Abstract
Primary dysmenorrhea (PD), as characterized by painful menstrual cramps without organic causes, is associated with central sensitization and brain function changes. Previous studies showed the integrated role of the default mode network (DMN) in the pain connectome and its key contribution on how an individual perceives and copes with pain disorders. Here, we aimed to investigate whether the cingulum bundle connecting hub regions of the DMN was disrupted in young women with PD. Diffusion tensor imaging was obtained in 41 PD patients and 41 matched healthy controls (HC) during their periovulatory phase. The production of prostaglandins (PGs) was obtained in PD patients during their pain‐free and pain phases. As compared with HC, PD patients had similar scores of pain intensity, anxiety, and depression in their pain‐free phase. However, altered white matter properties mainly located in the posterior section of the cingulum bundle were observed in PD. Besides PGs being related to menstrual pain, a close relationship was found between the white matter properties of the cingulum bundle during the pain‐free phase and the severity of the menstrual pain in PD patients. Our study suggested that PD had trait changes of white matter integrities in the cingulum bundle that persisted beyond the time of menstruation. We inferred that altered anatomical connections may lead to less‐flexible communication within the DMN, and/or between the DMN and other pain‐related brain networks, which may result in the central susceptibility to develop chronic pain conditions in PD's later life. Hum Brain Mapp 38:4430–4443, 2017. © 2017 Wiley Periodicals, Inc.
Keywords: primary dysmenorrhea, diffusion tensor imaging, cingulum bundle
INTRODUCTION
Primary dysmenorrhea (PD), or menstrual pain in the absence of any discernable macroscopic pelvic pathology, is the most prevalent gynecological disorder in young females. PD has been classified as a member of the central sensitivity syndromes together with several chronic pain conditions, including fibromyalgia [Giamberardino, 2008], irritable bowel syndrome (IBS) [Altman et al., 2006], painful bladder syndrome [Chung et al., 2014], migraine, low back pain, and so forth [Berkley, 2013]. Despite having high prevalence and worsening in the quality of life, PD is disregarded in the pain community [Berkley, 2013; Berkley and McAllister, 2011], receiving surprisingly slight scientific and clinical attention.
It is well known that the overproduction of uterine prostaglandins (PGs) plays an important role for the pathophysiology of PD. During menstruation, arachidonic acid released from endometrial sloughing is converted to PGs and leukotrienes [Sales and Jabbour, 2003], and abnormally enhanced release of PGs would induce uterine smooth muscle hypercontractions resulting in ischemia and hypoxia of the uterine muscle, which manifest as severe dysmenorrheic pain [Dawood, 1987]. While studies constructed a bridge between excessive release of PGs in the menstruation phase and dysmenorrheic pain in PD patients, there is mounting evidence that PD patients are hypersensitive to experimental pain not only when they are experiencing menstrual pain, but also during the pain‐free phase of the menstrual cycle [Iacovides et al., 2015]. Researchers pointed out that a vicious cycle of painful stimuli caused by uterine cramps may increase nociceptive‐related neuronal input in the brain [Iacovides et al., 2015; Li and Hu, 2016], which was associated with structural changes and functional reorganization in pain‐related brain networks [Bajaj et al., 2002]. This could be a viable explanation for the enhanced pain sensitivity even in the phase when PD patients are not experiencing menstrual pain [Iacovides et al., 2013; Vincent et al., 2011].
Initial neuroimaging studies suggested that the default mode network (DMN)—mainly comprised the posterior cingulate cortex (PCC)/precuneus, medial prefrontal cortex (mPFC), and lateral parietal lobe—was the primary network related to chronic pain [Kucyi and Davis, 2015]. Functional connectivity within the DMN measured by resting‐state functional magnetic resonance imaging (MRI) has been associated with individual differences in tendencies to attend to pain [Kucyi et al., 2013]. Kucyi et al. [2014] found the interrelationship between pain rumination and altered PCC–mPFC functional connectivity in chronic pain patients. The cingulum is a complex fiber system and a major constituent of the DMN [Emsell et al., 2013], which supports the transfer of neural information between the frontal cortex and posterior regions in the DMN [Jones et al., 2013]. Animal models of chronic pain found that the manipulation of the cingulate cortex and/or cingulum bundle could alter brain responses to pain [Donahue et al., 2001; Vaccarino and Melzack, 1989]. Recently, Liu et al. reported that the diffusion properties of white matter skeleton voxels around the cingulum bundle showed significant differences in women with PD as compared between HC [Liu et al., 2016]. Additionally, several studies also reported disrupted DMN connectivity in PD patients as compared with nondysmenorrheic women during menstruation and periovulatory phases [Tu et al., 2009; Wei et al., 2016; Wu et al., 2016]. As pain‐related impairments in functional connectivity are closely associated with disruptions in connecting white matter tracts [Mansour et al., 2013], we hypothesized that long‐range association bundles connecting hub regions of the DMN, such as the cingulum bundle, would be disrupted in women with PD.
To test this hypothesis, we collected various pathopsychophysiological data from 41 PD patients and 41 matched healthy controls (HC) during both menstruation and periovulatory phases, including pain intensity, anxiety, depression, and the production of PGs. In addition, diffusion tensor imaging (DTI) data were collected from all subjects during their periovulatory phase, and a tractography atlas‐based analysis (TABS) method, which combines spatial normalization of tensor images with a voxel‐wise statistical framework for tract‐oriented statistics for a single hypothesis test per tract [Goodlett et al., 2009; O'Donnell et al., 2009], was adopted to investigate microstructural properties of the cingulum bundle. The interrelationships among the severity of menstrual pain, the production of PGs, and the microstructural properties of the cingulum bundle were investigated using partial correlation analysis.
MATERIALS AND METHODS
All research procedures were approved by the Institutional Review Board of the First Affiliated Hospital of the Medical College in Xi'an Jiaotong University and were conducted in accordance with the Declaration of Helsinki. All subjects gave written, informed consent after the experimental procedures had been fully explained.
Participants
Inclusion criteria for the PD patient group were as follows: (1) according to the American College of Obstetricians and Gynecologists; (2) lower abdominal pain during menstruation that interfered with daily activities but without any underlying pathological abnormality within or outside the uterus; (3) regular menstrual cycle between 27 and 32 days; and (4) average cramping pain level in the past 6 months rated ≥4 (0 = not at all, 10 = the worst imaginable pain). PD patients were screened and diagnosed by a gynecologist at the Department of Obstetrics and Gynecology, First Affiliated Hospital of the Medical College of Xi'an Jiaotong University. Inclusion criteria for healthy controls (HC) were (1) regular menstrual cycle between 27 and 32 days and (2) without menstrual pain (pain with menstruation ≤2 on a visual analogue scale (VAS)).
