Abstract
Neurobehavioral models of pedophilia and child sexual offending suggest a pattern of temporal and in particular prefrontal disturbances leading to inappropriate behavioral control and subsequently an increased propensity to sexually offend against children. However, clear empirical evidence for such mechanisms is still missing. Using a go/nogo paradigm in combination with functional magnetic resonance imaging (fMRI) we compared behavioral performance and neural response patterns among three groups of men matched for age and IQ: pedophiles with (N = 40) and without (N = 37) a history of hands‐on sexual offences against children as well as healthy non‐offending controls (N = 40). As compared to offending pedophiles, non‐offending pedophiles exhibited superior inhibitory control as reflected by significantly lower rate of commission errors. Group‐by‐condition interaction analysis also revealed inhibition‐related activation in the left posterior cingulate and the left superior frontal cortex that distinguished between offending and non‐offending pedophiles, while no significant differences were found between pedophiles and healthy controls. Both areas showing distinct activation pattern among pedophiles play a critical role in linking neural networks that relate to effective cognitive functioning. Data therefore suggest that heightened inhibition‐related recruitment of these areas as well as decreased amount of commission errors is related to better inhibitory control in pedophiles who successfully avoid committing hands‐on sexual offences against children. Hum Brain Mapp 38:1092–1104, 2017. © 2016 Wiley Periodicals, Inc.
Keywords: pedophilia, fMRI, response inhibition, go/nogo, child sexual abuse, executive functioning
INTRODUCTION
According to the 10th Edition of the International Statistical Classification System of Diseases and Related Health Problems (ICD‐10) [World Health Organization, 1992], pedophilia is defined as a sexual preference characterized by sexual attraction towards prepubertal or early pubertal children. In popular usage, pedophilia is often equated with child molestation, but clinically both phenomena can be distinguished. Empirical investigations identified pedophilia to be a major risk factor for committing sexual offenses against children [Seto et al., 2006]. However, individuals with a diagnosis of pedophilia do not necessarily commit child sexual offenses (CSO), just as most of the persons who sexually offend against children do not fulfill diagnostic criteria for pedophilia [Beier et al., 2015]. In this line, previous studies suggested that only 40–50% of the juridical recorded cases of child sexual offenders are committed by pedophiles [Seto, 2008].
To further the understanding of potential neural dysfunctions underlying pedophilia and/or CSO the assessment of neuroimaging methods may be of high relevance. However, up to now, studies addressing the neurobiological correlates in this regard are scarce and most of them showed inconsistent results [Mohnke et al., 2014; Tenbergen et al., 2015]. The only replicated finding is limited to an amygdala volume reduction in offending pedophiles [Poeppl et al., 2013; Schiffer et al., 2007; Schiltz et al., 2007], although other authors using more stringent type‐1 error correction did not corroborate this result [Cantor et al., 2008; Cantor et al., 2015; Gerwinn et al., 2015b], suggesting insufficient effect sizes of the aberrant amygdala volume. In addition, there are studies reporting evidence for frontocortical neural dysfunction in pedophiles with a history of CSO (P + CSO) as indicated by regionally decreased brain volume [Mendez and Shapira, 2011; Schiffer et al., 2007], impaired neuro‐cognitive functions [Kruger and Schiffer, 2011; Schiffer and Vonlaufen, 2011], as well as altered activity in prefrontal areas comprising the orbitofrontal cortex (OFC) [Habermeyer et al., 2013a], dorsolateral prefrontal cortex (DLPFC) [Schiffer et al., 2008a], and the anterior cingulate cortex (ACC) [Schiffer et al., 2008b] during the processing of sexual stimuli. As normal brain function is highly dependent on appropriate communication within and across neural structures, recent studies investigated the pedophilia related differences in white matter axonal projections to other brain areas, which form the structural basis of connectivity in the brain. In this regard, Cantor et al. [2008, 2015] reported a partial dysconnectivity of regions that were identified to be reliably responsive to visual sexual stimuli [Poeppl et al., 2014; Stoléru et al., 2012]. While another study did not corroborate the latter findings [Gerwinn et al., 2015a], supportive evidence was provided by Poeppl et al. [2015] who identified a set of GM alterations related to offending pedophiles (including the right amygdala, left DLPFC, left insula, bilateral temporoparietal junction and the medial OFC) and demonstrated that these changes were functionally connected to key areas involved in the processing of sexual stimuli, nonsexual emotional functions, as well as in cognitive and executive functions. Differences in the composition of comparison groups regarding both experimental and control groups, mostly small sample sizes, and the usage of deliberate statistical thresholds among previous studies have been identified to be major methodological issues in the discussion of what might explain the contradictory findings. For an exhaustive discussion of this topic, please see Mohnke et al. [2014]. While some studies controlled for the influence of offence status by comparing groups of offending pedophiles with non‐sexual offenders [Cantor et al., 2008, 2015; Poeppl et al., 2011], other studies compared (mixed groups of non‐offending and) offending pedophiles with non‐pedophilic non‐offenders [Habermeyer et al., 2013a, 2013b; Schiffer et al., 2007]. Since none of the previous studies differed between offending and non‐offending groups of pedophiles, it remains largely unclear to what extent their results are related to a deviant sexual preference, i.e., pedophilia or aberrant behavior, i.e., CSO. Supporting the importance of this distinction, we recently reported a pattern of disrupted posterior cingulate cortex (PCC)‐PFC and amygdala‐PFC functional connectivity during resting state that distinguished between offending and non‐offending pedophiles [Kärgel et al., 2015].
Response inhibition, defined as the ability to withhold a pre‐potent response, represents a major aspect of executive function [Bari and Robbins, 2013] and has closely been associated to facets of impulsivity or self control, depending on the observed amount of errors and reaction times. The behavioral and neurobiological aspects of response inhibition have frequently been investigated by using go/nogo paradigms, during which participants are required to respond as quickly as possible to a frequently presented target stimulus and to withhold a pre‐potent response to a rarely presented distractor. Although there is evidence that neural activation related to response inhibition is task‐dependent [Simmonds et al., 2008], the neural network involved in response inhibition commonly include the supplementary motor area (SMA), premotor cortex, inferior parietal cortex, DLPFC, VLPFC, and the insula [Bari and Robbins, 2013; Chikazoe, 2010]. Poor task performance as well as altered activity within the former network has been confirmed by a variety of studies during response inhibition in populations showing impulsive behavior such as attention‐deficit/hyperactivity disorder, borderline personality disorder, antisocial personality disorder or drug abusers [Cubillo et al., 2010; Sebastian et al., 2014; Völlm et al., 2004; van Zutphen et al., 2015]. While non‐pedophilic child sexual offenders were also found to respond more impulsively [Eastvold, 2010; Schiffer and Vonlaufen, 2011], a recent review of the corresponding literature did not corroborate the hypothesis of poor performance on response inhibition in pedophilic offenders [Joyal et al., 2014]. Other recent studies demonstrated slower processing speed across a range of neuropsychological domains in offending pedophiles [Suchy et al., 2014, 2009] and provided evidence that this deficit may be related to fundamental neurocognitive impairments rather than a deliberate response style. A comprehensive overview on the neuropsychology of sex offenders including 23 studies can be found in Joyal et al. [2014]. Accordingly, the authors point to the importance to distinguish between specific subgroups of sex offenders, as for instance on the basis of criminological typologies.
