Abstract
Task‐switching performance relies on a broadly distributed frontoparietal network and declines in older adults. In this study, they investigated whether this age‐related decline in task switching performance was mediated by variability in global or regional white matter microstructural health. Seventy cognitively intact adults (43–87 years) completed a cued‐trials task switching paradigm. Microstructural white matter measures were derived using diffusion tensor imaging (DTI) analyses on the diffusion‐weighted imaging (DWI) sequence. Task switching performance decreased with increasing age and radial diffusivity (RaD), a measure of white matter microstructure that is sensitive to myelin structure. RaD mediated the relationship between age and task switching performance. However, the relationship between RaD and task switching performance remained significant when controlling for age and was stronger in the presence of cardiovascular risk factors. Variability in error and RT mixing cost were associated with RaD in global white matter and in frontoparietal white matter tracts, respectively. These findings suggest that age‐related increase in mixing cost may result from both global and tract‐specific disruption of cerebral white matter linked to the increased incidence of cardiovascular risks in older adults. Hum Brain Mapp 38:1588–1603, 2017. © 2016 Wiley Periodicals, Inc.
Keywords: task switching, executive function, cognitive control, aging, white matter, mixing cost, frontoparietal, radial diffusivity, tractography
INTRODUCTION
Cognitive control processes enable goal‐directed behavior by anticipating, detecting, and responding to important changes in one's environment. Cued‐trials task switching paradigms are often used to study cognitive control by manipulating the need to flexibly and efficiently anticipate, select, and implement action plans [see Karayanidis et al., 2010; Karayanidis and Jamadar, 2014]. Typically, these paradigms require rapid alternation between two or more choice response tasks. Within such mixed‐task blocks, switch cost refers to the finding that performance is slower and less accurate on switch trials (i.e., when the current trial requires a change in task) than repeat trials. Moreover, performance on repeat trials in these mixed‐task blocks is poorer compared with repeating the same task throughout the entire block (i.e., in single‐task blocks), a finding referred to as mixing cost. In cued‐trials paradigms, a cue provides valid information about whether the upcoming target requires a switch or repeat in task. Longer cue‐target intervals (CTIs) result in smaller switch cost and smaller mixing cost, indicating the engagement of proactive control processes that prepare the system to switch or repeat task (e.g., task‐set reconfiguration). However, a residual cost remains and this is taken as evidence that, even when proactive control processes are engaged, reactive control processes are required to resolve target‐driven interference arising from positive or negative priming processes [for review see Kiesel et al., 2010].
Functional neuroimaging studies have shown that cognitive control processes rely on broadly distributed frontoparietal networks [e.g., Corbetta and Shulman, 2002; Dosenbach et al., 2008]. In young adults, task switching performance is associated with activity in frontoparietal networks, including the dorsolateral and ventrolateral prefrontal cortex (dlPFC, vlPFC), the supplementary and pre‐supplementary motor areas (SMA, pre‐SMA), the anterior cingulate cortex (ACC), and the superior and inferior lobules of the parietal cortex [Badre and Wagner, 2006; Brass and von Cramon, 2004; Braver et al., 2003; Crone et al., 2006; Dove et al., 2000; Ruge et al., 2005; Slagter et al., 2006; Wager et al., 2004; Wylie et al., 2006; for reviews see Jamadar et al., 2015; Ruge et al., 2013]. This distributed frontoparietal network that is robustly associated with task switching in young adults appears to be less stable in older adults. Specifically, older adults tend to show less activation in task‐specific regions but recruitment of additional frontal regions that are not activated in younger adults [DiGirolamo et al., 2001; Gold et al., 2010; Milham et al., 2002]. In older adults, this pattern of reduced activation in task‐specific areas but greater frontal activation is also found in tasks assessing episodic memory, working memory and response inhibition [Cabeza et al., 2002]. In some studies, this increased frontal activation has been associated with improved performance and interpreted as evidence of recruitment of compensatory processes in order to preserve cognitive functioning [Davis et al., 2009; Reuter‐Lorenz and Cappell, 2008; Reuter‐Lorenz and Park, 2014]. However, the relationship between increased frontal activation and performance is not always straightforward [see Li et al., 2015; Spreng et al., 2010]. These functional neuroimaging findings have informed the revised scaffolding theory of aging and cognition [STAC‐r; Reuter‐Lorenz and Park, 2014], which posits that older adults use compensatory mechanisms (i.e., engagement of higher order task‐unrelated areas) to overcome inefficiencies in task‐specific networks. When switching between tasks, reduced efficiency of frontoparietal networks involved in anticipatory task‐set preparation is likely to lead to increased target‐driven interference. Consequently, greater compensatory recruitment of other frontal regions may be required to deal with the increased need for interference control in older adults. Such changes in the efficiency of frontoparietal networks may result from deterioration of white matter pathways that subserve task‐relevant neural circuits. These white matter pathways can be investigated in vivo using diffusion weighted imaging (DWI).
DWI is a magnetic resonance imaging (MRI) technique that is sensitive to the movement of water molecules in biological tissue. Using diffusion tensor imaging (DTI) to fit a mathematical model to DWI data, we can obtain measures of the directional dependence and rate of water molecule movement. The microstructural properties of the underlying tissue is inferred from these measures [Basser and Jones, 2002; Beaulieu, 2002; Le Bihan et al., 2001]. The degree of displacement (i.e., diffusion) of water molecules is directionally dependent and is indicated by fractional anisotropy (FA), a scalar value that describes the degree of anisotropy of a diffusion process and varies between zero (i.e., isotropic or random diffusion) and one (i.e., restricted diffusion along a single direction). DTI also provides measures of the rate of diffusion. Mean diffusivity (MD) is a measure of the overall magnitude of water diffusion. Axial diffusivity (AxD) and radial diffusivity (RaD) provide directional measures of principal diffusivity and cross‐sectional diffusivity along the main white matter axonal orientation, respectively. In animal studies, these directional diffusivity measures have been linked to white matter axonal structure and myelination. Specifically, axonal damage is associated with higher AxD values, whereas demyelination is associated with higher RaD values [Song et al., 2003; Sun et al., 2006]. At higher diffusion gradients (i.e., b > 2,880 vs. b < 1,280), radial diffusivity is the most sensitive DTI measure of white matter change, especially to changes in myelin content [Wu et al., 2011]. Hence, RaD is commonly interpreted as measuring myelin integrity, making it especially relevant to aging research. However, it is important to acknowledge that factors not related to pathology, such as the presence of crossing fibers and residual misalignment, can also impact RaD [Wheeler‐Kingshott and Cercignani, 2009].
In task switching studies, increasing age is associated with a robust increase in mixing cost but less consistent effects on switch cost [De Jong, 2001; Kramer et al., 1999, Expt 1; Kray, 2006; Mayr and Liebscher, 2001; Meiran et al., 2001, Expt 1; Whitson et al., 2012]. Few studies have examined whether age‐related changes in white matter microstructure impact task switching performance. Madden et al. [2009] examined whether the age‐related decline in the rate of information accrual in decision tasks [i.e., the drift rate parameter of the diffusion model, Ratcliff, 1978; Ratcliff and McKoon, 2008] is associated with FA changes in three white matter tracts. Mean drift rate (i.e., averaged over switch and repeat trials) was not correlated with FA in the superior longitudinal fasciculus (SLF), a long tract that traverses along the superior margin of the insula in an arc and projects predominantly from dorsal prefrontal regions to parietal, temporal and occipital cortices. However, FA in the genu and the splenium of the corpus callosum was positively correlated with mean drift rate, independently of age. Moreover, FA in these tracts accounted for a significant proportion of the age‐related variance in drift rate. This age‐related reduction in FA was driven almost entirely by changes in RaD, however, the direct influence of RaD on drift rate was not reported. Interestingly, switch cost (i.e., the difference in drift rate for switch vs. repeat trials) was not associated with any white matter microstructure measure. In a later study, Gold et al. [2010] found that decreased FA in SLF and pericallosal frontal white matter mediated the age‐related decline in general RT switch cost (i.e., poorer performance on mixed‐task vs. single‐task blocks—a measure very similar to mixing cost). Consistent with the STAC‐r model of cognitive aging, these findings suggest that reduced white matter efficiency in areas associated with the frontoparietal control network contribute to the age‐related decrease in task switching performance. However, these studies targeted only a small number of white matter tracts and did not control for variability in whole brain white matter. Therefore, it is not possible to determine whether the age‐related deficits in task switching performance resulted from a specific deterioration in these frontoparietal tracts or from global changes in white matter microstructure. Moreover, these studies did not specifically examine mixing cost, despite the fact that it shows the most robust age effect on task switching performance [e.g., Whitson et al., 2012].
Animal studies have shown that RaD is more sensitive than other DTI measures to demyelination [e.g., Wu et al., 2011], a process strongly implicated in age‐related brain deterioration [Bartzokis et al., 2012; Kemper, 1994; Marner et al., 2003; Minati et al., 2007; Sullivan and Pfefferbaum, 2006]. Age‐related changes in white matter microstructure are consistently larger for RaD than for FA [Bennett et al., 2010; Bhagat and Beaulieu, 2004; Borghesani et al., 2013; Burzynska et al., 2010; Davis et al., 2009; Haász et al., 2013]. Yet, RaD is not frequently reported in studies that investigate the relationship between white matter health and age‐related cognitive decline. The few studies do report RaD have found it to be more sensitive than other DTI measures to cognitive performance in older adults [Borghesani et al., 2013; Haász et al., 2013]. Jolly et al. [2016] showed that, compared with cortical atrophy, white matter hyperintensities and other DTI measures, RaD was the strongest predictor of scores on the Montreal Cognitive Assessment [MoCA; Nasreddine et al., 2005]. Moreover, whole brain RaD was a better predictor of MoCA scores than RaD in any one of the 18 white matter tracts extracted based on the probabilistic white matter tract atlas from the John Hopkins University [Hua et al., 2008]. In fact, the relationship between MoCA scores and RaD in each individual white matter tract did not remain significant when controlling for whole brain RaD [Jolly et al., 2016]. This is not surprising as the MoCA is a global cognitive screening tool that assesses memory, attention and executive functions.
