Abstract
Impaired cognitive empathy is a core social cognitive deficit in schizophrenia associated with negative symptoms and social functioning. Cognitive empathy and negative symptoms have also been linked to medial prefrontal and temporal brain networks. While shared behavioral and neural underpinnings are suspected for cognitive empathy and negative symptoms, research is needed to test these hypotheses. In two studies, we evaluated whether resting‐state functional connectivity between data‐driven networks, or components (referred to as, inter‐component connectivity), predicted cognitive empathy and experiential and expressive negative symptoms in schizophrenia subjects. Study 1: We examined associations between cognitive empathy and medial prefrontal and temporal inter‐component connectivity at rest using a group‐matched schizophrenia and control sample. We then assessed whether inter‐component connectivity metrics associated with cognitive empathy were also related to negative symptoms. Study 2: We sought to replicate the connectivity‐symptom associations observed in Study 1 using an independent schizophrenia sample. Study 1 results revealed that while the groups did not differ in average inter‐component connectivity, a medial‐fronto‐temporal metric and an orbito‐fronto‐temporal metric were related to cognitive empathy. Moreover, the medial‐fronto‐temporal metric was associated with experiential negative symptoms in both schizophrenia samples. These findings support recent models that link social cognition and negative symptoms in schizophrenia. Hum Brain Mapp 38:1111–1124, 2017. © 2016 Wiley Periodicals, Inc.
Keywords: resting‐state fMRI, mentalizing, avolition, anhedonia, apathy, alogia, psychosis
Abbreviations
- ACC
Anterior cingulate cortex
- DMN
Default mode network
- FoV
Field of view
- ICA
Independent component analysis
- OFC
Orbitofrontal cortex
- SANS
Scale for the assessment of negative symptoms
- SAPS
Scale for the assessment of positive symptoms
- STG
Superior temporal gyrus
- TE
Echo time
- TP
Temporal pole
- TPJ
Temporo‐parietal junction
- TR
Repetition time
INTRODUCTION
Empathy broadly refers to the sharing and understanding of the emotional experiences of others, and is composed of affective and cognitive subdomains [Shamay‐Tsoory, 2011]. More specifically, sharing the emotions of others reflects affective empathy, whereas understanding their emotional state is pertinent to cognitive empathy [Decety and Jackson, 2004]. Mentalizing, or the ability to infer the knowledge, emotions, beliefs, and intentions of others [Premack and Woodruff, 1978], also subdivides into affective and cognitive subdomains; knowledge about beliefs reflects cognitive mentalizing, and knowledge about the emotional state of another reflects affective mentalizing [Hooker et al., 2008; Shamay‐Tsoory et al., 2007b]. Both cognitive empathy and affective mentalizing recruit similar neural networks [Fan et al., 2011; Gonzalez‐Liencres et al., 2013; Hooker et al., 2008; Smith et al., 2015], and arguably describe synonymous behaviors [Walter, 2012]. Moreover, cognitive empathy (or affective mentalizing) is critical to navigating complex social interactions and developing meaningful interpersonal relationships [de Wall, 2012; Hooker et al., 2008; Zaki and Ochsner, 2012].
Cognitive empathy abilities are notably impaired in schizophrenia [Achim et al., 2011; Derntl et al., 2009; Horan et al., 2015], and contribute to poor social functioning [Couture et al., 2011; Fett et al., 2015; Michaels et al., 2014; Smith et al., 2012, 2014, 2015]. Deficits in cognitive empathy and mentalizing (more generally) have been linked with negative symptoms [Michaels et al., 2014; Shamay‐Tsoory et al., 2007; Smith et al., 2014], a core feature of schizophrenia. In particular, the tendency to “undermentalize” may impair affective mentalizing which infers that emotional states may be especially relevant to negative symptoms [Shamay‐Tsoory et al., 2007], whereas hypermentalizing about non‐emotional contexts may be linked with more severe positive symptoms [e.g., paranoia; Frith, 1992, 2004]. Negative symptoms (avolition, anhedonia) that reflect an individual's internal experiences (experiential negative symptoms) may be especially relevant to cognitive empathy, as both are processes focused on internal subjective states, either one's own or another's [Blanchard et al., 2011; Green et al., 2013; Lieberman, 2007]. In contrast, expressive negative symptoms (affective flattening, alogia) may be less relevant, as these symptoms reflect external communicative impairments. Although studies have reported inverse associations between cognitive empathy and negative symptoms [Bell and Mishara, 2006; Lincoln et al., 2011; Shamay‐Tsoory et al., 2007a; Ventura et al., 2013], the neural basis of such associations is not well understood. Thus, the current study aimed to elucidate the neural correlates of cognitive empathy, evaluate specific cognitive empathy‐negative symptom associations, and identify common neural substrates that support these processes.
Functional connectivity can be used to reveal shared neural substrates that underlie cognitive capacities and symptoms in schizophrenia. This approach can test the “dysconnectivity hypothesis,” which proposes that core schizophrenia symptoms emerge from aberrant communication within or between regions or networks that subserve key cognitive capacities [Bullmore et al., 1997; Friston and Frith, 1995; Friston, 1994; Frith, 1992; Repovs et al., 2011]. Several studies have found aberrant connectivity in the frontal cortex of schizophrenia subjects [Bluhm et al., 2007; Camchong et al., 2011; Ongür et al., 2010; Orliac et al., 2013; Salvador et al., 2010]. However, little is known about how these deviations relate to specific cognitive indices and symptoms [Pettersson‐Yeo et al., 2011]. Recently, Millan and colleagues (2014) suggested that social cognitive deficits and negative symptoms might involve abnormalities in common medial prefrontal and temporal networks, given behavioral parallels. For example, diminished emotional expressivity contributes to negative symptoms, while the interpretation of analogous non‐verbal behaviors (e.g., expressions/posturing of others) is fundamental to social cognition. We expanded this work to assess whether functional connectivity between medial prefrontal and temporal data‐driven networks (or components) predicted both cognitive empathy and negative symptoms.
Resting‐state functional connectivity methods are a valuable complement to task‐based approaches for elucidating the neural basis of specific behaviors or symptoms. Resting‐state functional connectivity is characterized by low frequency (<0.1 Hz) neuronal oscillations, which reveal temporal relations between proximal and distal regions [Biswal, Yetkin et al., 1995]. These connectivity patterns can be captured in the absence of task‐specific demands, i.e., when the subject is at rest. Even without task demands, intrinsic connectivity networks [ICNs; Biswal et al., 2010; Wisner et al., 2013a] at rest are similar to networks derived from task‐based data [Smith et al., 2009], and resting connectivity has been shown to predict individual differences in task‐evoked brain activity [Tavor et al., 2016]. One could then use resting‐state data to examine the neural connectivity supporting mental processes, or predispositions for mental processes. Moreover, individual differences in attention, effort, or comprehension do not confound resting‐state findings, which helps overcome such limitations from task‐based data. Likewise, there are fewer design constraints that could impede cross‐sample replications. Given these features, resting‐state methods can be used to investigate the common neural substrates of measured cognitive capacities and symptom domains.
Meta‐analytic studies of resting‐state connectivity have revealed numerous frontal and temporal ICNs that are consistently derived across datasets [Laird et al., 2011; Smith et al., 2009; Wisner et al., 2013a]. The social cognition literature suggests that frontal‐lobe ICNs that support cognitive empathy may include the medial prefrontal cortex (mPFC) or the orbitofrontal cortex [OFC; Carrington & Bailey, 2009; Denny et al., 2012; van der Meer et al., 2010]. The mPFC is involved in self‐ and other judgments relevant to cognitive empathy (Shamay‐Tsoory, 2011), whereas the OFC's role in tracking hedonic value and emotional valence may contribute to negative symptoms and cognitive empathy [Chevallier et al., 2012; Goodkind et al., 2012]. Functional abnormalities in these prefrontal regions have been observed in schizophrenia subjects [Pomarol‐Clotet et al., 2010], and linked to impaired cognitive empathy [Eack et al., 2013; Lee et al., 2011], as well as negative symptoms [Mazza et al., 2013; Millan et al., 2014; Orliac et al., 2013]. Temporal‐lobe ICNs that support cognitive empathy may include the superior temporal gyrus (STG), temporo‐parietal junction (TPJ), or the temporal poles [TP; Carrington and Bailey, 2009; Olson and Plotzker, 2007]. The STG has been associated with perceptual mechanisms (e.g., eye gaze) and mental attribution [Pinkham et al., 2003; Zilbovicius et al., 2006], the TPJ and TP with mental inference [Frith and Frith, 2006; Lombardo et al., 2011; Saxe and Kanwisher, 2003], and the TP with sensory‐emotional integration [Olson and Plotzker, 2007]. Activation and connectivity abnormalities in these temporal regions have been linked with cognitive empathy deficits in schizophrenia [Benedetti et al., 2009; Das et al., 2012; Lee et al., 2011; Smith et al., 2015]. Notably, while ample evidence relates temporal lobe dysfunction to negative symptoms [Anderson et al., 2002; Bodnar et al., 2014; Millan et al., 2014], studies have also linked these regions with positive symptoms [Allen et al., 2008]. Thus, the literature is unclear as to whether specific regions support these neural‐symptom associations. Moreover, these behaviors may not be sufficiently explained by one or a few isolated regions; rather, the dynamics across or between regions may help clarify the underlying neural substrates.
