Abstract
Effective regulation of negative affective states has been associated with mental health. Impaired regulation of negative affect represents a risk factor for dysfunctional coping mechanisms such as drug use and thus could contribute to the initiation and development of problematic substance use. This study investigated behavioral and neural indices of emotion regulation in regular marijuana users (n = 23) and demographically matched nonusing controls (n = 20) by means of an fMRI cognitive emotion regulation (reappraisal) paradigm. Relative to nonusing controls, marijuana users demonstrated increased neural activity in a bilateral frontal network comprising precentral, middle cingulate, and supplementary motor regions during reappraisal of negative affect (P < 0.05, FWE) and impaired emotion regulation success on the behavioral level (P < 0.05). Amygdala‐focused analyses further revealed impaired amygdala downregulation in the context of decreased amygdala–dorsolateral prefrontal cortex functional connectivity (P < 0.05, FWE) during reappraisal in marijuana users relative to controls. Together, the present findings could reflect an unsuccessful attempt of compensatory recruitment of additional neural resources in the context of disrupted amygdala–prefrontal interaction during volitional emotion regulation in marijuana users. As such, impaired volitional regulation of negative affect might represent a consequence of, or risk factor for, regular marijuana use. Hum Brain Mapp 38:4270–4279, 2017. © 2017 Wiley Periodicals, Inc.
Keywords: reappraisal, negative affect, marijuana use, prefrontal cortex, amygdala
INTRODUCTION
Emotion regulation allows individuals to modify their emotional experience and produce appropriate responses to environmental demands, including negative and distressing events [Gross and Munoz, 1995]. It is vital for successful everyday functioning and personal well‐being [Gross and John, 2003], and mental health [Min et al., 2013]. Efficient regulation of negative emotions is characterized by downregulation of activity in emotion processing regions, including the amygdala, in response to negative stimuli by prefrontal regulatory networks [Ochsner and Gross, 2005; Etkin et al., 2015]. Within these regulatory circuits, the anterior cingulate cortex (ACC) and adjacent ventromedial prefrontal cortex (vmPFC) are critically engaged in automatic control of emotions, whereas the lateral (lPFC) and dorsomedial (dmPFC) prefrontal cortex are engaged in their volitional control [Etkin et al., 2015; Wilcox et al., 2016].
Impaired regulation of stress and negative emotional states represents a risk factor for the initiation of problematic drug use [Quinn and Fromme, 2010] and the development of drug dependence [Cheetham et al., 2010], and individuals with substance use disorders demonstrate lower self‐reported emotion regulation success [Fox et al., 2007] and decreased prefrontal–amygdala coupling during reappraisal of negative emotions [Albein‐Urios et al., 2014] in comparison to healthy controls.
Worldwide, marijuana is among the most frequently used drug of potential abuse with estimated 9–25% of users developing problematic patterns of use [SAMHSA, 2010]. Marijuana's effects are predominately mediated by the two endocannabinoid CB(1) and CB(2) receptors, with the psychoactive effects being mainly dependent on the central CB(1) receptor. The CB(1) receptor has particularly high densities in striatal, hippocampal, amygdalar, and frontal regions [Mackie, 2008], which are vital for several functional domains, including emotional experience and emotion regulation [Etkin et al., 2015].
Previous studies in regular marijuana users revealed altered neural processing in key nodes of this circuitry and impaired performance in associated functions, including hippocampal learning (e.g. Becker et al. [2010]). Furthermore, studies have reported altered emotional experience and associated neural activity in regular marijuana users in the amygdala [Gruber et al., 2009], and frontal nodes critically engaged in emotion regulation, such as the medial prefrontal [Wesley et al., 2015] and orbitofrontal [Filbey et al., 2016] cortex. Moreover, regular marijuana users show impaired automatic emotion regulation in the domains of inhibitory and behavioral control [Battisti et al., 2010]. Despite inconsistent findings concerning behavioral indices (for intact behavioral inhibition performance in marijuana users, see, e.g., Smith et al. [2014]), studies applying neuroimaging methods consistently observed increased activity [Roberts and Garavan, 2010] and disrupted connectivity in the regulatory frontal networks [Filbey and Yezhuvath, 2013].
However, despite the fact that regular marijuana users often report gaining control of negative emotions as primary motivational drive to use marijuana [Simons et al., 2000], effects of regular marijuana use on the volitional cognitive regulation of negative emotions remain unclear. Against this background, this study examined the efficacy of cognitive emotion regulation and the integrity of the underlying neural networks in regular marijuana users by means of a validated reappraisal functional magnetic resonance imaging (fMRI) paradigm using the regulation strategy of distancing.
Based on previous findings on deficient automatic regulation of emotions [Battisti et al., 2010] in the context of frontal hyperactivity [Roberts and Garavan, 2010], we expected deficient reappraisal success and concomitantly increased frontal activity in regular marijuana users compared to matched nonusing controls. Moreover, based on a previous report on decreased prefrontal–amygdala coupling during volitional emotion regulation in substance users [Albein‐Urios et al., 2012], and the role of the dorsolateral prefrontal cortex (dlPFC) and (pre‐)supplementary motor area (pre‐SMA) in volitional control of emotions [Etkin et al., 2015], we expected decreased functional connectivity of these regions with the amygdala in the group of marijuana users.
MATERIALS AND METHODS
Subjects
Given that previous studies reported sex‐ and menstrual cycle‐dependent effects on emotion processing and regulation [Ricarte‐Trives et al., 2016], this study focused on male marijuana users only. In total, 23 regular recreational marijuana users (mean age ± SD = 21.24 ± 2.59) and 20 nonusing healthy control subjects (mean age ± SD = 21.10 ± 3.61) were recruited via advertisements.
