Abstract
A salience network (SN) anchored in the anterior insula (AI) and dorsal anterior cingulate cortex (dACC) plays a key role in switching between brain networks during salience detection and attention regulation. Previous fMRI studies have associated expectancy behaviors and SN activation with novelty seeking (NS) and reward dependence (RD) personality traits. To address the question of how functional connectivity (FC) in the SN is modulated by internal (expectancy‐related) salience assignment and different personality traits, 68 healthy participants performed a salience expectancy task using functional magnetic resonance imaging, and psychophysiological interaction analysis (PPI) was conducted to determine salience‐related connectivity changes during these anticipation periods. Correlation was then evaluated between PPI and personality traits, assessed using the temperament and character inventory of 32 male participants. During high salience expectancy, SN‐seed regions showed reduced FC to visual areas and parts of the default mode network, but increased FC to the central executive network. With increasing NS, participants showed significantly increasing disconnection between right AI and middle cingulate cortex when expecting high‐salience pictures as compared to low‐salience pictures, while increased RD also predicted decreased right dACC and caudate FC for high salience expectancy. Our findings suggest a direct link between personality traits and internal salience processing mediated by differential network integration of the SN. SN activity and coordination may therefore be moderated by novelty seeking and reward dependency personality traits, which are associated with risk of addiction. Hum Brain Mapp 38:4064–4077, 2017. © 2017 Wiley Periodicals, Inc.
Keywords: fMRI, salience network, expectancy, PPI, novelty seeking, reward dependence
INTRODUCTION
Human engagement with the world is highly influenced and regulated by attention, which relies on both internal and external factors. External stimuli can capture attention depending on their salience or significance, and attention can be facilitated through bottom–up, stimulus‐driven mechanisms [Beck and Kastner, 2005; Corbetta and Shulman, 2002]. Internally driven attention, such as expectation, employs higher cognitive processes and is mediated by a top–down goal‐directed mechanism [Dosenbach et al., 2008; Pessoa et al., 2003]. A large body of studies has shown that attention regulation can be affected by individual differences in personality traits that influence the manner in which people interact with external stimuli (reviewed in Canli and Amin [2002], Hamann and Canli [2004], and Calder et al. [2011]). For example, extroverted individuals are more likely to give high attention to positive mood, while neurotic individuals are susceptible to negative mood [Larsen and Ketelaar, 1991]. Canli et al. [2001, 2002] found such personality‐related emotional perception differences to have a strong neural basis, especially related to amygdala activity. However, few studies have focused on the relationship between goal‐directed attention regulation and personality traits, especially their neural mechanism.
Using EEG recordings and a greyscales task, in which participants were required to judge which of two left–right greyscale images appears darker (one greyscale image shaded from black on the left to white on the right and one greyscale image in the reverse direction), Tomer [2008] found that high novelty‐seeking (NS) participants showed a consistent attentional bias that favored the right‐sided greyscale stimuli, while the low NS participants showed a bias to the left (although the greyscale pairs are identical in overall luminance), suggesting hemispheric dopamine asymmetry and asymmetry in the novelty‐seeking behavioral approach. Importantly, NS has been found specifically to modulate expectancy periods (internally driven attention) but not picture perception periods (attention driven by external stimuli): participants with a higher NS personality score have shown an elevated blood oxygen level dependent (BOLD) signal in the medial prefrontal cortex (mPFC) during emotional compared with neutral expectancy [Bermpohl et al., 2008]. The harm avoidance (HA) personality has often been found to affect brain function and behaviors when negative emotional stimuli were used in tasks, even when the stimuli were only distractors [Most et al., 2006]. More specifically, high HA participants have been shown to produce a stronger amygdala response to irrelevant emotional distractors when they were unsure about what to expect [Most et al., 2006]. These previous studies gave rise to several interesting questions: (1) whether personality dimensions such as HA and NS played a major role in the above‐mentioned tasks due to individual differences in emotion regulation or rather due to the differing extent to which salience was perceived; (2) if the latter were the case, whether brain networks other than the emotion networks were involved, such as the salience network (SN); (3) whether personality not only affects brain function, but also contributes to brain network connectivity during certain tasks.
To investigate these questions, we first conducted a salience expectancy task and focused on the mechanisms underlying the SN. The anterior insula (AI) and dorsal anterior cingulate cortex (dACC), the main components of the SN, have been identified as playing a key role in switching between brain networks during salience detection and attention regulation [Bressler and Menon, 2010], in coordinating behavioral responses [Medford and Critchley, 2010], and in perceptual decision‐making [Lamichhane et al., 2016]. Previous studies investigating expectancy found the SN to be involved in internally and externally directed attention [Bermpohl et al., 2006; Herwig et al., 2007]. Moreover, salience‐related attention processing has been associated with reward and motivation [Ventura et al., 2007]. The SN processes sensory inputs that generate salience detection in the internal or the external world, and modulates other brain functional networks based on integration of this information [Bressler and Menon, 2010]. For instance, the right AI upregulates and downregulates the central‐executive network, a task‐related network including dorsolateral prefrontal cortex [dlPFC] and posterior parietal cortex brain areas, and the default‐mode network (DMN), a task‐inhibited network consisting of ventromedial prefrontal cortex [vmPFC] and posterior cingulate cortex areas, shifts brain activity patterns based on the condition (rest or specific tasks) [Menon and Uddin, 2010; Sridharan et al., 2008]. However, the functional connectivity (FC) between the structures supporting processing of salience expectancy and reward is not yet known, which motivated our current investigation of SN‐based FC.
Second, based on previous findings, we focused on the tridimensional Cloninger model (involving the following personality traits: NS, reward dependence [RD], HA; Cloninger [1994]), because their characteristics are crucially involved in personally relevant information processing (internal salience) and reward evaluation [Cohen et al., 2009; Enzi et al., 2009]. NS is related to behavioral activation, which is driven by looking for and feeling “rewarded” by new experiences, whereas RD is associated with behavioral maintenance that is driven by positive motivation such as social approval [Cloninger, 1994]. HA is often characterized by excessive worrying, shyness, being fearful, and greater sensitivity to negative stimuli [Cloninger, 1994]. Although negative stimuli may also be highly salient, in particular for individuals with elevated HA, detailed examination of negative salient information was beyond the scope of this study. We nevertheless include the dimension in our initial analysis. Previous studies have provided evidence of associations between personality traits and the brain's structural and functional variability. For example, NS predicted white matter fiber tract connectivity of the hippocampus, amygdala, and ventral striatum, whereas RD predicted the fiber tracts between prefrontal cortex and the striatum [Cohen et al., 2009]. Assessment of regional cerebral blood flow (rCBF) at rest revealed a positive association between NS and activations in ACC and right insula cortex, whereas RD was negatively correlated with ACC, AI, and neocortical region activations [Sugiura et al., 2000]. Using the same measurement (but investigating 7 different personality traits), Turner et al. [2003] found NS was related to motor cortex and somatic sensory cortex (the precentral and postcentral gyri) activation. RD, however, was related to activations in the frontal and the temporal lobes—including a region which was reported previously by Sugiura et al. [2000] to correlate negatively with RD. Owing to variability in methods and limitations in the studies performed so far, it is rather difficult, if not impossible, to draw far‐reaching conclusions about the relationship between personality traits—NS and RD—and brain mechanisms. Nevertheless, insula cortex is consistently reported in relation to personality traits, pointing toward a relationship between SN and personality traits. Moreover, a positron emission tomography (PET) study revealed a significant association between dopamine D2‐receptor binding in right AI and NS trait [Suhara et al., 2001], suggesting involvement of dopaminergic neurons of the AI in modulation of the NS personality.