Here, we employed two studies to test the hypothesis that shared medial prefrontal and temporal connectivity supports cognitive empathy deficits and experiential negative symptoms in schizophrenia, given that both reflect internal processes [Green et al., 2013; Lieberman, 2007]. In particular, we examined resting‐state functional connectivity between components (referred to as, inter‐component connectivity). Study 1: First, we assessed the association between cognitive empathy and symptoms among schizophrenia subjects. Second, we tested whether inter‐component connectivity of a priori selected medial prefrontal and temporal components differed between schizophrenia and control subjects. Third, we examined whether these connectivity metrics predicted cognitive empathy across the groups. Finally, we correlated the connectivity metrics that significantly predicted cognitive empathy with schizophrenia subjects' symptom ratings. We hypothesized that (i) cognitive empathy would be inversely correlated with experiential negative symptoms, but not other symptoms, (ii) schizophrenia subjects would show aberrant inter‐component connectivity between medial prefrontal and temporal components when compared with controls, and (iii) inter‐component connectivity between the same a priori medial prefrontal and temporal components would predict cognitive empathy and experiential negative symptoms, but not other symptoms. Study 2: Motivated by the results of Study 1, as well as the limitation of its small sample size, we investigated whether we could replicate the significant connectivity‐symptom associations in an independent schizophrenia sample.
STUDY 1: SCHIZOPHRENIA AND CONTROL SAMPLE
Materials and Methods
Subjects
Study 1 included schizophrenia and control subjects who were group‐matched for age, gender, and race. We retained 59 subjects (28 schizophrenia subjects, 31 controls) after excluding for: (i) a Diagnostic and Statistical Manual of Mental‐Disorders‐4th Edition (DSM‐IV) diagnosis of substance abuse or dependence within the past 6 months, (ii) a severe medical disorder, (iii) a head injury with neurological sequelae, (iv) excessive in‐scanner motion (mean absolute displacement above 1.5 mm, or any absolute displacement (translations or rotations) above 2.75 mm/degrees), or (v) behavioral performance more than two standard deviations below the group mean. We excluded controls with a lifetime history of a DSM‐IV axis I disorder or a first‐degree relative with a psychotic disorder. We recruited schizophrenia subjects using advertisements at outpatient treatment centers, surrounding neighborhoods, and the National Alliance for Mental Illness. We recruited controls via online and neighborhood advertisements. The Northwestern University Feinberg School of Medicine institutional review board approved the study. All subjects provided written informed consent.
Demographic and clinical measures
Diagnoses were determined using the Structured Clinical Interview for DSM‐IV [First et al., 2002]. We enrolled subjects following a diagnostic consensus between a masters (or doctorate) level research staff and a study psychiatrist. We converted antipsychotic medication dosages to chlorpromazine equivalents [CPZeq; Andreasen et al., 2010]. We assessed psychopathology using the Scale for the Assessment of Negative Symptoms (SANS) and the Scale for the Assessment of Positive Symptoms [SAPS; Andreasen et al., 1995]. Based on recent advances in our understanding of negative symptoms, we evaluated negative symptoms as two separable domains [Blanchard & Cohen, 2006; Horan et al., 2011; Strauss et al., 2013]. Specifically, we computed experiential negative symptom domain scores using the SANS global ratings for avolition and anhedonia and expressive negative symptom domain scores using the global ratings for affective flattening and alogia [Green et al., 2012; Rassovsky et al., 2011]. We also computed total symptom severity scores using the SAPS global ratings for hallucinations, delusions, formal thought disorder, and bizarre behavior, as well as the SANS global rating of attention (i.e., SANS and SAPS global scores not included in the experiential or expressive domains); we used these scores to test whether symptom associations were better explained by general psychopathology than experiential negative symptoms. Lastly, we assessed parental socioeconomic status (SES) using the Barrett Simplified Measure of Social Status [Barratt, 2005].
Cognitive empathy task
We assessed cognitive empathy using a computerized task outside of the scanner [Derntl et al., 2009; Smith et al., 2014]. Cognitive empathy [i.e., understanding the emotional perspective of others; Shamay‐Tsoory, 2011] involves mentalizing and is uniquely associated with social functioning in schizophrenia [Michaels et al., 2014; Smith et al., 2012, 2014, 2015]. For each trial, subjects viewed a picture for four seconds that displayed two people interacting with the face of a masked person (Supporting Information Fig. 1). Subjects were then presented with two emotional faces (or one neutral and one emotional face) and asked to select the emotional face (i.e., fear, anger, sadness, disgust, happiness) that best represented the masked face. Accuracy (correct trials/total completed trials) and response times (seconds) were recorded. We only used cognitive empathy accuracy for the analyses.
Figure 1.
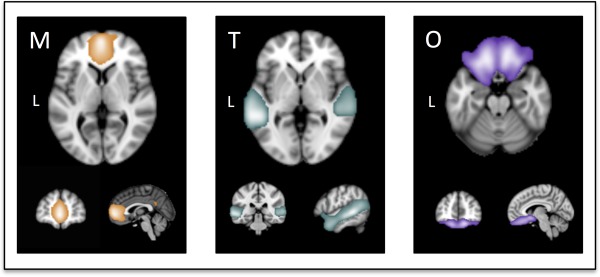
Illustrations of the targeted fronto‐temporal neural components [Abram et al., 2015]. M, medial prefrontal and anterior cingulate cortex component; T, superior temporal gyrus, temporo‐parietal junction, and temporal poles component; and O, orbitofrontal cortex component. [Color figure can be viewed at http://wileyonlinelibrary.com]
Cognitive assessment
We included global cognition as a covariate based on evidence linking social and cognitive deficits in schizophrenia [Fett et al., 2011; Ventura et al., 2013]. Subjects completed a comprehensive cognitive battery to generate four cognitive domain scores: crystalized intelligence, working memory, executive function, and episodic memory [Smith et al., 2012]. See Supporting Information for details. We computed a global cognition score by averaging the four domain scores.
fMRI data acquisition and preprocessing
Neuroimaging data were acquired on a 3T TIM Trio system (Siemens Medical Systems) at the Northwestern University Center for Translational Imaging. A high‐resolution T1‐weighted MPRAGE sequence was collected for registration [repetition time (TR)=2.4 ms; echo time (TE)=3.16 ms, flip = 8°, voxel size = 1 × 1 × 1 mm, matrix size = 256 × 256 mm, field of view (FoV)=256 × 256 mm]. Resting‐state parameters included: gradient‐echo echo‐planar imaging of 164 volumes; TR = 2.5 s; TE = 20 ms; flip = 80°; voxel size = 1.7 × 1.7 × 3 mm, matrix size = 128 × 120 mm, FoV = 220 × 206 mm. Temporal signal‐to‐noise (tSNR) maps and statistics for functional scans are presented in Supporting Information Fig. 2 [Murphy et al., 2007]. Standard preprocessing was completed using FMRIB Software Library (FSL 4.1.9), which included: brain extraction, motion correction, grand mean intensity normalization of the 4D dataset, high‐pass temporal filtering (threshold of 0.1 Hz), slice‐timing correction, and linear registration of resting scans to high‐resolution T1‐weighted structural images, as well as MNI152 standard space [Abram et al., 2015]; we did not employ low‐pass temporal filtering, as our higher‐level processing methods helped parse noise from high‐frequency fluctuations (e.g., cardiac and respiratory rhythms). Lastly, motion regression was completed on the preprocessed and registered resting scans.
Figure 2.
