Abstract
Healthy aging is associated with a decline in cognitive, executive, and motor processes that are concomitant with changes in brain activation patterns, particularly at high complexity levels. While speech production relies on all these processes, and is known to decline with age, the mechanisms that underlie these changes remain poorly understood, despite the importance of communication on everyday life. In this cross‐sectional group study, we investigated age differences in the neuromotor control of speech production by combining behavioral and functional magnetic resonance imaging (fMRI) data. Twenty‐seven healthy adults underwent fMRI while performing a speech production task consisting in the articulation of nonwords of different sequential and motor complexity. Results demonstrate strong age differences in movement time (MT), with longer and more variable MT in older adults. The fMRI results revealed extensive age differences in the relationship between BOLD signal and MT, within and outside the sensorimotor system. Moreover, age differences were also found in relation to sequential complexity within the motor and attentional systems, reflecting both compensatory and de‐differentiation mechanisms. At very high complexity level (high motor complexity and high sequence complexity), age differences were found in both MT data and BOLD response, which increased in several sensorimotor and executive control areas. Together, these results suggest that aging of motor and executive control mechanisms may contribute to age differences in speech production. These findings highlight the importance of studying functionally relevant behavior such as speech to understand the mechanisms of human brain aging. Hum Brain Mapp 38:2751–2771, 2017. © 2017 Wiley Periodicals, Inc.
Keywords: aging, speech production, movement time, primary motor cortex, premotor cortex, anterior insula, posterior cingulate gyrus, neural compensation, de‐differentiation
INTRODUCTION
Advancing age is associated with a decline in a number of cognitive functions, including episodic and working memory, attention and executive control [e.g., Park et al., 2002; Salthouse, 1996, 2009], as well as motor functions [e.g., Cerella, 1985; Spirduso, 1982; Ward, 2006; Ward and Frackowiak, 2003; Welford et al., 1969]. Several studies have shown that cognitive decline is associated with age‐related changes in the anatomy and functioning of the prefrontal cortex [Grady and Craik, 2000; Grady et al., 1994; Madden et al., 2002; Mattay et al., 2006; Nielson et al., 2002; Piefke et al., 2012]. In particular, “over‐activations” are frequently reported in the prefrontal cortex of older compared to younger adults during a variety of cognitive tasks. Over‐activations are also reported in brain‐imaging studies of older adults performing hand and finger motor tasks [e.g., Heuninckx et al., 2005, 2008; Marchand et al., 2011; Mattay et al., 2002; Riecker et al., 2006; Ward, 2006; Ward and Frackowiak, 2003; Wu and Hallett, 2005]. Several non‐mutually exclusive neurobiological hypotheses have been developed to account for these age‐related over‐activations, including the Compensation‐Related Utilization of Neural Circuit Hypothesis (CRUNCH), the Hemispheric Asymmetry Reduction in OLDer age (HAROLD) hypothesis, and the de‐differentiation hypothesis. The HAROLD model suggests that performance can be maintained in older adults through the recruitment of additional prefrontal regions that approximately mirror active sites in younger adults but in the opposite hemisphere [Cabeza et al., 2002]. The CRUNCH model proposes a more general explanation of the mechanisms of aging [Reuter‐Lorenz and Cappell, 2008; Reuter‐Lorenz and Mikels, 2006]. According to this view, several brain regions are over‐recruited in older adults compared to younger adults, even at low task complexity levels, reflecting neural processing inefficiency, and allowing performance to be maintained. However, as task demands increase, these compensatory effects reach their maximal capacity leading to insufficient processing and an age‐related decline in performance. The de‐differentiation hypothesis [Li and Lindenberger, 1999], in contrast, suggests that additional and less specialized neural regions are recruited during cognitive tasks in older adults, resulting in increased activation for older compared to younger adults. These increased activation, however, do not help maintain performance, and might reflect an age‐related difficulty in recruiting specialized neural mechanisms. De‐differentiation mechanisms can also take the form of a reduction in the selectivity of brain responses that is observed in older adults [Grady, 2008, 2012]. Hence, cognitive aging is associated with several kinds of neurobiological mechanisms.
In contrast to the rich literature on cognitive aging, much less is known about the neurobiological mechanisms associated with age‐related changes in spoken language functions, despite their importance in day‐to‐day communication and social interactions. Previous studies have documented a number of age‐related differences in various aspects of spoken language production including changes in voice production affecting the fundamental frequency (pitch) of the voice [Decoster and Debruyne, 1997; Honjo and Isshiki, 1980; Hunter et al., 2012; Linville, 1996; Mueller, 1997; Ramig, 1983], as well as, the stability [Lortie et al., 2015, 2016; Wilcox and Horii, 1980] and loudness of voice [Baker et al., 2001]. Other studies have reported age‐related differences in articulation and prosody, including a decrease in speech rate [Duchin and Mysak, 1987; Fozo and Watson, 1998; Searl et al., 2002; Wohlert and Smith, 1998], an increase in the duration of individual speech sounds and syllables during the repetition of words or sentences [Morris and Brown, 1987; Ryan and Burk, 1974; Smith et al., 1987], as well as a decrease in accuracy during the production of complex nonwords and non‐speech oro‐facial movements [Bilodeau‐Mercure et al., 2015a; Sadagopan and Smith, 2013]. Interestingly, a study from our group recently demonstrated that age differences in speech motor accuracy (but not speed) are only partly mediated by a decline in the endurance of the lips and not at all related to lip and tongue force or tactile sensitivity [Bilodeau‐Mercure and Tremblay, in press]. These results suggest that age‐related decline in speech production performance cannot entirely be accounted for by a decline at the level of the peripheral orofacial system and may instead reflect a decline in neuromotor control, consistent with the literature on the aging of manual movement control [Aoki and Fukuoka, 2010; Cacola et al., 2013; Krampe, 2002; Krampe et al., 2005; Niermeyer et al., 2016; Seidler et al., 2010]. The motor control literature suggests that motor sequencing, the process of sequencing motor commands to form smooth and unitary sequences, declines with advancing age [Krampe et al., 2005; Niermeyer et al., 2016], and that it is moderated by complexity, with more complex motor sequences posing higher challenges to older adults than simple sequences. Given that the production of speech is a highly complex form of sequential behavior, it is possible that declining sequencing mechanisms may affect speech production as well as other kinds of movements requiring fine motor control. Finally, there is also some evidence to suggest that age‐related decline in articulation and voice quality affect speech intelligibility [Parnell and Amerman, 1987; Shuey, 1989]. Clearly, spoken language production undergoes a range of changes throughout normal aging, although the underlying biological mechanisms are unknown. To the best of our knowledge, only a few studies have focused on imaging speech production mechanisms in the aging brain [Eckert et al., 2008; Harris et al., 2009; Sörös et al., 2011; Tremblay and Deschamps, 2016; Tremblay et al., 2013b]. Sörös et al. [2011] demonstrated widespread increase in activation in older compared to younger adults during a simple overt speech production task, including the precentral gyrus, anterior insula, supplementary motor area (SMA) as well as middle and inferior frontal gyri bilaterally. These results suggest that performance is maintained through compensatory activation within the motor system involved in speech production, consistent with the CRUNCH model of aging. A recent study from our group has shown that age‐related changes in speech movement time (MT) are associated with structural changes in several cortical and subcortical regions known for their involvement in speech production, including the bilateral anterior insula, the left primary motor area (M1), the rostral supramarginal gyrus, the right inferior frontal sulcus and the bilateral striatum [Tremblay and Deschamps, 2016]. These results suggest that the neural system supporting speech production undergoes both functional and structural changes throughout aging, which affect speech motor performance, but the specific neuromotor mechanisms involved remain unknown.
The general objective of the present study was to broaden current understanding of the aging of the neuromotor control of speech. To isolate motor from linguistic processes and to avoid potential top‐down confounding effects, participants were asked to produce meaningless sequences of syllables, a task requiring the grouping of several motor programs to form a smooth unitary sequence. To examine whether complexity modulates the impact of aging on speech production, we parametrically manipulated the sequential complexity of the sequences (repeating the same or different syllables) and the motor complexity of the syllables forming the sequences (simple vs. complex syllables), which allowed us to identify age effects on neuromotor processes associated with the ability to assemble syllables into sequences of different sequential and motor complexity. Experimental manipulations to these factors are known to modulate speech production performance in healthy adults and in adults with motor speech dysfunctions [e.g., Aichert and Ziegler, 2004; Baldo et al., 2011; Bohland and Guenther, 2006; Reilly and Spencer, 2013; Sadagopan and Smith, 2013; Tiffany, 1980]. Moreover, previous studies have shown that, behaviorally, syllable production tasks are age sensitive [Bilodeau‐Mercure and Tremblay, in press; Sadagopan and Smith, 2013]. Our protocol was adapted from Bohland and Guenther [2006], but it differed from it in that we did not compare go to no‐go trials, but instead asked participants to produce sequences of syllables in every experimental trial. This was done to reduce the cognitive load imposed by go‐no‐go paradigms, which require inhibitory control, an executive function known to decline with age [Langenecker et al., 2004; Nielson et al., 2002, 2004; Sommers and Danielson, 1999]. Based on previous studies, our general hypothesis was that the performance of older adults during a sequential speech production task would be lower than that of young adults, in terms of motor control (i.e., MT or accuracy), particularly at high complexity levels. Based on the cognitive aging literature, we hypothesized that age‐related speech production differences would be associated with activation patterns consistent with the compensatory and/or the de‐differentiation hypotheses, but that, contrary to the cognitive literature, these changes would occur within the neural system supporting speech production, including the SMA, the striatum, the cerebellum, the ventral M1 and the premotor cortex (PMv) [Bohland and Guenther, 2006; Ghosh et al., 2008; Peeva et al., 2010; Tremblay et al., 2013a; Tremblay and Small, 2011]. Based on the finding of a mediating role of the anterior insula in speech functions in aging, we also expected to find age‐related differences in this region [Bilodeau‐Mercure et al., 2015b; Tremblay et al., 2013b].
METHODS
Participants
The study comprised a total of thirty healthy adult native speakers of Canadian French, which were divided into two groups (young, older). Due to technical difficulties with audio recordings during the experiment, three participants were not included in the present study, which included 27 participants. The young adult group was comprised of 13 healthy right‐handed adults [Oldfield, 1971] (mean age 26.8 ± 4.48 SD; 8 females). The older adult group was comprised of 14 healthy right‐handed adults (mean age 68.2 ± 4; 10 females). Participants in both groups had normal or corrected‐to‐normal vision and no self‐reported history of speech, voice, language, psychological, neurological, or neurodegenerative disorder. All participants with corrected vision wore contact lenses during the MRI acquisition, or MRI compatible glasses. Participants were screened for depression using the Geriatric Depression Scale [Yesavage et al., 1982] and their cognitive functioning was evaluated using the Montreal Cognitive Assessment scale (MOCA) [Nasreddine et al., 2005]. Participants' characteristics are reported in Table 1. The study was approved by the Institutional Ethical Committee of the Institut Universitaire en Santé Mentale de Québec (#280‐2011).
Table 1.
Description of the participants
| Younger adults (N = 13) | Older adults (N = 14) | |||
|---|---|---|---|---|
| Mean (±SD) | Range | Mean | Range | |
| Age | 26.85 (±4.49) | 21–34 years | 68.24 (±4.07) | 61–74 years |
| Education (in number of years) | 17.61 (±0.5) | 15–21 years | 17.64 (±1.4) | 11–33 years |
| Gender ratio (M|F) | 5|8 | 4|10 | ||
| Handedness | 19.3 (±0.85) | 18–20 | 19.85 (±0.36) | 19–20 |
| MOCA (/30)a | 29.23 (±1.16) | 27–30 | 27.4 (±1.28) | 26–30 |
| Geriatric depression scale (/30)b | 2.53 (±2.18) | 0–6 | 0.93 (±1.59) | 0–5 |
The MOCA is a short cognitive test that is scored on a 30‐point scale. Higher scores indicate better cognitive functions. A score of ≥ 26 is considered normal. This score is adjusted for age and education. All participants scored normal at the test, but the young adults had higher scores than the older adults (t (25) = 3.5, P = 0.002).
The Geriatric depression scale (GDS) includes 30 questions. Each “negative” answer is worth one point; thus, a higher score indicates a more depressed state. For example, question one asks whether the person is globally satisfied with his/her life. A “no” answer is worth one point, whereas a “yes” answer is worth no point. Participants with global scores between 0 and 9 are considered normal, while global scores between 10 and 19 indicate a depression, and scores between 20 and 30 indicate a severe depression. All participants scored normal at the test, but the young adults were slightly less positive than the older adults (t (25) = 2.2, P = 0.037).
