Abstract
Studies indicate that both explicit and implicit processing of affectively charged stimuli may be reflected in specific behavioural markers and physiological signatures. Here, we investigated whether the pleasantness ratings of a neutral target were affected by the subliminal perception of a painful (a slap) or pleasant (a caress) touch delivered to others. In particular, we combined the continuous flash suppression technique with the affective misattribution procedure to explore subliminal processing of observed pain and pleasure in others. Results show that participants rated the neutral target as more or less likeable depending on whether they were subliminally primed with the pleasant or painful facial expression, respectively. The fMRI activity associated with painful and pleasant subliminal priming was mainly present in the anterior prefrontal cortex and the primary sensorimotor cortex, respectively. Thus, our study provides behavioural and neuro‐physiological evidence that: (i) emotional reactivity toward positive or negative states of others can occur at an entirely subliminal level; (ii) specific neural substrates underpin reactivity to positive‐ and negative‐valence of social emotions. Hum Brain Mapp 38:5562–5576, 2017. © 2017 Wiley Periodicals, Inc.
Keywords: empathy for pain, empathy for pleasant, stimuli, fMRI activity, implicit perception, continuous flash suppression, affective misattribution procedure
INTRODUCTION
Empathy is defined as the ability to perceive, understand, and react to the feelings, thoughts, or attitudes of others while maintaining a clear self‐other distinction [de Vignemont and Singer, 2006; Decety and Jackson, 2004; Decety and Lamm, 2006; Krishnan et al., 2016]. Importantly, empathy implies that the observation or imagination of another person in a particular emotional state may automatically activate the representation of that state in the observer/imaginer [Betti and Aglioti, 2016; Jackson et al., 2006; Lamm et al., 2011]. Thus, the vicarious sharing of others' states may rely both on implicit, largely automatic processes (e.g., mirroring, affective resonance, or emotional contagion) as well as on explicit higher‐order ones (e.g., emotion labelling and description, mentalizing, perspective taking, appraisal of the situation) [Singer, 2006; Singer and Lamm, 2009; Walter, 2012]. While there is a certain degree of agreement on the importance of both implicit and explicit components of empathy, some scholars give special importance to one over the other. Batson et al. [1997], for example, emphasize people's intentional role‐taking ability, which mainly taps into higher‐order, appraisal‐related, cognitive resources [Decety and Ickes, 2011]. In contrast, the perception‐action model [Gallese et al., 2004; Keysers and Gazzola, 2006; Preston and de Waal, 2002] conceptualizes empathy as a largely involuntary, vicarious response to the emotional state of another person, in which preconceptual phenomena, including simulation and imitation, have a clear role in the primitive understanding of another person's mind [Carr et al., 2003; Corradini and Antonietti, 2013]. The empathic sensorimotor reactivity to the suffering of others occurs rapidly and, possibly, involuntarily [Avenanti et al., 2005, 2006, 2010; Betti and Aglioti, 2016; Betti et al., 2009; Botvinick et al., 2005; Costantini et al., 2008; Han et al., 2009; Minio‐Paluello et al., 2006; Valeriani et al., 2008]. Much less is known about the neural network underlying the implicit mechanisms of empathic processes. Two studies have been conducted with the purpose of investigating the subliminal priming of suffering in other individuals [Ibanez et al., 2011; Yamada and Decety, 2009]. Authors in the first study [Ibanez et al., 2011] investigated whether processing of vicarious pain is other‐ rather than self‐related information, and whether it evokes signals of danger rather than empathy. To test this hypothesis, two experiments involving subliminal stimuli were designed. In both experiments, participants were presented with semantic expressions referring to neutral or painful events and previously primed with the faces of participants themselves, or those of others. Behavioural responses and electrical activity were then measured. Results showed that other‐face priming facilitated the detection of semantic pain expressions and modulated early (N1) and late (P3) cortical responses. These results illustrate the subliminal effect of empathy‐inducing stimuli and suggest that vicariously experienced pain acts as a negative affective prime, supporting the hypothesis of pain threat value in empathy. In another behavioural study, Yamada and Decety [2009] explored whether the affective valence of unconscious stimuli influences the detection of pain in others. In their study, the tendency to judge pain intensity was facilitated by unconscious negative affective processing, implying that detection of pain is primarily influenced by its inherent threat value. The spatiotemporal brain dynamics of empathy for pain and for happiness, instead, were recently investigated by a study [Wang et al., 2016], in which participants were primed by photographs of a stranger or a close friend. Their results suggested that only the priming of the close friend modulates both early‐ and late‐EEG signatures of pain and happiness processing. Together, these findings provide an incipient understanding of just how fundamental the unconscious detection of affective stimuli is to the processes involved in the experience of empathy and sympathy.
Neuroimaging and neurophysiological studies highlight both similarities and differences between first‐person and vicarious emotions such as disgust [Jabbi et al., 2007; Wicker et al., 2003], sadness [Rameson et al., 2012], or pleasant somatosensory and affective experiences [Francis et al., 1999; Lamm et al., 2015; McCabe et al., 2008; Rolls et al., 2003]. Pain, however, has been the focus of the majority of studies having to do with the neural basis of empathy [Engen and Singer, 2013; Krishnan et al., 2016; Lamm et al., 2011]. Although the somatic and vicarious pain representations are not entirely overlapping [Krishnan et al., 2016; Lamm et al., 2011], studies indicate that the explicit representation of self and others' pain may involve primary (S1) and secondary (S2) somatosensory areas, bilateral anterior insula (AI), supplementary motor area (SMA), anterior cingulate cortex (ACC), and midcingulate cortex (MCC) along with the underlying thalamic and brainstem regions.
Although the field is still in its infancy [Morelli et al., 2015a], the study of empathy for positive states (such as happiness) is rapidly increasing [Fusaro et al., 2016] to match the already established neuroscientific research on empathy for negative states such as pain or disgust. Given that the construct of positive empathy (PE) remains ill‐defined, different terms are used by different authors in reference to it (e.g., empathic joy, [Smith et al., 1989]; responsiveness to others' positive emotional disclosures, [Gable et al., 2006; Reis et al., 2010]; vicarious reward [Mobbs et al., 2009; Morelli et al., 2015a].
Generally speaking, PE refers to conditions in which imagining, recalling, observing, or learning of others' positive outcomes can trigger positive states in the empathizer. Operationally, PE has been described referring to the following three distinct empathic states: (i) empathic cheerfulness, which is the display of positive emotion to a suffering person in order to cheer that person up [Light et al., 2015]; (ii) empathic happiness, a joyful response to positive events that involve others [Batson et al., 1995]; and (iii) goodwill, the desire to see others in a happy state [Light et al., 2015], which may represent an innate attitude in promoting prosocial behaviours. While scarce, previous studies indicate that personal and vicarious rewards may be represented in both distinct and overlapping neural networks [Knutson et al., 2005; Krishnan et al., 2016; Liu et al., 2011]. In this vein, meta‐analytic evidence [Morelli et al., 2015b] suggests that vicarious reward is represented in neural regions that are also involved when directly experiencing reward. In particular, research on the neural correlates of positive empathy suggests that: (i) first‐hand and vicarious positive social touch (a caress) generates common activity in S1 and S2 [Ebisch et al., 2011]; (ii) depending on the tactile C afferents activity, also the posterior insular cortex has been found being involved in encoding of both self‐affective touch during social interaction and observed moving caresses [Morrison et al., 2011], (iii) the gender of a caresser and the concomitant perceived pleasantness of the touch influences neural activity in S1, hinting at this structure's role in coding the affective valence of touch in social contexts [Gazzola et al., 2012].
In the present study, we explored the neurophysiological correlates of the implicit perception of pain and pleasure in other individuals. We linked these correlates to empathy by measuring the BOLD responses of people tested with a novel experimental paradigm [Chiesa et al., 2015] that combines affective misattribution procedure (AMP) and continuous flash suppression task (CFS). AMP entails the brief presentation of a prime with negative or positive valence that influences the explicit likeness rating of neutral unfamiliar targets, for example, a Chinese logogram for non‐Chinese readers [Payne et al., 2005]. But rather than using a brief presentation, we presented a prime stimulus that was impenetrable to the consciousness by way of a CFS procedure. Introduced by Tsuchiya and Koch [2005], CFS is a procedure in which high‐contrast scrambled patterns are flashed to the dominant eye, thus rendering the low‐contrast stimulus presented to the nondominant eye undetectable by conscious awareness. Based on the assumption that the subliminal presentation of unpleasant, pleasant or neutral social stimuli to others may influence the direct likeness ratings [Nosek et al., 2011], we hypothesized that facial expressions of pain, pleasure or neutral touch might allow us to investigate whether subliminal primes with different valences activate different neural systems, as well as the degree of overlap between these neural systems and those activated during explicit observation and first‐hand experience of the same states.
