Abstract
Early stressors play a key role in shaping interindividual differences in vulnerability to various psychopathologies, which according to the diathesis‐stress model might relate to the elevated glucocorticoid secretion and impaired responsiveness to stress. Furthermore, previous studies have shown that individuals exposed to early adversity have deficits in emotion processing from faces. This study aims to explore whether early adversities associate with brain response to faces and whether this association might associate with the regional variations in mRNA expression of the glucocorticoid receptor gene (NR3C1). A total of 104 individuals drawn from the Northern Finland Brith Cohort 1986 participated in a face‐task functional magnetic resonance imaging (fMRI) study. A large independent dataset (IMAGEN, N = 1739) was utilized for reducing fMRI data‐analytical space in the NFBC 1986 dataset. Early adversities were associated with deviant brain response to fearful faces (MANCOVA, P = 0.006) and with weaker performance in fearful facial expression recognition (P = 0.01). Glucocorticoid receptor gene expression (data from the Allen Human Brain Atlas) correlated with the degree of associations between early adversities and brain response to fearful faces (R 2 = 0.25, P = 0.01) across different brain regions. Our results suggest that early adversities contribute to brain response to faces and that this association is mediated in part by the glucocorticoid system. Hum Brain Mapp 38:4470–4478, 2017. © 2017 Wiley Periodicals, Inc.
Keywords: fMRI, gene expression, cohort study, early adversity, glucocorticoid receptor
INTRODUCTION
The brain undergoes remodeling throughout childhood and youth [Paus et al., 2008]. Trajectories of brain development are influenced by a variety of factors, including the social environment. Early traumatic experiences play a key role in shaping interindividual differences in vulnerability and resilience to future adversity. Adversities in childhood and adolescence play a key role in shaping interindividual differences in vulnerability to many psychopathologies in later life including psychotic disorders [Matheson et al., 2013; Varese et al., 2012] and post‐traumatic stress disorder [Widom, 1999].
Previous work has shown that individuals exposed to early stressors have deficits in face processing [Cicchetti and Curtis, 2005; Curtis and Cicchetti, 2011; da Silva Ferreira et al., 2014; Fries and Pollak, 2004; Pollak et al., 2000]. Facial expressions are one of the key vehicles of interpersonal interactions, serving an important signaling role for different emotions and an entry point for a variety of social interactions [Becker et al., 2011]. Perception of faces involves both the ventral and dorsal pathways that project from the primary visual cortex [Goodale and Milner, 1992]. Brain regions contributing to the processing of faces have been further divided into two distinct neural systems, namely the core and extended systems [Haxby et al., 2000]. According to this hierarchical model, the core system—comprising the inferior occipital cortex, the posterior superior temporal sulcus (STS), and the lateral fusiform gyrus—contributes to early visual analysis of faces. Further processing of faces—for example, emotion, speech, eye‐gaze direction, and the person's name—is conducted in concert with other cortical and subcortical regions in the extended system.
The physiological response to stress, mediated by the hypothalamus–pituitary–adrenal (HPA) axis, involves secretion of cortisol from adrenal glands. Acting on glucocorticoid receptors, cortisol generates cascade of events throughout the body, including the cardiovascular, metabolic, immune, and nervous systems (for review, see Chrousos 2009). Previous studies have shown that early life stress affects the functioning of the glucocorticoid receptor (NR3C1), in part by epigenetic regulation of gene transcription [McGowan et al., 2009; van der Knaap et al., 2014]. Furthermore, previous research suggests that cortisol levels associate with the ability to recognize facial expressions accurately [Feeney et al., 2012; van Peer et al., 2009]. In the brain, the effects of glucocorticoids extend to both cortical and subcortical structures. According to the diathesis‐stress model, the HPA axis via cortisol response mediates the relationship between exposure to stressors and the onset of many psychopathologies including psychosis [Jones and Fernyhough, 2007; Walker and Diforio, 1997].
To understand the mechanisms underlying stress‐related modulation of face processing, we examined whether early adversity associates with brain response to faces and whether regional differences in the strength of this association vary as a function of inter‐regional variations in the expression of NR3C1 (a gene coding for glucocorticoid receptor). For this purpose, we administered a questionnaire about early life adversities and used functional magnetic resonance imaging (fMRI) to measure brain response to faces in a sample of young adults drawn from the Northern Finland Birth Cohort 1986 (NFBC 1986). We used a large independent sample (IMAGEN, N = 1739) for reducing data‐analytical space in the NFBC 1986. For glucocorticoid receptor NR3C1 mRNA expression, we used the publicly available Allen Brain Atlas dataset [Hawrylycz et al., 2012].