Although previous studies have already suggested an association between personality and the SN, no direct evidence has yet been identified to support the personality‐related SN function variation during actual salience processing. Using fMRI, the striatum has been shown to be involved in processing saliency that was related to reward quality, reflecting the involvement of the dopaminergic system in saliency mediation [Zink et al., 2004]. Dopaminergic drugs have been found to alter striatal connectivity to subcortical and cortical targets [Metzger et al., 2015], both during rest and during preceding attention, along with altered attentional responses during a similar experiment [Graf et al., 2013]. Furthermore, we previously found participants with low self‐directedness personality to be more distracted by high‐salience pictures as compared to low‐salience pictures, using the Attention Modulation by Salience Task, which suggested that personality trait‐shaped behavioral change [Dinica et al., 2015]. A remaining question is whether the personality traits modulate brain FC between the SN and other brain regions during internal salience expectation.
To this end, we employed a salience expectancy task to test the interaction between personality, internal salience generation (during expectancy), and SN FC. We hypothesized that participants' NS and RD scores would modulate specific connections of SN nodes both to distinct targets within the SN and to dopaminergic circuits.
METHODS
Participants
Sixty‐nine healthy participants (20 women, 49 men) between 22 and 52 years of age (mean age 31.85 ± 8.03 years old) without previous psychiatric, neurological, or medical illness participated in this study. All participants completed the mini‐international neuropsychiatric interview (MINI) to specifically exclude ICD‐10 psychiatric disorders [Sheehan et al., 1998]. Personality traits were assessed using the Temperament and Character Inventory (TCI), comprising a 240‐item questionnaire [Cloninger, 1994]. The TCI consists of seven subscales, including four temperament subscales (NS, RD, HA, and persistence) and three character subscales (self‐directedness, cooperativeness, and self‐transcendence). To test our hypothesis, we focused only on the tridimensional Cloninger model (NS, RD, HA). To enable the detection of a main effect of expectancy, we recruited a large mixed gender sample. However, to avoid confounding effects due to gender, and due to potential links between interindividual variability related to menstrual cycle phase [Ross et al., 2001] or Premenstrual Syndrome [Berlin et al., 2001] and personality traits, the between‐subject correlation was reported both for the full sample and for a male‐restricted group, which was therefore stratified to be larger than the female group when recruiting participants. One male participant was excluded due to massive head motion during fMRI scanning (rotation & translation > 5 mm), leaving 68 participants for the whole analysis.
The local ethics committee of the University of Magdeburg approved the study, and all participants provided written informed consent.
Expectancy Task
The salience expectancy paradigm employed visual cues to predict the different salience of the following pictures. A total of 40 pictures were selected from the International Affective Picture System (IAPS) [Lang et al., 2008] depicting human events or activities. To diminish the stress response, only positive or neutral pictures were used in the present study. These pictures were divided into high (n = 20) and low (n = 20) salience categories based on previous validation [Musolff, 2008]. We explained the salience as “a property inherent to the stimulus, intended to attract and maintain participants' attention to the stimulus.” Behavioral characterization of the salience effect of the stimuli used here has been provided by Dinica et al. [2015], who suggested that high salience (hs) stimuli are characterized by high stimulus complexity, high arousal, and a high degree of positive valence compared to low salience (ls) pictures, in which emotionally neutral content is depicted. Half of the selected pictures were preceded by a visual cue (expectancy period) and half were presented instantly following a fixation trial with no preceding visual cues (n = 10 hs and n = 10 ls pictures). The paradigm used in this study was adapted from Metzger et al. [2010] (Fig. 1). During the expectancy period, participants were asked to actively expect the subsequent pictures to be hs or ls when the visual cue was shown, and to view the following pictures passively. Participants were instructed to expect pictures to have the salience indicated by the preceding cue, to be aware of the predicted salience quality of the picture, and subsequently to look at the picture.
Figure 1.
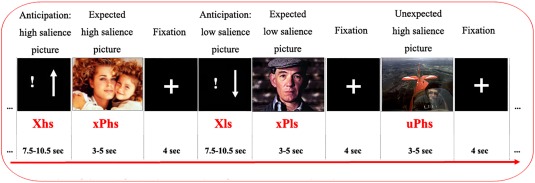
Salience expectancy task paradigm. [Color figure can be viewed at http://wileyonlinelibrary.com]
After the fMRI task, the participants were asked to rate the pictures that were displayed during the task, based on the pictures' salience. Finally, 27 participants completed a post hoc picture rating.
Data Acquisition and Analysis
A 3 T Siemens MAGNETOM Sonata MRI scanner equipped with an eight‐channel head coil was used to obtain 672 volumes using T2*‐weighted gradient echo planar imaging (EPI) volumes with BOLD contrast (repetition time (TR) = 1.25 s; echo time (TE) = 25 ms; flip angle 70°). Each volume consisted of 26 axial slices with a thickness of 5 mm recorded in ascending order. Each slice had a matrix size of 44 × 44 pixels.
The data were analyzed using Statistical Parametric Mapping Software (SPM8, FIL, Wellcome Trust Centre for Neuroimaging, UCL, London, UK) implemented in Matlab R2009a. The first 8 volumes were discarded to ensure signal stabilization. Functional images were realigned to the first image, mean‐adjusted by proportional scaling, resliced, and normalized to the standard EPI template with a 4 × 4 × 4 mm voxel size. Finally, the data were spatially smoothed using a Gaussian kernel of 6 mm (full‐width half‐maximum).
At the single‐subject level, we modeled six regressors of interest convolved with the canonical hemodynamic response function (HRF). Two of these regressors indicated the effect of expectancy during salience cues (expectancy of a hs cue or expectancy of a ls cue, marked as Xhs or Xls, accordingly) irrespective of whether a cue was followed by a picture or not. The other four regressors indicated the effect of expectancy and salience interaction during the picture display session (perception: expected hs pictures [xPhs], expected ls pictures [xPls], unexpected hs pictures [uPhs], unexpected ls pictures [uPls]). The voxel time series were high‐pass filtered at 1/128 Hz to account for nonphysiological slow drifts in the measured signal and modeled for temporal autocorrelation across scans with an autoregressive AR(1) model.
We conducted one‐sample t‐tests to check for salience effects during expectancy (hs cues − ls cues, marked as Xhs > Xls). Results were reported at cluster‐level P ≤ 0.05, with family‐wise error (FWE) correction for voxels surpassing a P < 0.001 initial voxel threshold.
Psychophysiological Interaction (PPI) Analysis
To investigate FC changes during salience expectancy periods, we conducted psychophysiological interaction (PPI) analysis. The PPI analysis explains neural responses in one brain area in terms of the interaction between influences of other brain regions and a cognitive/sensory process [Friston et al., 1997]. The PPI analysis employed a design matrix with three regressors: (i) the “psychological variable” representing the cognitive process of interest (the task contrast used for the analysis was [Xhs > Xls]); (ii) the “physiological variable” representing the neural response in the seed region and (iii) the interaction term of (i) and (ii). To quantify the physiological variable, we extracted the individual time series within a 10 mm radius sphere centered at the peak within predefined seed regions. Previous studies have indicated that the right AI is the core hub in the SN, and therefore only this region was likely to be active across multiple task domains [Menon and Uddin, 2010; Uddin et al., 2014; also see review in Uddin [2015]). To restrict our seed region to a relatively small area, two masks located in the right AI and the right dACC (taken from Horn et al. [2010] and Lord et al. [2012]) were used as our PPI seed regions.
The physiological factor was then multiplied by the psychological factor, yielding the interaction term. Subject‐specific contrast images resulting from the contrast [1 0 0], where the first column represents the interaction term, were then entered into a random effects group analysis using a one‐sample t‐test. The significance threshold used for PPI analyses was a cluster level of P ≤ 0.05 (after FWE correction).
Finally, we conducted a correlation analysis between the PPI maps and personality temperaments (NS and RD). Of the 68 participants, 40 participants had complete TCI data, including 8 female and 32 male participants. Out of the 32 male participants (mean age is 29.76 ± 4.75 years old), 2 participants' NS scores were identified as outliers: one was greater than the upper quartile plus 1.5 times the inter quartile range (IQR) and the other one was smaller than the bottom quartile minus 1.5 times the IQR. Therefore, in the following analysis, 40 participants (8 females and 32 males) were included for RD correlation analysis and 38 (8 females and 30 males) participants were included for NS correlation analysis. Of the 27 participants (including 15 males) who completed the rating task, 19 (4 females and 15 males) participants (mean age is 29.93± 3.63 years old) were included in the correlation analyses between RD and picture salience ratings, and 17 (4 females and 13 males) participants (mean age 30.08± 3.88 years) were included in the correlation analysis between NS and picture salience rating. Behavioral analysis was conducted using SPSS (PASW Statistics 18, Release Version 18.0.0). fMRI results were visualized using MRIcroN® (nonparametric mapping software [Rorden et al. 2007]).
