Abstract
The phantom sound of tinnitus is believed to be triggered by aberrant neural activity in the central auditory pathway, but since this debilitating condition is often associated with emotional distress and anxiety, these comorbidities likely arise from maladaptive functional connections to limbic structures such as the amygdala and hippocampus. To test this hypothesis, resting‐state functional magnetic resonance imaging (fMRI) was used to identify aberrant effective connectivity of the amygdala and hippocampus in tinnitus patients and to determine the relationship with tinnitus characteristics. Chronic tinnitus patients (n = 26) and age‐, sex‐, and education‐matched healthy controls (n = 23) were included. Both groups were comparable for hearing level. Granger causality analysis utilizing the amygdala and hippocampus as seed regions were used to investigate the directional connectivity and the relationship with tinnitus duration or distress. Relative to healthy controls, tinnitus patients demonstrated abnormal directional connectivity of the amygdala and hippocampus, including primary and association auditory cortex, and other non‐auditory areas. Importantly, scores on the Tinnitus Handicap Questionnaires were positively correlated with increased connectivity from the left amygdala to left superior temporal gyrus (r = 0.570, P = 0.005), and from the right amygdala to right superior temporal gyrus (r = 0.487, P = 0.018). Moreover, enhanced effective connectivity from the right hippocampus to left transverse temporal gyrus was correlated with tinnitus duration (r = 0.452, P = 0.030). The results showed that tinnitus distress strongly correlates with enhanced effective connectivity that is directed from the amygdala to the auditory cortex. The longer the phantom sensation, the more likely acute tinnitus becomes permanently encoded by memory traces in the hippocampus. Hum Brain Mapp 38:2384–2397, 2017. © 2017 Wiley Periodicals, Inc.
Keywords: tinnitus, effective connectivity, limbic system, functional connectivity, resting‐state fMRI
INTRODUCTION
Approximately 12% of adults have experienced subjective tinnitus, a phantom ringing or buzzing sensation [Jastreboff, 1990]. However, approximately 1% suffer from loud, incessant, and debilitating tinnitus, a condition for which they seek medical treatment [Shargorodsky et al., 2010; Sindhusake et al., 2003]. Patients with chronic tinnitus often suffer from sleep disturbances, depression, and anxiety, comorbid conditions that significantly impair the quality of daily life [Reynolds et al., 2004]. Tinnitus is generally associated with sensorineural hearing loss suggesting that the tinnitus generator might reside in the cochlea. However, since tinnitus often persists subsequent to auditory nerve transection [Jackler and Whinney, 2001], the prevailing view is that cochlear hearing loss induces aberrant neuroplastic changes in the central nervous system leading to chronic tinnitus, analogous to central pain [Bartels et al., 2007; Eggermont, 2005; Lockwood et al., 2002; Roussel et al., 2013]. Previous electrophysiological studies have suggested that tinnitus could arise from spontaneous hyperactivity, burst firing or enhance neural synchrony within the central auditory pathway [Eggermont and Roberts, 2012; Jastreboff, 1995; Kaltenbach et al., 2005; Llinás et al., 1999; Robertson and Mulders, 2012]. However, other studies suggest that tinnitus not only involves auditory structures, but also aberrant neural activity and interaction with other regions of the central nervous system associated with emotion, attention, distress, memory and motor activity [Chen et al., 2015a; Henry et al., 2014; Leaver et al., 2011, 2016a; Lockwood et al., 1998; Rauschecker et al., 2010]. Despite decades of research, the neural mechanism underlying the tinnitus generation still remains elusive.
Accumulating evidences, typically utilizing neuroimaging in humans, have proposed that reciprocal connections between the auditory regions and limbic system appear to be important for the development of chronic tinnitus [Chen et al., 2015a; Golm et al., 2013; Lanting et al., 2009; Leaver et al., 2011, 2016a; Rauschecker et al., 2010; Seydell‐Greenwald et al., 2014]. Since the auditory and limbic systems are interconnected, the phantom sound may influence the emotional and cognitive functions of the limbic system [Langguth, 2011; Winer, 2006]. The amygdala and hippocampus are two major limbic regions that receive either direct or indirect neuronal input from the central auditory system [Mohedano‐Moriano et al., 2007; Munoz‐Lopez et al., 2010; Sah et al., 2003]. In turn, there are direct or indirect projections from limbic system to auditory brain regions that may impact neuronal activity or regulate plasticity [Marsh et al., 2002; Weinberger, 2007]. However, the role of the limbic system and limbic–auditory interaction in tinnitus is far from clear.
Resting‐state low frequency (0.01–0.1Hz) fluctuations of blood oxygenation level‐dependent (BOLD) functional magnetic resonance imaging (fMRI) could provide new insights into how structurally segregated and functionally specialized cerebral networks are interconnected [Biswal et al., 1995; Lancaster et al., 2007]. Previous resting‐state fMRI studies have identified tinnitus‐related abnormalities in auditory, limbic and other brain regions [Carpenter‐Thompson et al., 2015; Leaver et al., 2016a]. A series of tinnitus models have been developed that can detect neural activity changes in non‐auditory limbic systems, such as the amygdala and the hippocampus [Jastreboff, 1990; Muhlau et al., 2006; Rauschecker et al., 2010]. Some have reported that tinnitus is associated with increased functional connectivity between the auditory network and the left amygdala, a region that assigns emotional significance to sensory experience [Kim et al., 2012]. Tinnitus was associated with enhanced connectivity between auditory cortices and the amygdala [Maudoux et al., 2012b]. On the other hand, decreased functional connectivity was observed between the right thalamus and the left amygdala in tinnitus patients [Zhang et al., 2015]. Furthermore, the hippocampus, important for memory and spatial navigation, is connected to the primary auditory cortex [Cenquizca and Swanson, 2007]. A major function of the hippocampal‐auditory system is the formation of long‐term auditory memories [Squire et al., 2001; Tamura et al., 1990]. Tinnitus is associated with loss of gray matter (GM) and white matter (WM) in the hippocampus [Gunbey et al., 2015; Landgrebe et al., 2009; Lanting et al., 2009]. Resting‐state fMRI has also indicated the abnormality of the hippocampus in salicylate‐induced tinnitus model of rats [Chen et al., 2015a]. Moreover, the parahippocampal area has been speculated to play a central role in memory recollection, sending information from the hippocampus to the association areas [Diederen et al., 2010]. Others have found increased functional connectivity between the left parahippocampus and the auditory resting‐state network in tinnitus patients [Schmidt et al., 2013] highlighting the role of parahippocampal regions in tinnitus physiopathology [Chen et al., 2015b; Leaver et al., 2016b; Maudoux et al., 2012a, 2012b]. While many studies have found that tinnitus is associated with changes in functional connectivity between different regions, it is impossible to discern the directionality or specificity of the disrupted connections in this disease.
To address this issue, we used Granger causality analysis (GCA) to identify differences in the direction of functional connectivity between tinnitus patients and controls. GCA has been widely used to reveal the causal effects among brain regions in various neurological or psychiatric disorders, such as Alzheimer's disease, depression, schizophrenia, and hepatic encephalopathy [Guo et al., 2014, 2015; Qi et al., 2013; Zhong et al., 2014]. Considering that the amygdala and hippocampus are two crucial limbic regions previously implicated in tinnitus, we selected the bilateral amygdala and hippocampus as seed regions and hypothesized that GCA and functional connectivity of these regions would be disrupted in chronic tinnitus patients. Moreover, the limbic–auditory disruption would be associated with specific tinnitus characteristics such as tinnitus distress. To our knowledge, this is the first study to use GCA to unravel the effective connectivity within the limbic system in tinnitus patients.
