Abstract
Mild cognitive impairment (MCI) is prevalent in 15%–40% of Parkinson's disease (PD) patients at diagnosis. In this investigation, we study brain intra‐ and inter‐network alterations in resting state functional magnetic resonance imaging (rs‐fMRI) in recently diagnosed PD patients and characterise them as either cognitive normal (PD‐NC) or with MCI (PD‐MCI). Patients were divided into two groups, PD‐NC (N = 62) and PD‐MCI (N = 37) and for comparison, healthy controls (HC, N = 30) were also included. Intra‐ and inter‐network connectivity were investigated from participants’ rs‐fMRIs in 26 resting state networks (RSNs). Intra‐network differences were found between both patient groups and HCs for networks associated with motor control (motor cortex), spatial attention and visual perception. When comparing both PD‐NC and PD‐MCI, intra‐network alterations were found in RSNs related to attention, executive function and motor control (cerebellum). The inter‐network analysis revealed a hyper‐synchronisation between the basal ganglia network and the motor cortex in PD‐NC compared with HCs. When both patient groups were compared, intra‐network alterations in RSNs related to attention, motor control, visual perception and executive function were found. We also detected disease‐driven negative synchronisations and synchronisation shifts from positive to negative and vice versa in both patient groups compared with HCs. The hyper‐synchronisation between basal ganglia and motor cortical RSNs in PD and its synchronisation shift from negative to positive compared with HCs, suggest a compensatory response to basal dysfunction and altered basal‐cortical motor control in the resting state brain of PD patients. Hum Brain Mapp 38:1702–1715, 2017. © 2016 Wiley Periodicals, Inc.
Keywords: FSL‐Nets, networks, Lewy body disease, fMRI, brain connectivity
INTRODUCTION
Parkinson's disease dementia (PDD) is a frequent complication of PD with a cumulative incidence approaching 80% [Hely et al., 2008]. Mild cognitive impairment associated with PD (PD‐MCI) is recognised as a clinical entity with a prevalence of 15%–40% at PD diagnosis and is a risk factor for the subsequent development of PDD [Yarnall et al., 2014]. Both PD‐MCI and PDD contribute to a poorer quality of life [Lawson et al., 2014], with PDD resulting in increased falls, neuropsychiatric disturbance, increased carer burden, loss of independence and higher likelihood of nursing home placement [Yarnall et al., 2013].
PD‐MCI as defined by the Movement Disorders Society (MDS) Task Force [Litvan et al., 2012] encompasses cognitive deficits of executive, attention, memory, language and visuospatial functions. The underlying pathology of the motor aspects of PD is relatively well understood with established evidence‐based therapies [Fox et al., 2011]. However, the aetiology underlying cognitive deficit in PD is less well known. At the cellular level, there is high variability in Lewy body‐related pathological load and in the distribution of the pathology in cortical and subcortical structures. Furthermore, there remains an undetermined role for additional concurrent pathologies such as neurofibrillary tangles, tau and amyloid‐β deposition in PD, although these are associated with cognitive decline [Halliday et al., 2011; Irwin et al., 2013] and dynamic changes in neurotransmitter pathways [Kehagia et al., 2010] are also a major feature. Consequently, there is a high degree of heterogeneity in the type and severity of non‐motor symptoms in patients with PD‐MCI and PDD [Galvin et al., 2006; Parkkinen et al., 2008].
Though the cellular level pathologies are diverse and complex, they do result in brain‐wide neural system dysfunctions which are more amenable to investigation [Gratwicke et al., 2015]. These macrolevel functional alterations have the potential to be biomarkers of disease progression, particularly when related to the cognitive changes seen in PD, and one methodology employed to measure this is resting state functional magnetic resonance imaging (rs‐fMRI). Independent component analysis (ICA), in particular, is a powerful data‐driven method that decomposes rs‐fMRI data into resting state networks (RSNs). These RSNs are associated with distinct brain functions [Baggio et al., 2015a], and can show changes or functional alterations which are specific to a particular disease [Dipasquale et al., 2015].
The most widely reported RSN, the default mode network (DMN), plays an active role in cognitive processing with previous studies showing impaired deactivation in PD‐MCI [Van Eimeren et al., 2009] and ‘over activity’ in PD [Shine et al., 2011, 2014]. It is dynamically linked to several other cognitive networks, including the dorsal attentional network (DAN) and ventral attention network (VAN), both of which also show connectivity changes in PD‐MCI [Baggio et al., 2015b; Peraza et al., 2015a; Shine et al., 2014]. As a collective, the DMN, DAN and VAN, together with several other RSNs, underlie specific cognitive processes such as attention, executive and motor functions.
The aim of this study was to quantify changes in rs‐fMRI intra and inter‐network connectivity in early PD and PD‐MCI using high dimensional ICA, dual regression and informative network connectivity measures [Filippini et al., 2009]. We hypothesised that participants with PD and, in particular, those with PD‐MCI, would show functional connectivity alterations in the DMN, DAN and VAN, and that there would be changes in connectivity both within and between these networks.
METHODS
Recruitment and Clinical Assessments
Participants with a new diagnosis of PD were recruited from clinics in Newcastle upon Tyne and Gateshead areas of the UK as part of the Incidence of Cognitive Impairment in Cohorts with Longitudinal Evaluation in PD (ICICLE‐PD) study [Yarnall et al., 2014]. The diagnosis of PD was made by a movement disorder specialist utilising the United Kingdom Parkinson's Disease Society Brain Bank Criteria for idiopathic PD [Hughes et al., 2002], with reconfirmation after 18 months from their first assessment. Exclusion criteria included Parkinsonism diagnosed before study launch, insufficient English proficiency to complete neuropsychological assessments, advanced cognitive impairment (Mini‐Mental State Examination, MMSE < 24) or dementia at diagnosis [Emre et al., 2007], and suggestive diagnosis of other brain conditions and Parkinsonian diseases such as dementia with Lewy bodies (DLB), progressive supranuclear palsy, and repeated strokes [Duncan et al., 2015; Yarnall et al., 2014]. Additionally, unrelated healthy control participants (HCs) were enrolled from community sources in Newcastle/Gateshead to provide normative data. The study was approved by the Newcastle and North Tyneside Research Ethics Committee and all subjects provided written informed consent.
Study participants completed a battery of neuropsychological tests: MMSE, the Montreal cognitive assessment (MoCA) [Nasreddine et al., 2005], selected tests from the Cambridge Neuropsychological Test Automated Battery [CANTAB: Tower of London, Paired Associates Learning (PAL), Spatial Recognition Memory (SRM), Pattern Recognition Memory (PRM), semantic fluency and phonetic fluency] [Robbins et al., 1994]. Power of attention (PoA) was estimated as a composite score of simple reaction time, choice reaction time and digit vigilance from the Cognitive Drug Research (CDR) battery. Motor disease severity was assessed with the revised Movement Disorder Society Unified Parkinson's Disease Rating Scale (MDS‐UPDRS) part III [Goetz et al., 2008]. The National Adult Reading Test (NART) [Mathias et al., 2007] was used to estimate premorbid levels of intelligence. Patients were assessed and underwent MRI scanning in the ‘ON’ state; that is, taking their usual dopaminergic medication, which was standardised to the Levodopa equivalent daily dose (LEDD) [Tomlinson et al., 2010].
In agreement with previous studies [Duncan et al., 2015; Mak et al., 2015; Yarnall et al., 2014], we applied recommended modified level II MDS criteria [Litvan et al., 2012] for the diagnosis of PD‐MCI with a threshold of 1.5 standard deviations (SD) below normative values (HCs). The level II MDS criteria establish that patients below this threshold in at least two neuropsychological tests assessing five cognitive domains (attention, execution, visuospatial, memory and language) would qualify for the diagnosis of PD‐MCI. A complete description of the participants’ clinical and neuropsychological assessments can be found in Yarnall et al. [2014].
