Abstract
Different lines of research suggest that anxiety‐related personality traits may influence the visual and vestibular control of balance, although the brain mechanisms underlying this effect remain unclear. To our knowledge, this is the first functional magnetic resonance imaging (fMRI) study that investigates how individual differences in neuroticism and introversion, two key personality traits linked to anxiety, modulate brain regional responses and functional connectivity patterns during a fMRI task simulating self‐motion. Twenty‐four healthy individuals with variable levels of neuroticism and introversion underwent fMRI while performing a virtual reality rollercoaster task that included two main types of trials: (1) trials simulating downward or upward self‐motion (vertical motion), and (2) trials simulating self‐motion in horizontal planes (horizontal motion). Regional brain activity and functional connectivity patterns when comparing vertical versus horizontal motion trials were correlated with personality traits of the Five Factor Model (i.e., neuroticism, extraversion‐introversion, openness, agreeableness, and conscientiousness). When comparing vertical to horizontal motion trials, we found a positive correlation between neuroticism scores and regional activity in the left parieto‐insular vestibular cortex (PIVC). For the same contrast, increased functional connectivity between the left PIVC and right amygdala was also detected as a function of higher neuroticism scores. Together, these findings provide new evidence that individual differences in personality traits linked to anxiety are significantly associated with changes in the activity and functional connectivity patterns within visuo‐vestibular and anxiety‐related systems during simulated vertical self‐motion. Hum Brain Mapp 38:715–726, 2017. © 2016 The Authors Human Brain Mapping Published by Wiley Periodicals, Inc.
Keywords: neuroticism, vestibular system, visuo‐vestibular interaction, functional connectivity, parieto‐insular vestibular cortex, virtual reality
INTRODUCTION
The influence of anxiety on visuo‐vestibular systems involved in balance control was initially described more than a century ago [Balaban and Jacob, 2001; Gowers, 1887], although only recent research has begun to examine the interactions across visual, vestibular, and anxiety systems [Balaban and Jacob, 2001; Carmona et al., 2009; Coelho and Balaban, 2015; Kapfhammer et al., 2014; Lopez, 2016; Mast et al., 2014].
First, animal studies have shown that rats with high levels of anxiety may display balance deficits when performing postural tasks that include visual threats, for example, walking on a spinning beam while passing throughout a rotating tunnel that simulates movement of the visual environment [Lepicard et al., 2000a; Rudrauf et al., 2004; Viaud‐Delmon et al., 2011]. At the same time, anxiolytic drugs (e.g., diazepam) have been found to reduce the number of falls in anxious rats during the same paradigm, while anxiogenic medication (e.g., methyl ß‐carboline‐3‐carboxylate “ß‐CCM”) has been shown to cause balance deficits in non‐anxious rats [Lepicard et al., 2000a, 2000b].
Second, behavioral studies in healthy people have suggested that individual differences in anxiety significantly affect balance control during postural challenges [Viaud‐Delmon et al., 2011]. For example, Bolmont et al. found a negative association between anxiety levels and balance measures during a postural task in which there was mismatch between visual and proprioceptive information [Bolmont et al., 2002]. In another study, high‐ relative to low‐anxious individuals displayed a shift from an allo‐centric to ego‐centric strategy when orienting themselves throughout a virtual reality navigation task [Viaud‐Delmon et al., 2002].
Third, clinical research in patients with vestibular and psychiatric disorders has supported the notion that visual, vestibular, and anxiety systems may closely interact during balance control [Balaban and Jacob, 2001; Staab et al., 2013]. More specifically, patients with vestibular or anxiety disorders have been found to rely more heavily than healthy persons on visual information during tasks requiring balance control [Cousins et al., 2014; Redfern et al., 2001, 2007]. Patients with vestibular disorders are also more likely to develop anxiety disorders than patients without vestibular dysfunctions [Best et al., 2009; Celestino et al., 2003; Eagger et al., 1992; Eckhardt‐Henn et al., 2003; Eckhardt et al., 1996]. Likewise, patients with anxiety and mood diseases frequently report hyper‐sensitivity to visual and vestibular stimuli during balance challenges [Hainaut et al., 2011; Staab et al., 2014]. Finally, exposure to moving visual stimuli during upright posture and head motion has been shown to exacerbate the sensation of unsteadiness in clinical conditions like height phobia and chronic subjective dizziness (CSD), two disorders in which the interplay between visual, vestibular, and anxiety systems plays a key pathogenetic role [Indovina et al., 2015b; Staab, 2012; Staab et al., 2013].
Together, these studies supported current theoretical models positing that there may be multiple pathways in the brain where anxiety influences the visual and vestibular systems and vice versa [Balaban, 2002]. Nevertheless, the core neural mechanisms underlying the interaction between these systems remain poorly understood and scarcely characterized. In particular, it is unknown whether and how individual differences in anxiety‐related personality traits modulate the brain activity and functional connectivity patterns in visual, vestibular, and anxiety systems.
In this study, we were specifically interested in the influence of trait‐ rather than state‐anxiety on the activity and connectivity across brain regions involved in the visuo‐vestibular control of balance. This is because trait‐anxiety (i.e., the potentially insidious innate tendency of an individual to worry) has not been studied as extensively as state‐anxiety (i.e., the severity of an individual's fear or nervousness at a given moment in time). Another reason why we focused on trait‐anxiety depended on the fact that trait‐anxiety appears to influence how normal individuals orient themselves in space and the thresholds at which they shift from low‐ to high‐risk postural control strategies [Hainaut et al., 2011; Hallam and Hinchcliffe, 1991; Viaud‐Delmon et al., 2002]. Last but not least, previous clinical studies found that patients with chronic dizziness were significantly more likely to possess high trait‐anxiety than patients with other chronic vestibular disorders (67% vs. 25%), even after controlling for the severity of state‐anxiety, depression, and dizziness handicap [Staab et al., 2014]. These results complemented an earlier study by Tschan et al., who showed that patients with resilient, optimistic, and self‐confident personality traits—which essentially are the opposite of high trait‐anxiety—had reduced likelihood of developing chronic dizziness after acute vestibular illnesses [Tschan et al., 2011]. All in all, we decided to use neuroticism and introversion as proxy measures of trait‐anxiety as there is considerable evidence that these personality traits are strongly related to anxiety‐related disorders [Barlow et al., 2014; Bienvenu et al., 2004; Costa and McCrae, 1992; Trull and Sher, 1994].