In regards to brain activity, we predicted that (i) pain expressions would engage the anterior cingulate cortex (ACC) and the insula, two regions known to be activated during first‐person and vicarious pain [e.g., Jackson et al., 2006; Lamm et al., 2011]; while (ii) observing pleasant situations would relate to activity in the somatosensory [Ebisch et al., 2011; Francis et al., 1999; Gazzola et al., 2012] and orbitofrontal regions [Morelli et al., 2015b].
MATERIALS AND METHOD
Participants
Twenty right‐handed volunteers gave written consent to participate in the study (13 female, mean age 24.7 ± 3.7 years). All of them were naïve as to the purposes of the experiment and free from any contraindication to MRI scanning. All volunteers were Italian, did not have any familiarity whatsoever with Chinese language, had normal or corrected‐to‐normal vision, and had not reported any neurological or psychiatric diseases. The study was approved by the independent Ethics Committee of the Santa Lucia Foundation (Scientific Institute for Research Hospitalization and Health Care) and was carried out in accordance with the principles of the 1964 Declaration of Helsinki.
Stimuli and Procedure
Primes consisted of pictures displaying faces of a stranger experiencing Neutral (a touch), Painful (a slap), or Pleasant (a caress) touch. There were 72 total greyscale images of faces (eight models, half females, with nine pictures for each model, three for each touch‐condition) cropped to show only the face, hair, and the acting hand (Fig. 1A). All images were matched for size (1,024 × 768 pixels), brightness, and contrast. To keep the primes subliminal, we used the CFS technique. In this powerful type of binocular rivalry, a series of high‐contrast patterns (the mask) are continuously flashed to the dominant eye, while the image of interest (the prime) is presented to the other eye (Fig. 1B). Under such binocular rivalry condition, the eye receiving the stronger input dominates the perception and suppresses the other stimulus. Participants wore red‐cyan anaglyph eyeglasses in order to filter one image to each eye, thus eliminating participants' awareness of the primes. In each trial, participants were presented with videos (duration = 1,500 ms) composed of (a) the prime, that is, one of the 72 desaturated cyan pictures, and (b) dynamic red masks made of high‐contrast neutral faces segmented into 128 × 128 pixels squares, randomly rearranged and rapidly (10 Hz) flashing [Tsuchiya and Koch, 2005]. The CFS method was coupled with a modified version of the AMP [Payne et al., 2005] that measured to what extent the implicit induction of affect (by a prime) brought about misattribution changes to the subsequently presented neutral stimuli, the Chinese logograms (target stimuli). Participants were instructed to classify the target as pleasant or unpleasant. The logic underlying the AMP procedure is that even when unaware of the affective‐laden prime pictures, participants may be more inclined to perceive a stimulus as pleasant if they have “implicitly” formed favourable feelings toward the target stimulus, which is neutral in and of itself . The priming's effect is measured in terms of the percentage of pleasant judgements toward the logograms. Each video (size: 19.5° × 14.6°, durations: 1,400 ms) was immediately followed by one of 72 Chinese pictograms. The pictogram/target was presented for 150 ms. The different target stimuli were presented in a fully randomized order, controlled by an in‐house Matlab script. For each trial, the participants were asked to respond to the following choices: (1) the target was pleasant or unpleasant; (2) the suppressed stimulus appeared to the Left/Right of the central fixation cross; (3) the priming was implicitly or explicitly perceived (see Fig. 1A).
Figure 1.
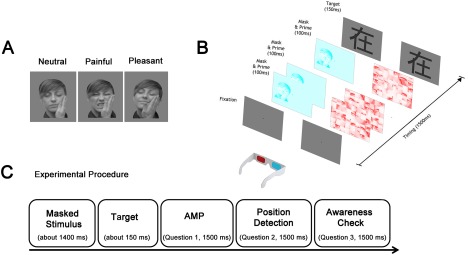
(A) Masking Procedure. Continuous Flash Suppression (CFS) consists in the simultaneous presentation of a flashing red mask into the dominant eye (on the right in the figure) and the cyan priming stimulus at low contrast (on the left). (B) Experimental Procedure. Illustration of a trial sequence after the fixation time, including the CFS masked video (∼1,400 ms), target presentation (Chinese Pictograph, 150 ms), Question 1 (AMP task, the target was Pleasant/Unpleasant), Question 2: Position detection (The prime has been presented on the Left/Right), Question 3: Awareness control (I have perceived the prime Consciously/Unconsciously). Participants were asked to respond to each question through a 2‐AFC method within 1,500 ms. [Color figure can be viewed at http://wileyonlinelibrary.com]
The last question was asked only when participants correctly identified the side on which the suppressed stimulus had appeared. All three answers were given within a two‐alternative forced choice task (2‐AFC). Trials in which participants reported to have consciously perceived the prime (Question 3) were discarded from the analyses. Furthermore, participants were informed about the presence of the prime but not about its content. In the case that a participant was able to see the prime explicitly, they were instructed not to let the image influence their explicit reports about the pleasantness of the target. A schematic representation of a trial is reported in Figure 1B.
Before scanning, volunteers entered a dark, quiet room where they underwent a brief familiarization task consisting in at least two sessions of 12 trials each. The familiarization procedure was a slightly modified version of the experimental task. The two main differences were the following: (i) primes were images of neutral objects (e.g., a bottle) instead of faces and (ii) catch trials, in which no prime appeared, were included in order to monitor the participant's comprehension of the task. To determine the ocular dominance and, consequently, the position of each lens of the anaglyph eyeglasses, the Miles test [Miles, 1930] was used in combination with a familiarization phase. The goal was to minimize the explicit detection of the priming images.
Each participant completed four fMRI runs lasting ∼13 min. Each run consisted of the presentation of 72 stimuli, 24 per condition (Neutral, Pleasant, and Painful), for a total of 288 trials for each session. Each trial was interleaved with a black fixation cross (dimension: 0.4°× 0.4°) of differing duration (mean 2,500 ms, range: 2,000–3,000 ms). A fully randomized event‐related design was used.
The entire protocol lasted about one hour in the scanner and about 30 additional minutes outside the scanner (for instruction, training and completion of the self‐administered questionnaires).
Behavioural Data Analysis
Based on the answers to Question 3, trials in which there was even partial explicit stimulus detection were excluded from all the behavioural analyses. The proportion of “like” ratings for each condition were then compared to investigate whether the stimuli had been subliminally processed. Specifically, the average percentage of “like” ratings was entered into a repeated measures analysis of variance (ANOVA) with the suppressed prime pictures condition (Painful, Neutral, Pleasant touches) as within‐subject factor. We expected the priming procedure to affect the explicit ratings of the neutral Chinese pictograms. More specifically, we expected the subliminal presentation of painful and pleasurable facial expressions to increase the likelihood of negative (“dislike”) and positive (“like”) evaluations, respectively. Participants were also asked to report the position of the prime on the screen (Question 2) as they had intuited it, as this is a valid method for indirectly evaluating the subjects' awareness of the primes [Greenwald et al., 1995]. Specifically, based on signal‐detection theory [Green and Swets, 1966], we calculated D‐primes to measure the participants' sensitivity in detecting the priming spatial position (Left vs. Right).
Prior to MRI scanning, participants were asked to fill the Italian version [Albiero et al., 2006] of the interpersonal reactivity index (IRI, [Davis, 1980] and, given the relationship between empathy and prosociality [Decety et al., 2016; Eisenberg and Miller, 1987], the prosocialness scale for adults (PSA) [Caprara et al., 2005]. The IRI is a 28‐item self‐report questionnaire assessing both the affective and the cognitive aspects of empathy based on the following four subscales: (1) empathic concern (EC), the tendency to have self‐oriented negative feelings in response to others' distress; (2) personal distress (PD), the extent to which an individual feels distress as a result of witnessing another's distress; (3) perspective taking (PT), which refers to the dispositional tendency of an individual to adopt the perspective of another; and (4) fantasy scale (FS), the individual's propensity to become imaginatively involved with fictional characters and situations. Each subscale had seven items scored on a 5‐point Likert scale (from 0 “it does not describe me at all” to 4 “it describes me very well”). The PSA questionnaire for assessing adult prosocialness contains 16 items. Participants were asked to indicate the degree to which they engage in different types of prosocial behaviours (e.g., “I try to help others”) on a 5‐point Likert scale (from 1 “never” to 5 “always”).
Trials Classification
In the present study, two methods were used to control whether participants consciously perceived the primes. The first was asking participants to report the position of the prime (left or right with respect to the fixation cross). The second was asking participants to report whether they had perceived any prime‐related clue (see above).