Up until now, there is only one study that addressed the neurobiological correlates of response inhibition in eleven pedophilic offenders from an outpatient treatment facility [Habermeyer et al., 2013b]. By utilizing a blocked go/nogo task during fMRI, Habermeyer et al. reported slower reaction time, less accurate visual target discrimination and attenuated deactivation of the left PCC/precuneus and the angular gyrus during the no‐go condition in pedophiles as compared to seven healthy controls. The finding of inhibition‐related alterations in brain areas which are part of the “Default Mode Network” (DMN) [Buckner et al., 2008] seems noteworthy, suggesting that pedophiles may have problems keeping their attention focused during cognitively challenging tasks. However, since the authors only assessed a small and mixed group of offending and non‐offending pedophiles (with six committing CSO and five consuming child pornography), the differential effect of sexual age preference and offensive behavior on neural correlates of inhibitory control remained unclear.
By using a letter based go/nogo task in combination with fMRI, the present study aimed at disentangling behavioral and neural characteristics of response inhibition processes associated with pedophilia or CSO. Although the consumption of child pornography is generally regarded as an offence, here we focused on hands‐on sexual contacts with children when referring to the offender status of a participant, which reflects a more severe and direct type of child sexual abuse and is of particular interest in the assessment of response inhibition. We therefore compared three groups of men: (1) pedophiles with a history of hands‐on sexual offending against children (P + CSO), (2) pedophiles who did not engage in hands‐on child sexual offending (P‐CSO), and (3) healthy controls without any criminal conviction or paraphilia (HC). Up until now, there is a lack of studies comparing the ability to inhibit pre‐potent response tendencies in pedophiles who engaged or not engaged in child sexual offending. Since only the latter group is characterized by the ability to avoid hands‐on child sexual offences, we expected to find a pattern of increased inhibitory control in non‐offending pedophiles as compared to offending pedophiles, as indicated by the number of commission errors. According to the finding that the VMPFC plays a critical role in inhibitory control [Bari and Robbins, 2013; Chikazoe, 2010] and, moreover, was reported to be altered in offending pedophiles [Kruger and Schiffer, 2011; Mendez and Shapira, 2011; Schiffer et al., 2007; Schiffer and Vonlaufen, 2011], we subsequently hypothesized that P + CSO as compared to both non‐offending groups would show different BOLD signal change during response inhibition in related medial prefrontal areas. Similarly, in P + CSO we expected aberrant BOLD activity in medial parietal regions [Habermeyer et al., 2013b; Kärgel et al., 2015] when compared to P‐CSO and HC.
METHODS
Participants
Participants of the present study were assessed within the framework of a German multi‐site research project called “Neural Mechanisms Underlying Pedophilia and Sexual Offending Against Children” (NeMUP; http://www.nemup.de), which comprises five collaborative research sites from the field of (forensic) psychiatry or sexual medicine located in Berlin, Bochum/Essen, Hanover, Kiel, and Magdeburg. Recruitment was carried out by providing study advertisement/information in diverse internet platforms and some correctional services in Germany. From the total NeMUP sample described elsewhere [Gerwinn et al., 2015b], fMRI data of a total of 117 male subjects matched with respect to age and IQ were analyzed. Accordingly, 77 subjects met ICD‐10 diagnostic criteria for pedophilia. Thirty‐seven pedophiles without a history of sexual offending against children (P‐CSO) were recruited from the community (n = 20) or the “Prevention Project Dunkelfeld” (PPD) [Beier et al., 2009] (n = 17). Forty pedophiles with a history of sexual offenses against children (P + CSO) were taken from the community (n = 16), and the PPD (n = 9), and from correctional institutions (n = 15). While four participants from the P + CSO group had been sentenced to probation, 11 participants were incarcerated serving a custodial sentence due to convictions for sexual offences against children. Each site contributed the following number of subjects. Berlin: P + CSO = 8, P‐CSO = 5, HC = 6; Bochum/Essen: P + CSO = 20, P‐CSO = 10, HC = 14; Hanover: P + CSO = 4, P‐CSO = 7, HC = 4; Kiel: P + CSO = 8, P‐CSO = 15, HC = 16. A history of CSO was defined as the individual involvement in at least one case of sexual offenses against children under the age of 14, which includes actions of touching or manipulating the child's naked body and/or genitals with the aim of sexually stimulating himself, penetrating the child anally/vaginally, or making the child touch or manipulate the offender's genitals or penetrating him. Exclusion criteria were neurological or acute psychiatric disorders other than pedophilia, acute episodes of alcohol or drug abuse/dependence, as well as past dependencies (past episodes of substance misuse were included), and current medication related to sexual functioning. None of the participants took psychotropic medication other than antidepressants for a period of at least 3 weeks before assessment. Antidepressant medication was evident in 1 P + CSO (SSNRI), 2 P‐CSO (SSRI, NDRI), and 2 healthy controls (SSNRI, anti‐convulsive). The healthy control group recruited from the community throughout advertisements in public institutions included 40 men without a history of criminal behavior or current psychiatric disorders. Among the subjects recruited in Bochum/Essen, 9 P + CSO, 5 P‐CSO, and 8 HC were also participants in another study [Kärgel et al., 2015]. The study was approved by the ethics committee of each research site separately and all participants gave written informed consent to the study protocol before being included.
Assessment
Data were acquired in two separate sessions distributed over 2 days. Initially (first session), the Structured Clinical Interview for the DSM (SCID) [Wittchen et al., 1997] accompanied by a semi‐structured clinical interview were completed to assess for DSM‐IV‐TR [American Psychiatric Association, 2000] Axis I and II disorders, as well as sexual age, gender, and interaction interest(s), child pornography consumption or offence history in general. Sexual gender and age orientation was confirmed by means of a modified version of the Kinsey scale for developmental stages [Kirk et al., 2000]. In cases of uncertainty regarding the sexual age orientation, a viewing‐time paradigm, legal information (if available) and individual case conferences were utilized to ensure valid clinical diagnoses. Global intelligence was estimated by means of 4 subtests (‘Similarities’, ‘Vocabulary’, ‘Block Design’, and ‘Matrix Reasoning’) derived from the German version of the Wechsler Adult Intelligence Scale, 4th Edition (WAIS‐IV) [Von Aster et al., 2006]. All assessments were carried out through experienced research associates, trained to use these instruments. In a second session, the MRI assessment was performed.
Go/Nogo Task
As part of a more extensive MRI examination, participants performed on an event‐related go/nogo task that was preceded by an alertness task comprised of go‐trials only. The latter task was implemented to allow the participants to familiarize themselves with the MRI environment and to provide a baseline measure for reaction times in response to simple target stimuli. The projection screen was localized in front (Hanover) or behind the magnet bore (Essen, Berlin, Kiel). Initially, an instruction screen was presented which informed the participants to respond as fast as possible to any of the presented alertness stimuli indicated by the letter “X”. After reading the instructions, the stimulus presentation of 50 alertness trials was initiated throughout an individual response. This condition was followed by a second instruction‐screen introducing the go/nogo condition comprised of 150 trials. Participants were required to respond as fast as possible to the frequently (80% of trials) presented go‐trials (indicated by the letter “X”) but were instructed to withhold their responses to the infrequently (20% of trials) occurring nogo‐trials (indicated by a “+” sign). Stimuli were presented using the Presentation® software package (Neurobehavioral Systems) and displayed in a pseudo‐randomized order, precluding the occurrence of two consecutively presented no‐go stimuli. Each stimulus was presented for 200 ms with an inter‐stimulus‐interval (ISI) of 1,500–2,500 milliseconds (ms). Prior to scanning, participants were instructed to respond with the right forefinger on the response box. To provide a behavioral measure of response inhibition, the amount of commission errors of nogo‐trials, and reaction times (RTs) to alertness as well as to go‐trials were used for further statistical analysis. Extreme‐values that differed more than threefold from the distance between the 25% and 75% percentile of the distribution were excluded from further analysis. Visual impairments were corrected by an MR compatible goggle system.
