Abstract
The ability to inhibit responses is a central sensorimotor function but only recently the importance of sensory processes for motor inhibition mechanisms went more into the research focus. In this regard it is elusive, whether there are differences between sensory modalities to trigger response inhibition processes. Due to functional neuroanatomical considerations strong differences may exist, for example, between the visual and the tactile modality. In the current study we examine what neurophysiological mechanisms as well as functional neuroanatomical networks are modulated during response inhibition. Therefore, a Go/NoGo‐paradigm employing a novel combination of visual, tactile, and visuotactile stimuli was used. The data show that the tactile modality is more powerful than the visual modality to trigger response inhibition processes. However, the tactile modality loses its efficacy to trigger response inhibition processes when being combined with the visual modality. This may be due to competitive mechanisms leading to a suppression of certain sensory stimuli and the response selection level. Variations in sensory modalities specifically affected conflict monitoring processes during response inhibition by modulating activity in a frontal parietal network including the right inferior frontal gyrus, anterior cingulate cortex and the temporoparietal junction. Attentional selection processes are not modulated. The results suggest that the functional neuroanatomical networks involved in response inhibition critically depends on the nature of the sensory input. Hum Brain Mapp 38:1941–1951, 2017. © 2017 Wiley Periodicals, Inc.
Keywords: response inhibition, sensory modalities, EEG, source localization, parietal cortex, frontal cortex
INTRODUCTION
The ability to inhibit responses is a central sensorimotor function that has intensively been investigated [Aron et al., 2004; Bari and Robbins, 2013; Diamond, 2013]. However, only recently the importance of perceptual processes for motor inhibition mechanisms went more into research focus [Huster et al., 2010; Shedden and Reid, 2001; Stock et al., 2015; Verbruggen et al., 2006]. Related to the question of the importance of perceptual processes for response inhibition is the question, whether sensory modalities differ in their efficiency to trigger response inhibition processes. For response inhibition processes, a frontostriatal network including inferior frontal cortices and medial frontal cortices as well as the supplementary motor area (SMA) is important [Bari and Robbins, 2013]. Interestingly, the SMA and the superior frontal cortex show strong axonal connections to somatosensory associations cortices [Borich et al., 2015; Fang et al., 2005], which has also been shown in neuroanatomical studies in macaques [Luppino et al., 1993]. Furthermore, parietal somatosensory regions show strong connections to posterior parietal areas [Borich et al., 2015] which are important for sensory motor integration [Andersen and Buneo, 2002] and have recently been shown to be involved during response inhibition [Dippel et al., 2016]. Finally, changes in somatosensory functions have a direct effect on motor cortical areas and the control of responses [Borich et al., 2015]. Due the outlined functional neuroanatomical considerations it is possible that the somatosensory modality is particularly powerful to trigger response inhibition processes. Such a short connection from primary sensory areas to functional neuroanatomical areas important for response inhibition is not evident for the visual modality. The visual cortex does not show direct structural anatomical connections to the SMA and adjacent areas in the superior frontal cortex [Hagmann et al., 2008]. In fact, the data by Hagmann et al. [2008] shows that the visual cortex shows strongest structural connections to the parietal cortex [Hagmann et al., 2008]. There are only indirect connections via different hub regions in the inferior and superior parietal cortex [Hagmann et al., 2008]. We therefore hypothesize that response inhibition processes are better when being triggered by tactile stimuli than by visual stimuli.
However, unlike conventional views of primary sensory areas several lines of evidence suggest that the (primary) somatosensory cortex is able to perform multisensory integration processes [Borich et al., 2015; Driver and Noesselt, 2008] and is involved in merging multimodal information, e.g. from the visual modality [Borich et al., 2015]. This is of importance, because recent results suggest that multisensory information has a strong impact on efficient response inhibition processes [Chmielewski et al., 2015]. For example, in line with co‐activation models [Gondan et al., 2010; Miller et al., 2004], it has been shown that the combination of auditory and visual stimuli to signal the need to inhibit a response leads to better response inhibition performance than a single visual or auditory stimulus [Chmielewski et al., 2015]. It may therefore be further hypothesized that visual stimuli, combined with tactile stimuli may be even more potent to trigger response inhibition processes, than single visual or tactile stimuli. This may be possible because the primary somatosensory cortex is able to perform multisensory integration processes [Borich et al., 2015; Driver and Noesselt, 2008]. Yet, it has also been suggested that the suitability of co‐activation models to explain the effects of multisensory input on response inhibition may depend on the nature of the stimuli [Gondan et al., 2010]. It is therefore also possible that there is no better response inhibition performance when combining visual and tactile stimuli.
In the current study we examine in how far there are differences between sensory modalities and a combination of sensory modalities (i.e. visuotactile stimuli) to trigger response inhibition processes and what neurophysiological mechanisms as well as functional neuroanatomical networks are involved. Therefore, we combine electrophysiological (EEG) with source localization techniques. The advantage of electrophysiological (EEG) techniques and event‐related potentials (ERPs) in particular is that using these techniques different cognitive‐neurophysiological subprocesses involved during information processing (and response inhibition) can be isolated on the basis of their temporal occurrence. In combination with source localization techniques this makes it possible to examine the functional neuroanatomical network related to these subprocesses that is modulated by the experimental manipulations. Concerning response inhibition processes it is possible to distinguish two subprocesses that are reflected by distinct ERPs: the Nogo‐N2 and the Nogo‐P3. It has repeatedly been shown that a frontal‐midline Nogo‐N2 event‐related potential (ERP) reflects processes like conflict monitoring or updating of the response program during response inhibition, while a Nogo‐P3 ERP reflects evaluative processing of the successful outcome of the inhibition [Beste et al., 2009, 2011, 2016, 2010; Huster et al., 2013], or the inhibition itself [Wessel and Aron, 2015]. It is likely that these processes are modulated when being triggered by tactile, compared to visual stimuli, or combined visuotactile stimuli. If the tactile modality is more efficient to trigger response inhibition this may be due to lower response conflicts during inhibition, which should be reflected in a smaller Nogo‐N2. However, it is also possible that motor inhibition processes (Nogo‐P3) become stronger, which should then lead to a higher Nogo‐P3 when tactile stimuli are used. Very likely, modulations in these processes are due changes in frontal brain areas like the right inferior frontal gyrus (IFG) and medial as well superior frontal areas. This because these regions are involved in response inhibition processes [Bari and Robbins, 2013]. However, also parietal areas may be modulated as these are known to be involved in sensory‐motor integration processes [Andersen and Buneo, 2002] and updating of internal representations based on new sensory information to initiate task‐appropriate actions [Geng and Vossel, 2013], i.e. the inhibition of responses [Dippel et al., 2016]. Yet, it has also been shown that perceptual and attentional selection processes are important to consider during response inhibition, which are also reflected by distinct ERPs components; i.e. the P1 and N1 ERP [Bonnefond et al., 2010; Chmielewski et al., 2015; Staub et al., 2014; Stock et al., 2015].