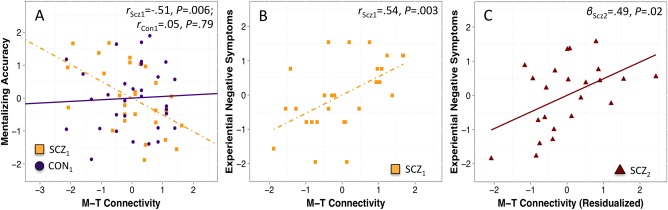
A: Group by M‐T inter‐component connectivity interaction when predicting cognitive empathy in Study 1 (i.e., SCZ Sample 1 and CON Sample 1); data illustrates the pairwise correlations for schizophrenia and control groups, separately. B: M‐T inter‐component connectivity predicts experiential negative symptoms for the schizophrenia group in Study 1. C: M‐T inter‐component connectivity predicts experiential negative symptoms for the independent schizophrenia sample in Study 2 (i.e., SCZ Sample 2); data has been residualized according to the covariates in Table III. Abbreviations: CON, control subjects; SCZ, schizophrenia subjects; M, medial prefrontal cortex and anterior cingulate cortex component; T, superior temporal gyrus, temporo‐parietal junction, and temporal poles component. [Color figure can be viewed at http://wileyonlinelibrary.com]
fMRI data higher‐level processing
ICNs were generated using independent component analysis (ICA), a data‐driven method that decomposes multivariate signals into functionally homogenous components [Poppe et al., 2013; Smith et al., 2009; Wisner et al., 2013a]. To calculate connectivity metrics for the current study, we employed ICA‐derived components previously generated from a large community sample [n = 218, mean age = 26 years (range 20–39), 49% male; Abram et al., 2015] instead of deriving components from the present samples. We adopted this approach for several reasons. First, due to differences in scanning parameters, we could not combine the two schizophrenia samples in the ICA process (such that each sample would contribute equally to ICN generation). Second, both samples in the current study were relatively small (each group with n < 35); larger samples likely produce more canonical components, as evidenced by the community maps showing strong correspondence with components documented in prior studies [e.g., Laird et al., 2011; Ray et al., 2013; Smith et al., 2009]. Third, the maps from the large community sample were more representative of the general population and thus not biased towards the control or schizophrenia samples employed in the current study. Thus, a spatial meta‐ICA pipeline was carried out on the community resting‐state scans as described previously [Abram et al., 2015] using FSL's MELODIC (Multivariate Exploratory Linear Optimized Decomposition in Independent Components) toolkit [http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/MELODIC; Biswal et al., 2010; Moodie et al., 2014; Poppe et al., 2013; Wisner et al., 2013a,b]; each ICA was constrained to a dimensionality of 60 based on prior neurometric research [Poppe et al., 2013]. See Supporting Information for details. From 60 total components, three medial prefrontal and temporal ICNs were selected for analysis. These components included hypothesized areas, as discussed in the introduction (Fig. 1): M, an mPFC and anterior cingulate cortex (ACC) component; T, an STG, TPJ, and TP component; O, an OFC component.
We applied the group‐level spatial maps derived from the community sample to the current resting‐state datasets using dual‐regression. Dual‐regression generated subject‐specific spatial maps (i.e., ICNs) and corresponding timeseries for each individual [Beckmann, Mackay, Filippini, & Smith, 2009; Filippini et al., 2009; Wisner et al., 2013b; Zuo et al., 2010]. This procedure included two steps: first, the complete set of group‐level spatial maps was used as spatial regressors onto each subject's 4D dataset to yield subject‐specific timeseries, one per group‐level spatial map, per subject. Next, the subject‐specific timeseries were used as temporal regressors onto the respective subjects' 4D dataset to derive a set of subject‐specific spatial maps, one per subject‐specific timeseries. For our analyses, we only used the subject‐specific timeseries data.
Inter‐component connectivity metric computations
To derive inter‐component connectivity metrics for each subject, we calculated Pearson correlations between the subject‐specific timeseries of each component pair. These values reflect the degree of temporal connectivity between component pairs [Wisner et al., 2013b]. We Fisher Z‐transformed all Pearson correlations. We computed group‐level means for each inter‐component connectivity metric using Fisher Z‐transformed values, and back transformed the mean values to Pearson r‐values for reporting in the demographic table. Based on the a priori ICN selection above, a total of three inter‐component connectivity metrics were derived, one for each pair of components.
Potential movement confounds
We calculated movement as the root mean square head position change (RMS mean absolute displacement); this statistic reflects the average displacement across six movement parameters that include three translational displacements across the X, Y, and Z axes, as well as three rotational displacements of pitch, yaw, and roll [Power et al., 2012]. As noted previously, we excluded subjects with RMS mean absolute displacement above 1.5 mm, or any absolute displacement (translations or rotations) above 2.75 mm/degrees. We correlated behavioral and connectivity metrics of interest with movement. We took this step to test for potential residual associations with movement, despite performing motion correction and motion regression as prior steps [Abram et al., 2015; Wisner et al., 2013b].
Movement did not correlate with cognitive empathy, inter‐component connectivity, or symptoms (all P ≥ 0.10; Supporting Information). Schizophrenia and control subjects did not differ with respect to average movement (t 58=−0.91, P = 0.36). Thus, to retain statistical power we did not include movement in the subsequent models.
Demographic and behavioral analyses
We tested for between‐group and between‐sample differences using t‐tests/ANOVAs for continuous variables and chi‐square (χ 2) for categorical variables. We used multiple imputation to compute missing parental SES data for the regression models [Rubin, 1987]; this approach is recommended over alternatives (e.g., mean imputation) as it uses sampling distributions to estimate missing values [Vaden et al., 2012].
Statistical analyses
To begin, we assessed whether cognitive empathy was negatively correlated with experiential negative symptoms among schizophrenia subjects using one‐tailed tests. Then, we investigated between‐group differences in the three inter‐component connectivity metrics using t‐tests. Next, we evaluated whether the same connectivity metrics predicted cognitive empathy and experiential negative symptoms in schizophrenia subjects. The latter analyses were carried out in two steps. First, we built a multivariate regression model to evaluate between‐group differences in the associations between inter‐component connectivity and cognitive empathy. This model included cognitive empathy as the dependent variable, the three connectivity metrics (M‐T, O‐T, and M‐O metrics) as the independent variables, and group status, global cognition, age, gender, and parental SES as the covariates; we included the latter three covariates given literature documenting age and gender‐related resting connectivity differences [Damoiseaux et al., 2008; Satterthwaite et al., 2014; Tian et al., 2011], as well as associations between SES and cognitive empathy‐related brain function [Muscatell et al., 2012]. The between‐group model also contained group‐by‐inter‐component connectivity interaction terms to assess whether connectivity metrics behaved differently across schizophrenia and control subjects. We then examined connectivity metrics that showed significant relations with cognitive empathy in the subsequent connectivity‐symptom analyses. In particular, we tested whether the significant connectivity metrics were related to experiential negative symptoms among schizophrenia subjects via Pearson correlations and partial correlations. To address antipsychotic treatment confounds, we re‐assessed any significant connectivity‐cognitive empathy and –symptom associations after including CPZeq as a covariate.
RESULTS
Subject Characteristics
Table 1 includes demographic information for Study 1 (i.e., CON Sample 1 and SCZ Sample 1). Control and schizophrenia subjects differed according to cognitive empathy accuracy and reaction time (both P ≤ 0.05). Between‐group comparisons for demographic variables with significant F‐statistics (i.e., age, parental SES, global cognition) showed that control and schizophrenia subjects did not differ in age (t 57=−1.10, P = 0.27), but control subjects had greater parental SES (t 55 = 2.79, P = 0.007) and global cognition scores (t 57 = 5.95, P < 0.001); correlations between parental SES and demographic variables can be found in Supporting Information. We also note that the experiential and expressive negative symptom distributions for SCZ Sample 1 were comparable with respect to mean (t 27 = 1.27, P = 0.22) and variance (F 1,27=.1.05, P = 0.90).
Table 1.
Demographics for samples 1 and 2
| CON (n = 31) Sample 1 | SCZ (n = 28) Sample 1 | SCZ (n = 23) Sample 2 | F/t/Χ 2 Statistica | |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 31.06 (7.87) | 33.17 (6.64) | 24.56 (3.58) | 11.82*** |
| Gender (% male) | 51.61 | 64.29 | 69.57 | 0.97 |
| Parental SES, yearsb | 30.25 (9.32) | 23.15 (9.87) | 26.75 (8.62) | 4.32* |
| Duration of Illness, yearsc | – | 14.57 (6.34) | 5.47 (3.10) | 6.04*** |
| CPZeq, mg | – | 329.79 (207.31) | – | – |
| Medicationd | ||||
| % Typical only | – | 85.71 | 73.91 | |
| % Atypical only | – | 0.00 | 0.00 | 1.83 |
| % Both | – | 14.29 | 21.74 | |
| % Neither | – | 0.00 | 4.35 | |
| Clinical symptom domainse | ||||
| Experiential negative | – | 3.02 (1.29) | 2.72 (1.03) | 0.91 |
| Expressive negative | – | 2.69 (1.26) | 1.52 (1.16) | 3.43** |
| Total symptom severity | – | 2.11 (1.42) | 1.55 (0.97) | 1.60 |
| Race | ||||
| % Caucasian | 51.61 | 42.86 | 65.22 | 5.35 |
| % African American | 32.26 | 39.29 | 34.78 | |
| % Other | 16.13 | 17.86 | 0.00 | |
| Cognitive function | ||||
| Global Cognitionf | 0.04 (0.62) | −0.89 (0.58) | −1.02 (0.61) | 26.20*** |
| Cognitive empathy task | ||||
| Accuracy | 0.86 (0.06) | 0.73 (0.10) | – | 5.87*** |
| Reaction time (sec) | 1.40 (0.26) | 1.57 (0.31) | – | −2.22* |
| Inter‐component connectivity | ||||
| M‐T Metric | 0.31 (0.22) | 0.29 (0.17) | 0.31 (0.20) | 0.09 |
| O‐T Metric | 0.22 (0.18) | 0.23 (0.14) | 0.15 (0.14) | 2.16 |
| M‐O Metric | 0.36 (0.17) | 0.35 (0.19) | 0.28 (0.14) | 1.62 |
ANOVA was used when all three groups had data for a demographic variable; t‐tests were used when only two groups had data for a demographic variable.
