Abstract
Initially identified during no‐task, baseline conditions, it has now been suggested that the default mode network (DMN) engages during a variety of working memory paradigms through its flexible interactions with other large‐scale brain networks. Nevertheless, its contribution to whole‐brain connectivity dynamics across increasing working memory load has not been explicitly assessed. The aim of our study was to determine which DMN hubs relate to working memory task performance during an fMRI‐based n‐back paradigm with parametric increases in difficulty. Using a voxel‐wise metric, termed the intrinsic connectivity contrast (ICC), we found that the bilateral angular gyri (core DMN hubs) displayed the greatest change in global connectivity across three levels of n‐back task load. Subsequent seed‐based functional connectivity analysis revealed that the angular DMN regions robustly interact with other large‐scale brain networks, suggesting a potential involvement in the global integration of information. Further support for this hypothesis comes from the significant correlations we found between angular gyri connectivity and reaction times to correct responses. The implication from our study is that the DMN is actively involved during the n‐back task and thus plays an important role related to working memory, with its core angular regions contributing to the changes in global brain connectivity in response to increasing environmental demands. Hum Brain Mapp 38:41–52, 2017. © 2016 Wiley Periodicals, Inc.
Keywords: default mode network, angular gyrus, intrinsic connectivity contrast, functional connectivity, working memory, behavioral performance, n‐back
INTRODUCTION
The past two decades have seen a surge in the number of neuroimaging studies characterizing the functional and structural organization of the human brain into distinct networks with diverse and interacting roles. Initially identified during resting‐state conditions, the exact cognitive relevance of these large‐scale brain networks (LSNs) remains to be elucidated (Vincent, 2009). However, their spatial similarity to known activation maps from controlled tasks suggests a potential significance in cognitive processing (Smith et al., 2009), which has given rise to the LSN nomenclature such as fronto‐parietal, dorsal attention, and somatomotor networks.
One key network that continues to be at the center of an on‐going debate on the significance of brain processing at rest (Morcom and Fletcher, 2007; Raichle and Snyder, 2007) is the default mode network (DMN) (Gusnard et al., 2001). Comprising the posterior cingulate, medial prefrontal cortices, and the bilateral angular gyri, the DMN has been reliably and consistently detected during passive or baseline conditions (Greicius et al., 2003; Laird et al., 2009). Nonetheless, recent findings have also highlighted the potential contribution of DMN to task execution (Vatansever et al., 2015b), especially in paradigms that require memory‐based processing (Buckner et al., 2008), which may further our understanding of behaviorally unconstrained rest states. Greater DMN activity/connectivity has been demonstrated in various experimental paradigms that span autobiographical memory retrieval, future planning, moral judgment, empathizing and reading comprehension (Andrews‐Hanna et al., 2014; Buckner et al., 2008; Smallwood et al., 2012). Moreover, recent studies increasingly implicate the DMN in core working memory processes (Fransson, 2006; Konishi et al., 2015; Piccoli et al., 2015; Spreng et al., 2014; Vatansever et al., 2015a), which may explain the observed engagement of this network in a wide variety of cognitive tasks.
Baddeley and Hitch described working memory as a cognitive system that guides behavior by enabling the temporary storage of information for on‐line retention, update, and manipulation (Baddeley and Hitch, 1974). Evaluating the potential involvement of the DMN in this core mental function, Spreng et al. demonstrated greater DMN activity while participants matched famous as compared to anonymous faces in a novel n‐back task. The results of this study indicated DMN engagement in working memory paradigms when the task goals were congruent with long‐term memory stores commonly associated with this network (Spreng et al., 2014). Further expanding this observation, Konishi et al. reported greater DMN activity during the 1‐Back in comparison to 0‐Back conditions of a perceptual n‐back task (Konishi et al., 2015), suggesting a DMN role that extends beyond its associations with long‐term memory and self‐referential processing.
From a functional connectivity perspective, most studies have compared resting state to n‐back task conditions, largely focusing on the seed‐based connectivity between posterior cingulate and medial prefrontal cortices. The connectivity strength between these two DMN hubs was shown to correlate with percent correct responses (Hampson et al., 2006), and such within network connectivity alterations were reported to persist beyond task disengagement (Gordon et al., 2014). Alternative methodological approaches (ICA) have likewise been used to further investigate within DMN hub connectivity during n‐back task execution, which suggested that alterations in anterior and posterior midline cingulate connections were positively related to the number of correct responses from 0‐Back to 2‐Back conditions (Esposito et al., 2009, 2006). Moreover, during working memory task execution greater interactions have been observed between the anterior/posterior DMN areas, and regions commonly associated with working memory such as bilateral inferior frontal gyri (Bluhm et al., 2011). In fact, such DMN interactions were shown to predict behavioral performance (Hampson et al., 2010) and to display variable dynamics during the three distinct working memory stages of encoding, maintenance, and retrieval (Piccoli et al., 2015).
Despite the mounting evidence for DMN engagement in working memory, what remains to be precisely assessed is the contribution of the core DMN regions to the alterations in global connectivity dynamics with parametric increase in working memory load. A recent study employing the Wason Selection Task (with serial card presentation) provided clues to this question by revealing greater cooperation between the angular gyri and striatum as the task complexity increased (Hearne et al., 2015), suggesting that the angular DMN regions may be mediating connectivity dynamics associated with the external demands on working memory. From a clinical viewpoint, age‐related alterations in the DMN were shown to affect working memory task performance (Sambataro et al., 2010) and DMN regions have been linked to Alzheimer's disease (Buckner et al., 2009). Therefore, delineating the precise functional involvement of DMN regions with greater working memory demands is crucial in furthering our understanding of the cognitive relevance of LSNs in healthy brain processing, and in expanding our knowledge on the DMN's potential breakdown in memory‐related disorders.
We investigated the changes in global functional connectivity during task execution in an fMRI‐based n‐back working memory paradigm with varying degrees of difficulty from 1‐Back to 3‐Back. Our major objectives in this study were to (a) identify the core DMN regions that displayed working memory load‐dependent changes in global functional connectivity by using an objective voxel‐based metric, termed intrinsic connectivity contrast (ICC) (Martuzzi et al., 2011), (b) investigate changes in the interactions of such regions with areas commonly associated with working memory, and finally (c) assess their correlation with task performance. We hypothesized that any observed decrease in working performance with increasing task difficulty would reveal global connectivity changes that would be reflected by task‐induced alterations in the DMN's widespread functional interactions.
