Abstract
Very preterm (VPT) birth (<32 weeks’ gestational age) has been implicated in social‐cognitive deficits including Theory of Mind (ToM); the ability to attribute mental states to others and understand that those beliefs can differ from one's own or reality. The neural bases for ToM deficits in VPT born children have not been examined. We used magnetoencephalography (MEG) for its excellent spatial and temporal resolution to determine the neural underpinnings of ToM in 24 VPT and 24 full‐term born (FT) children (7–13 years). VPT children performed more poorly on neuropsychological measures of ToM but not inhibition. In the MEG task, both FT children and VPT children recruited regions involved in false belief processing such as the rIFG (VPT: 275–350 ms, FT: 250–375 ms) and left inferior temporal gyrus (VPT: 375–450 ms, FT: 325–375 ms) and right fusiform gyrus (VPT: 150–200 ms, FT: 175–250 ms). The rIPL (included in the temporal‐parietal junction) was recruited in FT children (475–575 ms) and the lTPJ in VPT children (500–575 ms). However, activations in all regions were reduced in the VPT compared to the FT group. We suggest that with increasing social‐cognitive demands such as varying the type of scenarios in the standardized measure of ToM, reduced activations in the rIFG and TPJ in the VPT group may reflect the decreased performance. With access to both spatial and temporal information, we discuss the role of domain general and specific regions of the ToM network in both groups. Hum Brain Mapp 38:5577–5589, 2017. © 2017 Wiley Periodicals, Inc.
Keywords: magnetoencephalography, Theory of Mind, premature birth, cognition, neuroimaging
INTRODUCTION
In 2010, one in ten babies was born prematurely (<37/40 weeks' gestational age, GA) and 1/10 of these were very preterm (VPT) (<32/40 weeks' GA) [Blencowe et al., 2012]. The associated medical and neurological complications in a proportion of these children have garnered worldwide attention from health organizations [Howson et al., 2013]. In a recent study of 8,334 children born preterm [Manuck et al., 2016], 45.5% suffered from a major or minor morbidity, yet over 50% did not have any severe medical health concerns. However, even including those children born VPT without serious health problems, there is evidence for social, cognitive, and academic difficulties [Aarnoudse‐Moens et al., 2015; Anderson and Doyle, 2003; Hille et al., 2008]. Additionally, there is a negative relation between GA and associated difficulties, such that VPT children present with more serious [Bhutta et al., 2002] and persistent problems throughout development [de Kieviet et al., 2012; Moster et al., 2008; Nosarti et al., 2002; Spittle et al., 2009; Wehrle et al., 2016] compared to full‐term (FT) born children. Ritchie et al. [2015] showed that VPT children had poorer social competence than FT children, leading to poor scholastic, occupational and behavioral outcomes. To date, no study has directly measured the neural bases of these difficulties in VPT children.
Social‐cognitive skills allow processing information about oneself and others during social interactions and are required for social functioning according to Cavell's [1990] tri‐partite model. Theory of Mind (ToM), a key social‐cognitive ability to create mental representations of another person's mental state is often measured using a false belief task; a context in which a person must realize that another individual holds a belief about the world that is different from one's own and reality [Ruffman, 2014]. Although some argue that ToM can be observed around the first year of life [Onishi and Baillargeon, 2005], explicit ToM understanding likely develops between 4 and 5 years of age [Baron‐Cohen et al., 1985; Wellman et al., 2001]. Increased mastery of ToM ability continues through adolescence and adulthood [Blakemore, 2012; Lagattuta et al., 2016]. Williamson and Jakobson [2014] found that VPT children (ages 8–11 years) used fewer words denoting thoughts, beliefs, and perspectives (such as deceiving or cooperating) when describing socially relevant interactions between moving shapes. Where FT children attributed and understood certain movements to resemble social interactions, VPT children did not. Therefore, it is possible that differences in processing ToM stimuli characterize impairments in social functioning in children born VPT.
Numerous studies report that a ToM network is activated during the process of making theories about our thinking and about the thoughts/beliefs/perspectives of others [Carrington and Bailey, 2009]. In adults, this network involves the bilateral temporal‐parietal junction (TPJ; which includes the supramarginal and angular gyri and in some studies, the inferior parietal lobule [IPL]), thought to be involved in inferring the mental states of others, the medial prefrontal cortex (mPFC) involved in integrating and managing several representations, the superior temporal sulcus (STS) involved generally in recognizing social, goal‐directed motion [Schultz et al., 2004], and the temporal poles (TPs) involved in retrieving socially relevant information [Olson et al., 2013].
In children, similar activations in the ToM network have been reported. Kobayashi et al. [2007] found that adults (18–40 years) and children (8–12 years) elicited higher activity in the TPJ bilaterally, the right IPL and the right middle orbital gyrus on false belief tasks compared to the non‐ToM condition. Gweon et al. [2012] found activations in children and adults in the TPJ bilaterally, the precuneus and dorsal mPFC. In contrast, Saxe et al. [2009] found that younger children (6–8 years) recruited the rTPJ equally in ToM and non‐ToM conditions and older children recruited the rTPJ only in the ToM condition, resembling activations found in adults. These results suggested that regions involved in ToM in adults are recruited in children, but that an increased specialization of these ToM structures occurs with age.
Event‐related potential studies provide information about the temporal sequence of activation in the ToM network. Sabbagh and Taylor [2000] found left frontal activations in a false belief condition from 300 to 400 ms. Meinhardt et al. [2011] compared false and true belief and found an increased frontal late slow wave (adults: 600–900 ms; children: 750–1,450 ms) and a late positive complex similar to the P3 component associated with activity in the TPJ (adults and children: 300–600 ms). Other studies report similar findings in false belief tasks [Liu et al., 2004, 2009], but source localization from EEG recordings is less precise as the signal is distorted by conduction through brain tissue, skull and scalp.
In contrast, magnetoencephalography (MEG) measures the magnetic signal produced by neuronal currents, which is not distorted during volume conduction, and can provide both millisecond temporal information accompanied with millimetre spatial resolution, comparable to fMRI [Hari et al., 2000; Hari and Salmelin, 2012]. Vistoli et al. [2011] measured intention attribution using MEG in adults and found that from 200 to 600 ms, the rIPL, rSTS, and rTPJ were recruited. Our group [Mossad et al., 2016] used a false belief task to measure ToM and found that, adults recruited the rTPJ (150–225 ms), the right precuneus (275–375 ms), the right inferior frontal gyrus (IFG, 200–300 ms), and the superior frontal gyrus (SFG, 300–400 ms).
To date, the neural underpinnings of ToM have not been investigated in VPT populations. In the current study, we used MEG to determine the spatial‐temporal trajectory of ToM in VPT and FT children, and whether there were differences in neural, behavioral, and neuropsychological measures of ToM between groups.
Based on the findings from Kobayashi et al. [2007] and Moriguchi et al. [2007] regarding a similar pattern of activations between children and adults on ToM tasks, we predicted activations in the ToM network in FT born children. We expected that the temporal trajectory would follow previous studies: early activations in sensory regions corresponding to differences in contexts in the visual scenes between false and true belief conditions, followed by activations that correspond to the TPJ as early as 150 ms [Meinhardt et al., 2011; Mossad et al., 2016; Vistoli et al., 2011], and finally, frontal regions including the rIFG and mPFC would be involved in integrating discrepant mental states [Meinhardt et al., 2011; Mossad et al., 2016]. This temporal trajectory converges with a mechanism of false belief processing proposed by Samson et al. [2007] and Le Bouc et al. [2012] that involves inferring another's perspectives as well as inhibiting one's own perspectives.
