Abstract
Dyskinetic cerebral palsy (CP) has long been associated with basal ganglia and thalamus lesions. Recent evidence further points at white matter (WM) damage. This study aims to identify altered WM pathways in dyskinetic CP from a standardized, connectome‐based approach, and to assess structure‐function relationship in WM pathways for clinical outcomes. Individual connectome maps of 25 subjects with dyskinetic CP and 24 healthy controls were obtained combining a structural parcellation scheme with whole‐brain deterministic tractography. Graph theoretical metrics and the network‐based statistic were applied to compare groups and to correlate WM state with motor and cognitive performance. Results showed a widespread reduction of WM volume in CP subjects compared to controls and a more localized decrease in degree (number of links per node) and fractional anisotropy (FA), comprising parieto‐occipital regions and the hippocampus. However, supramarginal gyrus showed a significantly higher degree. At the network level, CP subjects showed a bilateral pathway with reduced FA, comprising sensorimotor, intraparietal and fronto‐parietal connections. Gross and fine motor functions correlated with FA in a pathway comprising the sensorimotor system, but gross motor also correlated with prefrontal, temporal and occipital connections. Intelligence correlated with FA in a network with fronto‐striatal and parieto‐frontal connections, and visuoperception was related to right occipital connections. These findings demonstrate a disruption in structural brain connectivity in dyskinetic CP, revealing general involvement of posterior brain regions with relative preservation of prefrontal areas. We identified pathways in which WM integrity is related to clinical features, including but not limited to the sensorimotor system. Hum Brain Mapp 38:4594–4612, 2017. © 2017 Wiley Periodicals, Inc.
Keywords: cerebral palsy, diffusion MRI, structural connectome, graph theory, network‐based statistic, white matter injury, fractional anisotropy, motor function, cognition
INTRODUCTION
Cerebral palsy (CP) encompasses a heterogeneous group of neuromotor disorders due to non‐progressive disturbances in the developing fetal or infant brain, causing activity limitation as well as impairments in sensation, cognition, and communication [Novak et al., 2012; Rosenbaum et al., 2007]. CP is the most common cause of physical disability in childhood with an estimated prevalence around 2 per 1,000 live births [Oskoui et al., 2013; Sellier et al., 2016]. This disabling condition is permanent throughout the individual's life and makes heavy and constant demands on health, educational, and social services [Krageloh‐Mann and Cans, 2009]. Dyskinetic is the second most common CP subtype after spastic CP. Its prevalence has been established at 0.14 per 1,000 live births [Himmelmann et al., 2009] and represents between 10% and 15% of the entire CP population [Bax et al., 2006; Himmelmann et al., 2009; Himmelmann and Uvebrant, 2014]. Dyskinetic CP is characterized by abnormal patterns of posture and/or movement accompanied by involuntary, uncontrolled, recurring, and occasionally stereotyped movements [Cans et al., 2007]. It is particularly disabling in comparison to other forms of CP: almost 60% uses a wheelchair for ambulation and more than half suffer accompanying impairments and disabilities, such as visual and hearing impairments or severe intellectual disability [Himmelmann et al., 2009; Novak et al., 2012]. At the cognitive level, there are not only disturbances in general cognitive performance but also difficulties in specific cognitive functions, being the visual‐perceptual impairments the most described so far [Ego et al., 2015]. This greater severity together with the lower frequency could explain why dyskinetic CP has been barely studied so far, which leads to a lack of knowledge about its distinctive features and, consequently, a lack of follow‐up programs and interventions adapted to their needs [Himmelmann, 2015].
Qualitative MRI studies showed that brain injury in dyskinetic CP is mainly located in the basal ganglia and thalamus, often found after brief, profound hypoxic insults in term or near‐term infants [Himmelmann and Uvebrant, 2011; Krageloh‐Mann and Cans, 2009; Yokochi, 1991]. Contrary, white matter (WM) injury has been commonly associated with preterm delivery or moderate‐intensity, prolonged hypoxic‐ischemic events, and thus normally linked to the development of spastic CP. Nevertheless, WM involvement has also been reported in dyskinetic cases although data vary widely among studies, with percentages ranging from 7% to 57% (Supporting Information, Table SI). Additional patterns may accompany the deep gray matter lesions, such as cortical injury. To a lesser extent, brain malformations can also be found (Supporting Information, Table SI). The percentage of cases with no apparent abnormality also varies depending on the study, with an average around 17%, which is in accordance with data found in the entire CP spectrum [Scheck et al., 2012]. As some authors highlight, the heterogeneity among studies may be due to not only small sample sizes but also differences in qualitative assessment based on clinical descriptions [Reid et al., 2014]. Another clear limitation of the use of qualitative MRI is its inability to probe microstructural damage of the brain tissue, which could also explain the high percentage of cases with no apparent abnormalities. There is consequently a great motivation to apply more advanced imaging techniques that help to objectively quantify the brain damage in CP [Scheck et al., 2012].
In line with the aforementioned, the first studies applying diffusion‐weighted MRI in dyskinetic CP have been carried out recently. Yoshida et al. [2011] concluded that subjects with dyskinetic CP had more severe and diffuse alterations in WM than do subjects with spastic CP after comparing nine manually drawn WM structures between 19 children with dyskinetic CP, 26 with spastic CP, and 31 healthy controls. Harlaar et al. [2013] analyzed the pyramidal and language tracts (arcuate fasciculus and the extreme capsule) in four subjects with severe dyskinetic CP and found smaller tract sizes and/or lower fractional anisotropy (FA) values than in controls. Furthermore, Park et al. [2014] found a FA reduction in areas adjacent to pyramidal tract in 23 adolescents and adults with dyskinetic CP. These studies have allowed identifying the potential involvement of WM in dyskinetic CP, but the results are limited by the use of a priori selected regions of interest (ROIs) that are normally related to sensorimotor function, leaving prefrontal, temporal and occipital WM areas poorly assessed [Scheck et al., 2012], and making it difficult to draw firm conclusions about the whole‐brain injury pattern underlying dyskinetic CP. There is one study that applied a whole‐brain approach in seven subjects with dyskinetic CP [Yoshida et al., 2013]. This study used an atlas‐based analysis in 205 predefined structures to describe the anatomical variability in different groups of CP, and concluded that WM changes were more severe in dyskinetic CP with a prominent FA decrease in the periventricular deep WM. The small number of dyskinetic CP subjects and the structure‐by‐structure analysis, which has low sensitivity to detect subtle abnormalities and low specificity on what connections are involved, are two limitations of this study.
The use of advanced, multi‐modal imaging techniques, combined with a graph theoretical approach, has made it possible to quantify and study the brain as a complex network of connections, a network also known as the human connectome [Sporns, 2010; Sporns et al., 2005]. Connectomics helps to describe the topology of structural brain networks with increased sensitivity and specificity [Bullmore and Sporns, 2009; van den Heuvel et al., 2016] and to explain how this substrate shapes and modulates brain function, which is not attributable to individual regions but rather emerges from the network as a whole [Sporns et al., 2005; van den Heuvel and Sporns, 2013]. Being able to describe the functional consequences of network perturbations will allow a better understanding of structural causes of dysfunction, and may permit the design and validation of intervention programs. This strategy has been scarcely implemented in the field of CP and always in spastic forms. In unilateral spastic CP subjects, Pannek et al. [2014] identified alterations in ipsilesional projection and association pathways and correlated the microstructure of these connections with the performance of the impaired hand. Englander et al. [2013], similarly, found a reduction in total WM connectivity throughout the brain in severe versus moderate bilateral spastic CP, including but not limited to regions associated with the sensorimotor system. A recent study applied a whole‐brain connectome analysis to investigate changes in connectivity following therapy and concluded that brain connectivity change can serve as a biomarker of functional improvement in CP [Englander et al., 2015].
The application of the connectome‐based analysis in the study of dyskinetic CP constitutes a promising way for elucidating pathways of WM injury from a whole‐brain approach, which can help to better understand the pathogenesis and the clinical impairments of this population. The analysis of the structure‐function relationships could lead to a better prediction of the clinical outcomes from image‐derived measures. In addition, changes in brain connectivity can become a useful tool to validate the effectiveness of clinical programs, as pointed out in spastic CP [Englander et al., 2015]. Thus, the aim of this study is twofold: (1) to identify and describe altered WM pathways associated with dyskinetic CP from the structural network of connections in a standardized, automated, connectome‐based approach, and (2) to assess structure‐function relationships in WM pathways for motor function and those cognitive functions commonly affected in these patients: intelligence and visuoperceptual abilities. Our a priory hypothesis is that brain connectivity would be reduced in dyskinetic CP compared to healthy controls reflecting an impaired WM organization, specifically among sensorimotor areas. Our second hypothesis is that the degree of impairment of some of these connections would correlate with measures of motor function [Arrigoni et al., 2016]. Finally, we further hypothesize that intelligence quotient (IQ) would correlate with connectivity measures in fronto‐striatal and parieto‐frontal connections, in accordance with a previous study [Ballester‐Plané et al., 2016]. We assume that visuoperceptual measures would correlate with the state of the visual processing circuitry, as found in previous studies of spastic CP [Ceschin et al., 2015; Fazzi et al., 2009].
MATERIALS AND METHODS
Participants
This study is part of a larger project that recruited participants with dyskinetic CP mainly from the Hospital Vall d'Hebron and the Hospital Sant Joan de Déu in Barcelona, Spain. The inclusion criteria for the general project were: (1) clinical diagnosis of CP with predominant dyskinetic features; (2) age over six years old; (3) an intelligible “yes”/“no” response system; and (4) being able to understand simple instructions, assessed by means of the Screening Test of Spanish Grammar (receptive part) [Toronto, 1973]. Participants with hearing abnormalities or severe visual difficulties that precluded cognitive assessment were excluded. Controls were ineligible if they were preterm, were suffering from a neurological or psychiatric disorder, or were substance‐abusing.
The project was approved by the University of Barcelona's (CBUB) Institutional Ethics Committee, Institutional Review Board (IRB 00003099, assurance number: FWA00004225; http://www.ub.edu/recerca/comissiobioetica.htm). The research was conducted in accordance with the Helsinki Declaration. Written informed consent was obtained from all participants, their parents or their legal guardians.
Clinical Measures
Motor status
Motor function was assessed following the recommendations of the Surveillance of CP in Europe: the Gross Motor Function Classification System (GMFCS) [Palisano et al., 2008] for lower limb function and the Manual Ability Classification System (MACS) [Eliasson et al., 2006] for the upper limbs. These scales were developed to characterize mobility and manual function in CP, respectively. They use a 5‐level classification system (from I to V). Higher scores indicate lower levels of motor functioning.
Cognitive assessment
The Raven's Colored Progressive Matrices (RCPM) test was used to assess intellectual ability [Raven et al., 2001]. The RCPM aims to measure the ability to deduct relationships and its use is recommended for people with physical disabilities, aphasia, deafness, or CP [Strauss et al., 2006]. It consists of 36 items of increasing difficulty, containing a pattern problem with one part removed and the participant has to choose which of the six alternatives completes the pattern. The raw scores were converted into IQ scores as described previously [Ballester‐Plané et al., 2016].
Visuoperceptual abilities were assessed by means of Benton's Facial Recognition Test (BFRT), which examines the ability to perceive faces [Benton, 1994]. On each item, subjects are presented with a target face above six test faces, and they are asked to indicate which of the six images matches the target face. We used the 27‐item short form. Short form scores were converted to long form scores (54 possible points) following the test instructions. Long form scores were then regressed by age and included in the analyses.