MATERIALS AND METHODS
Participants
Following the presentation of a study specific information pack, informed consent was obtained from 22 healthy participants (19–57 years old, mean = 35.0, SD = 11.2, 9/13 female‐to‐male ratio) with average scores of 117.1 (SD = 5.76) on the National Adult Reading Test (NART) and 29.33 (SD = 0.85) on the Mini Mental State Exam (MMSE), showing normal verbal intelligence and no signs of cognitive impairment. None of the participants had a history of drug or alcohol abuse, psychiatric or neurological disorders, or head injury.
Paradigm Specifications
Data from four fMRI paradigms were acquired as part of a larger study. For the n‐back working memory paradigm, the participants were presented with five cycles of four n‐back task blocks ranging in difficulty from 0 to 3‐Back, pseudo‐randomly interleaved with three fixation blocks. For the task blocks, single letters in white font were flashed on a black background (500 ms) followed by a fixation on a cross (2,500 ms). Instructions were displayed on the screen (10,000 ms) between each block. In the 0‐Back trials, the participants had to press a button when the letter Z appeared in a string of random letters presented serially. On the other hand, higher levels of n‐back difficulty required a button press when the current letter matched the letter presented one letter previously (1‐Back), two letters previously (2‐Back), and three letters previously (3‐Back), respectively. Furthermore, to ensure sustained attention throughout the experiment, the participants were required to respond to the nontargets by pressing a button under their middle finger.
The primary objective in this study was to investigate the DMN areas associated with the highest change in global brain connectivity with increasing working memory load in the n‐back paradigm. For this purpose, we omitted the fixation and control conditions that do not impose high demands on working memory load and instead analyzed the 1‐, 2‐, and 3‐Back conditions. Using a repeated measures ANCOVA with age as a covariate, these three blocks were assessed for an expected reduction in the d' metric based on the signal detection theory for task accuracy (Green and Swets, 1974), and an increase in latency to correct responses with greater task difficulty (Kitzbichler et al., 2011).
Image Acquisition
The MRI scanning was carried out using a Siemens MAGNETOM Tim Trio 3T scanner at the Wolfson Brain Imaging Centre, Addenbrooke's Hospital, Cambridge. In addition to a high resolution T 1‐weighted, magnetization‐prepared 180° radio‐frequency pulses, and rapid gradient‐echo (MPRAGE) structural scan [TR = 2300 ms; TE = 2.98 ms; TA = 9.14 min; flip angle = 9°; field of view (FOV) read = 256 mm; voxel size = 1.0 × 1.0 × 1.0 mm, slices per slab = 176], a whole‐brain echo planar imaging (EPI) sequence was performed for the N‐back paradigm (TR = 2000 ms; TE = 30 ms; flip angle = 78°; FOV read = 192 mm; voxel size = 3.0 × 3.0 × 3.0 mm; slices per volume= 32).
Spatial Preprocessing
All the preprocessing stages and statistical analyses of the acquired MRI data were performed using Statistical Parametric Mapping (SPM) Version 8.0 (http://www.fil.ion.ucl.ac.uk/spm/) and MATLAB Version 12a platforms (http://www.mathworks.co.uk/products/matlab/). To account for saturation effects and steady‐state magnetization we removed the first five volumes. Subsequently, the data were slice‐time adjusted and corrected for motion artifacts. Co‐registered to the mean fMRI image, structural images were segmented into gray/white matter and cerebrospinal fluid probability maps, and spatially normalized to Montreal Neurological Institute (MNI) space using the segmented high‐resolution structural gray matter image and an a priori template. This procedure utilized the unified segmentation–normalization framework, which combines tissue segmentation, bias correction, and spatial normalization in a single unified model (Ashburner and Friston, 2005). Finally, the data were smoothed with an 8‐mm FWHM Gaussian kernel and was carried on to further statistical analysis.
Confound Removal and Temporal Preprocessing
A strict nuisance regression method included motion artifacts, a linear detrending term and CompCor components attributable to the white matter and cerebrospinal fluid (Behzadi et al., 2007). This procedure eliminated the need for global signal normalization (Chai et al., 2012; Murphy et al., 2009). The subject‐specific six realignment parameters, the main effects of task conditions, and their first‐order derivatives were also included in the statistical analysis as potential confounds (Fair et al., 2007), and a temporal filter ranging between 0.009 and 0.08 Hz was applied (Fox et al., 2005).
Intrinsic Connectivity Contrast Analysis
The calculation of ICC maps and subsequent functional connectivity analyses were carried out utilizing the Conn functional connectivity toolbox (Whitfield‐Gabrieli and Nieto‐Castanon, 2012). Initially, we constructed regressors for each block type (fixation, 0, 1, 2, and 3‐Back) using onsets and durations. The block regressors for each task condition were then convolved with a rectified hemodynamic response function to account for the delay in hemodynamic response. Finally, time series for each block type were concatenated with the scans associated with nonzero effects and appropriately weighted. Such analysis pipeline de‐weights the beginning and end of each task block to eliminate the signal from nonbiological origins at the points of concatenation, minimizing the potential cross‐talk between adjacent task blocks (Whitfield‐Gabrieli and Nieto‐Castanon, 2012).
Subsequent to this time‐series concatenation procedure, voxel‐based global connectivity, based on the intrinsic connectivity contrast (ICC), was calculated for each condition. This novel measure utilizes graph theoretical metrics to objectively define how well each voxel is connected to the rest of the brain. An earlier version of this metric that solely relied on the network measure of degree has been previously employed in an Alzheimer's disease study to investigate the changes in the brain's cortical hubs (Buckner et al., 2009). Further extending this metric, Martuzzi et al. introduced a measure of connectivity strength by weighting the connections with their average r 2 value, therefore eliminating the need for an arbitrary threshold. The ICC score is a measure of the squared sum of mean correlations, with a greater ICC score representing greater average strength of the correlations in a given voxel. This method has been employed in a sedation study (Martuzzi et al., 2011) and produced connectivity results that were in agreement with the corresponding literature.
We created one ICC map for each participant at each level of n‐back task load ranging from 1‐Back to 3‐Back, which were entered into a within‐subject repeated‐measures ANOVA. The subsequent F‐contrast was conservatively corrected for multiple comparisons at the cluster level using family wise error (FWE) at the 0.05 level of significance (voxel level, 0.001 uncorrected).
Functional Connectivity and Behavioral Correlation Analyses
The statistically significant clusters from the ICC ANOVA (denoting the most significant global connectivity changes across task difficulty) were used as separate seed ROIs in functional connectivity analyses. The aim was to reveal the spatial extent of their connectivity changes and possible alterations in their relationship to other LSN regions with increasing task difficulty. For each level of difficulty, group level inferences were made using a one‐sample t‐test and the resulting statistical maps were cluster corrected at the FWE 0.05 level of significance (voxel level, 0.001 uncorrected). Furthermore, two repeated‐measures ANOVA were employed to identify the areas that showed connectivity changes to the seed ROIs with increasing task difficulty.