MRI Acquisition and Pre‐Processing
A total of 32 HC and 121 PD participants had brain resting state functional (rs‐fMRI) and structural (MRI) taken with a 3 Tesla Philips Intera Achieva scanner. Structural images were obtained with a magnetisation prepared rapid gradient‐echo sequence (MP‐RAGE), sagittal acquisition, echo time 4.6 ms, repetition time 8.3 ms, inversion time 1250 ms, flip angle = 8°, sensitivity encoding factor = 2, and in plane field of view of 240 × 240 mm with slice thickness of 1.0 mm. For the rs‐fMRI, participants were asked to lie inside of the scanner with their eyes closed during the recording. Functional images were acquired with a gradient‐echo planar imaging sequence with 25 contiguous axial slices, in‐plane resolution = 2 × 2 mm, slice thickness of 6 mm and repetition time of 3 seconds. A total of 128 fMRI volumes were obtained per participant.
Images were pre‐processed using FSL (FMRIB Software Library version 5.0, http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/). Functional images were slice timing and motion corrected. Additionally, to further reduce artefactual influences, the six movement parameters (rotations and translations), and the cerebrospinal fluid signal from the lateral ventricles were regressed out using the Resting‐State fMRI Data Analysis Toolkit (REST) [Song et al., 2011] in Matlab (MATLAB 7.14, The MathWorks Inc., Natick, MA). Finally, functional and structural images were linearly coregistered to the MNI (Montreal Neurology Institute) standard space. Functional images were spatially resampled to 4 × 4 × 4 mm voxel size, high pass filtered (150 seconds) and spatially smoothed with a 6 mm full width half maximum (FWHM) filter.
In order to maximise data quality for this study, we applied a conservative exclusion criteria for movement within the MRI scanner; those participants that moved greater than 2 mm translation or greater than 1° rotation were excluded from the analysis [Liao et al., 2010; Peraza et al., 2015b].
Independent Component Analysis and Dual Regression
The multivariate exploratory linear optimized decomposition into independent components (MELODIC) algorithm within FSL was implemented to estimate canonical RSNs [Beckmann et al., 2005]. For this, a high dimensional group concatenated MELODIC was run in the HC group to obtain 70 independent component maps; this number of components is recommended in order to obtain accurate maps for subcortical brain regions [Abou Elseoud et al., 2011; Dipasquale et al., 2015]. Component maps were visually analysed in their spatial and spectral content [Kelly et al., 2010] and 26 RSNs were identified as being of biological interest according to the previous literature [Agosta et al., 2012; Beckmann et al., 2005; Damoiseaux et al., 2008] (see Fig. 1).
Figure 1.
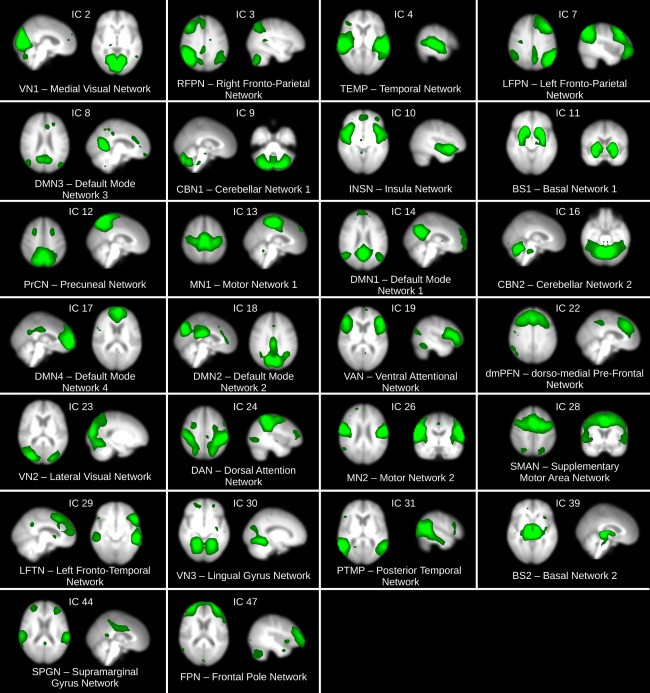
Resting state networks (RSNs) inferred from the HC group using group ICA (MELODIC). A total of 70 independent components were estimated and 26 of them identified as of biological interest according to the previous literature. Similarly RSN names were assigned following convention in previous literature when possible; otherwise names were given according to the highest positive region in the RSN map. Brain maps are shown in radiological convention. [Color figure can be viewed at http://wileyonlinelibrary.com]
FSL‐dual regression [Filippini et al., 2009] was implemented to assess intra‐RSN connectivity. Briefly, dual‐regression spatially regresses the canonical RSNs on each participant's fMRI to extract a representative time series per map (first regression). Posteriorly, these time series are regressed again onto each participant's fMRI (second regression) in order to obtain the individual RSN maps, which are used for group comparisons. Prior to dual‐regression, the 26 RSNs were concatenated in a single 4D file for the dual‐regression algorithm. In this way, the algorithm evaluates each RSN while the remaining 25 are used as spatial covariates.
Assessing Inter‐Network Connectivity
Inter‐network connectivity was investigated using FSL‐Nets (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FSLNets). This method takes the time series from the first regression in the dual‐regression analysis to create inter‐network connectivity matrices, which were estimated in this investigation using normalised covariance and transformed to Fisher‐z scores as implemented in the FSL‐Nets tool.
Statistical Analysis
Group differences in age were assessed with a one‐way ANOVA for the HC, PD‐NC and PD‐MCI groups. Gender differences were studied with a χ 2 test. Years of education and NART differences among the three groups were assessed with Kruskal–Wallis tests. Disease duration was assessed with a Mann–Whitney test between patient groups. LEDD, MDS‐UPDRS III, MMSE and MoCA were analysed for statistical comparisons with standard student t‐tests between both patient groups. The remaining clinical variables were compared with Mann–Whitney tests when variables did not show a Gaussian distribution.
Between‐group comparisons for the dual‐regressed maps were implemented using the general linear model (GLM) and significant differences were analysed with the FSL‐randomise function (10,000 permutations). All results from FSL‐randomise were corrected for multiple comparisons using threshold‐free cluster enhancement (TFCE) and were considered significant at a P‐value <0.05. Age, gender, and years of education were included as covariates of no interest in all GLM designs. For comparisons between patient groups, LEDD was also included as a covariate of no interest [Tahmasian et al., 2015].
For the inter‐network analysis with FSL‐Nets, between group differences were also assessed with FSL‐randomise (10,000 permutations) using the same strategy for the covariates of no interest as in the dual‐regression (see above). Inter‐network connectivity differences were taken as significant at a P‐value <0.05 FWE corrected for multiple comparisons.
Significant intra and inter‐network between group differences were investigated for associations with clinical variables that contributed to the MCI diagnostic criteria. For the intra‐network relations a voxelwise GLM was fitted using the fsl_glm function as follows; dual‐regression result ∼ clinical variable + age + gender + years of education + LEDD. For the results comparing against HCs only the patient groups were fitted to the GLM model and for the results comparing patient groups, the entire PD cohort was fitted to the model. Significance was assessed with nonparametric permutations (FSL‐randomise, 1,000 permutations) and TFCE correction for multiple voxel comparisons (P‐value <0.05 corrected). Similarly, the GLM was applied to investigate relationships between clinical variables and inter‐network connectivity differences from FSL‐Nets. The clinical variables investigated in were: MoCA, Power of Attention, Digit Vigilance Accuracy, PRM, SRM, UPDRS III, Tower of London, Animal naming, Language total score and PAL [Yarnall et al., 2014].
