Abstract
Childhood abuse has an enduring impact on the brain's stress system. Whether the effects of childhood abuse and adulthood stress are additive (cumulative stress hypothesis) or interactive (mismatch hypothesis) is widely disputed, however. The primary aim of this study was to test the utility of the cumulative stress and mismatch hypotheses in understanding brain and behaviour. We recruited 64 individuals (aged 14–26) from a specialised clinic for assessment and early intervention of mental health problems in young people. A T1‐weighted MRI, a resting state fMRI and clinical assessment were acquired from each participant. Grey matter estimates and resting state functional connectivity (rsFC) of the hippocampus, amygdala and anterior cingulate cortex (ACC) were determined using segmentation and seed‐to‐voxel rsFC analyses. We explored the effects of childhood abuse and recent stress on the structure and function of the regions of interest within general linear models. Worse psychiatric symptoms were significantly related to higher levels of life time stress. Individuals with mismatched childhood and recent stress levels had reduced left hippocampal volume, reduced ACC‐ventrolateral prefrontal cortex rsFC and greater ACC‐hippocampus rsFC, compared to individuals with matched childhood and recent stress levels. These results show specific utility of the cumulative stress hypothesis in understanding psychiatric symptomatology and of the mismatch hypothesis in modelling hippocampal grey matter, prefrontal rsFC, and prefrontal‐hippocampal rsFC. We provide novel evidence for the enduring impact of childhood abuse on stress reactivity in a clinical population, and demonstrate the distinct effects of stress in different systems. Hum Brain Mapp 38:2709–2721, 2017. © 2017 Wiley Periodicals, Inc.
Keywords: childhood abuse, stress, MRI, grey matter, resting state
INTRODUCTION
The brain regions involved in emotion and stress undergo both dendritic and synaptic remodelling in response to a psychologically traumatic event. The effects differ depending on the frequency, severity and timing of the stressor(s) (for review, see McEwen et al. [2015]). Human neuroimaging studies have found that adults with a history of childhood maltreatment and even those with a recent exposure to trauma have reduced hippocampal, amygdala and anterior cingulate cortex (ACC) grey matter, compared to individuals without any history of trauma [Aas et al., 2012, Ansell et al., 2012, Chaney et al., 2014, Cohen et al., 2006, Dannlowski et al., 2012, Hoy et al., 2012, Kuo et al., 2012, Malykhin et al., 2012, Opel et al., 2014, Papagni et al., 2011, Sodre et al., 2014, Vythilingam et al., 2002, Woon et al., 2010]. Childhood maltreatment has also been associated with reduced functional connectivity between regions involved in stress [Birn et al., 2014, Chugani et al., 2001, Herringa et al., 2013, Thomason et al., 2015]. In contrast, high adulthood stress has been linked to elevated hippocampal‐ventromedial prefrontal cortex connectivity [Admon et al., 2013a], as well as reduced anterior cingulate functional connectivity [Kennis et al., 2015].
Childhood abuse is strongly associated with adulthood mental illness. For example, a Canadian study of over 23,000 individuals found physical or sexual abuse in childhood tripled the risk of a psychiatric diagnosis in adulthood [Afifi et al., 2014]. Despite the compelling evidence that early life plays a large role in determining emotional processing, stress sensitivity and psychiatric risk in adulthood [Buchmann et al., 2014, Poon and Knight, 2012, van Vugt et al., 2014, Young and Widom, 2014, Scott et al., 2010, Spauwen et al., 2006], the interaction of childhood and adulthood stress on brain structure and function has not been thoroughly researched in humans. Two opposing views have emerged that attempt to describe how early life stress programs an individual's response to later life stress [Nederhof and Schmidt, 2012]. The cumulative stress hypothesis suggests greater exposure to trauma increases disease risk and neurobiological abnormalities in a dose dependent manner. Under this model, childhood and adulthood stress have an additive effect, which is supported by numerous reports of a correlation between lifetime stress and risk of mental illness [Kuhn et al., 2015, Myers et al., 2015, Toussaint et al., 2016, Vinkers et al., 2014]. On the other hand, the mismatch hypothesis suggests an individual is programmed by their early life environment to be suited to a similar environment later in life. The interactive effect of childhood and adulthood stress is central to this theory, in such that an individual with a history of childhood abuse would be at heighted disease risk only if their adulthood environment was not stressful [Santarelli et al., 2014]. Support or rejection of these hypotheses may enhance our understanding of stress sensitivity following childhood abuse. In turn, we can gain insight into the association of childhood abuse with enhanced psychiatric risk. Furthermore, by identifying the neurobiological consequences of childhood abuse and recent stress, we can provide a concrete basis for early intervention strategies and aid development of targeted treatment programs.
Previous neuroimaging studies have not provided clear support for either theory. Amongst veterans with a history of childhood maltreatment, greater combat exposure was correlated with reduced amygdala volume and reduced ACC thickness [Kuo et al., 2012, Woodward et al., 2013]. However, for veterans without childhood maltreatment, their combat exposure was not related to amygdala volume or ACC thickness. In another study, increased combat exposure and more severe childhood maltreatment were independently associated with enhanced blood oxygenation level dependent (BOLD) response of two anatomically distinct regions of the dorsal ACC upon angry face presentation [Herringa et al., 2013]. Together, this evidence suggests amygdala and ACC grey matter are more vulnerable to adulthood stress following childhood maltreatment, while the impacts of childhood maltreatment and adulthood stress on emotion induced functional reactivity of the ACC are independent.
Our primary aim was to test the utility of the cumulative stress and mismatch hypotheses in modelling hippocampal, amygdala and ACC structure and function. Our secondary aim was to extend research on the interaction of childhood and recent stress to individuals in the early stages of mental disorder. Given the instability and high co‐morbidity of psychiatric diagnoses in young people [Hafner et al., 2008], we followed the Research Domain Criteria recommendations of the National Institute of Mental Health [Cuthbert and Insel, 2013] and recruited a wide range of individuals from a specialised mental health clinic for young people. The advantages of using this trans diagnostic approach in research, in contrast to categorical diagnostic grouping, are discussed at length elsewhere [Casey et al., 2013, Cuthbert, 2014, Cuthbert and Insel, 2013]. Thirdly, we wished to ascertain whether variability in childhood trauma neuroimaging research stems from differences in recent stress. For example, childhood maltreatment has been linked to both increased and decreased amygdala volumes in adults [Aas et al., 2012, Cisler et al., 2013, Dean et al., 2014, Frodl et al., 2010, Pechtel et al., 2013, Aust et al., 2014, Hoy et al., 2012, Veer et al., 2015]. To do so, we designed a study in line with the proposal of Nederhof and Schmidt [2012]. Sixty‐four young people were split into four groups depending on exposure to childhood sexual or physical abuse (present/absent) and number of recent stressful events (high/low). Childhood sexual and physical abuse are particularly pervasive forms of childhood maltreatment. They have been associated with greater grey matter reductions [Paquola et al., 2016] and greater internalising problems [Pears et al., 2008], compared to other forms of childhood maltreatment. We assessed the independent and interactive effects of childhood abuse and recent stressful events on hippocampal, amygdala and ACC grey matter and resting state functional connectivity (rsFC), as well as psychiatric symptoms. We hypothesised, in line with Kuo et al. [2012] and Woodward et al. [2013], that grey matter volumes would be most reduced in individuals with childhood abuse and high levels of recent stressful events. We expected to detect abnormal ACC rsFC in individuals with childhood abuse and/or high stress, however, we did not expect to observe an interaction of childhood abuse and recent stress (in line with Herringa et al. [2013]). Finally, in support of the cumulative stress model, we expected psychiatric symptoms to be most severe in individuals with the greatest life stress.
