Abstract
The motivation to receive rewards enhances episodic memories, and the motivation is modulated by task difficulty. In episodic retrieval, however, functional neuroimaging evidence regarding the motivation that mediates interactions between reward and task difficulty is scarce. The present fMRI study investigated this issue. During encoding performed without fMRI, participants encoded Japanese words using either deep or shallow strategies, which led to variation in difficulty level during subsequent retrieval. During retrieval with fMRI, participants recognized the target words in either high or low monetary reward conditions. In the behavioral results, a reward‐related enhancement of memory was found only when the memory retrieval was difficult, and the rewarding effect on subjective motivation was greater in the retrieval of memories with high difficulty than those with low difficulty. The fMRI data showed that reward‐related increases in the activation of the substantia nigra/ventral tegmental area (SN/VTA), medial temporal lobe (MTL), dorsomedial prefrontal cortex (dmPFC), and dorsolateral prefrontal cortex (dlPFC) were greater during the retrieval of memories with high difficulty than those with low difficulty. Furthermore, reward‐related enhancement of functional connectivity between the SN/VTA and MTL and between the SN/VTA and dmPFC during the retrieval of memories with high difficulty was significantly correlated with reward‐related increases of retrieval accuracy and subjective motivation. The reward‐related enhancement of episodic retrieval and retrieval‐related motivation could be most effective when the level of retrieval difficulty is optimized. Such reward‐related enhancement of memory and motivation could be modulated by a network including the reward‐related SN/VTA, motivation‐related dmPFC, and memory‐related MTL. Hum Brain Mapp 38:3428–3443, 2017. © 2017 Wiley Periodicals, Inc.
Keywords: fMRI, reward, task difficulty, episodic memory, retrieval, dorsolateral prefrontal cortex, dorsomedial prefrontal cortex, hippocampus, substantia nigra, ventral tegmental area
INTRODUCTION
Motivation promotes learning and episodic memory in humans [Locke and Latham, 1990, 2002]. Previous studies have reported that motivation is modulated by the anticipation of rewards and that the processing of episodic memories is enhanced by the motivation to receive those rewards [Adcock et al., 2006; Murty and Adcock, 2014; Shigemune et al., 2014; Wolosin et al., 2012, 2013]. Additionally, motivation is increased in individuals performing difficult tasks [Anshel and Weinberg, 1992; Arkes, 1979; Shalley and Oldham, 1985]. These findings suggest that an interaction between the processing of reward and task difficulty could be mediated by the motivation in episodic memories. However, little is known regarding the neural mechanisms underlying how the effects of motivation modulated by rewards on memory processes are affected by the level of difficulty of memory tasks. The current functional MRI (fMRI) study investigated this issue.
Human cognitive processes, including episodic memory, are enhanced by the intention to earn rewards [Adcock et al., 2006; Murty and Adcock, 2014; Shigemune et al., 2014; Wolosin et al., 2012, 2013]. Previous studies have demonstrated that memory‐related activation of medial temporal lobe (MTL) regions including the hippocampus and parahippocampal gyrus (PHG) are significantly enhanced by the motivation of receiving rewards [Adcock et al., 2006; Dillon et al., 2014; Murty and Adcock, 2014; Shigemune et al., 2014; Wolosin et al., 2012, 2013] and that the reward‐related enhancement of memory was modulated by interactions between reward‐related regions, such as the substantia nigra/ventral tegmental area (SN/VTA) and the striatum, and memory‐related regions, such as the hippocampus [Shigemune et al., 2014]. There are also functional neuroimaging evidences in which activation of the dorsomedial prefrontal cortex (dmPFC) including the dorsal anterior cingulate cortex (dACC) and pre‐supplementary motor area (Pre‐SMA), and dorsolateral prefrontal cortex (dlPFC) occurred during the processing of monetary rewards [Etzel et al., 2016; Hartstra et al., 2010; Jimura et al., 2010; Satterthwaite et al., 2007; Wang et al., 2014]. In neurophysiological studies performed with experimental animals, the dmPFC region played an important role in seeking rewards [Ishikawa et al., 2008a, 2008b], the SMA and Pre‐SMA regions were active in representing reward‐dependent motivation for actions [Scangos and Stuphorn, 2010] and the dlPFC region was associated with cognitive control during the processing of tasks motivated by rewards [Donahue and Lee, 2015; Watanabe et al., 2002]. Given that activation of the dmPFC, including the ACC and the Pre‐SMA, was associated with the intention of voluntary movement [Haggard, 2008; Winterer et al., 2002] and that the dlPFC showed significant activation during cognitive control [D'Esposito et al., 1998; Owen, 1997; Smith and Jonides, 1999], the dmPFC and dlPFC activations identified in the reward‐related tasks could reflect the processing of motivation related to intention and cognitive control. Thus, the reward‐related enhancement of memory could be modulated by interacting mechanisms among the dmPFC and dlPFC, which are involved in motivation and cognitive control; SN/VTA and striatum, which are involved in the reward process; and MTL region (hippocampus and PHG), which is involved in the episodic memory process.
Motivation levels are associated with difficulty of cognitive tasks [Anshel and Weinberg, 1992; Arkes, 1979; LaPorte and Nath, 1976; Shalley and Oldham, 1985]. Functional neuroimaging studies have reported that the dmPFC and dlPFC show greater activation during the performance of difficult cognitive tasks than during easy cognitive tasks [Jaeggi et al., 2003; Kochan et al., 2011; Leung et al., 2007; Livesey et al., 2007; Prado and Noveck, 2007; Woodward et al., 2006]. In addition, activations of the SN/VTA and the striatum were modulated by task demands, even in the absence of rewards [Boehler et al., 2011]. Traditional psychological studies on episodic memory have demonstrated evidence to support the well‐known “levels‐of‐processing” theory in which the retrieval of memories encoded by “shallow” or perceptual processes are more difficult than those of memories encoded by “deep” or semantic processes [Craik and Lockhart, 1972; Craik and Tulving, 1975]. In functional neuroimaging studies investigating this theory, the dlPFC showed greater activation during encoding and/or retrieval through “shallow” processes than through “deep” processes [Buckner et al., 1998; Mandzia et al., 2004; Schott et al., 2013], and activation in this region reflected the successful encoding and retrieval of memories encoded by “shallow” processes [Henson et al., 2005]. In addition, activation of the dmPFC and dlPFC have been identified during high‐demand or high‐difficulty level retrieval tasks [Dobbins and Han, 2006; Reas and Brewer, 2013]. These findings suggest that activation of the dmPFC and dlPFC could increase during the retrieval of memories with high difficulty, compared to the retrieval of memories with low difficulty. Furthermore, interactions between the activation of the dmPFC/dlPFC, SN/VTA, striatum, and MTL could be critical in the retrieval of memories with high difficulty.
The relationship between reward and task difficulty in cognitive tasks has been demonstrated with the undermining effect in psychological studies [Cameron et al., 2004]. The undermining effect [Deci et al., 1999; Deci, 1971; Lepper et al., 1973; Morgan, 1984; Ryan et al., 1983], in which the intrinsic motivation to perform tasks is reduced by accepting extrinsic rewards such as money, was observed when performing low‐difficulty cognitive tasks. However, the intrinsic motivation increased when participants performed moderately difficult cognitive tasks [Cameron et al., 2004]. These reward‐task difficulty interactions have been found in several functional neuroimaging studies. For example, the beneficial effect of monetary rewards on performance of cognitive tasks was found only in tasks with high difficulty levels when the amount of monetary reward was adjusted to an optimal level, and activation of the dmPFC and reward‐related activation of the striatum during the anticipation of a reward was positively correlated with the amount of monetary reward [Chib et al., 2012]. Another fMRI study demonstrated that the enhancing effect of rewards on task performance was strongest when participants were performing a task requiring mid‐level cognitive control, and activation of the reward‐related midbrain significantly interacted with activation of the dlPFC [Bahlmann et al., 2015]. Other fMRI studies have consistently reported that activation of the dmPFC and dlPFC are regulated by the factors of reward and task difficulty in various cognitive tasks [Burke et al., 2013; Engelmann et al., 2009; Kouneiher et al., 2009; Krawczyk et al., 2007; Kurniawan et al., 2013; Pochon et al., 2002; Taylor et al., 2004]. However, there is little functional neuroimaging evidence regarding the brain‐behavior correlation and the inter‐regional connectivity underlying the interaction between the factors of reward and task difficulty in episodic memory. Based on these findings, we hypothesized that the enhancing effect of rewards on memory processes could be observed when the task difficulty of memory retrieval was adjusted to an optimal level by which the motivation was maximized, and the interaction between reward and task difficulty in memory processes could be supported by functional networks, including the dmPFC and dlPFC, which are involved in motivation and cognitive control; the SN/VTA and striatum, which are involved in reward processing; and the MTL, which is related to memory.
To investigate the neural mechanisms underlying the interaction between reward and task difficulty in the retrieval of episodic memories, we conducted an fMRI experiment with healthy young adults. The design of our fMRI experiment is illustrated in Figure 1. During the encoding phase, participants were required to learn Japanese words using two encoding strategies, which included deep (semantic) or shallow (perceptual) processes. By these encoding operations, memories encoded with the shallow strategy would be more difficult to retrieve than those encoded with the deep strategy. During the retrieval phase, which followed the encoding phase, participants recognized target words that were encoded using the two strategies. The words that were processed with deep encoding were easily retrieved, whereas the words processed with shallow encoding were retrieved with more difficulty. In addition, retrieval‐related activation was measured using fMRI. Thus, the design of our fMRI experiment enabled us to understand how the enhancement of reward‐related activation during memory retrieval was modulated by the task difficulty. Based on previous findings, we made three predictions for the present study. First, the reward‐related enhancement of motivation and memory retrieval would be greater during the retrieval of memories learned through a shallow encoding strategy (retrieval with high difficulty) than the retrieval of memories learned through a deep encoding strategy (retrieval with low difficulty). Second, a greater reward‐related increase in activation would be observed during the retrieval of memories with high difficulty than those with low difficulty in the dmPFC, dlPFC, SN/VTA, striatum, and MTL. Third, functional connectivity between these regions would be associated with the reward‐related enhancement of high‐difficulty memory retrieval.
