Abstract
Spontaneous thinking, an action to produce, consider, integrate, and reason through mental representations, is central to our daily experience and has been suggested to serve crucial adaptive purposes. Such thinking occurs among other experiences during mind wandering that is associated with activation of the default mode network among other brain circuitries. Whether and how such brain activation is linked to the experience of spontaneous thinking per se remains poorly known. We studied 51 healthy subjects using a comprehensive experience‐sampling paradigm during 3T functional magnetic resonance imaging. In comparison with fixation, the experiences of spontaneous thinking and spontaneous perception were related to activation of wide‐spread brain circuitries, including the cortical midline structures, the anterior cingulate cortex and the visual cortex. In direct comparison of the spontaneous thinking versus spontaneous perception, activation was observed in the anterior dorsomedial prefrontal cortex. Modality congruence of spontaneous‐experience‐related brain activation was suggested by several findings, including association of the lingual gyrus with visual in comparison with non‐verbal–non‐visual thinking. In the context of current literature, these findings suggest that the cortical midline structures are involved in the integrative core substrate of spontaneous thinking that is coupled with other brain systems depending on the characteristics of thinking. Furthermore, involvement of the anterior dorsomedial prefrontal cortex suggests the control of high‐order abstract functions to characterize spontaneous thinking per se. Hum Brain Mapp 38:3277–3288, 2017. © 2017 Wiley Periodicals, Inc.
Keywords: brain, default mode network, cortical midline structures, experience sampling, functional magnetic resonance imaging, thought, human
INTRODUCTION
“Cogito, ergo sum” (I think, therefore I am)
[Descartes]
As suggested by Descartes, spontaneous thinking is central to our daily experience, and human civilization would be difficult to imagine without ability of spontaneous thinking. Spontaneous thought has been defined as “a mental state, or a sequence of mental states, that arises relatively freely due to an absence of strong constraints on the contents of each state and on the transitions from one mental state to another” [Christoff et al., 2016]. Such definition includes process of spontaneous experience of percepts and feelings together with alteration between these experiences in addition to the experience of spontaneous thinking per se [Smallwood, 2013]. We focus here on the periods of time that are experienced as thinking (rather than perception or feeling). Although such thinking per se lacks clear‐cut scientific definition, several lines of reasoning point to characteristics of such an experience. According to Piaget, thinking operates on different modes and levels of abstraction that evolve during development [Piaget, 1951]. In line, Vygotsky saw thinking as partially separate phenomenon from inner speech that may operate on non‐verbal representations as well [Vygotsky, 1986]. Compared with perception, thinking is less coupled with the stimulus environment and more with personal goals and experience of agency. Spontaneous thought has been suggested to serve crucial adaptive functions including problem solving, creative insight, planning the future, and maintaining one's identity [Fox et al., 2013; Stawarczyk et al., 2011a], and these functions may be especially relevant for the experience of thinking per se. For example, subjects with more spontaneous future thinking develop more concrete goals during follow‐up [Medea et al., 2016]. Based on these considerations and general use of the word “thinking” in dictionaries and encyclopedias, we use here a working definition of spontaneous thinking per se as a spontaneous action to produce, consider, integrate, and reason through mental representations.
Neuronal correlates of the experience of spontaneous thinking per se remain poorly known. Brain functioning has been assessed during periods that fit the broad definition of spontaneous thought—that is, periods of not‐attending‐to‐any‐on‐demand task [Christoff et al., 2016; Fox et al., 2015]. As these periods include alteration of experiences of various perceptions and feelings with those of thinking per se [Heavey and Hurlburt, 2008; Stawarczyk et al., 2011a], brain correlates of spontaneous thinking per se nor of any other distinct spontaneous experience cannot be inferred from such brain functioning.
Spontaneous experiences are common during periods of resting state, and when compared with different on‐demand tasks, these periods are associated with the activation of the default mode network (DMN), including the precuneus‐posterior cingulate cortex, the lateral parieto‐temporal regions, and the medial prefrontal cortex [Raichle, 2015]. While such activation has been suggested to reflect intrinsic organization of the brain functioning [Raichle, 2015], it has also been shown to correlate with the experience of mind wandering [Fox et al., 2015; Mason et al., 2007]. In addition to the DMN, a recent meta‐analysis showed mind‐wandering‐related activation in several other brain regions, including the rostrolateral prefrontal cortex, the dorsal anterior cingulate cortex, the insula, the temporopolar cortex, the secondary somatosensory cortex, and the lingual gyrus [Fox et al., 2015]. Furthermore the experience of beginning of a spontaneous experiences, indicated by a button press, was related to activation of the medial temporal cortices [Ellamil et al., 2016].
In addition to thinking per se, mind‐wandering‐related brain activations may be related to different characteristics of thoughts and percepts. We aimed therefore to link the brain functioning to the common spontaneous percepts and dimensions of thinking. In particular, we aimed to test whether modality‐specific activation is associated with the experienced modality of thinking. While one study suggested association between language‐related regions and the experience of spontaneous inner speech [Hurlburt et al., 2016], and valence during mind wandering has been associated with activity in the emotion‐related medial orbitofrontal cortex [Tusche et al., 2014], little is known about the brain correlates of distinct spontaneous experiences. We used experience sampling during fMRI to study how the brain activation during mind wandering is coupled with such experiences, with the focus on the experience of spontaneous thinking per se.
METHODS AND MATERIALS
Participants
The ethics committee of the Helsinki and Uusimaa Hospital District approved the study, and each participant provided written informed consent before participation. We recruited 51 healthy participants aged 21–41 years (15 women; mean age for all participants 31 years) by word of mouth or as participants as healthy control subjects in a population‐based psychosis study [Mäntylä et al., 2015]. In total, 32 subjects underwent two 15‐min fMRI sessions, while 19 subjects completed a single session. We discarded one session each for four subjects who participated in two sessions due to head movement greater than 3 mm.
Experience Sampling
We asked participants to focus on the fixation cross during fMRI. They were told that, while the task was to attend to the fixation cross, other percepts and thoughts may capture one's attention. They practiced using the answer tool, and were introduced to the categories of experiences provided before imaging.