The final question to answer was whether the functional connectivity of these ROIs would correlate with behavioral performance. The behavioral measure we used was latency for correct responses, which was shown to be a reliable indicator of task performance in working memory paradigms (Kitzbichler et al., 2011). We used the maps obtained from the seed‐based functional connectivity analyses for each difficulty level in a repeated measures general linear model with the latencies as a covariate. The results were cluster corrected at the FWE 0.05 level of significance (voxel level, 0.001 uncorrected).
RESULTS
Change in Global Connectivity Centered on the Bilateral Angular Gyri
The initial analysis on the behavioral data using repeated‐measures ANCOVA with age as a covariate revealed the expected reduction in mean d' (F (2,19) = 15.24, P = 0.00013, mean 1‐Back = 3.25, 2‐Back = 2.96, 3‐Back = 2.19) and increase in mean reaction time to correct responses (F (2,19) = 9.72, P = 0.0012, mean 1‐Back = 756.50, 2‐Back = 921.44, 3‐Back = 958.12) with greater task difficulty, in line with previous publications (Owen et al., 2005). No significant interactions were detected between age and behavioral measures, suggesting no effect of age on our results.
Next, we used ICC to assess the changes in global brain connectivity across increasing task difficulty. First, the group ICC maps revealed that highly connected brain regions corresponded to LSNs as defined in the existing literature, e.g., default mode, dorsal attention, fronto‐parietal, visual, and somatomotor networks (Fig. 1A). An F‐contrast across the three experimental conditions (1‐, 2‐, 3‐Back) revealed two bilateral angular clusters (prominent DMN hubs) as the brain regions with the highest change in global connectivity with increasing working memory load (Fig. 1B). The left cluster [peak at MNIxyz: −30 −70 42] encompassed most of the angular gyrus but also extended to the middle occipital gyrus, and inferior/superior parietal gyri. The right cluster [peak at MNIxyz: 32 −74 42] spanned across the angular and middle/superior occipital gyri. It is worthwhile noting that the left angular cluster, which showed significant alterations in connectivity across task load in our study, overlaps with the dorsal subdivision of an angular system that is reportedly involved in a variety of semantic processing tasks (Noonan et al., 2013; Seghier et al., 2010). However, studies attributing similar functionality to the right angular cluster remain scarce.
Figure 1.
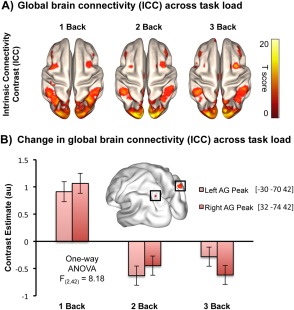
Global brain connectivity across increasing n‐back working memory load. (A) Group t‐tests for each memory load produced intrinsic connectivity contrast (ICC) maps illustrating the global brain connectivity profiles across three levels of working memory loads (1, 2, 3‐Back). The highly connected areas overlap with established LSNs from published literature. (B) The subsequent repeated measures ANOVA F‐contrast showed that the angular gyri (AG) were the brain areas with the greatest ICC change across the three experimental loads. All reported clusters are FWE corrected at the 0.05 level of significance (voxel 0.001 uncorrected) and are displayed on a smooth MNI152 brain. [Color figure can be viewed at http://wileyonlinelibrary.com.]
To confirm LSN membership of these clusters, we masked the result with an existing DMN template, from another study to avoid circularity (Thomason et al., 2011), and noted an overlap between the angular DMN components and the two clusters displaying maximum connectivity changes in response to the increase in task load.
Spatial Extent of Angular Gyri Connectivity across N‐Back Task Load
The next step aimed to reveal the spatial extent of the angular gyri functional connectivity, for which the seed ROIs were defined from significant clusters in the previous analysis. Our findings suggest that both left and right angular gyri were positively and negatively connected with aspects of the established LSNs (default mode, fronto‐parietal and dorsal attention networks) emphasizing their central role in global connectivity dynamics (Fig. 2A,B).
Figure 2.
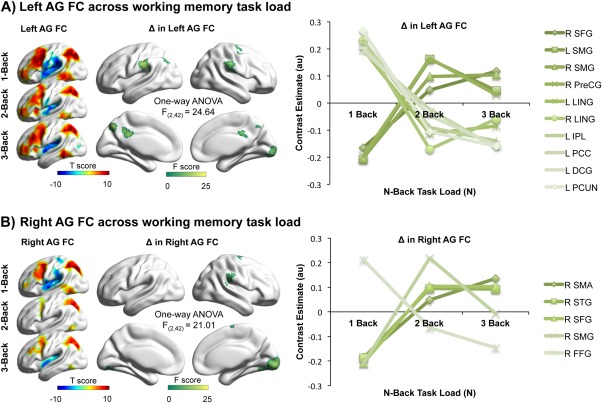
Functional connectivity (FC) of the (A) left and (B) right angular gyri (AG) across working memory load. Throughout the three n‐back conditions, both the left and the right angular gyri displayed strong functional connections with a variety of established LSN regions suggesting their central role in global connectivity dynamics. The red–yellow scale indicates positive correlation, while the green–blue scale shows negative correlations. All reported clusters are FWE corrected at the 0.05 level of significance (voxel 0.001 uncorrected). The one‐way ANOVA F‐contrast used to compute the change (Δ) in functional connectivity revealed divergent profiles for left and right seed ROIs (green‐white scale) with relative increases and decreases in their interactions with the rest of the brain. The brain regions that significantly changed their interaction with angular gyri are the superior frontal gyrus (SFG), supramarginal gyrus (SMG), precentral gyrus (PreCG), lingual gyrus (LING), inferior parietal lobule (IPL), posterior cingulate cortex (PCC), median cingulate gyrus (DCG), precuneus (PCUN), supplementary motor area (SMA), superior temporal gyrus (STG), and fusiform gyrus (FFG). [Color figure can be viewed at http://wileyonlinelibrary.com.]
Across the three task conditions, the left angular gyrus cluster revealed similar connectivity profiles correlating positively with fronto‐parietal network regions (dorsolateral frontal and posterior parietal cortices), as well as the dorsal posterior cingulate cortex and superior frontal gyrus, which are established DMN hubs. There were also negative connections from the same area to the insular and anterior cingulate cortices associated with the cingulo‐opercular network. Bilateral hippocampal connectivity persisted across the three experimental loads and correlated negatively with the left angular seed (Fig. 2A).
