Abstract
Introduction
Longstanding type 1 diabetes (T1DM) is associated with microangiopathy and poorer cognition. In the brain, T1DM is related to increased functional resting‐state network (RSN) connectivity in patients without, which was decreased in patients with clinically evident microangiopathy. Subcortical structure seems affected in both patient groups. How these localized alterations affect the hierarchy of the functional network in T1DM is unknown. Eigenvector centrality mapping (ECM) and degree centrality are graph theoretical methods that allow determining the relative importance (ECM) and connectedness (degree centrality) of regions within the whole‐brain network hierarchy.
Methods
Therefore, ECM and degree centrality of resting‐state functional MRI‐scans were compared between 51 patients with, 53 patients without proliferative retinopathy, and 49 controls, and associated with RSN connectivity, subcortical gray matter volume, and cognition.
Results
In all patients versus controls, ECM and degree centrality were lower in the bilateral thalamus and the dorsal striatum, with lowest values in patients without proliferative retinopathy (P FWE < 0.05). Increased ECM in this group versus patients with proliferative retinopathy was seen in the bilateral lateral occipital cortex, and in the right cuneus and occipital fusiform gyrus versus controls (P FWE < 0.05). In all patients, ECM and degree centrality were related to altered visual, sensorimotor, and auditory and language RSN connectivity (P FWE < 0.05), but not to subcortical gray matter volume or cognition (P FDR > 0.05).
Conclusion
The findings suggested reorganization of the hierarchy of the cortical connectivity network in patients without proliferative retinopathy, which is lost with disease progression. Centrality seems sensitive to capture early T1DM‐related functional connectivity alterations, but not disease progression. Hum Brain Mapp 38:3623–3636, 2017. © 2017 Wiley Periodicals, Inc.
Keywords: type 1 diabetes, eigenvector centrality mapping, graph theory, resting‐state fMRI, cognition
INTRODUCTION
Type 1 diabetes mellitus (T1DM) is a metabolic disorder in which exogenous insulin administration is vital due to the destruction of insulin producing pancreatic β‐cells by autoimmune reactions. Adults with longstanding T1DM are at risk of developing peripheral microvascular complications, such as retinopathy. Long‐term exposure to high blood glucose levels, or chronic hyperglycemic exposure, drives the development of retinopathy [Brownlee, 2005]. Retinopathy is the most prevalent microvascular complication in type 1 diabetes and one of the leading causes of blindness in the western world [Ding and Wong, 2012]. Retinopathy is a progressive complication that starts with small dot‐like bleedings of small retinal vessels. With increasing severity of such bleedings, hypoxia triggers the release of hormones to promote revascularization of the retina [Ding and Wong, 2012]. As these newly formed blood vessels are prone to leakage a vicious circle starts that will lead, if not interrupted, to blindness [Ding and Wong, 2012]. This last stage is called proliferative retinopathy. A 25‐year epidemiological study showed that virtually all T1DM patients developed retinopathy (97%), and that progression rate was 83% [Klein et al., 2008]. Forty‐two percent of the patients developed proliferative retinopathy, for which laser coagulation is required to prevent blindness [Klein et al., 2008].
Cognitively, studies have shown that T1DM is characterized by mental slowing, decreased mental flexibility, and lower attention [Brands et al., 2005], and some research has suggested an increased risk of dementia [Smolina et al., 2015]. Adult T1DM has also been found to affect gray and white matter structure and functional connectivity [Kodl et al., 2008; Musen et al., 2006; Ryan et al., 2015]. While these alterations in cognitive functioning, and structure and function of the brain have been found in the general T1DM patient population, many studies have shown that patients who have developed proliferative retinopathy show the most severe decrements and are most at risk to develop cerebral complications [Jacobson et al., 2011; Ryan et al., 2016; Wessels et al., 2008].
In our study, we have previously shown that T1DM patients with proliferative retinopathy showed decrements in cognitive functions depending on processing speed and attention in comparison with controls and their counterparts with uncomplicated T1DM, and decreased processing speed in patients without proliferative retinopathy relative to controls [Van Duinkerken et al., 2012]. Although we did not demonstrate any alterations in cortical thickness, we did show lower subcortical gray matter volume in the thalamus, nucleus accumbens, putamen, and caudate nucleus [Van Duinkerken et al., 2014]. Both patients with and without proliferative retinopathy showed these alterations, albeit the effect size was largest in the group with retinopathy. Assessment of functional resting‐state networks (RSNs) in this group using functional MRI showed a different pattern, where patients without complications had increased connectivity in the visual and sensorimotor networks compared with controls and their counterparts with proliferative retinopathy [Van Duinkerken et al., 2012]. Contrary, patients with proliferative retinopathy showed lower connectivity than controls in the auditory and language, ventral attention and left frontal‐parietal networks, where those without retinopathy had intermediate connectivity levels [Van Duinkerken et al., 2012]. This shows that indeed patients with proliferative retinopathy in our study are most prone to have developed alterations in cognition and brain structure and functioning. Functional connectivity shows a different pattern of alterations than that of cognition or subcortical gray matter volume, and may suggest a form of functional reorganization of the local RSN connectivity [Van Duinkerken et al., 2012], which is also found in children under 10 years of age with T1DM [Saggar et al., 2017].
However, by dividing the whole‐brain functional network into smaller sub‐networks that are assumed to function independently, the impact of RSN connectivity and structural alterations on the entire brain network is disregarded. Furthermore, dividing the whole‐brain functional network into smaller RSNs disregards the possibility that higher‐order (cognitive) functions rely on multiple RSNs at the same time, that is, that they need crosstalk between RSNs. Graph analytical methods have the advantage of analyzing the whole‐brain network at once and can thus overcome the above‐mentioned limitations, which may help in understanding cognitive deficits.
Eigenvector centrality mapping (ECM) is a graph theoretical approach. The voxel‐based ECM calculation assigns higher ECM values to voxels that are connected to more central voxels [Binnewijzend et al., 2014; Lohmann et al., 2010; Wink et al., 2012]. In other words, it calculates centrality of a node, which can be a brain region or a voxel, by adding up the centralities of its neighbors. Higher scores then indicate a more central or important role of the node in the functional network, as the node is connected to regions which themselves have higher centrality values. Except for considering the functional network as a whole and being easily computable, another advantage of ECM is that it is relatively insensitive to physiological effects, such as movement artifacts [Lohmann et al., 2010; Wink et al., 2012], which avoids the introduction of potential artifacts in the data [Power et al., 2015]. The difference between degree centrality, which integrates the number (or strengths) of connections a node receives, and eigenvector centrality, which integrates the centralities of a node's neighbors, is that eigenvector centrality is more sensitive to different levels of hierarchical networks. In type 2 diabetes, albeit being a fundamentally different disease related to obesity, insulin resistance and pancreatic β‐cell failure, degree centrality was lower in the lingual gyrus, but higher in the insula and anterior cingulate cortex when compared with controls [Cui et al., 2016].