On one hand, the first question showed us that subject responses were, on average, not random. On the other, the third question allowed us to exclude all trials in which the prime had not been completely suppressed. Nevertheless, the extent to which each given single‐trial was implicitly processed remains a question. The AMP is an indirect way to measure the mean effect of the priming on the subjects' affection, rather than the actual processing of each single prime. This was addressed as following: Two assumptions were considered: (i) that consecutively “consistent” judgements in respect to the prime, that is, “dislike” after painful stimuli or “like” after pleasant stimuli, suggests a higher probability that the affective information of those trials had been subliminally processed by the brain, and (ii) the more the participant's responses are consecutively “consistent” with the prime, the less probable it is that the stimulus‐response (S‐R) congruency can be explained by chance. Since it is not possible to select only the trials that are subliminally influencing participants' responses, we estimated the probability that prime had affected the response for each trial by estimating logistic “b” coefficients through a generalized linear model (GLM), with a logit link function for a binomial distribution associated to each trial. In particular, we estimated how the valence of the prime (pleasant, unpleasant, neutral) would predict the judgment of the logogram (like vs. dislike) in a sequence of 12 trials (the five preceding and the six following trials). Conventionally, we coded the binary outcome variable (i.e., the subject's judgment about the Chinese logogram), as “1” when participants' response was “like,” and “0” when it was “dislike.” The priming condition represented our predictor and its levels were coded as follows: the pleasant condition was coded as 1, the neutral conditions was coded as 0, and the unpleasant condition was coded as −1 (Table 1). In order to make the coefficients more easily interpretable, we transformed them into probabilities of giving a “consistent response” by applying the following formula p = (exp(b)/(1 + exp(b)), given that the b coefficient expresses the exponential of the odds.
Table 1.
Example of trial‐by‐trial stimulus/response‐coding, b‐coefficients and the associated weights for an entire run (i.e., 72 trials)
| Trial number | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Priming | PL | PL | PN | NT | PN | PN | PN | PN | PL | NT | NT | PL | PN | NT 1 | NT | NT | PL | PL |
| Predictor | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 |
| Judgment (resp.) | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 |
| Congruency | −1 | 1 | 1 | −1 | 1 | −1 | −1 | −1 | 1 | −1 | −1 | 1 | 1 | −1 | −1 | −1 | 1 | 1 |
| b coefficient | NaN | NaN | NaN | NaN | NaN | 0.29 | 13.35 | 13.35 | 0.49 | 0.2 | −12.15 | −12.15 | −13.35 | −14.26 | −14.26 | −13.35 | −13.35 | −13.35 |
| Weight | NaN | NaN | NaN | NaN | NaN | 0.56 | 0.71 | 0.71 | 0.60 | 0.54 | 0.33 | 0.33 | 0.00 | 0.00 1 | 0.00 | 0.00 | 0.00 | 0.00 |
| Trial number | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Priming | PN | PN | PL | PN | NT | NT | NT | PL | PN | PN | PL | PL | PN | PN | PN | NT | PL | PL |
| Predictor | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 |
| Judgment (resp.) | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Congruency | −1 | −1 | −1 | 1 | −1 | −1 | −1 | 1 | −1 | 1 | 1 | −1 | −1 | 1 | 1 | −1 | −1 | −1 |
| b coefficient | −14.26 | −14.54 | −13.77 | −13.56 | −13.56 | −14.26 | −13.56 | −12.86 | −12.86 | −13.06 | −0.36 | −0.2 | −0.2 | −0.2 | 0 | −0.2 | −0.49 | −0.49 |
| Weight | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.32 | 0.31 | 0.26 | 0.43 | 0.46 | 0.46 | 0.46 1 | 0.50 | 0.49 | 0.44 | 0.44 |
| Trial number | 37 | 38 | 39 | 40 | 41 | 42 | 43 | 44 | 45 | 46 | 47 | 48 | 49 | 50 | 51 | 52 | 53 | 54 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Priming | PN | PL | PN | PN | PL | PN | PN | NT | PL | NT | NT | NT | NT | PL | NT | NT | PN | PN |
| Predictor | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 1 | 0 | 0 | 0 | 0 |
| Judgment (resp.) | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Congruency | −1 | −1 | 1 | 1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | 1 | −1 | −1 | 1 | 1 |
| b coefficient | −0.49 | −0.29 | −0.13 | 0.16 | −0.29 | −0.57 | −0.57 | −0.29 | 0 | 0 | 0 | 0.49 | 0 | 0.2 1 | 0 | 0 | 12.35 | 12.64 |
| Weight | 0.44 | 0.50 | 0.51 | 0.54 | 0.46 | 0.39 | 0.39 | 0.46 | 0.50 | 0.50 | 0.50 | 0.56 | 0.50 | 0.52 1 | 0.50 | 0.50 | 0.61 | 0.69 |
| Trial number | 55 | 56 | 57 | 58 | 59 | 60 | 61 | 62 | 63 | 64 | 65 | 66 | 67 | 68 | 69 | 70 | 71 | 72 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Priming | NT | PL | PL | PL | PN | PL | PN | PN | NT | PL | NT | NT | NT | PL | NT | PL | PL | NT |
| Predictor | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 |
| Judgment (resp.) | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 |
| Congruency | −1 | −1 | −1 | −1 | −1 | −1 | 1 | 1 | −1 | −1 | −1 | −1 | −1 | 1 | −1 | −1 | −1 | −1 |
| b coefficient | 13.13 | 13.13 | 13.13 | 0 | 13.41 | 13.29 | 0 | 0 | 0 | −13.13 | −12.64 | 0 | NaN | NaN | NaN | NaN | NaN | NaN |
| Weight | 0.80 | 0.79 | 0.75 | 0.67 | 1.00 | 1.00 | 0.73 | 0.50 | 0.35 | 0.24 | 0.35 | 0.50 | NaN | NaN | NaN | NaN | NaN | NaN |
In a sequence of 12 trials, we established the relationship between the predictor priming (pleasant, neutral, and painful, that correspond to 1, 0, and 0, respectively), and the binary response participants provided about the Chinese logogram (like vs. dislike coded as 1 vs. 0, respectively), through a generalized linear model. In order to weight each trial, we transformed the coefficients into probabilities of giving a “consistent response” by applying the following formula p = (exp(b)/(1 + exp(b)). That probability (weight) has been used to separate the “high context‐consistency” (P > 0.50) from the “low context‐consistency” trials (P ≤ 0.50).
NT= neutral, PN = painful, PL = pleasant.
The choice of 12‐trials sequences was based on the theoretical consideration that 10–15 observations are needed for reliably estimating a parameter [Field, 2009], and the pragmatic consideration that the more we increase the temporal window (i.e., the number of trials used for estimating the logit function), the more we have to discard trials from the analysis. Consequently, the statistical power for the “Context × Prime” would be undermined. The choice of the number of trials and the procedure is not explicitly driven by previous literature, but follows the assumption that if an effect priming occurs in a trial, it is very likely that this trial is embedded in a sequence of events with a coherent effect. This Context‐consistency approach could be seen as a sort of smoothing procedure, something like a moving average but in the context of the estimation of a discrete outcome coming from a binomial distribution (see Table 1 for an example of an entire session).
Following this statistical approach, we separated trials with positive logit values, that is, those embedded in a sequence where the prime consistently affected the judgment, from those embedded in sequences where the responses were either provided by chance or were consistently incongruent. In other words, positive values of logit mean that a “consistent” judgement (i.e., “like” after pleasant or “dislike” after unpleasant) is more likely to occur (p consistency > 0.50). Based on this, we created two final trial types: the “high context‐consistency” trials (associated with p consistency > 0.50) and the “low context‐consistency” trials (associated with p consistency ≤ 0.50). To summarize, this coefficient indicates the strength of each trial's Context‐Consistency and, consequently, the probability (Low vs. High) of having been subliminally processed.
fMRI Data Acquisition
The fMRI study was carried out using a Siemens Allegra (Siemens Medical Systems, Erlangen, Germany) 3T scanner, and stimuli were presented with Cogent2000 (Cogent, http://www.vislab.ucl.ac.uk/Cogent/) for MATLAB (v 7.1, The MathWorks, Natick, MA) through a mirror mounted on an MRI headcoil on a monitor (1.024 × 768 resolution, and refresh rate 60 Hz). Head movement was minimized by mild restraint and cushioning. Each participant underwent four fMRI runs. Blood oxygenation level‐dependent (BOLD) contrast was obtained using gradient echo‐planar imaging sequence (repetition time = 2.08 s; time echo = 30 ms). Complete coverage of the cortex was obtained by 32 slices with a thickness of 2.5 mm. Given that the duration of the run depended on the response to the second question, a different number of volumes was obtained for each session of each participant (Mean = 279, SD = 5). The first four volumes of each run were discarded to insure a magnetization equilibrium.
fMRI Data Analysis
We used the statistical parametric mapping package SPM8 (http://www.fil.ion.ucl.ac.uk) implemented in MATLAB for data preprocessing and statistical analyses. The raw images were preprocessed according to the following steps: (i) realignment of acquired EPI images; (ii) correction of slice‐acquisition delays using the middle slice as reference; (iii) normalization to the standard MNI space; and, finally; (iv) smoothing of images with a Gaussian kernel of 8‐mm full‐width at half maximum (FWHM) to increase the signal‐to‐noise ratio. Two participants were excluded from the analysis due to excessive head motion during image acquisition (> 2.5 mm translation and/or 2.5° rotation). Realigned data were reassessed for the remaining eighteen participants using the Artifact Detection Tools software (ART; http://www.nitrc.org/projects/artifact_detect/), a graphic tool to detect excessive motion artifacts in fMRI data. Outlier scans were identified in the temporal sequence by comparing each scan with the adjacent ones using the following criteria in ART: Z‐threshold = 3.0, scan to scan movement threshold = 0.45 mm; rotation threshold = 0.02 rad. In addition to the six estimated motion parameters indicating the residual effects of head motion, these outliers were modelled in the first‐level analysis by including a regressor indicating each outlier scan for each run of each participant (< 5%) .