Compared to their FT peers, we expected VPT children to show reduced and/or delayed processing during the false belief condition. Neuropsychological measures of ToM and cognitive abilities may also elucidate any difficulties observed in the VPT group [Anderson et al., 2011; Jones et al., 2013; Nadeau et al., 2004].
METHOD
Participants
Children were recruited through records of preterm birth of children who were transferred to the neonatal intensive care unit at the Hospital for Sick Children (SickKids), Toronto, through advertisements in the hospital and on social media websites for parents of children born preterm. Of the children contacted, the participation rate was 33%. Thirty‐five VPT children (mean age = 9.9 ± 1.7 years) born at <32 weeks GA were recruited. One child did not complete the task. Two participants completed the MEG task but performed at ≤60% on all task conditions and eight participants were also excluded because of low accuracy on the false belief condition only. Thus, data from 12 females and 12 males born VPT (mean GA = 28.4 ± 2.06 weeks; mean birth weight = 1,162.8 ± 326.4g), (mean age = 10.25 ± 1.7 years, 20 right‐handed) were analyzed, Table 1.
Table 1.
Demographics of VPT and FT born groups
| VPT | FT | ||||
|---|---|---|---|---|---|
| n | 24 | 24 | |||
| Age (years) | Mean ± SD | 10.3 ± 1.7 | 10.3 ± 1.8 | ||
| Range | 7–12 | 7–13 | |||
| Sex | Male: Female | 12:12 | 12:12 | χ2(1) = 0 | P = 1 |
| Handedness | Right: Left | 20:4 | 23:1 | χ2(1) = 0 | P = 0.15 |
| Birthweight (g) | Mean ± SD | 1162.8 ± 326.4 | — | ||
| Gestational age (wk) | Mean ± SD | 28.4 ± 2.1 | ≥ 40 | ||
| n | 10 | 11 | |||
| Mother's education | Post‐secondary | Post‐secondary | |||
| Father's education | Post‐secondary | Post‐secondary |
Fifty‐three FT children were recruited through advertisements around the hospital and through flyers posted in local schools. Representativeness in this group is therefore influenced by self‐selection. Five children did not complete the task. One child was excluded because of low accuracy on all task conditions and seven were excluded because of low accuracy on only the false belief condition (≤60%). Of the remaining children, 24 (mean age = 10.25 ± 1.8 years, 23 right‐handed) were matched for age (t = −0.002, P = 0.998) and sex (12 females and 12 males) to the VPT born group.
Exclusion criteria included reports from parents on uncorrected hearing or visual impairments, psychiatric or neurological disorder, or standard contraindications for neuroimaging studies. Our protocol was to exclude participants with standard scores ≤70 on a vocabulary test; none needed to be excluded based this measure. Additionally, we did not include VPT children with severe medical conditions at birth such as grade 3 and 4 intraventricular haemorrhage. The protocol was approved by the SickKids research ethics board. Written informed consent was obtained from parents and verbal assent from children. Children completed the study in one visit.
Procedure
Assessments
Neuropsychological measures were used to assess working memory and inhibition as these abilities are required for success on many ToM tasks [Carlson et al., 2013; Schaafsma et al., 2015] and are likely involved in our false belief task. We used the forward and backward digit recall subtests of the Working Memory Test Battery for Children (WMTB) [Gathercole and Pickering, 2001] and the Inhibition subtest of the second edition of the Developmental NEuroPSYchological Assessment (NEPSY‐II) [Korkman et al., 2007]. Children also completed the ToM subtest of the NEPSY‐II and the vocabulary and matrix reasoning subtests of the Wechsler Abbreviated Scale of Intelligence (WASI) [Wechsler, 2002]. Parents completed the Behavior Rating Inventory of Executive Function (BRIEF) [Gioia et al., 2003] to assess executive functioning.
Theory of Mind task
The Jack and Jill false belief task used for this study was adapted from Dennis et al. [2012] for use in MEG. Images were projected on the centre of the screen with a black background at a viewing distance of 80 cm and subtended 6.8° of visual arc.
The task consisted of two images of cartoon drawings presented in sequence, followed by an inter‐stimulus interval, in which feedback was provided on whether the child responded correctly. The first image always included Jill watching Jack hold the ball over a blue hat (50% of trials) or a red hat (50% of trials). In the second image, Jack switched the location of the ball (66% of trials) or dropped it in the same hat (33%). For some trials (50%) Jill was present to witness where the ball was dropped or she was absent. This resulted in four different conditions: (1) An Unwitnessed Switch (UWS) condition where Jill is absent and does not see Jack switch the ball, (2) A Witnessed Switch (WS) condition where Jill witnessed Jack switch the location of the ball, (3) A Witnessed Non‐Switch (WNS) condition where Jill witnessed Jack drop the ball in the same location, and (4) An Unwitnessed Non‐Switch (UWNS) condition where Jill is absent and does not see Jack drop the ball in the same hat, see Figure 1.
Figure 1.
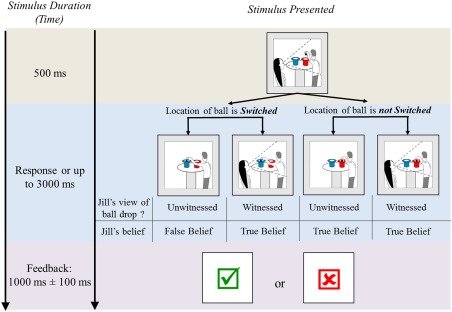
The Jack and Jill task. Each trial consisted of two frames immediately followed by feedback. The first image is common between all conditions showing Jack holding a ball over one of two hats and Jill watching him. The second image presented one of four conditions that differed on whether the location of the ball was switched and whether Jill witnessed the ball drop. Children responded to “Where does Jill think the ball is?” Inter‐trial intervals presented feedback with either a check mark “√” for a correct answer or an “×” for an incorrect response. The ToM contrast measured false belief understanding (Unwitnessed Switched > Witnessed Switched) and the control condition measured differences related to Jill's absence (Unwitnessed Non‐switched >Witnessed Non‐switched). Results are reported after spatially excluding the control contrast from the ToM contrast using a spatial mask.
False belief is measured in the UWS condition as Jill is unaware that the ball has been switched and has a false belief about the location of the ball. The other three conditions of the task measure true belief as they present scenarios where Jill is either present to see where Jack drops the ball (witnessed) or where Jack does not switch the ball (non‐switch conditions). We used the WS true belief condition to compare to the UWS false belief condition as it shares the switch component and matches on the number of trials. Hereinafter, we will use false belief and true belief to represent UWS and WS conditions respectively.
Prior to acquisition in the MEG, two practice sessions (40 trials) were conducted to ensure an understanding of the task. Children responded to the test question “Where does Jill think the ball is?” The MEG task was run until the child responded on at least 300 trials (100 UWS, 100 WS, 50 UWNS, 50 WNS). Trials were randomized within and between subjects. The software “Presentation” was used to project stimuli and record responses (Neurobehavioral Systems, Berkeley, CA). Participants responded using a button box, pressing left or right buttons, corresponding to where Jill thinks the ball is.
