Abstract
Positive symptoms of schizophrenia such as delusions and hallucinations are thought to arise from an alteration in predictive mechanisms of the brain. Here, we empirically tested the hypothesis that schizophrenia is associated with an enhanced signalling of higher‐level predictions that shape perception into conformity with acquired beliefs. Twenty‐one patients with schizophrenia and twenty‐eight healthy controls matched for age and gender took part in a functional magnetic resonance imaging (fMRI) experiment that assessed the effect of an experimental manipulation of cognitive beliefs on the perception of an ambiguous visual motion stimulus. At the behavioural level, there was a generally weaker effect of experimentally induced beliefs on perception in schizophrenia patients compared with controls, but a positive correlation between the effect of beliefs on perception and the severity of positive symptoms. At the neural level, belief‐related connectivity between a region encoding beliefs in the orbitofrontal cortex (OFC) and a region encoding visual motion in the visual cortex (V5) was higher in patients compared with controls, indicating a stronger impact of cognitive beliefs on visual processing in schizophrenia. We suggest that schizophrenia might be associated with a generally weaker acquisition of externally generated beliefs and a compensatory increase in the effect of beliefs on sensory processing. Our current results are in line with the notion that enhanced signalling of higher‐level predictions that shape perception into conformity with acquired beliefs might underlie positive symptoms in schizophrenia. Hum Brain Mapp 38:1767–1779, 2017. © 2017 Wiley Periodicals, Inc.
Keywords: schizophrenia, visual perception, functional magnetic resonance imaging, predictive coding, psychosis, hallucinations, delusions, placebo effect
INTRODUCTION
Hallucinations, percepts in the absence of a causative stimulus, and delusions, fixed beliefs that are not founded in the external reality, are the core symptoms of psychosis. Current conceptual models of psychotic symptoms elegantly explain these concomitant alterations of perception and beliefs in the framework of predictive coding [Friston, 2005; Kersten et al., 2004; Mumford, 1992]. Predictive coding draws on the idea that perception is an inferential process that combines sensory information with endogenous predictions based on prior experience in order to generate a stable and unitary representation of the world. Such endogenous predictions stem from an internal model representing beliefs about the outer world and are fed back from higher to lower levels of the information processing hierarchy, where they are integrated with incoming sensory information. The mismatch between the endogenous predictions and the incoming sensory information gives rise to a prediction error signal that is fed forward from lower to higher processing levels and drives learning by updating the internal belief model. Hence, predictive coding formalizes both perception and beliefs at the same time and therefore provides an excellent starting point for explaining hallucinations and delusions [Corlett et al., 2010; Fletcher and Frith, 2009].
In this context, psychosis is usually attributed to an altered integration of predictions with the sensory information [Adams et al., 2013; Hemsley, 2005], and the concomitant aberrant prediction error signals are thought to drive maladaptive belief updates [Corlett et al., 2009; Fletcher and Frith, 2009]. Hence, weakened or imprecise predictions are assumed to render sensory events surprising and salient, and the cognitive effort to make sense of such aberrant salience is thought to result in the adoption of delusional beliefs. The notion of a weakened influence of predictions on perception in psychosis is substantiated by a large body of evidence obtained in patients with schizophrenia [see Notredame et al., 2014 for a comprehensive review including also non‐confirmatory findings], a condition characterized by recurrent or persistent psychotic symptoms. For instance, we have previously shown that schizophrenia patients compared with healthy controls exhibit a weaker influence of stabilizing predictions on perception of an ambiguous visual stimulus [Schmack et al., 2015]. Moreover, schizophrenia patients compared with healthy controls are less susceptible to different visual illusions that depend on the integration of predictions with ambiguous sensory information, sometimes leading to more ‘veridical’ percepts [Dakin et al., 2005; Sanders et al., 2012; Schneider et al., 2002; Uhlhaas et al., 2004]. These findings are paralleled by visual cortex responses that are less biased by illusion‐related predictions [Seymour et al., 2013] as well as a weaker functional connectivity between higher‐level and lower‐level visual areas during viewing of a visual illusion [Dima et al., 2009], lending support to the notion that weakened predictive signalling is linked to psychosis.
However, while providing a powerful explanation for the formation of delusions, the outlined predictive coding framework fails to explain other core aspects of psychosis. In particular, delusions are characterized by a tendency to persist despite contrary evidence. This tenacity of delusions would rather suggest a strengthened than a weakened influence of predictions, thereby shaping sensory information into conformity with delusional beliefs [Adams et al., 2013; Corlett et al., 2009]. Along similar lines, hallucinations could be understood as the result of an increased effect of predictions on perception, such that sensory noise is transformed into hallucinatory percepts [Adams et al., 2013; Corlett et al., 2009; Powers et al., 2016]. These apparently contradictory notions of either a decrease or an increase of predictive signalling in psychosis can be reconciled by a hierarchical model that assumes that an imbalance between lower‐level and higher‐level predictive signalling lies at the core of psychosis [Schmack et al., 2013]. According to this model, reduced predictive signalling within lower sensory levels of the cortical processing hierarchy leads to unstable sensory representations, which results in the experience of overly salient sensory events and therefore explain the emergence of delusional misinterpretations. To compensate for these unstable sensory representations, predictive signalling from higher‐level non‐sensory brain circuits that encode beliefs is enhanced. Thereby, perception is stabilized by being sculpted into conformity with beliefs, which accounts for hallucinations and the tenacious maintenance of delusions. However, while previous work convincingly indicates a decreased signalling of lower‐level perceptual predictions in psychosis [Dakin et al., 2005; Dima et al., 2009; Sanders et al., 2012; Schmack et al., 2015; Schneider et al., 2002; Seymour et al., 2013; Uhlhaas et al., 2004], evidence with respect to the signalling of higher‐level predictions in psychosis is sparse.
Here, we sought to empirically test our predictive coding account of psychosis in a population of patients with schizophrenia and healthy controls. In an fMRI experiment, we used a placebo‐like manipulation to induce beliefs about the appearance of an ambiguous stimulus. We hypothesized that schizophrenia is associated with an enhanced signalling of such higher‐level predictions from the orbitofrontal cortex, a region previously implicated in the generation of experience‐induced beliefs [Bar et al., 2006; Petrovic et al., 2002; Schmack et al., 2013], and the visual cortex.
