Abstract
To analyze the involvement of different brain regions in behavioral inhibition and impulsiveness, differences in activation were investigated in fMRI data from a response inhibition task, the stop‐signal task, in 1709 participants. First, areas activated more in stop‐success (SS) than stop‐failure (SF) included the lateral orbitofrontal cortex (OFC) extending into the inferior frontal gyrus (ventrolateral prefrontal cortex, BA 47/12), and the dorsolateral prefrontal cortex (DLPFC). Second, the anterior cingulate and anterior insula (AI) were activated more on failure trials, specifically in SF versus SS. The interaction between brain region and SS versus SF activations was significant (P = 5.6 * 10−8). The results provide new evidence from this “big data” investigation consistent with the hypotheses that the lateral OFC is involved in the stop‐related processing that inhibits the action; that the DLPFC is involved in attentional processes that influence task performance; and that the AI and anterior cingulate are involved in emotional processes when failure occurs. The investigation thus emphasizes the role of the human lateral OFC BA 47/12 in changing behavior, and inhibiting behavior when necessary. A very similar area in BA47/12 is involved in changing behavior when an expected reward is not obtained, and has been shown to have high functional connectivity in depression. Hum Brain Mapp 38:3527–3537, 2017. © 2017 Wiley Periodicals, Inc.
Keywords: inhibition, impulsive behavior, orbitofrontal cortex, cingulate cortex, insula, depression
INTRODUCTION
Analyzing brain function in the stop‐signal task (SST) is of great interest, for this task is related to behavioral inhibition and impulsiveness, and its performance is impaired in attention‐deficit hyperactivity disorder (ADHD) [Aron et al., 2014]. The task has Go trials and Stop trials. During Go trials participants are presented with an arrow pointing either to the left or to the right, and are instructed to make a button response with their fingers corresponding to the direction of the arrow. In the unpredictable Stop trials (17%; 80 trials), the arrows pointing left or right are followed (on average 300 ms later) by an arrow pointing upwards, which instructs participants to inhibit their motor responses [Nymberg et al., 2013]. More details are provided in the Methods. The task design allows measurement of brain activation in relation to response inhibition and its failure. Not only is behavioral inhibition important in its own right and in relation to ADHD, but in addition this process is related to impulsiveness. Further, it is important in terms of understanding the underlying brain mechanisms to know whether the areas involved in behavioral inhibition are similar to or overlap with the areas of the lateral orbitofrontal cortex (OFC) area involved in reversing behavior to nonreward [Grabenhorst and Rolls, 2011; Rolls, 2014; Rolls and Grabenhorst, 2008; Thorpe et al., 1983], which have been thereby implicated in depression [Rolls, 2016c; Rolls, 2017].
The overall aim of the investigation described here is to analyze the activations that are measured with functional magnetic resonance imaging (fMRI) in the SST. The particular aims were as follows. First, previous investigations have highlighted the importance of the inferior frontal gyrus, based on the effects of brain damage [Aron et al., 2014] and fMRI [Aron et al., 2014; Boehler et al., 2010; Cai et al., 2014; Duann et al., 2009; Nymberg et al., 2013; Xue et al., 2008; Zhang and Li, 2012]. However, the inferior frontal gyrus may refer to a number of brain systems that may perform different functions. In particular, the lateral OFC Brodmann area 47/12 extends in humans round the inferior convexity where it adjoins posteriorly on the lateral surface areas BA 45, which is part of Broca's area on the left; and more anteriorly lateral area 10 [Öngür et al., 2003; Öngür and Price, 2000; Rolls et al., 2015]. A first aim of this investigation was therefore to identify the focus of the activation in the SST to clarify whether the focus was in the lateral orbitofrontal, areas in the inferior frontal gyrus, or whether the activation included both. We investigated this is a large group of 1709 individuals to obtain robust results and localization. The second particular aim was to investigate whether other areas that we and others found to be activated in the same task in brain regions connected to the lateral OFC/inferior frontal gyrus were activated in the same way, or differently which would imply different contributions to the task. The other areas included the anterior cingulate cortex (ACC), the insula [Cai et al., 2014], and the dorsolateral prefrontal cortex (DLPFC). We investigated this by contrasting the activations on stop‐success (SS) trials compared to stop‐failure (SF) trials. We reasoned that brain regions with larger activations on SS trials than SF trials might be involved in the computations involved in stopping the task. We reasoned that brain regions with larger activations on SF trials than SS trials might be involved in the emotional response to failure that would be expected to be more evident on SF trials. These particular aims make this a novel investigation.
The data we analyzed were from 1709 participants in the IMAGEN database [Nymberg et al., 2013]. The dataset is by far the largest one to include the SST, and should help to provide robust and well‐localized evidence on the neural processes involved in different aspects of the SST. This particular dataset has the interesting and useful property that all the participants were of a similar age, 14 years old, enabling processes at this important stage of development with respect to behavioral control and impulsiveness, important developments in adolescence [Nymberg et al., 2013], to be investigated, and ensuring a homogeneous population with respect to age. Individuals of this age were suitable for investigation of inhibition, for the SST was performed in a similar way to that described in older individuals [Cai et al., 2014]. A further advantage of this dataset is that all participants performed the same SST with similar imaging parameters.
A new key finding of this investigation is that a cortical area involved in the success of behavioral inhibition on the SST is the lateral OFC BA47/12. Another new key finding is that this function is dissociable from the functioning of the insula and ACC in the SST, which are activated more on failure trials.
The architectonic areas of the human OFC have been described by Price and coworkers [Öngür et al., 2003], and their analysis shows that the lateral OFC Brodmann area 47/12 continues round the inferior convexity to include the ventral part of the inferior temporal gyrus where it adjoins BA 45 posteriorly and lateral area 10 anteriorly. This area has been variously referred to as the ventrolateral prefrontal cortex (VLPFC), inferior frontal gyrus, and lateral OFC. The focus of the activations in SS–SF described in this article was in the lateral OFC BA 47/12 [Öngür et al., 2003], and when other terms are used in this article this is because they have been used in the literature.