Participants were asked to answer to the experience probes according to where their attention had been primarily focused during the several seconds prior to questioning. The experience samples were collected at random intervals between 12.0 and 40.5 s during imaging by using an answer tree that was based on the non‐imaging experience sampling findings [Heavey and Hurlburt, 2008; Stawarczyk et al., 2011a], general knowledge about common experiences in the scanner, and pilot studies (Fig. 1). Answering options were presented with Presentation software, reflected to a semitransparent screen. Subject had two buttons, one operated with the right and the other with the left thumb. By pressing the right hand button, the answering option next on the right side was highlighted and vice versa. Answer was registered after the selection remained unchanged for 2 s. Spontaneous thoughts were categorized according to those related and unrelated to the ongoing experiment after Stawarczyk et al. [2011a] and rated for valence and arousal. Thoughts unrelated to the experiment were divided into visual, verbal, and non‐visual–non‐verbal thought modalities [Heavey and Hurlburt, 2008].
Figure 1.
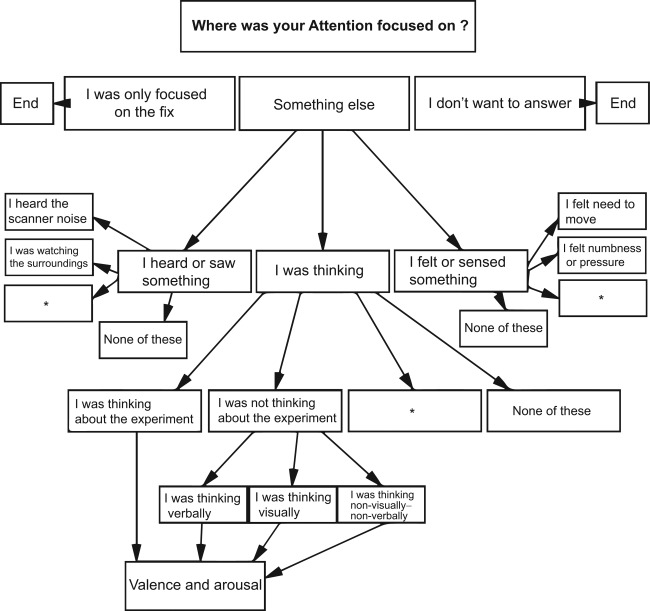
Answer tree. Response options in the boxes were followed by further options as illustrated by the arrows. Asterisks represent options for psychotic experiences, which are a part of an on‐going patient study. Valence and arousal ratings were prompted for all thoughts on a continuous visual‐analog scale ranging from 0 to 100 (VAS).
Imaging
For each session, 600 functional images were acquired using a Siemens Magnetom Skyra 3‐T system (Siemens AG, Erlangen, Germany) with a 30‐channel head coil at the Aalto University Advanced Magnetic Imaging Centre. The parameters for the gradient‐echo echo‐planar imaging sequence to acquire a blood oxygenation level‐dependent signal were as follows: TR, 1,500 ms; TE, 30 ms; flip angle, 75°; field of view, 24 cm; base resolution 64 × 64; and 36 slices resulting in a voxel size of 3.75 × 3.75 × 4 mm. The anatomical T1‐weighted images were acquired with a 1 × 1 × 1‐mm voxel size.
Analysis
Functional images for each participant were realigned to the first volume by linear rotation and translation, corrected for the slice timing, co‐registered to the anatomical image, normalized to a common Montreal Neurological Institute (MNI) space with 3 × 3 × 3 mm voxel size by using nonlinear transformation computed from the anatomical images [Ashburner, 2007], and smoothed with an 8‐mm FWHM Gaussian kernel. We analyzed the images using SPM12 (http://www.fil.ion.ucl.ac.uk/spm/software/spm12/), building a general linear model with a default high‐pass filter (cut off 128 s) and correcting for autocorrelation. Answering periods were modeled with boxcar functions convolved with the hemodynamic delay. We modeled the experience‐related brain activation without hemodynamic delay during three 1.5‐s whole‐head images: two images preceding the response and the first image during responding were included to avoid interference from the response‐related brain activation. Assuming a 5‐s hemodynamic delay, this model best captures the neuronal activity 3.5–8 s before the onset of responding. While the experience during this time window is likely to strongly correlate with the response about the current experience [Vanhaudenhuyse et al., 2011], such a model avoids any temporal overlap with the response period unlike a model with a hemodynamic delay. The functional images preceding the distinct non‐fixation answers were compared separately to those preceding fixation answers for each individual. In addition, visual, verbal, and non‐visual–non‐verbal thinking were compared pair‐wise. All thinking categories and all perception categories were pooled together and compared with fixation and to each other. The resulting individual contrast images were entered into the group‐level one‐sample t‐tests for those experiences that occurred in at least 20 subjects. The individual averages of valence and arousal related to thoughts were correlated between subjects with the individual contrast images for “pooled thinking categories versus fixation.” Due to limited data, we included all subjects who reported one or more occasions of an experience. While this increases noise, increasing number of subjects is considered to increase the statistical power more than increasing the number of time points [Friston et al., 2002].
Statistical Methods
We used two statistical thresholds to provide a comprehensive picture of our data. The first threshold comprised at least 10 contiguous voxels exceeding the voxel‐wise P < 0.005 in SPM parametric tests, as suggested by Lieberman and Cunningham [2009] to avoid too many false negative findings. The second threshold consisted of P < 0.05 familywise error (FWE) corrected for multiple comparisons within the entire brain volume based on a permutation test in SnPM extension of SPM (http://warwick.ac.uk/snpm) [Nichols and Holmes, 2002], as permutation test has been suggested to outperform parametric tests in correction for multiple comparisons [Eklund et al., 2016]. We computed 5,000 permutations [Nichols and Holmes, 2002] and considered corrected statistics both at the voxel‐level and at the cluster‐level with primary threshold of P < 0.01. Only the voxel‐level findings are reported, however, due to widespread activation and thus poor localization of the cluster‐level findings.
