Abstract
Knowledge about the recovery of oral intake after hemispheric stroke is important to guide therapeutic decisions, including the administration of enteral tube feeding and the choice of the appropriate feeding route. They aimed to determine the localization and connectivity of lesions in impaired recovery versus recovered swallowing after initially dysphagic stroke. Sixty‐two acute ischemic hemispheric stroke patients with impaired oral intake were included in a prospective observational cohort study. Voxel‐based lesion‐symptom mapping and probabilistic tractography were used to determine the association of lesion location and connectivity with impaired recovery of oral intake ≥7 days (indication for early tube feeding) and ≥4 weeks (indication for percutaneous endoscopic gastrostomy feeding) after stroke. Two distinct patterns influencing recovery of swallowing were recognized. Firstly, impaired recovery of oral intake after ≥7 days was significantly associated with lesions of the superior corona radiata (65% of statistical map, P < 0.05). The affected fibers were connected with the thalamus, primary motor, and supplemental motor areas and the basal ganglia. Secondly, impaired recovery of oral intake after ≥4 weeks significantly correlated with lesions of the anterior insula (54% of statistical map, P < 0.05), which was connected to adjacent operculo‐insular areas of deglutition. These findings indicate that early swallowing recovery is influenced by white matter lesions disrupting thalamic and corticobulbar projection fibers. Late recovery is determined by specific cortical lesions affecting association fibers. This knowledge may help clinicians to identify patients at risk of prolonged swallowing problems that would benefit from enteral tube feeding. Hum Brain Mapp 38:2165–2176, 2017. © 2017 Wiley Periodicals, Inc.
Keywords: stroke, deglutition disorders, dysphagia, magnetic resonance imaging, rehabilitation
INTRODUCTION
Swallowing disorders are a common and potentially life‐threatening consequence of ischemic hemispheric stroke. They occur in more than half of patients and can be the cause of complications including malnutrition, dehydration, and aspiration pneumonia [Foley et al., 2009; Martino et al., 2005]. Dysphagia can also lead to an increase of post‐stroke mortality and health‐care costs [Bonilha et al., 2014; Smithard et al., 2007].
A number of neuroimaging studies examined predictors of acute dysphagia after hemispheric stroke. They found acute dysphagia to be caused by lesions to a distributed swallowing network encompassing the primary sensorimotor cortex, frontal operculum, insula, and associated white matter tracts passing through the corona radiata and the posterior limb of the internal capsule [Galovic et al., 2013, 2016; Kim et al., 2016; Suntrup et al., 2015]. Severe impairment of swallowing in the acute phase was associated with lesions of the insula [Galovic et al., 2016] and the postcentral gyrus [Suntrup et al., 2015].
However, little knowledge exists about neuroanatomical predictors of the recovery of swallowing after stroke. Improved understanding of swallowing recovery is clinically important for several reasons. Firstly, patients with prolonged swallowing difficulties have worse functional outcome after stroke, that is, a higher risk of pneumonia or institutionalization and a longer duration of hospital stay [Broadley et al., 2003; Galovic et al., 2013]. Secondly, guidelines for enteral nutrition rely on the duration and severity of impaired oral intake as the main criterion to choose the appropriate feeding method [Corrigan et al., 2011; Gomes et al., 2014; Stroud et al., 2003; Wirth et al., 2013]. Enteral tube feeding should be established if oral intake is likely to be insufficient for at least 7 days [Corrigan et al., 2011; Stroud et al., 2003; Wirth et al., 2013]. Percutaneous endoscopic gastrostomy (PEG) feeding should be preferred if swallowing disturbances are likely to persist for more than 4 weeks [Corrigan et al., 2011; Gomes et al., 2014; Stroud et al., 2003; Wirth et al., 2013]. However, early enteral nutrition should be commenced within the first 72 hours [Dennis et al., 2005]. Hence, clinicians need to predict the duration of impaired oral intake to guide their therapeutic decisions. Finally, this knowledge might be helpful in advising patients and relatives and might facilitate the provision of healthcare services in a fiscally responsible manner.
Emerging evidence indicates that stroke location might influence the recovery of swallowing. Frontal and insular lobes were most commonly affected in patients with dysphagia persisting for 2 weeks [Broadley et al., 2003, 2005]. In a recent pilot study, we demonstrated the association between operculo‐insular lesions and risk of aspiration 7 days after stroke [Galovic et al., 2013]. However, these studies were merely statistical investigations that did not directly address the morphology of lesions in a voxel‐wise approach, did not focus on outcome parameters relevant for enteral tube feeding and study limitations included a short follow‐up and small sample size.
Only limited functional imaging data exists on the recovery of swallowing. Dysphagic stroke is associated with a widespread decrease of swallowing network activation in the ipsilesional hemisphere, whereas there is increased activation in the contralesional somatosensory cortex, middle frontal gyrus, and insula [Li et al., 2009]. Similarly, overactivation of the unaffected hemisphere was observed in stroke patients who recovered from dysphagia [Mihai et al., 2016]. Effective swallowing recovery was associated with functional reorganization of the contralesional primary motor cortex [Hamdy et al., 1998]. Similarly, there is some evidence in motor stroke indicating that increased activation of the contralesional cortex might contribute to effective recovery [Buetefisch, 2015].
We have performed a voxel‐based lesion‐symptom mapping (VLSM) and probabilistic tractography study of the association between lesion location and the persistence of impaired oral intake after ≥7 days and ≥4 weeks. From a clinical perspective, our aim was to find neuroanatomical predictors for the indication of early tube feeding (≥7 days) and PEG feeding (≥4 weeks) after hemispheric ischemic stroke.
METHODS
Participants
We screened 663 consecutive stroke patients admitted between January 2011 and June 2013 to a tertiary hospital in Switzerland (Fig. 1). Included were individuals without previous history of dysphagia who suffered acute first‐ever hemispheric ischemic stroke leading to impaired oral intake. Excluded were patients with no or minor impairment of functional oral intake (n = 350, see section “Impairment of Oral Intake”), recurrent stroke (n = 74), infratentorial stroke (n = 55), diagnosis other than ischemic stroke (n = 32), no magnetic resonance imaging (MRI) scan (n = 22), no evidence of stroke on imaging (n = 11), severe impairment of consciousness interfering with evaluation of swallowing (n = 10), patient arrival greater than 48 hours after stroke onset (n = 9), poor quality brain scan due to movement artifacts (n = 2) and pre‐existing dysphagia (n = 2). Some patients were lost to follow‐up (n = 20), died within the first 5 days after stroke (n = 12) or did not give consent (n = 2). The final study population consisted of 62 patients. Additionally, 24 healthy volunteers were explored for probabilistic tractography (see section “Probabilistic Tractography”). Patients and healthy volunteers gave informed consent according to the Declaration of Helsinki. Approval has been given by the local ethics committees.
Figure 1.