Exclusion criteria for all participants were as follows: (1) MRI scanning of the pelvis revealed organic pelvic diseases; (2) using oral contraceptives, hormonal supplements, Chinese herbal medicine, or any central‐acting medication (e.g., opioids, antiepileptics) within the past 6 months; (3) any physical illness such as a brain tumor, hepatitis, or epilepsy as assessed according to clinical evaluations and medical records; (4) existence of other comorbid chronic pain conditions (e.g.: tension type headache, fibromyalgia, etc.); (5) existence of a neurological disease or psychiatric disorder; (6) immediate plans for pregnancy or a positive pregnancy test; (7) history of childbirth; and (8) any contraindications to MRI.
Forty‐one right‐handed dysmenorrhea subjects and forty‐one, education‐ and age‐matched, healthy, right‐handed female HC were recruited. All participants were college students. Urine kits for luteinizing hormone were used to verify that the participants were in their periovulatory phase.
Psychological Assessment
Self‐rating anxiety scale (SAS) and self‐rating distress scale (SDS) were administered before MRI scanning to evaluate the subject's anxiety and depression levels. The VAS was performed in the PD and HC group during their periovulatory and menstruation phase to assess their current experience of menstrual pain.
Prostaglandin Measurement
Serum was extracted during the subjects' periovulatory and menstruation phases. The total serum concentrations were assayed using a radioimmunoassay technique for prostaglandin ( ).
Imaging Acquisition
The scanning was carried out in a 3.0 T Signa GE scanner with an 8‐channel phase array head coil. For each subject, a high‐resolution structural image was acquired by using a three‐dimensional MRI sequence with a voxel size of 1 × 1 × 1 mm using an axial Fast Spoiled Gradient Recalled sequence (FSGR) with the following parameters: repetition time (TR) = 4.0 ms; echo time (TE) =3.3 ms; data matrix = 256 × 256; field of view (FOV) = 240 × 240 mm.
DTI was obtained with a single‐shot echo‐planar imaging sequence. The diffusion sensitizing gradients were applied along two repeats of 30 noncollinear directions (b = 1000 s/mm2) with five repeats of the b0 (no diffusion weighted image) with a voxel size of 2 × 2 × 2 mm (TR = 9.4 s; TE = 84 ms).
Data Preprocessing
The diffusion‐weighted imaging data were corrected for motion, current distortions, and echo planar imaging distortion by using a freely available dMRI analysis software package ExploreDTI (http://www.exploredti.com) [Leemans et al., 2009]. The diffusion tensor and FA were calculated in native space.
The TABS Method
Automated TABS of diffusion MRI along with white matter fiber tracts is an important method to detect tract integrity and altered microstructural properties of cerebral white matter [Chen et al., 2015; Geng et al., 2012; Goodlett et al., 2009; O'Donnell et al., 2009]. The main contents of TABS are spatial normalization of tensor images (Fig. 1a,b) (this was performed by using the FMRIB Software Library v5.0, https://fsl.fmrib.ox.ac.uk/fsl/fslwiki) and quantitative tract analysis (Fig. 1c,d). Within its framework, the diffusion properties, such as FA, mean diffusivity (MD), radial diffusivity (RD), and axial diffusivity (AD), along fiber tracts are modeled as multivariate functions of arc length (Fig. 1e,f) [Goodlett et al., 2009].
Figure 1.
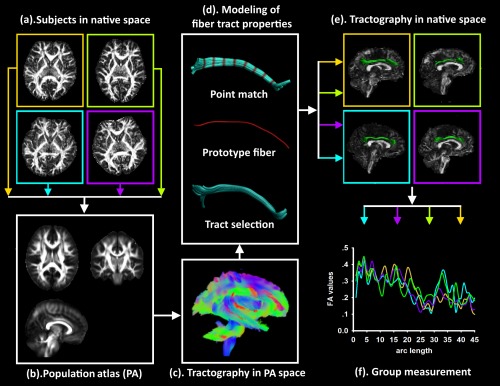
The pipeline of the tract‐based analysis using predefined tracts. With our TABS, we built a population‐specific diffusion tensor template and sensitively investigated the microstructural properties along the region of interest fibers to determine more characteristics. To provide spatial normalization for the analysis of diffusion values at corresponding locations, the tensor properties in the subjects' native space (a) were integrated to create a population‐specific tensor template (b). Based on the whole‐brain tractography maps in the population space (c), the tract of interest was selected and converted into a continuous function (d). Fibers in the population space were warped back into the individual's native space (e) to collect the diffusion measure (f). [Color figure can be viewed at http://wileyonlinelibrary.com]
The variance in diffusion tensor measured collectively may help to better understand how diffusion tensor is changing (e.g., axonal damage and myelination) and provide more specific biomarkers of white matter neuropathology [Alexander et al., 2007]. In more detail, FA decreased due to loss of coherence in the preferred direction of movement, and MD was higher as a result of increased free diffusion [Thomason and Thompson, 2011]. FA and MD are highly sensitive, but less specific to white matter changes. To yield a more comprehensive picture of different elements of white matter microstructure, AD and RD should be used for a conjoint analysis [Alexander et al., 2007]. It was demonstrated that AD could be a more specific marker for axonal change, while RD implicates the nature of myelin [Song et al., 2003]. The statistical analysis of these diffusion measurements using TABS is described as follows.
Construction of a Population Atlas (PA)
To provide spatial normalization for the analysis of diffusion values at corresponding locations, a population specific DTI template was created and the fiber tract analyses were employed in this PA space. Van Hecke et al. [2008] introduced a population‐based registration strategy that all subject data were iteratively aligned to each other to obtain the minimum mean deformation fields of each subject image to all other images. This strategy could obtain optimal spatial alignment results and the final diffusion tensor template had a higher accuracy and precision than the subject‐based template method [Van Hecke et al., 2008]. In this study, we followed a similar registration strategy in Van Hecke et al.'s [2008] study. Specifically, (1) the FA images of each subject were nonlinearly transformed to the MNI template, and the deformation fields ( … ) were obtained; (2) FA images in MNI space were iteratively aligned to each other to obtain minimal mean deformation fields of each subject image for all other images. The average deformation fields of each subject ( … ) were defined as the mean transformation to all other images [Van Hecke et al., 2008]; (3) the consecutive application of the deformation fields and was constructed and noted as . The multicomponent DT images of each subject in native space were transformed with one deformation field ( ) to the final PA space. Tensor reorientation was performed to rearrange the tensors with the direction information [Alexander et al., 2001]; and (4) finally, the recalculated DWIs were averaged to compose the PA (Fig. 1b). Notice that, only one tensor reorientation and one interpolation step were performed in Van Hecke et al.'s [2008] method.