In addition to the analysis in the entire brain volume, activation related to the experience of scanner noise was corrected for multiple comparisons in the volume of the bilateral auditory cortex to validate the method. The auditory cortex was defined from an automated meta‐analysis of 228 studies that included the key phrase “auditory cortex” [Yarkoni et al., 2011]. Due to a strong a priori assumption of the thinking‐related activation in the cortical midline structures, the activation was corrected for familywise error in these regions, whenever no whole‐brain‐corrected activation was observed. The regions of interest were modeled as spheres with 5‐mm radius, centered at the activation likelihood maxima in the meta‐analysis by Fox et al. on mind wandering [Fox et al., 2015] (x, y, z = 3, 61, 13 for the medial prefrontal cortex and −8, −56, 39 for the precuneus).
RESULTS
Figure 2 shows the spectrum of spontaneous experiences during fMRI. A mean of 98% of the 1,805 responses (mean 35.4, range 18–54 per subject) fit the defined categories. All 51 subjects reported an experience of attending to the fixation cross (mean 15.0, range 1–45 per subject), and 50 subjects thought about the ongoing experiment (mean 4.5, range 1–19 per subject). Among those thoughts reported independent of the ongoing experiment, 40 subjects reported verbal thoughts (mean 4.3, range 1–11 per subject), 39 reported visual thoughts (mean 3.1, range 1–18 per subject), and 25 reported non‐verbal–non‐visual thoughts (mean 2.6, range 1–8 per subject). Among other spontaneous experiences, 35 subjects reported scanner noise (mean 3.1, range 1–9 per subject), 21 reported needing to move (mean 1.5, range 1–3 per subject), and 20 reported numbness or pressure (mean 2.0, range 1–4 per subject). The emotional valence (mean ± SD on a 0–100 visual‐analog scale) was 65 ± 19 for experiment‐related thoughts, and differed between modalities of thoughts independent of the ongoing experiment (verbal mean ± SD = 64 ± 17, visual = 75 ± 18, and non‐verbal–non‐visual = 64 ± 17, P = 0.011, one‐way ANOVA). Visual thoughts were related to more positive valence than both verbal and non‐visual–non‐verbal thoughts (Scheffe post‐hoc test P = 0.02 and P = 0.05, respectively). Arousal ratings (22–27 ± 17–22) did not differ between the categories of thinking.
Figure 2.
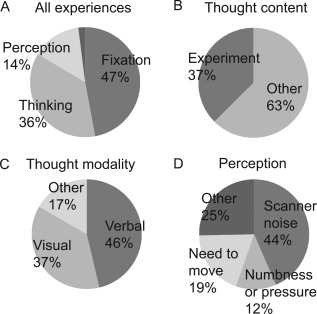
Experiences during scanning. (A) Proportions for major experience classes. The narrow section represents 2% of experiences not falling into any of the defined categories. Here, the category “perception” also refers to experience of feelings in order to aid clarity. (B) Proportions of thought contents (C) The modality of non‐experiment‐related thinking (D) Proportions of percepts. The category “other” included feelings, bodily sensations, and visual and auditory perceptions of the surroundings.
We compared the brain functioning preceding reports of all thoughts with fixation and perception to access brain correlates of the experience of thinking per se. To complement this analysis and to associate distinct characteristics of thinking to the brain functioning, we studied also association of the brain functioning with the thinking‐related modality, valence, and arousal. Figure 3A and Table 1 show that the brain activation preceding the reports of thoughts included bilaterally the precuneus‐posterior cingulate cortex, the medial prefrontal cortex‐anterior cingulate cortex, the visual cortex, the left inferior frontal gyrus and the inferior sensory‐motor cortices (liberal threshold P < 0.005 in more than 10 contiguous voxels). The dorsomedial prefrontal cortex and the lingual gyrus survived correction for multiple comparisons (Table 1). As controlling the comparison for age and sex had practically no influence on the findings and as all the comparisons were conducted within subject, we do not report controlled results. No activation was observed in the reverse contrast (fixation vs. thinking). Compared with the spontaneous perception, the spontaneous thinking associated with stronger activation of the dorsomedial prefrontal cortex (dmPFC; x, y, z = 3, 63, 15, P = 0.029, FWE‐corrected in the medial prefrontal region of interest; Fig. 4).
Figure 3.
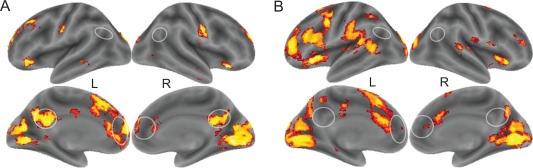
Brain activation related to spontaneous experience of thinking (A) and (B) perception (pooled activation across specific categories in comparison with fixation; thresholded at voxelwise P < 0.005). White circles indicate the main regions of the default mode network following an automated meta‐analysis of 516 studies that included the key phrase “default mode” (Yarkoni et al., 2011). [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 1.
