Abstract
Autism spectrum disorder (ASD) is typified as a brain connectivity disorder in which white matter abnormalities are already present early on in life. However, it is unknown if and to which extent these abnormalities are hard‐wired in (older) adults with ASD and how this interacts with age‐related white matter changes as observed in typical aging. The aim of this first cross‐sectional study in mid‐ and late‐aged adults with ASD was to characterize white matter microstructure and its relationship with age. We utilized diffusion tensor imaging with head motion control in 48 adults with ASD and 48 age‐matched controls (30–74 years), who also completed a Flanker task. Intra‐individual variability of reaction times (IIVRT) measures based on performance on the Flanker interference task were used to assess IIVRT‐white matter microstructure associations. We observed primarily higher mean and radial diffusivity in white matter microstructure in ASD, particularly in long‐range fibers, which persisted after taking head motion into account. Importantly, group‐by‐age interactions revealed higher age‐related mean and radial diffusivity in ASD, in projection and association fiber tracts. Subtle dissociations were observed in IIVRT‐white matter microstructure relations between groups, with the IIVRT‐white matter association pattern in ASD resembling observations in cognitive aging. The observed white matter microstructure differences are lending support to the structural underconnectivity hypothesis in ASD. These reductions seem to have behavioral percussions given the atypical relationship with IIVRT. Taken together, the current results may indicate different age‐related patterns of white matter microstructure in adults with ASD. Hum Brain Mapp 38:82–96, 2017. © 2016 Wiley Periodicals, Inc.
Keywords: autism, adults, DTI, white matter, underconnectivity, interference control, intra‐individual variability
Abbreviations
- AD
Axial diffusivity
- ASD
Autism spectrum disorder
- DTI
Diffusion tensor imaging
- DWI
Diffusion‐weighted imaging
- FA
Fractional anisotropy
- IIVRT
Intra‐individual variability of reaction time
- MD
Mean diffusivity
- MRT
Mean reaction times
- RD
Radial diffusion
- RI
Response incongruent
- SI
Stimulus incongruent
- TMI
Total motion index
- UNC
Uncinate fasciculus
INTRODUCTION
It is becoming widely accepted that autism spectrum disorder [ASD; (American Psychiatric Association, 2000)] is characterized by atypical structural brain connectivity (Vissers et al., 2012). Indeed, numerous diffusion tensor imaging (DTI) studies from childhood to young adulthood noticed disruptions to white matter microstructure in ASD [Aoki et al., 2013; Rane et al., 2015; Travers et al., 2012; Vissers et al., 2012], particularly among long‐range fibers (defined as connections between regions across different lobes and/or hemispheres). Together with reduced long‐range functional connectivity, disconnectivity of long‐range fibers is one of the suggested pathophysiological mechanisms in ASD, and led to the developmental underconnectivity hypothesis of ASD [Courchesne and Pierce, 2005; Just et al., 2004, 2012; Kana et al., 2011]. As with manifestations of autism symptomatology [Mukaetova‐Ladinska et al., 2012; Perkins and Berkman, 2012], white matter abnormalities may change across the first half of the lifespan [Travers et al., 2015], but these abnormalities still need to be quantified in (older) adults with ASD. Typical development of white matter microstructure shows a curvilinear pattern plateauing around mid‐adulthood and rapidly decreasing in old age [Westlye et al., 2010; Yeatman et al., 2014]. The question is if and how this interacts with ASD‐related white matter abnormalities in (late) adulthood.
DTI assesses the orientation and microstructure of white matter tracts in vivo [Basser et al., 1994; Le Bihan, 2003] and aids in understanding the underlying axonal architecture, which may facilitate the communication among brain regions [van den Heuvel et al., 2009]. White matter microstructure is quantified by fractional anisotropy (FA), mean, radial, and axial diffusivity (MD, RD, AD). Lower FA is suggested to indicate low tract coherence, likely due to damage in the tissue structural organization [Pierpaoli and Basser, 1996]. MD reflects overall diffusion magnitude, and could reflect altered cytoarchitecture (differences in cellular size and integrity [Basser et al., 1994; Pierpaoli et al., 1996], with higher MD thought to be indicative for immaturity or tissue degeneration [Sykova, 2004]. Studies in animal models have suggested that AD and RD provide a more specific interpretation of DTI results than FA and MD, and propose that AD is related to axonal integrity (damage or loss), whereas RD is related to myelination processes [Alexander et al., 2007; Kim et al., 2006; Song et al., 2003; Song et al., 2005]. While there's no simplistic one‐to‐one biological interpretation to these values to human studies, it is essential to include each of these measures to understand white matter microstructure of people with ASD.
White matter abnormalities in early adulthood in ASD appear to converge towards lower FA, and, despite being scarcely reported, also higher MD and RD in various tracts [Bloemen et al., 2010; Catani et al., 2008; Conturo et al., 2008; Gibbard et al., 2013; Itahashi et al., 2015; Langen et al., 2012; Mueller et al., 2013; Thakkar et al., 2008]. However, other studies reported no differences in FA and/or MD [Bakhtiari et al., 2012; Kirkovski et al., 2015; Pugliese et al., 2009; Thomas et al., 2011], or even higher FA [Roine et al., 2013] in young adults with ASD. These mixed results in DTI metrics may be explained by the heterogeneous nature of ASD, different methodological differences (voxel‐wise, whole‐brain or tractography approaches), small samples and the inclusion of adolescents, hampering interpretation of age‐related changes in adulthood [Aoki et al., 2013; Travers et al., 2012]. Furthermore, recent studies have shown that head motion can obscure reliability of diffusion‐weighted imaging (DWI) and lead to spurious results [Koldewyn et al., 2014; Yendiki et al., 2014]. To overcome a number of these issues, we used fiber tracking, including stringent data quality and motion control, to examine a relatively large sample of adults with ASD and matched controls (N = 96, aged 30–74 years). Fiber tracking takes into account the diffusion profile orientation to reconstruct major fiber bundle trajectories [Jones, 2008]. Here we used TRACULA [Tracts Constrained by Underlying Anatomy; (Yendiki et al., 2011)] to reliably extract 18 well‐known tracts, and quantify the microstructural organization to further the understanding of white matter abnormalities and anatomical connectivity in adults with ASD.
The aims of this study were threefold. First we tested if and to what extent white matter microstructure is impaired in adults with ASD, and second if these abnormalities are subject to atypical age‐related changes. Based on the available DTI evidence in ASD, albeit incomplete thus far for adults, we hypothesized reduced white matter microstructure primarily in long‐range association fibers [Aoki et al., 2013; Rane et al., 2015; Travers et al., 2012]. Furthermore, we expected age‐related changes of white matter microstructure to be more pronounced in ASD compared to controls [Kleinhans et al., 2012; Travers et al., 2015]. Given the well‐documented associations between white matter microstructure and intra‐individual variability of reaction times (IIVRT) in typical development and aging [MacDonald et al., 2009; Nilsson et al., 2014; Tamnes et al., 2012], and as meta‐analytical evidence up to age 30 suggests that people with ASD show increased IIVRT compared to controls [Karalunas et al., 2014], we additionally included IIVRT as a behavioral measure to test for the association with white matter microstructure. Thus thirdly, we expected differences in the IIVRT‐white matter microstructure association between groups, as disconnectivity in associative pathways can also increase IIVRT [MacDonald et al., 2006].
METHODS AND MATERIALS
Participants
Fifty individuals with ASD and 49 controls between 30–74 years were recruited from a cohort of participants (estimated IQ > 80) of a large‐scale behavioral study [Koolschijn and Geurts, 2016; Lever and Geurts, 2016]. Two individuals were excluded due to damaged DTI scans, one due to poor scan quality based on visual inspection. The final sample consisted of 48 participants with ASD (Mean age 51.3 (SD = 12.3), 33 males) and 48 controls (Mean age 50.5 (11.8), 31 males; see Table I). Details on inclusion criteria have been described earlier [Koolschijn and Geurts, 2016]. In short, all individuals with ASD received their clinical ASD diagnosis by a multidisciplinary team with clinicians experienced in the assessment of ASD. To verify the clinical diagnosis we used the following diagnostic inclusion criteria for ASD participants: (1) formal clinical diagnosis of ASD prior to inclusion; (2) confirmation of diagnosis with Autism Diagnostic Observation Schedule module 4 [Lord et al., 1989] and/or Autism‐Spectrum quotient, 50‐item list [Baron‐Cohen et al., 2001]: 31 individuals had a score above the cutoff of the ADOS (≥7) and those not scoring above this cut off did score above the clinical AQ cutoff (≥26) (for similar approaches see [Ecker et al. 2012; Lai et al. 2013]). Please note that, although the evaluation of childhood problems was part of the clinical assessment procedures, obviously, with the age‐range of our participants an interview such as the Autism Diagnostic Interview‐Revised (ADI‐R; [Lord et al. 1994]) is not feasible or reliable; (3) no self‐reported history of neurological disorders, chronic illness, learning disabilities or schizophrenia. Controls had to meet criterion #3; with additional exclusion criterion of ASD diagnosis or a first or second‐degree family member with ASD.
There were no between‐group differences for IQ, age, sex, and handedness (Table 1, additional demographics: Supporting Information Table I). Participants gave written informed consent and received participation fee and travel reimbursement. The university review board approved the study (#2013‐PN‐2668).
Table 1.