MATERIALS AND METHODS
Subjects
This study included 26 chronic tinnitus patients and 23 healthy subjects (all right handed, with at least 8 years of education) recruited through community health screening or newspaper advertisements. None of patients were excluded from the fMRI analysis because of excessive head motion during scanning. The patients were group‐matched in terms of age, sex and education. Twelve patients reported a predominantly left‐sided, six a predominantly right‐sided tinnitus, and eight patients described their tinnitus as bilateral or originating within the head. The severity of tinnitus and related distress were assessed by the Iowa version of the Tinnitus Handicap Questionnaires (THQ) [Kuk et al., 1990]. The hearing threshold was determined by pure tone audiometry (PTA) examination. All the participants had clinically normal hearing from 250 Hz to 8 kHz (hearing thresholds <25 dB). There were no significant differences in auditory thresholds between the tinnitus group and the control group (Supporting Information Fig. 1). None of the participants had depression and anxiety according to the Self‐Rating Depression Scale (SDS) and Self‐Rating Anxiety Scale (SAS) (overall scores <50, respectively) [Zung, 1971, 1986]. According to previous study [Khalfa et al., 2002], we used the Hyperacusis Questionnaire to exclude the participants with hyperacusis in the current study. Moreover, patients with Meniere's diseases were also excluded according to the previous diagnostic criteria [Lopez‐Escamez et al., 2015]. Participants were excluded if they suffered from pulsatile tinnitus, hyperacusis or Meniere's diseases or if they had a past history of severe alcoholism, smoking, head injury, stroke, Alzheimer's disease, Parkinson's disease, epilepsy, major depression, or other neurological or psychiatric illness, major medical illness (e.g., cancer, anemia, and thyroid dysfunction), MRI contraindications or severe visual loss. Table 1 summarizes the characteristics of the chronic tinnitus patients and healthy subjects. The study was approved by the Ethics Committee of Nanjing Medical University and informed consent was obtained from each participant.
Table 1.
Characteristics of tinnitus patients and healthy controls
|
Tinnitus patients (n = 26) |
Healthy controls (n = 23) |
P value | |
|---|---|---|---|
| Age (years) | 50.2 ± 13.0 | 44.4 ± 15.1 | 0.744 |
|
Gender (male: female) Education levels (years) |
9:17 12.5 ± 3.0 |
9:14 13.3 ± 3.7 |
0.509 0.405 |
|
Tinnitus duration (months) THQ score Hearing thresholds (left) Hearing thresholds (right) Hearing thresholds (mean) |
44.1 ± 38.5 50.0 ± 16.0 14.9 ± 3.0 16.2 ± 2.8 15.5 ± 2.1 |
– – 14.6 ± 2.5 15.1 ± 3.2 14.8 ± 1.7 |
– – 0.705 0.201 0.210 |
Data are represented as Mean ± SD. THQ, Tinnitus Handicap Questionnaire.
MRI Scanning
All subjects were scanned using a 3.0 T MRI scanner (Ingenia, Philips Medical Systems, Netherlands) with a 8‐channel receiver array head coil. Head motion and scanner noise were reduced using foam padding and earplugs. The earplugs (Hearos Ultimate Softness Series, The United States) were used to attenuate scanner noise by approximately 32 dB from the manufacture's data. The subjects were instructed to lie quietly with their eyes closed but not to fall asleep, and avoid thinking of anything particular during the scanning. Structural images were acquired with a three‐dimensional turbo fast echo (3D‐TFE) T1WI sequence with high resolution as follows: repetition time (TR)/echo time (TE) = 8.1/3.7 ms; slices = 170; thickness = 1 mm; gap = 0 mm; flip angle (FA) = 8°; acquisition matrix = 256 × 256; field of view (FOV) = 256 mm × 256 mm. The structural sequence took 5 minutes and 29 seconds. Functional images were obtained axially using a gradient echo‐planar imaging sequence as follows: TR = 2,000 ms; TE = 30 ms; slices = 36; thickness = 4 mm; gap = 0 mm; FOV = 240 mm × 240 mm; acquisition matrix = 64 × 64; and FA = 90°. The fMRI sequence took 8 minutes and 8 seconds. All scans were acquired with parallel imaging using sensitivity encoding (SENSE) technique, SENSE factor = 2.
Data Preprocessing
Data analyses were preprocessed using Data Processing Assistant for Resting‐State fMRI programs [Chao‐Gan and Yu‐Feng, 2010], which is based on Statistical Parametric Mapping (SPM8, http://www.fil.ion.ucl.ac.uk/spm) and resting‐state fMRI data analysis toolkit (REST, http://www.restfmri.net). The first 10 volumes were discarded and the remaining 230 consecutive volumes were used for data analysis. Slice‐timing and realignment for head motion correction were performed. Any subjects with a head motion greater than 2.0 mm translation or a 2.0° rotation in any direction were excluded. Data were spatial normalized to the Montreal Neurological Institute template (resampling voxel size = 3 × 3 × 3 mm3), smoothed with an isotropic Gaussian kernel [full width at half maximum (FWHM) = 4 mm], detrended and filtered (0.01–0.08 Hz).
Effective Connectivity Analysis
The bilateral amygdala or hippocampus was set as seed regions using the WFU_PickAtlas software (http://www.ansir.wfubmc.edu). Effective connectivity was analyzed using REST‐GCA in the REST toolbox [Zang et al., 2012]. In this study, the time series of the bilateral amygdala or hippocampus were defined as the seed time series x, and the time series y denotes the time series of all voxels in the brain. The linear direct influence of x on y (Fx → y), and the linear direct influence of y on x (Fy → x) were calculated voxel by voxel across the brain. Thus, two Granger causality maps were generated based on the influence measures for each subjects. The residual‐based F was normalized (F′) and standardized to Z score for each voxel (Zx → y and Zy → x, subtracting the global mean F′ values, divided by standard deviation).
Statistical Analysis
For the group analysis on the effective connectivity of the amygdala, mean values of Zx → y and Zy → x maps were computed for each group. All eight Granger causality maps were acquired, with four for each direction and four for each group (the left amygdala with Zx → y and Zy → x and the right amygdala with Zx → y and Zy → x for both the patient and healthy controls). These Granger causality maps were entered into a voxel‐wise two‐sample t‐test to determine the differences between tinnitus patients and healthy controls with age, sex, and education included as covariates. Thresholds were also set at a corrected P < 0.05, with multiple comparisons correction carried out using the AlphaSim program determined by Monte Carlo simulation (parameters were single voxel P value less than 0.05, a minimum cluster size of 85 voxels, FWHM = 4 mm, within a GM mask corresponding to the Automated Anatomical Labeling atlas). The statistical analysis for the effective connectivity of the hippocampus was similar to that used for the amygdala.
Between‐group t‐tests and χ 2‐tests were used to compare demographic data (statistical significance set at P < 0.05). To investigate the association between the clinical characteristic and the fMRI data, the clusters of the significant differences in effective connectivity of amygdala or hippocampus between groups were extracted. Mean z values within these clusters were correlated against each tinnitus characteristic using the Pearson's correlation analysis in SPSS software (version 18.0; SPSS, Chicago, IL). P< 0.05 was considered statistically significant, corrected for age, sex, education, and hearing thresholds. Bonferroni correction for multiple comparisons was applied in the correlation analysis.
RESULTS
Effective Connectivity from the Amygdala
Compared with healthy controls, patients with chronic tinnitus demonstrated significantly increased effective connectivity from the left amygdala to auditory areas in left superior temporal gyrus (STG) and non‐auditory brain regions, including the left anterior cingulate cortex (ACC), left precuneus, and right angular gyrus (AG). In addition, decreased effective connectivity was detected in the left cerebellar posterior lobe (Fig. 1A and Table 2). Furthermore, chronic tinnitus patients also showed significantly enhanced effective connectivity from the right amygdala to several brain regions that included the right STG, right ACC, right middle frontal gyrus (MFG), and right supramarginal gyrus (SMG). In contrast, reduced effective connectivity was observed in the right cerebellar posterior lobe (Fig. 1B and Table 3).
Figure 1.
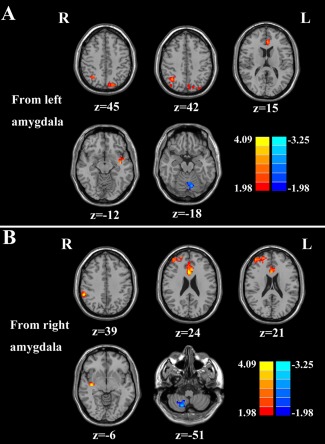
Altered effective connectivity from the amygdala to the whole brain regions in tinnitus patients compared with healthy controls. (A) From the left amygdala to the other brain regions. (B) From the right amygdala to the other brain regions. Thresholds were set at a corrected P < 0.05, determined by Monte Carlo simulation. Note that the left side corresponds to the right hemisphere.
Table 2.