Brain activation associated with common spontaneous experiences
| Experience | Region |
MNI coordinates (x, y, z) |
Minimum P | Extent (cm3) |
|---|---|---|---|---|
|
Thought n = 50 |
Bilateral occipital cortex and precuneus‐posterior cingulate cortex |
0, −63, −3 9, −66, 3 |
0.00003 0.036 |
55.5 |
| Left medial prefrontal and anterior cingulate gyrus |
–6, 60, 15 –9, 63, 21 |
0.00004 0.032 |
31.6 | |
| Right orbitofrontal cortex | 30, 21, −18 | 0.00002 | 1.6 | |
| Right lateral central sulcus | 45, −9, 27 | 0.0001 | 4.4 | |
| Right cerebellum |
21, −78, −36 12, −48, −45 |
0.0001 0.001 |
6.9 0.4 |
|
| Left inferior frontal gyrus | −39, 30, −12 | 0.0003 | 3.5 | |
| Left lateral central sulcus | −51, −12, 27 | 0.0003 | 1.2 | |
| Left middle temporal gyrus | −51, −36, −9 | 0.0006 | 0.4 | |
| Right inferior temporal cortex | 48, −15, −24 | 0.0007 | 0.5 | |
| Middle cingulate cortex | −6, −9, 30 | 0.002 | 0.4 | |
| Verbal thought n = 40 | Right lingual gyrus | 6, −66, 3 | 0.0003 | 1.7 |
| Right superior frontal gyrus | 21, 54, 27 | 0.0003 | 1.2 | |
| Right hippocampus | 27, −30, −3 | 0.0004 | 0.5 | |
| Right cerebellum | 21, −81, −42 | 0.0006 | 0.8 | |
| Left medial frontal gyrus | −9, −9, 66 | 0.0006 | 0.7 | |
| Left dorsal anterior cingulate cortex | −9, 12, 36 | 0.0007 | 0.3 | |
| Supplementary motor cortex | 0, 9, 57 | 0.002 | 0.4 | |
| Right cuneus | 15, −75, 9 | 0.002 | 0.3 | |
|
Visual thought n = 39 |
Left inferior frontal gyrus |
–54, 15, 0 –54, 15, 0 |
0.00002 0.02 |
3.3 |
| Bilateral occipital cortex and precuneus‐posterior cingulate cortex | 12, −72, 0 | 0.00005 | 8.8 | |
| Left anterior cingulate gyrus |
–6, 33, 12 –3, 18, 36 |
0.00005 0.04 |
4.3 | |
| Left medial frontal gyrus | −9, 45, −9 | 0.0001 | 6.9 | |
| Left lingual gyrus | −24, −51, −3 | 0.0001 | 1.6 | |
| Right middle temporal gyrus | −24, −51, −3 | 0.0002 | 3.9 | |
| Cerebellum |
15, −72, −36 –15, −90, −18 |
0.0002 0.001 |
44.0 | |
| Right precentral gyrus | 57, −12, 36 | 0.0002 | 0.6 | |
| Bilateral precuneus | −6, −69, 36 | 0.0003 | 3.1 | |
| Right inferior frontal gyrus | 30, 18, −21 | 0.0003 | 10.5 | |
| Right middle occipital gyrus | 36, −66, 6 | 0.0004 | 1.7 | |
| Left inferior parietal lobule | −45, −57, 45 | 0.0004 | 0.4 | |
| Left middle frontal gyrus |
–36, 27, 30 –36, 6, 45 –42, 12, 30 |
0.0005 0.002 0.003 |
3.3 | |
| Left middle temporal gyrus | −60, −27, −12 | 0.0007 | 2.8 | |
| Right insula | 39, 15, 9 | 0.001 | 0.5 | |
| Right superior frontal gyrus | 24, 54, 27 | 0.002 | 0.4 | |
| Right hippocampus | 21, −30, −6 | 0.002 | 1.0 | |
|
Non‐verbal–non‐visual thought |
Left precuneus | −9, −57, 24 | 0.0004 | 0.3 |
| n = 25 | Left medial frontal gyrus | −12, 63, 21 | 0.0007 | 0.8 |
|
Visual vs. non‐verbal–non‐visual thought n = 20 |
Left lingual gyrus | −24, −51, −9 | 0.0001 | 0.5 |
| Right middle temporal gyrus | 63, −30, −3 | 0.0002 | 0.5 | |
| Left inferior parietal lobe | −27, −42, 42 | 0.001 | 0.3 | |
|
Thought about the experiment n = 50 |
Left cuneus | −18, −93, 18 | 0.0001 | 0.4 |
| Left medial frontal gyrus | −3, 63, 21 | 0.0002 | 0.5 | |
| Right cerebellum | 9, −48, −42 | 0.002 | 0.6 | |
|
Thought‐related arousal n = 50 |
Left frontal operculum | −30, 33, 6 | 0.0001 | 1.3 |
| Left posterior temporal lobe | −42, −54, −6 | 0.0005 | 0.3 | |
| Right occipitotemporal cortex | 57, −69, −3 | 0.001 | 0.4 | |
| Right parietal cortex | 39, −54, 54 | 0.003 | 0.3 | |
|
Thought‐related valence n = 50 |
Right cerebellum | 18, −75, −36 | 0.001 | 0.7 |
|
Perception a n = 43 |
Left anterior insula and inferior frontal gyrus | –33, 27, −6 | 0.002 | 1.1 |
| Left occipital lobe and cerebellum |
–18, −93, 18 –12 −72 −12 |
0.004 0.04 |
0.5 0.03 |
|
| Left anterior cingulate cortex and supplementary motor area | –3, 15, 42 | 0.005 | 1.1 | |
| Right occipital lobe |
9, −69, 6 27, −90, 21 |
0.01 0.03 |
1.5 0.1 |
|
| Left superior frontal gyrus | –15, 9, 63 | 0.02 | 0.1 | |
| Right lingual gyrus | 12, −66, −9 | 0.02 | 0.1 | |
| Left lingual gyrus | –6, −84, 0 | 0.02 | 0.3 | |
| Right inferior frontal gyrus | 33, 27, −3 | 0.02 | 0.4 | |
| Left inferior frontal gyrus |
–48, 21, 24 –45, 18, −9 –39, 15, −3 |
0.03 0.04 0.04 |
0.2 0.03 0.03 |
|
| Left middle frontal gyrus |
–33, 51, 9 –39, 0, 39 |
0.03 0.04 |
0.2 0.1 |
|
| Left parahippocampal gyrus | –21, −27, −12 | 0.04 | 0.03 | |
|
Scanner noise n = 35 |
Left auditory cortex |
–54, −51, 5 –54, −51, 5 |
0.00001 0.006 b |
1.0 |
| Left inferior frontal gyrus | −48, 21, 27 | 0.0004 | 0.6 | |
| Left cingulate gyrus | −6, 24, 39 | 0.0004 | 0.3 | |
| Left putamen | −21, 15, −3 | 0.002 | 0.9 | |
| Occipital lobe | −12, −93, 12 | 0.002 | 4.2 | |
| Middle frontal gyrus | −24, 45, 12 | 0.002 | 1.7 | |
|
Need to move n = 20 |
Left anterior insula | −27, 24, −3 | 0.0003 | 0.3 |
| Bilateral cerebellum |
–12, −60, −42 9, −54, −30 21, −66, −51 |
0.0004 0.0005 0.0006 |
0.4 | |
| Left middle frontal gyrus |
–33, 45, 30 –27, 45, 12 |
0.0006 0.001 |
0.5 | |
| Left supplementary motor area | −15, 6, 66 | 0.0007 | 1.8 | |
| Left precuneus | −9, −75, 54 | 0.002 | 0.6 | |
| Occipital lobe |
–15, −66, 15 –12, −87, −3 |
0.002 0.002 |
1.5 | |
| Right inferior parietal cortex | 54, −18, 15 | 0.003 | 0.4 | |
|
Numbness or pressure n = 20 |
Left anterior insula | −30, 18, −9 | 0.0001 | 0.4 |
| Left supplementary motor area | −6, 24, 48 | 0.0003 | 0.6 | |
| Right inferior frontal gyrus | 51, 9, 9 | 0.0004 | 2.3 | |
| Left precuneus | −15, −66, 15 | 0.0004 | 0.4 | |
| Left anterior cingulate cortex | −9, 21, 21 | 0.0005 | 2.8 | |
| Left posterior insula | −39, −18, 6 | 0.0006 | 0.5 | |
| Left superior temporal gyrus | −60, −30, 9 | 0.0007 | 0.3 | |
| Right anterior insula | 36, 12, −12 | 0.0009 | 3.4 | |
| Left superior frontal gyrus | −24, −6, 63 | 0.001 | 1.3 | |
| Left inferior parietal cortex | −45, −30, 21 | 0.001 | 0.8 | |
| Left inferior frontal gyrus | −42, 54, 0 | 0.001 | 1.8 | |
| Right inferior parietal cortex | 66, −15, 24 | 0.002 | 3.3 |
With the exception of “visual vs. non–verbal–nonvisual thought,” all conditions are contrasted to fixation. Values in bold refer to corrected statistics from a permutation test, other values refer to parametric t‐test. Extent refers to the volume of the contiguous voxels (0.027 cm3 each) at P < 0.005, n = number of subjects in different contrasts.