Imaging acquisition
MEG data were recorded using a 151 channel CTF system (Coquitlam, BC) in a magnetically shielded room at SickKids. Data were recorded continuously with 600 Hz sampling rate, 0–150 Hz bandpass, and third‐order spatial gradient noise cancellation. Children lay supine with their head in the MEG helmet. Three fiducial coils placed at the left and right pre‐auricular points and the nasion were used to determine head position in the MEG helmet and to co‐register each participant's MEG data with their anatomical MRI. Data were acquired with continuous head localization to track head movements.
A 3T Siemens Tim Trio MR scanner with a 12‐channel head coil was used to obtain T1‐weighted MRIs with a 3D MRPAGE sequence acquired sagittally: TR/TE = 2,300/2.96 ms, a GRAPPA acceleration of 2, FA = 9°, 240 × 256 matrix, 192 slices, slice thickness of 1 mm isotropic voxels. MRIs were used as anatomical underlays for the MEG data for accurate source localization. A neuroradiologist at SickKids reviewed the images to exclude those with significant brain abnormalities.
MEG Data: Preprocessing and Source Reconstruction
Data were processed through SPM12 (Wellcome Department of Imaging Neuroscience, London, UK) run in MATLAB R2014b (The Mathworks, Natick, MA). First, data were bandpass filtered from 1 to 50 Hz. As the four conditions were presented in the second frame, data were epoched 200 ms before the onset of the second image until 600 ms post stimulus onset. The 200 ms window presented before the second image was baseline corrected. This epoch (–200 to 600 ms) was chosen to avoid motor‐related effects as response times varied from 600 to 1,700 ms across participants.
Trials were rejected if head motion exceeded 5 mm within trials and 10 mm between trials. Artefacts related to cardiac activity, eye blinks and eye movements, were removed using the independent component analysis (ICA) toolbox in FieldTrip [Oostenveld et al., 2011]. Trials that exceeded a threshold of 2,500 fT were rejected and removed. Trials within each condition were averaged for each participant. Whole brain, sensor data were examined and shown for a temporal electrode as an example, in Supporting Information Figure S1.
The position of the three fiducial markers was identified on each child's MRI and these coordinates were used to coregister the SPM12 cortical mesh template in Montreal Neurological Institute Standard Space to the sensor space [Mattout et al., 2007]. Forward computation of the gain matrix of the lead field model [Nolte, 2003] used a single shell head model. Sources of activation were reconstructed by inversion of the forward model using Empirical Bayesian Beamformer [Friston et al., 2006; Mattout et al., 2006]. Beamformer results were obtained for 19 time windows from 100 to 600 ms with 50 ms sliding overlapping time windows. Images were spatially smoothed with a 12 mm full‐width at half maximum of the Gaussian smoothing kernel.
Statistical Analyses
Assessments
Independent samples t‐tests were used to compare group differences on the assessments. Data were analyzed and corrected for multiple comparisons in SPSS [IBM Corp., 2013].
Jack and Jill task: behavioral analyses
A repeated measures ANOVA was used to compare accuracy and response times on false and true belief conditions between VPT and FT children.
Jack and Jill task: MEG analyses
Resulting images associated with each condition and time window were analyzed. To compute within group differences, a group by condition factorial was used and age and IQ were regressed out of the model to remove their effects on brain differences. Two contrasts: (1) ToM: UWS > WS (false belief > true belief) and (2) Control: UWNS > WNS (non‐switch true belief conditions) were analyzed within each group. This control contrast was created to exclude activations related to visual and attentional differences related to Jill's disappearance in the second image in the two unwitnessed conditions as well as the ball switch in the two switch conditions. In addition to visual differences between the unwitnessed and witnessed conditions, there is an added working memory load in the unwitnessed conditions where participants are required to recall where Jill last saw the ball. Removing activations related to these processes isolates activations related only to false belief. Therefore, we masked out the Control from the ToM contrast. We also measured group × condition interactions.
RESULTS
Assessments
FT children had a mean IQ score in the high average range (118.2 ± 12.29) whereas VPT children scored lower, but with a mean IQ score in the average range (109.4 ± 12.77; t = −2.43, P FWE = 0.02). VPT children performed more poorly on the ToM subtest (t = −2.49, P FWE = 0.02). However, these differences were not reflected in measures of executive functioning such as working memory (backward digit recall; t = −1.9, P = 0.08) or inhibitory control (t = −0.12, P = 0.9). VPT children were rated as having more working memory problems compared to FT children (P = 0.03) but not inhibition (P = 0.1), see Table 2.
Table 2.
Neuropsychological assessment results
| Preterm‐born children | Term‐born children | P | |
|---|---|---|---|
| n | 24 | 24 | |
| WASI (Standard Scores) | |||
| Two‐subtest IQ | 109.4 (12.8) | 118.2 (SD 12.2) | 0.019** |
| WMTB (Standard Scores) | |||
| Digit Recall | 107.0 (SD 15.8) | 109.9 (SD 20.5) | 0.60 |
| Backward Digit Recall | 89.6 (SD 16.2) | 99.3 (SD 18.3) | 0.08 |
| NEPSY | |||
| Theory of Mind (Raw) | 22.8 (SD 2.7) | 24.8 (SD 2.6) | 0.017** |
| Inhibition (Scaled Score) | 10.1 (SD 3.9) | 10.0 (SD 3.2) | 0.9 |
| BRIEF (problems) | |||
| Inhibition Problems | 48 (SD 10.2) | 44.3 (SD 6.7) | 0.1 |
| Working Memory Problems | 51.6 (SD 9.5) | 45.9 (SD 7.5) | 0.03** |
**P corrected < 0.05.
Jack and Jill Task: Behavioral Results
There were no significant condition x group interactions. Across groups, mean accuracy in the false belief condition (UWS) was significantly lower (Mean = 72%) than the three other conditions (WS, Mean = 86%, P < 0.001, UWNS, Mean = 88%, P < 0.001, and WNS, Mean = 96%, P < 0.001). Accuracy on the WNS condition was significantly higher than the WS (P < 0.001) and the UWNS (P < 0.001).
A similar pattern in performance was observed for response times. Although controls were generally faster than preterm born children in responding, the two groups were not significantly different. Across groups, mean response time was significantly higher in the false belief condition (UWS, Mean = 1.05 s, SD = 0.2 s) compared to the WS (Mean = 0.987 s, SD = 0.18 s, P < 0.001), UWNS (Mean = 0.995 s, SD = 0.256, P = 0.002) and WNS (Mean = 0.834 s, SD = 0.20 s, P < 0.001); see Figure 2.
Figure 2.
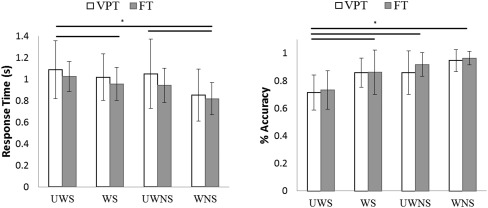
Accuracy and response times in the false belief condition and the three true belief conditions across VPT and FT born children. Overall, performance was significantly worse (lower accuracy and higher response time) on the false belief condition across both groups.