Stimuli and Procedures
The stimuli were sequences of six French syllables. Half of the syllables had a simple consonant vowel (CV) structure, and the other half had a more complex (articulatory/phonological) consonant‐consonant‐vowel (CCV) structure (see Supporting Information 1 for a list of all syllables). A total of 120 unique sequences were created. Half of these sequences were simple, consisting of one syllable repeated six times (e.g., /pa‐pa‐pa‐pa‐pa‐pa/), while the other half was complex consisting of three alternating syllables (e.g., /pa‐ta‐ka‐pa‐ta‐ka/). This resulted in a 2x2 experimental design with Motor complexity and Sequencing complexity as the within subject factors, each with two levels, for a total of four conditions (simple syllables within simple sequences [SS] (e.g., /do‐do‐do‐do‐do‐do/), simple syllables within complex sequences [SC] (e.g., /ge‐ve‐di‐ge‐ve‐di/), complex syllables within simple sequences [CS] (e.g., /vlo‐vlo‐vlo‐vlo‐vlo‐vlo/), and complex syllables within complex sequences [CC] (e.g., /tre‐gro‐ble‐tre‐gro‐ble/)).
During the functional magnetic resonance imaging (fMRI) session, participants were asked to produce meaningless sequences of syllables presented on the screen as quickly and accurately as possible. All sequences were produced during the silent period while the MRI gradients were switched off (see the MR image Acquisition section). Participants were instructed to stop speaking as soon as the syllables disappeared from the screen. The participants had 4 sec to read and produce the syllables. Participants' verbal responses were recorded using a high‐quality MRI compatible optical omnidirectional microphone (MO‐2000, Sennheiser). All stimuli were presented using Presentation Software (Neurobehavioral System, CA).
A resting condition (crosshair fixation) was also included as a baseline condition and interleaved with experimental trials. Within each run, the order of the conditions and the number and duration of rest trials were optimized using OPTseq2 (http://surfer.nmr.mgh.harvard.edu/optseq /).
The experimental consisted in 120 experimental trials and 40 baseline trials, separated into two runs of approximately 10 min each. In addition to this task, participants also performed a speech perception in noise task while in the MRI scanner. These results have been published elsewhere [Bilodeau‐Mercure et al., 2015b].
Acoustical and Behavioral Data Analysis
Three behavioral measures were extracted from the voice recordings to characterize motor speech performance in terms of motor control (motor accuracy, MT) and motor planning (reaction time [RT]). All acoustic analyses were performed using Praat software [Boersma and Weenink, 2017]. A semi‐automatic procedure was used for segmenting participants' responses. For each participant, the procedure involved the automatic segmentation of each sequence based on an intensity and duration algorithm detection. Based on minimal duration and low intensity energy parameters, the algorithm automatically established the sequence's boundaries. These boundaries were then manually adjusted, based on waveform and spectrogram information. For each participant and each condition, the mean RT and MT were computed. Given that participants were instructed to respond as soon as a cue appeared, and not as fast as they could, our RT measure is not a true measure of how fast participants could initiate a speech response. MT was calculated as the time from response onset to response offset. For the analyses of motor accuracy, a research assistant naive to the purpose of the study listened to the voice recordings, transcribed the responses and calculated the number of errors, including both omissions and commissions. A second research assistant validated the transcriptions. Whenever a disagreement occurred between the research assistants, a third research assistant, also naive to the purpose of the study, was asked to transcribe the response. All disagreements were thus resolved. A response was considered accurate and included in the analysis only if there was an agreement between at least two of the assistants.
To examine the effect of syllable and sequence complexity on motor performance (RT, MT, and accuracy), three mixed‐effect 2 × 2 × 2 ANOVAs were conducted (one per behavioral measure) with Motor complexity (simple, complex) and Sequence complexity (simple, complex) as the within subject factors and Group (young, elderly) as the between subject factor. For the ANOVAs, measures of effect sizes are provided in the form of partial eta squared ( ). When comparing two means, we report effect sizes in the form of Cohen d statistics.
MR Image Acquisition
The data were acquired on a whole‐body Philips 3.0 Tesla Achieva TX at the Clinic IRM Québec‐Mailloux in Québec City. Structural MR images were acquired with 3D T1‐weighted MPRAGE sequence (TR = 8.2 ms, TE = 3.7 ms, FoV= 250 mm, flip angle = 8°, 256 × 256 matrix, 180 slices/volume, slice thickness = 1 mm, no gap). Single‐shot EPI BOLD functional images were acquired using parallel imaging. Each functional EPI run began with six dummy scans to allow the magnetization to stabilize to a steady state. Ninety functional images were acquired per run in ∼10 min (40 interleaved 3 mm3 axial slices, no gap); SENSE = 2; TR = 6,500 ms; acquisition time = 2,140 ms, delay in TR = 4,360 ms; TE = 30 ms; FOV = 240 × 240 mm; 80 × 80 matrix; Flip angle: 90). Throughout the procedure, each participant's head was immobilized using a set of cushions and pads. To further mitigate motion concerns and to record verbal responses a sparse sampling parallel acquisition technique [Gracco et al., 2005] was used, which involved periods of 4,360 ms of silence (MRI gradients turned off) interleaved with periods of data acquisition (2,140 ms). The acquisition protocol is illustrated in Figure 1.
Figure 1.
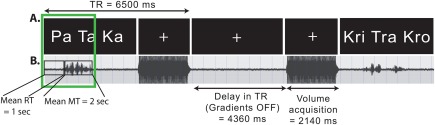
Experimental setup. (A) Illustration of the experimental design using a sparse‐sampling MRI data acquisition protocol. (B) Example of the acoustical recordings made during the MRI session. The green box represents the 3‐sec time window that was modeled in the within‐subject regression analysis. The group mean response time (RT) and MT are also provided. [Color figure can be viewed at http://wileyonlinelibrary.com]
MR Image Analyses
Head motion analysis
To determine whether head movements (i.e. translations and rotations) differed between young and older adults, we used the framewise displacement (FD) measure described in Power et al. [2012, 2014]. Briefly, FD is a measure that captures the movement of the head along the six rigid body parameters (translations and rotations along x, y, and z‐axes) from one volume to the next. It is calculated as the sum of the absolute difference in the six rigid body parameters between adjacent volumes. Rotational displacements are first converted from degrees to millimeters by calculating displacement on the surface of a sphere with a radius of 50 mm (this is the approximate distance from the cerebral cortex to the center of the head). The first volume of a run is set to 0 by convention. The rigid‐body translation and rotation algorithm implemented in AFNI's 3dvolreg outputs the adjustments needed to bring a volume back into alignment with the base [Cox and Jesmanowicz, 1999]. For each participant and each run, we calculated the average FD. Both runs FD values were averaged. A Mann‐Whitney U test was run to determine if there were differences in FD values between younger and older adults.
Pre‐processing
All data were converted to the AFNI file format, and visually inspected for artefacts. All time series were spatially registered to the first functional run, motion‐corrected, slice timing corrected, de‐spiked and mean‐normalized using AFNI [Cox, 1996]. All functional volumes acquired during excessive motion, defined as >1 mm, were excluded from the regression analysis using AFNI's censor function.
Subject‐level regression analysis
An ordinary least square regression approach was used to analyze subject data (10 regressors). Separate regressors were created for each participant for the correct and incorrect trials (regressors for incorrect trials were not considered in this article). Additional regressors were the mean, linear, and quadratic trend components, and the six motion parameters (x, y, z and roll, pitch and yaw). A 2‐parameter block shape model was used (AFNI model BLOCK, with a length 3 sec, representing the average time from stimulus onset to response offset [refer to Fig. 1]). The BLOCK function is created by convolving an incomplete gamma function (similar to a GAM function) with a boxcar function (equal to 1 over the stimulus duration, and 0 elsewhere). This model convolves to a maximum height of about 5.114, once the block length reaches about 15 sec. The BLOCK curve lasts about 15.8 sec longer than the stimulus duration (here 18.8 sec). This regression model was fit to each run separately (separate baseline models for each run). Because the behavioral analyses revealed age differences in MT (refer to Behavioral Data section), an additional regressor was included in the statistical model (implemented via AFNI programs 1dMarry and 3dDeconvolve with the stim_times_AM2 option). This additional predictor variable was the MT in seconds in each trial. The inclusion of this regressor allowed us to identify voxels whose activation level varied as a function of MT (BOLD‐MT) as well as to examine the stimulus‐locked BOLD response. Thus, for each participant, two effects were estimated for each trial type: (1) the stimulus‐locked BOLD response, reflecting response preparation and execution, and (2) the MT‐BOLD relationship.
Alignment and transformation to 2D (surface) space
Following regression, a surface representation of the participant's anatomy was created using FreeSurfer [Dale et al., 1999; Fischl et al., 1999] by inflating each hemisphere of the anatomical volumes to a surface representation and aligning it to a template of average curvature. These surfaces were exported into SUMA, AFNI's surface program [Saad et al., 2004]. The subject functional data (output from the first level regression) were then converted to the surface space (3dVol2Surf). Surface‐based alignment of the anatomical and functional data was conducted. Group‐level analyses were conducted on the surface, because surface analyses achieve better alignment between functional and structural data [Argall et al., 2006; Desai et al., 2005], and because spatial smoothing is more precise (see Smoothing section).
Smoothing
Following surface exportation, the results of the first‐level analysis (beta images) were smoothed on the surface to achieve a target smoothing value of 6 mm using a Gaussian FWHM filter. Smoothing on the surface as opposed to the volume ensures that white matter voxels are not included, and that functional data located in anatomically distant locations on the cortical surface are not averaged across sulci [Argall et al., 2006; Jo et al., 2007]. All data were visually inspected following exportation to surface space and after smoothing, as a standard measure of quality control. If a defect was detected or if the data were incorrectly aligned, the data were re‐exported. This occurred for two participants, which were successfully re‐exported.
Volumetric analyses
To analyze activation patterns within subcortical structures, in parallel to the surface analyses, we also conducted a standard volume analysis. Prior to the regression analysis, a moderate spatial smoothing was applied to the functional data (3 mm FWHM Gaussian filter), to prevent reduction of signal in small subcortical regions by volume averaging with larger surrounding regions of inactivity such as the white matter [Crosson et al., 2003]. The same statistical model was used to generate within‐subject statistical images for each of the conditions for both the surface and volume data. Following regression, anatomical and functional datasets were then spatially normalized to the MNI TT_N27 template using the 12‐parameter affine transform implemented in AFNI (@auto_tlrc program). The T1 image was first normalized to the template, and then, the T2 images were normalized to the normalized T1 images.
Group‐level voxel‐wise analyses
The first analysis examined the basic speech production network and served as a validity check. An average of all experimental conditions was computed (i.e., SS, SC, CS, CC) against the control condition (visual fixation) was compared against zero using a one‐sample t‐test (AFNI 3dTtest++). Next, the average of all conditions was compared for the younger and the older adults using a two‐sample t‐tests for independent samples. Because MT was included in the statistical model at the within‐subject level, these analyses represent the brain activation for speech production, controlling for MT.
Next, a series of analyses was conducted to examine the relationship between MT and the average BOLD signal across conditions (i.e., SS, SC, CS, CC). A one‐sample t‐test (against zero) was conducted to identify the voxels that were significantly correlated with MT. To identify whether the relationship between the BOLD signal and MT differs as a function of age, the average of all conditions was compared across groups using a two‐sample t‐test for independent samples. Finally, the same analysis was conducted separately for the most complex condition (CC), in which MT was the highest.
Following these analyses, a group‐level mixed effect ANOVA (AFNI 3dMVM program) [Chen et al., 2014] was computed across the whole brain, with Motor complexity (simple, complex) and Sequence complexity (simple, complex) as the within‐subject factors, Group as the between‐subject factor (young, older), and Subjects as a random factor. The dependent variable was the BOLD response independent of MT. This analysis allowed us to examine the relation between BOLD signal during speech production as a function of Group, Motor complexity, and Sequence complexity.
To decompose the interactions, we extracted, for each participant, the average regression coefficients (beta values) of all the clusters identified through the whole‐brain group analyses using a mask of the group result (AFNI 3dROIstats program). Functional regions were based on a mask of the group result, which averages the beta values across the whole cluster for each participant. These analyses are not independent from the ANOVA analyses, in the same way that post hoc analyses are not independent from the main effects. Thus, they are not meant to provide additional and independent information but rather clarify the ANOVA results and visualize the interactions.
The Destrieux atlas was used for cortical parcellation [Destrieux et al., 2010; Fischl et al., 2004] and to localize changes in BOLD signal in the cortex. For activation in the cerebellum, we used the MRI atlas of the Human Cerebellum [Schmahmann et al., 1999, 2000].