RESULTS
Descriptive and Post‐Test Picture Salience Rating Results
The post‐test picture salience rating from all 27 participants that completed the rating task indicated a clear distinction between high and low salience stimuli (hs mean rating score: 61.06 ± 11.19; ls mean rating score: 36.61 ± 13.26; paired‐sample t‐test: t(26) = 11.31, P < 0.001). Among these 27 participants, 13 male participants (hs pictures mean rating: 62.61 ± 8.58; ls pictures mean rating: 34.93 ± 10.72) and 4 female participants (hs pictures mean rating: 62.54 ± 8.51; ls pictures mean rating: 39.43 ± 11.23; NS mean: 18.00 ± 1.78; RD mean: 16.00 ± 1.73) also completed the TCI questionnaires and had NS scores (mean: 16.15 ± 3.96), while 15 male participants (hs pictures mean rating: 62.96 ± 8.79; ls pictures mean rating: 34.72 ± 10.07) and 4 females participants were included in the correlation with RD scores (mean: 14.20 ± 4.04).
When all male and female participants were included, we found both hs pictures (Pearson correlation r = 0.51, P = 0.03, two‐tailed) and ls pictures (Pearson correlation r = 0.46, P = 0.04, two‐tailed) were rated as having higher salience in high RD participants (for details, see Table 1).
Table 1.
Post‐test picture salience rating and personality correlation
| Mean ratings for high‐salience pictures | Mean ratings for low‐salience pictures | ||||
|---|---|---|---|---|---|
| R | P | r | P | ||
| Males only | NS | 0.12 | 0.697 | 0.343 | 0.252 |
| RD | 0.529 | 0.043* | 0.332 | 0.227 | |
| Male and female | NS | 0.13 | 0.62 | 0.366 | 0.149 |
| RD | 0.508 | 0.026* | 0.464 | 0.045* | |
* 0.01 < P < 0.05, Pearson correlation, significant level two‐tailed.
When only male participants were considered, neither hs (P = 0.70, two‐tailed) nor ls (P = 0.25, two‐tailed) rating scores showed significant correlation with NS. However, we found that males with higher RD scores rated the hs pictures as having significantly higher salience compared with the ratings of low RD participants (Pearson correlation r = 0.53, P = 0.04, two‐tailed), but this difference was not found for ls pictures (Pearson correlation r = 0.332, P = 0.23, two‐tailed).
Task‐Related Brain Activity in the Salience Network
When all male and female participants were included, we found that hs expectancy significantly increased brain activity in large areas of the brain (k = 6,318, P < 0.001 FWE correction), including the right inferior occipital gyrus (maximum peak [25 −102 8], Z = 6.52), middle cingulate cortex (MCC, maximum peak [−5 −27 38], Z = 6.38) and pregenual anterior cingulate cortex (pgACC, maximum peak [0 48 3], Z = 6.47), as compared with ls expectancy. Moreover, using right AI and right dACC masks, which were used later during PPI analysis, we evaluated specifically the brain activity within SN, and found that the right AI (maximum peak [30 13 −17], Z = 5.47, k = 49, P = 0.039 FWE correction; Fig. 2A) and the right dACC (maximum peak [5 23 23], Z = 5.89, k = 82, P = 0.009 FWE correction; Fig. 2B) showed increased activity during hs expectancy as compared with ls expectancy. No significant activation was found for the inverse condition (high < low salience expectancy).
Figure 2.
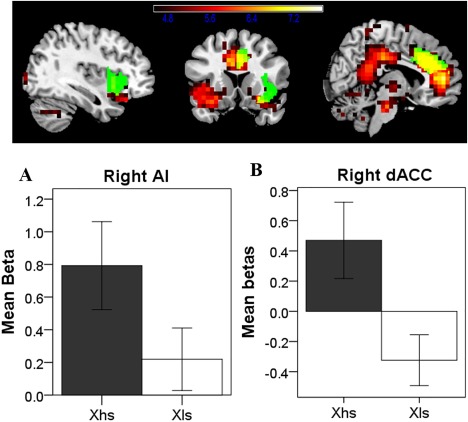
Greater BOLD (red) activity as a function of high salience expectancy than low salience expectancy, overlap with seed regions (right AI/dACC, green). Display threshold: P < 0.05 (FWE corrected, for better visualization). AI, anterior insula; dACC, anterior cingulate cortex; Xhs, high salience expectancy; Xls, low salience expectancy. [Color figure can be viewed at http://wileyonlinelibrary.com]
Similar results were obtained for the hs expectancy effect when only the 48 male participants were included: cluster 1 (k = 5,400, P < 0.001 FWE corrected), including a large part of frontal cortex and cingulate cortex; cluster 2 (k = 198, p < 0.001 FWE corrected), including the left superior occipital gyrus and inferior parietal cortex (maximum peak [−55 −67 23], Z = 4.53). Using right AI and right dACC masks, we found the right AI (maximum peak [30 13 −17], Z = 4.89, k = 43, P = 0.052 FWE correction) and the right dACC (maximum peak [0 28 28], Z = 5.85, k = 81, P = 0.009 FWE correction) showed increased activity during hs expectancy as compared with ls expectancy.
Task‐Related Functional Connectivity of the Salience Network
When all male and female participants were included, the PPI analysis revealed that hs expectancy induced positive right AI FC with hubs of SN (e.g., left AI, middle cingulate cortex [MCC]) and parts of the central executive networks (CEN, e.g., inferior and middle frontal gyrus) and the supplementary motor area (SMA), and negative right AI FC with visual areas (occipital cortex and lingual gyrus) and DMN (precuneus and ventral mPFC [vmPFC]) as compared to ls expectancy (Fig. 3A and Table 1).
Figure 3.

Functional connectivity during salience expectancy: (A) with right AI; (B) with right dACC. Warm‐colored clusters represent positive correlation; cold‐colored clusters represent negative correlation; green clusters represent seed regions (AI or dACC). AI, anterior insula; dACC, dorsal anterior cingulate cortex; IPL, inferior parietal lobule; IFG, inferior frontal gyrus; MFG, middle frontal gyrus; PG, precentral gyrus; AI, anterior insula; SMA, supplementary motor area; pgACC, pregenual anterior cingulate cortex; MTG, middle temporal gyrus; PrC, precuneus; L, left hemisphere; R, right hemisphere. Display threshold: P < 0.001 (uncorrected, for better visualization). [Color figure can be viewed at http://wileyonlinelibrary.com]
The right dACC showed similar positive FC with the CEN, and negative FC with visual areas (Fig. 3B and Table 2) as a function of high versus low salience expectancy. In addition, we found hs expectancy triggered significantly stronger dACC FC with bilateral inferior parietal lobule (IPL) than low salience expectancy.
Table 2.