Graph theoretical assessment of the functional network in T1DM has not been published before. Therefore, we first studied the functional whole‐brain network using voxel‐wise ECM and degree centrality in adult T1DM patients with and without proliferative retinopathy compared with controls. Based on the results of our previous RSN connectivity analysis, alterations were hypothesized strongest in patients without proliferative retinopathy. Next, in all patients, we aimed at identifying the relationship between potential alterations in ECM and degree centrality and previously observed cognitive decrements, altered RSN functional connectivity, and lower subcortical volume [Van Duinkerken et al., 2012, 2014]. To this end, we correlated ECM and degree centrality values with cognitive performance on the domains of general cognitive ability, information processing speed, and attention. The RSN functional connectivity of the sensorimotor, secondary visual, ventral attention, left frontal‐parietal, and auditory and language processing networks were included, as well as volume of the bilateral thalamus, putamen, caudate nucleus, and nucleus accumbens. As we did not observe any differences in cortical thickness, this was not included in the correlation analysis. Lastly, in all patients, the clinical relevance of ECM and degree centrality was determined by exploring associations with diabetes‐specific and demographic factors.
MATERIALS AND METHODS
Participants
This study was conducted in accordance with the Declaration of Helsinki and approved by the medical ethics committee of the VU University Medical Center. Written informed consent was obtained from all participants. One hundred and four participants with T1DM for at least 10 years were included, as well as 51 matched non‐diabetes controls. Inclusion criteria were right‐handedness, mastery of the Dutch language and age between 18 and 56 years. Exclusion criteria included cerebro‐ or cardiovascular disease, MRI‐contraindications, such as pregnancy, centrally acting medication use, (treatment of) psychiatric disorders, history of or current alcohol (men > 21; women > 14 units a week) or drug use, head trauma, and, for controls only, hypertension. Hypoglycemic episodes 24‐hours preceding examination resulted in rescheduling of the appointment. During the study days, blood glucose levels of patients had to range between 4 and 15 mmol/L (72–270 mg/dL). Glucose levels were checked regularly and corrected if necessary [Van Duinkerken et al., 2012].
Biomedical and Anthropometric Measures
In all participants, after a 15‐minute rest, blood pressure was measured three times with 5 minute intervals at the left arm, while in a seated position. Hypertension was defined as a mean systolic blood pressure ≥140 mm Hg, a mean diastolic blood pressure ≥90 mm Hg, or the use of antihypertensive drugs. Blood was drawn for routine analysis, including glycated hemoglobin (HbA1c). Depressive symptoms were evaluated with the Center for Epidemiological Studies Depression scale (CES‐D; [Radloff, 1977]). In patients, lifetime severe hypoglycemic events were self‐reported, based on standardized criteria [The Diabetes Control and Complications Trial Research Group, 1996].
Microvascular Complications
In patients, retinopathy status was ascertained by fundus photography, rated according to the EURODIAB classification [Aldington et al., 1995], albuminuria status was determined as a 24‐hour albumin:creatinine ratio >2.5 mg/mmol for men or >3.5 mg/mmol for women, and neuropathy status was ascertained by the most recent annual check‐up for neuropathy, which is incorporated into the medical records (n = 92), or self‐report if not available (n = 12) [Van Duinkerken et al., 2012]. Patients were included if they had proliferative retinopathy (EURODIAB level 4 or 5), which could be accompanied by albuminuria and/or neuropathy, or if they were free of clinically manifest microvascular complications.
MRI Acquisition
Patients underwent MRI‐scans on a 1.5T Siemens Sonata (Erlangen, Germany) MR‐system using an 8‐channel phased‐array head coil. Here, we used a 202 volume EPI‐based functional MRI sequence ([fMRI] repetition time 2,850 ms; echo time 60 ms; flip angle 90°; 211 × 211 mm2 field‐of‐view; isotropic 3.3 mm voxels; 36 axial slices), and a T1‐based magnetization prepared rapid acquisition gradient echo ([MPRAGE], repetition time 2,700 ms; echo time 5.17 ms; inversion time 950 ms; flip angle 8°; 256 × 256 mm2 field‐of‐view; 1.0 × 1.0 × 1.5 mm voxel size; 160 contiguous coronal partitions). Functional MRI scanning occurred in a darkened room, and subjects were asked to keep their eyes closed and not think of anything particular nor fall asleep.
Functional MRI Preprocessing
For preprocessing of the fMRI images, the MELODIC pipeline of FSL5.0.8 was used (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/; which has been described in detail in [Van Duinkerken et al., 2012]). In short, the first 2 volumes were discarded to reach a steady state of BOLD signal. The remaining volumes were brain extracted, slice timing and motion corrected, smoothed with a 5‐mm Gaussian kernel, and high‐pass filtered with a cut‐off of 150 seconds. Next, volumes were registered to the subject's high‐resolution T1‐MPRAGE scan by using affine boundary based registration, and non‐linearly registered to MNI152 standard space with a 10 mm warp resolution and resampling resolution of 4 mm isotropic. To further correct for motion scrubbing was applied. First, based on the raw fMRI‐scans, FSL's Motion Outliers script calculated the volumes to be scrubbed based on the Frame‐wise Displacement (FD) and DVARS (D referring to temporal derivative of time‐course and VARS to the root mean squared variance over voxels) combined [Power et al., 2012]. Next, the 3dTproject script of AFNI (https://afni.nimh.nih.gov/afni/) was used to scrub the preprocessed fMRI‐scans in 4 mm standard space. As removing volumes leads to fMRI‐scans of different length which can potentially confound between‐group analyses [Power et al., 2015], we interpolated the values of the to‐be scrubbed volumes from the neighboring volumes in time. This way, volumes with high motion were censored but not removed.
Group Specific Eigenvector Centrality Mapping Mask
A group‐specific mask was made excluding white matter and cerebrospinal fluid voxels, to determine ECM values only in gray matter voxels. First, for all participants gray matter masks obtained by FSL‐SIENAX [Smith et al., 2002] were merged with the participants' binarized subcortical segmentation from FSL‐FIRST [Patenaude et al., 2011], and subsequently binarized. They were individually non‐linearly registered into 4 mm standard space using the personal warp‐volume obtained from the MELODIC pipeline, summed and thresholded at >25% to obtain a gray matter mask with a relatively thick cortex. To make sure that the centrality networks were of the same size in everyone all fMRI‐volumes in 4 mm MNI‐space were merged into a single volume, binarized and summed together. This volume was subsequently thresholded at 153 (100%), only including voxels covered in every participant. Lastly, this fMRI‐mask was multiplied by the gray matter mask, generating a group specific mask including only gray matter voxels with 100% fMRI coverage (Fig. 1). In doing so equal network size in all participants was ensured, preventing a possible influence of size of the functional network on the between‐group results [Van Wijk et al., 2010].
Figure 1.
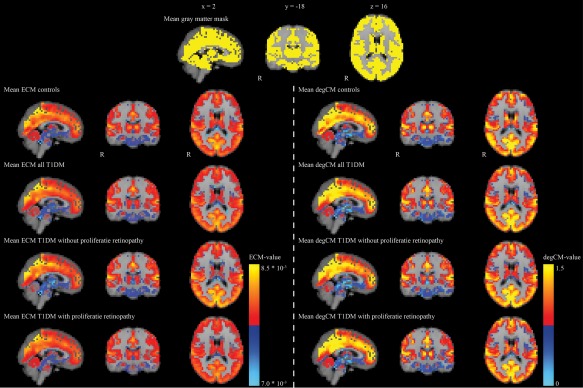
The common gray matter mask is shown in the top panel in yellow. Mean eigenvector centrality and degree centrality maps of all controls and patients, and separately the patients without and with proliferative retinopathy are given. Red and yellow colors indicate high centrality values, whereas blue colors indicate low centrality values. DegCM = degree centrality. ECM = eigenvector centrality mapping. T1DM = type 1 diabetes. Images are presented in radiological orientation. [Color figure can be viewed at http://wileyonlinelibrary.com]
Eigenvector Centrality Mapping
With ECM, centrality (i.e., relative importance) of each voxel in the functional network is calculated [Lohmann et al., 2010]. The fast‐ECM software (http://www.github.com/amwink/bias/tree/master/matlab/fastECM) that was used in this study is faster and computationally more efficient, as it computes matrix‐vector products without having to compute or store the connectivity matrix. Technical details of the fast‐ECM software can be found elsewhere [Wink et al., 2012]. In brief, eigenvector centrality considers a node more central if it is connected to more central nodes. This property corresponds to having a high coefficient in the dominant eigenvector of the connections matrix. Fast ECM uses an efficient formulation of power iteration to find the dominant eigenvector of the connections matrix:
- for data Y[NxT], voxel‐wise correlations can be computed as
where N = number of voxels, T = number of time points.