Clustering
All the analyses reported in this article are corrected for multiple comparisons. For the surface results, the Monte Carlo simulation procedure implemented in Freesurfer (mri_glmfit) was used to identify significant clusters of activated vertices taking into account the number of voxels and the amount of smoothing. The result indicated that, for an individual vertex threshold of P < 0.01, corrected for multiple comparisons to achieve a family‐wise error (FWE) rate of P < 0.05, the appropriate cluster size was ≥ 95 vertices. For the volumetric analyses, first we used AFNI's 3dfwhm program to estimate the actual smoothness of the data. This information was then used in AFNI's 3dClustSim program to determine the appropriate cluster size. The procedure returned a cluster size of ≥ 35 voxels [945 mm3].
RESULTS
Behavioral Data
There was no main effect of Group on response planning (RT) or motor control accuracy, nor any interaction with Group.1 The results of these analyses are reported in Supporting Information 2. For MT, first, the assumptions of normality and homogeneity of variances were verified. The assumption of normality for MT was satisfied for all groups and all experimental conditions, as assessed by Shapiro‐Wilk's test (P > 0.05). There was homogeneity of variances, as assessed by Levene's test for equality of variances (P > 0.05). The mixed effect 2 × 2 × 2 ANOVA, revealed a significant main effect of Group (F (1,25) = 8.47, P = 0.007, η 2 = 0.25), which was driven by shorter MT for the young compared to the older adults (means ± SE: 1.77 ± 0.08 sec vs. 2.07 ± 0.07 sec). The analysis also revealed a strong main effect of Motor complexity (F (1,25) = 270.16, P ≤ 0.0001, η 2 = 0.91), with shorter MT for the simple compared to the complex syllables (means ± SE: 1.78 ± 0.05 sec vs. 2.01 ± 0.05 sec). There was also a main effect of Sequence complexity (F (1,25) = 113.43, P ≤ 0.0001, η 2 = 0.82), with shorter MT for the simple compared to the complex sequences (means ± SE: 1.78 ± 0.05 sec vs. 2.05 ± 0.06 sec). The Motor by Sequential complexity interaction was significant (F (1,25) = 58.99, P ≤ 0.0001, η 2 = 0.70). The Group by Motor complexity interaction was significant (F (1,25) = 4.37, P = 0.047, η 2 = 0.15). To decompose this interaction, post hoc tests were conducted on Motor complexity scores [MT complex syllables – MT simple syllables]. This analysis revealed smaller complexity effect in the young compared to the older adults (means ± SE: 0.24 ± 0.02 sec vs. 0.31 ± 0.02 sec, t (25) = −2.09, P (one‐tailed) = 0.023, d = 0.83). This result is illustrated in Figure 2A (gray bars). The Group by Sequence complexity interaction was also significant (F (1,25) = 11.70, P = 0.002, η 2 = 0.32). Post hoc tests on syllable complexity scores [MT complex sequence – MT simple sequence] revealed smaller complexity effect in the young compared to the older adults (means ± SE: 0.19 ± 0.03 sec vs. 0.37 ± 0.04 sec, t (25) = −3.42, P (one‐tailed) = 0.001, d = 1.36). This result is also illustrated in Figure 2A (black bars). Finally, the 3‐way interaction between Group, Motor complexity and Sequence complexity was also significant (F (1,25) = 9.09, P = 0.006, η 2 = 0.27). Decomposition of this interaction, which is illustrated in Figure 2B, shows that the effect of Motor complexity was significant only at high sequence complexity level. This modulation was stronger for older compared to younger adults (t (25) = −3.01, P (one‐tailed) = 0.003, d = 1.29).
Figure 2.
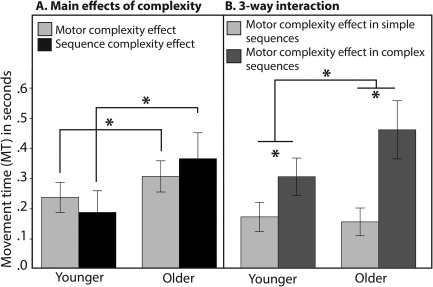
Behavioral results. The line charts present the results for the analysis of MT. In (A), the Complexity effects [Duration complex – Duration simple] are shown separately for each group (young, older) and each manipulation (syllable complexity, sequence complexity). In (B), the 3‐way (Group × Sequence complexity by Syllable Complexity) interaction is decomposed. The Syllable complexity effect (Duration complex syllable – Duration simple syllable) is shown separately for the simple and complex sequences, for the young and older adults. For both charts, error bars represent the standard error of the mean (SE). Asterisks indicate significance at P ≤ 0.05.
To decompose age differences in MT, a research assistant naive to the purpose of the study manually segmented the first run of all participants to extract the duration of the syllables, the duration of the pauses between the syllables as well as the duration of the pause occurring halfway through the sequence. The duration was then automatically extracted using Praat. Because of insufficient recording quality, three participants were excluded from this analysis, leaving 12 younger and 12 older adults. We first computed the mean of each measure and compared it across groups using independent sample t‐tests. For the duration of the pauses within syllable groups, across experimental conditions, the group difference was significant (means ± SE: 0.24 ± 0.02 sec vs. 0.36 ± 0.05 sec, t (22) = −2.11, P (one‐tailed) = 0.023, d = 0.9). For the duration of the pauses in between the two syllable groups, occurring halfway through the sequence, a group difference was also significant (means ± SE: 0.06 ± 0.001 sec vs. 0.15 ± 0.03 sec, t (22) = −2.83, P (one‐tailed) = 0.005, d = 1.2). There were no group differences for the duration of the syllables (means ± SE: 1.56 ± 0.06 sec vs. 1.71 ± 0.06 sec, t (22) = −1.62, P (one‐tailed) = 0.059, d = 0.69). Additional analyses are reported as Supporting Information 2B.
To further explore the nature of the age differences in MT, we examined the variability in MT. For each participant, we computed the standard deviation (SD) of MT in each experimental condition. Following a logarithmic (Log10), the assumption of normality for MT was satisfied for all groups and all experimental conditions, as assessed by Shapiro‐Wilk's test (P > 0.05). There was also homogeneity of variances, as assessed by Levene's test for equality of variances (P > 0.05), with the exception of the SC condition, in which variance was higher for the older compared to the younger adults. The ANOVA revealed a strong main effect of Group (F (1,25) = 37.27, P ≤ 0.001, η 2 = 0.59), but no interaction between Group and Motor complexity or between Group and Sequence complexity. As was expected, older adults produced responses that were more variables in duration than younger adults, with mean SD of 0.181 for the younger adults and 0.284 for the older adults, representing a 55% increase. The detail of this analysis is provided in Supporting Information 2A.
Head Motion
A Mann‐Whitney U test was run to determine if there were differences in FD values between younger and older adults. The analysis revealed no significant difference (U = 106, z = 0.73, P = 0.49).
BOLD‐MT Relationship
The network of regions active in the task within and across Groups, as well as group differences, are reported in Supporting Information 3. The basic network of regions that were sensitive to MT across experimental conditions and participants is shown in Figure 3A. As can be seen in the figure, a positive relationship between MT and the BOLD signal was found throughout the cortical motor system (more signal associated with slower responses), in the left hemisphere, including the central sulcus and precentral gyrus, the intraparietal sulcus and the medial part of the superior frontal gyrus (corresponding to the SMA). No activation was found in the basal ganglia or in the cerebellum. Next, we examined age differences in the relationship between BOLD and MT in the simplest (SS) and in the most complex (CC) tasks separately. For SS, no region survived correction. For CC, as shown in Figure 3B, age differences were found in several regions of the frontal lobe, including the central sulcus, inferior frontal gyrus, as well as in the intraparietal sulcus. A list of all activation is provided in Table 2 (A). To understand age differences in the relationships between the BOLD and MT, we examined the response patterns in each of these regions, separately for the younger and the older adults. These analyses revealed that BOLD response patterns varied in shape and direction across regions. Very few regions exhibited a response pattern akin to compensation, whereby a beneficial relationship between BOLD signal and speed was only present in older adults (left subcentral gyrus and sulcus, right cingulate gyrus). In the remaining regions identified through the whole‐brain analysis, we found various age‐dependent BOLD response patterns akin to de‐differentiation, mainly in task‐irrelevant areas. These response patterns took three different forms: (1) a detrimental relationship between BOLD signal and MT (slower responses associated stronger positive BOLD response) in older adults in the absence of a relationship between BOLD signal and MT in younger adults (right central sulcus [M1], right lateral occipital‐temporal sulcus, right lingual gyrus, and left transverse occipital sulcus). (2) The weakening of a relationship between BOLD signal and MT in older compared to younger adults (right inferior frontal sulcus, right marginalis part of the cingulate gyrus, right postcentral gyrus and sulcus, right inferior and middle occipital gyri, right cuneus, left middle frontal gyrus, left intraparietal sulcus, left paracentral gyrus and sulcus). (3) A change in the direction of the BOLD‐MT relationship (right precentral sulcus [PMv], right intraparietal sulcus, left inferior occipital sulcus, left inferior frontal sulcus). Supporting Information 4 provides a description of the response pattern in each of these regions.
Figure 3.
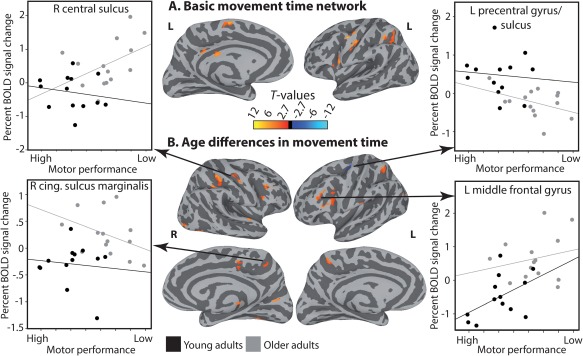
Motor control speed network. (A) Group‐level FWE‐corrected average of BOLD‐MT relationships across all condition for all the participants (B) Age‐differences in BOLD‐MT relationships. The pattern of age differences in the BOLD‐MT relationships is illustrated for four cortical regions in the form of scatter plots. The X axis shows MT in sec. Because long responses are considered to represent a low performance, these are labeled “LOW.” Faster responses represent a “HIGH” performance. Cortical activations are shown on the group average smoothed white matter folded surfaces. L = left hemisphere; R = right hemisphere. [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 2.
