ABSTRACT
The endothelium physically separates blood from surrounding tissue and yet allows for the regulated passage of nutrients, waste, and leukocytes into and out of the circulation. Trans-endothelium flux occurs across endothelial cells (transcellular) and between endothelial cells (paracellular). Paracellular endothelial barrier function depends on the regulation of cell-cell junctions. Interestingly, a functional relationship between cell-cell junctions and cell-matrix adhesions has long been appreciated but the molecular mechanisms underpinning this relationship are not fully understood. Here we review the evidence that supports the notion that cell-matrix interactions contribute to the regulation of cell-cell junctions, focusing primarily on the important adherens junction protein VE-cadherin. In particular, we will discuss recent insights gained into how integrin signaling impacts VE-cadherin stability in adherens junctions and endothelial barrier function.
KEYWORDS: Endothelial barrier, integrin, adherens junctions
Introduction
The endothelium acts as a dynamically regulated barrier which facilitates the transport of protein, fluid and blood cells throughout the body. Endothelial cells (ECs) respond to extracellular cues to restrict or promote the passage of circulating components into the tissue. Dysregulation of this process contributes to many common human pathological conditions including ischemia, cancer, diabetes, and sepsis.1,2 The permeability of this barrier can be regulated through both transcellular and paracellular mechanisms. Whereas transcellular permeability is dependent on vesicular transport of molecules across endothelial cells, paracellular permeability is regulated by changes in endothelial cell-cell adhesion. Distinct adhesive structures physically couple adjacent ECs including tight junctions (TJs) and adherens junctions (AJs).2 AJs are adhesive structures localized to the apical side of the endothelium and depend upon the function and stability of the adhesion molecule vascular endothelial cadherin (VE-cad).3 In inflammatory or pathological conditions, stimuli such as TNF-α, VEGF, and thrombin induce reversible weakening of the endothelial barrier by promoting cytoskeletal rearrangement that leads to AJ dissociation and VE-cad internalization.4
Considering that endothelial barrier function is responsive to a wide variety of biochemical and mechanical inputs, its regulation is, unsurprisingly, complex. One example of this complexity is the relationship between cell-matrix adhesions and cell-cell adhesions.5 Endothelial cells are anchored basally to the basement membrane, a sheet of extracellular matrix rich in laminin and collagen IV, primarily through integrin adhesion complexes commonly referred to as focal adhesions (FAs).6 The cross-regulation between AJs and FAs that contributes to endothelial cell adhesion and signaling has been discussed in several excellent reviews.5,7,8 Here, we will focus on the results of several recent studies that have highlighted how integrins contribute to the maintenance of AJs and vascular barrier function.
Adherens junctions regulation of the vascular barrier
Homophilic interactions between VE-cad expressed on adjacent ECs maintain vascular permeability.9,10 VE-cad is stabilized at junctions through its interaction with p120 catenin (p120) and is tethered to the actin cytoskeleton through an interaction with the catenin complex.11–14 β-catenin, in complex with actin-bound α-catenin, directly binds the cytoplasmic tail of VE-cad15,16 conferring mechanosensitivity of AJs to cytoskeletal perturbations17,18 (Figure 1). The catenin/actin linkage of VE-cad also acts as a local signaling hub for small GTPases which coordinate the stability of junctional cadherin complexes by regulating the stability of cortical actin stress fibers.32 The requirement of VE-cad stabilization in adherens junctions was eloquently demonstrated in vivo by generating mice expressing a VE-cad/α-catenin fusion protein which is retained and resistant to endocytosis from junctions.28 Mice expressing this VE-cad/α-catenin fusion protein were resistant to leukocyte extravasation in some tissues and agonist-induced models of hyperpermeability. We will briefly highlight the distinct mechanisms which promote hyperpermeability in disease states and their ultimate convergence in regulating the stability of junctional VE-cad.
Figure 1.
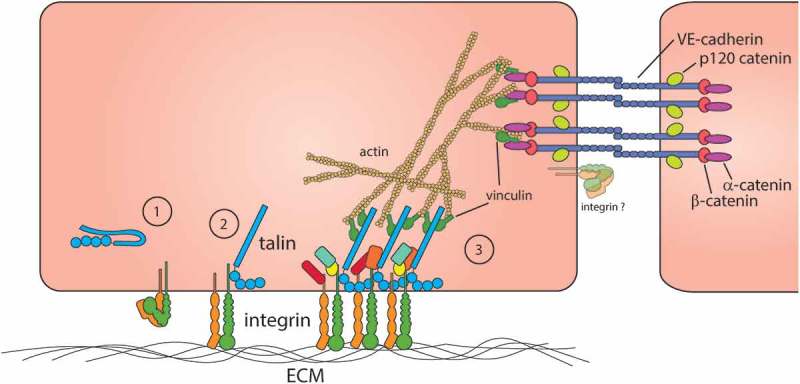
Molecular components of adherens junctions and integrin-containing cell-ECM adhesions in endothelial cells. (1) Cytoplasmic talin is thought to be adopt an auto-inhibited confirmation that precludes talin binding to integrin.19–22 (2) Binding of talin to the integrin β integrin cytoplasmic domain induces a conformational change in the integrin associated with increased integrin ligand binding, activation.23–27 (3) Ligated integrins cluster and promote the recruitment of adapter, signaling, and actin-binding proteins including talin and vinculin.25 Adherens junctions comprised principally of VE-cadherin mediate cell-cell adhesion and endothelial barrier function. VE-cadherin is linked indirectly to the actin cytoskeleton by binding to β-catenin that in turn binds to the actin-binding protein, α-catenin.2,4,9,28,29 Notably, both integrin and cadherin adhesion complexes share important components, including vinculin, that function to link these complexes to the actin cytoskeleton. Integrins themselves have been observed at the EC junctions30,31 but the significance of this observation is unclear.