Two different subject‐level models of analysis were run. First, the Painful (PN), Neutral (NT), and Pleasant (PL) conditions were considered with participants' judgements. Second, a new approach was used to highlight the neural activity in trials that behaviourally showed more context‐related consistency (cf. “Trials classification,” above).
In line with the literature on subliminal perception, we could not control the real awareness of the subjects. There has been much discussion as to whether subliminal stimuli are actually perceived (albeit at a very low level of consciousness), or completely overlooked [Dixon, 1971]. Aware of this problem in implicit processing, we used a rigorous method to give each stimulus an index as to the likelihood of being processed. Thus, in the present research, (i) we replicated the behavioural results of our group's previous study [Chiesa et al., 2015], giving robustness to this paradigm and (ii) we developed a method capable of estimating the likelihood that each stimulus had been processed by the brain. This method may be useful in future studies on brain processing of implicit stimuli. We reasoned that researchers have not always obtained results because it is nearly impossible in subliminal conditions to find the perfect threshold for each stimulus and each subject a priori. On the contrary, assigning statistical weight a posteriori to each stimulus for each subject allows for an analysis focused on the stimuli of interest.
The two analyses are described in detail below.
Prime‐response (P‐R)
The subject‐specific models included 12 event‐types produced by the crossing of the factors: Conditions [Painful, Neutral, and Pleasant]; Judgement on the Target [Like, Dislike]; Perception [Subliminal, Supraliminal]. These events were time‐locked at the onset of the video and convolved with the hemodynamic response function (HRF, event duration= 0).
The group‐level analyses (flexible‐factorial design) were carried out for subliminal trials only, providing a total of 6 contrasts of interest: Conditions [Painful (PN), Neutral (NT), Pleasant (PL)] × Judgement on the Target [Like, Dislike] (NTlike/PNlike/PLlike/NTdislike/PNdislike/PLdislike).
Context‐consistency
The first‐level models of this main analysis considered the stimulus condition and the trial context‐consistency, given a total of 12 events, corresponding to the new 3 × 2 × 2 factorial design (Conditions [Painful, Neutral, and Pleasant]; trials consistency [Low, High]; perception [Subliminal, Supraliminal]). 12 contrast images per participant were thus produced, but only the contrast images modelling the effect of the six conditions of interest for the subliminal trials (NTlow/PNlow/PLlow/NThigh/PNhigh/PLhigh) then underwent the second analysis. The supraliminal trials were not considered further for their inadequate number. The onsets corresponded to the onset of each video, with duration = 0. Averaged across the four fMRI runs, linear contrasts were used to determine responses for the six conditions of interest (NTlow/PNlow/PLlow/NThigh/PNhigh/PLhigh), which underwent the group random‐effect analysis (Penny and Holmes, 2004) using a flexible‐factorial design.
All the resulting SPM[T] maps were initially thresholded at P < 0.001 (voxel‐level, uncorrected). We considered cluster level effects to be significant at P < 0.05, corrected for multiple comparisons (Family Wise Correction, FWE).
Region of Interest Analysis
We carried out region of interest (ROI) analyses to delve deeper into the implicit perception of vicarious affective touch. And to increase the sensitivity of our analyses given the presence of a strong a priori hypothesis regarding the area related to the observation of both pleasant and painful touch in others, we pursued a small volume correction approach (SVC).
A recent meta‐analysis of 32 fMRI studies demonstrated the crucial role of the insular and medial/anterior cingulate cortex in empathy for pain [Lamm et al., 2011]. In studies in which participants observed others in pain, bilateral AI and ACC were consistently activated irrespective of the way in which empathy had been induced. Therefore, both the anterior cingulate cortex and the bilateral anterior insula were selected as anatomical ROIs for the pain condition. Additionally, given that the orbitofrontal cortex (OFC) emerged as the most likely region linking many types of reward and hedonic experience, we proceeded to investigate the region for any possible differences between pleasant and neutral priming.
ROIs were selected in the volumes of interest database [Nielsen and Hansen, 2002]. Significance threshold was set at P < 0.05 corrected for multiple comparisons.
Correlation Analysis
We sought to understand whether brain reactivity is dependent on such personality measures. We thus performed correlations between contrast estimates in identified areas and the behavioural indices, that is, those measured by the three subscales (EC, PD, and PT) of the IRI measure and the PSA questionnaire.
Based on the whole‐brain analyses, regions shared by the main effect and the interaction were then identified with the masking procedure in SPM. Two final ROIs were identified with Marsbar 0.4 (http://marsbar.sourceforge.net/, SPM toolbox): (1) a first cluster was identified within the anterior prefrontal cortex (PFC), which was activated by the subliminal presentation of the Pain‐Neutral condition in the high consistency trials, that is, the effect of the painful stimuli with high consistency only (PNhigh > NThigh) was masked inclusively for the interaction between “pain” and “consistency” [(PNhigh > NThigh) > (PNlow > Nlow)] and (2) the second cluster was identified within the left S1 as a result of the subliminal presentation of the Pleasant‐Neutral condition, that is, the effect of the pleasant priming in trials with high consistency (PLhigh > NThigh) was masked for the corresponding “pleasant × consistency” interaction [(PLhigh > NThigh) > (PLlow > NTlow)].
Parameter estimates of average BOLD signal were extracted from the peak of each ROI. Pearson's correlation analyses were run on SPSS software with significance threshold set at P < 0.05.
RESULTS
Behavioural Data
Trials in which participants reported to have seen the prime were removed from all the analyses (577 trials out of 5,760 total trials: about 10%). In keeping with our previous report [Chiesa et al., 2015], the AMP score, that is, the percentage of “like” response (Question 1), was higher in the Pleasant condition (M = 52.2%, 95% CI [46.6, 57.8]) and lower in the Pain condition (M = 45.1%, 95% CI [38.4, 51.9]) in regards to the Neutral condition (M = 50.4%, 95% CI [43.4, 57.4]). A one‐way repeated measures analysis of variance (ANOVA) revealed a significant effect of the suppressed prime [F(2,34) = 6.921, p = 0.003, η 2 = 0.29]. Pairwise comparisons showed that the Painful priming caused significantly lower “like” responses than both Neutral and Pleasant conditions (ts ≤ −3.09, ps ≤ 0.007), while these last two did not differ from one another (t(17) = 0.888, p = 0.387; see Fig. 2). All the significant comparisons survived the Bonferroni's correction for the FWE (P< 0.017).
Figure 2.
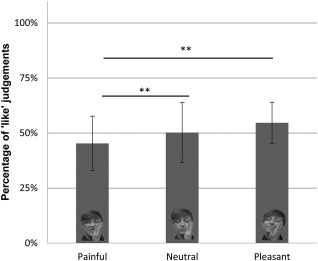
Mean and deviation standard for each priming condition (Painful, Neutral, and Pleasant) of the subliminal task. Note: higher values indicate higher proportions of positive responses. Results of the repeated‐measures ANOVA of percentage of “like” judgements in the AMP as a function of type of subliminal prime (painful, neutral or pleasant facial expressions) are shown. **P < 0.01.