Completed by n = 30 CON Sample 1 and n = 27 SCZ Sample 1.
Completed by n = 21 SCZ Sample 2.
CPZeq data was not available for Sample 2.
Symptom domain scores were obtained by summing the average global ratings for the respective domain, and dividing by the number of global ratings included.
Cognitive subtests were transformed to z‐scores using control data from the respective site.
P < 0.10, * P < 0.05, ** P < 0.01, *** P < 0.001.
Abbreviations: CON, control subjects; SCZ, schizophrenia subjects; CPZeq, chlorpromazine equivalents; M, medial prefrontal and anterior cingulate cortex component; T, superior temporal gyrus, temporo‐parietal junction, and temporal poles component; O, orbitofrontal cortex component.
Cognitive Empathy and Negative Symptom Associations
We found that cognitive empathy was negatively correlated with experiential negative symptoms at a trend level (r=−0.30, P = 0.06), but not with expressive negative symptoms (r = 0.06, P = 0.75) or total symptom severity (r = 0.03, P = 0.89). Here we Bonferroni‐corrected for three comparisons (i.e., P < 0.05/3 or P < 0.02). Moreover, the negative correlation between cognitive empathy and experiential negative symptoms was significantly stronger than that with expressive negative symptoms (Meng's z = −1.70, P = 0.04), and stronger than that with total symptom severity at a trend level (Meng's z = −1.43, P = 0.08).
Average Connectivity Differences
We did not detect between‐group differences for any of the inter‐component connectivity metrics (all P ≥ 0.10; Table 1).
Cognitive Empathy Between‐Group Analyses
Table 2 summarizes the between‐group cognitive empathy findings. The overall model evaluating group differences in the association between connectivity and cognitive empathy was significant (F 11,47 = 8.58, P ≤ 0.001). We observed significant main effects for the O‐T metric and global cognition, and a significant interaction between group and the M‐T metric (all P ≤ 0.05). A plot of the M‐T metric interaction indicated differential connectivity‐cognitive empathy associations across the groups; specifically, follow‐up within‐group analyses revealed that the M‐T metric was correlated with cognitive empathy for schizophrenia (r= −0.51, P = 0.006) but not control subjects (r = 0.05, P = 0.79; Fig. 2A). A partial correlation further revealed that the M‐T and cognitive empathy correlation remained significant when accounting for CPZeq (r= −0.43, P = 0.02).
Table 2.
Inter‐component connectivity predicts cognitive empathy (between‐group model)
| Predictor variable | β (SE) | t‐stat | P‐value |
|---|---|---|---|
| Inter‐component connectivity | |||
| M‐T metric | −0.44 (0.19) | −2.36 | 0.02 |
| O‐T metric | −0.42 (0.16) | −2.54 | 0.01 |
| M‐O metric | 0.03 (0.14) | 0.23 | 0.82 |
| Age | −0.01 (.09) | −0.11 | 0.92 |
| Gender | 0.21 (0.19) | 1.15 | 0.26 |
| Socioeconomic status | −0.02 (0.10) | −0.15 | 0.89 |
| Global cognition | 0.34 (0.13) | 2.67 | 0.01 |
| Group | 0.85 (0.22) | 3.87 | 0.0004 |
| Interaction terms | |||
| M‐T × Group | 0.52 (0.23) | 2.26 | 0.03 |
| O‐T × Group | 0.20 (0.21) | 0.96 | 0.34 |
| M‐O × Group | −0.03 (.19) | −0.17 | 0.87 |
Adjusted R 2 = 0.59, F 11, 47 = 8.58, P < 0.0001.
Abbreviations: M, medial prefrontal and anterior cingulate cortex component; T, superior temporal gyrus, temporo‐parietal junction, and temporal pole component; O, orbitofrontal cortex component.
Symptom Analyses
We used the cognitive empathy findings to inform our symptom analyses. Specifically, we examined whether connectivity metrics that predicted cognitive empathy (i.e., the M‐T and O‐T metrics) also contributed to symptom severity. M‐T connectivity was correlated with experiential negative symptoms (r = 0.54, P = 0.003; Fig. 2B), but not expressive negative symptoms (r = 0.14, P = 0.47) or total symptom severity (r = 0.24, P = 0.21). A partial correlation between the M‐T metric and experiential negative symptoms, while controlling for the other two symptom domains, supported the specificity of this association (partial r = 0.52, P = 0.003); the partial correlation also remained significant when accounting for CPZeq (partial r = 0.54, P = 0.002). Furthermore, the correlation with experiential negative symptoms was significantly greater than that with expressive negative symptoms (Meng's z =2.04, P = 0.04). In contrast to the M‐T metric, O‐T connectivity was not correlated with experiential negative symptoms (r = 0.17, P = 0.39), expressive negative symptoms (r= −0.30, P = 0.12), or total symptom severity (r= −0.17, P = 0.38).
Confound Analyses
As a last step, we assessed whether cognitive empathy accuracy attenuated the association between M‐T connectivity and experiential negative symptoms, given that cognitive empathy was correlated with M‐T connectivity and experiential negative symptoms at a trend level. We constructed a regression model that included experiential negative symptoms as the dependent variable, with the M‐T metric and cognitive empathy as the independent variables. Supporting Information Table 1 shows that M‐T connectivity predicted experiential negative symptoms over and above cognitive empathy (t 25 = 2.65, P = 0.01); in contrast, the cognitive empathy coefficient was not significant (t 25= −0.03, P = 0.88).
Summary of Study 1 Findings
We found that cognitive empathy accuracy was negatively correlated with experiential negative symptoms at a trend level, and this association was significantly stronger than that with expressive negative symptoms. Moreover, the two negative symptom domains did not differ with regard to distribution qualities (i.e., mean and variance), suggesting the observed differences were not better attributed to measurement issues. We also found that M‐T connectivity was related to cognitive empathy accuracy, and experiential negative symptoms (among individuals with schizophrenia) but not other symptoms, thus indicating the specificity of this connectivity‐symptom association. However, because of the small sample size, we were motivated to replicate this result. We therefore designed Study 2 to test whether we could replicate the observed connectivity‐symptom association in an independent schizophrenia sample.
STUDY 2: SECOND SCHIZOPHRENIA SAMPLE
Materials and Methods
Subjects
Study 2 evaluated an independent sample of schizophrenia subjects (n = 23) from an existing database at the Conte Center for the Neuroscience of Mental Disorders at Washington University in St. Louis. No subjects were excluded for excessive motion. The Washington University School of Medicine institutional review board approved the study procedures and subjects provided written informed consent. Specifically, we aimed to replicate the significant connectivity‐symptom association from Study 1. We applied the same exclusionary criteria as described under Study 1.
Demographics, clinical measures, and cognitive assessment
These procedures were identical to those described under Study 1.
fMRI data acquisition and preprocessing
Neuroimaging data were acquired on a 3T TIM Trio system at Washington University School of Medicine. These functional scans were also registered to a T1‐weighted MPRAGE sequence (TR = 2.4 ms; TE = 3.16 ms, flip = 8°, voxel size = 1 × 1 × 1 mm, matrix size = 256 × mm, FoV = 256 × mm). Resting‐state parameters included: gradient‐echo echo‐planar imaging of 164 volumes; TR = 2.2 s; TE = 27 ms; flip = 90°; voxel size = 4 × 4 × 4 mm, matrix size = 64 × 64 mm, FoV = 256 × 256 mm. tSNR maps and statistics for functional scans are presented in Supporting Information Fig. 2. As illustrated in Supporting Information Fig. 2, the samples did not differ in tSNR values despite scanning parameter differences (for all between‐sample comparisons P > 0.05), and the observed values fall within the range of recent studies [Griffanti et al., 2014; Smith et al., 2013]. Functional scans were pre‐processed according to the same procedures described under Study 1.
Inter‐component connectivity metric computations
Connectivity metrics were derived according to the same procedures described under Study 1.
Potential movement confounds
Movement was not associated with inter‐component connectivity or symptoms in the second sample (all P ≥ 0.10; Supporting Information). The Study 2 sample did not differ from the Study 1 sample on movement (t 49 = 0.23, P = 0.82).
Demographic and behavioral analyses
Analyses were comparable to Study 1; we used multiple imputation to derive missing duration of illness data for the regression model.
Statistical analyses
We aimed to replicate the significant connectivity‐symptom association identified in Study 1. In particular, we built a regression model that included experiential negative symptoms as the dependent variable and the M‐T metric as the independent variable. We also included demographic and clinical variables for which the two schizophrenia samples differed (i.e., SCZ Sample 1 and SCZ sample 2) as covariates (see Subject Characteristics).