In this study, we examine whether age‐related decline in task switching performance is associated with RaD in frontoparietal white matter pathways, over and above any global brain changes. We use a cued‐trials task switching paradigm that has been previously shown to produce a robust residual mixing cost but a small switch cost under prepared task conditions in highly practiced older adults [Whitson et al., 2012, see also Karayanidis et al., 2011]. If variability in mixing cost is specifically linked to white matter changes in frontoparietal networks, we expect that task switching performance will be more strongly associated with RaD in frontoparietal pathways and this relationship will remain significant when controlling for global RaD changes in cerebral white matter. Alternatively, as older adults often recruit additional brain regions than young adults when completing demanding cognitive tasks [Cabeza et al., 2002; Gold et al., 2010; Spreng et al., 2010], global white matter microstructural changes may be stronger predictors of decline in task switching performance than changes in any specific tract. We further hypothesize that the age effect on mixing cost will be reduced or eliminated when controlling for RaD in either frontoparietal networks or overall cerebral white matter, indicating that variability in white matter microstructure rather than age per se is responsible for cognitive control variability in older adults. In contrast, we expect that, consistent with a mediating role of white matter microstructure on task switching performance, the relationship between mixing cost and RaD in frontoparietal tracts will remain significant after controlling for age. Finally, we have previously shown that the relationship between brain white matter health and both global cognitive performance [Jolly et al., 2016] and brain arterial pulsatility [Jolly et al., 2013] is greater in the presence of cardiovascular risk factors. Therefore, we predict that the association between task switching performance and white matter RaD will be greater in adults with cardiovascular risk factors.
MATERIALS AND METHODS
Participants1
Seventy adults (aged 43–87 years; mean 66.79 ± SD 9.54) participated in this study. Thirty‐five participants were recruited from the Hunter Medical Research Institute (HMRI) Volunteer Register and had no history of neurological disorder. The remaining were recruited from the case list of a general neurology clinic at the John Hunter Hospital where they had been investigated by a neurologist (CL, MP) for possible mild stroke or a transient ischemic attack in the past 12 months. These participants were selected based on visual inspection of their pre‐existing clinical MRI scan to exclude cases with radiographical evidence of cortical or subcortical infarcts, marked cortical atrophy consistent with Alzheimer's pathology or a pattern of white matter pathology suggestive of multiple sclerosis. Participants from the two sources were pooled to create a sample that varied in degree of white matter pathology in the absence of a current neurological diagnosis. Participants gave written informed consent and received AU$60 for participation (Hunter New England Human Research Ethics Committee: 10/03/17/5.04). Five participants were excluded from the analysis of task switching data: four had an error rate exceeding 30% despite substantial task practice and one failed to complete testing. The remaining 65 participants scored within the high average range on full‐scale IQ [WASI; Wechsler, 1999] and working memory [Digit‐Span subtest from WAIS‐IV; Wechsler, 2008] and low average range on episodic memory [Logical Memory subtest I and 2 from WMS‐III; Wechsler, 1997]. More than half the participants (57%) reported having one or more vascular risk factors for which they were being medically treated, while 38% reported two or more risk factors (see Table 1). This study was embedded within a broader project, which involved a comprehensive neuropsychological assessment focusing on memory and executive function, as well as a stop‐signal task to assess inhibition processes.
Table 1.
Mean (standard deviation) for age, full‐scale IQ, digit‐span, logical memory I and 2, Montreal cognitive assessment (MoCA), cerebral volume, WMH, mean fractional anisotropy (FA), mean diffusivity (MD), as well as axial (AxD), and radial diffusivities (RaD) along with the clinical profile of participants
| Measure | Mean (SD) | Range |
|---|---|---|
| N = 65 (33M/32F) | ||
| Age (years) | 66.42 (9.38) | 43–82 |
| Full‐scale IQ | 112.85 (14.27) | 83–141 |
| Working Memory (scaled) | 10.80 (2.94) | 3–19 |
| Logical Memory I (scaled) | 9.72 (3.01) | 2–17 |
| Logical Memory II (scaled) | 9.16 (3.03) | 1–16 |
| MoCA | 26.31 (2.78) | 18–30 |
| Cerebral volume (% ICV) | 73.84 (4.67) | 63.26–84.58 |
| WMH (% ICV) | 0.426 (0.658) | 0.029–3.745 |
| FA | 0.396 (0.018) | 0.323–0.437 |
| MD | 0.582 (0.029) | 0.534–0.659 |
| AxD | 0.838 (0.029) | 0.787–0.915 |
| RaD | 0.455 (0.029) | 0.406–0.532 |
| Clinical profile | Yes | No |
| Vascular risk factors present | 37 (57%) 22M/15F | 28 (43%) 11M/17F |
| Hypertension | 24 (37%) 13M/11F | |
| Hypercholesterolemia | 21 (32%) 9M/12F | |
| Atrial fibrillation | 10 (15%) 7M/3F | |
| Diabetes | 6 (9%) 2M/4F | |
| Smoking | 3 (5%) 3M/0F | |
| Obesity | 6 (9%) 5M/1F | |
| Two vascular risk factors present | 18 (28%) 8M/10F | |
| Three vascular risk factors present | 6 (9%) 3M/3F | |
| Four vascular risk factors present | 1 (2%) 1M/0F |
% ICV, percentage of intracranial volume.
Stimuli and Tasks
The cued‐trials task switching paradigm [Fig. 1, see also Whitson et al., 2012] comprised two simple choice tasks: a letter classification task (i.e., vowel: A E I U or consonant: G K M R) and a number classification task (i.e., odd: 3 5 7 9 or even: 2 4 6 8; both Times New Roman, 60 × 60 pixels). Each task was mapped to a color scheme (hot or cold) and each choice to a left or right index finger response. An example of color‐task and response‐task mappings are shown in Figure 1.
Figure 1.
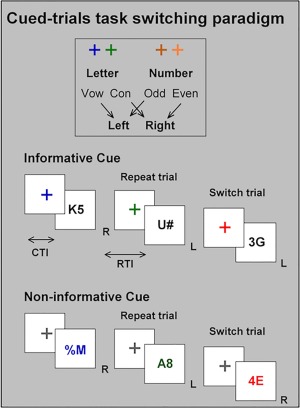
Schematic of the cued‐trials task switching paradigm. See Methods for more detail.
A grey outline of a square box (120 × 120 pixels) was continually presented on a black background in the center of a computer monitor. Each trial began with the presentation of the task cue (cross) followed by the target stimulus (cue‐target interval: CTI = 1,000 ms). The target was presented until response onset or 5,000 ms had elapsed. The next trial began 600 ms after the response (response‐target interval: 1,600 ms). Incorrect responses were followed by an error feedback tone.
The target consisted of two characters. One character was selected from the relevant task (e.g., a letter when the letter task was relevant). On 50% of trials, the other character was selected from the irrelevant task (e.g., a number when the letter task was relevant). These trials are referred to as incongruent trials because the relevant and irrelevant characters were incongruently mapped to response hand. For example, using the mapping in Figure 1, if the letter task was relevant and the letter was a consonant (e.g., K mapped to a right hand response), the other character would be an odd number (e.g., 5 mapped to a left hand response). On the remaining trials, the other character was a non‐alphanumeric symbol (i.e., %, #, and ?) that was not mapped to any response; these are referred to as neutral trials. The letter‐number pairs for incongruent targets and letter‐symbol or number‐symbol pairs for neutral targets were randomly selected from the full set of paired exemplars, with the restriction that the same exemplar could not be repeated on successive trials. Thus, the full stimulus set included 128 stimuli, 64 of which could have been preceded by two of the four cues (i.e., cue for number or letter), thereby forming 256 cue‐target associations. Response mapping was counterbalanced across participants.
On informative cue blocks, the cue was presented in a hot or a cold color that validly indicated the task to be performed on the upcoming target. The target was presented in black, in order to encourage participants to use the cue to prepare for the upcoming task. On non‐informative cue blocks, the cue was black, and the color of the target validly indicated the task to be performed. Cue color (for informative blocks) or target color (for non‐informative blocks) was never repeated on successive trials in order to eliminate any confounding effect of cue identity repetition [Logan and Bundesen, 2003]. For both cue types, mixed‐task blocks included 50% repeat trials (i.e., the current trial required performance of the same task as the preceding trial, e.g., trial n − 1 = letter, trial n = letter) and 50% switch trials (i.e., the current trial required a change in task, e.g., n − 1 = letter, n = number) presented in pseudorandom order. Only informative cues were used on single‐task blocks that required repeating the same task on all trials. To differentiate between repeat trials in single‐task and mixed‐task blocks, we refer to them as all‐repeat and mixed‐repeat trials, respectively.
Procedure
Participants completed three testing sessions spaced roughly 14 days apart. The first session included neuropsychological testing and training on the task switching paradigm. During training, response mapping for each task was explained, initially with neutral stimuli and then with mixed blocks of neutral and incongruent stimuli. Responses were made using buttons attached to the armrests. The second session included refresher training followed by behavioral and EEG data collection while completing the task switching paradigm followed by the stop‐signal task. The third session included the brain imaging protocol.
Task switching training included a total of 360 trials of single‐task and mixed‐task blocks spread over the two sessions. The task switching paradigm consisted of ten blocks of 70 trials, comprising two single‐task blocks with informative cues and eight mixed‐task blocks, that is, four with informative and four with non‐informative cues. This yielded 140 trials for each trial type: all‐repeat, informative mixed‐repeat, informative switch, non‐informative repeat, and non‐informative switch. Within each trial type, targets were approximately equally distributed across task (letter, number) and target type (neutral, incongruent). Participants were instructed to respond as quickly and as accurately as possible. Following each block, average RT and accuracy feedback was displayed and participants were encouraged to monitor and improve their performance.