Imaging Parameters and Processing
All images were acquired on 5 separate 3T MRI scanners equipped with 32 channel head coils: 2 x Siemens Skyra, 2 x Siemens Trio and 1 x Phillips Achiva. T2* weighted images were obtained using an echo planar imaging (EPI) sequence (slices = 38, field of view = 240 mm, voxel size = 2.3 × 2.3 × 3 mm, time of repetition = 2,400 ms, echo time 30 ms, flip angle = 80°, distance factor = 10%). T1 images were acquired by means of a MPRAGE sequence (slices = 192, FoV = 256 mm, voxel size = 1 × 1 × 1 mm, TR = 2,500 ms, TE = 4.37 ms, flip angle = 7°, distance factor = 50%). To preclude signal fluctuations across all sites, standardized MRI Phantom stability measures were conducted [Hellerbach et al., 2013]. Statistical Parametric Mapping software (SPM8, Welcome Department of Cognitive Neurology, London, UK) was used for functional imaging analysis. Initially, the first 4 images were discarded to account for T1 relaxation effects. Prior to statistical examination, functional volumes were (1) slice time corrected using the middle slice as reference, (2) realigned and unwarped, (3) co‐registered to the according T1 image, (4) spatially normalized into Montreal Neurological Institute (MNI) space utilizing the individual T1 image, and (5) smoothed with an isotropic Gaussian full width half maximum kernel of 8 mm.
Statistical Analysis
Behavioral analysis. SPSS v 22 (IBM Inc.) was used for statistical analysis. First, RTs to alertness and go‐trials were submitted to a group by condition repeated measures ANCOVA, to assess for behavioral effects of cognitive control. Second, univariate ANCOVA was used to investigate between‐group effects related to response inhibition indicated by the amount and reaction times of commission errors. Post‐error‐slowing was calculated, which is the reaction time differences between reactions times on correct go trials following commission errors as compared to reactions times on correct go trials following correct nogo trials. To quantify the ability of signal detection, we computed the parameter d‐prime, i.e., the difference between the z‐transformed proportion of hits (correct responses to go stimuli) and false alarms (failure to withhold a response to nogo stimuli) according to the formula d‐prime = z(hit‐rate)‐z(false alarm‐rate) [Ames et al., 2014]. Age and IQ‐scores were treated as covariates of no interest. Post‐hoc group analyses were corrected for multiple comparisons using the Bonferroni method.
fMRI Analysis
Functional volumes were analyzed using SPM8. For first‐level analysis, the following events were included as regressors in the design matrix: correct responses to (1) alertness‐ and (2) go‐trials, (3) successfully inhibited responses to nogo‐trials, and (4) false responses to nogo‐trials (commission errors). Event‐related responses were convolved with the canonical hemodynamic response function (HRF) and separate linear contrast images (vectors for each condition) were built. For second‐level random effects analysis, contrast images were submitted to a group (P + CSO, P‐CSO, HC) by condition (go‐trials, nogo‐trials) full factorial design including scanner site, age, sexual gender orientation and WAIS scores as covariates of no interest. To control for potential amplitude bias effects, i.e., when participants vary in their HRF peak latency, we also performed the whole analysis following the approach of Calhoun et al. [2004]. Accordingly, for each regressor at the first‐level the temporal derivative of this function was included, resulting in separate linear contrast images (vectors for each condition plus their temporal derivatives). Then, second‐level random effects analysis was performed using the non‐linear combinations of the effect regressors and their corresponding temporal derivatives in a full factorial design.
All functional images were filtered by means of a 128 seconds high‐pass filter to remove slow signal drifts. Statistical maps were computed to investigate the (1) within‐group main effect of task (nogo > go, go > nogo) and (2) group by condition interaction effects of response inhibition (nogo > go). Comparisons of fMRI volumes were computed within the framework of the general linear model. Because of differences in the applied statistical threshold of previous studies utilizing similar letter based go/nogo tasks [Simmonds et al., 2008], for all fMRI analyses in the present study we used a very conservative height threshold of P < 0.05 corrected for multiple comparisons after family‐wise error (FWE) in a whole brain analyses, to minimize false positive results as strictly as possible. Contrast estimates deriving from 3 mm spheres around the significant peak voxels of the group by condition analysis were used for correlation analysis with task‐related performance measures (RT alertness‐trials, RT go‐trials, commission errors nogo‐trials).
RESULTS
Characteristics of Study Groups
As shown in Table 1, there were no significant differences between study groups with respect to age, sexual gender orientation, handedness, and WAIS IQ score. Regarding prevalence of mental and personality disorders, there were no significant differences between both pedophilic groups (overall personality disorders: X2 1,75 = 0.57, P = 0.451; overall mental disorders: ,75 = 1.50, P = 0.221) but both pedophilic groups yielded higher rates then did healthy controls. Moreover, both pedophilic groups did not differ according to child pornography consumption (lifetime) and the ratio of exclusive/non‐exclusive type of pedophilia. Details on clinical diagnoses are provided in Table 1.
Table 1.
Characteristics of study participants and go/nogo task performance
| P+CSO | P‐CSO | HC | Statistics (P‐value) | |
|---|---|---|---|---|
| Characteristics | ||||
| Age, M(SD), range | 38.25 (8.54), 20–53 | 37.00 (8.84), 23–57 | 36.65 (10.13), 20–57 | F 2,114 = 0.32 (0.718) |
| hetero‐/homosexual | 22/18 | 27/10 | 26/14 | X2 2,114 = 2.72 (0.257) |
| EHI Index M(SD) | 67.25 (49.27) | 60.68 (55.10) | 69.75 (51.13) | X2 2,114 = 0.31 (0.733) |
| WAIS IQ estimates (SD) | 98 (18.87) | 105 (16.65) | 106 (18.22) | F 2,114 = 2.25 (0.110) |
| CPC lifetime | 31 (77.5%) | 25 (67.5%) | / | X2 1,75 = 0.96 (0.328) |
| Exclusive/non‐exclusive type | 17/23 | 10/27 | / | X2 1,75 = 2.02 (0.155) |
| Victims age, M(SD) | 10.04 (2.74) | / | / | / |
| DSM‐IV mental disorders (lifetime) | 25 | 18 | 11 | X2 2,114 = 9.99 (0.007)* |
| Depression | 17 | 15 | 3 | X2 2,114 = 14.60 (<0.001)** |
| Anxiety disorder | 7 | 8 | 6 | X2 2,114 = 0.58 (0.748) |
| Substance use disorder | 10 | 4 | 1 | X2 2,114 = 9.25 (0.010)* |
| Eating disorder | 2 | 0 | 0 | X2 2,114 = 3.92 (0.141) |
| Somatoform disorder | 1 | 3 | 0 | X2 2,114 = 3.98 (0.137) |
| Dissociative disorder | 0 | 0 | 1 | X2 2,114 = 1.94 (0.379) |
| Adaption disorder | 0 | 1 | 0 | X2 2,114 = 2.18 (0.336) |
| DSM‐IV personality disorders | 17 | 13 | 1 | X2 2,114 = 18.99 (<0.001)** |
| Antisocial PD | 3 | 0 | 0 | X2 2,114 = 5.92 (0.052)* |
| Avoidant PD | 7 | 7 | 0 | X2 2,114 = 8.30 (0.016) |
| Borderline PD | 4 | 0 | 0 | X2 2,114 = 8.00 (0.019) |
| Obsessive‐compulsive PD | 2 | 6 | 1 | X2 2,114 = 5.73 (0.057) |
| Narcissistic PD | 2 | 0 | 0 | X2 2,114 = 3.91 (0.141) |
| Paranoid PD | 1 | 0 | 0 | X2 2,114 = 1.94 (0.379) |
| Conduct disorder | 0 | 1 | 1 | X2 2,114 = 1.06 (0.588) |
| Go/nogo task performance | ||||
| RT Alertness, M(SD) | 252.50 (24.43) | 257.54 (35.71) | 257.53 (29.92) | F 2,110 = 0.63 (0.533) |
| RT Go, M(SD) | 393.04 (52.20) | 385.01 (52.63) | 383.83 (57.97) | F 2,113 = 0.18 (0.982) |
| Commission errors nogo, M(SD) | 7.78 (5.16) | 5.56 (2.90) | 7.4 (4.16) | F 2,111 = 3.25 (0.042)* |
| RT commission errors nogo, M(SD) | 327.66 (42.81) | 323.98 (43.36) | 335.39 (64.92) | F 2,114 = 0.75 (0.474) |
| RT post‐error slowing, M(SD) | 58.29 (61.67) | 50.99 (58.41) | 36.98 (71.23) | F 2,113 = 0.53 (0.592) |
| d‐prime, M(SD) | 2.94 (.83) | 3.09 (.69) | 2.88 (.71) | F 2,113 = 0.75 (0.476) |
Note: EHI = Edinburgh Handedness Inventory, WAIS = Wechsler Adult Intelligence Scale, CPC = child pornography consume, PD = personality disorder, RT = reaction time in milliseconds, P + CSO = pedophiles who engaged in child sexual abuse, P‐CSO = pedophiles who did not engage child sexual abuse, HC = healthy control group, * P < 0.05, ** P < 0.001.