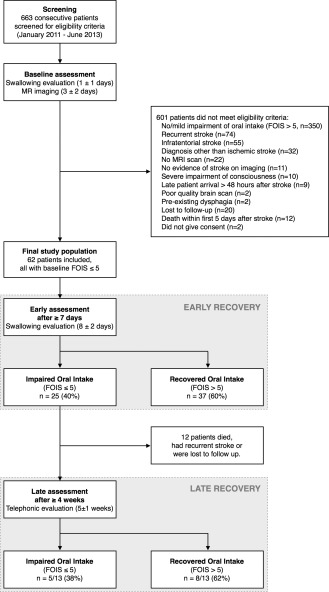
Study flow diagram.
Clinical Assessments
Swallowing was assessed within 48 hours of stroke onset (median 1 ± 1 days) and re‐evaluated ≥7 days after stroke (median 8 ± 2 days, Fig. 1). The evaluators were not aware of the MRI results. A telephonic follow‐up was performed at least 4 weeks after stroke (median 5 ± 1 weeks). In between all assessments routine therapeutic procedures were performed.
Swallowing assessment consisted of the examination of oral musculature strength, agility, and symmetry, as well as examination of protective reflexes and sensation by a speech‐language pathologist. Testing included the 50 mL water swallow test [DePippo et al., 1992] and Any 2 scale [Daniels et al., 1997] for risk of aspiration. Severity of dysphagia was quantified with the Parramatta Hospitals Assessment of Dysphagia [Broadley et al., 2005]. Additionally, Gugging Swallowing Screen [Trapl et al., 2007] was performed in some but not all cases. Instrumental testing with fiberoptic endoscopy was scored with the Fiberoptic Endoscopic Dysphagia Severity Scale (FEDSS) [Warnecke et al., 2009] and was performed in subjects with indeterminate results of the clinical swallowing evaluation or when deemed necessary by the treating physician. These evaluations were performed at baseline and at the early re‐assessment after ≥7 days (Fig. 1).
The late assessment after ≥4 weeks consisted of a standardized structured telephonic interview performed by a speech‐language pathologist (Fig. 1). The patient and/or medical staff were interviewed on the current diet modification, return to prestroke diet, restrictions on safe food and liquid consistencies, food preparations, compensation strategies, quantity of oral intake and enteral tube feeding.
Data at discharge was assessed by the treating physician and included the modified Rankin Scale (mRS) [Van Swieten et al., 1988], pneumonia during hospitalization, duration of hospital stay and institutionalization.
Impairment of Oral Intake
The duration of impaired oral intake is the decisive parameter to determine whether a patient should be tube‐fed and this parameter should be used to choose the appropriate feeding method (nasogastric vs. PEG feeding), as suggested by guidelines [Stroud et al., 2003; Wirth et al., 2013]. We scored the severity of impaired oral intake according to the Functional Oral Intake Scale (FOIS), a widely used, reliable and valid outcome measure to document a patient's safe and adequate functional oral intake [Crary et al., 2005]. The scale ranges from 1 (nothing by mouth) to 7 (total oral diet with no restrictions).
The evaluation is based on the level of oral intake or food and liquid consistency recommended by an objective swallow evaluation. To increase generalizability, a standardized guideline was established (Table 1). The main aspect of this guideline is the type and number of consistencies (solids, semisolids, liquids) that can be safely swallowed based on the results of clinical/instrumental testing. Additional parameters are the proportion of required intake that is safely consumed by mouth and quantitative results of clinical/instrumental swallowing evaluations. FOIS was derived based on the overall swallowing assessment at baseline and at the re‐evaluation after ≥7 days. At the late assessment after ≥4 weeks, FOIS was determined by a structured telephonic interview.
Table 1.
Guideline for Functional Oral Intake (FOIS) scoring based on a comprehensive swallow evaluation
| Safe swallowing possible for | |||||
|---|---|---|---|---|---|
| FOIS level | semisolids | liquids | solids | Quantity of oral intake | Supportive findings |
| Level 7 | Yes | Yes | Yes | Normal portion |
• 50 mL water swallow test negative • Any 2 < 2 • PHAD > 90 • GUSS = 20 • FEDSS = 1 |
| Level 6 | Yes | Yes | Yes | Normal portion |
• 50 mL water swallow test negative • Any 2 < 2 • PHAD ≤ 90 • GUSS 15 to 19 • FEDSS = 2 |
| Avoid challenging consistencies (e.g., mixed consistencies, crumby food) | |||||
| Level 5 | At least two consistencies can be swallowed securely | Normal portion | |||
| Level 4 | Yes | No | No | Normal portion |
• 50 mL water swallow test positive • Any 2 ≥ 2 • PHAD ≤ 80 • GUSS 10 to 14 • FEDSS 3–4 |
| Level 3 | Yes | No | No | ≤50% of normal portion | |
| Level 2 | Yes | No | No | Only few spoons or sips per mouth | |
| Level 1 | No | No | No | Nothing by mouth |
• 50 mL water swallow test positive • Any 2 ≥ 2 • PHAD ≤ 80 • GUSS ≤ 9 • FEDSS ≥ 4 |
“Safe swallowing” corresponds to consistencies that can be safely swallowed by a subject based on the results of the clinical/instrumental swallowing evaluation. “Portion” corresponds to the percent of required intake that is safely consumed by mouth.
To evaluate the recovery of swallowing, only patients with FOIS ≤5 at the initial assessment were included in the study, corresponding to a total oral diet with at least moderate or major restrictions. Subjects who recovered functional oral intake (FOIS > 5) at the early assessment ≥7 days after stroke were excluded from further follow‐up at the late assessment (Fig. 1). This was done to study swallowing recovery during the second to fourth week after stroke, hence, all subjects who have already recovered within the first week had to be excluded.
Statistical Analysis
Data is presented either as No. (%) for categorical variables or as median ± interquartile range for quantitative variables. The association of clinical variables and lesion load with swallowing recovery was calculated using univariate cumulative odds ordinal logistic regression after the assumption of proportional odds has been met. P values <0.05 were considered significant after correction for multiple testing with the Holm‐Bonferroni approach. Data were complete except for the modified Rankin Scale (mRS) at discharge, which was missing in 16 cases. Missing information in these 16 cases was imputed with the multiple imputations method. Analyses were carried out in SPSS 20.0 (IBM Corp.).
Brain Scan Acquisition
All subjects received one MRI scan performed within a median of 3 ± 2 days after stroke onset. Our standard stroke imaging protocol comprised transverse T2, T1, FLAIR (5 mm) and sagittal T2 images (4.5 mm), isotropic diffusion‐weighted imaging (DWI) sequences (b = 1,000 s/mm2) with transverse slices (4 mm) and integrated ADC map calculation and an intracranial arterial time‐of‐flight sequence acquired either on a 1.5T Siemens Avanto, 1.5T Siemens Symphony or a 3T Siemens Verio MRI system (Siemens, Erlangen, Germany). The whole brain was covered with all sequences.