FWE‐corrected whole brain results
| Effect | Region | Hemi | x | y | z | Area | Max F | Max P |
|---|---|---|---|---|---|---|---|---|
| A. Age differences in BOLD‐MT interactions (in the complex condition CC) | Part opercular of the inferior frontal gyrus and inferior part of the precentral sulcus | Left | −50 | 19 | 21 | 136 | 3.77 | 0.001 |
| Superior parietal lobule and intraparietal sulcus and transverse parietal sulci | Left | −29 | −58 | 59 | 102 | 4.74 | <0.001 | |
| Inferior occipital gyrus and sulcus | Left | −30 | −85 | −12 | 95 | 4.59 | <0.001 | |
| Superior occipital sulcus and transverse occipital sulcus | Left | −25 | −87 | 17 | 89 | 5.24 | <0.001 | |
| Precentral gyrus and superior part of the precentral sulcus | Left | −28 | −14 | 65 | 65 | −3.96 | 0.001 | |
| Middle frontal gyrus | Left | −42 | 29 | 26 | 60 | 4.19 | <0.001 | |
| Paracentral lobule and sulcus and superior parietal lobule | Left | −8 | −41 | 63 | 53 | 3.57 | 0.001 | |
| Subcentral gyrus and sulci | Left | −57 | −17 | 15 | 44 | 3.90 | 0.001 | |
| Inferior frontal sulcus | Left | −35 | 5 | 31 | 35 | 3.69 | 0.001 | |
| Supramarginal gyrus | Left | −61 | −27 | 22 | 21 | 3.72 | 0.001 | |
| Postcentral gyrus and sulcus | Right | 38 | −35 | 55 | 164 | 5.10 | <0.001 | |
| Central sulcus | Right | 37 | −14 | 51 | 90 | 3.94 | 0.001 | |
| Parieto‐occipital sulcus | Right | 14 | −67 | 16 | 60 | 4.15 | <0.001 | |
| Inferior occipital gyrus and sulcus | Right | 31 | −94 | 0 | 59 | 5.01 | <0.001 | |
| Inferior occipital gyrus and sulcus | Right | 46 | −73 | −7 | 59 | 4.09 | <0.001 | |
| Medial occipito‐temporal sulcus and lingual sulcus | Right | 30 | −64 | −7 | 59 | 4.57 | <0.001 | |
| Inferior frontal sulcus and middle frontal gyrus | Right | 42 | 22 | 27 | 45 | 5.01 | <0.001 | |
| Intraparietal sulcus and transverse parietal sulci | Right | 37 | −40 | 42 | 44 | 4.22 | <0.001 | |
| Inferior part of the precentral sulcus and inferior frontal sulcus | Right | 44 | 15 | 18 | 38 | 4.44 | <0.001 | |
| Marginal branch of the cingulate sulcus | Right | 15 | −45 | 61 | 37 | 3.50 | 0.002 | |
| Posterior ramus of the lateral sulcus | Right | 34 | −17 | 18 | 36 | 3.16 | 0.004 | |
| Lateral occipito‐temporal sulcus | Right | 52 | −38 | −18 | 30 | 3.76 | 0.001 | |
| Lateral aspect of the superior temporal gyrus | Right | 65 | −6 | 0 | 29 | 4.22 | <0.001 | |
| Middle‐posterior part of the cingulate gyrus and sulcus | Right | 12 | −11 | 50 | 27 | 3.02 | 0.006 | |
| Middle‐posterior part of the cingulate gyrus and sulcus | Right | 14 | −11 | 44 | 26 | 3.50 | 0.002 | |
| Intraparietal sulcus and transverse parietal sulci | Right | 43 | −49 | 45 | 22 | 4.31 | <0.001 | |
| B. Age Group × Sequence Complexity interaction | Inferior and middle occipital gyrus | Left | −43 | −86 | −1 | 36 | 18.09 | <0.001 |
| Anterior segment of the circular sulcus of the insula | Right | 30 | 31 | −2 | 283 | 110.01 | <0.001 | |
| Superior temporal sulcus | Right | 54 | −4 | −23 | 179 | 100.57 | <0.001 | |
| Medial orbital sulcus | Right | 15 | 28 | −21 | 135 | 73.13 | <0.001 | |
| Inferior and middle occipital gyrus and sulcus | Right | 33 | −94 | −2 | 142 | 64.12 | <0.001 | |
| Superior temporal sulcus | Right | 55 | −2 | −13 | 143 | 63.79 | <0.001 | |
| Sulcus intermedius primus (of Jensen) | Right | 56 | −45 | 28 | 97 | 20.22 | 0.000 | |
|
C. 3‐way interaction (Age Group × Sequence Complexity × Motor Complexity) |
Middle‐anterior part of cingulate gyrus and sulcus and superior frontal gyrus (medial) | Left | −12 | 15 | 36 | 230 | 19.59 | <0.001 |
| Marginal branch of the cingulate sulcus and superior frontal gyrus (medial part) | Left | −17 | −30 | 43 | 215 | 16.03 | <0.001 | |
| Inferior part of the precentral sulcus and inferior frontal sulcus | Left | −37 | 8 | 44 | 129 | 21.32 | <0.001 | |
| Supramarginal gyrus and posterior lateral fissure | Left | −54 | −36 | 27 | 85 | 24.17 | <0.001 | |
| Superior temporal sulcus | Left | −40 | −57 | 20 | 71 | 13.97 | 0.001 | |
| Superior frontal gyrus (medial part) | Left | −7 | 33 | 48 | 59 | 12.87 | 0.001 | |
| Central sulcus | Left | −33 | −21 | 48 | 55 | 16.72 | <0.001 | |
| Middle‐posterior part of the cingulate gyrus and sulcus | Left | −12 | −13 | 47 | 45 | 14.88 | 0.001 | |
| Central sulcus | Left | −41 | −15 | 41 | 45 | 15.31 | 0.001 | |
| Superior frontal gyrus (medial part) | Left | −6 | −24 | 68 | 40 | 13.40 | 0.001 | |
| Superior parietal lobule | Left | −14 | −73 | 62 | 33 | 13.49 | 0.001 | |
| Paracentral lobule and sulcus and marginal branch of the cingulate sulcus | Right | 12 | −42 | 68 | 212 | 38.06 | <0.001 | |
| Supramarginal gyrus and posterior ramus of the lateral sulcus | Right | 53 | −27 | 29 | 104 | 20.85 | <0.001 | |
| Intraparietal sulcus and transverse parietal sulci | Right | 31 | −53 | 41 | 75 | 14.52 | 0.001 | |
| Middle frontal gyrus | Right | 40 | 11 | 48 | 69 | 19.81 | <0.001 | |
| Postcentral sulcus | Right | 26 | −40 | 55 | 69 | 18.63 | <0.001 | |
| Middle‐posterior part of the cingulate gyrus and sulcus | Right | 14 | −10 | 47 | 65 | 13.33 | 0.001 | |
| Paracentral lobule and sulcus | Right | 5 | −31 | 74 | 44 | 14.90 | 0.001 | |
| Subparietal sulcus | Right | 15 | −53 | 38 | 42 | 13.27 | 0.001 |
Coordinates are in MNI space and represent the peak surface node for each of the cluster (FWE: P = 0.05, minimum cluster size: 95 contiguous surface nodes, each significant at P < 0.01). Cluster size is reported as area in mm2.
BOLD‐Motor Complexity Relationship
As shown in Figure 4, the direct comparison of complex and simple syllables revealed stronger activation for the complex syllables in several bilateral cortical and subcortical areas, including the ventral central sulcus and precentral gyrus (i.e., M1 and PMv), the left superior frontal gyrus (including the SMA and pre‐SMA), the middle anterior and posterior parts of the cingulate gyrus and sulcus, the cerebellum (lobules VI and V), as well as the calcarine sulcus and surrounding areas (cuneus and lingual gyrus). There was no Group by Motor complexity interaction.
Figure 4.
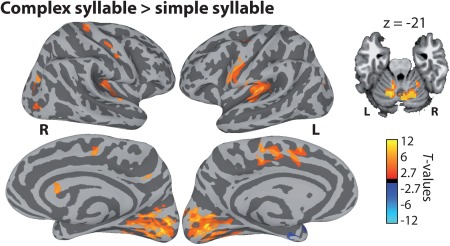
Motor complexity effects. Group‐level FWE‐corrected contrast of complex and simple syllables. Cortical activation is shown on the group average smoothed white matter folded surfaces. Cerebellar activation is shown on an axial slice of an MNI template (TT_N27). L = left hemisphere; R = right hemisphere. [Color figure can be viewed at http://wileyonlinelibrary.com]
BOLD‐Sequence Complexity Relationship
The direct comparison of complex and simple sequences (Fig. 5A) revealed strong positive activation bilaterally in the central sulcus, precentral gyrus and sulcus (including M1 and PMv), medial part of the superior frontal gyrus (including the SMA and pre‐SMA), the middle anterior part of the cingulate gyrus and sulcus, the intraparietal sulcus, and cerebellum (lobules VI and V and Crus I). There was also positive activation in the left inferior frontal sulcus. As can be seen in Figure 5B and detailed in Table 2 (B), several areas exhibited a Group by Sequence complexity interaction. Analyses of the activation in these regions revealed three distinct patterns: (1) a lesser deactivation in the older compared to the younger adults at high sequence complexity level (right superior temporal sulcus, right sulcus intermedius primus of Jensen). An example is provided in Figure 5B(i). (2) A lack of selectivity for the older compared to the younger adults (circular sulcus of the insula, right the inferior occipital gyrus and sulcus and left middle occipital gyrus). Examples are provided in Figure 5B(ii) and (iii). (3) A stronger complexity effect for the older compared to the younger adults (right superior temporal sulcus). To confirm that these response patterns were not related to MT, a standard multiple regression analysis was conducted on the BOLD signal (BOLD Complex sequences – BOLD Simple sequences) with Group and MT (MT complex sequences – MT Simple sequences) as the predictor variables, separately for each region identified through the whole brain analysis. In all analyses, as expected, there was a significant effect of Group (P ≤ 0.05) but MT did not significantly contribute to explaining the variance in the BOLD signal.
Figure 5.
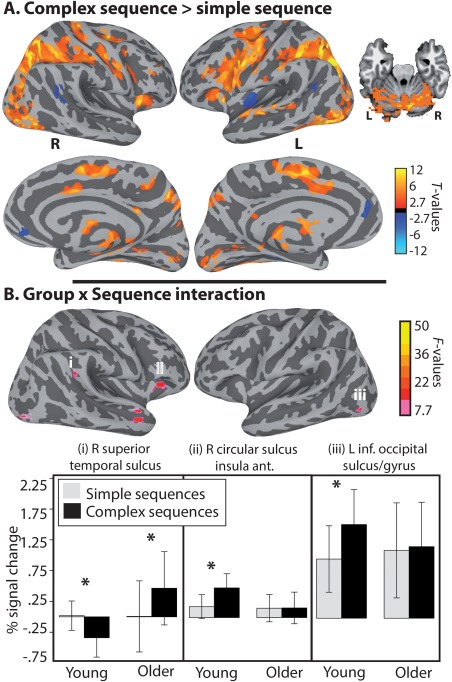
Sequence complexity effects. (A) Group‐level FWE‐corrected contrast of complex and simple sequences. (B) Group‐level FWE‐corrected 2‐way interaction between Group and Sequence complexity. The bar graphs illustrate the interaction in three regions, the right superior temporal sulcus, the right circular sulcus of the anterior insula and the left inferior occipital sulcus/gyrus, separately for the young (light gray bar) and older adults (black bar). The x‐axis represents the Sequence complexity effect (complex – simple sequence). The error bars represent the standard error of the mean (SE). Asterisks indicate significance at P ≤ 0.05. Cortical activation is shown on the group average smoothed white matter folded surfaces. Cerebellar activation is shown on an axial slice of an MNI template (TT_N27). L = left hemisphere; R = right hemisphere. [Color figure can be viewed at http://wileyonlinelibrary.com]
Interactions between Group, Motor Complexity, and Sequence Complexity
As illustrated in Figure 6A, the ANOVA revealed a 3‐way interaction in a distributed network of cortical areas (a complete list is provided in Table 2 (C)). To decompose this interaction, we examined the activation pattern in each of these regions (N = 19). We first computed a Motor complexity score [BOLD complex syllables – BOLD simple syllables] for the simple sequences and one for the complex sequences. A difference score was then calculated [BOLD complex sequences [complex syllables – simple syllables] − BOLD simple sequences [complex syllables – simple syllables]]. These difference scores were compared across Groups. The results demonstrate the same response pattern in all regions, that is, a stronger Motor complexity effect at high Sequence complexity for the older compared to the young adults, reflecting a stronger BOLD response. These results are summarized in Figure 6B in the form of a radar chart. Each dimension (radii) in the radar corresponds to a region. The longer the radii, the stronger the difference score (in percentage of BOLD signal change). Two detailed illustrations of this response pattern are provided in Figure 6C, in the form of line charts. To confirm that these response patterns were not related to MT, a standard multiple regression analysis was conducted on the BOLD signal ([complex sequence complex – simple syllables] – [simple sequence complex – simple syllables]) with Group and MT ([MT complex sequence complex – simple syllables] – [MT simple sequence complex – simple syllables]) as the predictor variables, separately for each region. In all analyses, as expected, there was a significant effect of Group (P ≤ 0.05) but MT did not significantly contribute to explaining the variance in the BOLD signal in any of the regions.
Figure 6.
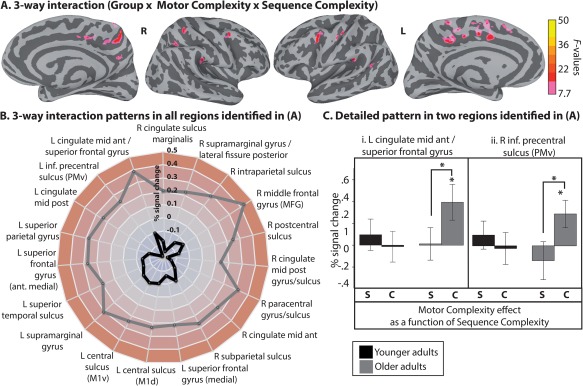
Three‐Way interactions. (A) Group‐level FWE‐corrected interaction between Group, Motor complexity and Sequence complexity. Activation is shown on the group average smoothed white matter folded surfaces. (B) A radar chart summarizes the response patterns that were found in regions identified in the group analysis illustrated in A. Each dimension (radii) in the radar chart corresponds to a region. The longer the radii, the stronger the difference score (in percentage of BOLD signal change), which represents the difference in Motor complexity effect across Sequence complexity levels [Complex sequence [complex– simple syllable] – Simple sequences [Complex– simple syllable]] separately for the young (black line) and older adults (gray line). As shown in the chart, the interaction was stronger for the older adults in all regions. (C) The detail of this response pattern is provided for two regions as examples. The x‐axis represents the Motor complexity effect (complex – simple syllable) in the simple sequence condition (S) and complex sequence (C), separately for the younger (black bars) and older adults (gray bars). The error bars represent the standard error of the mean (SE). Asterisks indicate significance at P ≤ 0.05. L = left hemisphere; R = right hemisphere. [Color figure can be viewed at http://wileyonlinelibrary.com]
DISCUSSION
The general objective of the present study was to extend current understanding of the aging of the neuromotor control of speech by examining age differences in brain activation patterns during a sequential speech production task in relation to behavioral data. Based on recent studies [Bilodeau‐Mercure et al., 2015a; Bilodeau‐Mercure and Tremblay, in press; Sadagopan and Smith, 2013], our general hypothesis was that the performance of older adults during sequential speech production would be lower than that of young adults, particularly at high complexity levels. Furthermore, we also hypothesized that age differences would be associated with brain activation patterns consistent with either the compensatory or the de‐differentiation hypotheses (or both), occurring primarily within the neural system supporting speech production, including M1, PMv, the striatum, and the cerebellum. As predicted, our results demonstrate age differences in behavior and brain activity, particularly when both sequential and motor complexity were high. At the behavioral level, this interaction was expressed as prolonged and more variable MT for older adults. These findings are discussed in the following sections.