Within‐group Pearsońs correlations were calculated between age and WAIS IQ score and behavioral performance (reaction times to go‐trials, commission errors, and errors to go‐trials). In the P + CSO group, WAIS IQ score was found to be negatively correlated with errors to go‐trials (r = −0.411, P = 0.008), i.e., the higher the IQ estimates, the less errors to go‐trials. Similarly, in the P‐CSO group WAIS IQ score was (marginally) negatively associated with errors to go‐trials (r = −0.322, P = 0.056). In healthy controls, age was positively related to reaction time to go‐trials (r = 0.395, P = 0.012) and commission errors (r = −0.376, P = 0.017), i.e., the older the controls are, the longer their reaction times and the more errors were produced. WAIS IQ score was negatively correlated with reaction time to go‐trials (r = −0.333, P = 0.036), i.e., higher IQ estimates were related to accelerated reaction times. There were no further correlations and none of the reported findings remained significant after Bonferroni correction.
Behavioral Results
As depicted in Figure 1a,b, there was a main effect of condition showing increased RTs in go as compared to alertness trials (F 2,114 = 565.08; P < 0.001), but no significant group‐by‐condition interaction effect, indicating no between‐group difference in any RT measure. However, as shown in Figure 1c, error analyses revealed a significant main effect of group (F 2,111 = 3.25; P = 0.042) indicating better inhibitory control in P‐CSO as compared to P + CSO (mean difference = −2.48 (SE = 1.00); P = 0.045), while the difference between P‐CSO and HC (MD = −1.82 (0.98); P = 0.190) as well as P + CSO and HC (MD = 0.65 (0.96); P > 0.999) failed to reach statistical significance. Groups did not significantly differ in post error slowing, d‐prime, and reaction times to commission errors. For details see Table 1.
Figure 1.
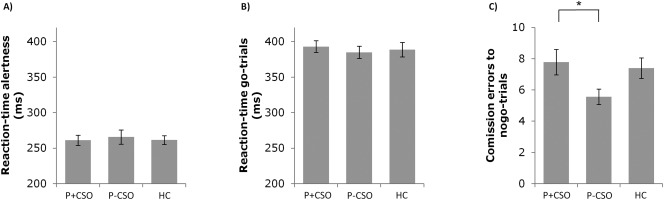
Behavioral variables of the go/nogo task. (A) Group specific reaction times in milliseconds and standard errors (SE) to alertness trials prior to the go/nogo task. (B) Group specific reaction times in milliseconds and SE to go‐trials. (C) Amount of commission errors to nogo‐trials and SE separately for each group: * = significant between‐group effect (post‐hoc), Bonferroni corrected.
fMRI Results
Analysis of the fMRI data was performed using two different approaches: a common approach were the event‐related responses were convolved with the canonical HRF only and another approach utilizing the canonical HRF plus the corresponding temporal derivative term to control for potential amplitude bias effects. In the following, we report the statistics from the common SPM analysis and it is indicated when any significant differences between both approaches were obtained.
As expected, main effect of task (nogo > go condition) revealed changes in BOLD responses in bilateral fronto‐parietal areas including the inferior parietal cortex, middle frontal cortex, PCC, medial frontal cortex including the ACC and supplementary motor cortex, middle temporal cortex, anterior insula, superior frontal cortex as well as the DLPFC and the left middle occipital cortex. For a detailed overview see Table 2 and Figure 2.
Table 2.
Brain regions showing significant inhibition‐related activity
| Contrast | Structure | H | BA | MNI (x,y,z) | Cluster | Statistics | ||
|---|---|---|---|---|---|---|---|---|
| main effect of condition | t‐value | |||||||
| nogo > go | inf parietal cortex | L | 40 | −50 | −57 | 49 | 1015 | 8.99 |
| mid temporal gyrus | L | 39 | −57 | −29 | −11 | 6.90 | ||
| inf parietal cortex | R | 40 | 51 | −52 | 52 | 1638 | 8.74 | |
| mid temporal cortex | R | 21 | 62 | −32 | −2 | 6.46 | ||
| ant Insula | R | 47 | 32 | 19 | −14 | 238 | 8.29 | |
| mid frontal cortex | L | 8 | −41 | 26 | 43 | 277 | 7.80 | |
| mid frontal cortex | R | 8 | 46 | 24 | 46 | 1378 | 7.52 | |
| DLPFC | R | 9 | 30 | 51 | 34 | 6.31 | ||
| suppl motor cortex | R | 6 | 5 | 10 | 61 | 5.72 | ||
| DLPFC | L | 9 | −25 | 54 | 34 | 129 | 7.19 | |
| ant Insula | L | 47 | −32 | 17 | −20 | 33 | 6.77 | |
| PCC | R | 24 | 5 | −22 | 34 | 56 | 5.94 | |
| ACC | R | 32 | 3 | 37 | 22 | 86 | 5.92 | |
| mid occipital cortex | L | 19 | −43 | −71 | −8 | 90 | 5.60 | |
| fusiform gyrus | L | 37 | −37 | −55 | −14 | 31 | 5.59 | |
| inf occipital cortex | R | 19 | 35 | −78 | −5 | 19 | 5.28 | |
| go > nogo | no region | |||||||
| group by condition interaction | F‐value | |||||||
| sup frontal cortex | L | 8 | −20 | 24 | 43 | 3 | 14.78 | |
| post‐hoc comparisons | t‐value | |||||||
| P‐CSO > P+CSO: | ||||||||
| nogo > go | PCC | L | 23 | −7 | −45 | 25 | 9 | 4.95 |
| PCC | L | 31 | −2 | −61 | 28 | 9 | 4.92 | |
| sup frontal cortex | L | 8 | −20 | 24 | 43 | 3 | 5.07 | |
| P+CSO > P‐CSO | no region | |||||||
| HC >|< P+CSO | no region | |||||||
| HC >|< P‐CSO | no region | |||||||
Note: H = Hemsiphere; ant = anterior, suppl = supplementary, sup = superior, inf = inferior, mid = middle, ACC = anterior cingulated, PCC = posterior cingulated cortex, DLPFC = dorsolateral prefrontal cortex, MNI = Montreal neurological institute, L = left, R = right, P + CSO = pedophiles who engaged in child sexual abuse, P‐CSO = pedophiles who did not engage child sexual abuse, HC = healthy control group, BA = Brodmann Area. All results were significant at P ≤ 0.05 corrected for family‐wise error (height threshold).