MRI Acquisition
MRI was performed on a Siemens Magnetom TRIO 3.0 T scanner (Erlangen, Germany) at the Hospital Universitari Vall d'Hebron (Barcelona, Spain). High‐resolution three‐dimensional T1‐weighted images were acquired in the sagittal plane with a MPRAGE sequence (parameters: repetition time 1,900 ms; echo time 2.46 ms; inversion time 900 ms; field of view 220 × 220 mm; flip angle 9°; and voxel size 0.7 mm × 0.7 mm × 1 mm). Two repetitions of the Diffusion Weighted Imaging (DWI) sequence were acquired at b = 1,000 s/mm2, along with one minimally diffusion weighted image (b = 0) (parameters: repetition time 8,400 ms; echo time 90 ms; field of view 240 × 240 mm; 65 axial slices; 30 diffusion directions; voxel size 2 × 2 × 2 mm with no gap).
To minimize movement during MRI, participants were offered the opportunity to listen to music or watch a movie at the time of the acquisition of the images with the aim of reducing the level of anxiety. Additionally, for those who could not remain still, a diazepam or pentobarbital with propofol was administered. The drug was prescribed by a physician in accordance with the protocol detailed and reviewed by the ethics committee.
Imaging Preprocessing
Anatomical T1‐weighted images were used for the selection of the nodes of the brain network. By means of the FreeSurfer software suite the gray matter tissue was parcelled into 68 cortical regions and 15 subcortical structures—bilateral thalamus, pallidum, caudate, putamen, accumbens, hippocampus, amygdala, and the brainstem [Desikan, 2006; Fischl et al., 2002]. In total, 83 brain regions were selected, representing the nodes of the individual brain networks. The accuracy of the cortical and subcortical parcellation was assessed visually. Those subjects not being adequately parcellated were excluded from the analysis and manual corrections were applied if needed.
For DWI data, subjects with scanner artefacts were visually identified and excluded from further analysis. Then, images were first corrected for eddy‐current distortions [Andersson and Skare, 2002] using FSL (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/). Second, volumes containing motion artefacts were visually identified and substituted by its counterpart in the other DWI repetition, as long as there were no errors, thus resulting in a corrected DWI set. Third, this DWI data was processed using a robust tensor fitting method to obtain the preferred diffusion direction within each voxel in the brain mask [Chang et al., 2012; de Reus and van den Heuvel, 2013a]. Eight streamline seeds were started in each WM voxel following the main projection direction from voxel to voxel by means of deterministic streamline tractography [Mori et al., 1999; Mori and Van Zijl, 2002]. Streamline end points were set when a tractography fiber reached a voxel with a FA value <0.1 (indicating a low level of preferred diffusion within that particular voxel), when the trajectory of the traced fiber left the brain mask or when the fiber tract made a sharp turn higher than 45°. The fiber reconstruction yielded a total set of 141,247 ± 30,258 (mean ± SD) interconnecting streamlines per individual subject.
Connectome Reconstruction
For each individual subject, a brain network was generated combining the collection of 83 segmented gray matter regions with the total set of reconstructed streamlines. The existence of an anatomical pathway (edges) and its level of connectivity strength between each pair of cortical and subcortical brain regions (nodes) was derived, examining for each possible pair of regions whether the total collection of fibers included streamlines that touched both regions [van den Heuvel and Sporns, 2011]. This resulted in the reconstruction of a structural undirected connectivity matrix of 83 by 83 regions. Connectivity weights were generated according to number of streamlines (NOS) and FA weighted values. NOS represents the number of reconstructed fiber tracts and is suggested to be correlated to tract volume [van den Heuvel et al., 2015a]. FA quantifies the fiber integrity of the connections [Basser and Pierpaoli, 1996] and was computed as the average FA over all values of the voxels that were traversed by the streamlines comprising the reconstructed pathway between the two regions.
Network Analyses
Analyses of brain network structure were performed in terms of graphs metrics, calculated using the Brain Connectivity Toolbox for MATLAB [Rubinov and Sporns, 2010]. For each subject, the organization of the brain network was analyzed by means of whole‐brain network connectivity, node‐level graph metrics, and edge‐level graph analysis. Whole‐brain network metrics included density – describing the fraction of present connections over possible connections (connection weights are ignored in calculations), global NOS – computed as the sum of all the connections in the network, and global FA – computed as the mean of all nonzero connections in the network. Given that NOS is a measure of WM volume, this measure was corrected as ratios by the total intracranial volume, which was extracted from the tissue parcellation performed with FreeSurfer, along with total gray matter volume. Node‐level graph metrics included degree – number of links connected to the node (connection weights are not taken into account in calculations), and strength – computed as the sum of weights of links connected to the node for NOS, and as the mean of nonzero links connected to the node in the case of FA. Edge‐level graph analyses were performed using the network‐based statistic (NBS) [Zalesky et al., 2010]. The NBS is a nonparametric statistical method to perform statistical analysis on large networks, dealing with the multiple comparisons problem on a graph. The NBS is the graph analogue of cluster‐based statistical methods used in mass univariate testing on all pixels in an image, letting us identify statistically significant components (“clusters” of connections). It has been widely used to identify connections and network components of the connectome associated with an experimental effect or a between‐group difference [Collin et al., 2014; Verstraete et al., 2011].
Statistical Analyses
A Chi‐square test, t‐test or Mann–Whitney analyses were applied depending on the characteristics of the variables to compare the demographic and clinical data between the dyskinetic CP and control groups.
For whole‐brain and node‐level measures, differences between CP and control groups were computed for every measure and the statistical significance of these differences was assessed by means of permutation testing. This involves generating a null distribution of differences observed after randomly permuting the members of the two groups (10,000 permutations) and then calculating the P‐value using this null distribution. Findings are considered significant at a P‐value smaller than 0.05. All node‐level analyses were corrected for multiple testing by means of the false discovery rate (FDR) method. Effect sizes (d) were computed as group mean differences divided by the average standard deviation of both samples and interpreted with cut‐off points of 0.2 for small, 0.5 for medium, and 0.8 for large effect sizes [Cohen, 1988].
Regarding the NBS analyses, a general linear model was used to identify differences in edge‐level metrics among dyskinetic CP subjects and typically‐developing controls for every connection. Only those connections found in more than 50% of the CP subjects and 50% of the controls were included in the analysis. A t‐statistic threshold of 2.5 (equivalent to a P‐value of 0.02) was applied for individual connections. We additionally applied the NBS analysis to assess the relationships between edge‐level metrics and clinical measures by means of Pearson and Spearman's rank correlation coefficients. In this case, only those connections found in more than 50% of the CP subjects were included in the analysis and an R‐threshold of ±0.3 along with a P‐threshold of 0.02 were used as a combined threshold for individual connections. In both analyses, the significance of the connected components in the resulting graph was estimated using permutation testing (10,000 permutations). Only significant clusters (P‐value of less than 0.05) were considered.
Due to the wide age‐range of the sample and to avoid any potential developmental effect, participants’ ages were regressed on all brain measures and we used the obtained residuals for all statistical analyses.
RESULTS
Participants
Out of a total of 53 subjects that met the inclusion and exclusion criteria and that accepted to participate, 11 were not included because they did not want to undergo an MRI (n = 3), had metal devices implanted which were incompatible with MRI (n = 2) or could not complete scans due to anxiety (n = 6). From the remaining 42 cases, 13 subjects were excluded due to scanner or movement artefacts or for not being adequately parcellated, thus good quality images were obtained in 29 cases. Small errors in the WM segmentation were corrected in four cases by means of control points. To reduce dispersion in the age range, the eldest four cases (47‐, 50‐, 51‐, and 60‐year old, respectively) were removed. Thus, the final sample comprised 25 cases with dyskinetic CP aged 11–42 years, without visual/auditory abnormalities, able to understand instructions and, at least, able to answer yes/no. The sample comprised 9 children (11–17 years), 13 young adults (18–30 years), and 3 adults older than 30‐year old (Table 1).
Table 1.
Demographic and clinical data of the sample
| CP group | HC group | T/U/χ 2(P) | |
|---|---|---|---|
| Age, mean ± SD | 21.55 ± 8.10 | 21.73 ± 8.6 | −0.075 (0.94) |
| Children (11 – 17y), n | 9 | 8 | |
| Young adults (18 – 30y), n | 13 | 13 | |
| Adults (>30y), n | 3 | 3 | |
| Gender, n | 13 m, 12 f | 12 m, 12 f | 0.02 (0.89) |
| IQ, median ± IQR (range) | 87 ± 42 (33 – 117) | 112 ± 6 (95 – 118) | 104.5 (<0.001) |
| BFRT, median ± IQR (range) | 41 ± 10 (28 – 50) | 49.5 ± 7 (36 – 54) | 90.5 (<0.001) |
| GMFCS, n | I: 10; II: 3; III: 1; IV: 4; V: 7 | n/a | |
| MACS, n | I: 3; II: 8; III: 4; IV: 3; V: 7 | n/a | |
| Motor distribution, n | Bilateral: 22; Unilateral: 3 | n/a | |
| Gestational age, n | <37 w: 3; ≥37 w: 22 | ≥37 w: 24 | |
| Aetiology, n | n/a | ||
| HIEa | 14 | ||
| Intra‐cranial haemorrhage/infarction | 1 | ||
| Kernicterus | 1 | ||
| Unclassifiable | 9b | ||
| Neuroimaging findings, n | n/a | ||
| Basal ganglia | 21 | ||
| Thalamus | 19 | ||
| White matter | 11 | ||
| Malformation | 1 | ||
| Cortical gray matter | 1 | ||
| Normal | 1 | ||
| Epilepsy status, n | n/a | ||
| No epilepsy | 13 | ||
| Active | 10 | ||
| Resolved | 2 |
HIE criteria were based on Himmelmann et al. [2010].
In three cases, the clinical history indicates a possible HIE but sufficient perinatal data are not available.
Bold indicates significant differences at P < 0.05.
Abbreviations: BFRT: Benton's Facial Recognition Test; CP: Cerebral palsy; f: Female; GMFCS: Gross Motor Function Classification System; HC: Healthy control; HIE: Hypoxic‐ischemic encephalopathy; IQ: Intelligence quotient; IQR: Interquartile range; m: Male; n/a: Not applicable; MACS: Manual Ability Classification System; SD: Standard deviation; w: Weeks; y: Years.
Importantly, there were no significant differences in age, gender, gestational age, motor function (GMFCS and MACS), IQ and visuoperceptual performance between CP subjects included and not‐included in the study (see Supporting Information, Table SII). Of the initial 25 typically developing controls matched by age, one subject was excluded due to scanner artefacts; thus, the control group consisted of 24 typically developing subjects.
Clinical and Radiological Findings
All levels of GMFCS and MACS were represented in the CP group (Table 1). The vast majority of the sample (n = 22) had tetraplegia and only three cases showed unilateral involvement (2 with left hemiparesis and 1 with right hemiparesis). Almost all subjects had received physiotherapy and speech therapy (n = 23 and n = 20, respectively) at some point. Fourteen of the CP subjects attended regular schools, 6 of which received some additional support at school, and the remaining 11 attended special education schools. Ten participants had active epilepsy and 14 were under pharmacological treatment: seven were taking antiepileptic drugs, five were taking drugs for dystonia and/or spasticity (mainly benzodiazepines, baclofen and anticholinergics), and two were taking both.
As shown in Table 1, most of the participants were born at term, suffered from hypoxic‐ischemic encephalopathy (HIE) and showed basal ganglia and/or thalamic lesions, as expected in this CP type [Himmelmann et al., 2007; Himmelmann et al., 2009; Rennie et al., 2007]. As shown in other MRI studies (Supporting Information, Table SI), the second radiological finding was the concomitant involvement of WM, seen in 11 subjects. In these cases, WM damage was mainly reported as perirolandic (n = 9), followed by periventricular (n = 4), subinsular (n = 1), and centrum semiovale (n = 1). Cerebral malformation was detected in one subject, which showed an incomplete inversion of the hippocampus, together with damage of basal ganglia and it corresponds to the subject with kernicterus. Finally, one subject had discrete hyperintense lesions in the frontal and occipital cortex and another one showed no apparent abnormality in the MRI, coinciding with a case of left hemiparesis.