Control of Movement Timing
The present study shows strong (d ≥ 0.9) and significant age differences in motor control during a sequential speech production task, with MT in older adults being longer than in younger adults, and more variable (by 55%). The average difference in MT between younger and older adults was ∼18%, and it reached ∼24% in the most complex condition (high sequential and high motor complexity) (CC). MT differences were related to longer pauses between syllables as well as longer pauses halfway through the sequence, but not to the production of longer syllables, suggesting that precise control over movement timing declines with age. The finding of an age difference in speech motor control is consistent with previous studies focusing on the production of syllables, nonwords and sentences, which have shown longer MT for older compared to younger adults [Bilodeau‐Mercure and Tremblay, in press; Sadagopan and Smith, 2013]. The finding of longer MT is also consistent with previous studies focusing on sequencing of finger movements [e.g., Aoki and Fukuoka, 2010; Cacola et al., 2013; Cousins et al., 1998; Loehrer et al., 2016; Ruiz et al., 2007]. In a recent study, we found that MT increased in older compared to younger adults in a simple sequential finger movement task, while response accuracy was stable [Bilodeau‐Mercure et al., 2015a]. It is possible that slowing was a strategic choice for older adults to emphasize movement accuracy at the cost of speed.
The group analysis shows that the relationship between the BOLD signal and MT varied as a function of age with important spatial differences, particularly in CC (high sequential and motor complexity). Interestingly, while the basic network for MT was left lateralized (Fig. 3A), age differences were found across both hemispheres (Fig. 3B), with a slight right predominance, consistent with the HAROLD model of hemispheric asymmetry in aging [Cabeza, 2002], which suggests that contralateral homologous regions are recruited in older ages to maintain performance. However, of all the regions that were modulated by MT in an age‐specific manner, only very few regions displayed clear compensatory‐like effects, while most regions exhibited a pattern of response akin to de‐differentiation. A clear de‐differentiation response pattern was found in the right M1, with stronger activation in older adults associated with longer MT, consistent with previous studies of manual motor control [e.g., Mattay et al., 2002; Naccarato et al., 2006; Ward and Frackowiak, 2003]. This suggests that, with age, M1 becomes less effective. A previous study has shown that de‐differentiation patterns in the motor system are associated with an increase in cortical‐subcortical functional connectivity within the sensorimotor network, including in M1 [Marchand et al., 2011]. In the current study, evidence of compensation‐like brain responses (stronger response in older adults associated with better performance) was also found, consistent with prior studies on motor aging [e.g., Mattay et al., 2002]. Such pattern was found in the right posterior cingulate cortex (PCC), a region involved in cognitive control. In this region, stronger activation in older adults was associated with better performance (shorter responses), while in the younger adults, there was no relationship between BOLD and MT. The PCC is a central component of the default mode network (DMN) [Buckner et al., 2008]. The DMN is a set of regions that are more active during “passive tasks” than during tasks demanding focused attention on external events [Raichle et al., 2001]. Because of its involvement in the DMN, the PCC is believed to play a role in internally‐directed cognition [Raichle et al., 2001]. However, there is also evidence to suggest that the PCC is involved in regulating the focus of attention [e.g., Leech et al., 2011; Leech and Sharp, 2014]. Given that our task was a demanding visually triggered sequential speech production task, an implication of the attention network is unsurprising. Analysis of the core speech network (Supporting Information 3) indeed revealed that the entire attention network was highly engaged, including the intraparietal sulcus, middle frontal gyrus (MFG), superior parietal cortex (SPL), anterior insula, and inferior frontal gyrus [Corbetta et al., 2008]. The finding of compensatory‐like activation in the right PCC suggests that allocation of more cognitive resources, such as increased focused attention, may contribute to maintaining motor timing in older age during demanding sequencing tasks. This interpretation is in line with the finding that motor sequencing during manual actions is correlated with executive functions in healthy older adults [Niermeyer et al., 2016]. A relationship between cognitive control and motor programming during a sequential motor task has also been shown in healthy older adults [Suchy and Kraybill, 2007]. The current finding of age differences in relation to speech performance in the PCC is consistent with a recent fMRI study by Heuninckx et al. [2008], which showed that an increase of activation was correlated to better coordination during an interlimb coordination task in a number of regions involved in cognitive/executive control, including the inferior frontal gyrus, the superior parietal gyrus, the anterior insula, and the MFG. Moreover, a recent study demonstrated age differences in the connectivity between the prefrontal cortex and M1 during complex bimanual finger movements [Loehrer et al., 2016]. Although these age differences were not correlated to performance, these findings add to the notion that complex motor behavior place high demands on the cognitive and executive systems of older adults, which suggest increased cognitive‐motor network integration in older adults. Taken together, the current findings suggest that age‐related plasticity can occur within and outside the prefrontal cortex to support fine motor control. These findings emphasize the need to study complex and highly functionally relevant motor behaviors such as speaking to understand age‐related alterations to complex brain functions.
Motor Sequencing
Motor sequencing is the planning of the order of each movement within a sequence, that is, for speech, the organization of movements into precise, smooth and coarticulated temporal sequences of movements of the lips, tongue, and jaw [Lashley, 1951]. In the current study, we manipulated the complexity of a sequential speech task by asking participants to either produce sequences of the same syllable repeated six times or to produce sequences of alternating syllables, which require a higher level of serial ordering. An effect of sequence complexity on BOLD signal was found in several regions including the bilateral medial frontal gyrus (corresponding to SMA and pre‐SMA), the left PMv, the bilateral anterior insula, and the anterior SPL. The SMA and pre‐SMA have been associated with response sequencing for speech and non‐speech movements [Bengtsson et al., 2005; Bohland and Guenther, 2006; Gerloff et al., 1997; Macar et al., 2002; Papoutsi et al., 2009], as well as speech sequence learning [Segawa et al., 2015]. The finding of stronger activation in these regions for more complex sequence supports the notion that they are involved in speech motor sequencing. The sequence complexity effect in the left PMv is consistent with the hypothesis that this region contains speech motor programs, as proposed in the DIVA model of speech production [Guenther et al., 2006]. To produce complex sequences, three different syllabic motor programs had to be activated, while the production of simple sequences required activation of only one program. It has been shown that as part of the process of learning new speech sequences, brain activation decreases in the left PMv with learning, suggesting that individual motor programs become merged into one, thus requiring the activation of only one motor program [Segawa et al., 2015]. In addition to a modulation in the PMv, we also found increased activation in the bilateral SPL, bilateral IPS and bilateral frontal eye field, three regions that are part of the dorsal attention system [Corbetta et al., 2008]. Modulations in these regions for complex finger movement sequences [Bengtsson et al., 2004], complex speech sequence production [Bohland and Guenther, 2006] and speech sequence learning [Segawa et al., 2015] have been reported. We thus suggest that the increase in activation in these regions during the production of complex visually triggered sequences of syllables may reflect beneficial increased visual attention to support performance [Corbetta et al., 2008]. This is consistent with the finding of a role for the SPL in visual attention in prior studies [Koenigs et al., 2009; Linden et al., 2003; Wojciulik and Kanwisher, 1999].
Interestingly, with the exception of the right anterior insula, regions that exhibited a Group by Sequence complexity interaction in the current study were not those that are typically associated with phonological and motor processes for speech production, which could suggest de‐differentiation. In three regions (bilateral anterior superior temporal sulcus and right sulcus of Jensen in the angular gyrus), older adults exhibited an increase in activation in relation to functional demands, with stronger activation for the complex compared to the simple sequences, suggesting compensatory‐like neural reorganization processes. However, given that activation in these regions did not differ across groups at the low complexity level, these patterns are only partly consistent with the CRUNCH model [Reuter‐Lorenz and Cappell, 2008; Reuter‐Lorenz and Mikels, 2006], which predicts higher activation in older adults at low complexity level. Further, the model suggests that, at high levels of complexity, compensatory mechanism becomes ineffective, leading to equivalent or less activation in older adults relative to young and a decline in performance [Grady, 2008, 2012]. In the current study, however, these BOLD response patterns were not related to behavioral performance. A compensatory pattern is sometimes invoked when older adults show stronger activation compared to younger adults while performing at the same level [Cabeza et al., 2002; Naccarato et al., 2006; Wu and Hallett, 2005]. However, other researchers have suggested that when performance is matched, higher activation levels reflect a less efficient use of neural resources, or increased effort, in older adults [Morcom et al., 2007]. The present results cannot distinguish between these two interpretations. Further studies are thus needed to tackle this important question. Having more than two complexity levels could help test the hypothesis of a gradient of activation as a function of task complexity in relation with CRUNCH.
In the other regions (right anterior insula, bilateral inferior occipital areas and right medial orbital sulcus), we found a different response pattern, that is, a lack of specificity in the BOLD response in relation to sequencing difficulty in older adults. Specifically, an increase in activation for the complex sequences was only found in the younger adults. This pattern of age difference is also inconsistent with the CRUNCH model [Reuter‐Lorenz and Cappell, 2008]. Instead, this pattern shares similarities with the de‐differentiation hypothesis, whereby less selective activity in task relevant regions was observed. Such activation pattern has been found in cognitive tasks such as visual processing [e.g., Grady et al., 1994] and attention [e.g., Townsend et al., 2006]. An age‐related de‐differentiation in motor‐evoked activity recorded from hand muscles has also been observed [Reuter et al., 2015]. Although it is unlikely that the anterior insula is involved in either speech‐specific or motor‐specific processes, it is a region that is active during demanding speech tasks [e.g., Ackermann and Riecker, 2004; Bilodeau‐Mercure et al., 2015b; Bohland and Guenther, 2006; Peeva et al., 2010]. It has been suggested that the anterior insula contributes to the attention orientation system [Corbetta et al., 2008] as well as to general executive processes involved in goal‐oriented tasks [Nelson et al., 2010]. The present results are consistent with this idea, with the need for monitoring/attention increasing as sequential complexity increases. This is supported by the behavioral data demonstrating slower and less accurate responses at high complexity level in all participants. In sum, in this study, we found increased activation in task‐irrelevant areas (bilateral anterior superior temporal sulcus and right sulcus of Jensen) in relation to sequence complexity, as well as less specific response in task‐relevant areas (insula), a pattern of response that is globally consistent with the de‐differentiation model.
