P‐value | |
|---|---|---|---|
| N | 15 | 15 | |
| Age (years) | 27.4 ± 1.7 | 27.7 ± 1.7 | 0.92 |
| Gender | 2 Males | 2 Males | |
| BMI (Kg/m2) | 21.9 ± 0.5 | 35.3 ± 0.9 | <10−6 |
| Education (years) | 16.3 ± 0.6 | 15.4 ± 0.4 | 0.21 |
| Hunger | 1.2 ± 1.3 | 0.1 ± 1.4 | 0.55 |
| Fullness | −4.7 ± 1.1 | −0.4 ± 1.3 | 0.02 |
| TFEQ‐Hunger | 6.1 ± 1.1 | 6.5 ± 0.9 | 0.74 |
| TFEQ‐Restraint | 6.6 ± 1.3 | 10.9 ± 1.3 | 0.03 |
| TFEQ‐Disinhibition | 4.6 ± 0.7 | 8.6 ± 0.8 | <10−3 |
| DEBQ‐EET | 2.0 ± 0.2 | 2.8 ± 0.1 | <10−3 |
| DEBQ‐EEDE | 2.5 ± 0.2 | 3.0 ±0.2 | 0.055 |
| DEBQ‐EECLE | 1.8 ± 0.2 | 2.7 ± 0.19 | <10−3 |
| DEBQ‐EE | 3.5 ± 0.11 | 3.3 ± 0.12 | 0.39 |
| DEBQ‐RE | 2.2 ± 0.2 | 3.0 ± 0.2 | 0.011 |
Abbreviations: DEBQ, Dutch Eating Behavior Questionnaire; EET, emotional eating total; EEDE, emotional eating diffuse emotions; EECLE, emotional eating clearly labeled emotions; EE, external eating; RE, restraint eating; TFEQ, Three Factor Eating Questionnaire.
Stimuli, Task and Studies Design
Participants in study 1 were presented with oral boluses of either a milkshake or a tasteless solution in the scanner with a total of 20 presentations of each stimulus [Geha et al., 2013]. Participants in study 2 were presented in each trial with either a low calorie flavored beverage, milkshake, or tasteless solution with a total of 20 presentations of each stimulus [Veldhuizen et al., 2013]. Stimuli, task designs and fMRI data acquisition for both Study 1 and 2 have been described in detail previously [Geha et al., 2013; Veldhuizen et al., 2013] and are reported here in the online Supporting Information.
fMRI Analysis for Experiment 1
Image preprocessing
Image analysis was performed on each subject's data using FMRIB Expert Analysis Tool (FEAT, Smith et al., 2004], http://www.fmrib.ox.ac.uk/fsl). The pre‐processing of each subject's time‐series of fMRI volumes encompassed: (i) skull extraction using BET; (ii) slice time correction; (iii) motion correction; (iv) spatial smoothing using a Gaussian kernel of full‐width‐half‐maximum 5 mm; (v) non‐linear high‐pass temporal filtering (128 seconds) and subtraction of the mean of each voxel time‐course from that time‐course. Anatomical and functional images were normalized to the standard Montreal Neurological Institute (MNI) template brain implemented in FMRIB Software Library (FSL). The six vectors for head motion, in addition to the global signal calculated as the average signal intensity of all brain voxels at each volume, were regressed out of the data to correct for head motion and fluctuations in whole brain signal intensity respectively [Fox et al., 2009]. Head motion can cause spurious but spatially structured changes in functional correlations [Power et al., 2012]. To minimize these effects, all subjects were movement‐scrubbed [Power et al., 2012; Power et al., 2014]. This procedure uses temporal masks to remove motion‐contaminated data from regression and correlation calculations by excising unwanted data. Frames in which collective displacement across all six rigid body movement correction parameters exceeded FD > 0.5 mm (assuming 50 mm cortical sphere radius) were identified. We excluded frames flagged by this criterion and replaced them with linear interpolation [Carp, 2013] since some of our data (experiment 1) had relatively short timeseries (135 time points) and we did not want to lose degrees of freedom. Subjects with 50% frames flagged were omitted from analyses.
Construction of brain graphs
In order to investigate whether BMI is associated with global changes in brain connectivity we used graph theory to explore brain networks. The basic elements of the networks (i.e., nodes) were defined as the voxels. Individual brain volumes were first transformed into an averaged whole brain mask. Within this mask voxels not shared by all subjects were zeroed to equate the number of nodes across subjects and thus not bias our analysis by underlying differences in gray matter volume. Finally, this mask was multiplied by a standard gray matter mask available in the FSL standard brains library. Only time‐series from voxels falling within this mask were used for construction of brain graphs. The first step was calculating pair‐wise Pearson correlation coefficients among all pairs of voxel‐wise time‐series. The whole timeseries was used without regressing out any of the task components. Graph theory constructs networks based on links between nodes. A link refers to a correlation above a certain threshold or “wiring cost” and multiple thresholds can be explored to reveal networks with different characteristics. Furthermore, since the actual degree of connectivity is unknown multiple thresholds are typically used [Buckner et al., 2009; Bullmore and Bassett, 2011; Tomasi and Volkow, 2010]. These thresholds are frequently chosen to be large enough to prevent false positive results but small enough to maintain the important connections. An un‐thresholded network will have every node connected to every other node. By using a wide range of network thresholds we are able to test for connectivity at different levels of wiring costs [Liang et al., 2013; Riedl et al., 2014; Tomasi et al., 2013]. Therefore, we calculated (1) pair‐wise Pearson correlation coefficients among all pairs of time‐series [Vi(t), V j(t)] where Vi(t) and Vj(t), corresponds to the BOLD signal of the i‐th and j‐th voxel respectively; (2) a link between a pair of voxels (i, j) is included in the network if the absolute value of the correlation Vi and Vj exceeds a specified threshold τ, |Vi(t), Vj(t)| ≥ τ. A randomly wired network with N nodes will have [N × (N − 1)/2] links where every node is connected to every other node. The wiring cost of the brain network of the same size N, is therefore a fraction of these possible edges. Accordingly, τ is determined for each run so that [N × (N − 1)/2] is equal to 0.5, 1, 2.5, 5, and 10% respectively [Power et al., 2013]. GBC was then obtained by assigning to each voxel the total number of links with a Pearson correlation ≥ τ in the corresponding network node. Next, we averaged GBC of different runs to obtain one map per subject. Images were then transformed into the standard MNI template for group analysis and resampled into a 4 × 4 × 4 mm space to reduce the number of voxels (i.e., nodes).
Comparison of brain graphs between groups
Within‐study effects of BMI on brain response to milkshake have been previously reported and are not described here [Babbs et al., 2013; Nolan‐Poupart et al., 2013; Stice et al., 2008b; Sun et al., 2015]. To examine between group differences in GBC we first performed singular value decomposition (SVD) to increase sensitivity [Horn and Johnson, 1985]. All functional correlation analyses are dominated by default mode network dynamics [Cole et al., 2010] and its slow time scale [Fox et al., 2005], which can obscure more subtle brain processes [Kelly et al., 2008]. We have adopted SVD as a strategy to cope with this problem [Andersen et al., 1999; Calhoun et al., 2001]. When GBC maps are decomposed into independent or uncorrelated patterns group comparisons are more sensitive to subtler, but often significant differences.
The construction of brain graphs yields a matrix M with m rows, where m = total number of voxels per subject, and n subjects, where n = total number of subjects—in this case the total number of healthy weight and obese participants. In order to identify brain spatial components showing significant differences between the groups (i.e., healthy weight vs. obese) we used SVD to reduce the dimensionality of M. Specifically, a linear decomposition of M was performed, such that , where U is an m × m unitary matrix whose columns represent the singular spatial components, V is an n × n unitary matrix whose columns correspond to the projection or weight of each component on each subject, and S is an m × n diagonal matrix, whose n elements are non‐negative and correspond to the relative weight of each component in explaining the variance of the data [Horn and Johnson, 1985]. The brain map for each individual k, represented as a vector over m voxels, can therefore be expressed as a linear combination of the singular components:
In order to identify components that discriminate between the healthy weight and obese groups sequential t‐tests were performed on each projected component. SVD produces many components, most of which portray noise, because the variance accounted for in each of the components decrease exponentially from component 1 to n. To account for multiple comparisons, we corrected the significance level for the P‐value of the projection on the i‐th component as . Therefore, P‐values are reported as significant only if they survived multiple comparison correction. Unless stated otherwise, the t‐tests were always calculated after correcting for gender and age. The component maps were illustrated after using mixture modeling with spatial enhancement as implemented in FSL to ensure that voxels with similar patterns of GBC form one network [Woolrich et al., 2005]. Mixture modeling uses a spatial Markov random field to regularize (smooth) the labeling of voxels into null, activated, or “de”‐activated, and hence allows us to answer the question: “Is the activation bigger than the overall level of background activation?” instead of answering the question “Is the activation zero or not?” Mixture modeling is a suitable method for illustrating component maps since the threshold of these maps is arbitrary. For our purpose we used a probability threshold P > 0.8 meaning that a voxel passes threshold only if its probability of belonging to activated or de‐activated voxels is > 0.8 compared to non‐activation/null distribution.
Seed based analysis
