Abstract
Decreased neural plasticity is observed with healthy ageing in the primary sensorimotor (SM1) cortex thought to participate in motor learning and memory consolidation processes. In the present magnetoencephalography study, the post‐training reorganization of resting‐state functional connectivity (rsFC) and its relation with motor learning and early consolidation in 14 young (19–30 years) and 14 old (66–70 years) healthy participants were investigated. At the behavioral level, participants were trained on a motor sequence learning task then retested 20–30 min later for transient offline gains in performance. Using a sensorimotor seed‐based approach, rsFC relying on beta band power envelope correlation was estimated immediately before and 10 min after the learning episode. Post‐training changes in rsFC (from before to after learning) were correlated with motor learning performance and with the offline improvement in performance within the hour after learning. Young and old participants exhibited differential patterns of sensorimotor‐related rsFC, bearing specific relationships with motor learning and consolidation. Our findings suggest that rsFC changes following learning reflect the offline processing of the new motor skill and contribute to the early memory consolidation within the hour after learning. Furthermore, differences in post‐training changes in rsFC between young and old participants support the hypothesis that ageing modulates the neural circuits underlying the learning of a new motor skill and the early subsequent consolidation stages. Hum Brain Mapp 38:923–937, 2017. © 2016 Wiley Periodicals, Inc.
Keywords: resting‐state functional connectivity, motor sequence learning, memory consolidation, ageing, brain plasticity, magnetoencephalography
INTRODUCTION
Motor skill learning and consolidation are instances of neural plasticity processes that can be altered with ageing. Behavioral studies show that even though old adults remain able to learn novel motor skills through practice, the consolidation of acquired representations can be compromised [Brown et al., 2009; Fogel et al., 2014; Spencer et al., 2007; Wilson et al., 2012]. These alterations may have deleterious consequences for quality of life and autonomy since a large part of our daily activities is subtended by the efficient implementation of cognitive and motor skills. It is therefore crucial to understand the neural and cognitive mechanisms by which these skills are acquired and consolidated, and how they are modified with age.
Neuroimaging studies indicate that the efficiency of plasticity mechanisms in the primary motor (M1) cortex decreases with ageing, which might contribute to motor decline [e.g., Freitas et al., 2011; Sawaki et al., 2003; Todd et al., 2010]. The reduction of experience‐induced motor plasticity with age additionally suggests post‐training consolidation deficits. Accordingly, we have shown using magnetoencephalography (MEG) that motor sequence learning induces post‐training reactivity changes in the central mu rhythm in young but not old adults [Mary et al., 2015]. The motor and sensorimotor cortical regions are part of a wider, distributed network in which plastic changes take place in the course of motor skill learning and consolidation in healthy young participants. Indeed, during task practice, inter‐regional connectivity changes are observed at various stages in the hippocampo‐cortical [Albouy et al., 2015], cortico‐cerebellar [Ma et al., 2010; Tamás Kincses et al., 2008] and cortico‐striatal [Albouy et al., 2015; Debas et al., 2014; Ma et al., 2010] networks, reflecting the creation and the progressive development of expertise‐related neural systems subtending motor performance.
Outside of task practice, motor learning‐dependent changes in resting state functional connectivity (rsFC) may evidence the functional reorganization of the brain networks supporting the offline consolidation of new motor skills [Sami et al., 2014]. Investigating rsFC, therefore, represents a promising tool to study memory consolidation mechanisms. The sensorimotor network includes a set of regions well known to be involved in motor sequence learning. Using functional magnetic resonance imaging (fMRI), this network has been shown to emerge from temporal correlations between the very slow (<0.1 Hz) fluctuations of the blood oxygen level dependent signal of M1 cortex and those of the primary somatosensory (SI) cortex, the secondary somatosensory (SII) cortex, the supplementary motor area (SMA), the premotor cortex, the putamen, the thalamus, and the cerebellum [Deco and Corbetta, 2011]. Recent studies conducted in young adults support the assumption that the sensorimotor network plays a role in motor memory consolidation. Indeed, motor learning‐related rsFC changes have been observed in young adults immediately [Albert et al., 2009; Gregory et al., 2014; Sami et al., 2014], hours [Sami et al., 2014; Vahdat et al., 2011] or even weeks [Ma et al., 2011; Sampaio‐Baptista et al., 2015] after initial learning. A fMRI study evidenced that improved behavioral performance after one day is associated with amplified resting‐state anti‐correlations within a cerebello‐fronto‐parietal network encompassing motor areas (M1 cortex and SMA), suggesting that spontaneous brain fluctuations are associated with the evolution of performance [Vahdat et al., 2011]. Beside, increased rsFC in fMRI between M1 cortex and bilateral sensorimotor and premotor cortices immediately after learning is positively correlated with performance improvement in motor sequence learning after a night of sleep [Gregory et al., 2014], indicating that functional brain modifications can precede and to some extent predict behavioral changes. However, Sami et al. [2014] found an enhanced rsFC in the sensorimotor network only 6 hours after explicit sequence learning whereas this enhancement was immediately present after implicit sequence learning. Other fMRI studies showed that the strength of spontaneous connectivity increases within the fronto‐parietal and cerebellar networks immediately after a visuo‐motor adaptation task [Albert et al., 2009]. Sampaio‐Baptista et al. [2015] reported rsFC changes in the motor network after a 6‐week practice on a complex motor task, characterized by an increased rsFC in the low intensity practice group and a decreased rsFC in the high intensity practice group. Finally, rsFC changes were reported in the right postcentral and the bilateral supramarginal gyri after 2 and 4 weeks of motor sequence learning [Ma et al., 2011].
The relation between sensorimotor network and motor consolidation may be affected by age. A variety of studies have reported age‐related decreases in the default‐mode network (DMN) rsFC as well as in the executive and attentional networks [for a review, see Sala‐Llonch et al., 2015]. However, results about the sensorimotor network are less consistent as some studies disclosed increased rsFC [e.g., Song et al., 2014], whereas others reported no detectable changes [e.g., Geerligs et al., 2015]. To the best of our knowledge, only one fMRI study focused on post‐learning rsFC modifications in ageing [Jacobs et al., 2015]. The authors reported an age‐related reorganization in the between‐networks coupling (i.e., between the DMN and the executive network, and between the DMN and the fronto‐parietal network) after episodic learning, which was related to memory retrieval performance 30 min later. Hence, age‐related experience‐dependent rsFC changes have been evidenced after episodic learning [Jacobs et al., 2015], but changes following motor sequence learning remain to be investigated.
This body of work suggests that investigating brain networks dynamics across the lifespan and following learning represents a promising approach to characterize age‐related changes in the evolution of memory consolidation processes. To date, fMRI remains the dominant imaging technique to study rsFC, although it is hampered by major caveats [Liu, 2013] such as age‐induced modifications in the neurovascular coupling [for a review, see, D'Esposito et al., 2003] that may bias the comparison in rsFC along the lifespan [for a review, see, Liu, 2013]. This limitation can be bypassed using electrophysiological techniques such as magnetoencephalography (MEG). Indeed, resting‐state networks spatially similar to those observed in fMRI studies have been reliably reconstructed from resting‐state MEG data using power envelope correlations [Brookes et al., 2011, 2012b; Hall et al., 2013; Hipp et al., 2012; de Pasquale et al., 2010; Wens et al., 2014a, 2014b]. Modulations of MEG activity within resting‐state networks have been disclosed during or after task performance [Betti et al., 2013; Brookes et al., 2012a] and after working memory training [Astle et al., 2015]. However, to the best of our knowledge, learning‐dependent as well as the associated age‐related changes in rsFC estimates per se have not yet been studied using MEG.
In the present study, we used MEG to investigate experience‐ and age‐related changes in sensorimotor rsFC associated with motor skill learning. To do so, resting‐state activity was obtained in young and old healthy participants in two sessions, before and 10 min after the repeated practice of a 5‐element motor sequence [i.e., the Finger Tapping Task (FTT); Karni et al., 1995]. Using the seed‐based connectivity method described in Wens et al. [2014a, 2014b], we then computed rsFC maps between a seed located in the right primary sensorimotor (rSM1) cortex and the rest of the brain, in the pre‐ and post‐ motor sequence learning sessions. Participants were also tested behaviorally 20–30 min after learning to measure the transient offline boost in behavioral performance consistently observed 5–30 min after motor learning [Albouy et al., 2006; Hotermans et al., 2006, 2008; Nettersheim et al., 2015; Schmitz et al., 2009], but not 4 hours after learning [Albouy et al., 2006; Hotermans et al., 2006; Nettersheim et al., 2015]. Two main analyses aimed at understanding the functional relevance of motor learning‐related changes in SM1‐based network. First, to determine how learning reorganizes spontaneous brain connectivity patterns, we investigated rsFC changes from before to after learning in relation to performance improvement during task practice, that is, motor learning. Second, we tested whether post‐learning changes in rsFC patterns are predictive of the offline improvement in performance 30 min after learning, which reflects the initial phase of memory consolidation. In young participants, we hypothesized that SM1 rsFC and its learning‐dependent changes would be observed within cortical regions involved in motor sequence learning and consolidation processes, including SMA, prefrontal and premotor cortices and cerebellum [e.g., Doyon et al., 2009; Penhune and Steele, 2012]. Given the evidence for age‐related changes in rsFC patterns and in the cortico‐striatal network involved in motor memory consolidation [for a review, see King et al., 2013], we also predicted differences in rsFC maps between young and old participants, before learning as well as during early consolidation.