Demographic variables
| Description | ASD | CTRL | Statistics |
|---|---|---|---|
| N=48 | N=48 | ||
| #Males (%) | 33 (69%) | 31 (65%) | χ2 = 0.19, P = 0.665 |
| Age (SD)[range] | 51.32 (12.29) [30.04–73.98] | 50.47 (11.83) [30.62–73.77] | F = 0.12, P = 0.733 |
| IQ (SD)[range] | 116.60 (16.04) [86–155] | 110.98 (15.34) [80–139] | F =3.08, P = 0.082 |
| Level of Educational Attainmenta | 1/16/22/9 | 1/11/26/10 | χ2=1.31, P = 0.726 |
| Handedness | χ2=1.23, P = 0.940 | ||
| Left | 5 | 4 | |
| Right | 40 | 41 | |
| Ambidexter | 3 | 3 | |
| Age first diagnosis | 45.70 (13.57) [11.22–68.04] | N.A. | |
| ADOS Total | 7.92 (3.35) [1–19] | N.A. | |
| ADOS cutoff (<7)b | 17 (35%) | N.A. | |
| AQ Total | 35.96 (6.62) | 13.00 (5.94) | F =319.83, P < 0.001 |
| [19–47] | [4–26] | ||
|
AQ‐cutoff (<26 ASD, >23 CTRL) |
4 (8%) | 0 | χ2=81.23, P < 0.001 |
| Medication N (%)c | 38 (79%) | 19 (40%) | χ2=15.59, P < 0.001 |
Note. Numbers in bold reflect significant between‐group differences.
Number of participants who had pre‐vocational education/junior general secondary or vocation education/senior general secondary education or vocation colleges/university education based on the Verhage scale(Verhage, 1964).
All participants below ADOS threshold scores, had scores above the AQ clinical cut‐off.
Detailed medication information is available in Supporting Information Table I.
Abbreviations: ASD, autism spectrum disorder; CTRL, controls; ADOS, Autism Diagnostic Observation Schedule; AQ, Autism‐Spectrum quotient; MMSE, Mini‐mental state examination; N.A., not applicable.
PROCEDURE AND EXPERIMENTAL DESIGN
Data Acquisition
Participants were trained to lie still in a mock‐scanner, and were scanned on a 3‐Tesla Philips Achieva MRI‐system (Best, The Netherlands). Two high‐resolution T1‐weighted scans were obtained: 3DFFE, multishot‐TFE: TR = 8.2 msec; TE = 3.8 msec, 220slices, voxel‐size = 1mm3, FOV = 240 × 188, matrix = 80, 2DSENSE: P(RL)=2.5, S(FH)=2). We collected two DWI measurements [TE(Echo Time)=86ms; TR(Repetition Time)=7542 ms; Flip Angle = 90°; 60 2mm transversal slices; FOV = 2242 mm; voxel‐size = 2 mm3, reconstruction matrix 1122, 32 directions, SENSE = 2, b0 = 1,000 s/mm2]. The second DWI set had identical parameters, except that it was acquired with a reversed k‐space readout direction, enabling removal of susceptibility artifacts during post‐processing [Andersson et al., 2003]. Head motion was restricted using foam inserts around the head.
Tract Reconstruction
Before tract reconstruction, diffusion scans were merged using FSL 5.0.8 [Jenkinson et al., 2012] and processed with a six‐stage processing‐stream: (1) FreeSurfer 5.3 segmentation and parcellation of T1‐weighted scans [Fischl, 2012] (gray matter segmentations will be presented elsewhere); (2) DTI‐preprocessing with software developed in‐house using the Neuroscience Gateway and the Dutch e‐Science Grid [Shahand et al., 2015]. Preprocessing included head motion and eddy current correction with affine registrations (all volumes were registered to the first volume using SPM8), gradient corrections [Leemans and Jones, 2009], Rician noise reduction [Caan et al., 2010] and diffusion tensor calculation using non‐linear least squares estimations. Subsequently, FA and MD‐maps were computed for each individual; (3) Ball‐and‐stick‐modeling using FSL [Jenkinson et al., 2012; Woolrich et al., 2009]; (4) Probabilistic tracking and track determination using TRACULA [Yendiki et al., 2011] on the Dutch e‐science grid through the Neuroscience Gateway [Shahand et al., 2015], including extraction of mean FA, MD, RD and AD for each tract; (5) Quality control. We automatically tested and visually inspected for incomplete or missing tracts using the number of reconstructed streamlines. In case of unsuccessful reconstruction, tract reinitialization was performed, followed by full reconstruction; (6) To account for potential confounding effects of head motion, we computed four head motion measures (average volume‐by‐volume translation and rotation, percentage of slices with signal dropout, and signal dropout severity; a feature in the TRACULA processing pipeline) and the total motion index (TMI) (a sum variable to estimate slower, between‐volume motion captured by the registration‐based measures, as well as more rapid, within‐volume motion, captured by the intensity‐based measures) for each participant as described in Yendiki et al. [2014].
IIVRT
Participants performed an adapted interference control paradigm [Flanker‐task; (Eriksen and Eriksen, 1974; van Veen et al., 2001)]. This paradigm is known to be challenging for both people with ASD [Geurts et al., 2014] and older adults [Lustig and Jantz, 2015], and is, therefore, likely to elicit a wide distribution of reaction times. Participants saw five horizontally presented letters (S, M, H, and/or P) and had to respond to the centrally located letter (target) while ignoring the other letters (flankers). There were three trial types: Congruent (CO, the target was flanked by identical letters); Stimulus incongruent (SI, target and flankers were non‐identical, but led to the same response); and Response incongruent (RI, target and flankers were mapped onto different responses). Participants were instructed to respond as fast as possible with their left or right index fingers; hand‐assignment was counterbalanced across participants (Supporting Information Methods). Mean reaction times (MRT), and accuracy were calculated for each participant overall and per trial type; IIVRT was calculated in terms of standard deviation of MRT (sdRT), and the coefficient of MRT variation (CV = sdRT/MRT), irrespective of performance. Both measures for quantifying IIVRT were included to be able to compare our results with current practices in the ASD literature [Karalunas et al., 2014)].
DATA ANALYSIS
Demographics
Demographics were compared between groups using ANOVAs, and χ2‐tests for categorical variables (Table 1).
White Matter Group and Age‐Comparisons
Given that we had specific hypotheses about group, and group‐by‐age interactions, and wanted to examine if these variables explained variance after taking head motion into account, we performed hierarchical linear regression analyses and examined average FA, AD, RD and MD for each of the 18 tracts. TMI was included to control for potential confounding effects of head motion [Koldewyn et al., 2014; Yendiki et al., 2014], followed by group (ASD and CTRL), age and group‐by‐age interaction as our variables of interest. Next, sex was added to examine possible white matter related sex‐differences [Beacher et al., 2012; Sacher et al., 2013]. Data were checked for outliers. All regression analyses were repeated with standardized residuals < −3 and >3 removed. Per outcome measure 0 to 3 cases were excluded to achieve that all residuals fell in the −3 to 3 ranges. If re‐analyses didn't change initial outcomes, data including outliers were reported. We considered results to be significant at P < 0.003, Bonferroni correction for 18 tracts. The same procedure was repeated without TMI to demonstrate possible motion‐related influences.
White Matter IIVRT Association
First, a partial correlation matrix, controlling for TMI, was built between DTI metrics from all tracts and measures derived from the Flanker task (MRT, sdRT, and CV overall and for each trial type), creating an 84x84 correlation matrix. MRT was included based on earlier associations with white matter microstructure [Gunning‐Dixon et al., 2009; Kennedy and Raz, 2009]. Partial correlations were considered significant with P < 0.00006 after Bonferroni correction (18 tracts, 4 DTI metrics, and 12 Flanker measures).
Second, we tested whether there were between‐group differences in white matter IIVRT‐associations. Partial correlation matrices, controlling for TMI, were built for each group. Subsequently, Fisher's r‐to‐z transformations were applied to obtain a comparable Z‐value instead of the original r. Z‐values were compared with:
P‐values were calculated for each ΔZ:
where normcdf is the normal cumulative distribution function (Matlab 8.4, The Mathworks Inc), and abs(ΔZ) the absolute value of ΔZ. See Supporting Information for behavioral Flanker analyses.
RESULTS
Movement and Tract Reconstruction
There were no differences in translational (M ASD = 0.40 vs. M CTRL = 0.26, F = 0.46, P = 0.50) or rotational movement parameters (M ASD = 0.19 vs. M CTRL = 0.28, F = 0.25, P = 0.62), or associations with age or group × age interactions (all P's > 0.22).
Out of the 1,728 reconstructed tracts, for 27 participants (18 ASD, 9 CTRL) a total of 44 tracts could not be (fully) reconstructed (2.5%), and were excluded from analyses (Supporting Information Table II).
Are There Differences in White Matter Microstructure Between ASD and CTRLS?
Table 2 shows the results from the hierarchical regression analyses comparing DTI metrics for each tract between groups. Here average values over the entire support of the path distributions are reported (results remained highly similar with weighted averages, Supporting Information Table III).
Table 2.