Figure 1.
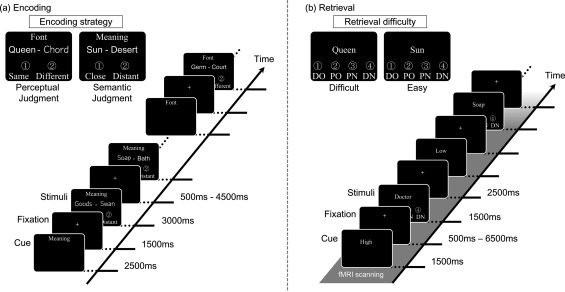
Experimental design of encoding and retrieval tasks. (a) During encoding, word pairs were presented one by one, and participants were instructed to encode the left word using two encoding strategies of either perceptual or semantic judgments. These encoding strategies were indicated by a cue presented before and during the experimental blocks to indicate whether participants should perform the perceptual or semantic judgments. The cue of “Font” corresponded to an encoding strategy with the perceptual judgments of word pairs, and the cue of “Meaning” referred to an encoding strategy with the semantic judgments. Responses of these judgments were recorded by pressing one of two buttons. In the perceptual judgments, participants were required to press the left button if the font of the target word was the same as that of the cue word and to press the right button if the fonts of two words were different. In the semantic judgments, participants pressed the left button if the target word was semantically close to the cue word and the right button if the target word was semantically distant from the cue word. (b) During retrieval, participants were randomly presented with either a target (old) word that was presented on the left side during encoding or a distracter (new) word, and they were required to judge whether each word was previously learned or not by selecting one of four response options (definitely old: DO, probably old: PO, probably new: PN, and definitely new: DN). In the retrieval phase, participants were required to recognize words in either the High or Low reward condition, which was instructed by a cue before the presentation of the words. All verbal labels were presented in Japanese. English is used here for illustration purposes only.
MATERIALS AND METHODS
Participants
Thirty‐three right‐handed undergraduate and graduate students recruited from the Tohoku University community participated in this study. Participants were healthy, native Japanese speakers with no history of neurological or psychiatric disease. All participants were paid for their participation in the experiment. The data from five participants were excluded from the analyses of behavioral and fMRI data because three of these participants could not complete the experimental tasks and two had fewer than two trials in one condition. In addition, the data from three participants who showed no enhancement by rewards in the subjective ratings of motivation were also excluded because it was highly possible that they would not show the modulatory effect of motivation by receiving rewards on memory retrieval. Thus, the data from 25 participants (10 men and 15 women; mean age 20.6 years; range 18–25 years) were analyzed in the present study. All participants gave informed consent, and the experimental protocol was approved by the Institutional Review Board (IRB) of Tohoku University School of Medicine.
Experimental Tasks
We prepared 768 two‐letter Japanese words that were used as the experimental stimuli in this study. The words were selected from a database of two‐letter Japanese Kanji words standardized by familiarity and imagery scores [Amano and Kondo, 1999]. These words were divided into three lists of 256 words, and each list was assigned as target words to be encoded, cue words to be paired with the target words, or distracter words to be used during the retrieval phase. Scores of familiarity and imagery were used for these words to ensure they were equal in terms of familiarity [F (2,765) = 0.04, P = 0.96, η 2 < 0.01] and imagery [F (2,765) = 0.01, P = 0.99, η 2 < 0.01] across the three lists. Each list of target and cue words were subdivided into four lists, across which the mean scores of familiarity [target words: F (3,252) = 0.01, P = 1.00, η 2 < 0.01, cued words: F (3,252) = 0.39, P = 0.76, η 2 < 0.01] and imagery [target words: F (3,252) = 0.05, P = 0.98, η 2 < 0.01, cued words: F (3,252) = 0.03, P = 0.99, η 2 < 0.01] were statistically equalized. In each list of words, word pairs were made with combinations between target and cue words, which were then categorized into semantically distant or close pairs by the experimenters. Ten young adults (recruited from the Tohoku University community and who did not participate in our fMRI tasks: five men and five women; mean age 22.9 years; range 20–28 years) confirmed the semantic distance of the distant or close pairs by evaluating the semantic similarities of the word pairs. To rate the semantic similarities, the participants were required to rate the semantic distance of word pairs with a seven‐level rating scale (1: distant to 7: close). The subjective ratings of semantic similarities revealed that there was no significant difference in the semantic distance across the four lists of word pairs [F (3,252) = 0.01, P = 1.00, η 2 < 0.01]. In each list of word pairs, half of the word pairs were distant‐meaning pairs and the remaining half were close‐meaning pairs. We performed a two‐way analysis of variance (ANOVA) for rating scores of semantic similarities using the factors of semantic distance and list group, and found a significant main effect of semantic distance [F (1,248) = 4103.06, P < 0.01, η p 2 = 0.94] but not of list group [F (3,248) = 0.16, P = 0.93, η p 2 < 0.01] and not an interaction between these factors [F (3,248) = 0.15, P = 0.93, η p 2 < 0.01]. Combinations between the four lists of word pairs and retrieval conditions were counterbalanced across participants.
The experimental tasks performed in this study were the encoding and retrieval tasks (see Fig. 1). Neural activation was measured only in the retrieval task using a hybrid (event‐related and blocked) fMRI design. During the encoding task, participants were presented with word pairs one by one and were required to encode the target words shown on the left side of the pairs using one of two different strategies. The first strategy was the perceptual judgment (shallow encoding) strategy, in which participants were instructed to judge whether the target word presented on the left side was written in the same font as the cue word presented in the right side. In this encoding condition, participants were required to press the left button if the font of the target word was the same as that of the cue word, and vice versa. The second strategy was the semantic judgment (deep encoding) strategy, in which participants were required to judge whether the target word on the left side was semantically close to or distant from the cue word presented on the right side. In this encoding condition, participants pressed the left button if the target word was semantically close to the cue word, and vice versa. Manipulating the encoding strategies enabled us to utilize two levels of task difficulty for the subsequent retrieval task, in which target words encoded through the shallow strategy were more difficult to retrieve than those encoded through the deep strategy. Participants were instructed that they would receive high (200 yen, or approximately 2 US dollars) or low (20 yen, or approximately 20 cents) monetary rewards for each target word that they successfully remembered in the subsequent retrieval task, and that they would lose 110 yen (i.e., 200 yen/2 + 20 yen/2) for each of the distractor words remembered falsely. During a run of the encoding task, participants performed eight blocks including 16 trials each, and the run was repeated twice with the different stimulus sets. During an encoding trial, a cue for the encoding strategy was presented before and during the blocks to indicate whether participants should perform either perceptual or semantic judgments. The cue of “Font” corresponded to a shallow encoding strategy with perceptual judgments of word pairs, whereas the cue of “Meaning” referred to a deep encoding strategy with semantic judgments. Within each block, the cue instructing the encoding strategy was first presented for 2,500 ms, which was followed by a visual fixation lasting 1,500 ms. After the instruction for the encoding strategy was presented, each word pair was presented for 3,000 ms and was followed by a visual fixation period during the interstimulus interval (ISI) that had a variable duration jittered between 500 and 4,500 ms.
Approximately 10 min after completing the encoding task, participants performed the retrieval task in the fMRI scanner. During the retrieval task, participants were randomly presented target (old) and distracter (new) words one by one and were required to judge whether each word was previously learned. The participants responded with one of four response options (definitely old: DO, probably old: PO, probably new: PN, and definitely new: DN). In the retrieval phase, participants were required to recognize words in either High or Low reward conditions, which were instructed by a cue before the presentation of the words. If participants successfully recognized target words, they were given 200 yen with a hit response in the High reward condition or 20 yen with a hit response in the Low reward condition. For a false alarm (FA) response, participants were penalized 110 yen (200 yen/2 + 20 yen/2) each in both High and Low reward conditions. In addition, target words had two levels of task difficulty for retrieval (Easy and Difficult) in which words encoded with the deep process were easier to retrieve than those encoded with the shallow process. Thus, retrieval trials for target (old) words were categorized into four conditions by combining the two factors of reward (High reward or Low reward) and task difficulty (Easy or Difficult). In each retrieval trial, a cue for indicating the High or Low reward condition was first presented for 1,500 ms and was followed by a visual fixation presented for a variable duration jittered between 500 ms and 6,500 ms. After the cue for the reward condition and visual fixation were presented, a word was presented for 1,500 ms and was followed by another visual fixation for 2,500 ms. In the retrieval phase, participants were required to perform four successive runs including 128 trials each. After the retrieval task, participants rated their subjective feelings of motivation in each retrieval condition by 10 cm visual analogue scales (VAS) (0: not motivated to 10: highly motivated) and were then told how much money they had won in the retrieval phase. The money was actually paid to participants.