The repeated‐measures ANOVA, F‐contrast across the three different working memory loads revealed connectivity changes between the left angular gyrus and the superior frontal, supramarginal, precentral, lingual, inferior parietal, the posterior and median cingulate gyri, and precuneus (Fig. 2A). While the right superior frontal, bilateral supramarginal, and right precentral gyri increased their connectivity to the left angular gyrus with increasing cognitive load, the left inferior parietal, bilateral lingual, left posterior and median cingulate gyri, and precuneus decreased their connectivity across the three conditions, suggesting a divergence in the relationship between this major DMN hub and the rest of the brain.
The right angular gyrus demonstrated a similar connectivity profile to the left cluster, i.e., positive connectivity to the dorsolateral prefrontal and parietal cortices, and negative correlation to the anterior cingulate and insular cortices (Fig. 2B). The repeated‐measures ANOVA F‐contrast across the three conditions indicated that the right supplementary motor area, superior temporal, superior frontal, and supramarginal gyri increased, whereas the right fusiform gyrus decreased its connectivity to the right angular gyrus cluster (Fig. 2B) with increasing task difficulty.
Behavioral Correlation of Angular Gyri Connectivity
The final question we asked was whether the connectivity of these two angular ROIs would relate to working memory performance measured during task execution. A whole‐brain general linear model analysis revealed that weaker connectivity between the left angular gyrus and posterior cingulate cortex (part of the DMN) across three levels of working memory load correlated with slower reaction times to correct responses (P = 0.039, R 2 = 0.18) (Fig. 3A). Similarly, weaker connectivity of the right angular gyrus cluster with the left calcarine cortex (part of the visual network) also correlated with slower reaction times to correct responses (P = 0.037, R 2 = 0.17) (Fig. 3B). Together the results suggest a behavioral relevance for the connectivity of angular gyri during the n‐back working memory task.
Figure 3.
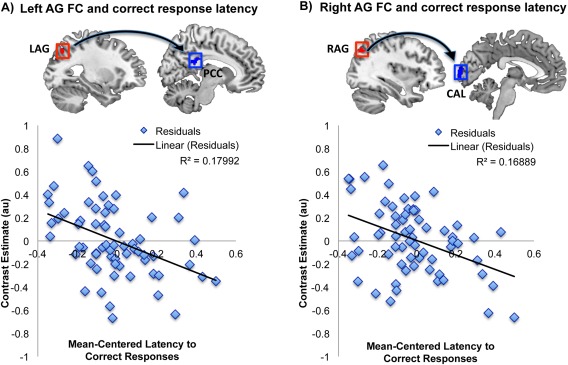
Behavioral significance of the (A) left and (B) right angular gyri (AG) functional connectivity across three levels of N‐back working memory task load. Using a repeated measures general linear model with latency to correct responses as the variable of interest the results indicated that across the three n‐back conditions weaker functional connectivity of the left angular gyrus to the posterior cingulate cortex (PCC) correlated with longer reaction times, thus worse performance (P = 0.039, R 2 = 0.18); the same relationship was observed between the right angular gyrus and left calcarine cortex (CAL) (P = 0.037, R 2 = 0.17). The reported results are FWE cluster corrected at the 0.05 level of significance (voxel 0.001 uncorrected). [Color figure can be viewed at http://wileyonlinelibrary.com.]
DISCUSSION
The aim of this study was to assess the precise involvement of the DMN in a working memory task with parametric increase in difficulty. We started data analysis with a functional connectivity technique (ICC) that does not require an a priori definition of a seed region, allowing us to examine the changes in global brain connectivity in response to increasing cognitive load. The ICC maps displayed connectivity profiles that overlapped with established LSNs, i.e., most of the regions implicated in default mode, salience, frontoparietal control, dorsal attention, somatomotor, auditory and visual networks displayed high temporal correlation with the rest of the brain. This confirmed that a relatively new technique produces sensible results, thus proving to be an effective voxel‐based metric for the assessment of global brain connectivity under varying experimental conditions, as was previously demonstrated in a sedation study by Martuzzi et al. (2011).
Working memory is a psychological construct that allows for the temporary storage, continuous update, and manipulation of information for cognitive processing. Meta‐analyses based on activation studies indicate that a distributed set of regions including the bilateral prefrontal, inferior parietal and superior temporal lobes are involved in the mediation of this function (Owen et al., 2005; Rottschy et al., 2012). Specific to the n‐back tasks, Owen et al. (2005) have provided evidence for greater activity observed on six main regions including the posterior parietal cortex, premotor cortex, dorsal cingulate, frontal poles, dorsolateral prefrontal cortex and mid ventrolateral prefrontal cortex during task execution in comparison with the chosen control conditions. Recently, a meta‐analysis by Rottschy et al. (2012) confirmed these findings using a wider range of working memory paradigms. They have indicated greater involvement of the left Broca's area (BA 44/45) in verbal as opposed to nonverbal working memory tasks, and greater engagement of the ventral Broca's area (BA 44/45), presupplementary motor area, left inferior temporal occipital cortex, lateral prefrontal cortex and premotor cortex in response to load‐dependent changes in working memory task demands. Thus, despite the variability that can be explained by the specifics of the employed working memory paradigm, converging evidence suggests the involvement of brain regions commonly associated with three main components of the working memory model, namely the phonological loop (specific to language processing), visuospatial sketchpad (visuospatial information) and central executive (Baddeley and Hitch, 1974; Owen et al., 2005; Rottschy et al., 2012).
From a functional connectivity perspective, such regions that are implicated in working memory make up an executive system that is known as the fronto‐parietal network (Vincent et al., 2008). However, recent studies also suggest an active DMN involvement in working memory paradigms (Esposito et al., 2009; Hampson et al., 2006; Spreng et al., 2014). In contrast to the false supposition on the task‐irrelevance of the DMN (Spreng, 2012), emerging studies suggest variable interactions between the DMN and LSNs during working memory task performance (Hampson et al., 2010; Piccoli et al., 2015; Vatansever et al., 2015a). Nevertheless, more studies are required to ascertain the specific contribution of DMN regions to the working memory load‐dependent changes in global brain connectivity. Given that the n‐back task in our study involved the rapid presentation of letters, we expected the engagement of fronto‐parietal network regions with greater task difficulty, and subsequent alterations in their interaction with the DMN regions.
To this end, a repeated‐measures ANOVA across the three experimental conditions revealed two main clusters with significant global connectivity changes centered on the left and right angular gyri, considered to be prominent DMN hubs (Buckner et al., 2008). The angular gyri are cross‐modal regions in the inferior parietal lobe, which together with other DMN regions, may act as connector hubs for global integration of information (de Pasquale et al., 2012). Anatomical connectivity data support this notion in that the angular regions display extensive structural connections with multiple remote brain regions (Hagmann et al., 2008). Specifically, they have direct connections to fronto‐opercular regions (Makris et al., 2005) as well as the posterior temporal areas (Makris et al., 2009), precuneus and superior frontal cortices (Makris et al., 2007), caudate and hippocampal regions (Uddin et al., 2010), and the supramarginal gyri (Lee et al., 2007).