Based on the proposed rational for the trial classification, in the ANOVA, we then added the Context‐Consistency factor (2 levels: high vs. low consistency) to the priming‐condition (3 levels: pain vs. neutral vs. pleasant). Results (Fig. 3) showed a main effect of the Prime [Prime: F(2,34) =4.399, p = 0.020, η 2 = 0.206; Context‐Consistency: F(1,17) = 1.129, p = 0.303, η 2 = 0.062] and, most importantly, a significant Prime X Context‐consistency interaction [Prime*Context‐Consistency: F(2,34)= 14.802, p < 0.001, η 2 = 0.465]. The latter demonstrated that indeed the effect of the prime was modulated by the context‐factor. Bonferroni‐corrected post hoc comparisons showed that in the Low consistency trials only the Pleasant condition differs from both Neutral [t = 3.977, p = 0.003] and Pain [t = 2.810, p = 0.036]. While, in the High‐Consistency trials all the priming stimuli significantly differ from each other [t > |2.760|, ps < 0.045].
Figure 3.
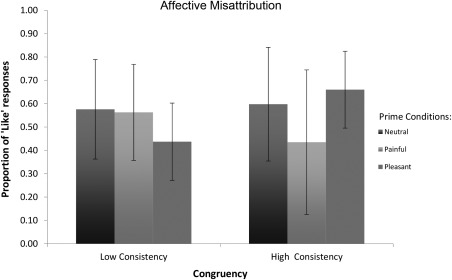
Mean and deviation standard for each priming condition (Painful, Neutral, and Pleasant) of the subliminal task based on the proposed trial classification criterion.
In order to exclude facilitation, suppression or habituation effects across all the four runs, we computed average values for each run and included them in an ANOVA with a four‐levels single factor (Run). The main effect was not statistically significant [F(3,51) = 2.18; p = 0.1; η 2 = 0.080], suggesting absence of a strong and systematic fluctuation in the priming effect. Since the classical frequentist approach cannot provide evidence in favour of the absence of an effect, we performed a multilevel Bayesian modelling analysis through the R package “brms” [Buerkner, 2017]. The prior for the fixed effect of the Run was set as a normal distribution centered on 0 and with a standard deviation of 0.20. We also modelled the dependence among the observations by setting participants as random intercepts and the random slope within each participant [Barr et al., 2013]. The null hypothesis of no effect of Run on the change in weights has a Bayes factor of 3.01, which represents positive evidence [Raftery, 1995] in favour of the null.
fMRI Results
Prime‐response
We modelled the subject's responses, that is, the judgements (Like, and Dislike) toward the Chinese logograms and the type of subliminal prime (Neutral, Painful, and Pleasant touches). We thus obtained six conditions: NTlike/PNlike/PLlike/NTdislike/PNdislike/PLdislike. Significant activations were only found for the main effect of the pleasant responses (Like > Dislike) in the right inferior temporal gyrus ([44, −64, −2], t = 4.93), left lingual gyrus ([–10, −80, −8], t = 4.71) and right cuneus ([18, −76, 36], t = 4.29.
Context‐consistency
Further analyses were successively run by considering the consistency (Low vs. High) of the responses in a subset of trials (1,953 and 1,950 trials, respectively). In other words, trials in these analyses were selected to model the strength of the supposed subliminal processing (see method session). At the individual level, the minimum number of trial per condition in the session was 19, including at least one trial per run (Supporting Information Tables SI and SII). Possible differences in error variance due to a different number of trials in the low versus high consistency conditions were accounted by the nonsphericity correction of the fMRI group analysis.
The neural correlates underlying each of the two emotional facial expressions were then investigated.
The subliminal presentation of pain stimuli that evoked coherent and consecutive responses over the neutral priming (contrast: (PNhigh > NThigh) > (PNlow > NTlow)) elicited the activation of the anterior PFC (Table 2). The activation peak coincides with the location of Brodmann's area 10 (BA10), with the cluster extending dorsally to the BA 9 and ventrally to BA 11 (see Fig. 4A). Our investigation of the neural correlates of the Pleasant priming presentation (contrast: (PLhigh > NThigh) > (PLlow > NTlow)) revealed the specific activity of the left sensorimotor region (see Fig. 4B and Table 2). None of the contrasts investigating the priming effect in the “low consistency” trials over the “high consistency” ones showed significant clusters in the group analyses.
Table 2.
shows the peak of maximal activation for each of the two affective priming [contrast Pain: (PNhigh > NThigh) > (PNlow > NTlow), contrast Pleasure: (PLhigh > NThigh) > (PLlow > NTlow)]
| Cluster | Coordinates (mm) | |||||||
|---|---|---|---|---|---|---|---|---|
| Contrasts | Brain region | BA | T value | |||||
| pFEW‐corr | k | x | y | z | ||||
| Pain | aPFC | 10 | 0.004 | 521 | 4.58 | 14 | 62 | 14 |
| Pleasure | ISM1 | 4 | 0.007 | 472 | 4.49 | −36 | −18 | 48 |
T‐Values estimated at uncorrected P < 0.001 with voxel cluster size threshold of 300 (P ≤ 0.05 FEW‐corrected), considering the whole brain as the volume of interest. MNI coordinates in millimetres. Note: aPFC= anterior prefrontal cortex; lSM1= left primary sensorimotor cortex.
Figure 4.
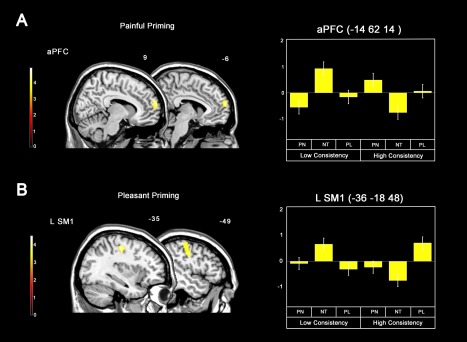
(A) Brain responses associated with effect of the painful priming selectively for high consistency trials (contrast: (PNhigh > NThigh) > (PNlow > NTlow)), and parameter estimates extracted from the cluster in the anterior prefrontal cortex (PFC). (B) Effect of the pleasant priming selectively for high consistency trials (contrast: (PLhigh > NThigh) > (PLlow > NTlow)) and parameter estimates extracted from the cluster in the left sensorimotor cortex. None of the contrasts investigating the priming effect in the “low consistency” trials over the “high consistence” ones showed significant clusters in the group analyses. P < 0.05 (FWE) at cluster level; cluster size k > 300 voxels. Note: aPFC= anterior prefrontal cortex; SM1= primary sensorimotor cortex. [Color figure can be viewed at http://wileyonlinelibrary.com]
We carried out ROI analyses to further explore the implicit responses specifically related to the painful and pleasant vicarious affective touch. ACC and AI were tested for the pain condition given the role in empathy for pain. We found that the implicit vicarious experience of painful emotions engages only the dorsal part of ACC (pFWE‐SVC = 0.017, [6 38 36], BA 32), see Figure 5. Bilateral OFC does not seem to be involved in the implicit perception of other individuals being stimulated by pleasant touch (pFWE‐SVC > 0.05).
Figure 5.
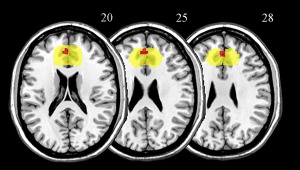
Axial representation of the brain response of the dorsal ACC associated with the painful priming selectively for high consistency trials (contrast: (PNhigh > NThigh) > (PNlow > NTlow), pFWE‐SVC = 0.017, in red), when the small volume correction (SVC) analysis is applied. The search volume of the anterior cingulate cortex (ACC) that was selected in the Volumes of Interest database (Nielsen and Hansen, 2002) and was used for the SVC analysis is highlighted in yellow. [Color figure can be viewed at http://wileyonlinelibrary.com]
Correlation Analysis
Other analyses assessed possible links between BOLD signal and personality traits. No significant correlations were found between the neural activity in regions shared by the main effect and the interaction when painful or pleasant stimuli were presented, and the IRI subscales or prosocialness measures (all ps > 0.05 corrected for multimple comparisons).
DISCUSSION
In this study, we investigated whether the implicit processing of affective social stimuli with negative (i.e., pain) or positive (i.e., pleasant touch) valence modulates neural representations involved in the vicarious representation of a given primed state. To do so, we combined fMRI with a novel experimental paradigm based on both a CFS procedure, tailored to render visual stimuli subliminal, and an AMP, that provides a behavioural index for the effects of affective priming stimuli. We observed increased brain activations in PFC and ACC—two important empathy‐related areas—in response to the implicit observation of pain in other individuals, and in the primary somatosensory region when participants were primed by pleasant faces.
Expanding on previous studies [Chiesa et al., 2015; Murphy and Zajonc, 1993; Payne et al., 2005), our behavioural results demonstrate that seeing a person receiving a painful, neutral or pleasant touch modifies likeability ratings of a neutral target according to affectively congruent responses. More specifically, we found that likeability ratings were lower following the subliminal presentation of suffering facial expressions when compared with both pleasant and neutral ones. On the contrary, like ratings of the target were more frequent when primed by an image of a person being pleasantly touched as opposed to painfully. Tellingly, the different experimental stimuli were matched in terms of their arousing power [Chiesa et al., 2015], thus ruling out the possibility that arousal alone influenced the results [Bradley et al., 2008; Lamm et al., 2015].