To test the hypothesis that anxiety‐related traits would affect the interplay between visuo‐vestibular and anxiety systems, we recruited n = 24 healthy people with variable levels of neuroticism and introversion. All participants were exposed to an immersive virtual reality rollercoaster task that has been previously developed to study vertical and horizontal heading in a visual gravity field [Indovina et al., 2013a, b, 2015a]. This paradigm allowed comparison of brain activity during trials that simulated forward self‐motion along a virtual vertical axis with brain responses associated with trials that simulated forward self‐motion along a virtual horizontal axis. The realistic and immersive virtual reality environment implemented in the rollercoaster task enabled us to investigate visually induced balance threat in a similar way to previously developed paradigms [Coelho et al., 2009; Diemer et al., 2015; Peperkorn and Muhlberger, 2013].
We hypothesized that, when comparing vertical versus horizontal motion trials, higher neuroticism and introversion scores would be associated with higher activity and functional connectivity patterns in cortical areas implicated in the visuo‐vestibular control of balance as: (1) the parieto‐insular vestibular cortex (PIVC); (2) the temporo‐parietal junction (TPJ), (3) and the human motion complex visual area (hMT+/V5) [Britten, 2008; De Waele et al., 2001; Indovina et al., 2005; Lopez and Blanke, 2011; Lopez et al., 2012; zu Eulenburg et al., 2012]. We also predicted that another group of brain regions critically involved in anxiety‐related behavior (i.e., inferior frontal gyrus, amygdala, and hippocampus) would show changes in the activity and functional connectivity patterns as a function of increasing levels of neuroticism and introversion. Together, these hypotheses may explain why people scoring high in anxiety‐related traits, relative to individuals scoring low in the same personality traits, display heightened sensitivity to visual stimuli that evoke balance threat [Staab et al., 2013].
PARTICIPANTS AND METHODS
Participants
Twenty‐four healthy volunteers (13 females, mean age: 32.4 ± 7.3 years) gave their written informed consent to participate in this study, which was approved by the Research Ethics Committee of Santa Lucia Foundation. Exclusion criteria included past or present otologic, neurologic or psychiatric disorders, chronic medical illnesses, pregnancy, medication use, smoking, and history of head injury. All participants were right‐handed, as assessed via the Edinburgh Handedness Inventory [Oldfield, 1971]. To evaluate neuroticism, introversion, and other non‐anxiety related personality traits (e.g., openness), all volunteers completed a computerized version of the Italian translation of the NEO‐PI‐R questionnaire, a highly standardized measure of the five factor model of personality [Costa and McCrae, 1997]. Raw scores for each personality factor were converted in T‐scores via a script written in SPSS (Statistical Package for Social Sciences, http://www.spss.it/) that used combined sex‐norms reported in the NEO‐PI‐R manual.
As motion sickness susceptibility (MSS) may influence the neural networks examined here [Napadow et al., 2013a, 2013b], we ensured that our results were not influenced by MSS and consequently included this measure as covariate of no interest in all the statistical models (see fMRI analysis sections below). MSS scores were assessed using the Motion Sickness Susceptibility Questionnaire (MSSQ) [Golding, 1998].
fMRI Task
The fMRI task was delivered via an MRI compatible VisualSystem (NordicNeuroLab, http://www.nordicneurolab.com/Products_and_Solutions/fMRI_Hardware/VisualSystem.aspx). This consists of goggles that have diopter correction and pupil distance adjustment, and provide immersion in virtual reality environments while isolating the participant from the external context. AVI videos were displayed via the PsychoToolbox 3.0.10 (https://github.com/Psychtoolbox-3/Psychtoolbox-3) running on Matlab 2012a (http://www.matworks.com), at 800 × 600 pixels, 30° × 23° visual angle, 60 frames per second.
A ride on a rollercoaster was simulated by showing first person perspective views of animated visual scenes compatible with forward self‐motion (Fig. 1). Participant's view was that of a passenger sitting in a front‐car and looking straight ahead. Previous studies have shown that, with this type of simulation, the time‐varying visual information prevails on static vestibular information to cue the visual vertical [Indovina et al., 2013a, 2013b]. A fixation cross was displayed at the center of the scene corresponding to the focus of expansion during rectilinear motion. The car traveled most of the time in the open air along tracks consisting of vertical and horizontal rectilinear sections, connected by curves (see Supporting Information for a video example of the visual stimulation). To avoid habituation phenomena, we changed the kinematic parameters across trials although these parameters were identical for horizontal and vertical motions trials. The optic flow expanded radially from the central fixation point in both types of trials and directional cues were provided by the visual scene. There were periods during which the car accelerated, decelerated, or moved at constant speed [Indovina et al., 2013a, 2013b]. See supplementary materials for further details about motion kinematics parameters. A total of 126 trials including vertical and horizontal conditions was presented. To equalize visual stimulation between vertical and horizontal conditions, a tunnel was shown at the end of each rectilinear segment as in a previous study [Indovina et al., 2013a, 2013b]. Nonetheless, it is important to note that here we investigated the correlations between brain responses associated with a rollercoaster ride simulation and personality traits, which were unlikely to be related to low level visual features.
Figure 1.

Visual virtual environment. Left panel: Still frames from animated visual stimuli at onsets of exemplificative vertical and horizontal trials. Right panel: Panoramic view. [Color figure can be viewed at http://wileyonlinelibrary.com]
The experiment included 3 sessions in total, each consisting of 6 movies with a mean duration of 48.25 s (minimum duration: 41.95 s, maximum duration: 56.63 s), presented in a random order. The first frame of each movie was static and lasted 15 s. The total duration of each session was 6 minutes and 20 s. To ensure that participants paid attention to the stimuli, they were instructed to fixate a cross at the center of the screen and press a button when the color of this fixation cross changed from blue to yellow. The color of the central cross changed six times during the whole experiment. At the end of the experiment, participants had to rate the “sense of presence” [Sanchez‐Vives and Slater, 2005], which corresponded to the perceived realism of the visual simulation. More specifically, they had to rate “how strong was their sensation of being on the moving rollercoaster car” on a Likert scale from 0 (“none”) to 10 (“as on a real rollercoaster”) [Indovina et al., 2013a]. Eye movements were recorded throughout the duration of the whole experiment for offline analyses.
Image Acquisition
Neuroimaging data were acquired on a 3 Tesla Unit and using an 8‐channels head coil (Discovery MR‐750, General Electric, Milwaukee, WI). Head movements were minimized using foam pads around participants' head. None of the participants had head movements greater than 2 mm. Whole‐brain fMRI data were acquired throughout echo planar images (EPI) sensitive to the blood oxygenation level‐dependent (BOLD) contrast (39 axial slices, 3‐mm thickness each; repetition time = 2,000 ms; echo time = 30 ms; voxel size: 3 × 3 × 3 mm).
Image Pre‐Processing
Data were pre‐processed with SPM8 (http://www.fil.ion.ucl.ac.uk/spm /). Slice‐acquisition delays were corrected using the first slice as reference (ascending order). EPIs were next realigned to the first scan by rigid body transformations to correct for head movements. Realigned scans were normalized to the standard template in the Montreal Neurological Institute (MNI) space using linear and nonlinear transformations, and finally images were smoothed with a Gaussian kernel of full width at half maximum of 8 mm [Penny et al., 2011; Worsley and Friston, 1995].