Behavioral Data Analysis
Behavioral data were analyzed using SPSS 18.0.0 software (SPSS Inc., Chicago, IL). To investigate the effects of reward and task difficulty on the participant's motivation to remember target words, subjective ratings of motivation measured by the VAS were analyzed using a two‐way repeated measures ANOVA with factors of reward (High reward, Low reward) and task difficulty (Easy, Difficult). All responses during retrieval were categorized by retrieval accuracy and confidence. Responses for target words were divided into hits with high confidence (High‐confidence Hit), hits with low confidence (Low‐confidence Hit), and misses (Miss) including both levels of confidence because there was only a small number of Miss trials with high confidence (mean = 1.7, SD = 1.2). Responses to distracter words included correct rejections (CR) and FA in both high and low confidence. To identify the effect of reward and task difficulty on High‐confidence Hit and Low‐confidence Hit rates, we separately performed two‐way repeated measures ANOVAs with factors of reward (High reward, Low reward) and task difficulty (Easy, Difficult). Response times (RTs) were analyzed by a three‐way repeated measures ANOVA with factors of reward (High reward, Low reward), task difficulty (Easy, Difficult), and retrieval performance (High‐confidence Hit, Low‐confidence Hit, and Miss). To confirm that no response bias existed between the High and Low reward conditions in the retrieval phase, FA rates were analyzed by a paired t‐test between the High reward and Low reward conditions.
fMRI Data Acquisition and Analysis
All MRI data were acquired using a 3T Philips Achieva scanner. Stimuli were visually presented on a screen back‐projected with a projector, and participants viewed the stimuli via a mirror attached to the head coil. Stimulus presentations were controlled by a program implemented in MATLAB (http://www.mathworks.com) on a Windows PC, and behavioral responses were recorded using a four‐button optic fiber response box (Current Designs, Inc., Philadelphia, PA). Scanner noise was absorbed by earplugs, and head motions were minimized by foam pads and a headband.
During MRI scanning, first, T1‐weighted sagittal scans were acquired for localizing functional scans. Second, functional images were acquired by echo‐planar functional images (EPIs) sensitive to blood‐oxygenation‐level dependent (BOLD) contrasts (64 × 64 matrix, TR = 2,000 ms, TE = 30 ms, flip angle = 70°, FOV = 24 × 24 cm, 34 slices, 3.75 mm slice thickness). Finally, high‐resolution T1‐weighted structural images were obtained (MPRAGE, 240 × 240 matrix, TR = 6.5 ms, TE = 3 ms, FOV = 24 × 24 cm, 162 slices, 1.0 mm slice thickness).
Preprocessing and statistical analyses of all images were performed by Statistical Parametric Mapping 8 (SPM 8‐Wellcome Trust Centre for Neuroimaging, London, UK) implemented in MATLAB. In the preprocessing of images, all functional images were corrected for slice timing and head motion, spatially normalized by an EPI template of the Montreal Neurological Institute (MNI) standard space of human brain (resampled resolution = 3.75 × 3.75 × 3.75 mm), and then spatially smoothed with a Gaussian kernel of 8‐mm full‐width at half‐maximum (FWHM).
In the statistical analysis, we performed two‐step statistics of the individual‐level fixed effects and group‐level random effects analyses. In the first (individual)‐level (fixed effects) analysis, trial‐by‐trial activations during retrieval were modeled by convolving a vector of word onsets with a canonical hemodynamic response function (HRF) in the context of the general linear model (GLM). Confounding factors (head motion and magnetic field drift) were included in the model. In the model of retrieval‐related activation, we set 12 conditions for target words, 2 conditions for distracter words, and 1 condition of no response. The 12 conditions associated with the processing of target words were defined by three factors, which included reward (High reward and Low reward), task difficulty (Easy and Difficult), and retrieval performance (High‐confidence Hit, Low‐confidence Hit, and Miss). The two conditions reflecting responses to distracter words were CR and FA for both high and low levels of confidence. One condition of no response included trials showing no response during encoding and/or retrieval. For illustration purposes in the Supporting Information, parameter estimates in the twelve experimental conditions for target words were extracted from a peak voxel of regions showing significant activations. Using the experimental conditions set for each participant, we identified retrieval success activations (RSAs) by comparing High‐confidence Hit with Miss in four conditions, which were defined by the factors of reward and task difficulty (Difficult‐High reward, Difficult‐Low reward, Easy‐High reward, and Easy‐Low reward). To identify whether RSAs were increased by monetary rewards, the reward‐related increase of RSAs was defined by comparing RSAs between High and Low rewards in each of the task difficulty conditions (Difficult and Easy). In addition, we identified contrasts reflecting simple effects of High reward, Low reward, Easy, Difficult, High‐confidence Hit, and Miss (see Supporting Information). These analyses yielded individual‐level t‐contrasts reflecting the reward‐related increase of RSAs in each difficulty condition.
In the second (group)‐level (random effects) analysis, we performed a paired t‐test using contrast images reflecting the reward‐related increase of RSAs identified in the first level analysis and compared the reward‐related increase of RSAs between Difficult and Easy. In addition, to identify regions reflecting simple effects of reward, task difficulty, and retrieval performance, paired t‐tests were performed between contrasts of High reward and Low reward, between Difficult and Easy, and between High‐confidence Hit and Miss (see Supporting Information). Activation identified at the threshold of P < 0.001 for the voxel level was corrected by whole‐brain multiple comparisons at the cluster level (FWE, P < 0.05) with a minimum cluster size of two successive voxels. In addition, we applied the small volume correction (SVC) method [Worsley et al., 1996] to regions of the SN/VTA, striatum, MTL, dmPFC, and dlPFC (FWE, P < 0.05), which were defined as a single region‐of‐interest (ROI) by previous studies mentioned above. The single ROI including these regions was formed by combining predefined ROIs of the striatum, MTL, dmPFC and dlPFC in the AAL ROI package [Tzourio‐Mazoyer et al., 2002] and a ROI of the SN/VTA defined in a previous study [Murty et al., 2014]. In the predefined ROIs, the striatum ROI included the caudate nucleus and putamen, the MTL ROI included the hippocampus and PHG, the dmPFC ROI included the superior medial frontal gyrus, supplementary motor area, and anterior cingulate gyrus, and the dlPFC ROI included the superior frontal gyrus and middle frontal gyrus. The SN/VTA ROI was provided by files downloaded from the website (https://web.duke.edu/adcocklab/index.html). The ROI files were defined by procedures employed in a previous study [Murty et al., 2014], in which the SN was defined by the following anatomical landmark: the inferior boundary was the most inferior horizontal section before the cerebral aqueduct merged with the fourth ventricle, the superior boundary was the most superior horizontal section that did not contain the third ventricle, the medial boundary of exclusion on each side was a straight line between the posterior edge of the cerebral peduncle and the posterior edge of the interpeduncular fossa, and the lateral boundary on each side was a curve from the peduncle's anterior medial edge to its posterior medial edge. The VTA was also defined by the following anatomical landmark: the anterior boundary was at the cerebral spinal fluid (CSF), the posterior boundary was at the coronal section that bisected the red nucleus, the superior boundary was at the top of the superior colliculus, the inferior boundary was at the bottom of the red nucleus, and the lateral boundary was in the sagittal slice connecting the peak of curvature of the interpeduncular fossa with the center of the colliculus [Murty et al., 2014]. These ROIs were combined into one ROI by the MarsBaR toolbox (http://marsbar.sourceforge.net/). Details of the statistical threshold and SVC method in the paired t‐tests for simple effects of reward, task difficulty, and retrieval performance were shown in the Supporting Information. Anatomical locations showing significant activations were primarily defined by the WFU Pick Atlas [Maldjian et al., 2004, 2003; Tzourio‐Mazoyer et al., 2002].
To identify regions showing a significant correlation between the reward‐related increase of RSAs and the retrieval performance or the subjective rating of motivation, we performed regression analyses for High‐confidence Hit rates and subjective ratings of motivation in each task difficulty of the Difficult and Easy retrieval condition. The rationale of this analysis was to investigate whether the relationship between the rewarding effects on RSA and behavioral performance was significant in each task difficulty. In each of these analyses, we used a model of regression analysis that included t‐contrasts reflecting the reward‐related increase of RSAs identified in the first‐level analysis and covariates of the differences in the High‐confidence Hit rates or subjective ratings of motivation between the High and Low reward conditions. In the regression analyses, regions reflecting significant correlations with the retrieval performance or the ratings of motivation were identified with the same significance threshold. For the ROI including the SN/VTA, striatum, MTL, dmPFC, and dlPFC, we also used the SVC methods (corrected by FWE, P < 0.05).
To identify the regions reflecting the modulatory effect of the reward‐related increases in retrieval performance or subjective motivation on functional connectivity with the SN/VTA regions identified in the previous analyses, we performed regression analyses for High‐confidence Hit rates or subjective ratings of motivation in contrasts related to the functional connectivity maps with the SN/VTA seed. The contrasts reflecting the functional connectivity map with the SN/VTA seed were acquired through a generalized form of psychophysiological interactions (gPPI) [McLaren et al., 2012]. The gPPI method enables us to convolve multiple conditions into one PPI model, while the standard PPI method is available in constructing one PPI model with a single condition [Friston et al., 1997; Gitelman et al., 2003]. Before performing the gPPI analyses, four retrieval runs were collapsed into one run. At the individual level (fixed effects), a new one‐run GLM of 15 conditions that included 12 conditions for target words, 2 conditions for distractor words, and 1 condition of no response, was produced. In this model, each of the left and right SN/VTA seeds was defined as a volume‐of‐interest (VOI) of a sphere with a 5 mm radius around the highest peak voxel. For each participant, the highest peak voxel was explored within the common region between the SN/VTA ROI defined by a previous study [Murty et al., 2014] and the ROI of a sphere with a 15 mm radius around the peak voxel identified in the prior paired t‐test (left SN/VTA: x = −3, y = −26, z = −16) or in the prior regression analysis (right SN/VTA: x = 5, y = −15, z = −20). The VOI data was extracted from sphere regions defined by the highest peak voxels included in the common regions. Data from one participant were excluded from the functional connectivity analysis with the right SN/VTA seed because the VOI of this region was not identified.