METHODS
Participants
Twenty‐one patients diagnosed with schizophrenia and twenty‐seven age‐ and gender‐matched healthy controls completed the study (see Table 1 for demographic and clinical characteristics). Patients were recruited at the Department of Psychiatry and Psychotherapy (Campus Charité Mitte) of the Charité‐Universitätsmedizin Berlin. All patients fulfilled the ICD‐10 criteria for paranoid schizophrenia and had no other psychiatric axis I disorder according to DSM IV [SCID I, First et al., 2002a]. Psychopathological symptoms were quantitatively assessed using the Positive and Negative Symptoms Scale (PANSS). Except one unmedicated patient, all patients had been on stable doses of second‐generation antipsychotic drugs for at least 4 weeks. Healthy volunteers had no axis I psychiatric disorder [SCID‐I, First et al., 2002b] and no family history of psychiatric disorders. Exclusion criteria in both groups were neurological disorders and drug abuse up to seven days before testing. All participants had normal or corrected‐to‐normal vision and gave written informed consent before participation. The study was approved by the local ethics committee of Charité‐Universitätsmedizin Berlin.
Table 1.
Demographic and clinical characteristics of schizophrenia patients and healthy controls
| Schizophrenia patients | Healthy controls | Two‐sided | |
|---|---|---|---|
| (n = 21) | (n = 28) | P‐value | |
| Age | 34.1 ± 6.7a | 31.5 ± 7.2a | 0.20b |
| Gender | 5 F–16 M | 8 F–20 M | 0.71c |
| Years of education | 14.2 ± 3.2a | 16.7 ± 3.0a | 0.01b |
|
Verbal intelligence (IQ in WST test) |
105.2 ± 8.8a | 107.4 ± 6.8a | <0.001b |
| Attention span (TN‐E in d2 test) | 152.0 ± 34.0a | 183.3 ± 43.3a | <0.001b |
| PDI | |||
| Yes/no | 17.2 ± 7.8a | 5.8 ± 5.6a | <0.001b |
| Conviction | 54.8 ± 33.4a | 15.0 ± 15.7a | <0.001b |
| Distress | 55.1 ± 32.4a | 10.9 ± 12.6a | <0.001b |
| Preoccupation | 51.3 ± 30.0a | 11.5 ± 12.9a | <0.001b |
| Sum | 178.4 ± 100.8a | 43.1 ± 45.4a | <0.001b |
| PANNS | |||
| Positive | 14.1 ± 4.8a | – | – |
| Negative | 13.6 ± 5.4a | – | – |
| General | 27.3 ± 9.4a | – | – |
| Medication | |||
| Daily olanzapine equivalent dose (mg) | 10.9 ± 5.2 | – | – |
Mean ± SD.
Two‐sample t‐test.
Chi‐squared test.
One additional healthy control participant completed the study, but was excluded from further analyses as visual inspection of the structural MRI scan revealed evidence of a macroscopic brain abnormality. Two additional schizophrenia patients were excluded from the study after a pre‐scanning behavioural experiment because of impaired stereopsis (see below).
Experimental Design
Inside the scanner, patients and controls performed a task in which we induced beliefs about a visual ambiguous stimulus using a placebo‐like manipulation [Schmack et al., 2013; Fig. 1A; Sterzer et al., 2008]. In brief, beliefs were induced in a learning phase by presenting stimuli that were unambiguous and truly biased in terms of their visual motion direction. However, participants were told that the stimuli were ambiguous and that the motion direction bias was caused by the glasses through which they viewed the stimuli. Crucially, in a subsequent test phase, participants retained their glasses (and beliefs about the prevalent direction of motion), and the stimuli actually presented were ambiguous and hence truly not biased. This enabled us to assess the impact of beliefs induced by prior experience on perception of an ambiguous and unbiased stimulus.
Figure 1.
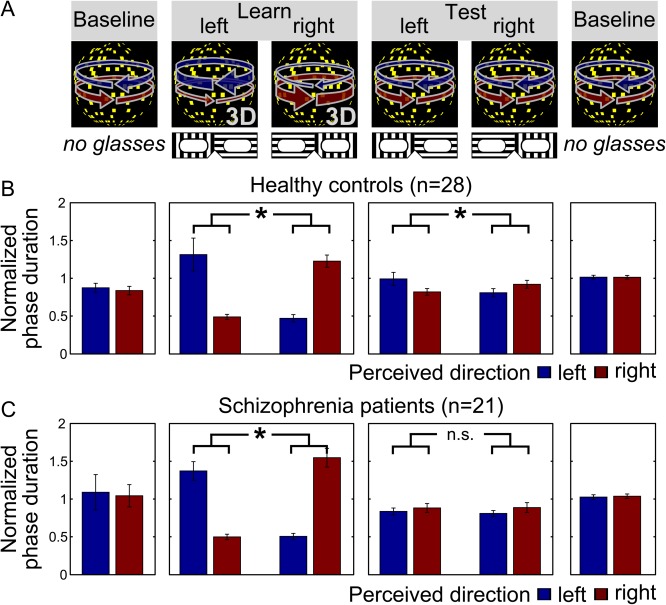
Behavioural effect of beliefs in healthy controls and schizophrenia patients. (A) Schematic illustration of the task. Higher‐level cognitive beliefs were induced by a placebo‐like experimental manipulation. During fMRI scanning, participants viewed a bistable stimulus that is alternately perceived as a sphere rotating to the right and a sphere rotating to the left, and indicated changes in perceived rotation direction by button presses. In the initial and final baseline phases, the rotation direction of the sphere was unbiased leading to comparable percept durations between right‐ and leftward rotation. In the learning phase, rotation direction was biased by stereoscopic depth cues but participants were made to believe that this bias was due to the glasses they were now wearing. In the test phase, participants retained their glasses and hence their beliefs about the prevalent motion direction, but were now presented with the unbiased sphere stimulus. (B + C) Effect of beliefs on perception in healthy controls and schizophrenia patients. In the learning phase, both controls (B) and patients (C) perceived the belief‐congruent percept for longer phase durations (P < 0.001 and P < 0.001, respectively, two‐sided paired t‐tests). Crucially, in the test phase, controls showed a significant difference between the belief‐congruent and belief‐incongruent percept (B, P = 0.04), whereas this effect of beliefs on perception was absent in patients (C, P = 0.79). Bars show the mean phase duration of each percept normalized with respect to the mean phase duration in the baseline runs. Error bars denote standard error. [Color figure can be viewed at http://wileyonlinelibrary.com]
Visual stimuli were presented using Matlab (MathWorks Inc.) and Cogent 2000 toolbox (http://www.vislab.ucl.ac.uk/cogent.php). They were projected onto a screen (screen size: 24.9° × 18.6°) inside the scanner bore by a projector (ProExtra Multiverse Projector, Sanyo Electric Co. Ltd; refresh rate 60 Hz). Participants viewed the screen via a mirror that was attached to the head coil directly above their eyes. Stimuli were dot‐kinematograms (DKs) that are perceived as a rotating sphere. In order to produce sphere stimuli that were truly biased in terms of their visual motion in the learning phase, all visual stimuli were presented dichoptically to produce stereoscopic vision. To this end, we used a MRI‐compatible system consisting of a plywood divider and prism lenses worn by the participants [Schurger, 2009].