Inclusion criteria for all participants were right‐handedness and age 18–40 years. Exclusion criteria for all participants included (1) history of psychiatric disorder according to DSM‐IV criteria (assessed using the Mini‐International Neuropsychiatric Interview (M.I.N.I.), Sheehan et al. [1998]), (2) regular or current use of psychoactive or cardiovascular medication, (3) >20 cigarettes per day, (4) positive urine screen for the substances cocaine, methamphetamine, amphetamine, or methadone (Drug‐Screen‐Multi 7TF, nal von minden GmbH, Moers, Germany), or (5) breath alcohol level >0.00‰ (assessed by breath sample using TM‐7500, Trendmedic, Penzberg, Germany). Additional inclusion criteria for marijuana users comprise marijuana use on at least 3 days per week (M = 5.74 days per week, SD = 1.35) during the previous 12 months (M = 50.46 months of regular use, SD = 32.57) and use on >200 lifetime occasions. Marijuana users were excluded if they reported having used other illicit substances on >50 lifetime occasions or during the 28 days prior to the experiment. To control for confounding acute marijuana effects, users had to remain abstinent during the 48 h prior to the fMRI experiment. Additional exclusion criteria for controls were use of any illicit substance including marijuana >10 lifetime occasions or during the 28 days prior to the experiment, or a positive urine screen for THC on the day of the fMRI experiment. Marijuana, nicotine, and alcohol use parameters were assessed using a validated structured interview based on self‐reported use [Becker et al., 2010]. To control for confounding effects of impulsivity, depression, anxiety, current mood, attention, and general intelligence on emotion regulation the Barratt Impulsiveness Scale (BIS‐11, Patton et al. [1995]), Beck's Depression Inventory (BDI‐II, Beck et al. [1996]), Positive and Negative Affect Schedule (PANAS, Watson et al. [1988]), State‐Trait Anxiety Inventory (STAI, Spielberger et al. [1983]), “Wortschatztest” (WST, Metzler and Schmidt [1992]), and d2 Test of Attention (“Aufmerksamkeits‐ und Belastungstest d2”, Brickenkamp [2002]) were administered to all participants prior to functional imaging.
Experimental Paradigm
Behavioral and neural indices of emotion regulation were assessed by means of a modified version of an evaluated event‐related cognitive reappraisal fMRI paradigm [Silvers et al., 2015a).
Briefly, the paradigm incorporated 30 neutral (valence mean ± SD: 5.41 ± 1.41, arousal mean ± SD: 3.54 ± 1.96) and 60 negative (30 “Spontan”: valence = 2.48 ± 1.53, arousal = 5.78 ± 2.16; 30 “Distanz”: valence = 2.46 ± 1.56, arousal = 5.59 ± 2.22) pictures from the International Affective Picture System (IAPS, National Institute of Mental Health Center for Emotion and Attention, University of Florida) database. Images of moderately negative valence were selected to avoid a ceiling effect and increase the sensitivity to capture effects of marijuana use on emotion regulation.
Each trial started with a 5–7 s (jittered) interval showing a fixation cross, followed by a 2 s display of the instruction (either “Spontan” (eng. spontaneous) (for negative and neutral images) or “Distanz” (eng. distance) (for negative images only), followed by a 2–4 s jittered interstimulus interval (ISI) and an 8 s presentation of the picture, during which participants were asked to either passively look at the picture (“Spontan”) or actively regulate their emotions by distancing (“Distanz”), depending on the preceding instruction. Each picture was followed by a 2–4 s jittered ISI and a subsequent rating scale for negative affect from 0 to 4. On this scale, 0 represents no negative affect, 2 a moderate negative affect, and 4 a strong negative affect. To decrease movement‐related artifacts, the paradigm was divided into two subsequent runs during the fMRI (45 stimuli per run). Valence and arousal of images were matched for the two negative conditions and between runs. Image presentation was randomized between subjects. The paradigm was presented using Presentation 14.9 (Neurobehavioral Systems Inc., Albany, CA, USA) and projected using a mirror‐system.
Prior to the task, participants were instructed how to respond during the paradigm. For the spontaneous condition, they were told to react naturally to the presented image without trying to regulate their emotions in any way. For the reappraisal condition, they were instructed to reduce their negative affect by distancing themselves from the presented image, for example, to take a step back from the image and to view the scene as an uninvolved bystander. After this instruction, participants were asked to describe the strategy in their own words and to give examples of how to implement it.
To control for potential influences of marijuana craving, the German version of the marijuana‐craving screening (CCS‐7) (range: “no craving” = 7 to “very strong craving” = 49) was administered pre‐ and post‐fMRI (Mean ± SD: CCS‐7 pre = 22.22 ± 8.00, CCS‐7 post = 23.91 ± 7.12, t (23) = 1.76, P = 0.09).
Behavioral Data Analysis
Behavioral data were analyzed using SPSS Statistics Version 22.0 (IBM Corp., Armonk, NY, USA). An initial analysis focused on examining emotion induction by the paradigm and potential group differences by means of a repeated measures ANOVA with emotion ratings per CONDITION (“spontaneous_negative”/“distance”/“neutral”) as dependent variable and GROUP (users vs controls) as between subject factor. Post‐hoc pairwise comparisons were corrected for multiple comparisons with Bonferroni corrections. To test our a priori hypothesis on reduced emotion regulation success in marijuana users, a planned contrast was used. In line with previous studies [Silvers et al., 2015b), emotion regulation success (reappraisal success) was defined as the mean decrease of the negative affect rating for reappraisal (“distance”) trials relative to ratings for emotional reactivity (“spontaneous_negative”). Between‐group differences in this planned comparison were evaluated by means of an independent t test.