Motor Complexity Effects
The main message of this study in relation to Motor complexity is that it is not a main factor of difficulty for older adults, at least not the kind of complexity that was manipulated here— the addition of a consonant cluster in the syllable onset to create complex syllables (e.g., /pa/vs./pra/). Moreover, the main effect of Motor complexity was also more circumscribed than the effect of Sequence complexity. It is possible that other kinds of complexity manipulations (e.g., place of articulation), or the use of more complex syllabic structure such as CCVC, could have resulted in stronger age dependent BOLD responses. The use of rare syllables could also have led to stronger BOLD responses, as complex but frequent syllables such as the one used in the current study are over‐learned, precompiled and therefore pose only limited demands on the motor system. In contrast, the complex sequences were new in this study, not precompiled; therefore, they likely posed higher demands on the motor system. Nevertheless, as expected, increasing the complexity of syllabic structure controlling for sequence complexity led to an increased BOLD response in several regions, including only the left medial premotor areas (SMA) and cingulate gyrus, the superior cerebellum, M1, and PMv but also bilaterally in the supratemporal cortex encompassing the transverse temporal gyrus and sulcus and the planum temporale. The increase in activation in the supratemporal cortex may reflect increased phonological processing. These regions are known to be activated during the perception of sublexical speech [Benson et al., 2001; Hugdahl et al., 2003; Rimol et al., 2005; Wilson and Iacoboni, 2006; Wilson et al., 2004; Deschamps and Tremblay, 2014]. The finding of an effect of motor complexity in the left pre‐SMA and SMA is consistent with prior brain imaging studies that have reported increase activation within the SMA/pre‐SMA for the production of multisyllabic words compared to monosyllabic words [Shuster and Lemieux, 2005], CCV compared to CV syllables [Bohland and Guenther, 2006] and disyllabic nonwords compared to monosyllable nonwords [Ghosh et al., 2008]. Taken together, these and the current findings support the notion that medial premotor areas are involved in the processing of syllable structure during speech production. A main effect of motor complexity was also found in the bilateral superior lateral cerebellum (lobules V and VI). Cerebellar activation has been reported in several speech production tasks [Bohland and Guenther, 2006; Chen and Desmond, 2005; Ghosh et al., 2008; Grabski et al., 2012; Peeva et al., 2010; Stoodley, 2012; Stoodley and Schmahmann, 2008; Stoodley et al., 2012]. Lobule VI contains a somatotopical map of the lips and tongue [Grodd et al., 2001], and, through cortico‐cerebellar‐thalamic loops, it connects with M1, SMA and PM [Hoover and Strick, 1999; Kelly and Strick, 2003]. The finding of cerebellar activation in the present study could therefore reflect a role in the preparation of complex speech utterances, consistent with the hypothesis of a role for this region in forming a pre‐articulatory verbal code and internal models of speech movements [Ackermann, 2008; Ackermann et al., 2007]. It could also reflect a role in sequencing [Ackermann, 2008; Molinari et al., 2008] at the syllable level. A role for the cerebellum in motor timing and sequencing has been proposed [Bengtsson et al., 2004, 2005; Ullen et al., 2005]. However, in the present study, neither sequence complexity nor MT was associated with cerebellar activation. Another possibility is that the role of the cerebellum is to provide executive support during speech production. Functional connections between the superior cerebellum, the prefrontal cortex and superior parietal areas have been found, suggestive of a role in executive control [Habas et al., 2009; Krienen and Buckner, 2009]. The notion of a cognitive cerebello‐cortical loop has gained support over the past two decades [Middleton and Strick, 1994, 1996, 1998a, 1998b, 2000]. However, if the role of the cerebellum was executive, we would have expected it to respond to both complexity manipulations. Future studies are needed to clarify the role of the cerebellum in speech production, for example, by exploring its functional connectivity using a psychophysiological approach [Friston et al., 1997; Gitelman et al., 2003]. Here, such analyses are not warranted because of the acquisition protocol that was used (sparse sampling) which resulted in a limited number of discontinuous time points per conditions, making the study of time‐course impossible.
The General Impact of Complexity
Importantly, although the analyses revealed no main effect of age on the BOLD response to Motor complexity alone, the analyses revealed widespread age differences at the behavioral and brain levels when response complexity was highest, that is, when both motor and sequential complexity were high. Behaviorally, we found that motor complexity effects in MT were stronger when sequence complexity was high, especially for the older adults (Fig. 2B). In contrast, sequence complexity effects were always present in older adults, suggesting that sequential complexity poses a higher challenge to the aging motor system than motor complexity alone. This may reflect vulnerability of response sequencing and motor timing processes.
Consistent with the MT data, the fMRI results show stronger BOLD‐MT relationship at the highest complexity level, as detailed in Control of Movement Timing section. Moreover, a stronger effect of motor complexity on BOLD signal was found at high sequence complexity for the older adults (Fig. 6). This pattern of response was found in several components of the motor system, including not only the left PMv, left M1, left SMA, and pre‐SMA but also in the right MFG and the bilateral PCC, and it was independent of MT. The finding of over‐activation in motor and cognitive/executive areas in older adults in relation to the functional demands of the task but not to performance may suggest a mechanism akin to neural compensation. However, classically, compensation is evoked when stronger activation is associated with better performance. The present compensatory‐like pattern is not entirely coherent with the CRUNCH model because over‐activation was only observed in the most difficult task, and not at low difficulty‐level. It is interesting to note that, in the present study, we found a de‐differentiation pattern in the right M1, and what appears to be a compensatory pattern in the left M1. Also interesting is the finding of compensatory‐like patterns in regions involved in cognitive control, including the right MFG and the bilateral PCC. This is consistent with the notion (discussed in Control of Movement Timing section) of increased cognitive‐motor integrations in aging at high complexity levels. The MFG is an important component of the attention network, involved in redirecting attention toward behaviorally relevant stimuli [Corbetta et al., 2008]. As previously discussed, the PCC is a central component of the DMN [Buckner et al., 2008]; evidence also suggests a role in regulating the focus of attention [e.g., Leech et al., 2011; Leech and Sharp, 2014]. These findings suggest that stronger attention‐related activation is associated with the production of fine motor actions at high complexity levels. Overall, these results demonstrate that the sensorimotor and executive systems are capable of adapting to decline in the central and peripheral nervous system to maintain speech motor performance.
LIMITS
The current study presents some limitations, including a small sample, a non‐ecological task, the absence of complete motor and cognitive assessments, the lack of a measure of vascularity and the absence of a correction for potential violations of the assumption of equal variance across groups in the whole‐brain fMRI analyses. The speech task was chosen because it eliminates the influence of linguistics factors (e.g., semantics) on speech production, thereby measuring “pure” maximal speech performance. Additionally, complete evaluations of oral non‐speech motor functions and cognition were not conducted (beyond the MOCA test, which was normal for all participants). It is thus possible that some of participants may have had slightly abnormal oral motor functions. However, participants did not report any respiratory, speech, language, swallowing, or neurodegenerative disorders. Moreover, all participants were able to perform the speech task. Another limitation of the present study is related to the absence of a vascular measure such as resting state T2 sequences with breath holding [e.g., Liu et al., 2013] or resting‐state fluctuation amplitude [Tsvetanov et al., 2015]. Aging is associated with alterations to vascular ultrastructure, vascular reactivity and resting state cerebral blood flow, all of which can affect the BOLD signal [e.g., D'Esposito et al., 2003, 2009]. Additionally, there is evidence that cerebrovascular reactivity varies across adults of the same age, and declines with increasing age [Lu et al., 2010]. Nevertheless, the pattern of results that we report is inconsistent with a global decline in the vascular system of older adults, given that we did not find a global decrease in BOLD response in older adults, but instead, several instances of BOLD signal increase in relation to functional demands or performance. Moreover, focusing on the age difference across complexity levels is a strategy that helps alleviate main effects of age such as vascular changes [D'Esposito et al., 2009]. Here, most of our analyses focused on the BOLD response at various complexity levels or on the BOLD‐MT relationship. It remains possible that our results slightly overestimate age differences [Tsvetanov et al., 2015]. Hence, future studies should acquire a vascular measure to ensure that age differences in BOLD signal are of neural origin.
CONCLUSIONS
Speaking is an extremely fast and intrinsically complex serial motor behavior that relies heavily on motor timing and sequencing mechanisms to achieve the smooth co‐articulated speech output that is necessary to communicate efficiently. The current study demonstrates age‐related behavioral and BOLD response differences in speech production. At the behavioral level, we show that MT is longer and more variable in older adults, which suggests that the motor control of timing during speech production declines with age. Our fMRI analyses reveal a range of age‐dependent BOLD response patterns within and outside of the sensorimotor network that typically supports speech functions, including executive control regions such as MFG and PCC. This suggests that, with advancing in age, performance on demanding speech tasks relies increasingly on resources beyond those typically involved in speaking in young adults. These findings offer an important snapshot into the neurobiology of aging and its effects on speech production, and highlight the need to investigate the relationship between executive and motor functions during the production of complex and functionally relevant actions such as speaking.
Supporting information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
Technical support for protocol development and data acquisition was provided by the “Consortium d'imagerie en neuroscience et santé mentale de Québec” (CINQ). Thanks to M. Bilodeau‐Mercure for her help with participant recruitment and testing, and to A.‐M. Audet for her help with the analysis of the behavioral data.
Footnote
RT, duration, and accuracy results, only for the sequence effects, have been reported somewhere else [Tremblay, P., Deschamps, I. (2016) Structural brain aging and speech production: a surface‐based brain morphometry study. Brain Struct Funct, 221:3275–3299]. However, the detailed analysis of the duration was never reported before.
REFERENCES
- Ackermann H (2008): Cerebellar contributions to speech production and speech perception: Psycholinguistic and neurobiological perspectives. Trends Neurosci 31:265–272. [DOI] [PubMed] [Google Scholar]
- Ackermann H, Riecker A (2004): The contribution of the insula to motor aspects of speech production: A review and a hypothesis. Brain Lang 89:320–328. [DOI] [PubMed] [Google Scholar]
- Ackermann H, Mathiak K, Riecker A (2007): The contribution of the cerebellum to speech production and speech perception: Clinical and functional imaging data. Cerebellum 6:202–213. [DOI] [PubMed] [Google Scholar]
- Aichert I, Ziegler W (2004): Syllable frequency and syllable structure in apraxia of speech. Brain Lang 88:148–159. [DOI] [PubMed] [Google Scholar]
- Aoki T, Fukuoka Y (2010): Finger tapping ability in healthy elderly and young adults. Med Sci Sports Exerc 42:449–455. [DOI] [PubMed] [Google Scholar]
- Argall BD, Saad ZS, Beauchamp MS (2006): Simplified intersubject averaging on the cortical surface using SUMA. Hum Brain Mapp 27:14–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KK, Ramig LO, Sapir S, Luschei ES, Smith ME (2001): Control of vocal loudness in young and old adults. J Speech Lang Hear Res 44:297–305. [DOI] [PubMed] [Google Scholar]
- Baldo JV, Wilkins DP, Ogar J, Willock S, Dronkers NF (2011): Role of the precentral gyrus of the insula in complex articulation. Cortex 47:800. [DOI] [PubMed] [Google Scholar]
- Bengtsson SL, Ehrsson HH, Forssberg H, Ullen F (2004): Dissociating brain regions controlling the temporal and ordinal structure of learned movement sequences. Eur J Neurosci 19:2591–2602. [DOI] [PubMed] [Google Scholar]
- Bengtsson SL, Ehrsson HH, Forssberg H, Ullen F (2005): Effector‐independent voluntary timing: Behavioural and neuroimaging evidence. Eur J Neurosci 22:3255–3265. [DOI] [PubMed] [Google Scholar]