Whole‐Brain Tractography
Fiber tracking was performed in the PA using a streamline tractography approach [Mori and van Zijl, 2002] (Fig. 1c). Tracking was initiated in every voxel and continued with a step size of 1 mm until one of the following thresholds was exceeded: fiber orientation distribution > 15°, angle > 45°, minimum length <20 mm, and maximum length >300 mm. A conservative spherical harmonic order, L max = 6, was used to prevent overfitting due to noise [Emsell et al., 2013].
Tract Selection in PA Space
Regions of interest (ROIs) were delineated from the whole‐brain tractography maps (Fig. 1c), and ROIs for tract selection and exclusion were placed manually in the PA. Based on expert neuroanatomical knowledge of known pathways derived from classical anatomical descriptions, a single‐rater defined the ROIs that were tailored to the PA. High intra‐ and inter‐rater reliability of segmenting fiber bundles using this approach has been successfully applied previously to improve tract delineation [Kristo et al., 2013; Reijmer et al., 2015; Tu et al., 2009]. In this study, the cingulum bundle was selected for TABS. The cingulum was divided into anterior dorsal, posterior dorsal, and parahippocampal sections.
Modeling of Fiber Tract Properties
To place coordinates on all fibers in a bundle, a prototype fiber was employed as a representative fiber that could propagate its coordinates to all other fibers within this bundle [O'Donnell et al., 2009]. Here, the prototype fiber was chosen as the fiber with the greatest length weighted according to the local fiber density which was proposed in O'Donnell et al. [2009]. The local density of a voxel was calculated as the number of tractography trajectories passing through that particular voxel, which could assess the degree of intersection of structural connectivity of a voxel in a fiber bundle. The fiber density was then integrated along each fiber (Fig. 1d).
To further characterize diffusion properties of the prototype fiber in PA space in the context of geometric models of fiber bundles, continuous function of the arc length was used to model the data within the prototype fiber [Goodlett et al., 2009]. In this study, the prototype fiber was generated for different ROIs respectively with discretizations of 2 mm. To transform the arc length coordinates defined by the prototype fiber to all other fibers, the optimal point match method was performed to solve a point correspondence to the matching problem [O'Donnell et al., 2009] (Fig. 1d).
Tractography in Native Space
After arc length correspondences were calculated, the ROI fibers in PA space were warped back into the individual's native space to collect the diffusion measures (Fig. 1e). Measurements from all subjects in the group are displayed in Figure 1f.
Statistical Analysis
The between‐group differences in the subjects' basic information (age, years of education, and VAS scores during the menstruation phase) were tested using a two‐sample t test. False discovery rate (FDR) was performed to account for multiple comparisons.
The between‐group differences in the subjects' diffusion properties were tested using the permutation‐based nonparametric inferences (5,000 random permutations). The threshold for the statistical significance was set as P < 0.01, using threshold‐free cluster enhancement (TFCE) with a family‐wise error (FWE) correction for multiple comparison corrections (5,000 permutations).
To investigate the relationship among the production of PGs, patients' diffusion properties during the periovulatory phase, and the VAS scores during the menstruation phase, a partial correlation analysis was performed. TFCE with the FWE correction was performed to account for multiple comparisons.
RESULTS
Clinical and Demographic Characteristics
In this study, there was no significant difference between PD and HC groups with regard to age, years of education, average days of one menstrual cycle, or age at menarche (P > 0.05, Table 1). All PD patients in our study had a long history of menstrual pain (mean ± se = 10.49 ± 1.1 years), with duration of pain 1–3 days in one menstrual cycle (mean ± se = 1.9 ± 0.38 days). During the periovulatory phase, all PD patients had similar scores of , VAS, SAS, and SDS, as compared with the HC (P > 0.05, FDR corrected, Table 1). During the menstruation phase, PD patients had a significantly higher level of and a higher VAS score (P < 0.05, FDR corrected) than HC, but no significant difference between the two groups was found in SAS and SDS (P > 0.05, FDR corrected, Table 1).
Table 1.
Demographic and Behavioral Data
| HC (n = 41) | PD (n = 41) | ||
|---|---|---|---|
| mean ± se | mean ± se | P value | |
| Age (years) | 23.09 ± 0.44 | 24.02 ± 0.51 | >0.05 |
| Education (years) | 18.01 ± 0.1 | 18.02 ± 0.13 | >0.05 |
| Cycles (days) | 29.21 ± 0.17 | 29.44 ± 0.19 | >0.05 |
| Age at menarche (years) | 12.21 ± 0.67 | 12.46 ± 0.84 | >0.05 |
| History of menstrual pain (years) | — | 10.49 ± 1.1 | — |
| Duration of menstrual pain (days) | — | 1.9 ± 0.38 | — |
| Drug taken (%) | — | 21% | — |
| Periovulatory phase | |||
| PGF | 7.22 ± 0.7 | 10.58 ± 1.33 | >0.05 |
| VAS | 0 | 0 | — |
| SAS | 26.5 ± 1.0 | 28.24 ± 1.28 | >0.05 |
| SDS | 27.1 ± 1.9 | 28.92 ± 1.16 | >0.05 |
| Menstruation phase | |||
| PGF | 6.96 ± 0.65 | 11.68 ± 1.16 | <0.00001 |
| VAS | 0.34 ± 0.12 | 6.5 ± 0.23 | <0.00001 |
| SAS | 27 ± 1.33 | 32.28 ± 1.8 | >0.05 |
| SDS | 28.8 ± 1.58 | 34.36 ± 1.95 | >0.05 |
PD, primary dysmenorrhea; HC, healthy controls; PGF , prostaglandins F ; VAS, visual analogue scale; SAS, self‐rating anxiety scale; SDS, self‐rating distress scale; se, standard error.
The comparisons of subjects' basic information were performed between PDM and HC groups using two‐sample t test. P < 0.05 was considered significant. False discovery rate (FDR) was used to correct for multiple comparisons.