MATERIALS AND METHODS
Participants
This study includes N = 24 healthy, right handed participants between 18 and 29 years of age (mean age 24.06 ± 3.17; 16 females). All participants had normal or corrected‐to‐normal vision and were free of any psychiatric and neurological symptoms. Informed consent was obtained from all individual participants included in the study. The study was approved by the institutional review board of the Medical faculty of the TU Dresden.
Task
A Go/NoGo‐paradigm was used employing a novel combination of visual, tactile and visuotactile stimuli in separate experimental conditions to examine the effects of stimulus modality on response inhibition processes. The experimental paradigm is outlined in Figure 1.
Figure 1.
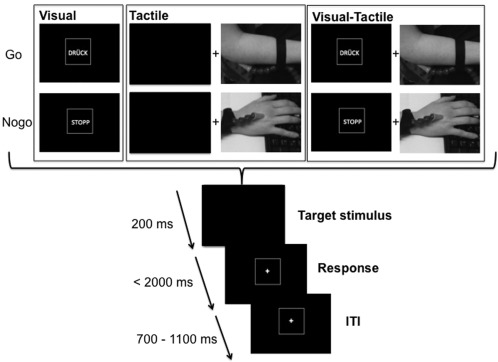
Schematic illustration of the Go/Nogo paradigm. The Figure shows the visual condition (left), the tactile condition (middle) and the visual‐tactile condition (right). In the visual‐tactile condition, the visual and tactile stimulus in Go and Nogo conditions was presented simultaneously. At the bottom of the figure, the timing of the stimuli, response deadlines and ITI is shown.
In all conditions the participants sat in front of a computer screen and were instructed to press a button with their right hand upon the presentation of the Go‐stimulus. Participants were instructed to respond as fast as possible. Go and Nogo trials were presented in a pseudorandom order. The experiment was divided into three blocks with 400 trials each (1,200 trials in total). The three blocks refer to the visual, tactile and visuotactile condition. Between the blocks the subjects were allowed to take a break. The order of the blocks was counterbalanced across the N = 24 subjects. In the visual and visuotactile condition the word “DRÜCK” (German for “press”) was the Go stimulus and the word “STOPP” (German for “stop”) the Nogo stimulus. In the tactile modality a short air puff on the right elbow served as the Go stimulus and the same air puff on the right dorsum of the hand served as the Nogo stimulus (refer Fig. 1). The locations for Go and Nogo stimulus presentations were counterbalanced across subjects. The different sites used for the tactile stimuli were used to ensure high discriminability of the Go and Nogo conditions. In the visuotactile condition the tactile Go and Nogo stimuli were presented simultaneously with the visual Go and Nogo stimuli. The tactile and visual information was given in a compatible manner (Visual Go and tactile Go, visual Nogo and tactile Nogo). In the tactile Go/Nogo conditions no visual stimuli were presented and participants were asked to look on a blank computer screen. The experiment began with a standardized instruction and an exercise of 10 trials. Every trial started with showing the Go and Nogo stimuli for 200 ms and was followed by a blank screen during response (for details, see Fig. 1). For a correct response on targets, the participants were asked to push a button with their right hand within 2,000 ms after the onset of target presentation. The intertrial interval (ITI) was jittered between 700 and 1,100 ms to avoid preparation effects. Stimulus presentation terminated whether the response was correct or not. To avoid repetition effects, the order of the Go and Nogo trials changed in a pseudorandomized manner; 70% of the trials applied to a Go‐stimulus and 30% to a Nogo‐stimulus.