- Benson RR, Whalen DH, Richardson M, Swainson B, Clark VP, Lai S, Liberman AM (2001): Parametrically dissociating speech and nonspeech perception in the brain using fMRI. Brain Lang 78:364–396. [DOI] [PubMed] [Google Scholar]
- Bilodeau‐Mercure M, Tremblay P: Speech production in aging: Linguistic and physiological factors. J Am Geriatr Soc (2016). [DOI] [PubMed] [Google Scholar]
- Bilodeau‐Mercure M, Kirouac V, Langlois N, Ouellet C, Gasse I, Tremblay P (2015a): Movement sequencing in normal aging: Speech, oro‐facial and finger movements. Age 37:37–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilodeau‐Mercure M, Lortie CL, Sato M, Guitton MJ, Tremblay P (2015b): The neurobiology of speech perception decline in aging. Brain Struct Funct 220:979–997. [DOI] [PubMed] [Google Scholar]
- Boersma P, Weenink D (2017): Praat: Doing phonetics by computer [Computer program]. Version 6.0.25, retrieved 11 February 2017 from http://www.praat.org/
- Bohland JW, Guenther FH (2006): An fMRI investigation of syllable sequence production. Neuroimage 32:821–841. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews‐Hanna JR, Schacter DL (2008): The brain's default network: Anatomy, function, and relevance to disease. Ann N Y Acad Sci 1124:1–38. [DOI] [PubMed] [Google Scholar]
- Cabeza R (2002): Hemispheric asymmetry reduction in older adults: The HAROLD model. Psychol Aging 17:85–100. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR (2002): Aging gracefully: Compensatory brain activity in high‐performing older adults. Neuroimage 17:1394–1402. [DOI] [PubMed] [Google Scholar]
- Cacola P, Roberson J, Gabbard C (2013): Aging in movement representations for sequential finger movements: A comparison between young‐, middle‐aged, and older adults. Brain Cogn 82:1–5. [DOI] [PubMed] [Google Scholar]
- Cerella J (1985): Information processing rates in the elderly. Psychol Bull 98:67–83. [PubMed] [Google Scholar]
- Chen SH, Desmond JE (2005): Cerebrocerebellar networks during articulatory rehearsal and verbal working memory tasks. NeuroImage 24:332–338. [DOI] [PubMed] [Google Scholar]
- Chen G, Adleman NE, Saad ZS, Leibenluft E, Cox RW (2014): Applications of multivariate modeling to neuroimaging group analysis: A comprehensive alternative to univariate general linear model. Neuroimage 99:571–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL (2008): The reorienting system of the human brain: From environment to theory of mind. Neuron 58:306–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins MS, Corrow C, Finn M, Salamone JD (1998): Temporal measures of human finger tapping: Effects of age. Pharmacol Biochem Behav 59:445–449. [DOI] [PubMed] [Google Scholar]
- Cox RW (1996): AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29:162–173. [DOI] [PubMed] [Google Scholar]
- Cox RW, Jesmanowicz A (1999): Real‐time 3D image registration for functional MRI. Magn Reson Med 42:1014–1018. [DOI] [PubMed] [Google Scholar]
- Crosson B, Benefield H, Cato MA, Sadek JR, Moore AB, Wierenga CE, Gopinath K, Soltysik D, Bauer RM, Auerbach EJ, Gokcay D, Leonard CM, Briggs RW (2003): Left and right basal ganglia and frontal activity during language generation: Contributions to lexical, semantic, and phonological processes. J Int Neuropsychol Soc 9: 1061–1077. [DOI] [PubMed] [Google Scholar]
- Deschamps I., Tremblay P. (2014): Sequencing at the syllabic and supra‐syllabic levels during speech perception: an fMRI study. Front Hum Neurosci 8:492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Esposito M, Deouell LY, Gazzaley A (2003): Alterations in the BOLD fMRI signal with aging and disease: A challenge for neuroimaging. Nat Rev Neurosci 4:863–872. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Jagust WJ, Gazzaley A (2009): Methodological and conceptual issues in the study of the aging brain In: Jagust WJ, D'Esposito M, editors. Imaging the Aging Brain. Oxford University Press, New York, USA. pp 11–25. [Google Scholar]
- Dale AM, Fischl B, Sereno MI (1999): Cortical surface‐based Analysis: I. Segmentation and surface reconstruction. Neuroimage 9:179–194. [DOI] [PubMed] [Google Scholar]
- Decoster W, Debruyne F (1997): The aging voice: Changes in fundamental frequency, waveform stability and spectrum. Acta Otorhinolaryngol Belg 51:105–112. [PubMed] [Google Scholar]
- Desai R, Liebenthal E, Possing ET, Waldron E, Binder JR (2005): Volumetric vs. surface‐based alignment for localization of auditory cortex activation. NeuroImage 26:1019–1029. [DOI] [PubMed] [Google Scholar]
- Destrieux C, Fischl B, Dale A, Halgren E (2010): Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage 53:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchin SW, Mysak ED (1987): Disfluency and rate characteristics of young adult, middle‐aged, and older males. J Commun Disord 20:245–257. [DOI] [PubMed] [Google Scholar]
- Eckert MA, Walczak A, Ahlstrom J, Denslow S, Horwitz A, Dubno JR (2008): Age‐related effects on word recognition: Reliance on cognitive control systems with structural declines in speech‐responsive cortex. J Assoc Res Otolaryngol 9:252–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM (1999): Cortical surface‐based analysis: II: Inflation, flattening, and a surface‐based coordinate system. Neuroimage 9:195–207. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM (2004): Automatically parcellating the human cerebral cortex. Cereb Cortex 14:11–22. [DOI] [PubMed] [Google Scholar]
- Fozo MS, Watson BC (1998): Task complexity effect on vocal reaction time in aged speakers. J Voice 12:404–414. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ (1997): Psychophysiological and modulatory interactions in neuroimaging. Neuroimage 6:218–229. [DOI] [PubMed] [Google Scholar]
- Gerloff C, Corwell B, Chen R, Hallett M, Cohen LG (1997): Stimulation over the human supplementary motor area interferes with the organization of future elements in complex motor sequences. Brain 120:1587–1602. [DOI] [PubMed] [Google Scholar]
- Ghosh SS, Tourville JA, Guenther FH (2008): A neuroimaging study of premotor lateralization and cerebellar involvement in the production of phonemes and syllables. J Speech Lang Hear Res 51:1183–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitelman DR, Penny WD, Ashburner J, Friston KJ (2003): Modeling regional and psychophysiologic interactions in fMRI: The importance of hemodynamic deconvolution. Neuroimage 19:200–207. [DOI] [PubMed] [Google Scholar]
- Grabski K, Lamalle L, Vilain C, Schwartz JL, Vallee N, Tropres I, Baciu M, Le Bas JF, Sato M (2012): Functional MRI assessment of orofacial articulators: Neural correlates of lip, jaw, larynx, and tongue movements. Hum Brain Mapp 33:2306–2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracco VL, Tremblay P, Pike B (2005): Imaging speech production using fMRI. Neuroimage 26:294–301. [DOI] [PubMed] [Google Scholar]
- Grady CL (2008): Cognitive neuroscience of aging. Ann N Y Acad Sci 1124:127–144. [DOI] [PubMed] [Google Scholar]
- Grady CL (2012): The cognitive neuroscience of aging. Nat Rev Neurosci 13:491–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, Craik FI (2000): Changes in memory processing with age. Curr Opin Neurobiol 10:224–231. [DOI] [PubMed] [Google Scholar]
- Grady CL, Maisog JM, Horwitz B, Ungerleider LG, Mentis MJ, Salerno JA, Pietrini P, Wagner E, Haxby JV (1994): Age‐related changes in cortical blood flow activation during visual processing of faces and location. J Neurosci 14:1450–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodd W, Hülsmann E, Lotze M, Wildgruber D, Erb M (2001): Sensorimotor mapping of the human cerebellum: fMRI evidence of somatotopic organization. Hum Brain Mapp 13:55–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenther FH, Ghosh SS, Tourville JA (2006): Neural modeling and imaging of the cortical interactions underlying syllable production. Brain Lang 96:280–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas C, Kamdar N, Nguyen D, Prater K, Beckmann CF, Menon V, Greicius MD (2009): Distinct cerebellar contributions to intrinsic connectivity networks. J Neurosci 29:8586–8594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KC, Dubno JR, Keren NI, Ahlstrom JB, Eckert MA (2009): Speech recognition in younger and older adults: A dependency on low‐level auditory cortex. J Neurosci 29:6078–6087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuninckx S, Wenderoth N, Debaere F, Peeters R, Swinnen SP (2005): Neural basis of aging: The penetration of cognition into action control. J Neurosci 25:6787–6796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuninckx S, Wenderoth N, Swinnen SP (2008): Systems neuroplasticity in the aging brain: Recruiting additional neural resources for successful motor performance in elderly persons. J Neurosci 28:91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honjo I, Isshiki N (1980): Laryngoscopic and voice characteristics of aged persons. Arch Otolaryngol (Chicago, Ill.: 1960) 106:149–150. [DOI] [PubMed] [Google Scholar]
- Hoover JE, Strick PL (1999): The organization of cerebellar and basal ganglia outputs to primary motor cortex as revealed by retrograde transneuronal transport of herpes simplex virus type 1. J Neurosci 19:1446–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugdahl K, Thomsen T, Ersland L, Rimol LM, Niemi J (2003): The effects of attention on speech perception: An fMRI study. Brain Lang 85:37–48. [DOI] [PubMed] [Google Scholar]
- Hunter EJ, Kapsner‐Smith M, Pead P, Engar MZ, Brown WR (2012): Age and speech production: A 50‐year longitudinal study. J Am Geriatr Soc 60:1175–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo HJ, Lee JM, Kim JH, Shin YW, Kim IY, Kwon JS, Kim SI (2007): Spatial accuracy of fMRI activation influenced by volume‐ and surface‐based spatial smoothing techniques. Neuroimage 34:550–564. [DOI] [PubMed] [Google Scholar]
- Kelly RM, Strick PL (2003): Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J Neurosci 23:8432–8444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M, Barbey AK, Postle BR, Grafman J (2009): Superior parietal cortex is critical for the manipulation of information in working memory. J Neurosci 29:14980–14986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krampe RT (2002): Aging, expertise and fine motor movement. Neurosci Biobehav Rev 26:769–776. [DOI] [PubMed] [Google Scholar]
- Krampe RT, Mayr U, Kliegl R (2005): Timing, sequencing, and executive control in repetitive movement production. J Exp Psychol Hum Percept Perform 31:379–397. [DOI] [PubMed] [Google Scholar]
- Krienen FM, Buckner RL (2009): Segregated fronto‐cerebellar circuits revealed by intrinsic functional connectivity. Cereb Cortex 19:2485–2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenecker SA, Nielson KA, Rao SM (2004): fMRI of healthy older adults during Stroop interference. Neuroimage 21:192–200. [DOI] [PubMed] [Google Scholar]
- Lashley KS (1951): The problem of serial order in behavior In: Jeffress LA, editor. Cerebral Mechanisms in Behavior. New York: Wiley; pp 112–131. [Google Scholar]
- Leech R, Sharp DJ (2014): The role of the posterior cingulate cortex in cognition and disease. Brain 137:12–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R, Kamourieh S, Beckmann CF, Sharp DJ (2011): Fractionating the default mode network: Distinct contributions of the ventral and dorsal posterior cingulate cortex to cognitive control. J Neurosci 31:3217–3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S‐C, Lindenberger U. (1999) Cross‐level unification: A computational exploration of the link between deterioration of neurotransmitter systems and dedifferentiation of cognitive abilities in old age In: Nilsson LG, Markowitsch HJ, editors. Cognitive Neuroscience of Memory. Kirkland, WA: Hogrefe & Huber; pp 103–146. [Google Scholar]
- Linden DEJ, Bittner RA, Muckli L, Waltz JA, Kriegeskorte N, Goebel R, Singer W, Munk MHJ (2003): Cortical capacity constraints for visual working memory: dissociation of fMRI load effects in a fronto‐parietal network. NeuroImage 20:1518–1530. [DOI] [PubMed] [Google Scholar]
- Linville SE (1996): The sound of senescence. J Voice 10:190–200. [DOI] [PubMed] [Google Scholar]
- Liu P, Hebrank AC, Rodrigue KM, Kennedy KM, Section J, Park DC, Lu H (2013): Age‐related differences in memory‐encoding fMRI responses after accounting for decline in vascular reactivity. Neuroimage 78:415–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loehrer PA, Nettersheim FS, Jung F, Weber I, Huber C, Dembek TA, Pelzer EA, Fink GR, Tittgemeyer M, Timmermann L (2016): Aging changes effective connectivity of motor networks during bimanual finger coordination. Neuroimage 143:325–342. [DOI] [PubMed] [Google Scholar]
- Lortie CL, Thibeault M, Guitton MJ, Tremblay P (2015): Effects of age on the amplitude, frequency and perceived quality of voice. Age 37:117. [DOI] [PMC free article] [PubMed]
- Lortie CL, Rivard J, Thibeault M, Tremblay P (2016): The moderating effect of frequent singing on voice aging. J Voice 31:112.e1–112.e12. [DOI] [PubMed]
