Abstract
Despite considerable research on experience‐dependent neuroplasticity in professional musicians, detailed understanding of an involvement of the insula is only now beginning to emerge. We investigated the effects of musical training on intrinsic insula‐based connectivity in professional classical musicians relative to nonmusicians using resting‐state functional MRI. Following a tripartite scheme of insula subdivisions, coactivation profiles were analyzed for the posterior, ventral anterior, and dorsal anterior insula in both hemispheres. While whole‐brain connectivity across all participants confirmed previously reported patterns, between‐group comparisons revealed increased insular connectivity in musicians relative to nonmusicians. Coactivated regions encompassed constituents of large‐scale networks involved in salience detection (e.g., anterior and middle cingulate cortex), affective processing (e.g., orbitofrontal cortex and temporal pole), and higher order cognition (e.g., dorsolateral prefrontal cortex and the temporoparietal junction), whereas no differences were found for the reversed group contrast. Importantly, these connectivity patterns were stronger in musicians who experienced more years of musical practice, including also sensorimotor regions involved in music performance (M1 hand area, S1, A1, and SMA). We conclude that musical training triggers significant reorganization in insula‐based networks, potentially facilitating high‐level cognitive and affective functions associated with the fast integration of multisensory information in the context of music performance. Hum Brain Mapp 38:4834–4849, 2017. © 2017 Wiley Periodicals, Inc.
Keywords: insula, musicians, experience‐dependent plasticity, sensorimotor, salience, executive control
INTRODUCTION
Playing a musical instrument places enormous demands on sensory, motor, and cognitive systems due to the continuous integration of complex perceptual inputs with precisely timed motor commands [Altenmüller, 2008; Zatorre et al., 2007]. Musical training has therefore been increasingly used as a framework for studying experience‐dependent neuroplasticity, providing compelling evidence that the repetitive performance of concurrent sensory and motor tasks modifies corresponding brain regions at both the functional and the structural levels [Bailey et al., 2014; Bermudez et al., 2009; Elbert et al., 1995; Gaser and Schlaug, 2003; Kleber et al., 2010, 2013; Lotze et al., 2003; Munte et al., 2002; Pantev et al., 2001; Schneider et al., 2002; Schulz et al., 2003; Steele et al., 2013]. A causal relationship with deliberate practice in sensitive periods has furthermore been suggested based on the observation that earlier commencement with musical training (<7 years) correlates with greater neural reorganization as well as enhanced planning, execution, and monitoring of music performance at the behavioral level [Bengtsson et al., 2005; Brown et al., 2015; Forgeard et al., 2008; Kleber et al., 2013; Penhune, 2011].
Most of the research concerned with the effects of musical training has focused either on auditory or sensorimotor systems, as both are fundamental for music perception and music performance (for review, see Herholz and Zatorre 2012). On the other hand, long‐term musical practice also enhances sensorimotor interactions as well as the integration of multisensory inputs [Musacchia et al., 2007; Petrini et al., 2009; Ragert et al., 2004; Zatorre et al., 2007; Zimmerman and Lahav, 2012], thus reducing auditory, tactile, and multisensory reaction times at a behavioral level [Landry and Champoux, 2017]. This suggests that music learning and practice affects not only uni‐ but also multisensory regions of the brain, such as the parietal lobe (perceptual–motor coordination), the superior temporal sulcus (action to sound mappings), and the prefrontal cortex (temporal integration) [Lappe et al., 2011; Lee and Noppeney, 2011; Paraskevopoulos et al., 2012; Zimmerman and Lahav, 2012]. However, one region that is most strongly and consistently implicated in sensory integration across different sources of input, including sound, touch, pain, and internal bodily sensations, is the insular cortex [Ackermann and Riecker, 2010; Bamiou et al., 2003; Craig, 2003, 2009a; Critchley et al., 2004; Karnath and Baier, 2010; Olausson et al., 2002]. Its vast cortical and subcortical connections and its participation in both stimulus detection and higher order cognitive control [Craig, 2009a; Deen et al., 2011; Dosenbach et al., 2007; Menon and Uddin, 2010] makes it perfectly suited to act as a critical hub for the coordination of large‐scale brain networks involved in sensory integration, affective processes, and executive functions [Cauda et al., 2011; Seeley et al., 2007; Uddin, 2014].
Insula activation has been reported for a wide range of musical tasks, such as tempo and melody processing (Platel et al., 1997; Thaut, 2003; Thaut et al., 2014), the acquisition of action–perception links during music learning [Mutschler et al., 2007], the gating of sensory motor information [Kleber et al., 2013], and the emotional processing of music (Blood et al. [1999]; for review, see also Koelsch [2014]). Similarly, cross‐sectional studies have associated musical training and aural skills with greater gray matter volume and its myelination in several brain regions including the insular cortices [Groussard et al., 2014; Shimizu and Sakai, 2015]. Lesions of the insula and its disconnection with the auditory cortex have furthermore been associated with musical anhedonia [Satoh et al., 2005; Sihvonen et al., 2016], whereas positive responses to pleasurable music correlated with enhanced activity in fronto‐insular areas in trained musicians [Brattico et al., 2015]. This is in line with a prominent theory that features the insula as the neural substrate of human awareness [Craig, 2002, 2009a, 2011]. Moreover, healthy professional musicians demonstrate enhanced interoceptive accuracy and greater sensitivity to somatosensory and pain signals [Schirmer‐Mokwa et al., 2015; Zamorano et al., 2014], which are integrated in the anterior insula [Critchley, 2004; Wiech et al., 2010]. These data may therefore provide a possible explanation for the increased prevalence of pain syndromes among professional musicians relative to the general population [Brandfonbrener, 2003; Steinmetz et al., 2014].
Despite accumulating evidence for experience‐dependent changes in the brain of musicians, we still know little about how musical training might affect the organization of large‐scale functional brain networks [Fauvel et al., 2014; Klein et al., 2016; Luo et al., 2012, 2014; Palomar‐García et al., 2017; Tanaka and Kirino, 2016a, 2016b]. For this purpose, we used resting‐state functional magnetic resonance imaging (rs‐fMRI) to assess spatial patterns of functional connectivity during spontaneous fluctuations of blood oxygenation level‐dependent (BOLD) activity, which may be closely related to neural subsystems revealed by task‐activation fMRI [Cole et al., 2014]. To date, only few such studies have been carried out with musicians. The available data suggest enhanced functional connectivity among predefined primary sensory and motor regions [Luo et al., 2012] and between regions with increased gray‐matter volume as a function of musical training [Fauvel et al., 2014]. Two recent studies reported greater functional connectivity at rest in musicians compared to nonmusicians encompassing constituents of the salience network [Luo et al., 2014] and between the precuneus and the insula [Tanaka and Kirino, 2016b]. Based on these reports and the notion that the insula represents a critical hub for sensory integration, we compared resting‐state fMRI connectivity patterns between musicians and nonmusicians within insula‐based networks.
According to Craig [2002, 2009a, 2011], sensory inputs are integrated along a posterior‐to‐mid‐to‐anterior insula progression scheme. In this scheme, the posterior insula (PI) provides the primary representation of the physiological condition of the body. A cognitive re‐representation of this input is performed and integrated in the mid insula and again in the anterior insula cortex, where subjective evaluation is performed in the ventral part of the anterior insula (vAI). In accordance with this model, the insula can be functionally divided into three subdivisions based on their patterns of whole‐brain functional connectivity during resting‐state fMRI: the posterior (PI), ventral anterior (vAI), and dorsal anterior (dAI) insula [Deen et al., 2011; Uddin et al., 2014]. These data associate the PI and portions of the mid insula with sensorimotor processes [Uddin et al., 2014]. In contrast, the dorsal anterior insula (dAI) was connected with areas involved in higher level cognitive control and the ventral anterior insula (vAI) was linked to regions involved in affective processes [Deen et al., 2011; Uddin et al., 2014].
Following this tripartite insula model, we hypothesized that trained musicians compared to nonmusicians would show enhanced functional connectivity between the PI and sensorimotor areas that are typically involved in music production, based on their extensive experience with fine‐grained sensorimotor coordination. We furthermore expected that the anterior insula of musicians would show greater connectivity with regions involved with affective, cognitive, and attentional processes, possibly reflecting enhanced top–down sensorimotor control in trained musicians. The presence of such effects in the absence of musical task performance may indicate lasting neuroplastic adaptation in these networks as a function of training. Therefore, we hypothesized that the proposed effects may be enhanced in more experienced musicians. Together, this study expands on previous knowledge about experience‐dependent neuroplasticity by providing novel data on how musical training can affect insula‐based networks involved in bottom–up and top–down processing of multisensory and motor signals.
MATERIALS AND METHODS
Participants
Eleven female professional classical musicians (mean age 31.4 ± 11.2 years) from different music schools and orchestras in the Balearic Islands (Spain) volunteered to participate in this study. All musicians were conservatory‐trained instrumentalists (5 string, 2 keyboard, and 4 wood instruments) with a long history of professional musical practice (20.5 ± 5.9 years) and an average of daily practice of 3 h (3.6 ± 2.2 h). A matched female control group was recruited from the University of the Balearic Islands, consisting of 12 right‐handed nonmusicians (mean age 28.1 ± 7.3 years) without any prior formal or informal music training. Details for both groups are provided in Table 1. Participants with neurological diseases, chronic pain, or pregnancy were not allowed in this study. One musician was eliminated from further analysis due to bad alignment during image preprocessing (see details in Image Acquisition section). The final number of professional musicians was therefore 10 (one left handed). All participants received verbal information about the scope of this study and provided written consent. The study was performed in accordance with the Declaration of Helsinki [1991] and approved by the Ethics Committee of the Balearic Islands.