Group and age‐related differences in white matter microstructure
| Tract | LH/RH | WM | N | Variable | ß | P | adj R2 | F | p‐F model | With TMI correctiona |
|---|---|---|---|---|---|---|---|---|---|---|
| fMajor | FA | 90 | 0.104 | 4.43 | 0.006 | |||||
| RD | 89 | 0.108 | 3.44 | 0.020 | ||||||
| fMinor | FA | 89 | Age | −0.354 | 0.010 | 0.24 | 7.94 | 0.001 | ||
| Sex | −0.317 | 0.001 | ||||||||
| MD | 89 | Group | 0.216 | 0.029 | 0.17 | 5.52 | 0.001 | |||
| Group × age | 0.286 | 0.045 | ||||||||
| Sex | 0.210 | 0.035 | ||||||||
| RD | 89 | Group | 0.192 | 0.047 | 0.206 | 6.72 | <0.001 | |||
| Sex | 0.265 | 0.007 | ||||||||
| AD | 89 | Group × age | 0.307 | 0.042 | 0.071 | 3.25 | 0.026 | |||
| ATR | LH | FA | 95 | Group | −0.240 | 0.012 | 0.182 | 6.23 | <0.001 | |
| Sex | −0.222 | 0.021 | ||||||||
| MD | 93 | Group | 0.259 | 0.005 | 0.271 | 9.55 | <0.001 | b | ||
| Group × age | 0.438 | 0.001 | b | |||||||
| RD | 93 | Group | 0.281 | 0.002 | 0.283 | 1.5 | <0.001 | |||
| Group × age | 0.413 | 0.002 | ||||||||
| RH | FA | 95 | Group | −0.213 | 0.020 | 0.235 | 1.62 | <0.001 | ||
| Age | −0.273 | 0.035 | ||||||||
| MD | 95 | Group | 0.227 | 0.012 | 0.269 | 12.51 | <0.001 | |||
| Group × age | 0.390 | 0.002 | ||||||||
| RD | 95 | Group | 0.252 | 0.004 | 0.330 | 16.44 | <0.001 | b | ||
| Group × age | 0.377 | 0.002 | b | |||||||
| CAB | RH | AD | 80 | Group | 0.246 | 0.027 | 0.066 | 2.87 | 0.042 | |
| CCG | LH | FA | 95 | 0.078 | 3.64 | 0.016 | ||||
| RD | 94 | Group | 0.21 | 0.031 | 0.149 | 6.44 | 0.001 | |||
| RH | MD | 96 | Group | 0.242 | 0.017 | 0.064 | 3.15 | 0.029 | ||
| RD | 96 | Group | 0.236 | 0.02 | 0.063 | 3.12 | 0.030 | |||
| CST | LH | FA | 95 | 0.081 | 3.76 | 0.014 | ||||
| RD | 95 | 0.088 | 4 | 0.010 | ||||||
| RH | FA | 95 | 0.087 | 3.97 | 0.010 | b | ||||
| MD | 95 | Group | 0.241 | 0.016 | 0.097 | 4.35 | 0.007 | b | ||
| RD | 95 | Group | 0.236 | 0.015 | 0.147 | 6.39 | 0.001 | b | ||
| ILF | LH | FA | 95 | Age | −0.293 | 0.035 | 0.155 | 6.75 | <0.001 | |
| MD | 95 | Group | 0.201 | 0.032 | 0.2 | 8.85 | <0.001 | b | ||
| Group × age | 0.379 | 0.005 | ||||||||
| RD | 95 | 0.166 | 7.17 | <0.001 | b | |||||
| AD | 95 | Group | 0.199 | 0.047 | 0.069 | 3.33 | 0.023 | |||
| Group × age | 0.346 | 0.018 | ||||||||
| RH | FA | 93 | Age | −0.454 | <0.001 | 0.325 | 15.77 | <0.001 | ||
| MD | 93 | Group | 0.286 | 0.002 | 0.268 | 12.24 | <0.001 | b | ||
| Group × age | 0.311 | 0.018 | b | |||||||
| RD | 93 | Group | 0.233 | 0.007 | 0.335 | 16.42 | <0.001 | b | ||
| Age | 0.313 | 0.013 | b | |||||||
| Group × age | 0.277 | 0.027 | b | |||||||
| AD | 93 | Group | 0.291 | 0.004 | 0.094 | 4.2 | 0.008 | b | ||
| SLFP | LH | FA | 95 | Group | −0.192 | 0.048 | 0.136 | 5.93 | 0.001 | |
| RD | 93 | 0.082 | 3.73 | 0.014 | ||||||
| AD | 91 | Age | −0.516 | <0.001 | 0.102 | 4.42 | 0.006 | b | ||
| Group × age | 0.363 | 0.013 | ||||||||
| RH | FA | 96 | Age | −0.296 | 0.03 | 0.177 | 7.8 | <0.001 | b | |
| MD | 96 | Group × age | 0.394 | 0.003 | 0.214 | 9.61 | <0.001 | |||
| RD | 96 | 0.137 | 5.88 | 0.001 | ||||||
| SLFT | LH | FA | 96 | 0.088 | 4.05 | 0.009 | ||||
| RD | 96 | Group | 0.261 | 0.008 | 0.134 | 5.9 | 0.001 | |||
| RH | FA | 96 | Group | −0.197 | 0.036 | 0.193 | 8.56 | <0.001 | b | |
| Age | −0.273 | 0.043 | ||||||||
| MD | 96 | Group | 0.233 | 0.009 | 0.272 | 12.85 | <0.001 | |||
| Group × age | 0.424 | 0.001 | b | |||||||
| RD | 96 | Group | 0.246 | 0.005 | 0.288 | 13.84 | <0.001 | b | ||
| Group × age | 0.379 | 0.003 | ||||||||
| AD | 96 | Group × age | 0.388 | 0.006 | 0.114 | 5.06 | 0.003 | |||
| UNC | LH | MD | 95 | 0.153 | 6.68 | <0.001 | ||||
| RD | 95 | 0.122 | 5.34 | 0.002 | ||||||
| AD | 95 | 0.121 | 5.33 | 0.002 | ||||||
| RH | FA | 93 | Group | −0.344 | 0.001 | 0.163 | 5.49 | <0.001 | ||
| Sex | −0.219 | 0.025 | ||||||||
| MD | 93 | Group | 0.357 | <0.001 | 0.239 | 10.61 | <0.001 | b | ||
| RD | 93 | Group | 0.406 | <0.001 | 0.283 | 10.06 | <0.001 | b | ||
| Sex | 0.198 | 0.029 | b | |||||||
| RH | AD | 94 | Group × age | 0.297 | 0.036 | 0.085 | 3.89 | 0.012 |
Note. Stepwise regression analyses were performed with (TMI as correction variable, then) group, age and group × age interaction, followed by sex as predictors. Numbers in bold reflect significant differences after correcting for multiple comparisons with P < 0.003.
Supporting Information Table IV provides the ß‐ and P‐values for the TMI‐corrected results.
Results remained significant after taking TMI into account.
Abbreviations: fMajor, Corpus callosum – forceps major; fMinor Corpus callosum – forceps minor; ATR, Anterior thalamic radiation; CAB, Cingulum – angular (infracallosal) bundle; CCG, Cingulum – cingulate gyrus (supracallosal) bundle; CST, Corticospinal tract; ILF, Inferior longitudinal fasciculus; SLFP, Superior longitudinal fasciculus – parietal bundle; SLFT, Superior longitudinal fasciculus – temporal bundle; UNC, Uncinate fasciculus; LH, left hemisphere; RH, right hemisphere; AD, axial diffusivity; FA, fractional anisotropy; MD, mean diffusivity; RD, radial diffusivity.
Two overall findings emerge: First, including TMI strongly influences significance (Supporting Informaton Table IV provides the β‐ and p‐values for the TMI‐corrected results). Second, we found consistently higher MD and RD in ASD compared to CTRLS for nearly all reported tracts.
For exploratory purposes, the between‐group analyses were also performed with the ADOS‐only group (i.e., those individuals with ADOS‐scores above cutoff (>7). Despite the smaller sample size in the ADOS (>7) group, results remained highly similar to the stringent TMI‐correct results (Supporting Information Table V).
Is White Matter Microstructure Differently Associated with Age in ASD and CTRLS?
Significant group‐by‐age interactions were found in bilateral ATR, right ILF, and SLFT (Table 2). The pattern was strikingly similar across the five tracts, showing higher MD and RD with increasing age in ASD relative to CTRLS (Fig. 1).
Figure 1.
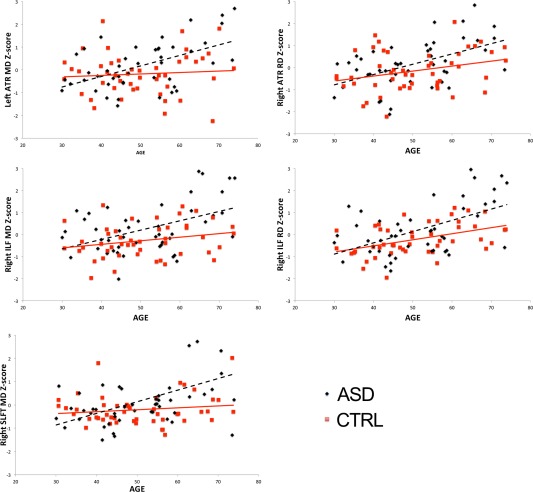
Group‐by‐age interactions for mean (MD) and radial diffusivity (RD). Figure 1 displays the significant group‐by‐age interactions, taking TMI into account, reported in Table II. Abbreviations: MD, mean diffusivity; RD, radial diffusion; ATR, Anterior Thalamic Radiation; ILF, Inferior Longitudinal Fasciculus; SLFT, Superior Longitudinal Fasciculus Temporal. [Color figure can be viewed at http://wileyonlinelibrary.com.]