Altered effective connectivity from the left amygdala to the other brain regions in tinnitus patients
| Brain region | BA |
MNI coordinatesx, y, z (mm) |
T score | Cluster size |
|---|---|---|---|---|
| L superior temporal gyrus | 41 | −39, −9, −12 | 4.4282 | 102 |
| L anterior cingulate cortex | 24 | −9, 18, 15 | 3.5999 | 92 |
|
R angular gyrus L precuneus |
19 7 |
36, −60, 42 −18, −81, 45 |
3.3214 3.1737 |
215 93 |
| L cerebellum posterior lobe | – | −9, −72, −18 | −3.8797 | 144 |
A corrected threshold of P < 0.05 determined by Monte Carlo simulation was taken as meaning that there was a significant difference between groups. BA, Brodmann's area; MNI: Montreal Neurological Institute; L, left; R, right; cluster size is in mm3.
Table 3.
Altered effective connectivity from the right amygdala to the other brain regions in tinnitus patients
| Brain region | BA |
MNI coordinatesx, y, z (mm) |
T score | Cluster size |
|---|---|---|---|---|
| R superior temporal gyrus | 41 | 39, −15, −6 | 4.5138 | 173 |
|
R anterior cingulate cortex R middle frontal gyrus |
24 10 |
3, 18, 24 24, 54, 21 |
4.4582 3.6235 |
366 280 |
| R supramarginal gyrus | 40 | 57, −42, 39 | 4.4161 | 112 |
| R cerebellum posterior lobe | – | 18, −66, −51 | −3.9077 | 135 |
A corrected threshold of P < 0.05 determined by Monte Carlo simulation was taken as meaning that there was a significant difference between groups. BA, Brodmann's area; MNI: Montreal Neurological Institute; L, left; R, right; cluster size is in mm3.
Effective Connectivity to the Amygdala
Compared with controls, chronic tinnitus patients showed enhanced effective connectivity from the right MFG, left middle temporal gyrus (MTG), left inferior frontal gyrus (IFG), and left postcentral gyrus (PoCG) to the left amygdala (Fig. 2A and Table 4). Moreover, the left ACC, left MTG, left MFG, right IFG, and right PoCG exhibited increased effective connectivity to the right amygdala in tinnitus patients compared with controls (Fig. 2B and Table 5).
Figure 2.
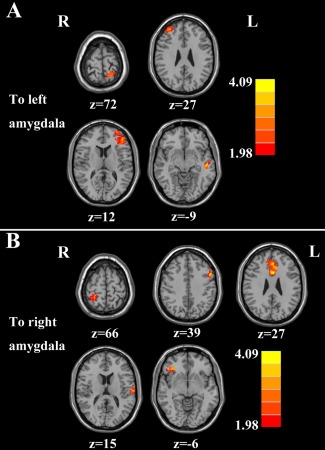
Altered effective connectivity from the whole brain regions to the amygdala in tinnitus patients compared with healthy controls. (A) From the other brain regions to the left amygdala. (B) From the other brain regions to the right amygdala. Thresholds were set at a corrected P < 0.05, determined by Monte Carlo simulation. Note that the left side corresponds to the right hemisphere.
Table 4.
Altered effective connectivity from the other brain regions to the left amygdala in tinnitus patients
| Brain region | BA |
MNI coordinatesx, y, z (mm) |
T score | Cluster size |
|---|---|---|---|---|
| R middle frontal gyrus | 10 | 36, 48, 27 | 3.9665 | 225 |
| L middle temporal gyrus | 21 | −54, −18, −9 | 4.0695 | 106 |
| L inferior frontal gyrus | 47 | −45, 42, 12 | 5.2759 | 666 |
| L postcentral gyrus | 3 | −18, −51, 72 | 3.2302 | 115 |
A corrected threshold of P < 0.05 determined by Monte Carlo simulation was taken as meaning that there was a significant difference between groups. BA, Brodmann's area; MNI: Montreal Neurological Institute; L, left; R, right; cluster size is in mm3.
Table 5.
Altered effective connectivity from the other brain regions to the right amygdala in tinnitus patients
| Brain region | BA |
MNI coordinatesx, y, z (mm) |
T score | Cluster size |
|---|---|---|---|---|
| L middle temporal gyrus | 21 | −54, −18, 15 | 4.1921 | 315 |
| L middle frontal gyrus | 10 | 36, 51, 39 | 4.0941 | 659 |
| L anterior cingulate cortex | 24 | −3, 15, 27 | 4.4820 | 1013 |
| R inferior frontal gyrus | 47 | 39, −15, −6 | 3.8851 | 101 |
| R postcentral gyrus | 3 | 27, −45, 66 | 3.2923 | 95 |
A corrected threshold of P < 0.05 determined by Monte Carlo simulation was taken as meaning that there was a significant difference between groups. BA, Brodmann's area; MNI: Montreal Neurological Institute; L, left; R, right; cluster size is in mm3.
Effective Connectivity from the Hippocampus
When compared with healthy controls, chronic tinnitus patients showed significantly increased effective connectivity from the left hippocampus to the left MTG and left PoCG whereas decreased effective connectivity was detected in left middle occipital gyrus (MOG) (Fig. 3A and Table 6). Furthermore, tinnitus patients displayed increased effective connectivity from the right hippocampus to several brain regions, including the left transverse temporal gyrus (TTG), right MTG, and right PoCG. Reduced effective connectivity was also detected in the right MOG (Fig. 3B and Table 7).
Figure 3.
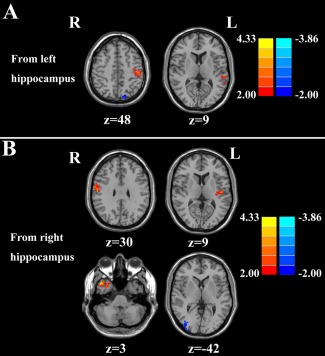
Altered effective connectivity from the hippocampus to the whole brain regions in tinnitus patients compared with healthy controls. (A) From the left hippocampus to the other brain regions. (B) From the right hippocampus to the other brain regions. Thresholds were set at a corrected P < 0.05, determined by Monte Carlo simulation. Note that the left side corresponds to the right hemisphere.
Table 6.
Altered effective connectivity from the left hippocampus to the other brain regions in tinnitus patients
| Brain region | BA |
MNI coordinatesx, y, z (mm) |
T score | Cluster size |
|---|---|---|---|---|
| L middle temporal gyrus | 21 | −36, 24, 9 | 3.6418 | 93 |
|
L postcentral gyrus L middle occipital gyrus |
3 31 |
63, −3, 48 −24, −72, 48 |
3.5776 −3.2747 |
128 102 |
A corrected threshold of P < 0.05 determined by Monte Carlo simulation was taken as meaning that there was a significant difference between groups. BA, Brodmann's area; MNI: Montreal Neurological Institute; L, left; R, right; cluster size is in mm3.
Table 7.
Altered effective connectivity from the right hippocampus to the other brain regions in tinnitus patients
| Brain region | BA |
MNI coordinatesx, y, z (mm) |
T score | Cluster size |
|---|---|---|---|---|
| L transverse temporal gyrus | 41 | −48, −18, 9 | 3.5434 | 153 |
| R middle temporal gyrus | 21 | 48, 12, −42 | 4.7906 | 243 |
|
R postcentral gyrus R middle occipital gyrus |
3 31 |
57, −12, 30 36, −81, 3 |
3.4788 −3.0669 |
114 158 |
A corrected threshold of P < 0.05 determined by Monte Carlo simulation was taken as meaning that there was a significant difference between groups. BA, Brodmann's area; MNI: Montreal Neurological Institute; L, left; R, right; cluster size is in mm3.
Effective Connectivity to the Hippocampus
Chronic tinnitus patients relative to controls demonstrated enhanced effective connectivity to the left hippocampus from several brain regions, including the right SFG, left parahippocampal gyrus and left insula (Fig. 4A and Table 8). Moreover, left and right MFG, left MTG, and left AG exhibited enhanced effective connectivity to the right hippocampus in tinnitus patients relative to controls (Fig. 4B and Table 9).
Figure 4.
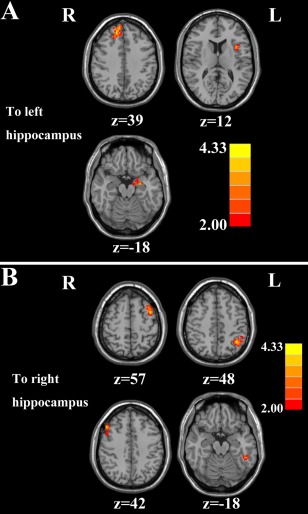
Altered effective connectivity from the whole brain regions to the hippocampus in tinnitus patients compared with healthy controls. (A) From the other brain regions to the left hippocampus. (B) From the other brain regions to the right hippocampus. Thresholds were set at a corrected P < 0.05, determined by Monte Carlo simulation. Note that the left side corresponds to the right hemisphere.