Table 1.
Sociodemographic and professional characteristics of musicians and nonmusicians
| Musicians (n = 10) | Nonmusicians (n = 12) | |
|---|---|---|
| Age (y) | 32.3 (11.4) | 28.1 (7.3) |
| Dominant hand (L/R) | 1/9 | 0/12 |
| Depression | 6.2 (6.7) | 4.6(3.8) |
| State anxiety | 12.1 (5.4) | 14.2 (11.6) |
| Trait anxiety | 12.4 (3.8) | 13.8 (8.4) |
Abbreviations: y, years; L, left; R, right.
All values represent mean and standard deviation (SD, in brackets).
Psychometric Assessment
To test possible differences in anxiety or mood states between groups, all participants completed the Spanish versions of Beck's Depression Inventory II [Beck et al., 1961; Sanz et al., 2005] and the State‐Trait Anxiety Inventory [Spielberger et al., 1970]. Manual dominance was determined with the Edinburgh Handedness Inventory [Oldfield, 1971].
Image Acquisition
MRI was performed using a GE 3 T scanner (General Electric Signa HDx, GE Healthcare, Milwaukee, WI). For each subject, 240 whole‐brain echo‐planar images were acquired over a period of 10 min with the eyes closed (32 transversal slices per volume; 3 mm slice thickness; 90° flip angle; repetition time [TR]: 2500 ms; echo time [TE]: 35 ms; 64 × 64 matrix dimensions; 200 mm field of view). The structural imaging data consisted of T1‐weighted images (256 slices per volume; repetition time [TR]: 7796 ms; echo time [TE]: 2.98 ms; matrix dimensions, 256 × 256; 240 mm field of view; 1 mm slice thickness; 12° flip angle). Scanner noise was passively reduced by −36 db using in‐ear hearing protection. In addition, MRI foam‐cushions were placed over the ears to restrict head motion and further to reduce the impact of scanner noise.
Neuroimaging Data Preprocessing
Image processing was performed with the Data Processing Assistant for Resting‐State fMRI (DPARSF; Chao‐Gan and Yu‐Feng 2010; Yan et al., 2016), which is based on the Statistical Parametric Mapping software package (SPM8; http://www.fil.ion.ucl.ac.uk/spm) and the toolbox for Data Processing & Analysis of Brain Imaging (DPABI; http://rfmri.org/DPABI DPARSF_V3.1_141101). The first 10 volumes from each resting dataset were discarded prior to data processing. Following slice‐time correction and co‐registration, gray and white matter were segmented from co‐registered T1 images using the unified segmentation model [Ashburner and Friston, 2005]. The resulting parameter file was used to normalize the functional images (3 mm3 voxel size) to standard Montreal Neurological Institute (MNI) stereotactic space and subsequently smoothed with an isotropic Gaussian kernel (FWHM: 6 mm3). Nuisance regression parameters included white matter (WM), cerebrospinal fluid (CSF), and six head motion parameters. WM and CSF masks were generated using SPM's a priori tissue probability maps (empirical thresholds: 90% for WM mask and 70% for CSF mask). No global signal regression was performed to avoid introducing distortions of BOLD signal [Murphy et al., 2009; Wong et al., 2016]. Head motion was below 2.0 mm maximum displacement or 2.0° of any angular motion for all participants. A temporal filter (0.006–0.1 Hz) was applied to reduce the impact of low‐frequency drifts and high‐frequency physiological noise.
Voxel‐Wise Functional Connectivity Analysis
Functionally segregated insular subdivisions representing the posterior (PI), ventral anterior (vAI), and dorsal anterior (dAI) insula were provided as template images by Deen et al. [2011] from their previous study. Mean coordinates of these insular subdivisions are shown in Table 2. The resulting six insular ROIs (i.e., left and right PI, vAI, and dAI) were used as seed‐regions of interest (ROI) to determine their individual connectivity pattern. The averaged time course from each seed region was then entered in a voxel‐wise correlation analysis to generate the functional connectivity maps. The correlation coefficient map was converted into z maps by Fisher's r‐to‐z transformation to improve normality [Rosner, 2011]. Statistical significance of functional connectivity was subsequently calculated with SPM8.
Table 2.
Mean coordinates of insular subdivisions in MNI152 space
| Left insula | Right insula | |||||
|---|---|---|---|---|---|---|
| Seed | Mean coordinates (mm) | Mean coordinates (mm) | ||||
| x | y | z | x | y | z | |
| Posterior insula | −38 | −6 | 5 | 35 | −11 | 6 |
| Dorsal anterior insula | −38 | 6 | 2 | 35 | 7 | 3 |
| Ventral anterior insula | −33 | 13 | −7 | 32 | 10 | −6 |
To validate the main insula ROI connectivity patterns against those published previously [Deen et al., 2011], we first entered individual z‐transformed connectivity maps from all participants in both groups into one‐sample t tests for each ROI. The significance threshold for voxel‐wise statistics was set to P < 0.05, familywise error (FWER) corrected for analyses across groups (N = 22).
To assess whole‐brain differences in insula‐based network connectivity between musicians and nonmusicians, we compared functional connectivity maps using independent two‐sample t tests for each ROI. A cluster‐extent based thresholding method was employed for these analyses, which is widely used in fMRI research and offers increased sensitivity to detect activations in studies with moderate sample sizes [Friston et al., 1994; Smith and Nichols, 2009; Woo et al., 2014]. Cluster‐extent based methods account for the fact that individual voxel activations are not independent of the activations of their neighboring voxels in spatially smoothed data [Friston et al., 2000; Heller et al., 2006; Wager et al., 2007] and effectively reduces the possibility of obtaining false positive clusters while improving the degree of confidence in inferences about specific voxels [Woo et al., 2014].
We determined cluster‐extent thresholds at P < 0.05 FWER correction for multiple comparisons using Monte‐Carlo simulation and the DPABI gray matter mask, as implemented in DPABI's instantiation [Song et al., 2011; Yan et al., 2016] of AlphaSim [Cox, 1996], following a stringent primary voxel‐level threshold of P < 0.001 and smoothness estimation based on the spatial correlation across voxels. Stringent primary thresholds are recommended to avoid inaccurate FWER correction [Woo et al., 2014]. Only those clusters surviving the FWER probability threshold were used for statistical inference. T values of significantly activated peak‐voxels refer to MNI coordinates. To measure the magnitude of the functional connectivity of insula subdivisions, we computed effect sizes using Cohen's d (d = 2t/sqrt(df)) [Cohen, 2013]. In addition, to control for a possible overestimation of the effect size due to the small sample size of our groups, we posteriorly adjusted Cohen's d by computing the unbiased Cohen's d: d unbiased = d [1 − (3/4df − 1)][Fritz et al., 2012].
Regression Analysis
To further examine changes in connectivity patterns in musicians as a function of musical practice, regression analyses were performed in SPM8 on the accumulated years of musical training for the connectivity maps for each insular ROI. We applied the P < 0.05 FWER correction based on cluster extent and a primary threshold of P < 0.001 using DPABI, as detailed above.
RESULTS
Demographic and Self‐Report Data
Chi‐square tests showed that the distribution of right and left dominant hand was similar in both groups X 2 (2, N = 22) = 1.257, P = 0.262). Moreover, t tests revealed no significant differences in age (t(1,20) = 0.713, P = 0.485), anxiety (STAI, state: t(1,19) = 0.989, P = 0.616; trait: t(1,19) = 5.28, P = 0.620], or depression (BDI, t(1,19) = 1.889, P = 0.485) between musicians and nonmusicians (Table 1).
Voxel‐Wise Functional Connectivity of Insula Subdivisions
Main connectivity patterns across all participants
Results from whole‐brain connectivity analyses across all participants replicated previously reported bilateral connectivity patterns of left and right pAI, dAI, and vAI [Cauda et al., 2011; Deen et al., 2011; Uddin et al., 2014].
The connectivity pattern of the posterior insula (PI; Fig. 1A) was centered on bilateral sensorimotor regions, including the precentral gyrus (M1) and premotor areas (SMA and pre‐SMA), the postcentral gyrus (primary and secondary somatosensory cortices; S1 and S2), temporal and parietal cortices (encompassing the supramarginal and Heschl's gyrus), and the cerebellum (Crus I and Crus II, Lobule VI, VIIb, and VIII). Furthermore, the PI was connected with the entire insula, frontal and rolandic operculae, and the middle cingulate cortex (MCC).
Figure 1.
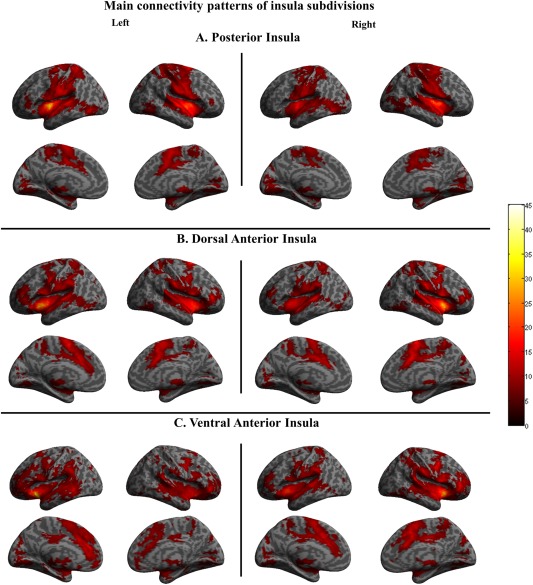
Main patterns of functional connectivity across all participants for each insular subdivision. Significance thresholds were set at P < 0.05, familywise error (FWER) corrected. (A) Posterior insula. (B) Dorsal anterior insula. (C) Ventral anterior insula. Connectivity maps for left insula seeds are shown on the left and connectivity maps for right insula seeds on the right. [Color figure can be viewed at http://wileyonlinelibrary.com]
The connectivity pattern of the dorsal anterior insula (dAI; Fig. 1B) was centered on the bilateral entire insula, the rolandic operculum, inferior frontal and orbitofrontal cortex, the ACC and MCC, premotor cortex, supplementary motor area (SMA), superior temporal gyrus and parietal cortices (encompassing the supramarginal and Heschl's gyrus), the precuneus, and the cerebellum (Crus I and Crus II, Lobule VI and VIII) (not displayed in Fig. 1B).