To examine potential confounders, between‐group differences in the control variables were examined using independent t tests. A paired t test was used to compare CCS‐7 scores pre‐ and post‐fMRI. To infer whether marijuana craving was associated with effective emotion regulation, the mean CCS‐7 score across both time points (due to no significant difference before and after fMRI) was entered into a bivariate correlation analysis with reappraisal success. Effects at P < 0.05 (two‐tailed) were considered statistically significant for behavioral emotion regulation indices and questionnaire data.
MRI Data Acquisition
Data were acquired on a Siemens Trio 3T MRI system (Siemens, Erlangen, Germany). Functional data were acquired using a T2* echo‐planar imaging (EPI) BOLD sequence (repetition time (TR) = 2500 ms, echo time (TE) = 30 ms, 37 slices, voxel size = 2.0 × 2.0 × 3.0 mm3, flip angle = 90°, field of view = 192 mm). To exclude subjects with apparent brain pathologies and facilitate normalization of the functional data, a high‐resolution T1‐weighted structural image was acquired in addition (TR = 1660 ms, TE = 2540 ms in 208 slices, field of view = 256 mm, voxel size = 0.8 × 0.8 × 0.8 mm3).
MRI Data Preprocessing
MRI data were processed using Statistical Parametric Mapping 12 (SPM 12, Wellcome Department of Imaging Neuroscience, University College London, UK) implemented in Matlab (MathWorks, Natick, MA, USA). The first five volumes of each subject and each session were discarded to allow for T1 equilibration. Subsequently, functional images were realigned to correct for head motion and registered to the T1 image. For normalization, a two‐step procedure was applied. Normalization parameters were first determined by segmenting the T1 image using the default tissue probability maps as priors. Next, normalization parameters were applied to normalize the functional images to the standard anatomical Montreal Neurological Institute (MNI) space resampled at 2.0 × 2.0 × 2.0 mm3. Normalized time‐series were smoothed with an 8 mm FWHM Gaussian kernel. Two control subjects were excluded from further fMRI analysis due to excessive head movement (>3 mm or >3°) or technical failure. This resulted in a sample size of 23 marijuana users and 18 control subjects for the fMRI analysis.
First‐Level Analysis
The first‐level matrix included session‐specific separate regressors for the cue presentation, the image presentation, and the rating period for each of the three conditions. Per session, this resulted in nine task regressors which were convolved with the hemodynamic response function plus 6 movement regressors (realignment parameters). Results were assessed using a general linear model (GLM) approach as implemented in SPM 12.
Given that previous studies reported altered neural activity in marijuana users during visual processing of neutral and negatively valenced visual stimuli [Wesley et al., 2015; Schwitzer et al., 2015], and that the main aim of this study was to determine alterations at the interface of emotional and cognitive processing the contrast “distance > baseline” was defined as main contrast of interest. In addition, the contrasts “neutral > baseline” and “spontaneous_negative > baseline” were specifically assessed to evaluate altered basic visual processing (“neutral > baseline”) and emotional reactivity to negative stimuli (“spontaneous_negative > baseline”).
Second‐Level Analysis
Group differences were assessed by entering the main single‐subject contrasts reflecting brain activation associated with basic processing of neutral images (“neutral > baseline”), emotional reactivity (“spontaneous_negative > baseline”), and emotion regulation (“distance > baseline”) into nonparametric independent t test permutation analyses (Statistical Non‐Parametric Mapping (SnPM 13), University of Warwick, UK, http://warwick.ac.uk/snpm). The number of permutations was set to 10,000. Pseudo t statistic was used incorporating variance smoothing with an 8 mm FWHM Gaussian kernel. The cluster‐forming threshold was T = 3.0902, and statistical significance was determined via cluster‐level inference at P FWE < 0.05. To specifically explore whether the decrease in amygdala activation upon reappraisal (“distance > baseline”) in marijuana users differed significantly from control subjects, we extracted parameter estimates from structurally defined masks of the left and right amygdala (MNI template Automated Anatomical Labeling implemented in the WFU PickAtlas). Estimates were entered into an independent t test and differences considered significant at P < 0.05 (two‐tailed).
Connectivity Analysis
Given the importance of the amygdala–frontal connectivity for effective emotion regulation [Etkin et al., 2015; Wilcox et al., 2016], between‐group differences in amygdala connectivity during “distance > baseline” were examined using structurally defined seed regions for the amygdala (MNI template Automated Anatomical Labeling implemented in the WFU PickAtlas) and a generalized form of context dependent psychophysiological interactions (gPPI, McLaren et al. [2012]). For this analysis, the same task regressors as specified for the BOLD level analysis were incorporated in the first level model. Between‐group differences were examined using an SPM independent t test for the contrasts “spontaneous_negative > baseline” and “distance > baseline.” Based on the importance of the dorsolateral PFC (dlPFC) and the pre‐SMA for explicit regulation of emotions [Etkin et al., 2015], the analysis focused on these regions of interest (ROIs). ROIs were anatomically defined using WFU PickAtlas (dlPFC: Brodmann areas 9/46; pre‐SMA: Brodmann area 6) and differences considered significant at P FWE < 0.05 (peak‐level inference) adjusted to the size of the ROIs.