MATERIALS AND METHODS
Participants
Fourteen young (7 females; 24.2 ± 3.5 years (mean ± std); range 19–30 years) and fourteen old (8 females; 69.1 ± 1.5 years; range 66–70 years) right‐handed healthy participants without any history of neuropsychiatric, sleep or movement disorders gave their written informed consent to participate in this study approved by the ULB‐Hospital Erasme Ethics Committee. Participants completed the Edinburgh handedness questionnaire [Oldfield, 1971] to confirm their right‐handedness (laterality score young: 75.4 ± 19.9, old: 89.3 ± 13.1). As the experimental task of this study consisted of a motor sequence learning, professional typists or musicians were not included. Participants did not take any medication known to affect sleep or memory. They were screened for depression using the Short version of the Beck Depression Inventory (BDI, [Beck et al., 1974]; French adaptation by [Collet and Cottraux, 1986]; score young: 2.6 ± 2.6, old: 2.3 ± 2.3; inclusion score ≤7) and for anxiety using the State‐Trait Anxiety Inventory (STAI, French version: [Bruchon‐Schweitzer and Paulhan, 1990]; score young: 33.7 ± 8.9, old: 29.2 ± 5.3; inclusion score ≤45). Level of education was calculated based on the International Standard Classification of Education and was similar in old (5.1 ± 2.4) and young (5.4 ± 2) participants (t(26) = −0.27, P = 0.79). Good sleep habits were assessed using the Pittsburgh Sleep Quality Index (PSQI, [Buysse et al., 1989]; young 4.2 ± 3.1 vs. old 3.4 ± 2.2, t(26) = 0.84; P = 0.41, inclusion score ≤8). Old adults averaged an intermediate morning‐type (60.8 ± 7.3) whereas young adults averaged a neutral type (49.8 ± 7.9; t(26) = −3.82, P < 0.001) at the Morningness–Eveningness Questionnaire ([Horne and Ostberg, 1976]; range young: 35–61, old: 51–76). Additionally, old participants scored within the normal range for the risk of dementia (Mattis Dementia Rating Scale, [Mattis, 1976]; score 141.5 ± 1.9, inclusion score >123). Sleep duration for the night before the MEG acquisition was similar in young (7.6 ± 1.3 hours) and old (7.2 ± 1.2 hours) adults (t(25) = 0.79; P = 0.44), as self‐reported from the St Mary's Hospital sleep questionnaire (adapted from [Ellis et al., 1981]).
Experimental Design
Figure 1 illustrates the experimental paradigm used in this study.
Figure 1.

Experimental design [adapted from Mary et al., 2015]. Participants began the experiment with a baseline resting state (REST 1; light gray), followed by a Simple Movement task (SMT‐1) and a Finger‐Tapping task (FTT‐1). Following a 10‐min break, participants completed a second resting state (REST 2; dark gray), followed by the SMT and FTT sessions (SMT‐2 and FTT‐2). MEG analyses were conducted on REST1 and REST2, and the SM1 seed for rsFC was determined based on the analysis of SMT‐1 data. Only behavioral measures were taken from FTT data.
Resting state data acquisitions lasted 5 min, during which participants were instructed to stay still and awake with their eyes open gazing at a fixation cross. Resting‐state sessions took place just before (Rest 1) and 10 min after (Rest 2) the execution of a Simple Movement Task (SMT‐1) and a motor sequence learning Finger‐Tapping Task (FTT‐1). After Rest 2, participants were administered again the SMT (SMT‐2) followed by the FTT (FTT‐2). Analysis of the mu rhythm during SMT‐1 and SMT‐2 has been previously reported, and SMT and FTT procedures were described in details [Mary et al., 2015]. Only essential information is provided here. During the SMT, participants performed 100 auditory‐cued key presses using all four left fingers simultaneously (index to little finger, interstimulus interval 5 sec). MEG data acquired during SMT1 were used to individually localize the right SM1 (rSM1) cortex seed for subsequent rsFC analyses (see below).
For each FTT ([Orban et al., 2010]; adapted from [Karni et al., 1995]) trial, participants were instructed to reproduce twice a 5‐element sequence of finger movements, as fast and accurately as possible, by pressing the four possible keys on a MEG‐compatible button box (fORP; Current Designs Inc.) with their left non‐dominant hand. The 5‐element sequence was as follows: little finger [4], index [1], ring [3], middle [2], and little finger [4]. The numeric representation of the sequence [4‐1‐3‐2‐4] was on permanent display to exclude any working memory component. During the learning phase (FTT‐1), 70 trials were administered. In the retest phase (FTT‐2) taking place 20–30 min after the end of the learning phase, 50 trials were administered. Following Hotermans et al. (2006, 2008), FTT performance was computed by considering the eight successive three‐element chunks (i.e., [413], [132], [324], [244], [441], [413], [132], [324]) that composed each trial (i.e., twice the sequence [4‐1‐3‐2‐4]). Speed performance was computed per trial as the mean execution time for correctly reproduced 3‐element chunks. Accuracy was not used as measure of learning because the error rate in this version of the FTT is extremely low [Mary et al., 2015; Orban et al., 2010]. The two first trials of the learning and retest sessions were excluded from statistical analyses to allow participants to get used to the task. Learning was then assessed by comparing the average execution times for the first and last 20 trials of the learning session. Performance gain during the retest phase was calculated by comparing the execution times for the 20 last trials of the learning session to the execution times for the 20 first trials of the retest session.
To investigate the relation between sequence learning and rsFC, a motor learning index was computed based on the assumption that performance improves with practice according to a power law [Newell and Rosenbloom, 1981]. The within‐session evolution of mean chunk execution times at chunk number was therefore individually fitted to a model using a logarithmic linear regression. The motor learning index was then estimated as the exponent , since higher values of indicate faster decreases in sequence execution time. The percentage of gain from the 20 last trials of learning to the 20 first trials of retest was also calculated to investigate the relation between the offline gain in performance at retest (i.e., boost effect; [Hotermans et al., 2006]) and the changes in rsFC after learning (see below).
MEG Acquisition and Preprocessing
MEG signals were recorded in a light‐weight magnetically shielded room using a whole‐scalp 306‐channel system (Vectorview & MaxshieldTM; Elekta Oy, Helsinki, Finland). Four head position indicator coils continuously monitored the subjects' head position. The locations of the coils and at least 300 head‐surface points (on scalp, nose, and face) were determined with respect to the three anatomical fiducials using an electromagnetic tracker (Fastrak, Polhemus, Colchester, VT). Electrooculogram (EOG) signals were obtained using bipolar electrodes located below the left outer canthus and above the right outer canthus. Electrocardiogram (ECG) was recorded with bipolar electrodes placed below the right collarbone and on the left last rib. Movements of the left hand were monitored based on surface electromyogram using bipolar electrodes placed on the left extensor carpi ulnaris muscle. Rest and SMT MEG data were pre‐processed using the signal space separation (SSS) method to suppress external interferences and correct for head movements [Taulu et al., 2005]. Cardiac, muscular and ocular artifacts were removed by visual inspection and an independent component approach [Vigário et al., 2000] combined with a correlation analysis between the independent components and EOG and ECG signals as described in [Mary et al., 2015]. After removal of the artifacts, MEG data were filtered in the beta‐band (12–30 Hz). Indeed, MEG‐based SM1 slow envelope correlation was shown to be frequency‐specific with the strongest correlation occurring in the alpha to beta frequency ranges [Brookes et al., 2011; Hipp et al., 2012]. Moreover, activity in the primary sensorimotor cortex can be assessed by measuring changes in the rolandic mu rhythm with dominant frequencies in alpha and beta bands [Cheyne, 2012; Hari and Salmelin, 1997; Jones et al., 2009; Mary et al., 2015]. The beta rhythm would originate in the pre‐central gyrus and would be involved in motor functions, whereas the alpha rhythm would be generated in the post‐central gyrus and would be involved in somatosensory processes [Hari and Salmelin, 1997; Mary et al., 2015]. Therefore, we computed the same rsFC analyses with the alpha frequency band (8–12 Hz). The results for the correlation between rsFC in the alpha band and behavioral performance are presented in Supporting Information.