- power iteration uses
which can be re‐formulated as
In this formulation, power iteration requires only a [Tx1] extra storage instead of [NxN]. As T < N, this is a great improvement in efficiency [Wink et al., 2012]. The connectivity matrix M is calculated as C + 1 (where C is the voxelwise correlation matrix), in which negative correlations range from 0 to 1 and positive correlations from 1 to 2, without the application of a threshold. As we used a group specific common mask and the fact that ECM does not rely on thresholding or binarizing of the matrix, all subjects' networks were of the same size. These individual eigenvector centrality maps in MNI152 standard space were then temporally concatenated into a single 4D‐file for statistical analysis.
Degree Centrality Mapping
Degree centrality was approximated using node strength, which integrates the connection strengths of each node. If eigenvector centrality is computed with power iteration and the estimate is initialized with a uniform vector, this corresponds to the result of the first iteration. As the connectivity matrices from the ECM analysis are C + 1 (for voxelwise correlation matrices C), the strength of the connections in this centrality measure per voxel is the sum of all correlations with that voxel plus a constant. Degree centrality maps were then normalized by dividing the maps through the mean of the map that was calculated using FSL‐stats. By normalizing the degree centrality maps they became more comparable with ECM, which is also a normalized measure, and it filters out potential global effects of disease [Eijlers et al., 2017; Finn et al., 2015].
Neuropsychological Assessment
Intelligence was estimated using the Dutch version of the National Adult Reading Test. The following tests were used to measure performance on the domains of memory, information processing speed, executive functions, attention, motor and psychomotor speed: Rey Auditory Verbal Learning Test, Wechsler Adult Intelligence Scale, 3rd edition revised Digit Span and Symbol Substitution Test, Stroop Color‐Word Test, Concept Shifting Task, Simple Auditory and Visual Reaction Time Tests, Computerized Visual Searching Task, D2‐test, Wisconsin Cart Sorting Test, Category Word Fluency Task, Tapping Test and Letter Digit Modality Test. General cognitive ability was based on the average of all tests (see Van Duinkerken et al. [2016] for tests). Test scores were normalized based on the mean and standard deviation of controls and, if necessary, transformed so that higher z‐values indicated better performance.
RSN Functional Connectivity
Independent Component Analysis (ICA) on the resting‐state fMRI data of this group identified 10 RSNs as we previously published [Van Duinkerken et al., 2012]. After ICA, dual‐regression was used which created personalized maps of each network for each subject by first creating the average time course within each network per subjects and then linearly modeling the group‐based network onto the subject's fMRI‐scan. Lastly, the personalized time‐course is regressed back onto the subject's fMRI‐scan [Beckmann et al., 2009].
Subcortical gray matter volume
As we did not find any cortical structural alterations in this group, only subcortical volume was used in the current study. Using the FIRST pipeline of FSL, volume of the bilateral thalamus, caudate nucleus, putamen, pallidum, hippocampus, nucleus accumbens, and amygdala was obtained, in native T1‐space [Patenaude et al., 2011; Van Duinkerken et al., 2014]. Correction for head size is necessary to allow for group comparisons. This correction was performed by multiplying all uncorrected volumes with the V‐scaling factor derived from the FSL‐SIENAX pipeline [Smith et al., 2002]. This factor is calculated through affine registering the skull image from FSL‐SIENAX to MNI152 standard space [Jenkinson et al., 2002; Jenkinson and Smith, 2001], and thus represents the scaling factor by which uncorrected volumes are multiplied to correct for differences in head size.
Statistical Analysis
Subject characteristics were analyzed using One‐Way ANOVA, Kruskal–Wallis test in the case of non‐normality, or χ2‐tests for categorical variables. Normality was checked using the Kolmogorov–Smirnov test and visual inspection of the histogram.
ECM and degree centrality values were first compared between all patients as 1 group and controls using a 2‐tailed t‐test permutation‐based statistical comparison (10.000 permutations; FSL‐PALM [Permutation Analysis of Linear Models] version alpha101 [Winkler et al., 2014, 2016]) with Threshold Free Cluster Enhancement (TFCE) settings, in which no minimum cluster size needs to be defined. In case of significant differences, both patient groups were compared with controls and to each other, as post‐hoc testing. Correlations between centrality and RSN connectivity were calculated at a voxel‐level using PALM, using the same settings as mentioned above. These analyses were corrected for age, sex, systolic blood pressure, and depressive symptoms. For all voxel‐based analyses Family Wise Error (FWE) correction for multiple comparisons was applied. A P FWE < 0.05 was considered statistically significant.
In all patients, potential clinical relevance of altered centrality was studied by correlating centrality measures to age, sex, diabetes duration, albumin:creatinine ratio, HbA1c, body mass index, and systolic blood pressure. Furthermore, in all patients linear regression was used to determine correlations between ECM/degree centrality and cognition and subcortical gray matter volume. In order to account for multiple testing, we corrected the P‐values of all correlations using the False Discovery Rate (FDR).
A P < 0.05 (FWE and FDR corrected) was considered to be statistically significant. All statistical tests were performed using IBM‐SPSS 20 (IBM‐SPSS, Chicago, IL), R (version 3.3.2, http://www.R-project.org), and FSL‐PALM.
RESULTS
Patient Characteristics
Due to artifacts induced by braces, fMRI‐scans of 2 control subjects could not be used, leaving 49 controls. As can be found in Table 1, all T1DM patients were on average 5 years older than the control participants (P = 0.009). They also had higher systolic blood pressure and reported more depressive symptoms (all P < 0.05). Patients with proliferative retinopathy drove these differences. Patients had, on average, a disease duration of 28 years with an early adolescent disease onset, with those patients with proliferative retinopathy having the longest disease duration and earliest onset age (all P < 0.05). In all T1DM patients a median of 9 fMRI‐volumes needed to be scrubbed, in the control group this was 10 (P = 0.729). After scrubbing the relative displacement was on average below 0.03 mm in all groups, and was not statistically significantly different between the groups (P > 0.05).
Table 1.