In the gPPI analysis of the present study, the gPPI toolbox version 13.1 (http://www.nitrc.org/projects/gppi) produced a GLM for each participant that included the PPI regressors of the 15 conditions, as well as the condition regressors of the 15 conditions and the BOLD signals in the left or right SN/VTA region as a seed. The PPI regressors reflected an interaction between the condition regressors and BOLD signals in the seed regions. In addition, six motion‐related regressors were included in the GLM. After the creation of this model, the gPPI toolbox estimated the model parameters and computed the linear contrasts. The brain regions showing a significant effect in the PPI regressor contrasts were considered to be functionally connected with the seed region on the basis of the significance threshold.
In the first‐level (fixed effects) analysis of the gPPI, PPI contrasts related to the reward‐related enhancement of retrieval success functional connectivity (RSC) were defined by two steps. First, PPI contrasts of High‐confidence Hit vs. Miss, which correspond to the RSC, were computed in four conditions (Difficult‐High reward, Difficult‐Low reward, Easy‐High reward, and Easy‐Low reward). Second, the reward‐related enhancement of the RSC was defined by comparing the RSC levels between High and Low rewards in each task difficulty condition (Difficult and Easy). In the second‐level (random effects) analysis, PPI contrasts related to the reward‐related enhancement of the RSC were analyzed by regression analyses with covariates related to the reward‐related enhancement of High‐confidence Hit rates or subjective ratings of motivation in each difficulty condition. Regions reflecting a significant correlation between the reward‐related increases of the RSC and of behavioral data were statistically identified at the same significance threshold (P < 0.001 at the voxel level and corrected by FWE, P < 0.05 for whole‐brain multiple comparisons at the cluster level with a minimum cluster size of two successive voxels). The SVC methods for ROI of the SN/VTA, striatum, MTL, dmPFC and dlPFC were also applied to the regression analyses at the same significance threshold (corrected by FWE, P < 0.05).
RESULTS
Behavioral Results
Consistent with our first prediction, the enhancing effect of rewards on motivation and successful recollection was significantly greater during the retrieval of memories with high difficulty than those with low difficulty (see Fig. 2). The details of the behavioral data are summarized in Table 1.
Figure 2.
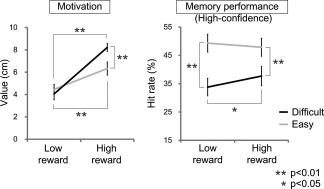
Behavioral results of subjective ratings of motivations using the visual analogue scale (VAS) and proportions (percentage) of retrieval accuracies for hit responses in high confidence (High‐confidence Hit). These data were analyzed by two‐way repeated measures ANOVAs with the factors of reward (High reward and Low reward) and task difficulty (Difficult and Easy). Error bars represent standard errors. **P < 0.01, *P < 0.05.
Table 1.
Behavioral results
| Easy (deep) | Difficult (shallow) | |||
|---|---|---|---|---|
| Low reward | High reward | Low reward | High reward | |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| Subjective motivation (cm) | 4.5 (2.2) | 6.3 (3.0) | 4.1 (2.6) | 8.2 (1.8) |
| Proportion of hit responses during retrieval (%) | ||||
| High‐confidence Hit | 49.3 (15.8) | 47.9 (15.8) | 33.8 (15.5) | 37.7 (17.0) |
| Low‐confidence Hit | 26.9 (12.2) | 26.2 (12.8) | 27.0 (11.6) | 25.5 (10.7) |
| No. trials | ||||
| High‐confidence Hit | 31.4 (10.1) | 30.4 (10.1) | 21.5 (9.9) | 23.9 (10.9) |
| Low‐confidence Hit | 17.1 (7.7) | 16.6 (8.1) | 17.2 (7.3) | 16.1 (6.7) |
| Miss | 15.1 (6.4) | 16.5 (6.6) | 24.9 (8.8) | 23.3 (8.7) |
| Response time (RT) during retrieval (ms) | ||||
| High‐confidence Hit | 1280.8 (236.2) | 1255.0 (246.1) | 1277.5 (219.2) | 1250.4 (220.7) |
| Low‐confidence Hit | 1708.7 (397.7) | 1725.1 (452.8) | 1679.1 (382.7) | 1766.7 (462.4) |
| Miss | 1690.7 (409.5) | 1754.5 (450.1) | 1686.2 (428.8) | 1701.4 (441.4) |
In the subjective ratings of motivation, an ANOVA with factors of reward and task difficulty showed a significant main effect of reward (High reward > Low reward) [F (1,24) = 59.03, P < 0.01, η p 2 = 0.71] and a significant interaction between reward and task difficulty [F (1,24) = 18.78, P < 0.01, η p 2 = 0.44]. A main effect of task difficulty was not significant [F (1,24) = 1.48, P = 0.23, η p 2 = 0.06]. In post‐hoc tests, we found that subjective ratings of motivation in both the Difficult and Easy retrieval conditions were significantly higher in the High reward condition than in the Low reward condition (P < 0.01 in both comparisons). Additionally, we found that only in the High reward condition, the motivation ratings were significantly higher in the Difficult retrieval condition than in the Easy retrieval condition (P < 0.01). In the Low reward condition, however, there was no significant difference in the motivation ratings between the Difficult and Easy retrieval conditions (P = 0.59).
For the High‐confidence Hit rates, an ANOVA with factors of reward and task difficulty demonstrated that a main effect of task difficulty (Easy > Difficult) [F (1,24) = 44.10, P < 0.01, η p 2 = 0.65] and an interaction between reward and task difficulty [F (1,24) = 4.27, P < 0.05, η p 2 = 0.15] were significant. However, a main effect of reward was not significant [F (1,24) = 0.72, P = 0.41, η p 2 = 0.03]. In post hoc tests, a significant enhancement of the High‐confidence Hit rates in the High reward condition compared to the Low reward condition was identified in the Difficult retrieval condition (P < 0.05) but not in the Easy retrieval condition (P = 0.48). Additionally, for both conditions of High and Low reward, the High‐confidence Hit rates in the Easy retrieval condition were higher than those in the Difficult retrieval condition (P < 0.01 in both comparisons). An ANOVA for Low‐confidence Hit rates showed no significant main effect of task difficulty [F (1,24) = 0.06, P = 0.81, η p 2 < 0.01], of reward [F (1,24) = 0.86, P = 0.36, η p 2 = 0.03] and no significant interaction between reward and task difficulty [F (1,24) = 0.12, P = 0.73, η p 2 < 0.01]. FA rates in each condition of the High and Low reward were also computed (High reward: mean = 18.2, SD = 11.7; Low reward: mean = 17.2, SD = 9.9) and were compared between these conditions with a paired t‐test. This analysis showed no significant difference in FA rates between the High and Low reward conditions [t (24) = 0.98, P = 0.34, r = 0.20].
ANOVAs for the RTs in the retrieval phase showed no significant main effect of task difficulty [F (1,24) = 0.45, P = 0.51, η p 2 = 0.02] and reward [F (1,24) = 3.06, P = 0.09, η p 2 = 0.11]. Significant interactions were not found between task difficulty and reward [F (1,24) = 0.08, P = 0.78, η p 2 < 0.01], between task difficulty and retrieval performance [F (2,48) = 0.79, P = 0.46, η p 2 = 0.03], and among reward, task difficulty, and retrieval performance [F (2,48) = 1.29, P = 0.28, η p 2 = 0.05]. A main effect of retrieval performance was significant [F (2,48) = 53.46, P < 0.01, η p 2 = 0.69], in which the RTs in High‐confidence Hit were significantly faster than those in Low‐confidence Hit and Miss. An interaction between reward and retrieval performance was significant [F (2,48) = 4.50, P < 0.05, η p 2 = 0.16], in which the RTs for Miss were significantly faster in the High reward condition than in the Low reward condition (P < 0.05), but not for High‐confidence Hit (P = 0.09) and Low‐confidence Hit (P = 0.06). Additionally, in both conditions of High and Low reward, the RTs in High‐confidence Hit were significantly faster than those in Low‐confidence Hit and Miss (P < 0.05 in all comparisons).
fMRI Results
Consistent with our second prediction, the reward‐related increase of RSAs in the SN/VTA, MTL, dlPFC, and dmPFC was significantly greater during the retrieval of memories with high difficulty than those with low difficulty (see Fig. 3a). The activation patterns were identified by the SVC method for ROIs in these regions (P < 0.001 for the voxel level and corrected by FWE, P < 0.05). The details regarding the regions showing significant activation are summarized in Table 2. In a paired t‐test for contrasts reflecting the reward‐related increase of RSAs between the Difficult and Easy retrieval conditions, we found that the reward‐related increase of RSAs in the SN/VTA, MTL (anterior and posterior PHG), dmPFC, and dlPFC was significantly greater in the Difficult retrieval condition than in the Easy retrieval condition. We did not find a significant reward‐related increase of RSAs in any regions when corrections were performed for the whole brain. Parameter estimates in each retrieval condition, which was defined by three factors of reward, task difficulty, and retrieval performance, were also extracted from peaks of these regions, and were shown in the Supporting Information (see Supporting Information Fig. S1). In addition, we investigated each simple effect of reward, task difficulty, and retrieval performance by paired t‐tests. In these tests, we found that activation in the SN/VTA and striatum was greater in the High reward condition than in the Low reward condition, and that the MTL (hippocampus and anterior PHG) showed greater activation in High‐confidence Hit than in Miss. A paired t‐test between the Difficult and Easy retrieval conditions showed no significant activation. Activations in the dmPFC and dlPFC reflected both effects of reward and retrieval performance, and activation in the MTL (hippocampus) was also significant in a paired t‐test between the High and Low reward conditions. Other regions reflecting a simple effect of retrieval performance were identified in the inferior frontal gyrus, orbitofrontal cortex, precentral gyrus, olfactory cortex, middle temporal gyrus, inferior temporal gyrus, fusiform gyrus, lingual gyrus, angular gyrus, posterior cingulate cortex, occipital cortex, calcarine sulcus, thalamus, and cerebellar hemisphere. Details of these results are shown in the Supporting Information (see Supporting Information Tables S1 and S2).