Our DK stimuli were an orthographic projection of a sphere rotating around a vertical axis (radius 5.1° of visual angle, rotation speed 1/18 revolutions/s). It consisted of 450 randomly distributed yellow square ‘dots’ (maximum 0.1° × 0.1° of visual angle), moving coherently left‐ or rightward on a black background with a central fixation cross. Stimuli were surrounded by a white square (5.2° × 5.2° of visual angle). Animation frames were updated every 0.04 s, and a sequence of 200 frames (8 s) was looped repeatedly to produce continuous motion. Dot lifetime was 25 display frames (1 s).
Depending on whether the stimulus was unbiased or truly biased, the rotation direction of the sphere was either ambiguous or unambiguous, respectively. The ambiguous sphere rotating around the vertical axis consisted of two identical DKs presented to each eye. The ambiguous sphere yields bistable perception alternating spontaneously between the two rotation directions. To produce the unambiguous sphere used for the experimental induction of beliefs in the learning phase of the experiment (see below), two slightly different DKs were displayed representing two different perspectives (maximal offset 0.5°). This interocular disparity minimizes the ambiguity of rotation direction. To mimic the spontaneous perceptual alternations that occur during viewing of the ambiguous sphere, the unambiguous sphere alternated between both rotation directions with an overall rate comparable to the subject's switch rate but with different dominance times (80% vs. 20% on average). A brief questionnaire after the experiment indicated that the majority of patients (17 out of 21) and controls (20 out of 28) did not notice a difference between the unbiased and the biased stimulus. Those who noticed a difference attributed it to subjective factors as habituation or fatigue, except one patient who assumed a ‘purpose behind it’ but assured after debriefing that he or she did not suspect a placebo‐like manipulation.
Before entering the scanner, participants were informed that they would perform an experiment on depth perception. They were told that they would view a bistable rotating sphere and were instructed to maintain fixation and indicate changes in perceived rotation direction by key presses. They were told that standard 3D‐glasses would be used in some runs of the experiment. These glasses, which were in reality completely transparent, would contain two different filters so that one eye would receive only horizontally polarized light and the other eye only vertically polarized light. Due to stereoscopic vision induced by these glasses participants' perception would be biased toward one rotation direction. Which rotation direction this was would depend on which eye looked through which filter, and orientation of the glasses would be manually reversed during the experiment. Participants were informed that the experimenter would come into the scanner room between the runs and manually reverse the orientation of the glasses.
During each experimental run, the stimulus was presented continuously for 212 s. The whole experiment consisted of four initial baseline runs, during which the ambiguous sphere was presented, followed by two unambiguous learning runs, four ambiguous test runs, and four final ambiguous baseline runs (Fig. 1). In the learning and test runs subjects wore the transparent glasses which they believed to contain polarizing filters. The actual (learning phase) or expected (test phase) dominant rotation directions in each run were contingent on the orientation of the transparent glasses and were switched between the runs resulting in one learning run and two test runs per orientation, respectively. The association between orientation and rotation direction was counterbalanced across participants to control for systematic effects of the glasses on perception. The order of dominant directions across runs was pseudo‐randomized and balanced across participants to preclude systematic effects of the dominant direction in the second learning run on the following test runs. Participants indicated perceptual transitions by key presses choosing between three response options: leftward rotation, rightward rotation and uncertain perceptual state.
To enable the successful induction of beliefs in the learning phase, we ensured that all included participants were able to distinguish between the leftward and rightward rotation of the unambiguous sphere. On a separate day prior to the scanning session, participants were presented outside the scanner with 40 unambiguous sphere stimuli rotating either to the left or to the right, and had to indicate the perceived rotation direction. All participants performed near‐perfectly in this task (mean percentage of correct trials 98.0%). Two additional schizophrenia patients were not able to reliably distinguish between left‐ and rightward rotation direction (percentage of correct trials 40% and 50%), and were therefore not invited to the fMRI scanning session.
Functional Localizer Scan
To identify areas responsive to visual motion, we performed two additional localizer runs of 235 s each. In order to identify the voxels that maximally responded to the stimulus used in the main experiment, the same moving ambiguous DK was alternated with static images of the DK. Each localizer run comprised five stimulation blocks of 13.5 s duration per condition (moving dots and stationary dots). The blocks were separated by rest periods of 9.0 s duration in which only the fixation cross and the fusion square were shown.
fMRI Acquisition
Functional MR scanning was performed on a 3 T scanner (TRIO Siemens) equipped with a 12‐channel head coil using a T2*‐weighted 2D‐GE‐EPI sequence (two‐dimensional gradient‐echo echo‐planar imaging, TR 2,120 ms, TE 25 ms, flip angle 90°, matrix size 64 × 64, FOV 192 mm, voxel size 3 × 3 × 3 mm). The 38 slices parallel to the calcarine sulcus were collected, covering the whole brain (slice thickness 2.5 mm, interslice gap 0.5 mm). Data were acquired in fourteen runs each comprising 100 volumes. Additionally, two functional localizer runs of 120 volumes each were acquired. For anatomical reference a structural image was collected using a T1‐weighted 3D‐MPRAGE sequence (three dimensional magnetization prepared rapid gradient‐echo, TR 1,900 ms, TE 2.52 ms, matrix size 256 × 256, FOV 256 mm, flip angle 9°, voxel size 1 × 1 × 1 mm).
Analysis of the Behavioural Data
To examine whether patients and controls differed in the effect of beliefs in perceptual inference, for each participant we calculated the belief‐induced bias by normalizing the ratio of belief‐congruent and belief‐incongruent mean percept duration with respect to the ratio from the learning phase. Higher values indicate a stronger belief‐induced bias, hence a stronger effect of beliefs in perceptual inference. As the distribution of the belief‐induced biases was skewed and differed significantly from a normal distribution (P = 0.01, Shapiro–Wilk test), we tested for group differences by a nonparametric Wilcoxon signed rank test (also known as independent samples Mann–Whitney U test). To test for a relationship between the severity of schizophrenia symptoms and the effect of beliefs on perception, we correlated the PANNS positive and negative subscales with the expectation‐induced bias by using nonparametric Spearman's rank correlations and Bonferroni correction for multiple testing.