METHODS
Task/Experimental Design
The SST is an event‐related task designed to study neural responses involved in successful and unsuccessful inhibitory control, with full details provided in [Nymberg et al., 2013], and an illustrative diagram in Figure 1. The task has Go trials (83%; 400 trials) and Stop trials (17%; 80 trials), with between three and seven go trials between two stop trials. It required participants to respond to visual go stimuli (arrows pointing left or right) and to withhold their motor response when the go stimulus was followed unpredictably by a stop signal (an arrow pointing upwards). A tracking algorithm changed the time interval (on average 300 ms with initial delay = 250 ms), that is, the stop signal delay (SSD), between the Go signal and Stop signal onsets on Stop trials adaptively to produce 50% successful and 50% unsuccessful response inhibition trials. The inter‐trial interval was 1,800 ms. The stop signal reaction time was calculated by subtracting the mean SSD (the average time between go and stop signal, at which the subject managed to inhibit to 50% of stop trials) from the mean reaction time to go trials.
Figure 1.
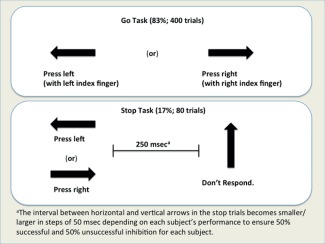
The SST. [Color figure can be viewed at http://wileyonlinelibrary.com]
SUBJECTS
Data were acquired from 1980, 14‐year‐old adolescents from the IMAGEN initiative across eight IMAGEN assessment sites [Nymberg et al., 2013], who had participated in the SST task. A total of 1709 participants from these passed quality controls for neuroimaging and behavioral tests and were included into further analysis [Nymberg et al., 2013]. (The dataset included 882 females, 827 males; 1493 were right‐handed, 192 left‐handed, and 24 had missing handedness records; the go reaction time had a mean of 470 ms, std 81 ms; the stop success reaction time had a mean of 234 ms, std 91 ms.) This dataset was used because it was homogeneous for age so that possible age differences did not need to be factored out; because impulsiveness and behavioral inhibition are key factors in adolescent development, and understanding how these processes operate at this developmental stage has potential clinical implications [Nymberg et al., 2013]; and because the large size of the cohort is an advantage in neuroimaging analyses to obtain robust results and accurate localization, and to enable whole‐brain analyses without selection of only some brain regions using a priori hypotheses. In these respects, this study is an advance beyond previous studies on behavioral inhibition investigated with the stop‐signal and similar tasks. The individuals understood and performed the task well in the scanner, as shown by the behavioral measures [Nymberg et al., 2013].
fMRI Data Acquisition and Preprocessing
fMRI data were acquired for the SST task with 3T MRI scanners. All data acquisition, preprocessing, and quality controls were performed by the IMAGEN initiative, with the detailed procedures and parameters provided in detail elsewhere [Nymberg et al., 2013; Whelan et al., 2012]. Briefly, functional MRI BOLD images were acquired with a gradient‐echo, echo‐planar imaging sequence. For the SST, 444 volumes were acquired for each subject, while each volume consisted of 40 slices aligned to the anterior commission/posterior commission line (2.4‐mm slice thickness, 1‐mm gap). The echo time was optimized (echo time = 30 ms, repetition time = 2200 ms) to provide reliable imaging of subcortical areas. Image processing and analysis were performed using SPM 8 (Statistical Parametric Mapping, http://www.fil.ion.ucl.ac.uk/spm). Time series data were corrected for slice timing, then for movement, non‐linearly warped onto MNI space using a custom EPI template, and Gaussian‐smoothed at 5‐mm full‐width half maximum. Estimated movement (three translations, three rotations, three translations shifted one volume acquisition before and three translations shifted one volume acquisition later) parameters were added as nuisance variables. Each fMRI time series underwent automatic spike detection and any artifactual time points were regressed out of each subject's data. Activation maps and contrast maps were computed using a general linear model with an autoregressive noise model. Based on behavioral records, each participant's design matrix included regressors for stop success trials, stop failure trials, trials on which the go response was too late, trials on which the go response was wrong (if any) and the nuisance variables. The regressors modeling the experimental conditions were convolved using SPM's default hemodynamic response function. A one‐sample t test was conducted, testing activity on stop success trials (and separately on stop fail trials), removing variance associated with the other regressors in the design matrix. Beta values of contrast maps were used for further analysis, and all the following analysis was performed using Matlab.
All the templates are in MNI space and voxel activations are presented in MNI coordinates. Results were analyzed for contrast maps that included SS–SF as this potentially provides evidence about the brain systems that implement behavioral inhibition; and SF–SS as this potentially provides evidence about brain systems activated by failure in the task.
The IMAGEN data come from multiple data collection sites. To test whether there was any significant variation between sites, we performed a two factor ANOVA with one factor the sites and the other factor the four brain regions considered here in SS–SF. No significant intersite variation was found (all P values were in the range 0.81–0.95).
RESULTS
First, in order to identify brain regions that are likely to be important in successful performance of the SST, we present the results for the contrast of SS–SF. Then we analyze the contrast SF–stop success, because this may reveal areas that may be more related to other processes, such as emotional responses to failure. Then, we present results for other contrasts. These analyses are based on data from 1709 participants. We emphasize results for areas implicated in behavioral change and emotion by lesion and much other evidence, and this includes brain regions such as the OFC and inferior frontal gyrus, ACC, and insula [Aron et al., 2014; Rolls, 2014, 2015a, 2016a,b].
Contrast of SS–SF
The results for the contrast SS–SF are illustrated in Figure 2a,b with further details in Table 1, with three regions described next. The first region is the VLPFC, with peak at [–42 50 −2] (t = 5.1, P = 3.58e‐7, significant under FDR correction for the whole brain), and with corresponding effects on the right [42 52 −4] (t = 2.8, P = 0.0052). This region includes the lateral OFC BA 47/12 which includes part of the inferior frontal gyrus [Öngür et al., 2003; Rolls et al., 2015].
Figure 2.