Brain Behavior Associations
To further explore associations between the neural indices with marijuana use parameters and negative affect ratings, parameter estimates were extracted from 6 mm spheres centered at the maximum t value of between‐group differences from the BOLD level and connectivity analysis using MarsBaR. Additionally, these neural indices were correlated with the mean CCS‐7 score before and after the MRI scan to investigate potential effects of craving on neural activity. Associations were examined using bivariate correlational analysis and considered significant at P < 0.05.
RESULTS
Subject Characteristics
Control subjects and marijuana users were comparable in age, years of education, general intelligence, impulsiveness, anxiety, current mood, attention, nicotine, and alcohol use (all P > 0.05, see Table 1).
Table 1.
Group characteristics and drug use parameters
| Measure | Marijuana users, M (SD) | Controls, M (SD) | P |
|---|---|---|---|
| Age | 21.24 (2.59) | 21.10 (3.61) | 0.88 |
| Years of education | 14.26 (1.84) | 15.38 (2.54) | 0.10 |
| WST | 29.34 (3.46) | 31.30 (2.99) | 0.06 |
| D2 | 183.96 (36.36) | 199.30 (53.05) | 0.27 |
| BIS‐11 | 65.74 (15.18) | 65.55 (11.50) | 0.96 |
| BDI‐II | 5.09 (4.27) | 4.05 (2.69) | 0.36 |
| PANAS positive | 32.04 (5.13) | 31.80 (4.66) | 0.87 |
| PANAS mood | 11.70 (1.80) | 12.05 (1.91) | 0.53 |
| STAI state | 33.17 (4.78) | 34.75 (4.92) | 0.29 |
| Age of first nicotine use |
N = 17 16.28 (2.14) |
N = 18 16.53 (1.81) |
0.70 |
| Years of nicotine use | 3.90 (3.12) | 4.05 (2.18) | 0.86 |
| Cigarettes per day | 7.38 (6.73) | 6.53 (5.18) | 0.65 |
|
Fagerström score Time since last cigarette (hours) |
1.94 (1.71) 12.53 (21.35) |
1.50 (2.09) 16.53 (21.64) |
0.50 0.58 |
| Age of first alcohol intake | 14.46 (3.58) | 15.70 (0.99) | 0.14 |
| Alcohol units per week | 9.24 (6.97) | 13.09 (14.44) | 0.26 |
|
Number of participants with past ecstasy use Lifetime occasions of ecstasy use |
11 7.45 (7.12) |
‐ | |
|
Number of participants with past cocaine use Lifetime occasions of cocaine use |
7 2.14 (1.07) |
‐ | |
|
Number of participants with past amphetamine use Lifetime occasions of amphetamine use |
10 3.75 (4.32) |
‐ | |
|
Number of participants with past hallucinogen use Lifetime occasions of hallucinogen use |
11 1.45 (0.93) |
1 1 |
|
|
Number of participants with past sedative use Lifetime occasions of sedative use |
2 15.5 (20.51) |
‐ | |
|
Number of participants with past opiate use Lifetime occasions of opiate use |
1 1 |
‐ | |
|
Number of participants with past solvents use Lifetime occasions of solvents use |
6 2.67 (1.97) |
‐ | |
|
Number of participants with past marijuana use % Lifetime marijuana dependence |
23 3 |
15 ‐ |
Marijuana users reported an age of onset of marijuana use at (mean ± SD) 16 ± 2 years (range: 13–23), an intake of 4.00 ± 3.67 gram per week (0.5–15), a duration of regular use of 4.28 ± 2.79 years (1–13), a number of lifetime occasions of 1,233.22 ± 797.89 (360–3600), and 85.89 ± 43.45 h since last use (48–240).
Behavioral Results
A repeated‐measures ANOVA with group (marijuana users vs controls) and affect rating (rating for spontaneous_negative vs distance vs neutral) as dependent factor yielded a main effect of condition (F (2,41) = 153.57, P < 0.001, η 2 = 0.79), but no main effect of group (F (1,41) < 1, P = 0.774) and no significant interaction (F (2,41) = 1.37, P = 0.260). Pairwise post‐hoc comparisons revealed that negative affect was significantly greater during “spontaneous_negative” (mean ± SD = 1.72 ± 0.70) relative to “neutral” (0.17 ± 0.20) (P < 0.001). These values are comparable with previous literature on emotion regulation [Silvers et al., 2015b), confirming a successful induction of moderate negative affect. Compared to “spontaneous_negative,” negative affect decreased during “distance” (1.14 ± 0.66) (P < 0.001), confirming a control of negative emotions.
However, relative to controls (0.74 ± 0.50), reappraisal success was significantly lower in marijuana users (0.46 ± 0.38) (t (41) = −2.053, P = 0.047, d = 0.65) (Fig. 1), suggesting impaired regulation of negative affect.
Figure 1.