METHOD
Participants
Sixty‐four young people (43 women, age range = 14–26 years) were recruited from a specialised clinic (headspace) for assessment and early intervention of mental health problems in young people [Scott et al., 2012] at the Brain & Mind Centre, Sydney, Australia. Inclusion criteria were a history of moderate‐severe childhood physical and/or sexual abuse, or no history of childhood maltreatment. All patients were receiving clinician‐based case management and relevant psychosocial interventions at the time of assessment. Primary diagnoses (as determined by a trained research psychologist via DSM‐IV criteria) included depressive disorder (n = 34), bipolar disorder (n = 15), psychotic disorder (n = 7) and anxiety disorder (n = 8). Seventy‐five percent of the participants presented with comorbid axis‐1 psychiatric diagnoses. To better characterise participants, we have noted the presence of any mood disorder, psychosis disorder or anxiety disorder in Table 1. Patients who were treated with psychotropic medications were assessed under ‘treatment as usual’ conditions, that is, medications were not interrupted in any way. At the time of assessment 29.7% of patients were not taking any psychotropic medications, 51.6% were taking a second‐generation antidepressant, 14.1% were taking mood stabilising medication and 23.4% were taking an atypical antipsychotic medication (Table 1). Exclusion criteria for all participants were medical instability (as determined by a psychiatrist, on the basis stability of treatment and symptoms), history of neurological disease (e.g. tumour, head trauma, epilepsy), medical illness known to impact cognitive and brain function (e.g. cancer, electroconvulsive therapy in the last 3 months), intellectual and/or developmental disability (a predicted IQ score < 70), insufficient English for testing or psychiatric assessment, and current substance dependence. The study was approved by the University of Sydney Human Research Ethics Committee and all participants gave written informed consent.
Table 1.
Group differences in demographic characteristics
|
NoCM‐LS [n =17] Group 1 |
NoCM‐HS [n =16] Group 2 |
CA‐LS [n =13] Group 3 |
CA‐HS [n =18] Group 4 |
One way ANOVA | Post hoc pairwise | |
|---|---|---|---|---|---|---|
| Age, years | 20.5 (2.9) | 20.1 (3.3) | 20.5 (2.9) | 20.1 (3.5) | ns | |
| Females | 12 | 10 | 10 | 11 | ns | |
| Any mood disorder | 15 | 14 | 13 | 18 | ns | |
| Any psychosis disorder | 4 | 4 | 1 | 4 | ns | |
| Any anxiety disorder | 12 | 10 | 6 | 12 | ns | |
| Illness duration, years | 5.4 (3.5) | 6.6 (3.6) | 4.7 (5.0) | 6.4 (4.8) | ns | |
| Antidepressant use | 9 | 9 | 7 | 6 | ns | |
| Mood stabilizer use | 2 | 3 | 1 | 4 | ns | |
| Antipsychotic use | 4 | 2 | 2 | 8 | ns | |
| CTQ score | 31.2 (5.3) | 33.6 (5.3) | 57.3 (14.7) | 62.1 (12.2) | F(3,60) = 42.01 | 1,2 < 3,4 |
| Sexual abuse score | 5.0 (0.0) | 5.1 (0.5) | 8.4 (6.6) | 9.3 (6.5) | F(3,60) = 3.99 | 1 < 3,4 2 < 4 |
| Physical abuse score | 5.0 (0.0) | 5.0 (0.0) | 11.5 (4.7) | 11.9 (4.6) | F(3,60) = 22.99 | 1,2 < 3,4 |
| Emotional abuse score | 7.3 (2.2) | 8.3 (1.9) | 14.4 (5.4) | 16.9 (4.3) | F(3,60) = 27.78 | 1,2 < 3,4 |
| Physical neglect score | 6.1 (1.4) | 6.1 (1.6) | 8.5 (3.4) | 9.1 (3.2) | F(3,60) = 6.75 | 1,2 < 3,4 |
| Emotional neglect score | 7.8 (2.7) | 9.1 (2.9) | 14.5 (6.9) | 14.8 (4.6) | F(3,60) = 10.97 | 1,2 < 3,4 |
| Recent stressful life events | 0.5 (0.5) | 2.8 (1.0) | 1.2 (0.8) | 3.9 (1.3) | F(3,60) = 42.38 | 1,3 < 2<4 |
| Intracranial volume, mm3 | 1553810 (141360) | 1472616 (173062) | 1455437 (146439) | 1511983 (116276) | ns |
Mean (standard deviation) or n provided where relevant. F, DoF and r are presented for contrasts in which P < 0.05 FDR corrected.
Legend: noCM: no childhood maltreatment. CA: childhood abuse. LS: low stress. HS: high stress. CTQ: Childhood trauma questionnaire. ns: not significant.
Clinical Assessment
The Childhood Trauma Questionnaire (CTQ) short form, a retrospective self‐report questionnaire, was used to measure exposure to maltreatment prior to the age of 16 [Bernstein et al., 1997]. The CTQ separately assesses experiences of sexual abuse, physical abuse, emotional abuse, physical neglect and emotional neglect, using a rating system along a five point Likert scale from 1 (never true) to 5 (very often true). Each participant produces a score from 5 to 25 for each subscale, and an additive score from 25 to 125 for total CTQ. Moderate‐severe cut‐offs for each subscore were used to classify the presence of childhood maltreatment [Bernstein et al., 1997]; sexual abuse ≥ 8, physical abuse ≥ 10, emotional abuse ≥ 13, physical neglect ≥ 10 and emotional neglect ≥ 15. Recent stressful events were measured with a brief checklist of threatening experiences [Brugha and Cragg, 1990]. Participants were asked to indicate (yes/no) whether they had experienced each of the twelve life events in the past 12 months.
In addition, a trained research psychologist conducted a clinical assessment (in a semi‐structured interview format) to inform the diagnostic classification and to determine the nature and history of any mental health problems. The assessment included the Hamilton Depression Rating Scale (HDRS, 17‐item) [Hamilton, 1967] to quantify current (over the last seven days) mood symptoms, the Brief Psychiatric Rating Scale (BPRS) [Overall and Gorham, 1962] to quantify current general psychiatric symptoms, the Kessler‐10 (K‐10) [Kessler et al., 2002], a brief instrument designed to detect psychological distress [Andrews and Slade, 2001] and the Overall Anxiety Severity and Impairment Scale (OASIS), a brief continuous measure of anxiety‐related severity and impairment [Norman et al., 2006].
Magnetic Resonance Imaging Acquisition
Participants underwent MRI scanning using a 3‐Tesla GE MR750 Discovery scanner (GE Medical Systems, Milwaukee, WI) at the Brain & Mind Centre, Sydney, Australia. From each participant we acquired a high resolution structural image (Customized MP‐RAGE 3D T1‐weighted sequence (0.9 mm isotropic resolution): repetition time (TR) = 7264ms; echo time (TE) = 2784ms; flip angle = 15º; coronal orientation; field of view (FOV) = 230 × 230 mm; matrix of 256 × 256; total slices =196) and resting state BOLD data (Echo planar imaging sequence: TR = 3000 ms; TE = 36 ms; slice thickness = 3.0 mm; flip angle = 90°; FOV = 240 × 240mm; matrix = 64 × 64; total slices = 20, total volumes = 273). Participants were instructed to rest comfortably with eyes closed without moving or falling asleep for the duration of the scans.
Structural Imaging Analysis
Volumetric analyses were performed using FMRIB Software Library (FSL) v5.0 [Smith et al., 2004]. Bilateral amygdalae and hippocampi were selected as regions of interest (ROI) based on extant literature and measured using FIRST software on T1‐weighted images [Patenaude et al., 2011]. Firstly, images were registered to MNI152 standard space using FMRIB's Linear Image Registration Tool (FLIRT). Then, the surface mesh of each subcortical structure was extracted and transformed back to original MRI space, filled and boundary corrected. Boundaries were visually inspected for gross errors. Additionally, total intracranial volume was estimated using SIENAX [Smith et al., 2002]. The process involved extraction of brain and skull images from a single whole head T1 weighted image, registration of the brain image to MNI152 standard space and tissue type segmentation with partial volume estimation. Finally, the volume of each structure for each subject was calculated in cubic millimetres and exported into SPSS 21.0 [SPSS Corp., 2012].