Imaging Protocols
Imaging was completed at the John Hunter Hospital, Newcastle on a 3T Siemens Verio scanner using a 32‐channel head coil. T1‐weighted images were acquired in the sagittal plan using ultrafast gradient echo 3D sequence (MPRAGE) with 1 mm isotropic voxel resolution (repetition time = 1,500 ms; echo time= 2.57 ms; inversion time = 900 ms; flip angle = 9°; slice thickness = 1 mm with no gap; TA =3 min 29 s). A 3D Fluid Attenuated Inversion Recovery (FLAIR) sequence with 1 mm isotropic voxels was acquired in the sagittal plane using a phase encoding acceleration factor of 2 (TR = 5,000 ms; TE = 395 ms; TI = 1,800 ms; 160 slices with slice thickness = 1 mm, TA = 5 min 52 s). Diffusion weighted images (DWI) were acquired in the axial plane using a twice refocused spin echo sequence with a phase encoding acceleration factor of 3 (128 × 128 matrix; TR = 11,200 ms; TE = 111 ms; 55 slices with a slice thickness = 2.2 mm; TA = 13 min 6 s). Diffusion was measured in 64 non‐collinear directions with a b‐value of 3,000 mm2/s along with a non‐diffusion weighted (b = 0) image.
Fiber tracking
Whole‐brain fiber tracking was performed using the MRtrix software package (Brain Research Institute, Melbourne, Australia, http://www.brain.org.au/software/). Constrained spherical deconvolution [CSD, Tournier et al., 2007] was used to model multiple fiber orientations, with a maximum harmonic order lmax = 8 being employed on these data. Probabilistic tracking was then performed using information from the derived fiber orientation distributions [Behrens et al., 2003]. Whole‐brain tracking was achieved by randomly seeding throughout a white matter mask based on segmentation of the T1‐weighted image. This white matter mask was created using the recon‐all function from FreeSurfer's automated cortical reconstruction process (http://surfer.nmr.mgh.harvard.edu/). The mask included cerebral white matter and excluded subtentorial regions. For each participant, their white matter mask was resampled into diffusion space by means of an affine registration with 12 degrees of freedom (dof). For tractography procedures, individual streamlines were discarded if they had a track length less than 10 mm or extended beyond the white matter mask. A total of 8,000,000 streamlines were generated for each participant to enable track density imaging [TDI; Calamante et al., 2010]. TDI was used to reconstruct white matter tracts with increased resolution by taking advantage of long‐range information contained in the diffusion MRI fiber‐tracks. A threshold on the whole‐brain TDI of 0.02% was used to remove any voxels that contained grey matter or suffered partial volume effects, resulting in a complete tractogram of the cerebral white matter.
In addition, 18 white matter tracts were extracted using the probabilistic white matter tract atlas from the John Hopkins University [Hua et al., 2008], including the anterior thalamic radiation (ATR), forceps minor (CCFmi) and forceps major (CCFma) of the corpus callosum, corticospinal tract (CST), cingulum of the cingulate gyrus (CgCin) and the hippocampus (CgHi), inferior fronto‐occipital fasciculus (IFO), inferior longitudinal fasciculus (ILF), superior longitudinal fasciculus (SLF), and the uncinate fasciculus (UNC). With the exception of the corpus callosum, the remaining tracts were reconstructed in both left and right hemispheres. These white matter tracts were aligned in standard space (Eve atlas) and resampled into each participant's native space via a non‐linear registration procedure [FNIRT; Andersson et al., 2007], which is part of the FMRIB software Library [FSL; Smith et al., 2004]. Using the resampled tracts, we further isolated each tract by constraining them based on the results from the whole‐brain tractography and the resulting volume was used to calculate our tract‐based DTI measures.
Quantifying measures of white matter radial diffusivity
Each participant's diffusion tensor was calculated using DTIfit within the FMRIB diffusion toolbox. Three eigenvectors that define the diffusion ellipsoid were calculated in each voxel from the diffusion tensor. These eigenvectors correspond to three eigenvalues, representing the magnitude of diffusivity in the three principal directions. Voxel‐wise maps of radial diffusivity (RaD) were calculated by taking the average of the second and third eigenvalue. Mean RaD was calculated for overall cerebral white matter as well as for each of the 18 white matter tracts. Since each white matter tract was included in the estimation of overall white matter, this could lead to a potential confound when investigating the relationship between task switching performance and RaD in specific tracts versus global white matter RaD. To avoid this, we created a separate control mask for each tract, so that the relationship between RaD and task switching performance could be examined after partialling out RaD in the remaining white matter (i.e., after excluding voxels relating to that tract). This was achieved by creating a unique overall white matter image that was used as a baseline image for each tract and consisted of the overall white matter RaD voxel‐wise map after removing the voxels pertaining to the specific tract. These were used as control variables in partial correlations.
Data Analysis
The first two trials of every block and trials faster than 200 ms or slower than the participant's mean RT + 3 SD were excluded from analyses. On average, 4.37% of trials were thus removed. Mean RT and error rate were computed for the remaining trials, and broken down by trial type (all‐repeat, mixed‐repeat, switch), target congruence (neutral, incongruent), and cue type (informative, non‐informative). Mixing cost was calculated by subtracting performance on all‐repeat from mixed‐repeat trials. Switch cost was calculated by subtracting performance on mixed‐repeat trials from switch trials. For accuracy scores, mixing cost and switch cost measures were inversed so that higher values represent poorer performance cost for both accuracy and RT.
For the informative cue condition, mixing cost and switch cost effects of trial type and target congruence on error rate and RT were investigated using repeated measures Analysis of Variance (ANOVA) with Greenhouse–Geisser correction for violations of the assumption of sphericity, as needed. Two planned comparisons defined mixing cost and switch cost. The effect of advance preparation on switch cost in mixed‐task blocks was examined using a cue type × trial type × target congruence repeated measures ANOVA. We examined the influence of age and white matter microstructure on task switching performance using Analysis of Covariance (ANCOVA) initially with age alone and then with both age and RaD as covariates.
Pearson correlations were used to measure the association between measures of task switching performance and white matter RaD across the whole brain and in separate tracts. All correlations were conducted with one‐tailed α = 0.05, as we expected higher white matter RaD to be associated with poorer task switching performance. Type 1 error rate was controlled by using a conservative Bonferroni family‐wise error rate correction, where the level of significance (α) was set to 0.05/10 (i.e., three trial types and two cost measures across two levels of target congruence for each family of 18 tracts). Finally, to investigate whether task switching performance was differentially sensitive to RaD within specific white matter tracts, we used partial correlations to control for variance associated with overall white matter RaD.
RESULTS
Prepared Mixing Cost and Switch Cost Performance
Figure 2a,b shows mean RT and error rate for neutral and incongruent targets in each trial type. Both Trial Type and Congruence had significant main effects on RT (F(2,128) = 76.28, P < 0.001, F(1,64) = 166.35, P < 0.001, respectively), and error rate (F(2,128) = 32.82, P < 0.001, F(1,64) = 44.02, P < 0.001, respectively), indicating slower RT and more errors for more difficult trials. The Trial Type x Congruence interaction was also significant for both RT (F(2,128) = 40.79, P < 0.001) and error rate (F(2,128) = 40.17, P < 0.001).
Figure 2.
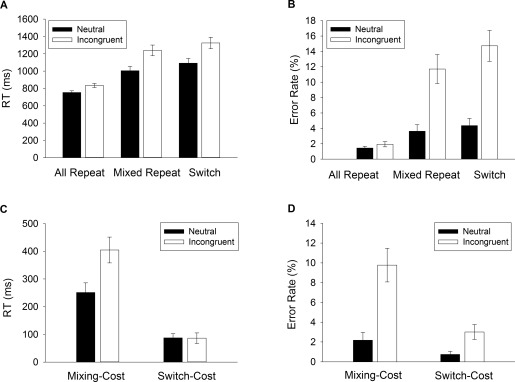
Task switching behavioral performance. Mean RT (A) and error rate (B) for each trial type (all‐repeat, mixed‐repeat and switch) depicted for neutral and incongruent targets. Mixing and switch costs for (C) RT and (D) error rate.
As shown in Figure 2c, RT showed a large mixing cost (319 ms, F(1,64) = 69.00, P < 0.001) and a smaller but still highly significant switch cost (86 ms, F(1,64) = 27.40, P < 0.001). RT mixing cost was significantly larger for incongruent than neutral targets (F(1,64) = 47.59, P < 0.001), but RT switch cost did not differ with Congruence (P > 0.942). A similar pattern was found for error rate (Fig. 2d); both mixing cost (F(1,64) = 27.40, P < 0.001) and switch cost (F(1,64) = 18.89, P < 0.001) were significantly greater for incongruent than neutral targets (F(1,64) = 31.99, P < 0.001, F(1,64) = 8.573, P = 0.004, respectively).
Age effects on mixing cost and switch cost
Age was a significant covariate on both mean RT (F(1,63) = 5.54, P = 0.022) and error rate (F(1,63) = 8.76, P < 0.001), indicating that increasing age was associated with slower RT and higher error rate. The effect of age on mean RT did not vary as a function of Trial Type, Congruence or their interaction (all P > 0.663). Thus, the age‐related increase in RT was ubiquitous, affecting all trial types and congruence conditions. In contrast, for error rate, age significantly interacted with Trial Type (F(2,126) = 8.03, P < 0.001). Specifically, increasing age was associated with a larger error mixing cost (F(1,63) = 5.30, P = 0.025) as well as a larger error switch cost (F(1,63) = 9.17, P = 0.004). Further, a significant Congruence × Age interaction (F(1,63) = 5.35, P = 0.024) indicated that the age effect on error rate was larger for incongruent than for neutral targets. There was also a significant Trial Type × Congruence × Age interaction for error rate (F(2,126) = 3.49, P = 0.046). Simple effects analyses showed that error switch cost increased with age for both incongruent and neutral trials, whereas the effect of age on error mixing cost was significant for incongruent trials only.