Figure 2.
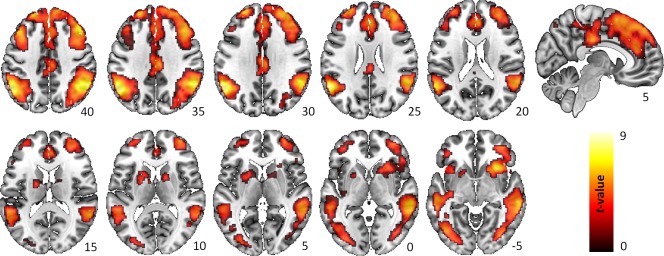
Main effect of response inhibition. Within group BOLD activations related to the effect of response inhibition in the contrast nogo > go. The statistical threshold map was set to P < 0.001 uncorrected (voxel level) and P < 0.05 FWE corrected (cluster level) for illustrative purposes. [Color figure can be viewed at http://wileyonlinelibrary.com]
There was a significant group‐by‐condition interaction effect (peak‐voxel at MNI −20, 24, 43, BA 8, cluster = 3 voxel, F‐value = 14.78). Using the temporal derivative approach, the peak voxel of this interaction effect moved to MNI −7, −45, 25, (BA 23, cluster = 9 voxel, F‐value = 13.66).
As illustrated in Figure 3, post‐hoc comparisons of the contrast P‐CSO > P + CSO revealed increased activation in the left PCC (MNI −7, −45, 25, BA 23, cluster = 9 voxel, t‐value = 4.95; MNI −2, −61, 28, BA 31, cluster = 9 voxel, t‐value = 4.92) and left superior frontal cortex (SFC) (MNI −20, 24, 43, BA 8, cluster = 3 voxel, t‐value = 5.07) during response inhibition (nogo > go‐trials). Notably, the latter effect in the SFC did not remain significant using the temporal derivative approach. No other between‐group comparisons (P + CSO > P‐CSO; HC P + CSO; HC P‐CSO) revealed statistically significant findings. The same was true for the group comparisons regarding the contrasts commission errors vs. baseline and commission errors vs. correct omitted nogo trials.
Figure 3.
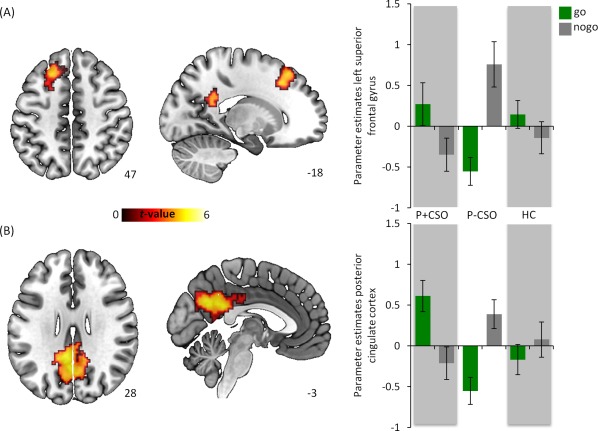
Between group fMRI analysis. Post‐hoc examination of the nogo > go contrast revealed a significantly increased activation in the (A) PCC (MNI x = −2, y = −59, z = 31) and (B) left superior frontal gyrus (MNI x = −20, y = 24, z = 43) in the P‐CSO group as compared to the P + CSO group. Parameter estimates and standard errors derived from 3mm spheres around the according peak voxels. The statistical threshold map was set to P < 0.001 uncorrected (height threshold) and P < 0.05 FWE corrected (extent threshold) for illustrative purposes. [Color figure can be viewed at http://wileyonlinelibrary.com]
The same was true for the correlation analysis. We did not find any significant association between behavioral performance and BOLD responses, neither across groups nor within groups.
DISCUSSION
This is the first study using event related fMRI in combination with a behavioral go/nogo paradigm, to analyze inhibitory control capacity and underlying inhibition‐related neural activation pattern with respect to pedophilia and/or sexual offending against children.
As compared to offending pedophiles, non‐offending pedophiles exhibited superior inhibitory control as reflected by a significantly lower rate of commission errors. In line with previous fMRI research [Blasi et al., 2006; Bari and Robbins, 2013; Simmonds et al., 2008], response inhibition led to stronger recruitment of a fronto‐parietal control network (FPCN). Congruent with our hypothesis, offending pedophiles revealed decreased activation of the medial parietal cortex including the left caudal PCC as well as the left SFC as compared to non‐offending pedophiles, but contrary to our hypotheses, showed no activation difference in prefrontal areas. Also as expected, non‐offending pedophiles and healthy controls did not differ with respect to any measure, and differences between offending pedophiles and healthy controls also did not reach significance.
Previous studies provided no clear evidence for the interpretation of increased impulsivity in pedophiles [Joyal et al., 2014]. More specifically, during the assessment of a letter based go/nogo task, increased frequencies of commission errors were documented in non‐pedophilic but only marginally in pedophilic child sexual offenders [Schiffer and Vonlaufen, 2011], while Habermeyer et al. [2013b] reported augmented amount of commission errors in pedophilic offenders relative to controls. Both former studies (and nearly all other studies in the field) included only mixed groups of offending pedophiles, thus precluding researchers from assessing whether differences relate to sexual preference, i.e., pedophilia or sexual deviant behavior, i.e., CSO. In contrast, in the current study, we had the very scarce opportunity to distinguish between offending and non‐offending pedophiles, which allowed for a more in depth analysis of the question, whether impulsivity relates to CSO rather than pedophilia. In the present study, non‐offending as compared to offending pedophiles revealed significantly decreased commission errors but both pedophilic groups did not differ from healthy controls. Accordingly, our results point to the interpretation of more elaborated self‐control abilities in pedophiles who did not act on their sexual preference, rather than a more impulsive response style in offending pedophiles.
The absence of executive abnormalities in the present group of offending pedophiles is in contrast to other studies [Suchy et al., 2014, 2009]. An explanation for this inconsistency might be related to the status of incarceration, which is likely to be associated with diverse deprivation effects [Lapornik et al., 1996] and could at least partly be responsible for executive deficiencies in previous (incarcerated) samples of offending pedophiles. However, in contrast to almost all previous studies, only every fourth offender in the current study was incarcerated at the time of assessment, while the majority of them were recruited from the community or the PPD, respectively. None of the aforementioned studies controlled for the effect of incarceration, so we assume that our more naturalistic and therefore less confounded sample reflect a more realistic picture of executive functioning in pedophilic offenders than did previous studies.