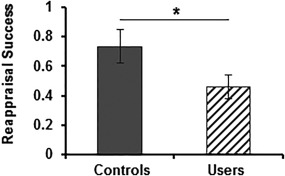
Difference in mean reappraisal success between controls and marijuana users. Relative to controls, marijuana users displayed impaired emotion regulation success during reappraisal of negative stimuli. Reappraisal success = mean arousal “spontaneous_negative” − mean arousal “distance.” Error bars indicate SEM. * significant at P < 0.05.
fMRI Results
A direct comparison of the groups did not reveal significant differences during the processing of neutral stimuli (“neutral”), arguing against general processing differences in marijuana users. In addition, there were no significant between‐group differences during processing of negative stimuli (“spontaneous_negative”) indicating intact emotional reactivity to negative stimuli in marijuana users. During cognitive regulation of emotions by means of distancing, however, marijuana users demonstrated higher activity compared to nonusing controls in a bilateral network spanning the bilateral precentral gyrus (40/−14/36, k = 430, P FWE = 0.026; −58/−2/24, k = 579, P FWE = 0.017), the right superior frontal gyrus (26/−8/70, k = 430, P FWE = 0.026), the left mid‐cingulate/SMA (−12/−22/40, k = 639, P FWE = 0.015), and the left precentral gyrus (−46/−14/56, k = 579, P FWE = 0.017) (Fig. 2a,b). Moreover, a direct comparison of parameter estimates extracted from the amygdala revealed that marijuana users showed a significantly higher right amygdala activity than control subjects (t (39) = 2.219, P = 0.032) during reappraisal, indicating that modulatory effects of the prefrontal cortex over the amygdala may be inefficient in marijuana users.
Figure 2.
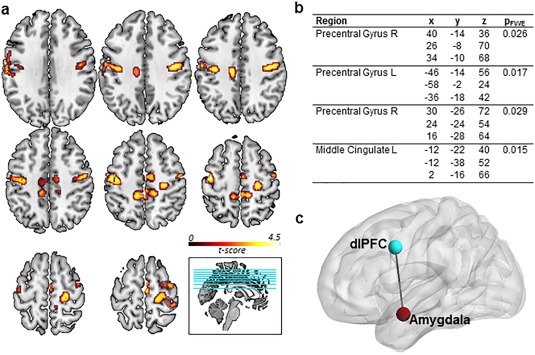
Neural differences between controls and marijuana users. (a) Greater fMRI activation during reappraisal in marijuana users relative to controls. P FWE < 0.05, cluster level inference. (b) Peak coordinates of greater activation during reappraisal in marijuana users relative to controls. (c) Regions of weaker connectivity during reappraisal of negative stimuli in marijuana users relative to controls. P FWE < 0.05.
Connectivity Results
Analysis of functional connectivity showed that marijuana users displayed decreased left amygdala–left dlPFC (−38/6/38, P FWE = 0.048) coupling during cognitive reappraisal relative to nonusing controls (Fig. 2c), suggesting impaired prefrontal–limbic communication during downregulation of negative affect.
Brain Behavior Associations
Our correlation analysis revealed no associations between neural indices related to emotion regulation and ratings of negative affect or reappraisal success. Moreover, parameters of marijuana use were not correlated with neural activity or ratings of negative affect (all P > 0.05).
Influence of Craving on Emotion Regulation
Exploring effects of craving on successful emotion regulation revealed that craving before and after the fMRI task were comparable (pre mean ± SD = 22.22 ± 7.98; post = 23.91 ± 7.12, t (22) = −1.76, P = 0.093). However, mean craving over both time points (mean ± SD = 23.07 ± 7.20) correlated with reappraisal success (r = −0.459, P = 0.028) (Fig. 3). Reappraisal success decreased with stronger craving, possibly reflecting an impact of craving‐associated distress on emotion regulation capacity. However, there were no significant associations between neural indices related to emotion regulation and craving (all P > 0.05).
Figure 3.
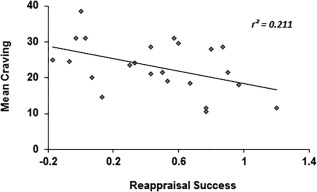
Association between craving and reappraisal success in marijuana users. Successful emotion regulation of negative stimuli decreases with higher subjective craving. (x) Reappraisal success = mean arousal “spontaneous_negative” − mean arousal “distance.” (y) CCS‐7 score. r = −0.459, P = 0.028.
DISCUSSION
In line with the previously proposed crucial role of deficient top–down control of emotions in the development and maintenance of substance use disorders [Wilcox et al., 2016], this study demonstrated impaired cognitive emotion regulation success in regular marijuana users with a distancing paradigm. On the neural level, regular marijuana users demonstrated altered neural activation and functional connectivity during reappraisal of negative stimuli. Marijuana users exhibited a hyperactive neural response during distancing in the bilateral precentral gyrus, SMA, and the middle cingulate cortex (MCC) compared to controls. Amygdala‐focused analyses further revealed increased amygdala activity and decreased amygdala–dlPFC coupling during distancing in marijuana users relative to controls, possibly associated with the inefficient reduction of negative affect. Together, these findings demonstrate that regular marijuana use is associated with inefficient volitional control of negative affect accompanied by increased activation in lateral and medial prefrontal regions and compromised fronto–limbic communication during cognitive reappraisal.
The neural network implicated in emotion regulation largely overlaps with fronto‐ and parietal‐cortical networks of cognitive control [Cole and Schneider, 2007]. Marijuana users displayed hyperactivity in core neural nodes of this regulatory circuitry and impaired volitional control of negative affect. More specifically, the SMA and MCC have been proposed as key regions of emotion–cognition integration [Shackman et al., 2011] and have been suggested as central executive components of ventrolateral PFC‐initiated regulatory control within the emotion regulation networks [Kohn et al., 2014]. In relation to this model, the present findings might imply that volitional emotion regulation is initiated appropriately in the ventrolateral PFC [Kohn et al., 2014] in marijuana users, with hyperactivity in the core executive modules such as the SMA and the MCC reflecting an increased, potentially compensatory, effort to exert control.