Global white matter RaD effects on mixing cost and switch cost
The inclusion of global white matter RaD as an additional covariate into the above analyses removed the main effects of Age on both RT and error rate2 across all levels of Trial Type and Congruence (all P > 0.608). Thus, controlling for RaD removed all age effects on task switching performance. Interestingly, even after accounting for the variability explained by age, increased RaD was still associated with higher RT and error rate (F(1,62) = 13.71, P < 0.001, F(1,62) = 18.40, P < 0.001, respectively; see Table 2). Furthermore, this effect of RaD on both RT and error rate varied across Trial Type (F(2,124) = 8.06, P < 0.001; F(2,124) = 13.96, P < 0.001), impacting especially on RT and error mixing costs (F(1,62) = 7.26, P = 0.009; F(1,62) = 13.85, P < 0.001, respectively). As seen in Figure 3, higher mixing cost for both RT and error rate was associated with higher RaD. In contrast, switch cost for either measure did not vary with RaD (P > 0.075).
Table 2.
Correlations between total white matter RaD and task switching performance
| Target | ||
|---|---|---|
| Trial type | Incongruent | Neutral |
| Error rate | ||
| All‐repeat | 0.424** | – |
| Mixed‐repeat | 0.532*** | 0.408** |
| Switch | 0.574*** | 0.501*** |
| Mixing cost | 0.503*** | 0.351* |
| Switch cost | – | 0.368* |
| Reaction time | ||
| All‐repeat | 0.511*** | 0.526*** |
| Mixed‐repeat | 0.416** | 0.499*** |
| Switch | 0.462*** | 0.506*** |
| Mixing cost | – | 0.415** |
| Switch cost | – | – |
Associations that remained significant after controlling for age are shaded.
Abbreviations: RaD, radial diffusivity; RT, response time. * P < 0.05, ** P < 0.01, *** P < 0.001 (after Bonferroni correction of α/10).
Figure 3.
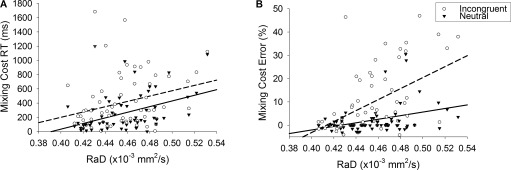
Relationship between white matter RaD with mixing cost for RT (A) and error rate (B) for neutral and incongruent target types. Solid fit lines represent neutral target types; dashed fit lines represent incongruent target types.
As shown in Figure 3, the effect of RaD on error mixing cost also varied with Congruence (RaD × Mixing Cost × Congruence: F(1,62) = 10.91, P = 0.002). Simple effects analyses showed that the interaction between RaD and error mixing cost was stronger for incongruent than for neutral targets (F(1,62) = 15.06, P < 0.001; F(1,62) = 5.18, P = 0.026).
Pearson correlations between RaD and task switching performance produced results consistent with the above covariate analyses. As seen in Table 2, higher total white matter RaD was significantly correlated with higher RT and higher error rate for almost all trial types, and these correlations remained significant even when controlling for age. RaD was associated with higher error mixing cost for incongruent targets and with higher RT mixing cost for neutral targets.
Effect of advance preparation on switch cost
On mixed‐task blocks, responding was significantly slower with non‐informative cues than with informative cues (F(1,64) = 74.95, P < 0.001). This effect of Cue Type was significantly larger for incongruent targets than for neutral targets (F(1,64) = 70.13, P < 0.001). Error rate showed no main effect of Cue Type or interaction between Cue Type and Trial Type or Congruence. The effect of Cue Type did not vary as a function of Age or RaD and therefore will not be discussed further.
In summary, increasing age was associated with an increase in error rate and RT during task switching. Error rate was particularly sensitive to aging, with an age‐related increase in both error mixing and switch costs. However, all age effects were eliminated when including RaD as an additional covariate. This indicates that the effect of age on task switching performance is better accounted by variability in white matter RaD. The effect of RaD was most evident for mixed‐repeat trials, as indicated by strong correlations between RaD and error mixing cost for incongruent targets and RT mixing cost for neutral targets. Importantly, the relationship between RaD and task switching performance remained significant when controlling for variability related to age. This pattern of findings suggests that global white matter RaD rather than age per se is a better predictor of task switching performance, and most specifically mixing cost. Hitherto, we have used a measure of RaD across whole brain white matter. Below, we test the hypothesis that white matter changes in specific frontoparietal tracts will more strongly predict task switching performance than global white matter changes.
Global versus tract specific effect of white matter microstructure on task switching
In order to determine whether changes in task switching performance were associated with microstructural changes in specific white matter tracts, we examined mean RaD across 18 prominent white matter tracts. With the exception of the left and right CgHi, RaD in each white matter tract showed a moderate to strong positive correlation with age (all r > 0.408, all P < 0.001)3. RaD in all 16 remaining white matter tracts was strongly correlated with overall white matter RaD (all r > 0.724, all P < 0.001). These 16 white matter tracts showed robust correlations with RT for all trial types (i.e., α = 0.05/10; 0.322 < r < 0.605, all P < 0.004) and 15 also correlated with error mixing cost (α = 0.05/10; 0.346 < r < 0.511, all P < 0.0024). The fact that task switching performance is associated with variability in RaD in most white matter tracts as well as across the whole brain suggest that task switching decline in older adults may be driven by global changes in RaD.
To determine whether RaD variability in any specific white matter tract had a greater effect on task switching performance compared with global white matter RaD, we re‐ran the above analyses for each tract using partial correlations to control for RaD in the remaining white matter (i.e., global white matter after excluding white matter related to that particular tract). Three white matter tracts in the left hemisphere produced significant correlations with task switching performance that could not be accounted for by changes in overall white matter RaD: the left inferior fronto‐occipital fasciculus (IFO) which connects the orbital and lateral frontal cortices to occipital cortex, the left inferior longitudinal fasciculus (ILF) which connects the occipital and temporal lobes, and the left superior longitudinal fasciculus (SLF) which connects perisylvian frontal, parietal, and temporal cortices.
As shown in Table 3, before partialling out overall white matter RaD, these three white matter tracts produced moderately strong correlations with both RT and error rate for most trial types and for mixing cost, with less consistent effects on switch cost. Including both overall white matter RaD and age as partial variables confirmed that RaD for each of these tracts explained a significant amount of variance in RT for all trial types and mixing cost (with the exception of SLF for all‐repeat trials). In contrast, this was not the case for error rate, suggesting that variability in error rate is best accounted for by overall white matter RaD. Thus, RT and RT mixing cost variability were sensitive to changes in RaD in three left‐lateralized white matter tracts (IFO, ILF, and SLF).
Table 3.
Correlations between task switching performance and RaD in IFO, ILF, and SLF
| Incongruent target | Neutral target | |||||
|---|---|---|---|---|---|---|
| IFO‐left | ILF‐left | SLF‐left | IFO‐left | ILF‐left | SLF‐left | |
| Error rate | ||||||
| All‐repeat | 0.467*** | 0.452*** | 0.457*** | 0.331* | 0.367* | 0.340* |
| Mixed‐repeat | 0.551*** | 0.530*** | 0.559*** | 0.418** | 0.363** | 0.407** |
| Switch | 0.590*** | 0.562*** | 0.582*** | 0.515*** | 0.469*** | 0.521*** |
| Mixing cost | 0.515*** | 0.495*** | 0.527*** | 0.357* | – | 0.342* |
| Switch cost | – | – | – | 0.382** | 0.394** | 0.431** |
| Reaction time | ||||||
| All‐repeat | 0.578*** | 0.588*** | 0.484*** | 0.610*** | 0.618*** | 0.510*** |
| Mixed‐repeat | 0.493*** | 0.501*** | 0.470*** | 0.583*** | 0.571*** | 0.547*** |
| Switch | 0.512*** | 0.515*** | 0.521*** | 0.578*** | 0.569*** | 0.571*** |
| Mixing cost | 0.357* | 0.362* | 0.375* | 0.487*** | 0.464*** | 0.493*** |
| Switch cost | – | – | – | – | – | – |
Shaded areas show those correlations that remained significant after partialling out overall white matter RaD.
Abbreviations: RaD, radial diffusivity; IFO, inferior fronto‐occipital fasciculus; ILF, inferior longitudinal fasciculus; SLF, superior longitudinal fasciculus. * P < 0.05, ** P < 0.01, *** P < 0.001 (after family‐wise Bonferroni correction of α/10).
These tracts show considerable spatial overlap. In fact, nearly half of the volume of the left IFO is located in white matter regions that also contain fibers belonging to the other two tracts (ILF and SLF). Specifically, 42% of IFO volume overlapped with IFL and 5% with SLF. Similarly, 59% of ILF volume overlapped with IFO and 9% with SLF. The SLF showed less overlap: 4% of its volume overlapped with IFO and 6% with ILF.
Given the extensive spatial overlap between IFO and ILF, the above analyses do not allow us to ascertain whether both of these tracts are equally important for task switching. To determine the distinct contribution of each tract to task switching performance, we compared performance to RaD in voxels unique to each tract. This was achieved by creating a mask that was based on the overlapping regions of both tracts. This overlapping region could be analyzed or removed from the individual tracts to analyze areas unique to that tract. We compared voxels specific to IFO (IFO‐only) or to IFL (IFL‐only) versus voxels common to both tracts (IFO and IFL). As shown in Table 4, RaD in voxels specific to the left IFO and voxels that spatially overlapped left IFO and left ILF accounted for significantly more variance in RT for each trial type and RT mixing cost than could be explained by global white matter RaD. However, RaD in left ILF‐only was no longer significantly associated with RT for any of the trial types or mixing cost after accounting for the global white matter RaD. Therefore, these findings suggest that left IFO is specifically associated with task switching performance whereas the involvement of the ILF appears likely to be due to areas of spatial overlap with IFO.