Seed based connectivity was determined using a well defined method [Fox et al., 2005]. To investigate whether brain networks correlated with specific regional activity (seed) as a function of group, seeds were defined as 10 mm spheres from maxima of GBC (“hubs”) where significant differences in GBC were found (see results section). Average BOLD time course of all voxels within the ROIs were extracted and then the correlation coefficient between this time course and the time variability of all brain voxels were computed using Matlab. Correlation coefficients were converted to a normal distribution using the Fisher z‐transform. These values were then converted to z‐scores (i.e., normalized correlation values) by dividing by the square root of the variance, estimated as 1/√df‐3, where df represents the degrees of freedom in our measurements (i.e., the number of volumes acquired). Because the BOLD time courses of consecutive samples are not statistically independent, the degrees of freedom were corrected by a factor according to Bartlet theory [Jenkins and Watts, 1968]. Group differences in seed based connectivity between healthy weight and obese subjects were identified using permutation based inference [Nichols and Holmes, 2002]) to allow rigorous comparisons of significance within the framework of the general linear model with P < 0.05. Group differences were tested against 5000 random permutations, which inherently accounts for multiple comparisons, using Randomise part of FSL [Winkler et al., 2014]. Group contrast clusters were identified using threshold‐free cluster enhancement (TFCE) method [Smith and Nichols, 2009], which bypasses the arbitrary threshold necessary in methods that use voxel‐based thresholding.
EXPERIMENTAL PROCEDURES FOR EXPERIMENT 2
The above analyses were based on data collected during consumption of a palatable and energy dense milkshake. To determine if BMI also influences global connectivity at rest we applied the same analysis described above to resting state data from the 1000 Functional Connectome Project [Nooner et al., 2012].
Participants
Resting state data was obtained from the 1000 Functional Connectome Project (http://fcon_1000.projects.nitrc.org/indi/pro/nki.html), which is part of an international neuroimaging data sharing initiative with available demographic data including BMI, and fasting blood glucose [Nooner et al., 2012]. From this data set we pre‐preprocessed 125 healthy adult subjects. We identified 33 healthy‐weight (19 females, average BMI = 23.1 ± 0.3 kg/m2), 28 obese (17 females, 32.8 ± 0.5 kg/m2) (Table 2). Individuals were not included if they had excessive head motion during scanning (exceeding 2 mm of movement in any direction), erroneous head positioning leading to brain regions being cut off, or if they had missing BMI values.
Table 2.
Demographic and metabolic characteristics of subjects in experiment 2
| Healthy weight | Obese | P‐value | |
|---|---|---|---|
| N | 32 | 22 | |
| Age (years) | 33.0 ± 2.8 | 43.6 ± 3.2 | 0.016 |
| Gender | 18 F | 17 F | 0.73 |
| BMI (Kg/m2) | 23.3 ± 0.3 | 32.8 ± 0.5 | <10−19 |
| BDI | 3.4 ± 0.6 | 2.8 ± 0.7 | 0.58 |
| TG (mg/dL) | 85.4 ± 6.7 | 124.9 ±24.6 | 0.18 |
| FBS (mg/dL) | 85.5 ± 1.6 | 88.3 ± 1.5 | 0.36 |
| TFEQ‐Hunger | 4.3 ± 0.6 | 4.6 ± 0.9 | 0.80 |
| TFEQ‐Disinhibition | 2.1 ± 0.4 | 3.5 ± 0.9 | 0.12 |
| TFEQ‐Restraint | 5.4 ± 0.8 | 6.6 ± 0.9 | 0.36 |
Abbreviations. BDI, Becks depression index; TG, Triglycerides; FBS, Fasting blood sugar. See also Table 1.
Task & fMRI Acquisition
Please see online Supporting Information.
FMRI Analyses for Experiment 2
As described in experiment 1.
RESULTS FOR EXPERIMENT 1
Demographics
By design, BMI differed significantly between healthy weight and obese groups pooled across the two studies (mean BMI ± standard error of the mean (SEM): healthy weight, 21.9 ± 0.5 Kg/m2; obese, 35.3 ± 0.9 Kg/m2, n = 15 per group; P < 10−6, unpaired t‐test). There was no group difference in age (healthy weight, 27.4 ± 1.7 years; obese, 27.7 ± 1.7 years, P = 0.92). Both healthy weight and obese groups included two males (Table 1). There was a significant difference between the two groups on TFEQ‐disinhibition (P < 10−3), and DEBQ emotional eating total (P < 10−3), emotional eating clearly labeled emotions (P < 10−3), and restrained eating (P = 0.011) indicating increased disinhibition and increased emotional eating with increasing BMI (Table 1).
Internal State Ratings
Hunger ratings did not differ between healthy weight and obese groups at the time of scanning (healthy weight, 1.2 ± 1.3; obese, 0.1 ± 1.4, P = 0.55). However the obese reported feeling more full than the healthy group (healthy weight, −4.7 ± 1.1; obese, −0.4 ± 1.3, P = 0.03) (Table 1).
Global Connectivity During Milkshake Consumption
GBC was calculated at 0.5%, 1%, 2.5%, 5% and 10% wiring costs. SVD decomposition resulted in 30 components for each wiring cost, 4 of which showed significant between group differences. Components reflect patterns of GBC that vary together or in the opposite direction. We depict these patterns in the figures by assigning the same colors and sign to regions where GBC covaries in the same direction (i.e., blue to light blue is negative and red to yellow is positive in Fig. 1A). For example, the mean projection of the healthy weight group is negative (Fig. 1A bar‐plot) indicating that their GBC decreases in areas depicted in red to yellow and increases in areas depicted in blue to light blue (note that the sign is arbitrary). Overall GBC was consistently decreased in the obese participants during milkshake consumption within brain areas implicated in feeding [Babbs et al., 2013; Cornier et al., 2015; de et al., 2003; Geha et al., 2013; Kringelbach et al., 2004; Kringelbach et al., 2003; Nolan‐Poupart et al., 2013; Smeets et al., 2006; Stice et al., 2011; Sun et al., 2015] and increased in visual, premotor and medial part of the superior parietal lobule (SPL).
Figure 1.
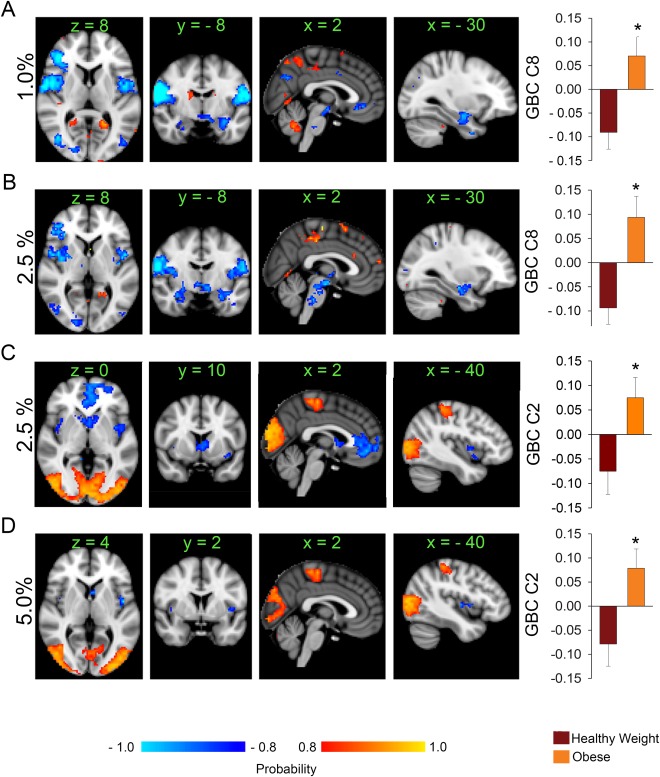
Brain network reorganization in obesity (n = 15 per group) during milkshake receipt. (A) The histogram plot shows the average (± SEM) projections of each group's GBC maps unto the brain component shown above obtained after SVD is applied to the data at 1% wiring cost. The obese group has an average positive projection onto component 8. This indicates therefore that the obese group has decreased GBC compared to the healthy weight group within the feeding circuit and the DMN (areas shown in blue to light blue). Areas in red to yellow have increased GBC in the obese compared to the healthy weight group. (B) and (C) At a higher wiring cost (2.5%) the obese group exhibits loss of GBC within the feeding circuit also, and in areas monitoring internal milieu. (D) At 5% wiring cost, the obese group still shows decreased GBC within areas that respond to milkshake like the insula and caudate. * P < 0.05, unpaired t‐test, corrected for multiple comparisons; color bar, heat map of probability values; numbers above slices, MNI coordinates in mm; orientation follows radiological convention. Percentages indicate network‐wiring cost. Abbreviations: GBC, global brain connectivity; C8, component 8; C2, component 2. [Color figure can be viewed at http://wileyonlinelibrary.com]
More specifically, at a wiring cost of 1% the groups (Fig. 1A; Supporting Information Fig. S1) showed significant differences on component 8 (mean healthy weight projection = −0.091 ± 0.035; mean obese projection = 0.07 ± 0.040, P = 0.0022). At a 2.5% wiring cost the groups showed significant differences on component 8 of this analysis (mean healthy weight projection = −0.094 ± 0.034; mean obese projection = 0.094 ± 0.040, P = 0.0022; Fig. 1B; Supporting Information Fig. S2). In both components (i.e., 8 from the 1% analysis and 8 from the 2.5% analysis) the midbrain, hypothalamus, thalamus, bilateral amygdala, hippocampi, putamen, visual cortices [Brodmann Areas (BA) 5,17, and 18], insula/parietal‐operculum, supra‐marginal gyri, primary and secondary sensory/motor cortices, and right ventral lateral prefrontal cortex decreased GBC (areas in blue to light blue) in the obese. An additional decrease in GBC in the obese group was also observed within the ventro‐medial prefrontal cortex and precuneus at 1% wiring cost. Both of these regions represent major hubs of the default mode network (DMN) [Buckner et al., 2008; Fox et al., 2005]. Increased GBC was observed in the obese group at 1% and 2.5% wiring costs in the cerebellum, a distinct region of the precuneus, the medial division of SPL, and the posterior cingulate gyrus (PCC, BA31). The middle frontal gyrus, bilateral supra‐marginal gyrus, and tails of the caudate showed increased GBC in the obese at 1% wiring cost only.
Group differences were also observed on component 2 at 2.5% wiring cost (mean healthy weight projection = −0.075 ± 0.047; mean obese projection = 0.075 ± 0.041, P = 0.024; Fig. 1C; Supporting Information Fig. S3). This component was characterized by (1) decreased GBC within the bilateral insula, bilateral caudate and the ventro‐medial prefrontal cortex, and (2) increased GBC in bilateral visual cortices, bilateral dorsal parietal areas, primary sensory cortices, and supplementary motor area (SMA, BA6) in the obese relative to the healthy weight group.
Finally, at 5% wiring cost the groups were significantly different on component 2 (mean healthy weight projection = −0.078 ± 0.047; mean obese projection = 0.078 ± 0.040, P = 0.017; Fig. 1D; Supporting Information Fig. S4). This component was very similar to component 2 seen at 2.5% wiring cost; however, only bilateral insula and caudate showed decreased GBC in the obese group.
Fullness
Given that the healthy weight subjects reported feeling significantly less full than the obese participants (Table 1), we repeated the previous analyses correcting for fullness. Differences remained significant on component 8 at 1% wiring costs (P = 0.003) and component 8 at 2.5% wiring cost (P = 0.003). However, components 2 at 2.5% and component 2 at 5% wiring cost were no longer significant (P = 0.041 at 2.5% and P = 0.048 at 5%) indicating that internal state influences GBC in these components. To further examine the possible effects of fullness on GBC we median split our participants into those with low (‐ 7.0 ± 0.5, n = 15) vs. high fullness ratings (1.8 ± 0.8, n = 15) (P < 10−6). We then compared the projections of the fullness groups on the components depicted in Figure 1 using a general linear model where group was an independent predictor and age and gender were variables of no interest. Fullness groupings did not result in any significant differences despite the fact there was a trend for the full group to have a lower BMI (P = 0.07), suggesting that the observed differences between healthy weight and obese groups are better accounted for by BMI than by internal state ratings.