Previous imaging studies have also observed heightened task‐dependent activation in marijuana users [Roberts and Garavan, 2010] in the context of intact task performance, indicating that additional resources may be recruited to maintain cognitive functioning despite marijuana‐associated alterations. However, in this study, increased prefrontal activity was accompanied by impaired emotion regulation success and associated amygdala downregulation. This suggests that these compensatory mechanisms fail at the interface of cognition and emotion, such as regulating the amygdala to volitionally control negative affect. Despite previous reports on both difficulties in emotion recognition [Bayrakci et al., 2015] and a decreased amygdala reactivity to emotional stimuli [Gruber et al, 2009], this study did not find changes in emotional reactivity per se in marijuana users, possibly related to differences in abstinence and addiction status compared to participants in previous studies [Bayrakci et al., 2015].
Increased prefrontal activity during reappraisal in users was accompanied by weaker functional coupling between the dlPFC and the amygdala relative to controls. Functional connectivity between the vmPFC/dlPFC and the amygdala has been associated with emotion regulation [Erk et al., 2010]. Specifically, stronger amygdala–prefrontal connectivity has been linked to higher reappraisal success and a greater ability to downregulate negative effect [Banks et al., 2007]. Successful volitional control of emotions therefore relies on intact communication between prefrontal regions and the amygdala. Decreased emotion regulation‐associated dlPFC–amygdala coupling in the present sample of marijuana users may therefore be at the core of the deficiency in regulating negative affect. In line with a dense localization of CB(1) receptors in the fronto–limbic neural circuitry [Eggan and Lewis, 2007], acute THC administration reduces dlPFC–amygdala coupling during volitional regulation of emotions [Gorka et al., 2016], emphasizing the relevance of the CB(1) system for this functional domain and suggesting that alterations in the cannabinoid system due to regular marijuana use may specifically affect this regulatory system. However, downregulation of CB(1) receptor availability upon regular marijuana exposure [Hirvonen et al., 2012] normalizes after 2 days [D'Souza et al., 2016]. Together with previous functional imaging studies implicating the serotonergic system in volitional control of emotions [Firk et al., 2013], this indicates that lasting functional alterations may more likely be related to downstream effects of long‐term CB(1) stimulation on other transmitter systems, such as serotonergic [Hill et al., 2006] neurotransmission.
We are the first to report reduced volitional regulation success of emotions alongside alterations at the interface of cognition and emotion in cognitive control brain circuits in regular marijuana users. The decrease of negative affect [Simons et al., 2000] and coping with stressors [Bonn‐Miller et al., 2007] have been reported as primary motivations for smoking marijuana, in line with findings linking stress and high negative affect to the initiation and escalation of substance use [Sinha, 2008]. Together, this suggests that the emotion regulation deficits may have preceded the onset of regular marijuana use. However, this cross‐sectional study design did not allow us to address this question directly.
Endocannabinoid signaling has been found to be involved in stress regulation [Volkow et al., 2016] and acute effects of cannabinoids such as THC include anxiolytic effects [Phan et al., 2008]. Marijuana use may thus downregulate negative affect. Therefore, substance use can become an emotion regulation tactic and marijuana use might represent a self‐medication to cope with lower emotion regulation efficacy. A lower capacity in effectively controlling and regulating emotions has been observed in various stages of substance use disorders (SUD) [Cheetham et al., 2010], highlighting the role of inefficient emotion regulation related to cognitive control deficits in substance use and abuse, including marijuana use.
Finally, although the emotion regulation task itself did not increase craving in the marijuana users, we found that higher task‐independent craving was associated with less efficient regulation of negative affect on the behavioral level. Emotional discomfort has been shown to interfere with deploying cognitive resources relevant for emotion regulation [Baumeister and Heatherton, 1996], suggesting that cognitive control mechanisms in the marijuana users may be challenged by craving‐related distress through interference with executive capacities for regulation, and the potential compensatory mechanism observed in marijuana users, thus diminishing the ability of effective regulation.
The findings from this study need to be considered in the context of some limitations. First, the behavioral and neural indices for assessing emotion regulation do not fully align. While reappraisal success was defined as the difference in ratings between spontaneous viewing of negative scenes and distancing, neural correlates of reappraisal were demonstrated using condition‐specific contrasts. The rationale to focus on condition‐specific contrasts was based on previous reports on altered visual processing of neutral and negatively valenced visual stimuli (see, e.g., Gruber et al. [2009], Schwitzer et al. [2015], and Wesley et al. [2015]) in marijuana users. Thus, group differences in the contrast between spontaneous viewing and regulation might not allow to clearly separate alterations in emotional reactivity and emotion regulation. Although both behavioral and neural alterations suggest emotion regulation alterations in marijuana users, the corresponding analyses might assess slightly different facets of emotion regulation. With regard to the design and participants of the present study some additional limitations need to considered. Most participants in this study were regular cigarette smokers. Although the groups did not differ in several tobacco use‐associated control variables and previous studies did not find strong evidence for distinct brain structural effects of marijuana and tobacco co‐use compared to marijuana alone [Wetherill et al., 2015, Filbey et al., 2015], we cannot completely rule out complex interaction effects between tobacco and marijuana use on the present findings. Moreover, the retrospective design of this study does not allow to disentangle whether deficient emotion regulation represents a predisposing risk factor for the development of regular marijuana use, or whether regular marijuana exposure disrupts the regulation of negative affect. Given that this study focused on male marijuana users, the present results cannot be generalized to female marijuana users. Finally, as cannabis users in this study reported a mean abstinence of 86 h, it remains unclear whether the observed alterations normalize during prolonged abstinence.