The connectivity pattern of the ventral anterior insula (vAI; Fig. 1C) showed bilateral connectivity with the insular cortices, the adjacent frontal operculae, frontal and the orbitrofrontal cortices, the anterior and middle cingulate cortex (ACC and MCC), premotor cortex, SMA, auditory temporal regions, parietal cortices, and the cerebellum (Crus I and Crus II, Lobule VI, VIIb, and VIII).
Difference connectivity maps between musicians and nonmusicians
T‐contrasts of connectivity maps for each of the insula subdivisions revealed increased functional connectivity in musicians compared to nonmusicians. The reversed contrast yielded no significant differences (Fig. 2 and Table 3).
Figure 2.
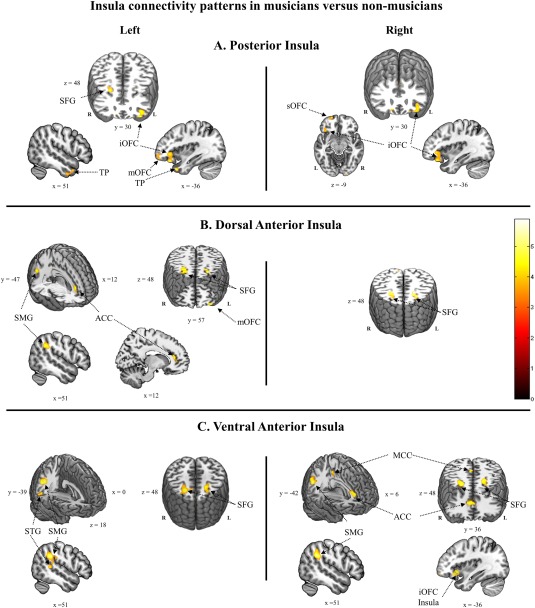
T‐maps showing significantly increased functional connectivity in musicians relative to nonmusicians. (A) Posterior insula. (B) Dorsal anterior insula. (C) Ventral anterior insula. Left insula seed connectivity is displayed on the left and right connectivity on the right. Cluster‐extent significance thresholds were set at P < 0.05 FWER correction for multiple comparisons using Monte Carlo simulation as implemented in DPABI's instantiation [Song et al., 2011; Yan et al., 2016] of AlphaSim [Cox, 1996], following a stringent primary voxel‐level threshold of P < 0.001 and smoothness estimation based on the spatial correlation across voxels. SMG, supramarginal gyrus; STG, superior temporal gyrus; MCC, middle cingulate cortex; ACC, anterior cingulate cortex; TP, temporal pole; SFG, superior frontal gyrus; sOFC, superior orbitofrontal; mOFC, middle orbitofrontal cortex; iOFC, inferior orbitofrontal cortex. [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 3.
Insula subdivision connectivity patterns in musicians versus nonmusicians
| Left insula | Right insula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Seed Connectivity region | (area) | Cluster size | Coordinates MNI | t | dunb | Cluster size | Coordinates MNI | t | dunb | |||||
| x | y | z | x | y | z | |||||||||
| PI | ||||||||||||||
| Superior frontal gyrus | (BA8) | R | 27 | 24 | 24 | 48 | 4.18 | 1.72 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Superior orbitofrontal | (BA10/Fp1) | L | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 28 | −21 | 66 | −9 | 4.80 | 1.97 |
| Middle orbitofrontal | (BA11/Fp1) | L | 60 | −33 | 57 | −15 | 4.99 | 2.05 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Inferior orbitofrontal | (BA47/Fo3) | L | 82 | −36 | 30 | −21 | 5.53 | 2.27 | 91 | −36 | 30 | −21 | 4.79 | 1.97 |
| Temporal pole | R | 28 | 51 | 12 | −33 | 4.19 | 1.72 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Temporal pole | (BA38) | L | 20 | −36 | 18 | −39 | 5.71 | 2.36 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| dAI | ||||||||||||||
| Superior frontal gyrus | (BA8) | R | 176a | 27 | 27 | 48 | 4.70 | 1.93 | 79a | 18 | 27 | 51 | 4.05 | 1.66 |
| Middle frontal gyrus | (BA6) | R | 176a | 30 | 12 | 51 | 4.56 | 1.87 | 79a | 24 | 6 | 45 | 4.45 | 1.83 |
| Superior frontal gyrus | (BA8) | L | 45a | −21 | 15 | 48 | 4.27 | 1.75 | 48a | −18 | 15 | 48 | 4.61 | 1.89 |
| Middle frontal gyrus | (BA8) | L | 45a | −27 | 27 | 48 | 5.35 | 2.20 | 48a | −27 | 27 | 45 | 4.39 | 1.81 |
| Middle orbitofrontal | (BA11/Fp1) | L | 27 | −33 | 57 | −15 | 5.59 | 2.30 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Angular gyrus | (PF) | R | 50a | 51 | −48 | 33 | 4.84 | 1.99 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Supramarginal gyrus | (PFm) | R | 50a | 48 | −39 | 30 | 4.65 | 1.91 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Anterior cingulate | (Area 33) | R | 42 | 12 | 39 | 12 | 4.89 | 2.01 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| vAI | ||||||||||||||
| Superior frontal gyrus | (BA8) | R | 144a | 21 | 18 | 48 | 5.37 | 2.20 | 204a | 24 | 18 | 48 | 5.15 | 2.12 |
| Middle frontal gyrus | R | 144a | 27 | 15 | 54 | 4.80 | 1.92 | 204a | 30 | 3 | 51 | 4.85 | 1.99 | |
| Superior frontal gyrus | L | 87a | −21 | 9 | 45 | 5.09 | 2.09 | 167a | −21 | 6 | 45 | 5.28 | 2.17 | |
| Middle frontal gyrus | (BA8) | L | 87a | −27 | −3 | 51 | 4.00 | 1.63 | 167a | −24 | 3 | 54 | 5.23 | 2.14 |
| Inferior orbitofrontal | (BA8) | L | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 62a | −36 | 27 | −12 | 4.74 | 1.95 |
| Insula | L | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 62a | −33 | 21 | −6 | 5.45 | 2.24 | |
| Angular gyrus | (PFm) | R | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 123a | 51 | −48 | 33 | 5.55 | 2.28 |
| Supramarginal gyrus | (PFm) | R | 215a | 51 | −42 | 30 | 5.68 | 2.34 | 123a | 51 | −42 | 30 | 5.68 | 2.34 |
| Superior temporal gyrus | (BA13/PFcm) | R | 215a | 51 | −36 | 18 | 4.12 | 1.67 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Anterior cingulate | (BA32/33) | R | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 109 | 6 | 36 | 15 | 5.02 | 2.11 |
| Medium cingulate | (BA31/5Ci) | R | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 34 | 3 | −33 | 48 | 4.58 | 1.88 |
MNI coordinates and local maxima of whole‐brain differences in insula‐based network connectivity during resting state in musicians compared to nonmusicians. A cluster‐extent‐based threshold at P < 0.05 FWER correction was employed to determine significance following a stringent primary voxel‐level threshold of P < 0.001 and smoothness estimation based on the spatial correlation across voxels. Only clusters that survived the FWER probability threshold are shown. T values of significantly activated peak‐voxels refer to MNI coordinates. Brodmann Areas (BA) labeling was performed using the Automatic Anatomic Labeling toolbox [Tzourio‐Mazoyer et al., 2002]. Probabilistic cytoarchitectonic maps for structure–function relationships in standard reference space were assigned using the Anatomy Toolbox [Eickhoff et al., 2005]. PI, posterior insula; dAI, dorsal anterior insula; vAI, ventral anterior insula; a = same cluster, dund = unbiased Cohen's d.
The left PI (Fig. 2A) showed increased functional connectivity with left orbitofrontal cortex, right superior frontal gyrus, and bilateral temporal pole. The right PI showed increased connectivity with left orbitofrontal cortex.
The left dAI (Fig. 2B) showed increased functional connectivity with left orbitofrontal cortex, bilateral superior and middle frontal gyrus, right ACC, and right angular and supramarginal gyrus. The right dAI showed increased functional connectivity with bilateral superior and middle frontal gyri only.
The left vAI (Fig. 2C) showed increased functional connectivity with right supramarginal gyrus, adjacent superior temporal gyrus, and bilateral superior and middle frontal gyrus. The right vAI showed increased connectivity in musicians with right supramarginal, angular and superior temporal gyrus, bilateral superior and middle frontal gyrus, and left orbitofrontal and left insular cortex, and right ACC/MCC.
Although similar regions appear to be connected to different insula subdivisions, a conjunction null analysis in SPM8 showed no evidence for jointly connected areas.