The fact that 44% of the participants showed concomitant WM involvement prompted us to additionally compare the two major lesional subgroups (those with basal ganglia and/or thalamic lesions without WM damage (BG, n = 13) and those with concomitant WM damage (BG ± WM, n = 11)) separately with their respective controls (see below).
Whole‐Brain Measures
As shown in Table 2, total intracranial and WM volume was significantly reduced in dyskinetic CP subjects compared to controls. No group effect was found in total gray matter volume. As regards whole‐brain network metrics, subjects with CP showed a decrease in density (5.5%) and global FA (3.5%) with a medium‐sized effect compared to controls, and a highly significant reduction in global NOS (16%).
Table 2.
Whole brain measures in dyskinetic CP subjects compared to healthy controls (HC)
| CP group mean (SD) | HC group mean (SD) | P | d | |
|---|---|---|---|---|
| Volumetric measures | ||||
| TIV | 1373 (169) | 1498 (130) | 0.005 | 0.84 |
| TGV/TIV | 0.452 (0.037) | 0.459 (0.028) | 0.273 | 0.2 |
| TWV/TIV | 0.267 (0.02) | 0.291 (0.016) | <0.001 | 1.34 |
| Whole brain network metrics | ||||
| Density | 0.244 (0.027) | 0.258 (0.02) | 0.045 | 0.6 |
| Global NOS/TIV | 0.09 (0.017) | 0.11 (0.012) | <0.001 | 1.23 |
| Global FA | 0.409 (0.03) | 0.422 (0.016) | 0.037 | 0.64 |
Bold indicates significant differences at P < 0.05.
Abbreviations: CP: Cerebral palsy; FA: Fractional anisotropy; HC: Healthy control; NOS: Number of streamlines; SD: Standard deviation; TGV: Total gray matter volume; TIV: Total intracranial volume; TWV: Total white matter volume.
Regarding the subgroup analyses based on radiological findings, BG group showed a reduction in total WM volume and global NOS compared to healthy controls, whereas the BG ± WM group showed a reduction in all whole‐brain measures except for total gray matter volume, as found in the general group (Supporting Information, Table SIII).
White Matter Involvement in Dyskinetic CP
Node‐level graph metrics
Group differences were also observed at the nodal‐level as measured by node‐specific graph metrics (Fig. 1, Table 3). Regarding the number of links connected to a node, the CP group showed a significant decrease in degree in: bilateral postcentral cortex, lingual cortex, pericalcarine cortex, and cuneus cortex; right precentral cortex, superior parietal cortex, and transverse temporal cortex; and left hippocampus. Furthermore, CP participants showed an increase in nodal degree in the right pallidum and bilateral supramarginal cortex as compared to controls (Fig. 1, Table 3). As this result was not expected, we further examined this finding. We computed the prevalence of all the connections of these regions across the entire group (controls and CP subjects), and then we compared the prevalence between subjects and controls using the Chi‐squared test. The right pallidum just showed an increased prevalence in the connection with the right supramarginal gyrus (χ2 = 9.69, P = 0.0019). As regards the right supramarginal gyrus, dyskinetic CP subjects also showed an increased prevalence of connections with the thalamus (χ2 = 7.84, P = 0.0051), caudate (χ2 = 6.56, P = 0.0104), and rostral middle frontal gyrus (χ2 = 5.95, P = 0.0147). Regarding the left supramarginal gyrus, CP participants showed an increased prevalence compared to controls in left caudal middle frontal gyrus (χ2 = 7.99, P = 0.0047) and putaminal (χ2 = 6.12, P = 0.0134) connections.
Figure 1.
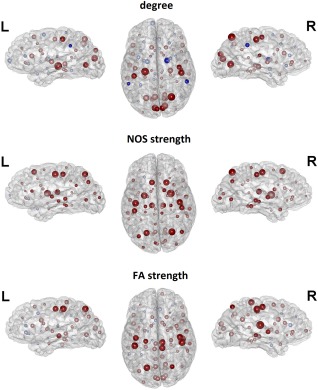
Results of the comparison of node‐level graph metrics between CP subjects and healthy controls (HC). The size of the nodes indicates the absolute difference among CP subjects and controls; warm colors indicate CP < HC and cold colors CP > HC; high‐intensity color indicates significant differences and low‐intensity color indicates non‐significant differences after applying FDR correction for multiple comparisons (P < 0.05); CP: Cerebral palsy; FA: Fractional anisotropy; HC: Healthy controls; L: Left; NOS: Number of streamlines; R: Right. BrainNet Viewer was used to create these figures [Xia et al., 2013]. [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 3.
Significant differences in node‐level graph metrics in dyskinetic CP subjects compared to healthy controls (HC)
| L Hemisphere | P | d | R Hemisphere | P | d |
|---|---|---|---|---|---|
| Degree | |||||
| Hippocampus L | <0.001 | 1.39 | |||
| Pallidum R† | 0.009 | 0.77 | |||
| Precentral R | 0.002 | 0.93 | |||
| Postcentral L | 0.002 | 0.93 | Postcentral R | <0.001 | 1.27 |
| Supramarginal L† | 0.003 | 0.89 | Supramarginal R† | 0.002 | 1 |
| Superior parietal R | 0.004 | 0.9 | |||
| Cuneus L | 0.009 | 0.79 | Cuneus R | <0.001 | 0.94 |
| Pericalcarine L | 0.002 | 0.91 | Pericalcarine R | <0.001 | 1.13 |
| Lingual L | 0.004 | 0.87 | Lingual R | <0.001 | 1.09 |
| Transverse temporal R | 0.007 | 0.8 | |||
| NOS strength | |||||
| Thalamus L | <0.001 | 1.35 | Thalamus R | <0.001 | 1.6 |
| Putamen L | 0.002 | 1.01 | Putamen R | <0.001 | 1.16 |
| Hippocampus L | <0.001 | 1 | Hippocampus R | <0.001 | 1.31 |
| Caudate R | 0.021 | 0.74 | |||
| Precentral L | <0.001 | 1.97 | Precentral R | <0.001 | 1.17 |
| Superior frontal L | 0.002 | 0.87 | Superior frontal R | 0.001 | 1.02 |
| Pars triangularis L | 0.012 | 0.68 | |||
| Paracentral R | 0.001 | 1.12 | |||
| Postcentral L | <0.001 | 1.51 | Postcentral R | <0.001 | 1.73 |
| Superior parietal L | <0.001 | 1.38 | Superior parietal R | <0.001 | 1.24 |
| Inferior parietal L | <0.001 | 1.59 | Inferior parietal R | 0.003 | 0.91 |
| Isthmus cingulated L | 0.015 | 0.74 | |||
| Lingual L | <0.001 | 0.99 | Lingual R | <0.001 | 1.17 |
| Lateral occipital L | 0.014 | 0.76 | Lateral occipital R | <0.001 | 0.91 |
| Pericalcarine L | 0.002 | 1 | Pericalcarine R | <0.001 | 1.13 |
| Cuneus L | 0.021 | 0.67 | |||
| Inferior temporal L | 0.012 | 0.75 | Inferior temporal R | <0.001 | 1.05 |
| Middle temporal L | 0.001 | 0.98 | Middle temporal R | 0.001 | 0.95 |
| Superior temporal L | 0.006 | 0.80 | Superior temporal R | 0.002 | 0.92 |
| Transverse temporal L | 0.001 | 0.95 | Transverse temporal R | <0.001 | 1.17 |
| Parahipocampal L | 0.015 | 0.74 | |||
| Fusiform R | 0.002 | 0.96 | |||
| Temporal pole R | 0.013 | 0.72 | |||
| FA strength | |||||
| Hippocampus L | 0.004 | 0.85 | |||
| Paracentral R | 0.005 | 0.92 | |||
| Precentral L | 0.001 | 1.05 | Precentral R | <0.001 | 1.14 |
| Postcentral L | <0.001 | 1.24 | Postcentral R | 0.001 | 1 |
| Superior parietal L | <0.001 | 1.05 | Superior parietal R | 0.002 | 0.86 |
| Posterior cingulate R | 0.004 | 0.85 | |||
| Middle temporal L | 0.007 | 0.78 | Middle temporal R | 0.009 | 0.82 |
| Superior temporal R | 0.002 | 0.94 | |||
Significant results after applying FDR correction for multiple comparisons (P < 0.05); All cases show CP < HC, except for † where CP > HC; CP: Cerebral palsy; FA: Fractional anisotropy; L: Left; NOS: Number of streamlines; R: Right.
Conversely, NOS weighted data revealed decreased connectivity widespread throughout the brain in CP subjects, including mainly putamen, thalamus, and hippocampus, dorsal and caudal areas of the frontal lobe (superior and perirolandic cortex), lateral parietal lobe (postcentral, superior, and inferior gyrus), and extensively the occipital and temporal lobes (Fig. 1, Table 3). Finally, FA weighted data revealed a more localized disruption of the fiber integrity encompassing bilateral precentral cortex, postcentral cortex, superior parietal cortex, and middle temporal cortex; right paracentral cortex, posterior cingulate, and superior temporal cortex; and left hippocampus (Fig. 1, Table 3).
We tested whether whole‐brain differences were driven by some nodes in particular or were more global affectations. We found that the global FA effect was mainly driven by some specific nodes rather than a general involvement of the brain (i.e., computing whole‐brain connectivity values as the average of all tracts of the brain, but now excluding those with reduced FA (density: P = 0.63, d = 0.18; global FA: P = 0.29, d = 0.34)). This is not the case of NOS, as a widespread reduction is observed in almost half of all brain regions. For this reason, the edge‐level analyses including NOS will be also regressed by global NOS.
Looking at the subgroups analyses, a less extensive involvement was observed in the BG group, as expected. The results did not reach statistical significance after correcting for multiple comparisons for degree nor FA strength (Supporting Information, Tables SIV and SVI). However, non‐corrected results included mainly subcortical nuclei and occipital areas (lingual, cuneus, and pericalcarine) and the pallidum and supramarginal gyrus showed again an increased degree compared to HC. BG group showed a significant reduction in NOS in putamen, peri‐rolandic areas (precentral and postcentral) and occipital regions (pericalcarine and lingual). Non‐corrected results also included other subcortical, parietal and temporal areas (Supporting Information, Table SV). Regarding the BG ± WM group, results were quite similar to those of the general group in all node‐level metrics (Supporting Information, Tables SIV, SV, and SVI). Regarding the degree, a more bilateral pattern was observed and some new areas with a reduced degree in the frontal and temporal lobes were added to the general findings. FA strength was the measure showing greater impairment compared to results of the overall group, with lower WM integrity again in frontal and some temporal areas.
Edge‐level graph metrics
NBS analysis comparing CP participants and controls did not identify any component with a significant reduction or increase in NOS after regressing for global NOS. The NBS analysis including FA weighted data revealed one component in which FA was significantly reduced in CP subjects. This component comprised 41 connections (37 intrahemispheric – 20 left and 17 right – and 4 interhemispheric connections) between 34 nodes (P = 0.0016). This component comprised connections among basal ganglia (mainly putamen and pallidum) and thalamus with the precentral and postcentral gyrus (27% of the total significant connections), intraparietal connections (20%), fronto‐parietal connections (15%), hippocampal connections (12%), other thalamic and basal ganglia connections (10%), fronto‐frontal connections (5%), other parietal connections (insula and amygdala, 5%), temporal connections (with precentral and lingual gyrus, 5%), and the connection between primary motor cortex and the brainstem (2%; Figs. 2 and 3). Similar results were observed when tested for a range of t‐threshold values (see Supporting Information, Fig. S1).