Jack and Jill Task: MEG Results
Coordinates for significant brain activations in the false belief greater than true belief are listed in Tables S1–S2 in Supporting Information.
Within‐group differences in FT and VPT children
The brain regions were identified using the AFNI atlas [Cox, 1996]. Activations within the FT and VPT groups encompassed occipital, temporal, parietal and frontal regions from 100 to 600 ms, Figure 3 and between group differences are shown in Figure 4. We found significantly different activations between the false and true belief conditions and reported activations that were significant at P FWEcorr < 0.05 to P ≤ 0.003.
Figure 3.
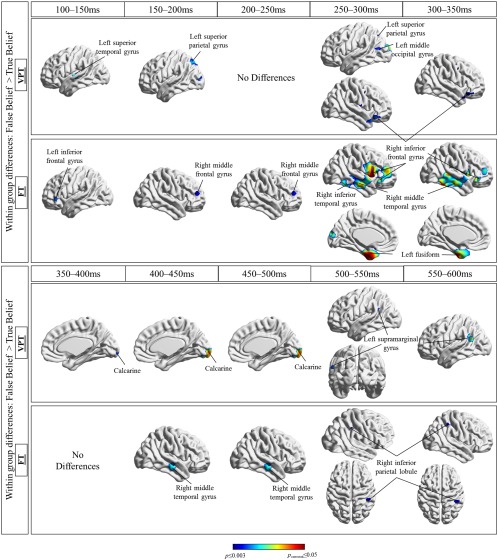
Summary of activations within FT and VPT born children in false belief > true belief, from 100 to 600 ms.
Figure 4.
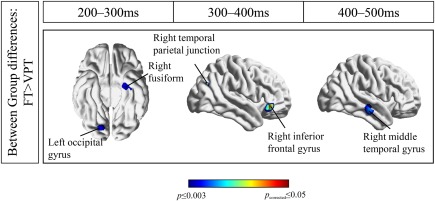
Between group differences in the false belief condition from 100 to 500 ms in FT > VPT.
Common activations
A number of occipital, temporal, and frontal areas showed significantly greater activity in the false belief > true belief conditions in both groups. These included the left and right calcarine starting at 100 ms and fusiform gyri starting at 150 ms, the right IFG at ∼250–350 ms, the inferior temporal gyri at ∼300–450 ms. Although the activations differed slightly between groups, they were most often similar in timing and location.
Unique activations
More interesting in terms of the research question, there were also brain regions more active in the false than true belief conditions in only one or the other of the two groups. Within the VPT group, these activations were in the left superior parietal lobule from 175 to 225 ms then 250 to 325 ms (P = 0.002) and the left supramarginal gyrus from 500 to 575 ms.
Within the FT group, these unique activations included frontal regions: the left inferior frontal (100–150 ms, P = 0.002), left middle frontal gyrus (175–225 ms, P = 0.003), and right middle frontal gyrus (175–325 ms, P ≤ 0.003). We also found differences in the FT group in the left fusiform gyrus (250–350 ms, P FWE=0.02), the right inferior temporal gyrus (300–375 ms, P FWE = 0.04), the right middle temporal gyrus (275–375 ms, P < 0.001), the left inferior occipital gyrus (450–500 ms, P = 0.003), and the right IPL (475–575, P = 0.003). Thus, the FT children showed more widespread activity during false belief processing, including frontal, temporal and posterior areas.
Condition × group interactions
We also analyzed between group differences (FT vs. VPT) across the two conditions (False vs. True belief). We found that the FT group showed significantly greater activity than the VPT group in the false belief condition in the right IFG from 275 to 325 ms (P ≤ 0.003), the left fusiform gyrus (225–275 ms, P < 0.001), the right fusiform gyrus (200–250 ms, P < 0.001), and the right middle temporal gyrus (400–475, P ≤ 0.002). Other regions included the right middle occipital gyrus (200–250 ms, P = 0.003), the left middle occipital gyrus (325–375 ms, P = 0.003), and medial occipital regions such as the right lingual gyrus (225–325, P ≤ 0.003). It is noteworthy that 41 voxels corresponding to the angular gyrus (rTPJ) were recruited only in the FT group in the false belief compared to the true belief condition from 375 to 425 ms, P = 0.006, but did not pass threshold for correction.
DISCUSSION
This study is the first to examine both spatial and temporal activations related to ToM in VPT children. Specifically, we investigated how VPT children reason about others' mental states using a false belief task. Our findings point to a less distributed pattern of activation in VPT children during ToM processing as well as a decreased activation in ToM regions compared to their FT peers. MEG localization results in the FT group converge with those previously reported in the literature and we offer implications regarding the mechanism of ToM.
Variability in Performance on Behavioral ToM Tests in VPT Children
We found no differences in inhibition between groups. On a measure of working memory, the VPT children performed worse and the difference was borderline significant. When executive functioning abilities were assessed on a parent questionnaire (BRIEF), parents showed that both groups had a comparable number of problems relating to inhibition but VPT children had significantly more problems in working memory. Deficits in executive functioning have been reported in VPT populations [Aarnoudse‐Moens et al., 2011] and other evidence suggests that with older children who had been born very prematurely, differences in those cognitive domains become apparent on tasks with increased executive functioning demands [Wehrle et al., 2016].
We found the expected group differences in performance on a ToM test from the NEPSY‐II. Williamson and Jakobson [2014] used a non‐verbal measure of ToM and found that children born preterm attributed intentions less accurately than their FT peers. It has been debated whether deficits in executive functioning contribute to deficits in ToM or simply co‐occur. Some studies report no differences in executive functioning in VPT compared to FT children [Degnan et al., 2015; Taylor et al., 2012] while others do [Anderson and Doyle, 2003; Duerden et al., 2013]. Our findings support the viewpoint that intact executive functioning skills, such as inhibition are not sufficient for an intact ToM ability [Mahy et al., 2014].
The results of the Jack and Jill task reflect the increased cognitive load required for success on false belief conditions, as suggested by Wellman et al. [2011]. Across groups, accuracy was lower and response times larger on false belief than true belief conditions. The lack of difference in performance between groups contrasts with the difference in performance on the ToM task on the NEPSY‐II that involved a variety of contexts requiring ToM processing. It is possible that with practice on the MEG task, VPT children were able to perform within a range that is similar to their FT peers. However, practicing false belief scenarios is not often possible during daily social interactions, therefore performance on the NEPSY‐II ToM subtest may be more predictive of real situations. Although behavioral performance was not significantly different between groups on the false belief task, the pattern of neural processing was different between groups.
ToM Regions Are Differentially Activated in VPT Children
Certain regions in the ToM network are known to be domain general, that is, brain regions that are recruited during ToM as well as with other cognitive processes. Others have argued that regions such as the rTPJ are selective for ToM processing [Saxe and Wexler, 2005]; we discuss these issues in detail below, focussing on the various regions implicated in ToM.