While both the right ventral ACC and bilateral amygdalae are known to be involved in supraliminal and subliminal fear stimuli processing [Williams et al., 2006], and a double dissociation between subliminal and supraliminal processing has been demonstrated using ERPs [Liddell et al., 2004] and fMRI [Williams et al., 2006], the overwhelming majority of studies on empathy have used supraliminal stimuli. The present study is thus unique for its use of subliminal stimuli to investigate empathic reactivity. Our analysis of brain activity indicates that painful and pleasurable subliminal information about others engages separate neural systems when compared with neutral faces, and that it does so consistently with the neural response expected for each of the tested social emotions. Specifically, we found that PFC and, ACC emerged from the ROI‐based approach, are two pivotal brain regions in the implicit perception of pain, in keeping with their established role in encoding pain in both self and others [Lamm et al., 2011; Ochsner et al., 2004; Singer et al., 2004; Zaki et al., 2016]. Although ACC has already been identified as a crucial node of the pain matrix called into play when individuals are empathizing with the pain of others [Lamm et al., 2011], no study has investigated thus far the involvement of the pain‐related brain areas when participants are not aware of the affective stimuli. In addition to ACC, increased blood flow in response to various noxious stimuli have been frequenly observed also in prefrontal cortices [Peyron et al., 2000]. Although the involvments of PFC is thought to reflect attentional and memory networks activated by noxious stimulation, here we showed that anterior PFC seems to play a role also in the subliminal processing of painful facial expressions in appropriate contexts (e.g., a slapped face). It is worth noting that while AI has been found involved in the vicarious processing of supraliminal stimuli [Lamm et al., 2011] and may be critical for interoceptive awareness [Craig, 2009], our results do not show that this region is also engaged in the subliminal processing of the pain empathic‐related stimuli.
Despite studies report that activity in reward related regions parallels the pleasure deriving from the pain of others (a complex emotion called schadenfreude) [Takahashi et al., 2009], much less is known about the neural correlates of seeing social pleasure in others. Thus, that subliminal processing of affectively positive touch brought about an increase of activity in S1 is one main point of novelty of the present study. Previous studies on the neural underpinnings of the vicarious processing elicited by explicitly processed somatosensory stimuli suggest that somatosensory areas may not only be involved in representing pleasant touch in others [Ebisch et al., 2011; Gazzola et al., 2012; Keysers et al., 2010; Lamm et al., 2015], but also in the mere observation of neutral [Blakemore et al., 2005; Schaefer et al., 2008] and painful touch [Bufalari et al., 2007; Cheng et al., 2008]. However, our “neutral touch” control condition allowed us to show that the reported effects are due to the specific social valence of the stimuli, rather than to touch per se. Thus, although the somatosensory cortices should be involved in mapping all types of touch (painful, neutral, and pleasant), in our study, we propose that the vicarious pleasant experience might be stronger than the other types of tactile sensations. It is also worth noting that the reported activation of S1 may reflect the mimicry response related to happy facial expression. This would be in keeping with the notion that facial mimicry of happy faces may also occur when people are not conscious that they are mirroring the seen expressions [Dimberg et al., 2000]. We propose that such an early processing may be a prerequisite for empathizing [Tamietto and de Gelder, 2009; Walter, 2012], and that it can be even more evident when stimuli remain subliminal. Similarly, we speculate that the implicit processing of others' pleasure does not highlight the role of medial orbitofrontal cortex (OFC), an area that is known to be heavily involved in the explicit coding of personal reward and feelings [Francis et al., 1999; Rolls et al., 2003]. This result is in line with the idea that OFC is related to rational and conscious evaluations of the hedonic quality of a stimulus [Berridge and Kringelbach, 2013]. Indeed, a recent voxel‐based morphometry analysis [Yue et al., 2016] demonstrates a relationship between trait positive empathy, measured by the Positive Empathy Scale, and the volume of grey matter in OFC. Not surprisingly, this finding may suggest that the abilities of explicit processing and regulating of emotion—not involved in the implicit task—are orchestrated by the prefrontal regions. However, although we did not find any significant involvement of OFC in the pleasant priming condition, it is worth noting that OFC is a region notoriously susceptible to distortions. Because the acquisition parameters used in our study were not optimized for detecting BOLD activity in OFC [Deichmann et al., 2003], we cannot exclude that OFC contributed to the processing of subliminal pleasant stimuli.
Analysis of correlations between BOLD signals and personality traits did not show associations between behavioral measures of individual empathy nor prosociality. One may note that the lack of correlation between empathy for pain and prosociality in our study is at odds from the above study. However, this difference could be explained by the possibility that real‐world donations are a more sensitive measure for prosocialness, as suggested by studies in which prosociality is measured by real‐world donations [Light et al., 2015] or actual helping behaviours [Waytz et al., 2012].
That our paradigm tests the effect of both positive and negative emotions displayed by others is an element of novelty that may in principle allow one to disentangle the emotional aspects of painful and pleasant experiences from a more sophisticated affective reaction related to empathy. While we believe that our study casts light on implicit empathy, some limitations of the design have to be acknowledged. For example, one potential criticism regards the possibility that the activity found in the present study only reflects the valence of the stimuli. We note, however, that the neural systems called into play by the subliminal processing of our stimuli involved regions known for being recruited in the affective processing of the same stimuli, rather than for their valence alone. For example, the mere valence of pleasantness should engage the OFC [Kringelbach, 2005; Kuhn and Gallinat, 2012]. On the contrary, we found that the implicit processing of the pleasant stimuli activates the left SM1, a region involved in a large variety of processes related to the social pleasure of being touched [Francis et al., 1999; Gazzola et al., 2012]. Also deserving of discussion is the potential to distinguish between the effect of social and nonsocial pain and pleasure, which would allow for conclusions to be made as to the specific effects of empathic mapping of the subliminally processed stimuli. Although the neural activity evoked by our stimuli is in accordance with what has been found in previous studies on empathy for pain and pleasure, we acknowledge that future investigations with a larger variety of stimuli should be performed.
CONCLUSIONS
Our study supports the notion of shared representations between first‐hand and vicarious experiences, and builds on previous studies by showing that specifically brain systems are engaged in mapping positive and negative experiences in others [Lamm et al., 2015]. Our novel paradigm may turn out to be adept at exploring the link between the subliminal perception of negative and positive social emotions and the ability to empathize in individuals with defective reactivity to the emotional, physical, and mental states of others, such as people on the autistic spectrum, or those with alexithymia [Bird et al., 2010]. Our approach may also be useful for exploring whether different nodes of the empathic or emotional network are recruited by painful and pleasant social versus nonsocial stimuli presented subliminally in people with neurotypical and nontypical development. Moreover, in accordance with previous findings on visuomotor priming [Ulrich and Kiefer, 2016], our paradigm may be useful for investigating the functional connectivity between task‐relevant brain regions, and thus for highlighting the neural mechanism underlying social priming.
Supporting information
Supporting Information Table 1.
Supporting Information Table 2.
Contributor Information
Patrizia Andrea Chiesa, Email: patrizia.chiesa@uniroma1.it.
Salvatore Maria Aglioti, Email: salvatoremaria.aglioti@uniroma.it.