Diffusion Tensor Imaging (DTI) scans in a cohort of 24 healthy individuals (see section “Probabilistic Tractography”) were acquired on a 3 tesla MRI scanner (Siemens Trio, Erlangen, Germany) equipped with an 18 channel head coil. The scans were obtained with the routine clinical sequence and consisted of a b = 0, 1,300 s/mm2 image (N = 42 directions, NEX = 2 averages, iPAT 2 using GRAPPA, 55 slices, pixel resolution 2.2 mm iso, TR/TE = 7,600/95 ms, bandwidth 1346 Hz/px). Additionally, anatomical scans were acquired using a T1‐weighted Modified Driven Equilibrium Fourier Transform (MDEFT) sequence.
Lesion‐Symptom Mapping
Established techniques of voxel‐based lesion‐symptom mapping (VLSM) were used. This is a structural approach to analyze the association of lesion location with impaired oral intake [Rorden and Karnath, 2004]. First, the individual lesion maps were manually outlined on DWI scans in MRIcron (http://www.mccauslandcenter.sc.edu/mricro/mricron/) by an evaluator blinded to patient outcome data. Second, T1 scans with co‐registered DWI sequences were spatially normalised with the unified segmentation algorithm of SPM8 using cost‐function masking to match the International Consortium for Brain Mapping (ICBM) standard template [Andersen et al., 2010; Ashburner and Friston, 2005; Rorden et al., 2012]. Next, individual volume of the normalized lesion maps was calculated. Finally, all lesion maps were flipped to one side in order to focus our analysis on lesion localization without regard to lateralization, as there were no significant differences between left and right hemispheric strokes in the baseline analysis.
The relationship between lesion location and the impairment of oral intake was calculated in the NPM software (http://www.mccauslandcenter.sc.edu/mricro/mricron/). Functional oral intake measured with FOIS exhibits a continuous spectrum of severity. To retain all available information of this parameter, it was introduced as an ordinal outcome variable without the need to specify a cut‐off value [Karnath and Smith, 2014]. Voxel‐wise analysis was done using the nonparametric Brunner–Munzel test [Rorden et al., 2007]. To reduce a type II error, only voxels affected in at least 10% of patients and a minimum cluster size of 30 voxels were considered. The obtained statistical maps were thresholded to a significance less than 0.05 after family‐wise error correction for multiple testing and related to anatomical structures using the AAL atlas [Tzourio‐Mazoyer et al., 2002] for gray matter and the JHU atlas [Wakana et al., 2004] for white matter.
In a further step, we performed a regions‐of‐interest (ROI) analysis to evaluate the impact of lesion load within selected ROIs on the recovery of swallowing. Based on the VLSM results we selected the superior corona radiata (JHU atlas) for early recovery of swallowing and the insular cortex (AAL atlas) for late recovery of swallowing. We extracted the proportion of voxels within these ROIs affected by a subject's ischemic lesion using MRIcron.
Probabilistic Tractography
Statistical lesion maps obtained in the VLSM analysis (Fig. 2A for early recovery and Fig. 3A for late recovery) may affect white matter fibers. To determine the statistical connectivity of these fibers with grey matter structures, we performed a probabilistic tractography using diffusion tensor imaging (DTI) data from a cohort of 24 healthy individuals (58% male, aged 63 ± 9 years).
Figure 2.
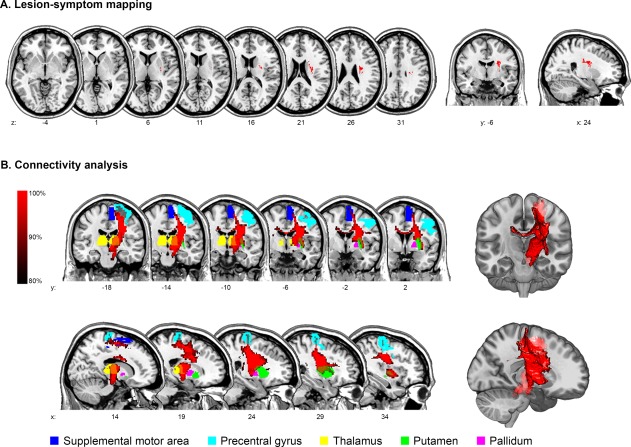
Imaging analysis after ≥7 days. (A) Voxel‐based lesion‐symptom mapping of impaired oral intake after ≥7 days. (B) Connectivity analysis of the statistical map. For illustration purposes, a mean path with a minimum frequency of 80% is displayed and significantly connected regions are overlaid. [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 3.
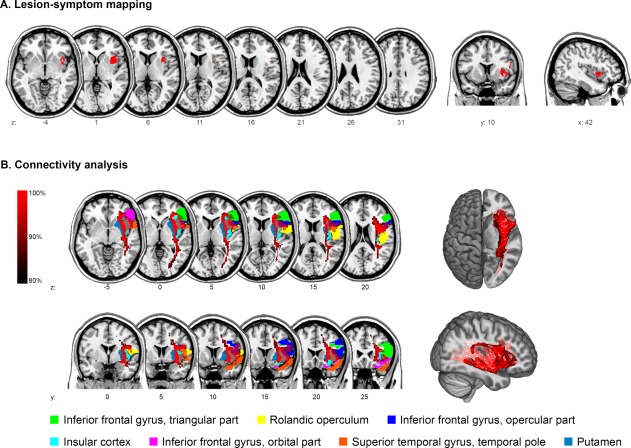
Imaging analysis after ≥4 weeks. (A) Voxel‐based lesion‐symptom mapping of impaired oral intake after ≥4 weeks. (B) Connectivity analysis of the statistical map. For illustration purposes, a mean path with a minimum frequency of 80% is displayed and significantly connected regions are overlaid. [Color figure can be viewed at http://wileyonlinelibrary.com]
We used FMRIB Software Library (FSL)'s Diffusion Toolkit (FDT) (http://www.fmrib.ox.ac.uk/fsl) for the preprocessing of the diffusion images and to estimate the diffusion tensor. Images underwent eddy current correction (using the eddy_correct tool), which corrects for spatial distortion and for simple head motion through affine transformation to a reference volume of each diffusion weighted image. Brain Extraction Tool (BET) with fractional threshold of 0.3 was used to delete non‐brain tissue from the images of the whole head.
All anatomical images (MDEFT) were registered to their respective DTI data using the linear FLIRT algorithm (FSL). In addition, the anatomical images were registered to the MNI standard space using both linear (FLIRT/FSL) and non‐linear (FNIRT/FSL) algorithms. The resulting transformations were inverted and combined to derive a mapping from native (DTI) to standard (MNI) space.