Differences in Tract Diffusivity Measures in the Cingulum Bundle
Before the fiber tract analysis in this study, tract‐based spatial statistical (TBSS) analysis for exploring regions with altered diffusion metrics without a hypothesis of an affected location was used (Supporting Information). In our findings, women with PD exhibited significantly lower FA values in the superior and posterior part of the corona radiata as compared with the HC (P < 0.05, FWE corrected, Supporting Information, Fig. S1). No significant between‐group differences were found in MD, RD, and AD for the whole‐brain analysis.
The cingulum bundle was constructed on the PA (Figs. 2 and 3). The diffusion values of these tracts were sampled along the template‐normalized arc length for each individual in the study for FA, MD, RD, and AD. As compared with HC, we found the cluster of the significant between‐group differences was located in the dorsal‐posterior cingulum in PD patients, with decreased FA (Fig. 4a), increased MD (Fig. 4b), decreased AD (Fig. 4c), and increased RD (Fig. 4d) (P < 0.01, FWE corrected). This pattern of diffusion measurements implied disrupted integrity of myelin sheaths with axonal loss, which may indicate decreased structural connectivity related to the posterior part of the cingulate cortex.
Figure 2.
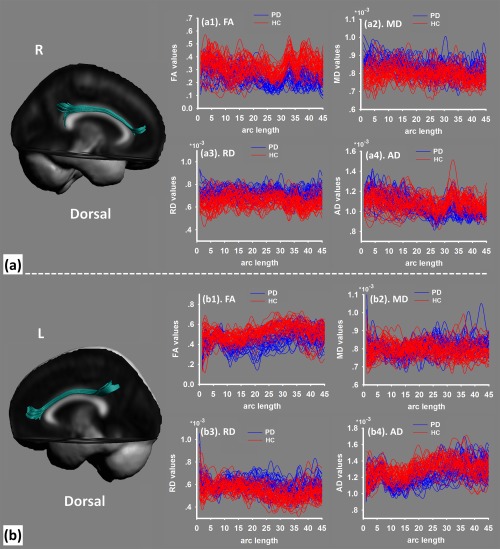
The dorsal part of the cingulum bundle (a, right side; b, left side) was extracted from the tensor template. The diffusion values are sampled along the normalized arc length for each individual in the study of fractional anisotropy (FA), mean diffusivity (MD), radial diffusivity (RD), and axial diffusivity (AD). [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 3.
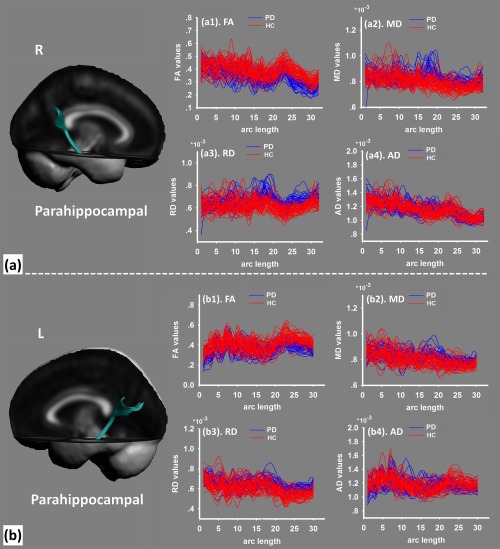
The parahippocampal sections of the cingulum bundle (a, right side; b, left side) were extracted from the tensor template. The diffusion values were sampled along the normalized arc length for each individual in the study of fractional anisotropy (FA), mean diffusivity (MD), radial diffusivity (RD), and axial diffusivity (AD). [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 4.
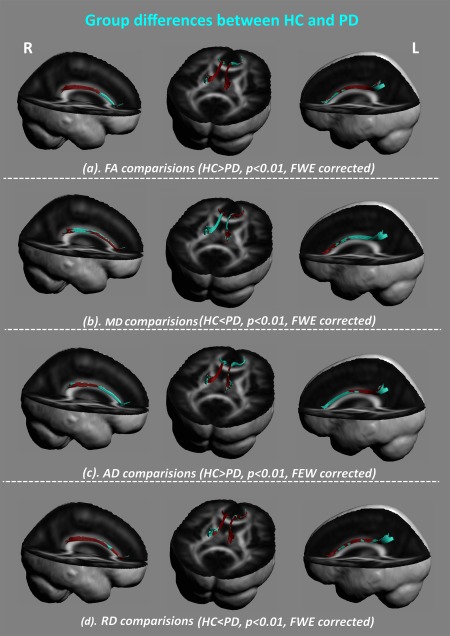
Between‐group differences of diffusion properties along the dorsal part of the cingulum bundle. As compared with the HC group, PD patients had altered white matter mainly located in the dorsal‐posterior cingulum in PD patients (P < 0.01, FWE corrected), with (a) decreased fractional anisotropy (FA), (b) increased mean diffusivity (MD), (c) decreased axial diffusivity (AD), and (d) increased radial diffusivity (RD). [Color figure can be viewed at http://wileyonlinelibrary.com]
For the parahippocampal section of the cingulum bundle in PD patients, it exhibited altered diffusion properties with decreased FA (Fig. 5a), increased MD (Fig. 5b), and increased RD than HC (Fig. 5c) (P < 0.01, FWE corrected). These altered diffusion properties converged in the posterior cingulate cortex, suggesting that the axons or dendritic arborizations were loosely packed (high MD) and lacked directional coherence (low FA) [Ellingson et al., 2013], indicating a reduction in projections and density of neural fibers.
Figure 5.
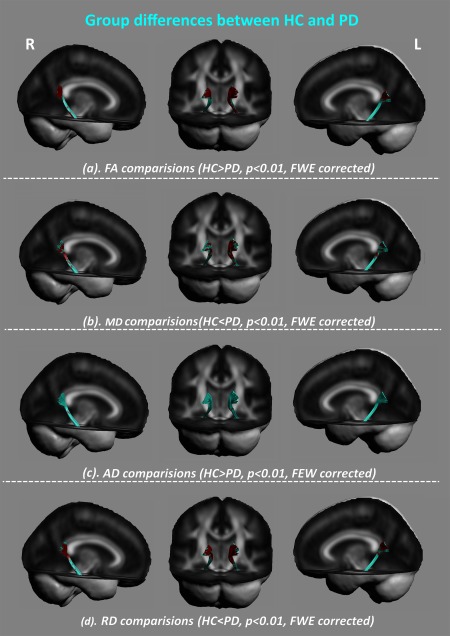
Between‐group differences of diffusion properties along the parahippocampal section. As compared with the HC group, PD patients had altered white matter mainly located in the posterior cingulum in PD patients (P < 0.01, FWE corrected) with (a) decreased fractional anisotropy (FA), (b) increased mean diffusivity (MD), and (c) increased radial diffusivity (RD). [Color figure can be viewed at http://wileyonlinelibrary.com]
As shown in Figures 6a and 7a, the overlap of the altered diffusion properties of the cingulum bundle was located in the posterior section.