Figure 3.
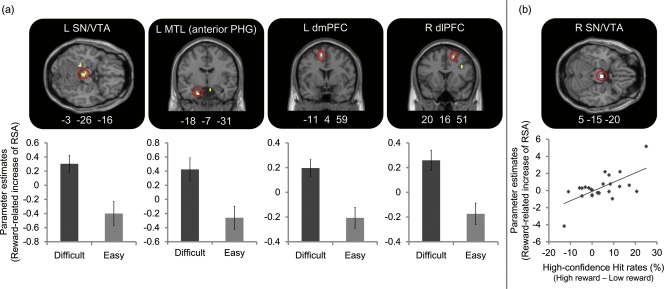
Regions reflecting the reward‐related activations modulated by task difficulty of Difficult and Easy. The small volume correction (SVC) method was applied to regions of the substantia nigra (SN)/ventral tegmental area (VTA), striatum, medial temporal lobe (MTL), dorsomedial prefrontal cortex (dmPFC), and dorsolateral prefrontal cortex (dlPFC) (FWE, P < 0.05). (a) fMRI results of a paired t‐test for contrasts of reward‐related increase in retrieval success activations (RSAs) between two levels of task difficulty (Difficult vs. Easy). Regions reflecting significantly greater reward‐related increase of RSAs in the Difficult condition than in the Easy condition were identified in the left SN/VTA, left MTL including anterior and posterior parahippocampal gyrus (PHG), left dmPFC, and right dlPFC. Parameter estimates in graphs were extracted from peaks of regions showing significant activations, and the mean values were computed in each of the Difficult and Easy retrieval conditions. Error bars represent standard errors. (b) fMRI results of the regression analysis for contrasts reflecting the reward‐related increase of RSAs with High‐confidence Hit rates in the Difficult retrieval condition. Activation in the right SN/VTA was significantly correlated with reward‐related increase of High‐confidence Hit rates in the Difficult retrieval condition. Parameter estimates in a graph were extracted from a peak of region showing a significant correlation in the Difficult retrieval condition.
Table 2.
Regions Showing Greater Reward‐Related Increase of Activations in the Retrieval of Memories With High Difficulty Than That With Low Difficulty
| Regions | L/R | BA | Z score | Cluster size | MNI coordinates | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| SN/VTA | L | 3.57 | 11 | −3 | −26 | −16 | |
| MTL (posterior PHG) | L | 30 | 3.22 | 2 | −22 | −37 | −13 |
| MTL (anterior PHG) | L | 36 | 3.98 | 4 | −18 | −7 | −31 |
| dlPFC | R | 8 | 3.41 | 3 | 20 | 16 | 51 |
| dmPFC | L | 6 | 3.64 | 4 | −11 | 4 | 59 |
SN/VTA, substantia nigra/ventral tegmental area; MTL, medial temporal lobe; PHG, parahippocampal gyrus; dlPFC, dorsolateral prefrontal cortex; dmPFC, dorsomedial prefrontal cortex; BA, Brodmann area; L, left; R, right.
To find regions reflecting the reward‐related increase of RSAs associated with retrieval performance or the subjective rating of motivation, we performed regression analyses for contrasts of the reward‐related increase of RSAs with regressors of the reward‐related increase of High‐confidence Hit rates and subjective ratings of motivation in each difficulty condition. As illustrated in Figure 3b, the reward‐related increase of RSAs in the right SN/VTA (x = 5, y = −15, z = −20, z score = 3.43, eight voxels) was significantly correlated with the reward‐related increase of High‐confidence Hit rates in the Difficult retrieval condition. In the Easy retrieval condition, however, this correlation was not observed in any region. The regression analysis showed no region reflecting a significant correlation between the reward‐related increase of RSAs and the subjective rating of motivation in both conditions of the Difficult and Easy retrieval. These results indicate that activation in the SN/VTA could contribute to the memory enhancement caused by reward and that the enhancement could be more effective when the difficulty of memory retrieval is high.
Consistent with our third prediction, the reward‐related enhancement of functional connectivity between the SN/VTA as a seed and MTL and between the SN/VTA as a seed and dmPFC during the retrieval of memories with high difficulty showed significant correlations with the reward‐related enhancement of retrieval performance and subjective motivation (see Fig. 4). In the Difficult retrieval condition, the reward‐related enhancement of RSC between activations in the left SN/VTA as a seed region and in the bilateral MTL regions (anterior PHG: x = 27, y = −3, z = −35, z score = 3.35, two voxels; posterior PHG: x = −18, y = −18, z = −28, z score = 3.32, two voxels) was significantly correlated with the reward‐related increase of High‐confidence Hit rates as a regressor. However, this correlation pattern was not observed in the Easy retrieval condition. In addition, the reward‐related enhancement of RSC between activations in the right SN/VTA and in the right dmPFC (x = 1, y = −3, z = 55, z score = 3.59, 13 voxels) showed a significant correlation with the reward‐related increase in subjective ratings of motivation in the Difficult retrieval condition, whereas the regression analysis showed no region reflecting a significant correlation between the reward‐related increase in RSC with the SN/VTA and subjective motivation in the Easy retrieval condition. The reward‐related enhancement of RSC as a seed of the left SN/VTA in both conditions of Difficult and Easy retrieval showed no region reflecting a significant correlation with the reward‐related increase in subjective ratings of motivation. In the reward‐related increase of RSC with the right SN/VTA seed during the Difficult and Easy retrieval condition, a significant correlation with the reward‐related enhancement of High‐confidence Hit rates was not identified in any region.
Figure 4.
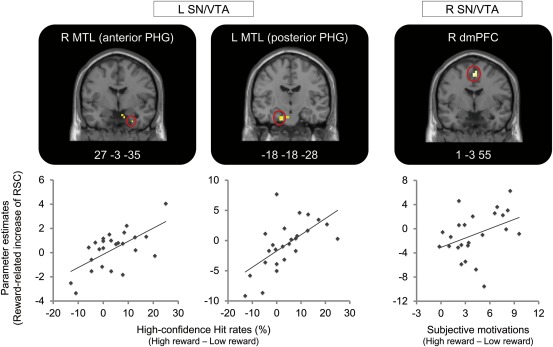
Regions showing a significant correlation between the reward‐related enhancement in functional connectivity with SN/VTA seeds and the High confidence Hit rates or the subjective ratings of motivation during retrieving memories with high difficulty. The small volume correction (SVC) method was applied to regions of the substantia nigra (SN)/ventral tegmental area (VTA), striatum, medial temporal lobe (MTL), dorsomedial prefrontal cortex (dmPFC), and dorsolateral prefrontal cortex (dlPFC) (FWE, P < 0.05). The regression analysis for PPI contrasts (left SN/VTA seed) reflecting the reward‐related increase of functional connectivity with the reward‐related increase of High‐confidence Hit rates identified the bilateral MTL. In the regression analysis for PPI contrasts (right SN/VTA seed), regions showing a significant correlation between reward‐related increase of functional connectivity and subjective motivation were identified in the right dmPFC. Parameter estimates in a graph were extracted from peaks of regions showing a significant correlation in the Difficult retrieval condition.
DISCUSSION
Three main findings emerged from the present study. First, the enhancing effect of a reward on subjective ratings of motivation and retrieval accuracies of memories was significantly larger during the retrieval of memories with high difficulty than those with low difficulty. Second, activations reflecting the effects of rewards during the successful retrieval of memories with high difficulty were identified in the SN/VTA, MTL (anterior and posterior PHG), dmPFC, and dlPFC. In addition, reward‐related increases in SN/VTA activation were significantly correlated with reward‐related increases in retrieval accuracy only in the high‐difficulty retrieval of memories. Third, the reward‐related enhancement of functional connectivity between the SN/VTA and MTL (anterior and posterior PHG) or between the SN/VTA and dmPFC was significantly correlated with the enhancing effect of rewards on retrieval accuracy and subjective motivation only in high‐difficulty memory retrieval. The reward‐related enhancement of memory retrieval, which was found only when the retrieval was highly difficult, could be modulated by the subjective motivation, and the functional modulation by motivation could be involved in the interacting mechanisms among the SN/VTA, the MTL, and the dmPFC.
Subjective Motivation and Memory Retrieval Enhanced by Rewards in the Retrieval of Memories With High Difficulty
The first main finding of our study was that reward‐related enhancement of subjective motivation and memory retrieval with high confidence was greater for the retrieval of memories with high difficulty than those with low difficulty (see Fig. 2). These findings suggest that the processing of rewards could more beneficially raise the subjective feelings of motivation when the task requirement is difficult than when it is easy, and increasing motivation by rewards in the retrieval of memories with high difficulty could contribute to the better remembering of memories.