Eating style
A similar procedure was followed for the eating style subscales that differed between the groups (Table 1). Including these subscales (i.e., TFEQ‐Disinhibition, DEBQ‐EET, DEBQ‐EECLE, DEBQ‐RE) into the main model rendered the effect of BMI no longer significant on any component. However, when we median split the groups according to high (> median) or low (< median) score no significant differences were observed on any of the components that were significantly influenced by BMI (Supporting Information Table S1). Collectively these analyses suggest that while fullness and eating style contributed to some of the effect of BMI, BMI was nevertheless the strongest predictor of differences in GBC.
Age
Since BMI and age are highly correlated [Reas et al., 2007], and age is associated with changes in GBC at rest [Hampson et al., 2012] we next explored the effect of age using the same procedures as above. Subjects from experiment 1 were split into a younger and an older group while matching for BMI and gender [younger: average age = 22.5 ± 0.6 years; older: 32.6 ± 0.8 years, P < 10−5; BMI (P = 0.16); two males each]. No significant effects of age were observed. Therefore, we conclude that in this relatively young cohort of subjects the effect of BMI on GBC captures more variance than that of age.
Motion
Finally, it is possible that obese and healthy weight subjects had different amounts of head motion, especially considering that this task involves swallowing. To rule out this possibility, we compared average head displacement. There was no significant difference between the groups in either study (repeated measures ANOVA, P = 0.25 for study 1 and P = 0.68 for study 2, Supporting Information Fig. S5A). In addition, we compared the percentage of frames flagged as affected by motion during movement scrubbing procedures (i.e., FD > 0.5 mm) between the groups. There was no significant difference: (%frames scrubbed in the healthy weight subjects = 1.3 ± 0.6; % frames scrubbed in obese subjects = 0.58 ± 0.37; P = 0.34, unpaired t‐test).
Seed Based Connectivity During Milkshake Consumption
The above analyses demonstrated different alterations in GBC as a function of BMI. We next tested the prediction that areas of decreased GBC within feeding circuitry might show enhanced “focal” reward circuit connectivity reflecting greater control of behavior by reward circuitry [Geha et al., 2013; Murdaugh et al., 2012; Stice et al., 2008a; Stice et al., 2008b; Stice et al., 2010; Stice et al., 2011]. ROIs were derived from the peak probability scores of the components depicted in Figure 1; including the hypothalamus, amygdala, hippocampus, insula, PCC and mPFC. Contrary to our hypothesis we observed significant increases in connectivity in the healthy weight compared to the obese group in the left amygdala/hippocampus (ROI centered around MNI coordinates in mm x = −26; y = −10; z = −20) and right hippocampus (ROI centered around MNI coordinates in mm x = 26; y = −10; z = −24). Both ROIs showed increased connectivity with putamen, amygdala, thalamus, midbrain, brainstem, right insula, and cerebellum in the healthy weight group (Fig. 2A,B). These results remained significant even after correcting separately for fullness, and eating style (P < 0.05, corrected).
Figure 2.
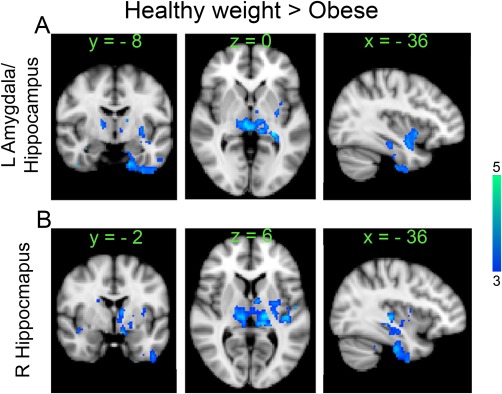
Whole brain seed based connectivity differences between healthy weight and obese groups (Healthy weight > Obese, unpaired t‐test, n =15 per group, P < 0.05 corrected) using activity in left amygdala/hippocampus (A), and right hippocampus (B) as seeds during milkshake consumption. Color bar, heat map of T‐values. [Color figure can be viewed at http://wileyonlinelibrary.com]
RESULTS FOR EXPERIMENT 2
Demographics
The healthy weight participants (n = 32) were on average significantly younger than the obese ones (n = 28) (Healthy Weight, 33.0 ± 2.8 years, 18 females; Obese, 43.6 ± 3.2 years P = 0.016, 17 females). No Group differences were observed on the Beck Depression Inventory (P = 0.83), fasting blood glucose (P = 0.86), Triglyceride (P = 0.10), TFEQ‐hunger scores (P = 0.33), TFEQ‐disinhibition (P = 0.14) or TFEQ‐restraint (P = 0.94). Demographic data for this cohort is presented in Table 2.
Global Connectivity at Rest
GBC calculations and SVD were performed as described in experiment 1. The healthy weight and obese groups showed significant differences on two components identified at wiring costs of 5% (component 10: mean projection of healthy weight = −0.055 ± 0.021; mean projection of obese = 0.063 ± 0.017; P < 10−4) and 10% (component 10: mean projection of healthy weight = −0.04 ± 0.02; mean projection of obese group = 0.046 ± 0.019). Decreased GBC in the obese group was observed in the ventromedial prefrontal cortex, bilateral ventral lateral prefrontal cortex and frontal operculum, insula, primary and secondary sensory cortices, parietal operculum, supra‐marginal gyri, and precuneus (areas in blue to light blue) (Fig. 3A.B, Supporting Information Figs. S6 and S7). At 10% wiring cost, we also observed decreased GBC in the caudate and putamen in the obese (Fig. 3B). Areas of increased GBC in the obese included the supplementary motor area (SMA BA6), bilateral superior frontal gyri (encompassing the frontal eye field), superior parietal lobules, visual cortices (BA 17), parahippocampal gyri/hippocampi (areas in red to yellow), and right putamen.
Figure 3.
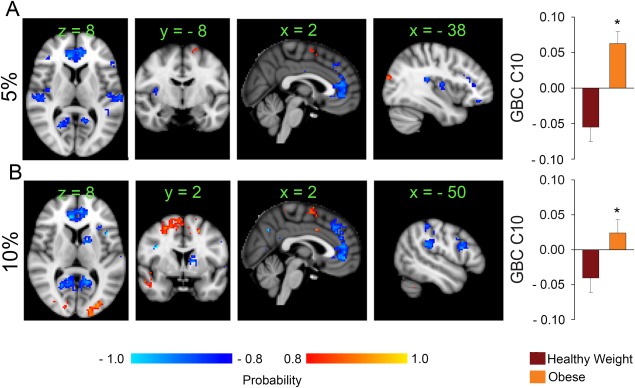
Brain networks reorganization in obesity during resting state scans. (A) At rest, the obese group shows decreased GBC within bilateral insula, lateral and ventral medial prefrontal cortex and visual areas (blue to light blue areas); increased GBC is observed within the supplementary motor area compared to the healthy weight group. (B) At a higher wiring cost, similar changes occur with additional decreases in GBC in the caudate and increases in the visual areas. [Color figure can be viewed at http://wileyonlinelibrary.com]
Since fullness ratings were unavailable and eating style did not differ between groups, follow‐up analyses were conducted to examine contributions of age and movement.
Age
Following the procedures outlined in study 1 we split our cohort at the median age, which was 34 years. The younger group had an average age of 23.7 ± 0.7 years (n = 30) and the older group of 52.3 ± 2.3 years (n = 30) (P < 10−12). The younger group had 15 females and the older group 20 females (P = 0.19), but BMI was significantly higher in the older group (younger average BMI = 26.1 ± 1.0 kg/m2, older group BMI = 29.4 ± 0.9 kg/m2, P = 0.014). We found an effect of age (corrected for BMI) at two wiring costs 1% and 5% respectively (Fig. 4A,B and Supporting Information Figs. S8–10 and Supporting Information Table S2). Age was associated with increased GBC in the bilateral striatum, hippocampi, posterior thalami, and temporal lobes (Fig. 4A). These results reproduce previously published effects of age on GBC (Hampson et al., 2012]. Furthermore, increased age was associated with decreased GBC in a widespread network of cortical areas including dorsal‐frontal parietal, sensory/motor, and DMN areas in addition to bilateral insula, thalamus, brainstem and cerebellum, young group (Fig. 4B).
Figure 4.
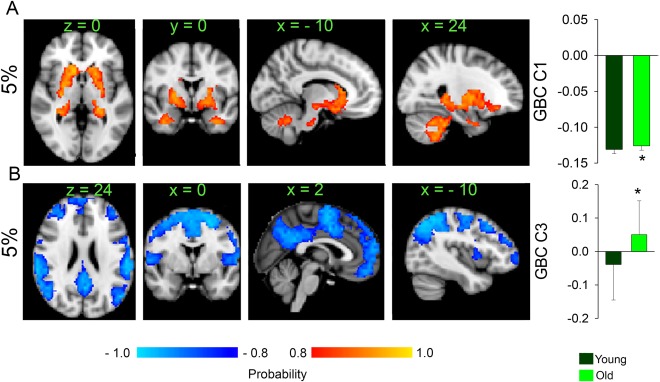
Changes in GBC with aging calculated at a network wiring cost of 5% (A) Component 1 where older show only increases in GBC compared to the younger participants in the whole striatum, hippocampi, amygdalae, brain stem and cerebellum. (B) Component 3 where older participants show loss of GBC in widespread fronto‐parietal and sensory/motor networks, including dorsal attention network, the DMN, bilateral insulae, and SMA. [Color figure can be viewed at http://wileyonlinelibrary.com]
Motion
To rule out the possibility that obese and healthy weight subjects had different amounts of head motion that could have contributed to our results we compared their average head displacement. There was no significant difference between the groups (repeated measures ANOVA, P = 0.83, Supporting Information Fig. S5B). However, there was a difference in the percentage of frames flagged as affected by motion (FD > 0.5 mm) between the groups (%frames scrubbed in healthy weight subjects = 1.4 ± 0.4; % frames scrubbed in obese subjects = 9.7 ± 2.2; P = 0.001, unpaired t‐test). These frames could not have affected the results though because they were excised and replaced by linear interpolation [Carp, 2013].
Seed Based Connectivity at Rest
Similar to the milkshake consumption data, we tested the prediction that areas with decreased GBC within the DMN might also show altered seed based connectivity. ROIs were derived from the peak probability scores of the component depicted in Figure 2; the list of seeds included the ventro‐medial prefrontal cortex, the rostral ACC (BA32) left middle frontal gyrus, right dorso‐lateral prefrontal cortex, the left and right insula, and the precuneus. The rostral ACC seed only (centered on x = 10; y = 38; z = 8 MNI coordinates in mm) showed significant differences between the healthy weight and obese groups (Fig. 5). The healthy weight group showed increased connectivity between rostral ACC and medial, dorso‐medial and right dorso‐lateral prefrontal cortex; in contrast, the obese group showed increased connectivity between rostral ACC and bilateral superior parietal lobules, bilateral premotor motor areas, SMA and mid‐ACC.
Figure 5.
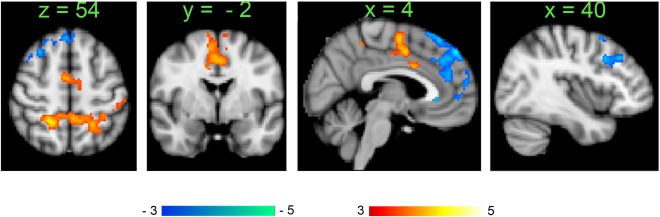
Seed based connectivity differences between healthy weight and obese groups (Healthy weight > Obese in blue to light blue; Obese > Healthy Weight in red to yellow unpaired t‐test, n = 32 healthy weight subjects, n = 28 obese subjects, P < 0.05 corrected) using activity in the rostral ACC as seed. Color bars, heat maps of T‐values. [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 3 summarizes the changes observed in GBC during both milkshake ingestion and during rest.
Table 3.
Summary of changes in GBC during milkshake ingestion and during rest
| Milkshake | Rest | |
|---|---|---|
| DMN (VMPFC & PCC) |