Eye‐Movement Analyses
As saccadic movements may indicate attentional biases and a lack of ability to fixate the central cross [Fischer and Breitmeyer, 1987], we computed the number of saccades (eye displacements lasting more than 100 ms and larger than 3 standard deviation from the baseline signal) and assessed whether there was a relationship between them and anxiety‐related personality scores. To calculate the number of saccadic movements, a video from the right eye (resolution: 320 × 240 pixels, number of frames: 11,700, frame‐rate: 30 frames per second) was recorded for the whole duration of the task using an EyeTracking Camera, which is integrated into the NordicLab VisualSystem (http://www.nordicneurolab.com/products/VisualSystem.html).
Eye movement data were analyzed via an in‐house script implemented in Matlab R2013a. Eye movement data of six participants could not be analyzed due to low quality of recording. The number of saccades was calculated per each session and each condition of interest (i.e., vertical and horizontal trials). An ANOVA test was performed to assess: (1) the main effect of session; (2) the main effect of motion direction, and (3) the interaction between session and motion direction. We also assessed whether there was any significant correlation between the number of saccades during vertical trials (relative to horizontal trials) and neuroticism and introversion scores. To this end, we subtracted the number of saccades during horizontal motion from the number of saccades during vertical motion and calculated the correlation between these values and subject‐specific personality scores.
fMRI Analysis of Regional Responses
For each participant, a general linear model (GLM) assessed regionally specific effects of task parameters on BOLD activations. Trials were modeled as epochs of variable duration and convolved with the SPM8 hemodynamic response function. First‐level GLMs included vertical, horizontal, and static conditions. Curves were modeled separately and excluded from the analyses. Six realignment parameters were included as effects of no interest to remove residual motion‐related variance. Low‐frequency signal drift was eliminated using a high‐pass filter (cut‐off, 128 s) and an autoregressive model (AR[1]) was applied to correct for voxels' autocorrelations.
The following contrasts were computed: (i) all motion versus static trials, and (ii) vertical versus horizontal motion trials. First, separate one‐sample t‐tests (one per each contrast of interest) were carried out to explore the brain responses that were associated with the main contrasts independently of personality traits as in our previous study [Indovina et al., 2013b]. Next, separate GLMs were generated for each of the Five Factor personality trait assessing the correlation between subject‐specific BOLD activity per each contrast and the individual scores in NEO‐PI‐R personality factors. To exclude the effect of motion sickness susceptibility on brain response evoked by self‐motion stimuli, individual MSSQ scores were entered into the GLM models as covariate of no interest.
Statistical maps were thresholded using two methods. First, we reported regions that met a threshold of P ≤ 0.05, with whole‐brain cluster level correction for multiple comparisons and P ≤ 0.005 to estimate minimum cluster size as in our earlier report [Indovina et al., 2013b]. This threshold was chosen to directly compare the current results from those previously reported in an independent sample [Indovina et al., 2013b]. Second, we employed a region of interest (ROI) approach using a threshold of P < 0.05, FWE small volume correction (svc) [Friston, 1997; Worsley et al., 1996]. To this end, we used functionally defined ROIs based on previous independent fMRI studies [Poldrack, 2007]. The ROIs were created using 6‐mm radius spheres for sub‐cortical regions and 8‐mm radius spheres for cortical areas. Next, all ROIs were combined into a unique mask which was in turn used to threshold the second level statistical maps.
The parieto‐insular vestibular cortex (PIVC), the human motion complex visual area (hMT+/V5), and the temporo‐parietal junction (TPJ) were defined as ROIs, given their key role in processing visual motion and vestibular stimuli [Bosco et al., 2008; Britten, 2008; Cardin and Smith, 2010; De Waele et al., 2001; Frank et al., 2014; Indovina et al., 2005; Lopez and Blanke, 2011; Lopez et al., 2012; zu Eulenburg et al., 2012]. To define the PIVC bilateral ROI, we used 8‐mm radius spheres centered on the coordinates reported in Cardin and Smith, transformed in the Montreal Neurological Institute (MNI) space (x: 41, y: −30, z: 18 and x: −40, y: −30, z: 20 for right and left PIVC, respectively) [Cardin and Smith, 2010]. This region has also been known as the Posterior Insular Cortex [Frank et al., 2016; Orban et al., 2003]. To define the hMT+/V5 and TPJ bilateral ROIs, we used 8‐mm radius spheres centered on the MNI coordinates reported in Bosco and colleagues (Right TPJ: x: 65, y: −36.6, z: 30.1; Left TPJ: x: −62.5, y: −36.2, z: 3; Right hMT+/V5: x: 55, y: −67.8, z: 1.8; Left hMT+/V5: x: −49.8, y: −71.0, z: 3.3) [Bosco et al., 2008].
The inferior frontal gyrus (IFg), amygdala, and hippocampus were also considered as ROIs given their critical role in anxiety‐related behavior [Bishop, 2007; Craig, 2009; Hüfner et al., 2011; Indovina et al., 2011; Kim et al., 2011; Lopez et al., 2012; Sylvester et al., 2012; zu Eulenburg et al., 2012]. The IFg and the bilateral amygdala were centered on the coordinates reported in Indovina and colleagues using 8‐mm and 6‐mm radius spheres respectively (Right IFg: x: 50, y: 26, z: 2; Left IFg: x: −42, y: 26, z: 12; Right Amygdala: x: 18, y: −2, z: −16; Left Amygdala: x: −18, y: −2, z: −16) [Indovina et al., 2014]. The bilateral hippocampus ROI was created centering the 6‐mm sphere on the MNI coordinates reported in Indovina et al. [2013b] (Right Hippocampus: x: 24, y: −16, z: −18; Left Hippocampus: x: −24, y: −16, z: −18) [Indovina et al., 2013b].
Functional Connectivity Analyses
Task‐dependent functional connectivity: psycho‐physiological interaction (PPI) in a GLM
To investigate how personality traits modulated functional connectivity changes within the visual, vestibular, and anxiety brain systems, Psycho‐Physiological Interaction (PPI) analyses were conducted. A PPI represents the change in connectivity (i.e., correlation) between couples of regions (i.e., the “seed” and the rest of the brain) that is induced by a specific task [Friston et al., 1997]. Overall, we sought to identify how personality traits modulated connectivity patterns between a seed region and the rest of the brain as a function of processing vertical versus horizontal motion trials (i.e., higher‐order PPIs) [Passamonti et al., 2008, 2009, 2012]. The left PIVC, which was derived from the analysis exploring the effect of neuroticism on regional brain responses (see “Results” section), was used as one of the main seed region. PPI analyses were also performed using the hMT+/V5 complex as seed whose MNI coordinates were derived from the local maxima of the main effect of the task (i.e., vertical vs. horizontal motion trials) independently of personality (X: −52, Y: −72, Z: 4).