Regression Analyses
Results from the regression analyses (Fig. 3 and Table 4) suggest a relationship between the insular subdivision connectivity patterns in professional musicians and years of music training. That is, more experienced musicians showed higher levels of resting‐state connectivity with the same regions found in the comparison between musicians and nonmusicians. Additionally, musicians with greater experience also showed greater insular connectivity with primary somatosensory cortex, primary motor cortex (hand‐area), primary auditory cortex, middle occipital gyrus, and the SMA.
Figure 3.
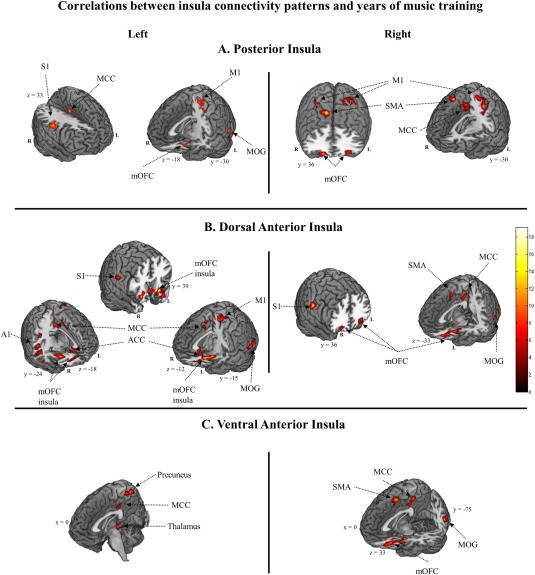
Results from regression analyses testing for correlations between spontaneous BOLD fluctuation connectivity patterns of insula subdivisions and accumulated years of training in professional musicians. Results are thresholded at P < 0.05 FWER correction for multiple comparisons using cluster‐extent‐based Monte Carlo simulation as implemented in DPABI's instantiation [Song et al., 2011; Yan et al., 2016] of AlphaSim [Cox, 1996], following a stringent primary voxel‐level threshold of P < 0.001 and smoothness estimation based on the spatial correlation across voxels. S1, primary somatosensory cortex; M1, primary motor cortex; A1, primary auditory cortex; MOG, middle occipital gyrus; SMA, supplementary motor area; MCC, middle cingulate cortex; ACC, anterior cingulate cortex; mOFC, middle orbitofrontal cortex. [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 4.
Correlations between insula connectivity patterns and years of music training
| Seed | Connectivity region | Left Insula | Right Insula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cluster size | Coordinates MNI | Assigned to area | Cluster size | Coordinates MNI | Assigned to area | |||||||||||
| x | y | z | t | R | x | y | z | t | R | |||||||
| PI | ||||||||||||||||
| MI | L | 152 | −30 | −27 | 66 | 10.73 | 0.967 | Area 4a | 166 | −39 | −24 | 63 | 9.58 | 0.959 | Area 4a/4p | |
| Medium cingulate | L | 64 | 0 | −27 | 39 | 7.81 | 0.940 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Middle temporal gyrus | L | 54a | −54 | −63 | 6 | 9.21 | 0.956 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Middle occipital gyrus | L | 54a | −42 | −72 | 12 | 6.13 | 0.908 | hOc4la | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Middle orbitofrontal | L | 35 | −12 | 21 | −18 | 6.15 | 0.909 | BA25/Fo2 | 86a | −33 | 36 | −21 | 7.63 | 0.936 | Fo3 | |
| Ventral anterior insula | L | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 86a | −27 | 18 | −15 | 7.22 | 0.961 | ‐ | |
| SI | R | 108 | 57 | −9 | 33 | 8.27 | 0.946 | Area 3b | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Supp. motor area | R | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 55 | 12 | 12 | 69 | 10.72 | 0.967 | ‐ | |
| Supp. motor area | R | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 204 | 6 | −12 | 54 | 8.14 | 0.945 | BA6 | |
| Middle orbitofrontal | R | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 103a | 27 | 36 | −21 | 5.04 | 0.945 | Fo3 | |
| Ventral anterior insula | R | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 103a | 30 | 18 | −12 | 7.47 | 0.935 | BA47 | |
| dAI | ||||||||||||||||
| MI | L | 65 | −33 | −24 | 72 | 5.25 | 0.932 | Area 4a | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Middle occipital gyrus | L | 80 | −45 | −72 | 12 | 10.19 | 0.922 | hOc4la | 52 | −48 | −75 | 12 | 10.39 | 0.917 | hOc4la | |
| Anterior Cingulate | L | 192a | −3 | 21 | −9 | 6.42 | 0.962 | s24 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Middle orbitofrontal | L | 192a | −27 | 36 | −15 | 7.84 | 0.972 | BA11/Fo3 | 144 | −33 | 36 | −12 | 8.23 | 0.946 | BA11/Fo3 | |
| Middle orbitofrontal | R | 66 | 27 | 39 | −18 | 6.23 | 0.963 | BA11/Fo3 | 32 | 27 | 36 | −18 | 5.55 | 0.939 | BA11/Fo3 | |
| SI | R | 41 | 60 | −15 | 33 | 6.32 | 0.913 | Area 1/3b | 77 | 57 | −15 | 33 | 12.75 | 0.976 | Area 1/3b | |
| Supp. motor area | R | 46 | 6 | 9 | 45 | 6.70 | 0.944 | ‐ | 33 | 9 | 3 | 54 | 10.24 | 0.964 | BA6 | |
| Medium cingulate | R | 94 | 6 | −39 | 45 | 9.62 | 0.959 | 5Ci & 5M | 41 | 0 | −24 | 39 | 7.19 | 0.931 | 5Ci | |
| Sup. temporal gyrus | R | 96 | 51 | −24 | 15 | 8.30 | 0.947 | TE1.0 & 1.1 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Inf. temporal gyrus | R | 41 | 51 | 0 | −33 | 11.21 | 0.969 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Lingual gyrus | R | 39 | 15 | −54 | ‐ 6 | 6.22 | 0.910 | BA19/ hOc2 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| vAI | ||||||||||||||||
| Middle orbitofrontal | L | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 204a | −24 | 33 | −15 | 8.37 | 0.956 | BA11/Fo3 | |
| Temporal pole | L | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 204a | −39 | 24 | −24 | 8.25 | 0.956 | ‐ | |
| Supp. motor area | L | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 73 | 0 | 3 | 57 | 9.10 | 0.955 | BA6 | |
| Middle occipital gyrus | L | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 36 | −48 | −75 | 12 | 10.49 | 0.917 | hOc4la | |
| Medium cingulate | L | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 44 | 0 | −36 | 48 | 7.87 | 0.941 | 5Ci | |
| Medium cingulate | R | 56 | 9 | −39 | 45 | 8.27 | 0.946 | 5Ci | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Precuneus | R | 49 | 6 | −57 | 57 | 19.16 | 0.989 | Area 7A | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Thalamus | R | 49 | 6 | −27 | −3 | 8.00 | 0.943 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
MNI coordinates from regression analyses testing for correlations between spontaneous BOLD fluctuation connectivity patterns of insula subdivisions and accumulated years of training in professional musicians. A cluster‐extent based thresholds at P < 0.05 FWER correction was employed to determine significance following a stringent primary voxel‐level threshold of P< 0.001 and smoothness estimation based on the spatial correlation across voxels. Only clusters that survived the FWER probability threshold are shown. T‐values of significantly activated peak‐voxels refer to MNI coordinates. Brodmann Areas (BA) labeling was performed using the Automatic Anatomic Labeling toolbox [Tzourio‐Mazoyer et al., 2002]. Probabilistic cytoarchitectonic maps for structure‐function relationships in standard reference space were assigned using the Anatomy Toolbox [Eickhoff et al., 2005]. S1, primary somatosensory cortex; M1, primary motor cortex.
DISCUSSION
This study investigated resting‐state connectivity of insula‐based networks as a function of musical expertise. The overall connectivity pattern of three insular subdivisions (i.e., posterior, dorsal anterior, and ventral anterior insula) across all participants confirmed the ones that have been demonstrated previously [Cauda et al., 2011; Deen et al., 2011; Uddin et al., 2014]. As predicted, professional musicians showed greater insular connectivity than nonmusicians with constituents of large‐scale brain networks involved with salience detection (i.e., anterior and medial cingulate cortex, ACC/MCC), executive control (i.e., dorsolateral prefrontal cortex, DLPFC, and the temporoparietal junction, TPJ), and affective processing (i.e., orbitofrontal cortex, OFC, and temporal pole, TP). The reversed comparison showed no differences. Moreover, years of musical training in musicians were positively correlated with connectivity between the three insular subdivisions (left and right) and sensorimotor regions involved in music production and perception (superior temporal gyrus, primary somatosensory, and motor cortices). Together, our current findings indicate substantial neuroplasticity within insula‐based networks as a function of music training, expanding prior notions of a substantial role of the insula in the practice and performance of music.
Involvement of the Insula in Music Processing
Although the insula is one of the most frequently activated regions in functional neuroimaging research in a broad range of domains, a detailed understanding of its involvement in music production and perception is still missing [Chang et al., 2013; Craig, 2009a]. Research suggests that both our sense of time [Meissner and Wittmann, 2011] and the acoustic processing of musical signals [Bamiou et al., 2003; Brown et al., 2004; Rogalsky et al., 2011; Wong et al., 2004] can be directly linked to activity in the insular cortex. Similarly, several music‐related studies reported insula co‐activation with frontal, temporal, and parietal regions [Kleber et al., 2007; Pleger et al., 2006; Zarate et al., 2010], contributing to the planning, execution, and emotional processing of musical performance [Blood et al., 1999; Chen et al., 2008; Platel et al., 1997; Thaut et al., 2014]. Distinct insular activation patterns have furthermore been associated with enhanced multisensory gating as a function of musicianship [Kleber et al., 2013, 2017; Zarate and Zatorre, 2008], supporting the accurate performance of musical tasks and the rapid acquisition of sound‐action associations [Mutschler et al., 2007].