Given our unexpected finding of lower belief‐induced biases in patients as compared with controls (see Results), we further explored whether antipsychotic medication or illness duration might have influenced the effect of beliefs on perception. To test for a potential influence of antipsychotic medication, we calculated the olanzapine equivalent doses of each patient's antipsychotic medication [Leucht et al., 2016]. To test for a potential influence of illness duration, we used age as a proxy for illness duration, given that we did not have access to reliable data on illness duration. Both of these two measures were then correlated to the expectation‐induced bias by using nonparametric Spearman's rank correlations. We further aimed to elucidate possibility that other factors that differed between the two groups might have contributed to the unexpected group difference of the effect of beliefs. We therefore compared age, gender, attention span (as measured by the d2 test), verbal intelligence (as measured by the vocabulary test ‘Wortschatztest’ [WST]) as well as total years of education between the two groups by two‐sample t‐tests. Each measure that significantly differed between the two groups was then correlated with the belief‐induced bias by nonparametric Spearman's rank correlations.
fMRI Data Analysis
fMRI data analysis was restricted to the experimental test runs of the experiment. fMRI data were pre‐processed and analysed using Statistical Parametric Mapping (SPM8). Images were slice‐time corrected and spatially realigned to the first volume. Each participant's structural T1 image was co‐registered to an individual mean EPI image. Transformation parameters were derived from normalizing the co‐registered structural image to a Montreal Neurological Institute (MNI) template, and the derived parameters were then applied to normalize the EPI volumes. Normalized images were spatially smoothed with a Gaussian kernel (FWHM = 8 × 8 × 8 mm).
Images from the test runs were analysed in an event‐related manner using the general linear model. On the first level, belief‐congruent and belief‐incongruent perceptual switches were modelled separately as regressors of interest. Other button presses indicating uncertainty were included as regressors of no interest. To account for the delay between a perceptual switch and the respective button press, 1,000 ms were subtracted from the times of the button presses. The evoked hemodynamic responses to the estimated perceptual switches were modelled as stick functions convolved with the canonical hemodynamic response function implemented in SPM8 and its first temporal derivative. Additional regressors of no interest were the convolved stick functions of button presses indicating uncertainty, the six movement parameters as well as one constant term per experimental run. Low‐frequency fluctuations were removed using a high‐pass filter with a cut‐off at 128 s. For each participant a contrast image that compared the parameter estimates for belief‐congruent and belief‐incongruent perceptual switches was computed and used for group level inference.
Prior to testing our main hypothesis, we aimed to replicate the previously shown effect of beliefs on fMRI activity in healthy individuals. To this end, we followed the analysis procedure as applied in previous work [Schmack et al., 2013]. In a second‐level analysis, the individual contrast images (‘belief‐congruent > belief‐incongruent’) from healthy controls were correlated with the belief‐induced bias. In a separate second‐level analysis, we also compared the effect of beliefs on fMRI activity between schizophrenia patients and healthy controls with a two‐sample t‐test between the contrast images (‘belief‐congruent > belief‐incongruent’). These analyses focused on left OFC, as previous work has consistently implicated this region in mediating the effect of beliefs and expectations on sensory processing [Bar et al., 2006; Petrovic et al., 2002; Schmack et al., 2013; Summerfield and Koechlin, 2008]. OFC was anatomically defined as left lateral inferior orbital gyrus based on the Talairach Daemon database (http://www.nitrc.org/projects/wfu_pickatlas/). We also performed an additional whole‐brain analysis. Results were considered statistically significant at P < 0.05, family‐wise‐error‐(FWE‐) corrected for multiple comparisons across all voxels within the OFC mask or across the whole brain.
To test our hypothesis that in schizophrenia perceptual inference relies more on predictions from higher‐level brain circuits that encode beliefs, we probed whether functional connectivity between left OFC and other brain regions differed between patients and controls. For this purpose, we analysed psychophysiological interactions (PPI) in SPM8. PPI is defined as the change in contribution of one brain area to another with the experimental or psychological context [Friston et al., 1997]. It computes whole‐brain connectivity on a voxel‐by‐voxel basis between the time series of a seed region and the time series of all other voxels, modulated by a context stimulus.
To preclude that the connectivity analysis was biased by potential group differences in fMRI activity, we defined the seed voxel based on results obtained by previous work. From the voxel that had exhibited the maximum belief‐related activity in OFC in a previous study [[−24 23 −17], Schmack et al., 2013], we extracted fMRI time series and deconvolved them to generate the neuronal signal for the seed region [Gitelman et al., 2003]. The PPI was then defined as the element‐by‐element product of the neuronal time series and a vector coding for the effect of beliefs. A general linear model was set up for each participant which included the following regressors of interest: the time series of the seed region (the physiological variable), the convolved stimulus stick function (the psychological variable: ‘belief‐congruent > belief‐incongruent’) and the reconvolved interaction term (the psychophysiological variable). Additional regressors of no interest were the convolved stick functions of button presses indicating uncertainty, six head movement parameters and one constant for each run. Contrasts of interest for the PPI were created.
To test for an increase in belief‐related connectivity in schizophrenia patients, the individual contrast images for the PPI were then compared between the groups with a two‐sample t‐test. To probe whether the severity of positive symptoms was related to the belief‐related connectivity, we conducted a separate correlation analysis in which the patients' individual contrast images for the PPI were regressed against the scores from the PANSS positive subscale. In both analyses, results were considered statistically significant at P < 0.05, FWE corrected for multiple comparisons across the whole brain or within our region‐of‐interest in visual cortex, the motion‐sensitive area hMT/V5. To define this region, the data from the localizer runs were used. In SPM8 these data were preprocessed applying slice‐time correction, motion adjustment, spatial normalization to the MNI template and spatial smoothing with a Gaussian Kernel (FWHM 8 × 8 × 8 mm). A first‐level analysis then included two regressors of interest (motion and stationary) and the six movement parameters as regressors of no interest. For the identification of the motion‐sensitive area hMT/V5 the individual contrast images ‘motion > stationary’ were subjected to a second‐level one‐sample t‐test. Using MRIcron a bilateral cluster on the occipitotemporal junction was identified and manually delineated (threshold P < 0.001 uncorrected, 496 voxels).
MVPA Analysis
Given our unexpected finding of lower belief‐induced biases in patients as compared with controls (see Results), we followed one of our reviewer's very helpful suggestion to further explore the possibility that healthy controls compared with schizophrenia patients might have been more readily reported their perception to be biased without showing their neural responses to be more biased, for example, due to a reporting bias induced by higher social conformity with the experimenter. We therefore performed multivoxel pattern analysis (MVPA) in order to analyse neural responses to left‐ and rightward rotation of our stimulus, as described in our previous work [Schmack et al., 2013].