Due to strong and widespread activation, only corrected voxel‐level P‐values are reported and extent refers to the volume of the contiguous voxels (0.027 cm3 each) at P < 0.05 corrected.
Corrected in the volume of the bilateral auditory cortex.
Figure 4.
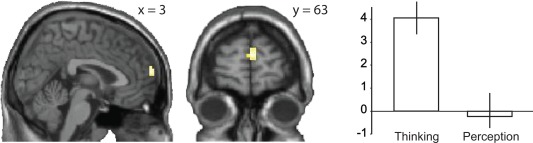
Brain activation in the contrast “spontaneous thinking versus spontaneous perception.” Thresholded at voxelwise P < 0.005. Bars on the right present parameter estimates (mean and SEM) in the contrasts thinking versus fixation and perception versus fixation. The eigenvariates of parameter estimates were extracted from whole the dorsomedial region of interest to avoid any circularity in the analysis. [Color figure can be viewed at http://wileyonlinelibrary.com]
Non‐visual–non‐verbal thinking was linked exclusively to the cortical midline structures. Activation related to the visual and non‐visual–non‐verbal thoughts overlapped in the posterior cingulate cortex and in the dmPFC (liberal threshold). Activation related to visual thought was stronger than activation related to the non‐visual–non‐verbal thought in the left lingual gyrus (liberal threshold; Fig. 5). Activation related to visual and verbal thoughts overlapped in the dorsal anterior cingulate cortex, in the left frontopolar cortex, and in the left lingual gyrus (liberal threshold). The strength of thought‐related brain activation correlated positively with the thought‐related valence in the right cerebellum and with thought‐related arousal in the left frontal operculum (liberal threshold; Table 1).
Figure 5.
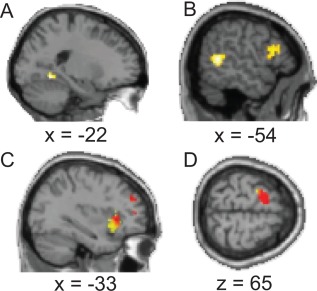
Modality‐congruent brain activation during spontaneous experience (P < 0.005). (A) Lingual gyrus activation in the contrast “visual thought versus non‐visual–non‐verbal thought” (B) Auditory cortex activation in the contrast “scanner noise versus fixation” (C) Activation of the left anterior insula in the contrast of experiencing numbness or pressure (yellow) and needing to move (red) versus fixation. The need to move was also associated with activation of the supplementary motor area (D). [Color figure can be viewed at http://wileyonlinelibrary.com]
We compared reports of the common spontaneous percepts, separately and pooled together, to fixation, to further access the relationship between the mind‐wandering‐related brain functioning and the current experience. Figure 3B and Table 1 show the wide‐spread brain activation in the spontaneous perception versus fixation contrast. No activation was observed in the contrast perception versus thinking. Activation of the left auditory cortex was related to the experience of scanner noise (corrected for multiple comparisons; Table 1 and Fig. 5). Activation of the left anterior insula was related to experiencing numbness or pressure as well as feeling need to move, and the need to move was also associated with activation of the supplementary motor area and the cerebellum (liberal threshold; Fig. 5 and Table 1).
DISCUSSION
We characterized brain functioning related to distinct experiences during mind‐wandering and found that in comparison with fixation, spontaneous thoughts and percepts were associated with widespread brain activation. Activation of the anterior dmPFC was stronger during spontaneous thinking than during spontaneous perception.
In accordance with an earlier study that simulated the scanning environment [Binder et al., 1999], our findings suggest that, during a simple fixation task, healthy subjects attend to the fixation cross less than half of the time. In agreement with a pioneering fMRI study among five subjects [Hurlburt et al., 2015], several experiences fell into different thoughts and percepts, similar to findings from non‐imaging experience samples [Heavey and Hurlburt, 2008]. The most prominent difference from the experience‐sampling findings in a natural environment [Heavey and Hurlburt, 2008] were the reported thoughts about the ongoing experiment, and the percepts surrounding scanner noise, numbness or pressure, and the need to move.