MATERIALS AND METHODS
Analytical Strategy and Samples
The workflow describing the analytical approach of this study is described in Figure 1. Study samples are described in detail in the Supporting Information. Local ethical committees approved the study protocol. First, we utilized a large sample of typically developing adolescents (IMAGEN, subsample of 1,110 adolescents, mean age = 14 years) [Schumann et al., 2010; Tahmasebi et al., 2012] that were recruited through local high schools in eight European cities. We used this sample to define regions of interest (ROIs) that engage in face processing. A total of 21 ROIs relevant for face processing (population probability 0.5) were identified using a probabilistic map as described in detail elsewhere [Tahmasebi et al., 2012]. For completeness, we added four contra‐lateral homologs, thus resulting in 25 ROIs. The advantage of the ROI approach is that it limits the analyses to brain regions most relevant to face processing, thereby reducing the risk of false‐positive and false‐negative results.
Figure 1.
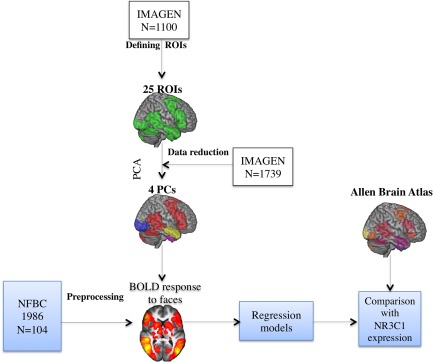
Flowchart depicting the analyses of our study. Abbreviations: ROI, region of interest; PCA, principle component analysis; BOLD, blood‐oxygen‐level dependent. [Color figure can be viewed at http://wileyonlinelibrary.com]
Next, we used an extension of the IMAGEN sample (N = 1739) to define principal components (PCs) of shared variance across the ROIs. This enabled us to reduce the number of statistical tests even further and to explore the clustering of different ROIs into independent functional entities. Factors acquired from the principal component analysis (PCA) were used for reducing fMRI data in the NFBC 1986 sample.
Third, we used an independent sample of participants in their early adulthood (NFBC 1986) [Veijola et al., 2013] (N = 104) to characterize the associations between early life adversities and brain response to faces. Participants of the NFBC 1986 subsample participated in fMRI while viewing four blocks of video clips of happy and four blocks of video clips of fearful facial expressions; these blocks were presented in pseudorandom order separated by blocks of a dynamic mosaic baseline (control stimulus) [Pulkkinen et al., 2015; Rahko et al., 2010]. The stimuli are described in detail in the Supporting Information. During the scan, participants were instructed to lay still and relaxed, and watch the stimuli on screen through a mirror positioned on the head coil. After the scanning, all participants completed a test of emotion recognition that involved viewing of 16 facial expressions on a computer screen. Childhood adversities were evaluated with the Trauma and Distress Scale (TADS) questionnaire [Patterson et al., 2002], which is a valid and reliable instrument for assessing retrospectively reported childhood traumas [Salokangas et al., 2016]. Total TADS scores were used as a measure of the level of adversity.
Last, we used NR3C1 expressions as a proxy of the glucocorticoid receptor density to evaluate whether the extent of the relationship between early adversities and brain response to faces (NFBC 1986 sample, N = 104) varies as a function of regional variations in this receptor's density across the different brain regions, thus testing for the specificity in this relationship. In a recent study, we have used the Allen Human Brain Atlas to explore inter‐regional variations in NR3C1 expression and age‐related cortical thinning during adolescence [Wong et al., 2017]. We acquired mRNA expression data for NR3C1 from Allen Human Brain Atlas, which contains postmortem measurements of gene expression obtained in six adult brains [Hawrylycz et al., 2012]. We merged 25 “face” ROIs with the original Allen Human Brain Atlas sample sites, excluding sample sites in white matter and cerebrospinal fluid. This resulted altogether 284 sample sites within 25 ROIs. Additional details of the study methods are provided in the Supporting Information.
Imaging Data Preprocessing in NFBC 1986
Detailed imaging and preprocessing description is provided in the Supporting Information. Neuroimaging data were analyzed with FSL [Jenkinson and Smith, 2001; Jenkinson et al., 2002; Smith, 2002; Woolrich et al., 2004, 2001; Worsley et al., 2001] and AFNI [Cox, 1996]. A standard preprocessing pipeline (before contrast modeling) was applied, including brain extraction, motion correction, spatial smoothing with FWHM 5.0 mm, linear co‐registration and nonlinear normalization to the 2 mm MNI‐152 template, high‐pass filtering (cutoff = 120 s) and prewhitening. The six motion correction parameters were included as additional regressors in the model. Regressors of interest were happy faces, fearful faces, and the baseline mosaic face was also modeled. Percent BOLD Signal Change (%BSC) for happy and fearful faces (relative to the dynamic mosaic, which was used as control stimulus) were extracted from 25 ROIs for each participant.