FreeSurfer version 5.1 (http://surfer.nmr.mgh.harvard.edu/) was used to obtain cortical thickness measurements. ACC thickness (instead of volume) was measured to make results comparable to extant literature. Processes included motion correction and averaging of two volumetric T1‐weighted images [Reuter et al., 2010], removal of non‐brain tissue [Segonne et al., 2004], alignment of scans to Talairach space, segmentation of the deep grey matter volumetric structures [Fischl et al., 2002, Fischl et al., 2004], intensity normalization [Sled et al., 1998], tessellation of the grey matter/white matter boundary, topology correction [Fischl et al., 2001, Segonne et al., 2007] and surface deformation to optimally place the grey/white and grey/cerebrospinal fluid borders [Dale et al., 1999, Dale and Sereno, 1993, Fischl and Dale, 2000]. Resulting cortical representations underwent surface inflation [Fischl et al., 1999a], registration to a spherical atlas to align individual cortical folding patterns with group cortical geometry [Fischl et al., 1999b], parcellation of the cortex into gyral and sulcal features [Desikan et al., 2006b, Fischl et al., 2004] and creation of cortical thickness statistical maps, calculated as the closest distance from the grey/white boundary to the grey/CSF boundary at each vertex on the tessellated surface [Fischl and Dale, 2000]. All images were visually inspected and any inaccuracies in segmentation and parcellation were manually edited. Finally, thickness measurements of bilateral rostral ACC (rACC) and caudal ACC (cACC) were extracted for each individual and exported to SPSS Version 21.0 [SPSS Corp., 2012].
Functional Imaging Analysis
Image preprocessing was performed using the Statistical Parametric Mapping (SPM12) software package (Wellcome Department of Imaging Neuroscience, London, UK; http://www.fil.ion.ucl.ac.uk/spm). Functional images were subjected to slice timing correction and motion realignment before being co‐registered to the structural image. Then both structural and functional scans were normalised to MNI space and resampled into a 2 × 2 × 2 mm3 voxel size. Segmentation was performed on the normalised structural image to yield grey matter, white matter and cerebrospinal fluid masks. Functional images were then smoothed using 8 mm full‐width at half‐maximum Gaussian.
Functional connectivity was measured via a seed‐based correlation method within the CONN‐fMRI functional connectivity toolbox [Whitfield‐Gabrieli and Nieto‐Castanon, 2012]. To mitigate the impact of movement and physiological noise confounds the CONN‐fMRI toolbox utilises anatomical component correction to regress principal components of white matter and cerebrospinal fluid from the BOLD time series at a voxel level before the resultant residual time‐series are band‐pass filtered (0.008 < f < 0.09 Hz). Seed regions were defined as the left hippocampus, right hippocampus, left amygdala, right amygdala, left rACC, right rACC, left cACC and right cACC (Fig. 1). Seed regions were masked using automated anatomical labelling [Desikan et al., 2006a, Tzourio‐Mazoyer et al., 2002]. Pearson's correlation coefficients were generated between the seed region time series and the time series of all other voxels, and converted to normally distributed z‐scores for second level general linear model analyses (described in the following section). Following statistical analysis, we extracted the z standardised correlation coefficients of each significant seed‐to‐voxel cluster pair for each participant. The correlation coefficients were exported to SPSS Version 21.0 [SPSS Corp., 2012] to estimate effect sizes and exported to GraphPad Prism [GraphPad, 2014] to illustrate group level effects (Fig. 3).
Figure 1.
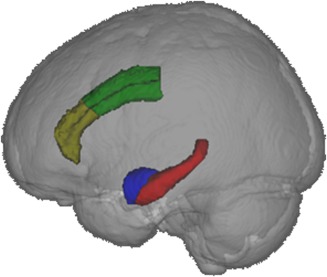
The left hippocampus (red), left amygdala (blue), left caudal ACC (green) and left rostral ACC (yellow) seed regions, presented from a left view in neurological convention. [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 3.
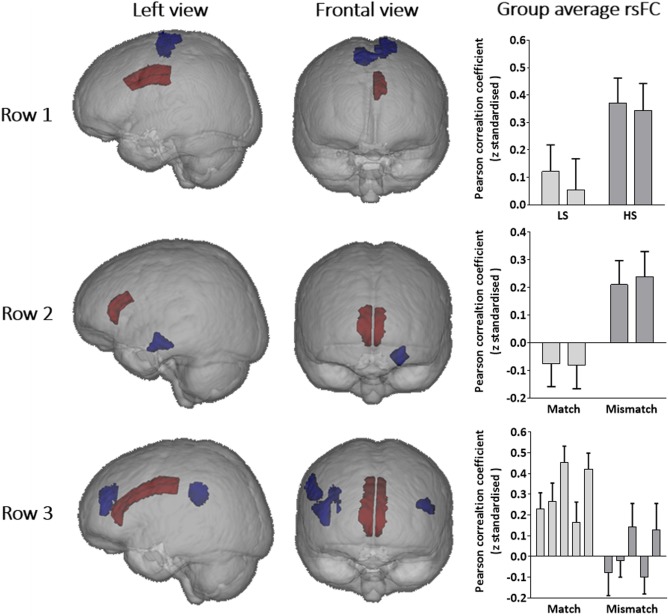
Each row represents a distinct effect of childhood abuse and/or recent stress on resting state functional connectivity (rsFC). Brains are presented in neurological convention with red representing seeds and blue signifying clusters in which rsFC with a seed region was significantly related to childhood abuse and/or recent stress. In the graphs, columns represent group average z scores of specific seed‐to‐voxel cluster rsFC, with 95% confidence intervals. Row 1: High recent stress was associated with enhanced rsFC of (column i) left cACC‐left pre/postcentral gyri and (column ii) left cACC‐right pre/postcentral gyri. Row 2: Mismatched child and adult stress levels was associated with enhanced rsFC of (column i) right rACC‐left hippocampus and (column ii) left rACC‐left hippocampus. Row 3: Mismatched child and adult stress levels was associated with decreased rsFC of (column i) right rACC‐left vlPFC, (column ii) right rACC‐right vlPFC, (column iii) right cACC‐right supramarginal gyrus, (column iv) left rACC‐ right vlPFC, (column v) left cACC‐right supramarginal gyrus. LS:low recent stress. HS: high recent stress. [Color figure can be viewed at http://wileyonlinelibrary.com]
Data Analysis
Participants with moderate‐severe childhood physical and/or sexual abuse were classed as the child abuse group (CA). Participants below the moderate‐severe cut‐off on all five subscores of the CTQ were classed as the no childhood maltreatment group (NoCM). Next, both the CA and NoCM groups were mean split into low recent stress (LS) and high recent stress (HS) groups based on the number of threatening life events experienced in the past year. As a preliminary analysis, we compared age, gender, diagnosis, illness duration, medication use, CTQ scores, number of recent stressful events and intracranial volume across groups using a one‐way ANOVA. Significant effects were further investigated with post hoc pairwise comparisons. We performed four statistical tests within general linear models to determine the relationship of clinical outcomes, grey matter and rsFC with childhood abuse and recent stress: (i) Main effect of childhood abuse (NoCM vs CA); (ii) Main effect of recent stressful events (LS vs HS); (iii) Cumulative stress hypothesis (main effect of the sum term of z standardised total CTQ and z standardised number of recent stressful events) and (iv) Mismatch hypothesis (NoCM‐LS and CA‐HS vs NoCM‐HS and CA‐LS). Age, gender, diagnosis, medication use and, in grey matter analyses, intracranial volume were entered as covariates in each analysis.
For clinical analyses, contrasts were considered significant if P < 0.05 following False Discovery Rate (FDR) correction across the four clinical outcomes (HDRS, BPRS, K10 and OASIS) and the four tests. For structural analyses, contrasts were considered significant if P < 0.05 following FDR correction across the eight ROIs and the four tests. In each case, FDR correction for multiple comparisons was performed within R Studio [R Team, 2015] using the method outlined by Benjamini and Hochberg [1995]. For rsFC analyses, we used conservative thresholds of P <0.001 for height (based on Bonferroni adjustment for eight seed regions and four tests) and P < 0.05 FDR corrected for cluster size. Pearson's r values were used as a measure of effect size.