Figure 2.
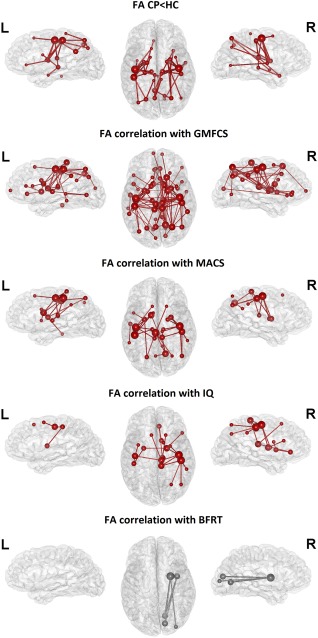
Results of the network‐based statistic analysis (NBS). Node and edge color indicates network membership; red indicates significance and gray indicates tendency (0.05 < P ≤ 0.1); the size of the nodes indicates the number of connections of this node that are included in the component. BFRT: Benton's Facial Recognition Test; CP: Cerebral palsy; FA: Fractional anisotropy; GMFCS: Gross Motor Function Classification System; HC: Healthy controls; IQ: Intelligence quotient; L: Left; MACS: Manual Ability Classification System; R: Right. BrainNet Viewer was used to create these figures [Xia et al., 2013]. [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 3.
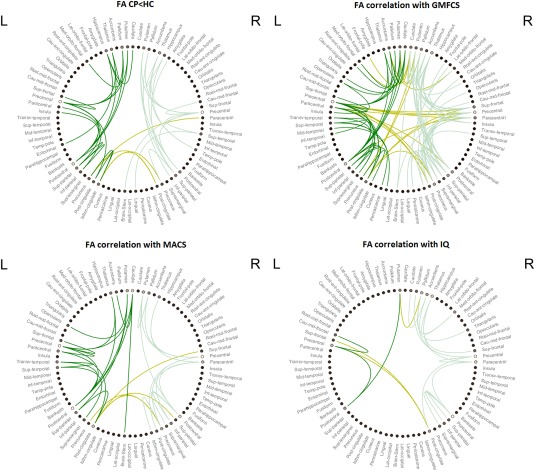
Circular graph of the results of the network‐based statistic analysis (NBS). Lines indicate significant connections for every kind of analysis. Edge colors separate intrahemispheric connections (dark green for the left hemisphere; soft green for the right hemisphere) and interhemispheric connections (green‐yellow). The color of the circle nodes indicates the number of connections of this node that are included in the component: the brighter means more connections. CP: Cerebral palsy; FA: Fractional anisotropy; GMFCS: Gross Motor Function Classification System; HC: Healthy controls; IQ: Intelligence quotient; L: Left; MACS: Manual Ability Classification System; R: Right. Schemaball was used to create this figure [Komarov, 2013]. [Color figure can be viewed at http://wileyonlinelibrary.com]
For the subgroup analyses, it was considered appropriate to use a more liberal P‐value threshold for the individual connections (0.05 instead of 0.02) due to the small size of the subgroups and the high statistical requirements used in the previous NBS analysis. The significance of the identified components remained the same (P‐value less than 0.05). NBS analysis including NOS weighted data identified one component comprising 20 connections (19 intrahemispheric – 15 left and 4 right – and 1 interhemispheric) in which NOS were significantly reduced in the BG group compared to controls (P = 0.002). This component mainly included left basal ganglia and thalamic connections among them, and with frontal and parietal regions (40%); occipital connections (30%); and temporal connections (15%; Supporting Information, Figure SII – A). There were no significant results with FA weighted data in the BG group. Finally, the NBS analyses comparing the BG ± WM group and the control group identified a significant reduction both in NOS and FA weighted data. As regards NOS, one bilateral component comprising 24 connections (20 intrahemispheric – 8 left and 12 right – and 4 interhemispheric) was identified (P = 0.001), including mainly basal ganglia and thalamic connections with precentral, postcentral and other parietal areas (50%); and parieto‐parietal and parieto‐temporal connections (33%; Supporting Information, Figure SII – B). FA weighted data identified one large, bilateral component of 134 connections (107 intrahemispheric – 52 left and 55 right – and 27 interhemispheric, P = 0.001) that mainly comprised parietal connections, mostly parieto‐parietal, and parieto‐frontal (40%); basal ganglia and thalamic connections, predominantly with frontal and parietal areas (36%), and fronto‐frontal connections (10%; Supporting Information, Figure SII – C).
White Matter Structure‐Function Correlations
Correlation with motor measures
The NBS analysis was also applied to identify clusters of connections that correlate with clinical measures. There were no significant results with NOS weighted data after regressing for global NOS. NBS analysis with FA weighted data revealed different networks related to clinical measures. One bilateral component was negatively correlated with GMFCS, associating lower levels of FA in these connections with a greater gross motor impairment. This component comprised 102 connections (83 intrahemispheric – 35 left and 48 right – and 19 interhemispheric connections) between 54 nodes (P < 0.001). Forty‐two percent of these connections included basal ganglia and thalamus and the remainder were cortico‐cortical connections. Regarding the basal ganglia and thalamic connections, 35% of these were directed to the precentral and postcentral cortex, 28% targeted the prefrontal cortex, 18% were connections among basal ganglia nuclei and thalamus, 14% targeted the parietal cortex (superior gyrus and precuneus), and 2 connections linking the right caudate with the insula and the brainstem (5%). With respect to cortico‐cortical connections, 41% were fronto‐parietal, 22% intraparietal, 14% fronto‐frontal, 10% included parieto‐temporal and parieto‐occipital connections, 7% were connections of the temporal lobe with the occipital, temporal and frontal lobes, 3% connected the insula and the prefrontal cortex, and there was one fronto‐occipital connection and the connection between primary motor cortex and the brainstem (3%; Figs. 2 and 3).
One bilateral component was negatively correlated with MACS, indicating that low levels of FA in these connections are related to a worse manual performance. This component comprised 34 connections (30 intrahemispheric – 17 left and 13 right – and 4 interhemispheric) between 29 nodes (P = 0.0067). Although smaller, there is an almost complete overlap of this component and the component correlating with GMFCS (91% of the connections included here were also present in the GMFCS component). Forty‐four percent of the connections comprised basal ganglia and thalamus, being the rest cortico‐cortical connections. Regarding the basal ganglia and thalamic connections, 60% of them were directed to the precentral and postcentral cortex and the remainder were connections among basal ganglia nuclei (13%), connections with the frontal cortex (13%), the parietal cortex (6.5%), and the brainstem (6.5%). As regards the cortico‐cortical connections, 37% were fronto‐parietal, 37% parieto‐parietal, 11% fronto‐frontal, and 16% temporo‐parietal and temporo‐temporal (Figs. 2 and 3). Similar findings were shown for GMFCS and MACS for a range of P‐threshold values (see Supporting Information, Fig. S1).
Correlation with cognitive measures
NBS showed a significant positive correlation with IQ measures in one component, showing that higher levels of FA in these edges are associated with better cognitive performance. The component comprised 20 connections (17 intrahemispheric – 3 left and 14 right – and 3 interhemispheric) between 20 nodes (P = 0.02). Of these connections, 25% linked the basal ganglia and thalamus with the frontal cortex, 25% were fronto‐parietal connections, 15% linked the basal ganglia with the parietal cortex, 15% were parieto‐parietal connections, 10% were connections among basal ganglia nuclei, and 10% were fronto‐frontal connections. Eighty percent of these connections correlating with IQ were shared with the GMFCS component (see above). Unshared connections (20%) involved mainly prefrontal and parietal areas.
Finally, a small component in the right hemisphere showed a trend toward significance (P = 0.051) in the positive correlational analysis with the visuoperceptual performance (BFRT), including five connections between putamen and occipital cortex (lateral occipital, lingual, and pericalcarine gyrus) and insula and occipital cortex (lingual and pericalcarine gyrus; Fig. 2). This component becomes significant (P = 0.028) with a lower P‐threshold value when tested for a range of P‐threshold values (see Supporting Information, Fig. S1).
DISCUSSION
White Matter Involvement in Dyskinetic CP
Significant differences were observed in the various whole‐brain network metrics and node‐level graph metrics computed, indicating a disruption in brain connectivity in subjects with dyskinetic CP. Even CP subjects with no radiological evidences of WM injury showed disrupted whole‐brain network and node‐level graph metrics. First, subjects with CP showed a significant reduction in network density. As density is a measure of gross connectedness, nodal degree analysis gives us more details of what brain regions are less interactive compared to controls, being mainly sensorimotor (precentral, postcentral, and superior parietal gyrus), visual (cuneus, lingual, and pericalcarine areas), and primary auditory areas (transverse temporal gyrus). Non‐corrected results for the CP subgroup with basal ganglia and/or thalamus injury and no WM involvement indicated a similar pattern. This pattern of involvement, which mainly comprises parieto‐occipital areas, coincides with previous findings on spastic CP [Ceschin et al., 2015; Nagae et al., 2007]. Ceschin et al. [2015] identified several parieto‐occipital nodes with a reduced nodal efficiency, local efficiency and clustering coefficient, and suggested that an altered occipito‐parietal network of disordered connectivity may underlie visuoperceptual dysfunction, an impairment that is estimated to occur in 40–50% of CP cases [Ego et al., 2015] and that has also been found in our sample (Table 1). A possible explanation for the involvement of sensorimotor areas could be related to the high metabolic rate of the primary sensory and motor cortex in the newborn period [Chugani, 1998; Rennie et al., 2007] becoming, among other structures, a target region in the event of acute brain asphyxia, which is a common cause of dyskinetic CP [Himmelmann et al., 2007]. Additionally to sensorimotor brain regions, primary visual and auditory areas – also impaired in our sample – have been found to be hubs in the organization of brain network during the neonatal period [Fransson et al., 2011; van den Heuvel et al., 2015b]. As it is in this period that brain damage occurs in dyskinetic CP patients, these hubs occupying a central position in the network might be more susceptible to brain damage than other not so central nodes [van den Heuvel and Sporns, 2013], which could explain the involvement of primary sensory areas in our sample.
Unexpectedly, two brain regions showed a significant higher nodal degree compared to controls: bilateral supramarginal gyrus and right pallidum. This result was also observed when comparisons were performed with the subgroups with and without WM involvement separately. Bilateral supramarginal gyrus presented an increased prevalence of connections with basal ganglia (including the right pallidum), thalamus, and middle frontal gyrus compared to controls. The supramarginal gyrus is part of the somatosensory association cortex which is involved in high‐level cognitive motor control, integrating different sensory data with motor signals and sending this information to premotor areas, where it will be used for motor programming and motor prediction [Fogassi and Luppino, 2005]. Delayed or disrupted sensorimotor integration has been described in CP as well as in other similar movement disorders such as focal dystonia, and it has been considered a key factor that underlies impaired motor function [Abbruzzese and Berardelli, 2003; Hoon et al., 2002, 2009; Nagae et al., 2007; Perruchoud et al., 2014; Rose et al., 2011; Tsao et al., 2015; Wingert et al., 2010]. It is known that an early brain injury can affect normal development promoting outgrowth of neural projections and generating abnormal connections and circuitry, which could lead to subsequent functional impairments [Giza and Prins, 2006; Johnston et al., 2002; Rocha‐Ferreira et al., 2016]. Thus, the observed overconnectivity in the inferior parietal lobule connections with basal ganglia and premotor areas could be a trigger for motor difficulties. The results presented by Wang et al. [2015] may point in this direction, as gray matter volume in the left supramarginal gyrus and bilateral pallidum, among other areas, significantly increased as aggravation of gross motor dysfunction in CP subjects. Reid et al. [2015a] also concluded that pallidal abnormality was a strong predictor of poor gross motor function in CP. Moreover, the finding of hyperconnected hubs in people with epilepsy has led to the conjecture that these aberrant connections may represent hyperexcitable circuits that initiate and maintain seizures [Bonilha et al., 2012]. It is worth noting that about half of our sample has a history of epilepsy, percentage that matches the recent population‐based data [Sellier et al., 2012]. Another hypothesis would be that this is a compensatory reorganization in which these connections counterbalance the effects of the altered sensorimotor system to convey the sensory feedback to motor areas using alternative pathways. There is evidence of reorganization of central motor pathways in people with CP, in which ipsilateral motor pathways are reinforced [Carr et al., 1993; Maegaki et al., 1999; Thomas et al., 2005]. In the somatosensory system, however, a different mechanism of postlesional reorganization is observed, in which “axonal bypasses” or alternative routes may be formed to achieve the original cortical destination [Little et al., 2009; for a review see Staudt, 2010; Thickbroom et al., 2001; Wilke et al., 2009]. However, the neurobiological mechanisms behind these neuroplastic processes are still unclear and warrant further study. Finally, another possible explanation for this finding may be methodological. The increase could be due to an effect of alterations in other brain areas, resulting in a better tractography of these specific regions. Pannek et al. [2014] also speculated that the finding of increased WM integrity in the temporal lobe of children with unilateral CP was artefactual.