PPI results
| Contrasts | Seed region | Correlation | Cluster size | FEW | Hemisphere | Brain regions | Brodmann area | Z | x | y | z |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Xhs > Xls | Right AI | Positive | 32 | 0.040 | Left | Precentral gyrus | 6 | 4.27 | −35 | −7 | 48 |
| 29 | 0.052 | Left | AI | 45 | 4.34 | −30 | 18 | 3 | |||
| IFG (opercularis) | 44 | 3.53 | −55 | 8 | 8 | ||||||
| 67 | 0.003 | Bilateral | Supplementary motor area (SMA) | 6 | 4.23 | 5 | 3 | 63 | |||
| 3.90 | −5 | −2 | 63 | ||||||||
| Middle cingulate cortex/caudal cingulate zone | 24 | 3.54 | −10 | 8 | 43 | ||||||
| 33 | 0.037 | Right | Precentral gyrus | 6 | 4.11 | 45 | −2 | 43 | |||
| Middle frontal gyrus | 3.72 | 40 | −2 | 58 | |||||||
| 34 | 0.033 | Left | Middle frontal gyrus | 45 | 3.94 | −30 | 38 | 18 | |||
| 3.21 | −35 | 43 | 33 | ||||||||
| 10 | 4.16 | −35 | 48 | 18 | |||||||
| Xhs > Xls | Right AI | Negative | 245 | <0.001 | Bilateral | Superior occipital gyrus, extended to lingual gyrus | 18 | 4.83 | −10 | −102 | 18 |
| 4.45 | −5 | −82 | −2 | ||||||||
| 4.43 | 20 | −102 | 13 | ||||||||
| 71 | 0.002 | Bilateral | Precuneus/posterior cingulate cortex | 30 | 4.31 | −5 | −62 | 13 | |||
| 4.07 | −10 | −57 | 8 | ||||||||
| 3.58 | −15 | −42 | −7 | ||||||||
| 35 | 0.031 | Bilateral | Medial prefrontal cortex/pgACC | 10 | 4.05 | −5 | 43 | −7 | |||
| 3.28 | 5 | 33 | −12 | ||||||||
| Xhs > Xls | Right dACC | Positive | 337 | <0.001 | Bilateral | Supplementary motor area (SMA) | 6 | 5.21 | 5 | 3 | 58 |
| 5.12 | −10 | 3 | 58 | ||||||||
| Precentral gyrus | 5.05 | 45 | 3 | 48 | |||||||
| 83 | 0.001 | Bilateral | Precuneus/posterior cingulate cortex | 31 | 4.74 | 0 | −22 | 23 | |||
| 214 | <0.001 | Bilateral | Inferior parietal lobule | 40 | 4.58 | 60 | −47 | 38 | |||
| 7 | 4.46 | 25 | −62 | 38 | |||||||
| 4.39 | 15 | −67 | 43 | ||||||||
| 39 | 0.028 | Right | Middle temporal gyrus | 21 | 4.43 | 50 | −22 | −12 | |||
| 93 | 0.001 | Left | Inferior parietal lobule | 40 | 4.39 | −40 | −52 | 43 | |||
| Supramarginal G (TPJ) | 4.22 | −45 | −47 | 33 | |||||||
| 42 | 0.022 | Left | Precentral gyrus | 6 | 4.32 | −40 | −2 | 48 | |||
| Xhs > Xls | Right dACC | Negative | 184 | <0.001 | Bilateral | Superior occipital gyrus, extended to lingual gyrus | 18 | 5.35 | −10 | −102 | 18 |
| 5.20 | 15 | −97 | 18 | ||||||||
| 5.16 | 20 | −102 | 13 |
FWEc, cluster‐level FWE correction; FWE, family‐wise error; AI, anterior insula; dACC, dorsal anterior cingulate cortex.
When only the 48 male participants were included, the PPI analysis showed only a tendency for hs expectancy to induce positive right AI FC with SMA ([0 3 58], k = 22, Z = 3.61, P = 0.085 FWE corrected), and negative right AI FC with visual areas (cluster 1, [−10 −97 13], k = 189, Z = 4.81, P < 0.001 FWE corrected; cluster 2, [15 −27 −17], k = 51, Z = 4.29, P = 0.006 FWE corrected; cluster 3, [−10 −57 8], k = 102, Z = 4.16, P < 0.001 FWE corrected).
The right dACC showed positive FC with the SMA ([−5 3 63], k = 68, Z = 4.43, P = 0.002 FWE corrected), and negative FC with visual areas ([15 −97 13], k = 427, Z = 5.57, P < 0.001 FWE corrected) as a function of high versus low salience expectancy.
Functional Connectivity and Personality Correlation
When both male and female participants were included, we found a negative correlation between NS and right AI PPI with right MCC during high vs low salience expectancy ([0 −2 48], K = 28, Z = 4.00, FWE P < 0.05 corrected). However, the dACC FC did not correlate significantly with RD.
When the 32 male participants who had both PPI and TCI data were included, we found a negative correlation between NS and right AI PPI with right MCC during high versus low salience expectancy ([10 3 43], k = 38, Z = 4.17, P < 0.05 FWE correction; Fig. 4A). This means that, with increasing NS, the FC between right AI and MCC is weaker when expecting hs pictures compared to expectancy of ls pictures (Fig. 4B).
Figure 4.

Novelty seeking (NS, top) and reward dependence (RD, bottom) correlated with PPI, as quantified by functional connectivity (FC) with predetermined seed regions: right AI and dACC (green). (A) Caudate (cold color). (C) Middle cingulate cortex (MCC) (cold color). Display threshold: P < 0.001 (uncorrected, for better visualization). Figures on the right represent the FC correlation with NS (B) and RD (D) under the conditions (Xhs > Xls). [Color figure can be viewed at http://wileyonlinelibrary.com]
Furthermore, we found a negative correlation between RD scores and right dACC PPI with caudate (striatum) ([−5 13 8], k = 30, Z = 3.9, P < 0.05 FWE correction; Fig. 4C) as a function of high versus low salience expectancy. More specifically, in subjects with low RD, right dACC to caudate FC was lower during expectancy of hs pictures, compared to expecting ls pictures, while this difference disappeared and even reversed for subjects with high RD (Fig. 4D).
No other significant correlation was found between HA and the PPI results.
Brain Activity/FC and Post‐Hoc Rating Correlation
Both BOLD data and ratings were available for 25 participants (including 8 females), but the whole‐brain correlation analysis within the full sample did not show any significant correlations. On subsequent ROI analysis (using AI and dACC masks as PPI seed regions), we evaluated BOLD‐rating correlations within the right AI and dACC and still found no significant correlations.
We then examined the correlation applying an ROI analysis in the 17 male participants, and found increased right AI ([25 23 −12], k = 39, P = 0.082 FWE corrected) and dACC ([5 23 28], k = 44, P = 0.155 FWE corrected) activity showed a tendency to be related to lower ratings for ls pictures.
To explore the PPI‐TCI findings further, we examined the PPI‐rating associations in the 25 participants (including 8 females). Whole‐brain correlation analysis did not reveal any significant correlations. We used small volume correction (SVC) to limit our investigations to prediction of AI‐MCC FC and dACC‐caudate FC. The search region was a 5‐mm‐radius sphere around the significant MCC and caudate peaks (based on the significant PPI‐TCI findings). The mean ratings for hs pictures tended to be positively correlated with AI‐MCC FC (hs rating: [5 −2 33], P = 0.052 FWE corrected) and mean ratings for ls pictures were negatively correlated with AI‐MCC FC (ls rating: [5 3 38], P = 0.041 FWE corrected). Furthermore, mean ratings for hs pictures showed a tendency to negative correlation with dACC‐caudate FC (hs rating: [15 13 8], P = 0.063 FWE corrected), and mean ratings for ls were positively correlated with dACC‐caudate FC (ls rating: [−5 23 8], P = 0.126 FWE corrected).
In the 17 male participants, the tendencies became weaker for AI‐MCC FC (hs rating: [5 −2 33], P = 0.078 FWE corrected; ls rating: [10 −2 43], P = 0.070 FWE corrected) but became stronger for dACC‐caudate FC (hs rating: [5 −2 13], P = 0.021 FWE corrected; ls rating: [0 8 3], P = 0.070 FWE corrected).
DISCUSSION
To investigate whether and how personality influences the FC of the SN underlying internal salience expectation, we conducted a salience expectancy task during fMRI scanning. We found that specific temperaments, that is, NS and RD, modulated either AI‐MCC decoupling or dACC‐caudate decoupling as functions of high versus low salience expectancy.