MVPA was spatially restricted to visual cortex, that was defined by an anatomically defined occipital lobe (http://www.nitrc.org/projects/wfu_pickatlas/) and the functionally defined motion‐sensitive area hMT/V5 (see above). As spatially non‐normalized data was used for MVPA (see below), visual cortex masks were created individually for each participant by applying the inverse transformation matrix resulting from the spatial normalization of the structural scan to the MNI template. From these visual cortex masks, the 2,000 voxels with the highest t‐values in a t‐test comparing rightward rotation to leftward rotation in the training data set (see below) were used for MVPA, as this voxel selection method had yielded the best results in our previous work [Schmack et al., 2013].
For MVPA, preprocessing only included slice time correction and spatial realignment, but no spatial normalization or spatial smoothing, and the first four scans were discarded to remove stimulus onset effects. BOLD signal time courses were extracted, linearly de‐trended and normalized to vectors of unit length. Each scan was then assigned to one of the two percepts (leftward rotation and rightward rotation) according to the perceptual dominance time course reported by the participants. To account for the hemodynamic delay (+4 s) and the time between a perceptual switch and the button press the perceptual time courses (−1 s) were shifted in time (+3 s). The resulting label contained a 1 for each scan corresponding to a leftward rotation (‘left’) and −1 for each scan corresponding to a rightward rotation (‘right’).
Classification was performed with a linear support‐vector‐machine (SVM) and the implementation LIBSVM (http://www.csie.ntu.edu.tw/%7ecjlin/libsvm/). A training subset of the fMRI and label data was used to train the classifier. The trained classifier then generated predictions of perceptual states for each time‐point from an independent test subset of fMRI data that was drawn from independent experimental runs (see below for details). Given that the number of scans labelled as ‘right’ and ‘left’ did not necessarily equal, we used different cost parameters c r and c l for each class to correct for this class imbalance and calculated the balanced accuracy to assess the performance of the classifier.
Two MVPAs were conducted. A first analysis was aimed to decoding perceived rotation direction from the baseline phase, in which participants viewed the ambiguous sphere in the absence of perceptual beliefs. The data from the baseline phase only were used for both training and testing the classifier in a run‐wise cross‐validation scheme. A second analysis then probed whether rotation direction could also be decoded from scans acquired during the test phase, when perception was biased by the participant's beliefs. The classifier was trained on all 8 runs from the baseline phase and tested on all 4 runs from the test phase.
To test for group and phase differences in decoding accuracies, we compared the prediction accuracy between the baseline phase, in which participants viewed the ambiguous sphere in the absence of perceptual beliefs, and the test phase, in which participants' perception was biased by beliefs. A classifier was trained on the first four baseline phase, and tested separately on the last four baseline runs and the four test runs. The decoding accuracies were then compared by a repeated measures 2 × 2 ANOVA with the factor group and phase.
RESULTS
Behavioural Results
Replicating our previous results [Schmack et al., 2013; Sterzer et al., 2008], we found an influence of higher‐level beliefs on perception in healthy controls (Fig. 1B). Belief‐congruent percepts were perceived for longer durations than belief‐incongruent percepts in the learning phase (t[27] = 12.7, P < 0.001, paired t‐tests), and, importantly, also in the test phase (t[27] = 2.2, P = 0.04, paired t‐test), indicating that participants' beliefs biased perception of the ambiguous sphere.
In schizophrenia patients, there was no significant influence of higher‐level beliefs on perception (Fig. 1C). In the learning phase patients behaved similar to controls and perceived belief‐congruent percepts for longer durations than belief‐incongruent percepts (t[20] = 13.2, P < 0.001, paired t‐test). In the test phase, however, patients showed no difference between belief‐congruent and belief‐incongruent percepts (t[20] = 0.3, P = 0.79).
When we directly compared the influence of higher‐level beliefs on perception between schizophrenia patients and healthy controls, we found that patients showed a weaker overall belief‐induced bias than controls (Fig. 2A, 0.32 [0.25–0.45] vs. 0.46 [0.34–0.62], median [interquartile distance], z = −2.21, P = 0.03, two‐sided Wilcoxon signed rank test). Notably, however, in the patient group we found a significant positive correlation between the severity of positive symptoms and the strength of the belief‐induced bias (Fig. 2B, r = 0.51, P = 0.04, two‐sided Spearman's rank correlation, P‐value Bonferroni‐corrected for n = 2 tests). We also found a positive correlation between the belief‐induced bias and the severity of negative symptoms that did not survive statistical correction for multiple testing (r = 0.45, P = 0.08, two‐sided Spearman's rank correlation, P‐value Bonferroni‐corrected for n = 2 tests).
Figure 2.
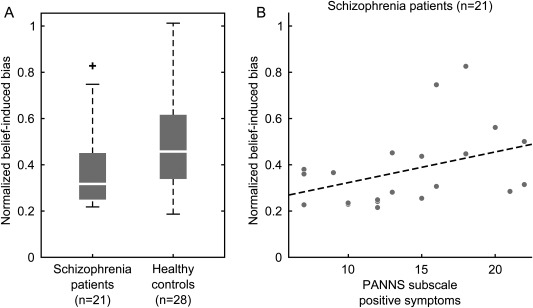
Behavioural belief‐induced bias in schizophrenia. (A) Schizophrenia patients showed a significantly lower belief‐induced bias than healthy controls (P = 0.03, two‐sided Wilcoxon signed rank test). The belief‐induced bias was calculated as the ratio of belief‐congruent and belief‐incongruent mean phase durations normalized with respect to the learning phase. Higher values indicate a stronger belief‐induced bias. Whiskers of the boxplots represent 1.5 interquartile distances. (B) In schizophrenia patients, the belief was correlated with the severity of positive symptoms (r = 0.51, P = 0.02, two‐sided Spearman's rank correlation). The severity of positive symptoms was quantified by the PANNS score on the positive symptoms scale. Each dot represents one individual, the dotted line is the least squares regression line.
Exploring further potential influences on the effect of beliefs on perception, we found no significant correlation between olanzapine equivalent dose and the belief‐induced bias (r = −0.02, P = 0.94, two‐sided Spearman's rank correlation across patients), but a trend‐wise negative correlation between age (as an indirect marker of illness duration) and the belief‐induced bias (r = −0.42, P = 0.06, two‐sided Spearman's rank correlation across patients). There was no relationship between age and the belief‐induced bias in the control group (r = 0.04, P = 0.85). Furthermore, although patients significantly differed from controls in verbal intelligence and years of education (see Table 1), there was no relationship between these measures and the belief‐induced bias (verbal intelligence r = −0.10, P = 0.51; years of education r = 0.07, P = 0.65; two‐sided Spearman's rank correlations across all participants). However, patients showed a significantly lower attention span than controls (see Table 1), and attention span was negatively correlated with the belief‐induced bias (r = 0.45, P = 0.001, two‐sided Spearman's rank correlation across all participants), even when controlling for group differences in attention span (F(46) = 5.28, P = 0.03, ANCOVA on box‐cox‐transformed belief‐induced bias).
fMRI Results
Based on the previously established role of the OFC in the encoding of perceptual beliefs [Schmack et al., 2013], we first established in the healthy control group that perceptual beliefs were associated with enhanced OFC activity. We found that fMRI activity evoked by the belief‐congruent percept, compared with the belief‐incongruent percept, was related to the strength of the belief‐induced perceptual bias in left lateral OFC ([−39 38 −5], t(27) = 3.88, P = 0.03, FWE‐corrected within OFC region of interest, Fig. 3), thereby replicating our previous results [Schmack et al., 2013]. There were no significant clusters in any other region of the brain.