Baseline characteristics of patient groups and non‐diabetes controls
| Non‐diabetes controls | Type 1 diabetes | P‐value | Type 1 diabetes without proliferative retinopathy | Type 1 diabetes with proliferative retinopathy | |
|---|---|---|---|---|---|
| N | 49 | 104 | – | 53 | 51 |
| Age (years) | 36.7 ± 11.2 | 41.1 ± 8.9 | 0.009 | 37.8 ± 9.2 | 44.5 ± 7.1*† |
| Sex (men/women; % men) | 19/30 (38.8) | 41/63 (39.4) | 0.999 | 20/33 (37.7) | 21/30 (41.2) |
| Estimated IQa | 108.5 ± 11.7 | 108.5 ± 12.4 | 0.978 | 107.0 ± 11.2 | 110.2 ± 13.4 |
| Depressive symptomsb | 4.0 (0 – 37) | 7.0 (0 – 42) | 0.029 | 5.0 (0 – 31) | 8.0 (0 – 42) *† |
| BMI (km/m2) | 24.3 ± 3.6 | 25.2 ± 3.8 | 0.140 | 24.8 ± 3.5 | 25.6 ± 4.1 |
| Systolic blood pressure (mm Hg) | 123.6 ± 11.3 | 131.3 ± 16.1 | 0.003 | 129.1 ± 14.7 | 133.6 ± 17.3* |
| Diastolic blood pressure (mm Hg) | 77.4 ± 7.2 | 77.1 ± 9.2 | 0.886 | 78.2 ± 9.5 | 76.0 ± 8.7 |
| HbA1c (mmol/mol) | 34.2 ± 2.6 | 63.2 ± 12.1 | <0.001 | 61.7 ± 9.8* | 64.7 ± 14.1* |
| HbA1c (%) | 5.3 ± 0.2 | 7.9 ± 1.1 | <0.001 | 7.8 ± 0.9* | 8.1 ± 1.3*† |
| Hypertension (%)c | – | 47 (45.2) | – | 13 (24.5) | 34 (66.7) † |
| Anti‐hypertensive medication (%) | – | 38 (36.5) | – | 6 (11.3) | 32 (62.7) † |
| Cholesterol medication (%) | – | 27 (26.0) | – | 11 (20.8) | 16 (31.4) |
| Diabetes duration (years) | – | 27.8 ± 10.7 | – | 21.6 ± 9.3 | 34.3 ± 7.9† |
| Diabetes onset age (years) | – | 13.3 ± 9.0 | – | 16.2 ± 9.7 | 10.2 ± 7.2† |
| Proliferative retinopathy (%)d | – | 51 (49.0) | – | – | 51 (100) |
| Albuminuria (%)e | – | 14 (13.5) | – | – | 25 (49.0) |
| Neuropathy (%)f | – | 25 (24.0) | – | – | 14 (27.5) |
| BG before MRI (mmol/L) | – | 9.8 ± 4.2 | – | 10.5 ± 4.7 | 9.2 ± 3.6 |
| BG before NPA (mmol/L) | – | 8.5 ± 4.0 | – | 8.4 ± 4.0 | 8.6 ± 4.0 |
| Severe hypoglycemic events (min–max)g | – | 2.0 (0–50) | – | 2.0 (0–50) | 2.0 (0–40) |
| Whole‐brain ECM (*10−3) | 7.9723 ± 0.008 | 7.9727 ± 0.008 | 0.805 | 7.9703 ± 0.010 | 7.9752 ± 0.005† |
| Relative displacement before scrubbing (mm) | 0.076 ± 0.04 | 0.080 ± 0.05 | 0.685 | 0.071 ± 0.04 | 0.088 ± 0.05 |
| Relative displacement after scrubbing (mm) | 0.026 ± 0.007 | 0.026 ± 0.008 | 0.976 | 0.025 ± 0.005 | 0.027 ± 0.009 |
| Scrubbed volumes (min–max) | 10 (1–36) | 9 (0–25) | 0.729 | 10 (2–23) | 9 (0–25) |
Data are means ± standard deviation, median with minimum and maximum or absolute numbers with percentages. NPA: neuropsychological assessment. BG: blood glucose.
*Significantly different from controls.
†Significantly different from patients without proliferative retinopathy.
Estimated IQ was measured using the Dutch version of the National Adult Reading Test (NART).
Depressive symptoms were measured using the Center for Epidemiological Studies scale for Depression.
Hypertension was defined as a systolic blood pressure of ≥140 mm Hg, a diastolic blood pressure of ≥90 mm Hg or the user of antihypertensive drugs. Controls with hypertension were excluded from the study.
Proliferative retinopathy was defined as a EURODIAB classification of 4 or 5.
Albuminuria was defined as an ACR >2.5 mg/mmol for men and >3.5 mg/mmol for women.
Neuropathy was based on medical records or, in case they were not available, based on self‐report.
Severe hypoglycemic events were self‐reported and defined as events for which the patient needs assistance from a third person to recuperate as a result of loss of consciousness or seriously deranged functioning, coma, or seizure owing to low glucose levels.
Eigenvector Centrality Mapping
Figure 1 shows the mean ECM values per group. The size of the ECM network was 15.696 voxels. All FSL‐PALM analyses were corrected for age, sex, systolic blood pressure, depressive symptoms, and multiple comparisons using TFCE and FWE. As can be found in the first column of Figure 2, all patients compared with controls showed significantly lower ECM values in a cluster comprising the bilateral thalamus, putamen, and caudate nucleus (P FWE < 0.05), without any areas of increased ECM. Post‐hoc group comparisons showed this effect was driven by patients without proliferative retinopathy. Relative to controls, this group showed lower ECM in the same subcortical areas, pallidum, cerebellum and brain stem, and increased ECM in the right cuneus and occipital fusiform gyrus (First column Fig. 2; P FWE < 0.05). Patients with proliferative retinopathy showed no differences with healthy controls. Directly comparing both patient groups, ECM was lowest in patients without versus with proliferative retinopathy in the brainstem, cerebellum, putamen, and thalamus. Higher ECM values in the group with uncomplicated T1DM were found in the bilateral occipital cortex and left superior temporal gyrus (First column Fig. 2; P FWE<0.05). Mean values of these clusters can be found in Table 2. As a difference in age, diabetes duration and disease onset age between the groups could affect the results, the analyses were repeated with groups matched for these factors. Results remained similar to the original analysis (First column Fig. 2).
Figure 2.
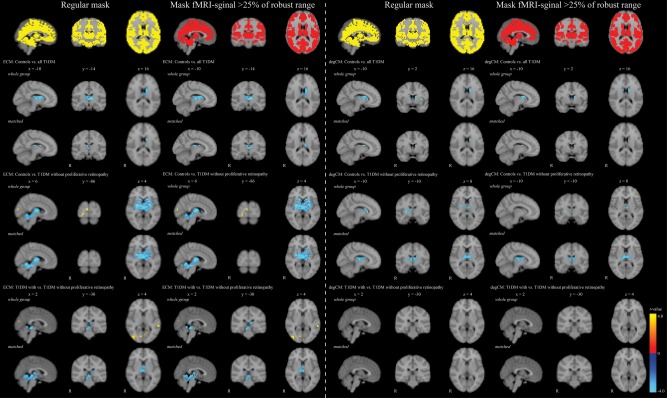
Clusters where eigenvector centrality and degree centrality values were different between groups. Results are presented on the 4 mm isotropic voxel MNI152 standard brain in radiological orientation. Represented are t‐values of the voxels that statistically significantly differed at P FWE < 0.05. The blue color indicates lower t‐values in the group that is mentioned last, red/yellow indicates higher t‐values in this group. The 1st and 3rd column represent the normal analysis, the 2nd and 4th column show the results of the analysis after removal of low quality signal. In the matched analysis 27 patients with proliferative retinopathy were included (11 men [40.7%]; mean age: 41.9 ± 7.8; mean diabetes duration: 29.0 ± 5.7; mean diabetes onset age: 12.9 ± 7.5). The group of patients without proliferative retinopathy included 29 participants (9 men [31.0%]; mean age: 40.3 ± 8.9; mean diabetes duration: 27.7 ± 8.2; mean diabetes onset age: 12.7 ± 8.4). These groups were compared with 38 controls (15 men [39.5%]; mean age: 40.8 ± 9.0). [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 2.