REFERENCES
- Albiero P, Ingoglia S, Lo Coco A (2006): Contributo all'adattamento italiano dell'Interpersonal Reactivity Index. TPM 13:107–125. [Google Scholar]
- Avenanti A, Bueti D, Galati G, Aglioti SM (2005): Transcranial magnetic stimulation highlights the sensorimotor side of empathy for pain. Nat Neurosci 8:955–960. [DOI] [PubMed] [Google Scholar]
- Avenanti A, Minio‐Paluello I, Bufalari I, Aglioti SM (2006): Stimulus‐driven modulation of motor‐evoked potentials during observation of others' pain. NeuroImage 32:316–324. [DOI] [PubMed] [Google Scholar]
- Avenanti A, Sirigu A, Aglioti SM (2010): Racial bias reduces empathic sensorimotor resonance with other‐race pain. Curr Biol 20:1018–1022. [DOI] [PubMed] [Google Scholar]
- Batson CD, Turk CL, Shaw LL, Klein TR (1995): Information function of empathic emotion: Learning that we value the other's welfare. J Pers Soc Psychol 68:300. [Google Scholar]
- Batson CD, Sager K, Garst E, Kang M, Rubchinsky K, Dawson K (1997): Is empathy‐induced helping due to self‐other merging? Journal of Personality & Social Psychology 73:495–509. [Google Scholar]
- Berridge KC, Kringelbach ML (2013): Neuroscience of affect: Brain mechanisms of pleasure and displeasure. Curr Opin Neurobiol 23:294–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betti V, Aglioti SM (2016): Dynamic construction of the neural networks underpinning empathy for pain. Neurosci Biobehav Rev 63:191–206. [DOI] [PubMed] [Google Scholar]
- Betti V, Zappasodi F, Rossini PM, Aglioti SM, Tecchio F (2009): Synchronous with your feelings: Sensorimotor {gamma} band and empathy for pain. J Neurosci 29:12384–12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird G, Silani G, Brindley R, White S, Frith U, Singer T (2010): Empathic brain responses in insula are modulated by levels of alexithymia but not autism. Brain 133:1515–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ, Bristow D, Bird G, Frith C, Ward J (2005): Somatosensory activations during the observation of touch and a case of vision‐touch synaesthesia. Brain 128:1571–1583. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Jha AP, Bylsma LM, Fabian SA, Solomon PE, Prkachin KM (2005): Viewing facial expressions of pain engages cortical areas involved in the direct experience of pain. NeuroImage 25:312–319. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Miccoli L, Escrig MA, Lang PJ (2008): The pupil as a measure of emotional arousal and autonomic activation. Psychophysiology 45:602–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bufalari I, Aprile T, Avenanti A, Di Russo F, Aglioti SM (2007): Empathy for pain and touch in the human somatosensory cortex. Cereb Cortex 17:2553–2561. [DOI] [PubMed] [Google Scholar]
- Bürkner PC (2017): An R Package for Bayesian Multilevel Models using Stan. Journal of Statistical Software. in press. [Google Scholar]
- Caprara GV, Steca P, Zelli A, Capanna C (2005): A new scale for measuring adults' prosocialness. Eur J Psychol Assess 21:77–89. [Google Scholar]
- Carr L, Iacoboni M, Dubeau MC, Mazziotta JC, Lenzi GL (2003): Neural mechanisms of empathy in humans: A relay from neural systems for imitation to limbic areas. Proc Natl Acad Sci USA 100:5497–5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Yang CY, Lin CP, Lee PL, Decety J (2008): The perception of pain in others suppresses somatosensory oscillations: A magnetoencephalography study. NeuroImage 40:1833–1840. [DOI] [PubMed] [Google Scholar]
- Chiesa PA, Liuzza MT, Acciarino A, Aglioti SM (2015): Subliminal perception of others' physical pain and pleasure. Exp Brain Res 233:2373–2382. [DOI] [PubMed] [Google Scholar]
- Corradini A, Antonietti A (2013): Mirror neurons and their function in cognitively understood empathy. Conscious Cogn 22:1152–1161. [DOI] [PubMed] [Google Scholar]
- Costantini M, Galati G, Romani GL, Aglioti SM (2008): Empathic neural reactivity to noxious stimuli delivered to body parts and non‐corporeal objects. Eur J Neurosci 28:1222–1230. [DOI] [PubMed] [Google Scholar]
- Craig AD (2009): How do you feel–now? The anterior insula and human awareness. Nat Rev Neurosci 10:59–70. [DOI] [PubMed] [Google Scholar]
- Davis MH (1980): A multidimensional approach to individual differences in empathy. Austin, TX: Univ of Texas. [Google Scholar]
- de Vignemont F, Singer T (2006): The empathic brain: How, when and why? Trends Cogn Sci 10:435–441. [DOI] [PubMed] [Google Scholar]
- Decety J, Ickes W (2011): The Social Neuroscience of Empathy. Cambridge MA: MIT Press. [Google Scholar]
- Decety J, Jackson PL (2004): The functional architecture of human empathy. Behav Cogn Neurosci Rev 3:71–100. [DOI] [PubMed] [Google Scholar]
- Decety J, Lamm C (2006): Human empathy through the lens of social neuroscience. ScientificWorldJournal 6:1146–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, Bartal IB, Uzefovsky F, Knafo‐Noam A (2016): Empathy as a driver of prosocial behaviour: Highly conserved neurobehavioural mechanisms across species. Philos Trans R Soc Lond B Biol Sci 371:20150077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deichmann R, Gottfried JA, Hutton C, Turner R (2003): Optimized EPI for fMRI studies of the orbitofrontal cortex. NeuroImage 19:430–441. [DOI] [PubMed] [Google Scholar]
- Dimberg U, Thunberg M, Elmehed K (2000): Unconscious facial reactions to emotional facial expressions. Psychol Sci 11:86–89. [DOI] [PubMed] [Google Scholar]
- Dixon NF (1971): Subliminal Perception: The Nature of a Controversy. London, New York: McGraw‐Hill. [Google Scholar]
- Ebisch SJ, Ferri F, Salone A, Perrucci MG, D'Amico L, Ferro FM, Romani GL, Gallese V (2011): Differential involvement of somatosensory and interoceptive cortices during the observation of affective touch. J Cogn Neurosci 23:1808–1822. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Miller PA (1987): The relation of empathy to prosocial and related behaviors. Psychol Bull 101:91. [PubMed] [Google Scholar]
- Engen HG, Singer T (2013): Empathy circuits. Curr Opin Neurobiol 23:275–282. [DOI] [PubMed] [Google Scholar]
- Francis S, Rolls ET, Bowtell R, McGlone F, O'Doherty J, Browning A, Clare S, Smith E (1999): The representation of pleasant touch in the brain and its relationship with taste and olfactory areas. Neuroreport 10:453–459. [DOI] [PubMed] [Google Scholar]
- Fusaro M, Tieri G, Aglioti SM (2016): Seeing Pain and Pleasure on self and others: Behavioural and psychophysiological reactivity in immersive virtual reality. J Neurophys 116:2656–2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gable SL, Gonzaga GC, Strachman A (2006): Will you be there for me when things go right? Supportive responses to positive event disclosures. J Pers Soc Psychol 91:904–917. [DOI] [PubMed] [Google Scholar]
- Gallese V, Keysers C, Rizzolatti G (2004): A unifying view of the basis of social cognition. Trends Cogn Sci 8:396–403. [DOI] [PubMed] [Google Scholar]
- Gazzola V, Spezio ML, Etzel JA, Castelli F, Adolphs R, Keysers C (2012): Primary somatosensory cortex discriminates affective significance in social touch. Proc Natl Acad Sci USA 109:E1657–E1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DM, Swets JA (1966): Signal Detection Theory and Psychophysics. New York: Wiley. [Google Scholar]
- Greenwald AG, Klinger MR, Schuh ES (1995): Activation by marginally perceptible (“subliminal”) stimuli: Dissociation of unconscious from conscious cognition. J Exp Psychol Gen 124:22–42. [DOI] [PubMed] [Google Scholar]
- Han S, Fan Y, Xu X, Qin J, Wu B, Wang X, Aglioti SM, Mao L (2009): Empathic neural responses to others' pain are modulated by emotional contexts. Hum Brain Mapp 30:3227–3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibanez A, Hurtado E, Lobos A, Escobar J, Trujillo N, Baez S, Huepe D, Manes F, Decety J (2011): Subliminal presentation of other faces (but not own face) primes behavioral and evoked cortical processing of empathy for pain. Brain Res 1398:72–85. [DOI] [PubMed] [Google Scholar]
- Jabbi M, Swart M, Keysers C (2007): Empathy for positive and negative emotions in the gustatory cortex. NeuroImage 34:1744–1753. [DOI] [PubMed] [Google Scholar]
- Jackson PL, Rainville P, Decety J (2006): To what extent do we share the pain of others? Insight from the neural bases of pain empathy. Pain 125:5–9. [DOI] [PubMed] [Google Scholar]
- Keysers C, Gazzola V (2006): Towards a unifying neural theory of social cognition. Prog Brain Res 156:379–401. [DOI] [PubMed] [Google Scholar]
- Keysers C, Kaas JH, Gazzola V (2010): Somatosensation in social perception. Nat Rev Neurosci 11:417–428. [DOI] [PubMed] [Google Scholar]
- Knutson B, Taylor J, Kaufman M, Peterson R, Glover G (2005): Distributed neural representation of expected value. J Neurosci 25:4806–4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML (2005): The human orbitofrontal cortex: Linking reward to hedonic experience. Nat Rev Neurosci 6:691–702. [DOI] [PubMed] [Google Scholar]