EEG Recording, Analysis and Source Reconstruction
The EEG was recorded with a sampling rate of 500 Hz using a 60‐channel system (BrainAmp, Brain Products Inc.) and electrode impedances were kept under 5 kΩ. We used LECTRON III‐10 (EasyCap Inc.) electrode gel. Passive Ag/AgCl‐electrodes (60 recording electrodes) were mounted in an elastic cap (EasyCap Inc.) and arranged in equidistant positions approximating the positions of the 10/20 system. The ground and reference electrode were placed at coordinates θ = 58, φ = 78 and θ = 90, φ = 90, respectively. After recording, data were down‐sampled to 256 Hz and filtered (band‐pass filter from 0.5 to 20 Hz, with a slope of 48 dB/oct each) using the BrainVision Analyzer 2 software package (BrainProducts Inc.). In a manual inspection of the data, technical artifacts were removed. Subsequently, horizontal blinks, pulse artifacts and vertical eye‐movements were corrected using independent component analysis (ICA, infomax algorithm). Then, the data was segmented for Go and Nogo trials in the three different experimental conditions (visual, tactile, visuotactile). Go trials were only included, if a correct response was given in a time window of 1,200 ms after stimulus presentation. Nogo trials were only included, if there was no response within 1,200 ms after stimulus onset. The segment length of trials was 1,400 ms, starting 200 ms before stimulus presentation (i.e. time point 0). Afterwards, an automated artifact rejection procedure was applied. In this procedure a maximal value difference of 200 μV in a 100 ms interval and an activity below 0.5 μV in a 100 ms period were used as rejection criteria. All EEG epochs in which one or both of these criteria were fulfilled were discarded from further data analysis. In order to eliminate the reference potential from the data a current source density (CSD) transformation [Nunez and Pilgreen, 1991] was applied to re‐reference the data. The resulting CSD values are stated in μV/m2. The CSD‐transformation was employed since it additionally serves as a spatial filter [Nunez and Pilgreen, 1991]. This makes it possible to identify electrodes that best reflect activity related to cognitive processes. Thereafter, a baseline correction from −200 ms to 0 ms (i.e. target onset) was applied. Averages of every participant and grand averages for each condition (Visual, tactile, visual‐tactile) and stimuli (Go/Nogo) were calculated. The P1 on Go and Nogo trials was quantified at electrodes P9 and P10 between 80 and 120 ms. The N1 on Go and Nogo trials was quantified at electrodes P9 and P10 between 180 and 220 ms. The N2 on Go and Nogo trials was quantified at electrode FCz between 250 and 310 ms. The P3 on Go and Nogo trials was quantified at electrode FCz, C3 and C4 between 350 ms and 430 ms. The above‐given time ranges are search windows. The quantification of peaks (amplitudes) and the latencies of these peaks were done semiautomatically. Since all of these search intervals were rather extended, peaks were manually relocated, if necessary. Doing so, for each participants and each ERP of interest the peak amplitude and latency was measured. The choice of electrodes by visual inspection including the selected time windows for data quantification was validated using statistical methods [Mückschel et al., 2014]: For each ERP component a search interval was defined (noted above), in which the ERP component is expected to be maximal. After this, we extracted the mean amplitude within each of these search intervals at each of the 60 electrode positions. This was done for the Nogo trials (which are the most important ones in a response inhibition study) and after CSD‐transformation of the data, because the CSD‐transformation has the effect of a spatial filter that accentuates scalp topography (Nunez and Pilgreen, 1991). Subsequently, we compared each electrode against an average of all other electrodes using Bonferroni‐correction for multiple comparisons (critical threshold P = 0.0007). Only electrodes that showed significantly larger mean amplitudes (i.e., negative for (No)go‐N2 potentials and positive for the (No)go‐P3 potentials) than the remaining electrodes were chosen.
Source localization was conducted using sLORETA (standardized low resolution brain electromagnetic tomography; [Pascual‐Marqui, 2002], which provides is a single linear solution for the inverse problem without localization bias [Marco‐Pallarés et al., 2005; Sekihara et al., 2005]. sLORETA reveals high convergence with fMRI data [Sekihara et al., 2005]. Evidence from EEG/TMS further confirm the validity of the sources estimated with sLORETA [Dippel and Beste, 2015]. In sLORETA, the standardized current density at each voxel is calculated in a realistic head model [Fuchs et al., 2002] using the MNI152 template. The voxel ‐ based sLORETA images (6239 voxels at 5 mm spatial resolution) of the intracerebral volume were compared between the conditions. The regularization parameter was kept constant for the different comparisons. For comparing the voxel‐based images, the sLORETA‐built‐in‐voxel‐wise randomization test with 2,000 permutations was used (based on statistical nonparametric mapping, SnPM). Voxels with significant differences (P < 0.01, corrected for multiple comparisons) between contrasted conditions were located in the MNI brain (http://www.unizh.ch/keyinst/NewLORETA/sLORETA/sLORETA.htm)
Statistical Analysis
Behavioral data, i.e. Go reaction times (RTs) and rate of Go hits was analyzed using repeated measures ANOVAs using “condition” (visual, tactile, and visuotactile) as within‐subject factors. The same was done for the rate of Nogo false alarms (i.e., button presses on the different Nogo trials). For the neurophysiological data (i.e. amplitudes and latencies), the factor “Go/Nogo,” “condition,” and the factor “electrode site” were modeled as within‐subject factors. Separate models were calculated for the amplitude and latency parameters. For all analyses Greenhouse‐Geisser correction was applied wherever appropriate and all post hoc tests and pair‐wise comparisons were Bonferroni‐corrected to correct for the number of tests performed e.g. on the values of the quantified ERP amplitudes or RT etc. All variables analyzed were normal distributed (all z < 0.12; P > 0.2), as indicated by Kolmogorov‐Smirnov tests. For the descriptive data, the mean and standard error of the mean (SEM) are given.
RESULTS
Behavioral Data
An analysis of the RTs on Go trials using a repeated measures ANOVA revealed a main effect condition [F (2,46) = 4.52; P = 0.03; η 2 = 0.164] showing that RTs were faster on visual‐tactile trials (351.93 ± 7.02) (P < 0.01), compared to the other conditions (visual: 376.22 ± 5.73; tactile: 378.43 ± 13.96), which did not differ from each other (P > 0.8) as indicated by Bonferroni‐corrected pair‐wise comparisons. There was no difference between conditions in the rate of missed go trials [F (2,46) = 1.63; P = 0.206]. Overall 0.79% ± 0.60 Go trials were missed.
The analysis of RTs on Nogo trials (i.e. the RT was defined as the onset of stimulus presentation and the onset of the erroneous button press in a given Nogo trial) revealed a main effect of condition [F (2,46) = 5.00, P = 0.030; η 2 = 0.179] showing that RTs increased from the visual‐tactile condition (305.59 ± 11.39) to the visual condition (335.38 ± 10.98) to the tactile condition (370.67 ± 32.67). Bonferroni‐corrected pair‐wise comparisons revealed that visual trials differed from visual‐tactile trials (P = 0.02) and that tactile trials differed from visual‐tactile trials (P = 0.049), while tactile and visual trails did not significantly differ (P > 0.4). However, the rate of false alarms (FA) is the most important behavioral parameter in Go/Nogo tasks. The ANOVA revealed a main effect of “condition” [F (2,46) = 3.71, P = 0.033; η 2 = 0.139]. Bonferroni‐corrected, pair‐wise comparisons showed that the rate of false alarms was lower in the tactile condition (15.63 ± 2.67) (P = 0.043), compared to the other conditions and did not differ between the visual‐tactile condition (20.25 ± 2.45) and the visual condition (21.21 ± 1.47) (P = 0.653). The inclusion of an additional between‐subject factor “sex” in the analyses did not change the pattern of results (P > 0.3).