RESULTS
Subject Characteristics
Table 1 also includes demographic information for Study 2 (i.e., SCZ Sample 2). SCZ Samples 1 and 2 differed with respect to duration of illness and expressive negative symptoms (both P ≤ 0.05). Follow‐up between‐group comparisons for the age and global cognition ANOVAs revealed significant differences for age (t 49 = 5.58, P < 0.001) but not global cognition (t 49 = 0.76, P = 0.45). We also note that parental SES was not associated with inter‐component connectivity or symptoms in SCZ Sample 2 (Supporting Information).
Average Connectivity Differences
SCZ Samples 1 and 2 did not differ with respect to inter‐component connectivity (all P ≥ 0.10; Table 1).
Symptom Analyses
Table 3 summarizes findings from the replication connectivity‐symptom regression model. The model fit for predicting experiential negative symptoms was significant (F4,18 = 5.37, P = 0.005). More specifically, the M‐T metric positively predicted experiential negative symptoms (t 18 = 2.66, P = 0.02), even when controlling for age, duration of illness, and expressive negative symptoms (i.e., covariates for which the schizophrenia samples differed). Expressive negative symptoms also predicted experiential negative symptoms (t 18 = 3.35, P = 0.004). Fig. 2C illustrates the positive connectivity‐symptom association for SCZ Sample 2 (comparable partial correlation for SCZ Sample 1 accounting for age, duration of illness, and expressive negative symptoms, r = 0.54, P = 0.002).
Table 3.
Inter‐component connectivity predicts experiential negative symptoms (replication)
| Predictor variable | β (SE) | t‐stat | P‐value |
|---|---|---|---|
| Inter‐component connectivity | |||
| M‐T metric | 0.49 (0.18) | 2.66 | 0.02 |
| Age | 0.06 (0.22) | 0.30 | 0.77 |
| Duration of illness | 0.38 (0.21) | 1.82 | 0.09 |
| Expressive negative symptoms | 0.57 (0.17) | 3.35 | 0.004 |
Adjusted R 2 = 0.46, F 4, 18 = 5.37, P = 0.005.
Abbreviations: M, medial prefrontal and anterior cingulate cortex component; T, superior temporal gyrus, temporo‐parietal junction, and temporal pole component.
DISCUSSION
The current study used resting‐state functional neuroimaging data to identify medial prefrontal and temporal component dynamics that predicted cognitive empathy deficits and negative symptoms in schizophrenia. Our data suggested that: (i) cognitive empathy and experiential negative symptoms were inversely correlated at a trend level, (ii) schizophrenia and control subjects did not differ in average inter‐component connectivity between a priori medial prefrontal and temporal components; however, (iii) the M‐T inter‐ component connectivity metric was related to cognitive empathy and experiential negative symptoms in the schizophrenia subjects, and (iv) this connectivity‐symptom association was replicated in a second independent sample of schizophrenia subjects at an earlier stage in their illness. Notably, these relationships were limited to experiential negative symptoms, and persisted when controlling for known correlates of social cognitive abilities.
Specifically, in Study 1, we found that two of the three inter‐component connectivity metrics predicted cognitive empathy. The O‐T connectivity was negatively associated with cognitive empathy in both groups, but not related to symptoms. In contrast, stronger M‐T connectivity predicted poorer cognitive empathy in schizophrenia, but failed to predict cognitive empathy in controls. Moreover, among schizophrenia subjects, stronger M‐T connectivity was associated with more severe experiential negative symptoms, but was not related to expressive negative symptoms or total symptom severity. Collectively these results speak to the specificity of the M‐T neural dynamics as related to experiential negative symptoms. Lastly, in Study 2, we found that the M‐T connectivity and experiential negative symptom association was present in an independent schizophrenia sample, even when accounting for demographic and clinical variables for which the samples differed.
Interestingly, the negative M‐T connectivity‐cognitive empathy association for schizophrenia subjects was divergent from controls; that is, higher connectivity levels predicted worse performance and greater symptomatology among schizophrenia subjects but did not predict performance among control subjects. We note this connectivity‐cognitive empathy association was also significant when controlling for cognition, SES, and medication. We therefore suggest that this association was not simply due to a generalized functioning impairment [or “negative manifold”; MacDonald, 2013; Sprong et al., 2007], where poorer performance on a task could be equally or better attributed to other detrimental illness features. Another noteworthy finding was a trend‐level association between cognitive empathy and experiential negative symptoms; while it is possible this was due to insufficient power, an alternative explanation is that these processes are indirectly related. For example, a path model proposed by Green and colleagues (2012) indicated that social cognition and experiential negative symptoms may only be indirectly related via defeatist beliefs. While the current study did not assess defeatist beliefs, future studies could test whether such a measure would influence associations between cognitive empathy, experiential negative symptoms, and neural connectivity.
Our results highlight resting‐state connectivity between an mPFC and ACC component with an STG, TPJ, and TP component. These component dynamics may support cognitive empathy and experiential negative symptoms given their roles in social perception, social cognition, and motivation [Green et al., 2012]. In particular, temporal areas may be tuned to the perceptual aspects of cognitive empathy [Lahnakoski et al., 2012; Olson & Plotzker, 2007; Pinkham et al., 2003; Zilbovicius et al., 2006], whereas medial prefrontal areas may be involved in the internal or emotional aspects of cognitive empathy [Lieberman, 2007]. Appropriate medial‐fronto‐temporal communication may facilitate the use of external social cues (temporal regions) when deducing the internal emotional states of others (medial prefrontal regions). With respect to experiential negative symptoms, the role of medial prefrontal areas in motivation and goal‐directed pursuits may be especially salient [Holroyd & Yeung, 2012; Ridderinkhof et al., 2004; Strauss et al., 2014], as these symptoms are largely defined by motivation [Blanchard et al., 2011]. Moreover, the successful integration of social perceptual information with motivational processes might support goal‐oriented behaviors. Thus, while temporal lobe‐based perceptual abilities may underlie a range of schizophrenia symptoms [e.g., auditory and visual hallucinations; Allen et al., 2008], medial prefrontal and temporal dynamics may be specific to experiential negative symptoms.
The medial prefrontal and temporal components may also relate to impaired social cognition in schizophrenia given their roles within larger networks, particularly the default mode network (DMN). For instance, the medial prefrontal component in the current study maps onto the anterior hub of the DMN [Andrews‐Hanna, 2012], which includes the ventral mPFC and ACC [Sheline et al., 2009]. The anterior DMN may support the self‐referential processes that are involved in higher‐order social cognition [Amodio & Frith, 2006; Gusnard et al., 2001; Mitchell et al., 2005; Spreng & Andrews‐Hanna, 2015]. Additionally, the temporal component shows overlap with the dorsal medial subsystem of the DMN, which includes the dorsal mPFC, lateral temporal cortex, TPJ, and TP [Andrews‐Hanna et al.,, 2010]. Emerging evidence suggests that the dorsal medial subsystem plays a role in introspective social processes, like mentalizing about others [Andrews‐Hanna et al., 2014; Andrews‐Hanna, 2012]. Alterations in the DMN have been observed among individuals with schizophrenia [Whitfield‐Gabrieli et al., 2009], and such abnormalities have been linked with poor social cognition in this population [Whitfield‐Gabrieli & Ford, 2012]. Hence, our findings can also be contextualized within the broader literature relating schizophrenia DMN abnormalities with social cognitive deficits.
This study has important implications for schizophrenia treatment. In particular, theories of therapeutic development suggest effective neuronal signatures are related to both disease mechanisms (e.g., cognitive deficits) and symptoms [Tregellas et al., 2014]. With respect to the current findings, therapeutic manipulation of M‐T connectivity might improve social cognitive abilities and reduce negative symptom severity. However, research is needed to evaluate whether reducing M‐T connectivity could normalize behavior in schizophrenia, given (i) this change would be dissimilar from the M‐T connectivity‐cognitive empathy pattern in controls, and (ii) schizophrenia subjects did not exhibit hyperconnectivity relative to controls. Alternatively, one could speculate that typical M‐T connectivity in schizophrenia might be insufficient for adept cognitive empathy. Rather, it might be beneficial to elevate connectivity above the levels of controls to normalize behavior, although additional research is needed to evaluate this alternative hypothesis. This perspective represents a shift from traditional patient/control comparisons, and instead emphasizes the value for gauging improvements within the schizophrenia population.
Limitations
Our findings should be interpreted in the context of some limitations. First, we observed a ceiling effect for controls on the cognitive empathy task, which may have restricted the observed range of healthy connectivity‐cognitive empathy associations. Second, our connectivity‐symptom correlations were based on scales that assess symptom domains via 2–5 global scores. These symptom scales are not consistent with psychometric principles that posit an average, over numerous items, yields more reliable construct measurement [Mathalon & Ford, 2012]. Lastly, the cognitive empathy task was only collected in one sample. Thus, we could not evaluate whether the observed connectivity‐cognitive empathy relationships replicated in the second sample.