In conditions that promote vascular permeability, AJs become destabilized, disassembled and VE-cad is endocytosed through a number of distinct mechanisms. In many cancers, increased permeability is triggered by the secretion of soluble vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF) and other pro-angiogenic factors. In a well-described process, VEGF binds to vascular endothelial growth factor 2 (VEGFR2) which leads to Src-dependent PAK-mediated phosphorylation of VE-cad at Ser665. VE-cad phosphorylation at Ser665 and Tyr685 is followed by β-arrestin2 binding which induces clathrin-dependent endocytosis of VE-cad.33,34 This pathway promotes cell migration, cytoskeletal rearrangement, and AJ disassembly. Pro-inflammatory molecules present in conditions of chronic inflammation or sepsis such as TNF-α and LPS can also promote endothelial permeability. Treatment of ECs in vitro with TNF-α generates tensile structures termed focal adherens junctions (FAJs) due to increased cell contraction.35 Secreted TNF-α is known to promote Fyn kinase-dependent phosphorylation of VE-cad cytoplasmic tail and VE-cad internalization which impairs pulmonary EC barrier function in vitro.36,37 Junctional disassembly induced by VEGF and TNF-α signal through independent pathways which converge at Src-family kinase-dependent VE-cad phosphorylation. Therefore, phosphatases like vascular endothelial protein tyrosine phosphatase (VE-PTP) play a critical role in maintaining junctional VE-cad38 as its interaction with VE-cad stabilizes junctional cadherin pools in resting and inflammatory states.39,40 The disassembly of cell-cell junctions in both pro-angiogenic and inflammatory contexts are coupled with the reorganization of the actin cytoskeleton. In resting states, a delicate tensional balance at cell-cell junctions permit the formation of linear AJs connected to circumferential actin that are regulated by small GTPases such as Rap1,41,42 Rac,33,43,44 and Rho.45,46 Agonist-induced cell contraction increases tension at AJs and leads to the formation of zipper-like AJs. Collectively, these data highlight the importance of VE-Cadherin stabilization at cell-cell contacts as well as the regulation of cell-cell tension in maintaining linear AJ organization in resting conditions.
Integrins signaling in ECs
Integrins are transmembrane adhesion receptors expressed on the surface of most mammalian cell types. Integrin subunits function as heterodimers consisting of one of 18 α- and one of the eight β-subunits which dictate ligand specificity.6 The most well-studied integrin classes in ECs engage ECM components by binding directly to collagen (α1β1, α2β1), fibronectin (α5β1, α4β1, αvβ3), vitronectin (αvβ3, avβ5) and laminin (α3β1, α6β1).47 In addition to functioning as important adhesion receptors, integrins are bi-directional signaling hubs involved in numerous fundamental cellular processes including cell migration, survival, and proliferation. On one hand, integrin affinity is regulated through so-called “inside-out” integrin signaling (integrin activation)48 whereby extracellular signals are transduced into the cell through cell-surface receptors (e.g. receptor tyrosine kinases, G-protein-coupled receptors) that in turn ultimately lead to the binding of cytoplasmic, integrin activating proteins such as talin23,24,49 and kindlin50 to the β-integrin cytoplasmic tail (Figure 1). On the other hand, integrin “outside-in” signaling occurs in response to integrin binding extracellular ligands and subsequent activation of cytoplasmic signaling pathways important for the maturation of adhesion complexes6,25 (Figure 1). For example, outside-in integrin signaling leads to the recruitment of actin-binding proteins (e.g. talin, vinculin, α-actinin, tensin, filamin) and adaptor proteins (of which there are more than 40) that either directly or indirectly link integrin to the actin cytoskeleton.51 In addition, in response to ligand binding, tyrosine phosphorylation of the β integrin cytoplasmic tail recruits adaptor proteins and non-receptor tyrosine kinases. In this way, integrins serve as a hub for signaling pathways essential to diverse and fundamental cellular functions including PI3K and MAPK.6 Integrins can also activate Ras and Rho family small GTPases that profoundly impact cytoskeletal organization and dynamics. Cytoskeletal reorganization requires the disassembly and reassembly of integrin adhesion complexes, processes that are regulated spatially and temporally by complex and context-dependent signaling events.52,53 The role of integrins and their subunit-specific requirements during embryogenesis and postnatal angiogenesis have been described previously in detail.54–56 However, new details on the distinct roles of β1 integrin and β3/β5 integrins in regulating the endothelial barrier have recently begun to be elucidated.