Neurophysiological Data
Perceptual and attentional processes
For all ERPs, peak to baseline amplitudes were entered into the repeated measures ANOVAs. The P1 and N1 are shown in Figure 2.
Figure 2.
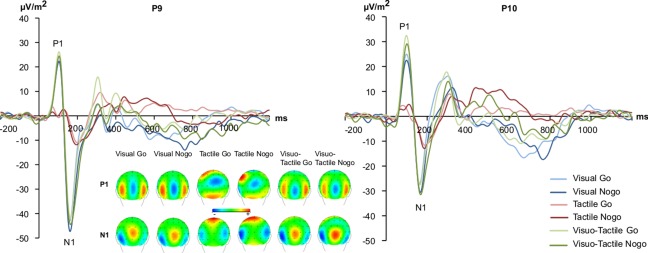
The P1 and N1 event related‐potentials shown at electrode P9 and P10. The y‐axis denotes μV/m2 and the x‐axis denotes time in ms. Time point zero denotes the time point of Go or Nogo stimulus presentation. Blue lines denote the ERPs in the visual, red in the tactile and green in the visual‐tactile condition; darker and brighter shading of these colours denote Nogo or Go trials, respectively. The scalp topography plots for Go and Nogo conditions are shown for the P1 and N1 ERPs in each experimental condition. Warm colors indicate positive and cold colors denote negative amplitudes. [Color figure can be viewed at http://wileyonlinelibrary.com]
There was a trivial main effect of “condition” [F (2,46) = 32.05, P < 0.001; η 2 = 0.582] with the P1 over occipital electrode leads and on tactile trials (8.43 ± 1.89) being smaller than on visual (27.19 ± 3.48) and visual‐tactile trials (33.03 ± 3.44) (all P < 0.001). No other main or interaction effects reached the level of significance (all F < 1.87, P > 0.185).
Concerning the N1 amplitudes over occipital electrode leads, a main effect of condition [F (2,46) = 28.11, P < 0.001; η 2 = 0.550] revealed a trivial effect that the N1 was smaller in the tactile (−12.62 ± 1.58) than in the other conditions (P < 0.001). The visual (−42.42 ± 5.47) and the visual‐tactile (−43.92 ± 5.74) condition did not differ in N1 amplitudes (P > 0.9). The main effect “Go/Nogo” was significant [F (1,23) = 10.12; P = 0.004; η 2 = 0.306] showing higher amplitudes on Nogo (−35.35 ± 4.02) than on Go trials (−30.62 ± 3.71). The main effect “electrode” [F (1,23) = 4.35; P = 0.048; η 2 = 0.159] revealed that the N1 was larger at electrode P9 (−36.85 ± 4.86) than at electrode P10 (−29.12 ± 3.47). The only significant interaction was an interaction “condition x electrode” [F (2,46) = 7.57; P = 0.007; η 2 = 0.248]. It is shown that there was no N1 amplitude difference between electrodes in the tactile condition (P > 0.9), while in the other conditions the N1 was larger at electrode P9 than at P10 (P < 0.01). There were no latency effects for the P1 and N1 (all F < 0.3, P > 0.7).
Response inhibition subprocesses
The N2 and P3 ERPs are shown in Figure 3.
Figure 3.
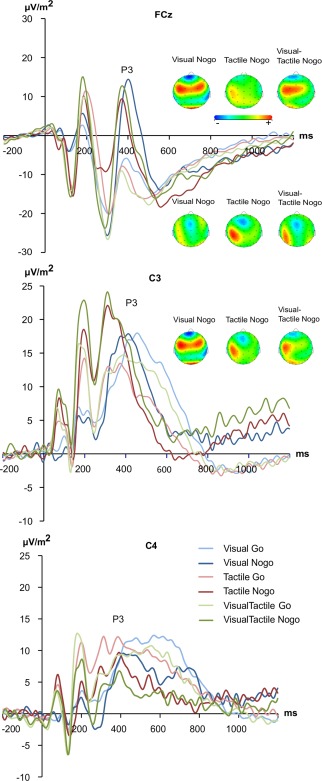
The N2 and P3 event related‐potentials shown at electrode FCz, C3, and C4. The y‐axis denotes μV/m2 and the x‐axis denotes time in ms. Time point zero denotes the time point of delivering Go or Nogo stimuli. Blue lines denote the ERPs in the visual, red in the tactile and green in the visual‐tactile condition; darker and brighter shading of these colours denote Nogo or Go trials, respectively. The scalp topography plots for Go and Nogo conditions are shown for each experimental condition. Warm colors indicate positive and cold colors denote negative amplitudes. [Color figure can be viewed at http://wileyonlinelibrary.com]
For the N2, peak to peak amplitudes were calculated as the preceding P2 peak showed strong variations between conditions (refer also Fig. 4)1. The repeated measures ANOVA revealed a main effect of “condition” [F (2,46) = 11.00, P < 0.001; η 2 = 0.324] with smaller N2s on visual (−19.31 ± 1.94) and tactile (−23.06 ± 2.64) than visual‐tactile trials (−29.00 ± 2.59) (all P < 0.004). The main effect “Go/Nogo” [F (1,23) = 72.63, P < 0.001; η 2 = 0.759] showed more negative amplitudes on Nogo (−40.30 ± 3.56) than on Go trials (7.28 ± 1.89). Importantly, there was a significant interaction “condition x Go/Nogo” [F (2,46) = 6.60, P = 0.003; η 2 = 0.223]. Post hoc tests for this interaction revealed no N2 amplitude differences between the conditions on Go trials (P > 0.7). However, on Nogo trials the tactile condition revealed the smallest Nogo‐N2 (−23.33 ± 1.55) differing from the visual (−32.12 ± 3.55) and visuotactile condition (−39.22 ± 4.11). The visual and the visuotactile condition did not differ in their Nogo‐N2 amplitudes (P > 0.2). Using sLORETA we examined what functional neuroanatomical structures relate to the observed effects for the Nogo‐N2 amplitudes; i.e. the sLORETA comparison was performed for the time point of the Nogo‐N2 peaks. The results of the sLORETA analyses are shown in Figure 4. Only significant (P < 0.01) activation differences are shown in the sLORETA maps (Fig. 4), which were corrected for multiple comparisons using randomization test with 2000 permutations based on statistical nonparametric mapping, SnPM.