RESULTS
Participant Characteristics
Demographic characteristics of the participants are reported in Table 1. The four groups did not differ significantly in terms of age, gender, diagnoses or medication use. Greater depressive symptoms (HDRS), general psychiatric symptoms (BPRS), current distress (K10) and anxiety symptoms (OASIS) were associated with higher levels of combined childhood abuse/recent stress (Table 2, Fig. 2). Additionally, childhood abuse was associated with significantly worse clinical outcomes (Table 2), and high recent stress was associated with greater depressive and general psychiatric symptoms (Table 2).
Table 2.
Relationship of childhood abuse and recent stress to clinical characteristics and grey matter volume/thickness
| NoCM‐LS [n =17] |
NoCM‐HS [n =16] |
CA‐LS [n =13] |
CA‐HS [n =18] |
Childhood abuse | Recent stressful events | Cumulative stress hypothesis | Mismatch hypothesis | |
|---|---|---|---|---|---|---|---|---|
| Hamilton Depression Rating Scale | 9.6 (7.0) | 15.5 (4.9) | 16.3 (6.6) | 16.9 (7.2) | F(1,56) = 9.51, r = 0.38 | F(1,56) = 6.39, r = 0.32 | F(1,56) = 14.73, r = 0.47 | Ns |
| Brief Psychiatric Rating Scale | 36.2 (9.9) | 40.4 (7.2) | 38.9 (5.9) | 47.6 (10.9) | F(1,56) = 7.84, r = 0.35 | F(1,56) = 9.21, r = 0.38 | F(1,56) = 9.52, r = 0.38 | Ns |
| Kessler 10 | 24.4 (8.7) | 29.4 (7.7) | 30.7 (30.7) | 33.0 (8.9) | F(1,56) = 11.45, r = 0.41 | ns | F(1,56) = 11.40, r = 0.44 | Ns |
| Overall Anxiety Severity and Impairment Scale | 6.2 (3.6) | 7.4 (4.4) | 8.1 (4.0) | 10.2 (5.2) | F(1,56) = 5.95, r = 0.31 | ns | F(1,56) = 14.14, r = 0.41 | Ns |
| Left amygdala, mm3 | 1365 (171) | 1410 (257) | 1347 (178) | 1380 (172) | ns | ns | ns | Ns |
| Right amygdala, mm3 | 1351 (186) | 1473 (283) | 1337 (193) | 1368 (164) | ns | ns | ns | Ns |
| Left hippocampus, mm3 | 3732 (396) | 3565 (460) | 3263 (457) | 3730 (289) | ns | ns | ns | F(1,61) = 11.71, r = 0.40 |
| Right hippocampus, mm3 | 3957 (429) | 3957 (556) | 3618 (411) | 3864 (295) | ns | ns | ns | Ns |
| Left cACC, mm2 | 2.86 (0.29) | 3.03 (0.22) | 2.81 (0.27) | 2.88 (0.22) | ns | ns | ns | Ns |
| Right cACC, mm2 | 2.71 (0.21) | 2.83 (0.17) | 2.83 (0.19) | 2.71 (0.28) | ns | ns | ns | Ns |
| Left rACC, mm2 | 3.05 (0.27) | 3.00 (0.30) | 3.06 (0.32) | 3.04 (0.28) | ns | ns | ns | Ns |
| Right rACC, mm2 | 3.05 (0.25) | 2.89 (0.23) | 3.22 (0.28) | 2.93 (0.25) | ns | F(1,61) = 10.88, r = 0.39 | ns | Ns |
Mean (standard deviation) or n provided where relevant. F, DoF and r are presented for contrasts in which P < 0.05 following FDR correction.
Legend: noCM: no childhood maltreatment. CA: childhood abuse. LS: low stress. HS: high stress. CTQ: Childhood trauma questionnaire. ns: not significant.
Figure 2.
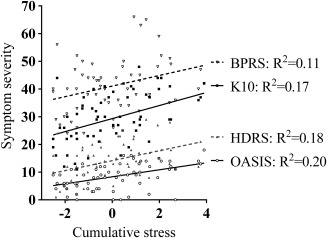
Greater cumulative life stress is associated with worse psychiatric symptom severity.
Structural Imaging
CA and NoCM groups did not significantly differ in terms of amygdala volume, hippocampal volume or ACC thickness (Table 2). Subjects reporting HS had approximately 7% thinner right rACC compared to subjects reporting LS (Table 2). Individuals with mismatched childhood and recent stress levels (NoCM‐HS and CA‐LS) had approximately 9% smaller left hippocampal volume compared to individuals with matched childhood and recent stress levels (NoCM‐LS and CA‐HS, Table 2).
Functional Imaging
Seed‐to‐voxel functional connectivity analyses were performed from each ROI (Fig. 1). Childhood maltreatment groups did not differ significantly in terms of rsFC from any ROI to any cluster of voxels. Compared to LS groups, individuals reporting HS had enhanced rsFC between the left cACC and bilateral precentral and postcentral gyri (Fig. 3 row 1, Table 3). Cumulative stress was not significantly related to rsFC from any ROI to and any cluster of voxels. Mismatched groups had greater rsFC between bilateral rACC and the left hippocampus, compared to matched groups (Fig. 3 row 2, Table 3). Additionally, several rsFC pathways from ACC seeds to bilateral ventrolateral prefrontal cortex (vlPFC) and the right supramarginal gyrus were reduced amongst mismatched groups, compared to matched groups (Fig. 3 row 3, Table 3).
Table 3.
Interactive and additive effects of childhood abuse and recent stressful events on seed‐to‐voxel resting state functional connectivity
| Test | Contrast | Seed region | Voxel cluster region | MNI coordinates | Cluster size | P value | r value |
|---|---|---|---|---|---|---|---|
| Main effect of childhood abuse | No significant effects | ||||||
| Main effect of adulthood stress | HS >LS | Left cACC | Left pre/postcentral | −18,‐38,66 | 199 | 0.003855 | 0.48 |
| Left cACC | Right pre/postcentral | 12,‐22,60 | 177 | 0.003855 | 0.45 | ||
| Effect of cumulative stress | No significant effects | ||||||
| Difference between matched and mismatched groups | Mismatch > Match | Right rACC | Left hippocampus | −24,‐12,‐18 | 147 | 0.021685 | 0.52 |
| Left rACC | Left hippocampus | −22,‐14,‐20 | 161 | 0.035854 | 0.52 | ||
| Match > Mismatch | Right rACC | Left vlPFC | −40,46,18 | 151 | 0.021685 | 0.57 | |
| Right rACC | Right vlPFC | 36,54,16 | 271 | 0.003016 | 0.52 | ||
| Right cACC | Right supramarginal | 58,‐50,36 | 389 | 0.000130 | 0.54 | ||
| Left rACC | Right vlPFC | 38,48,14 | 113 | 0.049409 | 0.52 | ||
| Left cACC | Right supramarginal | 56,‐46,36 | 270 | 0.003427 | 0.49 | ||
P value given at cluster level, FDR corrected.
Legend: LS = low stress. HS = high stress cACC = caudal anterior cingulate cortex. rACC = rostral anterior cingulate cortex, vlPFC = ventrolateral prefrontal cortex.
DISCUSSION
This is the first study to investigate the interaction of childhood abuse and recent stress on brain structure and function in young people. We endeavoured to understand how the cumulative stress and mismatch theories align with human neuroimaging research. In doing so, we provide support for a cumulative stress explanation of clinical symptoms and a mismatch explanation of right hippocampal volume and prefrontal rsFC. We hypothesise, as an extension of Nederhof and Schmidt's [2012] integrated model, that the dichotomy between the observed effects is related to the differential sensitivity of brain‐behaviour systems to early life programming. Mismatched early life and later life environments may be most problematic for systems with high sensitivity to early life programming, such as stress induced DNA methylation [Provençal and Binder, 2015]. In the hippocampus, for example, human and rodent studies have shown early life stress is associated with DNA methylation of promotors involved in stress reactivity (NR3C1; [McGowan et al., 2009, Suderman et al., 2012]), synaptic plasticity (protocadherins; [Suderman et al., 2012]) and neuronal differentiation (retinoic acid receptor α; [Boku et al., 2015]). On the other hand, systems with low sensitivity to early life programming are more likely to experience continuous wear and tear due to repeated use of adaptive responses to stress [Lupien et al., 2015].