RESULTS
From the cohort of participants, two HCs and 12 PD patients were excluded due to artefacts and vascular infarcts in their functional and structural MRIs respectively. Additionally, 10 PD patients were also excluded due to the motion exclusion criteria (>2 mm translation). After exclusions, 30 HCs and 99 PD patients remained for further functional analysis. From the cohort of PD patients, 62 were diagnosed as PD with normal cognition (PD‐NC) and 37 as PD‐MCI according to the MDS level II [Lawson et al., 2014].
The three groups were assessed for movement differences within the scanner. This test showed a significant difference in total translational movement (Kruskal–Wallis: χ 2 = 7.42, P‐value = 0.024, df = 2) but not in rotational movement. A post‐hoc t‐test revealed that this difference was driven by the PD‐NC group which, on average, moved more than the HCs (P‐value = 0.006) but not significantly more than the PD‐MCI group (P‐value = 0.83).
Statistical comparisons of demographic and clinical variables are shown in Table 1. Those with PD‐MCI were significantly older, had greater LEDD and had lower educational attainment compared with both the PD‐NC and HC groups. As expected, the PD‐MCI group performed worse in cognitive assessments when compared with PD‐NC and HCs and were more medicated compared with the PD‐NC.
Table 1.
Demographics and clinical variables
| Healthy Controls | PD‐NC; Mean (SD) | PD‐MCI; Mean (SD) | ||
|---|---|---|---|---|
| Number of participants | N = 30 | N = 62 | N = 37 | Significance, P‐value |
| Age | 64.05 (7.92) | 62.77 (10.83) | 70.40 (9.13) | F(2,126) = 7.39, P = 0.001a |
| Male/Female | 17/13 | 37/25 | 28/9 | Χ 2 = 3.36, P = 0.186 |
| Education (years) | 14.13 (3.65) | 14.32 (3.9) | 10.97 (3.24) | P < 0.001b |
| NART | 118.6(6.96) | 117.47(9.6), N = 61 | 112.52(9.5), N = 36 | P = 0.008b |
| Disease duration (months). | NA | 6.43 (4.76) | 5.81 (4.51) | P = 0.458c |
| LEDD | NA | 147.06 (112.0) | 201.59 (155.8) | t 97 = 2.01, P = 0.046 |
| MDS‐UPDRS III | NA | 24.59 (10.39) | 28.86 (10.97) | t 97 = 1.93, P = 0.056 |
| MMSE | 29.36 (0.88) | 29.01 (0.93) | 28.18 (1.48) | t 97 = 3.4, P = 0.001 |
| MoCA | 28.2 (1.78), N = 29 | 26.92 (2.30), N = 53 | 22.97 (3.64), N = 34 | t 85 = 6.2, P < 0.001 |
| Power of Attention | 1217.46 (101.175) | 1289.36 (126.98), N = 61 | 1400.65 (172.04) | t 96 = 3.6, P < 0.001 |
| Digit Vigilance Accuracy | 98.0 (2.5) | 97.59 (3.91), N = 61 | 83.9 (17.27) | P < 0.001c |
| PRM | 21.89 (1.61), N = 29 | 21.15 (2.2), N = 58 | 18.02 (2.8), N = 36 | P < 0.001c |
| SRM | 17.27 (1.22), N = 29 | 16.08 (1.7) N = 58 | 14.11 (2.5), N = 36 | P < 0.001c |
| Tower of London | 17.44 (1.76), N = 29 | 15.68 (2.25), N = 58 | 12.88 (4.1), N = 36 | P < 0.001c |
| Animal naming | NA | 23.9 (5.58), N = 60 | 17.57 (6.6), N = 35 | t 93 = 4.97, P = <0.001 |
| Language total score | NA | 24.45 (7.29), N = 50 | 19.29 (6.6), N = 32 | t 80 = 3.24, P = 0.002 |
| PAL | NA | 1.70 (0.43), N = 58 | 2.42 (0.83), N = 35 | P < 0.001c |
NART, national adult reading test; LEDD, Levodopa equivalent daily dose; MDS‐UPDRS III, Movement Disorder Society – unified Parkinson's disease rating scale part III; MMSE, mini‐mental state examination; MoCA, Montreal cognitive assessment; NA, not available; PRM, pattern recognition memory; SRM, spatial recognition memory; PAL, paired associates learning; SD, standard deviation.
ANOVA: healthy controls, PD‐NC, and PD‐MCI.
Χ 2 test Healthy controls, PD‐NC, and PD‐MCI.
Kruskal–Wallis test, Healthy controls, PD‐NC, PD‐MCI; Post‐hoc Mann–Whitney U: Age, PD‐NC < PD‐MCI P‐value <0.001; NART, PD‐NC > PD‐MCI P‐value = 0.005.
Mann–Whitney U test; PD‐NC and PD‐MCI.
Student t‐tests: PD‐NC and PD‐MCI.
Dual‐Regression and FSL‐Nets
The 26 RSNs of interest were investigated by dual‐regression for intra‐network related changes; representative contrast maps are shown in Figure 2. Significant clusters are shown in Table 2, labelled by RSN and cluster index for the three between‐group comparisons. In general, when comparing patient groups to HCs, we found a decrease in functional connectivity in the patient groups. When comparing HC and PD‐NC groups, lower functional synchronisations were found in the PD‐NC group for the RFPN, DAN, MN2 and FPN. Conversely, increased synchronisations in the PD‐NC group were found for the LFPN at the left subcallosal cortex when compared with HCs.
Figure 2.
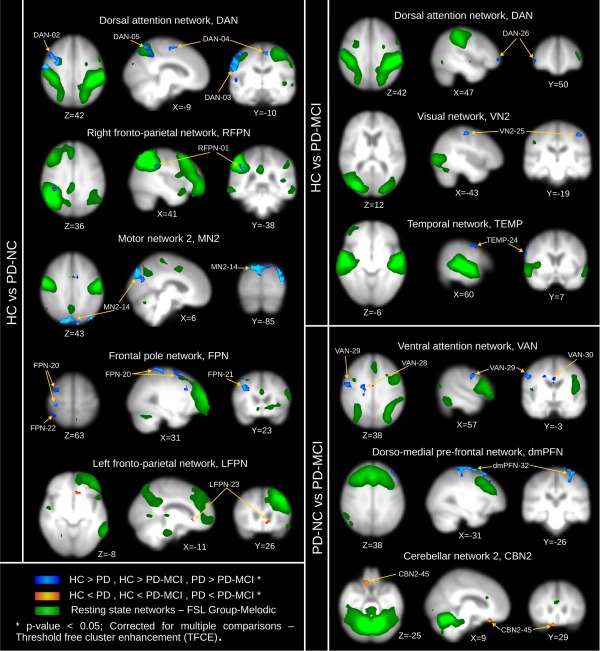
Dual‐regression results for between group comparisons: HC vs. PD‐NC, HC vs. PD‐MCI, and PD‐NC vs. PD‐MCI. Regions and MNI coordinates are described in Table 2. HC, healthy controls; PD‐NC, PD with normal cognition; PD‐MCI, PD with mild cognitive impairment; DAN, dorsal attention network; RFPN, right fronto‐parietal network; MN2, motor network 2; FPN, fronto‐polar network; LFPN, left fronto‐parietal network; VN2, visual network 2; TEMP, temporal network; dmPFN, dorso‐medial prefrontal network; CBN2, cerebellar network 2. Brain maps are presented in radiological convention, that is, right is left hemisphere. [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 2.