- Lu H, Yezhuvath US, Xiao G (2010): Improving fMRI sensitivity by normalization of basal physiologic state. Hum Brain Mapp 31:80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macar F, Lejeune H, Bonnet M, Ferrara A, Pouthas V, Vidal F, Maquet P (2002): Activation of the supplementary motor area and of attentional networks during temporal processing. Experimental brain research. Exp Hirnforsch Exp Cereb 142:475–485. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Turkington TG, Provenzale JM, Denny LL, Langley LK, Hawk TC, Coleman RE (2002): Aging and attentional guidance during visual search: Functional neuroanatomy by positron emission tomography. Psychol Aging 17:24–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand WR, Lee JN, Suchy Y, Garn C, Johnson S, Wood N, Chelune G (2011): Age‐related changes of the functional architecture of the cortico‐basal ganglia circuitry during motor task execution. Neuroimage 55:194–203. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Fera F, Tessitore A, Hariri AR, Das S, Callicott JH, Weinberger DR (2002): Neurophysiological correlates of age‐related changes in human motor function. Neurology 58:630–635. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Fera F, Tessitore A, Hariri AR, Berman KF, Das S, Meyer‐Lindenberg A, Goldberg TE, Callicott JH, Weinberger DR (2006): Neurophysiological correlates of age‐related changes in working memory capacity. Neurosci Lett 392:32–37. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL (1994): Anatomical evidence for cerebellar and basal ganglia involvement in higher cognitive function. Science 266:458–461. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL (1996): Basal ganglia and cerebellar output influences non‐motor function. Mol Psychiatry 1:429–433. [PubMed] [Google Scholar]
- Middleton FA, Strick PL (1998a): Cerebellar output: Motor and cognitive channels. Trends Cogn Sci 2:348–354. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL (1998b): The cerebellum: An overview. Trends Neurosci 21:367. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL (2000): Basal ganglia and cerebellar loops: Motor and cognitive circuits. Brain Res Brain Res Rev 31:236–250. [DOI] [PubMed] [Google Scholar]
- Molinari M, Chiricozzi FR, Clausi S, Tedesco AM, De Lisa M, Leggio MG (2008): Cerebellum and detection of sequences, from perception to cognition. Cerebellum 7:611–615. [DOI] [PubMed] [Google Scholar]
- Morcom AM, Li J, Rugg MD (2007): Age effects on the neural correlates of episodic retrieval: Increased cortical recruitment with matched performance. Cereb Cortex 17:2491–2506. [DOI] [PubMed] [Google Scholar]
- Morris R, Brown WS (1987): Age‐related voice measures among adult women. J Voice 1:43. [Google Scholar]
- Mueller PB (1997): The aging voice. Semin Speech Lang 18:159–168. quiz 168‐9. [DOI] [PubMed] [Google Scholar]
- Naccarato M, Calautti C, Jones PS, Day DJ, Carpenter TA, Baron JC (2006): Does healthy aging affect the hemispheric activation balance during paced index‐to‐thumb opposition task? An fMRI study. Neuroimage 32:1250–1256. [DOI] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H (2005): The Montreal Cognitive Assessment (MoCA): A brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53:695–699. [DOI] [PubMed] [Google Scholar]
- Nelson SM, Dosenbach NU, Cohen AL, Wheeler ME, Schlaggar BL, Petersen SE (2010): Role of the anterior insula in task‐level control and focal attention. Brain Struct Funct 214:669–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielson KA, Langenecker SA, Garavan H (2002): Differences in the functional neuroanatomy of inhibitory control across the adult life span. Psychol Aging 17:56–71. [DOI] [PubMed] [Google Scholar]
- Nielson KA, Langenecker SA, Ross TJ, Garavan H, Rao SM, Stein EA (2004): Comparability of functional MRI response in young and old during inhibition. Neuroreport 15:129–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niermeyer MA, Suchy Y, Ziemnik RE (2016): Motor sequencing in older adulthood: Relationships with executive functioning and effects of complexity. Clin Neuropsychol 17:1–21. [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9:97–113. [DOI] [PubMed] [Google Scholar]
- Papoutsi M, de Zwart JA, Jansma JM, Pickering MJ, Bednar JA, Horwitz B (2009): From phonemes to articulatory codes: An fMRI study of the role of Broca's area in speech production. Cereb Cortex 19:2156–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Lautenschlager G, Hedden T, Davidson NS, Smith AD, Smith PK (2002): Models of visuospatial and verbal memory across the adult life span. Psychol Aging 17:299–320. [PubMed] [Google Scholar]
- Parnell MM, Amerman JD (1987): Perception of oral diadochokinetic performances in elderly adults. J Commun Disord 20:339–351. [DOI] [PubMed] [Google Scholar]
- Peeva MG, Guenther FH, Tourville JA, Nieto‐Castanon A, Anton JL, Nazarian B, Alario FX (2010): Distinct representations of phonemes, syllables, and supra‐syllabic sequences in the speech production network. NeuroImage 50:626–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piefke M, Onur OA, Fink GR (2012): Aging‐related changes of neural mechanisms underlying visual‐spatial working memory. Neurobiol Aging 33:1284–1297. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE (2012): Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage 59:2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE (2014): Methods to detect, characterize, and remove motion artefact in resting state fMRI. NeuroImage 84:320–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL (2001): A default mode of brain function. Proc Natl Acad Sci USA 98:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramig LA (1983): Effects of physiological aging on vowel spectral noise. J Gerontol 38:223–225. [DOI] [PubMed] [Google Scholar]
- Reilly KJ, Spencer KA (2013): Sequence complexity effects on speech production in healthy speakers and speakers with hypokinetic or ataxic dysarthria. PLoS One 8:e77450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter EM, Behrens M, Zschorlich VR (2015): Age‐related differences in corticomotor facilitation indicate dedifferentiation in motor planning. Exp Gerontol 65:79–84. [DOI] [PubMed] [Google Scholar]
- Reuter‐Lorenz P, Cappell K (2008): Neurocognitive aging and the compensation hypothesis. Cur Dir Psych 17:177–182. [Google Scholar]
- Reuter‐Lorenz P, Mikels J (2006): The aging mind and brain: Implications of enduring plasticity for behavioral and cultural change. In: Lifespan Development and the Brain: The Perspective of Biocultural Co‐Constructivism. Cambridge University Press, Cambridge, England, pp 255–276. [Google Scholar]
- Riecker A, Groschel K, Ackermann H, Steinbrink C, Witte O, Kastrup A (2006): Functional significance of age‐related differences in motor activation patterns. Neuroimage 32:1345–1354. [DOI] [PubMed] [Google Scholar]
- Rimol LM, Specht K, Weis S, Savoy R, Hugdahl K (2005): Processing of sub‐syllabic speech units in the posterior temporal lobe: An fMRI study. NeuroImage 26:1059–1067. [DOI] [PubMed] [Google Scholar]
- Ruiz PJ, Bernardos VS, Bartolome M, Torres AG (2007): Capit timed tests quantify age‐related motor decline in normal subjects. J Neurol Sci 260:283–285. [DOI] [PubMed] [Google Scholar]
- Ryan WJ, Burk KW (1974): Perceptual and acoustic correlates of aging in the speech of males. J Commun Disord 7:181–192. [DOI] [PubMed] [Google Scholar]
- Saad Z, Reynolds R, Argall B, Japee S, Cox R (2004): SUMA: An interface for surface‐based intra‐and inter‐subject analysis with AFNI. IEEE International Symposium on Biomedical Imaging, Arlington, Virginia, USA. pp 1510–1513. [Google Scholar]
- Sadagopan N, Smith A (2013): Age differences in speech motor performance on a novel speech task. J Speech Lang Hear Res 56:1552–1566. [DOI] [PubMed] [Google Scholar]
- Salthouse TA (1996): The processing‐speed theory of adult age differences in cognition. Psychol Rev 103:403–428. [DOI] [PubMed] [Google Scholar]
- Salthouse TA (2009): Decomposing age correlations on neuropsychological and cognitive variables. J Int Neuropsychol Soc 15:650–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD, Doyon J, McDonald D, Holmes C, Lavoie K, Hurwitz AS, Kabani N, Toga A, Evans A, Petrides M (1999): Three‐dimensional MRI atlas of the human cerebellum in proportional stereotaxic space. Neuroimage 10:233–260. [DOI] [PubMed] [Google Scholar]
- Schmahmann J, Doyon J, Toga A, Evans A, Petrides M (2000): MRI Atlas of the Human Cerebellum. San Diego: Academic Press. [DOI] [PubMed] [Google Scholar]
- Searl JP, Gabel RM, Fulks JS (2002): Speech disfluency in centenarians. J Commun Disord 35:383–392. [DOI] [PubMed] [Google Scholar]
- Segawa JA, Tourville JA, Beal DS, Guenther FH (2015): The neural correlates of speech motor sequence learning. J Cogn Neurosci 27:819–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler RD, Bernard JA, Burutolu TB, Fling BW, Gordon MT, Gwin JT, Kwak Y, Lipps DB (2010): Motor control and aging: Links to age‐related brain structural, functional, and biochemical effects. Neurosci Biobehav Rev 34:721–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuey EM (1989): Intelligibility of older versus younger adults' CVC productions. J Commun Disord 22:437–444. [DOI] [PubMed] [Google Scholar]
- Shuster LI, Lemieux SK (2005): An fMRI investigation of covertly and overtly produced mono‐ and multisyllabic words. Brain Lang 93:20–31. [DOI] [PubMed] [Google Scholar]
- Smith BL, Wasowicz J, Preston J (1987): Temporal characteristics of the speech of normal elderly adults. J Speech Hear Res 30:522–529. [DOI] [PubMed] [Google Scholar]
- Sommers MS, Danielson SM (1999): Inhibitory processes and spoken word recognition in young and older adults: The interaction of lexical competition and semantic context. Psychol Aging 14:458–472. [DOI] [PubMed] [Google Scholar]
- Sörös P, Bose A, Sokoloff LG, Graham SJ, Stuss DT (2011): Age‐related changes in the functional neuroanatomy of overt speech production. Neurobiol Aging 32:1505–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spirduso WW. (1982): Physical fitness in relation to motor aging In: Mortimer JA, Pirozzolo FJ, Maletta GJ, editors. The Aging Motor System. New York: Praeger; pp 120–151. [Google Scholar]
- Stoodley CJ (2012): The cerebellum and cognition: Evidence from functional imaging studies. Cerebellum 11:352–365. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmann JD (2008): Functional topography in the human cerebellum: A meta‐analysis of neuroimaging studies. NeuroImage 44:489–501. [DOI] [PubMed]
- Stoodley CJ, Valera EM, Schmahmann JD (2012): Functional topography of the cerebellum for motor and cognitive tasks: An fMRI study. Neuroimage 59:1560–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchy Y, Kraybill M (2007): The relationship between motor programming and executive abilities: Constructs measured by the Push‐Turn‐Taptap task from the Behavioral Dyscontrol Scale‐Electronic Version. J Clin Exp Neuropsychol 29:648–659. [DOI] [PubMed] [Google Scholar]
- Tiffany WR (1980): The effects of syllable structure on diadochokinetic and reading rates. J Speech Hear Res 23:894–908. [DOI] [PubMed] [Google Scholar]
- Townsend J, Adamo M, Haist F (2006): Changing channels: An fMRI study of aging and cross‐modal attention shifts. Neuroimage 31:1682–1692. [DOI] [PubMed] [Google Scholar]
- Tremblay P, Deschamps I (2016): Structural brain aging and speech production: A surface‐based brain morphometry study. Brain Struct Funct 221:3275–3299. [DOI] [PubMed] [Google Scholar]
- Tremblay P, Small SL (2011): Motor response selection in overt sentence production: A functional MRI study. Front Psychol 2:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay P, Deschamps I, Gracco VL (2013a): Regional heterogeneity in the processing and the production of speech in the human planum temporale. Cortex 49:143–157. [DOI] [PubMed] [Google Scholar]
- Tremblay P, Dick AS, Small SL (2013b): Functional and structural aging of the speech sensorimotor neural system: Functional magnetic resonance imaging evidence. Neurobiol Aging 34:1935–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsvetanov KA, Henson RN, Tyler LK, Davis SW, Shafto MA, Taylor JR, Williams N, Cam C, Rowe JB (2015): The effect of aging on fMRI: Correction for the confounding effects of vascular reactivity evaluated by joint fMRI and MEG in 335 adults. Hum Brain Mapp 36:2248–2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullen F, Bengtsson SL, Ehrsson HH, Forssberg H (2005): Neural control of rhythmic sequences. Ann N Y Acad Sci 1060:368–376. [DOI] [PubMed] [Google Scholar]
- Ward NS (2006): Compensatory mechanisms in the aging motor system. Aging Res Rev 5:239–254. [DOI] [PubMed] [Google Scholar]
- Ward NS, Frackowiak RS (2003): Age‐related changes in the neural correlates of motor performance. Brain 126:873–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welford AT, Norris AH, Shock NW (1969): Speed and accuracy of movement and their changes with age. Acta Psychol 30:3–15. [DOI] [PubMed] [Google Scholar]
- Wilcox KA, Horii Y (1980): Age and changes in vocal jitter. J Gerontol 35:194–198. [DOI] [PubMed] [Google Scholar]
- Wilson SM, Iacoboni M (2006): Neural responses to non‐native phonemes varying in producibility: Evidence for the sensorimotor nature of speech perception. NeuroImage 33:316–325. [DOI] [PubMed] [Google Scholar]
- Wilson SM, Saygin AP, Sereno MI, Iacoboni M (2004): Listening to speech activates motor areas involved in speech production. Nat Neurosci 7:701–702. [DOI] [PubMed] [Google Scholar]
- Wohlert AB, Smith A (1998): Spatiotemporal stability of lip movements in older adult speakers. J Speech Lang Hear Res 41:41–50. [DOI] [PubMed] [Google Scholar]
- Wojciulik E, Kanwisher N (1999): The generality of parietal involvement in visual attention. Neuron 23:747–764. [DOI] [PubMed] [Google Scholar]
- Wu T, Hallett M (2005): The influence of normal human aging on automatic movements. J Physiol 562:605–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO (1982): Development and validation of a geriatric depression screening scale: A preliminary report. J Psychiatr Res 17:37–49. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information
Supporting Information
Supporting Information