Both the overall measure of WM volume as the total streamlines count indicated a significant reduction of WM volume in dyskinetic CP; result that was maintained in the subgroup analyses, even for those that did not present WM involvement at the radiological level. This result fits with the finding of Yoshida et al. [2013] which found a prominent decrease in WM volume in dyskinetic CP subjects. Harlaar et al. [2013] in a preliminary study with four subjects with severe dyskinetic CP showed that size of the pyramidal and language tracts was smaller than in controls. Looking at the node level to see what areas are involved, we observe a widespread reduction throughout the brain, including mainly putamen, thalamus and hippocampus, dorsal and caudal areas of the frontal lobe, lateral parietal areas, and extensively the temporal and occipital lobes. This pattern, although similar, was less extensive when analyzing the CP subgroup with damage in basal ganglia and/or thalamus and no WM involvement. It is well‐known that the basal ganglia and thalamus are the main affected areas and our results are consistent with a recent review showing specifically that putamen and thalamus are among the most commonly affected subcortical structures in dyskinetic CP [Aravamuthan and Waugh, 2016]. Moreover, widespread WM atrophy in infants with HIE may be secondary to disruption of the thalamo‐cortical connections or to a relative paucity of new WM growth [Mercuri et al., 2000; Rennie et al., 2007; Robertson and Perlman, 2006]. One noteworthy aspect is the relative preservation of the prefrontal areas in relation to the extensive involvement of the posterior regions. Ceschin et al. [2015] also identified a selective posterior regional vulnerability with a relative sparing of frontal regions in a group of children with spastic diplegic CP, and they attributed this finding to a reorganization of frontal–striatal and frontal–limbic pathways. This could be a manifestation of the more protracted developmental trajectory of the frontal lobe [Dennis and Thompson, 2013; Gogtay and Giedd, 2004; Wierenga et al., 2016], which could facilitate its reorganization after early brain damage. Analyses including FA data showed a global reduction of WM integrity in dyskinetic CP. However, node‐level FA strength data revealed a localized disruption of the fiber integrity encompassing posterior and medial regions, mainly sensorimotor and temporal areas. Yoshida et al. [2013] found a prominent reduction in FA in dyskinetic CP participants especially in the periventricular deep WM, and a smaller degree of FA decrease in peripheral WM regions. In a previous work of the same group, they found a FA reduction in several manually‐drawn WM tracts connecting areas with sensorimotor functions (corticospinal tract [CST], posterior thalamic radiation, or in the posterior limb of the internal capsule) [Yoshida et al., 2011]. The subgroup analysis may be indicating that the impairment in WM integrity occurs mainly and more extensively in the group with clear WM injury at the radiological level, as there were no significant results in the group without WM involvement. Nevertheless, we must consider these analyses as preliminary and analyze the results with caution, given such small samples.
In summary, results based in node‐level analyses have shown a pattern of disruptive brain connectivity characterized by a greater involvement of posterior regions with relative preservation of prefrontal areas. Based on these data, it is difficult to discern what alterations are caused by a primary lesion and what may be a developmental consequence of this primary damage. In this sense, it may be important to highlight those areas that have shown a significant decrease in all measures of connectivity (precentral, postcentral, superior parietal gyrus, and the hippocampus), as they may be areas of primary concern along with the well‐known deep gray matter nuclei (basal ganglia, mainly putamen, and thalamus). It is worth mentioning the involvement of the hippocampus, which was altered in all the connectivity measures. Although several studies of term infants suffering asphyxia report injury to the hippocampus [De Haan et al., 2006], this finding had not been consistently described in dyskinetic CP. Only the recent study of Park et al. showed a volume reduction in the hippocampus and parahippocampal gyrus in dyskinetic CP group compared to control group on a voxel‐based morphometry analysis [Park et al., 2014]. The known association between the hippocampus and memory and learning function [Squire and Wixted, 2011] points out the need to study this relationship in dyskinetic CP. It should be noted that a similar pattern is observed when groups with and without WM involvement at the radiological level are analyzed separately, with a more extensive involvement in the first subgroup, as could be expected.
The NBS analysis showed a bilateral symmetrical component with a significant reduction of WM FA in subjects with dyskinetic CP compared to healthy controls. Notably, almost 75% of the connections of this component comprised sensorimotor projections, being the precentral and postcentral gyrus the areas with more connections involved. In the first place, this is in agreement with a previous study analyzing FA in several manually‐drawn ROIs in people with dyskinetic CP that found a reduced FA in projection fibers connecting sensorimotor areas such as the posterior limb of the internal capsule or the posterior thalamic radiation [Yoshida et al., 2011]. Similar results, highlighting the involvement of the sensorimotor areas, have been found in spastic forms of CP [Arrigoni et al., 2016; Nagae et al., 2007; Pannek et al., 2014; Tsao et al., 2015], although with two remarkable differences: the contribution of basal ganglia and corticospinal connections. Although some alterations in basal ganglia have been described in cases with spastic CP [Krägeloh‐Mann et al., 2002], these are not very common [Bax et al., 2006; Himmelmann and Uvebrant, 2011] and most connectivity studies so far have not focused on these nuclei. Conversely, pyramidal tracts, and specifically the CST, are the most frequent assessed tracts in diffusion MRI studies in children with CP, mostly with spastic forms. Previous results repeatedly showed decreased integrity within this WM pathway compared to typically developing children, although thalamocortical connections are becoming increasingly important [Scheck et al., 2012]. In dyskinetic CP, results about the involvement of CST are contradictory. Harlaar et al. [2013] and Yoshida et al. [2011] showed a reduction in FA in the CST, while Park et al. [2014] did not. In our study, we found a FA reduction in the connections between right precentral gyrus and the brainstem, indicating some degree of impairment of the pyramidal tracts, but still far from the extensive involvement of the extrapyramidal tracts. This milder involvement of the pyramidal tracts could be responsible for the signs of spasticity sometimes observed concomitant with dystonia and choreoathetosis signs in some CP patients with predominant dyskinetic features. Second and consistent with the node‐level results, the WM pathway identified with a decreased FA mainly involves posterior brain regions with some preservation of the prefrontal areas. This is also observed at the level of commissural connections, as those showing a FA reduction link medial paracentral and parietal regions. This finding partially agrees with the study of Yoshida et al. [2011] who found a reduction of the WM integrity in the splenium of the corpus callosum, but also in the genu. Spastic CP studies also found an involvement of posterior parts of the corpus callosum [Arrigoni et al., 2016; Lee et al., 2011; Nagae et al., 2007; Thomas et al., 2005; Weinstein et al., 2014]. Finally, our findings confirm the aforementioned involvement of the hippocampus, as we find a decrease in FA in connections linking the hippocampus with other subcortical structures and medial and anterior areas of the temporal lobe.
When groups are analyzed separately, there is one component with a significant reduction in NOS in both groups with and without WM involvement at radiological level. In both groups, the reduction in NOS is mainly located in sensorimotor areas, emphasizing a greater involvement of occipital and temporal connections in the CP group without WM injury at the radiological level, and a greater involvement at the parietal level in the subgroup with WM involvement. In the latter group, there is also a reduction in the integrity of the WM, again with a high prevalence of parietal connections. The fact that there is no involvement of FA in the group with no radiological evidences of WM injury could be explained by different reasons. A first hypothesis is that there really is no alteration of the FA in this group, in which there is only a reduction in the number of fibers but not in the microstructural integrity of those that have remained. A second option would be that, in this group, the damage at FA level is much subtler and therefore a larger sample would be needed to detect these differences. Related to the latter, it could be that not all tracts that join two areas are affected in the same way or even different areas within the same tract. Finally, it is worth mentioning the role that can play the statistical requirements used in the NBS analysis in such a small sample. For all this, it is necessary to consider these subgroup analyses as preliminary and further studies are needed to analyse these differences with larger samples.
White Matter Structure‐Function Correlations
The second objective of this study was to analyze the structure‐clinical relationship underlying motor impairment and the most common cognitive deficits of this population, which are intellectual disability and visuoperceptual disturbances. With regard to motor function, we found a large bilateral component negatively correlated with GMFCS, indicating that lower levels of FA in these connections are related to greater gross motor impairment. This component comprised mainly basal ganglia and thalamic connections with primary sensorimotor areas, frontal and parietal areas, as well as fronto‐parietal and intraparietal connections. These results match those found in spastic CP in which thalamocortical and fronto‐parietal projections were associated with GMFCS, MACS, and other sensorimotor measures [Arrigoni et al., 2016; Pannek et al., 2014; Tsao et al., 2015]. If we compare this component with that found when compared with the control group, there are some points worth noting. First, there are a number of connections in which, although do not show a significant reduction of FA compared to controls, the degree of integrity of their WM fibers does relate to motor performance. This could mean that these connections, although not shown as affected in all CP subjects, could be injured in the most severe cases, which would have a larger brain injury. This would fit with qualitative MRI studies in which the extent of brain lesion has been strongly associated with motor impairment [Arnfield et al., 2013; Reid et al., 2015b]. Second, we observe a greater number of connections to the frontal lobe, specifically prefrontal areas, the left temporal lobe and the occipital lobe. As these areas are not solely or directly responsible for motor functions, they could be responsible for the diverse associated deficits observed in this population, as noted by some authors on cognitive impairment [Rai et al., 2013; Scheck et al., 2015; Tsao et al., 2015], visuoperceptual deficits [Arrigoni et al., 2016; Ego et al., 2015], or communication disturbances [Harlaar et al., 2013; Sigurdardottir and Vik, 2011]. This would also explain why the more severe the motor impairment, the larger the percentage of associated deficits [Himmelmann et al., 2009; Krägeloh‐Mann et al., 2002]. Results in this direction were found by Englander et al. [2013], who compared bilateral spastic CP cases according to their level of GMFCS and found differences in the sensorimotor network but also in many other connections (frontal, temporal, and occipital) related to perception and cognition, showing a decreased WM FA with increasing CP severity. Third, it is confirmed that interhemispheric connections involved in dyskinetic CP are those binding sensorimotor areas. In this analysis, the connections between bilateral primary motor areas are added to the somatosensory connections already present when compared to controls. This finding agrees with recent works proving that projections to primary and secondary motor and sensory areas are presumably located more posteriorly in the corpus callosum than previously suggested, probably including the posterior mid‐body, isthmus and splenium [Gooijers and Swinnen, 2014]. Finally, it also confirms the minor role of the brainstem connections in the motor impairment of people with dyskinetic CP, compared to spastic forms. Regarding fine motor function (MACS), a smaller component was identified in which more than 90% of the connections included were also correlated with GMFCS. This component mainly comprised basal ganglia and thalamocortical projections to sensorimotor areas, fronto‐parietal and parieto‐parietal connections. Arrigoni et al. [2016] also found a bigger component in which FA values correlated with GMFCS than with MACS in subjects with bilateral spastic CP, with a similar overlap in thalamocortical and fronto‐parietal connections although with a greater presence of corticospinal projections.