Table 4.
Correlation between RT on task switching paradigm and RaD in white matter specific to IFO, specific to ILF (i.e., after removing voxels that overlap) as well as in the overlapping IFO and ILF voxels that remained significant after correcting for multiple comparisons, overall white matter RaD (shaded)
| Incongruent target | Neutral target | |||||
|---|---|---|---|---|---|---|
| IFO‐only | ILF‐only | IFO and ILF | IFO‐only | ILF‐only | IFO and ILF | |
| All‐repeat | 0.546*** | 0.501*** | 0.585*** | 0.567*** | 0.504*** | 0.621*** |
| Mixed‐repeat | 0.463*** | 0.422** | 0.492*** | 0.551*** | 0.458*** | 0.574*** |
| Switch | 0.492*** | 0.450*** | 0.495*** | 0.555*** | 0.478*** | 0.556*** |
| Mixing‐cost | 0.333* | 0.302 | 0.353* | 0.466*** | 0.369* | 0.468*** |
Abbreviations: RaD, radial diffusivity; IFO, inferior fronto‐occipital fasciculus; ILF, inferior longitudinal fasciculus. * P < 0.05, ** P < 0.01, *** P < 0.001 (after Bonferroni correction of α/10).
Finally, as RaD in the left SLF and IFO were the strongest predictors of mixing cost, we performed mediation analyses to determine how much variance could be accounted for by changes in global white matter RaD. Global white matter RaD accounted for approximately 91% of the variance between RaD in the left SLF and error mixing cost (z = 2.31, P = 0.011, one‐tailed), but only approximately 44% of the variance for RT mixing cost (z = 0.71, P > 0.2, one‐tailed). A very similar pattern of results was found for the left IFO, with global white matter RaD accounting for approximately 92% (z = 2.34, P = 0.011, one‐tailed), and approximately 53% (z = 0.87, P > 0.15, one‐tailed) of the variance in error and RT mixing cost, respectively. These results confirm that the tract‐specific effect of RaD on mixing cost is exclusive to RT. In other words, RaD in these specific white matter tracts accounted for approximately twice the variance in RT mixing cost than can be explained by global white matter RaD.
Presence of Cardiovascular Risk Factors
As seen above, task switching performance was associated with age‐independent changes in white matter microstructure. As cardiovascular risk factors have been associated with subclinical pathological changes to white matter, we next examined whether the presence of cardiovascular risk factors influenced the relationship between white matter microstructure and task switching performance. Participants were divided into two groups: a CV‐risk group (i.e., at least one reported cardiovascular risk factor, n = 37) and a No‐risk group (i.e., no reported cardiovascular risk factors, n = 28; see Table 1). The CV‐risk group was significantly older (M = 69.76, SD = 8.39) and had significantly higher white matter RaD (M = 0.47, SD = 0.03) than the No‐risk group (M = 62.00, SD = 8.89; M = 0.44, SD = 0.02, respectively; independent samples t‐tests, all P < 0.001).
We performed a 2 group (CV‐risk, No‐risk) × 2 trial types (all‐repeat, mixed‐repeat) × 2 target type (incongruent, neutral) GML to examine whether mixing cost effects on RT and error rate were impacted by the presence of cardiovascular risk factors. The main effect of group was significant for both RT and error rate (F(1,63) = 16.96, P < 0.001, F(1,63) = 9.45, P = 0.003, respectively). The No‐risk group responded faster (802 ± 235 ms) and committed fewer errors (2.3% ± 4.6%) than the CV‐risk group (1,076 ± 438 ms, 6.6% ± 11%). Both RT and error mixing cost was larger for the CV‐risk than the No‐risk group (F(1,63) = 7.06, P = 0.010; F(1,63) = 7.41, P = 0.008, respectively). For error rate, the size of the congruence effect, especially for repeat trials in the mixed‐task block, also differed between groups (group x target type: F(1,63) = 5.75, P = 0.019; group x mixing x target type: (F(1,63) = 3.52, P = 0.065). Specifically, the CV‐risk group showed a larger error mixing cost for incongruent than neutral targets. In summary, participants who reported at least one CV risk factor were slower and more error prone, and had larger mixing cost than participants who reported no CV risk factor.
As participants in the CV‐risk group were significantly older and had significantly higher RaD compared with participants in the No‐CV‐risk group, it is important to determine whether age and RaD differences could account for group differences in task switching performance. To test this, we reran the above GLM analyses with age and global white matter RaD as covariates. With both covariates in the model, all group effects on error rate were eliminated and there was only an attenuated main effect of group on RT (F(1,61) = 6.82, P = 0.011). In conclusion, the differences in task switching performance between CV‐risk and No‐risk groups appear to be accounted for by group differences in whole brain RaD4.
DISCUSSION
In this study, we used a cued‐trials task switching paradigm to investigate whether age‐related decline in cognitive control ability can be attributed to whole brain or tract‐specific variability in white matter microstructural health. The most robust age effect in the task switching paradigm, the increase in mixing cost, was shown to be explained by variability in the microstructure of white matter at both global and tract‐specific levels. Specifically, error mixing cost was most strongly related to variability in global white matter microstructure, whereas RT mixing cost was related to variability in prominent frontoparietal white matter tracts. Hence, task switching performance in older adults was influenced by variability in both tract‐specific and global white matter microstructure. These results are consistent with earlier evidence that frontoparietal white matter is important for task switching in older adults [Gold et al., 2010; Madden et al., 2009]. Importantly, here we show that not all tract‐specific effects remain significant when controlling for global changes in white matter microstructure. This finding points to the need to control for global deterioration in white matter when seeking to identify the effects of tract‐specific deterioration on measures of cognitive aging [see also Salthouse et al., 2015]. A second important finding is that the relationship between mixing cost performance and white matter microstructure is more robust in the presence of cardiovascular risk factors. Taken together, these findings suggest that the robust increase in mixing cost often reported in older adults may in fact be due to the increased incidence of cardiovascular risks with increasing age and their impact on white matter health. These findings suggest that preventable and treatable risk factors can at least partly account for age‐related decline in cognitive control processes. They also highlight the importance of controlling the presence of cardiovascular risk factors when establishing normative patterns of cognitive aging. We discuss these findings in more detail below.
White Matter Microstructure Changes Mediate Age Effects on Task Switching
Consistent with previous studies, increasing age was associated with slower and more error prone performance on the cued‐trials task switching paradigm. While age affected both mixing cost and switch cost, age effects were most pronounced for mixing cost, especially for incongruent targets that had a high level of interference. These results are consistent with previous work showing that the age‐related increase in mixing cost is largest in tasks that involve stimulus and/or response level ambiguity [e.g., De Jong, 2001; Kray and Lindenberger, 2000; Meiran et al., 2001]. Interestingly, global white matter health mediated the effect of age on task switching performance. Therefore, the very robust effect of age on mixing cost [e.g., Kramer et al., 1999, Expt 1; Kray, 2006; Mayr and Liebscher, 2001; Meiran et al., 2001; Whitson et al., 2012] appears to result from the increased incidence of white matter microstructural changes among older adults, rather than represent a corollary of age per se.
Consistent with the above conclusion, the presence of cardiovascular risks impacted the relationship between aging and both cognitive and white matter decline. There is extensive evidence that cardiovascular health affects cognitive functioning and neural structure [Raz and Rodrigue, 2006; Salat, 2011]. For instance, cardiovascular risk factors are associated with poorer cognitive functioning [Cukierman et al., 2005; Elias et al., 2006; Harrington et al., 2000; Kivipelto et al., 2001; Swan et al., 1998; Tilvis et al., 2004] and increased risk of dementia [Chao et al., 2010; Whitmer et al., 2005]. Likewise, hypertension is associated with reduced white matter volume [Raz et al., 2003, 2005; Strassburger et al., 1997], increased white matter hyperintensities [Breteler et al., 1994; Goldstein et al., 2005; Murray et al., 2005; Raz et al., 2007] and changes in white matter microstructure [Burgmans et al., 2010; Hannesdottir et al., 2009; Huang et al., 2006; Kennedy and Raz, 2009; Leritz et al., 2010].
In the present study, participants who reported the presence of one or more cardiovascular risk factor performed more poorly on task switching and had poorer white matter health than participants without cardiovascular risk factors. Although cardiovascular risk factors were more prevalent in older adults, cardiovascular risk factors continued to be associated with poorer task switching performance even after controlling for age. In contrast, the effect of cardiovascular risk factors on task switching performance was removed after controlling for whole brain RaD. Similarly, controlling for RaD eliminated the effect of age on task switching performance, whereas controlling for age did not diminish the effect of RaD on task switching.
Recent work highlights that white matter deterioration in older adults may have a vascular origin. In the same cohort as the present study, Jolly et al. [2013] showed that decline in white matter microstructure was associated with increased brain arterial pulsatility, and that this relationship was stronger in the presence of cardiovascular risk factors. Fabiani et al. [2014] reported that arterial health in older adults was associated with reduced grey and white matter volumes and cognitive performance. Together, these findings suggest that poorer task switching performance in older adults may reflect changes in white matter microstructure that emerge because of the increasing prevalence of cardiovascular risk factors. Future work needs to examine the relative impact of different cardiovascular risk factors on cognitive and white matter changes, and whether these effects are prevented or reversed with optimal treatment regimes.