Such diverse anatomical connectivity also mirrors the variety of functional roles that have been attributed to the angular gyri [see Seghier (2013) for a comprehensive review]. In addition to numerical processing (Dehaene et al., 2003), the angular gyri have been consistently implicated in semantic cognition (Jefferies, 2013), especially in reading/comprehension tasks (Binder et al., 2005; Graves et al., 2010; Price and Mechelli, 2005), coding for concrete as opposed to abstract concepts (Wang et al., 2010). Functional subdivisions of the left angular gyrus into dorsal/middle/ventral sections were recently described based on their differential involvement in the search for semantic associations amongst perceptual stimuli (Seghier et al., 2010) as well as episodic and semantic retrieval (Bonnici et al., 2016). In contrast, a precise functional delineation of the right angular gyrus remains scarce, but a potential dorsal/ventral subdivision was observed in a number processing task (Cappelletti et al., 2010).
Additionally, structural studies have revealed learning‐based changes in angular anatomy (Draganski et al., 2004) as well as a significant correlation between angular cortical thickness and creativity (Jung et al., 2010). Another function attributed to the angular gyri is the shifting of attention (Corbetta et al., 2008) toward salient and rewarding events (Gottlieb, 2007), a process informed by the accumulation of task history (Taylor et al., 2011). Last but not least, these core DMN regions have also shown consistent activation in theory‐of‐mind or mentalizing tasks (Li et al., 2014; Lombardo et al., 2010; Mar, 2011). The overall message from these studies is that the bilateral angular gyri are involved in a wide range of cognitive paradigms, similar to other default mode regions (Andrews‐Hanna et al., 2014). Moreover, a recent study that investigated the effects of task complexity on brain connectivity revealed alterations in DMN and control network interactions, centered on the angular DMN and striatal regions (Hearne et al., 2015). In line with these findings, the angular gyri in our study displayed significant changes in their global connectivity profile during an n‐back working memory paradigm that parametrically increased the required cognitive demand for online retention, update, and manipulation of information.
Our seed‐based analysis illustrated that across the three levels of task difficulty the angular gyri positively connected with the fronto‐parietal as well as default mode networks, and negatively connected with the cingulo‐opercular network. Such data advocate a central role for the angular regions in terms of global connectivity dynamics and also implies the continuous interaction of DMN regions with known LSNs in this experimental context. These results overlap with recent reports on variable DMN interactions with areas commonly activated in working memory paradigms at particular stages of task execution. More specifically, greater positive interaction between the DMN and fronto‐parietal network regions have been observed at the retrieval stage of a working memory task (Piccoli et al., 2015).
Focusing on the areas that showed differential changes in their functional connectivity to the angular gyri across the three n‐back loads, the superior frontal gyrus is commonly implicated in working memory tasks as the area responsible for maintenance, manipulation of information and conflict‐resolution (du Boisgueheneuc et al., 2006). There are reports proposing that the superior frontal gyrus maintains a close relationship with the DMN, possibly bridging the default mode regions' connection to the fronto‐parietal network (Spreng et al., 2013). Moreover, in a memory retrieval task the activity of the left angular gyrus displayed a strong correlation with the superior frontal gyrus suggesting perhaps a central role of these connections in a memory related function (Nelson et al., 2010).
On the other hand, the supramarginal gyrus has been shown to activate in phonological decision making (Hartwigsen et al., 2010) and is thought to be a part of the phonological store and the articulatory rehearsal system, aided by the precentral gyrus and the supplementary motor areas (Chen and Desmond, 2005). Based on Baddeley's working memory model, visually presented stimuli, e.g., words or letters, are converted into auditory information and encoded into the phonological store by a process called articulatory control (Baddeley, 2003). Similarly, the superior temporal gyrus has been associated with auditory processing, and is thought to be important in silent, imagined speech (Buchsbaum et al., 2005). Together, the results allude to an increase in the angular gyri connectivity with the articulatory/phonological and auditory systems. Given the verbal nature of the n‐back utilized in this study, such a phonological strategy aided by the engagement of language‐related brain regions may have been employed by the participants in order to facilitate the processing of sequentially presented single letters.
In contrast to the above‐mentioned increases, the angular gyri functional connectivity to areas associated with visuospatial processing decreased with task difficulty. For instance, the left angular gyrus connectivity to the lingual gyrus, which is widely considered to be part of the visual system (Wang et al., 2008), illustrated a decrease across working memory load. Moreover, further decreases in connectivity were observed between the left angular gyrus and precuneus/posterior cingulate cortex. Considered a crucial hub of the DMN, the precuneus and posterior cingulate cortex have been associated with a number of functions extending from visual imagery (Hassabis and Maguire, 2007; Johnson et al., 2007) to retrieval of long‐term memory in mnemonic tasks (Shapira‐Lichter et al., 2013), “self” referential processing (Brewer et al., 2013) as well as attention and arousal (Leech and Sharp, 2014). Importantly, the posterior cingulate has also been discussed as a connector hub (Binder et al., 2009), evidenced by the “echoes” of other LSNs found in its sub regions (Leech et al., 2012). In this context, the posterior cingulate is believed to detect changes in the environment by integrating different sources of information and tracking the history of task outcomes (Pearson et al., 2011).
Taken together our results may suggest that with increasing n‐back task difficulty the participants rely more on articulatory/phonological strategies to cope with task demands as opposed to visuospatial information extracted from the presented letters alone. However, given the behavioral scores, the contribution of visuospatial information seems necessary for faster processing, since greater connectivity between the left angular gyrus and posterior cingulate, and right angular gyrus and calcarine cortex correlated with faster responses across the three n‐back conditions.
Overall, the results indicate that two important DMN hubs, the bilateral angular gyri, illustrate significant changes in their connectivity within the DMN as well as other brain areas, during an N‐back paradigm. Given their cross‐modal nature and their substantial structural/functional connections to the rest of the brain, the angular gyri may combine multimodal information based on the employed cognitive strategies, which they then transmit to frontal brain regions for further cognitive processing. In fact, recent evidence argues for angular involvement in the integration of visual and auditory information (Bernstein et al., 2008; Bonnici et al., 2016; Shannon and Buckner, 2004).