Dual‐regression results from the between group comparisons; corrected for multiple comparisons with threshold‐free cluster enhancement (TFCE, P‐value < 0.05)
| Dual‐regression, cluster index | Number of Voxels | Min P‐value | MNI coordinate | Brain regions—Harvard–Oxford atlas |
|---|---|---|---|---|
| HC > PD‐NC | ||||
| RFPN‐01 | 10 | 0.024 | 42,−38,36 | R supramarginal gyrus |
| DAN‐02 | 158 | 0.009 | 38,−2,40 | R precentral gyrus, R middle frontal gyrus |
| DAN‐03 | 144 | 0.005 | 58,−6,44 | R precentral gyrus, R postcentral gyrus |
| DAN‐04 | 43 | 0.017 | −6,−14,60 | L supplementary motor area, L precentral gyrus. |
| DAN‐05 | 34 | 0.022 | −10,−62,64 | L lateral occipital cortex, L precuneus cortex |
| DAN‐06 | 20 | 0.022 | 50,50,4 | R frontal pole |
| DAN‐07 | 18 | 0.036 | 18,−6,64 | R superior frontal gyrus |
| DAN‐08 | 17 | 0.038 | 62,−14,8 | R planum temporale |
| DAN‐09 | 8 | 0.04 | −14,−46,76 | L postcentral gyrus |
| DAN‐10 | 5 | 0.038 | −10,−78,52 | L lateral occipital cortex, superior division |
| DAN‐11 | 3 | 0.03 | −66,−50,36 | L supramarginal gyrus, posterior division |
| DAN‐12 | 2 | 0.048 | −18,−78,52 | L lateral occipital cortex, superior division |
| DAN‐13 | 1 | 0.048 | 42,−82,24 | R lateral occipital cortex, superior division |
| MN2‐14 | 601 | 0.001 | −42,−78,40 | L lateral occipital cortex, R cuneal cortex, R occipital cortex, L precentral gyrus, R lateral occipital cortex |
| MN2‐15 | 73 | 0.026 | 26,−54,72 | R superior parietal lobule |
| MN2‐16 | 32 | 0.013 | 34,26,16 | R inferior frontal gyrus, pars opercularis |
| MN2‐17 | 5 | 0.042 | −10,−18,36 | L cingulate gyrus, posterior division |
| MN2‐18 | 3 | 0.044 | 62,6,−4 | R superior temporal gyrus, anterior division |
| MN2‐19 | 2 | 0.05 | 2,−62,36 | R precuneus cortex |
| FPN‐20 | 192 | 0.013 | 38,−26,64 | R postcentral gyrus, R middle frontal gyrus, R superior parietal lobule, R precentral gyrus. |
| FPN‐21 | 25 | 0.014 | 38,22,32 | R middle frontal gyrus |
| FPN‐22 | 17 | 0.039 | 46,−46,60 | R superior parietal lobule |
| HC < PD‐NC | ||||
| LFPN‐23 | 9 | 0.031 | −10,26,−4 | L subcallosal cortex. |
| HC > PD‐MCI | ||||
| TEMP‐24 | 30 | 0.02 | 62,6,40 | R precentral gyrus. |
| VN2‐25 | 22 | 0.019 | −42,−18,56 | L precentral gyrus. |
| DAN‐26 | 8 | 0.031 | 50,50,0 | R frontal pole. |
| PD‐NC > PD‐MCI | ||||
| RFPN‐27 | 1 | 0.043 | −2,−62,36 | L precuneus cortex. |
| VAN‐28 | 22 | 0.025 | 18,−10,40 | R precentral gyrus, R cingulate gyrus. |
| VAN‐29 | 21 | 0.018 | 58,−2,40 | R precentral gyrus. |
| VAN‐30 | 9 | 0.046 | 2,−6,32 | R cingulate gyrus, anterior division. |
| VAN‐31 | 2 | 0.05 | −2,22,64 | L superior frontal gyrus. |
| dmPFN‐32 | 738 | 0.007 | −26,6,68 | L middle frontal gyrus, L precentral gyrus, L postcentral gyrus, R supplementary motor cortex. |
| dmPFN–33 | 56 | 0.017 | 14,62,−8 | R frontal pole, L frontal pole. |
| dmPFN–34 | 25 | 0.033 | 18,−54,76 | R superior parietal lobule. |
| dmPFN–35 | 9 | 0.031 | −26,50,36 | L frontal pole. |
| dmPFN–36 | 6 | 0.043 | 34,54,−16 | R frontal pole. |
| dmPFN–37 | 4 | 0.046 | 42,10,60 | R middle frontal gyrus. |
| dmPFN–38 | 4 | 0.04 | −6,54,44 | L frontal pole. |
| dmPFN–39 | 2 | 0.04 | 6,66,24 | R frontal pole. |
| dmPFN–40 | 2 | 0.046 | −22,66,16 | L frontal pole. |
| dmPFN–41 | 1 | 0.05 | 46,26,48 | R middle frontal gyrus. |
| dmPFN–42 | 1 | 0.05 | 54,18,44 | R middle frontal gyrus. |
| dmPFN–43 | 1 | 0.049 | 62,2,40 | R precentral gyrus. |
| PD‐NC < PD‐MCI | ||||
| INSN‐44 | 5 | 0.034 | −46,−70,48 | L lateral occipital cortex, superior division. |
| CBN2‐45 | 25 | 0.016 | 6,30,−28 | R subcallosal cortex, R frontal pole. |
L/R, right and left hemisphere.
When comparing HCs with the PD‐MCI group, lower functional synchronisations were found in PD‐MCI for the VN2, DAN and TEMP networks. Comparisons between both patient groups, PD‐NC and PD‐MCI, showed significantly lower synchronisation in the MCI group for the VAN, and dmPFN. Higher synchronisation in the PD‐MCI group when compared with PD‐NC was found for cerebellar and insular networks; CBN2 and INSN (see Table 2).
However, when analysing individual clusters, we noted that some of the significant decreases in functional synchronisation were, in actuality, negative synchronisations in functional connectivity or anticorrelations [Keller et al., 2013], that is, the absolute connectivity in these cases was higher in patients than in HCs; see for instance clusters FPN‐20, DAN‐08 or VN2‐25 whose summarised values are shown in the boxplots in Figure 3A,B. Comparisons between both patient groups, PD‐NC and PD‐MCI, also showed increased anticorrelations for the PD‐MCI patients, for example, cluster VAN‐29 in Figure 3C.
Figure 3.
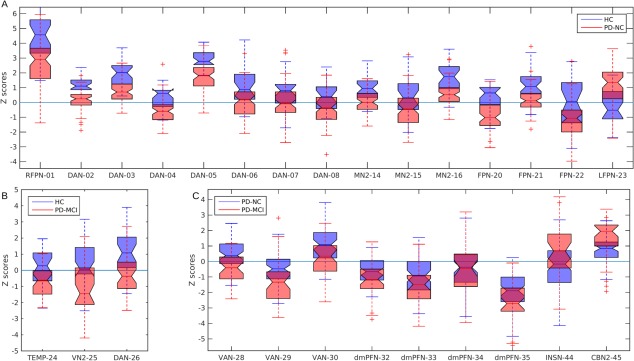
Cluster mean values from the significant differences shown in Figure 2 and Table 2 as boxplots. (A) HC vs. PD‐NC, (B) HC vs. PD‐MCI, and (C) PD‐NC vs. PD‐MCI. Note: although some results are reported as decreased synchronisations in the patient group, for example, HC > PD‐NC in panel (B), some of the findings relate to correlations shifting from positive in the healthy control (HC) group to negative correlations in the disease group, for example cluster VN2‐25 (visual network 2, cluster 25) in panel B. Resting state network acronyms and clusters codes are described in Figure 1 and Table 2, respectively. HC, healthy controls; PD‐NC, PD with normal cognition; PD‐MCI, PD with mild cognitive impairment. In each boxplot, the central mark is the sample median and the extremes are the 25th and 75th percentiles. [Color figure can be viewed at http://wileyonlinelibrary.com]
Inter‐network brain connectivity was assessed with FSL‐Nets, with the objective to determine differences in connectivity between the identified RSNs. Figure 4A–C show the within‐group mean connectivity matrices. Between‐group comparisons for the connectivity matrices revealed a significantly increased synchronisation in PD‐NC group compared with HCs between the basal network BS1 and the motor network MN1 (P‐value <0.05, FWE corrected for multiple comparisons). A post‐hoc analysis of these inter‐network values revealed that this increased synchronisation was a result of a shift from a negative to positive synchronisation in the PD‐NC group (Fig. 4D). In PD‐MCI, this synchronisation change although also present, did not reach significance after correction for multiple comparisons when compared against HCs.