Exploratory along‐the‐tract analyses were performed to appreciate the location of significant between‐group results (Supporting Information Fig. 1).
Is the IIVRT‐White Matter Microstructure Association Different in ASD and CTRLS?
Figure 2 and Table 3 represent the IIVRT‐white matter microstructure associations between groups. Higher MD and RD in bilateral CAB, ILF, SLFP and SLFT were associated with higher CV in ASD (positive association) compared to controls (negative to no association), but the opposite for CCG. Lower FA in bilateral ILF, and left SLFP were associated with higher CV in ASD, while in controls there was no such association. Finally, higher MD, RD and AD in left CAB were associated with higher overall MRT in controls, but the opposite was found for ASD. In addition, lower MD in left CST and lower AD in bilateral ILF were associated with higher MRT in ASD, while this association was absent in controls. Please note that when accuracy was taken into account, i.e. only correct trials were used for IIVRT‐white matter associations, the findings were changed towards larger between‐group differences in IIVRT‐white matter microstructure associations. Specifically, higher CV (for incongruent trials) was associated with higher MD, RD and AD in several tracts (Supporting Information Table VII and Supporting Information Fig. 2)
Figure 2.
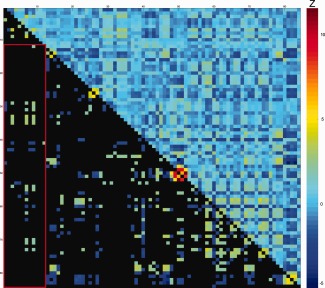
Difference scores of partial correlations between intra‐individual variability and white matter microstructure for ASD and controls. Values represent ΔZ‐scores, controlled for TMI. Upper diagonal: all ΔZ‐scores between ASD and CTRL for DTI parameters and Flanker measures. Lower diagonal: significant ΔZ‐scores ΔZ > |1.96|); non‐significant values in black. Warm colors indicate more positive partial‐correlation in ASD than in controls (e.g. rho‐ASD= −0.15 and rho‐CTRL = −0.44; or rho‐ASD = 0.44 and rho‐CTRL = 0.15; both result in positive ΔZ‐scores). Cool colors indicate more positive partial‐correlation in controls than in ASD (e.g. rho‐ASD = −0.44 and rho‐CTRL = −0.15; or rho‐ASD = 0.15 and rho‐CTRL = 0.44; both result in negative ΔZ‐scores). See Table 3 for exact values, also in‐text explanation and original partial‐correlations in Supporting Information Fig. 4. For denotation of rows/columns see Supporting Information Table VI. [Color figure can be viewed at http://wileyonlinelibrary.com.]
Table 3.
Significant difference scores of partial correlations between intra‐individual variability and white matter microstructure for ASD and controls
| MRT overall | sdRT overall | CV overall | MRT congruent correct | MRT stimulus incongruent correct | CV congruent | CV stimulus incongruent | CV response incongruent | sdRT response incongruent | |
|---|---|---|---|---|---|---|---|---|---|
| LH CAB AD | −2.06 | 2.22 | 2.18 | 2.77 | |||||
| LH CAB RD | −2.30 | 2.20 | |||||||
| LH CAB MD | −2.29 | 1.99 | 2.48 | ||||||
| RH CAB AD | 3.04 | 3.28 | 3.00 | ||||||
| RH CAB RD | 2.50 | 2.75 | 2.53 | ||||||
| RH CAB MD | 2.76 | 3.01 | 2.76 | ||||||
| RH CCG AD | −2.24 | −2.55 | −2.32 | 2.31 | |||||
| RH CCG MD | −2.17 | −2.20 | −2.48 | −2.05 | |||||
| LH CST MD | −1.99 | ||||||||
| LH ILF AD | −1.97 | −2.33 | |||||||
| LH ILF RD | 1.96 | 2.49 | 2.38 | ||||||
| LH ILF MD | 2.44 | 2.34 | |||||||
| LH ILF FA | −2.00 | ||||||||
| RH ILF AD | −2.11 | ||||||||
| RH ILF RD | 2.16 | ||||||||
| RH ILF FA | −2.08 | −1.98 | |||||||
| LH SLFP RD | 1.99 | ||||||||
| LH SLFP FA | −2.55 | ||||||||
| LH SLFT RD | 2.40 | 2.08 | |||||||
| LH SLFT MD | 2.24 | 2.10 | |||||||
| RH SLFT AD | 2.03 | 2.05 |
Values represent significant ΔZ‐scores. Abbreviations: CV, coefficient of variation; MRT, mean reaction time; sdRT, standard deviation MRT; LH/RH, left/right hemisphere; AD, axial diffusion; MD, mean diffusivity; RD, radial diffusion; ATR, Anterior thalamic radiation; CAB, Cingulum – angular (infracallosal) bundle; CCG, Cingulum – cingulate gyrus (supracallosal) bundle; CST, Corticospinal tract.
Supporting Information Fig. 3 shows the partial correlation matrix of the IIVRT‐white matter association across participants and Supporting Information Fig. 4 shows the partial correlations for each group. Higher IIVRT in terms of CV was associated with higher MD and RD most prominently in left ILF, and bilateral ATR and SLFP. However, after exclusion of incorrect trials, the IIVRT‐white matter associations across all participants (i.e. irrespective of diagnosis) were no longer significant across participants (Supporting Information Fig. 5 for partial correlations for each group).
Note that when comparing ASD and CTRLs on behavioral measures of the Flanker task (MRT, accuracy, sdRT, CV) there were no group differences. However, people with ASD were, independently of trial type, slower and more variable (sdRT) than CTRLs (for details see Supporting Information Table VIII).
DISCUSSION
The main objective was to examine white matter microstructure in ASD adulthood. This yielded several new findings. First, widespread disruptions of white matter architecture in long‐range fibers were found in ASD. Second, we report higher age‐related white matter diffusivity in ASD for bilateral anterior thalamic radiation and right inferior and superior longitudinal fasciculi compared to age‐matched controls. Finally, effects of diagnosis on the IIVRT‐white matter microstructure association were predominantly accounted for by higher CV and lower white matter microstructure. These findings may indicate different age‐related patterns of white matter microstructure in adults with ASD.
White Matter in ASD
Higher MD and RD mainly characterized reduced white matter microstructure in major association fibers in (older) adults with ASD. These results convincingly confirm and build upon the general view of white matter disruptions in ASD, specifically those in long‐range tracts, accruing evidence for the underconnectivity hypothesis in ASD. Whereas earlier DWI studies revealed spurious group differences due to head motion in ASD [Koldewyn et al., 2014; Yendiki et al., 2014], these results remained significant after controlling for head motion parameters, hence showing robustness of our findings. Nevertheless, the number of between‐group differences failing significance after controlling for head motion, particularly those related to FA, stresses the importance of taking motion parameters into account in ASD research.
Our results indicate that white matter abnormalities in adults with ASD are related to higher directional diffusivities. However it is difficult to pinpoint the origin of these higher diffusivities. For instance, the interpretation for AD varies, with reduced AD found to be associated with axonal damage in animal studies [Kim et al., 2006; Song et al., 2003; Song et al., 2005], but the opposite in human studies [Bastin et al., 2009; Klawiter et al., 2011; Salat et al., 2010; Sullivan et al., 2010; Vernooij et al., 2008; Vernooij et al., 2009]. Thus, DTI metrics are sensitive for white matter microstructure, but the relationship may be indirect and not a one‐to‐one association with the actual physical properties [Walhovd et al., 2014]. Nonetheless, evidence from scant post‐mortem studies confirms myelin‐related abnormalities to be associated with disconnection of long‐distance pathways and neighboring connectivity [Broek et al., 2014; Zikopoulos and Barbas, 2010]. Regardless of deciphering the direct physical associations, our findings of higher diffusivity are consistent with the notion that underconnectivity in ASD is related to altered axonal microstructure [McFadden and Minshew, 2013].
When specifically focusing on long‐range fibers and their relationship with ASD, we see that reduced fiber coherence in bilateral Anterior Thalamic Radiation (ATR; left higher MD, right higher RD) expands earlier ASD reports showing reduced ATR fiber microstructure from infancy to young adulthood [Bloemen et al., 2010; Cheng et al., 2010; Itahashi et al., 2015; Jou et al., 2011; Keller et al., 2007; Kleinhans et al., 2012; Lazar et al., 2014; Shukla et al., 2011; Wolff et al., 2012]. ATR is a projection fiber connecting thalamic nuclei with prefrontal and anterior cingulate cortices [Schmahmann and Pandya, 2006] and abnormalities contribute to cognitive [Mamah et al., 2010; Van der Werf et al., 2003] and affective [Coenen et al., 2012; Spalletta et al., 2013; Torso et al., 2015] dysfunctions, but no studies to date explicitly tested these behavioral associations in ASD. The ILF is a long‐range tract connecting the occipital cortex with temporal structures, and the observed reduced fiber microstructure confirms earlier ASD reports in childhood and young adulthood [Kleinhans et al., 2008; Koldewyn et al., 2014; Pugliese et al., 2009], and extends those to older ages. ILF disconnectivity has been associated with impaired face and emotion recognition [Philippi et al., 2009], visual perception, and language functions [Catani and Thiebaut de Schotten, 2008], which are affected in ASD [Weigelt et al., 2012]. The SLF findings are partly in line with earlier reports in young adults [Bloemen et al., 2010; Mueller et al., 2013] and build upon meta‐analytical evidence of reduced SLF microstructure in ASD [Aoki et al., 2013]. This major cortical association fiber pathway facilitates a network essential for language processing [Makris et al., 2005; Schmahmann et al., 2008], and other core processes such as attention, memory, and emotion [Urger et al., 2015], all of which have historically been associated with ASD. Finally, we showed higher MD and RD in the right uncinate fasciculus (UNC), a prominent white matter tract connecting inferior frontal and temporal lobe regions [Catani et al., 2002; Catani and Thiebaut de Schotten, 2008; Ebeling and von Cramon, 1992]. Although the exact functions of the UNC remain matter of debate, its functions lie at the intersection of memory and social–emotional processes [Von Der Heide et al., 2013] and may contribute to social impairments in ASD [Ameis and Catani, 2015].