Mean values per group of the eigenvector centrality clusters, sub‐network functional connectivity, and subcortical volume and integrity
| Non‐diabetes controls | Type 1 diabetes | P‐valuea | Type 1 diabetes without proliferative retinopathy | Type 1 diabetes with proliferative retinopathy | |
|---|---|---|---|---|---|
| Eigenvector centrality (*10−3) | |||||
| Cluster all patients vs. controls | 8.02 ± 0.19 | 7.83 ± 0.28 | <0.001 | 7.77 ± 0.34* | 7.90 ± 0.18*† |
| Cluster patients without retinopathy vs. controls (neg) | 7.89 ± 0.15 | 7.77 ± 0.25 | <0.001 | 7.70 ± 0.31* | 7.85 ± 0.15† |
| Cluster patients without retinopathy vs. controls (pos) | 8.02 ± 0.27 | 8.19 ± 0.27 | 0.002 | 8.30 ± 0.28* | 8.09 ± 0.22† |
| Cluster patients without vs. with retinopathy (neg) | 7.62 ± 0.25 | 7.58 ± 0.36 | 0.493 | 7.43 ± 0.42* | 7.74 ± 0.18† |
| Cluster patients without vs. with retinopathy (pos) | 8.20 ± 0.21 | 8.25 ± 0.24 | 0.289 | 8.35 ± 0.24* | 8.14 ± 0.17† |
| Degree centrality | |||||
| Cluster all patients vs. controls | 1.07 ± 0.28 | 0.83 ± 0.25 | <0.001 | 0.82 ± 0.27* | 0.83 ± 0.24* |
| Cluster patients without retinopathy vs. controls | 1.07 ± 0.31 | 0.82 ± 0.28 | <0.001 | 0.76 ± 0.29* | 0.88 ± 0.27* |
| Normalized Subcortical gray matter volume (mL) | |||||
| Bilateral thalamus | 10.5 ± 0.81 | 10.0 ± 0.83 | 0.026 | 10.2 ± 0.94 | 9.89 ± 0.69 |
| Bilateral putamen | 6.88 ± 0.70 | 6.44 ± 0.70 | 0.003 | 6.53 ± 0.67* | 6.36 ± 0.71* |
| Bilateral caudate nucleus | 4.78 ± 0.51 | 4.55 ± 0.55 | 0.201 | 4.54 ± 0.56 | 4.55 ± 0.55 |
| Bilateral nucleus accumbens | 0.75 ± 0.10 | 0.69 ± 0.13 | 0.011 | 0.72 ± 0.13 | 0.67 ± 0.13* |
Values are presented as means with standard deviation.
P‐values are corrected for age, sex, systolic blood pressure and depressive symptoms.
*Significantly different from control participants.
†Significantly different relative to type 1 diabetes patients without proliferative retinopathy.
Normalized Degree Centrality
Mean degree centrality values are provided in Figure 1. As can be seen in the third column of Figure 2, degree centrality was lower in all T1DM patients relative to controls in the bilateral caudate nucleus, putamen, and thalamus (P FWE < 0.05), although results are spatially more widespread in the left hemisphere. This effect was driven by the patients without proliferative retinopathy, who had, in comparison with controls, lower degree centrality in the same regions (P FWE < 0.05). There were no differences between patients with proliferative retinopathy and controls or between both patients groups, and results were similar in the matched analysis. No increased degree centrality was observed.
Recalculating Centrality After Removal of Low Quality fMRI‐Signal
It is possible that fMRI‐signal decreases in quality at the extremities of the brain, such as the subcortical, cerebellar and brain stem region, which are the regions of altered centrality in this T1DM group. To eliminate low quality signal the individual raw fMRI‐scans were thresholded at 25% of the robust range of the non‐zero voxels, leaving only high‐quality signal. The subsequent gray matter mask was created as described in the “Materials and Methods” section. The second and forth column of Figure 2 show that low quality signal was mainly present in the temporal and frontal regions, and to a lesser extend in the brain stem. Recalculation of ECM and degree centrality and rerunning the statistical analyses did not show any significant changes in the results (2nd and 4th column Fig. 2).
Correlations between Centrality, Local RSN Functional Connectivity and Subcortical Gray Matter Volume
Given the overlap in different clusters of centrality differences in the three group comparisons (Fig. 2), the mean value per participant was extracted of all voxels showing higher or lower ECM and lower degree centrality across the group comparisons. This significantly reduced the number of statistical tests and prevented selection bias by choosing one contrast for higher and one for lower ECM/degree centrality.
The results of the voxel‐wise analysis between centrality and the secondary visual, sensorimotor, left frontal‐parietal, ventral attention, and auditory and language RSNs can be found in Figure 3. Both lower subcortical/cerebellar ECM and subcortical degree centrality were related to higher connectivity in the auditory and language network, secondary visual, and sensorimotor networks (all P FWE < 0.05; Fig. 3). Higher occipital ECM was related to higher connectivity in the same networks, although the spatial extent of this correlation for the auditory and language network was limited (all P FWE < 0.05; Fig. 3). The mean R 2 ranged between 0.30 and 0.40, indicating that between 30% and 40% of the variance is explained by the used model. There were no correlations between centrality and the left frontal‐parietal or ventral attention networks (all P FWE > 0.05).
Figure 3.
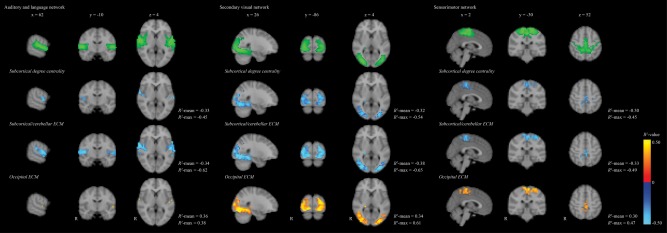
Schematic representation of the correlation between lower subcortical/cerebellar and higher occipital eigenvector centrality and lower subcortical degree centrality with functional connectivity in the auditory and language, secondary visual and sensorimotor RSNs in all T1DM patients. The top shows in green the RSN networks thresholded at z < 3.9, overlaid on a 4 mm standard brain. The voxels showing statistically significant correlations between centrality and functional connectivity were thresholded at P FWE < 0.05. The correlation coefficient was calculated as the R 2 for the model of centrality, corrected for age, sex, systolic blood pressure, and depressive symptoms. Lighter blue colors indicate higher negative R 2‐values, whereas more yellow colors indicate higher positive R 2‐values. ECM = eigenvector centrality mapping, T1DM = type 1 diabetes. [Color figure can be viewed at http://wileyonlinelibrary.com]
Neither ECM, nor degree centrality were related to subcortical gray matter volume (all P FDR > 0.05).
Clinical Relevance of Centrality Alterations
In all patients, uncorrected for confounding factors and multiple comparisons, higher ECM values in the occipital cortices were related to better general cognitive ability (β = 0.204; P = 0.038). This was no longer statistically significant after correction for confounding factors and multiple comparisons (P FDR > 0.05). There were no correlations with other cognitive domains or with degree centrality.
Clinically, lower ECM in the subcortical/cerebellar region was related lower age (β = 0.262; P FDR = 0.049; Figure 4), whereas lower ECM in the occipital cortices was related to higher age (β = −0.266; P FDR = 0.049; Figure 4) and longer diabetes duration (β = −0.283; P FDR = 0.049; Figure 4). There were no correlations with degree centrality.