Future Directions
Future studies may incorporate task‐based imaging or therapeutic manipulations to directly evaluate changes in medial prefrontal and temporal connectivity as it relates to cognitive empathy and symptoms. Researchers may also add neurophysiological measures (e.g., eye‐tracking) to assess associations between visual processing, cognitive empathy, symptoms, and social functioning in schizophrenia. Such research could reveal whether comparable medial prefrontal and temporal connectivity is implicated in the basic perceptual processes that underlie cognitive empathy and everyday social interactions.
CONCLUSIONS
This study suggests that resting‐state connectivity dynamics between medial prefrontal and temporal neural components may support both cognitive empathy deficits and experiential negative symptoms in schizophrenia subjects. Moreover, these findings provide brain‐based evidence for recent path models that indirectly link social cognition and experiential negative symptoms with social functioning in schizophrenia [Couture et al., 2011; Galderisi, Rossi, & Rocca, 2014; Green et al., 2012; Rassovsky et al., 2011]. This work has important implications for developing biomarker‐based treatments aimed to improve negative symptoms and social functioning in schizophrenia.
Supporting information
Supporting Information Figures.
Supporting Information Table 1.
Supporting Information
ACKNOWLEDGMENTS
The authors thank the research staff at the Northwestern University Schizophrenia Research Group and Washington University in St. Louis for study coordination and data collection. They also want to acknowledge the participants for volunteering their time. Lastly, they thank Dr. Colin G. DeYoung for allowing them to apply his ICA‐derived spatial maps in their analyses.
REFERENCES
- Abram SV, Wisner KM, Grazioplene RG, Krueger RF, MacDonald AW, DeYoung CG (2015): Functional coherence of insula networks is associated with externalizing behavior. J Abnorm Psychol 124:1079–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achim AM, Ouellet R, Roy MA, Jackson PL (2011): Assessment of empathy in first‐episode psychosis and meta‐analytic comparison with previous studies in schizophrenia. Psychiatry Res 190:3–8. [DOI] [PubMed] [Google Scholar]
- Allen P, Larøi F, McGuire PK, Aleman A (2008): The hallucinating brain: A review of structural and functional neuroimaging studies of hallucinations. Neurosci Biobehav Rev 32:175–191. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Frith CD (2006): Meeting of minds: The medial frontal cortex and social cognition. Nat Rev Neurosci 7:268–277. [DOI] [PubMed] [Google Scholar]
- Anderson JE, Wible CG, McCarley RW, Jakab M, Kasai K, Shenton ME (2002): An MRI study of temporal lobe abnormalities and negative symptoms in chronic schizophrenia. Schizophr Res 58:123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC, Arndt S, Miller D, Flaum M, Nopoulos P (1995): Correlational studies of the scale for the assessment of negative symptoms and the scale for the assessment of positive symptoms: An overview and update. Psychopathology 28:7–17. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC (2010): Antipsychotic dose equivalents and dose‐years: A standardized method for comparing exposure to different drugs. Biol Psychiatry 67:255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews‐Hanna JR (2012): The brain's default network and its adaptive role in internal mentation. Neuroscientist 18:251–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews‐Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL (2010): Functional‐anatomic fractionation of the brain's default network. Neuron 65:550–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews‐Hanna JR, Smallwood J, Spreng RN (2014): The default network and self‐generated thought: Component processes, dynamic control, and clinical relevance. Ann N Y Acad Sci 1316:29–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barratt, W. (2005). The Barratt Simplified Measure of Social Status (BSMSS): Measuring SES. Terre Haute, IN: Indiana State University, Department of Educational Leadership, Administration, and Foundations. [Google Scholar]
- Beckmann C, Mackay C, Filippini N, Smith SM (2009): Group comparison of resting‐state FMRI data using multi‐subject ICA and dual regression. NeuroImage 47:S148. [Google Scholar]
- Bell MD, Mishara AL (2006): Does negative symptom change relate to neurocognitive change in schizophrenia? Implications for targeted treatments. Schizophr Res 81:17–27. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Bernasconi A, Bosia M, Cavallaro R, Dallaspezia S, Falini A, Poletti S, Radaelli D, Riccaboni R, Scotti G, Smeraldi E (2009): Functional and structural brain correlates of theory of mind and empathy deficits in schizophrenia. Schizophr Res 114:154–160. [DOI] [PubMed] [Google Scholar]
- Biswal BB, Yetkin FZ, Haughton VM, Hyde JS (1995): Functional connectivity in the motor cortex of resting human brain using echo‐planar mri. Brain 34:537–541. [DOI] [PubMed] [Google Scholar]
- Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, Beckmann CF, Adelstein JS, Buckner RL, Colcombe S, Dogonowski AM, Ernst M, Fair D, Hampson M, Hoptman MJ, Hyde JS, Kiviniemi VJ, Kötter R, Li SJ, Lin CP, Lowe MJ, Mackay C, Madden DJ, Madsen KH, Margulies DS, Mayberg HS, McMahon K, Monk CS, Mostofsky SH, Nagel BJ, Pekar JJ, Peltier SJ, Petersen SE, Riedl V, Rombouts SA, Rypma B, Schlaggar BL, Schmidt S, Seidler RD, Siegle GJ, Sorg C, Teng GJ, Veijola J, Villringer A, Walter M, Wang L, Weng XC, Whitfield‐Gabrieli S, Williamson P, Windischberger C, Zang YF, Zhang HY, Castellanos FX, Milham MP (2010): Toward discovery science of human brain function. Proc Natl Acad Sci U S A 107:4734–4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard JJ, Cohen AS (2006): The structure of negative symptoms within schizophrenia: Implications for assessment. Schizophr Bull 32:238–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard JJ, Kring AM, Horan WP, Gur R (2011): Toward the next generation of negative symptom assessments: The collaboration to advance negative symptom assessment in schizophrenia. Schizophr Bull 37:291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluhm RL, Miller J, Lanius RA, Osuch EA, Boksman K, Neufeld RW, Théberge J, Schaefer B, Williamson P (2007): Spontaneous low‐frequency fluctuations in the BOLD signal in schizophrenic patients: Anomalies in the default network. Schizophr Bull 33:1004–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar M, Hovington CL, Buchy L, Malla AK, Joober R, Lepage M (2014): Cortical thinning in temporo‐parietal junction (TPJ) in non‐affective first‐episode of psychosis patients with persistent negative symptoms. PloS One 9:e101372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore ET, Frangou S, Murray RM (1997): The dysplastic net hypothesis: An integration of developmental and dysconnectivity theories of schizophrenia. Schizophr Res 28:143–156. [DOI] [PubMed] [Google Scholar]
- Camchong J, MacDonald AW, Bell C, Mueller BA, Lim KO (2011): Altered functional and anatomical connectivity in schizophrenia. Schizophr Bull 37:640–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington SJ, Bailey AJ (2009): Are there theory of mind regions in the brain? A review of the neuroimaging literature. Hum Brain Mapp 30:2313–2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevallier C, Kohls G, Troiani V, Brodkin ES, Schultz RT (2012): The social motivation theory of autism. Trend Cogn Sci 16:231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couture SM, Granholm EL, Fish SC (2011): A path model investigation of neurocognition, theory of mind, social competence, negative symptoms and real‐world functioning in schizophrenia. Schizophr Res 125:152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Beckmann CF, Arigita EJ, Barkhof F, Scheltens P, Stam CJ, Smith SM, Rombouts SA (2008): Reduced resting‐state brain activity in the “default network” in normal aging. Cereb Cortex 18:1856–1864. [DOI] [PubMed] [Google Scholar]
- Das P, Calhoun V, Malhi GS (2012): Mentalizing in male schizophrenia patients is compromised by virtue of dysfunctional connectivity between task‐positive and task‐negative networks. Schizophr Res 140:51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wall FBM (2012): The antiquity of empathy. Science 336:874–876. [DOI] [PubMed] [Google Scholar]
- Decety J, Jackson P (2004): The functional architecture of human empathy. Behav Cogn Neurosci Rev 3:71–100. [DOI] [PubMed] [Google Scholar]
- Denny BT, Kober H, Wager TD, Ochsner KN (2012): A meta‐analysis of functional neuroimaging studies of self‐ and other judgments reveals a spatial gradient for mentalizing in medial prefrontal cortex. J Cogn Neurosci 24:1742–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derntl B, Finkelmeyer A, Toygar TK, Hülsmann A, Schneider F, Falkenberg DI, Habel U (2009): Generalized deficit in all core components of empathy in schizophrenia. Schizophr Res 108:197–206. [DOI] [PubMed] [Google Scholar]