Our clinical data was suggestive of greater lifetime stress being related to greater depressive, general psychiatric, distress and anxiety symptoms. Similarly, Kuhn et al. [2015] found that the additive effect of childhood maltreatment and a greater number of recent stressful events was related to increased depressive and anxious temperament in a sample of 1158 healthy young adults. Our findings support and extend upon this work by showing that the cumulative effect of stress on psychiatric symptoms is also evident in a real‐world clinical setting. Our results, like those of Kuhn et al. [2015], are correlative in nature however and causal relations are difficult to determine. Although our neuroimaging data did not support the cumulative stress hypothesis, greater life stress has been associated with enhanced stress‐induced prefrontal‐limbic‐striatal BOLD response [Seo et al., 2014] and decreased prefrontal BOLD response during emotion regulation [Kim et al., 2013]. Future studies should investigate whether task‐oriented brain function mediates the effect of cumulative stress on psychiatric symptoms.
Young people with mismatched childhood and recent stress levels were shown to have abnormal rsFC in pathways responsible for social and cognitive functioning. The ACC, vlPFC and supramarginal gyrus (as part of the posterior parietal cortex) are important nodes of the brain's social cognition network [Satpute and Lieberman, 2006]. The communication of these regions, alongside the medial temporal lobe and medial prefrontal cortex, is important for self‐reflection and self‐agency. Reduced rsFC of the ACC with the vlPFC and supramarginal gyrus, as reported in the mismatched groups, may reflect downregulation of this system, and has been specifically linked to worse metacognitive abilities elsewhere [Baird et al., 2013]. In addition, precise communication of the ACC with the hippocampus is necessary for effective learning. Greater rsFC between the rACC and hippocampus has been shown to predict worse learning transfer [Gerraty et al., 2014] and has been associatied with greater difficulty in integrating novel information [van Kesteren et al., 2010]. In sum, reduced rsFC between the ACC and vlPFC combined with enhanced rsFC between the ACC and the hippocampus may provide the neural basis for decreased sociality and hippocampal memory, as has been detected in rodent models of the mismatch hypothesis [Ricon et al., 2012, Santarelli et al., 2014]. Furthermore, the mismatch hypothesis may be especially applicable to social and cognitive brain networks due to their high sensitivity to programming in early life [Marquez et al., 2013, Tzanoulinou et al., 2014].
A mismatch of childhood and recent stress levels was also related to abnormalities in hippocampal structure. This may reflect adaptive programming following childhood abuse, in which hippocampal grey matter is primed for high stress. Preclinical research has shown hormones [Renard et al., 2010, Renard et al., 2007], cell adhesion molecules [van der Kooij et al., 2015] and epigenetic modifications [Kinnally et al., 2011] mediate the hippocampus' stress sensitivity following early life adversity.
Interestingly, childhood abuse has been repeatedly linked to smaller hippocampal volumes in adults from 18 to 50 years of age [Andersen et al., 2008, Bremner et al., 1997, Bremner et al., 2003, Sala et al., 2011, Stein et al., 1997, Vythilingam et al., 2002, Weniger et al., 2008] but in our study no main effect of childhood abuse was noted. Additionally, in psychosis cohorts, a clear association of childhood maltreatment with hippocampal volume has not been detected [Hoy et al., 2012, Sheffield et al., 2013]. Our study may shed some light on this discrepancy (despite diagnosis not affecting the findings). One possible explanation stems from individuals with psychotic disorders experiencing high levels of trauma [Beards et al., 2013]. Thus, individuals with psychosis may align with the HS groups of our study. In contrast, previous studies which have reported an association of childhood abuse with decreased hippocampal volume may have been primarily composed of individuals with low levels of recent stress. At this point, such an assertion is speculative and further testing of groups matched for psychiatric diagnosis and recent stress is necessary.
In line with Woodward et al. [2013], we found that high recent stress was associated with reduced ACC thickness amongst individuals with childhood maltreatment and that ACC thickness was not related to recent stress amongst individuals without childhood maltreatment. This pattern of results is suggestive of enhanced vulnerability of ACC grey matter to adulthood stress following childhood trauma. Admon et al. [2013b] postulated that reduced rACC following adulthood trauma represents a risk factor for post‐traumatic stress disorder. The rACC also plays a central role in emotion regulation [Etkin et al., 2011], and decreased rACC grey matter is associated with anxiety disorders [Shin and Liberzon, 2010] and depressive symptoms [Chen et al., 2007; Lim et al., 2012, Webb et al., 2014]. Given this evidence, abnormal structural remodelling of the rACC in response to stress in CA individuals may underpin enhanced psychiatric vulnerability. To our knowledge, no study has investigated the interaction of childhood trauma on ACC grey matter and subsequent mental health (as has been tested longitudinally in the hippocampus by Rao et al. [2010]).
We detected an association between high recent stress and enhanced rsFC of the cACC with the precentral gyrus. However, this finding contradicts a previous study. Kennis et al. [2015] found that combat exposure was related to reduced rsFC of the cACC and the precentral gyrus, which they proposed was related to motor cortex development following military training. Although our sample differs in numerous ways from the aforementioned study in the type of recent stress experienced, age, gender ratio and psychiatric morbidity, further research is required to understand this anomalous finding.
One limitation of this study is the small size of the four groups. Future studies require larger sample sizes, especially to investigate disorder specific effects. The psychiatric heterogeneity of this cohort is reflective of the clinical service from which participants were recruited [Scott et al., 2012]. However, due to the novelty of this study and the large representation of mood disorders in the cohort, it is difficult to eliminate any potential influence of diagnosis. Replications in other psychiatric cohorts and healthy cohorts are essential to validate the generalizability of these findings. Additionally, the retrospective nature of the CTQ could lead to misrepresentation of childhood maltreatment. The CTQ is a widely used and an effective research tool for cross sectional studies, however, and has been shown to be equally valid to psychiatrist led interviews [Bernstein et al., 1997, Karos et al., 2014, Spinhoven et al., 2014]. Third, susceptibility to early life programming differs greatly between individuals [Ellis et al., 2011], and as such genetic susceptibility should be explored in the future.
CONCLUSION
These results show specific utility of the cumulative stress hypothesis in relation to psychiatric symptoms while the mismatch hypothesis appears to relate to social and cognitive brain systems with high sensitivity to early life programming. These findings highlight the necessity of community‐based and clinical programs that reduce stress and improve coping abilities in individuals with a history of childhood abuse, particularly those with major psychiatric disorders. Additionally, the sensitivity of prefrontal‐hippocampal networks to stress could be mediated by early intervention social and cognitive training. We hope future studies can continue this avenue of research with the inclusion of genetic susceptibility, which is a key component of the Nederhof and Schmidt [2012] model, as well as longitudinal designs and balanced clinical interventions for fuller characterisation of the effects.
ACKNOWLEDGEMENTS
The authors have no conflict of interest to report.