FDT's DTIfit was then used for voxel‐wise calculation of the diffusion tensor within the brain. Before probabilistic tracking, Markov Chain Monte Carlo sampling was run to local modeling distributions of diffusion parameters at each voxel using BEDPOST (Bayesian Estimation of Diffusion Parameters Obtained using Sampling Techniques).
Probabilistic tracking using FDT's Probtrackx2 was performed using the seed mask corresponding to the statistical map obtained with VLSM for early recovery (statistical map in Fig. 2A) and for late recovery (statistical map in Fig. 3A). The seed mask in standard space was transformed into each subject's native space using an affine linear and non‐linear transformation (FLIRT, FNIRT). After probabilistic tracking in each subject, the resulting paths were transformed back to MNI space and a mean path was generated.
Connectivity analyses from diffusion MRI are confounded by factors such as distance between points and curvature along the connection, complicating quantitative interpretations of which regions are connected [Jones, 2010]. To account for these confounding factors, we have used a previously proposed technique [Morris et al., 2008] that corrects for the probability of connections occurring from a purely random distribution. After applying this correction, the tractography results in each voxel could be termed as giving the “connectivity probability” between the statistical lesion map (Fig. 2A for early recovery and Fig. 3A for late recovery) and each voxel in the image. For each AAL region, the median connectivity probability was calculated, and all regions with a “median connectivity” value equal to or higher than 0.25 were termed “significantly connected” [Morris et al., 2008].
RESULTS
Early Assessment After ≥7 Days
Clinical data
The study population comprised 62 acute ischemic stroke patients (45% male, aged 75 ± 21 years) with impaired oral intake in the first 48 hours after stroke (median FOIS 4 ± 2). After ≥7 days, median FOIS increased to 6 ± 2 and 25 (40%) patients had a FOIS ≤5. Failed recovery of oral intake after ≥7 days correlated with poor outcome after stroke (Table 2). There was no association with stroke lateralization or lesion size.
Table 2.
Association of impaired oral intake after ≥7 days with outcome and complications after stroke
|
Impaired after ≥7 days (n = 25) |
Recovered after ≥7 days (n = 37) |
OR (95% CI) | P value | |
|---|---|---|---|---|
| Pneumonia during hospital stay | 3 (12%) | 2 (5%) | 4.9 (0.9–25.8) | 0.06 |
| mRS at discharge | 4 ± 2 | 2 ± 2 | 1.9 (1.2–2.8) | 0.005a |
| Duration of hospital stay (days) | 15 ± 9 | 12 ± 5 | 1.1 (1.0–1.2) | 0.006a |
| Institutionalisation | 24 (96%) | 27 (73%) | 4.2 (1.2–14.8) | 0.03 |
Data is presented as N (%) or median ± IQR. The ORs and P values denote whether poor oral intake after ≥7 days was associated with poor outcome after stroke.
Significant P value after Holm–Bonferroni correction. mRS, modified Rankin Scale; OR, odds ratio; CI, confidence interval.
Lesion analysis
The statistical map (Fig. 2A) of voxels associated with impaired oral intake after ≥7 days affected white matter structures in 89% with a center of maximum overlap over the superior corona radiata (MNI coordinates x = 27, y = −7, z = 24). The largest proportion of significant voxels covered the superior corona radiata (65%) and to a lesser extent the superior longitudinal fascicle (12%), external capsule (8%) and putamen (7%, Table 3A).
Table 3.
Anatomical regions associated with impaired oral intake after ≥7 days and ≥4 weeks
| Proportion of statistical map affected (% of voxels) |
Center of statistical map (MNI coordinates) |
|||
|---|---|---|---|---|
| x | y | z | ||
| A. Impaired oral intake after ≥7 days | ||||
| Superior corona radiate | 65 | 26 | −7 | 23 |
| Superior longitudinal fascicle | 12 | 31 | −16 | 33 |
| External capsule | 8 | 28 | −5 | 16 |
| Putamen | 7 | 26 | −4 | 16 |
| B. Impaired oral intake after ≥4 weeks | ||||
| Insular cortex | 54 | 39 | −2 | −10 |
| Putamen | 10 | 34 | −6 | −13 |
| Inferior frontal gyrus, opercular part | 9 | 50 | 14 | −1 |
Only regions affecting ≥5% of the statistical maps are reported. MNI, Montreal Neurological Institute.
Connectivity analysis
Probabilistic tractography in Figure 2B shows that the statistical lesion map (Fig. 2A) mainly affected projection fibers and, to a lesser degree, commissural fibers to the contralateral thalamus. Highest median connectivity (MC) was observed with the ipsilateral thalamus (MC 0.88). The ipsilateral supplemental motor area and precentral gyrus had the highest proportion of connected voxels (82% and 70%, respectively). Other significantly connected regions were the contralateral thalamus, ipsilateral putamen, and ipsilateral pallidum (Table 4A).
Table 4.
Anatomical regions significantly connected to the statistical lesion map after ≥7 days and ≥4 weeks
| Proportion of region connected (% of voxels) | Median connectivity | |
|---|---|---|
| A. Impaired oral intake after ≥7 days | ||
| Supplemental motor area | 82 | 0.33 |
| Precentral gyrus | 70 | 0.25 |
| Thalamus ipsilateral | 60 | 0.88 |
| Thalamus contralateral | 56 | 0.29 |
| Putamen | 54 | 0.50 |
| Pallidum | 49 | 0.25 |
| B. Impaired oral intake after ≥4 weeks | ||
| Inferior frontal gyrus, triangular part | 80 | 0.33 |
| Rolandic operculum | 78 | 0.29 |
| Inferior frontal gyrus, opercular part | 74 | 0.79 |
| Insular cortex | 66 | 0.75 |
| Inferior frontal gyrus, orbital part | 63 | 0.29 |
| Superior temporal gyrus, temporal pole | 60 | 0.29 |
| Putamen | 57 | 0.71 |
Anteroposterior‐localization
Motor fibers passing through the corona radiata are organized somatotopically with tracts representing facial muscles located anteriorly, the upper limb in the middle, and the lower limb posteriorly. The location of a lesion can be quantified with the CP (distance between the center of the lesion and the posterior pole of the lateral ventricle) to AP (distance between the anterior and posterior poles of the lateral ventricle) ratio [Kim and Pope, 2005]. The center of the statistical map in the corona radiata had a CP/AP ratio of 0.53. This indicates a location immediately anterior to the facial fibers [CP/AP 0.44 ± 0.08 according to Kim and Pope, 2005].
Lesion load in the corona radiata
Based on the above results, we performed a post‐hoc analysis of the association of lesion load in the superior corona radiata with early swallowing recovery. The proportion of damaged voxels in the superior corona radiata negatively correlated with the degree of oral intake after ≥7 days (P = 0.001). About 83% (5/6) of subjects in whom more than 50% of the corona radiata was affected had impaired oral intake after ≥7 days. In comparison, oral intake was impaired in 57% (8/14) of those with damage to 25%–50% of the corona radiata and in 29% of those with less than 25% damage.