Statistical Analyses
For statistical analyses, we used R (http://cran.r-project.org) version 3.1.1 [R Core Team, 2014]. All statistical analyses were adjusted for sex. In the IMAGEN sample, we performed PCA. In the NFBC 1986 sample, we first performed MANCOVA analysis to assess multivariate relationships between adversities and brain response to fearful/happy faces using factors acquired from the PCA in the IMAGEN sample. Linear regression, Pearson correlation and Spearman correlation were also used as specified in the Results section below. TADS scores were normalized using a square root transformation. Screening for possible multivariate outliers was conducted using the Mahalanobis distance statistic at P < 0.001. Normality of residuals and homoscedasticity were visually confirmed using histograms and residuals vs. fitted plots in histograms. Correlation coefficients (e.g., correlation between adversity and BOLD response in Amygdala) were converted to z using Fisher's r to z.
RESULTS
Demographics
Demographic data for the NFBC 1986 are presented in Table 1. The IMAGEN dataset was younger than the NFBC 1986; its age ranged from 13 to 17 years, with mean age of 14.6 (standard deviation [sd] 0.44) and 52% of this sample being female adolescents.
Table 1.
Demographic characteristics of the NFBC 1986
| Variable | NFBC 1986 (N = 104) |
|---|---|
| Sex (females) | 57% |
| Age [M (SD)] | 22.8 (0.82) |
| Handedness, right | 90% |
| IQ [M (SD)] | 113 (21.6) |
| GAF [M (SD)] | 82 (8.5) |
| Education | |
| Comprehensive (9 school years) | 31% |
| Matriculation (12 school years) | 69% |
GAF, global assessment of functioning; M, mean; SD, standard deviation.
PCA (IMAGEN)
We used parallel analysis for the determination of number of components in the IMAGEN dataset. Parallel analysis suggested four‐component solution (68% of the variance), which was further varimax rotated. Factor loadings are presented in Figure 2, which depicts their regional distribution across the 25 ROIs. Loadings >0.5 on specific ROIs were considered as main contributors to a specific factor. Using this criterion, we evaluated the spatial representation of each component, which is presented in Figure 2. The four factors were labeled as follows: (1) “Main” (Frontal lobe/FFA/Posterior STS/Putamen/Cerebellum, 29% of variance), (2) “Visual” (V2V3/LOC, 15% of variance), (3) “Gaze/Affect” (Amygdala/Anterior STS, 14% of variance), and (4) “Rhinal sulcus (9% of variance)”.
Figure 2.
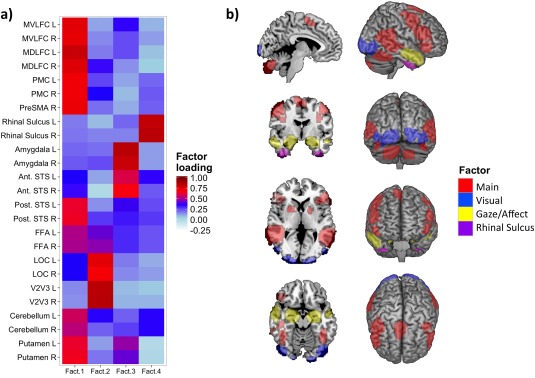
(a) Factor loadings for %BSC in 25 ROIs in the IMAGEN dataset. Abbreviations: MVLFC, mid‐ventrolateral frontal cortex; MDLFC, mid‐dorsolateral frontal cortex; PMC, premotor cortex; PreSMA, pre‐supplementary motor area; STS, superior temporal sulcus; FFA, fusiform face area; LOC, lateral occipital cortex; L, left; R, right; Ant, anterior; Post, posterior. (b) Spatial representation of each factor (loadings > 0.5). [Color figure can be viewed at http://wileyonlinelibrary.com]
Overlap Between the 25 ROIs and BOLD Response to Faces (NFBC 1986)
We evaluated how the overall BOLD response to faces in the NFBC 1986 dataset overlaps with the original 25 ROIs (defined in the IMAGEN sample). For this purpose, we first conducted univariate GLM to assess BOLD response to happy and fearful faces (thresholded at z > 2.3, P = 0.05 corrected, dynamic mosaic as a baseline stimulus). We then evaluated the overall response to faces in NFBC 1986, excluding voxels responding specifically to happy or fearful facial expression. The overall response to faces in NFBC 1986 sample involved 85% voxels of the 25 ROIs. This overlap was even higher for happy and fearful facial expressions: 86% and 89%, respectively. Supporting Information, Figure 1 depicts the regional representation of the overlap.