The time‐series for each participant seed was computed using the first eigenvariate from all voxels' time series within a 8‐mm sphere and next deconvolved to estimate the neuronal time series [Gitelman et al., 2003]. Separate PPIs were carried out for the contrast vertical versus horizontal motion trials. The PPI regressor was calculated as the element‐by‐element product of the neuronal time series of the seed region and a vector coding the contrast of interest. The first‐level GLM included the main effect of task, the “seed” time‐series, and six movement parameters as effects of no interest. Subject‐specific PPI contrast images were computed and finally entered into second‐level GLMs that identified the brain areas for which the change in connectivity with the seed was modulated by individual differences in personality scores. The same statistical approach used for regional analyses was also employed for second‐level PPI maps.
RESULTS
Behavioral Results
Participants' age, NEO‐PI‐R personality scores, MSSQ scores, and other behavioral data (e.g. sense of presence during the rollercoaster simulation) are summarized in Table 1. A positive correlation was found between the “sense of presence” (i.e., the perceived realism of the visual simulation) and neuroticism (r = 0.45, P = 0.03, uncorrected for multiple comparisons). No significant correlations were found across personality traits (r's < 0.24, P's > 0.25) or between personality traits and motion sickness scores (r's < 0.35, P's > 0.08). Finally, no participant reported sense of nausea throughout the task.
Table 1.
Demographic, psychological and behavioral data of the sample. Data are expressed as Mean (SD).
| N° subjects | 24 (13 F, 11M) | |
|---|---|---|
| Mean (SD) | Range | |
| Age | 32.5 (7.2) | 22–52 |
| NEO PI‐R | ||
| Neuroticism | 48.9 (10.1) | 31.6–66.9 |
| Extraversion | 54.4 (9.7) | 35.7–73.2 |
| Openness | 55.2 (9.8) | 34.0–71.6 |
| Agreeablenness | 47.2 (7.4) | 28.9–59.3 |
| Conscientiousness | 52.5 (8.2) | 33.5–68.1 |
| Motion sickness susceptibility | 11.2 (11.4) | 0–38.6 |
| Behavioral data | ||
| Sense of presence on the Rollercoaster (1‐10) | 4.7 (2.8) | 1–9 |
Eye Movements' Results
The number of saccadic movements did not differ as a function of the session (F (2,102) = 1.3, P = 0.28), motion direction (F (1,102) = 0.002, P = 0.97) or as a function of the session by direction interaction (F (2,102) = 1, P = 0.37). Furthermore, no significant correlations between the number of saccades, neuroticism and introversion scores were found (r's < 0.03, P's > 0.87).
Local Brain Responses Independently of Personality
For the sake of completeness, we also report the brain responses associated with the same contrasts examined in Indovina et al. [2013b], at the same statistical threshold (P < 0.05 FWE whole brain cluster level corrected). Similar to the previous findings, the contrast of all rectilinear visual motion versus static condition showed activations in the PIVC, occipital and parietal cortices, as well as in the middle cingulate cortex, cerebellum (lobule VII, VIII, IX), postcentral gyrus, precentral gyrus, hippocampus, and thalamus (Supporting Information Fig. S1 and Supporting Information Table S1 for details).
Regional activations for the main effect of vertical motion direction relative to horizontal motion direction are reported in Supporting Information Figure S2 and Supporting Information Table S2. Similar to Indovina et al. [2013b], we found activations in the postcentral gyrus and precentral gyrus (Supporting Information Table S2). Additional activations were found in the calcarine sulcus, superior occipital gyrus, middle temporal gyrus, and supplementary motor area. Regional effects for the main effect of motion direction horizontal greater than vertical are reported in Supporting Information Table S3. Again, highly overlapping activations with those reported in Indovina et al. [2013b] were found in the lingual gyrus, fusiform gyrus and middle occipital gyrus (Supporting Information Fig. S3).
Effect of Personality Traits on Local Brain Responses
We found positive correlations between neuroticism scores and activity in the left PIVC (MNI coordinates: x: −42, y: −28, z: 20; Z‐score: 3.91, P = 0.027 FWE svc) when comparing vertical versus horizontal motion trials (Fig. 2, panel A). This result was not significant at the whole brain level of correction but survived svc, FWE in a combined mask of six ROIs. The PIVC region identified in this analysis also partially overlapped with the PIVC area found when comparing all visual motion trials relative to a static condition (Fig. 2, panel B). No negative correlation between local brain activity was found with neuroticism nor any positive or negative correlation was detected between regional brain responses and introversion or other personality traits (P's > 0.28 after svc). Finally, no significant correlation, either positive or negative, was found between brain responses and personality traits for the contrast “all motion versus rest” (P's > 0.19 after svc).
Figure 2.
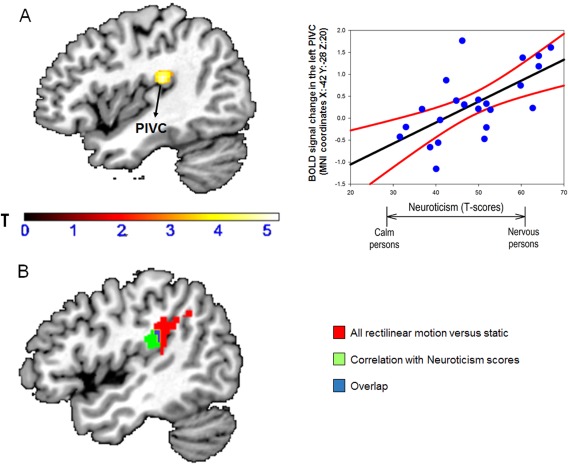
Panel A: Effect of individual differences in neuroticism on brain activations during vertical versus horizontal motion. Neuroticism scores were positively associated with brain responses in the left Parieto‐Insular Vestibular Cortex (PIVC). For visualization purposes only, we displayed results at a statistical threshold of P < 0.005, uncorrected. The coordinates (X, Y, Z) are in the Montreal Neurological Institute (MNI) space. Color bars represent T‐statistics. In the scatterplot, each dot represents individual mean BOLD responses within the displayed cluster, while black lines represent the regression lines and red lines are the 95% confidence interval. Panel B: Overlap between the PIVC clusters. The red PIVC cluster represents the activation found as main effect of all rectilinear motion versus static condition (independently of personality); the green cluster represents the cluster found as a function of Neuroticism, while the blue region represents the overlap between the red and green clusters. BOLD: blood oxygenation level dependent. [Color figure can be viewed at http://wileyonlinelibrary.com]
Effect of Personality Traits on Functional Connectivity Patterns
The brain region showing statistically significant correlations with neuroticism (i.e., left PIVC) for the vertical versus horizontal comparison was then used as seed region for the PPI analysis exploring influences of personality traits on functional connectivity patterns. This analysis showed that neuroticism was positively correlated with connectivity changes between the left PIVC and right amygdala (MNI coordinates: x: 18, y: 2, z: −14; Z‐score: 3.86, P = 0.033 after svc) (Fig. 3). In contrast, no significant main effect of PPI (i.e., independently of personality) was found. Finally, when testing for connectivity effects using the hMT+/V5 as seed region, we found increasing coupling of this region with the left PIVC as a function of increasing neuroticism (MNI coordinates: x: −40, y: −24, z: 24, Z‐score: 3.36, for the vertical vs. horizontal contrast). However, this result was statistically borderline after svc at FWE so we did not discuss it further (P‐svc = 0.13).