Table 8.
Altered effective connectivity from the other brain regions to the left hippocampus in tinnitus patients
| Brain region | BA |
MNI coordinatesx, y, z (mm) |
T score | Cluster size |
|---|---|---|---|---|
| R superior frontal gyrus | 9 | 15, 42, 39 | 4.3330 | 466 |
| L parahippocampa gyrus | 36 | −27, −9, −18 | 3.4124 | 87 |
| L insula | 13 | −36, 12, 12 | 3.5545 | 182 |
A corrected threshold of P < 0.05 determined by Monte Carlo simulation was taken as meaning that there was a significant difference between groups. BA, Brodmann's area; MNI: Montreal Neurological Institute; L, left; R, right; cluster size is in mm3.
Table 9.
Altered effective connectivity from the other brain regions to the right hippocampus in tinnitus patients
| Brain region | BA |
MNI coordinatesx, y, z (mm) |
T score | Cluster size |
|---|---|---|---|---|
|
L middle temporal gyrus R middle frontal gyrus |
21 10 |
−51, −42, −18 51, 24, 42 |
3.4336 4.0547 |
115 178 |
| L middle frontal gyrus | 10 | −39, 6, 57 | 4.8569 | 827 |
| L angular gyrus | 39 | −39, −54, 48 | 4.4139 | 481 |
A corrected threshold of P < 0.05 determined by Monte Carlo simulation was taken as meaning that there was a significant difference between groups. BA, Brodmann's area; MNI: Montreal Neurological Institute; L, left; R, right; cluster size is in mm3.
Correlation Analysis
Pearson's correlation analyses revealed that THQ scores were positively correlated with the increased effective connectivity from the left amygdala to the left STG (r = 0.570, P = 0.005), and from the right amygdala to the right STG (r = 0.487, P = 0.018). In addition, the enhanced effective connectivity from the right hippocampus to the left TTG was positively associated with the tinnitus duration (r = 0.452, P = 0.030) (Fig. 5). These correlations had been corrected for age, sex, and education. Other measures of increased effective connectivity were independent of tinnitus duration or THQ scores. None of the disrupted effective connectivity was correlated with SAS or SDS score. Supporting Information Table 1 presented the significant correlations before and after correction for age, sex, education, and hearing thresholds.
Figure 5.
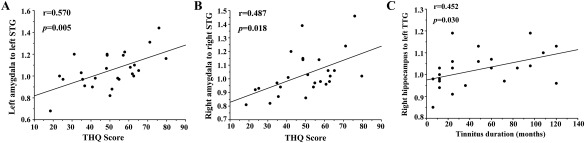
Correlations between abnormal effective connectivity and tinnitus characteristics. (A) Correlations between the THQ scores and the increased effective connectivity from the left amygdala to the left STG (r = 0.570, P = 0.005). (B) Correlations between the THQ scores and the increased effective connectivity from the right amygdala to the right STG (r = 0.487, P = 0.018). (C) Correlations between the tinnitus duration and the enhanced effective connectivity from the right hippocampus to the left TTG (r = 0.452, P = 0.030). The correlations were corrected for age, sex, and education. THQ, Tinnitus Handicap Questionnaires; STG, Superior Temporal Gyrus; TTG, Transverse Temporal Gyrus.
Structural Data
Supporting Information Table 2 showed the comparisons of the brain volumes (GM volume, WM volume, and brain parenchyma volume) between the chronic tinnitus patients and healthy controls. The GM, WM, and brain parenchyma volumes in subjects with tinnitus were not significantly different from healthy controls.
DISCUSSION
To our knowledge, this is the first study to use GCA to identify changes in the direction of effective connectivity in two regions strongly implicated in tinnitus, the hippocampus and amygdala. In tinnitus patients, significant changes in effective connectivity occurred in a relatively circumscribed network emanating from and projecting to the amygdala or the hippocampus. In most cases, tinnitus was associated with an increase in effective connectivity rather than a decrease [Chen et al., 2016; Hong et al., 2016; Zobay et al., 2015]. A major finding of this study was that tinnitus distress was strongly correlated with a bilateral increase in effective connectivity from the amygdala to the STG. This suggests that the amygdala sends a strong negative emotional signal to the auditory cortex which influences how acoustic information is interpreted [LeDoux, 2007]. The second major finding was that tinnitus duration was strongly correlated with increased effective connectivity from the right hippocampus to the left TTG. One interpretation of this result is that the hippocampus relays the memory of the phantom sound to the auditory cortex where acoustic image is consolidated to a chronic state [Halford and Anderson, 1991; Kraus and Canlon, 2012].
Disrupted Effective Connectivity from and to the Amygdala
The amygdala attaches emotional significance to our sensory experiences [Herry and Johansen, 2014; Resnik and Paz, 2015]. The amygdala receives and sends auditory information directly or indirectly to the medial geniculate body and auditory cortex and is well‐positioned to relay emotional attributes of a sound to the auditory cortex [Chen et al., 2012; LeDoux, 2007]. Prior resting‐state fMRI studies have revealed aberrant functional coupling between auditory cortex and amygdala in tinnitus patients [Kim et al., 2012; Maudoux et al., 2012b; Moller, 2006; Zhang et al., 2015] as well as rats with salicylate‐induced tinnitus [Chen et al., 2015a]. Infusion of salicylate directly into amygdala increases sound‐evoked activity in the auditory cortex, illustrating the potent modulatory effect that the amygdala exerts on auditory processing carried out by the auditory cortex [Chen et al., 2012]. Collectively, these results reinforce the view that the amygdala contributes to the fear and anxiety experienced by many tinnitus patients [Cima et al., 2011; Halford and Anderson, 1991]. Our tinnitus patients showed enhanced coupling in each hemisphere from the amygdala to the STG which was correlated with tinnitus distress. The amygdala is capable of modulating auditory cortex activity and plasticity. Fear conditioning, which includes activation of amygdala, alters the functional properties of the auditory cortex [Froemke and Martins, 2011; Headley and Weinberger, 2013] such that even non‐auditory conditioned stimuli can activate auditory cortex [Ide et al., 2013]. In addition, a feedback pathway from the amygdala to the auditory cortex may suppress the tinnitus signal at a subcortical level before it reaches auditory cortex and consciousness [Rauschecker et al., 2010]. Diffusion tensor imaging (DTI) revealed significant differences in the strength of the fiber tracts connecting the auditory cortex and amygdala [Crippa et al., 2010]. Our results are consistent with previous reports showing that tinnitus patients manifest increased functional connectivity between the auditory system and the amygdala in several resting‐state fMRI studies [Kim et al., 2012; Maudoux et al., 2012b].
Our tinnitus patients also exhibited increased bidirectional connectivity between the amygdala and ACC consistent with previous structural and functional data [Bush et al., 2000]. The ACC plays a crucial role in a form of attention regulating emotional and cognitive functions [Bush et al., 2000] and therefore could be important as an emotional and attentional regulator of tinnitus. Decreased GM volume has been reported in the ACC of tinnitus patients [Aldhafeeri et al., 2012] and electroencephalography (EEG) suggests that the dorsal ACC might be involved in persistent attention to tinnitus [De Ridder et al., 2011; Vanneste et al., 2010a]. Tinnitus distress is linked to increased beta activity in the dorsal ACC and the amount of distress correlates with an alpha activity in several brain regions such as the amygdala, insula, and parahippocampus [Vanneste et al., 2010a]. Others have reported abnormal ACC function in tinnitus patients using positron emission tomography (PET) [Mirz, 2000], magnetoencephalography (MEG) [Schlee et al., 2009b], and resting‐state fMRI [Chen et al., 2015c]. Taken together, these results suggest that enhanced reciprocal functional connectivity between the amygdala and ACC likely contributes to the emotional and attentional aspects of tinnitus.