Age‐Related White Matter Differences
Age‐related patterns were strikingly similar to the group‐analyses, showing higher diffusivity primarily in right fiber bundles, confirming our second hypothesis of higher age‐related white matter microstructure decline in ASD. Again, these alterations were limited to projection and association fibers (ATR, ILF, SLF). Importantly, the age‐related findings of the healthy controls were similar to the trajectories reported by two large DTI lifespan studies in healthy individuals [Westlye et al., 2010; Yeatman et al., 2014], with MD showing minor increases within our age‐range. The moderate increase of RD with advancing age in ILF was similar to these large studies, whereas the age‐related trajectories for RD in ATR were less steep compared to typical aging patterns [Westlye et al., 2010].
Prior white matter cross‐sectional studies in ASD have revealed rather mixed results with increasing age [Cheng et al., 2010; Keller et al., 2007; Kleinhans et al., 2012; Lazar et al., 2011; Lee et al., 2007; Lisiecka et al., 2015; Pugliese et al., 2009; Shukla et al., 2011]. Sparse evidence from longitudinal studies suggests atypical postnatal development of fiber microstructure, followed by blunted development leading to lower coherence around age two [Wolff et al., 2012]. Similarly, in an older sample, atypical high FA characterized the corpus callosum before age 10, after which group differences stabilized up to age 40 [Travers et al., 2015]. In an animal model of ASD, atypical growth trajectories in FA (low) and MD (high) were found in limbic, motor, and frontal brain regions suggestive for impaired connectivity [Kumar et al., 2012]. This might suggest that there are at least three marked postnatal developmental stages of WM in ASD: (1) early infancy; (2) transition from childhood to adolescence/young adulthood; (3) mid adulthood, which may be the period of a more premature transition into aging as compared to the onset of typical aging [Giorgio et al., 2010; Yap et al., 2013].
IIVRT‐White Matter Associations
The observed association of higher IIVRT (CV), with higher MD and RD in prominent major fiber bundles, irrespective of diagnosis, is consistent with reports on typical development and aging [Fjell et al., 2011; Grydeland et al., 2013; Tamnes et al., 2012]. Moreover, the tracts associated with IIVRT were identical to those reported in older adults [Mella et al., 2013]. However, when including only trials with correct responses, the associations were no longer significant, which has been reported previously [Mazerolle et al., 2013]. Thus, these findings provide limited evidence for the hypothesis that lower white matter microstructure generates performance variability [MacDonald et al., 2006, 2009, 2009].
Subtle dissociations in IIVRT‐white matter microstructure associations were observed between groups, even when incorrect trials were excluded from the analyses. The patterns in people with ASD converge towards those associations often reported in cognitive aging [Fjell et al., 2011; MacDonald et al., 2009; Mella et al., 2013]. For example, reduced fiber coherence in callosal fibers was associated with higher CV in ASD, but not in controls. These results in ASD are in line with the general idea that IIVRT reflects neural noise and disruptions of action potentials associated with reduced structural connectivity [MacDonald et al., 2009; Russell et al., 2006; Walhovd and Fjell, 2007]. Although at first sight this might not fit our observations in the controls, recent evidence from a lifespan study suggested that IIVRT‐white matter microstructure relations are more prominent with advancing age, and that aging‐related altered axonal microstructure contributes to higher IIVRT [Grydeland et al., 2013]. Thus, the strength of these associations might indeed be stronger in older ages, but less strong or absent within the age‐range of our controls. Hence, we provide evidence, although speculative, for different age‐related patterns of white matter microstructure in ASD.
Strengths and Limitations
The study has some potential limitations. While the sample is relatively large compared to the current DWI‐literature in ASD, a question can be raised concerning the extent to which the sample represents the general ASD population, as this is first DWI study to include older adults. Nevertheless, our reported widespread white matter microstructural differences resemble previous meta‐analytical findings [Aoki et al., 2013; Travers et al., 2012] and thus indicate robustness of white matter abnormalities in ASD across the spectrum. Some may argue that our sample is mildly affected based on relatively low AQ and ADOS‐scores in some participants and IQ‐scores above 80. Prior studies have shown that ASD symptoms reduce with increasing age, but ASD‐related impairments do remain pervasive across the lifespan [Perkins and Berkman, 2012]. Moreover, adults with an established ASD diagnosis tend to have lower scores on self‐report questionnaires [Bishop and Seltzer, 2012], though our mean AQ‐scores match those from a large recent study [Ruzich et al., 2015], and, if reported, from most adult DTI studies [Bakhtiari et al., 2012; Gibbard et al., 2013; Itahashi et al., 2015; Kirkovski et al., 2015; Mueller et al., 2013; Roine et al., 2013]. In addition, it is known that those with a formal diagnosis of ASD in childhood are not always meeting an ADOS cutoff in adulthood [Anderson et al. 2014; Fein et al. 2013; McGovern and Sigman 2005] and it seems that diagnoses based on a combination of history/caregiver information and clinical observation are significantly more stable over time than results from any single instrument such as the ADOS [Bastiaansen et al. 2011; Jones and Lord 2013; Lord et al. 2006]).
Psychotropic medication exposure, such as antipsychotics, may be an important potential confounder in adult ASD research as it is rather commonly prescribed (see for example Starkstein et al., 2015 where 78% of the adult ASD sample was using some form of neuroleptics) and might impact white matter microstructure (e.g. in schizophrenia: Szesko et al. 2014, but see [Kanaan et al. 2009; Tamnes & Agartz et al. 2016], and Bipolar disorder [Hafeman et al. 2012] for contrasting findings). As also a part of our ASD group was on psychotropic medication this might have had an effect on the observed pattern of findings. However, when excluding those on antipsychotic treatment the majority of our findings remained comparable (Supporting Information Table IX), despite a smaller sample size and exclusion of a relatively large number of adults with ADOS‐scores >7. From an IQ‐perspective, the current sample does not represent the entire ASD population (i.e., including those with intellectual disability), given that IQ‐scores were above 80. However, in MRI‐studies, and particularly current adult DTI studies, IQ‐scores are often above 80 to ensure full participation in the study, and to be able to follow procedures and understand cognitive tasks. Thus, even if our sample has “lower” symptom severity, this not only affects the autism community investigating adults in general, but it also demonstrates the robustness of structural white matter abnormalities reported in ASD.
CONCLUSION
Taken together, our results provide substantial evidence for widespread reductions in white matter microstructure in (older) adults with ASD, lending support to the structural underconnectivity hypothesis. Most importantly, group‐by‐age interactions revealed higher age‐related white matter diffusivity in ASD, and the IIVRT‐white matter association pattern in ASD resembles observations in cognitive aging. Thus speculatively, our cross‐sectional findings may indicate different age‐related patterns of white matter microstructure in ASD. However, whether this will already have repercussions in daily life within this age‐range remains to be tested in a longitudinal study, as earlier cross‐sectional reports on neuropsychological behavioral tasks did not reveal premature aging patterns [Lever and Geurts, 2016; Lever et al., 2015; Ring et al., 2015].
Supporting information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
The funding body had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The authors acknowledge all involved with data acquisition, and the support of SURF Foundation for the computational resources of the Dutch e‐science Grid.