Association Between Adversities and Brain Response to Faces (NFBC 1986)
Next, using PCs defined in the IMAGEN dataset (N = 1739), we characterized multivariate associations between early adversity and brain response to faces. We observed that early adversities (Wilks’ ∧ = 0.86, F(4,98) = 3.9, P = 0.006) had multivariate associations with brain response to fearful faces. Furthermore, as family socioeconomic status correlates often with the level of adversity [Biederman et al., 1995], we used family income at the age of 16 as a proxy of family socioeconomic status in MANCOVA model. The multivariate relationship between early adversity and BOLD response to fearful faces remained significant after this adjustment (P = 0.02), which indicates that this relationship cannot be explained by family socioeconomic status. No associations were discovered between adversities and brain response to happy faces (P = 0.18).
Post hoc linear regressions revealed a number of brain–behavior relationships, which are described in Figure 3. First, we discovered that early adversities had a negative relationship with the BOLD response of the “Main” factor and “Rhinal sulcus.” Second, early adversities predicted a stronger BOLD response of the “Visual” factor.
Figure 3.
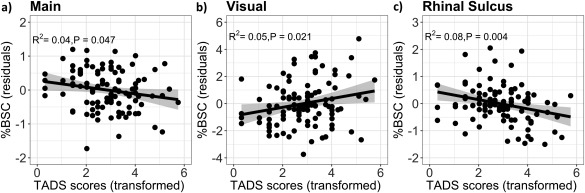
(a) Regression analyses between early adversities (TADS scores) and brain response to fearful faces (“Main” factor). (b) Regression analyses between early adversities (TADS scores) and brain response to fearful faces (“Visual” factor). (c) Regression analyses between early adversities (TADS scores) and brain response to fearful faces (“Rhinal Sulcus” factor). Plots are shown in standardized residuals (covariating for sex). Regression lines are plotted with shaded 95% CIs.
Facial Recognition Test and Behavioral Results (NFBC 1986)
Recognition of happy facial expressions (median 100%; range 67–100%) was significantly better than that of fearful facial expressions (median 80%, range 0–100%) P < 0.001 (paired Wilcoxon Rank Sum test). We observed a negative correlation between TADS scores and the recognition of fearful facial expression (Spearman's ρ = −0.25, P = 0.01). TADS scores predicted negatively Global Assessment of Functioning (GAF) (Spearman's ρ = −0.27, P = 0.006). The detailed frequency distributions of TADS scores (varied 0 to 33) are provided in Supporting Information, Figure 2.
BOLD Response to Faces and Early Adversities as a Function of NR3C1 Gene Expression (NFBC 1986 and Allen Brain Atlas)
Last, we examined whether the magnitude of the relationship between adversity and brain response to fearful faces (Fisher's Z transformed correlation coefficients) varied as a function of regional differences in NR3C1 expression across the 25 ROIs. In this analysis (presented in Fig. 4), NR3C1 expression correlated across the 25 ROIs with the magnitude of associations between adversity and BOLD response to fearful faces (r = 0.50, P = 0.01).
Figure 4.
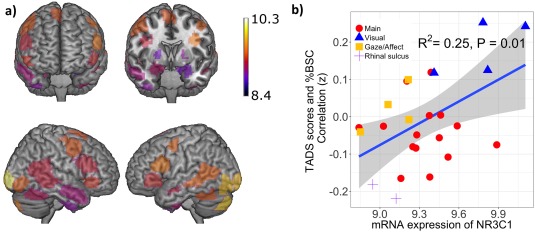
(a) Spatial representation of the mRNA expression of NR3C1 across the 25 ROIs. (b) Correlation of the NR3C1 mRNA expression—across the 25 ROIs—with the magnitude of associations between adversity and brain response to fearful faces. Regression line is plotted with shaded 95% CI. [Color figure can be viewed at http://wileyonlinelibrary.com]
DISCUSSION
This study explored whether early life adversities associate with brain response to faces. We also explored whether this association varies as a function of regional differences in mRNA expression of glucocorticoid receptor gene NR3C1.
We utilized previously defined ROIs that were acquired from a large population‐based sample [Tahmasebi et al., 2012]. These ROIs had high population probability and were originally constructed from probabilistic maps of the face‐processing neural network engaged consistently and robustly while viewing the ambiguous faces. Assessment of the overlapping voxels between the ROIs and the response to faces in the NFBC 1986 suggested highly similar engagements between the two datasets despite the use of different video clips of faces, and slight differences in the age of the IMAGEN and the NFBC 1986 participants, thereby supporting our approach.
Our PCA‐based clustering in the IMAGEN sample revealed interesting face‐processing entities in the 25 ROIs. The “Main” factor encompassed most of the ROIs, which may indicate representation of the core and extended systems after early visual attention [Haxby et al., 2000]. The “Visual” factor represented posterior occipital regions, which might be modulated by visual attention in the early stage of face perception [Kastner et al., 1999]. The “Gaze/Affect” factor represented the amygdala and anterior STS, which might support perception of eye gaze and emotion from faces [Adolphs, 2008; De Souza et al., 2005]. Finally, located in the inferior temporal lobe, the “Rhinal sulcus” might represent part of the ventral visual stream and provide discriminating and filtering functions during face perception [Murray and Mishkin, 1986].