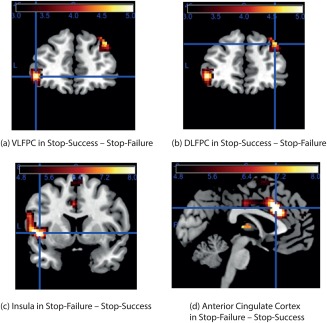
Contrast maps for SS – SF; and for SF – SS. All contrast maps were thresholded at P < 0.05 with Bonferroni correction across the whole brain, corresponding to t = 4.75. The calibration bar in this and subsequent figures shows the t value for the contrast. (a) The contrast SS – SF for VLPFC including the Lateral OFC, with peak at [–42 50 −2] (t = 5.1, P = 3.58e‐7, significance reported unless otherwise stated using FDR correction for the whole brain). There were corresponding effects on the right [42 52 −4] (t = 2.8, P = 0.0052). (b) The contrast SS – SF for DLPFC, with peak at [38 44 38] (t = 5.0, P = 6.02e‐7). (c) The AI cortex in the contrast SF – SS showed effects ([–46 8 −2] t = 8.3, P = 2.22e‐16 at crosshairs; [46 12 2] t = 5.3, P = 1.23e‐7). (d) The supracallosal ACC in the contrast SF – SS showed effects ([–2 18 38] t = 9.2, P = 6.06e‐20 at crosshairs; [4 30 26] t = 8.5, P = 2.80e‐17). [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 1.
Contrasts for SS – SF
| ROI | Full name of ROI | Cluster center coordinates | Highest t‐score (P‐value) |
|---|---|---|---|
| 1 | VLPFC/Lateral OFC | (–42,50,–2) | 5.1 (3.58e‐7) |
| 2 | DLPFC | (38,44,38) | 5.0 (6.02e‐7) |
| 3 | Ventral Striatum | (22,12,–8) | 8.0 (1.78e‐15) |
| 4 | Premotor Cortex | (30,–8,64) | 5.0 (6.02e‐7) |
| 5 | Inferior Temporal Gyrus | (54,–58,–8) | 3.9 (9.80e‐5) |
| 6 | Parietal | (–48,–58,50) | 4.6 (4.38e‐6) |
| 7 | Brodmann Area 19 | (28,–80,–14) | 4.3 (1.76e‐5) |
The second region is the DLPFC, with peak at [38 44 38] (t = 5.0, P = 6.02e‐7, significant under FDR correction for the whole brain).
Another interesting region is the pars opercularis of the right inferior frontal gyrus, with a peak at [50,16,34] (t = 3.0, P = 0.0028), which is probably BA 45 or 44 [Öngür et al., 2003; Rolls, et al., 2015].
These regions are of particular interest for the analysis of this task because the lateral OFC is involved in changing behavior when nonreward or punishment is received [Kringelbach and Rolls, 2003; Rolls, 2014, 2016c; Rolls and Grabenhorst, 2008]; and lesions of the (right) inferior frontal gyrus that are close to the contrast peaks just described impair the performance of the SST [Aron et al., 2014]. (They described the region as BA 44 inferior frontal gyrus, opercular part in the AAL atlas [Tzourio‐Mazoyer et al., 2002].) In addition to these contrasts of especial interest, effects were also found as shown in Table 1 in the ventral striatum (which receives inputs from the OFC and other parts of the prefrontal cortex [Haber and Knutson, 2010]), premotor cortex (which may reflect the change of movement), inferior temporal gyrus (and related visual areas), and parietal cortex area 7 (both of which receive back projections from the prefrontal cortex [Pandya et al., 2015; Rolls, 2016a]).
Contrast of SF–SS
In the contrast SF – SS, the anterior insular (AI) cortex showed effects ([–46 8 −2] t = 8.3, P = 2.22e‐16; [46 12 2] t = 5.3, P = 1.23e‐7), as did the supracallosal ACC ([–2 18 38] t = 9.2, P = 6.06e‐20; [4 30 26] t = 8.5, P = 2.80e‐17) (see Fig. 2c,d and Table 2). It is of interest that these activations were largest on the left of the brain. The effects in both these areas may be related to the emotional responses to the failure, for this AI region is implicated in autonomic effects [Critchley and Harrison, 2013; Rolls, 2016b], and this part of the ACC is implicated in the representation of aversive/unpleasant events [Grabenhorst and Rolls, 2011; Rolls, 2014; Rolls and Grabenhorst, 2008].
Table 2.
Contrasts for SF – SS
| ROI | Full name of ROI | Cluster center coordinates | Highest t‐score (P‐value) |
|---|---|---|---|
| 1 | ACC | (–2,18,36) | 9.2 (6.06e‐20) |
| 2 | Insula | (–46,8,–2) | 8.3 (2.22e‐16) |
| 3 | Brodmann Area 4 (primary somatomotor area) | (–44,–2,8) | 6.9 (6.18e‐12) |
| 4 | Postcentral gyrus | (–58,–18,46) | 7.2 (7.36e‐13) |
| 5 | Postcentral gyrus | (–60,–18,22) | 7.5 (8.08e‐14) |
| 6 | Posterior Thalamus | (–2,–22,6) | 8.1 (6.66e‐16) |
In summary, SF activates the AI more than SS, especially the ventral AI (see Fig. 2d); and further, both types of stop trials activate the AI more than Go trials (two sample t‐test between SS and Go, with SS > Go, t = 28.98, P = 2.54e‐162; two sample t‐test between SF and Go, with SF > Go, t = 30.56, P = 3.45e‐178). Activation on Go trials was found in the posterior insula. Also shown in Table 2 are effects in movement‐related cortical areas such as the precentral and postcentral gyrus, which are likely given the functions of these regions to be related to the motor responses which are different on SF trials (on which a response is completed) than on SS trials (on which a movement is not completed).
OTHER CONTRASTS
Figure 3a shows the activation produced in the SS condition. This shows that the area activated extends from the OFC though the lateral OFC Brodmann area 47/12 [Öngür et al., 2003] to the inferior frontal gyrus, and up to the DLPFC.
Figure 3.