Figure 6.
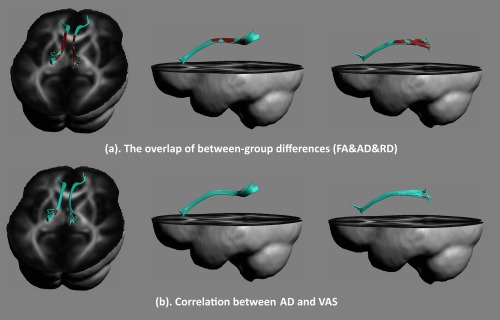
Altered diffusion measures of the dorsal part of the cingulum bundle were overlapped in PD patients (a). After controlling for the amount of prostaglandin F 2α production, the correlation between diffusion properties in the cingulum bundle during the periovulatory phase and the severity of menstrual pain during the menstrual period in PD patients was performed (b). A negative correlation between VAS scores and the AD in the dorsal‐posterior cingulum was found (P < 0.05, FWE corrected). [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 7.
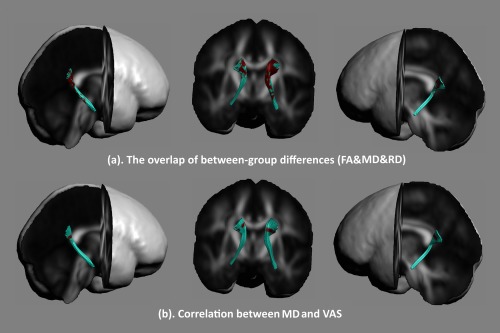
Altered diffusion measures of the parahippocampal section were overlapped in PD patients (a). After controlling the amount of prostaglandin F 2α production, a correlation between diffusion properties in the cingulum bundle during the periovulatory phase and the severity of menstrual pain during the menstrual period in PD patients were performed (b). A positive correlation between VAS scores and MD in the parahippocampal section was found (P < 0.05, FWE corrected). [Color figure can be viewed at http://wileyonlinelibrary.com]
Correlation Analysis Among Diffusion Properties During Periovulation, , and VAS During the Menstruation Phase
A significant correlation between the degree of dysmenorrheic severity and production was found in PD patients during the menstruation phase (r = 0.56, P = 0.005). In addition, significant correlations were observed between diffusion properties in the cingulum bundle during the periovulatory phase and the severity of menstrual pain during the menstrual period in PD patients (Figs. 6b and 7b; P < 0.05, FWE corrected). A negative correlation was observed between VAS score and AD in the dorsal‐posterior cingulum (Fig. 6b), and a significantly positive correlation was observed between VAS score with MD in the parahippocampal section (Fig. 7b). More importantly, even when controlling for the level of , a significant correlation was still found. It should be noted that no significant correlation was observed between diffusion properties in the cingulum bundle and production. All correlation results suggested that, independent of the production of PGs, the altered white matter tract integrity of the cingulum bundle had an important influence on the intensity of pain perception for PD patients during their pain phase.
DISCUSSION
In this study, we investigated the alteration of diffusion properties along the cingulum bundle in PD patients relative to healthy controls during their periovulatory phase. Our primary findings demonstrated that long‐term cyclic dysmenorrhea was associated with the white matter properties of the cingulum bundle in PD patients even in the pain‐free phase. It was important to note that PD patients who had more severe alteration of white matter integrity in their pain‐free phase were more vulnerable to perceive higher intensity of menstrual pain. Our findings suggested that the abnormal white matter integrity of the cingulum bundle in PD patients may underpin the central susceptibility to dysmenorrheic pain.
Altered White Matter Integrity in PD Patients
In our study, the most prominent microstructural findings differentiating patients with PD from HC were observed in the posterior section of the cingulum bundle. The posterior cingulate cortex, as the rear part of the cingulate cortex and the upper part of the limbic lobe, has been shown to have abnormal brain metabolism in PD patients [Tu et al., 2009]. For patients with PD, peripheral nociception during the menstrual cycle is magnified, which causes hyperexcitability in the somato‐visceral spinal pathways causing heightened pain perception [Bajaj et al., 2002; Granot et al., 2001]. On the basis of tract‐tracing and cytoarchitectonic studies of the macaque brain [Shipp et al., 2013], it was proposed that the human brain contained an interoceptive system and the cingulate cortex was an important node in this brain circuit [Barrett and Simmons, 2015]. Similar white matter changes, specifically lower FA in the posterior cingulate cortex, have been observed in other recurrent experiences in abdominal pain conditions, including IBS [Ellingson et al., 2013]. This finding was consistent with our results and indicated that patients with chronically recurring visceral pain had altered white matter integrity in the brain.
Previously, several studies reported abnormal brain function of the DMN in PD patients throughout the menstrual cycle. For example, Wu et al. [2016] reported the reduction of local functional connectivities in the DMN in PD patients during the periovulatory phase; during this pain‐free phase, decreased functional connectivity between the posterior cingulate cortex and periaqueductal gray was also found in PD patients [Wei et al., 2016]. These findings may implicate maladaptive neuroplasticity of the endogenous pain control systems in PDM [Wei et al., 2016]. Considering that white matter integrity in the cingulum bundle reflects the anatomical connections for functional connectivity in the DMN [Hirsiger et al., 2016], altered diffusion properties in the cingulum bundle may be related to the dysfunctional connection within the DMN, and/or between the DMN and other pain modulation systems. Hence, our findings appeared to be consistent with the dysregulated functional connection associated with communication between the DMN and antinociceptive system in PD patients. The altered white matter properties related to the DMN during the periovulatory phase could also explain why heightened sensitivity to experimental pain existed in women with PD during nonpainful phases. Our results extended previous findings of alterations in the brain function in the DMN in PD patients [Tu et al., 2010] by showing impaired white matter properties of the cingulum bundle beyond the time of menstruation.