Focusing visual attention on potential cues in the environment is a fundamental mechanism that serves an important survival function. Our discovery of dose‐dependent heightened %BSC in the “Visual” factor with relation to adversities could therefore represent a bottom–up driven, heightened sensitivity to visual cues of social significance. According to previous research, threatening faces (vs friendly faces) elicits a stronger event‐related potential (ERP) response in occipital cortex [Weymar et al., 2011], and individuals with conditioning to certain types of threat (e.g., spiders) have higher ERPs in the occipital cortex compared with nonanxious individuals irrespective of fearful and nonfearful target contents [Weymar et al., 2014]. In this light, our results might indicate that early adversities associate with bottom–up‐driven visual alertness in the early stages of face perception.
We discovered negative relationships between early adversities and factors representing brain regions linked to further processing of faces after initial representation in the visual cortex (i.e., “Main” and “Rhinal Sulcus”). In line with the previous studies reporting deficits in facial expression processing in individuals with history of adversities [Curtis and Cicchetti, 2011; da Silva Ferreira et al., 2014], we speculate that face‐processing systems may be especially vulnerable to early life adversities. This is consistent with the observed, albeit weak, negative correlation between adversities and fearful facial expression recognition performance.
In this study, we evaluated whether associations between early adversity and brain responses to dynamic facial expressions in early adulthood varied as a function of regional differences in the expression of NR3C1, a gene related to the HPA axis. As hypothesized, inter‐regional variations in early adversity‐related BOLD responses were positively associated with inter‐regional variations in mRNA expression levels of NR3C1. Specifically, the strongest associations between adversities and BOLD response to fearful faces were in brain regions with higher NR3C1 mRNA expression levels. The highest expression of NR3C1 is found in occipital regions and the lowest in temporal regions.
Excessive secretion of glucocorticoids is a potential mediator between early adversities and many psychopathologies [Walker and Diforio, 1997; Widom, 1999]. Previous studies have reported that patients with psychotic disorders manifest HPA dysregulation, such as high levels of baseline cortisol and adenocorticotropic hormone [Ryan et al., 2004; Walsh et al., 2005]. Excessive glucocorticoid secretion is also associated with impaired cognitive processing [McEwen et al., 2015]. The fact that the NR3C1 expression correlated—across the 25 ROIs—with both the magnitude of associations between adversity and brain response to fearful faces suggests that the above influences may be, in part, mediated by cortisol acting via glucocorticoid receptors. Nonetheless, only experimental studies can confirm this possibility.
There were several strengths in our study. First, we utilized a large population‐based sample to provide robust ROIs and principle components for data reduction. Second, we utilized a unique data setting in NFBC 1986: participants were born in the same geographical region, had a similar ethnic and cultural background, and were of the same age at the time of the study. Longitudinal data of the NFBC 1986 were utilized for participants’ health record data and earlier status and family background. Participants of the NFBC 1986 were in their early 20s, which is the peak age for developing many mental disorders including psychotic disorders [Hafner et al., 1998; Paus et al., 2008].
This study also has limitations that should be addressed in future studies. First, our study was cross‐sectional, which prevented us from exploring the developmental aspects of brain response to faces. In addition, questionnaires that measure subjectively childhood and youth experiences may be affected by recall bias. We did not use video‐based real‐time eye tracking or reaction time measures during fMRI. Another limitation is the relatively moderate reporting of childhood adversities in our study sample, which prevented us from exploring the possible detailed effect of specific adversity type on brain response to faces. The Allen Human Brain Atlas is based on only six donors. Nonetheless, we observed high consistency of NR3C1 expression profiles across the six donors (donor‐to‐median correlation of 0.84), and high similarity of median profiles obtained from the Allen Brain Atlas and another database, namely the BrainSpan atlas (r = 0.82; Wong et al., 2017).
In this research, we employed a data‐reduction procedure (PCA of values from the 25 ROIs) using a dataset of adolescents, and applied the resulting PCA‐based factors in early adulthood. There is, however, no other dataset with the same size that we are aware of that could be used in a similar manner to provide robust principal components. Note, nonetheless, that brain regions engaged by the adolescents are not qualitatively different from those observed here in the adult sample or from those identified by a meta‐analysis based on multiple imaging studies carried out mostly in adults [Fusar‐Poli et al., 2009]
CONCLUSIONS
Our work has examined whether regional variations in the association between adversity and BOLD response to faces vary as function of the expression of glucocorticoid receptor gene. Based on our results, the link between early adversities and brain response to fearful faces might be mediated by the glucocorticoid system. Further research is, however, needed to replicate these findings. Altogether, our results support the conceptualizations of higher HPA axis activation leading to the dysfunction of the brain function, which may, in part, lead to individual differences in mental health disorders in later life [Jones and Fernyhough, 2007; Walker and Diforio, 1997].