Compared with fixation, thinking in general, and visual and non‐verbal–non‐visual thinking in particular, were associated with activation of the precuneus‐posterior cingulate cortex and of the medial prefrontal cortex, corresponding to the cortical midline structures of the DMN. Furthermore, activation of the dmPFC in contrast to spontaneous perception suggests selectivity for the experience of thinking. Medial prefrontal cortex activation has already been associated with post‐scanning reports on the spontaneous experiences in an early brain imaging study [McGuire et al., 1996] and several subsequent studies have linked mind wandering to the activation of the precuneus‐posterior cingulate cortex and the medial prefrontal cortex among other regions [Fox et al., 2015]. Our findings further converge with a study by Stawarczyk et al. who found the cortical midline structures to be related to task‐ and stimulus‐independent experiences [Stawarczyk et al., 2011b], and with a recent finding of an association between the DMN and brain activation in the contrast spontaneous thought versus perception [Van Calster et al., 2017]. Furthermore, resting‐state connectivity of the posterior cingulate cortex [Smallwood et al., 2016] and the medial prefrontal cortex [Karapanagiotidis et al., 2016] has been shown to reflect contents of spontaneous thought. Our findings agree with and extend the literature linking spontaneous thought to the cortical midline structures of the DMN.
Several findings illustrate the potential functional roles of the DMN in spontaneous thinking. The DMN includes brain regions that have the most numerous connections to other parts of the brain [Van den Heuvel and Sporns, 2013]. Such hubs are able to integrate multiple modalities and representations, and have been suggested to support global integration for conscious experience [Vatansever et al., 2015]. Furthermore, the long distance, both anatomically and in functional connectivity measures, suggests these regions to be highly stimulus independent [Margulies et al., 2016]. In line, the DMN is involved in multiple high‐order abstract functions, including episodic and semantic memory, social cognition, goal‐directed working memory, reward‐bound decision making, planning the future, and construction of subjective meaning [Fox et al., 2015; Margulies et al., 2016; Stawarczyk and D'Argembeau, 2015]. Dorsomedial PFC in particular has been related to complex decision making, control of decision strategy [Venkatraman et al., 2009], delayed reward during decision making [Wang et al., 2014], intentional mind‐wandering [Golchert et al., 2016], and reflection on both self and others [Van der Meer et al., 2010]. Furthermore, the dmPFC is organized along posterior‐to‐anterior gradient of increasing abstraction [Venkatraman et al., 2009; Wang et al., 2014]. In this context, our findings suggest the anterior‐dmPFC‐related control of abstract cognition to characterize the spontaneous experience of thinking. Highly integrative nature of the dmPFC supports component process view that proposes complex mental states to depend on combination of multiple subprocesses [Andrews‐Hanna et al., 2014; Smallwood and Schooler, 2015].
In addition to the cortical midline hubs of the DMN, activation of multiple brain regions was more strongly related to the spontaneous thinking than to fixation. However, similar pattern of activation was observed in the contrast between spontaneous perception and fixation. Thus, these activations may stem from deactivation during fixation or processes that are common to mind‐wandering in general. Based on the literature, several of the observed regions maybe, however, of interest for further studies on spontaneous thinking. The anterior cingulate cortex has been associated with cognitive control and choice value in the context of internal models about the environment [Kolling et al., 2016], functions that agree well with the suggested role of spontaneous thinking in problem solving and planning the future [Fox et al., 2013; Medea et al., 2016; Stawarczyk et al., 2011a]. Association of the lingual gyrus with spontaneous cognition is supported by the activation during sleep‐state dreaming [Fox et al., 2013] and the finding that lesions to this region may inhibit dreaming and visual imagery [Bischof and Bassetti, 2004]. Thinking is often considered verbal, and in addition to the association with thinking in general in the present study, the left inferior frontal gyrus has been related to spontaneous inner speech [Hurlburt et al., 2016]. Furthermore, as subliminal or embodied vocalization maybe recruited during on‐demand inner speech [Oppenheim and Dell, 2010], present finding of the activation in the regions corresponding to the motor representation of vocalization during spontaneous thinking maybe of interest.
Some of our findings suggest that functionally congruent brain circuitries are recruited during spontaneous experiences. This was particularly clear in the auditory‐cortex activation related to experiencing scanner noise and visual cortex activation related to visual versus non‐verbal–non‐visual thinking. Furthermore, interoceptive experiences were related to the left anterior insula, previously associated with interoception [Craig, 2009]. The need to move associated with the motor system, and emotional arousal and valence related to the frontal operculum and the cerebellar region that have been linked to emotional processing [Kirby and Robinson, 2015]. There were discrepancies as well, including the visual cortex activation during verbal thought. Such discrepancies may stem from introspective errors, gaze differences between verbal thinking and fixation, or from the dynamics of intrinsic organization of the brain during mind‐wandering [Raichle, 2015]. Taken together, our findings together with the previous ones [Hurlburt et al., 2016; Tusche et al., 2014] suggest that despite its limitations [Krueger et al., 2014], introspection may capture distinct spontaneous experiences, even at an accuracy of some modalities of spontaneous thought.
A common limitation of experience sampling studies is that amount of data points during the periods of interest is relatively small resulting in compromised statistical power. We note that most of the present findings are based on a relatively liberal statistical threshold to balance between false negative and false positive findings [Lieberman and Cunningham, 2009]. The risk of false positives for some findings is thus larger than in studies that use more conservative thresholds (Note that several findings survived strict correction for multiple comparisons). In comparison, most voxel‐wise P‐values in this study are less than 0.001 (see Table 1), the threshold applied in some earlier experience‐sampling fMRI studies [Christoff et al., 2009; Shergill et al., 2000]. Especially the contrasts with a small number of subjects with few reports of the particular experience should be interpreted with caution.
Because all of the participants did not report all the experiences, number of the contrast images differs between the contrasts in Table 1, and one should avoid comparing the findings. We do not see this as a major limitation; however, as in any study with multiple statistical tests, results between tests should be compared with caution due to threshold effects. To compare different experiences, direct comparisons should be used, but this was limited in the present study by statistical power. While the contribution of most of the present findings is to inform about the brain activation that is associated with distinct spontaneous experiences beyond mind wandering in general, further studies are needed to test for specificity of these findings. More data per subject may be necessary to obtain sufficient statistical power. One could also collect experience samples first outside of the scanner to consider individual variability of the experience in the study design and analysis.