In the context of resting state, the insula is typically attributed to salience and central executive control networks. Particularly, it has been proposed that the insula contributes to the dynamic coordination of large‐scale networks as an integral hub for salience detection and its top–down regulation [Craig, 2009a; Critchley et al., 2004; Karnath and Baier, 2010; Menon and Uddin, 2010; Seeley et al., 2007; Uddin et al., 2014]. In line with this conceptual framework, a recent resting‐state study suggests greater connectivity among the constituents of the salience network in musicians compared to nonmusicians [Luo et al., 2014]. Based on these data, this study aimed at providing a more detailed investigation into insula connectivity in musicians.
Insula Connectivity Patterns in Musicians and Nonmusicians
Following a tripartite insula model, the main patterns of coactivation for each insula subdivision across all participants replicated previously reported results [Deen et al., 2011; Uddin et al., 2014]. That is, the dAI showed a “cognitive” frontoparietal connectivity pattern, whereas the vAI showed predominant coactivation with regions involved in affective processing (e.g., the orbitofrontal cortex), and the PI mainly with sensory areas (Fig. 1). In line with our hypothesis, direct group comparisons showed significantly enhanced connectivity across all insula subdivisions in professional musicians relative to nonmusicians. The reversed comparison showed no differences. A certain degree of overlap between coactivated regions among insula subdivisions (e.g., frontal lobe and cingulate cortex) may be expected, as the anterior insula can be involved in both differential (i.e., domain specific) and common contributions to fundamental aspects of cognitive processes [Uddin et al., 2014].
Posterior insula connectivity in musicians
Craig [2002, 2009a, 2011] suggested a model of insula function in which the sensory input is integrated along a posterior‐to‐anterior processing scheme, generating subjective feelings from the integration of intero‐ and exteroceptive sensory information. In this model, afferent sensory inputs representing the physiological condition of the body travel from receptive cells in lamina I of the spinal cord via brainstem (solitary tract and spinal trigeminal) nuclei and the thalamus to the posterior insular cortex. The PI is therefore considered to contain the primary interoceptive representation of visceral and sensorial feelings from the body [Pugnaghi et al., 2011]. We found that the PI subdivision (containing also parts of the mid‐insula, see Uddin et al., 2014) showed greater connectivity in musicians compared to nonmusicians, particularly with higher level multisensory regions (i.e., the orbitofrontal cortex, OFC, and the temporal pole, TP). This is also consistent with previous structural and functional neuroimaging studies that suggest a causal involvement of the PI, TP, and the OFC in music‐evoked emotions, such as visceral reactions during emotionally laden moments (for reviews, see Koelsch [2014] and Zatorre [2015]).
Neurofunctional studies indicate enhanced connectivity of the TP with the OFC and the insula during the coupling of highly processed perceptual inputs with visceral emotional responses [Roesch and Olson, 2007]. In addition, the OFC is related to the discrimination of positive and negative reward values during the experience of pleasantness/unpleasantness in music and other hedonic tasks [Blood et al., 1999; Blood and Zatorre, 2001; Francis et al., 1999; Small et al., 2001]. Together, enhanced posterior (and mid‐) insula connectivity in musicians might facilitate the integration of visceral interoceptive inputs with other neural inputs from higher order sensory regions (e.g., the temporal pole), forming a combined representation of salient features in an individual's internal and external environment [Karnath and Baier, 2010; Mutschler et al., 2007]. In the same vein, more accentuated visceral reactions in professional musicians have been associated with behavioral modifications in respiratory and cardiovascular patterns in response to increased tempo and emotionally relevant music sequences [Bernardi et al., 2006; Blood and Zatorre, 2001; Haas et al., 1986]. Enhanced integration of respiratory and cardiovascular signals could therefore also facilitate the control of musical tempo, harmony, timbre, and intensity. In broader terms, our data suggest that musical training alters the regulation and integration of interoceptive information in the PI [Bernardi et al., 2006; Blood et al., 1999; Craig, 2002, 2009b; Haas et al., 1986; Koelsch, 2014].
The anterior insula in musicians
Several models have proposed a functional distinction between the dorsal and ventral anterior insula, in which the dorsal anterior insula is associated with higher level cognitive processes and the ventral anterior insula with the subjective evaluation of feelings states [Craig, 2002; Dosenbach et al., 2007; Nelson et al., 2010; Seeley et al., 2007]. Previous resting‐state studies furthermore suggest that the vAI and dAI interface between the emotional and cognitive aspects of novelty detection [Menon and Uddin, 2010; Seeley et al., 2007; Uddin et al., 2014]. Analogous to this functional distinction, the AI is described as a switch‐node between the “central‐executive network” and the “salience network,” thus modulating the top–down control of behaviorally relevant signals across sensory modalities [Craig, 2009a, 2009b; Crottaz‐Herbette and Menon, 2006; Menon and Uddin, 2010; Seeley et al., 2007].
Both the ventral and the dorsal AI showed enhanced functional connectivity in musicians relative to nonmusicians, particularly with regions involved with salience detection, subjective evaluation, and cognition. However, in contrast to the functional organization previously proposed for dorsal (cognitive) and ventral (affective) anterior insula [Menon and Uddin, 2010; Uddin et al., 2014], we found a partly reversed and partly overlapping pattern in the left anterior insula in professional musicians relative to nonmusicians. That is, the left vAI was connected with bilateral DLPFC, right SMG (cognitive network), and right STG, whereas the left dAI showed additional connectivity with right ACC and the left OFC (salience and affective networks, respectively). Increased connectivity between the left vAI with auditory regions and the cognitive network on one hand and between the left dAI with regions of the affective and salience networks on the other hand might facilitate a shift between salience and executive processing, thereby supporting domain‐general access to attention and control systems [Menon and Uddin, 2010; Uddin, 2014]. As resting‐state networks are closely related to neural subsystems revealed by task‐activation fMRI [Cole et al., 2014], this could reflect altered central representations of bodily information in musicians to generate more appropriate behavioral responses in a music performance context [Craig, 2002; Fauvel et al., 2014; Luo et al., 2014; Menon and Uddin, 2010; Schirmer‐Mokwa et al., 2015; Seeley et al., 2007; Strait and Kraus, 2011; Taylor et al., 2009].
We also found that co‐activation patterns differed between the right and the left anterior insula. That is, in addition to both left and right vAI showing increased functional connectivity with the bilateral DLPFC and the right TPJ, the right vAI also showed increased functional connectivity with other regions involved in interoception and bodily perception such as the ACC, the MCC, the insula, and the OFC (Fig. 2C). The right AI cortex anticipates the sensory and affective consequences of touch [Lovero et al., 2009], yet functional asymmetry of the insula has also been ascribed to differences in ascending and descending connections [Allen et al., 2016; Bastos et al., 2015]. Intriguingly, unique functions have been ascribed to the right ventral AI [Craig, 2009a; Critchley et al., 2004; Kleber et al., 2013; Nieuwenhuys, 2012; Seeley et al., 2011; Uddin, 2014], based on a unique abundance of von Economo neurons (VENs) in both the right ventral AI and the ACC (Seeley et al., 2011). The large axons of VENs enhance the communication between both regions, which in turn facilitates the interaction with frontal and temporal areas to benefit the intuitive assessment of complex situations [Allman et al., 2010]. As multisensory integration and the subjective evaluation of sensory feedback are defining characteristics of professional musicians [Altenmüller, 2008], an increased number of coactivated regions with the right vAI could be related to increased attention to musically relevant sensory information and increased self‐evaluation in musicians [Strait and Kraus, 2011; Thaut et al., 2014; Zatorre et al., 1994, 1999]. This may in turn explain enhanced sensorimotor integration [Kleber et al., 2013; Riecker et al., 2000] and movement awareness [Baier and Karnath, 2008; Karnath and Baier, 2010] in musicians. The right vAI is moreover crucial for subjective evaluation (Craig 2002, 2009), which is in good agreement with recent behavioral studies that demonstrated similar subjective pain ratings to thermal and pressure stimulation in healthy musicians and chronic pain patients [Zamorano et al., 2014] and enhanced interoceptive accuracy in musicians [Schirmer‐Mokwa et al., 2015].
Insula Connectivity As a Function of Musical Training
Despite the moderate sample size, regression analyses revealed that musicians with more years of musical experience also showed greater functional connectivity with the PI, the dAI, and the vAI. That is, experience increased insular co‐activation with lateral and contralateral regions of sensorimotor (i.e., primary somatosensory and motor cortices, SMA, MCC), auditory systems (i.e., middle and superior temporal gyrus), in addition to the middle occipital cortex (MOG), which is closely related to visual‐motor actions [Astafiev et al., 2004], and the bilateral OFC. These results expand prior evidence for more efficient interhemispheric communication [Hughes and Franz, 2007] and greater neural plasticity in more experience musicians to the insular cortex [Brown et al., 2015; Herholz and Zatorre, 2012]. We propose that insula networks are far more sensitive to functional reorganization as a consequence of long‐term musical practice than previously acknowledged.