REFERENCES
- Aas M, Navari S, Gibbs A, Mondelli V, Fisher HL, Morgan C, Morgan K, Maccabe J, Reichenberg A, Zanelli J, Fearon P, Jones PB, Murray RM, Pariante CM, Dazzan P (2012): Is there a link between childhood trauma, cognition, and amygdala and hippocampus volume in first‐episode psychosis? Schizophr Res 137:73–79. [DOI] [PubMed] [Google Scholar]
- Admon R, Leykin D, Lubin G, Engert V, Andrews J, Pruessner J, Hendler T (2013a): Stress‐induced reduction in hippocampal volume and connectivity with the ventromedial prefrontal cortex are related to maladaptive responses to stressful military service. Hum Brain Mapp 34:2808–2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Admon R, Milad MR, Hendler T (2013b): A causal model of post‐traumatic stress disorder: disentangling predisposed from acquired neural abnormalities. Trends Cogn Sci 17:337–347. [DOI] [PubMed] [Google Scholar]
- Afifi TO, Macmillan HL, Boyle M, Taillieu T, Cheung K, Sareen J (2014): Child abuse and mental disorders in Canada. Can Med Assoc J 186:E324–E332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL, Tomada A, Vincow ES, Valente E, Polcari A, Teicher Mh (2008): Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. J Neuropsychiatry Clin Neurosci 20:292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews G, Slade T (2001): Interpreting scores on the Kessler Psychological Distress Scale (K10). Aust N Z J Public Health 25:494–497. [DOI] [PubMed] [Google Scholar]
- Ansell EB, Rando K, Tuit K, Guarnaccia J, Sinha R (2012): Cumulative adversity and smaller gray matter volume in medial prefrontal, anterior cingulate, and insula regions. Biol Psychiatry 72:57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aust S, Stasch J, Jentschke S, Alkan Härtwig E, Koelsch S, Heuser I, Bajbouj M (2014): Differential effects of early life stress on hippocampus and amygdala volume as a function of emotional abilities. Hippocampus 24:1094–1101. [DOI] [PubMed] [Google Scholar]
- Baird B, Smallwood J, Gorgolewski KJ, Margulies DS (2013): Medial and lateral networks in anterior prefrontal cortex support metacognitive ability for memory and perception. J Neurosci 33:16657–16665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beards S, Gayer‐Anderson C, Borges S, Dewey ME, Fisher HL, Morgan C (2013): Life events and psychosis: a review and meta‐analysis. Schizophr Bull 39:740–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y (1995): Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc Ser B (Methodological) 57:289–300. [Google Scholar]
- Bernstein DP, Ahluvalia T, Pogge D, Handelsman L (1997): Validity of the childhood trauma questionnaire in an adolescent psychiatric population. J Am Acad Child Adolesc Psychiatry 36:340–348. [DOI] [PubMed] [Google Scholar]
- Birn RM, Patriat R, Phillips ML, Germain A, Herringa RJ (2014): Childhood maltreatment and combat posttraumatic stress differentially predict fear‐related fronto‐subcortical connectivity. Depress Anxiety 31:880–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boku S, Toda H, Nakagawa S, Kato A, Inoue T, Koyama T, Hiroi N, Kusumi I (2015): Neonatal maternal separation alters the capacity of adult neural precursor cells to differentiate into neurons via methylation of retinoic acid receptor gene promoter. Biol Psychiatry 77:335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Randall P, Vermetten E, Staib L, Bronen RA, Mazure C, Capelli S, McCarthy G, Innis RB, Charney DS (1997): Magnetic resonance imaging‐based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse ‐ A preliminary report. Biol Psychiatry 41:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Southwick SM, McGlashan T, Nazeer A, Khan S, Vaccarino LV, Soufer R, Garg PK, Ng CK, Staib LH, Duncan JS, Charney DS (2003): MRI and PET study of deficits in hippocampal structure and function in women with childhood sexual abuse and posttraumatic stress disorder. Am J Psychiatry 160:924–932. [DOI] [PubMed] [Google Scholar]
- Brugha TS, Cragg D (1990): The list of threatening experiences: the reliability and validity of a brief life events questionnaire. Acta Psychiatr Scand 82:77–81. [DOI] [PubMed] [Google Scholar]
- Buchmann AF, Holz N, Boecker R, Blomeyer D, Rietschel M, Witt SH, Schmidt MH, Esser G, Banaschewski T, Brandeis D, Zimmermann US, Laucht M (2014): Moderating role of FKBP5 genotype in the impact of childhood adversity on cortisol stress response during adulthood. Eur Neuropsychopharmacol 24:837–845. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Craddock N, Cuthbert BN, Hyman SE, Lee FS, Ressler KJ (2013): DSM‐5 and RDoC: progress in psychiatry research? Nat Rev Neurosci 14:810–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaney A, Carballedo A, Amico F, Fagan A, Skokauskas N, Meaney J, Frodl T (2014): Effect of childhood maltreatment on brain structure in adult patients with major depressive disorder and healthy participants. J Psychiatry Neurosci 39:50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C‐H, Ridler K, Suckling J, Williams S, Fu CHY, Merlo‐Pich E, Bullmore E (2007): Brain imaging correlates of depressive symptom severity and predictors of symptom improvement after antidepressant treatment. Biol Psychiatry 62:407–414. [DOI] [PubMed] [Google Scholar]
- Chugani HT, Behen ME, Muzik O, Juhasz C, Nagy F, Chugani DC (2001): Local brain functional activity following early deprivation: a study of postinstitutionalized Romanian orphans. Neuroimage 14:1290–1301. [DOI] [PubMed] [Google Scholar]
- Cisler JM, James GA, Tripathi S, Mletzko T, Heim C, Hu XP, Mayberg HS, Nemeroff CB, Kilts Cd (2013): Differential functional connectivity within an emotion regulation neural network among individuals resilient and susceptible to the depressogenic effects of early life stress. Psychol Med 43:507–518. [DOI] [PubMed] [Google Scholar]
- Cohen RA, Grieve S, Hoth KF, Paul RH, Sweet L, Tate D, Gunstad J, Stroud L, McCaffery J, Hitsman B, Niaura R, Clark CR, Macfarlane A, Bryant R, Gordon E, Williams LM (2006): Early life stress and morphometry of the adult anterior cingulate cortex and caudate nuclei. Biol Psychiatry 59:975–982. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN (2014): Translating intermediate phenotypes to psychopathology: the NIMH Research Domain Criteria. Psychophysiology 51:1205–1206. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Insel TR (2013): Toward the future of psychiatric diagnosis: the seven pillars of RDoC. Bmc Med 11: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale A, Fischl B, Sereno MI (1999): Cortical surface‐based analysis: I. Segmentation and surface reconstruction. Neuroimage 9:179– 194. [DOI] [PubMed] [Google Scholar]
- Dale AM, Sereno MI (1993): Improved localization of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: a linear approach. J Cogn Neurosci 5:162–176. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Lenzen T, Grotegerd D, Domschke K, Hohoff C, Ohrmann P, Bauer J, Lindner C, Postert C, Konrad C, Arolt V, Heindel W, Suslow T, Kugel H (2012): Limbic scars: long‐term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol Psychiatry 71:286–293. [DOI] [PubMed] [Google Scholar]
- Dean AC, Kohno M, Hellemann G, London ED (2014): Childhood maltreatment and amygdala connectivity in methamphetamine dependence: a pilot study. Brain Behav 4:867–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ (2006a): An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31:968–980. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Boyce WT, Belsky J, Bakermans‐Kranenburg MJ, Van Ijzendoorn MH (2011): Differential susceptibility to the environment: An evolutionary–neurodevelopmental theory. Dev Psychopathol 23:7–28. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R (2011): Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci 15:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Dale AM (2000): Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A 97:11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Liu A, Dale AM (2001): Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Med Imag 20:70–80. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, Van Der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM (2002): Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33:341–355. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Van Der Kouwe AJW, Makris N, Ségonne F, Quinn BT, Dale AM (2004): Sequence‐independent segmentation of magnetic resonance images. Neuroimage 23:S69– S84. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM (1999a): Cortical surface‐based analysis. II: Inflation, flattening, and a surface‐based coordinate system. NeuroImage 9:195–207. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM (1999b): High‐resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp 8:272–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl T, Reinhold E, Koutsouleris N, Reiser M, Meisenzahl EM (2010): Interaction of childhood stress with hippocampus and prefrontal cortex volume reduction in major depression. J Psychiatr Res 44:799–807. [DOI] [PubMed] [Google Scholar]