In summary, the present findings provide the first evidence of impaired volitional emotion regulation in marijuana users and associated prefrontal hyperactivity and reduced amygdala–dlPFC coupling. The results are in line with previous data suggesting compensatory recruitment of prefrontal neural resources to overcome marijuana‐associated alterations during cognitive processing. The results also demonstrate that the compensatory mechanism fails at the interface of emotion and cognition. The lower emotion regulation success was additionally accompanied by decreased amygdala–dlPFC coupling, a pathway critical to the implementation of successful regulation of negative affect. Taken together, these results suggest that deficient emotion regulation in regular marijuana users related to disrupted regulatory control circuits might present a consequence of marijuana use, or predispose individuals to consume marijuana to cope with negative affective states.
ACKNOWLEDGMENTS
The authors thank Paul Jung and Laura Schinabeck for their excellent technical support.
REFERENCES
- Albein‐Urios N, Verdejo‐Román J, Asensio S, Soriano‐Mas C, Martínez‐González JM, Verdejo‐García A (2014): Re‐appraisal of negative emotions in cocaine dependence: Dysfunctional corticolimbic activation and connectivity. Addict Biol 19:415–426. [DOI] [PubMed] [Google Scholar]
- Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Phan KL (2007): Amygdala‐frontal connectivity during emotion regulation. Soc Cogn Affect Neurosci 2:303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battisti RA, Roodenrys S, Johnstine SJ, Pesa N, Hermens DF, Solowij N (2010): Chronic cannabis users show altered neuropsychological functioning on Stroop task conflict resolution. Psychopharmacology (Berl) 212:613–624. [DOI] [PubMed] [Google Scholar]
- Baumeister RF, Heatherton TF (1996): Self‐regulation failure: An overview. Psychol Inquiry 7:1–15. [Google Scholar]
- Bayrakci A, Sert E, Zorlu N, Erol A, Sarıçiçek A, Mete L (2015): Facial emotion recognition deficits in abstinent cannabis dependent patients. Compr Psychiatry 58:160–164. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK (1996): Beck Depression Inventory‐II. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Becker B, Wagner D, Gouzoulis‐Mayfrank E, Spuentrup E, Daumann J (2010): Altered parahippocampal functioning in cannabis users is related to the frequency of use. Psychopharmacology (Berl) 209:361–374. [DOI] [PubMed] [Google Scholar]
- Bonn‐Miller MO, Zvolensky MJ, Bernstein A (2007): Marijuana use motives: Concurrent relations to frequency of past 30‐day use and anxiety sensitivity among young adult marijuana smokers. Addict Behav 32:49–62. [DOI] [PubMed] [Google Scholar]
- Brickenkamp R (2002): d2 Aufmerksamkeits‐Belastungstest. Hogrefe: Göttingen. [Google Scholar]
- Cheetham A, Allen NB, Yücel M, Lubman DI (2010): The role of affective dysregulation in drug addiction. Clin Psychol Rev 30:621–634. [DOI] [PubMed] [Google Scholar]
- Cole MW, Schneider W (2007): The cognitive control network: Integrated cortical regions with dissociable functions. Neuroimage 37:343–360. [DOI] [PubMed] [Google Scholar]
- D'Souza DC, Cortes‐Briones JA, Ranganathan M, Thurnauer H, Creatura G, Surti T, Planeta B, Neumeister A, Pittman B, Normandin M, Kapinos M, Ropchan J, Huang Y, Carson RE, Skosnik PD (2016): Rapid changes in CB1 receptor availability in cannabis dependent males after abstinence from cannabis. Biol Psychiatry Cogn Neurosci Neuroimag 1:60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggan SM, Lewis DA (2007): Immunocytochemical distribution of the cannabinoid CB1 receptor in the primate cortex: A regional and laminar analysis. Cereb Cortex 17:175–191. [DOI] [PubMed] [Google Scholar]
- Erk S, Mikschl A, Stier S, Ciaramidaro A, Gapp V, Weber B, Walter H (2010): Acute and sustained effects of cognitive emotion regulation in major depression. J Neurosci 30:15726–15734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Büchel C, Gross JJ (2015): The neural bases of emotion regulation. Nat Rev Neurosci 16:603–700. [DOI] [PubMed] [Google Scholar]
- Filbey F, Yezhuvath U (2013): Functional connectivity in inhibitory control networks and severity of cannabis use disorder. Am J Drug Alcohol Abuse 39:382–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey FM, McQueeny T, Kadamangudi S, Bice C, Ketcherside A (2015): Combined effects of marijuana and nicotine on memory performance and hippocampal volume. Behav Brain Res 293:46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey FM, Dunlop J, Ketcherside A, Baine J, Rhinehardt T, Kuhn B, DeWitt S, Alvi T (2016): fMRI study of neural sensitization to hedonic stimuli in long‐term, daily cannabis users. Hum Brain Mapp 37:3431–3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firk C, Siep N, Markus CR (2013): Serotonin transporter genotype modulates cognitive reappraisal of negative emotions: A functional magnetic resonance imaging study. Soc Cogn Affect Neurosci 8:247–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Axelrod SR, Paliwal P, Sleeper J, Sinha R (2007): Difficulties in emotion regulation and impulse control during cocaine abstinence. Drug Alcohol Depend 89:298–301. [DOI] [PubMed] [Google Scholar]
- Gorka SM, Phan KL, Lyons M, Mori S, Angstadt M, Rabinak CA (2016): Cannabinoid modulation of frontolimbic activation and connectivity during volitional regulation of negative affect. Neuropsychopharmacology 41:1888–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ, Muñoz RF (1995): Emotion regulation and mental health. Clin Psychol Sci Pract 2:151–164. [Google Scholar]