Limitations
This study may comprise some limitations. A first issue arises from the fact that only female trained musicians volunteered for this fMRI study. Previous studies have demonstrated gender differences during neural processing of music and music‐related stimuli, showing that females have a greater level of bilateral brain functioning compared to males, who show a predominant asymmetrical laterality [Koelsch et al., 2003a, 2003b]. Therefore, we cannot generalize our results to male musicians. In addition, the participants were classical‐trained musicians and we cannot rule out the possibility that insula‐based connectivity differs in other musical genres. However, as musicians in this study belonged to several different instrumental groups (string, keyboard, and woodwind), our data could reflect common consequences of musical training rather than specialized instrument‐specific effects. Another issue is that resting‐state fMRI exposes participants to continuous scanner noise. Therefore, we cannot completely rule out that differences in insular connectivity patterns might have been biased by auditory processes in musicians and nonmusicians. However, this is unlikely as recent studies have not found between‐group differences in auditory network activation during resting state [Palomar‐García et al., 2017] nor when comparing standard continuous acquisition with sparse acquisition [Yakunina et al., 2016]. Another consideration concerns global signal removal. There is currently no consensus on the removal of global signal when computing resting‐state functional connectivity, as this may not only introduce distortions (i.e., artefactual autocorrelations) in BOLD signals [Murphy and Fox, 2017] but could also remove neural information related to vigilance awareness [Wong et al., 2016, 2013]. Therefore, we did not perform global signal regression and our results must be interpreted in the context of the method applied. Finally, correlations between spontaneous BOLD fluctuation and years of musical training are based on a limited number of participants. Future studies should replicate these results in bigger samples.
CONCLUSIONS
To the best of our knowledge, these data provide first evidence that the training and performance of music can lead to profound neuroplastic changes in insula‐based networks involved in bottom–up and top–down processing of multisensory and motor signals [Chang et al., 2013]. The connectivity pattern of insular subdivisions in musicians encompassed constituents of large‐scale networks implicated in salience detection, emotional experience, and higher level cognitive control. As resting‐state fMRI activity is associated with neural subsystems revealed by task‐activation fMRI [Cole et al., 2014], our results lend support to a critical role of the insula subserving the integration of sensorimotor information, emotional expression, and cognitive control during music performance [Kleber et al., 2013, 2017; Koelsch, 2014; Thaut et al., 2014]. In the light of recent findings of maladaptive sensory processes in trained musicians [Zamorano et al., 2014], increased insular connectivity could furthermore represent a potential neural correlate that facilitates the development of pain syndromes [Kuner and Flor, 2016]. Future studies on music processing should therefore consider the insula as a central gateway for multisensory integration in music perception and production.
ACKNOWLEDGMENTS
We acknowledge the support provided by teachers and directors of the Superior Music Conservatory of the Balearic Islands. We also thank Dr Benjamin Deen and Dr Kevin Pelphrey for kindly providing us with the insula subdivisions from their earlier work. We are also grateful to Juan Gea for his help with data acquisition, and to Dr Ralf Veit and Dr Stephanie Kullmann for their support with data analyses.
REFERENCES
- Ackermann H, Riecker A (2010): The contribution(s) of the insula to speech production: a review of the clinical and functional imaging literature. Brain Struct Funct 214:419–433. [DOI] [PubMed] [Google Scholar]
- Allen M, Fardo F, Dietz MJ, Hillebrandt H, Friston KJ, Rees G, Roepstorff A (2016): Anterior insula coordinates hierarchical processing of tactile mismatch responses. NeuroImage 127:34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allman JM, Tetreault NA, Hakeem AY, Manaye KF, Semendeferi K, Erwin JM, Park S, Goubert V, Hof PR (2010): The von Economo neurons in frontoinsular and anterior cingulate cortex in great apes and humans. Brain Struct Funct 214:495–517. [DOI] [PubMed] [Google Scholar]
- Altenmüller E (2008): Neurology of musical performance. Clin Med 8:410–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ (2005): Unified segmentation. NeuroImage 26:839–851. [DOI] [PubMed] [Google Scholar]
- Association WM (1991): Declaration of Helsinki. Law Med Health Care 19:264–265. [PubMed] [Google Scholar]
- Astafiev SV, Stanley CM, Shulman GL, Corbetta M (2004): Extrastriate body area in human occipital cortex responds to the performance of motor actions. Nat Neurosci 7:542–548. [DOI] [PubMed] [Google Scholar]
- Baier B, Karnath H‐O (2008): Tight link between our sense of limb ownership and self‐awareness of actions. Stroke 39:486–488. [DOI] [PubMed] [Google Scholar]
- Bailey JA, Zatorre RJ, Penhune VB (2014): Early Musical Training Is Linked to Gray Matter Structure in the Ventral Premotor Cortex and Auditory–Motor Rhythm Synchronization Performance. ?J Cognitive Neurosci 26:755–767. [DOI] [PubMed] [Google Scholar]
- Bamiou DE, Musiek FE, Luxon LM (2003): The insula (Island of Reil) and its role in auditory processing: Literature review. Brain Res Rev 42:143–154. [DOI] [PubMed] [Google Scholar]
- Bastos AM, Vezoli J, Bosman CA, Schoffelen J‐M, Oostenveld R, Dowdall JR, De Weerd P, Kennedy H, Fries P (2015): Visual areas exert feedforward and feedback influences through distinct frequency channels. Neuron 85:390–401. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward C, Mendelson M (1961): Beck depression inventory (BDI). Arch Gen Psychiatry 4:561–571. [DOI] [PubMed] [Google Scholar]
- Bengtsson SL, Nagy Z, Skare S, Forsman L, Forssberg H, Ullén F (2005): Extensive piano practicing has regionally specific effects on white matter development. Nat Neurosci 8:1148–1150. [DOI] [PubMed] [Google Scholar]
- Bermudez P, Lerch JP, Evans AC, Zatorre RJ (2009): Neuroanatomical correlates of musicianship as revealed by cortical thickness and voxel‐based morphometry. Cereb Cortex 19:1583–1596. [DOI] [PubMed] [Google Scholar]
- Bernardi L, Porta C, Sleight P (2006): Cardiovascular, cerebrovascular, and respiratory changes induced by different types of music in musicians and non‐musicians: the importance of silence. Heart 92:445–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blood a. J, Zatorre RJ (2001): Intensely pleasurable responses to music correlate with activity in brain regions implicated in reward and emotion. Proc Natl Acad Sci USA 98:11818–11823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blood AJ, Zatorre RJ, Bermudez P, Evans AC (1999): Emotional responses to pleasant and unpleasant music correlate with activity in paralimbic brain regions. Nat Neurosci 2:382–387. [DOI] [PubMed] [Google Scholar]
- Brandfonbrener AG (2003): Musculoskeletal problems of instrumental musicians. Hand Clin 19:231–239. v–vi. [DOI] [PubMed] [Google Scholar]
- Brattico E, Bogert B, Alluri V, Tervaniemi M, Eerola T, Jacobsen T (2015): It's sad but I like it: The neural dissociation between musical emotions and liking in experts and laypersons. Front Hum Neurosci 9:676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RM, Zatorre RJ, Penhune VB (2015): Expert music performance: cognitive, neural, and developmental bases. Prog Brain Res 217:57–86. [DOI] [PubMed] [Google Scholar]
- Brown S, Martinez MJ, Parsons LM (2004): Passive music listening spontaneously engages limbic and paralimbic systems. Neuroreport 15:2033–2037. [DOI] [PubMed] [Google Scholar]
- Cauda F, D'Agata F, Sacco K, Duca S, Geminiani G, Vercelli A (2011): Functional connectivity of the insula in the resting brain. NeuroImage 55:8–23. [DOI] [PubMed] [Google Scholar]
- Chang LJ, Yarkoni T, Khaw MW, Sanfey AG (2013): Decoding the role of the insula in human cognition: functional parcellation and large‐scale reverse inference. Cereb Cortex 23:739–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao‐Gan Y, Yu‐Feng Z (2010): DPARSF: A MATLAB toolbox for “Pipeline” data analysis of resting‐state fMRI. Front Syst Neurosci 4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JL, Penhune VB, Zatorre RJ (2008): Listening to musical rhythms recruits motor regions of the brain. Cereb Cortex 18:2844–2854. [DOI] [PubMed] [Google Scholar]
- Cohen J (2013): Statistical Power Analysis for the Behavioral Sciences. Academic Press. [Google Scholar]
- Cole MW, Bassett DS, Power JD, Braver TS, Petersen SE (2014): Intrinsic and task‐evoked network architectures of the human brain. Neuron 83:238–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW (1996): AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29:162–173. [DOI] [PubMed] [Google Scholar]
- Craig AD (2002): How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci 3:655–666. [DOI] [PubMed] [Google Scholar]
- Craig AD (2003): Interoception: The sense of the physiological condition of the body. Curr Opin Neurobiol 13:500–505. [DOI] [PubMed] [Google Scholar]
- Craig AD (2009a): How do you feel — now? The anterior insula and human awareness. Nat Rev Neurosci 10:59–70. papers3://publication/uuid/2CB3E0C6‐D12A‐4CD4–976C‐4EB906C77CF1. [DOI] [PubMed] [Google Scholar]
- Craig AD (2009b): Emotional moments across time: a possible neural basis for time perception in the anterior insula. Philos Trans R Soc Lond B Biol Sci 364:1933–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD (2011): Significance of the insula for the evolution of human awareness of feelings from the body. Ann N Y Acad Sci 1225:72–82. [DOI] [PubMed] [Google Scholar]