Requirements of β3 and β5 integrins in EC barrier function
Although the requirement of β3 integrin in developmental and postnatal angiogenesis has been well-studied,57–60 considerably less is known about the function of β3 integrin in EC barrier function. Early evidence indicated that the deletion of β3 in mice resulted in increased VEGF and LPS-induced Evan’s Blue leakage in dermal and lung/intestinal vessels, respectively,61 whereas baseline permeability appeared similar relative to control littermates. Eloquent studies by Su et al.62 showed that the permeability-inducing effects of VEGF, TGF-β and thrombin on pulmonary ECs were exacerbated by pre-treatment with αvβ3 blocking antibodies consistent with the notion that β3 integrin promotes EC barrier. Furthermore, treating HUVECs with the barrier-enhancing agent sphingosine 1-phosphate promoted αvβ3 localization to cell-cell contacts and sites of cortical actin and this was inhibited by pre-treatment with an αvβ3 function-blocking antibody. Interestingly, activation of αvβ3 with low doses of the cyclic RGD peptide, cilengitide, reduced HUVEC monolayer permeability likely by promoting Src kinase-mediated phosphorylation of VE-cad at Y731 and Y658 thereby promoting the internalization of VE-cad.63 The mechanism(s) underlying these observed effects of cilengitide on HUVEC barrier function are unclear but could include indirectly inhibiting β1 integrin through trans-dominant inhibition.63,64 Interestingly, the role of αvβ5 in response to sepsis-induced leak contrasts with the barrier-protective role of αvβ3 in LPS-induced leak. Antibody blockade of HUVEC αvβ5 attenuated LPS-induced barrier dysfunction in vitro while β5 knockout mice exhibit increased survival in a cecal ligation model of sepsis relative to WT mice.65 It was proposed that blockade of β5 integrin mitigates the induction of cytoskeletal contraction in these contexts thereby stabilizing cell-cell junctions. As β5 integrin was deleted globally in the mice used in this study, the relative contributions of β5 integrin in ECs versus other cell types remains an open question. Collectively, these data point to important, and likely distinct, roles for β3 and β5 integrin in regulating the endothelial barrier in physiological and pathophysiological conditions.
Role of β1 integrins in EC barrier function
A role of β1 integrins in endothelial barrier function was first discovered when antibodies specific to α5β1 integrin revealed a localization pattern of this receptor at cell-cell junctions in addition to its well-described localization to FAs.30 In contrast, cell-cell junction localization of αvβ3 integrin was not observed. Furthermore, antibody blockade of α5β1, but not αvβ3, impaired monolayer permeability in vitro. A recent study by Yamamoto et al. demonstrated that EC-specific deletion of β1 integrin during postnatal development promoted VE-cadherin internalization and cell-cell junction disassembly.66 These investigators convincingly demonstrated that junctional disassembly in β1 integrin-deficient ECs was due to reduced Rap1/MRCK and Rho/Rho-kinase activity that impaired VE-cad trafficking to cell-cell junctions. Intriguingly, work by Hakanpaa et al. showed that the deletion of a single β1 integrin allele in ECs of established blood vessels did not alter basal permeability but rather protected β1 EC heterozygous mice from LPS-induced hyperpermeability compared to wild type littermates.67 Furthermore, pharmacological inhibition of β1 integrin also mitigated LPS-induced tracheal permeability. Prior to LPS treatment, wildtype mice were prophylactically administered either a β1 blocking antibody (HMβ1-1) or an isotype nonimmune IgG control with the former group showing reduced levels of FITC-dextran extravasation relative to control.67 It was therefore proposed that in quiescent endothelium β1 integrin predominantly localizes to FAs whereas inflammatory molecules acting in an angiopoietin-2-dependent manner induce β1 integrin association with tensin at fibrillar adhesions. β1 integrin-positive fibrillar adhesions in turn promote cytoskeletal tension that alters cell-cell junctions and increases vascular permeability. Interestingly, deletion of talin1 (Tln1), a key modulator of integrin activation, in the ECs of established vessels induced intestinal vascular leak, cell-cell junction disassembly and lethality 16–21 days after Tln1 deletion.31 Loss of EC Tln1 reduced β1 integrin activation and cell-cell junctions of tln1-depleted ECs displayed significant VE-cadherin disorganization. Deletion of tln1 in vitro resulted in reduced monolayer barrier function measured by electrical cell-substrate impedance sensing (ECIS). Changes in junctional integrity were in part due to increased cytoskeletal contraction in response to increased Rho/MLC activity in talin-deficient cells. These changes in junctional organization and diminished barrier function in talin-depleted ECs were rescued by antibody-mediated β1 integrin activation and phenocopied when ECs were treated with β1 integrin blocking antibody. Together, the results above point to an important role of β1 integrin in maintaining vascular permeability in both quiescent and pathological states. These findings collectively implicate the importance of β1 integrin activation in maintaining junctional integrity but it is likely that the absence of EC talin in established vessels inhibits the activation of integrin subclasses in addition to β1 that may be required for intestinal microvascular stability.
Integrin adhesion signaling-dependent regulation of barrier function
All aspects of integrin function depend on interactions with cytoplasmic adaptor proteins. Therefore, important insights into the role of integrins in EC barrier function can be gleaned from studies focused on integrin-associated proteins and protein complexes. Integrin adhesion promotes the activation of focal adhesion kinase (FAK), a non-receptor tyrosine kinase that also functions as an important signaling hub required for FA maturation and disassembly during cell migration.68–70 Chen and colleagues discovered that inducible EC-specific FAK deletion or expression of a kinase-dead mutant FAK inhibited VEGF-induced dermal permeability.71 This study determined that VEGF treatment promoted the localization of FAK to cell-cell junctions where it interacts with VE-Cad to phosphorylate β-catenin thus promoting junctional disassembly. Recent work exploring the role of kindlin2, a β-integrin interacting protein implicated in integrin bidirectional signaling,72 revealed a similar interaction with β and γ-catenin that is necessary for the maintenance of the vascular barrier in tracheal vessels.73 Mice heterozygous for kindlin-2 exhibited exacerbated leak and disassembly of cell-cell junctions in response to LPS relative to WT littermates. It is important to note that the interaction of kindlin-2 with the catenin-complex occurs through a different FERM subdomain than those required for its interaction with the β-integrin tail. Indeed, kindlin2 mutations that disrupt integrin interactions did not alter endothelial barrier function in vitro.73 Consistent with these observations, kindlin2 biotin proximity labeling indicated that kindlin2 may interact with proteins in epithelial cell-cell junctions and observed kindlin2 localized to the junctions of MDCK cells.74 Another important regulator of EC integrin activation is the small GTPase Rap1, specifically the isoforms Rap1A/B.75 Lakshmikanthan et al. reported that ECs lacking Rap1B exhibit reduced integrin activation and reduced VEGF-VEGFR2 signaling. Specifically, the ablation of Rap1B reduced αvβ3 integrin-mediated co-activation of VEGFR2 signaling.76 Recent work from this group revealed that while Rap1B is the predominant isoform required for angiogenesis, Rap1B and Rap1A have distinct roles in normal barrier maintenance and agonist-induced hyperpermeability.77 Deletion of EC Rap1A, but not Rap1B, reduced basal permeability in lung vasculature, while EC-specific ablation of Rap1B attenuated vessel leak in a VEGF-induced model of hyperpermeability. Curiously, deletion of either isoform increased lethality in an LPS-induced sepsis model relative to vehicle-treated mice. These findings will require future studies to determine the context-specific roles of Rap1 (pro-angiogenic vs. pro-inflammatory vs. homeostatic barrier maintenance) and to establish to what extent Rap1 function in EC barrier regulation is integrin-dependent.