Given the angular connectivity profile to the regions associated with articulatory/phonological and visual processes, it may be possible to assign an “integrator” role to the angular gyri (Binder et al., 2009). Such explanation is also rooted in Baddeley's working memory model, which has recently been revised to include an “episodic buffer” that is hypothesized to combine auditory and visual information, as well as long‐term memory (Baddeley, 2003; Sestieri et al., 2011). In another line of thought, such convergence zones in the brain have been related to a global workspace implicated in consciousness (Baars, 2002). In this framework, in addition to the long‐range fronto‐parietal network connections, the DMN is thought to provide a supplementary site for global integration of information necessary for conscious processing (Dehaene and Changeux, 2011; Smallwood et al., 2012). To this end, we have recently shown the expansion of this psychological construct with greater working memory load in an n‐back paradigm, and a potential role for the DMN in facilitating this global functional integration (Vatansever et al., 2015a). Such results concur with the hypothesized episodic buffer component of the working memory model (Baars and Franklin, 2003; Baddeley, 2000).
Working memory is an executive function that is commonly assessed in neuroimaging studies, and constitutes a key process in maintaining cognitive health (Cocchi et al., 2009; McLean et al., 2004). Similarly, based on the wider association of altered DMN activity/connectivity in a number of neurodegenerative and psychiatric disorders, such as Alzheimer's disease (Buckner et al., 2009), traumatic brain injury (Bonnelle et al., 2011), depression and schizophrenia (Greicius et al., 2007; Whitfield‐Gabrieli et al., 2009), it is of paramount importance for us to decipher the exact contribution of this network to working memory processes, and future work should aim to directly test its role in active experimental paradigms.
ACKNOWLEDGMENTS
The funding for this study was provided by the Evelyn Trust (RUAG/018). In addition, DV received funding from the Yousef Jameel Academic Program, DKM was funded by the NIHR Cambridge Biomedical Centre (RCZB/004), and an NIHR Senior Investigator Award (RCZB/014) and EA Stamatakis was supported by the Stephen Erskine Fellowship Queens' College Cambridge. The authors thank Dr. Sanja Abbott for programming the stimulus delivery, as well as Dr. Guy Williams, Victoria Lupson and the rest of the staff in the Wolfson Brain Imaging Centre (WBIC) at Addenbrooke's Hospital for their assistance in scanning. Last but not least, they thank all the participants for their contribution to this study.
REFERENCES
- Andrews‐Hanna JR, Smallwood J, Spreng RN (2014): The default network and self‐generated thought: Component processes, dynamic control, and clinical relevance. Ann N Y Acad Sci 1316:29–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ (2005): Unified segmentation. Neuroimage 26:839–851. [DOI] [PubMed] [Google Scholar]
- Baars BJ (2002): The conscious access hypothesis: Origins and recent evidence. Trends Cogn Sci 6:47–52. [DOI] [PubMed] [Google Scholar]
- Baars BJ, Franklin S (2003): How conscious experience and working memory interact. Trends Cogn Sci 7:166–172. [DOI] [PubMed] [Google Scholar]
- Baddeley A (2000): The episodic buffer: A new component of working memory? Trends Cogn Sci 4:417–423. [DOI] [PubMed] [Google Scholar]
- Baddeley A (2003): Working memory: Looking back and looking forward. Nat Rev Neurosci 4:829–839. [DOI] [PubMed] [Google Scholar]
- Baddeley AD, Hitch GJ (1974): Working memory In: Bower GH, editor. The psychology of learning and motivation, Vol 8. London: Academic Press. [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT (2007): A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 37:90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein LE, Auer ET Jr, Wagner M, Ponton CW (2008): Spatiotemporal dynamics of audiovisual speech processing. Neuroimage 39:423–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL (2009): Where is the semantic system? A critical review and meta‐analysis of 120 functional neuroimaging studies. Cereb Cortex 19:2767–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Medler DA, Desai R, Conant LL, Liebenthal E (2005): Some neurophysiological constraints on models of word naming. Neuroimage 27:677–693. [DOI] [PubMed] [Google Scholar]
- Bluhm RL, Clark CR, McFarlane AC, Moores KA, Shaw ME, Lanius RA (2011): Default network connectivity during a working memory task. Hum Brain Mapp 32:1029–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnelle V, Leech R, Kinnunen KM, Ham TE, Beckmann CF, De Boissezon X, Greenwood RJ, Sharp DJ (2011): Default mode network connectivity predicts sustained attention deficits after traumatic brain injury. J Neurosci 31:13442–13451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnici HM, Richter FR, Yazar Y, Simons JS (2016): Multimodal Feature Integration in the Angular Gyrus during Episodic and Semantic Retrieval. J Neurosci 36:5462–5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JA, Garrison KA, Whitfield‐Gabrieli S (2013): What about the “Self” is processed in the posterior cingulate cortex? Front Hum Neurosci 7:647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchsbaum BR, Olsen RK, Koch P, Berman KF (2005): Human dorsal and ventral auditory streams subserve rehearsal‐based and echoic processes during verbal working memory. Neuron 48:687–697. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews‐Hanna JR, Schacter DL (2008): The brain's default network: Anatomy, function, and relevance to disease. Ann N Y Acad Sci 1124:1–38. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, Andrews‐Hanna JR, Sperling RA, Johnson KA (2009): Cortical hubs revealed by intrinsic functional connectivity: Mapping, assessment of stability, and relation to Alzheimer's disease. J Neurosci 29:1860–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappelletti M, Lee HL, Freeman ED, Price CJ (2010): The role of right and left parietal lobes in the conceptual processing of numbers. J Cogn Neurosci 22:331–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai XJ, Castanon AN, Ongur D, Whitfield‐Gabrieli S (2012): Anticorrelations in resting state networks without global signal regression. Neuroimage 59:1420–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SH, Desmond JE (2005): Temporal dynamics of cerebro‐cerebellar network recruitment during a cognitive task. Neuropsychologia 43:1227–1237. [DOI] [PubMed] [Google Scholar]
- Cocchi L, Walterfang M, Testa R, Wood SJ, Seal ML, Suckling J, Takahashi T, Proffitt TM, Brewer WJ, Adamson C, Soulsby B, Velakoulis D, McGorry PD, Pantelis C (2009): Grey and white matter abnormalities are associated with impaired spatial working memory ability in first‐episode schizophrenia. Schizophr Res 115:163–172. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL (2008): The reorienting system of the human brain: From environment to theory of mind. Neuron 58:306–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Pasquale F, Della Penna S, Snyder AZ, Marzetti L, Pizzella V, Romani GL, Corbetta M (2012): A cortical core for dynamic integration of functional networks in the resting human brain. Neuron 74:753–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S, Changeux JP (2011): Experimental and theoretical approaches to conscious processing. Neuron 70:200–227. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Piazza M, Pinel P, Cohen L (2003): Three parietal circuits for number processing. Cogn Neuropsychol 20:487–506. [DOI] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A (2004): Neuroplasticity: Changes in grey matter induced by training. Nature 427:311–312. [DOI] [PubMed] [Google Scholar]