Although the PCC has not been reported consistently in the context of response inhibition, previous fMRI studies demonstrated aberrant PCC activity in clinical populations showing inhibitory control deficits, as for instance in ADHD [Rubia et al., 2005] or Williams Syndrome [Mobbs et al., 2007]. Steele and colleagues [2014], who assessed a go/nogo task in a large sample of healthy participants, proposed a rather indirect association between the PCC and response inhibition, since this region has been related to consciousness, episodic memory, and self‐referential mental processes and therefore was thought to play an important role in the interplay of various neural networks [Leech and Sharp, 2014]. The PCC has been reported as a key structure of the DMN, showing highly correlated activity during rest and reactive deactivation when attention is directed externally [Gusnard et al., 2001; Raichle et al., 2001]. The magnitude of hemodynamic responses in the PCC have been found to be associated with cognitive load in the healthy brain, while the inability of appropriate deactivation is related to disturbed cognitive function in the healthy as well as in the damaged brain [Leech and Sharp, 2014]. Moreover, aberrant PCC responses were reported in pedophile offenders during rest [Kärgel et al., 2015] as well as during the processing of preferred sexual stimuli [Poeppl et al., 2011].
Our finding of inhibition‐related PCC activation in a pedophilic sub‐sample is largely in line with previous findings by Habermeyer et al. [2013b], who proposed an attenuated deactivation of the DMN in pedophiles during the cognitive demanding nogo condition. Consequently, they suggested that pedophiles might be involved in self‐referential mental activities more than in applying task‐related cognitive control. However, since the authors assessed a mixed group of offending pedophiles, it is not possible to conclude from this study whether this activation pattern relates to pedophilia or CSO.
In contrast to the DMN, enhanced BOLD activity has been observed in the FPCN during cognitive demanding tasks where attention is directed to external information [Leech and Sharp, 2014]. It is suggested that neural responses of both networks are tightly coupled and exhibit an anti‐correlated activity/connectivity pattern [Kelly et al., 2008; Leech and Sharp, 2014]. In the present study no differential PCC activity was observed between both conditions (nogo > go) in healthy controls. Notably, during the cognitively more demanding nogo‐trials offending pedophiles exhibited decreased activity in the PCC as compared to non‐offending pedophiles, whereas an opposite response pattern was found during the processing of go‐trials. Following the idea that the PCC plays a central role in linking the abovementioned neural networks thus allowing for efficient cognitive function, our results might indicate dysfunctional or diminished engagement of the FPCN in pedophilic offenders during cognitive demanding tasks on the one hand and inappropriately increased FPCN involvement during the less challenging cognitive tasks on the other hand. By contrast, pedophiles who did not engage in CSO seem to show a neural mechanism with probably compensatory function. This mechanism that also utilizes parts of the PCC, results in an appropriate inhibition‐related activation of the FPCN (i.e., during the nogo condition). This notion is strongly supported by the fact that the left SFC, which is also part of the FPCN [Niendam et al., 2012] showed a similar response pattern. The functional significance of the SFC is thought to be related to executive functioning, particularly to working memory function [du Boisgueheneuc et al., 2006; Rowe et al., 2000]. This domain, which comprises an essential aspect of response inhibition allows an individual to appropriately maintain internal representations of task goals. The hypothesis that “compensatory” engagement of the FPCN might help preventing pedophiles from sexual offending against children is quite speculative, but warrants further investigation. Interestingly, our findings of neurofunctional group differences in neural structures essential for efficient cognitive functioning may support the idea of a general processing deficit in offending pedophiles as proposed by Suchy et al. [2014], which, however, seems to be related to sexual offending behavior rather than aberrant sexual interests in general.
Disinhibition has been discussed as a predictor of sexual offending within diverse theories, i.e., the self‐regulation model [Ward et al., 1998] or the motivation‐facilitation model [Seto, 2008]. It is suggested that only individuals who can overcome their inhibitions, e.g., worries about potential negative consequences on the victim, or fear to violate against social norms, would show an increased propensity to sexually approach victims. Although disinhibition has been reported a relevant risk factor for sexual recidivism in general [Hanson and Morton‐Bourgon, 2005; Mann et al., 2010], in line with previous research on personality traits [Cohen and Galynker, 2012] and cognitive functioning in pedophilia [Schiffer and Vonlaufen, 2011], our findings indicate that response inhibition abilities are not reduced in pedophilia per se. The present study rather extends the existing literature by providing some evidence that in pedophilia the ability not to offend against children might be associated with an elevated level of inhibitory control as well as an increased inhibition‐related recruitment of the FPCN.
Strengths and limitations
Inconsistent localization of stimulus projection screen and unbalanced sample distribution across fMRI scanners may contribute to a potential site confound of our findings. Thus, a control analysis was conducted including N = 87 controls (n = 24 Berlin; n = 27 Essen; n = 15 Hanover; n = 21 Kiel) to compare them with regard to percent signal change (PSC) values extracted from relevant structures associated with response inhibition. Our results demonstrate that PSC values taken from specific ROIs including the ACC, bilateral Insula, and inferior parietal cortex do not systematically differ across sites. Accordingly, and moreover due to the observation that inclusion vs. exclusion of site as a covariate did not significantly influence the present results, a site confound is rather unlikely to account for the findings at hand. The recruitment of pedophiles with a history of sexual offending against children from community and the PPD represented a principle strength of the present investigation, however, to conclusively disentangle the response inhibition related correlates of pedophilia from those associated with sexual offending against children, the inclusion of a non‐pedophilic CSO group would have been an important addition. Another limitation was inherent to the proportions of participants suffering from (other) mental or personality disorders. Both pedophilic groups showed higher rates of both mental and personality disorders than did healthy controls. Nevertheless, since the effects that we reported here were restricted to differences between both pedophilic groups, and both pedophilic groups were similar with respect to number of patients suffering from other mental or personality disorders, this limitation could not have affected the findings discussed here.
CONCLUSIONS
Taken together, our results suggest that (1) pedophiles without a history of CSO may have increased self‐control abilities, (2) pedophiles in general are not likely to be more impulsive than controls, and (3) pedophiles who did not commit hands‐on offenses against children might deploy a neural compensatory mechanism involving parts of the FPCN facilitating efficient response inhibition or cognitive control in general. Our present fMRI study, of course, cannot fully explain the discrepant findings of previous investigations assessing for the neurobiological correlates of pedophilia and CSO but, nonetheless, might provide essential methodological indications reflecting the importance to separate pedophilic hands‐on offenders from those that have not sexually offended against children. The finding that groups differed more according to their deviant sexual behavior than according to their sexual preference pattern may represent an important shift in how these groups are considered. From a clinical point of view, the present data thus indicate that intervention strategies aimed at fostering basic inhibitory control abilities might be useful for preventing CSO in both pedophiles who already engaged in CSO as well as those at risk.
ROLE OF FUNDING SOURCE
This work was funded by grants from the German Research Foundation (DFG): Schi 1034/3‐1 to BS & THCK and from the Federal Ministry of Education and Research (BMBF) : 01KR1205 to BS, MW, HW, THCK, JP & KB.
DISCLOSURE OF BIOMEDICAL FINANCIAL INTERESTS AND POTENTIAL CONFLICTS OF INTEREST
All authors stated no conflict of interest
ACKNOWLEDGEMENTS
The authors thank Nils Vahle for his assistance with the fMRI recordings and technical support.
Contributor Information
Christian Kärgel, Email: christian.kaergel@uni-due.
Boris Schiffer, Email: deboris.schiffer@rub.de.