CONTRIBUTORS
Vesa Kiviniemi, Jouko Miettunen, Jenny Barnett, Graham K. Murray, Peter Jones, Irma Moilanen, Tanja Nordström, Pirjo Mäki, and Juha Veijola planned the NFBC 1986 substudy data collection. Vesa Kiviniemi conducted the brain imaging in NFBC 1986 sample. Tanja Nordström, Pirjo Mäki, Jouko Miettunen, Juha Veijola, and Jenni Koivukangas performed the data collection of NFBC 1986. Johannes Lieslehto analyzed the fMRI data and conducted statistical analyses. Johannes Lieslehto, Tomas Paus, and Juha Veijola wrote the first draft of the manuscript and all authors contributed to its subsequent revisions. All authors have accepted the final version of the manuscript.
CONFLICT OF INTEREST
All the authors declared no conflicts of interest.
Supporting information
Supporting Information
Supporting Information
Supporting Information
ACKNOWLEDGEMENTS
The facial stimuli used in this study were contributed by professor Mikko Sams (Department of Biomedical Engineering and Computational Science (BECS), The Aalto University School of Science and Technology, Helsinki, Finland). The authors also thank Daniel Joel Shaw from the Central European Institute of Technology for valuable comments.
Members of the IMAGEN consortium (http://www.imagen-europe.com): Eric Artiges, Tobias Banaschewski, Gareth Barker, Arun L.W. Bokde, Uli Bromberg, Christian Büchel, Anna Cattrell, Patricia J.Conrod, Sylvane Desrivières, Herta Flor, Vincent Frouin, Jürgen Gallinat, Hugh Garavan,Penny Gowland, Andreas Heinz, Bernd Ittermann, Claire Lawrence, Herve Lemaitre, Jean‐Luc Martinot, Eva Mennigen, Frauke Nees, Dimitri Papadopoulos‐Orfanos, Tomáš Paus, Zdenka Pausova, Luise Poustka, Sarah Rodehacke, Gunter Schumann, Michael N. Smolka, Nora C. Vetter, Henrik Walter, and Robert Whelan.
REFERENCES
- Adolphs R (2008): Fear, faces, and the human amygdala. Curr Opin Neurobiol 18:166–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker DV, Neel R, Srinivasan N, Neufeld S, Kumar D, Fouse S (2011): The vividness of happiness in dynamic facial displays of emotion. PLoS One 7:e26551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J, Milberger S, Faraone SV, Kiely K, Guite J, Mick E, Ablon S, Warburton R, Reed E (1995): Family‐environment risk factors for attention‐deficit hyperactivity disorder. A test of Rutter's indicators of adversity. Arch Gen Psychiatry 52:464–470. [DOI] [PubMed] [Google Scholar]
- Chrousos GP (2009): Stress and disorders of the stress system. Nat Rev Endocrinol 5:374–381. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Curtis WJ (2005): An event‐related potential study of the processing of affective facial expressions in young children who experienced maltreatment during the first year of life. Dev Psychopathol 17:641–677. [DOI] [PubMed] [Google Scholar]
- Cox RW (1996): AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29:162–173. [DOI] [PubMed] [Google Scholar]
- Curtis WJ, Cicchetti D (2011): Affective facial expression processing in young children who have experienced maltreatment during the first year of life: An event‐related potential study. Dev Psychopathol 23:373–395. [DOI] [PubMed] [Google Scholar]
- da Silva Ferreira GC, Crippa JA, de Lima OF (2014): Facial emotion processing and recognition among maltreated children: A systematic literature review. Front Psychol 5:1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Souza WC, Eifuku S, Tamura R, Nishijo H, Ono T (2005): Differential characteristics of face neuron responses within the anterior superior temporal sulcus of macaques. J Neurophysiol 94:1252–1266. [DOI] [PubMed] [Google Scholar]
- Feeney J, Gaffney P, O'Mara SM (2012): Age and cortisol levels modulate judgment of positive and negative facial expressions. Psychoneuroendocrinology 37:827–835. [DOI] [PubMed] [Google Scholar]
- Fries AB, Pollak SD (2004): Emotion understanding in postinstitutionalized Eastern European children. Dev Psychopathol 16:355–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar‐Poli P, Placentino A, Carletti F, Landi P, Allen P, Surguladze S, Benedetti F, Abbamonte M, Gasparotti R, Barale F, Perez J, McGuire P, Politi P (2009): Functional atlas of emotional faces processing: A voxel‐based meta‐analysis of 105 functional magnetic resonance imaging studies. J Psychiatry Neurosci 34:418–432. [PMC free article] [PubMed] [Google Scholar]