|

|
| Dorsal Attention Network |

|

|
| Ventral Attention Network |

|

|
| VMPFC | – |

|
| SMA |

|

|
| Insula |

|

|
| Thalamus |

|

|
| Dorsal Striatum |

|
– |
| Ventral Striatum |

|
– |
| Hippocampus |

|
– |
| Amygdala |

|
– |
| Hypothalamus |

|
– |
| Mid‐brain |

|

|
Arrows indicate direction of change of GBC in obesity.
Dorsal attention network includes: Superior parietal lobule, fronal eye fields (premotor area), visual areas. Ventral Attention network includes: right frontal eye field, ventral lateral prefrontal cortex, and right Temporo‐parietal junction (supra‐marginal gyrus).
Abbreviations: DMN, default mode network; SMA, supplementary motor area; VMPFC, ventro‐medial prefrontal cortex.
DISCUSSION
Our results demonstrate alterations of GBC in obese individuals. In the resting state and during milkshake consumption GBC is consistently decreased in the ventromedial and ventrolateral prefrontal cortex, insula and caudate nucleus, and increased in the premotor areas, superior parietal lobule, and visual cortex (Table 1, 3; Figs. 1 and 3). Condition specific effects are also present. While at rest, but not while consuming milkshake, GBC in the dorsal and ventral lateral prefrontal cortex and caudate nucleus is decreased in the obese group. In contrast, during milkshake consumption, but not at rest, GBC is decreased in feeding‐related circuitry of obese individuals including the primary somatosensory/motor cortices, bilateral insula, amygdala, anterior hippocampus, hypothalamus, midbrain and brainstem [Babbs et al., 2013; Cornier et al., 2015; de et al., 2003; Geha et al., 2013; Kringelbach et al., 2004; Kringelbach et al., 2003; Nolan‐Poupart et al., 2013; Smeets et al., 2006; Stice et al., 2011; Sun et al., 2015]. Collectively these findings suggest that obesity is associated with a state‐independent alteration in the balance of GBC between regions monitoring the oral sensory and rewarding properties of food (ventral medial prefrontal and insular cortex) and those orienting to stimuli in the external world (premotor, parietal and visual areas). Unexpectedly, GBC in the fusiform gyrus differed depending on BMI group and condition. Greater connectivity in the obese was observed during milkshake consumption and lower connectivity at rest.
We also examined seed based connectivity to determine whether the widespread loss of global influence on the limbic system is replaced by enhanced local influences from other limbic regions. Contrary to our hypothesis during milkshake consumption, seed based connectivity was also decreased in the obese group such that the amygdala and the hippocampus were less connected to the midbrain, thalamus, amygdala, putamen and insula. Similarly, the rostral ACC showed decreased connectivity to the medial, medio‐dorsal prefrontal cortex and dorso‐lateral prefrontal cortex while at rest in the obese group. However, increased seed based connectivity was observed in the obese while at rest between the rostral ACC and the dorsal attention network and salience network [Corbetta et al., 2008; Corbetta and Shulman, 2002; Menon and Uddin, 2010] including bilateral superior frontal gyrus specifically in the premotor areas, and bilateral superior parietal lobules, the SMA and the mid‐ACC. Altered seed connectivity at rest in the obese group is consistent with the changes in GBC observed during both milkshake consumption and rest where we observed loss of GBC in the medial and lateral prefrontal cortex and increased GBC in the dorsal attention network.
Implications of Altered GBC in Obesity
Although brain organization has been previously examined in relation to obesity [Babiloni et al., 2011; Garcia‐Garcia et al., 2012; Garcia‐Garcia et al., 2013; Kullmann et al., 2011; Le et al., 2007; Marques‐Iturria et al., 2015; McFadden et al., 2013; Stanek et al., 2011; Stoeckel et al., 2009; Tregellas et al., 2011; Verstynen et al., 2012; Xu et al., 2013] the current study is the first to show alterations during food consumption and to compare network organization at rest and during eating. Further, since subjects were neither hungry nor full we capture GBC at a time when the alterations in function are more likely to be related to eating in the absence of hunger. GBC is associated with cognitive function [van den Heuvel and Sporns, 2013] and cerebral metabolism [Fulcher and Fornito, 2016; Liang et al., 2013; Tomasi et al., 2013], both of which are compromised in obesity [Coppin et al., 2014; Grundy, 2016; Heni et al., 2015; Stoeckel et al., 2016]. For example, GBC is directly correlated with measures of cerebral blood flow [Liang et al., 2013], neuronal glucose uptake [Tomasi et al., 2013], and with transcription of genes regulating oxidative brain metabolism and ATP synthesis [Fulcher and Fornito, 2016]. Obesity is associated with brain insulin resistance [Heni et al., 2015], insensitivity to a variety of nutrients, hormones and peptides and abnormal brain glucose uptake [Marques et al., 2014; Orava et al., 2014; Volkow et al., 2008]. Consequently, altered GBC in obesity may reflect altered brain metabolism and insensitivity to metabolic signals. Since prefrontal hubs are critical for efficient information transfer supporting key cognitive functions [Cole et al., 2010; Tomasi and Volkow, 2010; van den Heuvel and Sporns, 2013] it is possible that altered GBC provides a link between metabolic dysfunction and the cognitive and behavioral deficits consistently observed in obesity [Coppin et al., 2014; Gunstad et al., 2007; Vainik et al., 2013]. For instance, dorso‐lateral prefrontal cortex GBC, which we demonstrate is decreased in obesity, positively predicts executive control [Cole et al., 2012], which is the most consistent cognitive deficit observed in studies of the neuropsychology of obesity [Stoeckel et al., 2016].
The current findings also agree with a host of studies showing altered brain function and structure with increased body weight. Obese individuals exhibit shrinkage of grey matter [Gunstad et al., 2008; Marques‐Iturria et al., 2013; Pannacciulli et al., 2006; Raji et al., 2010], decreased directed water flow in white matter [Verstynen et al., 2012; Xu et al., 2013], and decreased activation and cerebral blood flow in the prefrontal cortex [Le et al., 2006; Le et al., 2007; Willeumier et al., 2011]. Results also accord with findings from studies using different methods to assess connectivity in obesity. Independent component analyses revealed decreased DMN and increased striatal connectivity in obesity [Garcia‐Garcia et al., 2012; Garcia‐Garcia et al., 2013] while examination of eigenvector centrality (a measure of hub connectivity) identified decreased connectivity in the same region of dorsolateral prefrontal cortex [Garcia‐Garcia et al., 2015] where we observed decreases in GBC. This same method was also used to show a negative relationship between visceral adipose tissue and eigenvector centrality from resting state data in the primary somatosensory/motor areas and cerebellum [Raschpichler et al., 2013] in older participants. We did see decreased GBC in somatosensory/motor regions at rest and during milkshake consumption. In contrast, we observed increased cerebellar GBC during milkshake consumption only, albeit in a different region. The reason for the discrepant finding is not clear; however, it could be related to age or the use of BMI vs. adiposity, differences in participants' hunger and fullness levels between the two studies, or the use use of data collected at rest vs. during milkshake consumption.
Extending prior literature the overall pattern of change in GBC that we observe indicates that the balance in GBC is altered between regions monitoring the internal milieu (i.e., ventro‐ medial prefrontal cortex and insula) and those monitoring the external world (i.e superior frontal gyrus, lateral‐prefrontal cortex, mid‐ACC, SMA, superior parietal lobule, supra‐marginal gyri and visual cortices). The areas showing increased GBC and increased seed based connectivity in obesity are part of the dorsal attention network (superior parietal lobule, middle frontal gyrus, and visual cortices) [Corbetta et al., 2008; Corbetta and Shulman, 2002], which is involved in top‐down control of attention and orienting to relevant stimuli in the external world [Corbetta et al., 2000]. Furthermore, the SMA and mid‐ACC are part of a network responding to stimulus salience [Menon and Uddin, 2010]. Interestingly, GBC was decreased in the ventral attention network (supra‐marginal gyrus, ventral lateral prefrontal cortex/anterior insula) which is involved in reorienting attention following breaches of expectation [Corbetta et al., 2008]. Hence our results suggest that obese individuals have a disruption in monitoring the internal milieu and reduced flexibility in switching behavior despite unexpected outcomes [Davis, 2009; Vainik et al., 2013].
Implications for disease states