Right inferior frontal gyrus
Previous studies have shown that intact executive functioning skills are required for developing false belief understanding, and of those skills, inhibition was the most correlated with ToM abilities [Rothmayr et al., 2011]. Van der Meer et al. [2011] showed that the rIFG was domain‐general by testing participants using videos of false belief scenarios and a response inhibition task. Although some regions were specific to ToM processing, the bilateral IFG were recruited in both tasks. In the present context, the rIFG was involved as participants inhibited their own beliefs to understand Jill's. The timing of activation of the rIFG in both groups (VPT: 275–350 ms, FT: 250–375 ms) overlapped but occurred slightly later than timing previously reported in an MEG study using the current false belief paradigm in adults (200–300 ms) [Mossad et al., 2016]. The timing of the rIFG also overlapped with studies focusing specifically on inhibition (230–260 ms) [Vara et al., 2014; Vidal et al., 2012] that included adolescents and adults. Our findings point to a reliable rIFG activation during false belief reasoning that occurs at 250 ms reflecting the inhibition of one's own mental states (beliefs). Inhibition is a skill that was not impaired in our sample of VPT children and correspondingly, we did not find differences in neural processing.
Temporal‐parietal junction
The junction of the IPL, the supramarginal and the right angular gyrus forms this region. Comparing the two groups, we found that the rTPJ (right angular gyrus) was recruited in the contrast FT > VPT from 325 to 375 ms, at P = 0.006. Within the FT group, the rIPL was recruited (from 475 to 575 ms) and within the VPT group, the left supramarginal gyrus from 500 to 575 ms. TPJ recruitment has been extensively reported in false belief conditions [van Veluw and Chance, 2014]. Kobayashi et al. [2007] reported bilateral TPJ in children for inferring the beliefs of others, as did Gweon et al. [2012] who found TPJ activations in children on a ToM compared to a non‐ToM condition. These fMRI studies suggest that typical processing of the mental states of others uses processes that are similar in children and adults. In a prior study using the same Jack and Jill paradigm in adults, we found that the right angular gyrus was recruited from 150 to 225 ms. Therefore, in this study the rTPJ was recruited later in children than previously reported.
The lateralization of the TPJ activity is variable across studies, with some reporting right (false vs. true belief) [Sommer et al., 2007], left (ToM vs. non‐ToM) [Kobayashi et al., 2007] as well as bilateral TPJ activations [false belief vs. false photograph, Dodell‐Feder et al., 2011 and Aichhorn et al., 2009; false belief vs. physical stories, Gobbini et al., 2007; ToM vs. non‐ToM, Saxe and Kanwisher, 2003]. We found that VPT children recruited the left, and FT children recruited the right TPJ. Saxe et al., [2009] showed that with age, children selectively recruit the rTPJ in a ToM compared to a non‐ToM condition, whereas younger children recruited both the rTPJ and lTPJ. It is possible that VPT children would recruit the rTPJ if tested later in development. The right hemisphere dominance in activations in the FT children is consistent with the right hemisphere dominance we found with this task in adults [Mossad et al., 2016].
The exact role of the rTPJ in false belief and ToM is still debated. Mitchell [2008] argues that the rTPJ is not specific to ToM but also involved in reorienting attention to visual stimuli. Rothmayr et al. [2011] propose a possible functional distinction between the dorsal and ventral TPJ, where the ventral region of the left TPJ was exclusively recruited for false belief reasoning whereas the dorsal region was recruited for both false belief and an inhibition task. Others have suggested that the TPJ is involved in updating one's “mental model” [Meinhardt et al., 2011]—that a false belief condition would cause a larger change in the mental model (discrepant mental states) compared to a true belief context and therefore the TPJ would be more highly involved.
Middle frontal gyrus
We found greater rMFG activity in the false belief than true belief condition in the FT group from 175 to 325 ms. The rMFG is a domain general region that is activated across different tasks. Rothmayr et al. [2011] showed that the rMFG was involved both in an inhibition and a false belief task and suggested that it has a role in working memory. Other fMRI studies show that the MFG is related to spatial working memory processing, storing information [Leung et al., 2002] and in orienting attention [Japee et al., 2015]. In our task, it is difficult to dissociate whether the rMFG was involved in working memory processes related to trying to recall where Jill last saw the ball or re‐orienting to Jill's disappearance from the scene. We included a control condition in our analysis to exclude activity related to working memory and attention but this region survived this manipulation. As the rMFG is functionally connected in networks like the ventral attention, dorsal attention, and ToM networks, a connectivity analysis of this node may provide more information about its function during this task.
Inferior temporal and fusiform gyri
The inferior temporal and fusiform gyri have been reported in false belief tasks [Schurz et al., 2014], while Sabbagh et al., [2009] reported that resting state activations in the inferior temporal and fusiform gyri were positively related to false belief performance. We found that both groups recruited the fusiform gyri and then the inferior temporal regions. Evidence suggests that the anterior aspects of the temporal lobe are involved in representing and retrieving information about a person's social traits and concepts [Olson et al., 2013]. Gainotti [2015] proposed that the right anterior temporal lobe is involved in representing non‐verbal, social information while the left is involved in lexical representations. Subsequently, our results suggest that both groups processed the task as involving social information, but the FT also used language‐related representation.
Occipital activations
We found that a number of occipital regions were recruited more in false belief compared to the true belief condition in both groups from 100 to 400 ms. We found similar occipital recruitment when we compared the two groups, but the regions were only recruited in the contrast FT > VPT. Sabbagh et al. [2009] found similar activations in the middle occipital gyrus and the cuneus and that performance on a ToM task was correlated with the cuneus activation. These differences in the occipital lobe were also reported in adults doing the same task [Mossad et al., 2016]. Others have also argued that the cuneus is involved in mental imagery, which is required in the false belief condition to reimagine Jill and her perspective of the location of the ball [Kosslyn et al., 1999; Sabbagh et al., 2009].
Our findings provide early evidence for atypical ToM processing in school age VPT children. However, it is important to consider some methodological limitations in this study. As it was important to exclude participants with severe brain injury to establish a benchmark for how VPT children without serious medical conditions process ToM information, our sample was only representative of those preterm children [Manuck et al., 2016]. A consequence was the observed average IQ and unimpaired executive functioning abilities, in the VPT children, which is in contrast to the literature showing impairments on average in this population. In the future, preterm children with severe medical conditions at birth should be included and compared to those without. Another possible methodological constraint is low power. Although future studies should focus on increasing the sample size, when neuropsychological results were re‐analyzed with a larger sample (with 10 extra participants in each group who were excluded due to the neuroimaging portion), we found that results were stable. Only the group differences on the backward digit recall subtest went from not significant (P = 0.08) to significantly different (P = 0.02).
SUMMARY AND CONCLUSION
VPT birth (<32 weeks gestation) introduces numerous stressors at birth and throughout development that increase the risks for cognitive and social difficulties [Aarnoudse‐Moens et al., 2015; Jones et al., 2013; Kuzniewicz et al., 2014] compared to FT born individuals. Although the neural bases for the cognitive and psychiatric problems are beginning to be studied, very little is known about how neural processing atypicalities can lead to the socio‐cognitive difficulties. ToM ability develops from preschool into adulthood and improvements are thought to depend on continued maturation and specialization of the ToM network [Apperly et al., 2004; Gweon et al., 2012; Sabbagh et al., 2009] as well as mastery of other abilities such as executive functioning, language and emotion processing [Schaafsma et al., 2015].