Figure 4.
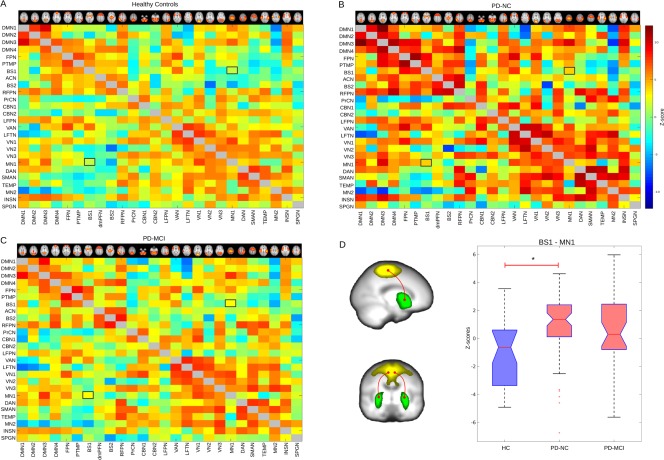
FSL‐Net group connectivity matrices. Panels A to C show FSL‐Net normalised covariance matrices from the three participant groups; healthy controls (HCs), PD with normal cognition (PD‐NC) and with mild cognitive impairment (PD‐MCI); for displaying purposes, matrices are not adjusted for confounding covariates. (D) The PD‐NC group showed significant higher connectivity between basal network BS1 and motor network MN1 with a shift from negative to positive synchronisation (*P‐value < 0.05 FWE corrected for multiple comparisons, and corrected for age, gender, levodopa equivalent daily dose and years for education which were included as covariates of not interest). [Color figure can be viewed at http://wileyonlinelibrary.com]
Several intra‐network connectivity clusters (Table 2) significantly related to the clinical variables (see Table 3). Spatial and Pattern recognition memory (SRM, PRM), Power of Attention (PoA), paired associates learning (PAL), MoCA and Tower of London test significantly correlated with dual‐regressed results. The most significant relation occurred between cluster FPN‐20 and the SRM variable. This cluster demonstrated a disconnection between the frontal pole network and the right middle frontal gyrus. For the only significant inter‐network difference (BS1‐MN1), we detected a significant positive relation between this inter‐network connectivity and PAL (coefficient B1 = 1.59, P‐value = 0.045, R 2 = 0.25, SE = 2.18).
Table 3.
Multiple regression results between significant cluster from the dual‐regression analysis and clinical variables from the MCI diagnostic criteria
| Dual‐regression | TFCE GLM corrected clusters | GLM: Voxel ∼ B1*variable + age + gender + YoE + LEDD | ||||
|---|---|---|---|---|---|---|
|
HC vs. PD‐NC |
Variable | Number of voxels | B1 | Min P‐value | MNI coordinate | Brain region |
| LFPN‐23 | PRM | 6 | 0.23716 | 0.0028 | −6,30,−8 | L subcallosal cortex |
| LFPN‐23 | ToL | 1 | 0.18869 | 0.0074 | −6,26,−4 | L subcallosal cortex |
| MN2‐14 | PoA | 7 | 0.005 | 0.0012 | −30,−94,24 | L occipital pole |
| MN2‐14 | PoA | 3 | 0.0059 | 0.00053 | 34,−86,40 | R cerebral cortex |
| MN2‐14 | PoA | 1 | 0.00591 | 0.00063 | −46,−82,28 | L cerebral cortex |
| FPN‐20 | SRM | 4 | 0.47865 | 9.10E‐005 | 38,18,56 | R middle frontal gyrus |
| FPN‐20 | SRM | 3 | 0.41244 | 0.0011 | 18,−46,76 | R superior parietal lobule |
| FPN‐20 | SRM | 2 | 0.40269 | 0.0029 | 38,−2,68 | R precentral gyrus |
| FPN‐20 | SRM | 1 | 0.36416 | 0.0023 | 22,−26,80 | R postcentral gyrus |
| FPN‐22 | UPDRS | 2 | −0.073 | 0.00046 | 42,−46,68 | R superior parietal lobule |
| FPN‐22 | UPDRS | 2 | −0.08 | 0.00053 | 42,−50,68 | R superior Parietal lobule |
|
HC vs. PD‐MCI |
||||||
| LFPN‐23 | SRM | 1 | −0.2610 | 0.0025 | −30,38,24 | L frontal pole |
| TEMP‐24 | PAL | 1 | −0.7557 | 0.0021 | 62,6,36 | R precentral gyrus |
| VN2‐25 | PAL | 3 | −0.9386 | 0.0088 | −46,−18,52 | L postcentral gyrus |
| PD‐NC vs. PD‐MCI | ||||||
| VAN‐28 | MoCA | 2 | 0.13936 | 0.002277 | 26,−10,40 | R precentral gyrus |
| VAN‐28 | MoCA | 1 | 0.11588 | 0.0042 | 18,−18,40 | R cingulate gyrus, posterior division |
| VAN‐28 | PRM | 10 | 0.16192 | 0.0002 | 18,−14,40 | R cingulate gyrus, anterior division |
| dmPFN‐32 | ToL | 13 | 0.0036 | 0.00064 | −34,−10,76 | L precentral gyrus |
| dmPFN‐32 | ToL | 6 | 0.0037 | 0.0022 | −30,−38,72 | L postcentral gyrus |
Cluster significance was assessed with FSL‐randomise with threshold free cluster enhancement (TFCE) correction for multiple comparisons.
YoE, years of education; LEDD, Levodopa equivalent daily dose; GLM, general linear model; PoA, power of attention; ToL, Tower of London; R/L, right and left hemisphere.
DISCUSSION
We studied functional connectivity in patients diagnosed with PD with normal cognition (PD‐NC) and PD‐MCI. In general, our dual regression analyses found lower functional synchronisations in the patient groups when compared with HCs. An analysis of the significant clusters revealed that some of the apparent synchronisation decreases in the patient groups were actually a reflection of increases in negative synchronisations which are commonly referred to as anticorrelations [Keller et al., 2013]. Our analysis examining connectivity between networks with FSL‐Nets found increased synchronisation between one of the identified basal ganglia networks, BS1, and motor networks, MN1 in both patient groups compared with HCs.
Lower and Higher Functional Synchronisations in PD‐NC and PD‐MCI
The PD‐NC group demonstrated lower synchronisations for RSNs related to attention (RFPN, DAN), motor control (MN2), and memory recall and execution (FPN) when compared with HCs. The RFPN showed a decreased connectivity in the right supramarginal gyrus, a region related to space perception and limb location [De Schotten et al., 2005]. The DAN network showed significantly reduced connectivity in the right precentral gyrus that suggests a disconnection between this primarily attentional network and the right sensorimotor and frontal cortices. A similar finding was found in the PD‐MCI group but with a smaller cluster in the right frontal pole.
For the motor network MN2, we found reduced connectivity with occipital and parietal cortices. Lower connectivity between this network and the occipital and parietal regions was also reported by Peraza et al. [2014] in dementia with Lewy bodies (DLB). This dementia is also characterised by the presence of Parkinsonism and visuospatial and attentional impairments. Specifically, significant regions from the MN2 related with PoA which assesses attention function. Similarly, the FPN‐20 cluster significantly related with SRM at several brain regions such as the right middle frontal gyrus, which aligns again with expected attention and executive deficits [Talati and Hirsch, 2005].