Effects of frontoparietal Versus Global White Matter Microstructural Changes on Task Switching Performance
Previous DTI studies [Gold et al., 2010; Madden et al., 2009] showed that white matter microstructure in frontoparietal regions was related to task switching performance, with weaker associations present in the genu and bilateral pericallosal frontal regions. However, these studies did not establish the spatial specificity of these effects when controlling for change in global brain white matter [Salthouse et al., 2015]. In the present study, we extend these findings by showing that (a) when controlling for age, diffuse (global) and regional (tract‐specific) changes in white matter are sensitive to distinct aspects of task switching performance, and (b) not all regional changes survive correction for global brain white matter changes. Only three of the 16 white matter tracts that were significantly correlated with task switching performance survived after correcting for global white matter.
The three anterior–posterior white matter tracts that survived after controlling for global white matter RaD were all associated with RT mixing cost; all correlations with error mixing cost failed to reach significance after correction. Moreover, after controlling for spatial overlap between two of these tracts, the relationship between RT mixing cost and RaD was localized to two left frontoparietal tracts (IFO and SLF). Both the IFO and SLF are long association tracts that connect frontal and parietal cortices [Ashtari et al., 2007; Jellison et al., 2004; Martino et al., 2010], making this finding consistent with neuroimaging evidence that distributed frontoparietal neural networks underpin effective task switching [Badre and Wagner, 2006; Brass and von Cramon, 2004; Braver et al., 2003; Dove et al., 2000; Reynolds et al., 2004; see Jamadar et al., 2015]. Thus, degradation of frontoparietal white matter appears to underlie the robust age effect on RT mixing cost supporting the notion that white matter forms the “backbone” of the frontoparietal cognitive control network [Corbetta and Shulman, 2002; Dosenbach et al., 2008]. Interestingly, the IFO tract has additional occipital connections and has been previously reported to be specifically associated with set‐shifting in the Trail Making Task [Perry et al., 2009]. Future work needs to examine the directionality and specificity of these frontoparietal and other anterior–posterior tract effects.
Interestingly, while RT mixing cost varied with RaD within frontoparietal tracts, error mixing cost was sensitive to global white matter RaD. The RT effects have been previously reported by Gold et al. [2010] and Madden et al. [2009]. However, these studies did not report effects on error rate or control for global white matter decline. It is also possible that our older adults sample which included participants with cardiovascular risk factors is more representative of the typical aging population and more sensitive to differential global and regional white matter changes than the more homogenous healthy older samples typically used in aging studies.
Further systematic work is needed to replicate the novel dissociation between global vs. frontoparietal white matter RaD and error versus RT mixing cost, respectively. It appears that response speed is sensitive to subtle changes in frontoparietal networks even under highly practiced task conditions. In contrast, response accuracy appears sensitive to more white matter diffuse changes arising from the sustained presence of cardiovascular risk factors. If this is the case, studies using healthy older samples may not have sufficient variability in white matter disruption to produce noticeable impairments to cognitive control (as evidenced by increase in error rate). Aging‐related increase in RT is a most robust phenomenon [Salthouse, 2000] and older adults show a preference for accurate versus speeded performance [Ratcliff and McKoon, 2008; Whitson et al., 2012, 2014]. Thus, RT effects may be more ubiquitous, whereas error effects can be masked by changes in speed‐accuracy trade‐off and emerge only when these compensatory processes fail. This explanation is consistent with our earlier findings in this sample. In Jolly et al. [2016], global white matter microstructure was strongly associated with performance in the Montreal Cognitive Assessment (MoCA) and better accounted for MoCA performance than white matter in any regional tract, especially in the presence of CV risk factors. Moreover, increased arterial pulsatility, a measure of CV health, was more strongly associated with global than regional white matter health [Jolly et al., 2013]. Thus, increasingly broad changes to white matter microstructure, as a result of presence of CV risk factors, may produce broad‐based cognitive decline (detected by broad screening tests), but also impact specific high level cognitive control processes when compensatory mechanisms are no longer efficient.
Relevance of Findings to Models of Cognitive Aging
In line with the revised scaffolding theory of cognitive aging [STAC‐r; Reuter‐Lorenz and Park, 2014], the present findings show that reduced integrity in frontoparietal regions that are associated with task switching performance can account for the age‐related increase in mixing cost that is robustly reported in the literature. Disruption of these task‐specific networks would necessitate compensatory recruitment of non‐task‐specific regions. However, when the localized frontoparietal changes are accompanied by reduced global white matter integrity, the efficiency of such compensatory scaffolding may be compromised. In the context of task switching, aging has been shown to reduce efficiency of proactive control processes involved in anticipatory task‐set reconfiguration and increase engagement of reactive control processes involved in interference control [e.g., Karayanidis et al., 2011]. Disruption of frontoparietal control networks is likely to reduce the efficiency of proactive control, resulting in greater target‐driven interference and consequently greater reliance on compensatory processes. However, global white matter decline may reduce the efficiency of this compensatory implementation of reactive control processes. This interpretation is consistent with functional neuroimaging studies which show that older adults tend to show more spatially extended cortical activation compared with younger adults [Cabeza et al., 2002; Spreng et al., 2010] and that increased connectivity in prefrontal areas is associated with better task performance in older adults [Hakun et al., 2015].
Radial Diffusivity Versus Other Measures of White Matter Microstructure
Finally, in the present study, we showed that the relationship between task switching performance and white matter microstructure was most pronounced for RaD, compared with FA that is more frequently reported in DTI studies. As FA and RaD are sensitive to distinct diffusion properties (i.e., anisotropy vs. cross‐sectional water movement), our findings suggest that particular properties of white matter may be uniquely related to aging‐related changes in cognition. It has been previously argued that AxD and RaD measures are unable to distinguish between axon‐ and myelin‐related processes [Wheeler‐Kingshott and Cercignani, 2009]. However, recent evidence suggests that RaD is sensitive to myelin properties of white matter [e.g., Janve et al., 2013] and that this sensitivity may vary at different diffusion gradients [Counsell et al., 2006; Hui et al., 2010]. For instance, Wu et al. [2011] showed that diffusion gradient values similar to those used in the present study (i.e., b > 2,880 mm2/s) are more sensitive to demyelination than lower gradients. These findings suggest that age effects on white matter microstructure may be largely due to demyelination processes and point to the importance of customizing DWI protocols to the specific mechanisms likely to produce variability in white matter microstructure in different age groups [Jones et al., 2013].
CONCLUSION
Advancing age is typically associated with decline in higher order cognitive control functions. Here we report novel evidence that age‐related decline in task switching performance is mediated by variability in the white matter architecture of the aging brain that may result from the presence of cardiovascular risk factors. Importantly, we show that the robust age‐related increase in mixing cost can be linked to disruption of white matter organization at both global brain and tract‐specific pathways. Specifically, degradation of frontoparietal white matter microstructure was linked to increased RT mixing cost, whereas error mixing cost varied with whole brain white matter organization. These white matter effects were most strongly evident in radial diffusivity, probably reflecting demyelination linked to changes in the cardiovascular system.
ACKNOWLEDGMENTS
We thank the HMRI Research Register for recruitment of healthy participants and Kristy Morris for recruitment from the neurology clinic. We also thank Vanessa Case for scheduling testing of participants.
Footnotes
This study uses data collected as part of a larger testing protocol. We have already reported data examining the relationship between white matter measures and (1) brain blood pulsatility [Jolly et al., 2013], and (2) a cognitive screening task [Jolly et al., 2016].
RaD was more strongly associated with task switching performance than any of the other DTI measures. However, the same (albeit somewhat weaker) pattern of correlations were found between FA and task switching performance.
The fact that only CgH tracts did not show a significant relationship may be due to its size. CgH has a very small volume and therefore fewer number of voxels across which to take measurement (less than half the volume of any of the other tracts investigated). Small variation in tract reconstruction between individuals would therefore have a greater impact on this small tract, which may explain why the association with age was not as robust.
To examine the impact of risk factor severity, we reran the analyses with three groups: no CV risk, one CV risk factor (n = 12), two or more CV risk factors (n = 25). The effect of global white matter RaD was greater in the presence of two or more CV risk factors. However, as the number of participants per group was small, differences between the one CV and two or more CV factors are not reported separately.
REFERENCES
- Andersson JL, Jenkinson M, Smith S (2007): Non‐linear registration, aka Spatial normalisation FMRIB technical report TR07JA2FMRIB Analysis Group of the University of Oxford.