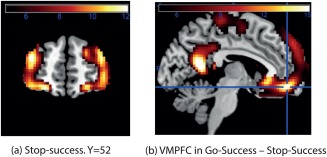
Further neuroimaging results. (a) The activation for SS thresholded at P < 1e‐8, corresponding to t = 5.75, to show the regions of the lateral OFC and DLPFC that were activated. (b). Contrast of Go trials ‐ stop trials. The whole of the ventromedial prefrontal cortex (VMPFC) was more strongly activated than the lateral OFC areas. [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 3b shows that on the Go‐success trials, the ventromedial prefrontal cortex, including the medial OFC BA13, is activated. This is consistent with its role in representing many different rewards [Grabenhorst and Rolls, 2011; Rolls, 2014], with the reward in this case the simple completion of a Go trial which corresponds to correct performance of the typical trial type in this task. It is noted that in the Go‐Success condition, the activation was much lower than on SS in the lateral OFC 47/12/inferior frontal gyrus region (t = −22.54, P = 2.09e‐97), than in the DLPFC (t = −39.20, P = 1.06e‐237), than in the ACC (t = −49.27, P < 4.94 e‐324, and than in the insula (t = −28.98, P = 2.54e‐162). This indicates that these four regions have activations that are related to the stop trials, rather than the Go trials.
Dissociation between Brain Regions Involved in SS and SF
To investigate whether the activations in the areas of interest are different on stop success–stop failure trials (which may reflect engagement of brain regions necessary to change the behavior) from the activations on SF–SS trials, we performed a two way analysis to test for significant interaction between these contrasts and brain regions.
Figure 4 illustrates the dissociation between the greater activations in SS in the Lateral OFC and DLPFC, versus the greater activations in SF in the AI and ACC, with the dissociation confirmed by a significant interaction effect in a two‐factor ANOVA involving the 4 brain regions × the two contrasts F[3,13664] = 12.21, with P‐value = 5.61 * 10−8.
Figure 4.
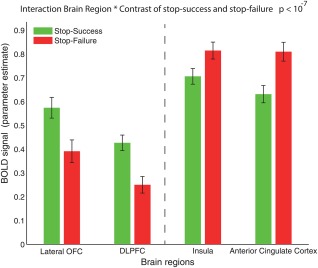
The BOLD signal activations (parameter estimates; mean over the significant voxels using Bonferroni correction +/– SEM) for SS (green) and for SF (red) for the four main brain regions, Lateral OFC BA47/12, DLPFC, Insula, and ACC. [Color figure can be viewed at http://wileyonlinelibrary.com]
Comparing brain regions of particular interest, the interaction statistic for Lateral OFC and Insula vs contrast is F[1,6832] = 13.04, with P‐value = 3.08 * 10−4. This shows that the Lateral OFC and the insula are activated differently as a function of these two contrasts. The interaction statistic for Lateral OFC and ACC versus contrast is F[1, 6832] = 18.53, with P‐value = 1.69 * 10−5. This shows that the Lateral OFC and the ACC are activated differently as a function of these two contrasts.
DISCUSSION
Some of the new findings and advances made in this article, made possible by the large sample size, whole‐brain analysis with no a priori regions of interest, and the identical SST performed by all 1709 participants, include the following which are discussed below. These attributes of this investigation enable it to go beyond previous studies which had fewer subjects and/or used a priori regions and/or which combined results from several tasks [Aron et al., 2014; Boehler et al., 2010; Cai et al., 2014; Duann et al., 2009; Xue et al., 2008; Zhang and Li, 2012]. First, the peak of the activation in successful trials of the SST in the inferior frontal gyrus/inferior convexity prefrontal cortex (Fig. 2a) is in an area that is part of area 47/12, a lateral OFC region [Öngür et al., 2003]. This is more clearly shown to be lateral OFC area 47/12 than in some earlier studies [Aron et al., 2014; Boehler et al., 2010; Cai et al., 2014; Duann et al., 2009; Xue et al., 2008; Zhang and Li, 2012]. This is important, for it helps to identify this system as a lateral OFC region activated in other types of task in which behavior must change, namely tasks in which a nonreward signals that behavior should change, and in which punishers/aversive stimuli are received [Grabenhorst and Rolls, 2011; Rolls, 2014, 2016c]. Second, a dissociation of brain areas activated in the SST was found, with the finding for the first time that the lateral OFC and DLPFC are activated relatively more on successful than on failure trials, whereas the insula and ACC are relatively more activated on failure than on reward trials. This again is important, for this finding provides evidence on which brain systems are especially important in performing the SST correctly, and on other brain areas that respond more with failure. The new findings help to provide a framework for understanding the functions of these four areas in the SST and in behavioral inhibition, and are discussed next.
First, we consider areas activated in the contrast SS–SF. Areas identified in this contrast are candidates for contributing to the successful implementation of stopping the action when the Stop signal is received. One of these areas is the lateral OFC area BA 47/12, which extends into the inferior frontal gyrus (Fig. 2a and Table 2) as defined cytoarchitectonically in humans [Öngür et al., 2003], and thus includes more than the opercular part of the inferior frontal gyrus that is often emphasized as being involved in the stop‐signal response inhibition task [Aron et al., 2014; Rae et al., 2015]. This region is of particular interest for the analysis of this task because the lateral part of the OFC is activated by many aversive stimuli, and when behavior must be changed when reward is not received [Grabenhorst and Rolls, 2011; Kringelbach and Rolls, 2003; O'Doherty et al., 2001; Rolls, 2014; Rolls and Grabenhorst, 2008], and lesions of the (right) inferior frontal gyrus that are close to the contrast peaks in this region impair the performance of the SST [Aron et al., 2014]. (They described the region as inferior frontal gyrus, opercular part in the AAL atlas [Rolls et al., 2015; Tzourio‐Mazoyer et al., 2002].)