GBC has been repeatedly shown to be altered in disease states [Rubinov and Bullmore, 2013a; Rubinov and Bullmore, 2013b). In addition, a current and very hotly debated hypothesis postulates that major psychiatric disorders and aging are associated with abnormalities of brain networks characterized mostly by disconnection [Liang et al., 2006] and hub disruption [Rubinov and Bullmore, 2013a; Rubinov and Bullmore, 2013b). Most mental conditions in which these disruptions have been demonstrated are highly associated with obesity [Barnes and Yaffe, 2011; Egan et al., 2013; Tiihonen et al., 2009]; however, most, if not all of these studies failed to control for BMI [Alexander‐Bloch et al., 2013; Anticevic et al., 2013; Anticevic et al., 2014; Baliki et al., 2014; Bassett et al., 2008; Bassett et al., 2012; Fornito et al., 2011; Seeley et al., 2009; van den Heuvel et al., 2010; van den Heuvel et al., 2013; Wang et al., 2012; Zhang et al., 2012; Zhou et al., 2012]. Schizophrenia in particular has been recently characterized by brain hub disruption and “disconnection” [Fornito et al., 2012] in regions overlapping with the ones we show to be less connected in obese subjects such as lateral and medial prefrontal cortex and striatum [Anticevic et al., 2013; Bassett et al., 2012; Rubinov and Bullmore, 2013b). However, the prevalence of obesity and metabolic derangements in schizophrenic patients is much higher than the general population [Brown et al., 2010; De Hert et al., 2006; De et al., 2012]. Our data suggests that differences in BMI between healthy control groups and schizophrenic patients could contribute to these findings. It is therefore imperative that BMI be accounted for when examining GBC in disease states.
Decreased GBC and Seed Connectivity in Brain Circuits Regulating Ingestive Behavior
During milkshake consumption, but not at rest GBC was reduced in the midbrain, hypothalamus, amygdala and hippocampus. However, during both rest and milkshake consumption GBC was decreased in the ventral medial and ventral lateral prefrontal cortex, insula and caudate. All of these regions play critical roles in regulating ingestive behavior [Abizaid et al., 2006; Dagher, 2012; Saper et al., 2002] and are frequently activated in neuroimaging studies examining brain response to food consumption [Babbs et al., 2013; Cornier et al., 2015; de et al., 2003; Geha et al., 2013; Kringelbach et al., 2004; Kringelbach et al., 2003; Nolan‐Poupart et al., 2013; Smeets et al., 2006; Stice et al., 2011; Sun et al., 2015]. Contrary to our hypothesis this decreased global connectivity was also accompanied by decreased seed based connectivity between ingestive behavior nodes. Specifically, the hippocampus and amygdala showed reduced connectivity with midbrain, thalamus, amygdala, striatum and insula during milkshake consumption.
The amygdala and hippocampus play important roles in regulating food intake [Davidson et al., 2009; Petrovich, 2011] and animal models have implicated both regions in overeating. For example, the amygdala has been associated with hunger‐enhanced memory for food stimuli [Morris and Dolan, 2001], is sensitive to the orexigenic hormone ghrelin [Alvarez‐Crespo et al., 2012], is hyper‐responsive to food cues in Prader‐Willis syndrome, which is characterized by extreme hyperphagia [Holsen et al., 2012], and the enhanced activation is associated with weight gain [Sun et al., 2015].
The hippocampus projects to the hypothalamus [Cenquizca and Swanson, 2006; Cenquizca and Swanson, 2007], nucleus accumbens and ventral medial prefrontal cortex [Haber and Knutson, 2010] and expresses receptors for many metabolic hormones including leptin, ghrelin, and insulin [Davidson et al., 2009; Ferrini et al., 2009; Lathe, 2001]. Davidson and colleagues have argued that hippocampal representations of internal state serve as contextual cues during food selection so that responding for food is commensurate with homeostatic need [Davidson et al., 2007]. As such, lesions of the hippocampus lead to weight gain in animals [Davidson et al., 2009] and insensitivity to internal state in humans [Hebben et al., 1985]. The famous patient H.M. who had bilateral hippocampal resection for the treatment of epilepsy and consequent anterograde amnesia, was also insensitive to internal state. For example, 10 minutes following a meal H. M. would happily consume a second lunch as he had neither awareness of fullness nor memory of eating. There is also evidence that hippocampal modulation by satiety remains abnormal in the post‐obese, suggesting a causal role for this region in obesity [DelParigi et al., 2004]. Recently work in animals has also shown that exposure to a “western” diet can damage hippocampal circuits and impair hippocampal‐dependent functions [Kanoski and Davidson, 2011], thus leading to a vicious cycle of both obesity and cognitive decline [Kanoski, 2012]. Our finding of reduced global and focal hippocampal connectivity is consistent with this argument and compliments prior work showing reduced hippocampal volume in obese individuals [Mueller et al., 2012; Raji et al., 2010].
Age and BMI
A unique aspect of our study is that we evaluated GBC as a function of age and BMI. Age influences a variety of measures of brain structure and function including GBC [Hampson et al., 2012]. More specifically, GBC decreases in cortical regions and increases in subcortical regions [Hampson et al., 2012]. Here we reproduce this finding in the resting state data (Fig. 4) and show that the association between GBC and BMI remains after correcting for age suggesting the existence of different mechanisms leading to a similar phenotype (i.e., loss of GBC). We did not observe an effect of age on GBC during milkshake consumption but this is likely because our sample was relatively young. Most importantly our data indicate that BMI and age produce independent effects on GBC.
SUMMARY
In conclusion, we demonstrate that obesity is associated with decreased GBC within the lateral and medial prefrontal cortex, insula and limbic system, and increased GBC within visual, parietal and premotor areas. This finding adds to the emerging literature supporting an association between obesity and brain dysfunction, and provides a potential link between cognitive dysfunction and brain changes observed in obesity. Our approach points to a new biomarker of brain changes with obesity and emphasize the importance of accounting for BMI when studying brain networks.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
The authors received partial funding from a donation made to David Kessler, who then awarded the fund John B. Pierce Laboratory to study the effects of BMI on brain response to food. Dr. Kessler played no role in the design, conduct, analysis or interpretation of the study. However, he did develop a questionnaire, which we administered to our subjects.
REFERENCES
- Abizaid A, Gao Q, Horvath TL (2006): Thoughts for food: Brain mechanisms and peripheral energy balance. Neuron 51:691–702. [DOI] [PubMed] [Google Scholar]
- Alexander‐Bloch AF, Vertes PE, Stidd R, Lalonde F, Clasen L, Rapoport J, Giedd J, Bullmore ET, Gogtay N (2013): The anatomical distance of functional connections predicts brain network topology in health and schizophrenia. Cerebral Cortex 23:127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez‐Crespo M, Skibicka KP, Farkas I, Molnar CS, Egecioglu E, Hrabovszky E, Liposits Z, Dickson SL (2012): The amygdala as a neurobiological target for ghrelin in rats: Neuroanatomical, electrophysiological and behavioral evidence. PloS One 7:e46321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen AH, Gash DM, Avison MJ (1999): Principal component analysis of the dynamic response measured by fMRI: A generalized linear systems framework. Magn Reson Imaging 17:795–815. [DOI] [PubMed] [Google Scholar]
- Anticevic A, Brumbaugh MS, Winkler AM, Lombardo LE, Barrett J, Corlett PR, Kober H, Gruber J, Repovs G, Cole MW, Krystal JH, Pearlson GD, Glahn DC (2013): Global prefrontal and fronto‐amygdala dysconnectivity in bipolar I disorder with psychosis history. Biol Psychiatry 73:565–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Hu S, Zhang S, Savic A, Billingslea E, Wasylink S, Repovs G, Cole MW, Bednarski S, Krystal JH, Bloch MH, Li CS, Pittenger C (2014): Global resting‐state functional magnetic resonance imaging analysis identifies frontal cortex, striatal, and cerebellar dysconnectivity in obsessive‐compulsive disorder. Biol Psychiatry 75:595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babbs RK, Sun X, Felsted J, Chouinard‐Decorte F, Veldhuizen MG, Small DM (2013): Decreased caudate response to milkshake is associated with higher body mass index and greater impulsivity. Physiol Behav 121:103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiloni C, Marzano N, Lizio R, Valenzano A, Triggiani AI, Petito A, Bellomo A, Lecce B, Mundi C, Soricelli A, Limatola C, Cibelli G, Del Percio C (2011): Resting state cortical electroencephalographic rhythms in subjects with normal and abnormal body weight. Neuroimage 58:698–707. [DOI] [PubMed] [Google Scholar]
- Baliki MN, Mansour AR, Baria AT, Apkarian AV (2014): Functional reorganization of the default mode network across chronic pain conditions. PloS One 9:e106133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes DE, Yaffe K (2011): The projected effect of risk factor reduction on Alzheimer's disease prevalence. Lancet Neurol 10:819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett DS, Bullmore E, Verchinski BA, Mattay VS, Weinberger DR, Meyer‐Lindenberg A (2008): Hierarchical organization of human cortical networks in health and schizophrenia. J Neurosci 28:9239–9248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett DS, Nelson BG, Mueller BA, Camchong J, Lim KO (2012): Altered resting state complexity in schizophrenia. Neuroimage 59:2196–2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S, Kim M, Mitchell C, Inskip H (2010): Twenty‐five year mortality of a community cohort with schizophrenia. Br J Psychiatry 196:116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews‐Hanna JR, Schacter DL (2008): The brain's default network: Anatomy, function, and relevance to disease. Ann N Y Acad Sci 1124:1–38. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, Andrews‐Hanna JR, Sperling RA, Johnson KA (2009): Cortical hubs revealed by intrinsic functional connectivity: Mapping, assessment of stability, and relation to Alzheimer's disease. J Neurosci 29:1860–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore ET, Bassett DS (2011): Brain graphs: Graphical models of the human brain connectome. Ann Rev Clin Psychol 7:113–140. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pearlson GD, Pekar JJ (2001): A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp 14:140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao M, Wang JH, Dai ZJ, Cao XY, Jiang LL, Fan FM, Song XW, Xia MR, Shu N, Dong Q, Milham MP, Castellanos FX, Zuo XN, He Y (2014): Topological organization of the human brain functional connectome across the lifespan. Dev Cogn Neurosci 7:76–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp J (2013): Optimizing the order of operations for movement scrubbing: Comment on Power et al. Neuroimage 76:436–438. [DOI] [PubMed] [Google Scholar]