The Relationship Between White Matter Alternation, Production of PGs, and Intensity of Menstrual Pain in PD Patients
In our results, the white matter properties of the cingulum bundle during the periovulatory phase were significantly correlated with the intensity of menstrual pain as rated by patients during the subsequent menstrual period. As we know, one of the main factors responsible for the pathogenesis of PD is the overproduction of uterine PGs [Dawood, 2006] which are controlled by both estrogen and progesterone [Alam et al., 1976; Castracane and Jordan, 1975]. Animal studies demonstrated that estrogen and progesterone could stimulate neurite outgrowth, synapse number, dendritic branching and myelination [Cooke and Woolley, 2005; Sá et al., 2009], forming the basis of white matter connectivity in the central nervous system [Peper et al., 2011]. Hence, there is a possibility that abnormal production of uterine PGs may be directly or indirectly involved in the mechanisms of altered white matter integrity in our study. However, in our results, we did not find any significant correlation between PGs production and diffusion properties along the cingulum bundle. This finding suggested that the underlying PGs production differed in PD patients and may not contribute to the plastic changes in the cingulum bundle.
Growing evidence reported the amplification of the peripheral nociceptive message generated by the reproductive organs in women with dysmenorrhea, thus causing an increased excitability of somatovisceral convergent neurons in the spinal cord subcortical areas and cortical areas and ultimately increased pain perception and altered pain modulation [Bajaj et al., 2002; Granot et al., 2001]. It is conceivable that the altered white matter integrity of the cingulum may be due to the increased viscerosensory input from the periphery into the central nervous system. Notably, in our findings, enhanced release of PGs was closely related to the severity of menstrual pain. When controlling for PG production, significant correlations between white matter properties and the severity of ongoing menstrual pain still remain. This relationship indicated that recurrent experience of dysmenorrheic pain into the central nervous system may result in white matter modifications, thus causing increased pain perception. Our findings provided important and novel insights into the diffusion properties along the cingulum bundle and its association with dysmenorrheic pain in PD patients.
Glahn et al. [2010] provided direct evidence that connectivity within the default mode network was influenced by genetic factors, and studies also pointed out that white‐matter tract microstructure was heritable [Chiang et al., 2009; Kochunov et al., 2009]. Given that genetic factors have a significant etiological contribution to both chronic pain conditions and experimentally‐induced pain [Kato et al., 2006; Norbury et al., 2007; Williams et al., 2012], the possibility also exists that altered white matter in the cingulum bundle may predate the development of PD. One theory stated that different subjects were prone to developing chronic pain due to genetic and development forces and these predisposing factors may be captured by the limbic brain anatomy and physiology [Baliki and Apkarian, 2015]. Studies in the future need to focus on teenagers with primary dysmenorrhea following menarche or in those that are predisposed to developing dysmenorrhea to identify the origin of the inherent sensitivity to pain prior to developing primary dysmenorrhea.
The High Comorbidity in Later Life of PD Patients With Many Functional Pain Disorders
Recently, as increased sensitivity to experimental pain was a potential marker for transformation to a chronic pain state [Carli et al., 2002; Hu and Iannetti, 2016; Staud et al., 2003], several researchers pointed out that dysmenorrhea may predispose women to a chronic pain state [Tu et al., 2013; Wei et al., 2016]. Indeed, there is a high comorbidity in later life for PD with many functional pain disorders and chronic pain conditions, such as chronic pelvic pain conditions [Baranowski et al., 2012], IBS [Olafsdottir et al., 2012], and many others [Berkley, 2013]. As these disorders are predominant in females [Mogil, 2012], abnormal pain perception in young PD women may account for this phenomenon. Kucyi and Davis [2015] have recently introduced the concept of a dynamic pain connectome in the brain, where the spatiotemporal signature of brain network communication represents the integration of all cognitive, affective, and sensorimotor aspects of pain. They pointed out that the DMN may act as an “exchange hinge” for pain perception and modulation, and is a crucial component of the pain connectome that plays an important role in the individual's ongoing dynamics of pain [Kucyi et al., 2014; Loggia et al., 2013]. In our findings, the altered white matter integrity of the cingulum bundle may lead to abnormal information transformation within the DMN, which may be closely associated with the intensity of dysmenorrheic pain from one visceral domain. It is conceivable that these changes, appear to be related to spontaneous communication within the pain connectome, which may also be amplified by the nociceptive input from the second visceral location [Brumovsky and Gebhart, 2010], and may make young PD patients vulnerable to other functional pain disorders later on in life.
Supporting information
Supporting Information
Supporting Figure 1
Conflict of interest: None declared.
Contributor Information
Li Hu, Email: huli@psych.ac.cn.
Ming Zhang, Email: profzmmri@gmail.com.
REFERENCES
- Alam N, Russell P, Tabor M, Moulton B (1976): Progesterone and estrogen control of uterine prostaglandin dehydrogenase activity during deciduomal growth. Endocrinology 98:859–863. [DOI] [PubMed] [Google Scholar]
- Alexander AL, Lee JE, Lazar M, Field AS (2007): Diffusion tensor imaging of the brain. Neurotherapeutics 4:316–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander DC, Pierpaoli C, Basser PJ, Gee JC (2001): Spatial transformations of diffusion tensor magnetic resonance images. IEEE T Med Imaging 20:1131–1139. [DOI] [PubMed] [Google Scholar]
- Altman G, Cain KC, Motzer S, Jarrett M, Burr R, Heitkemper M (2006): Increased symptoms in female IBS patients with dysmenorrhea and PMS. Gastroenterol Nurs 29:4–11. [DOI] [PubMed] [Google Scholar]
- Bajaj P, Bajaj P, Madsen H, Arendt‐Nielsen L (2002): A comparison of modality‐specific somatosensory changes during menstruation in dysmenorrheic and nondysmenorrheic women. Clin J Pain 18:180–190. [DOI] [PubMed] [Google Scholar]
- Baliki M, Apkarian AV (2015): Nociception, pain, negative moods, and behavior selection. Neuron 87:474–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranowski A, Abrams P, Berger R, Buffington A, Collett B, Emmanuel A, Fall M, Hanno P, Howard F, Hughes J (2012): Taxonomy of Pelvic Pain. Classification of Chronic Pain.