REFERENCES
- American Psychiatric Association (2000): Diagnostic and Statistical Manual of Mental Disorders: DSM‐IV‐TR. Washington, DC: American Psychiatric Association. [Google Scholar]
- Ames SL, Wong SW, Bechara A, Cappelli C, Dust M, Grenard JL, Stacy AW (2014): Neural correlates of a Go/NoGo task with alcohol stimuli in light and heavy young drinkers. Behavi Brain Res 274:382–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari A, Robbins TW (2013): Inhibition and impulsivity: Behavioral and neural basis of response control. Prog Neurobiol 108:44–79. [DOI] [PubMed] [Google Scholar]
- Beier KM, Ahlers CJ, Goecker D, Neutze J, Mundt IA, Hupp E, Schaefer GA (2009): Can pedophiles be reached for primary prevention of child sexual abuse? First results of the Berlin Prevention Project Dunkelfeld (PPD). J Foren Psychi Psych 20:851–867. [Google Scholar]
- Beier KM, Grundmann D, Kuhle LF, Scherner G, Konrad A, Amelung T (2015): The German Dunkelfeld project: A pilot study to prevent child sexual abuse and the use of child abusive images. J Sex Med 12:529–542. [DOI] [PubMed] [Google Scholar]
- Blasi G, Goldberg TE, Weickert T, Das S, Kohn P, Zoltick B, Bertolino A, Callicott JH, Weinberger DR, Mattay VS (2006): Brain regions underlying response inhibition and interference monitoring and suppression. Eur J Neurosci 23:1658–1664. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews‐Hanna JR, Schacter DL (2008): The brain's default network: Anatomy, function, and relevance to disease. Ann N Y Acad Sci 1124:1–38. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Stevens MC, Pearlson GD, Kiehl KA (2004): fMRI analysis with the general linear model: Removal of latency‐induced amplitude bias by incorporation of hemodynamic derivative terms. NeuroImage 22:252–257. [DOI] [PubMed] [Google Scholar]
- Cantor JM, Kabani N, Christensen BK, Zipursky RB, Barbaree HE, Dickey R, Klassen PE, Mikulis DJ, Kuban ME, Blak T, Richards BA, Hanratty MK, Blanchard R (2008): Cerebral white matter deficiencies in pedophilic men. J Psychiatr Res 42:167–183. [DOI] [PubMed] [Google Scholar]
- Cantor, J.M. , Lafaille, S. , Soh, D.W. , Moayedi, M. , Mikulis, D.J. , Girard, T.A. (2015) Diffusion Tensor Imaging of pedophilia. Arch Sex Behav 44:2161. [DOI] [PubMed]
- Chikazoe J (2010): Localizing performance of go/no‐go tasks to prefrontal cortical subregions. Curr Opin Psychiatry 23:267–272. [DOI] [PubMed] [Google Scholar]
- Cohen LJ, Galynker I (2012): Identifying psychological traits potentially subserving aberrant motivation or inhibitory failure in pedophilic behavior. Isr J Psychiatry Relat Sci 49:280–290. [PubMed] [Google Scholar]
- Cubillo A, Halari R, Ecker C, Giampietro V, Taylor E, Rubia K (2010): Reduced activation and inter‐regional functional connectivity of fronto‐striatal networks in adults with childhood Attention‐Deficit Hyperactivity Disorder (ADHD) and persisting symptoms during tasks of motor inhibition and cognitive switching. J Psychiatr Res 44:629–639. [DOI] [PubMed] [Google Scholar]
- du Boisgueheneuc F, Levy R, Volle E, Seassau M, Duffau H, Kinkingnehun S, Samson Y, Zhang S, Dubois B (2006): Functions of the left superior frontal gyrus in humans: A lesion study. Brain 129:3315–3328. [DOI] [PubMed] [Google Scholar]
- A Eastvold, Y Suchy, D Strassberg (2011): Executive function profiles of pedophilic and nonpedophilic child molesters. JINS 17:295–307. [DOI] [PubMed] [Google Scholar]
- Gerwinn H, Pohl A, Granert O, van Eimeren T, Wolff S, Jansen O, Deuschl G, Huchzermeier C, Stirn A, Siebner HR, Ponseti J (2015a): The (in)consistency of changes in brain macrostructure in male paedophiles: A combined T1‐weighted and diffusion tensor imaging study. J Psychiatr Res 68:246–253. [DOI] [PubMed] [Google Scholar]
- Gerwinn, H. , Weiß, S. , Tenbergen, G. , Amelung, T. , Foedisch, C. , Pohl, A. , Massau, C. , Mohnke, S. , Kärgel, C. , Wittfoth, M. , Jung, S. , Drumkova, K. , Walter, M. , Beier, K.M. , Walter, H. , Ponseti, J. , Schiffer, B. , Kruger, T.H.C. (2015b) Paedophilia and child sexual offending: Clinical characteristics and offence associated factors in the NeMUP‐study. Submitted manuscript.
- Gusnard DA, Raichle ME, Raichle ME (2001): Searching for a baseline: Functional imaging and the resting human brain. Nat Rev Neurosci 2:685–694. [DOI] [PubMed] [Google Scholar]
- Habermeyer B, Esposito F, Handel N, Lemoine P, Klarhofer M, Mager R, Dittmann V, Seifritz E, Graf M (2013a): Immediate processing of erotic stimuli in paedophilia and controls: A case control study. BMC Psychiatry 13:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habermeyer B, Esposito F, Händel N, Lemoine P, Kuhl HC, Klarhöfer M, Mager R, Mokros A, Dittmann V, Seifritz E, Graf M (2013b): Response inhibition in pedophilia: An fMRI pilot study. Neuropsychobiology 68:228–237. [DOI] [PubMed] [Google Scholar]
- Hanson RK, Morton‐Bourgon KE (2005): The characteristics of persistent sexual offenders: A meta‐analysis of recidivism studies. J Consult Clin Psychol 73:1154–1163. [DOI] [PubMed] [Google Scholar]
- Hellerbach A, Schuster V, Jansen A, Sommer J (2013): MRI phantoms ‐ are there alternatives to agar?. PLoS One 8:e70343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyal CC, Beaulieu‐Plante J, de Chantérac A (2014): The neuropsychology of sex offenders: A meta‐analysis. Sex Abuse 26:149–177. [DOI] [PubMed] [Google Scholar]
- Kärgel C, Massau C, Weiss S, Walter M, Kruger TH, Schiffer B (2015): Diminished functional connectivity on the road to child sexual abuse in pedophilia. J Sex Med 12:783–795. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP (2008): Competition between functional brain networks mediates behavioral variability. Neuroimage 39:527–537. [DOI] [PubMed] [Google Scholar]
- Kirk KM, Bailey JM, Dunne MP, Martin NG (2000): Measurement models for sexual orientation in a community twin sample. Behav Genet 30:345–356. [DOI] [PubMed] [Google Scholar]
- Kruger TH, Schiffer B (2011): Neurocognitive and personality factors in homo‐ and heterosexual pedophiles and controls. J Sex Med 8:1650–1659. [DOI] [PubMed] [Google Scholar]
- Lapornik R, Lehofer M, Moser M, Pump G, Egner S, Posch C, Hildebrandt G, Zapotoczky HG (1996): Long‐term imprisonment leads to cognitive impairment. Forensic Sci Int 82:121–127. [DOI] [PubMed] [Google Scholar]
- Leech R, Sharp DJ (2014): The role of the posterior cingulate cortex in cognition and disease. Brain 137:12–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann RE, Hanson RK, Thornton D (2010): Assessing risk for sexual recidivism: Some proposals on the nature of psychologically meaningful risk factors. Sex Abuse 22:191–217. [DOI] [PubMed] [Google Scholar]