- Krishnan A, Woo CW, Chang LJ, Ruzic L, Gu X, Lopez‐Sola M, Jackson PL, Pujol J, Fan J, Wager TD (2016): Somatic and vicarious pain are represented by dissociable multivariate brain patterns. eLife 5: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn S, Gallinat J (2012): The neural correlates of subjective pleasantness. NeuroImage 61:289–294. [DOI] [PubMed] [Google Scholar]
- Lamm C, Decety J, Singer T (2011): Meta‐analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. NeuroImage 54:2492–2502. [DOI] [PubMed] [Google Scholar]
- Lamm C, Silani G, Singer T (2015): Distinct neural networks underlying empathy for pleasant and unpleasant touch. Cortex 70:79–89. [DOI] [PubMed] [Google Scholar]
- Liddell BJ, Williams LM, Rathjen J, Shevrin H, Gordon E (2004): A temporal dissociation of subliminal versus supraliminal fear perception: An event‐related potential study. J Cogn Neurosci 16:479–486. [DOI] [PubMed] [Google Scholar]
- Light SN, Moran ZD, Swander L, Le V, Cage B, Burghy C, Westbrook C, Greishar L, Davidson RJ (2015): Electromyographically assessed empathic concern and empathic happiness predict increased prosocial behavior in adults. Biol Psychol 104:116–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Hairston J, Schrier M, Fan J (2011): Common and distinct networks underlying reward valence and processing stages: A meta‐analysis of functional neuroimaging studies. Neurosci Biobehav Rev 35:1219–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe C, Rolls ET, Bilderbeck A, McGlone F (2008): Cognitive influences on the affective representation of touch and the sight of touch in the human brain. Soc Cogn Affect Neurosci 3:97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles WR (1930): Ocular dominance in human adults. J Gen Psychol 3:412–430. [Google Scholar]
- Minio‐Paluello I, Avenanti A, Aglioti SM (2006): Left hemisphere dominance in reading the sensory qualities of others' pain? Soc Neurosci 1:320–333. [DOI] [PubMed] [Google Scholar]
- Mobbs D, Yu R, Meyer M, Passamonti L, Seymour B, Calder AJ, Schweizer S, Frith CD, Dalgleish T (2009): A key role for similarity in vicarious reward. Science 324:900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli SA, Lieberman MD, Zaki J (2015a): The emerging study of positive empathy. Soc Personal Psychol Compass 9:57–68. [Google Scholar]
- Morelli SA, Sacchet MD, Zaki J (2015b): Common and distinct neural correlates of personal and vicarious reward: A quantitative meta‐analysis. NeuroImage 112:244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison I, Björnsdotter M, Olausson H (2011): Vicarious responses to social touch in posterior insular cortex are tuned to pleasant caressing speeds. J Neurosci 31:9554–9562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy ST, Zajonc RB (1993): Affect, cognition, and awareness: Affective priming with optimal and suboptimal stimulus exposures. J Pers Soc Psychol 64:723–739. [DOI] [PubMed] [Google Scholar]
- Nielsen FA, Hansen LK (2002): Automatic anatomical labelling of Talairach coordinates and generation of volumes of interest via tbe BrainMap database. In: NeuroImage, 16(2), 8th International Conference on Functional Mapping of the Human Brain, June 2–6, 2002, Sendai, Japan.
- Nosek BA, Hawkins CB, Frazier RS (2011): Implicit social cognition: From measures to mechanisms. Trends Cogn Sci 15:152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Knierim K, Ludlow DH, Hanelin J, Ramachandran T, Glover G, Mackey SC (2004): Reflecting upon feelings: An fMRI study of neural systems supporting the attribution of emotion to self and other. J Cogn Neurosci 16:1746–1772. [DOI] [PubMed] [Google Scholar]
- Payne BK, Cheng CM, Govorun O, Stewart BD (2005): An inkblot for attitudes: Affect misattribution as implicit measurement. J Pers Soc Psychol 89:277–293. [DOI] [PubMed] [Google Scholar]
- Penny W, Holmes P (2004): Random‐effects analysis. Human Brain Function. San Diego: Elsevier, pp. 843–850. [Google Scholar]
- Peyron R, Laurent B, Garcia‐Larrea L (2000): Functional imaging of brain responses to pain. A review and meta‐analysis (2000). Neurophysiol Clin 30:263–288. [DOI] [PubMed] [Google Scholar]
- Preston SD, de Waal FB (2002): Empathy: Its ultimate and proximate bases. Behav Brain Sci 25:1–20. discussion 20–71. [DOI] [PubMed] [Google Scholar]
- Raftery AE (1995): Bayesian model selection in social research In: Marsden PV, editor. Sociological methodology Cambridge, MA: Blackwell; pp. 111–196. [Google Scholar]
- Rameson LT, Morelli SA, Lieberman MD (2012): The neural correlates of empathy: Experience, automaticity, and prosocial behavior. J Cogn Neurosci 24:235–245. [DOI] [PubMed] [Google Scholar]
- Reis HT, Smith SM, Carmichael CL, Caprariello PA, Tsai FF, Rodrigues A, Maniaci MR (2010): Are you happy for me? How sharing positive events with others provides personal and interpersonal benefits. J Pers Soc Psychol 99:311–329. [DOI] [PubMed] [Google Scholar]
- Rolls ET, O'Doherty J, Kringelbach ML, Francis S, Bowtell R, McGlone F (2003): Representations of pleasant and painful touch in the human orbitofrontal and cingulate cortices. Cereb Cortex 13:308–317. [DOI] [PubMed] [Google Scholar]
- Schaefer M, Heinze HJ, Rotte M (2008): Observing the touched body magnified alters somatosensory homunculus. Neuroreport 19:901–905. [DOI] [PubMed] [Google Scholar]
- Singer T (2006): The neuronal basis and ontogeny of empathy and mind reading: Review of literature and implications for future research. Neurosci Biobehav Rev 30:855–863. [DOI] [PubMed] [Google Scholar]
- Singer T, Lamm C (2009): The social neuroscience of empathy. Ann N Y Acad Sci 1156:81–96. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O'Doherty J, Kaube H, Dolan RJ, Frith CD (2004): Empathy for pain involves the affective but not sensory components of pain. Science 303:1157–1162. [DOI] [PubMed] [Google Scholar]
- Smith KD, Keating JP, Stotland E (1989): Altruism reconsidered: The effect of denying feedback on a victim's status to empathic witnesses. J Pers Soc Psychol 57:641. [Google Scholar]
- Takahashi H, Kato M, Matsuura M, Mobbs D, Suhara T, Okubo Y (2009): When your gain is my pain and your pain is my gain: Neural correlates of envy and schadenfreude. Science 323:937–939. [DOI] [PubMed] [Google Scholar]
- Tamietto M, de Gelder B (2009): Neural bases of the non‐conscious perception of emotional signals. Nat Rev Neurosci 11:697–709. [DOI] [PubMed] [Google Scholar]
- Tsuchiya N, Koch C (2005): Continuous flash suppression reduces negative afterimages. Nat Neurosci 8:1096–1101. [DOI] [PubMed] [Google Scholar]
- Ulrich M, Kiefer M (2016): The Neural Signature of Subliminal Visuomotor Priming: Brain Activity and Functional Connectivity Profiles. Cereb Cortex 26:2471–2482. [DOI] [PubMed] [Google Scholar]
- Valeriani M, Betti V, Le Pera D, De Armas L, Miliucci R, Restuccia D, Avenanti A, Aglioti SM (2008): Seeing the pain of others while being in pain: A laser‐evoked potentials study. NeuroImage 40:1419–1428. [DOI] [PubMed] [Google Scholar]
- Walter H (2012): Social cognitive neuroscience of empathy: Concepts, circuits, and genes. Emot Rev 4:9–17. [Google Scholar]
- Wang Y, Song J, Guo F, Zhang Z, Yuan S, Cacioppo S (2016): Spatiotemporal brain dynamics of empathy for pain and happiness in friendship. Front Behav Neurosci 10:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waytz A, Zaki J, Mitchell JP (2012): Response of dorsomedial prefrontal cortex predicts altruistic behavior. J Neurosci 32:7646–7650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicker B, Keysers C, Plailly J, Royet JP, Gallese V, Rizzolatti G (2003): Both of us disgusted in My insula: The common neural basis of seeing and feeling disgust. Neuron 40:655–664. [DOI] [PubMed] [Google Scholar]
- Williams LM, Liddell BJ, Kemp AH, Bryant RA, Meares RA, Peduto AS, Gordon E (2006): Amygdala‐prefrontal dissociation of subliminal and supraliminal fear. Hum Brain Mapp 27:652–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Decety J (2009): Unconscious affective processing and empathy: An investigation of subliminal priming on the detection of painful facial expressions. Pain 143:71–75. [DOI] [PubMed] [Google Scholar]
- Yue T, Pan W, Huang X (2016): The relationship between trait positive empathy and brain structure: A voxel‐based morphometry study. Neuroreport 27:422–426. [DOI] [PubMed] [Google Scholar]
- Zaki J, Wager TD, Singer T, Keysers C, Gazzola V (2016): The anatomy of suffering: Understanding the relationship between nociceptive and empathic pain. Trends Cogn Sci 20:249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Table 1.
Supporting Information Table 2.