A hypothesis that is consistent with these findings and with the literature [Grabenhorst and Rolls, 2011; Kringelbach and Rolls, 2003; Rolls, 2014, 2016c; Rolls and Grabenhorst, 2008] is that this lateral OFC/inferior frontal gyrus BA47/12 region is involved in the stop‐related processing that inhibits the action. Indeed, the activation of the lateral OFC/VLPFC in SS–SF (Fig. 2a) is very similar indeed to that in the lateral OFC on reversal trials in visual discrimination reversal, when nonreward is obtained and behavior must change [Kringelbach and Rolls, 2003] (with a similar lateral OFC area activated in macaques [Chau et al., 2015]). At the neuronal level, error‐related neurons that respond when behavior must be changed due to nonreward or punishment are found in the OFC [Thorpe et al., 1983], and are part of the mechanism for the change of behavior, in that reversal learning is impaired by damage to this region [Grabenhorst and Rolls, 2011; Hornak et al., 2004; Rolls, 2014; Rolls and Grabenhorst, 2008; Rolls et al., 1994]. The functional connectivity of this region with the temporal cortical visual areas [Cheng et al., 2016] provides interesting evidence on the region of the VLPFC that receives visual inputs, consistent with anatomical evidence [Pandya et al., 2015; Petrides et al., 2012; Yeterian et al., 2012], and indeed is likely to be the route via which the visual stop signal reaches the VLPFC BA 47/12. Possible routes for this lateral OFC system to influence action include (1) via the opercular and nearby parts of the inferior frontal gyrus (area 44) which in turn has connections with the preSMA (the presupplementary motor area, referred to in this article as medial prefrontal cortex/area 8), both of which are implicated in this response inhibition task [Rae et al., 2015]; (2) via subcortical structures such as the striatum/basal ganglia (caudate, putamen, ventral striatum, globus pallidus), which are activated (Tables 1 and 2) and implicated [Rae et al., 2015] in this task; and (3) via connections to the ACC [Grabenhorst and Rolls, 2011; Rolls, 2014]. It is notable that aspects of social cognition dependent on this inferior frontal gyrus region are developing rapidly during the stage of adolescence investigated here [Kilford et al., 2016].
A second of these areas activated by the contrast SS–SF is the DLPFC (Fig. 2b). A hypothesis that is consistent with the literature [Fuster, 2001, 2008; Goldman‐Rakic, 1996; Rolls, 2008, 2016a] is that this region is involved in short‐term memory and attention, so that greater activation of this region on SS–SF may reflect the influence of good attention on the performance of the SST. In more detail, the DLPFC (BA9/10/46) and VLPFC (BA44/45) regions are implicated in working memory [Goldstein et al., 2011], with VLPFC/BA44/45 maintaining information and DLPFC monitoring and manipulating information [Petrides, 1994, 1995]. The monitoring function of the DLPFC is reported to serve behavior control [Cieslik et al., 2013], such as when we need to adapt our behavior to a changing environment, override habitual responses, or shift between different tasks [Hoshi, 2006; Mansouri et al., 2009; Miller and Cohen, 2001; Passingham and Sakai, 2004], which supports the hypothesis that the DLPFC plays an important role in the SST. Further, the right DLPFC is especially implicated in working memory [Goldstein et al., 2011; Kane and Engle, 2002], monitoring [Shallice, 2004; Vogt et al., 2007], and resolution of conflict during motor response execution [Aron et al., 2003, 2004; Nee, et al., 2007], all of which are consistent with research linking hemispheric lateralization with type of task (e.g., spatial with right, verbal with left) [Petrides, 1994, 1995]. This evidence is consistent with the findings of the present analysis with larger effects in the right DLPFC, for the SST involves processing of spatial information provided by arrows on the screen that direct responses made by the hand.
Second, we consider areas that were activated in the strength order SF > SS. More precisely, we performed a two‐sample t‐test between the pair of conditions (SF, SS) for the average activation strength in the regions of AI and ACC separately. For AI, SF > SS (t = 2.7, P = 0.0063). For ACC, SF > SS (t = 3.7, P = 2.1* 10−4). We also performed the interaction analysis illustrated in Figure 4, which strongly supports the dissociation (P‐value = 5.61 * 10−8). These areas of especial interest in this task were the anterior cingulate region and the AI. The fact that these areas had activations in the order just described is consistent with the hypothesis that they are involved in the emotional processes that occur when failure occurs, and in the emotional processes that occur when an action must be aborted, with the smallest activation on trials on which the action proceeds to completion. On the failure trials, an error in an action has occurred, and this may produce an emotional response. This hypothesis is consistent with the evidence that the part of the ACC just dorsal and posterior to the genu of the corpus callosum is the cingulate region in which many aversive and unpleasant events are represented, including unpleasant tastes, odors and flavors, painful stimuli, and changing behavior when an expected visual stimulus is not obtained in the reversal of a visual discrimination task [de Araujo et al., 2003; Grabenhorst and Rolls, 2011; Kringelbach and Rolls, 2003; Rolls, 2014; Rolls et al., 2003a, 2003b]. The anterior and midcingulate cortex are implicated in action‐outcome learning, that is learning whether actions are associated with reward or punishment [Grabenhorst and Rolls, 2011; Rolls, 2014; Rushworth et al., 2011], and contains neurons that respond when errors in actions occur and rewards are reduced [Niki and Watanabe, 1979; Shima and Tanji, 1998]. The pregenual cingulate cortex in contrast was not activated in the SST, consistent with the evidence that for comparison its activations are related to rewarding, subjective pleasant stimuli [Grabenhorst and Rolls, 2011; Rolls, 2014; Rolls and Grabenhorst, 2008], and not to correcting errors such as having to change behavior, in for example reversal, or to aversive stimuli [Grabenhorst and Rolls, 2011; Kringelbach and Rolls, 2003; Rolls, 2014]. Parts of the AI located anterior and ventral to the insular taste cortex are part of the visceral cortex involved in autonomic responses [Critchley, 2005; Critchley and Harrison, 2013; Rolls, 2014, 2015b, 2016b; Simmons et al., 2013], which will be engaged by emotional states such as failure in a task. This anterior cingulate – AI system has also been termed a “saliency” network [Menon and Uddin, 2010], and salient stimuli may often be aversive, and are likely to produce autonomic responses [Rolls, 2016b]. Indeed, we note that the hypothesis described here is different from (and more parsimonious than) the hypothesis of Menon et al. [Cai et al., 2014; Menon and Uddin, 2010], that the AI is involved in “salience detection.” (It is noted that activations to “salience detection” might alternatively be cast as activations to rewarding, punishing, and novel stimuli all of which might elicit behavioral and autonomic output [Rolls, 2014, 2016b].)