DISCUSSION
In this study, we aimed to detect differences in ECM and degree centrality at a voxel level, between well‐characterized patients with longstanding T1DM with and without proliferative retinopathy and controls. We showed that ECM and degree centrality were lower in all T1DM patients as one group relative to controls in the thalamus and dorsal striatal regions (putamen and caudate nucleus), with lowest values in the group without proliferative retinopathy. In these patients versus controls ECM, but not degree centrality, was increased in the right cuneus and occipital fusiform gyrus. Comparing patients without to patients with proliferative retinopathy this increase was spatially more widespread and also found in the left occipital cortex and superior temporal gyrus. Low quality signal did not influence these results. Functionally, altered ECM and degree centrality were related to altered connectivity in the visual, sensorimotor, and auditory and language RSNs, but it was not related to volume of subcortical nuclei or cognition. Clinically, increased occipital ECM was related to shorter disease duration and younger age. Younger age was also related to decreased subcortical/cerebellar ECM.
Centrality analyses, by considering the whole brain as one network, show which regions are relatively more or less central and connected in the global network. Thus, our results indicate that in patients without proliferative retinopathy the thalamus, dorsal striatum, cerebellum and brain stem were less central and connected, whereas the bilateral occipital cortex was more central and connected within this entire functional brain network. Using the same voxel‐based ECM approach, lower occipital and higher frontal ECM was found in Alzheimer's disease [Binnewijzend et al., 2014], and increased thalamic and precuneus ECM with decreased occipitotemporal and sensorimotor ECM in multiple sclerosis [Schoonheim et al., 2014]. In patients with the human immunodeficiency virus ECM remained relatively unaffected, although no voxel‐based approach was used [Thomas et al., 2015]. Degree centrality in patients with type 2 diabetes was lower in the lingual gyrus, but higher in the insula and anterior cingulate cortex [Cui et al., 2016].
Although the effect of lower ECM and degree centrality was robust and spatially widespread in patients without proliferative retinopathy, there was only focally increased ECM, and no increased degree centrality. As both are a relative approach, increases in other regions may have been expected, such as was seen in multiple sclerosis [Schoonheim et al., 2014]. Its relative absence may suggest that increased ECM is diffusely distributed throughout the network, and thus failed to reach statistical significance. In our RSN analysis we previously found increased connectivity within the visual and sensorimotor areas in patients without proliferative retinopathy [Van Duinkerken et al., 2012], the first spatially overlapping with increased occipital ECM. It may suggest that connectivity is rerouted towards a more cortico‐cortical flow at the expense of the more common thalamo‐cortico‐thalamic circuit. In the more progressive stage of the disease, the normalization of cortically increased RSN connectivity may then lead to a normalization of subcortical and cerebellar ECM and degree centrality values. Alternatively, early subclinical changes in retinal and peripheral nerve structure and function, which have been found previously in patients without clinically manifest retinopathy or neuropathy [Almeida et al., 2008; Van Dijk et al., 2010], may result in altered input to the thalamus and dorsal striatum, which in turn may result in lower centrality of these structures. Taken together these results suggest an alteration of functional gray matter network organization in patients with yet uncomplicated T1DM in the absence of extensive gray or white matter damage or cognitive decrements. Centrality may thus serve as an early marker of cortical functional reorganization. When assessing local RSN functional connectivity in this group of patients we previously showed a similar pattern of alterations between the groups using both resting‐state fMRI [Van Duinkerken et al., 2012], and magnetoencephalography [Demuru et al., 2014]. This supports the hypothesis that functional connectivity in T1DM is a sensitive tool to detect cortical reorganization early in the disease. This is further supported by findings of functional reorganization in children below the age of 10 years with T1DM, that is, in those with only a short disease duration [Saggar et al., 2017]. This and our previous studies also show the limited capacity of functional connectivity analyses to discriminate between patients with and without proliferative retinopathy. It seems thus less useful in determining disease progression.
There was a small correlation found between higher occipital ECM and better cognitive performance in T1DM patients, but not with subcortical/cerebellar ECM or degree centrality, which did not survive FDR‐correction. This is in agreement with our previous findings showing increased visual RSN connectivity being related to better cognition [Van Duinkerken et al., 2012], and an absence of a correlation between thalamus or putamen volume and cognition in this group [Van Duinkerken et al., 2014]. This may suggest that the cognitive decrements in this group are not driven by subcortical/cerebellar, but rather by occipital alterations in function. Similar associations between occipital ECM values and cognition were also found in Alzheimer's disease and multiple sclerosis [Binnewijzend et al., 2014; Schoonheim et al., 2014], possibly suggesting altered posterior centrality is important for cognition.
Decreased ECM in the occipital cortex in the T1DM groups was related to longer disease duration and older age, whereas decreased ECM in the subcortical/cerebellar regions was related to lower age. As can be seen in Figure 4, these correlations are the resultant of combining the groups with and without proliferative retinopathy, as the correlation coefficients would not be statistically significant in the groups separately. However, as both groups have the same disease and share the same pathophysiology to develop retinopathy (i.e., chronic hyperglycemic exposure) we decided to combine the groups and increase the power to identify correlations.
Figure 4.
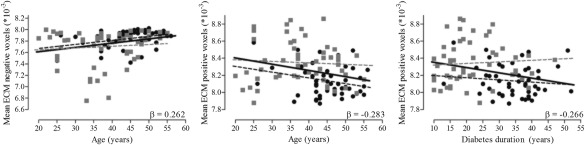
Scatter plots of the association between eigenvector centrality, age, and diabetes duration. The gray squares represent the patients without proliferative retinopathy and the black circles depict the patients with proliferative retinopathy. The solid black line indicates the regression line for all type 1 diabetes patients. Gray dashed lines represent the regression line of the patients without proliferative retinopathy and dashed black lines those of the patients with proliferative retinopathy.
In our study, ECM and degree centrality were strongly correlated and showed substantial overlap in the between‐group results. Despite these similarities, only ECM showed correlations with cognition and disease variables, which suggests that ECM is more sensitive to subtle T1DM‐related network architecture alterations than degree centrality. How other graph theoretical metrics are affected by T1DM and whether they are better capable of detecting disease progression should be determined in future studies.
Increased occipital cortex ECM was related to longer disease duration. However, lower subcortical and cerebellar ECM was, similarly to RSN connectivity in this group [Van Duinkerken et al., 2012], not related to any diabetes‐related factors, but it was related to lower age. Degree centrality was not related to any clinical factor. These correlations between clinical factors and centrality were relatively low, but in line with correlations we have previously found [Demuru et al., 2014; Van Duinkerken et al., 2009, 2012]. This suggests that there are other more prominent factors, as mentioned above, that have a stronger relationship with altered centrality. Furthermore, that centrality and connectivity alterations are present before the development of microvascular complications and are only marginally related to diabetes duration, as demonstrated here and in the previous RSN article [Van Duinkerken et al., 2012], suggests that other factors related to hyperglycemic exposure may be related to these alterations. It is known that chronic hyperglycemia leads to a cascade of changes, including increased advanced glycation endproduct formation, and increased inflammatory and oxidative stress responses [Brownlee, 2005]. Future studies need to unravel this.