Figure 3.

Effect of individual differences in neuroticism on brain functional connectivity during vertical versus horizontal motion. Neuroticism scores were positively associated with the functional connectivity between the left PIVC (“seed” region), and the right amygdala. For visualization purposes only, we displayed the results at a statistical threshold of P < 0.005, uncorrected. The coordinates (X, Y, Z) are in the Montreal Neurological Institute (MNI) space. Color bars represent T‐statistics. In the scatterplot, each dot represents the individual mean BOLD responses within the displayed cluster; black lines represent the regression lines, while red lines are the 95% confidence interval. BOLD: blood oxygenation level dependent. [Color figure can be viewed at http://wileyonlinelibrary.com]
DISCUSSION
To our knowledge, this is the first fMRI study demonstrating that individual differences in neuroticism (but not other personality traits including introversion) modulated brain activity and functional connectivity patterns between visuo‐vestibular and anxiety regions during an immersive virtual reality task simulating self‐motion in a gravity field. More specifically, when comparing vertical versus horizontal motion trials, we found that neuroticism influenced the activity in the left PIVC, one of the most important vestibular cortical areas [Indovina et al., 2005; Lopez et al., 2012; zu Eulenburg et al., 2012]. For the same contrast, neuroticism levels also modulated the task‐dependent connectivity between the left PIVC and right amygdala.
All these findings were independent of motion sickness susceptibility (MSS) which is important as there is evidence that MSS itself may influence visuo‐vestibular brain systems [Napadow et al., 2013a, 2013b]. Furthermore, no significant correlations were found between neuroticism and the number of saccadic eye movements recorded throughout the task which indicates that the attentional level or ability to fixate the central cross during the experiment did not affect the main results. Nonetheless, we found a positive association (although at an uncorrected level) between neuroticism and the sense of presence, which suggests that people with higher levels of neuroticism perceived the rollercoaster paradigm as more immersive and realistic than people with lower neuroticism scores.
The PIVC is a cortical region which is known to respond to different types of visual and vestibular information including visual stimuli that simulate vertical motion within a gravity field [Bense et al., 2001; Bottini et al., 1994; Indovina et al., 2005, 2013b; Lacquaniti et al., 2013; Lopez, 2016; Lopez et al., 2012; Maffei et al., 2010; zu Eulenburg et al., 2012]. There is also evidence that patients with focal PIVC lesions may display unsteadiness and impairments in the visual perception of verticality [Baier et al., 2012; Brandt et al., 1994]. Furthermore, increased PIVC activity has been associated to coherent optic flow compatible with self‐motion [Antal et al., 2008; Cardin and Smith, 2010; Grusser et al., 1990], although reduced PIVC activation has been found in similar contexts [Brandt et al., 1998; Kleinschmidt et al., 2002]. Finally, local electrical stimulation of the PIVC during neurosurgical procedures as well as epileptic discharges originating in the PIVC have been linked to strong sensations of self‐motion [Isnard et al., 2004; Nguyen et al., 2009]. Hence, the increased PIVC activity as a function of higher neuroticism scores may provide a mechanistic explanation of why people with anxiety‐related traits display enhanced susceptibility to visual motion stimuli [Staab, 2012, 2014, 2013].
Interestingly, when comparing vertical versus horizontal motion trials, we also found that neuroticism modulated the connectivity changes between the PIVC and amygdala, a key sub‐cortical “hub” in anxiety and other affective behaviors [Kim et al., 2011; LeDoux, 1998, 2003]. This indicates that vertical self‐motion simulation may evoke heightened interactions between vestibular and anxiety systems in people with high levels of neuroticism. An altered interplay between vestibular and anxiety systems may thus mediate the increased sensitivity to balance threats that has been described in anxious individuals [Staab et al., 2013]. Our connectivity data were also consistent with studies in monkeys showing that PIVC and amygdala are anatomical connected [Mufson et al., 1981], although a recent study in humans has found that the anterior insula and not the PIVC may show enhanced connectivity with the amygdala as a function of higher anxiety levels [Baur et al., 2013]. Nevertheless, the connectivity analyses used here were not only able to identify functional interplays between pairs of brain regions directly connected (i.e., via a single synapsis) but could also identify interactions between regions that may be mediated by additional areas [Friston et al., 1997]. This implies that “in‐between” regions as the anterior insula, which is directly linked with both the PIVC and amygdala [Almashaikhi et al., 2014a, 2014b], may have mediated the effects observed between the PIVC and amygdala. Finally, the viscero‐motor response to interoceptive inputs (including vestibular stimuli) may be orchestrated by reverberating circuits that include the anterior insula, PIVC, and amygdala [Craig, 2010; Menon and Uddin, 2010]. These feed‐forward and feed‐back loops are thought to be critical for adjusting the autonomic functions associated with vestibular stimulation and in modulating the early sensory processing of vestibular inputs [Craig, 2010; Menon and Uddin, 2010].
We also note that no correlation between introversion and brain activity or connectivity patterns was found in this study, in contrast with predictions based on our previous work using sound‐evoked vestibular stimulation [Indovina et al., 2014]. It may be that introversion exerts a different influence on activity in visual‐vestibular‐anxiety pathways in response to different types of motion‐related stimuli, perhaps being more sensitive to stimuli indicating actual self‐motion (i.e., vestibular and proprioceptive inputs) than virtual motion in the external world (i.e., visual inputs). Alternatively, the two stimuli may have elicited different levels of instinctive threat. More specifically, the sound‐evoked vestibular stimulus was a sudden input that lacked context from other motion cues, whereas the virtual rollercoaster task included less sudden motion stimuli that were immersed in a full visual context mimicking a consciously recognizable experience. In our previous study [Indovina et al., 2014], introversion was primarily correlated with activity in anxiety regions in the brain, making the instantaneous threat explanation more plausible than a differential effect of vestibular versus visual inputs.