In line with previous findings [Bressler and Menon, 2010; Menon and Uddin, 2010], our event‐related fMRI results confirmed that the SN is important for internal salience evaluation: more specifically, hs expectancy induces stronger AI and dACC activation than low salience expectancy. We note that a negative BOLD response in dACC while responding to the low salience expectancy condition was found in most of our participants, in contrast with the high salience expectancy condition, which induced a positive BOLD response. This finding is in accord with several previous studies, in which the dACC was often activated by cognitive tasks relating to the continuous internal monitoring of action, attention and self‐reflection [Amodio and Frith, 2006; Northoff et al., 2006], and task‐relevant interferences [Stawarczyk et al., 2011]. A recent study showed that when actively monitoring their thoughts (heightened cognitive self‐consciousness), participants showed a positive BOLD response in the dACC. However, when participants were asked to notice the distractor (external monitoring), they showed a negative BOLD response in dACC [Bonhage et al., 2016]. When expecting positive stimuli, patients with major depression showed an abnormal negative BOLD response in the dACC region, suggesting that the dACC is responsible for emotional/salience‐related internal expectancy behaviors [Zhang et al., 2017]. It is possible that the high salience expectancy led to increased internal monitoring, constituting greater task demands, and the stronger positive BOLD response represented a heightened cognitive self‐consciousness, which induced more cognitive resource allocation. On the other hand, the low expectancy cues did not trigger such strong task demands and therefore did not occupy more cognitive resources. Instead, a negative BOLD response was exhibited, as a consequence of high versus low expectancy resource competition. Our BOLD‐rating correlation results (although not significant) could lend some support to the theory: the ratings (especially the ratings for low salience pictures) might predict the differences between AI and dACC activities that were induced by Xhs versus Xls; more specifically, when participants rated the low salience pictures with lower scores, it was easier for the participants' SN to distinguish between the high and low expectancy conditions. In other words, in this study, the more the participants rated the low salience pictures as meaningful, the more cognitive resources they would engage during expectancy of high salience stimuli.
Using PPI analysis, we then found that in responding to high‐ compared with low‐salience cues (goal‐relevant stimuli), the FC among hubs of the SN (AI, dACC) and the hubs of the CEN was significantly increased, but the FC among hubs of the SN and the hubs of the DMN was significantly decreased. Our findings provide direct evidence in support of the large‐scale network theory, which proposes that the switching mechanisms in large‐scale networks could be regarded as saliency filters that help to differentially amplify a stimulus [Menon and Uddin, 2010]. Menon [2011] proposed a unifying triple network model of psychiatric dysfunctions, where both disconnections within the SN and deviant interaction between the SN, and particularly the DMN and also a central executive network, are considered. For instance, by engaging the right AI, a stimulus can preferentially access the brain's attentional and working memory resources. Conjoint activity of ACC and AI would thus act as input and output regions to produce subjective emotional feelings and to mediate responses (self‐awareness) to internal and external events [Medford and Critchley, 2010]. SN (especially the right AI) is responsible for switching the CEN and the DMN on or off during cognitive information processing depending on the tasks [Fox et al., 2005; Menon and Uddin, 2010]. Therefore, the SN acts as a “causal outflow hub,” receiving and processing internal and external signal inputs, detecting salient events, and initiating the corresponding cognitive controls, which in turn regulate the homeostatic state and human behaviors [Menon and Uddin, 2010; Uddin et al., 2009]. Resting‐state FC (RSFC) analysis suggests that the SN controls goal‐directed behavior through stable maintenance of task mode and strategy [Dosenbach et al., 2007]; high concentration of von Economo neurons (large layer V projection bipolar neurons) in the SN as a result of human evolution might explain the unique role of the SN in intuition‐based choice in social situations [Robbins et al., 2012] and reflect stable set‐maintenance in goal‐directed behavior in humans [Dosenbach et al., 2008]. During the expectancy session, the participants were instructed to actively expect the subsequent pictures according to the cues, which means that they would engage more cognitive resources in response to the high salience cues (representing exciting and higher significance events to follow) but allocate less cognitive resources to the low salience cues (nothing interesting to follow). Therefore, a high salience expectancy condition can be regarded as a stronger goal‐relevant condition, which we found to be related to stronger SN to CEN connectivity in order to prepare the brain for cognitive processing. On the other hand, a low salience expectancy condition can be regarded as a goal‐irrelevant condition, which we found to be related to stronger SN to DMN connectivity indicative of “mind wandering.” Through the present salience expectancy task, we provide potential support for the notion that the SN hubs exhibit increased FC with executive systems, because the manipulation of high salience expectancy was goal‐relevant, but the same regions would “toggle” resources to the default network if the ensuing material was goal‐irrelevant (low salience). Structural or functional impairment of the SN “switching” functions could therefore be a risk factor for psychiatric disorders [Krishnadas et al., 2014; Medford and Critchley, 2010; Modinos et al., 2009]. Indeed, decreased FC was recently observed between the bilateral insula and the DMN in cocaine‐dependent individuals during rest, and the organizational disruptions within the SN in these individuals have been associated with greater alexithymia [Liang et al., 2015]. RSFC in depressive patients has also been found to alter to other target networks [Zhang et al., 2016]. Furthermore, the FC between dorsomedial prefrontal cortex (dACC) and posterior DMN showed an abnormal disconnection when depressive patients were expecting positive stimuli [Zhang et al., 2017].
In addition to the CEN, we also found that SN was positively correlated with other brain regions, for example the SMA. The posterior medial frontal cortex (pMFC), also known as the SMA, is well‐recognized as a “performance monitoring” region, which is especially involved in reward anticipation, monitoring, and comparing performance with internal goals [Ridderinkhof et al., 2004]. Uddin et al. [2014] used a meta‐analytic approach and found that the dorsal AI was regarded as a critical functional hub in the human brain, co‐activating with several brain regions regardless the type of tasks, including the SMA (very similar to our SMA cluster). According to the theory of embodied emotion [Niedenthal, 2007; Niedenthal and Maringer, 2009], “perceiving and thinking about emotion involve perceptual, somatovisceral, and motoric re‐experiencing of the relevant emotion in one's self.” Previous studies have suggested that the SMA acts as an “interface” between emotion systems (e.g., limbic system) and motor systems (e.g., the primary motor area), to regulate emotionally triggered movements [Oliveri et al., 2003]. The SMA mediates network changes related to emotional reactivity when processing content reflecting insecure attachment [Borchardt et al., 2015], and in controlling internally triggered movements [Elangovan et al., 2016]. Specifically, it has been proposed that FC between the SMA and brain areas responsive to emotion in others reflects an empathic response to negative emotional stimuli, in which an impulse to act results in an internalized motor response [Pratumvinit et al., 2016]. Parallel to these findings, salient cues in a paradigm involving mental actions have led to enhanced FC between reward centers and motor cortex [Mendelsohn et al., 2014], and the SN has been specifically proposed to play a role in motor response planning [Spencer and Rupp, 2009]. Taken together, in our study, the increased SMA‐AI and SMA‐dACC FC with expectancy of high compared with low salience stimuli is in line with these suggestions, and a plausible interpretation of the current finding is that the increased FC between SMA and SN nodes during high salience expectancy (expecting highly arousing pictures) reflects internal preparation to react to emotionally salient stimuli. Our results add further evidence that the SN is crucial for the control of goal‐directed behavior by building a bridge between the salience signal input (salience detection) and performance output (physiological response).
Reduced FC between AI/dACC and visual areas triggered by goal‐relevant stimuli (high salience pictures) may further reflect decreased vigilance toward external visual information during expectancy. While we are not aware of any studies similar to the present work, we identified indirect evidence that may support our interpretation. A recent study found that migraine patients with aura showed decreased FC between the AI and occipital regions (the same region as in our finding) during rest as compared with healthy controls and migraine patients without aura, supporting the notion that such FC is responsible for detection and reorientation toward salient and behaviorally relevant stimuli [Niddam et al., 2008]. In particular, such reduced FC between AI and occipital regions was negatively related to stronger headache severity, suggesting an evaluation process of the impact of sensory stimuli on the body state (self‐referential event). However, a limitation for such an interpretation is that it is difficult to measure the actual mental processes during anticipation, especially as the sensory input of high and low salience cues was quite similar. Future studies should implement ways to investigate how visual cues are related to internal attention regulation.