A highlight of this study is that the large number of participants enabled voxel‐level analysis of the data in a purely data driven way, with no a priori hypotheses on particular brain regions that could limit the results. This makes the results very robust, as shown by the significance values obtained.
In the context of previous research, differences of the present study from previous investigations of the SST enable it to go beyond previous studies which had in general far fewer participants and/or used a priori regions and/or which combined results from several tasks [Aron et al., 2014; Boehler et al., 2010; Cai et al., 2014; Duann et al., 2009; Xue et al., 2008; Zhang and Li, 2012]. Further, differences from a previous investigation of inhibition [Cai et al., 2014] are that in the present investigation meta‐analysis was not needed to identify a priori regions of interest for the analysis as there is a single set of 1709 participants in the present study so enabling an unbiased brain‐wide voxel‐level study, allowing very much more statistical power and potentially significance of the findings; that our analysis is entirely based on task‐related data with 1709 participants; and in that we focused on analysis in a single task, rather than including data from a number of different tasks. This investigation differs from an earlier investigation of an overlapping dataset [Whelan et al., 2012] by focusing on how different brain systems contribute to different processes involved in response inhibition, and its failure. Another strength of the present investigation is that use of the IMAGEN database allowed the data to be collected from a large cohort of individuals all of a similar adolescent age, so that age‐related factors did not need to be regressed out of the analysis.
A limitation of this investigation is that it would have been of interest to include a different type of task requiring behavioral change, to allow direct comparison. The SST required a response to be changed: stopped. A reward reversal task requires the association of a stimulus with reward to be changed, and this will change whether that stimulus is selected in future. This type of learning is stimulus‐reward association and reversal, and requires a stimulus‐stimulus association to be changed, where the second stimulus is the reward outcome [Rolls, 2014; Rolls and Deco, 2016]. Neurons in the primate OFC respond to this nonreward signal, which is a mismatch between what is expected and what is obtained as the outcome; and the reward expectation neurons reverse the stimulus to which they respond [Thorpe et al., 1983]. The crucial part of the OFC for this reward reversal as shown by activation studies is the lateral OFC [Chau et al., 2015; Kringelbach and Rolls, 2003; Rolls, 2014, 2016c]. This concept of a non‐reward and punishment system involving the lateral OFC [Rolls, 2016a] has been developed into a theory of depression in which the nonreward attractor‐related firing of the OFC neurons is over‐responsive in depression [Rolls, 2016c, 2017]. The interesting point here is whether there is a similar system in the lateral OFC for nonreward detection, which indicates that a reward association should be changed, and for the detection of a stop or error signal informing the participant that a response should be changed. Although it is a limitation that this could not be addressed in this analysis by a direct comparison in the same participants, this study does make the important new point that the SST and reward reversal are tasks that require similar computations of an error, which in turn leads to behavioral change, and both involved lateral OFC areas. It will be interesting in future work to examine this relationship further, by including both tasks in the same study, or be recording single neuron activity in the OFC in both types of task.
The findings are of interest in that performance in the SST provides evidence about behavioral inhibition and impulsiveness, and the performance of the task is impaired in ADHD [Aron et al., 2014]. The finding described here that the lateral OFC is a region implicated in behavioral change of the response inhibition type is important for this helps to focus attention on this brain region as a key region involved in a number of behavioral change, nonreward, and reward reversal functions that are fundamental to social and emotional behavior, and in impulsive behavior [Aron et al., 2014; Rolls, 2014, 2016c]. An important finding of this study for the understanding of the mechanisms of behavioral change is that the region most implicated in this is a ventrolateral part of the prefrontal cortex which is lateral OFC area BA47/12 [Öngür et al., 2003; Öngür and Price, 2000]. This is relevant for example to the brain mechanisms that are different in patients with depression, who have increased functional connectivity of parts of BA47/12 with the precuneus, angular gyrus, and inferior temporal cortex [Cheng et al., 2016], consistent with the hypothesis of overactive networks in depression in this region which is implicated in nonreward and behavioral change [Rolls, 2016c].
AUTHOR CONTRIBUTIONS
WD performed the analyses of the data, and cowrote the article. ETR worked with WD throughout the data analyses, and cowrote the article. XJ performed the initial analyses of the data. JF initiated and directed the research and the methodological analyses. TWR participated in discussions on the results. Members of the IMAGEN consortium performed the data acquisition.
DISCLOSURES
Dr. Banaschewski has served as an advisor or consultant to Bristol‐Myers Squibb, Desitin Arzneimittel, Eli Lilly, Medice, Novartis, Pfizer, Shire, UCB, and Vifor Pharma; he has received conference attendance support, conference support, or speaking fees from Eli Lilly, Janssen McNeil, Medice, Novartis, Shire, and UCB; and he is involved in clinical trials conducted by Eli Lilly, Novartis, and Shire; the present work is unrelated to these relationships. Dr. Gallinat has received research funding from the German Federal Ministry of Education and Research, AstraZeneca, Eli Lilly, Janssen‐Cilag, and Bristol‐Myers Squibb; he has received speaking fees from AstraZeneca, Janssen‐Cilag, and Bristol‐Myers Squibb. The other authors report no biomedical financial interests or potential conflicts of interest.