Finally, it is worth mentioning that our study had a number of strengths including the use a previously developed fMRI paradigm to evoke activations in brain visuo‐vestibular areas, and a sample of participants with a full range of personality traits. In terms of potential shortcomings, we acknowledge that this is the first evidence showing that neuroticism modulated responses to a visual motion stimulus in visuo‐vestibular and anxiety‐related areas, implying that our results should be interpreted with caution until they will be replicated in future studies. Another limitation of this study is the absence of objective measures to assess autonomic nervous system response (e.g., skin conductance). Although in our previous study we did not find evidence that the sense of presence changed when comparing vertical relative to horizontal motion trials [Indovina et al. 2013b], it is still possible that the vertical condition caused greater arousal and autonomic reactivity, particularly in people with high neuroticism scores. Consequently, assessing how autonomic reactivity during a rollercoaster ride varies in relation to visuo‐vestibular and anxiety systems and as a function of neuroticism remains an interesting open question for future research.
In conclusion, we envisage that the results of the current study may inspire forthcoming research into the neural basis of the interplay between visual, vestibular, and anxiety systems both in healthy people and in patients with anxiety and vestibular disorders.
Supporting information
Supporting Information
None of the authors have conflict of interests.
REFERENCES
- Almashaikhi T, Rheims S, Jung J, Ostrowsky‐Coste K, Montavont A, De Bellescize J, Arzimanoglou A, Keo Kosal P, Guenot M, Bertrand O, Ryvlin P (2014a): Functional connectivity of insular efferences. Hum Brain Mapp 35:5279–5294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almashaikhi T, Rheims S, Ostrowsky‐Coste K, Montavont A, Jung J, De Bellescize J, Arzimanoglou A, Keo Kosal P, Guenot M, Bertrand O, Ryvlin P (2014b): Intrainsular functional connectivity in human. Hum Brain Mapp 35:2779–2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antal A, Baudewig J, Paulus W, Dechent P (2008): The posterior cingulate cortex and planum temporale/parietal operculum are activated by coherent visual motion. Vis Neurosci 25:17–26. [DOI] [PubMed] [Google Scholar]
- Baier B, Suchan J, Karnath HO, Dieterich M (2012): Neural correlates of disturbed perception of verticality. Neurology 78:728–735. [DOI] [PubMed] [Google Scholar]
- Balaban CD (2002): Neural substrates linking balance control and anxiety. Physiol Behav 77:469–475. [DOI] [PubMed] [Google Scholar]
- Balaban CD, Jacob RG (2001): Background and history of the interface between anxiety and vertigo. J Anxiety Disord 15:27–51. [DOI] [PubMed] [Google Scholar]
- Barlow DH, Sauer‐Zavala S, Carl JR, Bullis JR, Ellard KK (2014): The nature, diagnosis, and treatment of neuroticism back to the future. Clin Psychol Sci 2:344–365. [Google Scholar]
- Baur V, Hanggi J, Langer N, Jancke L (2013): Resting‐state functional and structural connectivity within an insula‐amygdala route specifically index state and trait anxiety. Biol Psychiatry 73:85–92. [DOI] [PubMed] [Google Scholar]
- Bense S, Stephan T, Yousry TA, Brandt T, Dieterich M (2001): Multisensory cortical signal increases and decreases during vestibular galvanic stimulation (fMRI). J Neurophysiol 85:886–899. [DOI] [PubMed] [Google Scholar]
- Best C, Eckhardt‐Henn A, Tschan R, Dieterich M (2009): Psychiatric morbidity and comorbidity in different vestibular vertigo syndromes. Results of a prospective longitudinal study over one year. J Neurol 256:58–65. [DOI] [PubMed] [Google Scholar]
- Bienvenu OJ, Samuels JF, Costa PT, Reti IM, Eaton WW, Nestadt G (2004): Anxiety and depressive disorders and the five‐factor model of personality: A higher‐ and lower‐order personality trait investigation in a community sample. Depress Anxiety 20:92–97. [DOI] [PubMed] [Google Scholar]
- Bishop SJ (2007): Neurocognitive mechanisms of anxiety: An integrative account. Trends Cogn Sci 11:307–316. [DOI] [PubMed] [Google Scholar]
- Bolmont B, Gangloff P, Vouriot A, Perrin PP (2002): Mood states and anxiety influence abilities to maintain balance control in healthy human subjects. Neurosci Lett 329:96–100. [DOI] [PubMed] [Google Scholar]
- Bosco G, Carrozzo M, Lacquaniti F (2008): Contributions of the human temporoparietal junction and MT/V5+ to the timing of interception revealed by transcranial magnetic stimulation. J Neurosci 28:12071–12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottini G, Sterzi R, Paulesu E, Vallar G, Cappa SF, Erminio F, Passingham RE, Frith CD, Frackowiak RS (1994): Identification of the central vestibular projections in man: A positron emission tomography activation study. Exp Brain Res 99:164–169. [DOI] [PubMed] [Google Scholar]
- Brandt T, Dieterich M, Danek A (1994): Vestibular cortex lesions affect the perception of verticality. Ann Neurol 35:403–412. [DOI] [PubMed] [Google Scholar]
- Brandt T, Bartenstein P, Janek A, Dieterich M (1998): Reciprocal inhibitory visual‐vestibular interaction. Visual motion stimulation deactivates the parieto‐insular vestibular cortex. Brain 121: 1749–1758. [DOI] [PubMed] [Google Scholar]
- Britten KH (2008): Mechanisms of self‐motion perception. Annu Rev Neurosci 31:389–410. [DOI] [PubMed] [Google Scholar]
- Cardin V, Smith AT (2010): Sensitivity of human visual and vestibular cortical regions to egomotion‐compatible visual stimulation. Cereb Cortex 20:1964–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona JE, Holland AK, Harrison DW (2009): Extending the functional cerebral systems theory of emotion to the vestibular modality: A systematic and integrative approach. Psychol Bull 135:286. [DOI] [PubMed] [Google Scholar]
- Celestino D, Rosini E, Carucci ML, Marconi PL, Vercillo E (2003): Meniere's disease and anxiety disorders. Acta Otorhinolaryngol Ital 23:421–427. [PubMed] [Google Scholar]
- Coelho CM, Balaban CD (2015): Visuo‐vestibular contributions to anxiety and fear. Neurosci Biobehav Rev 48:148–159. [DOI] [PubMed] [Google Scholar]
- Coelho CM, Waters AM, Hine TJ, Wallis G (2009): The use of virtual reality in acrophobia research and treatment. J Anxiety Disord 23:563–574. [DOI] [PubMed] [Google Scholar]