Tinnitus patients showed increased effective connectivity from the amygdala to the SMG, AG, and precuneus. Prior EEG, PET, or fMRI studies have indicated that the SMG may be involved in tinnitus [Chen et al., 2015c; Mirz et al., 1999; Weiler et al., 2000]. Schmidt et al. demonstrated that the dorsal attention network, with seed regions in the bilateral intraparietal sulci, showed decreased correlations with the right SMG in tinnitus patients [Schmidt et al., 2013]. Thus, the increased effective connectivity to the SMG in tinnitus may alter the connectivity in the dorsal attention network. Furthermore, the AG and precuneus belong to the default mode network (DMN). The DMN, consisting of nodes in the AG, posterior cingulate/precuneus, medial temporal gyrus and medial prefrontal gyrus, is most active at rest and shows reduced activity when a subject enters a task‐based state involving attention or goal‐directed behavior [Mantini et al., 2007; Raichle et al., 2001]. Previous resting‐state fMRI studies have also found aberrant functional connectivity within the DMN in tinnitus patients compared with healthy controls [Burton et al., 2012; Chen et al., 2015c; Maudoux et al., 2012b; Schmidt et al., 2013]. Nevertheless, the source of abnormal neural activity within specific DMN regions due to tinnitus still remains unknown. Our results suggest that increased effective connectivity to AG and precuneus might be responsible for disrupting the DMN in tinnitus patients.
Neuroimaging studies have suggested that abnormal coupling between the frontal cortex and other regions contributes to tinnitus disability [Chen et al., 2014, 2015c; Lanting et al., 2016; Leaver et al., 2016b; Mirz, 2000; Schlee et al., 2009a; Vanneste and De Ridder, 2012; Vanneste et al., 2010a; Zhang et al., 2015]. Rauschecker et al. developed a model to demonstrate structural and functional differences in ventromedial prefrontal cortex that were associated with tinnitus subjective loudness, indicating the contribution of frontal cortex to certain perceptual features of tinnitus [Rauschecker et al., 2010]. Activity in the frontal cortex increases during periods of active listening [Hall et al., 2000; Voisin et al., 2006] and auditory attention while listening to angry prosody, strong activates a network involving the auditory cortex, amygdala and frontal cortex [Ceravolo et al., 2016]. Our tinnitus patients showed enhanced connectivity between the frontal cortex (MFG and IFG) and the amygdala. Chen et al. observed significantly enhanced functional connectivity within the executive control of attention network, including the MFG and IFG [Chen et al., 2015c]. Moreover, the increased functional connectivity between bilateral insula and MFG was positively correlated with tinnitus distress. Furthermore, the IFG serves as the core region of response inhibition and IFG activity might mirror the attempt to control the bottom‐up attention allocation to the tinnitus percept in a top‐down manner [Aron et al., 2014]. In one hypothetical model, the IFG acts as executive control components in the attention system that regulates dorsal and ventral attention networks [Shulman et al., 2009]. These results suggest that the attentional networks in the frontal cortex enhance the negative attributes of tinnitus supplied by the amygdala.
We observed increased connectivity from the PoCG to the amygdala phantom somatosensory perceptions or somatic tinnitus. Possible neural correlates of somatosensory modulation of tinnitus were assessed [Murray et al., 2005], which was consistent with previous fMRI studies showing abnormal neural activity in somatosensory subnetworks in tinnitus [Chen et al., 2015a; Maudoux et al., 2012a]. Moreover, reduced effective connectivity from the amygdala to the cerebellum was observed in tinnitus patients. Although the cerebellum is primarily involved in motor planning and fine motor control, some cerebellar regions such as the paraflocculus and vermis receive inputs from auditory centers [Petacchi et al., 2005] and are activated by simple and complex sounds [Lockwood et al., 1998, 2002]. During residual inhibition of tinnitus, decreased activity was observed in the right cerebellum [Osaki et al., 2005]. Animal studies of tinnitus have suggested that the parafloccular lobe of the cerebellum acts as a gain control mechanism comparing the afferent input from the cochlea with descending signals from the cerebral cortex [Bauer et al., 2013]. Ablation or inactivation of the paraflocculus abolished noise‐induced tinnitus suggesting that parts of the cerebellum may be involved in regulation the sensation or emotional features of tinnitus.
Disrupted Effective Connectivity from and to the Hippocampus
The hippocampus, important for memory and spatial navigation, is connected to the auditory cortex [Cenquizca and Swanson, 2007]. Effective connectivity from the right hippocampus to the left primary auditory cortex was enhanced in our tinnitus patients and was strongly correlated with tinnitus duration. Tinnitus was also associated with enhanced connectivity from the hippocampus to the association auditory cortex. The hippocampus receives sensory auditory input from primary auditory cortex directly or indirectly via the parahippocampal cortex or the perirhinal cortex, or via other forebrain pathways including amygdala insula or medial prefrontal cortex [Mohedano‐Moriano et al., 2007; Munoz‐Lopez et al., 2010]. In turn, the auditory association cortex receives indirect input from hippocampus via parahippocampal cortex or perirhinal cortex [O'Mara, 2005]. Animal study also indicated a direct connection from hippocampus to the auditory association cortex and even to the primary auditory cortex [Cenquizca and Swanson, 2007]. The hippocampal‐auditory network is critically important for the formation of long‐term auditory memories. Patients with severe bilateral hippocampal damage perform poorly on auditory recognition tests [Squire et al., 2001], and studies with monkeys suggest that auditory cues play a role in spatial memory [Tamura et al., 1990]. Among patients who could modulate tinnitus loudness with a jaw clench (somatic tinnitus), a decrease in tinnitus loudness was correlated with decreased activity in the hippocampus and auditory cortex [Lockwood et al., 1998]. Consistent with our results, regional global connectivity of the hippocampus was positively correlated with tinnitus loudness [Ueyama et al., 2013].
Interactions between the hippocampus and the insula as well as the parahippocampus have been observed in tinnitus patients. Increased insular response may be an indication of successful adaption to the tinnitus perception [Haller et al., 2010]. The insula cortex can provide executive control that switches attention between tinnitus and other conditions. Furthermore EEG studies showed that alpha activity in the anterior insula was observed in patients with severe tinnitus‐related distress who can or cannot cope with these phantom sounds [Vanneste et al., 2014; Vanneste and De Ridder, 2012; Vanneste et al., 2010a]. In addition, chronic tinnitus patients showed enhanced spontaneous neuronal activity and functional connectivity in bilateral anterior insula revealed by resting‐state fMRI [Chen et al., 2015c]. The parahippocampal area plays a pivotal role in memory recollection and transferring information from the hippocampus to the association areas [Diederen et al., 2010]. Tinnitus distress correlated with bilateral activation of the posterior parahippocampal‐hippocampal interface [Schecklmann et al., 2013]. EEG studies demonstrated that the involvement of the parahippocampal area in tinnitus might be linked with the constant updating of the tinnitus percept from memory thereby preventing habituation [De Ridder et al., 2006; Vanneste and De Ridder, 2012]. Additionally, narrow band noise tinnitus patients have increased activity in the parahippocampus in comparison to pure tone tinnitus patients at the gamma frequency band [Vanneste et al., 2010b]. Prior resting‐state fMRI studies also provided further support linking tinnitus physiopathology with parahippocampal region [Chen et al., 2015b; Leaver et al., 2016b; Maudoux et al., 2012a].
The right SFG showed enhanced effective connectivity to the left hippocampus in tinnitus. Based on the previous fMRI studies, the SFG has been regarded as a major integrative hub of the tinnitus network architecture [Chen et al., 2014, 2016]. Wunderlich et al. found the activation of the SFG due to acoustic stimulation in a pitch discrimination task, suggesting the perception of auditory inputs in a more emotional context in tinnitus [Wunderlich et al., 2010]. While it is difficult to establish conclusive interpretations of our results, we suggest that the SFG may be responsible for the integration of multi‐sensory information, including the auditory sensation and pathophysiology of tinnitus perception. Interestingly, reduced effective connectivity from the hippocampus to the MOG was found in tinnitus, which may be due to compensatory mechanisms in visual regions associated with hearing a phantom sound. The multisensory connections between auditory and visual regions make it possible for external sounds or the phantom sound of tinnitus to alter brain activity in the visual areas [Cate et al., 2009]. Previous fMRI studies also showed aberrant function in visual network in tinnitus patients [Burton et al., 2012; Chen et al., 2014, 2015c; Maudoux et al., 2012b]. As such, tinnitus can be regarded as the consequence of multisensory interactions between auditory, limbic, and visual regions.