- Critchley HD (2004): The human cortex responds to an interoceptive challenge. Proc Natl Acad Sci USA 101:6333–6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ (2004): Neural systems supporting interoceptive awareness. Nat Neurosci 7:189–195. [DOI] [PubMed] [Google Scholar]
- Crottaz‐Herbette S, Menon V (2006): Where and when the anterior cingulate cortex modulates attentional response: combined fMRI and ERP evidence. J Cogn Neurosci 18:766–780. [DOI] [PubMed] [Google Scholar]
- Deen B, Pitskel NB, Pelphrey K. a (2011): Three systems of insular functional connectivity identified with cluster analysis. Cereb Cortex 21:1498–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Fair D. a, Miezin FM, Cohen AL, Wenger KK, Dosenbach R. a T, Fox MD, Snyder AZ, Vincent JL, Raichle ME, Schlaggar BL, Petersen SE (2007): Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci USA 104:11073–11078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbert T, Pantev C, Wienbruch C, Rockstroh B, Taub E (1995): Increased cortical representation of the fingers of the left hand in string players. Science 270:305–307. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K (2005): A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage 25:1325–1335. [DOI] [PubMed] [Google Scholar]
- Fauvel B, Groussard M, Chételat G, Fouquet M, Landeau B, Eustache F, Desgranges B, Platel H (2014): Morphological brain plasticity induced by musical expertise is accompanied by modulation of functional connectivity at rest. NeuroImage 90:179–188. [DOI] [PubMed] [Google Scholar]
- Forgeard M, Winner E, Norton A, Schlaug G (2008): Practicing a musical instrument in childhood is associated with enhanced verbal ability and nonverbal reasoning. PLoS One 3:e3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis S, Rolls ET, Bowtell R, McGlone F, O'Doherty J, Browning A, Clare S, Smith E (1999): The representation of pleasant touch in the brain and its relationship with taste and olfactory areas. Neuroreport 10:453–459. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Jezzard P, Turner R (1994): Analysis of functional MRI time‐series. Hum Brain Mapp 1:153–171. [Google Scholar]
- Friston KJ, Josephs O, Zarahn E, Holmes AP, Rouquette S, Poline J‐B (2000): To smooth or not to smooth? NeuroImage 12:196–208. [DOI] [PubMed] [Google Scholar]
- Fritz CO, Morris PE, Richler JJ (2012): Effect size estimates: Current use, calculations, and interpretation. J Exp Psychol Gen 141:2–18. [DOI] [PubMed] [Google Scholar]
- Gaser C, Schlaug G (2003): Brain structures differ between musicians and non‐musicians. J Neurosci 23:9240–9245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groussard M, Viader F, Landeau B, Desgranges B, Eustache F, Platel H (2014): The effects of musical practice on structural plasticity: The dynamics of grey matter changes. Brain Cogn 90C:174–180. [DOI] [PubMed] [Google Scholar]
- Haas F, Distenfeld S, Axen K (1986): Effects of perceived musical rhythm on respiratory pattern. J Appl Physiol 61:1185–1191. [DOI] [PubMed] [Google Scholar]
- Heller R, Stanley D, Yekutieli D, Rubin N, Benjamini Y (2006): Cluster‐based analysis of FMRI data. NeuroImage 33:599–608. [DOI] [PubMed] [Google Scholar]
- Herholz SC, Zatorre RJ (2012): Musical training as a framework for brain plasticity: behavior, function, and structure. Neuron 76:486–502. [DOI] [PubMed] [Google Scholar]
- Hughes CML, Franz EA (2007): Experience‐dependent effects in unimanual and bimanual reaction time tasks in musicians. J Mot Behav 39:3–8. [DOI] [PubMed] [Google Scholar]
- Karnath H‐O, Baier B (2010): Right insula for our sense of limb ownership and self‐awareness of actions. Brain Struct Funct 1–7. [DOI] [PubMed] [Google Scholar]
- Kleber B, Birbaumer N, Veit R, Trevorrow T, Lotze M (2007): Overt and imagined singing of an Italian aria. NeuroImage 36:889–900. [DOI] [PubMed] [Google Scholar]
- Kleber B, Veit R, Birbaumer N, Gruzelier J, Lotze M (2010): The brain of opera singers: experience‐dependent changes in functional activation. Cereb Cortex (New York, NY 1991) 20:1144–1152. [DOI] [PubMed] [Google Scholar]
- Kleber B, Zeitouni AG, Friberg A, Zatorre RJ (2013): Experience‐dependent modulation of feedback integration during singing: role of the right anterior insula. J Neurosci 33:6070–6080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleber B, Friberg A, Zeitouni A, Zatorre R (2017): Experience‐dependent modulation of right anterior insula and sensorimotor regions as a function of noise‐masked auditory feedback in singers and nonsingers. NeuroImage 147:97–110. [DOI] [PubMed] [Google Scholar]
- Klein C, Liem F, Hänggi J, Elmer S, Jäncke L (2016): The “silent” imprint of musical training. Hum Brain Mapp 37:536–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelsch S (2014): Brain correlates of music‐evoked emotions. Nat Rev Neurosci 15:170–180. [DOI] [PubMed] [Google Scholar]
- Koelsch S, Grossmann T, Gunter TC, Hahne A, Schroger E, Friederici AD (2003a): Children processing music: electric brain responses reveal musical competence and gender differences. J Cogn Neurosci 15:683–693. [DOI] [PubMed] [Google Scholar]
- Koelsch S, Maess B, Grossmann T, Friederici AD (2003b): Electric brain responses reveal gender differences in music processing. Neuroreport 14:709–713. [DOI] [PubMed] [Google Scholar]
- Kuner R, Flor H (2016): Structural plasticity and reorganisation in chronic pain. Nat Rev Neurosci 18:20–30. [DOI] [PubMed] [Google Scholar]
- Landry SP, Champoux F (2017): Musicians react faster and are better multisensory integrators. Brain Cogn 111:156–162. [DOI] [PubMed] [Google Scholar]
- Lappe C, Trainor LJ, Herholz SC, Pantev C (2011): Cortical plasticity induced by short‐term multimodal musical rhythm training. PLoS One 6:e21493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Noppeney U (2011): Long‐term music training tunes how the brain temporally binds signals from multiple senses. Proc Natl Acad Sci USA 108:E1441–E1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotze M, Scheler G, Tan H‐R, Braun C, Birbaumer N (2003): The musician's brain: functional imaging of amateurs and professionals during performance and imagery. NeuroImage 20:1817–1829. [DOI] [PubMed] [Google Scholar]
- Lovero KL, Simmons AN, Aron JL, Paulus MP (2009): Anterior insular cortex anticipates impending stimulus significance. NeuroImage 45:976–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo C, Guo Z, Lai Y, Liao W, Liu Q, Kendrick KM, Yao D, Li H (2012): Musical training induces functional plasticity in perceptual and motor networks: insights from resting‐state FMRI. PLoS One 7:e36568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo C, Tu S, Peng Y, Gao S, Li J, Dong L, Li G, Lai Y, Li H, Yao D (2014): Long‐term effects of musical training and functional plasticity in salience system. Neural Plast 2014:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner K, Wittmann M (2011): Body signals, cardiac awareness, and the perception of time. Biol Psychol 86:289–297. [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin LQ (2010): Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct 214:655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munte TF, Altenmüller E, Jancke L (2002): The musician's brain as a model of neuroplasticity. Nat Rev 3:473–478. [DOI] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA (2009): The impact of global signal regression on resting state correlations: Are anti‐correlated networks introduced? NeuroImage 44:893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K, Fox MD (2017): Towards a consensus regarding global signal regression for resting state functional connectivity MRI. NeuroImage 154:169–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musacchia G, Sams M, Skoe E, Kraus N (2007): Musicians have enhanced subcortical auditory and audiovisual processing of speech and music. Proc Natl Acad Sci USA 104:15894–15898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutschler I, Schulze‐Bonhage A, Glauche V, Demandt E, Speck O, Ball T (2007): A rapid sound‐action association effect in human insular cortex. PLoS One 2:e259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SM, Dosenbach NUF, Cohen AL, Wheeler ME, Schlaggar BL, Petersen SE (2010): Role of the anterior insula in task‐level control and focal attention. Brain Struct Funct 214:669–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuys R (2012): The insular cortex: a review. Prog Brain Res 195:123–163. [DOI] [PubMed] [Google Scholar]
- Olausson H, Lamarre Y, Backlund H, Morin C, Wallin BG, Starck G, Ekholm S, Strigo I, Worsley K, Vallbo AB, Bushnell MC (2002): Unmyelinated tactile afferents signal touch and project to insular cortex. Nat Neurosci 5:900–904. [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971): The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9:97–113. [DOI] [PubMed] [Google Scholar]
- Palomar‐García M‐Á, Zatorre RJ, Ventura‐Campos N, Bueichekú E, Ávila C (2017): Modulation of functional connectivity in auditory‐motor networks in musicians compared with nonmusicians. Cereb Cortex 27:2768–2778. [DOI] [PubMed] [Google Scholar]
- Pantev C, Roberts LE, Schulz M, Engelien A, Ross B (2001): Timbre‐specific enhancement of auditory cortical representations in musicians. Neuroreport 12:169–174. [DOI] [PubMed] [Google Scholar]
- Paraskevopoulos E, Kuchenbuch A, Herholz SC, Pantev C, Veeraswamy S (2012): Evidence for training‐induced plasticity in multisensory brain structures: An MEG study. Ed. Ramesh Balasubramaniam. PLoS One 7:e36534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penhune VB (2011): Sensitive periods in human development: evidence from musical training. Cortex 47:1126–1137. [DOI] [PubMed] [Google Scholar]