NBS correlational analysis including cognitive measures identified clusters of connections positively correlated with WM integrity. As regards to intelligence, a component was identified with a remarkable proportion of frontal projections (60% of all connections involved), linked to basal ganglia, thalamus, parietal areas and, to a lesser extent, other frontal areas. The large overlap of the IQ component with the one found with GMFCS was totally expected, as poorer motor function has been strongly associated with greater cognitive impairment in dyskinetic CP [Himmelmann et al., 2009]. This relationship is not surprising as an increasing amount of evidence shows the relevant role that basal ganglia connectivity also play in cognition [Burgaleta et al., 2014; Rhein et al., 2014; Sandman et al., 2014], especially for their prefrontal connections [Leisman et al., 2014]. Interestingly, those connections included in the IQ component and non‐shared with the GMFCS component involved prefrontal areas such as the pars triangularis of the inferior frontal gyrus, the caudal part of the middle frontal gyrus or the superior frontal gyrus. Fronto‐parietal projections were also included, which is consistent with distributed models of intelligence that emphasize the crucial role of WM tracts underlying parieto‐frontal association cortices [Jung and Haier, 2007]. Accordingly with these findings, our results suggest that the state of WM connections in fronto‐striatal and parieto‐frontal circuitry may have a role in intellectual performance in people with dyskinetic CP. As a final point, FA in a small right component was associated with visuoperceptual performance, fitting with the results found by the same authors of the test [Benton and Van Allen, 1968], as well as subsequently works supporting this test as a good measure of right hemisphere functioning [Tranel et al., 2009]. Furthermore, our findings indicate that visuoperceptual performance in dyskinetic CP is linked to microstructural integrity of the visual afferent pathways and visual processing circuitry. Specifically, the component included connections between putamen, insula and visual areas of the occipital lobe (pericalcarine and lingual gyrus). The primary visual cortex is located around the calcarine sulcus and the lingual gyrus is also known to be involved in basic visual information processing and visual memory functions, areas that are gathered in the visuoperceptual test used. The projections of these visual areas to more rostral structures could be related to the additional connections that have been associated with the ventral visual pathway, which is an occipito‐temporal pathway supporting the processing of object quality or identity [Kravitz et al., 2014]. In spastic CP, involvement of the ventral stream of visual perception has also been detected in preterm children with periventricular leukomalacia [Fazzi et al., 2009]. Additionally, a FA reduction has also been found in longitudinal visual association tracts such as inferior fronto‐occipital fasciculus, inferior longitudinal fasciculus, optic radiation, and posterior thalamic radiation in spastic diplegic CP subjects [Ceschin et al., 2015].
Strengths and Limitations
This study demonstrates that the application of the connectome‐based approach for the first time to the study of dyskinetic CP helps to elucidate WM injury in this population, identifying pathways of altered connectivity, as well as to increase knowledge about the structure‐function relationship. It is further demonstrated as a valid tool for detecting microstructural WM impairment that cannot be identified by conventional MRI. Identifying the particular features of individuals with dyskinetic CP is crucial for a better understanding of this little‐known CP subtype, and may contribute to the design of more appropriate interventions and follow‐up programs. Moreover, changes in brain connectivity may potentially help to determine the underlying mechanisms of the observed functional improvements produced by these interventions.
However, the current study is not without limitations. First, as DWI is an indirect reconstruction of WM rather than a direct axonal measure, data should be interpreted as an estimation of anatomical connectivity [Jones, 2010]. Second, deterministic tractography algorithms are criticized for their failure to resolve crossing‐fiber geometries, which predominantly results in false negatives, especially in short, local tracts between adjacent brain regions [Jbabdi and Johansen‐Berg, 2011]. Although connectome sensitivity can be improved with probabilistic tractography algorithms, this is at the expense of lowering specificity due to the introduction of false positives, which may be not recommended as connectome specificity has been found to be more important than connectome sensitivity, particularly with respect to analyses of topological properties of brain networks [Zalesky et al., 2016]. It should be added that there is evidence showing that diffusion tractography derived connectome using deterministic fiber tracking form a valid in vivo approximation of WM tract in terms of strength [van den Heuvel et al., 2015a]. Another methodological aspect of parcellation‐based connectomes is the impact of template choice on the spatial and topological organization of the resulting brain networks. Care should be taken when comparing results of studies that use different parcellation schemes [de Reus and van den Heuvel, 2013b]. One specific limitation of the parcellation scheme used in this work is that the cerebellum was not included. As the cerebellum has been involved in dystonia [Shakkottai et al., 2017], it would be interesting for future works to study its role in dyskinetic CP.
Finally, the sample also requires some considerations. Although sample size may be considered large taking into account the characteristics of the sample and previous DWI studies, it is small in terms of statistical power. Results at the subgroup level must be considered preliminary and future studies with larger samples should confirm these findings. Second, the standardized and automated approach carried out here increases reproducibility, but it prevents the inclusion of significantly atypical brains due to problems in the automated parcellation. This, together with the fact that those subjects with severe sensory or comprehension deficits cannot be included in a study of this nature may limit the generalizability of the results. Another limitation is the lack of a well‐established measure of the dyskinesia component in CP, hence our study was only based on general clinical criteria. Furthermore, it cannot be excluded that the relationship between brain structure and clinical function may be modulated by the pharmacological treatment [Haneef et al., 2015; Vaessen et al., 2012], which is very common in CP. Finally, the wide age range of the sample should also be noted. This might also be affecting the relationship between brain structure and clinical functions, even though this variable was controlled for in all analyses. However, it should be noted that the clinical measures used have been found to remain quite stable by the age of our sample [Franić et al., 2014; McKone et al., 2009; Schalke et al., 2013].
CONCLUSION
The present findings indicate that connectome‐based analyses can identify a disruption in brain connectivity in subjects with dyskinetic CP. This disruption is characterized by a widespread reduction of WM volume but a more localized involvement in terms of fiber integrity (FA) and number of links per node (degree), generally involving parieto‐occipital areas with a relative preservation of the prefrontal lobe. Hippocampal connectivity was also shown to be reduced in CP subjects. Bilateral supramarginal gyrus, however, showed higher degree compared to controls, with an increased prevalence of connections with basal ganglia, thalamus and frontal areas. At the network level, we identified a bilateral symmetrical pathway in which FA was reduced in CP subjects compared to controls, mostly comprising connections among basal ganglia and thalamus with sensorimotor areas, parieto‐parietal connections, fronto‐parietal connections as well as hippocampal connections. Preliminary analyses showed a similar, although smaller, pattern of WM involvement in subjects with no evidence of WM injury at the radiological level regarding measures of WM volume. No significant reduction was observed is this group compared to controls in terms of WM integrity. Finally, we also identified pathways in which FA was related to clinical features. Gross and fine motor function correlated with fiber integrity in a bilateral pathway comprising connections among the sensorimotor system, but gross motor function was also significantly correlated with prefrontal, temporal, and occipital connections. As regards cognitive measures, IQ was correlated with FA in a network with a remarkable prevalence of fronto‐striatal and parieto‐frontal connections, and visuoperceptual performance was linked to right occipital projections.
Conflict of interests
None of the authors have any conflict of interest to report.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
The authors wish to thank all participants and their families for their collaboration. We would also like to thank the association ASPACE for providing patients enrolled in this study as well as the members of the Dutch Connectome Lab from the Brain Center Rudolf Magnus (UMC Utrecht) for their collaboration and help in this work.
REFERENCES
- Abbruzzese G, Berardelli A (2003): Sensorimotor integration in movement disorders. Mov Disord 18:231–240. [DOI] [PubMed] [Google Scholar]
- Andersson JLR, Skare S (2002): A model‐based method for retrospective correction of geometric distortions in diffusion‐weighted EPI. Neuroimage 16:177–199. [DOI] [PubMed] [Google Scholar]
- Aravamuthan BR, Waugh JL (2016): Localization of basal ganglia and thalamic damage in dyskinetic cerebral palsy. Pediatr Neurol 54:11–21. [DOI] [PubMed] [Google Scholar]
- Arnfield E, Guzzetta A, Boyd R (2013): Relationship between brain structure on magnetic resonance imaging and motor outcomes in children with cerebral palsy: A systematic review. Res Dev Disabil 34:2234–2250. [DOI] [PubMed] [Google Scholar]
- Arrigoni XF, Peruzzo XD, Gagliardi XC, Maghini XC, Colombo XP, Iammarrone XFS, Pierpaoli XC, Triulzi XF (2016): Whole‐brain DTI assessment of white matter damage in children with bilateral cerebral palsy: Evidence of involvement beyond the primary target of the anoxic insult. Pediatrics 37:1347–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballester‐Plané J, Laporta‐Hoyos O, Macaya A, Póo P, Meléndez‐Plumed M, Vázquez É, Delgado I, Zubiaurre‐Elorza L, Narberhaus A, Toro‐Tamargo E, Russi ME, Tenorio V, Segarra D, Pueyo R (2016): Measuring intellectual ability in cerebral palsy: The comparison of three tests and their neuroimaging correlates. Res Dev Disabil 56:83–98. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C (1996): Microstructural and physiological features of tissues elucidated by quantitative‐diffusion‐tensor MRI. J Magn Reson Series B 1:209–219. [DOI] [PubMed] [Google Scholar]
- Bax M, Tydeman C, Flodmark O (2006): Clinical and MRI correlates of cerebral palsy: The European Cerebral Palsy Study. JAMA 296:1602–1608. [DOI] [PubMed] [Google Scholar]
- Benton AL (1994): Contributions to Neuropsychological Assessment: A Clinical Manual, Vol. 2 New York: Oxford University Press. [Google Scholar]