Subcortical volume, which we have shown to be lower with respect to controls in this patient sample [Van Duinkerken et al., 2014], was not related to the observed centrality alterations. This is similar to findings in Alzheimer's disease, where only marginal overlap was seen between altered cortical gray matter volume and ECM [Binnewijzend et al., 2014]. On the other hand, we found strong correlations between altered ECM and degree centrality values and altered local visual, sensorimotor, and auditory and language RSN functional connectivity. In these first two networks, connectivity was highest in patients without proliferative retinopathy, whereas in the latter network connectivity was highest in controls, intermediate in patients without and lowest in patients with proliferative retinopathy [Van Duinkerken et al., 2012]. These networks are comprised of regions homogenous in terms of functions and spatial location, with direct afferent and efferent anatomical and functional connections with subcortical nuclei [Behrens et al., 2003; Schoonheim et al., 2014]. Functional connectivity of the left frontal‐parietal and ventral attention RSNs were not related to subcortical ECM alterations. The relationship between RSN connectivity and the whole‐brain functional network is still poorly understood and needs further study. However, the networks that did not show a correlation are larger in size and comprise multiple areas in different parts of the brain, thus being spatially less homogenous than the ones showing a correlation. This may affect the correlation with ECM and degree centrality.
Several limitations of this study should be mentioned. Firstly, patients with proliferative retinopathy were oldest, had the highest systolic blood pressure and level of depressive symptoms, and the longest disease duration and subsequently the earliest disease onset age. Although our main findings were in the other patient group, many of these variables can still confound the results; hence all analyses were corrected for these factors, except for diabetes duration and onset age. To control for these factors, we repeated the analyses with groups matched for age, diabetes duration and onset age, showing similar results. We have included two distinct groups of patients, and can therefore not extrapolate our results to groups of patients with moderate levels of retinopathy or other microvascular complications. We did not regress out motion parameters, as previous research has shown that ECM is relatively insensitive to these noise parameters [Lohmann et al., 2010; Wink et al., 2012], movement in this group was limited, and that rigorously removing these noise parameters may actually degrade data quality [Yan et al., 2013]. Instead we used scrubbing. Within graph theory, different network sizes can account for differences in ECM and degree centrality values between groups. Here network size was the same in every participant as the functional scans were non‐linearly registered to standard space and a common gray matter mask was used. Using this mask allowed us to determine ECM and degree centrality in gray matter only, thus regressing out white matter and cerebrospinal fluid signal was not necessary. There are different approaches to filtering of raw fMRI‐signal, with some suggesting no filtering, only high‐pass filtering (common in FSL), and some band‐pass filtering [Power et al., 2015]. Until now there is no gold standard. We followed FSL's recommendation to only use a high‐pass filter on fMRI data, as low‐pass filtering might introduce artificial correlations in the data [Davey et al., 2013].
CONCLUSION
To conclude, we demonstrated that subcortical/cerebellar ECM and degree centrality were lower and bilateral occipital ECM higher in T1DM patients without proliferative retinopathy. Functionally, this was associated with altered sensorimotor, visual, and auditory and language RSN functional connectivity, but not with volumetric alterations in subcortical nuclei or with cognition. It suggests reorganization of cortical connectivity pathways in patients without proliferative retinopathy, which is lost with disease progression. How these alterations develop over time, their relationship with cognition and the underlying mechanisms should be determined in future studies.
ACKNOWLEDGMENTS
EvD received a personal grant of the Brazilian National Council for Scientific and Technological Development (CNPq). No potential conflicts of interest relevant to this manuscript were reported.
EvD participated in the design of the study, included participants, performed the MRI‐scans and neuropsychological assessments, analyzed the data and wrote the manuscript. MMS supervised the fMRI preprocessing and Independent Component Analysis. RGIJ participated in the design of the study and obtained EFSD funding. ACM rated all fundus photographs. JLF provided part of the analysis infrastructure. MK participated in the design of the study and supervised the neuropsychological assessment. MD was principal investigator of the whole study, obtained DDRF and EFSD funding, and participated in the design of this study. FJS participated in the design of this study and supervised the psychological measures within the whole study. FB participated in the design of the study, supervised MRI data collection and clinically rated all structural MRI scans. AMW developed the eigenvector centrality mapping program. All authors, except for MD, have critically reviewed the results and have made critical revisions to the manuscript. EvD and AMW had full access to the data and had final responsibility for the decision to submit the manuscript.
REFERENCES
- Aldington SJ, Kohner EM, Meuer S, Klein R, Sjølie AK (1995): Methodology for retinal photography and assessment of diabetic retinopathy: the EURODIAB IDDM Complications Study. Diabetologia 38:437–444. [DOI] [PubMed] [Google Scholar]
- Almeida S, Riddell MC, Cafarelli E (2008): Slower conduction velocity and motor unit discharge frequency are associated with muscle fatigue during isometric exercise in type 1 diabetes mellitus. Muscle Nerve 37:231–240. [DOI] [PubMed] [Google Scholar]
- Beckmann C, Mackay C, Filippini N, Smith S (2009): Group comparison of resting‐state FMRI data using multi‐subject ICA and dual regression. NeuroImage 47:S39–S41. [Google Scholar]
- Behrens TE,J‐BH, Woolrich MW, Smith SM, Wheeler‐Kingshott CA, Boulby PA, Barker GJ, Sillery EL, Sheehan K, Ciccarelli O, Thompson AJ, Brady JM, Matthews PM (2003): Non‐invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci 6:750–757. [DOI] [PubMed] [Google Scholar]
- Binnewijzend MAA, Adriaanse SM, Van der Flier WM, Teunissen CE, de Munck JC, Stam CJ, Scheltens P, van Berckel BNM, Barkhof F, Wink AM (2014): Brain network alterations in Alzheimer's disease measured by Eigenvector centrality in fMRI are related to cognition and CSF biomarkers. Hum Brain Mapp 35:2383–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brands AM, Biessels GJ, de Haan EH, Kappelle LJ, Kessels RP (2005): The effects of type 1 diabetes on cognitive performance: a meta‐analysis. Diabetes Care 28:726–735. [DOI] [PubMed] [Google Scholar]
- Brownlee M (2005): The pathobiology of diabetic complications: a unifying mechanism. Diabetes 54:1615–1625. [DOI] [PubMed] [Google Scholar]
- Cui Y, Li S‐F, Gu H, Hu Y‐Z, Liang X, Lu C‐Q, Cai Y, Wang C‐X, Yang Y, Teng G‐J (2016): Disrupted Brain Connectivity Patterns in Patients with Type 2 Diabetes. AJNR Am J Neuroradiol 37:2115–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey CE, Grayden DB, Egan GF, Johnston LA (2013): Filtering induces correlation in fMRI resting state data. NeuroImage 64:728–740. [DOI] [PubMed] [Google Scholar]
- Demuru M, Van Duinkerken E, Fraschini M, Marrosu F, Snoek FJ, Barkhof F, Klein M, Diamant M, Hillebrand A (2014): Changes in MEG resting‐state networks are related to cognitive decline in type 1 diabetes mellitus patients. NeuroImage Clin 5:69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J, Wong TY (2012): Current epidemiology of diabetic retinopathy and diabetic macular edema. Curr Diab Rep 12:346–354. [DOI] [PubMed] [Google Scholar]