Limitations
Our moderate small sample size may have reduced our ability to detect causal relationships between abnormal effective connectivity and tinnitus characteristics. Furthermore, we only selected the amygdala and hippocampus as seed regions to investigate the effective connectivity of limbic structures with tinnitus. The current GCA approach could be extended to the subdivisions of the amygdala and the hippocampus, and other limbic regions such as parahippocampus, cingulate gyrus and hypothalamus. Moreover, the subjects in the current study showed no hearing loss, which is not representative for most tinnitus patients. Finally, the unavoidable MR scanner noise could have affected our effective connectivity analysis [Logothetis et al., 2009]. The concept of resting state is somewhat problematic in our study because the auditory pathway is likely to be activated by scanner noise which is nearly impossible to completely eliminate even with earplugs or active noise reduction. Indeed, scanner noise has been shown to cause some suppression of the DMN [Perrachione and Ghosh, 2013]. The existence of scanner noise may make the internal sound of tinnitus less salient thereby reducing the differences in resting‐state functional connectivity between tinnitus and control groups. However, this limitation applies to virtually all resting‐state studies in the literatures. Nevertheless, this confounding factor should be taken into consideration for all the auditory fMRI studies.
CONCLUSIONS
Despite these limitations, our results identified disrupted effective connectivity networks in the limbic regions of tinnitus patients. Tinnitus severity was positively correlated with a bilateral increase in effective connectivity from the amygdala to the auditory cortex on the same side. In addition, tinnitus duration was positively correlated with enhanced effective connectivity from the right hippocampus to the left auditory cortex. These findings mainly emphasized the crucial role of limbic system and limbic–auditory interaction in tinnitus patients, which could help enhance our understanding of the neuropathological mechanisms underlying tinnitus.
Supporting information
Supporting Information Figure 1
Supporting Information Table 1
ACKNOWLEDGMENTS
Y‐C C and WX designed the experiment, collected the data, performed the analysis, and wrote the article. HC, YF, and J‐J X helped collect the data and perform the analysis. J‐P G, RS and XY contributed to the discussion and manuscript revision.
Yu‐Chen Chen and Wenqing Xia have contributed equally to this work.
The authors declare that there is no conflict of interests.
Contributor Information
Yu‐Chen Chen, Email: chenyuchen1989@126.com.
Xindao Yin, Email: y.163yy@163.com.
REFERENCES
- Aldhafeeri FM, Mackenzie I, Kay T, Alghamdi J, Sluming V (2012): Neuroanatomical correlates of tinnitus revealed by cortical thickness analysis and diffusion tensor imaging. Neuroradiology 54:883–892. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA (2014): Inhibition and the right inferior frontal cortex: One decade on. Trends Cogn Sci 18:177–185. [DOI] [PubMed] [Google Scholar]
- Bartels H, Staal MJ, Albers FW (2007): Tinnitus and neural plasticity of the brain. Otol Neurotol 28:178–184. [DOI] [PubMed] [Google Scholar]
- Bauer CA, Kurt W, Sybert LT, Brozoski TJ (2013): The cerebellum as a novel tinnitus generator. Hear Res 295:130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Zerrin Yetkin F, Haughton VM, Hyde JS (1995): Functional connectivity in the motor cortex of resting human brain using echo‐planar mri. Magn Reson Med 34:537–541. [DOI] [PubMed] [Google Scholar]
- Burton H, Wineland A, Bhattacharya M, Nicklaus J, Garcia KS, Piccirillo JF (2012): Altered networks in bothersome tinnitus: A functional connectivity study. BMC Neurosci 13:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI (2000): Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci 4:215–222. [DOI] [PubMed] [Google Scholar]
- Carpenter‐Thompson JR, Schmidt SA, Husain FT (2015): Neural plasticity of mild tinnitus: An fmri investigation comparing those recently diagnosed with tinnitus to those that had tinnitus for a long period of time. Neural Plastic 2015:161478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cate AD, Herron TJ, Yund EW, Stecker GC, Rinne T, Kang X, Petkov CI, Disbrow EA, Woods DL (2009): Auditory attention activates peripheral visual cortex. PloS One 4:e4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenquizca LA, Swanson LW (2007): Spatial organization of direct hippocampal field CA1 axonal projections to the rest of the cerebral cortex. Brain Res Rev 56:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceravolo L, Frühholz S, Grandjean D (2016): Modulation of auditory spatial attention by angry prosody: An fMRI auditory dot‐probe study. Front Neurosci 10:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao‐Gan Y, Yu‐Feng Z (2010): DPARSF: A MATLAB toolbox for “pipeline” data analysis of resting‐state fMRI. Front Syst Neurosci 4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GD, Manohar S, Salvi R (2012): Amygdala hyperactivity and tonotopic shift after salicylate exposure. Brain Res 1485:63–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YC, Zhang J, Li XW, Xia W, Feng X, Gao B, Ju SH, Wang J, Salvi R, Teng GJ (2014): Aberrant spontaneous brain activity in chronic tinnitus patients revealed by resting‐state functional MRI. NeuroImage: Clin 6:222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y‐C, Li X, Liu L, Wang J, Lu C‐Q, Yang M, Jiao Y, Zang F‐C, Radziwon K, Chen G‐D (2015a): Tinnitus and hyperacusis involve hyperactivity and enhanced connectivity in auditory‐limbic‐arousal‐cerebellar network. Elife 4:e06576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YC, Xia W, Feng Y, Li X, Zhang J, Feng X, Wang CX, Cai Y, Wang J, Salvi R (2015b): Altered interhemispheric functional coordination in chronic tinnitus patients. BioMed Res Int 2015:345647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YC, Zhang J, Li XW, Xia W, Feng X, Qian C, Yang XY, Lu CQ, Wang J, Salvi R (2015c): Altered intra‐and interregional synchronization in resting‐state cerebral networks associated with chronic tinnitus. Neural Plastic 2015:475382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YC, Feng Y, Xu JJ, Mao CN, Xia W, Ren J, Yin X (2016): Disrupted brain functional network architecture in chronic tinnitus patients. Front Aging Neurosci 8:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cima RF, Crombez G, Vlaeyen JW (2011): Catastrophizing and fear of tinnitus predict quality of life in patients with chronic tinnitus. Ear Hear 32:634–641. [DOI] [PubMed] [Google Scholar]
- Crippa A, Lanting CP, Dijk Pv, Roerdink JB (2010): A diffusion tensor imaging study on the auditory system and tinnitus. Open Neuroimaging J 4:16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ridder D, Fransen H, Francois O, Sunaert S, Kovacs S, Van De Heyning P (2006): Amygdalohippocampal involvement in tinnitus and auditory memory. Acta Oto‐Laryngol 5:50–53. [DOI] [PubMed] [Google Scholar]
- De Ridder D, Vanneste S, Congedo M (2011): The distressed brain: A group blind source separation analysis on tinnitus. PloS One 6:e24273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diederen KM, Neggers SF, Daalman K, Blom JD, Goekoop R, Kahn RS, Sommer IE (2010): Deactivation of the parahippocampal gyrus preceding auditory hallucinations in schizophrenia. Am J Psychiatry 167:427–435. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ (2005): Tinnitus: Neurobiological substrates. Drug Discov Today 10:1283–1290. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ, Roberts LE (2012): The neuroscience of tinnitus: Understanding abnormal and normal auditory perception. Front Syst Neurosci 6:5–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froemke RC, Martins ARO (2011): Spectrotemporal dynamics of auditory cortical synaptic receptive field plasticity. Hear Res 279:149–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golm D, Schmidt‐Samoa C, Dechent P, Kroner‐Herwig B (2013): Neural correlates of tinnitus related distress: An fMRI‐study. Hear Res 295:87–99. [DOI] [PubMed] [Google Scholar]