- Ashtari M, Cottone J, Ardekani BA, Cervellione K, Szeszko PR, Wu J, Kumra S (2007): Disruption of white matter integrity in the inferior longitudinal fasciculus in adolescents with schizophrenia as revealed by fiber tractography. Arch Gen Psychiatry 64:1270–1280. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD (2006): Computational and neurobiological mechanisms underlying cognitive flexibility. Proc Natl Acad Sci 103:7186–7191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G, Lu PH, Heydari P, Couvrette A, Lee GJ, Kalashyan G, Altshuler LL (2012): Multimodal magnetic resonance imaging assessment of white matter aging trajectories over the lifespan of healthy individuals. Biol Psychiatry 72:1026–1034. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Jones DK (2002): Diffusion‐tensor MRI: Theory, experimental design and data analysis–a technical review. NMR Biomed 15:456–467. [DOI] [PubMed] [Google Scholar]
- Beaulieu C (2002): The basis of anisotropic water diffusion in the nervous system–a technical review. NMR Biomed 15:435–455. [DOI] [PubMed] [Google Scholar]
- Behrens TEJ, Johansen‐Berg H, Woolrich MW, Smith SM, Wheeler‐Kingshott CAM, Boulby PA, Matthews PM (2003): Non‐invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci 6:750–757. [DOI] [PubMed] [Google Scholar]
- Bennett IJ, Madden DJ, Vaidya CJ, Howard DV, Howard JH (2010): Age‐related differences in multiple measures of white matter integrity: A diffusion tensor imaging study of healthy aging. Hum Brain Mapp 31:378–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagat YA, Beaulieu C (2004): Diffusion anisotropy in subcortical white matter and cortical gray matter: Changes with aging and the role of CSF‐suppression. J Magn Reson Imaging 20:216–227. [DOI] [PubMed] [Google Scholar]
- Borghesani PR, Madhyastha TM, Aylward EH, Reiter MA, Swarny BR, Schaie KW, Willis SL (2013): The association between higher order abilities, processing speed, and age are variably mediated by white matter integrity during typical aging. Neuropsychologia 51:1435–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brass M, von Cramon DY (2004): Selection for cognitive control: A functional magnetic resonance imaging study on the selection of task‐relevant information. J Neurosci 24:8847–8852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, Reynolds JR, Donaldson DI (2003): Neural mechanisms of transient and sustained cognitive control during task switching. Neuron 39:713–726. [DOI] [PubMed] [Google Scholar]
- Breteler MMB, Van Swieten JC, Bots ML, Grobbee DE, Claus JJ, Van Den Hout JHW, Hofman A (1994): Cerebral white matter lesions, vascular risk factors, and cognitive function in a population‐based study The Rotterdam Study. Neurology 44:1246–1246. [DOI] [PubMed] [Google Scholar]
- Burgmans S, van Boxtel MP, Gronenschild EHBM, Vuurman EFPM, Hofman P, Uylings HBM, Raz N (2010): Multiple indicators of age‐related differences in cerebral white matter and the modifying effects of hypertension. Neuroimage 49:2083–2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burzynska AZ, Preuschhof C, Bäckman L, Nyberg L, Li SC, Lindenberger U, Heekeren HR (2010): Age‐related differences in white matter microstructure: Region‐specific patterns of diffusivity. Neuroimage 49:2104–2112. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR (2002): Aging gracefully: Compensatory brain activity in high‐performing older adults. Neuroimage 17:1394–1402. [DOI] [PubMed] [Google Scholar]
- Calamante F, Tournier JD, Jackson GD, Connelly A (2010): Track‐density imaging (TDI): super‐resolution white matter imaging using whole‐brain track‐density mapping. Neuroimage 53:1233–1243. [DOI] [PubMed] [Google Scholar]
- Chao LL, Buckley ST, Kornak J, Schuff N, Madison C, Yaffe K, Weiner MW (2010): ASL perfusion MRI predicts cognitive decline and conversion from MCI to dementia. Alzheim Dis Assoc Disord 24:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counsell SJ, Shen Y, Boardman JP, Larkman DJ, Kapellou O, Ward P, Rutherford MA (2006): Axial and radial diffusivity in preterm infants who have diffuse white matter changes on magnetic resonance imaging at term‐equivalent age. Pediatrics 117:376–386. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL (2002): Control of goal‐directed and stimulus‐driven attention in the brain. Nat Rev Neurosci 3:201–215. [DOI] [PubMed] [Google Scholar]
- Crone EA, Donohue SE, Honomichl R, Wendelken C, Bunge SA (2006): Brain regions mediating flexible rule use during development. J Neurosci 26:11239–11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cukierman T, Gerstein HC, Williamson JD (2005): Cognitive decline and dementia in diabetes—systematic overview of prospective observational studies. Diabetologia 48:2460–2469. [DOI] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Buchler NG, White LE, Madden DJ, Cabeza R (2009): Assessing the effects of age on long white matter tracts using diffusion tensor tractography. Neuroimage 46:530–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jong R (2001): Adult age differences in goal activation and goal maintenance. Eur J Cogn Psychol 13:71–89. [Google Scholar]
- DiGirolamo GJ, Kramer AF, Barad V, Cepeda NJ, Weissman DH, Milham MP, McAuley E (2001): General and task‐specific frontal lobe recruitment in older adults during executive processes: A fMRI investigation of task‐switching. Neuroreport 12:2065–2071. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE (2008): A dual‐networks architecture of top‐down control. Trends Cogn Sci 12:99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dove A, Pollmann S, Schubert T, Wiggins CJ, Yves von Cramon D (2000): Prefrontal cortex activation in task switching: An event‐related fMRI study. Cogn Brain Res 9:103–109. [DOI] [PubMed] [Google Scholar]
- Elias MF, Sullivan LM, Elias PK, Vasan RS, D'Agostino RB, Seshadri S, Benjamin EJ (2006): Atrial fibrillation is associated with lower cognitive performance in the Framingham offspring men. J Stroke Cerebrovasc Dis 15:214–222. [DOI] [PubMed] [Google Scholar]
- Fabiani M, Low KA, Tan CH, Zimmerman B, Fletcher MA, Schneider‐Garces N, Gratton G (2014): Taking the pulse of aging: Mapping pulse pressure and elasticity in cerebral arteries with optical methods. Psychophysiology 51:1072–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold BT, Powell DK, Xuan L, Jicha GA, Smith CD (2010): Age‐related slowing of task switching is associated with decreased integrity of frontoparietal white matter. Neurobiol Aging 31:512–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein IB, Bartzokis G, Guthrie D, Shapiro D (2005): Ambulatory blood pressure and the brain A 5‐year follow‐up. Neurology 64:1846–1852. [DOI] [PubMed] [Google Scholar]
- Haász J, Westlye ET, Fjær S, Espeseth T, Lundervold A, Lundervold AJ (2013): General fluid‐type intelligence is related to indices of white matter structure in middle‐aged and old adults. Neuroimage 83:372–383. [DOI] [PubMed] [Google Scholar]
- Hakun JG, Zhu Z, Johnson NF, Gold BT (2015): Evidence for reduced efficiency and successful compensation in older adults during task switching. Cortex 64:352–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannesdottir K, Nitkunan A, Charlton RA, Barrick TR, MacGregor GA, Markus HS (2009): Cognitive impairment and white matter damage in hypertension: A pilot study. Acta Neurol Scand 119:261–268. [DOI] [PubMed] [Google Scholar]
- Harrington F, Saxby BK, McKeith IG, Wesnes K, Ford GA (2000): Cognitive performance in hypertensive and normotensive older subjects. Hypertension 36:1079–1082. [DOI] [PubMed] [Google Scholar]
- Hua K, Zhang J, Wakana S, Jiang H, Li X, Reich DS, Mori S (2008): Tract probability maps in stereotaxic spaces: Analyses of white matter anatomy and tract‐specific quantification. Neuroimage 39:336–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Ling XY, Liu SR (2006): Diffusion tensor imaging on white matter in normal adults and elderly patients with hypertension. Chin Med J 119:1304. [PubMed] [Google Scholar]
- Hui ES, Cheung MM, Chan KC, Wu EX (2010): B‐value dependence of DTI quantitation and sensitivity in detecting neural tissue changes. Neuroimage 49:2366–2374. [DOI] [PubMed] [Google Scholar]
- Jamadar S, Thienel R, Karayanidis F (2015): Task switching In: Toga AW, Poldrack RA, editors. Brain Mapping: An Encyclopedic Reference. Amsterdam: Elsevier. [Google Scholar]
- Janve VA, Zu Z, Yao SY, Li K, Zhang FL, Wilson KJ, Gochberg DF (2013): The radial diffusivity and magnetization transfer pool size ratio are sensitive markers for demyelination in a rat model of type III multiple sclerosis (MS) lesions. Neuroimage 74:298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellison BJ, Field AS, Medow J, Lazar M, Salamat MS, Alexander AL (2004): Diffusion tensor imaging of cerebral white matter: A pictorial review of physics, fiber tract anatomy, and tumor imaging patterns. Am J Neuroradiol 25:356–369. [PMC free article] [PubMed] [Google Scholar]
- Jolly TA, Bateman GA, Levi CR, Parsons MW, Michie PT, Karayanidis F (2013): Early detection of microstructural white matter changes associated with arterial pulsatility. Front Hum Neurosci 7:782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly TA, Cooper PS, Wan Ahmadul Badwi SA, Phillips NA, Rennie JL, Levi CR, Drysdale KA, Parsons MW, Michie PT, Karayanidis F (2016): Microstructural white matter changes mediate age‐related cognitive decline on the Montreal Cognitive Assessment (MoCA). Psychophysiology 53:258–267. [DOI] [PubMed] [Google Scholar]
- Jones DK, Knösche TR, Turner R (2013): White matter integrity, fiber count, and other fallacies: The do's and don'ts of diffusion MRI. Neuroimage 73:239–254. [DOI] [PubMed] [Google Scholar]
- Karayanidis F, Jamadar SD (2014): Event‐related potentials reveal multiple components of proactive and reactive control in task switching In: Granje JA, Houghton G, editors. Task Switching and Cognitive Control. Oxford: Oxford University Press; [Google Scholar]
- Karayanidis F, Jamadar S, Ruge H, Phillips N, Heathcote A, Forstmann BU (2010): Advance preparation in task‐switching: Converging evidence from behavioral, brain activation, and model‐based approaches. Cognition 1:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karayanidis F, Whitson LR, Heathcote A, Michie PT (2011): Variability in proactive and reactive cognitive control processes across the adult lifespan. Front Psychol 2:318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper TL (1994): Neuroanatomical and neuropathological changes during aging and dementia In: Albert ML, Knoefel JE, editors. Clinical Neurology of Aging, 2nd ed New York: Oxford University Press; pp 3–67. [Google Scholar]