- Carter S, Caron A, Richard D, Picard F (2013): Role of leptin resistance in the development of obesity in older patients. Clin Interv Aging 8:829–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenquizca LA, Swanson LW (2006): Analysis of direct hippocampal cortical field CA1 axonal projections to diencephalon in the rat. J Comp Neurol 497:101–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenquizca LA, Swanson LW (2007): Spatial organization of direct hippocampal field CA1 axonal projections to the rest of the cerebral cortex. Brain Res Rev 56:1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Pathak S, Schneider W (2010): Identifying the brain's most globally connected regions. Neuroimage 49:3132–3148. [DOI] [PubMed] [Google Scholar]
- Cole MW, Yarkoni T, Repovs G, Anticevic A, Braver TS (2012): Global connectivity of prefrontal cortex predicts cognitive control and intelligence. J Neurosci 32:8988–8999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppin G, Nolan‐Poupart S, Jones‐Gotman M, Small DM (2014): Working memory and reward association learning impairments in obesity. Neuropsychologia 65:146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL (2002): Control of goal‐directed and stimulus‐driven attention in the brain. Nat Rev Neurosci 3:201–215. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Ollinger JM, McAvoy MP, Shulman GL (2000): Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nat Neurosci 3:292–297. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL (2008): The reorienting system of the human brain: From environment to theory of mind. Neuron 58:306–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornier MA, Shott ME, Thomas EA, Bechtell JL, Bessesen DH, Tregellas JR, Frank GK (2015): The effects of energy balance, obesity‐proneness and sex on the neuronal response to sweet taste. Behav Brain Res 278:446–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossley NA, Mechelli A, Vertes PE, Winton‐Brown TT, Patel AX, Ginestet CE, McGuire P, Bullmore ET (2013): Cognitive relevance of the community structure of the human brain functional coactivation network. Proc Natl Acad Sci U S A 110:11583–11588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagher A (2012): Functional brain imaging of appetite. Trend Endocrinol Metabo 23:250–260. [DOI] [PubMed] [Google Scholar]
- Davidson TL, Kanoski SE, Schier LA, Clegg DJ, Benoit SC (2007): A potential role for the hippocampus in energy intake and body weight regulation. Curr Opin Pharmacol 7:613–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson TL, Chan K, Jarrard LE, Kanoski SE, Clegg DJ, Benoit SC (2009): Contributions of the hippocampus and medial prefrontal cortex to energy and body weight regulation. Hippocampus 19:235–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C (2009): Psychobiological traits in the risk profile for overeating and weight gain. Int J Obesity 33 Suppl 2:S49–S53. [DOI] [PubMed] [Google Scholar]
- de A, I , Kringelbach ML, Rolls ET, Hobden P (2003): Representation of umami taste in the human brain. J.Neurophysiol 90:313–319. [DOI] [PubMed] [Google Scholar]
- De Hert MA, van WR, Van ED, Hanssens L, Wampers M, Scheen A, Peuskens J (2006): Prevalence of the metabolic syndrome in patients with schizophrenia treated with antipsychotic medication. Schizophr Res 83:87–93. [DOI] [PubMed] [Google Scholar]
- De HM, Detraux J, van WR, Yu W, Correll CU (2012): Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol 8:114–126. [DOI] [PubMed] [Google Scholar]
- DelParigi A, Chen K, Salbe AD, Hill JO, Wing RR, Reiman EM, Tataranni PA (2004): Persistence of abnormal neural responses to a meal in postobese individuals. Int J Obes Relat Metab Disord 28:370–377. [DOI] [PubMed] [Google Scholar]
- Egan AM, Dreyer ML, Odar CC, Beckwith M, Garrison CB (2013): Obesity in young children with autism spectrum disorders: Prevalence and associated factors. Childhood Obesity 9:125–131. [DOI] [PubMed] [Google Scholar]
- Eguiluz VM, Chialvo DR, Cecchi GA, Baliki M, Apkarian AV (2005): Scale‐free brain functional networks. Phys Rev Lett 94:018102. [DOI] [PubMed] [Google Scholar]
- Erion JR, Wosiski‐Kuhn M, Dey A, Hao S, Davis CL, Pollock NK, Stranahan AM (2014): Obesity elicits interleukin 1‐mediated deficits in hippocampal synaptic plasticity. J Neurosci 34:2618–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrini F, Salio C, Lossi L, Merighi A (2009): Ghrelin in central neurons. Curr Neuropharmacol 7:37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito A, Yoon J, Zalesky A, Bullmore ET, Carter CS (2011): General and specific functional connectivity disturbances in first‐episode schizophrenia during cognitive control performance. Biol Psychiatry 70:64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito A, Zalesky A, Pantelis C, Bullmore ET (2012): Schizophrenia, neuroimaging and connectomics. Neuroimage 62:2296–2314. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME (2005): The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A 102:9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Zhang D, Snyder AZ, Raichle ME (2009): The global signal and observed anticorrelated resting state brain networks. J Neurophysiol 101:3270–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis H, Stevenson R (2013): The longer‐term impacts of Western diet on human cognition and the brain. Appetite 63:119–128. [DOI] [PubMed] [Google Scholar]
- Fulcher BD, Fornito A (2016): A transcriptional signature of hub connectivity in the mouse connectome. Proc Natl Acad Sci U S A 113:1435–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia‐Garcia I, Jurado MA, Garolera M, Segura B, Marques‐Iturria I, Pueyo R, Vernet‐Vernet M, Sender‐Palacios MJ, Sala‐Llonch R, Ariza M, Narberhaus A, Junque C (2012): Functional connectivity in obesity during reward processing. Neuroimage 66C:232–239. [DOI] [PubMed] [Google Scholar]
- Garcia‐Garcia I, Jurado MA, Garolera M, Segura B, Sala‐Llonch R, Marques‐Iturria I, Pueyo R, Sender‐Palacios MJ, Vernet‐Vernet M, Narberhaus A, Ariza M, Junque C (2013): Alterations of the salience network in obesity: A resting‐state fMRI study. Hum Brain Mapp 34:2786–2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia‐Garcia I, Jurado MA, Garolera M, Marques‐Iturria I, Horstmann A, Segura B, Pueyo R, Sender‐Palacios MJ, Vernet‐Vernet M, Villringer A, Junque C, Margulies DS, Neumann J (2015): Functional network centrality in obesity: A resting‐state and task fMRI study. Psychiatry Res 233:331–338. [DOI] [PubMed] [Google Scholar]
- Geha PY, Aschenbrenner K, Felsted J, O'malley SS, Small DM (2013): Altered hypothalamic response to food in smokers. Am J Clin Nutr 97:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales MM, Tarumi T, Miles SC, Tanaka H, Shah F, Haley AP (2010): Insulin sensitivity as a mediator of the relationship between BMI and working memory‐related brain activation. Obesity 18:2131–2137. [DOI] [PubMed] [Google Scholar]
- Grundy SM (2016): Metabolic syndrome update. Trends Cardiovasc Med 26:364–373. [DOI] [PubMed] [Google Scholar]
- Gunstad J, Paul RH, Cohen RA, Tate DF, Spitznagel MB, Gordon E (2007): Elevated body mass index is associated with executive dysfunction in otherwise healthy adults. Comprehensive Psychiatry 48:57–61. [DOI] [PubMed] [Google Scholar]
- Gunstad J, Paul RH, Cohen RA, Tate DF, Spitznagel MB, Grieve S, Gordon E (2008): Relationship between body mass index and brain volume in healthy adults. Int J Neurosci 118:1582–1593. [DOI] [PubMed] [Google Scholar]
- Gupta A, Mayer EA, Sanmiguel CP, Van Horn JD, Woodworth D, Ellingson BM, Fling C, Love A, Tillisch K, Labus JS (2015): Patterns of brain structural connectivity differentiate normal weight from overweight subjects. NeuroImage Clin 7:506–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B (2010): The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology 35:4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Tokoglu F, Shen X, Scheinost D, Papademetris X, Constable RT (2012): Intrinsic brain connectivity related to age in young and middle aged adults. PloS One 7:e44067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harriger L, van den Heuvel MP, Sporns O (2012): Rich club organization of macaque cerebral cortex and its role in network communication. PloS One 7:e46497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebben N, Corkin S, Eichenbaum H, Shedlack K (1985): Diminished ability to interpret and report internal states after bilateral medial temporal resection: Case H.M. Behav Neurosci 99:1031–1039. [DOI] [PubMed] [Google Scholar]