Thoughts can be classified in many ways, including but not limited to temporal dimension (past and future), and reference to self or others, in addition to the sensory modality (visual, verbal, non‐verbal–non‐visual). The last dimension was selected in the present study after Heavey and Hurlburt [2008], but in future studies, other dimensions need to be assessed to understand human spontaneous thinking per se and its neuronal substrates more thoroughly.
We asked about experiences during a time window of several seconds before the probe, and the reported experiences were linked to fMRI signal that reflects neuronal activation approximately 3.5–8 s before the probe. During such a long period of time, experience can alternate. Based on the experience sampling data, Vanhaudenhuyse et al. computed the frequency of spontaneous alteration between the internal and external focus of attention to be on average 0.05 Hz (range 0.1–0.01 Hz) [Vanhaudenhuyse et al., 2011]. Assuming the periods of internal focus to be related to the experience of continuous thinking per se, the experiences reported in the present study are likely to be coupled with the fMRI measures.
While the validity of the present findings obtained in an imaging environment may be questioned in a natural setting, our data suggest that it is possible to differentiate between thoughts and percepts related to the ongoing experiment from other experiences which more likely share similarities with those occurring in a natural setting. For the interpretation of the brain imaging findings, it is essential to understand the brain correlates of spontaneous experiences that frequently occur in an imaging environment. Our findings show that while fixation is a common comparison condition in brain imaging studies, brain activation depends strongly on the common spontaneous thoughts and percepts during the fixation task.
In conclusion, our findings propose that, while the DMN is too narrow a view, its midline hubs—the anterior dmPFC in particular—are involved in neuronal substrates of spontaneous thinking per se. Other circuitries may then be recruited depending on the characteristics of thinking. Selective involvement of the anterior dmPFC suggests that control for high‐order abstract functions characterize the spontaneous thinking per se.
ACKNOWLEDGMENTS
We thank Riitta Hari, Marita Kattelus, Teemu Mäntylä, Eva Rikandi, Jaana Suvisaari, and Antti Takalahti for their support, expert help, and advice. Authors report no conflict of interest.
REFERENCES
- Andrews‐Hanna JR, Smallwood J, Spreng RN (2014): The default network and self‐generated thought: Component processes, dynamic control, and clinical relevance. Ann N Y Acad Sci 1316:29–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J (2007): A fast diffeomorphic image registration algorithm. Neuroimage 38:95–113. [DOI] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Bellgowan PS, Rao SM, Cox RW (1999): Conceptual processing during the conscious resting state. A functional MRI study. J Cogn Neurosci 11:80–95. [DOI] [PubMed] [Google Scholar]
- Bischof M, Bassetti CL (2004): Total dream loss: A distinct neuropsychological dysfunction after bilateral PCA stroke. Ann Neurol 56:583–586. [DOI] [PubMed] [Google Scholar]
- Christoff K, Gordon AM, Smallwood J, Smith R, Schooler JW (2009): Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proc Natl Acad Sci U S A 106:8719–8724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoff K, Irving ZC, Fox KC, Spreng RN, Andrews‐Hanna JR (2016): Mind‐wandering as spontaneous thought: A dynamic framework. Nat Rev Neurosci 17:718–731. [DOI] [PubMed] [Google Scholar]
- Craig AD (2009): How do you feel—now? The anterior insula and human awareness. Nat Rev Neurosci 10:59–70. [DOI] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, Knutsson H (2016): Cluster failure: Why fMRI inferences for spatial extent have inflated false‐positive rates. Proc Natl Acad Sci U S A 113:7900–7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- M Ellamil, KC Fox, ML Dixon, S Pritchard, RM Todd, E Thompson, K Christoff (2016): Dynamics of neural recruitment surrounding the spontaneous arising of thoughts in experienced mindfulness practitioners. Neuroimage 136:186–196. [DOI] [PubMed] [Google Scholar]
- Fox KC, Nijeboer S, Solomonova E, Domhoff GW, Christoff K (2013): Dreaming as mind wandering: Evidence from functional neuroimaging and first‐person content reports. Front Hum Neurosci 7:412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox KC, Spreng RN, Ellamil M, Andrews‐Hanna JR, Christoff K (2015): The wandering brain: Meta‐analysis of functional neuroimaging studies of mind‐wandering and related spontaneous thought processes. Neuroimage 111:611–621. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Glaser DE, Henson RN, Kiebel S, Phillips C, Ashburner J (2002): Classical and Bayesian inference in neuroimaging: Applications. Neuroimage 16:484–512. [DOI] [PubMed] [Google Scholar]
- Golchert J, Smallwood J, Jefferies E, Seli P, Huntenburg JM, Liem F, Lauckner ME, Oligschlager S, Bernhardt BC, Villringer A, Margulies DS (2016): Individual variation in intentionality in the mind‐wandering state is reflected in the integration of the default‐mode, fronto‐parietal, and limbic networks. Neuroimage 146:226–235. [DOI] [PubMed] [Google Scholar]
- Heavey CL, Hurlburt RT (2008): The phenomena of inner experience. Cons Cogn 17:798–810. [DOI] [PubMed] [Google Scholar]
- Hurlburt RT, Alderson‐Day B, Fernyhough C, Kuhn S (2015): What goes on in the resting‐state? A qualitative glimpse into resting‐state experience in the scanner. Front Psychol 6:1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlburt RT, Alderson‐Day B, Kuhn S, Fernyhough C (2016): Exploring the ecological validity of thinking on demand: Neural correlates of elicited vs. spontaneously occurring inner speech. PloS One 11:e0147932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karapanagiotidis T, Bernhardt BC, Jefferies E, Smallwood J (2016): Tracking thoughts: Exploring the neural architecture of mental time travel during mind‐wandering. Neuroimage 147:272–281. [DOI] [PubMed] [Google Scholar]
- Kirby LA, Robinson JL (2015): Affective mapping: An activation likelihood estimation (ALE) meta‐analysis. Brain Cogn pii:S0278. [DOI] [PubMed] [Google Scholar]