- Kennedy KM, Raz N (2009): Aging white matter and cognition: Differential effects of regional variations in diffusion properties on memory, executive functions, and speed. Neuropsychologia 47:916–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiesel A, Steinhauser M, Wendt M, Falkenstein M, Jost K, Philipp AM, Koch I (2010): Control and interference in task switching—A review. Psychol Bull 136:849. [DOI] [PubMed] [Google Scholar]
- Kivipelto M, Helkala EL, Hänninen T, Laakso MP, Hallikainen M, Alhainen K, Nissinen A (2001): Midlife vascular risk factors and late‐life mild cognitive impairment A population‐based study. Neurology 56:1683–1689. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Hahn S, Gopher D (1999): Task coordination and aging: Explorations of executive control processes in the task switching paradigm. Acta Psychol 101:339–378. [DOI] [PubMed] [Google Scholar]
- Kray J (2006): Task‐set switching under cue‐based versus memory‐based switching conditions in younger and older adults. Brain Res 1105:83–92. [DOI] [PubMed] [Google Scholar]
- Kray J, Lindenberger U (2000): Adult age differences in task switching. Psychol Aging 15:126. [DOI] [PubMed] [Google Scholar]
- Le Bihan D, Mangin JF, Poupon C, Clark CA, Pappata S, Molko N, Chabriat H (2001): Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging 13:534–546. [DOI] [PubMed] [Google Scholar]
- Leritz EC, Salat DH, Milberg WP, Williams VJ, Chapman CE, Grande LJ, McGlinchey RE (2010): Variation in blood pressure is associated with white matter microstructure but not cognition in African Americans. Neuropsychology 24:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HJ, Hou XH, Liu HH, Yue CL, Lu GM, Zuo XN (2015): Putting age‐related task activation into large‐scale brain networks: A meta‐analysis of 114 fMRI studies on healthy aging. Neurosci Biobehav Rev 57:156–174. [DOI] [PubMed] [Google Scholar]
- Logan GD, Bundesen C (2003): Clever homunculus: Is there an endogenous act of control in the explicit task‐cuing procedure?. J Exp Psychol 29:575. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Spaniol J, Costello MC, Bucur B, White LE, Cabeza R, Huettel SA (2009): Cerebral white matter integrity mediates adult age differences in cognitive performance. J Cogn Neurosci 21:289–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marner L, Nyengaard JR, Tang Y, Pakkenberg B (2003): Marked loss of myelinated nerve fibers in the human brain with age. J Comp Neurol 462:144–152. [DOI] [PubMed] [Google Scholar]
- Martino J, Brogna C, Robles SG, Vergani F, Duffau H (2010): Anatomic dissection of the inferior fronto‐occipital fasciculus revisited in the lights of brain stimulation data. Cortex 46:691–699. [DOI] [PubMed] [Google Scholar]
- Mayr U, Liebscher T (2001): Is there an age deficit in the selection of mental sets? Eur J Cogn Psychol 13:47–69. [Google Scholar]
- Meiran N, Gotler A, Perlman A (2001): Old age is associated with a pattern of relatively intact and relatively impaired task‐set switching abilities. J Gerontol Series B 56:88–102. [DOI] [PubMed] [Google Scholar]
- Milham MP, Erickson KI, Banich MT, Kramer AF, Webb A, Wszalek T, Cohen NJ (2002): Attentional control in the aging brain: Insights from an fMRI study of the stroop task. Brain Cogn 49:277–296. [DOI] [PubMed] [Google Scholar]
- Minati L, Grisoli M, Bruzzone MG (2007): MR spectroscopy, functional MRI, and diffusion‐tensor imaging in the aging brain: A conceptual review. J Geriatr Psychiatry Neurol 20:3–21. [DOI] [PubMed] [Google Scholar]
- Murray AD, Staff RT, Shenkin SD, Deary IJ, Starr JM, Whalley LJ (2005): Brain white matter hyperintensities: Relative importance of vascular risk factors in nondemented elderly people. Radiology 237:251–257. [DOI] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Chertkow H (2005): The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53:695–699. [DOI] [PubMed] [Google Scholar]
- Perry ME, McDonald CR, Hagler DJ, Jr. , Gharapetian L, Kuperman JM, Koyama AK, Dale AM, McEvoy LK (2009): White matter tracts associated with set‐shifting in healthy aging. Neuropsychologia 47:2835–2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff R (1978): A theory of memory retrieval. Psychol Rev 85:59. [DOI] [PubMed] [Google Scholar]
- Ratcliff R, McKoon G (2008): The diffusion decision model: Theory and data for two‐choice decision tasks. Neural Comput 20:873–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM (2006): Differential aging of the brain: Patterns, cognitive correlates and modifiers. Neurosci Biobehav Rev 30:730–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Acker JD (2003): Hypertension and the brain: Vulnerability of the prefrontal regions and executive functions. Behav Neurosci 117:1169. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Acker JD (2005): Regional brain changes in aging healthy adults: General trends, individual differences and modifiers. Cereb Cortex 15:1676–1689. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Kennedy KM, Acker JD (2007): Vascular health and longitudinal changes in brain and cognition in middle‐aged and older adults. Neuropsychology 21:149. [DOI] [PubMed] [Google Scholar]
- Reynolds JR, Donaldson DI, Wagner AD, Braver TS (2004): Item‐and task‐level processes in the left inferior prefrontal cortex: Positive and negative correlates of encoding. Neuroimage 21:1472–1483. [DOI] [PubMed] [Google Scholar]
- Reuter‐Lorenz PA, Cappell KA (2008): Neurocognitive aging and the compensation hypothesis. Curr Directions in Psychol Sci 17:177–182. [Google Scholar]
- Reuter‐Lorenz PA, Park DC (2014): How does it STAC up? Revisiting the scaffolding theory of aging and cognition. Neuropsychol Rev 24:355–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruge H, Brass M, Koch I, Rubin O, Meiran N, von Cramon DY (2005): Advance preparation and stimulus‐induced interference in cued task switching: Further insights from BOLD fMRI. Neuropsychologia 43:340–355. [DOI] [PubMed] [Google Scholar]
- Ruge H, Jamadar S, Zimmermann U, Karayanidis F (2013): The many faces of preparatory control in task switching: Reviewing a decade of fMRI research. Hum Brain Mapp 34:12–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salat DH (2011): The declining infrastructure of the aging brain. Brain Connect 1:279–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA (2000): Aging and measures of processing speed. Biol Psychol 54:35–54. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Habeck C, Razlighi Q, Barulli D, Gazes Y, Stern Y (2015): Breadth and age‐dependency of relations between cortical thickness and cognition. Neurobiol Aging 36:3020–3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slagter HA, Weissman DH, Giesbrecht B, Kenemans JL, Mangun GR, Kok A, Woldorff MG (2006): Brain regions activated by endogenous preparatory set shifting as revealed by fMRI. Cogn Affect Behav Neurosci 6:175–189. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen‐Berg H, Matthews PM (2004): Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23:S208–S219. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH (2003): Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage 20:1714–1722. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Wojtowicz M, Grady CL (2010): Reliable differences in brain activity between young and old adults: A quantitative meta‐analysis across multiple cognitive domains. Neurosci Biobehav Rev 34:1178–1194. [DOI] [PubMed] [Google Scholar]
- Strassburger TL, Lee HC, Daly EM, Szczepanik J, Krasuski JS, Mentis MJ, Alexander GE (1997): Interactive effects of age and hypertension on volumes of brain structures. Stroke 28:1410–1417. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A (2006): Diffusion tensor imaging and agingNeuroscience and. Biobehav Rev 30:749–761. [DOI] [PubMed] [Google Scholar]
- Sun SW, Liang HF, Le TQ, Armstrong RC, Cross AH, Song SK (2006): Differential sensitivity of in vivo and ex vivo diffusion tensor imaging to evolving optic nerve injury in mice with retinal ischemia. Neuroimage 32:1195–1204. [DOI] [PubMed] [Google Scholar]
- Swan GE, DeCarli C, Miller BL, Reed T, Wolf PA, Jack LM, Carmelli D (1998): Association of midlife blood pressure to late‐life cognitive decline and brain morphology. Neurology 51:986–993. [DOI] [PubMed] [Google Scholar]
- Tilvis RS, Kähönen‐Väre MH, Jolkkonen J, Valvanne J, Pitkala KH, Strandberg TE (2004): Predictors of cognitive decline and mortality of aged people over a 10‐year period. J Gerontol Ser a 59:M268–M274. [DOI] [PubMed] [Google Scholar]
- Tournier J, Calamante F, Connelly A (2007): Robust determination of the fibre orientation distribution in diffusion MRI: Non‐negativity constrained super‐resolved spherical deconvolution. Neuroimage 35:1459–1472. [DOI] [PubMed] [Google Scholar]
- Wager TD, Jonides J, Reading S (2004): Neuroimaging studies of shifting attention: A meta‐analysis. Neuroimage 22:1679–1693. [DOI] [PubMed] [Google Scholar]
- Wechsler D (1997): Wechsler Memory Scale (WMS‐III). San Antonio, TX: Psychological Corporation. [Google Scholar]
- Wechsler D (1999): Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Wechsler D (2008): WAIS‐IV: Wechsler Adult Intelligence Scale. Baltimore: Pearson. [Google Scholar]
- Wheeler‐Kingshott CA, Cercignani M (2009): About “axial” and “radial” diffusivities. Magn Reson Med 61:1255–1260. [DOI] [PubMed] [Google Scholar]
- Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K (2005): Midlife cardiovascular risk factors and risk of dementia in late life. Neurology 64:277–281. [DOI] [PubMed] [Google Scholar]
- Whitson LR, Karayanidis F, Michie PT (2012): Task practice differentially modulates task‐switching performance across the adult lifespan. Acta Psychol 139:124–136. [DOI] [PubMed] [Google Scholar]
- Whitson LR, Karayanidis F, Fulham R, Provost A, Michie PT, Heathcote A, Hsieh S (2014): Reactive control processes contributing to residual switch cost and mixing cost across the adult lifespan. Front Psychol 5:383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YC, Field AS, Duncan ID, Samsonov AA, Kondo Y, Tudorascu D, Alexander AL (2011): High b‐value and diffusion tensor imaging in a canine model of dysmyelination and brain maturation. Neuroimage 58:829–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie GR, Javitt DC, Foxe JJ (2006): Jumping the gun: Is effective preparation contingent upon anticipatory activation in task‐relevant neural circuitry?. Cereb Cortex 16:394–404. [DOI] [PubMed] [Google Scholar]