Earlier studies of correlation between temperament and alcohol‐seeking behavior have suggested distinct personality‐related behavior patterns. For instance, people with low NS personality are described as rigid, orderly, and attentive to details, while people with high NS personality are described as impulsive, disorderly, and distractible; people with low RD personality as socially detached, emotionally insensitive, and independently self‐willed, while people with high RD personality are emotionally dependent, sympathetic, sensitive to social cues, and persistent [Cloninger, 1987]. Bermpohl et al. [2008] used an emotional expectancy task and found that participants with higher NS scores showed greater BOLD signals in the mPFC during emotional expectancy as compared to neutral expectancy, but the actual perception of emotional pictures did not reveal any such correlation. Contrary to Bermpohl's finding, Enzi et al. [2009] found the activity of right dACC and left AI was negatively mediated by NS when participants perceived stimuli with low personal relevance. They concluded that high novelty seekers were not able to react properly to low salience stimuli, whereas low novelty seekers were better able to react in an appropriate way even to events without highly self‐relevant information.
Our PPI and personality correlation confirmed our hypothesis and revealed that right AI‐MCC FC related to high salience expectancy was negatively modulated by NS. The MCC is the ventral part of the pMFC, and as described above, the right AI is strongly connected to pMFC/SMA when expecting high rather than low salience stimuli. More specifically, the cluster within the MCC is also known as the caudal cingulate zone (CCZ), which is adjacent to the SMA and anatomically connects the SMA and spinal cord to regulate body‐related movement. Moreover, the gray matter volume of this region has been found to be positively correlated with NS [Gardini et al., 2009]. However, the FC was only evident in participants with lower NS scores; for higher NS participants, the FC was even reversed, such that low rather than high salience picture expectation was related to stronger FC. We postulate that the higher FC in participants with lower NS scores reflects a requirement for greater engagement of performance monitoring systems when expecting a high salience picture in those who habitually avoid novelty. This enhanced engagement might relate to their subsequent tendency to rate the high salience pictures with higher preference scores. On the other hand, participants with higher NS personality might not be as well able to disconnect the AI‐MCC FC for low salience expectancy as the lower NS personality participants. We interpret the inability to disconnect this brain network as resulting in greater cost in terms of brain resources that would otherwise have contributed to other networks, resulting in a lack of increase in their right AI to MCC FC for high salience expectancy. The latter finding might relate to their subsequent tendency to rate the low salience pictures with higher preference scores. Therefore, whether participants had a high or low NS personality led to employment of differing salience expectancy processing mechanisms. Within a relatively small group size (n = 13), we did not find significant correlation between NS and picture salience rating, indicating that NS may not influence the judgment of saliency in our participants, or the processing of salience perception. Rather, NS personality engaged distinct AI‐MCC FC disconnection to high salience expectancy, which is in line with the notion that the MCC/CCZ plays a top–down role, influencing AI activity because of actively expecting information of high saliency rather than low saliency. High NS participants, however, showed a “deficiency” in this effect; instead, they showed a significant AI‐MCC FC disconnection when actively expecting information of high salience. Our findings again suggest that the right AI is the key region of the SN and acts as a salience filter [Menon and Uddin, 2010] to identify highly self‐relevant and emotionally arousing (salient) information based on self‐reflection and temperament. Indeed, evidence suggests a right AI to pMFC (SMA‐CCZ) loop is involved in regulating salience expectancy behavior [Modinos et al., 2009], and atypical engagement of the dorsal AI and SMA/MCC could result in misappropriated salience detection and changed attentional processes, which has been related to several psychiatric conditions (reviewed in Uddin [2015]).
In contrast to NS, we observed that participants with lower RD scores showed stronger dACC to caudate FC as a function of high salience expectancy rather than low salience expectancy, although our initial PPI results did not reveal significant SN to caudate/striatum FC changes during high versus low salience expectancy. Interestingly, participants with lower RD scores also rated the high salience pictures with lower scores. A weak link between salience rating and the dACC‐caudate FC suggested that increased high salience expectancy related dACC‐caudate FC might predict higher rating scores for low salience pictures, but predict lower rating scores for high salience pictures. It seems that the AI‐MCC FC and dACC‐caudate FC showed opposite prediction direction to the ratings, but one should be cautious about the conclusion when the findings were based on only tendencies from SVC analysis. Nevertheless, if the tendencies were truly representative, participants with higher RD showed decreased dACC‐caudate FC during high salience expectancy as compared with low salience expectancy, but they tended to regard the high salience pictures as being more salient than the low RD participants did. A possible explanation is that compared to the NS personality, which is more sensitive to the expectancy (seeking) period, the RD personality is more sensitive to the consequences of an action—e.g., perception period (actually seeing the stimuli), therefore they would have stronger feelings toward the high‐salience pictures. This hypothesis can be supported by an fMRI study from Krebs et al. [2009], in which they found that NS was positively correlated with reward system activation elicited by novel cues that did not predict reward, whereas RD was related to activations elicited by novel cues that predicted reward. An addiction study also found that smokers who scored higher on RD showed stronger craving reactions to both smoking cues and stress cues [Michalowski and Erblich, 2014].
Judging by the previous studies, it might be confusing that high RD participants showed decreased dACC to caudate FC in relation to high salience expectancy—if the high salience stimuli were regarded as higher reward (higher salience rating), one might expect the opposite. But the question is, does higher dACC‐caudate FC represent higher reward expectancy? Indeed, the RD personality is often found to be structurally or functionally related to the prefrontal/anterior limbic area and striatum (caudate) regions. For example, white matter (WM) fiber tracking from the PFC region to the striatum predicted RD [Cohen et al., 2009], and this finding has been replicated in a later study using a larger sample size in which increased RD was associated with decreased WM architecture coherence in frontal cortex [Bjornebekk et al., 2012]. A negative correlation between RD and gray matter density of frontostriatal and limbic areas has also been identified using MRI [Gardini et al., 2009; Van Schuerbeek et al., 2011]. Studies of addiction behaviors have suggested that addiction is correlated with decreased dopaminergic functioning (due to reduced dopaminergic receptor levels and dopamine release) in the mesocorticolimbic pathways that include striatum and cingulate gyrus [Volkow et al., 2003, 2005], and high RD populations have a higher risk of addiction behaviors [Schreckenberger et al., 2008]. It is therefore likely that participants with higher RD, which is often accompanied with cingulate cortex and caudate dysfunction, have a weaker connection from the SN to the caudate under the influence of high salience expectancy. Such reduced connectivity might relate to the inability of the high RD population to control their desire for highly rewarding events to follow.
In summary, both NS and RD may play important roles in salience expectancy but with different neurobiological mechanisms. Higher NS personality might relate to insufficient disconnection between the AI and the midline cingulate cortex when expecting low‐salience pictures and the tendency to perceive low‐salience stimuli with higher significance. In contrast, higher RD personality might relate to insufficient connectivity between the dACC and the caudate when expecting high salience pictures and to having stronger feelings when perceiving high salience stimuli.
Future studies should gather more behavioral and FC evidence to test these hypotheses. Moreover, due to the limited number of subsamples with overlapping useful personality ratings and fMRI data, separate correlational analyses were only possible in the male group, while for females, the overlap of 4 subjects was too small. Accordingly, we critically limit the correlational part of our findings to an effect in males, which deserves future investigation in female individuals.
CONCLUSION
In summary, our PPI and personality correlation findings provide strong evidence for a specific modulation of FC between the SN and the affective/reward system by personality traits when expecting high salience events. Both high NS and high RD participants had insufficient connection/disconnection between the SN and affective/reward networks, via distinct routes, as a function of high/low salience information expectancy. Our findings support the hypothesis that addiction behavior results in part from dysfunctions during salience expectancy, through connection/disconnection between the SN and the affective/reward system when distinguishing between events with differing salience, depending on the dimensions of the personality.