Deconstructing ToM into subcomponent processes helps define the necessary skills that must develop before ToM ability can fully emerge. In general, the pattern of activation in the preterm group can be described as (1) fewer differences between false and true belief conditions and (2) less distributed pattern of activation, involving fewer and mainly left lateralized activations. Our findings occurred within the time windows from previously reported MEG and EEG ToM studies (100–600 ms, compared to 200–600 ms in Vistoli et al. [2011] and 100–400 ms in Mossad et al. [2016]. The pattern of activation in both groups began early on, in occipital regions, then the IFG was recruited, and finally, the TPJ was recruited in the last time window, 500–600 ms. Two differences regarding the TPJ emerge; first that the TPJ is recruited later in FT children compared to adults [Mossad et al., 2016] reflecting an increase in the cognitive resources required to update their mental states of Jill's false belief. Second, FT children recruit the rTPJ more than VPT in false belief when the groups are compared. Future studies could examine the TPJ across various cognitive tasks to differentiate the neurocognitive function of anatomically distinct regions of the TPJ.
Researchers have suggested that ToM processing requires (1) a mental representation of reality, (2) mental representation of oneself and others' beliefs, and (3) inhibition of own beliefs [Le Bouc et al., 2012]. Our findings suggest that, after inhibiting one's own beliefs, children recruit the TPJ about 100 ms later to enable them to succeed in inferring others' beliefs. Therefore, we suggest that in children, an additional recruitment of the TPJ is involved in false belief understanding after inhibition. Future studies should examine whether VPT children retain this pattern of ToM processing throughout development and whether ToM training [e.g., Lecce et al., 2014] can evoke recruitment of the rTPJ in VPT children in false belief, as observed in FT children and adults, as well as improve performance on standardized measures of ToM.
Supporting information
Supporting Information Tables and Figure
ACKNOWLEDGMENT
We thank our participants and their families, Michelle AuCoin‐Power, Tamara Powell, and Marc Lalancette, for their assistance with data collection.
REFERENCES
- Aarnoudse‐Moens CSH, Duivenvoorden HJ, Weisglas‐Kuperus N, Van Goudoever JB, Oosterlaan J (2011): The profile of executive function in very preterm children at 4 to 12 years. Dev Med Child Neurol 54:247–253. [DOI] [PubMed] [Google Scholar]
- Aarnoudse‐Moens CSH, van Goudoever JB, Oosterlaan J (2015): Meta‐analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics 124: 717–728. [DOI] [PubMed] [Google Scholar]
- Aichhorn M, Perner J, Weiss B, Kronbichler M, Staffen W, Ladurner G (2009): Temporo‐parietal junction activity in theory‐ of‐mind tasks: Falseness, beliefs, or attention. J Cogn Neurosci 21:1179–1192. [DOI] [PubMed] [Google Scholar]
- Anderson P, Doyle LW (2003): Neurobehavioral outcomes of school‐age children born extremely low birth weight or very preterm in the 1990s. JAMA 289:3264–3272. [DOI] [PubMed] [Google Scholar]
- Anderson PJ, De Luca CR, Hutchinson E, Spencer‐Smith MM, Roberts G, Doyle LW (2011): Attention problems in a representative sample of extremely preterm/extremely low birth weight children. Dev Neuropsychol 36:57–73. [DOI] [PubMed] [Google Scholar]
- Apperly IA, Samson D, Chiavarino C, Humphreys GW (2004): Frontal and temporo‐parietal lobe contributions to theory of mind: Neuropsychological evidence from a false‐belief task with reduced language and executive demands. J Cogn Neurosci 16:1773–1784. [DOI] [PubMed] [Google Scholar]
- Baron‐Cohen S, Leslie AM, Frith U (1985): Does the autistic child have a “theory of mind”? Cognition 21:37–46. [DOI] [PubMed] [Google Scholar]
- Bhutta AT, Cleves MA, Casey PH, Cradock MM, Anans KJS (2002): Cognitive and behavioural outcome of school‐aged children who were born preterm. A meta‐analysis. JAMA 288:728–737. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ (2012): Development of the social brain in adolescence. J R Soc Med 105:111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blencowe H, Cousens S, Oestergaard M, Chou D, Moller AB, Narwal R, Adler A, Garcia CV, Rohde S, Say L, Lawn JE (2012): National, regional and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: A systematic analysis and implications. Lancet 379:2162–2172. [DOI] [PubMed] [Google Scholar]
- Carlson SM, Claxton LJ, Moses LJ (2013): The relation between executive function and Theory of Mind is more than skin deep. J Cogn Dev 16: 186–197. [Google Scholar]
- Carrington SJ, Bailey AJ (2009): Are there theory of mind regions in the brain? A review of the neuroimaging literature. Hum Brain Mapp 30:2313–2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavell TA (1990): Social adjustment, social performance, and social skills: A tri‐component model of social competence. J Clin Child Psychol 19:111–122. [Google Scholar]
- Cox RW (1996): AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29:162–173. [DOI] [PubMed] [Google Scholar]
- De Kieviet JF, Zoetebier L, Van Elburg RM, Vermeulen RJ, Oosterlaan J (2012): Brain development of very preterm and very low birthweight children in childhood and adolescence: A meta‐analysis. Dev Med Child Neurol 54:313–323. [DOI] [PubMed] [Google Scholar]
- Degnan AJ, Wisnowski JL, Choi S, Ceshin R, Bhushan C, Leahy RM, Corby P, Schmithorst VJ, Panigrahy A (2015): Altered structural and functional connectivity in late preterm preadolescence: An anatomic seed‐based study of resting state networks related to the posteromedial and lateral parietal cortex. PLoS One 10:e0130686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M, Simic N, Gerry Taylor H, Bigler ED, Rubin K, Vannatta K, Gerhardt CA, Stancin T, Roncadin C, Yeates KO (2012): Theory of mind in children with traumatic brain injury. JINS 18:908–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodell‐Feder D, Koster‐Hale J, Bedny M, Saxe R (2011): fMRI item analysis in a theory of mind task. NeuroImage 55:705–712. [DOI] [PubMed] [Google Scholar]
- Duerden EG, Card D, Lax I, Donner EJ, Taylor MJ (2013): Alterations in frontostriatal pathways in children born very preterm. Dev Med Child Neurol 55:952–958. [DOI] [PubMed] [Google Scholar]
- Friston K, Henson R, Phillips C, Mattout J (2006): Bayesian estimation of evoked and induced responses. Hum Brain Mapp 27:722–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gathercole S, Pickering S (2001): Working Memory Test Battery for Children. London, GB: Pearson Assessment. [Google Scholar]
- Gainotti G (2015): Is the difference between right and left ATLs due to the distinction between general and social cognition or between verbal and non‐verbal representations? Neurosci Biobehav Rev 51:296–312. [DOI] [PubMed] [Google Scholar]
- Gioia GA, Espy KA, Isquith PK (2003): BRIEF‐P: Behavior Rating Inventory of Executive Function–Preschool Version. Lutz, FL: Psychological Assessment Resources.