The only network that showed higher connectivity in the PD‐NC group compared with HCs was the LFPN network with the left subcallosal cortex. The LFPN is one of the attentional networks with the highest activity in the supramarginal gyrus which links this network to spatial attention. Its increased synchronisation with the subcallosal cortex is difficult to interpret in PD. However, this region showed a positive relation with the PRM and Tower of London tests, the latter test being related to executive function. The higher connectivity in PD‐NC patients and the positive relation with the clinical variables suggests a possible compensation mechanism in cognitively intact PD patients.
For the PD‐MCI group when comparing to HCs, differences were found for RSNs related to vision (VN2), attention (DAN) and hearing (TEMP). Both the VN2 and TEMP networks showed apparent lower connectivity with the primary motor cortices while the DAN had apparent lower connectivity with the right frontal pole, which a post‐hoc analysis revealed to be a result of negative synchronisations. Functional alterations in PD‐MCI between the DAN and frontal regions have been reported before [Baggio et al., 2015b] and would map onto attention/executive deficits in PD‐MCI, although we did not find a significant relation with any clinical variables in the current cohort. The lower functional connectivity between auditory and visual related networks with the pre‐ and postcentral cortices, related negatively with the PAL test. The PAL test assesses new learning through a visual recognition task and this may map onto visual and memory impairments in PD‐MCI.
When we compared both patient groups, the RSN that showed the broadest differences was the dorso‐medial pre‐frontal network (dmPFN) with the left frontal cortex, mainly the middle frontal and precentral gyri. This network is part of the fronto‐basal‐ganglia system which previous investigations related to motor control and cognition [Aron et al., 2007; Aron and Poldrack, 2006]. This aligned with the positive relationship between dmPFN clusters in the pre‐ and postcentral gyri and the Tower of London test which assesses executive function and planning. The MoCA also related with the VAN‐28 cluster at the right precentral and cingulate gyri which may reflect the cognitive deficits in PD.
The PD‐MCI group demonstrated increased synchronisations for the insula network INSN and cerebellar network CBN2 with the lateral occipital and subcallosal cortices, when compared with PD‐NC patients. The insula network, also referred to as the salience network, is commonly associated with orienting attention to a stimulus [Seeley et al., 2007]. The insula has been reported as functionally impaired in the Lewy body diseases [Roquet et al., 2016] and it has been associated with complex visual hallucinations [Blanc et al., 2014] and thus despite the absence of visual hallucinations in our PD‐MCI cohort early changes in the insula network may be an important pre‐requisite to these phenomena particularly as cognitive symptoms progress.
Negative Synchronisations in Parkinson's Disease
The negative synchronisations observed in the present study (see Fig. 3) revealed by our dual‐regression analyses show a characteristic that is commonly neglected in many functional studies: anticorrelations. Positive synchronisations can be interpreted as a function of direct communication between two brain regions working in synchrony and mediated by excitatory connections [Keller et al., 2013]. However, negative synchronisations or anticorrelations are not easily interpreted [Chai et al., 2012] and shifts from positive to negative correlations in patient groups are even more challenging to understand. Nevertheless, recent evidences have shown that negative synchronisations in fMRI have a biological origin [Keller et al., 2013; Liang et al., 2012], with current hypotheses suggesting that they are an expression of inhibitory brain mechanisms between two or more neuronal systems driven by allocation of functional resources [Coombs Iii et al., 2014; Schafer et al., 2012], or a dynamic self‐regulatory process which optimises brain function in a constantly changing sensory environment, such as the dynamic interrelation between the anticorrelated DAN and DMN [Fox et al., 2005].
Shifting from positive to negative synchronisations or vice versa in patient groups might be driven by compensatory mechanisms in the presence of the disease. Previous investigations have reported the spreading of RSN synchronisations in neurodegenerative patient groups, and have been interpreted as an attempt by the RSN in question to control resources of a secondary system altered by disease [Gorges et al., 2015]. Our observations of negative synchronisations or anticorrelations certainly demand further investigation in order to understand their role in neurodegenerative diseases.
Hyper Cortico‐Basal Network Connectivity in PD‐NC
We observed that the PD‐NC group presented with higher mean connectivity than HCs and PD‐MCI group. However, FSL‐Nets only showed a significant increase in functional connectivity between the BS1 and MN1 networks in the PD‐NC group compared with HCs and this significantly related positively with PAL test. Recent research suggests that in Lewy body diseases, increased connectivity can occur at an early stage in the disease process [Gorges et al., 2015]. This may reflect the recruitment of additional neuronal circuitry needed to compensate for the presence of neuropathology in the primary neural network responsible for a given function, as explained previously. Furthermore, we observed that the cortico‐basal connectivity between BS1 and MN1 was significantly greater in PD‐NC than the HC group, which corresponded to a shift from negative to positive connectivity, although connectivity in neither group was significantly different to zero. A similar finding was reported by Yu et al. in 2013 using a ROI analysis between the putamen and the SMA [Yu et al., 2013]. As discussed in the previous section, functional anticorrelations in a healthy brain might relate to self‐regulatory interactions between neural networks. Following this logic, the shift from negative to positive synchronisation observed in this study between the basal ganglia and motor cortex in PD, may represent a negotiating mechanism of motor control that has become dysfunctional in the presence of the pathology.
LIMITATIONS
There are some limitations within our present study. First, our patients were on dopaminergic medication and scanned in their ‘ON’ state, which might alter functional connectivity. However, previous investigations have shown that Levodopa replacement therapy tends to normalise functional activity toward HC levels [Tahmasian et al., 2015]; despite this, we still found significant functional connectivity alterations. Related to this, our patients were also assessed with a battery of neuropsychological tests which were used for the diagnosis of MCI. As demonstrated in previous reports on this cohort [Yarnall et al. 2014] and consistent with findings in other cohorts [Cholerton et al., 2014] there is substantial heterogeneity in cognitive subdomain deficits experienced by PD‐MCI patients which raises difficulties in terms of interpretation. Study of the different MCI subtype profiles in our cohort will require a stratified statistical analysis which is beyond the scope of the current study although it is a focus of our ongoing work.
Another concern is years of education, which differ between the patient groups especially PD‐NC and PD‐MCI, which might be an important confounder related to cognitive reserve and functional brain activity. We reduced the influence of years of education and medication (LEDD) by including these variables as covariates of no interest in our analyses. A third factor is motion within the scanner; total head movement index was higher in the PD‐NC group than in HCs. However, our PD‐NC group moved less than two mm within the scanner which was within our motion inclusion criteria, and correction for motion was applied by the FSL toolbox. Additionally, we regressed the six motion covariates from the functional images to further reduce its effects.
There are concerns about the impact of cortical atrophy in functional connectivity studies. Cortical density differences between groups were not broad in our participants due to their early disease stage and were only significant when comparing HCs and PD‐MCI patients (HC > PD‐MCI) and delimited within the insula cortices which agrees with previous structural studies [Duncan et al., 2015] (see Supporting Information Material). Previous investigations in rs‐fMRI have proven that cortical density differences do not alter the position of the significant functional clusters, although their size may be slightly decreased [Damoiseaux et al., 2012]. Hence, we are confident that the small structural differences between HCs and PD‐MCI patients did not affect our functional findings.
Finally in the present study we did not carry out any reliability testing of our probabilistic group ICA procedure which is a potential limitation [Abou‐Elseoud et al., 2010]. There are, however, emerging reliability procedures (albeit it is not established by consensus which approach is better) which may be useful to apply in the future to our data and others to confirm the reliability of the group ICA maps.