Figure 3.
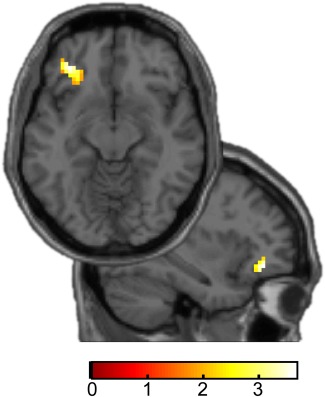
Belief‐related activity in healthy controls. In left OFC, switch‐related activity (belief‐congruent minus belief‐incongruent perceptual switches) in the test phase was associated with the behavioural belief‐induced perceptual bias (t[27] = 3.88, P = 0.03, FWE‐corrected) within OFC ROI). Axial and sagittal slices show voxels within the respective ROIs. For display purposes t‐maps are thresholded at P < 0.05, uncorrected. Colour bars indicate t‐values. [Color figure can be viewed at http://wileyonlinelibrary.com]
To test whether this belief‐related activity in OFC was altered in schizophrenia, we compared the fMRI activity in the test phase evoked by belief‐congruent versus belief‐incongruent percepts between healthy controls and schizophrenia patients. There were no significant group differences in OFC nor in any other region of the brain, thus not providing any evidence for a differential encoding of perceptual beliefs in schizophrenia patients compared with healthy controls.
Most importantly, we then probed whether schizophrenia was associated with an increased functional coupling between higher‐level areas encoding beliefs and lower‐level visual areas. Remarkably, we found that schizophrenia patients compared with healthy controls exhibited a stronger belief‐related connectivity between OFC and right motion‐sensitive area V5 in visual cortex ([42 −55 7], T = 3.7, P = 0.03, corrected within functionally defined visual cortex, Fig. 4). A contralateral cluster in left V5 showed a similar trend but failed to reach significance after correction for multiple comparisons ([−42 −70 10], T = 2.2, P = 0.02, uncorrected). There was no correlation between the severity of positive symptoms and the belief‐related connectivity between OFC and bilateral V5.
Figure 4.
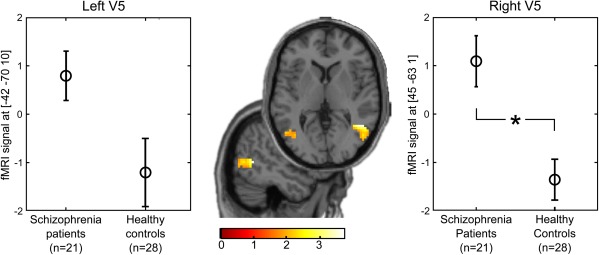
Increased belief‐related connectivity between OFC and visual cortex V5 in schizophrenia. Schizophrenia patients showed a significantly increased psychophysiological interaction (OFC × belief‐congruent minus belief‐incongruent perceptual switches) compared with healthy controls in right motion‐sensitive visual cortex (t = 3.7, P = 0.03, corrected within V5 ROI). A contralateral cluster showed a similar trend but failed to reach significance after correction for multiple comparisons (T = 2.2, P = 0.02, uncorrected). Axial and sagittal slices show voxels with a significant group difference in the psychophysiological interaction (patients > controls) within the V5 ROI. For display purposes t‐maps are thresholded at P < 0.05, uncorrected. Colour bars indicate t‐values. [Color figure can be viewed at http://wileyonlinelibrary.com]
MVPA Results
Using MVPA to explore whether the group differences in the reported effect of beliefs were explained by group differences in the encoding of leftward and rightward rotation direction in visual cortex, we found that decoding accuracies were significantly above chance in both patients and controls both when testing on the baseline and on the test phase (patients baseline 55.9% ± 1.1 [mean ± s.e.m], controls baseline 61.2% ± 1.4, patients test 56.6% ± 1.6, controls test 62.0% ± 1.7, all P < 0.001 in paired t‐tests against chance level of 50%). Moreover, when comparing decoding accuracies between groups and phases, there was a significant main effect of group with higher decoding accuracies in the control group (F(94,1) = 12.3, P < 0.001), but neither a main effect of phase (F(94,1) = 0.2, P = 0.62) nor an interaction between phase and group (F(94,1) = 0.0, P = 0.98). These results indicate that controls compared with patients showed closer correspondence between reported perception and visual cortex responses, thereby not providing any evidence for a stronger reporting bias due to social conformity in controls.
DISCUSSION
Our current study was designed to empirically test a predictive coding model of psychosis. Our central finding is that schizophrenia patients compared with healthy controls showed increased belief‐related connectivity from OFC to visual cortex. This finding confirms previous work showing a positive relationship between psychosis‐proneness of healthy individuals and belief‐related connectivity from OFC to visual cortex by the use of a very similar experimental design [Schmack et al., 2013]. Our current work therefore provides further support to the idea that psychosis is associated with an enhanced feedback signalling of higher‐level predictions from non‐sensory areas to sensory areas. Such enhanced feedback signalling could represent a mechanism by which sensory processing is shaped into conformity with beliefs, which might account for hallucinations and persistent delusions.
Interestingly, our finding of an increased belief‐related connectivity in schizophrenia was not paralleled at the behavioural level by an increased belief‐induced bias on perception in schizophrenia. On the contrary, patients compared with controls showed a reduced belief‐induced bias on reported perception. At the same time, in patients the belief‐induced bias on reported perception correlated positively with the severity of positive symptoms. This latter finding is in line with previous work that has associated psychosis‐related phenomena with an enhanced impact of higher‐level predictions on reported perception: we have previously established a link between psychosis‐proneness in healthy individuals and an increased belief‐induced bias on reported perception by the use of a very similar experimental design [Schmack et al., 2013]. Similarly, psychosis‐proneness in healthy individuals and at‐risk‐mental state for psychosis have been related to an increased use of experimentally induced prior knowledge during perception of ambiguous images [Teufel et al., 2015]. Hence, our behavioural results provide further evidence for the hypothesized link between an enhanced impact of higher‐level predictions on perception and psychotic symptoms.