The reward‐related enhancement of subjective motivation was significantly greater when memory retrieval was difficult than when it was easy. This finding is consistent with previous findings in which the subjective motivation was significantly enhanced when the motor and cognitive tasks were difficult rather than easy [Anshel and Weinberg, 1992; LaPorte and Nath, 1976; Shalley and Oldham, 1985]. For example, one psychological study reported that participants showed higher scores of intrinsic motivation when they attained a difficult goal of assembling helicopter models than when they did not attain that goal or attained an easier goal [Shalley and Oldham, 1985]. However, other studies have shown that the subjective motivation was significantly lower during the performance of difficult tasks than of easy tasks [Arkes, 1979; Hom and Maxwell, 1983]. This inconsistency may be explained by two possible theories. One is the goal setting theory [Locke and Latham, 2002], which states that subjective motivations are positively correlated with goal levels when the goal is regarded as achievable but not when the goal is too high to reach. The other theory is the optimal level theory [Arkes and Clark, 1975; Berlyne, 1960; Duffy, 1957; Hebb, 1955; Malmo, 1959; Yerkes and Dodson, 1908], which states that subjective motivations and preferences are highest when stimulus variables such as complexity, novelty, uncertainty, conflict, and difficulty are maintained at an optimal level. Thus, the present findings, in which the rewarding effect on subjective motivations was significantly higher in the Difficult retrieval condition than in the Easy retrieval condition, suggest that both conditions of the Difficult and Easy retrieval could be appropriately adjusted to achievable levels of difficulty and that the level of task difficulty in the Difficult retrieval condition could be closer to the optimal level than that in the Easy retrieval condition.
In the present study, the reward‐related enhancement of memory retrieval was identified only in the memory retrieval with high difficulty, and the interacting effect between reward and task difficulty was found in the successful retrieval with high confidence. These findings suggest that the reward‐related enhancement of subjective motivation in the Difficult retrieval condition, in which the task difficulty was modulated optimally to maximize the subjective motivation, could be good enough to increase the retrieval of memories and that this effect could contribute to the retrieval of memory details related to word items. The importance of rewards or punishments in performing cognitive tasks adjusted to an optimal level of task difficulty has been reported in previous studies for experimental animals and humans. For example, a previous study using experimental animals reported that the improvement in task performance resulting from electrical shocks delivered as a punishment was larger in a discrimination task with high difficulty than that with low difficulty, and the optimal level of the shock intensity differed according to the task difficulty [Yerkes and Dodson, 1908]. In previous studies with human participants, responses in acquiring rewards and avoiding punishments were faster when participants predicted the need for greater efforts to perform the tasks [Kurniawan et al., 2013], and the reward‐related enhancement of a motor task under the optimal level of reward was found only when the task was difficult to complete [Chib et al., 2012]. Other studies have demonstrated that the enhancing effect of reward on the performance of cognitive tasks is highest when the cognitive control demands are of intermediate difficulty [Bahlmann et al., 2015] and that there is no reward‐related enhancement when the retrieval performance is sufficiently high in reward‐motivated memory retrieval tasks in which participants are shown reward values associated with the target stimuli just before the retrieval phase [Elward et al., 2015]. Taken together, the effect of reward on behavioral performance in cognitive tasks, including episodic memory, could be most effective when the task difficulty is adjusted to optimal levels, and the reward‐related enhancement of memory retrieval could be mediated by a reward‐related increase in subjective motivation at the optimal levels of task difficulty.
Reward‐Related Increase in Activation of the SN/VTA, MTL, dmPFC, and dlPFC During the Retrieval of Memories With High Difficulty
The second main finding of our study was that reward‐related increases in activation of the SN/VTA, MTL (anterior and posterior PHG), dmPFC, and dlPFC were greater during the retrieval of memories with high difficulty than those with low difficulty (see Fig. 3a). In addition, during high‐difficulty memory retrieval, the reward‐related increase in SN/VTA activation was significantly correlated with the reward‐related enhancement of retrieval accuracy with high confidence (see Fig. 3b). These findings suggest that the SN/VTA, MTL, dmPFC, and dlPFC could contribute to the enhancing effects by rewards on memory retrieval when the retrieval of memories is difficult, and the reward‐related enhancement of memories with high difficulty could be modulated mainly by SN/VTA activation.
In the present study, we found that reward‐related increases in activation of the SN/VTA and MTL were modulated by task difficulty. These results are consistent with functional neuroimaging studies linking activation of the SN/VTA, striatum, and MTL to the reward‐related enhancement of memories [Adcock et al., 2006; Dillon et al., 2014; Krebs et al., 2009; Murty and Adcock, 2014; Shigemune et al., 2014; Wittmann et al., 2008, 2005; Wolosin et al., 2012, 2013]. For example, source memories were enhanced by monetary rewards or punishments, and this process was associated with an interaction between the activation of the reward‐ and punishment‐related SN/VTA and striatal regions and the activation of the memory‐related hippocampus [Shigemune et al., 2014]. Furthermore, the reward‐related enhancement of memories for item‐related associations was modulated by interacting mechanisms between the memory‐related MTL regions, including the hippocampus and PHG, and the reward‐related SN/VTA [Dillon et al., 2014; Wolosin et al., 2012, 2013]. The present finding, in which the reward‐related increase in SN/VTA activation was significantly correlated with the reward‐related enhancement of retrieval accuracy during high‐difficulty memory retrieval, is consistent with functional neuroimaging evidence showing a significant correlation between SN/VTA activation and memory performance during the anticipation of rewards [Adcock et al., 2006]. One fMRI study reported that reward‐related improvement in motor tasks was observed only when the task demands were highly difficult and the reward‐related modulation was associated with activation in the striatum [Chib et al., 2012]. Given that an anterior part of the PHG is involved in the memory processes of item‐related details [Staresina and Davachi, 2008, 2010] and that a posterior part of the PHG contributes to the memory processes of spatial information [Buffalo et al., 2006; Manelis et al., 2012; Ploner et al., 2000; Sommer et al., 2005], the SN/VTA and MTL (anterior and posterior PHG) identified in the present study could play an important role in enhancing the effect of monetary rewards on memory for item‐related details, such as the arrangement of encoded word pairs. The contributions of these regions could be the most influential when the difficulty level of memory tasks is adjusted to be optimally difficult.
The present finding that reward‐related increases in activation of the dmPFC and dlPFC were modulated by task difficulty has been consistently reported in functional neuroimaging studies [Bahlmann et al., 2015; Burke et al., 2013; Cameron et al., 2004; Chib et al., 2012; Engelmann et al., 2009; Kouneiher et al., 2009; Krawczyk et al., 2007; Kurniawan et al., 2013; Pochon et al., 2002; Taylor et al., 2004]. For example, one fMRI study demonstrated that the beneficial effect of rewards on task performance was strongest when participants performed a cognitive task with a median level of task difficulty, and the activation in the dmPFC and dlPFC reflected the interaction between reward and task difficulty [Bahlmann et al., 2015]. Functional neuroimaging studies have shown a possible role of the dmPFC, including the pre‐SMA and ACC, in voluntary movement and intention [Haggard, 2008; Winterer et al., 2002], as well as a role of the dlPFC in cognitive control [D'Esposito et al., 1998; Owen, 1997; Smith and Jonides, 1999]. Thus, the dmPFC and dlPFC could contribute to reward‐related enhancement of highly demanding episodic memories by the motivation‐driven intentions and cognitive controls of receiving rewards.
Functional Connectivity Between the SN/VTA and the MTL, and the dmPFC
The third main finding of our study was that the modulatory effect of reward on the functional connectivity of the SN/VTA as a seed region with the MTL (anterior and posterior PHG) and dmPFC was significantly correlated with the reward‐related enhancement of retrieval performance and subjective motivation only when the retrieval of memories was difficult. This finding suggests that interacting mechanisms among the SN/VTA, MTL, and dmPFC could contribute to the reward‐related enhancement of memory retrieval when the task difficulty is adjusted to an optimal level.
The present finding of functional connectivity between the SN/VTA and MTL (anterior and posterior PHG) is supported by evidence from previous studies indicating anatomical and intrinsic functional connections between the SN/VTA and MTL [Beckstead, 1978; Haber and Knutson, 2010; Kahn and Shohamy, 2013]. In addition, functional neuroimaging studies have revealed that task‐related functional connectivity between the SN/VTA and MTL during the processing of episodic memories is significantly strengthened by monetary rewards [Adcock et al., 2006; Shigemune et al., 2014; Wolosin et al., 2012]. Together with the present finding that the reward‐related enhancement of functional connectivity between activations in the SN/VTA and MTL was significantly correlated with the memory performance during high‐difficulty memory retrieval, these findings suggest that interactions between activations in the SN/VTA and MTL could contribute more to the reward‐related modulation of the retrieval of memories for item‐related details than that for the simple recognition of memory items.
In the present study, the regression analyses of PPI contrasts with subjective ratings of motivation demonstrated that the reward‐related enhancement of functional connectivity between the SN/VTA and dmPFC was significantly correlated with the enhancing effects of reward on subjective ratings of motivation during the retrieval of memories with high difficulty. This finding is consistent with previous findings that have demonstrated the anatomical and functional connection between the SN/VTA and the dmPFC [Haber and Knutson 2010; Murty and Adcock 2014]. For example, one fMRI study has reported that functional connectivity between the SN/VTA and dmPFC is modulated by the reward‐motivated enhancement of MTL activations in a reaction time task in which participants encounter goal‐irrelevant expectancy violations [Murty and Adcock, 2014]. Given that the SN/VTA is associated with the processing of rewards [D'Ardenne et al., 2008], and that the dmPFC is important in the processing of motivation [Kouneiher et al., 2009], the present findings on functional connectivity suggest that the motivation of receiving rewards under an optimal level of task difficulty could be related to the interaction between the SN/VTA and the dmPFC. Thus, functional networks including the SN/VTA, MTL, and dmPFC could contribute to the reward‐related enhancement of memory retrieval for item‐related details, and the enhancement could be most effective when the task difficulty is optimally adjusted.