Figure 4.
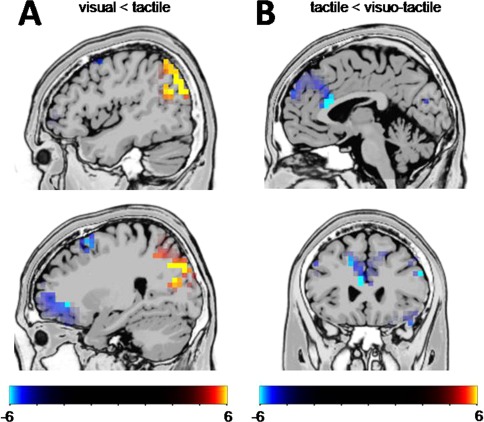
(A) Results from the sLORETA analysis contrasting the Nogo visual against the Nogo tactile condition for the time point of the Nogo‐N2 peaks. The differences in activations are located in the right hemisphere. (B) Results from the sLORETA analysis contrasting the Nogo tactile against the Nogo visuotactile condition. The images show critical t‐values. [Color figure can be viewed at http://wileyonlinelibrary.com]
Contrasting the visual and the tactile condition (Fig. 4A) it is shown that areas in the right posterior parietal cortex including the right angular gyrus (BA39) and right inferior parietal lobe (BA7, BA39) and hence the right temporoparietal junction (TPJ) are stronger activated in the tactile condition than in the visual condition. However, the sLORETA analysis further revealed that areas in the middle (MFG) and superior frontal gyrus (SFG) (BA6), as well as the right inferior frontal gyrus (IFG) (BA44) showed weaker activation in the tactile than in the visual condition. Contrasting the visuotactile and the tactile condition (Fig. 4B) it is shown that activation differences were seen in the left anterior cingulate cortex (ACC) (BA32) and the left medial frontal gyrus (BA9) with the tactile condition showing weaker neuronal activity in this area compared to the visuotactile condition.
Concerning the P3 amplitudes, a main effect “electrode” [F (2,46) = 16.79, P < 0.001; η 2 = 0.425] revealed that the P3 was larger at electrode C3 (17.02 ± 2.51) than electrode C4 (7.65 ± 2.29) and electrode FCz (−0.335 ± 1.70) (all P < 0.019). The main effect “Go/Nogo” [F (1,23) = 84.99, P < 0.001; η 2 = 0.787] revealed higher amplitudes on Nogo (12.67 ± 1.57) than on Go trials (3.55 ± 1.30). The only significant interaction was an interaction “electrode x Go/Nogo” [F (2,46) = 41.91, P < 0.001; η 2 = 0.646]. On Go trials the P3 amplitude was significantly larger at electrode C3 than on the other electrodes, while electrode C4 was larger than electrode FCz (all P < 0.001). The P3 amplitudes on Nogo trials were larger at electrode C3 than on electrode FCz (P = 0.046) and electrode C4 (P = 0.001). No other main or interaction effects reached the level of significance [all F < 3.07, P > 0.056]; i.e. there were no amplitude differences between visual (8.13 ± 1.63), tactile (7.98 ± 1.43), and visual‐tactile trials (7.99 ± 1.67). There were no latency effects for the Nogo‐N2 and Nogo‐P3 (all F < 0.9, P > 0.2). As with the behavioral data, the inclusion of an additional between‐subject factor “sex” in the analyses did not change the pattern of results (P > 0.4).
DISCUSSION
In the current study we examined the question in how far sensory modalities (i.e. the tactile and the visual) differ in their efficiency to trigger response inhibition processes. This was motivated by functional neuroanatomical considerations.