- Goodale MA, Milner AD (1992): Separate visual pathways for perception and action. Trends Neurosci 15:20–25. [DOI] [PubMed] [Google Scholar]
- Hafner H, an der Heiden W, Behrens S, Gattaz WF, Hambrecht M, Loffler W, Maurer K, Munk‐Jorgensen P, Nowotny B, Riecher‐Rossler A, Stein A (1998): Causes and consequences of the gender difference in age at onset of schizophrenia. Schizophr Bull 24:99–113. [DOI] [PubMed] [Google Scholar]
- Hawrylycz MJ, Lein ES, Guillozet‐Bongaarts AL, Shen EH, Ng L, Miller JA, van de Lagemaat LN, Smith KA, Ebbert A, Riley ZL, Abajian C, Beckmann CF, Bernard A, Bertagnolli D, Boe AF, Cartagena PM, Chakravarty MM, Chapin M, Chong J, Dalley RA, Daly BD, Dang C, Datta S, Dee N, Dolbeare TA, Faber V, Feng D, Fowler DR, Goldy J, Gregor BW, Haradon Z, Haynor DR, Hohmann JG, Horvath S, Howard RE, Jeromin A, Jochim JM, Kinnunen M, Lau C, Lazarz ET, Lee C, Lemon TA, Li L, Li Y, Morris JA, Overly CC, Parker PD, Parry SE, Reding M, Royall JJ, Schulkin J, Sequeira PA, Slaughterbeck CR, Smith SC, Sodt AJ, Sunkin SM, Swanson BE, Vawter MP, Williams D, Wohnoutka P, Zielke HR, Geschwind DH, Hof PR, Smith SM, Koch C, Grant SG, Jones AR (2012): An anatomically comprehensive atlas of the adult human brain transcriptome. Nature 489:391–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI (2000): The distributed human neural system for face perception. Trends Cogn Sci 4:223–233. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S (2002): Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17:825–841. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S (2001): A global optimisation method for robust affine registration of brain images. Med Image Anal 5:143–156. [DOI] [PubMed] [Google Scholar]
- Jones SR, Fernyhough C (2007): A new look at the neural diathesis–stress model of schizophrenia: The primacy of social‐evaluative and uncontrollable situations. Schizophr. Bull 33:1171–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner S, Pinsk MA, De Weerd P, Desimone R, Ungerleider LG (1999): Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron 22:751–761. [DOI] [PubMed] [Google Scholar]
- Matheson SL, Shepherd AM, Pinchbeck RM, Laurens KR, Carr VJ (2013): Childhood adversity in schizophrenia: A systematic meta‐analysis. Psychol Med 43:225–238. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Bowles NP, Gray JD, Hill MN, Hunter RG, Karatsoreos IN, Nasca C (2015): Mechanisms of stress in the brain. Nat Neurosci 18:1353–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan PO, Sasaki A, D'Alessio AC, Dymov S, Labonte B, Szyf M, Turecki G, Meaney MJ (2009): Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci 12:342–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA, Mishkin M (1986): Visual recognition in monkeys following rhinal cortical ablations combined with either amygdalectomy or hippocampectomy. J Neurosci 6:1991–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson P, Skeate A, Birchwood M (2002): TADS‐EPOS 1.2. Birmingham: University of Birmingham. [Google Scholar]
- Paus T, Keshavan M, Giedd JN (2008): Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci 9:947–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak SD, Cicchetti D, Hornung K, Reed A (2000): Recognizing emotion in faces: Developmental effects of child abuse and neglect. Dev Psychol 36:679–688. [DOI] [PubMed] [Google Scholar]
- Pulkkinen J, Nikkinen J, Kiviniemi V, Maki P, Miettunen J, Koivukangas J, Mukkala S, Nordstrom T, Barnett JH, Jones PB, Moilanen I, Murray GK, Veijola J (2015): Functional mapping of dynamic happy and fearful facial expressions in young adults with familial risk for psychosis ‐ Oulu Brain and Mind Study. Schizophr Res 164:242–249. [DOI] [PubMed] [Google Scholar]
- R Core Team (2014): R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; URL: http://www.R-project.org/. [Google Scholar]
- Rahko J, Paakki JJ, Starck T, Nikkinen J, Remes J, Hurtig T, Kuusikko‐Gauffin S, Mattila ML, Jussila K, Jansson‐Verkasalo E, Katsyri J, Sams M, Pauls D, Ebeling H, Moilanen I, Tervonen O, Kiviniemi V (2010): Functional mapping of dynamic happy and fearful facial expression processing in adolescents. Brain Imag Behav 4:164–176. [DOI] [PubMed] [Google Scholar]
- Ryan MC, Sharifi N, Condren R, Thakore JH (2004): Evidence of basal pituitary‐adrenal overactivity in first episode, drug naive patients with schizophrenia. Psychoneuroendocrinology 29:1065–1070. [DOI] [PubMed] [Google Scholar]