- Gobbini MI, Koralek AC, Bryan RE, Montgomery KJ, Haxby JV (2007): Two takes on the social brain: A comparison of theory of mind tasks. J Cogn Neurosci 19:1803–1814. [DOI] [PubMed] [Google Scholar]
- Gweon H, Dodell‐Feder D, Bedny M, Saxe R (2012): Theory of Mind performance in children correlates with functional specialization of a brain region for thinking about thoughts. Child Dev 83:1853–1868. [DOI] [PubMed] [Google Scholar]
- Hari R, Salmelin R (2012): Magnetoencephalography: From SQUIDs to neuroscience. Neuroimage 61:386–396. [DOI] [PubMed] [Google Scholar]
- Hari R, Levänen S, Raij T (2000): Timing of human cortical functions during cognition: Role of MEG. Trends Cogn Sci 4:455–462. [DOI] [PubMed] [Google Scholar]
- Hille ETM, Dorrepaal C, Perenbcom R, Gravenhorst JB, Brand R, Verloove‐Vanhorick S (2008): Social lifestyle, risk‐taking behaviour and psychopathology in young adults born very preterm or with a very low birthweight. J Pediatr 152:793–800. [DOI] [PubMed] [Google Scholar]
- Howson CP, Kinney MV, McDougall L, Lawn JE (2013): Born too soon: Preterm birth matters. Reprod Health 10:(1):S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBM Corp (2013): IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp. [Google Scholar]
- Japee S, Holiday K, Satyshur MD, Mukai I, Ungerleider LG (2015): A role of right middle frontal gyrus in reorienting of attention: A case study. Front Syst Neurosci 9:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KM, Champion PR, Woodward LJ (2013): Social competence of preschool children born very preterm. Early Hum Dev 89:795–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi C, Glover GH, Temple E (2007): Children's and adults' neural bases of verbal and nonverbal ‘theory of mind’. Neuropsychologia 45:1522–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkman M, Kirk U, Kemp S (2007): NEPSY‐II: A Developmental Neuropsychological Assessment. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Kosslyn SM, Pascual‐Leone A, Felician O, Camposano S, Keenan JP, Thompson WL, Ganis G, Sukel KE, Alpert NM (1999): The role of Area 17 in visual imagery. Science 284:167–170. [DOI] [PubMed] [Google Scholar]
- Kuzniewicz MW, Wi S, Qian Y, Walsh EM, Armstrong MA, Croen LA (2014): Prevalence and neonatal factors associated with autism spectrum disorders in preterm infants. J Pediatr 164:20–25. [DOI] [PubMed] [Google Scholar]
- Lagattuta KH, Elrod NM, Kramer HJ (2016): How do thoughts, emotions, and decisions align? A new way to examine theory of mind during middle childhood and beyond. J Exp Child Psychol 149:116–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bouc R, Lenfant P, Delbeuck X, Ravasi L, Lebert F, Semah F, Pasquier F (2012): My belief or yours? Differential theory of mind deficits in frontotemporal dementia and Alzheimer's disease. Brain 135:3026–3038. [DOI] [PubMed] [Google Scholar]
- Lecce S, Bianco F, Devine RT, Hughes C, Banerjee R (2014): Promoting theory of mind during middle childhood: A training program. J Exp Child Psychol 126:52–67. [DOI] [PubMed] [Google Scholar]
- Leung HC, Gore JC, Goldman‐Rakic PS (2002): Sustained mnemonic response in the human middle frontal gyrus during on‐line storage of spatial memoranda. J Cogn Neurosci 14:659–671. [DOI] [PubMed] [Google Scholar]
- Liu D, Sabbagh MA, Gehring WJ, Wellman HM (2004): Decoupling beliefs from reality in the brain: An ERP study of theory of mind. Neuroreport 15:991–995. [DOI] [PubMed] [Google Scholar]
- Liu D, Sabbagh MA, Gehring WJ, Wellman HM (2009): Neural correlates of children's theory of mind development. Child Dev 80:318–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahy CEV, Moses LJ, Pfeifer JH (2014): How and where: Theory‐of‐mind in the brain. Dev Cogn Neurosci 9:68–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuck TA, Rice MM, Bailit JL, Grobman WA, Reddy UM, Wapner RJ, Thorp JM, Caritis SN, Prasad M, Tita ATN, Saade GR, Sorokin Y, Rouse DJ, Blackwell SC, Tolosa JE, Varner M, Hill K, Sowles A, Postma J, Alexander S, Andersen G, Scott V, Morby V, Jolley K, Miller J, Berg B, Talucci M, Zylfijaj M, Reid Z, Leed R, Benson J, Forester S, Kitto C, Davis S, Falk M, Perez C, Dorman K, Mitchell J, Kaluta E, Clark K, Spicer K, Timlin S, Wilson K, Leveno K, Moseley L, Santillan M, Price J, Buentipo K, Bludau V, Thomas T, Fay L, Melton C, Kingsbery J, Benezue R, Simhan H, Bickus M, Fischer D, Kamon T, Deangelis D, Mercer B, Milluzzi C, Dalton W, Dotson T, McDonald P, Brezine C, McGrail A, Latimer C, Guzzo L, Johnson F, Gerwig L, Fyffe S, Loux D, Frantz S, Cline D, Wylie S, Iams J, Wallace M, Northen A, Grant J, Colquitt C, Rouse D, Andrews W, Mallett G, Ramos‐Brinson M, Roy A, Stein L, Campbell P, Collins C, Jackson N, Dinsmoor M, Senka J Paychek K, Peaceman A, Moss J, Salazar A, Acosta A, Hankins G, Hauff N, Palmer L, Lockhart P, Driscoll D, Wynn L, Sudz C, Dengate D, Girard C, Field S, Breault P, Smith F, Annunziata N, Allard D, Silva J, Gamage M, Hunt J, Tillinghast J, Corcoran N, Jimenez M, Ortiz F, Givens P, Rech B, Moran C, Hutchinson M, Spears Z, Carreno C, Heaps B, Zamora G, Seguin J, Rincon M, Snyder J, Farrar C, Lairson E, Bonino C, Smith W, Beach K, Van Dyke S, Butcher S, Thom E, Zhao Y, McGee P, Momirova V, Palugod R, Reamer B, Larsen M, Williams T, Spangler T, Lozitska A, Spong C, Tolivaisa S, Vandorsten JP (2016): Preterm neonatal morbidity and mortality by gestational age: A contemporary cohort. Am J Obstet Gynecol 215:103e–1031–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattout J, Phillips C, Penny WD, Rugg MD, Friston KJ (2006): MEG source localization under multiple constraints: An extended Bayesian framework. NeuroImage 30:753–767. [DOI] [PubMed] [Google Scholar]
- Mattout J, Henson RN, Friston KJ (2007): Canonical source reconstruction for MEG. Comput Intell Neurosci 2007:67613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhardt J, Sodian B, Thoermer C, Do¨hnel K, Sommer M (2011): True‐and false‐belief reasoning in children and adults: An event‐related potential study of theory of mind. Developmental 1:67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JP (2008): Activity in right temporo‐parietal junction is not selective for theory‐of‐mind. Cereb Cortex 18:262–271. [DOI] [PubMed] [Google Scholar]
- Moriguchi Y, Ohnishi T, Mori T, Matsuda H, Komaki G (2007): Changes of brain activity in the neural substrates for theory of mind during childhood and adolescence. Psychiatry Clin Neurosci 61:355–363. [DOI] [PubMed] [Google Scholar]