- N Kolling, T Behrens, MK Wittmann, M Rushworth (2016): Multiple signals in anterior cingulate cortex. Curr Opin Neurobiol 37:36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger J, Bernini M, Wilkinson S (2014): Introspection, isolation, and construction: Mentality as activity. Commentary on Hurlburt, Heavey & Kelsey (2013). “Toward a phenomenology of inner speaking”. Cons Cogn 25:9–10. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Cunningham WA (2009): Type I and Type II error concerns in fMRI research: Re‐balancing the scale. Soc Cogn Affect Neurosci 4:423–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies DS, Ghosh SS, Goulas A, Falkiewicz M, Huntenburg JM, Langs G, Bezgin G, Eickhoff SB, Castellanos FX, Petrides M, Jefferies E, Smallwood J (2016): Situating the default‐mode network along a principal gradient of macroscale cortical organization. Proc Natl Acad Sci U S A 113:12574–12579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN (2007): Wandering minds: The default network and stimulus‐independent thought. Science 315:393–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire PK, Paulesu E, Frackowiak RS, Frith CD (1996): Brain activity during stimulus independent thought. Neuroreport 7:2095–2099. [PubMed] [Google Scholar]
- Medea B, Karapanagiotidis T, Konishi M, Ottaviani C, Margulies D, Bernasconi A, Bernasconi N, Bernhardt BC, Jefferies E, Smallwood J (2016): How do we decide what to do? Resting‐state connectivity patterns and components of self‐generated thought linked to the development of more concrete personal goals. Exp Brain Res 16:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäntylä T, Mantere O, Raij TT, Kieseppä T, Laitinen H, Leiviskä J, Torniainen M, Tuominen L, Vaarala O, Suvisaari J (2015): Altered activation of innate immunity associates with white matter volume and diffusion in first‐episode psychosis. PloS One 10:e0125112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP (2002): Nonparametric permutation tests for functional neuroimaging: A primer with examples. Hum Brain Mapp 15:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim GM, Dell GS (2010): Motor movement matters: The flexible abstractness of inner speech. Mem Cognit 38:1147–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piaget J (1951): Psychology of Intelligence. London: Routledge and Kegan Paul. [Google Scholar]
- Raichle ME (2015): The brain's default mode network. Ann Rev Neurosci 38:433–447. [DOI] [PubMed] [Google Scholar]
- Shergill SS, Brammer MJ, Williams SC, Murray RM, McGuire PK (2000): Mapping auditory hallucinations in schizophrenia using functional magnetic resonance imaging. Arch Gen Psychiatry 57:1033–1038. [DOI] [PubMed] [Google Scholar]
- Smallwood J (2013): Distinguishing how from why the mind wanders: A process‐occurrence framework for self‐generated mental activity. Psychol Bull 139:519–535. [DOI] [PubMed] [Google Scholar]
- Smallwood J, Schooler JW (2015): The science of mind wandering: Empirically navigating the stream of consciousness. Ann Rev Psychol 66:487–518. [DOI] [PubMed] [Google Scholar]
- Smallwood J, Karapanagiotidis T, Ruby F, Medea B, de Caso I, Konishi M, Wang HT, Hallam G, Margulies DS, Jefferies E (2016): Representing representation: Integration between the temporal lobe and the posterior cingulate influences the content and form of spontaneous thought. PloS One 11:e0152272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stawarczyk D, D'Argembeau A (2015): Neural correlates of personal goal processing during episodic future thinking and mind‐wandering: An ALE meta‐analysis. Hum Brain Mapp 36:2928–2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stawarczyk D, Majerus S, Maj M, Van der Linden M, D'Argembeau A (2011a): Mind‐wandering: Phenomenology and function as assessed with a novel experience sampling method. Acta Psychol 136:370–381. [DOI] [PubMed] [Google Scholar]
- Stawarczyk D, Majerus S, Maquet P, D'Argembeau A (2011b): Neural correlates of ongoing conscious experience: Both task‐unrelatedness and stimulus‐independence are related to default network activity. PloS One 6:e16997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tusche A, Smallwood J, Bernhardt BC, Singer T (2014): Classifying the wandering mind: Revealing the affective content of thoughts during task‐free rest periods. Neuroimage 97:107–116. [DOI] [PubMed] [Google Scholar]
- Van Calster L, D'Argembeau A, Salmon E, Peters F, Majerus S (2017): Fluctuations of attentional networks and default mode network during the resting state reflect variations in cognitive states: Evidence from a novel resting‐state experience sampling method. J Cogn Neurosci 29:95–113. [DOI] [PubMed] [Google Scholar]
- Van den Heuvel MP, Sporns O (2013): Network hubs in the human brain. Trends Cogn Sci 17:683–696. [DOI] [PubMed] [Google Scholar]
- Van der Meer L, Costafreda S, Aleman A, David AS (2010): Self‐reflection and the brain: A theoretical review and meta‐analysis of neuroimaging studies with implications for schizophrenia. Neurosci Biobehav Rev 34:935–946. [DOI] [PubMed] [Google Scholar]
- Vanhaudenhuyse A, Demertzi A, Schabus M, Noirhomme Q, Bredart S, Boly M, Phillips C, Soddu A, Luxen A, Moonen G, Laureys S (2011): Two distinct neuronal networks mediate the awareness of environment and of self. J Cogn Neurosci 23:570–578. [DOI] [PubMed] [Google Scholar]
- Vatansever D, Menon DK, Manktelow AE, Sahakian BJ, Stamatakis EA (2015): Default mode dynamics for global functional integration. J Neurosci 35:15254–15262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatraman V, Rosati AG, Taren AA, Huettel SA (2009): Resolving response, decision, and strategic control: Evidence for a functional topography in dorsomedial prefrontal cortex. J Neurosci 29:13158–13164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vygotsky LS (1986): Thought and Language. Cambridge, MA: MIT Press. [Google Scholar]
- Wang Q, Luo S, Monterosso J, Zhang J, Fang X, Dong Q, Xue G (2014): Distributed value representation in the medial prefrontal cortex during intertemporal choices. J Neurosci 34:7522–7530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD (2011): Large‐scale automated synthesis of human functional neuroimaging data. Nat Methods 8:665–670. [DOI] [PMC free article] [PubMed] [Google Scholar]