- Gerraty RT, Davidow JY, Wimmer GE, Kahn I, Shohamy D (2014): Transfer of learning relates to intrinsic connectivity between hippocampus, ventromedial prefrontal cortex, and large‐scale networks. J Neurosci 34:11297–11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAPHPAD 2014. . GraphPad Prism In: INC G. S. (ed.) 6.04 for Windows ed. San Diego; California USA. [Google Scholar]
- Hafner H, An Der Heiden W, Maurer K (2008): Evidence for separate diseases? Stages of one disease or different combinations of symptom dimensions? Eur Arch Psychiatry Clin Neurosci 258:85–96. [DOI] [PubMed] [Google Scholar]
- Hamilton M (1967): Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol 6:278–296. [DOI] [PubMed] [Google Scholar]
- Herringa RJ, Birn RM, Ruttle PL, Burghy CA, Stodola DE, Davidson RJ, Essex MJ (2013): Childhood maltreatment is associated with altered fear circuitry and increased internalizing symptoms by late adolescence. Proc Natl Acad Sci U S A 110:19119–19124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoy K, Barrett S, Shannon C, Campbell C, Watson D, Rushe T, Shevlin M, Bai F, Cooper S, Mulholland C (2012): Childhood trauma and hippocampal and amygdalar volumes in first‐episode psychosis. Schizophr Bull 38:1162–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karos K, Niederstrasser N, Abidi L, Bernstein DP, Bader K (2014): Factor structure, reliability, and known groups validity of the german version of the childhood trauma questionnaire (short‐form) in swiss patients and nonpatients. J Child Sex Abuse 23:418–430. [DOI] [PubMed] [Google Scholar]
- Kennis M, Rademaker AR, Van Rooij SJH, Kahn RS, Geuze E (2015): Resting state functional connectivity of the anterior cingulate cortex in veterans with and without post‐traumatic stress disorder. Hum Brain Mapp 36:99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Andrews G, Colpe LJ, Hiripi E, Mroczek DK, Normand SLT, Walters EE, Zaslavsky AM (2002): Short screening scales to monitor population prevalences and trends in non‐specific psychological distress. Psychol Med 32:959–976. [DOI] [PubMed] [Google Scholar]
- Kim P, Evans GW, Angstadt M, Ho SS, Sripada CS, Swain JE, Liberzon I, Phan KL (2013): Effects of childhood poverty and chronic stress on emotion regulatory brain function in adulthood. Proc Natl Acad Sci U S A 110:18442–18447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnally EL, Feinberg C, Kim D, Ferguson K, Leibel R, Coplan JD, John Mann J (2011): DNA methylation as a risk factor in the effects of early life stress. Brain Behav Immunity 25:1548–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn M, Scharfenort R, Schumann D, Schiele MA, Munsterkotter AL, Deckert J, Domschke K, Haaker J, Kalisch R, Pauli P, Reif A, Romanos M, Zwanzger P, Lonsdorf TB (2015): Mismatch or allostatic load? Timing of life‐adversity differentially shapes gray matter volume and anxious‐temperament. Soc Cogn Affect Neurosci 11:537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo JR, Kaloupek DG, Woodward SH (2012): Amygdala volume in combat‐exposed veterans with and without posttraumatic stress disorder: A cross‐sectional study. Arch Gen Psychiatry 69:1080–1086. [DOI] [PubMed] [Google Scholar]
- Lim HK, Jung WS, Ahn KJ, Won WY, Hahn C, Lee SY, Kim I, Lee CU (2012): Regional cortical thickness and subcortical volume changes are associated with cognitive impairments in the drug‐naive patients with late‐onset depression. Neuropsychopharmacology 37:838–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, Ouellet‐morin I, Hupbach , A, Tu MT, Buss C, Walker D, Pruessner J, McEwen BS ( 2015): Beyond the Stress Concept: Allostatic Load—A Developmental Biological and Cognitive Perspective Developmental Psychopathology. Hoboken, NJ, USA: Wiley. [Google Scholar]
- Malykhin NV, Carter R, Hegadoren KM, Seres P, Coupland NJ (2012): Fronto‐limbic volumetric changes in major depressive disorder. J Affect Disord 136:1104–1113. [DOI] [PubMed] [Google Scholar]
- Marquez C, Poirier GL, Cordero MI, Larsen MH, Groner A, Marquis J, Magistretti PJ, Trono D, Sandi C (2013): Peripuberty stress leads to abnormal aggression, altered amygdala and orbitofrontal reactivity and increased prefrontal MAOA gene expression. Transl Psychiatry 3:e216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Nasca C, Gray JD (2015): Stress effects on neuronal structure: hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology 41:3–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan PO, Sasaki A, D'alessio AC, Dymov S, Labonte B, Szyf M, Turecki G, Meaney MJ (2009): Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci 12:342–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers HF, Wyatt GE, Ullman JB, Loeb TB, Chin D, Prause N, Zhang M, Williams JK, Slavich GM, Liu H (2015): Cumulative burden of lifetime adversities: Trauma and mental health in low‐SES African Americans and Latino/as. Psychol Trauma 7:243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nederhof E, Schmidt MV (2012): Mismatch or cumulative stress: toward an integrated hypothesis of programming effects. Physiol Behav 106:691–700. [DOI] [PubMed] [Google Scholar]
- Norman SB, Cissell SH, Means‐Christensen AJ, Stein MB (2006): Development and validation of an overall anxiety severity and impairment scale (OASIS). Depress Anxiety 23:245–249. [DOI] [PubMed] [Google Scholar]
- Opel N, Redlich R, Zwanzger P, Grotegerd D, Arolt V, Heindel W, Konrad C, Kugel H, Dannlowski U (2014): Hippocampal atrophy in major depression: a function of childhood maltreatment rather than diagnosis? Neuropsychopharmacology 39:2723–2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overall JE, Gorham DR (1962): The brief psychiatric rating scale. Psychol Rep 10:799–812. [Google Scholar]
- Papagni SA, Benetti S, Arulanantham S, McCrory E, McGuire P, Mechelli A (2011): Effects of stressful life events on human brain structure: a longitudinal voxel‐based morphometry study. Stress 14:227–232. [DOI] [PubMed] [Google Scholar]
- Paquola C, Bennett MR, Lagopoulos J (2016): Understanding heterogeneity in grey matter research of adults with childhood maltreatment—A meta‐analysis and review. Neurosci Biobehav Rev 69:299–312. [DOI] [PubMed] [Google Scholar]
- Patenaude B, Smith SM, Kennedy DN, Jenkinson M (2011): A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage 56:907–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pears KC, Kim HK, Fisher PA (2008): Psychosocial and cognitive functioning of children with specific profiles of maltreatment. Child Abuse Negl 32:958–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechtel P, Teicher MH, Anderson CM, Lyons‐Ruth K (2013): Sensitive periods of amygdala development: the role of adversity in preadolescence. Biol Psychiatry 73:83s–83s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon CY, Knight BG (2012): Emotional reactivity to network stress in middle and late adulthood: the role of childhood parental emotional abuse and support. Gerontologist 52:782–791. [DOI] [PubMed] [Google Scholar]
- Provençal N, Binder EB (2015): The effects of early life stress on the epigenome: From the womb to adulthood and even before. Exp Neurol 268:10–20. [DOI] [PubMed] [Google Scholar]
- R TEAM . 2015. RStudio: Integrated Development for R. Boston, MA: RStudio, Inc. [Google Scholar]
- Rao U, Chen LA, Bidesi AS, Shad MU, Thomas MA, Hammen CL (2010): Hippocampal changes associated with early‐life adversity and vulnerability to depression. Biol Psychiatry 67:357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renard GM, Rivarola MA, Suarez MM (2007): Sexual dimorphism in rats: effects of early maternal separation and variable chronic stress on pituitary‐adrenal axis and behavior. Int J Dev Neurosci 25:373–379. [DOI] [PubMed] [Google Scholar]