Late Assessment After ≥4 Weeks
Clinical data
Out of 25 patients eligible for late assessment after ≥4 weeks, twelve had died, had recurrent stroke or were lost to follow‐up (Fig. 1). About 13 remaining patients were included in final analysis (31% male, aged 76 ± 17 years). Median FOIS at the late assessment was 6 ± 1 and five (38%) patients had a FOIS ≤5. Outcome was mostly unfavorable with mRS of 4 ± 2, institutionalization in 92% and pneumonia in 15%. There were no differences in lesion size and stroke laterality.
Lesion analysis
The statistical lesion map associated with impaired oral intake after ≥4 weeks covered gray matter in 76% (Fig. 3A). The center of maximum overlap was over the anterior insular cortex (MNI coordinates x = 39, y = 10, z = 2). Significant proportions of the statistical map affected the insula (54%) and to a lesser extent the putamen (10%) and the inferior frontal operculum (9%, Table 3B).
Connectivity analysis
The statistical lesion map (Fig. 3A) was significantly connected through association fibers with several operculo‐insular regions, including the Rolandic operculum, insular cortex, and the inferior frontal gyrus, as shown in Figure 3B. The highest median connectivity was measured in the opercular part of the inferior frontal gyrus (MC 0.79) and the insular cortex (MC 0.75). Other significantly connected regions were the superior temporal gyrus and the putamen (Table 4B).
Lesion load in the insular cortex
A post‐hoc analysis showed that the proportion of damaged voxels in the insular cortex negatively correlated with the degree of oral intake after ≥4 weeks (P = 0.04). All subjects (4/4) in whom ischemic damage affected more than 50% of the insula had impaired oral intake after ≥4 weeks. In contrast, oral intake was impaired in 50% (1/2) of those with damage to 25%–50% of the insula and none (0/7) of those with less than 25% damage.
DISCUSSION
This study analyzed the association of lesion location and connectivity with the recovery of initially impaired oral intake 7 days and 4 weeks after hemispheric stroke. We have demonstrated that impaired recovery of swallowing function within 7 days is influenced by white matter lesions affecting mainly projection fibers within the corona radiata. In contrast, swallowing recovery within 4 weeks is determined by cortical lesions affecting the anterior insular cortex and adjacent association fibers.
Early Recovery After ≥7 Days
Traditionally, acute swallowing disturbances after stroke have been related to lesions of the brainstem or the cerebral cortex [Flowers et al., 2011] and the role of subcortical structures has not been recognized for a long time. However, lesions of the periventricular white matter were recently shown to be associated with acute dysphagia [Cola et al., 2010] or acute risk of aspiration [Daniels and Foundas, 1999; Galovic et al., 2013] and bilateral corona radiata infarcts were observed to cause the Foix–Chavany–Marie syndrome with inability to swallow [Bradley et al., 2014]. These authors assumed a disconnection of corticobulbar fibers as the most likely mechanism of disturbed deglutition.
In addition to these findings, our novel results demonstrate that subcortical structures not only cause acute dysphagia but also interfere with recovery of swallowing. Subcortical lesions can cause pronounced deactivation of large cortical areas due to the phenomenon of diaschisis [Finger et al., 2004] leading to a wide‐ranging disconnection of the overlying cortical network [Rijntjes, 2006]. We assume that diaschisis due to lesions of the corona radiata might cause a breakdown of the cortical swallowing network. This down‐regulation is likely to last from several days up to one week [Rijntjes, 2006] and might markedly influence the recovery of functional swallowing.
Connectivity analysis of the subcortical statistical lesion map (Fig. 2A) showed significant interconnection with a distributed network involving the ipsilateral and contralateral thalamus, the primary motor and premotor cortex, and the basal ganglia (Fig. 2B, Table 4A). The thalamus plays a role in the sensorimotor integration of swallowing through thalamocortical and thalamostriatal fibers [Mosier and Bereznaya, 2001; Mosier et al., 1999]. Sensory afferent input is necessary for processing the structural aspects of the bolus being swallowed as well as for timing of consecutive stages of deglutition. This has been elegantly underlined in studies of oropharyngeal anesthesia, which caused disturbed swallowing [Chee et al., 2005; Ertekin et al., 2000] and reduced cortical activation during deglutition [Teismann et al., 2007]. It can be assumed that a disruption of thalamic fibers within the corona radiata might lead to a sensory and motor disintegration of swallowing. Interestingly, lesions in the corona radiata were not only connected to the ipsilateral but also to the contralateral thalamus. This finding implies that a unilateral subcortical lesion could affect the swallowing network in both hemispheres.
The statistical map (Fig. 2A) also interconnects with the primary motor and premotor cortex, the caudal parts of which have been shown to represent swallowing musculature (Table 4A) [Hamdy et al., 1996]. Projection fibers in the corona radiata are organized somatotopically with facial tracts located anteriorly and lower limb fibers posteriorly. In this study, the anteroposterior location of the statistical map in the corona radiata had a CP/AP ratio of 0.53, signifying a location immediately anterior to the facial fibers [Kim and Pope, 2005]. Extending the principle of somatotopical organization, the affected pyramidal tracts likely involve corticobulbar fibers of the pharyngeal musculature. Disrupting these fibers would lead to disconnection of the swallowing centers in the cortex and the brainstem. Additionally, the post‐hoc analysis suggests that larger strokes affecting bigger parts of the corona radiata are more likely to disrupt these fibers. However, location of the lesion might be more important than its size as also some subjects with small lesion load (<25%) had impaired swallowing recovery if stroke specifically damaged these corticobulbar fibers.
Late Recovery After ≥4 Weeks
Our findings indicate that influence on swallowing recovery shifts from subcortical to cortical regions during the second to fourth week after stroke. This observation is consistent with knowledge on motor and language recovery after stroke. Whereas the first phase of rehabilitation is characterized by a widespread depression of the cortical network, the second phase is associated with an up‐regulation and over‐activation, possibly due to the onset of cortical plasticity [Rijntjes, 2006]. Additionally, our connectivity analysis suggests that the affected type of fibers shifts from projection to association fibers.
It is well known that insular stroke commonly leads to swallowing disturbances [Daniels and Foundas, 1997; Galovic et al., 2013; Riecker et al., 2009]. However, it is a new finding that lesions of the anterior insula modulate swallowing recovery during the second to fourth week after stroke. We propose three explanations for this observation.
Firstly, sensory information about bolus structure and quality is a prerequisite for proper timing of deglutition and for secure swallowing, as described in the sections above. The anterior insular cortex, in addition to the thalamus, is regarded as a central area of sensory integration during swallowing [Aziz et al., 1997; Humbert and Joel, 2012]. The multisensory integrative nature of the insula is underlined by the probabilistic tractography, which shows a high connectivity throughout the ipsilateral hemisphere through association fibers. Some of these fibers extend as far as the occipital and parietal association cortices, most likely being part of the interior fronto‐occipital fasciculus (Fig. 3B) [Catani et al., 2012]. Thus, damage to the insula might slow recovery of oral intake because of sensorimotor disintegration of swallowing. Similar findings were demonstrated in hemiparetic cortical stroke, as motor recovery was impaired due to disturbed afferent input from lesioned sensory cortices [Abela et al., 2012].