REFERENCES
- Amodio DM, Frith CD (2006): Meeting of minds: The medial frontal cortex and social cognition. Nat Rev Neurosci Vol. 7:268–277. [DOI] [PubMed] [Google Scholar]
- Beck DM, Kastner S (2005): Stimulus context modulates competition in human extrastriate cortex. Nat Neurosci 8:1110–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin RE, Raju JD, Schmidt PJ, Adams LF, Rubinow DR (2001): Effects of the menstrual cycle on measures of personality in women with premenstrual syndrome: A preliminary study. J Clin Psychiatry 62:337–342. [DOI] [PubMed] [Google Scholar]
- Bermpohl F, Pascual‐Leone A, Amedi A, Merabet LB, Fregni F, Gaab N, Alsop D, Schlaug G, Northoff G (2006): Dissociable networks for the expectancy and perception of emotional stimuli in the human brain. NeuroImage 30:588–600. [DOI] [PubMed] [Google Scholar]
- Bermpohl F, Pascual‐Leone A, Amedi A, Merabet LB, Fregni F, Wrase J, Schlagenhauf F, Bauer M, Heinz A, Schlaug G, et al. (2008): Novelty seeking modulates medial prefrontal activity during the anticipation of emotional stimuli. Psychiatry Res 164:81–85. [DOI] [PubMed] [Google Scholar]
- Bjornebekk A, Westlye LT, Fjell AM, Grydeland H, Walhovd KB (2012): Social reward dependence and brain white matter microstructure. Cereb Cortex 22:2672–2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchardt V, Krause AL, Li M, van Tol MJ, Demenescu LR, Buchheim A, Metzger CD, Sweeney‐Reed CM, Nolte T, Lord AR, Walter M (2015): Dynamic disconnection of the supplementary motor area after processing of dismissive biographic narratives. Brain Behav 5:e00377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressler SL, Menon V (2010): Large‐scale brain networks in cognition: Emerging methods and principles. Trends Cogn Sci 14:277–290. [DOI] [PubMed] [Google Scholar]
- Calder AJ, Ewbank M, Passamonti L (2011): Personality influences the neural responses to viewing facial expressions of emotion. Phil Trans R Soc B 366:1684–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Zhao Z, Desmond JE, Kang E, Gross J, Gabrieli JDE (2001): An fMRI study of personality influences on brain reactivity to emotional stimuli. Behav Neurosci 115:33–42. [DOI] [PubMed] [Google Scholar]
- Canli T, Sivers H, Gotlib IH, Gabrieli JDE (2002): Amygdala activation to happy faces as a function of extraversion. Science 296:2191. [DOI] [PubMed] [Google Scholar]
- Canli T, Amin Z (2002): Neuroimaging of emotion and personality: Scientific evidence and ethical considerations. Brain Cogn 50:414–431. [DOI] [PubMed] [Google Scholar]
- Cloninger CR (1987): Neurogenetic adaptive mechanisms in alcoholism. Science 236:410–416. [DOI] [PubMed] [Google Scholar]
- Cloninger CR (1994): Temperament and personality. Curr Opin Neurobiol 4:266–273. [DOI] [PubMed] [Google Scholar]
- Cohen MX, Schoene‐Bake JC, Elger CE, Weber B (2009): Connectivity‐based segregation of the human striatum predicts personality characteristics. Nat Neurosci 12:32–34. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL (2002): Control of goal‐directed and stimulus‐driven attention in the brain. Nat Rev Neurosci 3:201–215. [DOI] [PubMed] [Google Scholar]
- Bonhage C, Weber F, Exner C, Kanske P (2016): Thinking about thinking: Neural mechanisms and effects on memory. Neuroimage 127:203–214. [DOI] [PubMed] [Google Scholar]
- Dinica K, Demenescu LR, Lord A, Krause AL, Kaiser R, Horn D, Metzger CD, Walter M (2015): Self‐directedness and the susceptibility to distraction by saliency. Cogn Emot 1–9. [DOI] [PubMed] [Google Scholar]
- Dosenbach NUF, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RAT, Fox MD, Snyder AZ, Vincent JL, Raichle ME, Schlaggar BL, Petersen SE (2007): Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci USA 104:11073–11078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE (2008): A dual‐networks architecture of top‐down control. Trends Cogn Sci 12:99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elangovan S, Venugopalan SR, Srinivasan S, Karimbux NY, Weistroffer P, Allareddy V (2016): Integration of basic‐clinical sciences, PBL, CBL, and IPE in U.S. dental schools' curricula and a proposed integrated curriculum model for the future. J Dent Educ 80:281–290. [PubMed] [Google Scholar]
- Enzi B, de Greck M, Prosch U, Tempelmann C, Northoff G (2009): Is our self nothing but reward? Neuronal overlap and distinction between reward and personal relevance and its relation to human personality. PLoS One 4:e8429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME (2005): The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA 102:9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ (1997): Psychophysiological and modulatory interactions in neuroimaging. Neuroimage 6:218–229. [DOI] [PubMed] [Google Scholar]
- Gardini S, Cloninger CR, Venneri A (2009): Individual differences in personality traits reflect structural variance in specific brain regions. Brain Res Bull 79:265–270. [DOI] [PubMed] [Google Scholar]
- Graf H, Abler B, Hartmann A, Metzger CD, Walter M (2013): Modulation of attention network activation under antidepressant agents in healthy subjects. Int J Neuropsychopharmacol 16:1219–1230. [DOI] [PubMed] [Google Scholar]
- Hamann SB, Canli T (2004): Individual differences in emotion processing. Curr Opin Neurobiol 14:233–238. [DOI] [PubMed] [Google Scholar]
- Herwig U, Abler B, Walter H, Erk S (2007): Expecting unpleasant stimuli – An fMRI study. Psychiatry Res Neuroimag 154:1–12. [DOI] [PubMed] [Google Scholar]
- Horn DI, Yu C, Steiner J, Buchmann J, Kaufmann J, Osoba A, Eckert U, Zierhut KC, Schiltz K, He H, Biswal B, Bogerts B, Walter M (2010): Glutamatergic and resting‐state functional connectivity correlates of severity in major depression ‐ The role of pregenual anterior cingulate cortex and anterior insula. Front Syst Neurosci 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs RM, Schott BH, Düzel E (2009): Personality traits are differentially associated with patterns of reward and novelty processing in the human substantia nigra/ventral tegmental area. Biol Psychiatry 65:15: 103–110 [DOI] [PubMed] [Google Scholar]
- Krishnadas R, Palaniyappan L, Lang J, McLean J, Cavanagh J (2014): Psychoticism and salience network morphology. Personality Individ Diff 57:37–42. [Google Scholar]
- Lamichhane B, Adhikari BM, Dhamala M (2016): Salience network activity in perceptual decisions. Brain Connect. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert B.N. (2008): International affective picture system (IAPS): Affective ratings of pictures and instruction manual. Technical Report A‐8. [Google Scholar]
- Larsen RJ, Ketelaar T (1991): Personality and susceptibility to positive and negative emotional states. J Personal Social Psychol 61:132–140. [DOI] [PubMed] [Google Scholar]
- Liang X, He Y, Salmeron BJ, Gu H, Stein EA, Yang Y (2015): Interactions between the salience and default‐mode networks are disrupted in cocaine addiction. J Neurosci 35:8081–8090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord A, Horn D, Breakspear M, Walter M (2012): Changes in community structure of resting state functional connectivity in unipolar depression. PLoS One 7:e41282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medford N, Critchley HD (2010): Conjoint activity of anterior insular and anterior cingulate cortex: Awareness and response. Brain Struct Funct 214:535–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn A, Pine A, Schiller D (2014): Between thoughts and actions: Motivationally salient cues invigorate mental action in the human brain. Neuron 81:207–217. [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin LQ (2010): Saliency, switching, attention and control: A network model of insula function. Brain Struct Funct 214:655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V (2011): Large‐scale brain networks and psychopathology: A unifying triple network model. Trends Cogn Sci 15:483–506. [DOI] [PubMed] [Google Scholar]