- Barrett LF, Simmons WK (2015): Interoceptive predictions in the brain. Nat Rev Neurosci 16:419–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkley KJ (2013): Primary dysmenorrhea: An urgent mandate. Pain 1. [Google Scholar]
- Berkley KJ, McAllister SL (2011): Don't dismiss dysmenorrhea! Pain 152:1940–1941. [DOI] [PubMed] [Google Scholar]
- Brumovsky P, Gebhart G (2010): Visceral organ cross‐sensitization—an integrated perspective. Auton Neurosci 153:106–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carli G, Suman AL, Biasi G, Marcolongo R (2002): Reactivity to superficial and deep stimuli in patients with chronic musculoskeletal pain. Pain 100:259–269. [DOI] [PubMed] [Google Scholar]
- Castracane VD, Jordan VC (1975): The effect of estrogen and progesterone on uterine prostaglandin biosynthesis in the ovariectomized rat. Biol Reprod 13:587–596. [DOI] [PubMed] [Google Scholar]
- Chen YJ, Lo YC, Hsu YC, Fan CC, Hwang TJ, Liu CM, Chien YL, Hsieh MH, Liu CC, Hwu HG (2015): Automatic whole brain tract‐based analysis using predefined tracts in a diffusion spectrum imaging template and an accurate registration strategy. Hum Brain Mapp 36:3441–3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang MC, Barysheva M, Shattuck DW, Lee AD, Madsen S, Avedissian C, Klunder AD, Toga AW, Mcmahon KL, Zubicaray GID (2009): Genetics of brain fiber architecture and intellectual performance. J Neurosci 29:2212–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung SD, Liu SP, Lin HC, Kang JH (2014): Association of dysmenorrhea with interstitial cystitis/bladder pain syndrome: A case–control study. Obstet Gynecol Scand 93:921–925. [DOI] [PubMed] [Google Scholar]
- Cooke BM, Woolley CS (2005): Gonadal hormone modulation of dendrites in the mammalian CNS. J Neurobiol 64:34–46. [DOI] [PubMed] [Google Scholar]
- Dawood MY (1987): Dysmenorrhea and Prostaglandins. Gynecol Endocrinol: Springer; pp 405–421. [Google Scholar]
- Dawood MY (2006): Primary dysmenorrhea: Advances in pathogenesis and management. Obstet Gynecol 108:428–441. [DOI] [PubMed] [Google Scholar]
- Donahue RR, Lagraize SC, Fuchs PN (2001): Electrolytic lesion of the anterior cingulate cortex decreases inflammatory, but not neuropathic nociceptive behavior in rats. Brain Res 897:131–138. [DOI] [PubMed] [Google Scholar]
- Ellingson BM, Mayer E, Harris RJ, Ashe‐McNally C, Naliboff BD, Labus JS, Tillisch K (2013): Diffusion tensor imaging detects microstructural reorganization in the brain associated with chronic irritable bowel syndrome. Pain 154:1528–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsell L, Leemans A, Langan C, Van Hecke W, Barker GJ, McCarthy P, Jeurissen B, Sijbers J, Sunaert S, Cannon DM (2013): Limbic and callosal white matter changes in euthymic bipolar I disorder: An advanced diffusion magnetic resonance imaging tractography study. Biol Psychiat 73:194–201. [DOI] [PubMed] [Google Scholar]
- Geng X, Gouttard S, Sharma A, Gu H, Styner M, Lin W, Gerig G, Gilmore JH (2012): Quantitative tract‐based white matter development from birth to age 2years. Neuroimage 61:542–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giamberardino MA (2008): Women and visceral pain: Are the reproductive organs the main protagonists? Mini‐review at the occasion of the “European Week Against Pain in Women 2007”. Eur J Pain 12:257–260. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Winkler AM, Kochunov P, Almasy L, Duggirala R, Carless MA, Curran JC, Olvera RL, Laird AR, Smith SM (2010): Genetic control over the resting brain. Proc Natl Acad Sci USA 107:1223–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodlett CB, Fletcher PT, Gilmore JH, Gerig G (2009): Group analysis of DTI fiber tract statistics with application to neurodevelopment. Neuroimage 45:S133–S142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granot M, Yarnitsky D, Itskovitz‐Eldor J, Granovsky Y, Peer E, Zimmer E (2001): Pain perception in women with dysmenorrhea. Obstet Gynecol 98:407–411. [DOI] [PubMed] [Google Scholar]
- Hirsiger S, Koppelmans V, Mérillat S, Liem F, Erdeniz B, Seidler RD, Jäncke L (2016): Structural and functional connectivity in healthy aging: Associations for cognition and motor behavior. Hum Brain Mapp 37:855–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Iannetti GD (2016): Painful issues in pain prediction. Trends Neurosci 39:212–220. [DOI] [PubMed] [Google Scholar]
- Iacovides S, Avidon I, Baker FC (2015): What we know about primary dysmenorrhea today: A critical review. Hum Reprod Update 21:762–778. [DOI] [PubMed] [Google Scholar]
- Iacovides S, Baker FC, Avidon I, Bentley A (2013): Women with dysmenorrhea are hypersensitive to experimental deep muscle pain across the menstrual cycle. J Pain 14:1066–1076. [DOI] [PubMed] [Google Scholar]
- Jones DK, Christiansen K, Chapman R, Aggleton J (2013): Distinct subdivisions of the cingulum bundle revealed by diffusion MRI fibre tracking: Implications for neuropsychological investigations. Neuropsychologia 51:67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Sullivan PF, Evengård B, Pedersen NL (2006): Importance of genetic influences on chronic widespread pain. Arthritis Rheum 54:1682–1686. [DOI] [PubMed] [Google Scholar]
- Kochunov P, Winkler A, Blangero J, Lancaster JL, Almasy L, Fox PT, Glahn D (2009): Genetics of DTI‐derived parameters of cerebral white matter. A track‐based heritability and linkage study in extended pedigree. Neuroimage 47:S139. [Google Scholar]
- Kristo G, Leemans A, Raemaekers M, Rutten GJ, Gelder B, Ramsey NF (2013): Reliability of two clinically relevant fiber pathways reconstructed with constrained spherical deconvolution. Magnet Reson Med 70:1544–1556. [DOI] [PubMed] [Google Scholar]
- Kucyi A, Davis KD (2015): The dynamic pain connectome. Trends Neurosci 38:86–95. [DOI] [PubMed] [Google Scholar]
- Kucyi A, Moayedi M, Weissman‐Fogel I, Goldberg MB, Freeman BV, Tenenbaum HC, Davis KD (2014): Enhanced medial prefrontal‐default mode network functional connectivity in chronic pain and its association with pain rumination. J Neurosci 34:3969–3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucyi A, Salomons TV, Davis KD (2013): Mind wandering away from pain dynamically engages antinociceptive and default mode brain networks. Proc Natl Acad Sci USA 110:18692–18697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leemans A, Jeurissen B, Sijbers J, Jones D (2009): ExploreDTI: A graphical toolbox for processing, analyzing, and visualizing diffusion MR data. p 3537.