- Petrini K, Dahl S, Rocchesso D, Waadeland CH, Avanzini F, Puce A, Pollick FE (2009): Multisensory integration of drumming actions: musical expertise affects perceived audiovisual asynchrony. Exp Brain Res 198:339–352. [DOI] [PubMed] [Google Scholar]
- Platel H, Price C, Baron JC, Wise R, Lambert J, Frackowiak RSJ, Lechevalier B, Eustache F (1997): The structural components of music perception. A functional anatomical study. Brain 120:229–243. [DOI] [PubMed] [Google Scholar]
- Pleger B, Ruff CC, Blankenburg F, Bestmann S, Wiech K, Stephan KE, Capilla A, Friston KJ, Dolan RJ (2006): Neural coding of tactile decisions in the human prefrontal cortex. J Neurosci 26:12596–12601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugnaghi M, Meletti S, Castana L, Francione S, Nobili L, Mai R, Tassi L (2011): Features of somatosensory manifestations induced by intracranial electrical stimulations of the human insula. Clin Neurophysiol 122:2049–2058. [DOI] [PubMed] [Google Scholar]
- Ragert P, Schmidt A, Altenmüller E, Dinse HR (2004): Superior tactile performance and learning in professional pianists: evidence for meta‐plasticity in musicians. Eur J Neurosci 19:473–478. [DOI] [PubMed] [Google Scholar]
- Riecker A, Ackermann H, Wildgruber D, Dogil G, Grodd W (2000): Opposite hemispheric lateralization effects during speaking and singing at motor cortex, insula and cerebellum. Neuroreport 11:1997–2000. [DOI] [PubMed] [Google Scholar]
- Roesch MR, Olson CR (2007): Neuronal activity related to anticipated reward in frontal cortex: does it represent value or reflect motivation?. Ann N Y Acad Sci 1121:431–446. [DOI] [PubMed] [Google Scholar]
- Rogalsky C, Rong F, Saberi K, Hickok G (2011): Functional anatomy of language and music perception: temporal and structural factors investigated using functional magnetic resonance imaging. J Neurosci 31:3843–3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner B (2011): Fundamentals of Biostatistics. Brooks, Cole: Cengage Learning. [Google Scholar]
- Sanz J, García‐Vera MP, Espinosa R, Fortún M, Vázquez C (2005): Adaptación española del Inventario para la Depresión de Beck‐II (BDI‐II): 3. Propiedades psicométricas en pacientes con trastornos psicológicos. Clínica y Salud 16:121–142. [Google Scholar]
- Satoh M, Takeda K, Murakami Y, Onouchi K, Inoue K, Kuzuhara S (2005): A case of amusia caused by the infarction of anterior portion of bilateral temporal lobes. Cortex 41:77–83. [DOI] [PubMed] [Google Scholar]
- Schirmer‐Mokwa K, Fard PR, Zamorano AM, Finkel S, Birbaumer N, Kleber BA (2015): Evidence for enhanced interoceptive accuracy in professional musicians. Front Behav Neurosci 9:349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider P, Scherg M, Dosch HG, Specht HJ, Gutschalk A, Rupp A (2002): Morphology of Heschl's gyrus reflects enhanced activation in the auditory cortex of musicians. Nat Neurosci 5:688–694. [DOI] [PubMed] [Google Scholar]
- Schulz M, Ross B, Pantev C (2003): Evidence for training‐induced crossmodal reorganization of cortical functions in trumpet players. Neuroreport 14:157–161. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD (2007): Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Merkle FT, Gaus SE, Craig AD, Allman JM, Hof PR, Economo CV (2011): Distinctive neurons of the anterior cingulate and frontoinsular cortex: A historical perspective. Cereb Cortex 22:245–250. [DOI] [PubMed] [Google Scholar]
- Shimizu Y, Sakai KL (2015): Visualization of gray matter myelin and fiber bundles critical for relative pitch: A role of the left posterior long insular cortex. Brain Nerve 67:1147–1155. [DOI] [PubMed] [Google Scholar]
- Sihvonen AJ, Ripollés P, Leo V, Rodríguez‐Fornells A, Soinila S, Särkämö T (2016): Neural basis of acquired amusia and its recovery after stroke. J Neurosci 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small DM, Zatorre RJ, Dagher a, Evans a. C, Jones‐Gotman M (2001): Changes in brain activity related to eating chocolate: from pleasure to aversion. Brain 124:1720–1733. [DOI] [PubMed] [Google Scholar]
- Smith SM, Nichols TE (2009): Threshold‐free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage 44:83–98. [DOI] [PubMed] [Google Scholar]
- Song X‐W, Dong Z‐Y, Long X‐Y, Li S‐F, Zuo X‐N, Zhu C‐Z, He Y, Yan C‐G, Zang Y‐F (2011): REST: a toolkit for resting‐state functional magnetic resonance imaging data processing. PLoS One 6:e25031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE (1970): Manual for the state‐trait anxiety inventory.
- Steele CJ, Bailey JA, Zatorre RJ, Penhune VB (2013): Early musical training and white‐matter plasticity in the corpus callosum: evidence for a sensitive period. J Neurosci 33:1282–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz A, Stang A, Kornhuber M, Röllinghoff M, Delank K‐S, Altenmüller E (2014): From embouchure problems to embouchure dystonia? A survey of self‐reported embouchure disorders in 585 professional orchestra brass players. Int Arch Occup Environ Health 87:783–792. [DOI] [PubMed] [Google Scholar]
- Strait DL, Kraus N (2011): Can you hear me now? Musical training shapes functional brain networks for selective auditory attention and hearing speech in noise. Front Psychol 2:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S, Kirino E (2016a): Functional connectivity of the dorsal striatum in female musicians. Front Hum Neurosci 10:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S, Kirino E (2016b): Functional connectivity of the precuneus in Female University students with long‐term musical training. Front Hum Neurosci 10:328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor KS, Seminowicz D. a, Davis KD (2009): Two systems of resting state connectivity between the insula and cingulate cortex. Hum Brain Mapp 30:2731–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaut M (2003): Neural basis of rhythmic timing networks in the human brain. Ann N Y Acad Sci 999:364–373. [DOI] [PubMed] [Google Scholar]
- Thaut M, Trimarchi P, Parsons L (2014): Human brain basis of musical rhythm perception: Common and distinct neural substrates for meter, tempo, and pattern. Brain Sci 4:428–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio–Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single–subject brain. Neuroimage 15:273–289. [DOI] [PubMed] [Google Scholar]
- Uddin LQ (2014): Salience processing and insular cortical function and dysfunction. Nat Rev Neurosci 16:55–61. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Kinnison J, Pessoa L, Anderson ML (2014): Beyond the tripartite cognition‐emotion‐interoception model of the human insular cortex. J Cogn Neurosci 26:16–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Lindquist M, Kaplan L (2007): Meta‐analysis of functional neuroimaging data: current and future directions. Soc Cogn Affect Neurosci 2:150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiech K, Lin C, Brodersen KH, Bingel U, Ploner M, Tracey I (2010): Anterior insula integrates information about salience into perceptual decisions about pain. J Neurosci 30:16324–16331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CW, DeYoung PN, Liu TT (2016): Differences in the resting‐state fMRI global signal amplitude between the eyes open and eyes closed states are related to changes in EEG vigilance. NeuroImage 124:24–31. [DOI] [PubMed] [Google Scholar]
- Wong CW, Olafsson V, Tal O, Liu TT (2013): The amplitude of the resting‐state fMRI global signal is related to EEG vigilance measures. NeuroImage 83:983–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong PCM, Parsons LM, Martinez M, Diehl RL (2004): The role of the insular cortex in pitch pattern perception: The effect of linguistic contexts. J Neurosci 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo CW, Krishnan A, Wager TD (2014): Cluster‐extent based thresholding in fMRI analyses: pitfalls and recommendations. NeuroImage 91:412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakunina N, Kim TS, Tae WS, Kim SS, Nam EC (2016): Applicability of the sparse temporal acquisition technique in resting‐state brain network analysis. Am J Neuroradiol 37:515–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C‐G, Wang X‐D, Zuo X‐N, Zang Y‐F (2016): DPABI: Data processing & analysis for (resting‐state) brain imaging. Neuroinformatics 14:339–351. [DOI] [PubMed] [Google Scholar]
- Zamorano AM, Riquelme I, Kleber B, Altenmüller E, Hatem SM, Montoya P (2014): Pain sensitivity and tactile spatial acuity are altered in healthy musicians as in chronic pain patients. Front Hum Neurosci 8:1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate JM, Delhommeau K, Wood S, Zatorre RJ (2010): Vocal accuracy and neural plasticity following micromelody‐discrimination training. PLoS One 5:e11181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate JM, Zatorre RJ (2008): Experience‐dependent neural substrates involved in vocal pitch regulation during singing. NeuroImage 40:1871–1887. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ (2015): Musical pleasure and reward: mechanisms and dysfunction. Ann N Y Acad Sci 1337:202–211. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Chen JL, Penhune VB (2007): When the brain plays music: auditory‐motor interactions in music perception and production. Nat Rev Neurosci 8:547–558. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Evans A, Meyer E (1994): Neural mechanisms underlying melodic perception and memory for pitch. J Neurosci 14:1908–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatorre RJ, Mondor TA, Evans AC (1999): Auditory attention to space and frequency activates similar cerebral systems. NeuroImage 10:544–554. [DOI] [PubMed] [Google Scholar]
- Zimmerman E, Lahav A (2012): The multisensory brain and its ability to learn music. Ann N Y Acad Sci 1252:179–184. [DOI] [PubMed] [Google Scholar]