The results show that response inhibition processes are better when being triggered by the tactile modality, compared to the visual modality, because the rate of false alarms was lower in tactile than in the visual modality. However, when tactile and visual stimuli were presented simultaneously, performance in response inhibition (as indicated by the false alarm data), was comparable to the level when using single visual stimuli. This shows that even though two modalities are combined, which has been shown to foster response inhibition processes [Cavina‐Pratesi et al., 2001; Chmielewski et al., 2015; Gondan et al., 2010], this does not necessarily lead to better response inhibition performance (refer false alarm data). The results obtained regarding response inhibition performance (false alarm data) in the current study are therefore not in line with co‐activation models stating that a similar multisensory input facilitates subsequent cognitive processes [Miller et al., 2004]. Effects of multisensory (visual‐auditory) information on performance in Go/Nogo tasks can be interpreted with co‐activation models [Chmielewski et al., 2015; Gondan et al., 2010]. However, Gondan et al. [2010] suggests that the suitability of co‐activation models to explain the effects of multisensory input on response inhibition may depend on the nature of the stimuli. The current results are in line with this. It seems that for the tactile modality the co‐activation account is not suitable when response inhibition processes are concerned—as otherwise the false alarm rate shall be better than in the single visual or single tactile condition. Yet, the RT data on Go trials is in line with co‐activation models. Together, the behavioral data on response inhibition, and especially the false alarm data, suggest that even though the tactile modality is more efficient to trigger response inhibition processes than the visual modality it loses this property when being combined with the visual modality. Another explanation for the increased rate of false alarms in the visuotactile condition relates to the increase in the speed of responding on Go trials. False alarm rates are not independent from reaction times [Gondan et al., 2010]. As the Go process has, on average, become faster in the visuotactile condition, and the no‐go process has still the same speed it cannot “catch up.” As a result, more false alarms are committed. The fact that subjects respond significantly faster during Go trials in the visuotactile condition than in the tactile condition supports this alternative hypothesis. The neurophysiological data provides insights into the mechanisms that relate to these processes:
Response inhibition processes are reflected by the Nogo‐N2 and Nogo‐P3 [Huster et al., 2013]. For the Nogo‐N2 it has been suggested that it reflects either a conflict between executing or to inhibiting a particular response [Nieuwenhuis et al., 2004]. For the (Nogo)‐N2 the results show that in the visual condition the N2 amplitude was larger on Nogo than on Go trials, which is well in line with the literature [Huster et al., 2013]. In the tactile condition, however, this pattern was reversed since the N2 amplitude was stronger on Go than on Nogo trials. In the visuotactile condition no amplitude difference between Go and Nogo trials was observed. Interestingly, the Nogo‐N2 amplitude in the tactile condition was smaller than in the visual and visuotactile condition. The Nogo‐N2 amplitude was therefore smallest in the condition with best response inhibition performance. This pattern of results is in line with the functional interpretation of the (Nogo)‐N2 to reflect a response conflict [Nieuwenhuis et al., 2004]. When being triggered by the tactile modality, the conflict to execute or inhibit a response may be smaller which leads to better response inhibition performance (i.e. fewer false alarms). Of note the sLORETA analyses (Fig. 4A), showing underlying sources using randomization tests and SnPM, suggest that posterior parietal areas including the angular gyrus (BA39) and inferior parietal lobe (BA7, BA39) and hence the temporoparietal junction (TPJ) are stronger activated in the tactile condition than in the visual condition. It has been suggested that the functional role of the TPJ relates to “contextual updating” in the sense that the TPJ updates internal representations based on new sensory information to initiate task‐appropriate actions [Geng and Vossel, 2013]. This role has recently been shown to be important for response inhibition [Dippel et al., 2016]. It is seems that the tactile modality is more powerful to update internal representations and to initiate task‐appropriate actions, which leads to the better response inhibition performance. Critically, the sLORETA analysis further suggests that when compared to the visual condition, areas in the middle (MFG) and superior frontal gyrus (SFG) (BA6), right inferior frontal gyrus (IFG) (BA44) showed weaker activation in the tactile than in the visual condition. Areas including the MFG and especially the SFG are known to be modulated by the degree of cognitive conflict [Rushworth et al., 2004]. The lower activations seen in these regions in the tactile condition is in line with the above interpretation of a reduced cognitive conflict. However, these areas and the rIFG have moreover frequently been implicated to mediate response inhibition processes [Bari and Robbins, 2013]. The lower activation of brain regions known to constitute a response inhibition network together with better behavioral response inhibition performance suggests that response inhibition networks become more efficient when being triggered by the tactile modality. Interestingly the identified brain regions showing weaker activation in the tactile than in the visual condition, especially the SFG (BA6), have been shown to yield strong structural neuroanatomical connections to somatosensory association cortices [Borich et al., 2015; Fang et al., 2005]. It may be speculated that this structural neuroanatomical property is important for the effects observed in the SFG and MFG in the tactile experimental condition. Yet, not only cortico‐cortical, but also subcortico‐cortical connections could be important in response inhibition processes because there are direct projections from e.g. visual cortices via tectal and thalamic structures to the basal ganglia [Coizet et al., 2009; Redgrave et al., 2010] and hence fronto‐striatal circuits, which play an important role in response inhibition [Bari and Robbins, 2013]. Future studies shall evaluate in how far one of these possible pathway may be involved.
When comparing the tactile with the visuotactile condition activation differences were seen in the anterior cingulate cortex (ACC) (BA32) and the medial frontal gyrus (BA9) with the tactile condition showing weaker neuronal activity in this area compared to the visuotactile condition (Fig. 4B). Using source localization, the ACC has repeatedly been shown to underlie modulations in the Nogo‐N2 [Huster et al., 2013; Kropotov et al., 2011; Pandey et al., 2012]. The behavioral data clearly shows that in the visuotactile condition response inhibition performance (cf. false alarm data) was worse compared to the tactile condition and at the same level as in the visual condition. It therefore appears that there is a multisensory conflict despite the information conveyed via the visual and tactile modality was identical. There is thus no gain by redundant information, but rather costs of redundant visuotactile information when it comes to response inhibition processes. Such redundancy costs emerge when stimuli compete for neural representation, suppressing the encoding of any single modality. Competitive mechanisms are common to sensory processes where competition leads to suppression of certain sensory stimuli [Desimone and Duncan, 1995]. However, it has been shown that such perceptual conflicts also modulate medial frontal regions including the ACC [Labrenz et al., 2012; Westerhausen et al., 2010]. The results suggest that there is a multisensory conflict or competition in the ACC that compromises response inhibition performance (cf. false alarm data). It may be speculated that the visual information wins this competition in medial frontal areas and determines response inhibition processes, because performance in the visuotactile condition was comparable to the condition with pure visual stimuli to trigger response inhibition.
Because it was especially the Nogo‐N2 which was differentially modulated across conditions, the data suggest that the effect of stimulus modality on response inhibition emerge at the level of conflict monitoring processes during inhibitory control. In fact, the results are quite specific since no specific modulations of Go and Nogo trials were observed for the (Nogo)‐P3 and hence processes related to the evaluative processing of the successful outcome of the inhibition [Beste et al., 2009, 2011, 2010; Huster et al., 2013], or the inhibition itself [Wessel and Aron, 2015]. For perceptual and attentional selection processes (i.e. P1 and N1 potentials) only trivial effects were obtained showing that these potentials measured over occipital leads were smaller in the tactile than in the other conditions including visual stimuli. This suggests that attentional selection processes are not relevant for the effects of stimulus modality on response inhibition processes.