- Benton AL, Van Allen MW (1968): Impairment in facial recognition in patients with cerebral disease. Trans Am Neurol Assoc 93:38–42. [PubMed] [Google Scholar]
- Bonilha L, Nesland T, Martz GU, Joseph JE, Spampinato MV, Edwards JC, Tabesh A (2012): Medial temporal lobe epilepsy is associated with neuronal fibre loss and paradoxical increase in structural connectivity of limbic structures. J Neurol Neurosurg Psychiatry 83:903–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore E, Sporns O (2009): Complex brain networks: Graph theoretical analysis of structural and functional systems. Nat Rev Neurosci 10:186–198. [DOI] [PubMed] [Google Scholar]
- Burgaleta M, Macdonald P. a, Martínez K, Román FJ, Álvarez‐Linera J, González AR, Karama S, Colom R (2014): Subcortical regional morphology correlates with fluid and spatial intelligence. Hum Brain Mapp 35:1957–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cans C, Dolk H, Platt MJ, Colver A, Prasauskiene A, Krägeloh‐Mann I, SCPE Collaborative Group (2007): Recommendations from the SCPE collaborative group for defining and classifying cerebral palsy. Dev Med child Neurol 109:35–38. [DOI] [PubMed] [Google Scholar]
- Carr LJ, Harrison LM, Evans AL, Stephens JA (1993): Patterns of central motor reorganization in hemiplegic cerebral palsy. Brain 116:1223–1247. [DOI] [PubMed] [Google Scholar]
- Ceschin R, Lee VK, Schmithorst V, Panigrahy A (2015): Regional vulnerability of longitudinal cortical association connectivity Associated with structural network topology alterations in preterm children with cerebral palsy. NeuroImage Clin 9:322–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang LC, Walker L, Pierpaoli C (2012): Informed RESTORE: A method for robust estimation of diffusion tensor from low redundancy datasets in the presence of physiological noise artifacts. Magn Reson Med 68:1654–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugani HT (1998): A critical period of brain development: Studies of cerebral glucose utilization with PET. Prev Med (Baltim) 27:184–188. [DOI] [PubMed] [Google Scholar]
- Cohen J (1988): Statistical Power Analysis for the Behavioural Sciences, 2nd ed New York: Academic Press. [Google Scholar]
- Collin G, Kahn RS, Reus MA , De Cahn W , van den Heuvel MP (2014): Impaired rich club connectivity in unaffected siblings of schizophrenia patients. Schizophr Bull 40:438–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haan M, Wyatt JS, Roth S, Vargha‐Khadem F, Gadian D, Mishkin M (2006): Brain and cognitive‐behavioural development after asphyxia at term birth. Dev Sci 9:350–358. [DOI] [PubMed] [Google Scholar]
- de Reus MA, van den Heuvel MP (2013a): Estimating false positives and negatives in brain networks. Neuroimage 70:402–409. [DOI] [PubMed] [Google Scholar]
- de Reus MA, van den Heuvel MP (2013b): The parcellation‐based connectome: Limitations and extensions. Neuroimage 80:397–404. [DOI] [PubMed] [Google Scholar]
- Dennis EL, Thompson PM (2013): Typical and atypical brain development: A review of neuroimaging studies. Dialogues Clin Neurosci 15:359–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS (2006): An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31:968–980. [DOI] [PubMed] [Google Scholar]
- Ego A, Lidzba K, Brovedani P, Belmonti V, Gonzalez‐Monge S, Boudia B, Ritz A, Cans C (2015): Visual‐perceptual impairment in children with cerebral palsy: A systematic review. Dev Med Child Neurol 57:46–51. [DOI] [PubMed] [Google Scholar]
- Eliasson A‐C, Krumlinde‐Sundholm L, Rösblad B, Beckung E, Arner M, Ohrvall A‐M, Rosenbaum P (2006): The Manual Ability Classification System (MACS) for children with cerebral palsy: Scale development and evidence of validity and reliability. Dev Med Child Neurol 48:549–554. [DOI] [PubMed] [Google Scholar]
- Englander ZA, Pizoli CE, Batrachenko A, Sun J, Worley G, Mikati M. a, Kurtzberg J, Song AW (2013): Diffuse reduction of white matter connectivity in cerebral palsy with specific vulnerability of long range fiber tracts. NeuroImage Clin 2:440–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englander ZA, Sun J, Case L, Mikati MA, Kurtzberg J, Song AW (2015): Brain structural connectivity increases concurrent with functional improvement: Evidence from diffusion tensor MRI in children with cerebral palsy during therapy. NeuroImage Clin 7:315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazzi E, Bova S, Giovenzana A, Signorini S, Uggetti C, Bianchi P (2009): Cognitive visual dysfunctions in preterm children with periventricular leukomalacia. Dev Med Child Neurol 51:974–981. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM (2002): Whole brain segmentation: Neurotechnique automated labeling of neuroanatomicalstructures in the human brain. Neuron 33:341–355. [DOI] [PubMed] [Google Scholar]
- Fogassi L, Luppino G (2005): Motor functions of the parietal lobe. Curr Opin Neurobiol 15:626–631. [DOI] [PubMed] [Google Scholar]
- Franić S, Dolan CV, van Beijsterveldt CEM, Pol HEH, Bartels M, Boomsma DI (2014): Genetic and environmental stability of intelligence in childhood and adolescence. Twin Res Hum Genet 17:151–163. [DOI] [PubMed] [Google Scholar]
- Fransson P, Åden U, Blennow M, Lagercrantz H (2011): The functional architecture of the infant brain as revealed by resting‐state fMRI. Cereb Cortex 21:145–154. [DOI] [PubMed] [Google Scholar]
- Giza CC, Prins ML (2006): Is being plastic fantastic? Mechanisms of altered plasticity after developmental traumatic brain injury. Dev Neurosci 28:364–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd J (2004): Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA 101:8174–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooijers J, Swinnen SP (2014): Interactions between brain structure and behavior: The corpus callosum and bimanual coordination. Neurosci Biobehav Rev 43:1–19. [DOI] [PubMed] [Google Scholar]
- Haneef Z, Levin HS, Chiang S (2015): Brain graph topology changes associated with anti‐epileptic drug use. Brain Connect 5:284–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlaar L, Pouwels PJ, Vermeulen RJ, Oostrom K, Barkhof F (2013): Language comprehension in young people with severe cerebral palsy in relation to language tracts: A diffusion tensor imaging study. Neuropediatrics 44:286–290. [DOI] [PubMed] [Google Scholar]
- Himmelmann K (2015): The quest for patterns in dyskinetic cerebral palsy. Dev Med Child Neurol 58:112. [DOI] [PubMed] [Google Scholar]
- Himmelmann K, Uvebrant P (2011): Function and neuroimaging in cerebral palsy: A population‐based study. Dev Med Child Neurol 53:516–521. [DOI] [PubMed] [Google Scholar]
- Himmelmann K, Uvebrant P (2014): The panorama of cerebral palsy in Sweden. XI. Changing patterns in the birth‐year period 2003–2006. Acta Paediatr 103:618–624. [DOI] [PubMed] [Google Scholar]
- Himmelmann K, Hagberg G, Wiklund LM, Eek MN, Uvebrant P (2007): Dyskinetic cerebral palsy: A population‐based study of children born between 1991 and 1998. Dev Med Child Neurol 49:246–251. [DOI] [PubMed] [Google Scholar]
- Himmelmann K, Hagberg G, Uvebrant P (2010): The changing panorama of cerebral palsy in Sweden. X. Prevalence and origin in the birth-year period 1999–2002. Acta Paediatr Int J Paediatr 99:1337–1343. [DOI] [PubMed] [Google Scholar]
- Himmelmann K, McManus V, Hagberg G, Uvebrant P, Krägeloh‐Mann I, Cans C, SCPE collaboration (2009): Dyskinetic cerebral palsy in Europe: Trends in prevalence and severity. Arch Dis Child 94:921–926. [DOI] [PubMed] [Google Scholar]
- Hoon AH, Lawrie WT, Melhem ER, Reinhardt EM, Van Zijl PCM, Solaiyappan M, Jiang H, Johnston MV, Mori S, Hoon AH Jr, Lawrie WT Jr (2002): Diffusion tensor imaging of periventricular leukomalacia shows affected sensory cortex white matter pathways. Neurology 59:752–756. [DOI] [PubMed] [Google Scholar]
- Hoon AH Jr, Stashinko EE, Nagae LM, Lin DDM, Keller J, Bastian A, Campbell ML, Levey E, Mori S, Johnston MV, Hoon AH (2009): Sensory and motor deficits in children with cerebral palsy born preterm correlate with diffusion tensor imaging abnormalities in thalamocortical pathways. Dev Med Child Neurol 51:697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jbabdi S, Johansen‐Berg H (2011): Tractography: Where do we go from here? Brain Connect 1:169–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston MV, Nakajima W, Hagberg H (2002): Mechanisms of hypoxic neurodegeneration in the developing brain. Neuroscientist 8:212–220. [DOI] [PubMed] [Google Scholar]
- Jones DK (2010): Precision and accuracy in diffusion tensor magnetic resonance imaging. Top Magn Reson Imaging 21:87–99. [DOI] [PubMed] [Google Scholar]
- Jung RE, Haier RJ (2007): The Parieto‐Frontal Integration Theory (P‐FIT) of intelligence: Converging neuroimaging evidence. Behav Brain Sci 30:135–154‐187. [DOI] [PubMed] [Google Scholar]
- Komarov O (2013): Schemaball, MATLAB Central File Exchange. Available at: https://es.mathworks.com/matlabcentral/fileexchange/42279-schemaball, accessed on October 4, 2016.
- Krageloh‐Mann I, Cans C (2009): Cerebral palsy update. Brain Dev 31:537–544. [DOI] [PubMed] [Google Scholar]
- Krägeloh‐Mann I, Helber A, Mader I, Staudt M, Wolff M, Groenendaal F, DeVries L, Krageloh‐Mann I (2002): Bilateral lesions of thalamus and basal ganglia: Origin and outcome. Dev Med Child Neurol 44:477–484. [DOI] [PubMed] [Google Scholar]
- Kravitz DJ, Saleem KS, Baker CI, Ungerleider LG, Mishkin M (2014): The ventral visual pathway: An expanded neural framework for the processing of object quality. Trends Cogn Sci 17:26–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JD, Park HJ, Park ES, Oh MK, Park B, Rha DW, Cho SR, Kim EY, Park JY, Kim CH, Kim DG, Park CI (2011): Motor pathway injury in patients with periventricular leucomalacia and spastic diplegia. Brain 134:1199–1210. [DOI] [PubMed] [Google Scholar]
- Leisman G, Braun‐Benjamin O, Melillo R (2014): Cognitive‐motor interactions of the basal ganglia in development. Front Syst Neurosci 8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little GE, López‐Bendito G, Rünker AE, García N, Piñon MC, Chédotal A, Molnár Z, Mitchell KJ (2009): Specificity and plasticity of thalamocortical connections in Sema6A mutant mice. Ed. William A Harris. PLoS Biol 7:e1000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maegaki Y, Maeoka Y, Ishii S, Eda I, Ohtagaki A, Kitahara T, Suzuki N, Yoshino K, Ieshima A, Koeda T, Takeshita K (1999): Central motor reorganization in cerebral palsy patients with bilateral cerebral lesions. Pediatr Res 45:559–567. [DOI] [PubMed] [Google Scholar]
- McKone, E. , Crookes, K. , & Kanwisher N (2009): The cognitive and neural development of face recognition in humans In: Gazzaniga MS, editor. The Cognitive Neurosciences, 4th ed Cambridge: MIT Press. [Google Scholar]
- Mercuri E, Ricci D, Cowan FM, Lessing D, Frisone MF, Haataja L, Counsell SJ, Dubowitz LM, Rutherford M. a (2000): Head growth in infants with hypoxic‐ischemic encephalopathy: Correlation with neonatal magnetic resonance imaging. Pediatrics 106:235–243. [DOI] [PubMed] [Google Scholar]
- Mori S, Van Zijl PCM (2002): Fiber tracking: Principles and strategies ‐ A technical review. NMR Biomed 15:468–480. [DOI] [PubMed] [Google Scholar]
- Mori S, Crain BJ, Chacko VP, Van Zijl PCM (1999): Three‐dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol 45:265–269. [DOI] [PubMed] [Google Scholar]
- Nagae LM, Hoon AH, Jr Stashinko E, Lin D, Zhang W, Levey E, Wakana S, Jiang H, Leite CC, Lucato LT, van Zijl PC, Johnston MV, Mori S (2007): Diffusion tensor imaging in children with periventricular leukomalacia: Variability of injuries to white matter tracts. AJNRAmerican J Neuroradiol 28:1213–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak I, Hines M, Goldsmith S, Barclay R (2012): Clinical prognostic messages from a systematic review on cerebral palsy. Pediatrics 130:e1285–e1312. [DOI] [PubMed] [Google Scholar]