Secondly, mounting evidence supports a key role of the insular cortex in the planning and preparation of volitional swallowing. Insular activation starts a long time prior to the onset of deglutition [Watanabe et al., 2004]. Several but not all studies found it to specifically trigger volitional swallowing, whereas insular activation was not observed during reflexive swallows or simple tongue movements [Dziewas et al., 2003; Kern et al., 2001]. Hence, the insular cortex may represent a premotor module, integrating multisensory afferences into premotor areas for deglutition. Damage of this complex might disrupt access to the primary swallowing cortex and impair rehabilitation.
Finally, infarctions of the primary swallowing cortex in the caudal precentral gyrus commonly lead to dysphagia [Galovic et al., 2016; Suntrup et al., 2015]. The neuronal rehabilitation of these defects relies on the recruitment of unaffected ipsilesional [Fridman et al., 2004] or contralesional [Hamdy et al., 1998] areas. Cortical plasticity sets in within 2 weeks after stroke [Dijkhuizen et al., 2001] and a higher degree of cortical reorganization leads to a better outcome [Thickbroom et al., 2004]. Results of our connectivity analysis (Fig. 3B, Table 4B) show a high interconnection of the insular lesion map with several opercular regions, pointing to a central role of the insula in the cortical swallowing network [Lowell et al., 2012]. The disruption of this central swallowing node might impair ipsilesional recruitment of cortical reorganization and, consequently, slow rehabilitation of oral intake. Hence, the insula might be a crucial structure that drives neuronal plasticity and effective recovery from dysphagic stroke.
In addition, our results suggest that the extent of ischemic damage within the insula might be a promising prognostic marker for late swallowing recovery. Although our sample size was small, all subjects with lesions affecting ≥50% of the insular cortex had impaired oral intake after ≥4 weeks, whereas all of those with lesions affecting less than 25% of the insula recovered.
Limitations
Some limitations have to be considered. Twelve patients were lost to follow‐up during the late assessment after ≥4 weeks. Although there were no differences in baseline characteristics, lesion volume or stroke lateralization compared with the remaining population, the findings of the late assessment have to be interpreted with caution and their generalizability needs to be validated in a bigger patient group.
Late assessment was done with a structured telephonic interview. Although a standardized approach was used, a telephonic interview has its obvious limitations. FOIS levels obtained at the late assessment might not be directly translatable to the FOIS levels determined using objective evaluations at previous evaluations (baseline assessment and early re‐assessment).
It needs to be noted that several factors that are not strictly swallowing‐related might also lead to impaired oral intake. Oral intake as a construct is multifactorial and can be influenced by cognitive and communicative competence, sensory and motor information processing, and level of consciousness. Hence, regions established by the VLSM method might have influenced the recovery of oral intake not only through interference with swallowing but also through influence on these factors. Future research should address these points and analyze the influence of corona radiata infarctions or insular lesions on specific swallowing functions in a large cohort followed up for at least 4 weeks.
There are some inherent limitations of the VLSM method and DTI analysis. Some areas might be falsely positive due to their close proximity with truly positive neighboring regions. However, these vascular‐anatomical effects were almost completely reduced by using a control group of unaffected patients [Rorden and Karnath, 2004]. The post‐hoc probabilistic tractography was fitted on a group of healthy subjects and the results cannot consider individual differences in patients. DTI tractography is less likely to follow the corona radiata tracts into the more inferior and lateral parts of the motor and premotor cortices, which might have underestimated the connectivity to these areas.
CONCLUSIONS
Prognostication of swallowing problems and recovery plays a significant role in management of acute stroke patients and remains an imprecise science despite its importance to stroke related complications and outcomes. This study provides evidence that the location and connectivity of lesions modulate the recovery of swallowing function after stroke.
Our results are consistent with the observation that the dynamics of rehabilitation occur in two distinct phases [Rijntjes, 2006]. During the initial phase within the first week, impaired recovery of oral intake is mainly influenced by white matter lesions, in particular those of the superior corona radiata, which disrupt thalamic and corticobulbar projection fibers. This likely leads to an acute wide‐ranging breakdown of a distributed swallowing network. Such an acute network suppression can be overcome within one week [Rijntjes, 2006]. The second phase of rehabilitation during the second to fourth week is dominated by damage to specific cortical nodes of swallowing interconnected with association fibers. If such a central node, that is, the anterior insula, is affected, recovery tends to be particularly slow.
Knowledge about the prognosis of swallowing is crucial to guide therapeutic decisions, that is, the administration of early enteral tube feeding, because the choice of the appropriate feeding route depends on the duration of impaired oral intake [Corrigan et al., 2011; Gomes et al., 2014; Stroud et al., 2003; Wirth et al., 2013]. Our results directly address this issue. Affections of the superior corona radiata impair recovery within the first 7 days and clinicians might consider early enteral tube feeding in these patients. Those with lesions of the anterior insular cortex might be at risk of long‐term impairment and they might benefit from PEG feeding.
DISCLOSURES
Dr. Kägi reports membership on advisory boards of Bayer, Boehringer‐Ingelheim, Nestle SA, GE healthcare, Merz, and receiving a travel grant from Bayer.
Other authors report nothing to disclose.
ACKNOWLEDGMENTS
MG, NL, MM, JW, GK, and BJW had major responsibilities in the design and implementation of the study. MG, NL and MM were responsible for acquiring data. MG, MW, MZ, and SBV analyzed the data and interpreted it together with NL, FB, JW, GK, and BJW. MG drafted the manuscript and all other authors critically revised it for important intellectual content. Funding was obtained and the study was supervised by MG, GK, and BJW.