- Mossad SI, AuCoin‐Power M, Urbain C, Smith ML, Pang EW, Taylor MJ (2016): Thinking about the thoughts of others; temporal and spatial neural activation during false belief reasoning. NeuroImage 134:320–327. [DOI] [PubMed] [Google Scholar]
- Moster D, Lie RT, Markestad T (2008): Long‐term medical and social consequences of preterm birth. The N Engl J Med 359:262–273. [DOI] [PubMed] [Google Scholar]
- Nadeau L, Tessier R, Lefebvre F, Robaey P (2004): Victimization: A newly recognized outcome of prematurity. Dev Med Child Neurol 46:508–513. [DOI] [PubMed] [Google Scholar]
- Nolte G (2003): The magnetic lead field theorem in the quasi‐static approximation and its use for magnetoencephalography forward calculation in realistic volume conductors. Phys Med Biol 48:3637–3652. [DOI] [PubMed] [Google Scholar]
- Nosarti C, Al‐Asady MHS, Frangou S, Stewart AL, Rifikin L, Murray RM (2002): Adolescents who were born very preterm have decreased brain volumes. Brain 125:1616–1623. [DOI] [PubMed] [Google Scholar]
- Olson IR, McCoy D, Klobusicky E, Ross LA (2013): Social cognition and the anterior temporal lobes: A review and theoretical framework. SCAN 8:123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishi K, Baillargeon R (2005): Do 15‐month‐old infants understand false beliefs? Science 308:255–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostenveld R, Fries P, Maris E, Schoffelen JM (2011): FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci 2011:156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie K, Bora S, Woodward L (2015): Social development of children born very preterm: A systematic review. Dev Med Child Neurol 57:899–918. [DOI] [PubMed] [Google Scholar]
- Rothmayr C, Sodian B, Hajak G, Do¨hnel K, Meinhardt J, Sommer M (2011): Common and distinct neural networks for false‐belief reasoning and inhibitory control. NeuroImage 56:1705–1713. [DOI] [PubMed] [Google Scholar]
- Ruffman T (2014): To belief or nor belief: Children's theory of mind. Dev Rev 34:265–293. [Google Scholar]
- Sabbagh MA, Taylor M (2000): Research Report: Neural correlates of theory of mind reasoning: An event‐related potential study. Psychol Sci 11:46–50. [DOI] [PubMed] [Google Scholar]
- Sabbagh MA, Bowman LC, Evraire LE, Ito JMB (2009): Neurodevelopmental correlates of theory of mind in preschool children. Child Dev 80:1147–1162. [DOI] [PubMed] [Google Scholar]
- Samson D, Apperly IA, Humphreys GW (2007): Error analyses reveal contrasting deficits in “theory of mind”: Neuropsychological evidence from a 3‐option false belief task. Neuropsychologia 45:2561–2569. [DOI] [PubMed] [Google Scholar]
- Saxe R, Kanwisher N (2003): People thinking about thinking people: The role of the temporo‐parietal junction in “theory of mind.”. NeuroImage 19:1835–1842. [DOI] [PubMed] [Google Scholar]
- Saxe R, Wexler A (2005): Making sense of another mind: The role of temporo‐parietal junction. Neuropsychologia 43:1391–1399. [DOI] [PubMed] [Google Scholar]
- Saxe RR, Whitfield‐Gabrieli S, Scholz J, Pelphrey KA (2009): Brain regions for perceiving and reasoning about other people in school‐aged children. Child Dev 80:1197–1209. [DOI] [PubMed] [Google Scholar]
- Schaafsma SM, Pfaff DW, Spunt RP, Adolphs R (2015): Deconstructing and reconstructing theory of mind. Trends Cogn Sci 19:65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz J, Imamizu H, Kawato M, Frith CD (2004): Activation of the human superior temporal gyrus during observation of goal attribution by intentional objects. J Cogn Neurosci 16:1695–1705. [DOI] [PubMed] [Google Scholar]
- Schurz M, Radua J, Aichhorn M, Richlan F, Perner J (2014): Fractionating theory of mind: A meta‐analysis of functional brain imaging studies. Neurosci Biobehav Rev 42:9–34. [DOI] [PubMed] [Google Scholar]
- Sommer M, Do¨hnel K, Sodian B, Meinhardt J, Thoermer C, Hajak G (2007): Neural correlates of true and false belief reasoning. NeuroImage 35:1378–1384. [DOI] [PubMed] [Google Scholar]
- Spittle AJ, Treyvaud K, Doyle LW, Roberts G, Lee KJ, Inder TE, Cheong JLY, Hunt RW, Newnham CA, Anderson PJ (2009): Early emergence of behavior and socio‐emotional problems in very preterm infants. J Am Acad Child Adolesc Psychiatry 48:909–919. [DOI] [PubMed] [Google Scholar]
- Taylor MJ, Donner EJ, Pang EW (2012): fMRI and MEG in the study of typical and atypical cognitive development. Clinical Neurophysiology 42:19–25. [DOI] [PubMed] [Google Scholar]
- Van der Meer L, Groenewold NA, Nolen WA, Pijnenborg M, Aleman A (2011): Inhibit yourself and understand the other: Neural basis of distinct processes underlying Theory of Mind. NeuroImage 56:2364–2374. [DOI] [PubMed] [Google Scholar]
- Van Veluw SJ, Chance SA (2014): Differentiating between self and others: An ALE meta‐analysis of fMRI studies of self‐recognition and theory of mind. Brain Imaging Behav 8:24–38. [DOI] [PubMed] [Google Scholar]
- Vara AS, Pang EW, Vidal J, Anagnostou E, Taylor MJ (2014): Neural mechanisms of inhibitory control continue to mature in adolescence. Dev Cogn Neurosci 10:129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal J, Mills T, Pang EW, Taylor MJ (2012): Response inhibition in adults and teenagers: Spatiotemporal differences in the prefrontal cortex. Brain Cogn 79:49–59. [DOI] [PubMed] [Google Scholar]
- Vistoli D, Brunet‐Gouet E, Baup‐Bobin E, Hardy‐Bayle M, Passerieux C (2011): Anatomical and temporal architecture of theory of mind: A MEG insight into the early stages. NeuroImage 54:1406–1414. [DOI] [PubMed] [Google Scholar]
- Wechsler D (2002): Wechsler Abbreviated Scales of Intelligence. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Wehrle FM, Kaufmann L, Benz LD, Huber R, O'Gorman RL, Latal B, Hagmann CF (2016): Very preterm adolescents show impaired performance with increasing demands in executive function tasks. Early Hum Dev 92:37–43. [DOI] [PubMed] [Google Scholar]
- Wellman H, Cross D, Watson J (2001): Meta‐analysis of theory of mind development: The truth about false belief. Child Dev 72:655–684. [DOI] [PubMed] [Google Scholar]
- Wellman H, Fang F, Peterson C (2011): Sequential progressions in a Theory‐of‐Mind scale: Longitudinal perspectives. Child Dev 82:780–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson KE, Jakobson LS (2014): Social attribution skills of children born preterm at very low birthweight. Dev Psychopathol 26:889–900. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Tables and Figure