CONCLUSION
We reported changes in functional connectivity in patients diagnosed with PD‐NC and PD‐MCI using the level II MDS criteria. Our findings suggest that functional alterations in RSNs are more evident in our PD‐NC group than in our PD‐MCI group when compared with each other and against HCs. Most of these alterations were in RSNs associated to attention, motor control, visual and executive functions.
Altered functional synchronisations were found between RSN activity and several brain regions in PD‐NC. When assessing connectivity among RSNs with FSL‐Nets, the PD‐NC group showed higher synchronisation values for cortico‐basal networks, which was also present in the PD‐MCI group although not to a significant level. Finally, we observed shifts in the sign of the synchronisation in the PD groups which might be related to the disease stage and compensatory brain mechanisms. Longitudinal studies observing this transition will shed more light on the pathophysiological alterations in brain connectivity that are driven by PD.
Supporting information
Supporting Information
ACKNOWLEDGEMENTS
The authors thank NIHR NE‐DeNDRON for assistance with identification and recruitment of study participants. The authors are also grateful to colleagues from the neurology and geriatric medicine departments at Newcastle upon Tyne Hospitals NHS Foundation Trust and the Queen Elizabeth Hospital, Gateshead. The research was supported by the National Institute for Health Research (NIHR) Newcastle Biomedical Research Centre based at Newcastle upon Tyne Hospitals NHS Foundation Trust and Newcastle University. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Conflict of interest: None.
REFERENCES
- Abou‐Elseoud A, Starck T, Remes J, Nikkinen J, Tervonen O, Kiviniemi V (2010): The effect of model order selection in group PICA. Hum Brain Mapp 31:1207–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abou Elseoud A, Littow H, Remes J, Starck T, Nikkinen J, Nissilä J, Tervonen O, Timonen M, Kiviniemi VJ (2011): Group‐ICA model order highlights patterns of functional brain connectivity. Front Syst Neurosci 5:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agosta F, Pievani M, Geroldi C, Copetti M, Frisoni GB, Filippi M (2012): Resting state fMRI in Alzheimer's disease: Beyond the default mode network. Neurobiol Aging 33:1564–1578. [DOI] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA (2006): Cortical and subcortical contributions to stop signal response inhibition: Role of the subthalamic nucleus. J Neurosci 26:2424–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Durston S, Eagle DM, Logan GD, Stinear CM, Stuphorn V (2007): Converging evidence for a fronto‐basal‐ganglia network for inhibitory control of action and cognition. J Neurosci 27:11860–11864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggio HC, Segura B, Junque C (2015a): Resting‐state functional brain networks in parkinson's disease. CNS Neurosci Therap 21:793–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggio HC, Segura B, Sala‐Llonch R, Marti MJ, Valldeoriola F, Compta Y, Tolosa E, Junque C (2015b): Cognitive impairment and resting‐state network connectivity in Parkinson's disease. Hum Brain Mapp 36:199–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM (2005): Investigations into resting‐state connectivity using independent component analysis. Philos Trans R Soc Lond Ser B, Biol Sci 360:1001–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanc F, Noblet V, Philippi N, Cretin B, Foucher J, Armspach JP, Rousseau F, The Alzheimer's Disease Neuroimaging Investigators (2014): Right anterior insula: Core region of hallucinations in cognitive neurodegenerative diseases. PloS One 9:e114774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai XJ, Castanon AN, Ongur D, Whitfield‐Gabrieli S (2012): Anticorrelations in resting state networks without global signal regression. NeuroImage 59:1420–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cholerton BA, Zabetian CP, Wan JY, Montine TJ, Quinn JF, Mata IF, Chung KA, Peterson A, Espay AJ, Revilla FJ, Devoto J, Watson GS, Hu S‐C, Leverenz JB, Edwards KL (2014): Evaluation of mild cognitive impairment subtypes in Parkinson's disease. Mov Disord 29:756–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs Iii G, Loggia ML, Greve DN, Holt DJ (2014): Amygdala perfusion is predicted by its functional connectivity with the ventromedial prefrontal cortex and negative affect. PloS One 9:e97466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Beckmann CF, Arigita EJ, Barkhof F, Scheltens P, Stam CJ, Smith SM, Rombouts SA (2008): Reduced resting‐state brain activity in the “default network” in normal aging. Cereb Cortex 18:1856–1864. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Prater KE, Miller BL, Greicius MD (2012): Functional connectivity tracks clinical deterioration in Alzheimer's disease. Neurobiol Aging 33:828 e19–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Schotten MT, Urbanski M, Duffau H, Volle E, Lévy R, Dubois B, Bartolomeo P (2005): Direct evidence for a parietal‐frontal pathway subserving spatial awareness in humans. Science 309:2226–2228. [DOI] [PubMed] [Google Scholar]
- Dipasquale O, Griffanti L, Clerici M, Nemni R, Baselli G, Baglio F (2015): High‐dimensional ica analysis detects within‐network functional connectivity damage of default‐mode and sensory‐motor networks in alzheimer's disease. Front Human Neurosci 9:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan GW, Firbank MJ, Yarnall AJ, Khoo TK, Brooks DJ, Barker RA, Burn DJ, O'Brien JT (2015): Gray and white matter imaging: A biomarker for cognitive impairment in early Parkinson's disease? Mov Disord 31:103–110. [DOI] [PubMed] [Google Scholar]
- Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y, Broe GA, Cummings J, Dickson DW, Gauthier S, Goldman J, Goetz C, Korczyn A, Lees A, Levy R, Litvan I, McKeith I, Olanow W, Poewe W, Quinn N, Sampaio C, Tolosa E, Dubois B (2007): Clinical diagnostic criteria for dementia associated with Parkinson's disease. Mov Disord 22:1689–1707. [DOI] [PubMed] [Google Scholar]
- Filippini N, MacIntosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, Matthews PM, Beckmann CF, Mackay CE (2009): Distinct patterns of brain activity in young carriers of the APOE‐ε4 allele. Proc Natl Acad Sci 106:7209–7214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME (2005): The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A 102:9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox SH, Katzenschlager R, Lim SY, Ravina B, Seppi K, Coelho M, Poewe W, Rascol O, Goetz CG, Sampaio C (2011): The movement disorder society evidence‐based medicine review update: Treatments for the motor symptoms of Parkinson's disease. Mov Disord 26 Suppl 3:S2–41. [DOI] [PubMed] [Google Scholar]
- Galvin JE, Pollack J, Morris JC (2006): Clinical phenotype of Parkinson disease dementia. Neurology 67:1605–1611. [DOI] [PubMed] [Google Scholar]
- Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez‐Martin P, Poewe W, Sampaio C, Stern MB, Dodel R, Dubois B, Holloway R, Jankovic J, Kulisevsky J, Lang AE, Lees A, Leurgans S, LeWitt PA, Nyenhuis D, Olanow CW, Rascol O, Schrag A, Teresi JA, van Hilten JJ, LaPelle N (2008): Movement Disorder Society‐sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS‐UPDRS): scale presentation and clinimetric testing results. Mov Disord 23:2129–2170. [DOI] [PubMed] [Google Scholar]
- Gorges M, Müller HP, Lulé D, Pinkhardt EH, Ludolph AC, Kassubek J (2015): To rise and to fall: Functional connectivity in cognitively normal and cognitively impaired patients with Parkinson's disease. Neurobiol Aging 36:1727–1735. [DOI] [PubMed] [Google Scholar]
- Gratwicke J, Jahanshahi M, Foltynie T (2015): Parkinson's disease dementia: A neural networks perspective. Brain: J Neurol 138:1454–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday G, Holton J, Revesz T, Dickson D (2011): Neuropathology underlying clinical variability in patients with synucleinopathies. Acta Neuropathol 122:187–204. [DOI] [PubMed] [Google Scholar]
- Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG (2008): The Sydney multicenter study of Parkinson's disease: The inevitability of dementia at 20 years. Mov Disord 23:837–844. [DOI] [PubMed] [Google Scholar]
- Hughes AJ, Daniel SE, Ben‐Shlomo Y, Lees AJ (2002): The accuracy of diagnosis of parkinsonian syndromes in a specialist movement disorder service. Brain: J Neurol 125:861–870. [DOI] [PubMed] [Google Scholar]
- Irwin DJ, Lee VMY, Trojanowski JQ (2013): Parkinson's disease dementia: Convergence of [alpha]‐synuclein, tau and amyloid‐[beta] pathologies. Nat Rev Neurosci 14:626–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehagia AA, Barker RA, Robbins TW (2010): Neuropsychological and clinical heterogeneity of cognitive impairment and dementia in patients with Parkinson's disease. Lancet Neurol 9:1200–1213. [DOI] [PubMed] [Google Scholar]
- Kelly RE, Jr , Alexopoulos GS, Wang Z, Gunning FM, Murphy CF, Morimoto SS, Kanellopoulos D, Jia Z, Lim KO, Hoptman MJ (2010): Visual inspection of independent components: Defining a procedure for artifact removal from fMRI data. J Neurosci Methods 189:233–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller CJ, Bickel S, Honey CJ, Groppe DM, Entz L, Craddock RC, Lado FA, Kelly C, Milham M, Mehta AD (2013): Neurophysiological Investigation of Spontaneous Correlated and Anticorrelated Fluctuations of the BOLD Signal. J Neurosci 33:6333–6342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson RA, Yarnall AJ, Duncan GW, Khoo TK, Breen DP, Barker RA, Collerton D, Taylor JP, Burn DJ (2014): Severity of mild cognitive impairment in early Parkinson's disease contributes to poorer quality of life. Parkinsonism Relat Disord 20:1071–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Z, King J, Zhang N (2012): Anticorrelated resting‐state functional connectivity in awake rat brain. NeuroImage 59:1190–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao W, Chen H, Feng Y, Mantini D, Gentili C, Pan Z, Ding J, Duan X, Qiu C, Lui S, Gong Q, Zhang W (2010): Selective aberrant functional connectivity of resting state networks in social anxiety disorder. NeuroImage 52:1549–1558. [DOI] [PubMed] [Google Scholar]
- Litvan I, Goldman JG, Tröster AI, Schmand BA, Weintraub D, Petersen RC, Mollenhauer B, Adler CH, Marder K, Williams‐Gray CH, Aarsland D, Kulisevsky J, Rodriguez‐Oroz MC, Burn DJ, Barker RA, Emre M (2012): Diagnostic criteria for mild cognitive impairment in Parkinson's disease: Movement Disorder Society Task Force guidelines. Mov Disord 27:349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak E, Su L, Williams GB, Firbank MJ, Lawson RA, Yarnall AJ, Duncan GW, Owen AM, Khoo TK, Brooks DJ, Rowe JB, Barker RA, Burn DJ, O'Brien JT (2015): Baseline and longitudinal grey matter changes in newly diagnosed Parkinson's disease: ICICLE‐PD study. Brain: J Neurol 138:2974–2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias JL, Bowden SC, Barrett‐Woodbridge M (2007): Accuracy of the Wechsler Test of Adult Reading (WTAR) and National Adult Reading Test (NART) when estimating IQ in a healthy Australian sample. Aust Psychol 42:49–56. [Google Scholar]
- Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H (2005): The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53:695–699. [DOI] [PubMed] [Google Scholar]
- Parkkinen L, Pirttila T, Alafuzoff I (2008): Applicability of current staging/categorization of alpha‐synuclein pathology and their clinical relevance. Acta Neuropathol 115:399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peraza LR, Kaiser M, Firbank M, Graziadio S, Bonanni L, Onofrj M, Colloby SJ, Blamire A, O'Brien J, Taylor JP (2014): fMRI resting state networks and their association with cognitive fluctuations in dementia with Lewy bodies. NeuroImage: Clin 4:558–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peraza LR, Colloby SJ, Firbank MJ, Greasy GS, McKeith IG, Kaiser M, O'Brien J, Taylor JP (2015a): Resting state in Parkinson's disease dementia and dementia with Lewy bodies: Commonalities and differences. Int J Geriatr Psychiatry 30:1135–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peraza LR, Taylor JP, Kaiser M (2015b): Divergent brain functional network alterations in dementia with Lewy bodies and Alzheimer's disease. Neurobiol Aging 36:2458–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, James M, Owen AM, Sahakian BJ, McInnes L, Rabbitt P (1994): Cambridge Neuropsychological Test Automated Battery (CANTAB): a factor analytic study of a large sample of normal elderly volunteers. Dementia (Basel, Switzerland) 5:266–281. [DOI] [PubMed] [Google Scholar]
- Roquet D, Sourty M, Botzung A, Armspach JP, Blanc F (2016): Brain perfusion in dementia with Lewy bodies and Alzheimer's disease: An arterial spin labeling MRI study on prodromal and mild dementia stages. Alzheim Res Ther 8:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer K, Blankenburg F, Kupers R, Gruner JM, Law I, Lauritzen M, Larsson HB (2012): Negative BOLD signal changes in ipsilateral primary somatosensory cortex are associated with perfusion decreases and behavioral evidence for functional inhibition. NeuroImage 59:3119–3127. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD (2007): Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine JM, Halliday GM, Naismith SL, Lewis SJ (2011): Visual misperceptions and hallucinations in Parkinson's disease: Dysfunction of attentional control networks? Mov Disord 26:2154–2159. [DOI] [PubMed] [Google Scholar]
- Shine JM, Halliday GM, Gilat M, Matar E, Bolitho SJ, Carlos M, Naismith SL, Lewis SJ (2014): The role of dysfunctional attentional control networks in visual misperceptions in Parkinson's disease. Hum Brain Mapp 35:2206–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song XW, Dong ZY, Long XY, Li SF, Zuo XN, Zhu CZ, He Y, Yan CG, Zang YF (2011): REST: A toolkit for resting‐state functional magnetic resonance imaging data processing. PloS One 6:e25031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahmasian M, Bettray LM, van Eimeren T, Drzezga A, Timmermann L, Eickhoff CR, Eickhoff SB, Eggers C (2015): A systematic review on the applications of resting‐state fMRI in Parkinson's disease: Does dopamine replacement therapy play a role? Cortex 73:80–105. [DOI] [PubMed] [Google Scholar]
- Talati A, Hirsch J (2005): Functional specialization within the medial frontal gyrus for perceptual go/no‐go decisions based on “what,” “when,” and “where” related information: An fMRI study. J Cogn Neurosci 17:981–993. [DOI] [PubMed] [Google Scholar]
- Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE (2010): Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord 25:2649–2653. [DOI] [PubMed] [Google Scholar]
- Van Eimeren T, Monchi O, Ballanger B, Strafella AP (2009): Dysfunction of the default mode network in Parkinson disease: A functional magnetic resonance imaging study. Arch Neurol 66:877–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarnall AJ, Rochester L, Burn DJ (2013): Mild cognitive impairment in Parkinson's disease. Age Ageing 42 :567–576. [DOI] [PubMed] [Google Scholar]
- Yarnall AJ, Breen DP, Duncan GW, Khoo TK, Coleman SY, Firbank MJ, Nombela C, Winder‐Rhodes S, Evans JR, Rowe JB, Mollenhauer B, Kruse N, Hudson G, Chinnery PF, O'Brien JT, Robbins TW, Wesnes K, Brooks DJ, Barker RA, Burn DJ (2014): Characterizing mild cognitive impairment in incident Parkinson disease: The ICICLE‐PD study. Neurology 82:308–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu R, Liu B, Wang L, Chen J, Liu X (2013): Enhanced functional connectivity between putamen and supplementary motor area in Parkinson's disease patients. PloS One 8:e59717. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