Limitations
In the present study, there are several potential limitations. The first potential limitation is that d‐primes were not available in each retrieval condition. Given that the retrieval difficulty was modulated by the encoding operations of “deep” (Easy retrieval) or “shallow” (Difficult retrieval) encoding strategy, responses for distracter (new) words were not categorized by a factor of task difficulty. Thus, it would be inappropriate to calculate d‐prime scores by High‐confidence Hit rates including both factors of reward and task difficulty and FA rates including only a factor of reward. As shown in a paired t‐test for FA rates of the behavioral results, we did not find the possibility of response bias between the High and Low reward conditions in the retrieval phase. Given that FA rates for both conditions of task difficulty were common and identical in the present study, however, it was difficult to examine whether High‐confidence Hit rates were modulated by different effects of response bias between the Difficult and Easy retrieval conditions. Further analyses of the different response criterion between the two task difficulty conditions would be required by defining FA responses for each task difficulty in a new experimental paradigm.
The second potential limitation is that cluster sizes identified in several regions were small, although activation and functional connectivity in these regions were still significant after the SVC on the statistical threshold of P < 0.001 at the voxel level and corrected by FWE at the cluster level (P < 0.05). Activation and functional connectivity patterns in these regions should be interpreted with some caution and be carefully replicated in future studies. Given that these results were consistent with our hypothesis, however, the importance of these findings should be emphasized in the present study. Further studies would be required to strengthen these results.
CONCLUSIONS
In the present fMRI study, we investigated the neural mechanisms underlying the reward‐related enhancement of memory during the retrieval of episodic memories with high difficulty. The experiment in this study yielded three major findings. First, subjective ratings of motivation were significantly increased by monetary rewards in the retrieval of memories with high difficulty, and the reward‐related enhancement of memories was found in the retrieval of memories with high difficulty, but not with low difficulty. Second, activation associated with the rewarding effect on retrieving memories with high difficulty was identified in the SN/VTA, MTL, dmPFC, and dlPFC. Further, the reward‐related increase in SN/VTA activation was significantly correlated with the reward‐related increase in the accuracy of high‐difficulty memory retrieval. Third, the regression analyses for PPI contrasts with retrieval accuracy and subjective ratings of motivation showed that the reward‐related increases of functional connectivity between the SN/VTA and MTL and between the SN/VTA and dmPFC were significantly correlated with the reward‐related enhancement of retrieval accuracy and motivation ratings during high‐difficulty memory retrieval. These findings suggest that the reward‐related enhancement of episodic memories could be greatest when the memory retrieval is appropriately difficult, and that the rewarding effects on memory retrieval in an optimal level of task difficulty could be involved in the interacting mechanisms of the reward‐related SN/VTA, memory‐related MTL and motivation‐related dmPFC.
CONFLICT OF INTEREST
None of authors have a potential conflict of interest.
Address correspondence to Yayoi Shigemune at
Department of Psychology, Graduate School of Letters, Chuo University, Room 3420, 742‐1 Higashinakano, Hachioji‐shi, Tokyo 192‐0393 Japan. E‐mail: gemune@gmail.com
Supporting information
Supporting Information
REFERENCES
- Adcock RA, Thangavel A, Whitfield‐Gabrieli S, Knutson B, Gabrieli JD (2006): Reward‐motivated learning: Mesolimbic activation precedes memory formation. Neuron 50:507–517. [DOI] [PubMed] [Google Scholar]
- Amano S, Kondo T (1999): NTT Database Series: Nihongo‐No goitokusei (Lexical Properties of Japanese). Tokyo: Sanseido. [Google Scholar]
- Anshel HM, Weinberg R (1992): The effect of goal difficulty and task complexity on intrinsic motivation and motor performance. J Sport Behav 15:159–176. [Google Scholar]
- Arkes HR (1979): Competence and the overjustification effect. Motiv Emotion 3:143–150. [Google Scholar]
- Arkes HR, Clark P (1975): Effects of task difficulty on subsequent preference for visual complexity. Percept Mot Skills 41:395–399. [DOI] [PubMed] [Google Scholar]
- Bahlmann J, Aarts E, D'Esposito M (2015): Influence of motivation on control hierarchy in the human frontal cortex. J Neurosci 35:3207–3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckstead RM (1978): Afferent connections of the entorhinal area in the rat as demonstrated by retrograde cell‐labeling with horseradish peroxidase. Brain Res 152:249–264. [DOI] [PubMed] [Google Scholar]
- Berlyne ED (1960): Conflict, Arousal, and Curiosity. McGraw‐Hill Series in Psychology. New York: McGraw‐Hill Book Company. [Google Scholar]
- Boehler CN, Hopf JM, Krebs RM, Stoppel CM, Schoenfeld MA, Heinze HJ, Noesselt T (2011): Task‐load‐dependent activation of dopaminergic midbrain areas in the absence of reward. J Neurosci 31:4955–4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Koutstaal W, Schacter DL, Wagner AD, Rosen BR (1998): Functional‐anatomic study of episodic retrieval using fMRI. I. Retrieval effort versus retrieval success. Neuroimage 7:151–162. [DOI] [PubMed] [Google Scholar]
- Buffalo EA, Bellgowan PS, Martin A (2006): Distinct roles for medial temporal lobe structures in memory for objects and their locations. Learn Mem 13:638–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke CJ, Brunger C, Kahnt T, Park SQ, Tobler PN (2013): Neural integration of risk and effort costs by the frontal pole: only upon request. J Neurosci 33:1706–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron J, Pierce W, So S (2004): Rewards, task difficulty, and intrinsic motivation: A test of learned industriousness theory. Alberta J Educ Res 50:317–320. [Google Scholar]
- Chib VS, De Martino B, Shimojo S, O'Doherty JP (2012): Neural mechanisms underlying paradoxical performance for monetary incentives are driven by loss aversion. Neuron 74:582–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craik IMF, Lockhart SR (1972): Levels of processing: A framework for memory research. J Verbal Learn Verbal Behav 11:671–684. [Google Scholar]
- Craik IMF, Tulving E (1975): Depth of processing and the retention of words in episodic memory. J Exp Psychol Gen 104:268–294. [Google Scholar]
- D'Ardenne K, McClure SM, Nystrom LE, Cohen JD (2008): BOLD responses reflecting dopaminergic signals in the human ventral tegmental area. Science 319:1264–1267. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Aguirre GK, Zarahn E, Ballard D, Shin RK, Lease J (1998): Functional MRI studies of spatial and nonspatial working memory. Brain Res Cogn Brain Res 7:1–13. [DOI] [PubMed] [Google Scholar]
- Deci EL, Koestner R, Ryan RM (1999): A meta‐analytic review of experiments examining the effects of extrinsic rewards on intrinsic motivation. Psychol Bull 125:627–668; discussion 692‐700. [DOI] [PubMed] [Google Scholar]
- Deci LE (1971): Effects of externally mediated rewards on intrinsic motivation. J Pers Soc Psychol 18:105–115. [Google Scholar]
- Dillon DG, Dobbins IG, Pizzagalli DA (2014): Weak reward source memory in depression reflects blunted activation of VTA/SN and parahippocampus. Soc Cogn Affect Neurosci 9:1576–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbins IG, Han S (2006): Isolating rule‐ versus evidence‐based prefrontal activity during episodic and lexical discrimination: A functional magnetic resonance imaging investigation of detection theory distinctions. Cereb Cortex 16:1614–1622. [DOI] [PubMed] [Google Scholar]
- Donahue CH, Lee D (2015): Dynamic routing of task‐relevant signals for decision making in dorsolateral prefrontal cortex. Nat Neurosci 18:295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy E (1957): The psychological significance of the concept of arousal or activation. Psychol Rev 64:265–275. [DOI] [PubMed] [Google Scholar]
- Elward RL, Vilberg KL, Rugg MD (2015): Motivated memories: Effects of reward and recollection in the core recollection network and beyond. Cereb Cortex 25:3159–3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann JB, Damaraju E, Padmala S, Pessoa L (2009): Combined effects of attention and motivation on visual task performance: Transient and sustained motivational effects. Front Hum Neurosci 3:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etzel JA, Cole MW, Zacks JM, Kay KN, Braver TS (2016): Reward motivation enhances task coding in frontoparietal cortex. Cereb Cortex 26:1647–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ (1997): Psychophysiological and modulatory interactions in neuroimaging. Neuroimage 6:218–229. [DOI] [PubMed] [Google Scholar]
- Gitelman DR, Penny WD, Ashburner J, Friston KJ (2003): Modeling regional and psychophysiologic interactions in fMRI: The importance of hemodynamic deconvolution. Neuroimage 19:200–207. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B (2010): The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology 35:4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggard P (2008): Human volition: Towards a neuroscience of will. Nat Rev Neurosci 9:934–946. [DOI] [PubMed] [Google Scholar]
- Hartstra E, Oldenburg JF, Van Leijenhorst L, Rombouts SA, Crone EA (2010): Brain regions involved in the learning and application of reward rules in a two‐deck gambling task. Neuropsychologia 48:1438–1446. [DOI] [PubMed] [Google Scholar]
- Hebb DO (1955): Drives and the C.N.S. (conceptual nervous system). Psychol Rev 62:243–254. [DOI] [PubMed] [Google Scholar]
- Henson RN, Hornberger M, Rugg MD (2005): Further dissociating the processes involved in recognition memory: An FMRI study. J Cogn Neurosci 17:1058–1073. [DOI] [PubMed] [Google Scholar]
- Hom LH, Maxwell RF (1983): The impact of task difficulty expectations on intrinsic motivation. Motiv Emotion 7:19–24. [Google Scholar]