- du Boisgueheneuc F, Levy R, Volle E, Seassau M, Duffau H, Kinkingnehun S, Samson Y, Zhang S, Dubois B (2006): Functions of the left superior frontal gyrus in humans: A lesion study. Brain 129:3315–3328. [DOI] [PubMed] [Google Scholar]
- Esposito F, Aragri A, Latorre V, Popolizio T, Scarabino T, Cirillo S, Marciano E, Tedeschi G, Di Salle F (2009): Does the default‐mode functional connectivity of the brain correlate with working‐memory performances? Arch Ital Biol 147:11–20. [PubMed] [Google Scholar]
- Esposito F, Bertolino A, Scarabino T, Latorre V, Blasi G, Popolizio T, Tedeschi G, Cirillo S, Goebel R, Di Salle F (2006): Independent component model of the default‐mode brain function: Assessing the impact of active thinking. Brain Res Bull 70:263–269. [DOI] [PubMed] [Google Scholar]
- Fair DA, Schlaggar BL, Cohen AL, Miezin FM, Dosenbach NU, Wenger KK, Fox MD, Snyder AZ, Raichle ME, Petersen SE (2007): A method for using blocked and event‐related fMRI data to study “resting state” functional connectivity. Neuroimage 35:396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME (2005): The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA 102:9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P (2006): How default is the default mode of brain function? Further evidence from intrinsic BOLD signal fluctuations. Neuropsychologia 44:2836–2845. [DOI] [PubMed] [Google Scholar]
- Gordon EM, Breeden AL, Bean SE, Vaidya CJ (2014): Working memory‐related changes in functional connectivity persist beyond task disengagement. Hum Brain Mapp 35:1004–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb J (2007): From thought to action: The parietal cortex as a bridge between perception, action, and cognition. Neuron 53:9–16. [DOI] [PubMed] [Google Scholar]
- Graves WW, Desai R, Humphries C, Seidenberg MS, Binder JR (2010): Neural systems for reading aloud: A multiparametric approach. Cereb Cortex 20:1799–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DM, Swets JA (1974): Signal Detection Theory and Psychophysics. Huntington, NY: R. E. Krieger Pub. Co. xiii, 479 p. p. [Google Scholar]
- Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, Reiss AL, Schatzberg AF (2007): Resting‐state functional connectivity in major depression: Abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry 62:429–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V (2003): Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc Natl Acad Sci USA 100:253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME (2001): Medial prefrontal cortex and self‐referential mental activity: Relation to a default mode of brain function. Proc Natl Acad Sci USA 98:4259–4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, Sporns O (2008): Mapping the structural core of human cerebral cortex. PLoS Biol 6:e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Driesen N, Roth JK, Gore JC, Constable RT (2010): Functional connectivity between task‐positive and task‐negative brain areas and its relation to working memory performance. Magn Reson Imaging 28:1051–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Driesen NR, Skudlarski P, Gore JC, Constable RT (2006): Brain connectivity related to working memory performance. J Neurosci 26:13338–13343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwigsen G, Baumgaertner A, Price CJ, Koehnke M, Ulmer S, Siebner HR (2010): Phonological decisions require both the left and right supramarginal gyri. Proc Natl Acad Sci USA 107:16494–16499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassabis D, Maguire EA (2007): Deconstructing episodic memory with construction. Trends Cogn Sci 11:299–306. [DOI] [PubMed] [Google Scholar]
- Hearne L, Cocchi L, Zalesky A, Mattingley JB (2015): Interactions between default mode and control networks as a function of increasing cognitive reasoning complexity. Hum Brain Mapp 36:2719–2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferies E (2013): The neural basis of semantic cognition: Converging evidence from neuropsychology, neuroimaging and TMS. Cortex 49:611–625. [DOI] [PubMed] [Google Scholar]
- Johnson MR, Mitchell KJ, Raye CL, D'Esposito M, Johnson MK (2007): A brief thought can modulate activity in extrastriate visual areas: Top‐down effects of refreshing just‐seen visual stimuli. Neuroimage 37:290–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung RE, Segall JM, Jeremy Bockholt H, Flores RA, Smith SM, Chavez RS, Haier RJ (2010): Neuroanatomy of creativity. Hum Brain Mapp 31:398–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitzbichler MG, Henson RN, Smith ML, Nathan PJ, Bullmore ET (2011): Cognitive effort drives workspace configuration of human brain functional networks. J Neurosci 31:8259–8270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi M, McLaren DG, Engen H, Smallwood J (2015): Shaped by the past: The default mode network supports cognition that is independent of immediate perceptual input. PLoS One 10:e0132209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Li K, Robin Da, Glahn DC, Fox PT (2009): Investigating the functional heterogeneity of the default mode network using coordinate‐based meta‐analytic modeling. J Neurosci 29:14496–14505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Devlin JT, Shakeshaft C, Stewart LH, Brennan A, Glensman J, Pitcher K, Crinion J, Mechelli A, Frackowiak RS, Green DW, Price CJ (2007): Anatomical traces of vocabulary acquisition in the adolescent brain. J Neurosci 27:1184–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R, Braga R, Sharp DJ (2012): Echoes of the brain within the posterior cingulate cortex. J Neurosci 32:215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R, Sharp DJ (2014): The role of the posterior cingulate cortex in cognition and disease. Brain 137:12–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Mai X, Liu C (2014): The default mode network and social understanding of others: What do brain connectivity studies tell us. Front Hum Neurosci 8:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo MV, Chakrabarti B, Bullmore ET, Wheelwright SJ, Sadek SA, Suckling J, Consortium MA, Baron‐Cohen S (2010): Shared neural circuits for mentalizing about the self and others. J Cogn Neurosci 22:1623–1635. [DOI] [PubMed] [Google Scholar]
- Makris N, Kennedy DN, McInerney S, Sorensen AG, Wang R, Caviness VS Jr, Pandya DN (2005): Segmentation of subcomponents within the superior longitudinal fascicle in humans: A quantitative, in vivo, DT‐MRI study. Cereb Cortex 15:854–869. [DOI] [PubMed] [Google Scholar]
- Makris N, Papadimitriou GM, Kaiser JR, Sorg S, Kennedy DN, Pandya DN (2009): Delineation of the middle longitudinal fascicle in humans: A quantitative, in vivo, DT‐MRI study. Cereb Cortex 19:777–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Papadimitriou GM, Sorg S, Kennedy DN, Caviness VS, Pandya DN (2007): The occipitofrontal fascicle in humans: A quantitative, in vivo, DT‐MRI study. Neuroimage 37:1100–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mar RA (2011): The neural bases of social cognition and story comprehension. Annu Rev Psychol 62:103–134. [DOI] [PubMed] [Google Scholar]