REFERENCES
- Alexander AL, Lee JE, Lazar M, Field AS (2007): Diffusion tensor imaging of the brain. Neurotherapeutics 4:316–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameis SH, Catani M (2015): Altered white matter connectivity as a neural substrate for social impairment in Autism Spectrum Disorder. Cortex 62:158–181. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (2000) Diagnostic and Statistical Manual of Mental Disorders, 4th ed., text rev. Washington DC: American Psychiatric Association. Arlington, VA, USA [Google Scholar]
- Andersson JL, Skare S, Ashburner J (2003): How to correct susceptibility distortions in spin‐echo echo‐planar images: Application to diffusion tensor imaging. Neuroimage 20:870–888. [DOI] [PubMed] [Google Scholar]
- Aoki Y, Abe O, Nippashi Y, Yamasue H (2013): Comparison of white matter integrity between autism spectrum disorder subjects and typically developing individuals: A meta‐analysis of diffusion tensor imaging tractography studies. Mol Autism 4:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakhtiari R, Zurcher NR, Rogier O, Russo B, Hippolyte L, Granziera C, Araabi BN, Nili Ahmadabadi M, Hadjikhani N (2012): Differences in white matter reflect atypical developmental trajectory in autism: A tract‐based spatial statistics study. NeuroImage Clin 1:48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron‐Cohen S, Wheelwright S, Skinner R, Martin J, Clubley E (2001): The autism‐spectrum quotient (AQ): Evidence from Asperger syndrome/high‐functioning autism, males and females, scientists and mathematicians. J Autism Dev Disord 31:5–17. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, Lebihan D (1994): Mr Diffusion Tenser Spectroscopy and Imaging. Biophys J 66:259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastiaansen JA, Meffert H, Hein S, Huizinga P, Ketelaars C, Pijnenborg M, Bartels A, Minderaa R, Keysers C, de Bildt A (2011): Diagnosing autism spectrum disorders in adults: the use of Autism Diagnostic Observation Schedule (ADOS) module 4. J Autism Dev Disord 41:1256–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastin ME, Clayden JD, Pattie A, Gerrish IF, Wardlaw JM, Deary IJ (2009): Diffusion tensor and magnetization transfer MRI measurements of periventricular white matter hyperintensities in old age. Neurobiol Aging 30:125–136. [DOI] [PubMed] [Google Scholar]
- Beacher FD, Minati L, Baron‐Cohen S, Lombardo MV, Lai MC, Gray MA, Harrison NA, Critchley HD (2012): Autism attenuates sex differences in brain structure: A combined voxel‐based morphometry and diffusion tensor imaging study. AJNR Am J Neuroradiol 33:83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop SL, Seltzer MM (2012): Self‐reported autism symptoms in adults with autism spectrum disorders. J Autism Dev Disord 42:2354–2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloemen OJ, Deeley Q, Sundram F, Daly EM, Barker GJ, Jones DK, van Amelsvoort TA, Schmitz N, Robertson D, Murphy KC, Murphy DG (2010): White matter integrity in Asperger syndrome: A preliminary diffusion tensor magnetic resonance imaging study in adults. Autism Res 3:203–213. [DOI] [PubMed] [Google Scholar]
- Broek JA, Guest PC, Rahmoune H, Bahn S (2014): Proteomic analysis of post mortem brain tissue from autism patients: Evidence for opposite changes in prefrontal cortex and cerebellum in synaptic connectivity‐related proteins. Mol Autism 5:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caan MW, Khedoe G, Poot D, den Dekker A, Olabarriaga S, Grimbergen K, van Vliet L, Vos F (2010): Adaptive noise filtering for accurate and precise diffusion estimation in fiber crossings. Med Image Comput Comput Assist Interv 13:167–174., [DOI] [PubMed] [Google Scholar]
- Catani M, Howard RJ, Pajevic S, Jones DK (2002): Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage 17:77–94. [DOI] [PubMed] [Google Scholar]
- Catani M, Thiebaut de Schotten M (2008): A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex 44:1105–1132. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, Daly E, Embiricos N, Deeley Q, Pugliese L, Curran S, Robertson D, Murphy DG (2008): Altered cerebellar feedback projections in Asperger syndrome. Neuroimage 41:1184–1191. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Chou KH, Chen IY, Fan YT, Decety J, Lin CP (2010): Atypical development of white matter microstructure in adolescents with autism spectrum disorders. Neuroimage 50:873–882. [DOI] [PubMed] [Google Scholar]
- Coenen VA, Panksepp J, Hurwitz TA, Urbach H, Madler B (2012): Human medial forebrain bundle (MFB) and anterior thalamic radiation (ATR): Imaging of two major subcortical pathways and the dynamic balance of opposite affects in understanding depression. J Neuropsychiatry Clin Neurosci 24:223–236. [DOI] [PubMed] [Google Scholar]
- Conturo TE, Williams DL, Smith CD, Gultepe E, Akbudak E, Minshew NJ (2008): Neuronal fiber pathway abnormalities in autism: An initial MRI diffusion tensor tracking study of hippocampo‐fusiform and amygdalo‐fusiform pathways. J Int Neuropsychol Soc 14:933–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E, Pierce K (2005): Why the frontal cortex in autism might be talking only to itself: Local over‐connectivity but long‐distance disconnection. Curr Opin Neurobiol 15:225–230. [DOI] [PubMed] [Google Scholar]
- Ebeling U, von Cramon D (1992): Topography of the uncinate fascicle and adjacent temporal fiber tracts. Acta Neurochir (Wien) 115:143–148. [DOI] [PubMed] [Google Scholar]
- Eriksen BA, Eriksen CW (1974): Effects of noise letters upon the identification of a target letter in a nonsearch task. Perception & Psychophysics 16:143–149. [Google Scholar]
- Fischl B (2012): FreeSurfer. Neuroimage 62:774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Amlien IK, Walhovd KB (2011): Reduced white matter integrity is related to cognitive instability. J Neurosci 31:18060–18072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geurts HM, van den Bergh SF, Ruzzano L (2014): Prepotent response inhibition and interference control in autism spectrum disorders: Two meta‐analyses. Autism Res 7:407–420. [DOI] [PubMed] [Google Scholar]
- Gibbard CR, Ren J, Seunarine KK, Clayden JD, Skuse DH, Clark CA (2013): White matter microstructure correlates with autism trait severity in a combined clinical‐control sample of high‐functioning adults. NeuroImage Clinical 3:106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgio A, Santelli L, Tomassini V, Bosnell R, Smith S, De Stefano N, Johansen‐Berg H (2010): Age‐related changes in grey and white matter structure throughout adulthood. Neuroimage 51:943–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grydeland H, Walhovd KB, Tamnes CK, Westlye LT, Fjell AM (2013): Intracortical myelin links with performance variability across the human lifespan: Results from T1‐ and T2‐weighted MRI myelin mapping and diffusion tensor imaging. J Neurosci 33:18618–18630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning‐Dixon FM, Brickman AM, Cheng JC, Alexopoulos GS (2009): Aging of cerebral white matter: A review of MRI findings. Int J Geriatr Psychiatry 24:109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafeman DM, Chang KD, Garrett AS, Sanders EM, Phillips ML (2012): Effects of medication on neuroimaging findings in bipolar disorder: an updated review. Bipolar Disord 14:375–410. [DOI] [PubMed] [Google Scholar]
- Itahashi T, Yamada T, Nakamura M, Watanabe H, Yamagata B, Jimbo D, Shioda S, Kuroda M, Toriizuka K, Kato N, Hashimoto R (2015): Linked alterations in gray and white matter morphology in adults with high‐functioning autism spectrum disorder: a multimodal brain imaging study. NeuroImage Clin 7:155–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM (2012): Fsl. Neuroimage 62:782–790. [DOI] [PubMed] [Google Scholar]
- Jones DK (2008): Studying connections in the living human brain with diffusion MRI. Cortex 44:936–952. [DOI] [PubMed] [Google Scholar]
- Jones RM, Lord C (2013): Diagnosing autism in neurobiological research studies. Behav Brain Res 251:113–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jou RJ, Mateljevic N, Kaiser MD, Sugrue DR, Volkmar FR, Pelphrey KA (2011): Structural neural phenotype of autism: Preliminary evidence from a diffusion tensor imaging study using tract‐based spatial statistics. AJNR Am J Neuroradiol 32:1607–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Minshew NJ (2004): Cortical activation and synchronization during sentence comprehension in high‐functioning autism: Evidence of underconnectivity. Brain 127:1811–1821. [DOI] [PubMed] [Google Scholar]
- Just MA, Keller TA, Malave VL, Kana RK, Varma S (2012): Autism as a neural systems disorder: A theory of frontal‐posterior underconnectivity. Neurosci Biobehav Rev 36:1292–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaan R, Barker G, Brammer M, Giampietro V, Shergill S, Woolley J, Picchioni M, Toulopoulou T, McGuire P (2009): White matter microstructure in schizophrenia: effects of disorder, duration and medication. Br J Psychiatry 194:236–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana RK, Libero LE, Moore MS (2011): Disrupted cortical connectivity theory as an explanatory model for autism spectrum disorders. Phys Life Rev 8:410–437. [DOI] [PubMed] [Google Scholar]
- Karalunas SL, Geurts HM, Konrad K, Bender S, Nigg JT (2014): Annual research review: Reaction time variability in ADHD and autism spectrum disorders: Measurement and mechanisms of a proposed trans‐diagnostic phenotype. J Child Psychol Psychiatry 55:685–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller TA, Kana RK, Just MA (2007): A developmental study of the structural integrity of white matter in autism. Neuroreport 18:23–27. [DOI] [PubMed] [Google Scholar]
- Kennedy KM, Raz N (2009): Aging white matter and cognition: Differential effects of regional variations in diffusion properties on memory, executive functions, and speed. Neuropsychologia 47:916–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Budde MD, Liang HF, Klein RS, Russell JH, Cross AH, Song SK (2006): Detecting axon damage in spinal cord from a mouse model of multiple sclerosis. Neurobiol Dis 21:626–632. [DOI] [PubMed] [Google Scholar]
- Kirkovski M, Enticott PG, Maller JJ, Rossell SL, Fitzgerald PB (2015): Diffusion tensor imaging reveals no white matter impairments among adults with autism spectrum disorder. Psychiatry Res 233:64–72. [DOI] [PubMed] [Google Scholar]
- Klawiter EC, Schmidt RE, Trinkaus K, Liang HF, Budde MD, Naismith RT, Song SK, Cross AH, Benzinger TL (2011): Radial diffusivity predicts demyelination in ex vivo multiple sclerosis spinal cords. Neuroimage 55:1454–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinhans NM, Richards T, Sterling L, Stegbauer KC, Mahurin R, Johnson LC, Greenson J, Dawson G, Aylward E (2008): Abnormal functional connectivity in autism spectrum disorders during face processing. Brain 131:1000–1012. [DOI] [PubMed] [Google Scholar]
- Kleinhans NM, Pauley G, Richards T, Neuhaus E, Martin N, Corrigan NM, Shaw DW, Estes A, Dager SR (2012): Age‐related abnormalities in white matter microstructure in autism spectrum disorders. Brain Res 1479:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koldewyn K, Yendiki A, Weigelt S, Gweon H, Julian J, Richardson H, Malloy C, Saxe R, Fischl B, Kanwisher N (2014): Differences in the right inferior longitudinal fasciculus but no general disruption of white matter tracts in children with autism spectrum disorder. Proc Natl Acad Sci U S A 111:1981–1986., [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolschijn PC, Geurts HM (2016): Gray matter characteristics in mid and old aged adults with ASD. J Autism Dev Disord 46:2666–2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M, Kim S, Pickup S, Chen R, Fairless AH, Ittyerah R, Abel T, Brodkin ES, Poptani H (2012): Longitudinal in‐vivo diffusion tensor imaging for assessing brain developmental changes in BALB/cJ mice, a model of reduced sociability relevant to autism. Brain Res 1455:56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langen M, Leemans A, Johnston P, Ecker C, Daly E, Murphy CM, Dell'acqua F, Durston S, Consortium A, Murphy DG (2012): Fronto‐striatal circuitry and inhibitory control in autism: Findings from diffusion tensor imaging tractography. Cortex 48:183–193. [DOI] [PubMed] [Google Scholar]
- Lazar M, Miles L, Donaldson J, Jensen JH, Ming JC. Atypical gray and white matter microstructure in autism spectrum disorders. In International Society for Magnetic Resonance in Medicine, 2011. Conference in Montreal, Canada. p 350.