Future considerations
Integrins are attractive therapeutic targets particularly in hematopoietic cells.78 A better understanding of the mechanisms underlying integrin signaling in the maintenance and regulation of vascular permeability may reveal opportunities for pharmacological intervention in a number of human diseases. We conclude by highlighting fundamental topics that should be considered in future endeavors. First, does the repertoire of integrin expressed in particular endothelium differ in a) specific organs, b) blood vessel types (arteries/veins/capillaries), c) endothelium types (continuous, sinusoidal and fenestrated)? If so, what is the functional significance of these specifications? Second, as discussed above, our lab has reported that regulation of integrin affinity modulation (integrin activation) contributes to the regulation of endothelial barrier function in vitro. Is endothelial cell integrin activation important in vivo? Finally, examining the role of integrin expression and signaling in the context of vascular inflammation will be an important area of investigation going forward.
As vascular organization and function are organ-specific,79,80 it is likely that endothelial integrins may also have organ-specific roles in regulating the vascular barrier. An example of this can be found in recent work by Bix and colleagues in which they induced deletion of EC α5 integrin, which has been previously shown to have no effect on established vessels, to find that mice lacking α5 integrin in endothelium were resistant to experimental ischemic stroke.81 EC α5 knockout mice exhibited a less permeable blood-brain-barrier in response to stroke as measured by dye extravasation into cerebral tissue. Similarly, studies examining the roles of β1, β3 and β5 integrins in barrier function described above appear to have specificity, as noted, for intestinal, dermal and lung vascular barrier function. An additional level of vascular specification can be found in capillary endothelium in different organs:82–84 continuous (brain, lung), fenestrated (intestinal, endocrine organs)85 and sinusoidal (liver). Continuous endothelium in the brain is required for the tight barrier of the BBB whereas fenestrated endothelium in the intestine or sinusoidal endothelium in the liver accommodate the passage of blood cells and circulating components into interstitial tissues. A more recent example of vascular-bed specific barrier regulation is also observed in the blood-testis-barrier wherein Rap1 has been implicated in the regulation of this barrier.86,87 Given the spectrum of barrier tightness in these contexts, it is not surprising that integrin function may differ in specific types of endothelium.
The mechanisms underlying how integrins and integrin-associated proteins (talin, kindlin2, Rap1) modulate EC barrier function are yet to be clearly defined. Clearly, each of these proteins can function at cell-ECM adhesion complexes to alter cytoskeleton dynamics and intracellular tension that can then impact VE-cadherin organization, stability, and function in adherens junctions32,35,88,89 (Figure 1). Alternatively, integrins or these integrin-associated proteins may function at the cell-cell junction to more directly impact VE-cadherin. Of course, these two concepts are not mutually exclusive and going forward it will be interesting to delineate the relative contribution of these pathways on EC barrier function. In addition, questions remain regarding how different integrin heterodimer classes (αxβ1, αvβ3, αvβ5) contribute to the regulation of the endothelial barrier. For example, whereas inhibition of β1 integrin appears to protect against LPS-induced vascular leak, shRNA-mediated deletion of α5 integrin in thrombin-stimulated cells phenocopied β1 integrin deletion in these cells whereas prophylactic treatment of mice with anti-α4 integrin antibody in the LPS-model of hyperpermeability does not mirror the protective effects seen in β1-heterozygous mice/β1 integrin blocking antibody.67 Collectively, this suggests specificity for α5β1 integrin in this context whereas α4β1 appears to be dispensable in regulation of the barrier in response to LPS. However, it may also be important to differentiate between integrin activation and integrin expression in regulation of the barrier. Indeed, EC-tln1 knockout mice in which EC β1 integrin activation is impaired, but express normal levels of EC β1 integrin surface protein, exhibit pathological leak in the intestinal microvasculature. It is therefore likely that integrin activation must be finely tuned in certain physiological and pathological contexts, a hypothesis that should be explored in future investigations.
Funding Statement
This work was supported in part by National Institutes of Health (NIH) National Heart, Lung, and Blood Institute grant [HL117061 (B.G.P.), F31HL136194 (F.E.P.)].