- Heni M, Kullmann S, Preissl H, Fritsche A, Haring HU (2015): Impaired insulin action in the human brain: Causes and metabolic consequences. Nat Rev Endocrinol 11:701–711. [DOI] [PubMed] [Google Scholar]
- Holsen LM, Savage CR, Martin LE, Bruce AS, Lepping RJ, Ko E, Brooks WM, Butler MG, Zarcone JR, Goldstein JM (2012): Importance of reward and prefrontal circuitry in hunger and satiety: Prader‐Willi syndrome vs simple obesity. Int J Obesity 36:638–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn RA, Johnson CR (1985): Matrix Analysis. Cambridge Cambridgeshire; New York: Cambridge University Press; xiii, 561 p. [Google Scholar]
- Jenkins GM, Watts DG (1968): Spectral Analysis and Its Applications. San Francisco: Holden‐Day; xviii, 525 p. p. [Google Scholar]
- Kanoski SE (2012): Cognitive and neuronal systems underlying obesity. Physiol Behav 106:337–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanoski SE, Davidson TL (2011): Western diet consumption and cognitive impairment: Links to hippocampal dysfunction and obesity. Physiol Behav 103:59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson HK, Tuulari JJ, Hirvonen J, Lepomaki V, Parkkola R, Hiltunen J, Hannukainen JC, Soinio M, Pham T, Salminen P, Nuutila P, Nummenmaa L (2013): Obesity is associated with white matter atrophy: A combined diffusion tensor imaging and voxel‐based morphometric study. Obesity 21:2530–2537. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP (2008): Competition between functional brain networks mediates behavioral variability. Neuroimage 39:527–537. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, O'Doherty J, Rolls ET, Andrews C (2003): Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness. Cereb.Cortex 13:1064–1071. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, de A, I , Rolls ET (2004): Taste‐related activity in the human dorsolateral prefrontal cortex. Neuroimage 21:781–788. [DOI] [PubMed] [Google Scholar]
- Kroemer NB, Krebs L, Kobiella A, Grimm O, Vollstadt‐Klein S, Wolfensteller U, Kling R, Bidlingmaier M, Zimmermann US, Smolka MN (2013): (Still) longing for food: Insulin reactivity modulates response to food pictures. Hum Brain Mapp 34:2367–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann S, Heni M, Veit R, Ketterer C, Schick F, Haring HU, Fritsche A, Preissl H (2011): The obese brain: Association of body mass index and insulin sensitivity with resting state network functional connectivity. Hum.Brain Mapp 33:1052–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann S, Pape AA, Heni M, Ketterer C, Schick F, Haring HU, Fritsche A, Preissl H, Veit R (2013): Functional network connectivity underlying food processing: Disturbed salience and visual processing in overweight and obese adults. Cereb Cortex 23:1247–1256. [DOI] [PubMed] [Google Scholar]
- Lathe R (2001): Hormones and the hippocampus. J Endocrinol 169:205–231. [DOI] [PubMed] [Google Scholar]
- Le DS, Pannacciulli N, Chen K, Del Parigi A, Salbe AD, Reiman EM, Krakoff J (2006): Less activation of the left dorsolateral prefrontal cortex in response to a meal: A feature of obesity. Am J Clin Nutr 84:725–731. [DOI] [PubMed] [Google Scholar]
- Le DS, Pannacciulli N, Chen K, Salbe AD, Del Parigi A, Hill JO, Wing RR, Reiman EM, Krakoff J (2007): Less activation in the left dorsolateral prefrontal cortex in the reanalysis of the response to a meal in obese than in lean women and its association with successful weight loss. Am J Clin Nutr 86:573–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- le Roux CW, Batterham RL, Aylwin SJ, Patterson M, Borg CM, Wynne KJ, Kent A, Vincent RP, Gardiner J, Ghatei MA, Bloom SR (2006): Attenuated peptide YY release in obese subjects is associated with reduced satiety. Endocrinology 147:3–8. [DOI] [PubMed] [Google Scholar]
- Liang M, Zhou Y, Jiang T, Liu Z, Tian L, Liu H, Hao Y (2006): Widespread functional disconnectivity in schizophrenia with resting‐state functional magnetic resonance imaging. Neuroreport 17:209–213. [DOI] [PubMed] [Google Scholar]
- Liang X, Zou Q, He Y, Yang Y (2013): Coupling of functional connectivity and regional cerebral blood flow reveals a physiological basis for network hubs of the human brain. Proc Natl Acad Sci U S A 110:1929–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques EL, Halpern A, Correa Mancini M, de Melo ME, Horie NC, Buchpiguel CA, Martins Novaes Coutinho A, Ono CR, Prando S, Santo MA, Cunha‐Neto E, Fuentes D, Cercato C (2014): Changes in neuropsychological tests and brain metabolism after bariatric surgery. J Clin Endocrinol Metab 99:E2347–E2352. [DOI] [PubMed] [Google Scholar]
- Marques‐Iturria I, Pueyo R, Garolera M, Segura B, Junque C, Garcia‐Garcia I, Jose Sender‐Palacios M, Vernet‐Vernet M, Narberhaus A, Ariza M, Jurado MA (2013): Frontal cortical thinning and subcortical volume reductions in early adulthood obesity. Psychiatry Res 214:109–115. [DOI] [PubMed] [Google Scholar]
- Marques‐Iturria I, Scholtens LH, Garolera M, Pueyo R, Garcia‐Garcia I, Gonzalez‐Tartiere P, Segura B, Junque C, Sender‐Palacios MJ, Vernet‐Vernet M, Sanchez‐Garre C, de Reus MA, Jurado MA, van den Heuvel MP (2015): Affected connectivity organization of the reward system structure in obesity. Neuroimage 111:100–106. [DOI] [PubMed] [Google Scholar]
- McFadden KL, Cornier MA, Melanson EL, Bechtell JL, Tregellas JR (2013): Effects of exercise on resting‐state default mode and salience network activity in overweight/obese adults. Neuroreport 24:866–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Uddin LQ (2010): Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct 214:655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JS, Dolan RJ (2001): Involvement of human amygdala and orbitofrontal cortex in hunger‐enhanced memory for food stimuli. J Neurosci 21:5304–5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller K, Sacher J, Arelin K, Holiga S, Kratzsch J, Villringer A, Schroeter ML (2012): Overweight and obesity are associated with neuronal injury in the human cerebellum and hippocampus in young adults: A combined MRI, serum marker and gene expression study. Transl Psychiatry 2:e200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdaugh DL, Cox JE, Cook EW, III , Weller RE (2012): fMRI reactivity to high‐calorie food pictures predicts short‐ and long‐term outcome in a weight‐loss program. Neuroimage 59:2709–2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP (2002): Nonparametric permutation tests for functional neuroimaging: A primer with examples. Hum Brain Mapp 15:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan‐Poupart S, Veldhuizen MG, Geha P, Small DM (2013): Midbrain response to milkshake correlates with ad libitum milkshake intake in the absence of hunger. Appetite 60:168–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nooner KB, Colcombe SJ, Tobe RH, Mennes M, Benedict MM, Moreno AL, Panek LJ, Brown S, Zavitz ST, Li Q, Sikka S, Gutman D, Bangaru S, Schlachter RT, Kamiel SM, Anwar AR, Hinz CM, Kaplan MS, Rachlin AB, Adelsberg S, Cheung B, Khanuja R, Yan C, Craddock CC, Calhoun V, Courtney W, King M, Wood D, Cox CL, Kelly AM, Di Martino A, Petkova E, Reiss PT, Duan N, Thomsen D, Biswal B, Coffey B, Hoptman MJ, Javitt DC, Pomara N, Sidtis JJ, Koplewicz HS, Castellanos FX, Leventhal BL, Milham MP (2012): The NKI‐Rockland sample: A model for accelerating the pace of discovery science in psychiatry. Front Neurosci 6:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olde Dubbelink KT, Felius A, Verbunt JP, van Dijk BW, Berendse HW, Stam CJ, Delemarre‐van de Waal, HA (2008): Increased resting‐state functional connectivity in obese adolescents; a magnetoencephalographic pilot study. PLoS.One 3:e2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orava J, Nummenmaa L, Noponen T, Viljanen T, Parkkola R, Nuutila P, Virtanen KA (2014): Brown adipose tissue function is accompanied by cerebral activation in lean but not in obese humans. J Cereb Blood Flow Metab 34:1018–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page KA, Seo D, Belfort‐DeAguiar R, Lacadie C, Dzuira J, Naik S, Amarnath S, Constable RT, Sherwin RS, Sinha R (2011): Circulating glucose levels modulate neural control of desire for high‐calorie foods in humans. J Clin Invest 121:4161–4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannacciulli N, Del Parigi A, Chen K, Le DS, Reiman EM, Tataranni PA (2006): Brain abnormalities in human obesity: A voxel‐based morphometric study. Neuroimage 31:1419–1425. [DOI] [PubMed] [Google Scholar]
- Petrovich GD (2011): Learning and the motivation to eat: Forebrain circuitry. Physiol Behav 104:582–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE (2012): Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59:2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Schlaggar BL, Lessov‐Schlaggar CN, Petersen SE (2013): Evidence for hubs in human functional brain networks. Neuron 79:798–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE (2014): Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage 84:320–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raji CA, Ho AJ, Parikshak NN, Becker JT, Lopez OL, Kuller LH, Hua X, Leow AD, Toga AW, Thompson PM (2010): Brain structure and obesity. Hum Brain Mapp 31:353–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschpichler M, Straatman K, Schroeter ML, Arelin K, Schlogl H, Fritzsch D, Mende M, Pampel A, Bottcher Y, Stumvoll M, Villringer A, Mueller K (2013): Abdominal fat distribution and its relationship to brain changes: The differential effects of age on cerebellar structure and function: A cross‐sectional, exploratory study. BMJ Open 3: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reas DL, Nygard JF, Svensson E, Sorensen T, Sandanger I (2007): Changes in body mass index by age, gender, and socio‐economic status among a cohort of Norwegian men and women (1990‐2001). BMC Public Health 7:269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedl V, Bienkowska K, Strobel C, Tahmasian M, Grimmer T, Forster S, Friston KJ, Sorg C, Drzezga A (2014): Local activity determines functional connectivity in the resting human brain: A simultaneous FDG‐PET/fMRI study. J Neurosci 34:6260–6266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M, Sporns O (2010): Complex network measures of brain connectivity: Uses and interpretations. Neuroimage 52:1059–1069. [DOI] [PubMed] [Google Scholar]