- Lazar M, Miles LM, Babb JS, Donaldson JB (2014): Axonal deficits in young adults with high functioning autism and their impact on processing speed. NeuroImage Clin 4:417–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bihan D (2003): Looking into the functional architecture of the brain with diffusion MRI. NatRevNeurosci 4:469–480. [DOI] [PubMed] [Google Scholar]
- Lee JE, Bigler ED, Alexander AL, Lazar M, DuBray MB, Chung MK, Johnson M, Morgan J, Miller JN, McMahon WM, Lu J, Jeong EK, Lainhart JE (2007): Diffusion tensor imaging of white matter in the superior temporal gyrus and temporal stem in autism. Neurosci Lett 424:127–132. [DOI] [PubMed] [Google Scholar]
- Leemans A, Jones DK (2009): The B‐matrix must be rotated when correcting for subject motion in DTI data. Magn Reson Med 61:1336–1349. [DOI] [PubMed] [Google Scholar]
- Lever AG, Geurts HM (2016): Age‐related differences in cognition across the adult lifespan in autism spectrum disorder. Autism Res 9:666–676., [DOI] [PubMed] [Google Scholar]
- Lever AG, Werkle‐Bergner M, Brandmaier AM, Ridderinkhof KR, Geurts HM (2015): Atypical working memory decline across the adult lifespan in autism spectrum disorder? J Abnorm Psychol 124:1014–1026. [DOI] [PubMed] [Google Scholar]
- Lisiecka DM, Holt R, Tait R, Ford M, Lai MC, Chura LR, Baron‐Cohen S, Spencer MD, Suckling J (2015): Developmental white matter microstructure in autism phenotype and corresponding endophenotype during adolescence. Translational Psychiatry 5:e529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, Goode S, Heemsbergen J, Jordan H, Mawhood L, Schopler E (1989): Autism diagnostic observation schedule: A standardized observation of communicative and social behavior. J Autism Dev Disord 19:185–212. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, DiLavore PS, Shulman C, Thurm A, Pickles A (2006): Autism from 2 to 9 years of age. Arch Gen Psychiatry 63:694–701. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A (1994): Autism Diagnostic Interview‐Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord 24:659–685. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, DiLavore PS, Shulman C, Thurm A, Pickles A (2006): Autism from 2 to 9 years of age. Arch Gen Psychiatry 63:694–701. [DOI] [PubMed] [Google Scholar]
- Lustig C, Jantz T (2015): Questions of age differences in interference control: When and how, not if? Brain Res 1612:59–69. [DOI] [PubMed] [Google Scholar]
- MacDonald SW, Nyberg L, Backman L (2006): Intra‐individual variability in behavior: Links to brain structure, neurotransmission and neuronal activity. Trends Neurosci 29:474–480. [DOI] [PubMed] [Google Scholar]
- MacDonald SW, Li SC, Backman L (2009): Neural underpinnings of within‐person variability in cognitive functioning. Psychol Aging 24:792–808. [DOI] [PubMed] [Google Scholar]
- Makris N, Kennedy DN, McInerney S, Sorensen AG, Wang R, Caviness VS Jr, Pandya DN (2005): Segmentation of subcomponents within the superior longitudinal fascicle in humans: A quantitative, in vivo, DT‐MRI study. Cereb Cortex 15:854–869. [DOI] [PubMed] [Google Scholar]
- Mamah D, Conturo TE, Harms MP, Akbudak E, Wang L, McMichael AR, Gado MH, Barch DM, Csernansky JG (2010): Anterior thalamic radiation integrity in schizophrenia: A diffusion‐tensor imaging study. Psychiatry Res 183:144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazerolle EL, Wojtowicz MA, Omisade A, Fisk JD (2013): Intra‐individual variability in information processing speed reflects white matter microstructure in multiple sclerosis. NeuroImage Clinical 2:894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden K, Minshew NJ (2013): Evidence for dysregulation of axonal growth and guidance in the etiology of ASD. Front Hum Neurosci 7:671., [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mella N, de Ribaupierre S, Eagleson R, de Ribaupierre A (2013): Cognitive intraindividual variability and white matter integrity in aging. Sci World J 2013:350623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller S, Keeser D, Samson AC, Kirsch V, Blautzik J, Grothe M, Erat O, Hegenloh M, Coates U, Reiser MF, Hennig‐Fast K, Meindl T (2013): Convergent findings of altered functional and structural brain connectivity in individuals with high functioning autism: A multimodal MRI study. PLoS One 8:e67329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukaetova‐Ladinska EB, Perry E, Baron M, Povey C, Autism Ageing Writing G (2012): Ageing in people with autistic spectrum disorder. Int J Geriatr Psychiatry 27:109–118. [DOI] [PubMed] [Google Scholar]
- Nilsson J, Thomas AJ, O'Brien JT, Gallagher P (2014): White matter and cognitive decline in aging: A focus on processing speed and variability. J Int Neuropsychol Soc 20:262–267. [DOI] [PubMed] [Google Scholar]
- Perkins EA, Berkman KA (2012): Into the unknown: Aging with autism spectrum disorders. Am J Intellect Dev Disabil 117:478–496. [DOI] [PubMed] [Google Scholar]
- Philippi CL, Mehta S, Grabowski T, Adolphs R, Rudrauf D (2009): Damage to association fiber tracts impairs recognition of the facial expression of emotion. J Neurosci 29:15089–15099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierpaoli C, Basser PJ (1996): Toward a quantitative assessment of diffusion anisotropy. Magn Reson Med 36:893–906. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di Chiro G (1996): Diffusion tensor MR imaging of the human brain. Radiology 201:637–648. [DOI] [PubMed] [Google Scholar]
- Pugliese L, Catani M, Ameis S, Dell'Acqua F, Thiebaut de Schotten M, Murphy C, Robertson D, Deeley Q, Daly E, Murphy DG (2009): The anatomy of extended limbic pathways in Asperger syndrome: A preliminary diffusion tensor imaging tractography study. Neuroimage 47:427–434. [DOI] [PubMed] [Google Scholar]
- Rane P, Cochran D, Hodge SM, Haselgrove C, Kennedy DN, Frazier JA (2015): Connectivity in autism: A review of MRI connectivity studies. Harv Rev Psychiatry 23:223–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ring M, Gaigg SB, Bowler DM (2015): Object‐location memory in adults with autism spectrum disorder. Autism Res 8:609–619. [DOI] [PubMed] [Google Scholar]
- Roine U, Roine T, Salmi J, Nieminen‐Von Wendt T, Leppamaki S, Rintahaka P, Tani P, Leemans A, Sams M (2013): Increased coherence of white matter fiber tract organization in adults with Asperger syndrome: A diffusion tensor imaging study. Autism Res 6:642–650. [DOI] [PubMed] [Google Scholar]
- Russell VA, Oades RD, Tannock R, Killeen PR, Auerbach JG, Johansen EB, Sagvolden T (2006): Response variability in attention‐deficit/hyperactivity disorder: A neuronal and glial energetics hypothesis. Behav Brain Funct 2:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzich E, Allison C, Smith P, Watson P, Auyeung B, Ring H, Baron‐Cohen S (2015): Measuring autistic traits in the general population: A systematic review of the Autism‐Spectrum Quotient (AQ) in a nonclinical population sample of 6,900 typical adult males and females. Mol Autism 6:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacher J, Neumann J, Okon‐Singer H, Gotowiec S, Villringer A (2013): Sexual dimorphism in the human brain: Evidence from neuroimaging. Magn Reson Imaging 31:366–375. [DOI] [PubMed] [Google Scholar]
- Salat DH, Tuch DS, van der Kouwe AJ, Greve DN, Pappu V, Lee SY, Hevelone ND, Zaleta AK, Growdon JH, Corkin S, Fischl B, Rosas HD (2010): White matter pathology isolates the hippocampal formation in Alzheimer's disease. Neurobiol Aging 31:244–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN (2006): Fiber Pathways of the Brain. New York: Oxford University Press. [Google Scholar]
- Schmahmann JD, Smith EE, Eichler FS, Filley CM (2008): Cerebral white matter: Neuroanatomy, clinical neurology, and neurobehavioral correlates. Ann N Y Acad Sci 1142:266–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahand S, Benabdelkader A, Jaghoori MM, Mourabit Ma, Huguet J, Caan MWA, van Kampen AHC, Olabarriaga SD (2015): A data‐centric neuroscience gateway: Design, implementation, and experiences. Concurrency Comput Pract Exp 27:489–506. [Google Scholar]