Source Reconstruction
Individual 3D‐T1 weighted MRIs were acquired using a 1.5 T MRI scanner (Intera, Philips, The Netherlands) and segmented using the Freesurfer software (Martinos Center for Biomedical Imaging, Massachusetts). Co‐registration between MEG and MRI coordinate systems was realized manually using the three anatomical landmarks and the head‐surface points. Individual MEG forward models were then computed using the Boundary Element Method implemented in the MNE software suite (Martinos Center for Biomedical Imaging, Massachusetts). To facilitate inter‐subject comparisons, the models were computed on a source grid obtained from a homogeneous 5‐mm grid source space covering the whole Montreal Neurological Institute (MNI) brain, which was then transformed onto individual MRIs using the non‐linear spatial‐normalization algorithm implemented in Statistical Parametric Mapping (SPM8, Wellcome Department of Cognitive Neurology, London). Source reconstruction was finally performed using a band‐specific Minimum Norm Estimation (MNE) [Dale and Sereno, 1993]. To avoid possible issues associated with SSS regarding resting‐state networks [Luckhoo et al., 2012], MNE was restricted to the planar gradiometers. Their sensor‐space noise covariance matrix was estimated in the beta band from 5 min of artifact‐free data recorded from an empty room, and the MNE regularization parameter was set as in Hämäläinen et al. [2010]. Each estimated three‐dimensional source was projected onto its direction of maximum variance and the analytic signal was computed using the Hilbert transform.
1. Seed‐Based Resting‐State Functional Connectivity
Seed‐based rsFC maps were estimated using the slow Hilbert envelope correlation analysis adapted from the seminal works of MEG rsFC [Brookes et al., 2012b; Hipp et al., 2012] and previously described in Wens et al. [2014a, 2014b]. Briefly, the instantaneous orthogonalization method of [Hipp et al., 2012] was first applied to sources' analytic signals in order to limit the spurious rsFC due to the spatial leakage effects in MNE [Wens, 2015; Wens et al., 2015]. Slow envelope fluctuations (∼1 Hz) were then extracted by averaging their Hilbert envelope over sliding windows (size: 1 s, step: 0.5 s) and their Pearson's correlation coefficient between the seed and orthogonalized sources were finally computed. Individual SM1 networks were derived for both resting‐state sessions (i.e., Rest 1 and Rest 2) as the resulting rsFC maps based on a rSM1 seed.
In this work, the rSM1 seed location was determined individually using a functional localizer [as in Brookes et al., 2012b] on the basis of SMT event‐related mu‐rhythm post‐movement enhancement or resynchronization. Post‐movement resynchronization or rebound was used rather than movement suppression or desynchronization because it was better localized in terms of time and frequency and because it was associated with experience‐induced modulation in our prior study [Mary et al., 2015]. Event‐related time‐frequency power analysis was performed on SMT data using a Morlet wavelet decomposition with a standard time‐frequency compromise [Tallon‐Baudry et al., 1996]. Power was averaged over a pre‐selection of 9 pairs of gradiometers overlying the rSM1 cortex [Kim and Chung, 2007]. Post‐movement time (young 2653.3 ± 672.9 ms; old 2279.3 ± 707.6 ms) and frequency (young 21.1 ± 2.5 Hz; old 19.1 ± 4.1 Hz) of maximum power were then selected. The wavelet coefficients at selected time and frequency were then source projected as described above. The MNE depth bias was corrected using the sLORETA noise normalization [Pascual‐Marqui, 2002]. The seed location was finally selected as the local maximum peak in the rSM1 cortex contralateral to hand movement.
rsFC and Behavioral Performance
To investigate post‐training changes in rsFC, difference maps between Rest 2 and Rest 1 rsFC (Rest 2–Rest 1) were computed for each subject. We first tested the existence of a relation between the FTT‐1 motor learning index and the post‐training changes in rsFC using inter‐subject Pearson's correlations at each source to characterize learning‐related plastic changes in the SM1 network. In a second step, correlations using the performance gain at retest (i.e., the boost effect) were performed to investigate whether rsFC post‐training changes are associated with the motor improvement observed 20–30 min after the end of learning. A positive (respectively, negative) correlation between motor performance (i.e., learning or boost) and rsFC changes (Rest 2–Rest 1) in a given brain area indicates that an increased (respectively, decreased) connectivity between rSM1 cortex and this region after learning is associated with a better performance.
The above correlations were performed separately for the young and the old groups. Reported results were significant at P corr < 0.05 after correction for the massive multiple comparisons involved in testing source‐level statistical maps [Barnes et al., 2011]. Correction for multiple comparisons was applied using a Bonferroni correction for the number of spatial degrees of freedom involved in MNE reconstructions, estimated as in [Wens et al., 2015]. In practice, the corrected significance level corresponded to P < 0.0009.
Finally, we tested whether the correlation coefficients between motor performance and rsFC changes (Rest 2–Rest 1) differed between the young and the old groups (t(11)‐tests on Fisher transformed peak r scores). We assessed statistical significance for each source location in the brain and we reported results significant at P corr < 0.05 after correction for the multiple comparisons involved in testing source‐level statistical maps [Barnes et al. 2011; Wens et al., 2015]. In practice, the corrected significance level corresponded to P < 0.0009. For the sake of completeness, we reported when the P uncorr < 0.01 for the brain sources found significant in the above group analyses (Tables 1 and 2).
Table 1.
Correlations between rsFC post‐training changes (Rest 2–Rest 1) and motor learning
| MNI coordinates (mm) | ||||||
|---|---|---|---|---|---|---|
| Location of maxima | x | y | z | Peak r‐scores | P‐value | |
| Young participants | Young | Old | ||||
| L parietal operculum (SII) | −64 | −21 | 20 | 0.83 | −0.27 | # |
| L precentral gyrus (premotor area) | −50 | 4 | 10 | −0.89 | −0.053 | |
| L superior frontal gyrus (SMA) | −9 | −21 | 79 | 0.8 | −0.35 | # |
| R superior frontal gyrus (SMA) | 16 | −2 | 75 | 0.79 | −0.61 | * |
| R lingual gyrus | 16 | −57 | −6 | −0.83 | 0.35 | # |
| R superior frontal gyrus (pre‐SMA) | 21 | 19 | 60 | 0.88 | 0.076 | # |
| R cerebellum (crus I) | 45 | −81 | −31 | −0.79 | 0.14 | |
| Old participants | Old | Young | ||||
| R superior temporal gyrus | 38 | 9 | −21 | −0.8 | 0.26 | # |
| R inferior frontal gyrus | 33 | 22 | −11 | −0.83 | −0.28 | |
| R lingual gyrus (V1) | 8 | −91 | −5 | −0.85 | −0.2 | # |
| R cerebellum (superior vermis) | 6 | −56 | −15 | −0.8 | −0.17 | |
| L cuneus (V2) | −10 | −86 | 30 | −0.85 | 0.05 | # |
| L superior temporal gyrus | −44 | −12 | −6 | 0.83 | 0.61 | |
| L superior parietal lobule (BA7) | −44 | −56 | 55 | −0.85 | 0.24 | # |
| L inferior parietal lobule | −64 | −26 | 37 | −0.79 | 0.19 | # |
Brain regions where rsFC changes from Rest 1 to Rest 2 are significantly correlated with the learning index, in young (A) and old (B) participants. MNI coordinates locate the correlation peak in each region of significance. Peak r scores show the correlation coefficients in the group for which a significant correlation has been identified (young or old) and the corresponding values in the other group (old or young). Positive (respectively, negative) values indicate that rsFC post‐training increases (respectively, decreases) are associated with better learning performance. The P‐values assess the differences between young and old individuals in the peak r scores. The family‐wise error was controlled for the number of spatial degrees of freedom involved in MNE reconstructions using a Bonferroni correction (* indicates P < 0.0009; # indicates P uncor r < 0.01). SII, secondary somatosensory cortex; SMA, supplementary motor area; V1, primary visual cortex; V2, secondary visual cortex.
Table 2.