- Oskoui M, Coutinho F, Dykeman J, Jetté N, Pringsheim T (2013): An update on the prevalence of cerebral palsy: A systematic review and meta‐analysis. Dev Med Child Neurol 55:509–519. [DOI] [PubMed] [Google Scholar]
- Palisano RJ, Rosenbaum P, Bartlett D, Livingston MH (2008): Content validity of the expanded and revised Gross Motor Function Classification System. Dev Med Child Neurol 50:744–750. [DOI] [PubMed] [Google Scholar]
- Pannek K, Boyd RN, Fiori S, Guzzetta A, Rose SE (2014): Assessment of the structural brain network reveals altered connectivity in children with unilateral cerebral palsy due to periventricular white matter lesions. NeuroImage Clin 5:84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park BH, Park SH, Seo JH, Ko MH, Chung GH (2014): Neuroradiological and Neurophysiological characteristics of patients with dyskinetic cerebral palsy. Ann Rehabil Med 38:189–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perruchoud D, Murray MM, Lefebvre J, Ionta S (2014): Focal dystonia and the Sensory‐Motor Integrative Loop for Enacting (SMILE). Front Hum Neurosci 8:458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai Y, Chaturvedi S, Paliwal VK, Goyal P, Chourasia A, Singh Rathore RK, Yadav A, Pandey CM, Lalla RS, Garg RK, Gupta RK (2013): DTI correlates of cognition in term children with spastic diplegic cerebral palsy. Eur J Paediatr Neurol 17:294–301. [DOI] [PubMed] [Google Scholar]
- Raven JC, Court JH, Seisdedos Cubero N (2001): Raven: Matrices progresivas: Escalas: CPM color, SPM general, APM superior. Madrid: TEA. [Google Scholar]
- Reid SM, Dagia CD, Ditchfield MR, Carlin JB, Reddihough DS (2014): Population‐based studies of brain imaging patterns in cerebral palsy. Dev Med Child Neurol 56:222–232. [DOI] [PubMed] [Google Scholar]
- Reid SM, Dagia CD, Ditchfield MR, Reddihough DS (2015a): Grey matter injury patterns in cerebral palsy: Associations between structural involvement on MRI and clinical outcomes. Dev Med Child Neurol 57:1159–1167. [DOI] [PubMed] [Google Scholar]
- Reid SM, Ditchfield MR, Bracken J, Reddihough DS (2015b): Relationship between characteristics on magnetic resonance imaging and motor outcomes in children with cerebral palsy and white matter injury. Res Dev Disabil 45–46:178–187. [DOI] [PubMed] [Google Scholar]
- Rennie JM, Hagmann CF, Robertson NJ (2007): Outcome after intrapartum hypoxic ischaemia at term. Semin Fetal Neonatal Med 12:398–407. [DOI] [PubMed] [Google Scholar]
- Rhein C, Mühle C, Richter‐Schmidinger T, Alexopoulos P, Doerfler A, Kornhuber J (2014): Neuroanatomical correlates of intelligence in healthy young adults: The role of basal ganglia volume. PLoS One 9:e93623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson CMT, Perlman M (2006): Follow‐up of the term infant after hypoxic‐ischemic encephalopathy. Paediatr Child Health (Oxford) 11:278–282. [PMC free article] [PubMed] [Google Scholar]
- Rocha‐Ferreira E, Hristova M, Rocha‐Ferreira E, Hristova M (2016): Plasticity in the neonatal brain following hypoxic‐ischaemic injury. Neural Plast 2016:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose S, Guzzetta A, Pannek K, Boyd R (2011): MRI structural connectivity, disruption of primary sensorimotor pathways, and hand function in cerebral palsy. Brain Connect 1:309–316. [DOI] [PubMed] [Google Scholar]
- Rosenbaum P, Paneth N, Leviton A, Goldstein M, Bax M, Damiano D, Dan B, Jacobsson B (2007): A report: The definition and classification of cerebral palsy April 2006. Dev Med Child Neurol Suppl 109:8–14. [PubMed] [Google Scholar]
- Rubinov M, Sporns O (2010): NeuroImage Complex network measures of brain connectivity: Uses and interpretations. Neuroimage 52:1059–1069. [DOI] [PubMed] [Google Scholar]
- Sandman CA, Head K, Muftuler LT, Su L, Buss C, Davis EP (2014): Shape of the basal ganglia in preadolescent children is associated with cognitive performance. Neuroimage 99:93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalke D, Brunner M, Geiser C, Preckel F, Keller U, Spengler M, Martin R (2013): Stability and change in intelligence from age 12 to age 52: Results from the Luxembourg MAGRIP study. Dev Psychol 49:1529–1543. [DOI] [PubMed] [Google Scholar]
- Scheck SM, Boyd RN, Rose SE (2012): New insights into the pathology of white matter tracts in cerebral palsy from diffusion magnetic resonance imaging: A systematic review. Dev Med Child Neurol 54:684–696. [DOI] [PubMed] [Google Scholar]
- Scheck SM, Pannek K, Raffelt DA, Fiori S, Boyd RN, Rose SE (2015): Structural connectivity of the anterior cingulate in children with unilateral cerebral palsy due to white matter lesions. Neuroimage Clin 9:498–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellier E, Uldall P, Calado E, Sigurdardottir S, Torrioli MG, Platt MJ, Cans C (2012): Epilepsy and cerebral palsy: Characteristics and trends in children born in 1976–1998. Eur J Paediatr Neurol 16:48–55. [DOI] [PubMed] [Google Scholar]
- Sellier E, Platt MJ, Andersen GL, Krägeloh‐Mann I, De La Cruz J, Cans C, Surveillance of Cerebral Palsy Network (2016): Decreasing prevalence in cerebral palsy: A multi‐site European population‐based study, 1980 to 2003. Dev Med Child Neurol 58:85–92. [DOI] [PubMed] [Google Scholar]
- Shakkottai VG, Batla A, Bhatia K, Dauer WT, Dresel C, Niethammer M, Eidelberg D, Raike RS, Smith Y, Jinnah HA, Hess EJ, Meunier S, Hallett M, Fremont R, Khodakhah K, LeDoux MS, Popa T, Gallea C, Lehericy S, Bostan AC, Strick PL (2017): Current opinions and areas of consensus on the role of the cerebellum in dystonia. Cerebellum 16:577–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdardottir S, Vik T (2011): Speech, expressive language, and verbal cognition of preschool children with cerebral palsy in Iceland. Dev Med Child Neurol 53:74–80. [DOI] [PubMed] [Google Scholar]
- Sporns O (2010): Networks of the Brain. Cambridge: MIT Press. [Google Scholar]
- Sporns O, Tononi G, Kötter R (2005): The human connectome: A structural description of the human brain. PLoS Comput Biol 1:e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Wixted JT (2011): The cognitive neuroscience of human memory since H.M. Annu Rev Neurosci 34:259–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudt M (2010): Reorganization after pre‐ and perinatal brain lesions. J Anat 217:469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss E, Spreen O, Sherman EMS (2006): A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary, Vol. 3 Oxford: Oxford University Press. [Google Scholar]
- Thickbroom GW, Byrnes ML, Archer SA, Nagarajan L, Mastaglia FL (2001): Differences in sensory and motor cortical organization following brain injury early in life. Ann Neurol 49:320–327. [PubMed] [Google Scholar]
- Thomas B, Eyssen M, Peeters R, Molenaers G, Van Hecke P, De Cock P, Sunaert S (2005): Quantitative diffusion tensor imaging in cerebral palsy due to periventricular white matter injury. Brain 128:2562–2577. [DOI] [PubMed] [Google Scholar]
- Toronto AS (1973): Screening Test of Spanish Grammar, ed. Evenston, IL: Northwestern University Press. [Google Scholar]
- Tranel D, Vianna E, Manzel K, Damasio H, Grabowski T (2009): Neuroanatomical correlates of the benton facial. J Clin Exp Neuropsychol 31:219–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao H, Pannek K, Boyd RN, Rose SE (2015): Changes in the integrity of thalamocortical connections are associated with sensorimotor deficits in children with congenital hemiplegia. Brain Struct Funct 220:307–318. [DOI] [PubMed] [Google Scholar]
- Vaessen MJ, Jansen JFA, Vlooswijk MCG, Hofman PAM, Majoie HJM, Aldenkamp AP, Backes WH (2012): White matter network abnormalities are associated with cognitive decline in chronic epilepsy. Cereb Cortex 22:2139–2147. [DOI] [PubMed] [Google Scholar]
- Verstraete E, Veldink JH, Mandl RCW, Berg LH Van Den, Den MP Van (2011): Impaired structural motor connectome in amyotrophic lateral sclerosis. PLoS One 6:e24239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Sporns O (2011): Rich‐club organization of the human connectome. J Neurosci 31:15775–15786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Sporns O (2013): Network hubs in the human brain. Trends Cogn Sci 17:683–696. [DOI] [PubMed] [Google Scholar]
- van den Heuvel MP, de Reus MA, Feldman Barrett L, Scholtens LH, Coopmans FMT, Schmidt R, Preuss TM, Rilling JK, Li L (2015a): Comparison of diffusion tractography and tract‐tracing measures of connectivity strength in rhesus macaque connectome. Hum Brain Mapp 36:3064–3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Kersbergen KJ, de Reus MA, Keunen K, Kahn RS, Groenendaal F, de Vries LS, Benders MJNL (2015b): The neonatal connectome during preterm brain development. Cereb Cortex 25:3000–3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Bullmore ET, Sporns O (2016): Comparative connectomics. Trends Cogn Sci 20:345–361. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wang H, Yu Y, Xu L, Chen Y, Wu D (2015): [A voxel‐based morphometric study on change of gray matter structures in cerebral palsy]. Chinese J Pediatr 53:696–700. [PubMed] [Google Scholar]
- Weinstein M, Green D, Geva R, Schertz M, Fattal‐Valevski A, Artzi M, Myers V, Shiran S, Gordon AM, Gross‐Tsur V, Ben BD (2014): Interhemispheric and intrahemispheric connectivity and manual skills in children with unilateral cerebral palsy. Brain Struct Funct 219:1025–1040. [DOI] [PubMed] [Google Scholar]
- Wierenga LM, van den Heuvel MP, van Dijk S, Rijks Y, de Reus MA, Durston S (2016): The development of brain network architecture. Hum Brain Mapp 37:717–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke M, Staudt M, Juenger H, Grodd W, Braun C, Krägeloh‐Mann I (2009): Somatosensory system in two types of motor reorganization in congenital hemiparesis: Topography and function. Hum Brain Mapp 30:776–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingert JR, Sinclair RJ, Dixit S, Damiano DL, Burton H (2010): Somatosensory‐evoked cortical activity in spastic diplegic cerebral palsy. Hum Brain Mapp 31:1772–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia M, Wang J, He Y (2013): BrainNet viewer: A network visualization tool for human brain connectomics. PLoS One 8:e68910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokochi K (1991): MRI in athetotic cerebral palsied children. Acta Paediatr Scand 80:818–823. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Hayakawa K, Oishi K, Mori S, Kanda T, Yamori Y, Yoshida N, Hirota H, Iwami M, Okano S, Matsushita H (2011): Athetotic and spastic cerebral palsy: Anatomic characterization based on diffusion‐tensor imaging. Radiology 260:511–520. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Faria AV, Oishi K, Kanda T, Yamori Y, Yoshida N, Hirota H, Iwami M, Okano S, Hsu J, Li X, Jiang H, Li Y, Hayakawa K, Mori S (2013): Anatomical characterization of athetotic and spastic cerebral palsy using an atlas‐based analysis. J Magn Reson Imaging 38:288–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalesky A, Fornito A, Bullmore ET (2010): Network‐based statistic: Identifying differences in brain networks. Neuroimage 53:1197–1207. [DOI] [PubMed] [Google Scholar]
- Zalesky A, Fornito A, Cocchi L, Gollo LL, van den Heuvel MP, Breakspear M (2016): Connectome sensitivity or specificity: Which is more important? Neuroimage 142:407–420. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