- Costa PT, McCrae RR (1992): Normal personality assessment in clinical practice: The NEO Personality Inventory. Psychol Assess 4:5. [Google Scholar]
- Costa PT, Jr , McCrae RR (1997): Stability and change in personality assessment: The revised NEO Personality Inventory in the year 2000. J Pers Assess 68:86–94. [DOI] [PubMed] [Google Scholar]
- Cousins S, Cutfield NJ, Kaski D, Palla A, Seemungal BM, Golding JF, Staab JP, Bronstein AM (2014): Visual dependency and dizziness after vestibular neuritis. PLoS One 9:e105426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD (2009): How do you feel–now? The anterior insula and human awareness. Nat Rev Neurosci 10:59–70. [DOI] [PubMed] [Google Scholar]
- Craig AD (2010): The sentient self. Brain Struct Funct 214:563–577. [DOI] [PubMed] [Google Scholar]
- De Waele C, Baudonniere PM, Lepecq JC, Tran Ba Huy P, Vidal PP (2001): Vestibular projections in the human cortex. Exp Brain Res 141:541–551. [DOI] [PubMed] [Google Scholar]
- Diemer J, Lohkamp N, Muhlberger A, Zwanzger P (2015): Fear and physiological arousal during a virtual height challenge‐effects in patients with acrophobia and healthy controls. J Anxiety Disord 37:30–39. [DOI] [PubMed] [Google Scholar]
- Eagger S, Luxon LM, Davies RA, Coelho A, Ron MA (1992): Psychiatric morbidity in patients with peripheral vestibular disorder: A clinical and neuro‐otological study. J Neurol Neurosurg Psychiatry 55:383–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhardt‐Henn A, Breuer P, Thomalske C, Hoffmann SO, Hopf HC (2003): Anxiety disorders and other psychiatric subgroups in patients complaining of dizziness. J Anxiety Disord 17:369–388. [DOI] [PubMed] [Google Scholar]
- Eckhardt A, Tettenborn B, Krauthauser H, Thomalske C, Hartmann O, Hoffmann SO, Hopf HC (1996): Vertigo and anxiety disorders–results of interdisciplinary evaluation. Laryngorhinootologie 75:517–522. [DOI] [PubMed] [Google Scholar]
- Fischer B, Breitmeyer B (1987): Mechanisms of visual attention revealed by saccadic eye movements. Neuropsychologia 25:73–83. [DOI] [PubMed] [Google Scholar]
- Frank SM, Baumann O, Mattingley JB, Greenlee MW (2014): Vestibular and visual responses in human posterior insular cortex. J Neurophysiol 112:2481–2491. [DOI] [PubMed] [Google Scholar]
- Frank SM, Wirth AM, Greenlee MW (2016): Visual‐vestibular processing in the human Sylvian fissure. J Neurophysiol 116:263–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ (1997): Testing for anatomically specified regional effects. Hum Brain Mapp 5:133–136. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ (1997): Psychophysiological and modulatory interactions in neuroimaging. Neuroimage 6:218–229. [DOI] [PubMed] [Google Scholar]
- Gitelman DR, Penny WD, Ashburner J, Friston KJ (2003): Modeling regional and psychophysiologic interactions in fMRI: The importance of hemodynamic deconvolution. Neuroimage 19:200–207. [DOI] [PubMed] [Google Scholar]
- Golding JF (1998): Motion sickness susceptibility questionnaire revised and its relationship to other forms of sickness. Brain Res Bull 47:507–516. [DOI] [PubMed] [Google Scholar]
- Gowers WR (1887): A manual of diseases of the nervous system. J Nervous Mental Dis 14:123–125. [Google Scholar]
- Grusser OJ, Pause M, Schreiter U (1990): Vestibular neurones in the parieto‐insular cortex of monkeys (Macaca fascicularis): visual and neck receptor responses. J Physiol 430:559–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainaut JP, Caillet G, Lestienne FG, Bolmont B (2011): The role of trait anxiety on static balance performance in control and anxiogenic situations. Gait Posture 33:604–608. [DOI] [PubMed] [Google Scholar]
- Hallam R, Hinchcliffe R (1991): Emotional stability; its relationship to confidence in maintaining balance. J Psychosom Res 35:421–430. [DOI] [PubMed] [Google Scholar]
- Hüfner K, Strupp M, Smith P, Brandt T, Jahn K (2011): Spatial separation of visual and vestibular processing in the human hippocampal formation. Ann N Y Acad Sci 1233:177–186. [DOI] [PubMed] [Google Scholar]
- Indovina I, Maffei V, Bosco G, Zago M, Macaluso E, Lacquaniti F (2005): Representation of visual gravitational motion in the human vestibular cortex. Science 308:416–419. [DOI] [PubMed] [Google Scholar]
- Indovina I, Robbins TW, Nunez‐Elizalde AO, Dunn BD, Bishop SJ (2011): Fear‐conditioning mechanisms associated with trait vulnerability to anxiety in humans. Neuron 69:563–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indovina I, Maffei V, Lacquaniti F (2013a): Anticipating the effects of visual gravity during simulated self‐motion: Estimates of time‐to‐passage along vertical and horizontal paths. Exp Brain Res 229:579–586. [DOI] [PubMed] [Google Scholar]
- Indovina I, Maffei V, Pauwels K, Macaluso E, Orban GA, Lacquaniti F (2013b): Simulated self‐motion in a visual gravity field: Sensitivity to vertical and horizontal heading in the human brain. Neuroimage 71:114–124. [DOI] [PubMed] [Google Scholar]
- Indovina I, Riccelli R, Staab JP, Lacquaniti F, Passamonti L (2014): Personality traits modulate subcortical and cortical vestibular and anxiety responses to sound‐evoked otolithic receptor stimulation. J Psychosom Res 77:391–400. [DOI] [PubMed] [Google Scholar]
- Indovina I, Mazzarella E, Maffei V, Cesqui B, Passamonti L, Lacquaniti F (2015a): Sound‐evoked vestibular stimulation affects the anticipation of gravity effects during visual self‐motion. Exp Brain Res 233:2365–2371. [DOI] [PubMed] [Google Scholar]
- Indovina I, Riccelli R, Chiarella G, Petrolo C, Augimeri A, Giofrè L, Lacquaniti F, Staab JP, Passamonti L (2015b): Role of the insula and vestibular system in patients with chronic subjective dizziness: An fMRI study using sound‐evoked vestibular stimulation. Front Behav Neurosci 9:334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isnard J, Guenot M, Sindou M, Mauguiere F (2004): Clinical manifestations of insular lobe seizures: A stereo‐electroencephalographic study. Epilepsia 45:1079–1090. [DOI] [PubMed] [Google Scholar]