In apparent contradiction to this notion, however, our behavioural results also show that high‐level predictions affect reported perception to a lower degree in schizophrenia as compared with healthy individuals. This latter finding is unexpected given the previous and current evidence for an enhanced signalling of higher‐level predictions in psychosis and psychosis‐proneness [Schmack et al., 2013; Teufel et al., 2015]. One potential explanation for this unexpected finding might be that our current study examined clinically stable patients on antipsychotic medication. It is conceivable that illness‐related factors and/or antipsychotic medication rendered patients in our current study less susceptible than controls to the experimental induction of beliefs, such that high‐level predictions were not acquired to a degree sufficient to impact perception. Interestingly, we found a negative relationship between attention span and the effect of beliefs as well as a lower attention span in patients compared with controls, raising the possibility that patients might have had difficulties in learning the high‐level predictions due to attention deficits. Hence, the weaker effect of beliefs on reported perception in patients compared with controls could reflect learning impairments rather than altered predictive perception. In this context, it is tempting to speculate that during the course of the illness patients become more reluctant toward incorporating new predictions about the world due to the continuous experience of a mismatch between their predictions and their experience. This speculation is supported by the trend‐wise negative relationship between age (as a proxy for illness duration) and the effect of beliefs in patients but not in controls. Moreover, although we did not find a significant correlation between antipsychotic dose and the effect of beliefs, it is still conceivable that antipsychotic medication might have contributed to a decreased learning of the high‐level predictions in our experiment. This would be in line with the causal role of dopamine in reinforcement learning [Steinberg et al., 2013], which suggests that dopamine‐antagonistic antipsychotic drugs might impair learning, as well as with the well‐established finding of more rigid reinforcement learning in schizophrenia [see Deserno et al., 2013 for a comprehensive review]. However, it is unclear whether attention‐, illness‐ and/or medication‐related learning abnormalities might interfere with the acquisition of beliefs in our current experimental design where learning demands were minimal and independent from reinforcement. Future work in medication‐free individuals at early stages of their illness and with experimental designs that allow to control for attention and learning is needed.
Nevertheless, our fMRI results showing an increased belief‐related connectivity in patients compared with controls point to enhanced signalling of higher‐level predictions during perception in schizophrenia. At first sight this finding seems difficult to reconcile with the behaviourally weaker effect of beliefs on reported perception in patients compared with controls. However, it is conceivable that impaired learning diminished belief acquisition in patients, but did not abolish it completely. This interpretation is supported by the observed relationship between the severity of positive symptoms and the effect of beliefs on reported perception, which can only be explained by some degree of belief acquisition in psychotic patients. Hence, the increased belief‐related connectivity between OFC and visual cortex could be a manifestation of an increased effect on neural coupling of generally less strongly acquired beliefs. It is tempting to speculate that the increased signalling of higher‐level predictions at the neural level might be a compensatory mechanism for the decreased learning of such predictions. The interpretation of our current findings as enhanced signalling of higher‐level predictions nicely dovetail with previous neuroimaging work on visual perception in schizophrenia that has investigated signalling of lower‐level predictions by the use of visual illusions or related phenomena. For instance, visual cortex responses to a centre stimulus are less strongly suppressed by a suppressive surround stimulus in schizophrenia patients compared with healthy controls [Seymour et al., 2013], which can be attributed to reduced signalling of local predictions within visual cortex. Another study has examined the neural underpinnings of the reduced schizophrenia‐related susceptibility to the hollow‐mask illusion, where a concave face is perceived as convex due to the effect of the learned prediction that faces are convex: schizophrenia patients compared with controls showed reduced effective connectivity between higher‐order visual areas in parietal cortex and object‐sensitive visual cortex during viewing of this illusion [Dima et al., 2009], which again can be interpreted to reduced predictive signalling within earlier stages of processing. According to predictive coding accounts of psychosis, such weakened predictive signalling within earlier stages of processing might give rise to unstable sensory representations. The resulting experience of a changing and unpredictable world renders sensory events overly salient, and the cognitive effort to make sense of this aberrant salience results in the formation of delusions [Heinz, 2002; Kapur, 2003]. Crucially, our current results showing an enhanced signalling of higher‐level predictions point to an additional alteration of the brain's predictive mechanisms in psychosis: in order to cope with the continuous instability of sensory representations, perception may rely more on predictions from higher‐level non‐sensory brain circuits that stabilize processing in lower‐level sensory areas. As a result, perception is shaped into conformity with higher‐level beliefs, which would explain the tenacity of delusions and for hallucinations [Corlett et al., 2009; Schmack et al., 2013].
However, our fMRI results showing an increased belief‐related connectivity in patients compared with controls might also be interpreted as an inefficient neural coupling between frontal and visual areas. This alternative interpretation would provide an explanation for the behaviourally weaker effect of beliefs on reported perception in patients compared with controls, thereby following a similar logic as interpretations of previous imaging work on working memory in schizophrenia: here, patients compared with controls have been found to exhibit stronger task‐related neural signals despite a weaker behavioural task performance which has been interpreted as an inefficient neural functioning [Manoach et al., 1999]. Hence, although our finding of an increased belief‐related connectivity between OFC and visual cortex is in line with our initial hypothesis of an increased signalling of higher‐level predictions in schizophrenia, given our behavioural findings it might reflect a rather attenuated role of higher‐level predictions during perception in schizophrenia. In this case, our current work in clinically stable patients on antipsychotic medication would contrast with previous work indicating a strengthened role of higher‐level predictions in psychosis‐proneness in both healthy at‐risk‐mental state for psychosis individuals [Schmack et al. 2013; Teufel et al. 2015]. To reconcile these findings, one might speculate that the role of higher‐level predictions might weaken over the course of the illness. Future studies in patients at earlier stages of their illness or using longitudinal designs will help to elucidate this possibility.
Taken together, our current work illustrates how alterations of the brain's predictive mechanisms might give rise to both hallucinatory percepts and delusional beliefs, thereby expanding our current mechanistic understanding of psychosis.
The authors declare that they have no conflict of interest.