- Metzger CD, Eckert U, Steiner J, Sartorius A, Buchmann JE, Stadler J, Tempelmann C, Speck O, Bogerts B, Abler B, Walter M (2010): High field fMRI reveals thalamocortical integration of segregated cognitive and emotional processing in mediodorsal and intralaminar thalamic nuclei. Front Neuroanat 4: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger CD, Wiegers M, Walter M, Abler B, Graf H (2015): Local and global resting state activity in the noradrenergic and dopaminergic pathway modulated by reboxetine and amisulpride in healthy subjects. Int J Neuropsychopharmacol 19:pii: pyv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalowski A, Erblich J (2014): Reward dependence moderates smoking‐cue‐ and stress‐induced cigarette cravings. Addict Behav 39:1879–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modinos G, Ormel J, Aleman A (2009): Activation of anterior insula during self‐reflection. PLoS One 4:e4618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Most SB, Chun MM, Johnson MR, Kiehl KA (2006): Attentional modulation of the amygdala varies with personality. NeuroImage 31:934–944. [DOI] [PubMed] [Google Scholar]
- Musolff, N. (2008): Vergleich von subjectiven und objektiven Korrelaten der Salienz: eine kombinierte behaviorale und 7t‐fMRT‐Studie [Comparing subjective and objective salience correlated – A combined behavioral 7t fMRI study] (unpublished diploma thesis). Otto‐von‐Guericke University, Magdeburg, Germany. [Google Scholar]
- Niddam DM, Lai K, Fuh J, Chuang CN, Chen W, Wang S (2016): Reduced functional connectivity between salience and visual networks in migraine with aura. Cephalalgia 36:53–66. [DOI] [PubMed] [Google Scholar]
- Niedenthal PM (2007): Embodying emotion. Science 316:1002–1005. [DOI] [PubMed] [Google Scholar]
- Niedenthal PM, Maringer M (2009): Embodied emotion considered. Emot Rev 1:122–128. [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J (2006): Self‐referential processing in our brain — A meta‐analysis of imaging studies on the self. NeuroImage 31:440–457. [DOI] [PubMed] [Google Scholar]
- Oliveri M, Babiloni C, Filippi MM, Caltagirone C, Babiloni F, Cicinelli P, Traversa R, Palmieri MG, Rossini PM (2003): Influence of the supplementary motor area on primary motor cortex excitability during movements triggered by neutral or emotionally unpleasant visual cues. Exp Brain Res 149:214–221. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Kastner S, Ungerleider LG (2003): Neuroimaging studies of attention: From modulation of sensory processing to top‐down control. J Neurosci 23:3990–3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratumvinit B, Charoenkoop N, Niwattisaiwong S, Kost GJ, Tientadakul P (2016): The effects of temperature and relative humidity on point‐of‐care glucose measurements in hospital practice in a tropical clinical setting. J Diabetes Sci Technol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S (2004): The role of the medial frontal cortex in cognitive control. Science 306:443–447. [DOI] [PubMed] [Google Scholar]
- Robbins JM, Ford MT, Tetrick LE (2012): Perceived unfairness and employee health: A meta‐analytic integration. J Appl Psychol 97:235–272. [DOI] [PubMed] [Google Scholar]
- Rorden C, Karnath HO, Bonilha L (2007): Improving lesion‐symptom mapping. J Cogn Neurosci 19:1081–1088. [DOI] [PubMed] [Google Scholar]
- Ross C, Coleman G, Stojanovska C (2001): Relationship between the NEO personality inventory revised neuroticism scale and prospectively reported negative affect across the menstrual cycle. J Psychosom Obstet Gynaecol 22:165–176. [DOI] [PubMed] [Google Scholar]
- Schreckenberger M, Klega A, Grunder G, Buchholz HG, Scheurich A, Schirrmacher R, Schirrmacher E, Muller C, Henriksen G, Bartenstein P (2008): Opioid receptor PET reveals the psychobiologic correlates of reward processing. J Nucl Med 49:1257–1261. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC (1998): The Mini‐International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM‐IV and ICD‐10. J Clin Psychiatry 59 Suppl 20:22–33. quiz 34–57. [PubMed] [Google Scholar]
- Spencer S, Rupp DE (2009): Angry, guilty, and conflicted: Injustice toward coworkers heightens emotional labor through cognitive and emotional mechanisms. J Appl Psychol 94:429–444. [DOI] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V (2008): A critical role for the right fronto‐insular cortex in switching between central‐executive and default‐mode networks. Proc Natl Acad Sci USA 105:12569–12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stawarczyk D, Majerus S, Maquet P, D'Argembeau A (2011): Neural correlates of ongoing conscious experience: Both task‐unrelatedness and stimulus‐independence are related to default network activity. PLoS One 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura M, Kawashima R, Nakagawa M, Okada K, Sato T, Goto R, Sato K, Ono S, Schormann T, Zilles K, Fukuda H (2000): Correlation between human personality and neural activity in cerebral cortex. NeuroImage 11:541–546. [DOI] [PubMed] [Google Scholar]
- Suhara T, Yasuno F, Sudo Y, Yamamoto M, Inoue M, Okubo Y, Suzuki K (2001): Dopamine D2 receptors in the insular cortex and the personality trait of novelty seeking. NeuroImage 13:891–895. [DOI] [PubMed] [Google Scholar]
- Tomer R (2008): Attentional bias as trait: Correlations with novelty seeking. Neuropsychologia 46:2064–2070. [DOI] [PubMed] [Google Scholar]
- Turner RM, Hudson IL, Butler PH, Joyce PR (2003): Brain function and personality in normal males: A SPECT study using statistical parametric mapping. NeuroImage 19:1145–1162. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Kelly AM, Biswal BB, Castellanos FX, Milham MP (2009): Functional connectivity of default mode network components: Correlation, anticorrelation, and causality. Hum Brain Mapp 30:625–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Kinnison J, Pessoa L, Anderson ML (2014): Beyond the tripartite cognition–emotion–interoception model of the human insular cortex. J Cogn Neurosci 26:16–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ (2015): Salience processing and insular cortical function and dysfunction. Nat Rev Neurosci Vol. 16: 55–61. [DOI] [PubMed] [Google Scholar]
- Van Schuerbeek P, Baeken C, De Raedt R, De Mey J, Luypaert R (2011): Individual differences in local gray and white matter volumes reflect differences in temperament and character: A voxel‐based morphometry study in healthy young females. Brain Res 1371:32–42. [DOI] [PubMed] [Google Scholar]
- Ventura R, Morrone C, Puglisi‐Allegra S (2007): Prefrontal/accumbal catecholamine system determines motivational salience attribution to both reward‐ and aversion‐related stimuli. Proc Natl Acad Sci USA 104:5181–5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ (2003): The addicted human brain: Insights from imaging studies. J Clin Invest 111:1444–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Ma Y, Fowler JS, Wong C, Ding YS, Hitzemann R, Swanson JM, Kalivas P (2005): Activation of orbital and medial prefrontal cortex by methylphenidate in cocaine‐addicted subjects but not in controls: Relevance to addiction. J Neurosci 25:3932–3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Li M, Qin W, Demenescu LR, Metzger CD, Bogerts B, Yu C, Walter M (2016): Altered functional connectivity density in major depressive disorder at rest. Eur Arch Psychiatry Clin Neurosci 266:239–248. [DOI] [PubMed] [Google Scholar]
- Zhang B, Li S, Zhuo C, Li M, Safron A, Genz A, Qin W, Yu C, Walter M (2017): Altered task‐specific deactivation in the default mode network depends on valence in patients with major depressive disorder. J Affect Disord 207:377–383. [DOI] [PubMed] [Google Scholar]
- Zink CF, Pagnoni G, Martin‐Skurski ME, Chappelow JC, Berns GS (2004): Human striatal responses to monetary reward depend on saliency. Neuron 42:509–517. [DOI] [PubMed] [Google Scholar]