The results of this study have clinical implications for neurological disorders like Tourette's Syndrom, showing a hypersensitivity of the somatosensory system. These somatosensory areas as well medial frontal regions with the ACC are involved in Tic generation and premonitory perceptions [Bohlhalter et al., 2006]. It is therefore likely that the dependence of inhibitory control processes by sensory modalities may be useful to understand peculiarities in sensory as well as executive control functions neuropsychiatric disorders. Furthermore, future studies may investigate the effects of age, because white matter and their impact on structural connectivity is subject to considerable maturational effects.
A limitation of the study is that we have not included a condition using visual Go trials and tactile Nogo trials, or a condition using visual Nogo trials and tactile Go trials. However, the main focus of this study was on the impact of different sensory modalities on response inhibition processes and not generally on a bias towards the tactile system. Regarding this, a Go‐tactile/Nogo‐visual condition as well as a Go‐visual/Nogo‐tactile are not too central, because the visual and the tactile dimension variation on Nogo trials has been incorporated in the paradigm.
In summary, the study shows that sensory modalities differ in their efficiency to trigger response inhibition processes. The tactile modality is more powerful than the visual modality. However, the tactile modality loses this efficacy when being combined with the visual modality. This may be due to competitive mechanisms leading to a suppression of certain sensory stimuli. Variations in the sensory modality specifically affected conflict monitoring processes during response inhibition, but not perceptual and attentional selection processes, resource allocation and the motor inhibition process per se. The data shows that differences between modalities and combined visuotactile stimuli are due to processing changes in a frontoparietal network encompassing middle and superior frontal areas, the right inferior frontal gyrus and the temporoparietal junction. This suggests that the functional neuroanatomical networks involved is response inhibition critically depends on the nature of the sensory input.
Footnotes
However, the same results were for the N2 were obtained when the statistics were calculated of the peak to baseline amplitudes.
REFERENCES
- Andersen RA, Buneo CA (2002): Intentional maps in posterior parietal cortex. Annu Rev Neurosci 25:189–220. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA (2004): Inhibition and the right inferior frontal cortex. Trends Cogn Sci 8:170–177. [DOI] [PubMed] [Google Scholar]
- Bari A, Robbins TW (2013): Inhibition and impulsivity: behavioral and neural basis of response control. Prog Neurobiol 108:44–79. [DOI] [PubMed] [Google Scholar]
- Beste C, Dziobek I, Hielscher H, Willemssen R, Falkenstein M (2009): Effects of stimulus‐response compatibility on inhibitory processes in Parkinson's disease. Eur J Neurosci 29:855–860. [DOI] [PubMed] [Google Scholar]
- Beste C, Ness V, Falkenstein M, Saft C (2011): On the role of fronto‐striatal neural synchronization processes for response inhibition–evidence from ERP phase‐synchronization analyses in pre‐manifest Huntington's disease gene mutation carriers. Neuropsychologia 49:3484–3493. [DOI] [PubMed] [Google Scholar]
- Beste C, Stock A‐K, Epplen JT, Arning L (2016): Dissociable electrophysiological subprocesses during response inhibition are differentially modulated by dopamine D1 and D2 receptors. Eur Neuropsychopharmacol 26:1029‐1036. [DOI] [PubMed] [Google Scholar]
- Beste C, Willemssen R, Saft C, Falkenstein M (2010): Response inhibition subprocesses and dopaminergic pathways: Basal ganglia disease effects. Neuropsychologia 48:366–373. [DOI] [PubMed] [Google Scholar]
- Bohlhalter S, Goldfine A, Matteson S, Garraux G, Hanakawa T, Kansaku K, Wurzmann R, Hallett M (2006): Neural correlates of tic generation in Tourette syndrome: An event‐related functional MRI study. Brain 129:2029–2037. [DOI] [PubMed] [Google Scholar]
- Bonnefond A, Doignon‐Camus N, Touzalin‐Chretien P, Dufour A (2010): Vigilance and intrinsic maintenance of alert state: An ERP study. Behav Brain Res 211:185–190. [DOI] [PubMed] [Google Scholar]
- Borich MR, Brodie SM, Gray WA, Ionta S, Boyd LA (2015): Understanding the role of the primary somatosensory cortex: Opportunities for rehabilitation. Neuropsychologia 79:246–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavina‐Pratesi C, Bricolo E, Prior M, Marzi CA (2001): Redundancy gain in the stop‐signal paradigm: implications for the locus of coactivation in simple reaction time. J Exp Psychol Hum Percept Perform 27:932–941. [PubMed] [Google Scholar]
- Chmielewski WX, Mückschel M, Dippel G, Beste C (2015): Concurrent information affects response inhibition processes via the modulation of theta oscillations in cognitive control networks. Brain Struct Funct 221:3949–3961. [DOI] [PubMed] [Google Scholar]
- Coizet V, Graham JH, Moss J, Bolam JP, Savasta M, McHaffie JG, Redgrave P, Overton PG (2009): Short‐latency visual input to the subthalamic nucleus is provided by the midbrain superior colliculus. J Neurosci 29:5701–5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R, Duncan J (1995): Neural mechanisms of selective visual attention. Annu Rev Neurosci 18:193–222. [DOI] [PubMed] [Google Scholar]
- Diamond A (2013): Executive functions. Annu Rev Psychol 64:135–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dippel G, Beste C (2015): A causal role of the right inferior frontal cortex in implementing strategies for multi‐component behaviour. Nat Commun 6:6587. [DOI] [PubMed] [Google Scholar]
- Dippel G, Chmielewski W, Mückschel M, Beste C (2016): Response mode‐dependent differences in neurofunctional networks during response inhibition: an EEG‐beamforming study. Brain Struct Funct 221:4091–4101. [DOI] [PubMed] [Google Scholar]