References
- 1.Dejana E, Giampietro C.. Vascular endothelial-cadherin and vascular stability. Curr Opin Hematol. 2012;19:1–9. [DOI] [PubMed] [Google Scholar]
- 2.Dejana E. Tournier-Lasserve E and Weinstein BM. The control of vascular integrity by endothelial cell junctions: molecular basis and pathological implications. Dev Cell. 2009;16:209–221. [DOI] [PubMed] [Google Scholar]
- 3.Giannotta M, Trani M, Dejana E. VE-cadherin and endothelial adherens junctions: active guardians of vascular integrity. Dev Cell. 2013;26:441–454. [DOI] [PubMed] [Google Scholar]
- 4.Lampugnani MG, Dejana E, Giampietro C. Vascular endothelial (VE)-cadherin, endothelial adherens junctions, and vascular disease. Cold Spring Harb Perspect Biol. 2018;10:pii: a029322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mui KL, Chen CS, Assoian RK. The mechanical regulation of integrin-cadherin crosstalk organizes cells, signaling and forces. J Cell Sci. 2016;129:1093–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz MA, DeSimone DW. Cell adhesion receptors in mechanotransduction. Curr Opin Cell Biol. 2008;20:551–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ross TD, Coon BG, Yun S, Baeyens N, Tanaka K, Ouyang M, Schwartz MA. Integrins in mechanotransduction. Curr Opin Cell Biol. 2013;25:613–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carmeliet P, Lampugnani MG, Moons L, Breviario F, Compernolle V, Bono F, Balconi G, Spagnuolo R, Oosthuyse B, Dewerchin M, et al. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell. 1999;98:147–157. [DOI] [PubMed] [Google Scholar]
- 10.Crosby CV, Fleming PA, Argraves WS, Corada M, Zanetta L, Dejana E, Drake CJ. VE-cadherin is not required for the formation of nascent blood vessels but acts to prevent their disassembly. Blood. 2005;105:2771–2776. [DOI] [PubMed] [Google Scholar]
- 11.Tanihara H, Kido M, Obata S, Heimark RL, Davidson M, St John T, Suzuki S. Characterization of cadherin-4 and cadherin-5 reveals new aspects of cadherins. J Cell Sci. 1994;107:1697–1704. [DOI] [PubMed] [Google Scholar]
- 12.Peifer M, Berg S, Reynolds AB. A repeating amino acid motif shared by proteins with diverse cellular roles. Cell. 1994;76:789–791. [DOI] [PubMed] [Google Scholar]
- 13.Chiasson CM, Wittich KB, Vincent PA, Faundez V, Kowalczyk AP. p120-catenin inhibits VE-cadherin internalization through a Rho-independent mechanism. Mol Biol Cell. 2009;20:1970–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nanes BA, Chiasson-MacKenzie C, Lowery AM, Ishiyama N, Faundez V, Ikura M, Vincent PA, Kowalczyk AP. p120-catenin binding masks an endocytic signal conserved in classical cadherins. J Cell Biol. 2012;199:365–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Desai R, Sarpal R, Ishiyama N, Pellikka M, Ikura M, Tepass U. Monomeric alpha-catenin links cadherin to the actin cytoskeleton. Nat Cell Biol. 2013;15:261–273. [DOI] [PubMed] [Google Scholar]
- 16.Pokutta S, Choi HJ, Ahlsen G, Hansen SD, Weis WI. Structural and thermodynamic characterization of cadherin.beta-catenin.alpha-catenin complex formation. J Biol Chem. 2014;289:13589–13601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Breviario F, Caveda L, Corada M, Martin-Padura I, Navarro P, Golay J, Introna M, Gulino D, Lampugnani MG, Dejana E. Functional properties of human vascular endothelial cadherin (7B4/cadherin-5), an endothelium-specific cadherin. Arterioscler Thromb Vasc Biol. 1995;15:1229–1239. [DOI] [PubMed] [Google Scholar]
- 18.Vestweber D, Winderlich M, Cagna G, Nottebaum AF. Cell adhesion dynamics at endothelial junctions: VE-cadherin as a major player. Trends Cell Biol. 2009;19:8–15. [DOI] [PubMed] [Google Scholar]
- 19.Goksoy E, Ma YQ, Wang X, Kong X, Perera D, Plow EF, Qin J. Structural basis for the autoinhibition of talin in regulating integrin activation. Mol Cell. 2008;31:124–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goult BT, Xu XP, Gingras AR, Swift M, Patel B, Bate N, Kopp PM, Barsukov IL, Critchley DR, Volkmann N, et al. Structural studies on full-length talin1 reveal a compact auto-inhibited dimer: implications for talin activation. J Struct Biol. 2013;184:21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goult BT, Bate N, Anthis NJ, Wegener KL, Gingras AR, Patel B, Barsukov IL, Campbell ID, Roberts GC, Critchley DR. The structure of an interdomain complex that regulates talin activity. J Biol Chem. 2009;284:15097–15106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dedden D, Schumacher S, Kelley CF, Zacharias M, Biertumpfel C, Fassler R, Mizuno N. The architecture of Talin1 reveals an autoinhibition mechanism. Cell. 2019;179:e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calderwood DA, Zent R, Grant R, Rees DJ. Hynes RO and Ginsberg MH. The Talin head domain binds to integrin beta subunit cytoplasmic tails and regulates integrin activation. J Biol Chem. 1999;274:28071–28074. [DOI] [PubMed] [Google Scholar]
- 24.Tadokoro S, Shattil SJ, Eto K, Tai V, Liddington RC, de Pereda JM, Ginsberg MH, Calderwood DA. Talin binding to integrin beta tails: a final common step in integrin activation. Science. 2003;302:103–106. [DOI] [PubMed] [Google Scholar]