- Renard GM, Rivarola MA, Suarez MM (2010): Gender‐dependent effects of early maternal separation and variable chronic stress on vasopressinergic activity and glucocorticoid receptor expression in adult rats. Dev Neurosci 32:71–80. [DOI] [PubMed] [Google Scholar]
- Reuter M, Rosas HD, Fischl B (2010): Highly accurate inverse consistent registration: a robust approach. Neuroimage 53:1181–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricon T, Toth E, Leshem M, Braun K, Richter‐Levin G (2012): Unpredictable chronic stress in juvenile or adult rats has opposite effects, respectively, promoting and impairing resilience. Stress 15:11–20. [DOI] [PubMed] [Google Scholar]
- Sala M, Caverzasi E, Lazzaretti M, Morandotti N, De Vidovich G, Marraffini E, Gambini F, Isola M, De Bona M, Rambaldelli G, D'allio G, Barale F, Zappoli F, Brambilla P (2011): Dorsolateral prefrontal cortex and hippocampus sustain impulsivity and aggressiveness in borderline personality disorder. J Affect Disord 131:417–421. [DOI] [PubMed] [Google Scholar]
- Santarelli S, Lesuis SL, Wang XD, Wagner KV, Hartmann J, Labermaier C, Scharf SH, Muller MB, Holsboer F, Schmidt MV (2014): Evidence supporting the match/mismatch hypothesis of psychiatric disorders. Eur Neuropsychopharmacol 24:907–918. [DOI] [PubMed] [Google Scholar]
- Satpute AB, Lieberman MD (2006): Integrating automatic and controlled processes into neurocognitive models of social cognition. Brain Res 1079:86–97. [DOI] [PubMed] [Google Scholar]
- Scott EM, Hermens DF, Glozier N, Naismith SL, Guastella AJ, Hickie IB (2012): Targeted primary care‐based mental health services for young Australians. Med J Aust 196:136–140. [DOI] [PubMed] [Google Scholar]
- Scott KM, Smith DR, Ellis PM (2010): Prospectively ascertained child maltreatment and its association with dsm‐iv mental disorders in young adults. Arch Gen Psychiatry 67:712–719. [DOI] [PubMed] [Google Scholar]
- Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, Fischl B (2004): A hybrid approach to the skull stripping problem in MRI. Neuroimage 22:1060– 1075. [DOI] [PubMed] [Google Scholar]
- Segonne F, Pacheco J, Fischl B (2007): Geometrically accurate topology‐correction of cortical surfaces using nonseparating loops. IEEE Trans Med Imag 26:518–529. [DOI] [PubMed] [Google Scholar]
- Seo D, Tsou KA, Ansell EB, Potenza MN, Sinha R (2014): Cumulative adversity sensitizes neural response to acute stress: association with health symptoms. Neuropsychopharmacology 39:670–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield JM, Williams LE, Woodward ND, Heckers S (2013): Reduced gray matter volume in psychotic disorder patients with a history of childhood sexual abuse. Schizophr Res 143:185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Liberzon I (2010): The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology 35:169–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC (1998): A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imag 17:87–97. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen‐Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM (2004): Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23 Suppl 1:S208–S219. [DOI] [PubMed] [Google Scholar]
- Smith SM, Zhang Y, Jenkinson M, Chen J, Matthews PM, Federico A, De Stefano N (2002): Accurate, robust, and automated longitudinal and cross‐sectional brain change analysis. Neuroimage 17:479–489. [DOI] [PubMed] [Google Scholar]
- Sodre LA, Vasconcelos‐Moreno MP, Vianna‐Sulzbach M, Goi PD, Duarte JA, Polita SRL, Massuda R, Czepielewski LS, Goldfeld P, Reckziegel RFX, Kauer‐Sant'anna M, Gama CS (2014): Amygdala volume is decreased in individuals with bipolar disorder and childhood trauma. Biol Psychiatry 75:237S–237S. [Google Scholar]
- Spauwen J, Krabbendam L, Lieb R, Wittchen H‐U, Van Os J (2006): Impact of psychological trauma on the development of psychotic symptoms: relationship with psychosis proneness. Br J Psychiatry 188:527–533. [DOI] [PubMed] [Google Scholar]
- Spinhoven P, Penninx BW, Hickendorff M, Van Hemert AM, Bernstein DP, Elzinga BM (2014): Childhood trauma questionnaire: Factor structure, measurement invariance, and validity across emotional disorders. Psychol Assess 26:717–729. [DOI] [PubMed] [Google Scholar]
- SPSS CORP., I . 2012. IBM SPSS Statistics for Windows. 21.0 ed. Armonk, New York: IBM Corp. [Google Scholar]
- Stein MB, Koverola C, Hanna C, Torchia MG, McClarty B (1997): Hippocampal volume in women victimized by childhood sexual abuse. Psychol Med 27:951–959. [DOI] [PubMed] [Google Scholar]
- Suderman M, McGowan PO, Sasaki A, Huang TC, Hallett MT, Meaney MJ, Turecki G, Szyf M (2012): Conserved epigenetic sensitivity to early life experience in the rat and human hippocampus. Proc Natl Acad Sci U S A 109 Suppl 2:17266–17272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason ME, Marusak HA, Tocco MA, Vila AM, McGarragle O, Rosenberg DR (2015): Altered amygdala connectivity in urban youth exposed to trauma. Soc Cogn Affect Neurosci 10:1460–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toussaint L, Shields GS, Dorn G, Slavich GM (2016): Effects of lifetime stress exposure on mental and physical health in young adulthood: How stress degrades and forgiveness protects health. J Health Psychol 21:1004–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzanoulinou S, Garcia‐Mompo C, Castillo‐Gomez E, Veenit V, Nacher J, Sandi C (2014): Long‐term behavioral programming induced by peripuberty stress in rats is accompanied by GABAergic‐related alterations in the amygdala. PLoS One 9:e94666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. Neuroimage 15:273–289. [DOI] [PubMed] [Google Scholar]
- Van Der Kooij MA, Grosse J, Zanoletti O, Papilloud A, Sandi C (2015): The effects of stress during early postnatal periods on behavior and hippocampal neuroplasticity markers in adult male mice. Neuroscience 311:508–518. [DOI] [PubMed] [Google Scholar]
- Van Kesteren MTR, Fernández G, Norris DG, Hermans EJ (2010): Persistent schema‐dependent hippocampal‐neocortical connectivity during memory encoding and postencoding rest in humans. Proc Natl Acad Sci U S A 107:7550–7555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Vugt E, Lanctot N, Paquette G, Collin‐Vezina D, Lemieux A (2014): Girls in residential care: from child maltreatment to trauma‐related symptoms in emerging adulthood. Child Abuse Negl 38:114–122. [DOI] [PubMed] [Google Scholar]
- Veer IM, Oei NY, Van Buchem MA, Spinhoven P, Elzinga BM, Rombouts SA (2015): Evidence for smaller right amygdala volumes in posttraumatic stress disorder following childhood trauma. Psychiatry Res 233:436–442. [DOI] [PubMed] [Google Scholar]
- Vinkers CH, Joels M, Milaneschi Y, Kahn RS, Penninx BW, Boks MP (2014): Stress exposure across the life span cumulatively increases depression risk and is moderated by neuroticism. Depress Anxiety 31:737–745. [DOI] [PubMed] [Google Scholar]
- Vythilingam M, Heim C, Newport J, Miller AH, Anderson E, Bronen R, Brummer M, Staib L, Vermetten E, Charney DS, Nemeroff CB, Bremner JD (2002): Childhood trauma associated with smaller hippocampal volume in women with major depression. Am J Psychiatry 159:2072–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb CA, Weber M, Mundy EA, Killgore WD (2014): Reduced gray matter volume in the anterior cingulate, orbitofrontal cortex and thalamus as a function of mild depressive symptoms: a voxel‐based morphometric analysis. Psychol Med 44:2833–2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weniger G, Lange C, Sachsse U, Irle E (2008): Amygdala and hippocampal volumes and cognition in adult survivors of childhood abuse with dissociative disorders. Acta Psychiatr Scand 118:281–290. [DOI] [PubMed] [Google Scholar]
- Whitfield‐Gabrieli S, Nieto‐Castanon A (2012): Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect 2:125–141. [DOI] [PubMed] [Google Scholar]
- Woodward SH, Kuo JR, Schaer M, Kaloupek DG, Eliez S (2013): Early adversity and combat exposure interact to influence anterior cingulate cortex volume in combat veterans. Neuroimage Clin 2:670–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woon FL, Sood S, Hedges DW (2010): Hippocampal volume deficits associated with exposure to psychological trauma and posttraumatic stress disorder in adults: a meta‐analysis. Prog Neuropsychopharmacol Biol Psychiatry 34:1181–1188. [DOI] [PubMed] [Google Scholar]
- Young JC, Widom CS (2014): Long‐term effects of child abuse and neglect on emotion processing in adulthood. Child Abuse Negl 38:1369–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]