- Driver J, Noesselt T (2008): Multisensory interplay reveals crossmodal influences on “sensory‐specific” brain regions, neural responses, and judgments. Neuron 57:11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang P‐C, Stepniewska I, Kaas JH (2005): Ipsilateral cortical connections of motor, premotor, frontal eye, and posterior parietal fields in a prosimian primate, Otolemur garnetti. J Comp Neurol 490:305–333. [DOI] [PubMed] [Google Scholar]
- Fuchs M, Kastner J, Wagner M, Hawes S, Ebersole JS (2002): A standardized boundary element method volume conductor model. Clin Neurophysiol off J Int Fed Clin Neurophysiol 113:702–712. [DOI] [PubMed] [Google Scholar]
- Geng JJ, Vossel S (2013): Re‐evaluating the role of TPJ in attentional control: contextual updating?. Neurosci Biobehav Rev 37:2608–2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gondan M, Götze C, Greenlee MW (2010): Redundancy gains in simple responses and go/no‐go tasks. Atten Percept Psychophys 72:1692–1709. [DOI] [PubMed] [Google Scholar]
- Hagmann R, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, Sporns O (2008): Mapping the structural core of the human cerebral cortex. PLoS Biol 6:e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huster RJ, Enriquez‐Geppert S, Lavallee CF, Falkenstein M, Herrmann CS (2013): Electroencephalography of response inhibition tasks: functional networks and cognitive contributions. Int J Psychophysiol off J Int Organ Psychophysiol 87:217–233. [DOI] [PubMed] [Google Scholar]
- Huster RJ, Westerhausen R, Pantev C, Konrad C (2010): The role of the cingulate cortex as neural generator of the N200 and P300 in a tactile response inhibition task. Hum Brain Mapp 31:1260–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropotov JD, Ponomarev VA, Hollup S, Mueller A (2011): Dissociating action inhibition, conflict monitoring and sensory mismatch into independent components of event related potentials in GO/NOGO task. Neuroimage 57:565–575. [DOI] [PubMed] [Google Scholar]
- Labrenz F, Themann M, Wascher E, Beste C, Pfleiderer B (2012): Neural correlates of individual performance differences in resolving perceptual conflict. PLoS One 7:e42849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luppino G, Matelli M, Camarda R, Rizzolatti G (1993): Corticocortical connections of area F3 (SMA‐proper) and area F6 (pre‐SMA) in the macaque monkey. J Comp Neurol 338:114–140. [DOI] [PubMed] [Google Scholar]
- Marco‐Pallarés J, Grau C, Ruffini G (2005): Combined ICA‐LORETA analysis of mismatch negativity. Neuroimage 25:471–477. [DOI] [PubMed] [Google Scholar]
- Miller J, Kühlwein E, Ulrich R (2004): Effects of redundant visual stimuli on temporal order judgments. Percept Psychophys 66:563–573. [DOI] [PubMed] [Google Scholar]
- Mückschel M, Stock A‐K, Beste C (2014): Psychophysiological mechanisms of interindividual differences in goal activation modes during action cascading. Cereb Cortex (NY, 1991) 24:2120–2129. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Yeung N, Cohen JD (2004): Stimulus modality, perceptual overlap, and the go/no‐go N2. Psychophysiology 41:157–160. [DOI] [PubMed] [Google Scholar]
- Nunez PL, Pilgreen KL (1991): The spline‐Laplacian in clinical neurophysiology: A method to improve EEG spatial resolution. J Clin Neurophysiol Off Publ Am Electroencephalogr Soc 8:397–413. [PubMed] [Google Scholar]
- Pandey AK, Kamarajan C, Tang Y, Chorlian DB, Roopesh BN, Manz N, Stimus A, Rangaswamy M, Porjesz B (2012): Neurocognitive deficits in male alcoholics: an ERP/sLORETA analysis of the N2 component in an equal probability Go/NoGo task. Biol Psychol 89:170–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual‐Marqui RD (2002): Standardized low‐resolution brain electromagnetic tomography (sLORETA): Technical details. Methods Find Exp Clin Pharmacol 24(Suppl D):5–12. [PubMed] [Google Scholar]
- Redgrave P, Coizet V, Comoli E, McHaffie JG, Leriche M, Vautrelle N, Hayes LM, Overton P (2010): Interactions between the midbrain superior colliculus and the basal ganglia. Front Neuroanat 4:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth MFS, Walton ME, Kennerley SW, Bannerman DM (2004): Action sets and decisions in the medial frontal cortex. Trends Cogn Sci 8:410–417. [DOI] [PubMed] [Google Scholar]
- Sekihara K, Sahani M, Nagarajan SS (2005): Localization bias and spatial resolution of adaptive and non‐adaptive spatial filters for MEG source reconstruction. Neuroimage 25:1056–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shedden JM, Reid GS (2001): A variable mapping task produces symmetrical interference between global information and local information. Percept Psychophys 63:241–252. [DOI] [PubMed] [Google Scholar]
- Staub B, Doignon‐Camus N, Bacon É, Bonnefond A (2014): The effects of aging on sustained attention ability: an ERP study. Psychol Aging 29:684–695. [DOI] [PubMed] [Google Scholar]
- Stock A‐K, Popescu F, Neuhaus AH, Beste C (2015): Single‐subject prediction of response inhibition behavior by event‐related potentials. J Neurophysiol 115:1252–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F, Liefooghe B, Vandierendonck A (2006): The effect of interference in the early processing stages on response inhibition in the stop signal task. Q J Exp Psychol 59:190–203. [DOI] [PubMed] [Google Scholar]
- Wessel JR, Aron AR (2015): It's not too late: The onset of the frontocentral P3 indexes successful response inhibition in the stop‐signal paradigm. Psychophysiology 52:472–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerhausen R, Moosmann M, Alho K, Belsby S‐O, Hämäläinen H, Medvedev S, Specht K, Hugdahl K (2010): Identification of attention and cognitive control networks in a parametric auditory fMRI study. Neuropsychologia 48:2075–2081. [DOI] [PubMed] [Google Scholar]