- Eack SM, Wojtalik JA, Newhill CE, Keshavan MS, Phillips ML (2013): Prefrontal cortical dysfunction during visual perspective‐taking in schizophrenia. Schizophr Res 150:491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Duncan NW, de Greck M, Northoff G (2011): Is there a core neural network in empathy? An fMRI based quantitative meta‐analysis. Neurosci Biobehav Rev 35:903–911. [DOI] [PubMed] [Google Scholar]
- Fett A, Viechtbauer W, Dominguez M, de G, Penn DL, van Os J, Krabbendam L (2011): The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: A meta‐analysis. Neuroscience and Biobehav Rev 35:573–588. [DOI] [PubMed] [Google Scholar]
- Fett A, Shergill SS, Krabbendam L (2015): Social neuroscience in psychiatry: Unravelling the neural mechanisms of social dysfunction. Psychol Med 45:1145–1165. [DOI] [PubMed] [Google Scholar]
- Filippini N, MacIntosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, Matthews PM, Beckmann CF, Mackay CE (2009): Distinct patterns of brain activity in young carriers of the APOE‐epsilon4 allele. Proc Natl Acad Sci U S A 106:7209–7214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First, M. B. et, Spitzer, R. L. , Gibbon, M. , & Williams, J. B. W. (2002). Structured Clinical Interview for DSM‐IV‐TR Axis I Disorders, Research Version, Non‐patient Edition. for DSMIV. New York, NY: Biometrics Research, New York State Psychiatric Institute. [Google Scholar]
- Friston KJ (1994): Functional and effective connectivity in neuroimaging: A synthesis. Hum Brain Mapp 2:56–78. [Google Scholar]
- Friston KJ, Frith CD (1995): Schizophrenia: A disconnection syndrome? Clin Neurosci 3:89–97. [PubMed] [Google Scholar]
- Frith, C. D. (1992). The cognitive neuropsychology of schizophrenia. Essays in Cognitive Psychology; The cognitive neuropsychology of schizophrenia. Hove, UK: Lawrence Erlbaum Associates. [Google Scholar]
- Frith CD (2004): Schizophrenia and theory of mind. Psychol Med 34:385–389. [DOI] [PubMed] [Google Scholar]
- Frith CD, Frith U (2006): The neural basis of mentalizing. Neuron 50:531–534. [DOI] [PubMed] [Google Scholar]
- Galderisi S, Rossi A, Rocca P (2014): The influence of illness‐related variables, personal resources and context‐related factors on real‐life functioning of people with schizophrenia. World Psychiatry 13:275–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez‐Liencres C, Shamay‐Tsoory SG, Brüne M (2013): Towards a neuroscience of empathy: Ontogeny, phylogeny, brain mechanisms, context and psychopathology. Neuroscience and Biobehav Rev 37:1537–1548. [DOI] [PubMed] [Google Scholar]
- Goodkind MS, Sollberger M, Gyurak A, Rosen HJ, Rankin KP, Miller B, Levenson R (2012): Tracking emotional valence: The role of the orbitofrontal cortex. Hum Brain Mapp 33:753–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Hellemann G, Horan WP, Lee J, Wynn JK (2012): From perception to functional outcome in schizophrenia: Modeling the role of ability and motivation. Arch Gen Psychiatry 69:1216–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, M. F. , Horan, W. P. , Mathis, K. I. , & Wynn, J. K. (2013). Neurocognition and functional outcome in schizophrenia: Filling in the gaps In Harvey PD, editor, Cognitive Impairment in Schizophrenia. Cambridge University Press; pp. 85–97. [Google Scholar]
- Griffanti L, Salimi‐Khorshidi G, Beckmann CF, Auerbach EJ, Douaud G, Sexton CE, Zsoldos E, Ebmeier KP, Filippini N, Mackay CE, Moeller S, Xu J, Yacoub E, Baselli G, Ugurbil K, Miller KL, Smith SM (2014): ICA‐based artefact and accelerated fMRI acquisition for improved resting state network imaging. NeuroImage 95:232–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME (2001): Medial prefrontal cortex and self‐referential mental activity: Relation to a default mode of brain function. Proc Natl Acad Sci U S A 98:4259–4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd CB, Yeung N (2012): Motivation of extended behaviors by anterior cingulate cortex. Trend Cogn Sci 16:122–128. [DOI] [PubMed] [Google Scholar]
- Hooker CI, Verosky SC, Germine LT, Knight RT, D'Esposito M (2008): Mentalizing about emotion and its relationship to empathy. Soc Cogn Affect Neurosci 3:204–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan WP, Kring AM, Gur RE, Reise SP, Blanchard JJ (2011): Development and psychometric validation of the Clinical Assessment Interview for Negative Symptoms (CAINS). Schizophr Res 132:140–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan WP, Reise SP, Kern RS, Lee J, Penn DL, Green MF (2015): Structure and correlates of self‐reported empathy in schizophrenia. J Psychiatr Res 66‐67:60–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahnakoski JM, Glerean E, Salmi J, Jääskeläinen IP, Sams M, Hari R, Nummenmaa L (2012): Naturalistic fMRI mapping reveals superior temporal sulcus as the hub for the distributed brain network for social perception. Front Hum Neurosci 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Fox PM, Eickhoff SB, Turner JA, Ray KL, McKay DR, Glahn DC, Beckmann CF, Smith SM, Fox PT (2011): Behavioral interpretations of intrinsic connectivity networks. J Cogn Neurosci 23:4022–4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Quintana J, Nori P, Green MF (2011): Theory of mind in schizophrenia: Exploring neural mechanisms of belief attribution. Soc Neurosci 6:569–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD (2007): Social cognitive neuroscience: A review of core processes. Ann Rev Psychol 58:259–289. [DOI] [PubMed] [Google Scholar]
- Lincoln TM, Mehl S, Kesting ML, Rief W (2011): Negative symptoms and social cognition: Identifying targets for psychological interventions. Schizophr Bull 37, Suppl 2:S23–S32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo MV, Chakrabarti B, Bullmore ET, Baron‐Cohen S (2011): Specialization of right temporo‐parietal junction for mentalizing and its relation to social impairments in autism. NeuroImage 56:1832–1838. [DOI] [PubMed] [Google Scholar]
- MacDonald, A. W. (2013). What kind of a thing is schizophrenia? Specific causation and general failure modes In: Silverstein SM, Moghaddam B, & Wykes T, Editors. Schizophrenia: Evolution and Synthesis, Vol. 13 The MIT Press; pp. 25–41. [PubMed] [Google Scholar]
- Mathalon DH, Ford JM (2012): Neurobiology of schizophrenia: Search for the elusive correlation with symptoms. Front Hum Neurosci 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza M, Catalucci A, Pino MC, Casacchia M, Gallucci M (2013): Dysfunctional neural networks associated with impaired social interactions in early psychosis: An ICA analysis. Brain Imaging Behav 7:248–259. [DOI] [PubMed] [Google Scholar]
- Michaels TM, Horan WP, Ginger EJ, Martinovich Z, Pinkham AE, Smith MJ (2014): Cognitive empathy contributes to poor social functioning in schizophrenia: Evidence from a new self‐report measure of cognitive and affective empathy. Psychiatry Res 220:803–810. [PubMed] [Google Scholar]
- Millan MJ, Fone K, Steckler T, Horan WP (2014): Negative symptoms of schizophrenia: Clinical characteristics, pathophysiological substrates, experimental models and prospects for improved treatment. Eur Neuropsychopharmacol 24:645–692. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Banaji MR, Macrae CN (2005): The link between social cognition and self‐referential thought in the medial prefrontal cortex. J Cogn Neurosci 17:1306–1315. [DOI] [PubMed] [Google Scholar]
- Moodie CA, Wisner KM, MacDonald AW (2014): Characteristics of canonical intrinsic connectivity networks across tasks and monozygotic twin pairs. Hum Brain Mapp 35:5532–5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K, Bodurka J, Bandettini PA (2007): How long to scan? The relationship between fMRI temporal signal to noise ratio and necessary scan duration. NeuroImage 34:565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscatell KA, Morelli SA, Falk EB, Way BM, Pfeifer JH, Galinsky AD, Lieberman MD, Dapretto M, Eisenberger NI (2012): Social status modulates neural activity in the mentalizing network. NeuroImage 60:1771–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson IR, Plotzker A (2007): The Enigmatic temporal pole: A review of findings on social and emotional processing. Brain 1718–1731. [DOI] [PubMed] [Google Scholar]
- Ongür D, Lundy M, Greenhouse I, Shinn AK, Menon V, Cohen BM, Renshaw PF (2010): Default mode network abnormalities in bipolar disorder and schizophrenia. Psychiatry Res 183:59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orliac F, Naveau M, Joliot M (2013): Links among resting‐state default‐mode network, salience network, and symptomatology in schizophrenia. Schizophr Res 148:74–80. [DOI] [PubMed] [Google Scholar]
- Pettersson‐Yeo W, Allen P, Benetti S, Mcguire P, Mechelli A (2011): Dysconnectivity in schizophrenia: Where are we now? Neurosci Biobehav Rev 35:1110–1124. [DOI] [PubMed] [Google Scholar]
- Pinkham AE, Penn DL, Perkins DO, Lieberman J (2003): Implications for the neural basis of social cognition for the study of schizophrenia. Am J Psychiatry 160:815–824. [DOI] [PubMed] [Google Scholar]