- 25.Calderwood DA, SJ S, Ginsberg MH. Integrins and actin filaments: reciprocal regulation of cell adhesion and signaling. J Biol Chem. 2000;275:22607–22610. [DOI] [PubMed] [Google Scholar]
- 26.Garcia-Alvarez B, de Pereda JM, Calderwood DA, Ulmer TS, Critchley D, Campbell ID, Ginsberg MH, Liddington RC. Structural determinants of integrin recognition by talin. Mol Cell. 2003;11:49–58. [DOI] [PubMed] [Google Scholar]
- 27.Petrich BG, Marchese P, Ruggeri ZM, Spiess S, Weichert RA, Ye F, Tiedt R, Skoda RC, Monkley SJ, Critchley DR, et al. Talin is required for integrin-mediated platelet function in hemostasis and thrombosis. J Exp Med. 2007;204:3103–3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schulte D, Kuppers V, Dartsch N, Broermann A, Li H, Zarbock A, Kamenyeva O, Kiefer F, Khandoga A, Massberg S, et al. Stabilizing the VE-cadherin-catenin complex blocks leukocyte extravasation and vascular permeability. Embo J. 2011;30:4157–4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corada M, Mariotti M, Thurston G, Smith K, Kunkel R, Brockhaus M, Lampugnani MG, Martin-Padura I, Stoppacciaro A, Ruco L, et al. Vascular endothelial-cadherin is an important determinant of microvascular integrity in vivo. Proc Natl Acad Sci U S A. 1999;96:9815–9820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lampugnani MG, Resnati M, Dejana E, Marchisio PC. The role of integrins in the maintenance of endothelial monolayer integrity. J Cell Biol. 1991;112:479–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pulous FE, Grimsley-Myers CM, Kansal S, Kowalczyk AP, Petrich BG. Talin-dependent integrin activation regulates VE-cadherin localization and endothelial cell barrier function. Circ Res. 2019;124:891–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dorland YL, Malinova TS, van Stalborch AM, Grieve AG, van Geemen D, Jansen NS, de Kreuk BJ, Nawaz K, Kole J, Geerts D, et al. The F-BAR protein pacsin2 inhibits asymmetric VE-cadherin internalization from tensile adherens junctions. Nat Commun. 2016;7:12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gavard J, Gutkind JS. VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nat Cell Biol. 2006;8:1223–1234. [DOI] [PubMed] [Google Scholar]
- 34.Wallez Y, Cand F, Cruzalegui F, Wernstedt C, Souchelnytskyi S, Vilgrain I, Huber P. Src kinase phosphorylates vascular endothelial-cadherin in response to vascular endothelial growth factor: identification of tyrosine 685 as the unique target site. Oncogene. 2007;26:1067–1077. [DOI] [PubMed] [Google Scholar]
- 35.Huveneers S, Oldenburg J, Spanjaard E, van der Krogt G, Grigoriev I, Akhmanova A, Rehmann H, de Rooij J. Vinculin associates with endothelial VE-cadherin junctions to control force-dependent remodeling. J Cell Biol. 2012;196:641–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Angelini DJ, Hyun SW, Grigoryev DN, Garg P, Gong P, Singh IS, Passaniti A, Hasday JD, Goldblum SE. TNF-alpha increases tyrosine phosphorylation of vascular endothelial cadherin and opens the paracellular pathway through fyn activation in human lung endothelia. Am J Physiol Lung Cell Mol Physiol. 2006;291:L1232–45. [DOI] [PubMed] [Google Scholar]
- 37.Adam AP, Lowery AM, Martino N, Alsaffar H, Vincent PA. Src family kinases modulate the loss of endothelial barrier function in response to TNF-alpha: crosstalk with p38 signaling. PLoS One. 2016;11:e0161975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nottebaum AF, Cagna G, Winderlich M, Gamp AC, Linnepe R, Polaschegg C, Filippova K, Lyck R, Engelhardt B, Kamenyeva O, et al. VE-PTP maintains the endothelial barrier via plakoglobin and becomes dissociated from VE-cadherin by leukocytes and by VEGF. J Exp Med. 2008;205:2929–2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Broermann A, Winderlich M, Block H, Frye M, Rossaint J, Zarbock A, Cagna G, Linnepe R, Schulte D, Nottebaum AF, et al. Dissociation of VE-PTP from VE-cadherin is required for leukocyte extravasation and for VEGF-induced vascular permeability in vivo. J Exp Med. 2011;208:2393–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frye M, Dierkes M, Kuppers V, Vockel M, Tomm J, Zeuschner D, Rossaint J, Zarbock A, Koh GY, Peters K, et al. Interfering with VE-PTP stabilizes endothelial junctions in vivo via Tie-2 in the absence of VE-cadherin. J Exp Med. 2015;212:2267–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fukuhara S, Sakurai A, Sano H, Yamagishi A, Somekawa S, Takakura N, Saito Y, Kangawa K, Mochizuki N. Cyclic AMP potentiates vascular endothelial cadherin-mediated cell-cell contact to enhance endothelial barrier function through an Epac-Rap1 signaling pathway. Mol Cell Biol. 2005;25:136–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Noda K, Zhang J, Fukuhara S, Kunimoto S, Yoshimura M, Mochizuki N. Vascular endothelial-cadherin stabilizes at cell-cell junctions by anchoring to circumferential actin bundles through alpha- and beta-catenins in cyclic AMP-Epac-Rap1 signal-activated endothelial cells. Mol Biol Cell. 2010;21:584–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Wetering S, van Buul JD, Quik S, Mul FP, Anthony EC, Ten Klooster JP, Collard JG, Hordijk PL. Reactive oxygen species mediate Rac-induced loss of cell-cell adhesion in primary human endothelial cells. J Cell Sci. 2002;115:1837–1846. [DOI] [PubMed] [Google Scholar]