Correlations between rsFC post‐training changes (Rest 2–Rest 1) and the boost of performance
| MNI coordinates (mm) | ||||||
|---|---|---|---|---|---|---|
| Location of maxima | x | y | z | Peak r‐scores | P‐value | |
| Young participants | Young | Old | ||||
| L inferior frontal gyrus | −54 | 24 | 14 | −0.84 | −0.2 | |
| L superior parietal lobule (BA5) | −4 | −61 | 70 | 0.79 | 0.6 | |
| R middle frontal gyrus (DLPFC) | 39 | 24 | 50 | 0.83 | 0.25 | |
| R inferior temporal gyrus | 50 | −41 | −25 | −0.79 | 0.33 | # |
| R middle temporal gyrus | 66 | −26 | −5 | −0.80 | 0.1 | # |
| Old participants | Old | Young | ||||
| L caudate nucleus | −10 | −1 | 15 | 0.79 | −0.19 | # |
| L lingual gyrus (V2) | −4 | −91 | −11 | −0.84 | 0.044 | # |
| L paracentral lobule | 11 | −11 | 80 | −0.81 | −0.046 | |
| R cerebellum (lobule V) | 20 | −34 | −22 | −0.82 | −0.26 | |
| R superior temporal gyrus | 66 | −6 | 1 | 0.84 | 0.02 | # |
Brain regions where rsFC changes from Rest 1 to Rest 2 significantly correlated with the boost of performance at retest, in young (A) and old (B) participants. MNI coordinates locate the correlation peak in each region of significance. Peak r scores show the correlation coefficients in the group for which a significant correlation has been identified (young or old) and the corresponding values in the other group (old or young). Positive (respectively, negative) values indicate that post‐training rsFC increases (respectively, decreases) are associated with a stronger boost effect. The P‐values assess the differences between young and old individuals in the peak r score and no group differences survive to correction for multiple comparisons (all P > 0.0009; # indicates P uncorr < 0.01). DLPFC, dorsolateral prefrontal cortex; BA5, somatosensory association cortex; V2, secondary visual cortex.
RESULTS
Finger Tapping Task
Figure 2 illustrates group‐averaged execution times during learning and retest for young and old participants.
Figure 2.
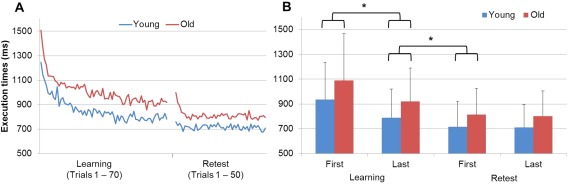
Finger Tapping Task performance, averaged for young (blue) or old (red) participants. A. Mean execution times (ms) for the correctly generated 3‐element chunks per trial (8 successive 3‐element chunks per trial) are plotted as a function of the 70 trials during the learning (FTT‐1) and the 50 trials during the retest (FTT‐2) sessions (see Experimental Design for further details). B. Mean execution times (ms) for the first 20 trials (3–23) and last 20 trials during the learning (FTT‐1) and retest (FTT‐2) sessions. [Color figure can be viewed at http://wileyonlinelibrary.com]
A repeated measures ANOVA on mean execution time with session (learning vs. retest) and trial type (20 first vs. 20 last) as within‐subject factors and group (young vs. old) as between‐subject factor yielded main effects of session (F(1,26) = 20.27; P < 0.001) and trial type (F(1,26) = 37.55; P < 0.001), and a session by trial interaction effect (F(1,26) = 36.31; P < 0.001). The group and other interaction effects were non‐significant (P > 0.05). Tukey post‐hoc analyses revealed faster execution times in the last than in the first trials during the learning session (1013 ± 343 ms vs. 855 ± 255 ms, P < 0.001) and faster execution times in the first trials during the retest as compared with the last trials during learning (855 ± 255 ms vs. 765 ± 211 ms, P < 0.001). Hence, both young and old participants learned the sequence and exhibited a performance boost at retest (Fig. 2B).
In addition to the mean execution time, we tested the accuracy by comparing the average of the first 20 trials and last 20 trials of the session. A repeated measures ANOVA on the percentage of correctly executed chunks yielded a main effect of group (F(1,26) = 4.38; P < 0.05). Although accuracy was higher than 95% in both groups, old adults (95.7 ± 5.8) were globally less accurate than young adults (98.2 ± 2.2).
Resting‐State Functional Connectivity in Relation to Motor Performance
Correlations between rsFC post‐training changes and motor learning
Figure 3 and Table 1 summarize the significant correlations disclosed between the motor learning index and rsFC post‐training changes (Rest 2–Rest 1).
Figure 3.
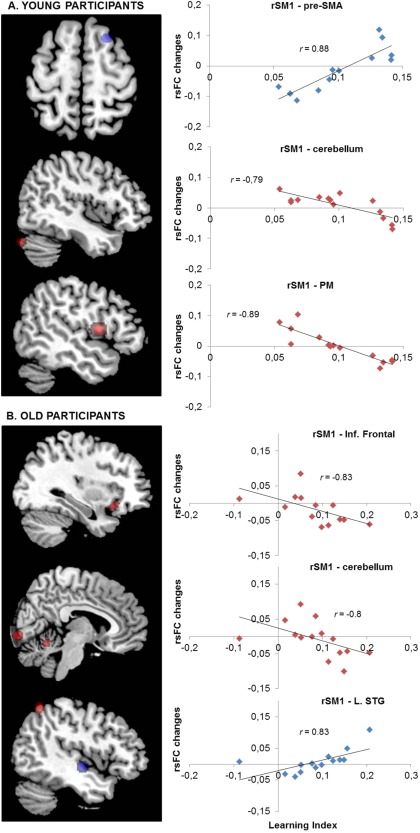
Post‐training changes in rsFC (Rest 2–Rest 1) in relation to motor learning index in young [A] (top panels) and old [B] (lower panels) participants. Color‐coded statistical maps (P corr < 0.05) show positive (blue) and negative (red) correlations between the rsFC changes and the motor learning index. The right panels represent the linear trend between individual rsFC changes and the associated learning index in the significant regions (see Table I for MNI coordinates). The value r represents the Pearson's correlation coefficient. rSM1, right primary sensorimotor cortex; SMA, supplementary motor area; PM, premotor area; Inf., Inferior; L., left; STG, superior temporal gyrus. (See Supporting Information data for all correlations: Figs. S1 and S2). [Color figure can be viewed at http://wileyonlinelibrary.com]
In young participants, positive correlations were identified at SII cortex, SMA and pre‐SMA, and negative correlations at premotor area, lingual gyrus and cerebellum (Crus I) (Fig. 3A).
In old participants, a positive correlation was identified at the left superior temporal gyrus (STG), and negative correlations at the right STG, inferior frontal gyrus, cerebellum (superior vermis), visual areas (V1 and V2) and left superior parietal lobule (BA7) (Fig. 3B).
Comparisons between young and old adults revealed correlational differences between learning index and seed‐based rsFC changes between rSM1 and the right superior frontal gyrus (SMA, peak MNI coordinates (x, y, z): 16 −1 76 mm; t(25) = −4.19, P corr < 0.05). Young adults exhibited a positive correlation between performance and the rSM1‐SMA rsFC, whereas old adults exhibited a negative correlation (see Table 1 and Fig. 3).
Correlations between rsFC post‐training changes and motor performance boost at retest
Figure 4 and Table 2 summarize the significant correlations disclosed between the motor performance improvement at retest, 20–30 min after learning (i.e., boost effect), and rsFC post‐training changes (Rest 2–Rest 1).
Figure 4.
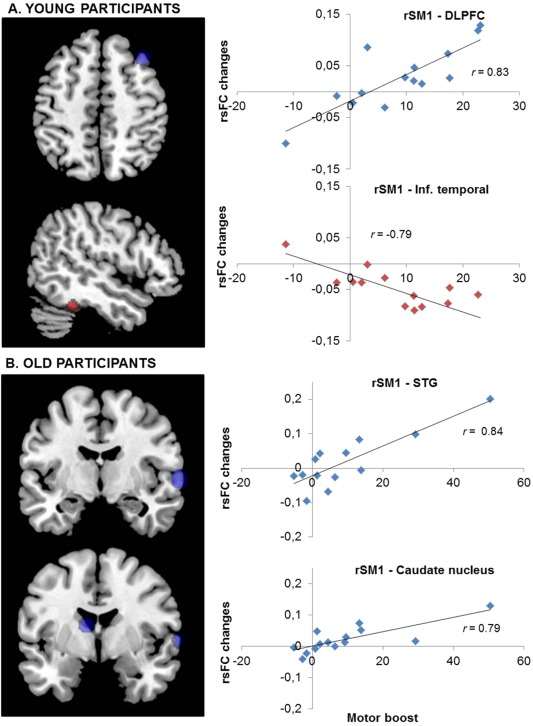
Post‐training changes in rsFC (Rest 2–Rest 1) in relation to the offline improvement in performance (motor boost) in young [A] (top panels) and old [B] (lower panels) participants. Statistical maps (P corr < 0.05) show positive (blue) and negative (red) correlations between the rsFC changes and the amplitude of the behavioral boost of performance. The right panels represent the linear trend between individual changes in rsFC and the associated behavioral boost in the significant regions (see Table II for MNI coordinates). The value r represents the Pearson's correlation coefficient. rSM1, right primary sensorimotor cortex; DLPFC, dorsolateral prefrontal cortex; Inf., Inferior; STG, superior temporal gyrus. (See Supporting Information data for all correlations: Figs. S3 and S4). [Color figure can be viewed at http://wileyonlinelibrary.com]
In young participants, positive correlations were identified at dorsolateral prefrontal cortex (DLPFC) and superior parietal lobule (BA5), negative correlations at inferior frontal gyrus and at middle and inferior temporal gyri (Fig. 4A).