- Pomarol‐Clotet E, Canales‐Rodríguez EJ, Salvador R, Sarró S, Gomar JJ, Vila F, Ortiz‐Gil J, Iturria‐Medina Y, Capdevila A, McKenna PJ (2010): Medial prefrontal cortex pathology in schizophrenia as revealed by convergent findings from multimodal imaging. Mol Psychiatry 15:823–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppe AB, Wisner K, Atluri G, Lim KO, Kumar V, Macdonald AW (2013): Toward a neurometric foundation for probabilistic independent component analysis of fMRI data. Cogn, Affect Behav Neurosci 13:641–659. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE (2012): Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage 59:2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premack D, Woodruff G (1978): Does the chimpanzee have a theory of mind?. Behav Brain Sci 1:515–526. [Google Scholar]
- Rassovsky Y, Horan WP, Lee J, Sergi MJ, Green MF (2011): Pathways between early visual processing and functional outcome in schizophrenia. Psychol Med 41:487–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray KL, McKay DR, Fox PM, Riedel MC, Uecker AM, Beckmann CF, Smith SM, Fox PT, Laird AR (2013): ICA model order selection of task co‐activation networks. Front Neurosc 7:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repovs G, Csernansky JG, Barch DM (2011): Brain network connectivity in individuals with schizophrenia and their siblings. Biol Psychiatry 69:967–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S (2004): The role of the medial frontal cortex in cognitive control. Science (New York, N.Y.) 306:443–447. [DOI] [PubMed] [Google Scholar]
- Rubin, D. B. (1987). Multiple imputation for nonresponse in surveys, Vol. 81 New York, NY: J Wiley & Sons. [Google Scholar]
- Salvador R, Sarró S, Gomar JJ, Ortiz‐Gil J, Vila F, Capdevila A, Bullmore E, McKenna PJ, Pomarol‐Clotet E (2010): Overall brain connectivity maps show cortico‐subcortical abnormalities in schizophrenia. Hum Brain Mapp 31:2003–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Wolf DH, Roalf DR, Ruparel K, Erus G, Vandekar S, Gennatas ED, Elliott MA, Smith A, Hakonarson H, Verma R, Davatzikos C, Gur RE, Gur RC (2014): Linked sex differences in cognition and functional connectivity in youth. Cerebral Cortex (New York, N.Y.: 1991) 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe R, Kanwisher N (2003): People thinking about thinking people: The role of the temporo‐parietal junction in “theory of mind.”. NeuroImage 19:1835–1842. [DOI] [PubMed] [Google Scholar]
- Shamay‐Tsoory SG (2011): The neural bases for empathy. Neuroscientist 17:18–24. [DOI] [PubMed] [Google Scholar]
- Shamay‐Tsoory SG, Aharon‐peretz J, Levkovitz Y (2007a): The neuroanatomical basis of affective mentalizing in schizophrenia: Comparison of patients with schizophrenia and patients with localized prefrontal lesions. Schizophr Res 90:274–283. [DOI] [PubMed] [Google Scholar]
- Shamay‐Tsoory SG, Shur S, Barcai‐Goodman L, Medlovich S, Harari H, Levkovitz Y (2007b): Dissociation of cognitive from affective components of theory of mind in schizophrenia. Psychiatry Res 149:11–23. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, Mintun MA, Wang S, Coalson RS, Raichle ME (2009): The default mode network and self‐referential processes in depression. Proc Natl Acad Sci U S A 106:1942–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, Beckmann CF (2009): Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci U S A 106:13040–13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MJ, Horan WP, Karpouzian TM, Abram SV, Cobia DJ, Csernansky JG (2012): Self‐reported empathy deficits are uniquely associated with poor functioning in schizophrenia. Schizophr Res 137:196–202. [DOI] [PubMed] [Google Scholar]
- Smith SM, Beckmann CF, Andersson J, Auerbach EJ, Bijsterbosch J, Douaud G, Duff E, Feinberg DA, Griffanti L, Harms MP, Kelly M, Laumann T, Miller KL, Moeller S, Petersen S, Power J, Salimi‐Khorshidi G, Snyder AZ, Vu AT, Woolrich MW, Xu J, Yacoub E, Uğurbil K, Van Essen DC, Glasser MF; WU‐Minn HCP Consortium (2013): Resting‐state fMRI in the Human Connectome Project. NeuroImage 80:144–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MJ, Horan WP, Cobia DJ, Karpouzian TM, Fox JM, Reilly JL, Breiter HC (2014): Performance‐based empathy mediates the influence of working memory on social competence in Schizophrenia. Schizophr Bull 40:824–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MJ, Schroeder MP, Abram SV, Goldman MB, Parrish TB, Wang X, Derntl B, Habel U, Decety J, Reilly JL, Csernansky JG, Breiter HC (2015): Alterations in brain activation during cognitive empathy are related to social functioning in schizophrenia. Schizophr Bull 41:211–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Andrews‐Hanna JR (2015): The default network and social cognition In: Toga AW, editor. Brain Mapping: An Encyclopedic Reference. Academic Press: Elsevier: pp. 165–169. [Google Scholar]
- Sprong M, Schothorst P, Vos E, Hox J, van Engeland H (2007): Theory of mind in schizophrenia: Meta‐analysis. Br J Psychiatry 191:5–13. [DOI] [PubMed] [Google Scholar]
- Strauss GP, Horan WP, Kirkpatrick B, Fischer BA, Keller WR, Miski P, Buchanan RW, Green MF, Carpenter WT Jr (2013): Deconstructing negative symptoms of schizophrenia: Avolition‐apathy and diminished expression clusters predict clinical presentation and functional outcome. J Psychiatr Res 47:783–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, Waltz JA, Gold JM (2014): A review of reward processing and motivational impairment in schizophrenia. Schizophr Bull 40(S2):107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavor I, Jones OP, Mars RB, Smith SM, Behrens TE, Jbabdi S (2016): Task‐free MRI predicts individual differences in brain activity during task performance. Science 352:216–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, Wang J, Yan C, He Y (2011): Hemisphere‐ and gender‐related differences in small‐world brain networks: A resting‐state functional MRI study. NeuroImage 54:191–202. [DOI] [PubMed] [Google Scholar]
- Tregellas JR, Smucny J, Harris JG, Olincy A, Maharajh K, Kronberg E, Eichman LC, Lyons E, Freedman R (2014): Intrinsic hippocampal activity as a biomarker for cognition and symptoms in schizophrenia. Am J Psychiatry 171:549–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaden KI, Gebregziabher M, Kuchinsky SE, Eckert MA (2012): Multiple imputation of missing fMRI data in whole brain analysis. NeuroImage 60:1843–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Meer L, Costafreda S, Aleman A, David AS (2010): Self‐reflection and the brain: A theoretical review and meta‐analysis of neuroimaging studies with implications for schizophrenia. Neurosci Biobehav Rev 34:935–946. [DOI] [PubMed] [Google Scholar]
- Ventura J, Wood RC, Hellemann GS (2013): Symptom domains and neurocognitive functioning can help differentiate social cognitive processes in schizophrenia: A meta‐analysis. Schizophr Bull 39:102–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura J, Ered A, Subotnik KL, Horan WP, Hellemann GS (2015): Theory of mind in the early course of schizophrenia: Stability, symptom and neurocognitive correlates, and relationship with functioning. Psychol Med 45:2031–2043. [DOI] [PubMed] [Google Scholar]
- Walter H (2012): Social cognitive neuroscience of empathy – Concepts, circuits and genes. Emotion Rev 4:9–17. [Google Scholar]
- Whitfield‐Gabrieli S, Ford JM (2012): Default mode network activity and connectivity in psychopathology. Ann Rev Clin Psychol 8:49–76. [DOI] [PubMed] [Google Scholar]
- Whitfield‐Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraon SV, McCarley RW, Shenton ME, Green AI, Nieto‐Castanon A, LaViolette P, Wojcik J, Gabrieli JDE, Seidman LJ (2009): Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first‐degree relatives of persons with schizophrenia. Proc Natal Acad Sci U S A 106:1279–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisner KM, Atluri G, Lim KO, Macdonald AW (2013a): Neurometrics of intrinsic connectivity networks at rest using fMRI: Retest reliability and cross‐validation using a meta‐level method. NeuroImage 76:236–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisner KM, Patzelt EH, Lim KO, MacDonald AW (2013b): An intrinsic connectivity network approach to insula‐derived dysfunctions among cocaine users. Am J Drug Alcohol Abuse 39:403–413. [DOI] [PubMed] [Google Scholar]
- Zaki J, Ochsner K (2012): The neuroscience of empathy: Progress, pitfalls and promise. Nat Neurosci 15:675–680. [DOI] [PubMed] [Google Scholar]
- Zilbovicius M, Meresse I, Chabane N, Brunelle F, Samson Y, Boddaert N (2006): Autism, the superior temporal sulcus and social perception. Trend Neurosci 29:359–366. [DOI] [PubMed] [Google Scholar]
- Zuo XN, Kelly C, Adelstein JS, Klein DF, Castellanos FX, Milham MP (2010): Reliable intrinsic connectivity networks: Test‐retest evaluation using ICA and dual regression approach. NeuroImage 49:2163–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Figures.
Supporting Information Table 1.
Supporting Information