- 44.Timmerman I, Heemskerk N, Kroon J, Schaefer A, van Rijssel J, Hoogenboezem M, van Unen J, Goedhart J, Gadella TW Jr., Yin T, et al. A local VE-cadherin and Trio-based signaling complex stabilizes endothelial junctions through Rac1. J Cell Sci. 2015;128:3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gorovoy M, Neamu R, Niu J, Vogel S, Predescu D, Miyoshi J, Takai Y, Kini V, Mehta D, Malik AB, et al. RhoGDI-1 modulation of the activity of monomeric RhoGTPase RhoA regulates endothelial barrier function in mouse lungs. Circ Res. 2007;101:50–58. [DOI] [PubMed] [Google Scholar]
- 46.Mikelis CM, Simaan M, Ando K, Fukuhara S, Sakurai A, Amornphimoltham P, Masedunskas A, Weigert R, Chavakis T, Adams RH, et al. RhoA and ROCK mediate histamine-induced vascular leakage and anaphylactic shock. Nat Commun. 2015;6:6725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stupack DG, Cheresh DA. ECM remodeling regulates angiogenesis: endothelial integrins look for new ligands. Sci STKE. 2002;2002:pe7. [DOI] [PubMed] [Google Scholar]
- 48.Shattil SJ, Kim C, Ginsberg MH. The final steps of integrin activation: the end game. Nat Rev Mol Cell Biol. 2010;11:288–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wegener KL, Partridge AW, Han J, Pickford AR, Liddington RC, Ginsberg MH, Campbell ID. Structural basis of integrin activation by talin. Cell. 2007;128:171–182. [DOI] [PubMed] [Google Scholar]
- 50.Ma YQ, Qin J, Wu C, Plow EF. Kindlin-2 (Mig-2): a co-activator of beta3 integrins. J Cell Biol. 2008;181:439–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zaidel-Bar R, Itzkovitz S, Ma’ayan A, Iyengar R, Geiger B. Functional atlas of the integrin adhesome. Nat Cell Biol. 2007;9:858–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Horton ER, Byron A, Askari JA, Ng DHJ, Millon-Fremillon A, Robertson J, Koper EJ, Paul NR, Warwood S, Knight D, et al. Definition of a consensus integrin adhesome and its dynamics during adhesion complex assembly and disassembly. Nat Cell Biol. 2015;17:1577–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Humphries JD, Chastney MR, Askari JA, Humphries MJ. Signal transduction via integrin adhesion complexes. Curr Opin Cell Biol. 2019;56:14–21. [DOI] [PubMed] [Google Scholar]
- 54.Avraamides CJ, Garmy-Susini B, Varner JA. Integrins in angiogenesis and lymphangiogenesis. Nat Rev Cancer. 2008;8:604–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hynes RO, Lively JC, McCarty JH, Taverna D, Francis SE, Hodivala-Dilke K, Xiao Q. The diverse roles of integrins and their ligands in angiogenesis. Cold Spring Harb Symp Quant Biol. 2002;67:143–153. [DOI] [PubMed] [Google Scholar]
- 56.Stupack DG, Cheresh DA. Integrins and angiogenesis. Curr Top Dev Biol. 2004;64:207–238. [DOI] [PubMed] [Google Scholar]
- 57.Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 1994;264:569–571. [DOI] [PubMed] [Google Scholar]
- 58.Mahabeleshwar GH, Feng W, Phillips DR, Byzova TV. Integrin signaling is critical for pathological angiogenesis. J Exp Med. 2006;203:2495–2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Steri V, Ellison TS, Gontarczyk AM, Weilbaecher K, Schneider JG, Edwards D, Fruttiger M, Hodivala-Dilke KM, Robinson SD. Acute depletion of endothelial beta3-integrin transiently inhibits tumor growth and angiogenesis in mice. Circ Res. 2014;114:79–91. [DOI] [PubMed] [Google Scholar]
- 60.Reynolds LE, Wyder L, Lively JC, Taverna D, Robinson SD, Huang X, Sheppard D, Hynes RO, Hodivala-Dilke KM. Enhanced pathological angiogenesis in mice lacking beta3 integrin or beta3 and beta5 integrins. Nat Med. 2002;8:27–34. [DOI] [PubMed] [Google Scholar]
- 61.Robinson SD, Reynolds LE, Wyder L, Hicklin DJ, Hodivala-Dilke KM. Beta3-integrin regulates vascular endothelial growth factor-A-dependent permeability. Arterioscler Thromb Vasc Biol. 2004;24:2108–2114. [DOI] [PubMed] [Google Scholar]
- 62.Su G, Atakilit A, Li JT, Wu N, Bhattacharya M, Zhu J, Shieh JE, Li E, Chen R, Sun S, et al. Absence of integrin alphavbeta3 enhances vascular leak in mice by inhibiting endothelial cortical actin formation. Am J Respir Crit Care Med. 2012;185:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alghisi GC, Ponsonnet L, Ruegg C. The integrin antagonist cilengitide activates alphaVbeta3, disrupts VE-cadherin localization at cell junctions and enhances permeability in endothelial cells. PLoS One. 2009;4:e4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gonzalez AM, Bhattacharya R, deHart GW, Jones JC. Transdominant regulation of integrin function: mechanisms of crosstalk. Cell Signal. 2010;22:578–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Su G, Hodnett M, Wu N, Atakilit A, Kosinski C, Godzich M, Huang XZ, Kim JK, Frank JA, Matthay MA, et al. Integrin alphavbeta5 regulates lung vascular permeability and pulmonary endothelial barrier function. Am J Respir Cell Mol Biol. 2007;36:377–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yamamoto H, Ehling M, Kato K, Kanai K, van Lessen M, Frye M, Zeuschner D, Nakayama M, Vestweber D, Adams RH. Integrin beta1 controls VE-cadherin localization and blood vessel stability. Nat Commun. 2015;6:6429. [DOI] [PubMed] [Google Scholar]