In old participants, positive correlations were identified at left caudate nucleus and right STG, and negative correlations at right cerebellum (lobule V), left lingual gyrus (V2) and left paracentral lobule (Fig. 4B).
Coefficient correlations between motor performance boost and seed‐based rsFC changes did not significantly differ between young and old adults (P corr > 0.05).
DISCUSSION
The current study aimed at investigating the rsFC changes following motor sequence learning and how ageing affect those changes. This is, to the best of our knowledge, the first MEG study investigating specific age‐related rsFC modulations following motor sequence learning. Functional connectivity with respect to a seed located in the rSM1 cortex, was measured during a resting state before and after explicit motor sequence learning involving the left hand in young and old healthy participants. Correlations between rsFC changes (from before to after learning) and the evolution of behavioral performance (i.e., during learning or after a delayed offline period) were identified in regions previously shown to be involved in motor sequence learning. The correlations observed in both young and old adults suggest first, that motor learning induces a functional reorganization within the resting brain immediately after learning and second, that practice‐related changes in the sensorimotor network promote early motor performance improvement. Moreover, the presence of age‐related differences in the plastic rsFC modulations supports an age‐dependent reorganization of the neural circuits underlying learning and early consolidation stages of a new motor skill.
At the behavioral level, performance on the Finger Tapping Task (FTT) improved during learning. Execution times were also faster at retest than at the end of learning, indicating an offline boost in performance in both young and old participants. Given prior evidence for a decline in motor memory consolidation with ageing [Brown et al., 2009; Fogel et al., 2014; Spencer et al., 2007; Wilson et al., 2012], an absence or at least a decreased amplitude of the boost effect may have been expected in old as compared with young participants. This was not the case in the present study, which is likely due to the Simple Movement Task (SMT‐2) that occurred immediately before the retest phase (FTT‐2). Since the motor boost effect is transient and exhausted with continued motor practice [Hotermans et al., 2006], it is likely that part of the beneficial effect observed in young participants was captured during the elementary motor SMT‐2. Accordingly, we previously showed that administering the FTT without the intermediate SMT leads to a higher boost effect in young than old participants (see results of the behavioral‐only condition in [Mary et al., 2015]). Finally, although we did not evidence a decrease of the boost effect in old as compared with young adults in the MEG procedure, we might expect an age‐related decrease in the ability to consolidate new motor skills as previously reported [Brown et al., 2009; Fogel et al., 2014; Spencer et al., 2007; Wilson et al., 2012]. Moreover, resting state might prepare new motor skills for later consolidation during sleep, as rsFC post‐learning changes were related to sleep‐dependent memory improvement in young adults [Gregory et al., 2014]. However, sleep‐dependent memory consolidation was not investigated in the present study. Therefore, it would be interesting in a future study to investigate how immediate rsFC changes in young and old adults are related to sleep‐dependent memory decline in ageing.
In a first step, we investigated rsFC changes in relation to motor learning performance, to determine the links between learning experience and the reorganization of functional connectivity patterns. In young adults, better learning performance was associated with increased post‐training rsFC in the SII cortex, the SMA and the pre‐SMA. The correlation between rsFC changes with the SMA and learning in the young group was significantly different from the corresponding correlation obtained in the same region in the old group The supplementary motor complex (SMA and pre‐SMA) plays, among others, a role in the learning of sequential movements [for a review, see Nachev et al., 2008]. Importantly, motor cortical regions such as the SMA and the pre‐SMA might be engaged in planning the individual movements constituting the sequence, in order to produce fast and accurate sequences [Doyon et al., 2009]. Some authors propose that M1 cortex and SMA play a similar role in motor sequence acquisition [Kim and Shin, 2014; Tamaki et al., 2013]. Indeed, Kim and Shin [2014] reported that high beta frequency repetitive transcranial magnetic stimulations applied over M1 cortex or SMA reduce response latency to execute explicit and implicit sequences. Our findings support the proposal that increased connectivity between rSM1 cortex and the supplementary motor complex facilitates sequential learning, and that increased connectivity between rSM1 and SII cortices promotes sensorimotor integration of the sequence. However, contrarily to Gregory et al. [2014], we found decreased connectivity between rSM1 and premotor cortices. In the Wu et al. [2014] study, increased connectivity between M1 and premotor cortices before learning was predictive of reduced skill learning. According to the authors, an increased coupling between these regions may represent an inefficient motor system, as premotor cortex activation is associated with higher cognitive demands. Additionally, decreased connectivity after learning between rSM1 cortex and the cerebellum was associated with better performance during learning in young and old adults in the present study. Previous neuroimaging studies have reported a gradually decreasing activity in the cerebellum, associated with increasing activity in motor frontal regions with practice [Grafton et al., 2002; Lehericy et al., 2005; Penhune and Doyon, 2005]. In addition, Ma et al. [2010] showed that connectivity between cerebellum and M1 cortex gradually decreases within four weeks of daily motor sequence training. In agreement with our results, motor performance was associated with an increased negative correlation between cerebellum and motor frontal regions (M1 cortex and SMA) during the resting state following learning [Vahdat et al., 2011]. The cerebellum plays an important role during early learning but with the automatization of the motor sequence, it becomes less necessary to produce accurate movements [Penhune and Steele, 2012]. In the present study, the reduced error rates in the FTT even at the beginning of the practice might explain a reduced involvement of the cerebellum and a decreased need to perform error detection and correction.
In old adults, we found that decreased post‐training rsFC between rSM1 cortex and the right STG, inferior frontal gyrus, the cerebellum, visual areas (V1 and V2) and the left superior and inferior parietal lobules was associated with better motor learning. Contrarily to these findings, Albert et al. [2009] reported increased connectivity strength in the frontoparietal and the cerebellar networks during resting state after motor adaptation learning. The requirements of the task in the Albert et al. [2009] study were different however, as they used a visuo‐motor adaptation task known to rely on different neural substrates than the FTT [Doyon et al., 2009]. Our findings are however consistent with other studies that reported gradually decreasing activity in a fronto‐parieto‐cerebellar network during motor sequence practice [Tamás Kincses et al., 2008] and increased negative connections within the cerebello‐fronto‐parietal network after learning [Vahdat et al., 2011].
In addition to motor learning, the fronto‐parietal network has been associated with goal‐directed attentional processing [Fox et al., 2006]. A decreased coupling between rSM1 cortex and the attentional network at rest suggests that older participants recruiting less attentional resources to perform the sequence of movements developed better skills. In line with Jacobs et al. [2015], this result additionally suggests that the age‐related formation of a novel memory mainly depends on a dynamic reorganization between large‐scale networks, rather than within networks. A decreased rsFC between rSM1 cortex and STG, V1 and the superior parietal lobule was also observed in old adults. The medial superior temporal region and the temporoparietal junction are involved in visual motion processing [Bosco et al., 2008] and the superior parietal lobule (BA7) has been suggested to integrate visual and somatosensory inputs during motor learning [Hardwick et al., 2013]. In the present study, we noticed that several old adults encountered difficulties to perform the motor sequence without watching their hand. The connection between rSM1 cortex and these areas might therefore be stronger in participants who need a visual feedback from their hand to execute the sequence. In addition, increased connectivity after learning between rSM1 and the left STG was associated with increased learning performance. Still, the presence of increased rsFC between rSM1 and the left STG associated with a decreased rsFC between rSM1 and the right STG remains unclear. An increased involvement of the medial temporal network (including the superior and middle temporal gyri) has been found after implicit motor sequence learning, with the assumption that the medial temporal lobe is involved in the learning of longer and more complex sequences [Sami et al., 2014]. Partially in line with this hypothesis, FTT sequences might be perceived as more complex by old than young adults, which may explain the increased/decreased involvement of temporal regions in old/younger adults, respectively.