ACKNOWLEDGMENTS
J. Feng is a Royal Society Wolfson Research Merit Award holder.
REFERENCES
- Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW (2003): Stop‐signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat Neurosci 6:115–116. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA (2004): Inhibition and the right inferior frontal cortex. Trends Cogn Sci 8:170–177. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA (2014): Inhibition and the right inferior frontal cortex: One decade on. Trends Cogn Sci 18:177–185. [DOI] [PubMed] [Google Scholar]
- Boehler CN, Appelbaum LG, Krebs RM, Hopf JM, Woldorff MG (2010): Pinning down response inhibition in the brain–conjunction analyses of the Stop‐signal task. Neuroimage 52:1621–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W, Ryali S, Chen T, Li CS, Menon V (2014): Dissociable roles of right inferior frontal cortex and anterior insula in inhibitory control: Evidence from intrinsic and task‐related functional parcellation, connectivity, and response profile analyses across multiple datasets. J Neurosci 34:14652–14667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau BK, Sallet J, Papageorgiou GK, Noonan MP, Bell AH, Walton ME, Rushworth MF (2015): Contrasting roles for orbitofrontal cortex and amygdala in credit assignment and learning in macaques. Neuron 87:1106–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng W, Rolls ET, Qiu J, Liu W, Tang Y, Huang CC, Wang X, Zhang J, Lin W, Zheng L, Pu J, Tsai SJ, Yang AC, Lin CP, Wang F, Xie P, Feng J (2016): Medial reward and lateral non‐reward orbitofrontal cortex circuits change in opposite directions in depression. Brain 139:3296–3309. [DOI] [PubMed] [Google Scholar]
- Cieslik EC, Zilles K, Caspers S, Roski C, Kellermann TS, Jakobs O, Langner R, Laird AR, Fox PT, Eickhoff SB (2013): Is there “one” DLPFC in cognitive action control? Evidence for heterogeneity from co‐activation‐based parcellation. Cereb Cortex 23:2677–2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD (2005): Neural mechanisms of autonomic, affective, and cognitive integration. J Comp Neurol 493:154–166. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Harrison NA (2013): Visceral influences on brain and behavior. Neuron 77:624–638. [DOI] [PubMed] [Google Scholar]
- de Araujo IET, Kringelbach ML, Rolls ET, Hobden P (2003): The representation of umami taste in the human brain. J Neurophysiol 90:313–319. [DOI] [PubMed] [Google Scholar]
- Duann JR, Ide JS, Luo X, Li CS (2009): Functional connectivity delineates distinct roles of the inferior frontal cortex and presupplementary motor area in stop signal inhibition. J Neurosci 29:10171–10179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster JM (2001): The prefrontal cortex ‐ an update: Time is of the essence. Neuron 30:319–333. [DOI] [PubMed] [Google Scholar]
- Fuster JM (2008) The Prefrontal Cortex. London: Academic Press. [Google Scholar]
- Goldman‐Rakic PS (1996): The prefrontal landscape: Implications of functional architecture for understanding human mentation and the central executive. Philos Trans R Soc B 351:1445–1453. [DOI] [PubMed] [Google Scholar]
- Goldstein KE, Hazlett EA, Savage KR, Berlin HA, Hamilton HK, Zelmanova Y, Look AE, Koenigsberg HW, Mitsis EM, Tang CY, McNamara M, Siever LJ, Cohen BH, New AS (2011): Dorso‐ and ventro‐lateral prefrontal volume and spatial working memory in schizotypal personality disorder. Behav Brain Res 218:335–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabenhorst F, Rolls ET (2011): Value, pleasure, and choice in the ventral prefrontal cortex. Trends Cogn Sci 15:56–67. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B (2010): The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology 35:4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornak J, O'Doherty J, Bramham J, Rolls ET, Morris RG, Bullock PR, Polkey CE (2004): Reward‐related reversal learning after surgical excisions in orbitofrontal and dorsolateral prefrontal cortex in humans. J Cogn Neurosci 16:463–478. [DOI] [PubMed] [Google Scholar]
- Hoshi E (2006): Functional specialization within the dorsolateral prefrontal cortex: A review of anatomical and physiological studies of non‐human primates. Neurosci Res 54:73–84. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Engle RW (2002): The role of prefrontal cortex in working‐memory capacity, executive attention, and general fluid intelligence: An individual‐differences perspective. Psychon Bull Rev 9:637–671. [DOI] [PubMed] [Google Scholar]
- Kilford EJ, Garrett E, Blakemore SJ (2016): The development of social cognition in adolescence: An integrated perspective. Neurosci Biobehav Rev 70:106–120. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET (2003): Neural correlates of rapid reversal learning in a simple model of human social interaction. Neuroimage 20:1371–1383. [DOI] [PubMed] [Google Scholar]
- Mansouri FA, Tanaka K, Buckley MJ (2009): Conflict‐induced behavioural adjustment: A clue to the executive functions of the prefrontal cortex. Nat Rev Neurosci 10:141–152. [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin LQ (2010): Saliency, switching, attention and control: A network model of insula function. Brain Struct Funct 214:655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Cohen JD (2001): An integrative theory of prefrontal cortex function. Annu Rev Neurosci 24:167–202. [DOI] [PubMed] [Google Scholar]
- Nee DE, Wager TD, Jonides J (2007): Interference resolution: Insights from a meta‐analysis of neuroimaging tasks. Cogn Affect Behav Neurosci 7:1–17. [DOI] [PubMed] [Google Scholar]
- Niki H, Watanabe M (1979): Prefrontal and cingulate unit activity during timing behavior in the monkey. Brain Res 171:213–224. [DOI] [PubMed] [Google Scholar]
- Nymberg C, Jia T, Lubbe S, Ruggeri B, Desrivieres S, Barker G, Buchel C, Fauth‐Buehler M, Cattrell A, Conrod P, Flor H, Gallinat J, Garavan H, Heinz A, Ittermann B, Lawrence C, Mann K, Nees F, Salatino‐Oliveira A, Paillere Martinot ML, Paus T, Rietschel M, Robbins T, Smolka M, Banaschewski T, Rubia K, Loth E, Schumann G, Consortium I (2013): Neural mechanisms of attention‐deficit/hyperactivity disorder symptoms are stratified by MAOA genotype. Biol Psychiatry 74:607–614. [DOI] [PubMed] [Google Scholar]