- Mendez M, Shapira JS (2011): Pedophilic behavior from brain disease. J Sex Med 8:1092–1100. [DOI] [PubMed] [Google Scholar]
- Mobbs D, Eckert MA, Mills D, Korenberg J, Bellugi U, Galaburda AM, Reiss AL (2007): Frontostriatal dysfunction during response inhibition in Williams syndrome. Biol Psychiatry 62:256–261. [DOI] [PubMed] [Google Scholar]
- Mohnke S, Müller S, Amelung T, Krüger TH, Ponseti J, Schiffer B, Walter M, Beier KM, Walter H (2014): Brain alterations in paedophilia: A critical review. Prog Neurobiol 122:1–23. [DOI] [PubMed] [Google Scholar]
- Niendam TA, Laird AR, Ray KL, Dean YM, Glahn DC, Carter CS (2012): Meta‐analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn Affect Behav Neurosci 12:241–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poeppl TB, Eickhoff SB, Fox PT, Laird AR, Rupprecht R, Langguth B, Bzdok D (2015): Connectivity and functional profiling of abnormal brain structures in pedophilia. Hum Brain Mapp 36:2374–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poeppl TB, Langguth B, Laird AR, Eickhoff SB (2014): The functional neuroanatomy of male psychosexual and physiosexual arousal: A quantitative meta‐analysis. Hum Brain Mapp 35:1404–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poeppl TB, Nitschke J, Dombert B, Santtila P, Greenlee MW, Osterheider M, Mokros A (2011): Functional cortical and subcortical abnormalities in pedophilia: A combined study using a choice reaction time task and fMRI. J Sex Med 8:1660–1674. [DOI] [PubMed] [Google Scholar]
- Poeppl TB, Nitschke J, Santtila P, Schecklmann M, Langguth B, Greenlee MW, Osterheider M, Mokros A (2013): Association between brain structure and phenotypic characteristics in pedophilia. J Psychiatr Res 47:678–685. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL (2001): A default mode of brain function. Proc Natl Acad Sci U S A 98:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe JB, Toni I, Josephs O, Frackowiak RS, Passingham RE (2000): The prefrontal cortex: Response selection or maintenance within working memory? Science 288:1656–1660. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Brammer MJ, Toone B, Taylor E (2005): Abnormal brain activation during inhibition and error detection in medication‐naive adolescents with ADHD. Am J Psychiatry 162:1067–1075. [DOI] [PubMed] [Google Scholar]
- Schiffer B, Krueger T, Paul T, de Greiff A, Forsting M, Leygraf N, Schedlowski M, Gizewski E (2008a): Brain response to visual sexual stimuli in homosexual pedophiles. J Psychiatry Neurosci 33:23–33. [PMC free article] [PubMed] [Google Scholar]
- Schiffer B, Paul T, Gizewski E, Forsting M, Leygraf N, Schedlowski M, Kruger TH (2008b): Functional brain correlates of heterosexual paedophilia. Neuroimage 41:80–91. [DOI] [PubMed] [Google Scholar]
- Schiffer B, Peschel T, Paul T, Gizewski E, Forsting M, Leygraf N, Schedlowski M, Krueger TH (2007): Structural brain abnormalities in the frontostriatal system and cerebellum in pedophilia. J Psychiatr Res 41:753–762. [DOI] [PubMed] [Google Scholar]
- Schiffer B, Vonlaufen C (2011): Executive dysfunctions in pedophilic and nonpedophilic child molesters. J Sex Med 8:1975–1984. [DOI] [PubMed] [Google Scholar]
- Schiltz K, Witzel J, Northoff G, Zierhut K, Gubka U, Fellmann H, Kaufmann J, Tempelmann C, Wiebking C, Bogerts B (2007): Brain pathology in pedophilic offenders: evidence. of volume reduction in the right amygdala and related diencephalic structures. Arch Gen Psychiatry 64:737–746. [DOI] [PubMed] [Google Scholar]
- Sebastian A, Jung P, Krause‐Utz A, Lieb K, Schmahl C, Tuscher O (2014): Frontal dysfunctions of impulse control ‐ a systematic review in borderline personality disorder and attention‐deficit/hyperactivity disorder. Front Hum Neurosci 8:698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto, M.C. (2008) Pedophilia and Sexual Offending Against Children: Theory, Assessment, and intervention. Washington, DC: American Psychological Association. [Google Scholar]
- Seto MC, Cantor JM, Blanchard R (2006): Child pornography offenses are a valid diagnostic indicator of pedophilia. J Abnorm Psychol 115:610–615. [DOI] [PubMed] [Google Scholar]
- Simmonds DJ, Pekar JJ, Mostofsky SH (2008): Meta‐analysis of Go/No‐go tasks demonstrating that fMRI activation associated with response inhibition is task‐dependent. Neuropsychologia 46:224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele VR, Claus ED, Aharoni E, Harenski C, Calhoun VD, Pearlson G, Kiehl KA (2014): A large scale (N=102) functional neuroimaging study of error processing in a Go/NoGo task. Behav Brain Res 268:127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoléru S, Fonteille V, Cornélis C, Joyal C, Moulier V (2012): Functional neuroimaging studies of sexual arousal and orgasm in healthy men and women: A review and meta‐analysis. Neurosci Biobehav Rev 36:1481–1509. [DOI] [PubMed] [Google Scholar]
- Suchy Y, Eastvold AD, Strassberg DS, Franchow EI (2014): Understanding processing speed weaknesses among pedophilic child molesters: Response style vs. neuropathology. J Abnorm Psychol 123:273–285. [DOI] [PubMed] [Google Scholar]
- Suchy Y, Whittaker JW, Strassberg DS, Eastvold A (2009): Neurocognitive differences between pedophilic and nonpedophilic child molesters. J Int Neuropsychol Soc 15:248–257. [DOI] [PubMed] [Google Scholar]
- Tenbergen G, Wittfoth M, Frieling H, Ponseti J, Walter M, Walter H, Beier KM, Schiffer B, Kruger TH (2015): The neurobiology and psychology of pedophilia: Recent advances and challenges. Front Hum Neurosci 9:344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zutphen L, Siep N, Jacob GA, Goebel R, Arntz A (2015): Emotional sensitivity, emotion regulation and impulsivity in borderline personality disorder: A critical review of fMRI studies. Neurosci Biobehav Rev 51:64–76. [DOI] [PubMed] [Google Scholar]
- Völlm B, Richardson P, Stirling J, Elliott R, Dolan M, Chaudhry I, Del Ben C, McKie S, Anderson I, Deakin B (2004): Neurobiological substrates of antisocial and borderline personality disorder: Preliminary results of a functional fMRI study. Crim Behav Ment Health 14:39–54. [DOI] [PubMed] [Google Scholar]
- Von Aster, M. , Neubauer, A. , Horn, R. (2006) Wechsler Intelligenztest für Erwachsene (WIE). Deutschsprachige Bearbeitung und Adaptation des WAIS‐III von David Wechsler. Frankfurt. Harcourt Test Services.
- Ward T, Hudson SM, Keenan T (1998): A self‐regulation model of the sexual offense process. Sexual. Abuse 10:141–157. [Google Scholar]
- Wittchen, H.U. , Zaudig, M. , Fydrich, T. (1997) SKID Strukturiertes Klinisches Interview für DSM‐IV. Achse I und II. Göttingen. Hogrefe.
- World Health Organization . (1992) The ICD‐10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. Geneva.