- Gross JJ, John OP (2003): Individual differences in two emotion regulation processes: Implications for affect, relationships, and well‐being. J Pers Soc Psychol 85:348–362. [DOI] [PubMed] [Google Scholar]
- Gruber SA, Rogowska J, Yurgelun‐Todd DA (2009): Altered affective response in marijuana smokers: An FMRI study. Drug Alcohol Depend 105:139–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Sun JC, Tse MT, Gorzalka BB (2006): Altered responsiveness of serotonin receptor subtypes following long‐term cannabinoid treatment. Int J Neuropsychopharmacol 9:277–286. [DOI] [PubMed] [Google Scholar]
- Hirvonen J, Goodwin RS, Li CT, Terry GE, Zoghbi SS, Morse C, Pike VW, Volkow ND, Huestis MA, Innis RB (2012): Reversible and regionally selective downregulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Mol Psychiatry 17:642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn N, Eickhoff SB, Scheller M, Laird AR, Fox PT, Habel U (2014): Neural network of cognitive emotion regulation – an ALE meta‐analysis and MACM analysis. Neuroimage 87:345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie K (2008): Cannabinoid receptors: Where they are and what they do. J Neuroendocrinol 20:10–14. [DOI] [PubMed] [Google Scholar]
- McLaren DG, Ries ML, Xu G, Johnson SC (2012): A generalized form of context‐dependent psychophysiological interactions (gPPI): A comparison to standard approaches. Neuroimage 61:1277–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzler P, Schmidt KH (1992): Wortschatztest (WST). Weinheim: Beltz Test GmbH. [Google Scholar]
- Min JA, Yu JJ, Lee CU, Chae JH (2013): Cognitive emotion regulation strategies contributing to resilience in patients with depression and/or anxiety disorders. Compr Psychiatry 54:1190–1197. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ (2005): The cognitive control of emotion. Trends Cogn Sci 9:242–249. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES (1995): Factor structure of the Barratt Impulsiveness scale. J Clin Psychol 51:768–764. [DOI] [PubMed] [Google Scholar]
- Phan KL, Angstadt M, Golden J, Onyewuenyi, Popovska A, de Wit H (2008): Cannabinoid modulation of amygdala reactivity to social signals of threat in humans. J Neurosci 28:2313–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn PD, Fromme K (2010): Self‐regulation as a protective factor against risky drinking and sexual behavior. Psychol Addict Behav 24:376–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricarte‐Trives JJ, Navarro Bravo B, Latorre Postigo JM, Ros Segura L, Watkins E (2016): Age and gender differences in emotion regulation strategies: Autobiographical memory, rumination, problem solving and distraction. Span J Psychol 19:E43. [DOI] [PubMed] [Google Scholar]
- Roberts GM, Garavan H (2010): Evidence of increased activation underlying cognitive control in ecstasy and cannabis users. Neuroimage 52:429–435. [DOI] [PubMed] [Google Scholar]
- Schwitzer T, Schwan R, Angioi‐Duprez K, Ingster‐Moati I, Lalanne L, Giersch A, Laprevote V (2015): The cannabinoid system and visual processing: A review on experimental findings and clinical presumptions. Eur Neuropsychopharmacol 25:100–112. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ (2011): The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci 12:154–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Harnett Sheehan K, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC (1998): The Mini‐International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM‐IV and ICD‐10. J Clin Psychiatry 20:22–33. [PubMed] [Google Scholar]
- Silvers JA, Wager TD, Weber J, Ochsner KN (2015a): The neural bases of uninstructed negative emotion modulation. Soc Cogn Affect Neurosci 10:10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers JA, Weber J, Wager TD, Ochsner KN (2015b): Bad and worse: Neural systems underlying reappraisal of high and low intensity negative emotions. Soc Cogn Affect Neurosci 10:172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons J, Correia CJ, Carey KB (2000): A comparison of motives for marijuana and alcohol use among experienced users. Addict Behav 25:153–160. [DOI] [PubMed] [Google Scholar]
- Sinha R (2008): Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci 1141:105–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JL, Mattick RP, Jamadar SD, Iredale JM (2014): Deficits in behavioural inhibition in substance abuse and addiction: A meta‐analysis. Drug Alcohol Depend 145:1–33. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA (1983): Manual for the State‐Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA) (2010): Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings. http://archive.samhsa.gov/data/NSDUH/2k11Results/NSDUHresults2011.htm
- Volkow ND, Hampson AJ, Baler R (2017): Don't worry, be happy: Endocannabinoids and cannabis at the intersection of stress and reward. Annu Rev Pharmacol Toxicol 57:285–308. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A (1988): Development and validation of brief measures of positive and negative affect: The PANAS scales. J Pers Soc Psychol 54:1063. [DOI] [PubMed] [Google Scholar]
- Wesley MJ, Lile JA, Hanlon CA, Porrino LJ (2015): Abnormal medial prefrontal cortex activity in heavy cannabis users during conscious emotional evaluation. Psychopharmacology (Berl) 233:1035–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill RR, Jagannathan K, Hager N, Childress AR, Rao H, Franklin TR (2015): Cannabis, cigarettes, and their co‐occuring use: Disentangling differences in gray matter volume. Int J Neuropsychopharmacol 18:pyv061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox CE, Pommy JM, Adinoff B (2016): Neural circuitry of impaired emotion regulation in substance use disorders. Am J Psychiatry 173:344–361. [DOI] [PMC free article] [PubMed] [Google Scholar]