- Gunbey HP, Gunbey E, Aslan K, Bulut T, Unal A, Incesu L (2015): Limbic‐auditory interactions of tinnitus: An evaluation using diffusion tensor imaging. Clin Neuroradiol [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Guo W, Liu F, Liu J, Yu L, Zhang J, Zhang Z, Xiao C, Zhai J, Zhao J (2014): Abnormal causal connectivity by structural deficits in first‐episode, drug‐naive schizophrenia at rest. Schizophr Bull 41:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Liu F, Zhang Z, Liu J, Yu M, Zhang J, Xiao C, Zhao J (2015): Unidirectionally affected causal connectivity of cortico‐limbic‐cerebellar circuit by structural deficits in drug‐naive major depressive disorder. J Affect Disord 172:410–416. [DOI] [PubMed] [Google Scholar]
- Halford JB, Anderson SD (1991): Anxiety and depression in tinnitus sufferers. J Psychosom Res 35:383–390. [DOI] [PubMed] [Google Scholar]
- Hall DA, Haggard MP, Akeroyd MA, Summerfield AQ, Palmer AR, Elliott MR, Bowtell RW (2000): Modulation and task effects in auditory processing measured using fMRI. Hum Brain Mapp 10:107–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller S, Birbaumer N, Veit R (2010): Real‐time fMRI feedback training may improve chronic tinnitus. Eur Radiol 20:696–703. [DOI] [PubMed] [Google Scholar]
- Headley DB, Weinberger NM (2013): Fear conditioning enhances gamma oscillations and their entrainment of neurons representing the conditioned stimulus. J Neurosci 33:5705–5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry JA, Roberts LE, Caspary DM, Theodoroff SM, Salvi RJ (2014): Underlying mechanisms of tinnitus: Review and clinical implications. J Am Acad Audiol 25:5–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herry C, Johansen JP (2014): Encoding of fear learning and memory in distributed neuronal circuits. Nat Neurosci 17:1644–1654. [DOI] [PubMed] [Google Scholar]
- Hong SK, Park S, Ahn MH, Min BK (2016): Top‐down and bottom‐up neurodynamic evidence in patients with tinnitus. Hear Res 342:86–100. [DOI] [PubMed] [Google Scholar]
- Ide Y, Takahashi M, Lauwereyns J, Sandner G, Tsukada M, Aihara T (2013): Fear conditioning induces guinea pig auditory cortex activation by foot shock alone. Cogn Neurodyn 7:67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackler RK, Whinney D (2001): A century of eighth nerve surgery. Otol Neurotol 22:401–416. [DOI] [PubMed] [Google Scholar]
- Jastreboff PJ (1990): Phantom auditory perception (tinnitus): mechanisms of generation and perception. Neurosci Res 8:221–254. [DOI] [PubMed] [Google Scholar]
- Jastreboff PJ (1995): Salicylate‐induced abnormal activity in the inferior colliculus of rats. Hear Res 82:158–178. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, Zhang J, Finlayson P (2005): Tinnitus as a plastic phenomenon and its possible neural underpinnings in the dorsal cochlear nucleus. Hear Res 206:200–226. [DOI] [PubMed] [Google Scholar]
- Khalfa S, Dubal S, Veuillet E, Perez‐Diaz F, Jouvent R, Collet L (2002): Psychometric normalization of a hyperacusis questionnaire. ORL 64:436–442. [DOI] [PubMed] [Google Scholar]
- Kim JY, Kim YH, Lee S, Seo JH, Song HJ, Cho JH, Chang Y (2012): Alteration of functional connectivity in tinnitus brain revealed by resting‐state fMRI?: A pilot study. Int J Audiol 51:413–417. [DOI] [PubMed] [Google Scholar]
- Kraus KS, Canlon B (2012): Neuronal connectivity and interactions between the auditory and limbic systems. Effects of noise and tinnitus. Hear Res 288:34–46. [DOI] [PubMed] [Google Scholar]
- Kuk FK, Tyler RS, Russell D, Jordan H (1990): The psychometric properties of a tinnitus handicap questionnaire. Ear Hear 11:434–445. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Tordesillas ‐Gutiérrez D, Martinez M, Salinas F, Evans A, Zilles K, Mazziotta JC, Fox PT (2007): Bias between MNI and Talairach coordinates analyzed using the ICBM‐152 brain template. Hum Brain Mapp 28:1194–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgrebe M, Langguth B, Rosengarth K, Braun S, Koch A, Kleinjung T, May A, de Ridder D, Hajak G (2009): Structural brain changes in tinnitus: Grey matter decrease in auditory and non‐auditory brain areas. NeuroImage 46:213–218. [DOI] [PubMed] [Google Scholar]
- Langguth B (2011): A review of tinnitus symptoms beyond 'ringing in the ears': a call to action. Curr Med Res Opin 27:1635–1643. [DOI] [PubMed] [Google Scholar]
- Lanting C, De Kleine E, Van Dijk P (2009): Neural activity underlying tinnitus generation: Results from PET and fMRI. Hear Res 255:1–13. [DOI] [PubMed] [Google Scholar]
- Lanting C, Wozniak A, van Dijk P, Langers DR (2016): Tinnitus‐ and task‐related differences in resting‐state networks. Adv Exp Med Biol 894:175–187. [DOI] [PubMed] [Google Scholar]
- Leaver AM, Renier L, Chevillet MA, Morgan S, Kim HJ, Rauschecker JP (2011): Dysregulation of limbic and auditory networks in tinnitus. Neuron 69:33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaver AM, Seydell‐Greenwald A, Rauschecker JP (2016a): Auditory–limbic interactions in chronic tinnitus: Challenges for neuroimaging research. Hear Res 334:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaver AM, Turesky TK, Seydell‐Greenwald A, Morgan S, Kim HJ, Rauschecker JP (2016b): Intrinsic network activity in tinnitus investigated using functional MRI. Hum Brain Mapp 37:2717–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J (2007): The amygdala. Curr Biol 17:R868–R874. [DOI] [PubMed] [Google Scholar]
- Llinás RR, Ribary U, Jeanmonod D, Kronberg E, Mitra PP (1999): Thalamocortical dysrhythmia: A neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc Natl Acad Sci 96:15222–15227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood AH, Salvi RJ, Coad ML, Towsley ML, Wack DS, Murphy BW (1998): The functional neuroanatomy of tinnitus: Evidence for limbic system links and neural plasticity. Neurology 50:114–120. [DOI] [PubMed] [Google Scholar]
- Lockwood AH, Salvi RJ, Burkard RF (2002): Tinnitus. N Engl J Med 347:904–910. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Murayama Y, Augath M, Steffen T, Werner J, Oeltermann A (2009): How not to study spontaneous activity. NeuroImage 45:1080–1089. [DOI] [PubMed] [Google Scholar]
- Lopez‐Escamez JA, Carey J, Chung WH, Goebel JA, Magnusson M, Mandala M, Newman‐Toker DE, Strupp M, Suzuki M, Trabalzini F, Bisdorff A (2015): Diagnostic criteria for Meniere's disease. J Vestib Res 25:1–7. [DOI] [PubMed] [Google Scholar]
- Mantini D, Perrucci MG, Del Gratta C, Romani GL, Corbetta M (2007): Electrophysiological signatures of resting state networks in the human brain. Proc Natl Acad Sci U S A 104:13170–13175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh RA, Fuzessery ZM, Grose CD, Wenstrup JJ (2002): Projection to the inferior colliculus from the basal nucleus of the amygdala. J Neurosci 22:10449–10460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maudoux A, Lefebvre P, Cabay JE, Demertzi A, Vanhaudenhuyse A, Laureys S, Soddu A (2012a): Auditory resting‐state network connectivity in tinnitus: A functional MRI study. PloS One 7:e36222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maudoux A, Lefebvre P, Cabay JE, Demertzi A, Vanhaudenhuyse A, Laureys S, Soddu A (2012b): Connectivity graph analysis of the auditory resting state network in tinnitus. Brain Res 1485:10–21. [DOI] [PubMed] [Google Scholar]
- Mirz F (2000): Cortical networks subserving the perception of tinnitus‐a PET study. Acta Oto‐Laryngol 120:241–243. [DOI] [PubMed] [Google Scholar]
- Mirz F, Pedersen B, Ishizu K, Johannsen P, Ovesen T, Stodkilde‐Jorgensen H, Gjedde A (1999): Positron emission tomography of cortical centers of tinnitus. Hear Res 134:133–144. [DOI] [PubMed] [Google Scholar]
- Mohedano‐Moriano A, Pro‐Sistiaga P, Arroyo‐Jimenez MM, Artacho‐Perula E, Insausti AM, Marcos P, Cebada‐Sanchez S, Martinez‐Ruiz J, Munoz M, Blaizot X, Martinez‐Marcos A, Amaral DG, Insausti R (2007): Topographical and laminar distribution of cortical input to the monkey entorhinal cortex. J Anat 211:250–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller AR (2006): Neural plasticity in tinnitus. Prog Brain Res 157:365–372. [DOI] [PubMed] [Google Scholar]
- Muhlau M, Rauschecker JP, Oestreicher E, Gaser C, Rottinger M, Wohlschlager AM, Simon F, Etgen T, Conrad B, Sander D (2006): Structural brain changes in tinnitus. Cereb Cortex 16:1283–1288. [DOI] [PubMed] [Google Scholar]