- Martuzzi R, Ramani R, Qiu M, Shen X, Papademetris X, Constable RT (2011): A whole‐brain voxel based measure of intrinsic connectivity contrast reveals local changes in tissue connectivity with anesthetic without a priori assumptions on thresholds or regions of interest. Neuroimage 58:1044–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean A, Dowson J, Toone B, Young S, Bazanis E, Robbins TW, Sahakian BJ (2004): Characteristic neurocognitive profile associated with adult attention‐deficit/hyperactivity disorder. Psychol Med 34:681–692. [DOI] [PubMed] [Google Scholar]
- Morcom AM, Fletcher PC (2007): Does the brain have a baseline? Why we should be resisting a rest. Neuroimage 37:1073–1082. [DOI] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA (2009): The impact of global signal regression on resting state correlations: Are anti‐correlated networks introduced? Neuroimage 44:893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SM, Cohen AL, Power JD, Wig GS, Miezin FM, Wheeler ME, Velanova K, Donaldson DI, Phillips JS, Schlaggar BL, Petersen SE (2010): A parcellation scheme for human left lateral parietal cortex. Neuron 67:156–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan KA, Jefferies E, Visser M, Lambon Ralph MA (2013): Going beyond inferior prefrontal involvement in semantic control: Evidence for the additional contribution of dorsal angular gyrus and posterior middle temporal cortex. J Cogn Neurosci 25:1824–1850. [DOI] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E (2005): N‐back working memory paradigm: A meta‐analysis of normative functional neuroimaging studies. Hum Brain Mapp 25:46–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson JM, Heilbronner SR, Barack DL, Hayden BY, Platt ML (2011): Posterior cingulate cortex: Adapting behavior to a changing world. Trends Cogn Sci 15:143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccoli T, Valente G, Linden DE, Re M, Esposito F, Sack AT, Di Salle F (2015): The default mode network and the working memory network are not anti‐correlated during all phases of a working memory task. PLoS One 10:e0123354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Mechelli A (2005): Reading and reading disturbance. Curr Opin Neurobiol 15:231–238. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Snyder AZ (2007): A default mode of brain function: A brief history of an evolving idea. NeuroImage 37:1083–1090; discussion 1097‐9. [DOI] [PubMed] [Google Scholar]
- Rottschy C, Langner R, Dogan I, Reetz K, Laird AR, Schulz JB, Fox PT, Eickhoff SB (2012): Modelling neural correlates of working memory: A coordinate‐based meta‐analysis. Neuroimage 60:830–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambataro F, Murty VP, Callicott JH, Tan HY, Das S, Weinberger DR, Mattay VS (2010): Age‐related alterations in default mode network: Impact on working memory performance. Neurobiol Aging 31:839–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier ML (2013): The angular gyrus: Multiple functions and multiple subdivisions. Neuroscientist 19:43–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier ML, Fagan E, Price CJ (2010): Functional subdivisions in the left angular gyrus where the semantic system meets and diverges from the default network. J Neurosci 30:16809–16817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sestieri C, Corbetta M, Romani GL, Shulman GL (2011): Episodic memory retrieval, parietal cortex, and the default mode network: Functional and topographic analyses. J Neurosci 31:4407–4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon BJ, Buckner RL (2004): Functional‐anatomic correlates of memory retrieval that suggest nontraditional processing roles for multiple distinct regions within posterior parietal cortex. J Neurosci 24:10084–10092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapira‐Lichter I, Oren N, Jacob Y, Gruberger M, Hendler T (2013): Portraying the unique contribution of the default mode network to internally driven mnemonic processes. Proc Natl Acad Sci USA 110:4950–4955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood J, Brown K, Baird B, Schooler JW (2012): Cooperation between the default mode network and the frontal‐parietal network in the production of an internal train of thought. Brain Res 1428:60–70. [DOI] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, Beckmann CF (2009): Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci USA 106:13040–13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN (2012): The fallacy of a “task‐negative” network. Front Psychol 3:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, DuPre E, Selarka D, Garcia J, Gojkovic S, Mildner J, Luh WM, Turner GR (2014): Goal‐congruent default network activity facilitates cognitive control. J Neurosci 34:14108–14114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Sepulcre J, Turner GR, Stevens WD, Schacter DL (2013): Intrinsic architecture underlying the relations among the default, dorsal attention, and frontoparietal control networks of the human brain. J Cogn Neurosci 25:74–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PC, Muggleton NG, Kalla R, Walsh V, Eimer M (2011): TMS of the right angular gyrus modulates priming of pop‐out in visual search: Combined TMS‐ERP evidence. J Neurophysiol 106:3001–3009. [DOI] [PubMed] [Google Scholar]
- Thomason ME, Dennis EL, Joshi AA, Joshi SH, Dinov ID, Chang C, Henry ML, Johnson RF, Thompson PM, Toga AW, Glover GH, Van Horn JD, Gotlib IH (2011): Resting‐state fMRI can reliably map neural networks in children. Neuroimage 55:165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Amin H, Rykhlevskaia E, Nguyen DA, Greicius MD, Menon V (2010): Dissociable connectivity within human angular gyrus and intraparietal sulcus: Evidence from functional and structural connectivity. Cereb Cortex 20:2636–2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatansever D, Menon DK, Manktelow AE, Sahakian BJ, Stamatakis EA (2015a): Default mode dynamics for global functional integration. J Neurosci 35:15254–15262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatansever D, Menon DK, Manktelow AE, Sahakian BJ, Stamatakis EA (2015b): Default mode network connectivity during task execution. Neuroimage 122:96–104. [DOI] [PubMed] [Google Scholar]
- Vincent JL (2009): Learning and memory: While you rest, your brain keeps working. Curr Biol 19:R484–R486. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL (2008): Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol 100:3328–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Conder JA, Blitzer DN, Shinkareva SV (2010): Neural representation of abstract and concrete concepts: A meta‐analysis of neuroimaging studies. Hum Brain Mapp 31:1459–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Jiang T, Yu C, Tian L, Li J, Liu Y, Zhou Y, Xu L, Song M, Li K (2008): Spontaneous activity associated with primary visual cortex: A resting‐state FMRI study. Cereb Cortex 18:697–704. [DOI] [PubMed] [Google Scholar]
- Whitfield‐Gabrieli S, Nieto‐Castanon A (2012): Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect 2:125–141. [DOI] [PubMed] [Google Scholar]
- Whitfield‐Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, Shenton ME, Green AI, Nieto‐Castanon A, LaViolette P, Wojcik J, Gabrieli JD, Seidman LJ (2009): Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first‐degree relatives of persons with schizophrenia. Proc Natl Acad Sci USA 106:1279–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]