- Rubinov M, Bullmore E (2013a): Fledgling pathoconnectomics of psychiatric disorders. Trend Cogn Sci 17:641–647. [DOI] [PubMed] [Google Scholar]
- Rubinov M, Bullmore E (2013b): Schizophrenia and abnormal brain network hubs. Dialogues Clin Neurosci 15:339–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB, Chou TC, Elmquist JK (2002): The need to feed: Homeostatic and hedonic control of eating. Neuron 36:199–211. [DOI] [PubMed] [Google Scholar]
- Scheinost D, Benjamin J, Lacadie CM, Vohr B, Schneider KC, Ment LR, Papademetris X, Constable RT (2012): The intrinsic connectivity distribution: A novel contrast measure reflecting voxel level functional connectivity. Neuroimage 62:1510–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD (2009): Neurodegenerative diseases target large‐scale human brain networks. Neuron 62:42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiri R, Karppinen J, Leino‐Arjas P, Solovieva S, Viikari‐Juntura E (2010): The association between obesity and low back pain: A meta‐analysis. Am J Epidemiol 171:135–154. [DOI] [PubMed] [Google Scholar]
- Shiri R, Solovieva S, Husgafvel‐Pursiainen K, Taimela S, Saarikoski LA, Huupponen R, Viikari J, Raitakari OT, Viikari‐Juntura E (2008): The association between obesity and the prevalence of low back pain in young adults: The Cardiovascular Risk in Young Finns Study. Am J Epidemiol 167:1110–1119. [DOI] [PubMed] [Google Scholar]
- Smeets PA, de GC, Stafleu A, van Osch MJ, Nievelstein RA, van der GJ (2006): Effect of satiety on brain activation during chocolate tasting in men and women. Am J Clin Nutr 83:1297–1305. [DOI] [PubMed] [Google Scholar]
- Smith SM, Nichols TE (2009): Threshold‐free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 44:83–98. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen‐Berg H, Bannister P, De Luca CJ, Drobnjak I, Flitney DE, Nianzy R, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Mathews PM (2004): Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23(S1):208–219. [DOI] [PubMed] [Google Scholar]
- Stanek KM, Grieve SM, Brickman AM, Korgaonkar MS, Paul RH, Cohen RA, Gunstad JJ (2011): Obesity is associated with reduced white matter integrity in otherwise healthy adults. Obesity 19:500–504. [DOI] [PubMed] [Google Scholar]
- Stice E, Spoor S, Bohon C, Small DM (2008a): Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science 322:449–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Spoor S, Bohon C, Veldhuizen MG, Small DM (2008b): Relation of reward from food intake and anticipated food intake to obesity: A functional magnetic resonance imaging study. J Abnorm Psychol 117:924–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Yokum S, Blum K, Bohon C (2010): Weight gain is associated with reduced striatal response to palatable food. J Neurosci 30:13105–13109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Yokum S, Burger KS, Epstein LH, Small DM (2011): Youth at risk for obesity show greater activation of striatal and somatosensory regions to food. J Neurosci 31:4360–4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckel LE, Kim J, Weller RE, Cox JE, Cook EW, 3rd , Horwitz B (2009): Effective connectivity of a reward network in obese women. Brain Res Bull 79:388–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckel LE, Arvanitakis Z, Gandy S, Small D, Kahn CR, Pascual‐Leone A, Pawlyk A, Sherwin R, Smith P (2016): Complex mechanisms linking neurocognitive dysfunction to insulin resistance and other metabolic dysfunction. F1000Res 5:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stunkard AJ, Messick S (1985): The three‐factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res 29:71–83. [DOI] [PubMed] [Google Scholar]
- Sun X, Kroemer NB, Veldhuizen MG, Babbs AE, de Araujo IE, Gitelman DR, Sherwin RS, Sinha R, Small DM (2015): Basolateral amygdala response to food cues in the absence of hunger is associated with weight gain susceptibility. J Neurosci 35:7964–7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiihonen J, Lonnqvist J, Wahlbeck K, Klaukka T, Niskanen L, Tanskanen A, Haukka J (2009): 11‐year follow‐up of mortality in patients with schizophrenia: A population‐based cohort study (FIN11 study). Lancet 374:620–627. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND (2010): Functional connectivity density mapping. Proc Natl Acad Sci U S A 107:9885–9890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Wang GJ, Volkow ND (2013): Energetic cost of brain functional connectivity. Proc Natl Acad Sci U S A 110:13642–13647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tregellas JR, Wylie KP, Rojas DC, Tanabe J, Martin J, Kronberg E, Cordes D, Cornier MA (2011): Altered default network activity in obesity. Obesity 19:2316–2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainik U, Dagher A, Dube L, Fellows LK (2013): Neurobehavioural correlates of body mass index and eating behaviours in adults: a systematic review. Neurosci Biobehav Rev 37:279–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Sporns O (2013): Network hubs in the human brain. Trend Cogn Sci 17:683–696. [DOI] [PubMed] [Google Scholar]
- van den Heuvel MP, Mandl RC, Stam CJ, Kahn RS, Hulshoff Pol HE (2010): Aberrant frontal and temporal complex network structure in schizophrenia: A graph theoretical analysis. J Neurosci 30:15915–15926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Sporns O, Collin G, Scheewe T, Mandl RC, Cahn W, Goni J, Hulshoff Pol HE, Kahn RS (2013): Abnormal rich club organization and functional brain dynamics in schizophrenia. JAMA Psychiatry 70:783–792. [DOI] [PubMed] [Google Scholar]
- Van ST, Frijters JER, Bergers GPA, Defares PB (1986): The Dutch Eating Behavior Questionnaire (Debq) for assessment of restrained, emotional, and external eating behavior. Int J Eat Disord 5:295–315. [Google Scholar]
- Veldhuizen MG, Nachtigal DJ, Flammer LJ, de Araujo IE, Small DM (2013): Verbal descriptors influence hypothalamic response to low‐calorie drinks. Mol Metab 2:270–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstynen TD, Weinstein AM, Schneider WW, Jakicic JM, Rofey DL, Erickson KI (2012): Increased body mass index is associated with a global and distributed decrease in white matter microstructural integrity. Psychosom Med 74:682–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Thanos PK, Logan J, Alexoff D, Ding YS, Wong C, Ma Y, Pradhan K (2008): Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: Possible contributing factors. Neuroimage 42:1537–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Su TP, Zhou Y, Chou KH, Chen IY, Jiang T, Lin CP (2012): Anatomical insights into disrupted small‐world networks in schizophrenia. Neuroimage 59:1085–1093. [DOI] [PubMed] [Google Scholar]
- Wijngaarden MA, Veer IM, Rombouts SA, van Buchem MA, Willems van Dijk K, Pijl H, van der Grond J (2015): Obesity is marked by distinct functional connectivity in brain networks involved in food reward and salience. Behav Brain Res 287:127–134. [DOI] [PubMed] [Google Scholar]
- Willeumier KC, Taylor DV, Amen DG (2011): Elevated BMI is associated with decreased blood flow in the prefrontal cortex using SPECT imaging in healthy adults. Obesity 19:1095–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE (2014): Permutation inference for the general linear model. Neuroimage 92:381–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TE, Beckmann CF, Smith SM (2005): Mixture models with adaptive spatial regularization for segmentation with an application to FMRI data. IEEE Trans Med Imaging 24:1–11. [DOI] [PubMed] [Google Scholar]
- Xu J, Li Y, Lin H, Sinha R, Potenza MN (2013): Body mass index correlates negatively with white matter integrity in the fornix and corpus callosum: A diffusion tensor imaging study. Hum Brain Mapp 34:1044–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Hao Y, Manor B, Novak P, Milberg W, Zhang J, Fang J, Novak V (2015): Intranasal insulin enhanced resting‐state functional connectivity of hippocampal regions in type 2 diabetes. Diabetes 64:1025–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Lin L, Lin CP, Zhou Y, Chou KH, Lo CY, Su TP, Jiang T (2012): Abnormal topological organization of structural brain networks in schizophrenia. Schizophr Res 141:109–118. [DOI] [PubMed] [Google Scholar]
- Zhou J, Gennatas ED, Kramer JH, Miller BL, Seeley WW (2012): Predicting regional neurodegeneration from the healthy brain functional connectome. Neuron 73:1216–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigman JM, Bouret SG, Andrews ZB (2016): Obesity Impairs the Action of the Neuroendocrine Ghrelin System. Trend Endocrinol Metab 27:54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