- Munoz‐Lopez M, MohedanoMoriano A, Insausti R (2010): Anatomical pathways for auditory memory in primates. Front Neuroanat 4:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray MM, Molholm S, Michel CM, Heslenfeld DJ, Ritter W, Javitt DC, Schroeder CE, Foxe JJ (2005): Grabbing your ear: Rapid auditory‐somatosensory multisensory interactions in low‐level sensory cortices are not constrained by stimulus alignment. Cereb Cortex 15:963–974. [DOI] [PubMed] [Google Scholar]
- O'Mara S (2005): The subiculum: What it does, what it might do, and what neuroanatomy has yet to tell us. J Anat 207:271–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osaki Y, Nishimura H, Takasawa M, Imaizumi M, Kawashima T, Iwaki T, Oku N, Hashikawa K, Doi K, Nishimura T (2005): Neural mechanism of residual inhibition of tinnitus in cochlear implant users. Neuroreport 16:1625–1628. [DOI] [PubMed] [Google Scholar]
- Perrachione TK, Ghosh SS (2013): Optimized design and analysis of sparse‐sampling FMRI experiments. Front Neurosci 7:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petacchi A, Laird AR, Fox PT, Bower JM (2005): Cerebellum and auditory function: An ALE meta‐analysis of functional neuroimaging studies. Hum Brain Mapp 25:118–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi R, Zhang LJ, Zhong J, Zhang Z, Ni L, Jiao Q, Liao W, Zheng G, Lu G (2013): Altered effective connectivity network of the basal ganglia in low‐grade hepatic encephalopathy: A resting‐state fMRI study with Granger causality analysis. PloS One 8:e53677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL (2001): A default mode of brain function. Proc Natl Acad Sci 98:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauschecker JP, Leaver AM, Muhlau M (2010): Tuning out the noise: Limbic‐auditory interactions in tinnitus. Neuron 66:819–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnik J, Paz R (2015): Fear generalization in the primate amygdala. Nat Neurosci 18:188–190. [DOI] [PubMed] [Google Scholar]
- Reynolds P, Gardner D, Lee R (2004): Tinnitus and psychological morbidity: A cross‐sectional study to investigate psychological morbidity in tinnitus patients and its relationship with severity of symptoms and illness perceptions. Clin Otolaryngol Allied Sci 29:628–634. [DOI] [PubMed] [Google Scholar]
- Robertson D, Mulders W (2012): The Inferior Colliculus: Involvement in Hyperactivity and Tinnitus. Tinnitus: Springer; pp. 121–135. [Google Scholar]
- Roussel NA, Nijs J, Meeus M, Mylius V, Fayt C, Oostendorp R (2013): Central sensitization and altered central pain processing in chronic low back pain: Fact or myth?. Clin J Pain 29:625–638. [DOI] [PubMed] [Google Scholar]
- Sah P, Faber ES, Lopez De Armentia M, Power J (2003): The amygdaloid complex: Anatomy and physiology. Physiol Rev 83:803–834. [DOI] [PubMed] [Google Scholar]
- Schecklmann M, Landgrebe M, Poeppl TB, Kreuzer P, Männer P, Marienhagen J, Wack DS, Kleinjung T, Hajak G, Langguth B (2013): Neural correlates of tinnitus duration and distress: A positron emission tomography study. Hum Brain Mapp 34:233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlee W, Hartmann T, Langguth B, Weisz N (2009a): Abnormal resting‐state cortical coupling in chronic tinnitus. BMC Neurosci 10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlee W, Mueller N, Hartmann T, Keil J, Lorenz I, Weisz N (2009b): Mapping cortical hubs in tinnitus. BMC Biol 7:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt SA, Akrofi K, Carpenter‐Thompson JR, Husain FT (2013): Default mode, dorsal attention and auditory resting state networks exhibit differential functional connectivity in tinnitus and hearing loss. PloS One 8:e76488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seydell‐Greenwald A, Raven EP, Leaver AM, Turesky TK, Rauschecker JP (2014): Diffusion imaging of auditory and auditory‐limbic connectivity in tinnitus: Preliminary evidence and methodological challenges. Neural Plastic 2014:145943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shargorodsky J, Curhan GC, Farwell WR (2010): Prevalence and characteristics of tinnitus among US adults. Am J Med 123:711–718. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Astafiev SV, Franke D, Pope DL, Snyder AZ, McAvoy MP, Corbetta M (2009): Interaction of stimulus‐driven reorienting and expectation in ventral and dorsal frontoparietal and basal ganglia‐cortical networks. J Neurosci 29:4392–4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindhusake D, Mitchell P, Newall P, Golding M, Rochtchina E, Rubin G (2003): Prevalence and characteristics of tinnitus in older adults: The Blue Mountains Hearing Study. Int J Audiol 42:289–294. [DOI] [PubMed] [Google Scholar]
- Squire LR, Schmolck H, Stark SM (2001): Impaired auditory recognition memory in amnesic patients with medial temporal lobe lesions. Learn Mem 8:252–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura R, Ono T, Fukuda M, Nakamura K (1990): Recognition of egocentric and allocentric visual and auditory space by neurons in the hippocampus of monkeys. Neurosci Lett 109:293–298. [DOI] [PubMed] [Google Scholar]
- Ueyama T, Donishi T, Ukai S, Ikeda Y, Hotomi M, Yamanaka N, Shinosaki K, Terada M, Kaneoke Y (2013): Brain regions responsible for tinnitus distress and loudness: A resting‐state fMRI study. PloS One 8:e67778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanneste S, De Ridder D (2012): The auditory and non‐auditory brain areas involved in tinnitus. An emergent property of multiple parallel overlapping subnetworks. Front Syst Neurosci 6:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanneste S, Plazier M, der Loo E, de Heyning PV, Congedo M, De Ridder D (2010a): The neural correlates of tinnitus‐related distress. Neuroimage 52:470–480. [DOI] [PubMed] [Google Scholar]
- Vanneste S, Plazier M, van der Loo E, Van de Heyning P, De Ridder D (2010b): The differences in brain activity between narrow band noise and pure tone tinnitus. PloS One 5:e13618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanneste S, Congedo M, De Ridder D (2014): Pinpointing a highly specific pathological functional connection that turns phantom sound into distress. Cereb Cortex 24:2268–2282. [DOI] [PubMed] [Google Scholar]
- Voisin J, Bidet‐Caulet A, Bertrand O, Fonlupt P (2006): Listening in silence activates auditory areas: A functional magnetic resonance imaging study. J Neurosci 26:273–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler EW, Brill K, Tachiki KH, Wiegand R (2000): Electroencephalography correlates in tinnitus. Int Tinnitus J 6:21–24. [PubMed] [Google Scholar]
- Weinberger NM (2007): Associative representational plasticity in the auditory cortex: A synthesis of two disciplines. Learn Mem 14:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer JA (2006): Decoding the auditory corticofugal systems. Hear Res 212:1–8. [DOI] [PubMed] [Google Scholar]
- Wunderlich AP, Schonfeldt‐Lecuona C, Wolf RC, Dorn K, Bachor E, Freund W (2010): Cortical activation during a pitch discrimination task in tinnitus patients and controls–an fMRI study. Audiol Neuro‐Otol 15:137–148. [DOI] [PubMed] [Google Scholar]
- Zang ZX, Yan CG, Dong ZY, Huang J, Zang YF (2012): Granger causality analysis implementation on MATLAB: A graphic user interface toolkit for fMRI data processing. J Neurosci Meth 203:418–426. [DOI] [PubMed] [Google Scholar]
- Zhang J, Chen YC, Feng X, Yang M, Liu B, Qian C, Wang J, Salvi R, Teng GJ (2015): Impairments of thalamic resting‐state functional connectivity in patients with chronic tinnitus. Eur J Radiol 84:1277–1284. [DOI] [PubMed] [Google Scholar]
- Zhong Y, Huang L, Cai S, Zhang Y, von Deneen KM, Ren A, Ren J, Initiative AsDN (2014): Altered effective connectivity patterns of the default mode network in Alzheimer's disease: An fMRI study. Neurosci Lett 578:171–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zobay O, Palmer AR, Hall DA, Sereda M, Adjamian P (2015): Source space estimation of oscillatory power and brain connectivity in tinnitus. PloS One 10:e0120123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zung WW (1971): A rating instrument for anxiety disorders. Psychosomatics 12:371–379. [DOI] [PubMed] [Google Scholar]
- Zung W (1986): Zung Self‐Rating Depression Scale and Depression Status Inventory. Assessment of Depression. New York: Springer; pp. 221–231. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Figure 1
Supporting Information Table 1