- O'Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C (2001): Abstract reward and punishment representations in the human orbitofrontal cortex. Nat Neurosci 4:95–102. [DOI] [PubMed] [Google Scholar]
- Öngür D, Price JL (2000): The organisation of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex 10:206–219. [DOI] [PubMed] [Google Scholar]
- Öngür D, Ferry AT, Price JL (2003): Architectonic division of the human orbital and medial prefrontal cortex. J Comp Neurol 460:425–449. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Seltzer B, Petrides M, Cipolloni PB (2015) Cerebral Cortex: Architecture, Connections, and the Dual Origin Concept. New York: Oxford University Press. [Google Scholar]
- Passingham D, Sakai K (2004): The prefrontal cortex and working memory: Physiology and brain imaging. Curr Opin Neurobiol 14:163–168. [DOI] [PubMed] [Google Scholar]
- Petrides M (1994): Frontal lobes and working memory: Evidence from investigations of the effects of cortical excisions in nonhuman primates. Handbook Neuropsychol 9:59–82. [Google Scholar]
- Petrides M (1995): Functional organization of the human frontal cortex for mnemonic processing. Evidence from neuroimaging studies. Ann N Y Acad Sci 769:85–96. [DOI] [PubMed] [Google Scholar]
- Petrides M, Tomaiuolo F, Yeterian EH, Pandya DN (2012): The prefrontal cortex: Comparative architectonic organization in the human and the macaque monkey brains. Cortex 48:46–57. [DOI] [PubMed] [Google Scholar]
- Rae CL, Hughes LE, Anderson MC, Rowe JB (2015): The prefrontal cortex achieves inhibitory control by facilitating subcortical motor pathway connectivity. J Neurosci 35:786–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET (2008): Memory, Attention, and Decision‐Making: A Unifying Computational Neuroscience Approach. Oxford: Oxford University Press. [Google Scholar]
- Rolls ET (2014): Emotion and Decision‐Making Explained. Oxford: Oxford University Press. [Google Scholar]
- Rolls ET (2015a): Limbic systems for emotion and for memory, but no single limbic system. Cortex 62:119–157. [DOI] [PubMed] [Google Scholar]
- Rolls ET (2015b): Taste, olfactory, and food reward value processing in the brain. Prog Neurobiol 127‐128:64–90. [DOI] [PubMed] [Google Scholar]
- Rolls ET (2016a): Cerebral Cortex: Principles of Operation. Oxford: Oxford University Press. [Google Scholar]
- Rolls ET (2016b): Functions of the anterior insula in taste, autonomic, and related functions. Brain Cogn 110:4–19. [DOI] [PubMed] [Google Scholar]
- Rolls ET (2016c): A non‐reward attractor theory of depression. Neurosci Biobehav Rev 68:47–58. [DOI] [PubMed] [Google Scholar]
- Rolls ET (2017): The roles of the orbitofrontal cortex via the habenula in non‐reward and depression, and in the responses of serotonin and dopamine neurons. Neurosci Biobehav Rev 75:331–334. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Deco G (2016): Non‐reward neural mechanisms in the orbitofrontal cortex. Cortex 83:27–38. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Grabenhorst F (2008): The orbitofrontal cortex and beyond: From affect to decision‐making. Prog Neurobiol 86:216–244. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Hornak J, Wade D, McGrath J (1994): Emotion‐related learning in patients with social and emotional changes associated with frontal lobe damage. J Neurol Neurosurg Psychiatry 57:1518–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, Kringelbach ML, de Araujo IET (2003a): Different representations of pleasant and unpleasant odors in the human brain. Eur J Neurosci 18:695–703. [DOI] [PubMed] [Google Scholar]
- Rolls ET, O'Doherty J, Kringelbach ML, Francis S, Bowtell R, McGlone F (2003b): Representations of pleasant and painful touch in the human orbitofrontal and cingulate cortices. Cereb Cortex 13:308–317. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Joliot M, Tzourio‐Mazoyer N (2015): Implementation of a new parcellation of the orbitofrontal cortex in the automated anatomical labeling atlas. Neuroimage 122:1–5. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Noonan MP, Boorman ED, Walton ME, Behrens TE (2011): Frontal cortex and reward‐guided learning and decision‐making. Neuron 70:1054–1069. [DOI] [PubMed] [Google Scholar]
- Shallice T. (2004) The Fractionation of Supervisory Control. Cambridge, MA: MIT Press. [Google Scholar]
- Shima K, Tanji J (1998): Role for cingulate motor area cells in voluntary movement selection based on reward. Science 282:1335–1338. [DOI] [PubMed] [Google Scholar]
- Simmons WK, Avery JA, Barcalow JC, Bodurka J, Drevets WC, Bellgowan P (2013): Keeping the body in mind: Insula functional organization and functional connectivity integrate interoceptive, exteroceptive, and emotional awareness. Hum Brain Mapp 34:2944–2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe SJ, Rolls ET, Maddison S (1983): Neuronal activity in the orbitofrontal cortex of the behaving monkey. Exp Brain Res 49:93–115. [DOI] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. Neuroimage 15:273–289. [DOI] [PubMed] [Google Scholar]
- Vogt S, Buccino G, Wohlschlager AM, Canessa N, Shah NJ, Zilles K, Eickhoff SB, Freund HJ, Rizzolatti G, Fink GR (2007): Prefrontal involvement in imitation learning of hand actions: Effects of practice and expertise. Neuroimage 37:1371–1383. [DOI] [PubMed] [Google Scholar]
- Whelan R, Conrod PJ, Poline JB, Lourdusamy A, Banaschewski T, Barker GJ, Bellgrove MA, Buchel C, Byrne M, Cummins TD, Fauth‐Buhler M, Flor H, Gallinat J, Heinz A, Ittermann B, Mann K, Martinot JL, Lalor EC, Lathrop M, Loth E, Nees F, Paus T, Rietschel M, Smolka MN, Spanagel R, Stephens DN, Struve M, Thyreau B, Vollstaedt‐Klein S, Robbins TW, Schumann G, Garavan H, Consortium I (2012): Adolescent impulsivity phenotypes characterized by distinct brain networks. Nat Neurosci 15:920–925. [DOI] [PubMed] [Google Scholar]
- Xue G, Aron AR, Poldrack RA (2008): Common neural substrates for inhibition of spoken and manual responses. Cereb Cortex 18:1923–1932. [DOI] [PubMed] [Google Scholar]
- Yeterian EH, Pandya DN, Tomaiuolo F, Petrides M (2012): The cortical connectivity of the prefrontal cortex in the monkey brain. Cortex 48:58–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Li CS (2012): Functional networks for cognitive control in a stop signal task: Independent component analysis. Hum Brain Mapp 33:89–104. [DOI] [PMC free article] [PubMed] [Google Scholar]