- Ishikawa A, Ambroggi F, Nicola SM, Fields HL (2008a): Contributions of the amygdala and medial prefrontal cortex to incentive cue responding. Neuroscience 155:573–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa A, Ambroggi F, Nicola SM, Fields HL (2008b): Dorsomedial prefrontal cortex contribution to behavioral and nucleus accumbens neuronal responses to incentive cues. J Neurosci 28:5088–5098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeggi SM, Seewer R, Nirkko AC, Eckstein D, Schroth G, Groner R, Gutbrod K (2003): Does excessive memory load attenuate activation in the prefrontal cortex? Load‐dependent processing in single and dual tasks: Functional magnetic resonance imaging study. Neuroimage 19:210–225. [DOI] [PubMed] [Google Scholar]
- Jimura K, Locke HS, Braver TS (2010): Prefrontal cortex mediation of cognitive enhancement in rewarding motivational contexts. Proc Natl Acad Sci USA 107:8871–8876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn I, Shohamy D (2013): Intrinsic connectivity between the hippocampus, nucleus accumbens, and ventral tegmental area in humans. Hippocampus 23:187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochan NA, Valenzuela M, Slavin MJ, McCraw S, Sachdev PS, Breakspear M (2011): Impact of load‐related neural processes on feature binding in visuospatial working memory. PLoS One 6:e23960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouneiher F, Charron S, Koechlin E (2009): Motivation and cognitive control in the human prefrontal cortex. Nat Neurosci 12:939–945. [DOI] [PubMed] [Google Scholar]
- Krawczyk DC, Gazzaley A, D'Esposito M (2007): Reward modulation of prefrontal and visual association cortex during an incentive working memory task. Brain Res 1141:168–177. [DOI] [PubMed] [Google Scholar]
- Krebs RM, Schott BH, Schutze H, Duzel E (2009): The novelty exploration bonus and its attentional modulation. Neuropsychologia 47:2272–2281. [DOI] [PubMed] [Google Scholar]
- Kurniawan IT, Guitart‐Masip M, Dayan P, Dolan RJ (2013): Effort and valuation in the brain: The effects of anticipation and execution. J Neurosci 33:6160–6169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPorte ER, Nath R (1976): Role of performance goals in prose learning. J Educ Psychol 68:260–264. [Google Scholar]
- Lepper RM, Greene D, Nisbett ER (1973): Undermining children's intrinsic interest with extrinsic rewards: A test of the “overjustification” hypothesis. J Pers Soc Psychol 28:129–137. [Google Scholar]
- Leung HC, Oh H, Ferri J, Yi Y (2007): Load response functions in the human spatial working memory circuit during location memory updating. Neuroimage 35:368–377. [DOI] [PubMed] [Google Scholar]
- Livesey AC, Wall MB, Smith AT (2007): Time perception: manipulation of task difficulty dissociates clock functions from other cognitive demands. Neuropsychologia 45:321–331. [DOI] [PubMed] [Google Scholar]
- Locke AE, Latham PG (1990): A Theory of Goal Setting & Task Performance. Englewood Cliffs, NJ: Prentice Hall. [Google Scholar]
- Locke EA, Latham GP (2002): Building a practically useful theory of goal setting and task motivation. A 35‐year odyssey. Am Psychol 57:705–717. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Burdette JH (2004): Precentral gyrus discrepancy in electronic versions of the Talairach atlas. Neuroimage 21:450–455. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH (2003): An automated method for neuroanatomic and cytoarchitectonic atlas‐based interrogation of fMRI data sets. Neuroimage 19:1233–1239. [DOI] [PubMed] [Google Scholar]
- Malmo RB (1959): Activation: A neuropsychological dimension. Psychol Rev 66:367–386. [DOI] [PubMed] [Google Scholar]
- Mandzia JL, Black SE, McAndrews MP, Grady C, Graham S (2004): fMRI differences in encoding and retrieval of pictures due to encoding strategy in the elderly. Hum Brain Mapp 21:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manelis A, Reder LM, Hanson SJ (2012): Dynamic changes in the medial temporal lobe during incidental learning of object‐location associations. Cereb Cortex 22:828–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren DG, Ries ML, Xu G, Johnson SC (2012): A generalized form of context‐dependent psychophysiological interactions (gPPI): A comparison to standard approaches. Neuroimage 61:1277–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan M (1984): Reward‐induced decrements and increments in intrinsic motivation. Rev Educ Res 54:5–30. [Google Scholar]
- Murty VP, Adcock RA (2014): Enriched encoding: Reward motivation organizes cortical networks for hippocampal detection of unexpected events. Cereb Cortex 24:2160–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murty VP, Shermohammed M, Smith DV, Carter RM, Huettel SA, Adcock RA (2014): Resting state networks distinguish human ventral tegmental area from substantia nigra. Neuroimage 100:580–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM (1997): The functional organization of working memory processes within human lateral frontal cortex: The contribution of functional neuroimaging. Eur J Neurosci 9:1329–1339. [DOI] [PubMed] [Google Scholar]
- Ploner CJ, Gaymard BM, Rivaud‐Pechoux S, Baulac M, Clemenceau S, Samson S, Pierrot‐Deseilligny C (2000): Lesions affecting the parahippocampal cortex yield spatial memory deficits in humans. Cereb Cortex 10:1211–1216. [DOI] [PubMed] [Google Scholar]
- Pochon JB, Levy R, Fossati P, Lehericy S, Poline JB, Pillon B, Le Bihan D, Dubois B (2002): The neural system that bridges reward and cognition in humans: An fMRI study. Proc Natl Acad Sci USA 99:5669–5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado J, Noveck IA (2007): Overcoming perceptual features in logical reasoning: A parametric functional magnetic resonance imaging study. J Cogn Neurosci 19:642–657. [DOI] [PubMed] [Google Scholar]
- Reas ET, Brewer JB (2013): Retrieval search and strength evoke dissociable brain activity during episodic memory recall. J Cogn Neurosci 25:219–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan MR, Mims V, Koestner R (1983): Relation of reward contingency and interpersonal context to intrinsic motivation: A review and test using cognitive evaluation theory. J Pers Soc Psychol 45:736–750. [Google Scholar]
- Satterthwaite TD, Green L, Myerson J, Parker J, Ramaratnam M, Buckner RL (2007): Dissociable but inter‐related systems of cognitive control and reward during decision making: Evidence from pupillometry and event‐related fMRI. Neuroimage 37:1017–1031. [DOI] [PubMed] [Google Scholar]
- Scangos KW, Stuphorn V (2010): Medial frontal cortex motivates but does not control movement initiation in the countermanding task. J Neurosci 30:1968–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott BH, Wustenberg T, Wimber M, Fenker DB, Zierhut KC, Seidenbecher CI, Heinze HJ, Walter H, Duzel E, Richardson‐Klavehn A (2013): The relationship between level of processing and hippocampal‐cortical functional connectivity during episodic memory formation in humans. Hum Brain Mapp 34:407–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalley CE, Oldham GR (1985): Effects of goal difficulty and expected external evaluation on intrinsic motivation: A laboratory study. Acad Manage J 28:628–640. [Google Scholar]
- Shigemune Y, Tsukiura T, Kambara T, Kawashima R (2014): Remembering with gains and losses: Effects of monetary reward and punishment on successful encoding activation of source memories. Cereb Cortex 24:1319–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, Jonides J (1999): Storage and executive processes in the frontal lobes. Science 283:1657–1661. [DOI] [PubMed] [Google Scholar]
- Sommer T, Rose M, Weiller C, Buchel C (2005): Contributions of occipital, parietal and parahippocampal cortex to encoding of object‐location associations. Neuropsychologia 43:732–743. [DOI] [PubMed] [Google Scholar]
- Staresina BP, Davachi L (2008): Selective and shared contributions of the hippocampus and perirhinal cortex to episodic item and associative encoding. J Cogn Neurosci 20:1478–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staresina BP, Davachi L (2010): Object unitization and associative memory formation are supported by distinct brain regions. J Neurosci 30:9890–9897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SF Taylor, RC Welsh, TD Wager, KL Phan, KD Fitzgerald, WJ Gehring (2004): A functional neuroimaging study of motivation and executive function. Neuroimage 21:1045–1054. [DOI] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. Neuroimage 15:273–289. [DOI] [PubMed] [Google Scholar]
- Wang Q, Luo S, Monterosso J, Zhang J, Fang X, Dong Q, Xue G (2014): Distributed value representation in the medial prefrontal cortex during intertemporal choices. J Neurosci 34:7522–7530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Hikosaka K, Sakagami M, Shirakawa S (2002): Coding and monitoring of motivational context in the primate prefrontal cortex. J Neurosci 22:2391–2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterer G, Adams CM, Jones DW, Knutson B (2002): Volition to action–an event‐related fMRI study. Neuroimage 17:851–858. [PubMed] [Google Scholar]
- Wittmann BC, Schiltz K, Boehler CN, Duzel E (2008): Mesolimbic interaction of emotional valence and reward improves memory formation. Neuropsychologia 46:1000–1008. [DOI] [PubMed] [Google Scholar]
- Wittmann BC, Schott BH, Guderian S, Frey JU, Heinze HJ, Duzel E (2005): Reward‐related FMRI activation of dopaminergic midbrain is associated with enhanced hippocampus‐dependent long‐term memory formation. Neuron 45:459–467. [DOI] [PubMed] [Google Scholar]
- Wolosin SM, Zeithamova D, Preston AR (2012): Reward modulation of hippocampal subfield activation during successful associative encoding and retrieval. J Cogn Neurosci 24:1532–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolosin SM, Zeithamova D, Preston AR (2013): Distributed hippocampal patterns that discriminate reward context are associated with enhanced associative binding. J Exp Psychol Gen 142:1264–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward TS, Cairo TA, Ruff CC, Takane Y, Hunter MA, Ngan ET (2006): Functional connectivity reveals load dependent neural systems underlying encoding and maintenance in verbal working memory. Neuroscience 139:317–325. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC (1996): A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp 4:58–73. [DOI] [PubMed] [Google Scholar]
- Yerkes MR, Dodson DJ (1908): The relation of strength of stimulus to rapidity of habit‐formation. J Comp Neurol Psychol 18:459–482. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