- 67.Hakanpaa L, Kiss EA, Jacquemet G, Miinalainen I, Lerche M, Guzman C, Mervaala E, Eklund L, Ivaska J, Saharinen P. Targeting beta1-integrin inhibits vascular leakage in endotoxemia. Proc Natl Acad Sci U S A. 2018;115:E6467–E6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lawson C, Lim ST, Uryu S, Chen XL, Calderwood DA, Schlaepfer DD. FAK promotes recruitment of talin to nascent adhesions to control cell motility. J Cell Biol. 2012;196:223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nader GP, Ezratty EJ, Gundersen GG. FAK, talin and PIPKIgamma regulate endocytosed integrin activation to polarize focal adhesion assembly. Nat Cell Biol. 2016;18:491–503. [DOI] [PubMed] [Google Scholar]
- 70.Abedi H, Zachary I. Vascular endothelial growth factor stimulates tyrosine phosphorylation and recruitment to new focal adhesions of focal adhesion kinase and paxillin in endothelial cells. J Biol Chem. 1997;272:15442–15451. [DOI] [PubMed] [Google Scholar]
- 71.Chen XL, Nam JO, Jean C, Lawson C, Walsh CT, Goka E, Lim ST, Tomar A, Tancioni I, Uryu S, et al. VEGF-induced vascular permeability is mediated by FAK. Dev Cell. 2012;22:146–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bledzka K, Bialkowska K, Sossey-Alaoui K, Vaynberg J, Pluskota E, Qin J, Plow EF. Kindlin-2 directly binds actin and regulates integrin outside-in signaling. J Cell Biol. 2016;213:97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pluskota E, Bledzka KM, Bialkowska K, Szpak D, Soloviev DA, Jones SV, Verbovetskiy D, Plow EF. Kindlin-2 interacts with endothelial adherens junctions to support vascular barrier integrity. J Physiol. 2017;595:6443–6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dong JM, Tay FP, Swa HL, Gunaratne J, Leung T, Burke B, Manser E. Proximity biotinylation provides insight into the molecular composition of focal adhesions at the nanometer scale. Sci Signal. 2016;9:rs4. [DOI] [PubMed] [Google Scholar]
- 75.Chrzanowska-Wodnicka M. Rap1 in endothelial biology. Curr Opin Hematol. 2017;24:248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lakshmikanthan S, Sobczak M, Chun C, Henschel A, Dargatz J, Ramchandran R, Chrzanowska-Wodnicka M. Rap1 promotes VEGFR2 activation and angiogenesis by a mechanism involving integrin alphavbeta(3). Blood. 2011;118:2015–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lakshmikanthan S, Sobczak M, Li Calzi S, Shaw L, Grant MB, Chrzanowska-Wodnicka M. Rap1B promotes VEGF-induced endothelial permeability and is required for dynamic regulation of the endothelial barrier. J Cell Sci. 2018;131:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ley K, Rivera-Nieves J, Sandborn WJ, Shattil S. Integrin-based therapeutics: biological basis, clinical use and new drugs. Nat Rev Drug Discov. 2016;15:173–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Aird WC. Endothelial cell heterogeneity. Cold Spring Harb Perspect Med. 2012;2:a006429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Aird WC. Endothelium in health and disease. Pharmacol Rep. 2008;60:139–143. [PubMed] [Google Scholar]
- 81.Roberts J, de Hoog L, Bix GJ. Mice deficient in endothelial alpha5 integrin are profoundly resistant to experimental ischemic stroke. J Cereb Blood Flow Metab. 2017;37:85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Aird WC. Endothelium and haemostasis. Hamostaseologie. 2015;35:11–16. [DOI] [PubMed] [Google Scholar]
- 83.Aird WC. Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circ Res. 2007;100:174–190. [DOI] [PubMed] [Google Scholar]
- 84.Aird WC. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res. 2007;100:158–173. [DOI] [PubMed] [Google Scholar]
- 85.Stan RV, Tse D, Deharvengt SJ, Smits NC, Xu Y, Luciano MR, McGarry CL, Buitendijk M, Nemani KV, Elgueta R, et al. The diaphragms of fenestrated endothelia: gatekeepers of vascular permeability and blood composition. Dev Cell. 2012;23:1203–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Berruti G, Ceriani M, Martegani E. Dynamic of VE-cadherin-mediated spermatid-Sertoli cell contacts in the mouse seminiferous epithelium. Histochem Cell Biol. 2018;150:173–185. [DOI] [PubMed] [Google Scholar]
- 87.Aivatiadou E, Mattei E, Ceriani M, Tilia L, Berruti G. Impaired fertility and spermiogenetic disorders with loss of cell adhesion in male mice expressing an interfering Rap1 mutant. Mol Biol Cell. 2007;18:1530–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Majno G, Shea SM, Leventhal M. Endothelial contraction induced by histamine-type mediators: an electron microscopic study. J Cell Biol. 1969;42:647–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Oldenburg J, de Rooij J. Mechanical control of the endothelial barrier. Cell Tissue Res. 2014;355:545–555. [DOI] [PubMed] [Google Scholar]