In a second step, we examined the relation between the motor boost of performance observed at retest 20–30 min after learning and learning‐related changes in rsFC, to determine how this functional reorganization may promote early offline consolidation processes. Young adults exhibited increased connectivity between rSM1 cortex and the DLPFC and the somatosensory association cortex/superior parietal lobule (BA5) in relation to performance improvement, and decreased connectivity between rSM1 and the inferior frontal, inferior temporal and middle temporal gyri. Increased connectivity between rSM1 cortex and the somatosensory and dorsal prefrontal regions confirm the proposal that sensorimotor integration and attentional processing might be critical during the early stages of the consolidation of motor sequences [Ma et al., 2011]. On the other hand, the lateral temporal cortex (LTC) is known to be involved in the DMN, even though its involvement is less robust than other areas [Buckner et al., 2008]. In the context of the present study, we suggest that participants with increased cross‐network rsFC between rSM1 cortex and DLPFC, and decreased connectivity between rSM1 and LTC are more able to both remain focused on the task and less focused on internal processes, which is beneficial to the boost of performance. Some participants reported mentally vocalizing the sequence of digits during training, probably to guide their sequential finger movements in the first stage of motor practice. In the resting scan after learning, the decreased connectivity between SM1 cortex and a Broca‐compatible area in the inferior frontal gyrus may reflect a gradual decrease in the verbal rehearsal of the sequence, following a progressive automatization of the motor sequence. In old participants, increased rsFC between rSM1 cortex and the caudate nucleus after learning was positively related to the boost of motor performance, suggesting that old participants with stronger connections within the cortico‐striatal network after learning exhibit a higher offline gain of performance. Consistent with such findings, it was proposed that the deterioration of the cortico‐striatal network contributes to an age‐related decline in motor memory consolidation [see King et al., 2013, for a review]. Additionally, increased rsFC between rSM1 and the STG was associated with increased performance. Conversely, decreased rsFC between rSM1 cortex and the cerebellum and V2 was related to improved performance boost in old adults, like learning in our prior analysis. Altogether, these findings demonstrate that changes in rsFC are associated with an early offline boost of performance in both young and old adults, which suggests that resting brain fluctuations contribute to early consolidation.
Some limitations could modulate the interpretation of our results. First, the subjects sample size (14 young and 14 old subjects) is relatively small to conduct correlational analyses. In practice, small sample leads to large statistical fluctuations on correlation estimates and so to high statistical thresholds of significance. These statistical limitations explain that only restricted areas are displayed in our maps of correlations (Figs. 3 and 4). Second, we did not include a control session in which participants did not perform a motor learning task. It might be claimed that some of the observed rsFC changes are due to motor activity, rather than motor learning. However, the results of the Albert et al. [2009] study using a motor control task suggest that rsFC changes taking place after motor learning are actually specific to motor memory consolidation. Moreover, the results of our correlational analyses support the functional relevance of the identified resting state networks and their post‐learning changes for early motor memory consolidation. Third, our analyses were restricted to one seed located in the rSM1 area. The choice of this seed was based on previous works that identified changes driven by motor memory in the sensorimotor network [Gregory et al., 2014; Ma et al., 2011; Sami et al., 2014; Sampaio‐Baptista et al., 2015; Vahdat et al., 2011] and on our previous study evidencing age‐related plasticity change in SM1 cortex following learning [Mary et al., 2015]. We are well aware that the selection of one specific seed constrains the functional connectivity analysis. Therefore, we cannot exclude the possibility that other networks might be related to motor sequence learning. The spatial constraint inherent to seed‐based envelope correlation method may be bypassed using other approaches such as ICA, which is frequently used in MEG to identify resting‐state networks. However, we decided to use a seed‐based approach to focus our analysis on the sensorimotor network. Fourth, the cortico‐striatal network was sparsely related to performance in our study, whereas it was demonstrated to be strongly involved in the initial and the consolidation phases of motor sequence learning [see Doyon et al., 2009, for a review]. Considering that the spatial resolution of the MEG decreases as a function of the source depth [Hillebrand and Barnes, 2002], it is not surprising that subcortical regions (such as the striatum or the hippocampus) were more difficult to localize with MEG. Notwithstanding the fact that a stronger involvement of the cortico‐striatal network may have been undetected in our study, our results are consistent with [Tamás Kincses et al., 2008] who did not find correlated activity with the striatum in the early learning phase. Finally, although the ability of MEG to detect subcortical and cerebellar activity is a matter of debate, an increasing number of MEG studies have reported task‐related activity modulation at the cerebellum [Bourguignon et al., 2013; Jerbi et al., 2007; Martin et al., 2006; Muthuraman et al., 2014; Stancak et al., 2011] and the caudate nucleus [Altamura et al., 2014], which supports the present rsFC findings.
CONCLUSIONS
To sum up, our findings suggest that plastic changes induced by the acquisition of a new motor skill modulate spontaneous activity patterns within the resting brain, with ageing as an additional modulatory factor. Experience‐dependent changes in resting state networks may reflect the offline processing of newly acquired memories that contribute to memory consolidation [Albert et al., 2009; Gregory et al., 2014; Peigneux et al., 2006; Sami et al., 2014; Vahdat et al., 2011]. Indeed, acquisition of a new motor skill induces functional reorganization at rest immediately after learning. This reorganization is associated with an improvement of motor performance following wakefulness, within the hour after the end of learning. As expected, modulations in learning‐related networks have been identified within the sensorimotor network [Deco and Corbetta, 2011] and in regions involved in motor learning and motor memory consolidation [e.g., Doyon et al., 2009]. Nonetheless, different sensorimotor‐related rsFC patterns and relationships with behavioral performance in young and old participants support the proposal of age‐related changes in the neural circuits underlying learning and early consolidation of novel motor skills. Finally, this MEG study highlights the functional role of intra‐ and inter‐hemispheric power envelope correlation within the SM1 resting‐state network outside the classical coupling between SM1 homologous regions.
Supporting information
Supporting Figure 1
Supporting Figure 2
Supporting Figure 3
Supporting Figure 4
Supporting Figure 5
Supporting Figure 6
Supporting Figure 7
Supporting Figure 8
Supporting Information
ACKNOWLEDGMENTS
This research project was financially supported by the Belgian Fonds de la Recherche Scientifique (FRS‐FNRS grant reference #7020836), and the ULB Action de Recherche Concertée (ARC) grant “Pathophysiology of Brain Plasticity Processes in Memory Consolidation.” Alison Mary (Research Fellow) was supported by the FNRS (grant reference 1.A092.12F) and by the Fonds David et Alice Van Buuren (ULB). Xavier De Tiège (Postdoctoral Clinical Master Specialist) and Vincent Wens (Research Logistic Collaborator) are supported by the FRS‐FNRS (Belgium). Rachel Leproult was supported by a “Brains Back to Brussels” grant. Philippe Peigneux is ULB Francqui Research Professor 2013–2016.
Contributor Information
Alison Mary, Email: alismary@ulb.ac.be.
Philippe Peigneux, Email: philippe.peigneux@ulb.ac.be.