- Shukla DK, Keehn B, Muller RA (2011): Tract‐specific analyses of diffusion tensor imaging show widespread white matter compromise in autism spectrum disorder. J Child Psychol Psychiatry 52:286–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH (2003): Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage 20:1714–1722. [DOI] [PubMed] [Google Scholar]
- Song SK, Yoshino J, Le TQ, Lin SJ, Sun SW, Cross AH, Armstrong RC (2005): Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage 26:132–140. [DOI] [PubMed] [Google Scholar]
- Spalletta G, Fagioli S, Caltagirone C, Piras F (2013): Brain microstructure of subclinical apathy phenomenology in healthy individuals. Hum Brain Mapp 34:3193–3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkstein S, Gellar S, Parlier M, Payne L, Piven J (2015): High rates of parkinsonism in adults with autism. J Neurodev Disord 7:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Rohlfing T, Pfefferbaum A (2010): Quantitative fiber tracking of lateral and interhemispheric white matter systems in normal aging: Relations to timed performance. Neurobiol Aging 31:464–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykova E (2004): Extrasynaptic volume transmission and diffusion parameters of the extracellular space. Neuroscience 129:861–876. [DOI] [PubMed] [Google Scholar]
- Tamnes CK, Agartz I (2016): White Matter Microstructure in Early‐Onset Schizophrenia: A Systematic Review of Diffusion Tensor Imaging Studies. J Am Acad Child Adolesc Psychiatry 55:269–279. [DOI] [PubMed] [Google Scholar]
- Tamnes CK, Fjell AM, Westlye LT, Ostby Y, Walhovd KB (2012): Becoming consistent: Developmental reductions in intraindividual variability in reaction time are related to white matter integrity. J Neurosci 32:972–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakkar KN, Polli FE, Joseph RM, Tuch DS, Hadjikhani N, Barton JJ, Manoach DS (2008): Response monitoring, repetitive behaviour and anterior cingulate abnormalities in autism spectrum disorders (ASD). Brain 131:2464–2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C, Humphreys K, Jung KJ, Minshew N, Behrmann M (2011): The anatomy of the callosal and visual‐association pathways in high‐functioning autism: A DTI tractography study. Cortex 47:863–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torso M, Serra L, Giulietti G, Spano B, Tuzzi E, Koch G, Caltagirone C, Cercignani M, Bozzali M (2015): Strategic lesions in the anterior thalamic radiation and apathy in early Alzheimer's disease. PLoS One 10:e0124998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers BG, Adluru N, Ennis C, Tromp do PM, Destiche D, Doran S, Bigler ED, Lange N, Lainhart JE, Alexander AL (2012): Diffusion tensor imaging in autism spectrum disorder: a review. Autism Res 5:289–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers BG, Tromp do PM, Adluru N, Lange N, Destiche D, Ennis C, Nielsen JA, Froehlich AL, Prigge MB, Fletcher PT, Anderson JS, Zielinski BA, Bigler ED, Lainhart JE, Alexander AL (2015): Atypical development of white matter microstructure of the corpus callosum in males with autism: A longitudinal investigation. Mol Autism 6:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urger SE, De Bellis MD, Hooper SR, Woolley DP, Chen SD, Provenzale J (2015): The superior longitudinal fasciculus in typically developing children and adolescents: Diffusion tensor imaging and neuropsychological correlates. J Child Neurol 30:9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Mandl RC, Kahn RS, Hulshoff Pol HE (2009): Functionally linked resting‐state networks reflect the underlying structural connectivity architecture of the human brain. Hum Brain Mapp 30:3127–3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Werf YD, Jolles J, Witter MP, Uylings HB (2003): Contributions of thalamic nuclei to declarative memory functioning. Cortex 39:1047–1062. [DOI] [PubMed] [Google Scholar]
- van Veen V, Cohen JD, Botvinick MM, Stenger VA, Carter CS (2001): Anterior cingulate cortex, conflict monitoring, and levels of processing. Neuroimage 14:1302–1308. [DOI] [PubMed] [Google Scholar]
- Verhage, F. (1964) Intelligentie en leeftijd: onderzoek bij Nederlanders van twaalf tot zevenenzeventig jaar [Intelligence and age: Study with Dutch people from age 12 to 77]. Assen. Van Gorcum.
- Vernooij MW, de Groot M, van der Lugt A, Ikram MA, Krestin GP, Hofman A, Niessen WJ, Breteler MM (2008): White matter atrophy and lesion formation explain the loss of structural integrity of white matter in aging. Neuroimage 43:470–477. [DOI] [PubMed] [Google Scholar]
- Vernooij MW, Ikram MA, Vrooman HA, Wielopolski PA, Krestin GP, Hofman A, Niessen WJ, Van der Lugt A, Breteler MM (2009): White matter microstructural integrity and cognitive function in a general elderly population. Arch Gen Psychiatry 66:545–553. [DOI] [PubMed] [Google Scholar]
- Vissers ME, Cohen MX, Geurts HM (2012): Brain connectivity and high functioning autism: A promising path of research that needs refined models, methodological convergence, and stronger behavioral links. Neurosci Biobehav Rev 36:604–625. [DOI] [PubMed] [Google Scholar]
- Von Der Heide RJ, Skipper LM, Klobusicky E, Olson IR (2013): Dissecting the uncinate fasciculus: Disorders, controversies and a hypothesis. Brain 136:1692–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM (2007): White matter volume predicts reaction time instability. Neuropsychologia 45:2277–2284. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Johansen‐Berg H, Karadottir RT (2014): Unraveling the secrets of white matter–bridging the gap between cellular, animal and human imaging studies. Neuroscience 276:2–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigelt S, Koldewyn K, Kanwisher N (2012): Face identity recognition in autism spectrum disorders: A review of behavioral studies. Neurosci Biobehav Rev 36:1060–1084. [DOI] [PubMed] [Google Scholar]
- Westlye LT, Walhovd KB, Dale AM, Bjornerud A, Due‐Tonnessen P, Engvig A, Grydeland H, Tamnes CK, Ostby Y, Fjell AM (2010): Life‐span changes of the human brain white matter: Diffusion tensor imaging (DTI) and volumetry. Cereb Cortex 20:2055–2068. [DOI] [PubMed] [Google Scholar]
- Wolff JJ, Gu H, Gerig G, Elison JT, Styner M, Gouttard S, Botteron KN, Dager SR, Dawson G, Estes AM, Evans AC, Hazlett HC, Kostopoulos P, McKinstry RC, Paterson SJ, Schultz RT, Zwaigenbaum L, Piven J, Network I (2012): Differences in white matter fiber tract development present from 6 to 24 months in infants with autism. Am J Psychiatry 169:589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich MW, Jbabdi S, Patenaude B, Chappell M, Makni S, Behrens T, Beckmann C, Jenkinson M, Smith SM (2009): Bayesian analysis of neuroimaging data in FSL. Neuroimage 45:S173–S186. [DOI] [PubMed] [Google Scholar]
- Yap QJ, Teh I, Fusar‐Poli P, Sum MY, Kuswanto C, Sim K (2013): Tracking cerebral white matter changes across the lifespan: Insights from diffusion tensor imaging studies. J Neural Transm (Vienna) 120:1369–1395. [DOI] [PubMed] [Google Scholar]
- Yeatman JD, Wandell BA, Mezer AA (2014): Lifespan maturation and degeneration of human brain white matter. Nat Commun 5:4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yendiki A, Panneck P, Srinivasan P, Stevens A, Zollei L, Augustinack J, Wang R, Salat D, Ehrlich S, Behrens T, Jbabdi S, Gollub R, Fischl B (2011): Automated probabilistic reconstruction of white‐matter pathways in health and disease using an atlas of the underlying anatomy. Front Neuroinform 5:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yendiki A, Koldewyn K, Kakunoori S, Kanwisher N, Fischl B (2014): Spurious group differences due to head motion in a diffusion MRI study. Neuroimage 88:79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zikopoulos B, Barbas H (2010): Changes in prefrontal axons may disrupt the network in autism. J Neurosci 30:14595–14609. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information