REFERENCES
- Adams RA, Stephan KE, Brown HR, Frith CD, Friston KJ (2013): The computational anatomy of psychosis. Front Psychiatry 4:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar M, Kassam KS, Ghuman AS, Boshyan J, Schmid AM, Schmidt AM, Dale AM, Hämäläinen MS, Marinkovic K, Schacter DL, Rosen BR, Halgren E (2006): Top‐down facilitation of visual recognition. Proc Natl Acad Sci U S A 103:449–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corlett PR, Frith CD, Fletcher PC (2009): From drugs to deprivation: A Bayesian framework for understanding models of psychosis. Psychopharmacology (Berl) 206:515–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corlett PR, Taylor JR, Wang X‐J, Fletcher PC, Krystal JH (2010): Toward a neurobiology of delusions. Prog Neurobiol 92:345–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakin S, Carlin P, Hemsley D (2005): Weak suppression of visual context in chronic schizophrenia. Curr Biol CB 15:R822–R824. [DOI] [PubMed] [Google Scholar]
- Deserno L, Boehme R, Heinz A, Schlagenhauf F (2013): Reinforcement learning and dopamine in schizophrenia: Dimensions of symptoms or specific features of a disease group?. Front Psychiatry 4:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dima D, Roiser JP, Dietrich DE, Bonnemann C, Lanfermann H, Emrich HM, Dillo W (2009): Understanding why patients with schizophrenia do not perceive the hollow‐mask illusion using dynamic causal modelling. NeuroImage 46:1180–1186. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB (2002a): Structured Clinical Interview for DSM‐IV‐TR Axis I Disorders, Research Version, Patient Edition. (SCID‐I/P). N Y Biom Res N Y State Psychiatr Inst.
- First MB, Spitzer RL, Gibbon M, Williams JB (2002b): Structured Clinical Interview for DSM‐IV‐TR Axis I Disorders, Research Version, Non‐patient Edition. (SCID‐I/NP). N Y Biom Res N Y State Psychiatr Inst.
- Fletcher PC, Frith CD (2009): Perceiving is believing: A Bayesian approach to explaining the positive symptoms of schizophrenia. Nat Rev Neurosci 10:48–58. [DOI] [PubMed] [Google Scholar]
- Friston K (2005): A theory of cortical responses. Philos Trans R Soc Lond B Biol Sci 360:815–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ (1997): Psychophysiological and modulatory interactions in neuroimaging. NeuroImage 6:218–229. [DOI] [PubMed] [Google Scholar]
- Gitelman DR, Penny WD, Ashburner J, Friston KJ (2003): Modeling regional and psychophysiologic interactions in fMRI: The importance of hemodynamic deconvolution. NeuroImage 19:200–207. [DOI] [PubMed] [Google Scholar]
- Heinz A (2002): Dopaminergic dysfunction in alcoholism and schizophrenia–psychopathological and behavioral correlates. Eur Psychiatry J Assoc Eur Psychiatr 17:9–16. [DOI] [PubMed] [Google Scholar]
- Hemsley DR (2005): The schizophrenic experience: Taken out of context?. Schizophr Bull 31:43–53. [DOI] [PubMed] [Google Scholar]
- Kapur S (2003): Psychosis as a state of aberrant salience: A framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry 160:13–23. [DOI] [PubMed] [Google Scholar]
- Kersten D, Mamassian P, Yuille A (2004): Object perception as Bayesian inference. Annu Rev Psychol 55:271–304. [DOI] [PubMed] [Google Scholar]
- Leucht S, Samara M, Heres S, Davis JM (2016): Dose equivalents for antipsychotic drugs: The DDD method. Schizophr Bull 42 Suppl 1:S90–S94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoach DS, Press DZ, Thangaraj V, Searl MM, Goff DC, Halpern E, Saper CB, Warach S (1999): Schizophrenic subjects activate dorsolateral prefrontal cortex during a working memory task, as measured by fMRI. Biol Psychiatry 45:1128–1137. [DOI] [PubMed] [Google Scholar]
- Mumford D (1992): On the computational architecture of the neocortex. II. The role of cortico‐cortical loops. Biol Cybern 66:241–251. [DOI] [PubMed] [Google Scholar]
- Notredame C‐E, Pins D, Deneve S, Jardri R (2014): What visual illusions teach us about schizophrenia. Front Integr Neurosci 8:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovic P, Kalso E, Petersson KM, Ingvar M (2002): Placebo and opioid analgesia– imaging a shared neuronal network. Science 295:1737–1740. [DOI] [PubMed] [Google Scholar]
- Powers AR, III Kelley M, Corlett PR (2016): Hallucinations as Top‐Down Effects on Perception. Biol Psychiatry Cogn Neurosci Neuroimaging 1:393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders LLO, Muckli L, de Millas W, Lautenschlager M, Heinz A, Kathmann N, Sterzer P (2012): Detection of visual events along the apparent motion trace in patients with paranoid schizophrenia. Psychiatry Res 198:216–223. [DOI] [PubMed] [Google Scholar]
- Schmack K, Gòmez‐Carrillo de Castro A, Rothkirch M, Sekutowicz M, Rössler H, Haynes J‐D, Heinz A, Petrovic P, Sterzer P (2013): Delusions and the role of beliefs in perceptual inference. J Neurosci Off J Soc Neurosci 33:13701–13712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmack K, Schnack A, Priller J, Sterzer P (2015): Perceptual instability in schizophrenia: Probing predictive coding accounts of delusions with ambiguous stimuli. Schizophr Res Cogn 2:72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider U, Borsutzky M, Seifert J, Leweke FM, Huber TJ, Rollnik JD, Emrich HM (2002): Reduced binocular depth inversion in schizophrenic patients. Schizophr Res 53:101–108. [DOI] [PubMed] [Google Scholar]
- Schurger A (2009): A very inexpensive MRI‐compatible method for dichoptic visual stimulation. J Neurosci Methods 177:199–202. [DOI] [PubMed] [Google Scholar]
- Seymour K, Stein T, Sanders LLO, Guggenmos M, Theophil I, Sterzer P (2013): Altered contextual modulation of primary visual cortex responses in schizophrenia. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol 38:2607–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg EE, Keiflin R, Boivin JR, Witten IB, Deisseroth K, Janak PH (2013): A causal link between prediction errors, dopamine neurons and learning. Nat Neurosci 16:966–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterzer P, Frith C, Petrovic P (2008): Believing is seeing: Expectations alter visual awareness. Curr Biol CB 18:R697–R698. [DOI] [PubMed] [Google Scholar]
- Summerfield C, Koechlin E (2008): A neural representation of prior information during perceptual inference. Neuron 59:336–347. [DOI] [PubMed] [Google Scholar]
- Teufel C, Subramaniam N, Dobler V, Perez J, Finnemann J, Mehta PR, Goodyer IM, Fletcher PC (2015): Shift toward prior knowledge confers a perceptual advantage in early psychosis and psychosis‐prone healthy individuals. Proc Natl Acad Sci U S A 112:13401–13406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Silverstein SM, Phillips WA, Lovell PG (2004): Evidence for impaired visual context processing in schizotypy with thought disorder. Schizophr Res 68:249–260. [DOI] [PubMed] [Google Scholar]