REFERENCES
- Albert NB, Robertson EM, Miall RC (2009): The resting human brain and motor learning. Curr Biol CB 19:1023–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albouy G, Ruby P, Phillips C, Luxen A, Peigneux P, Maquet P (2006): Implicit oculomotor sequence learning in humans: Time course of offline processing. Brain Res 1090:163–171. [DOI] [PubMed] [Google Scholar]
- Albouy G, Fogel S, King BR, Laventure S, Benali H, Karni A, Carrier J, Robertson EM, Doyon J (2015): Maintaining vs. enhancing motor sequence memories: Respective roles of striatal and hippocampal systems. NeuroImage 108:423–434. [DOI] [PubMed] [Google Scholar]
- Altamura M, Carver FW, Elvevåg B, Weinberger DR, Coppola R (2014): Dynamic cortical involvement in implicit anticipation during statistical learning. Neurosci Lett 558:73–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astle DE, Barnes JJ, Baker K, Colclough GL, Woolrich MW (2015): Cognitive training enhances intrinsic brain connectivity in childhood. J Neurosci Off J Soc Neurosci 35:6277–6283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes GR, Litvak V, Brookes MJ, Friston KJ (2011): Controlling false positive rates in mass‐multivariate tests for electromagnetic responses. NeuroImage 56:1072–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Rial WY, Rickels K (1974): Short form of depression inventory: Cross‐validation. Psychol Rep 34:1184–1186. [PubMed] [Google Scholar]
- Betti V, Della Penna S, de Pasquale F, Mantini D, Marzetti L, Romani GL, Corbetta M (2013): Natural scenes viewing alters the dynamics of functional connectivity in the human brain. Neuron 79:782–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco G, Carrozzo M, Lacquaniti F (2008): Contributions of the human temporoparietal junction and MT/V5+ to the timing of interception revealed by transcranial magnetic stimulation. J Neurosci Off J Soc Neurosci 28:12071–12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguignon M, De Tiège X, Op de Beeck M, Van Bogaert P, Goldman S, Jousmäki V, Hari R (2013): Primary motor cortex and cerebellum are coupled with the kinematics of observed hand movements. NeuroImage 66:500–507. [DOI] [PubMed] [Google Scholar]
- Brookes MJ, Woolrich M, Luckhoo H, Price D, Hale JR, Stephenson MC, Barnes GR, Smith SM, Morris PG (2011): Investigating the electrophysiological basis of resting state networks using magnetoencephalography. Proc Natl Acad Sci 108:16783–16788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes MJ, Liddle EB, Hale JR, Woolrich MW, Luckhoo H, Liddle PF, Morris PG (2012a): Task induced modulation of neural oscillations in electrophysiological brain networks. NeuroImage 63:1918–1930. [DOI] [PubMed] [Google Scholar]
- Brookes MJ, Woolrich MW, Barnes GR (2012b): Measuring functional connectivity in MEG: A multivariate approach insensitive to linear source leakage. NeuroImage 63:910–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R, Robertson E, Press D (2009): Sequence skill acquisition and off‐line learning in normal aging. PLoS One 4:e6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchon‐Schweitzer M, Paulhan I (1990): Manuel de l'inventaire d'Anxiété trait‐état (forme Y) Laboratoire de psychologie de la santé. France: Université de Bordeaux II. [Google Scholar]
- Buckner RL, Andrews‐Hanna JR, Schacter DL (2008): The brain's default network: Anatomy, function, and relevance to disease. Ann N Y Acad Sci 1124:1–38. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ (1989): The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res 28:193–213. [DOI] [PubMed] [Google Scholar]
- Cheyne DO (2013): MEG studies of sensorimotor rhythms: A review. Exp Neurol 245:27–39. [DOI] [PubMed] [Google Scholar]
- Collet L, Cottraux J (1986): The shortened Beck Depression Inventory: Study of the concurrent validity with the hamilton depression rating scale and the widlöcher retardation rating scale. Encéphale Rev Psychiatr Clin Biol Thérapeutique 12:77–79. [PubMed] [Google Scholar]
- Dale AM, Sereno MI (1993): Improved localization of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: A Linear Approach. J Cogn Neurosci 5:162–176. [DOI] [PubMed] [Google Scholar]
- Debas K, Carrier J, Barakat M, Marrelec G, Bellec P, Tahar AH, Karni A, Ungerleider LG, Benali H, Doyon J (2014): Off‐line consolidation of motor sequence learning results in greater integration within a cortico‐striatal functional network. NeuroImage 99:50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deco G, Corbetta M (2011): The dynamical balance of the brain at rest. Neurosci Rev J Bringing Neurobiol Neurol Psychiatry 17:107–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Esposito M, Deouell LY, Gazzaley A (2003): Alterations in the BOLD fMRI signal with ageing and disease: A challenge for neuroimaging. Nat Rev Neurosci 4:863–872. [DOI] [PubMed] [Google Scholar]
- Doyon J, Bellec P, Amsel R, Penhune V, Monchi O, Carrier J, Lehéricy S, Benali H (2009): Contributions of the basal ganglia and functionally related brain structures to motor learning. Behav Brain Res 199:61–75. [DOI] [PubMed] [Google Scholar]
- Ellis BW, Johns MW, Lancaster R, Raptopoulos P, Angelopoulos N, Priest RG (1981): The St. Mary's Hospital sleep questionnaire: A study of reliability. Sleep 4:93–97. [DOI] [PubMed] [Google Scholar]
- Fogel SM, Albouy G, Vien C, Popovicci R, King BR, Hoge R, Jbabdi S, Benali H, Karni A, Maquet P, Carrier J, Doyon J (2014): fMRI and sleep correlates of the age‐related impairment in motor memory consolidation. Hum Brain Mapp 35:3625–3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME (2006): Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci 103:10046–10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas C, Perez J, Knobel M, Tormos JM, Oberman L, Eldaief M, Bashir S, Vernet M, Peña‐Gómez C, Pascual‐Leone A (2011): Changes in Cortical Plasticity Across the Lifespan. Front Aging Neurosci 3:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerligs L, Renken RJ, Saliasi E, Maurits NM, Lorist MM (2015): A brain‐wide study of age‐related changes in functional connectivity. Cereb Cortex 25:1987–1999. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Hazeltine E, Ivry RB (2002): Motor sequence learning with the nondominant left hand. A PET functional imaging study. Exp Brain Res 146:369–378. [DOI] [PubMed] [Google Scholar]
- Gregory MD, Agam Y, Selvadurai C, Nagy A, Vangel M, Tucker M, Robertson EM, Stickgold R, Manoach DS (2014): Resting state connectivity immediately following learning correlates with subsequent sleep‐dependent enhancement of motor task performance. NeuroImage 102 Pt 2:666–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall EL, Woolrich MW, Thomaz CE, Morris PG, Brookes MJ (2013): Using variance information in magnetoencephalography measures of functional connectivity. NeuroImage 67:203–212. [DOI] [PubMed] [Google Scholar]
- Hämäläinen MS, Lin F‐H, Mosher JC (2010): Anatomically and functionally constrained minimum‐norm estimates In: Hansen P, Kringelbach M, Salmelin R, editors. MEG: An Introduction to Methods. Oxford: Oxford University Press; pp 186–215. [Google Scholar]
- Hardwick RM, Rottschy C, Miall RC, Eickhoff SB (2013): A quantitative meta‐analysis and review of motor learning in the human brain. NeuroImage 67:283–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hari R, Salmelin R (1997): Human cortical oscillations: A neuromagnetic view through the skull. Trends Neurosci 20:44–49. [DOI] [PubMed] [Google Scholar]
- Hillebrand A, Barnes GR (2002): A quantitative assessment of the sensitivity of whole‐head meg to activity in the adult human cortex. NeuroImage 16:638–650. [DOI] [PubMed] [Google Scholar]
- Hipp JF, Hawellek DJ, Corbetta M, Siegel M, Engel AK (2012): Large‐scale cortical correlation structure of spontaneous oscillatory activity. Nat Neurosci 15:884–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne JA, Ostberg O (1976): A self‐assessment questionnaire to determine morningness‐eveningness in human circadian rhythms. Int J Chronobiol 4:97–110. [PubMed] [Google Scholar]
- Hotermans C, Peigneux P, de Noordhout AM, Moonen G, Maquet P (2006): Early boost and slow consolidation in motor skill learning. Learn Mem 13:580–583. [DOI] [PubMed] [Google Scholar]
- Hotermans C, Peigneux P, de Noordhout AM, Moonen G, Maquet P (2008): Repetitive transcranial magnetic stimulation over the primary motor cortex disrupts early boost but not delayed gains in performance in motor sequence learning. Eur J Neurosci 28:1216–1221. [DOI] [PubMed] [Google Scholar]
- Jacobs HIL, Dillen KNH, Risius O, Göreci Y, Onur OA, Fink GR, Kukolja J (2015): Consolidation in older adults depends upon competition between resting‐state networks. Front Aging Neurosci 6:344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerbi K, Lachaux J‐P, N'Diaye K, Pantazis D, Leahy RM, Garnero L, Baillet S (2007): Coherent neural representation of hand speed in humans revealed by MEG imaging. Proc Natl Acad Sci U S A 104:7676–7681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SR, Pritchett DL, Sikora MA, Stufflebeam SM, Hämäläinen M, Moore CI (2009): Quantitative analysis and biophysically realistic neural modeling of the MEG mu rhythm: Rhythmogenesis and modulation of sensory‐evoked responses. J Neurophysiol 102:3554–3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karni A, Meyer G, Jezzard P, Adams MM, Turner R, Ungerleider LG (1995): Functional MRI evidence for adult motor cortex plasticity during motor skill learning. Nature 377:155–158. [DOI] [PubMed] [Google Scholar]
- Kim JS, Chung CK (2007): Robust source analysis of oscillatory motor cortex activity with inherently variable phase delay. NeuroImage 37:518–529. [DOI] [PubMed] [Google Scholar]
- Kim YK, Shin SH (2014): Comparison of effects of transcranial magnetic stimulation on primary motor cortex and supplementary motor area in motor skill learning (randomized, cross over study). Front Hum Neurosci 8:937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King BR, Fogel SM, Albouy G, Doyon J (2013): Neural correlates of the age‐related changes in motor sequence learning and motor adaptation in older adults. Front Hum Neurosci 7:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehericy S, Bardinet E, Tremblay L, Van de Moortele P‐F, Pochon J‐B, Dormont D, Kim D‐S, Yelnik J, Ugurbil K (2005): Motor control in basal ganglia circuits using fMRI and brain atlas approaches. Cereb Cortex 16:149–161. [DOI] [PubMed] [Google Scholar]
- Liu TT (2013): Neurovascular factors in resting‐state functional MRI. NeuroImage 80:339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckhoo, H. , Brookes, M.J. , Heise, V. , Mackay, C.E. , Ebmeier K., Morris, P.G. , & Woolrich, M.W. (2012): Extracting resting state networks from Elekta Neuromag MEG data using independent component analysis. Poster at the 18th Annual Meeting of the Organization for Human Brain Mapping, Beijing, China.