REFERENCES
- Abela E, Missimer J, Wiest R, Federspiel A, Hess C, Sturzenegger M, Weder B (2012): Lesions to primary sensory and posterior parietal cortices impair recovery from hand paresis after stroke. PLoS ONE 7:e31275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SM, Rapcsak SZ, Beeson PM (2010): Cost function masking during normalization of brains with focal lesions: Still a necessity?. Neuroimage 53:78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ (2005): Unified segmentation. Neuroimage 26:839–851. [DOI] [PubMed] [Google Scholar]
- Aziz Q, Andersson JL, Valind S, Sundin A, Hamdy S, Jones AK, Foster ER, Långström B, Thompson DG (1997): Identification of human brain loci processing esophageal sensation using positron emission tomography. Gastroenterology 113:50–59. [DOI] [PubMed] [Google Scholar]
- Bonilha HS, Simpson AN, Ellis C, Mauldin P, Martin‐Harris B, Simpson K (2014): The one‐year attributable cost of post‐stroke dysphagia. Dysphagia 29:545–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley N, Hannon N, Lebus C, O'Brien E, Khadjooi K (2014): Bilateral corona radiata infarcts: A new topographic location of Foix‐Chavany‐Marie syndrome. Int J Stroke 9:E39–E39. [DOI] [PubMed] [Google Scholar]
- Broadley S, Croser D, Cottrell J, Creevy M, Teo E, Yiu D, Pathi R, Taylor J, Thompson PD (2003): Predictors of prolonged dysphagia following acute stroke. J Clin Neurosci 10:300–305. [DOI] [PubMed] [Google Scholar]
- Broadley S, Cheek A, Salonikis S, Whitham E, Chong V, Cardone D, Alexander B, Taylor J, Thompson P (2005): Predicting prolonged dysphagia in acute stroke: The Royal Adelaide Prognostic Index for Dysphagic Stroke (RAPIDS). Dysphagia 20:303–310. [DOI] [PubMed] [Google Scholar]
- Buetefisch CM (2015): Role of the contralesional hemisphere in post‐stroke recovery of upper extremity motor function. Front Neurol 6:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Dell'acqua F, Vergani F, Malik F, Hodge H, Roy P, Valabregue R, Thiebaut de Schotten M (2012): Short frontal lobe connections of the human brain. Cortex 48:273–291. [DOI] [PubMed] [Google Scholar]
- Chee C, Arshad S, Singh S, Mistry S, Hamdy S (2005): The influence of chemical gustatory stimuli and oral anaesthesia on healthy human pharyngeal swallowing. Chem Senses 30:393–400. [DOI] [PubMed] [Google Scholar]
- Cola MG, Daniels SK, Corey DM, Lemen LC, Romero M, Foundas AL (2010): Relevance of subcortical stroke in dysphagia. Stroke 41:482–486. [DOI] [PubMed] [Google Scholar]
- Corrigan ML, Escuro AA, Celestin J, Kirby DF (2011): Nutrition in the stroke patient. Nutr Clin Pract 26:242–252. [DOI] [PubMed] [Google Scholar]
- Crary MA, Mann GDC, Groher ME (2005): Initial psychometric assessment of a functional oral intake scale for dysphagia in stroke patients. Arch Phys Med Rehabil 86:1516–1520. [DOI] [PubMed] [Google Scholar]
- Daniels SK, Foundas AL (1997): The role of the insular cortex in dysphagia. Dysphagia 12:146–156. [DOI] [PubMed] [Google Scholar]
- Daniels SK, Foundas AL (1999): Lesion localization in acute stroke patients with risk of aspiration. J Neuroimaging 9:91–98. [DOI] [PubMed] [Google Scholar]
- Daniels S, McAdam C, Brailey K, Foundas A (1997): Clinical assessment of swallowing and prediction of dysphagia severity. Am J Speech Lang Pathol 6:17–24. [Google Scholar]
- Dennis MS, Lewis SC, Warlow C, FOOD Trial Collaboration (2005): Effect of timing and method of enteral tube feeding for dysphagic stroke patients (FOOD): a multicentre randomised controlled trial. Lancet 365:764–772. [DOI] [PubMed] [Google Scholar]
- DePippo KL, Holas MA, Reding MJ (1992): Validation of the 3‐oz water swallow test for aspiration following stroke. Arch Neurol 49:1259–1261. [DOI] [PubMed] [Google Scholar]
- Dijkhuizen RM, Ren J, Mandeville JB, Wu O, Ozdag FM, Moskowitz MA, Rosen BR, Finklestein SP (2001): Functional magnetic resonance imaging of reorganization in rat brain after stroke. Proc Natl Acad Sci U S A 98:12766–12771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziewas R, Sörös P, Ishii R, Chau W, Henningsen H, Ringelstein EB, Knecht S, Pantev C (2003): Neuroimaging evidence for cortical involvement in the preparation and in the act of swallowing. Neuroimage 20:135–144. [DOI] [PubMed] [Google Scholar]
- Ertekin C, Kiylioglu N, Tarlaci S, Keskin A, Aydogdu I (2000): Effect of mucosal anaesthesia on oropharyngeal swallowing. Neurogastroenterol Motil 12:567–572. [DOI] [PubMed] [Google Scholar]
- Finger S, Koehler PJ, Jagella C (2004): The Monakow concept of diaschisis: Origins and perspectives. Arch Neurol 61:283–288. [DOI] [PubMed] [Google Scholar]
- Flowers HL, Skoretz SA, Streiner DL, Silver FL, Martino R (2011): MRI‐based neuroanatomical predictors of dysphagia after acute ischemic stroke: A systematic review and meta‐analysis. Cerebrovasc Dis 32:1–10. [DOI] [PubMed] [Google Scholar]
- Foley NC, Martin RE, Salter KL, Teasell RW (2009): A review of the relationship between dysphagia and malnutrition following stroke. J Rehabil Med 41:707–713. [DOI] [PubMed] [Google Scholar]
- Fridman EA, Hanakawa T, Chung M, Hummel F, Leiguarda RC, Cohen LG (2004): Reorganization of the human ipsilesional premotor cortex after stroke. Brain 127:747–758. [DOI] [PubMed] [Google Scholar]
- Galovic M, Leisi N, Müller M, Weber J, Abela E, Kägi G, Weder B (2013): Lesion location predicts transient and extended risk of aspiration after supratentorial ischemic stroke. Stroke 44:2760–2767. [DOI] [PubMed] [Google Scholar]
- Galovic M, Leisi N, Muller M, Weber J, Tettenborn B, Brugger F, Abela E, Weder B, Kägi G (2016): Neuroanatomical correlates of tube dependency and impaired oral intake after hemispheric stroke. Eur J Neurol 23:926–934. [DOI] [PubMed] [Google Scholar]
- Gomes F, Hookway C, Weekes CE, Royal College of Physicians Intercollegiate Stroke Working Party (2014): Royal College of Physicians Intercollegiate Stroke Working Party evidence‐based guidelines for the nutritional support of patients who have had a stroke. J Hum Nutr Diet 27:107–121. [DOI] [PubMed] [Google Scholar]
- Hamdy S, Aziz Q, Rothwell JC, Singh KD, Barlow J, Hughes DG, Tallis RC, Thompson DG (1996): The cortical topography of human swallowing musculature in health and disease. Nat Med 2:1217–1224. [DOI] [PubMed] [Google Scholar]