- Salokangas RK, Schultze‐Lutter F, Patterson P, von Reventlow HG, Heinimaa M, From T, Luutonen S, Hankala J, Kotimaki M, Tuominen L (2016): Psychometric properties of the Trauma and Distress Scale, TADS, in an adult community sample in Finland. Eur J Psychotraumatol 7:30062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann G, Loth E, Banaschewski T, Barbot A, Barker G, Buchel C, Conrod PJ, Dalley JW, Flor H, Gallinat J, Garavan H, Heinz A, Itterman B, Lathrop M, Mallik C, Mann K, Martinot JL, Paus T, Poline JB, Robbins TW, Rietschel M, Reed L, Smolka M, Spanagel R, Speiser C, Stephens DN, Strohle A, Struve M IMAGEN Consortium (2010): The IMAGEN study: Reinforcement‐related behaviour in normal brain function and psychopathology. Mol Psychiatry 15:1128–1139. [DOI] [PubMed] [Google Scholar]
- Smith SM (2002): Fast robust automated brain extraction. Hum Brain Mapp 17:143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahmasebi AM, Artiges E, Banaschewski T, Barker GJ, Bruehl R, Büchel C, Conrod PJ, Flor H, Garavan H, Gallinat J, Heinz A, Ittermann B, Loth E, Mareckova K, Martinot J, Poline J, Rietschel M, Smolka MN, Ströhle A, Schumann G, Paus T The IMAGEN Consortium (2012): Creating probabilistic maps of the face network in the adolescent brain: A multicentre functional MRI study. Hum Brain Mapp 33:938–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Knaap LJ, Riese H, Hudziak JJ, Verbiest MM, Verhulst FC, Oldehinkel AJ, van Oort FV (2014): Glucocorticoid receptor gene (NR3C1) methylation following stressful events between birth and adolescence. The TRAILS study. Transl Psychiatry 4:e381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Peer JM, Spinhoven P, van Dijk JG, Roelofs K (2009): Cortisol‐induced enhancement of emotional face processing in social phobia depends on symptom severity and motivational context. Biol Psychol 81:123–130. [DOI] [PubMed] [Google Scholar]
- Varese F, Smeets F, Drukker M, Lieverse R, Lataster T, Viechtbauer W, Read J, van Os J, Bentall RP (2012): Childhood adversities increase the risk of psychosis: A meta‐analysis of patient‐control, prospective‐ and cross‐sectional cohort studies. Schizophr Bull 38:661–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veijola J, Maki P, Jaaskelainen E, Koivukangas J, Moilanen I, Taanila A, Nordstrom T, Hurtig T, Kiviniemi V, Mukkala S, Heinimaa M, Lindholm P, Jones PB, Barnett JH, Murray GK, Miettunen J (2013): Young people at risk for psychosis: Case finding and sample characteristics of the Oulu Brain and Mind Study. Early Interv Psychiatry 7:146–154. [DOI] [PubMed] [Google Scholar]
- Walker EF, Diforio D (1997): Schizophrenia: A neural diathesis‐stress model. Psychol Rev 104:667–685. [DOI] [PubMed] [Google Scholar]
- Walsh P, Spelman L, Sharifi N, Thakore JH (2005): Male patients with paranoid schizophrenia have greater ACTH and cortisol secretion in response to metoclopramide‐induced AVP release. Psychoneuroendocrinology 30:431–437. [DOI] [PubMed] [Google Scholar]
- Weymar M, Keil A, Hamm AO (2014): Timing the fearful brain: Unspecific hypervigilance and spatial attention in early visual perception. Soc Cogn Affect Neurosci 9:723–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weymar M, Löw A, Öhman A, Hamm AO (2011): The face is more than its parts — Brain dynamics of enhanced spatial attention to schematic threat. Neuroimage 58:946–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widom CS (1999): Posttraumatic stress disorder in abused and neglected children grown up. Am J Psychiatry 156:1223–1229. [DOI] [PubMed] [Google Scholar]
- Wong AP, French L, Leonard G, Michel P, Pike GB, Richer L, Veillette S, Pausova Z, Paus T (2017): Inter‐regional variations in gene expression and age‐related cortical thinning in the adolescent brain. Cereb Cortex 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TEJ, Beckmann CF, Jenkinson M, Smith SM (2004): Multilevel linear modelling for FMRI group analysis using Bayesian inference. Neuroimage 21:1732–1747. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM (2001): Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage 14:1370–1386. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Jezzard P, Matthews PM, Smith SM (2001): Statistical analysis of activation images, Ch 14. In: Functional MRI: An Introduction to Methods. OUP. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information
Supporting Information