- Ma L, Wang B, Narayana S, Hazeltine E, Chen X, Robin DA, Fox PT, Xiong J (2010): Changes in regional activity are accompanied with changes in inter‐regional connectivity during 4 weeks motor learning. Brain Res 1318:64–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Narayana S, Robin DA, Fox PT, Xiong J (2011): Changes occur in resting state network of motor system during 4 weeks of motor skill learning. NeuroImage 58:226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin T, Houck JM, Bish JP, Kičić D, Woodruff CC, Moses SN, Lee DC, Tesche CD (2006): MEG reveals different contributions of somatomotor cortex and cerebellum to simple reaction time after temporally structured cues. Hum Brain Mapp 27:552–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mary A, Bourguignon M, Wens V, Op de Beeck M, Leproult R, De Tiège X, Peigneux P (2015): Aging reduces experience‐induced sensorimotor plasticity. A magnetoencephalographic study. NeuroImage 104:59–68. [DOI] [PubMed] [Google Scholar]
- Mattis S (1976): Mental Status Examination For Organic Mental Syndrome In The Elderly Patient. Geriatric Psychiatry. New York: Grune and Stratton; pp 77–121. [Google Scholar]
- Muthuraman M, Hellriegel H, Hoogenboom N, Anwar AR, Mideksa KG, Krause H, Schnitzler A, Deuschl G, Raethjen J (2014): Beamformer source analysis and connectivity on concurrent EEG and MEG data during voluntary movements. PloS One 9:e91441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachev P, Kennard C, Husain M (2008): Functional role of the supplementary and pre‐supplementary motor areas. Nat Rev Neurosci 9:856–869. [DOI] [PubMed] [Google Scholar]
- Nettersheim A, Hallschmid M, Born J, Diekelmann S (2015): The role of sleep in motor sequence consolidation: Stabilization rather than enhancement. J Neurosci off J Soc Neurosci 35:6696–6702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell A, Rosenbloom PS (1981): Mechanisms of skill acquisition and the power law of practice In: Anderson JR. Cognitive Skills and Their Acquisition. Hillsdale, NJ: Lawrence Erlbaum; pp 1–55. [Google Scholar]
- Oldfield RC (1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9:97–113. [DOI] [PubMed] [Google Scholar]
- Orban P, Peigneux P, Lungu O, Albouy G, Breton E, Laberenne F, Benali H, Maquet P, Doyon J (2010): The multifaceted nature of the relationship between performance and brain activity in motor sequence learning. NeuroImage 49:694–702. [DOI] [PubMed] [Google Scholar]
- Pascual‐Marqui RD (2002): Standardized low‐resolution brain electromagnetic tomography (sLORETA): technical details. Methods Find Exp Clin Pharmacol 24 Suppl D:5–12. [PubMed] [Google Scholar]
- de Pasquale F. d, Penna SD, Snyder AZ, Lewis C, Mantini D, Marzetti L, Belardinelli P, Ciancetta L, Pizzella V, Romani GL, Corbetta M (2010): Temporal dynamics of spontaneous MEG activity in brain networks. Proc Natl Acad Sci 107:6040–6045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peigneux P, Orban P, Balteau E, Degueldre C, Luxen A, Laureys S, Maquet P (2006): Offline persistence of memory‐related cerebral activity during active wakefulness. PLoS Biol 4:e100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penhune VB, Doyon J (2005): Cerebellum and M1 interaction during early learning of timed motor sequences. NeuroImage 26:801–812. [DOI] [PubMed] [Google Scholar]
- Penhune VB, Steele CJ (2012): Parallel contributions of cerebellar, striatal and M1 mechanisms to motor sequence learning. Behav Brain Res 226:579–591. [DOI] [PubMed] [Google Scholar]
- Sala‐Llonch R, Bartrés‐Faz D, Junqué C (2015): Reorganization of brain networks in aging: A review of functional connectivity studies. Quant Psychol Meas 6:663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sami S, Robertson EM, Miall RC (2014): The time course of task‐specific memory consolidation effects in resting state networks. J Neurosci 34:3982–3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampaio‐Baptista C, Filippini N, Stagg CJ, Near J, Scholz J, Johansen‐Berg H (2015): Changes in functional connectivity and GABA levels with long‐term motor learning. NeuroImage 106:15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawaki L, Yaseen Z, Kopylev L, Cohen LG (2003): Age‐dependent changes in the ability to encode a novel elementary motor memory. Ann Neurol 53:521–524. [DOI] [PubMed] [Google Scholar]
- Schmitz R, Schabus M, Perrin F, Luxen A, Maquet P, Peigneux P (2009): Recurrent boosting effects of short inactivity delays on performance: An ERPs study. BMC Res Notes 2:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Birn RM, Boly M, Meier TB, Nair VA, Meyerand ME, Prabhakaran V (2014): Age‐related reorganizational changes in modularity and functional connectivity of human brain networks. Brain Connect 4:662–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer RMC, Gouw AM, Ivry RB (2007): Age‐related decline of sleep‐dependent consolidation. Learn Mem 14:480–484. [DOI] [PubMed] [Google Scholar]
- Stancak A, Alghamdi J, Nurmikko TJ (2011): Cortical activation changes during repeated laser stimulation: A magnetoencephalographic study. PloS One 6:e19744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallon‐Baudry C, Bertrand O, Delpuech C, Pernier J (1996): Stimulus specificity of phase‐locked and non‐phase‐locked 40 Hz visual responses in human. J Neurosci 16:4240–4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaki M, Huang T‐R, Yotsumoto Y, Hämäläinen M, Lin F‐H, Náñez JE, Watanabe T, Sasaki Y (2013): Enhanced spontaneous oscillations in the supplementary motor area are associated with sleep‐dependent offline learning of finger‐tapping motor‐sequence task. J Neurosci Off J Soc Neurosci 33:13894–13902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamás Kincses Z, Johansen‐Berg H, Tomassini V, Bosnell R, Matthews PM, Beckmann CF (2008): Model‐free characterization of brain functional networks for motor sequence learning using fMRI. NeuroImage 39:1950–1958. [DOI] [PubMed] [Google Scholar]
- Taulu S, Simola J, Kajola M (2005): Applications of the signal space separation method. IEEE Trans Signal Process 53:3359– 3372. [Google Scholar]
- Todd G, Kimber TE, Ridding MC, Semmler JG (2010): Reduced motor cortex plasticity following inhibitory rTMS in older adults. Clin Neurophysiol Off J Int Fed Clin Neurophysiol 121:441–447. [DOI] [PubMed] [Google Scholar]
- Vahdat S, Darainy M, Milner TE, Ostry DJ (2011): functionally specific changes in resting‐state sensorimotor networks after motor learning. J Neurosci 31:16907–16915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigário R, Särelä J, Jousmäki V, Hämäläinen M, Oja E (2000): Independent component approach to the analysis of EEG and MEG recordings. IEEE Trans Biomed Eng 47:589–593. [DOI] [PubMed] [Google Scholar]
- Wens V (2015): Investigating complex networks with inverse models: Analytical aspects of spatial leakage and connectivity estimation. Phys Rev E Stat Nonlin Soft Matter Phys 91:012823. [DOI] [PubMed] [Google Scholar]
- Wens V, Bourguignon M, Goldman S, Marty B, Op de Beeck M, Clumeck C, Mary A, Peigneux P, Van Bogaert P, Brookes MJ, De Tiège X (2014a): Inter‐ and intra‐subject variability of neuromagnetic resting state networks. Brain Topogr 27:620–634. [DOI] [PubMed] [Google Scholar]
- Wens V, Mary A, Bourguignon M, Goldman S, Marty B, Op de Beeck M, Van Bogaert P, Peigneux P, De Tiège X (2014b): About the electrophysiological basis of resting state networks. Clin Neurophysiol 125:1711–1713. [DOI] [PubMed] [Google Scholar]
- Wens V, Marty B, Mary A, Bourguignon M, Op de Beeck M, Goldman S, Van Bogaert P, Peigneux P, De Tiège X (2015): A geometric correction scheme for spatial leakage effects in MEG/EEG seed‐based functional connectivity mapping. Hum Brain Mapp 36:4604–4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JK, Baran B, Pace‐Schott EF, Ivry RB, Spencer RMC (2012): Sleep modulates word‐pair learning but not motor sequence learning in healthy older adults. Neurobiol Aging 33:991–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Srinivasan R, Kaur A, Cramer SC (2014): Resting‐state cortical connectivity predicts motor skill acquisition. NeuroImage 91:84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Figure 1
Supporting Figure 2
Supporting Figure 3
Supporting Figure 4
Supporting Figure 5
Supporting Figure 6
Supporting Figure 7
Supporting Figure 8
Supporting Information