- Li X, Hu L (2016): The role of stress regulation on neural plasticity in pain chronification. Neural Plast 2016:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Wang G, Liu Y, Yu Q, Yang F, Jin L, Sun J, Yang X, Qin W, Calhoun VD (2016): White matter microstructure alterations in primary dysmenorrhea assessed by diffusion tensor imaging. Sci Rep 6:25836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loggia ML, Kim J, Gollub RL, Vangel MG, Kirsch I, Kong J, Wasan AD, Napadow V (2013): Default mode network connectivity encodes clinical pain: An arterial spin labeling study. Pain 154:24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour AR, Baliki MN, Huang L, Torbey S, Herrmann KM, Schnitzer TJ, Apkarian AV (2013): Brain white matter structural properties predict transition to chronic pain. Pain 154:2160–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogil JS (2012): Sex differences in pain and pain inhibition: Multiple explanations of a controversial phenomenon. Nat Rev Neurosci 13:859–866. [DOI] [PubMed] [Google Scholar]
- Mori S, van Zijl P (2002): Fiber tracking: Principles and strategies–A technical review. NMR Biomed 15:468–480. [DOI] [PubMed] [Google Scholar]
- Norbury TA, Macgregor AJ, Urwin J, Spector TD, Mcmahon SB (2007): Heritability of responses to painful stimuli in women: A classical twin study. Brain 130:3041–3049. [DOI] [PubMed] [Google Scholar]
- O'Donnell LJ, Westin C‐F, Golby AJ (2009): Tract‐based morphometry for white matter group analysis. Neuroimage 45:832–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olafsdottir LB, Gudjonsson H, Jonsdottir HH, Björnsson E, Thjodleifsson B (2012): Natural history of irritable bowel syndrome in women and dysmenorrhea: A 10‐year follow‐up study. Gastroent Res Pract 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peper JS, van den Heuvel MP, Mandl RC, Pol HEH, van Honk J (2011): Sex steroids and connectivity in the human brain: A review of neuroimaging studies. Psychoneuroendocrino 36:1101–1113. [DOI] [PubMed] [Google Scholar]
- Reijmer Y, Schultz A, Leemans A, O'sullivan M, Gurol M, Sperling R, Greenberg S, Viswanathan A, Hedden T (2015): Decoupling of structural and functional brain connectivity in older adults with white matter hyperintensities. Neuroimage 117:222–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sá S, Lukoyanova E, Madeira M (2009): Effects of estrogens and progesterone on the synaptic organization of the hypothalamic ventromedial nucleus. Neuroscience 162:307–316. [DOI] [PubMed] [Google Scholar]
- Sales KJ, Jabbour HN (2003): Cyclooxygenase enzymes and prostaglandins in pathology of the endometrium. Reproduction 126:559–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipp S, Adams RA, Friston KJ (2013): Reflections on agranular architecture: Predictive coding in the motor cortex. Trends Neurosci 36:706–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S‐K, Sun S‐W, Ju W‐K, Lin S‐J, Cross AH, Neufeld AH (2003): Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage 20:1714–1722. [DOI] [PubMed] [Google Scholar]
- Staud R, Robinson ME, Vierck CJ, Cannon RC, Mauderli AP, Price DD (2003): Ratings of experimental pain and pain‐related negative affect predict clinical pain in patients with fibromyalgia syndrome. Pain 105:215–222. [DOI] [PubMed] [Google Scholar]
- Thomason ME, Thompson PM (2011): Diffusion imaging, white matter, and psychopathology. Ann Rev Clin Psychol 7:63–85. [DOI] [PubMed] [Google Scholar]
- Tu C‐H, Niddam DM, Chao H‐T, Chen L‐F, Chen Y‐S, Wu Y‐T, Yeh T‐C, Lirng J‐F, Hsieh J‐C (2010): Brain morphological changes associated with cyclic menstrual pain. Pain 150:462–468. [DOI] [PubMed] [Google Scholar]
- Tu C‐H, Niddam DM, Chao H‐T, Liu R‐S, Hwang R‐J, Yeh T‐C, Hsieh J‐C (2009): Abnormal cerebral metabolism during menstrual pain in primary dysmenorrhea. Neuroimage 47:28–35. [DOI] [PubMed] [Google Scholar]
- Tu C‐H, Niddam DM, Yeh T‐C, Lirng J‐F, Cheng C‐M, Chou C‐C, Chao H‐T, Hsieh J‐C (2013): Menstrual pain is associated with rapid structural alterations in the brain. Pain 154:1718–1724. [DOI] [PubMed] [Google Scholar]
- Vaccarino AL, Melzack R (1989): Analgesia produced by injection of lidocaine into the anterior cingulum bundle of the rat. Pain 39:213–219. [DOI] [PubMed] [Google Scholar]
- Van Hecke W, Sijbers J, D'Agostino E, Maes F, De Backer S, Vandervliet E, Parizel PM, Leemans A (2008): On the construction of an inter‐subject diffusion tensor magnetic resonance atlas of the healthy human brain. Neuroimage 43:69–80. [DOI] [PubMed] [Google Scholar]
- Vincent K, Warnaby C, Stagg CJ, Moore J, Kennedy S, Tracey I (2011): Dysmenorrhoea is associated with central changes in otherwise healthy women. Pain 152:1966–1975. [DOI] [PubMed] [Google Scholar]
- Wei S‐Y, Chao H‐T, Tu C‐H, Li W‐C, Low I, Chuang C‐Y, Chen L‐F, Hsieh J‐C (2016): Changes in functional connectivity of pain modulatory systems in women with primary dysmenorrhea. Pain 157:92–102. [DOI] [PubMed] [Google Scholar]
- Williams FM, Scollen S, Cao D, Memari Y, Hyde CL, Zhang B, Sidders B, Ziemek D, Shi Y, Harris J (2012): Genes contributing to pain sensitivity in the normal population: An exome sequencing study. PLoS Genet 8:1208–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T‐H, Tu C‐H, Chao H‐T, Li W‐C, Low I, Chuang C‐Y, Yeh T‐C, Cheng C‐M, Chou C‐C, Chen L‐F (2016): Dynamic changes of functional pain connectome in women with primary dysmenorrhea. Sci Rep 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Figure 1