- Eijlers AJ, Meijer KA, Wassenaar TM, Steenwijk MD, Uitdehaag BM, Barkhof F, Wink AM, Geurts JJG, Schoonheim MM (2017): Increased default‐mode network centrality in cognitively impaired multiple sclerosis patients. Neurology 88:952–960. [DOI] [PubMed] [Google Scholar]
- Finn ES, Shen X, Scheinost D, Rosenberg MD, Huang J, Chun MM, Papademetris X, Constable RT (2015): Functional connectome fingerprinting: identifying individuals using patterns of brain connectivity. Nat Neurosci 18:1664–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson AM, Ryan CM, Cleary PA, Waberski BH, Weinger K, Musen G, Dahms W (2011): Biomedical risk factors for decreased cognitive functioning in type 1 diabetes: an 18 year follow‐up of the Diabetes Control and Complications Trial (DCCT) cohort. Diabetologia 54:245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Smith S (2001): A global optimisation method for robust affine registration of brain images. Med Image Anal 5:143–156. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S (2002): Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage 17:825–841. [DOI] [PubMed] [Google Scholar]
- Klein R, Knudtson MD, Lee KE, Gangnon R, Klein BEK (2008): The wisconsin epidemiologic study of diabetic retinopathy XXII. Ophthalmology 115:1859–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodl CT, Franc DT, Rao JP, Anderson FS, Thomas W, Mueller BA, Lim KO, Seaquist ER (2008): Diffusion Tensor Imaging (DTI) identifies deficits in white matter microstructure in subjects with type 1 diabetes mellitus that correlate with reduced neurocognitive function. Diabetes 27:3083–3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmann G, Margulies DS, Horstmann A, Pleger B, Lepsien J, Goldhahn D, Schloegl H, Stumvoll M, Villringer A, Turner R (2010): Eigenvector centrality mapping for analyzing connectivity patterns in fMRI data of the human brain. PLoS ONE 5:e10232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musen G, Lyoo IK, Sparks CR, Weinger K, Hwang J, Ryan CM, Jimerson DC, Hennen J, Renshaw PF, Jacobson AM (2006): Effects of type 1 diabetes on gray matter density as measured by voxel‐based morphometry. Diabetes 55:326–333. [DOI] [PubMed] [Google Scholar]
- Patenaude B, Smith SM, Kennedy DN, Jenkinson M (2011): A Bayesian model of shape and appearance for subcortical brain segmentation. NeuroImage 56:907–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE (2012): Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage 59:2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Schlaggar BL, Petersen SE (2015): Recent progress and outstanding issues in motion correction in resting state fMRI. NeuroImage 105:536–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS (1977): The CES‐D scale: a self‐report depression scale for research in the general population. Appl Psychol Meas 1:385–401. [Google Scholar]
- Ryan JP, Aizenstein HJ, Orchard TJ, Ryan CM, Saxton JA, Fine DF, Nunley KA, Rosano C (2015): Age of childhood onset in type 1 diabetes and functional brain connectivity in midlife. Psychosom Med 77:622–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan CM, Van Duinkerken E, Rosano C (2016): Neurocognitive consequences of diabetes. Am Psychol 71:563–576. [DOI] [PubMed] [Google Scholar]
- Saggar M, Tsalikian E, Mauras N, Mazaika P, White NH, Weinzimer S, Buckingham B, Hershey T, Reiss AL (2017): Compensatory hyperconnectivity in developing brains of young children with type 1 diabetes. Diabetes 66:754–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoonheim M, Geurts J, Wiebenga O, De Munck J, Polman C, Stam C, Barkhof F, Wink A (2014): Changes in functional network centrality underlie cognitive dysfunction and physical disability in multiple sclerosis. Mult Scler 20:1058–1065. [DOI] [PubMed] [Google Scholar]
- Smith SM, Zhang Y, Jenkinson M, Chen J, Matthews PM, Federico A, De Stefano N (2002): Accurate, robust, and automated longitudinal and cross‐sectional brain change analysis. NeuroImage 17:479–489. [DOI] [PubMed] [Google Scholar]
- Smolina K, Wotton CJ, Goldacre MJ (2015): Risk of dementia in patients hospitalised with type 1 and type 2 diabetes in England, 1998–2011: a retrospective national record linkage cohort study. Diabetologia 58:942–950. [DOI] [PubMed] [Google Scholar]
- The Diabetes Control and Complications Trial Research Group (1996): Effects of intensive diabetes therapy on neuropsychological function in adults in the diabetes control and complications trial. Ann Intern Med 124:379–388. [DOI] [PubMed] [Google Scholar]
- Thomas JB, Brier MR, Ortega M, Benzinger TL, Ances BM (2015): Weighted brain networks in disease: centrality and entropy in human immunodeficiency virus and aging. Neurobiol Aging 36:401–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk HW, Verbraak FD, Kok PHB, Garvin MK, Sonka M, Lee K, de Vries H, J, Michels RPJ, van Velthoven MEJ, Schlingemann RO, Abramoff MD (2010): Decreased retinal ganglion cell layer thickness in patients with type 1 diabetes. Invest Ophthalmol Vis Sci 51:3660–3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Duinkerken E, Klein M, Schoonenboom NS, Hoogma RP, Moll AC, Snoek FJ, Stam CJ, Diamant M (2009): Functional brain connectivity and neurocognitive functioning in patients with longstanding type 1 diabetes mellitus with and without microvascular complications: a magnetoencephalography study. Diabetes 58:2335–2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Duinkerken E, Schoonheim MM, Sanz‐Arigita EJ, IJzerman RG, Moll AC, Snoek FJ, Ryan CM, Klein M, Diamant M, Barkhof F (2012): Resting‐state brain networks in type 1 diabetes patients with and without microangiopathy and their relation with cognitive functions and disease variables. Diabetes 61:1814–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Duinkerken E, Schoonheim MM, Steenwijk MD, Klein M, Ijzerman RG, Moll AC, Heijmans MW, Snoek FJ, Barkhof F, Diamant M (2014): Ventral striatum, but not cortical volume loss is related to cognitive dysfunction in type 1 diabetes patients with and without microangiopathy. Diabetes Care 37:2483–2490. [DOI] [PubMed] [Google Scholar]
- Van Duinkerken E, Ryan CM, Schoonheim MM, Barkhof F, Klein M, Moll AC, Diamant M, IJzerman RG, Snoek FJ (2016): Subgenual cingulate cortex functional connectivity in relation to depressive symptoms and cognitive functioning in type 1 diabetes mellitus patients. Psychosom Med 78:740–749. [DOI] [PubMed] [Google Scholar]
- Van Wijk BCM, Stam CJ, Daffertshofer A (2010): Comparing brain networks of different size and connectivity density using graph theory. PLoS ONE 5:e13701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels AM, Scheltens P, Barkhof F, Heine RJ (2008): Hyperglycaemia as a determinant of cognitive decline in patients with type 1 diabetes. Eur J Pharmacol 585:88–96. [DOI] [PubMed] [Google Scholar]
- Wink AM, de Munck JC, van der Werf YD, van den Heuvel OA, Barkhof F (2012): Fast eigenvector centrality mapping of voxel‐wise connectivity in functional magnetic resonance imaging: implementation, validation, and interpretation. Brain Connect 2:265–274. [DOI] [PubMed] [Google Scholar]
- Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE (2014): Permutation inference for the general linear model. NeuroImage 92:381–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler AM, Webster MA, Brooks JC, Tracey I, Smith SM, Nichols TE (2016): Non‐parametric combination and related permutation tests for neuroimaging. Hum Brain Mapp 37:1486–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C‐G, Cheung B, Kelly C, Colcombe S, Craddock RC, Di Martino A, Li Q, Zuo X‐N, Castellanos FX, Milham MP (2013): A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. NeuroImage 76:183–201. [DOI] [PMC free article] [PubMed] [Google Scholar]