- Hamdy S, Aziz Q, Rothwell JC, Power M, Singh KD, Nicholson DA, Tallis RC, Thompson DG (1998): Recovery of swallowing after dysphagic stroke relates to functional reorganization in the intact motor cortex. Gastroenterology 115:1104–1112. [DOI] [PubMed] [Google Scholar]
- Humbert IA, Joel S (2012): Tactile, gustatory, and visual biofeedback stimuli modulate neural substrates of deglutition. Neuroimage 59:1485–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DK (2010): Challenges and limitations of quantifying brain connectivity in vivo with diffusion MRI. iim 2:341–355. [Google Scholar]
- Karnath H‐O, Smith DV (2014): The next step in modern brain lesion analysis: Multivariate pattern analysis. Brain 137:2405–2407. [DOI] [PubMed] [Google Scholar]
- Kern MK, Jaradeh S, Arndorfer RC, Shaker R (2001): Cerebral cortical representation of reflexive and volitional swallowing in humans. Am J Physiol Gastrointest Liver Physiol 280:G354–G360. [DOI] [PubMed] [Google Scholar]
- Kim JS, Pope A (2005): Somatotopically located motor fibers in corona radiata: Evidence from subcortical small infarcts. Neurology 64:1438–1440. [DOI] [PubMed] [Google Scholar]
- Kim B‐R, Moon W‐J, Kim H, Jung E, Lee J (2016): Association of dysphagia with supratentorial lesions in patients with middle cerebral artery stroke. Ann Rehabil Med 40:637–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Luo C, Yu B, Yan B, Gong Q, He C, He L, Huang X, Yao D, Lui S, Tang H, Chen Q, Zeng Y, Zhou D (2009): Functional magnetic resonance imaging study on dysphagia after unilateral hemispheric stroke: A preliminary study. J Neurol Neurosurg Psychiatr 80:1320–1329. [DOI] [PubMed] [Google Scholar]
- Lowell SY, Reynolds RC, Chen G, Horwitz B, Ludlow CL (2012): Functional connectivity and laterality of the motor and sensory components in the volitional swallowing network. Exp Brain Res 219:85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino R, Foley N, Bhogal S, Diamant N, Speechley M, Teasell R (2005): Dysphagia after stroke: Incidence, diagnosis, and pulmonary complications. Stroke 36:2756–2763. [DOI] [PubMed] [Google Scholar]
- Mihai PG, Otto M, Domin M, Platz T, Hamdy S, Lotze M (2016): Brain imaging correlates of recovered swallowing after dysphagic stroke: A fMRI and DWI study. Neuroimage Clin 12:1013–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris DM, Embleton KV, Parker GJM (2008): Probabilistic fibre tracking: Differentiation of connections from chance events. Neuroimage 42:1329–1339. [DOI] [PubMed] [Google Scholar]
- Mosier K, Bereznaya I (2001): Parallel cortical networks for volitional control of swallowing in humans. Exp Brain Res 140:280–289. [DOI] [PubMed] [Google Scholar]
- Mosier KM, Liu WC, Maldjian JA, Shah R, Modi B (1999): Lateralization of cortical function in swallowing: A functional MR imaging study. AJNR Am J Neuroradiol 20:1520–1526. [PMC free article] [PubMed] [Google Scholar]
- Riecker A, Gastl R, Kühnlein P, Kassubek J, Prosiegel M (2009): Dysphagia due to unilateral infarction in the vascular territory of the anterior insula. Dysphagia 24:114–118. [DOI] [PubMed] [Google Scholar]
- Rijntjes M (2006): Mechanisms of recovery in stroke patients with hemiparesis or aphasia: New insights, old questions and the meaning of therapies. Curr Opin Neurol 19:76–83. [DOI] [PubMed] [Google Scholar]
- Rorden C, Karnath H‐O (2004): Using human brain lesions to infer function: A relic from a past era in the fMRI age?. Nat Rev Neurosci 5:813–819. [DOI] [PubMed] [Google Scholar]
- Rorden C, Karnath H‐O, Bonilha L (2007): Improving lesion‐symptom mapping. J Cogn Neurosci 19:1081–1088. [DOI] [PubMed] [Google Scholar]
- Rorden C, Bonilha L, Fridriksson J, Bender B, Karnath H‐O (2012): Age‐specific CT and MRI templates for spatial normalization. Neuroimage 61:957–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithard DG, Smeeton NC, Wolfe CDA (2007): Long‐term outcome after stroke: Does dysphagia matter?. Age Ageing 36:90–94. [DOI] [PubMed] [Google Scholar]
- Stroud M, Duncan H, Nightingale J, British Society of Gastroenterology (2003): Guidelines for enteral feeding in adult hospital patients. Gut 52:vii1–vii12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suntrup S, Kemmling A, Warnecke T, Hamacher C, Oelenberg S, Niederstadt T, Heindel W, Wiendl H, Dziewas R (2015): The impact of lesion location on dysphagia incidence, pattern and complications in acute stroke. Part 1: dysphagia incidence, severity and aspiration. Eur J Neurol 22:832–838. [DOI] [PubMed] [Google Scholar]
- Teismann IK, Steinstraeter O, Stoeckigt K, Suntrup S, Wollbrink A, Pantev C, Dziewas R (2007): Functional oropharyngeal sensory disruption interferes with the cortical control of swallowing. BMC Neurosci 8:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thickbroom GW, Byrnes ML, Archer SA, Mastaglia FL (2004): Motor outcome after subcortical stroke correlates with the degree of cortical reorganization. Clin Neurophysiol 115:2144–2150. [DOI] [PubMed] [Google Scholar]
- Trapl M, Enderle P, Nowotny M, Teuschl Y, Matz K, Dachenhausen A, Brainin M (2007): Dysphagia bedside screening for acute‐stroke patients: The Gugging Swallowing Screen. Stroke 38:2948–2952. [DOI] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. Neuroimage 15:273–289. [DOI] [PubMed] [Google Scholar]
- Van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J (1988): Interobserver agreement for the assessment of handicap in stroke patients. Stroke 19:604–607. [DOI] [PubMed] [Google Scholar]
- Wakana S, Jiang H, Nagae‐Poetscher LM, van Zijl PCM, Mori S (2004): Fiber tract‐based atlas of human white matter anatomy. Radiology 230:77–87. [DOI] [PubMed] [Google Scholar]
- Warnecke T, Ritter MA, Kr ouml ger B, Oelenberg S, Teismann I, Heuschmann PU, Ringelstein EB, Nabavi DG, Dziewas R (2009): Fiberoptic Endoscopic Dysphagia Severity Scale Predicts Outcome after Acute Stroke. Cerebrovasc Dis 28:283–289. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Abe S, Ishikawa T, Yamada Y, Yamane G‐Y (2004): Cortical regulation during the early stage of initiation of voluntary swallowing in humans. Dysphagia 19:100–108. [DOI] [PubMed] [Google Scholar]
- Wirth R, Smoliner C, Jäger M, Warnecke T, Leischker AH, Dziewas R, DGEM Steering Committee* (2013): Guideline clinical nutrition in patients with stroke. Exp Transl Stroke Med 5:14. [DOI] [PMC free article] [PubMed] [Google Scholar]