- Kapfhammer HP, Huppert D, Grill E, Fitz W, Brandt T (2014): Visual height intolerance and acrophobia: Clinical characteristics and comorbidity patterns. Eur Arch Psychiatry Clin Neurosci 265:375–385. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Loucks RA, Palmer AL, Brown AC, Solomon KM, Marchante AN, Whalen PJ (2011): The structural and functional connectivity of the amygdala: From normal emotion to pathological anxiety. Behav Brain Res 223:403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschmidt A, Thilo KV, Buchel C, Gresty MA, Bronstein AM, Frackowiak RS (2002): Neural correlates of visual‐motion perception as object‐ or self‐motion. Neuroimage 16:873–882. [DOI] [PubMed] [Google Scholar]
- Lacquaniti F, Bosco G, Indovina I, La Scaleia B, Maffei V, Moscatelli A, Zago M (2013): Visual gravitational motion and the vestibular system in humans. Front Integr Neurosci 7:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J (1998): Fear and the brain: Where have we been, and where are we going?. Biol Psychiatry 44:1229–1238. [DOI] [PubMed] [Google Scholar]
- LeDoux J (2003): The emotional brain, fear, and the amygdala. Cell Mol Neurobiol 23:727–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepicard EM, Joubert C, Hagneau I, Perez‐Diaz F, Chapouthier G (2000a): Differences in anxiety‐related behavior and response to diazepam in BALB/cByJ and C57BL/6J strains of mice. Pharmacol Biochem Behav 67:739–748. [DOI] [PubMed] [Google Scholar]
- Lepicard EM, Venault P, Perez‐Diaz F, Joubert C, Berthoz A, Chapouthier G (2000b): Balance control and posture differences in the anxious BALB/cByJ mice compared to the non anxious C57BL/6J mice. Behav Brain Res 117:185–195. [DOI] [PubMed] [Google Scholar]
- Lopez C (2016): The vestibular system: Balancing more than just the body. Curr Opin Neurol 29:74–83. [DOI] [PubMed] [Google Scholar]
- Lopez C, Blanke O (2011): The thalamocortical vestibular system in animals and humans. Brain Res Rev 67:119–146. [DOI] [PubMed] [Google Scholar]
- Lopez C, Blanke O, Mast FW (2012): The human vestibular cortex revealed by coordinate‐based activation likelihood estimation meta‐analysis. Neuroscience 212:159–179. [DOI] [PubMed] [Google Scholar]
- Maffei V, Macaluso E, Indovina I, Orban G, Lacquaniti F (2010): Processing of targets in smooth or apparent motion along the vertical in the human brain: An fMRI study. J Neurophysiol 103:360–370. [DOI] [PubMed] [Google Scholar]
- Mast FW, Preuss N, Hartmann M, Grabherr L (2014): Spatial cognition, body representation and affective processes: The role of vestibular information beyond ocular reflexes and control of posture. Front Integr Neurosci 8:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Uddin LQ (2010): Saliency, switching, attention and control: A network model of insula function. Brain Struct Funct 214:655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mufson EJ, Mesulam MM, Pandya DN (1981): Insular interconnections with the amygdala in the rhesus monkey. Neuroscience 6:1231–1248. [DOI] [PubMed] [Google Scholar]
- Napadow V, Sheehan J, Kim J, Dassatti A, Thurler AH, Surjanhata B, Vangel M, Makris N, Schaechter JD, Kuo B (2013a): Brain white matter microstructure is associated with susceptibility to motion‐induced nausea. Neurogastroenterol Motil 25:448–e303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napadow V, Sheehan JD, Kim J, LaCount LT, Park K, Kaptchuk TJ, Rosen BR, Kuo B (2013b): The brain circuitry underlying the temporal evolution of nausea in humans. Cereb Cortex 23:806–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen DK, Nguyen DB, Malak R, Leroux JM, Carmant L, Saint‐Hilaire JM, Giard N, Cossette P, Bouthillier A (2009): Revisiting the role of the insula in refractory partial epilepsy. Epilepsia 50:510–520. [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9:97–113. [DOI] [PubMed] [Google Scholar]
- Orban GA, Fize D, Peuskens H, Denys K, Nelissen K, Sunaert S, Todd J, Vanduffel W (2003): Similarities and differences in motion processing between the human and macaque brain: Evidence from fMRI. Neuropsychologia 41:1757–1768. [DOI] [PubMed] [Google Scholar]
- Passamonti L, Rowe JB, Ewbank M, Hampshire A, Keane J, Calder AJ (2008): Connectivity from the ventral anterior cingulate to the amygdala is modulated by appetitive motivation in response to facial signals of aggression. Neuroimage 43:562–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passamonti L, Rowe JB, Schwarzbauer C, Ewbank MP, von dem Hagen E, Calder AJ (2009): Personality predicts the brain's response to viewing appetizing foods: The neural basis of a risk factor for overeating. J Neurosci 29:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passamonti L, Crockett MJ, Apergis‐Schoute AM, Clark L, Rowe JB, Calder AJ, Robbins TW (2012): Effects of acute tryptophan depletion on prefrontal‐amygdala connectivity while viewing facial signals of aggression. Biol Psychiatry 71:36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penny WD, Friston KJ, Ashburner JT, Kiebel SJ, Nichols TE (2011): Statistical Parametric Mapping: The Analysis of Functional Brain Images. New York: Academic Press. [Google Scholar]
- Peperkorn HM, Muhlberger A (2013): The impact of different perceptual cues on fear and presence in virtual reality. Stud Health Technol Inform 191:75–79. [PubMed] [Google Scholar]
- Poldrack RA (2007): Region of interest analysis for fMRI. Soc Cogn Affect Neurosci 2:67–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redfern MS, Yardley L, Bronstein AM (2001): Visual influences on balance. J Anxiety Disord 15:81–94. [DOI] [PubMed] [Google Scholar]
- Redfern MS, Furman JM, Jacob RG (2007): Visually induced postural sway in anxiety disorders. J Anxiety Disord 21:704–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudrauf D, Venault P, Cohen‐Salmon C, Berthoz A, Jouvent R, Chapouthier G (2004): A new method for the assessment of spatial orientation and spatial anxiety in mice. Brain Res Brain Res Protoc 13:159–165. [DOI] [PubMed] [Google Scholar]
- Sanchez‐Vives MV, Slater M (2005): From presence to consciousness through virtual reality. Nat Rev Neurosci 6:332–339. [DOI] [PubMed] [Google Scholar]
- Staab JP (2012): Chronic subjective dizziness. Continuum (Minneap Minn) 18:1118–1141. [DOI] [PubMed] [Google Scholar]
- Staab JP (2014): The influence of anxiety on ocular motor control and gaze. Curr Opin Neurol 27:118–124. [DOI] [PubMed] [Google Scholar]
- Staab JP, Balaban CD, Furman JM (2013): Threat assessment and locomotion: Clinical applications of an integrated model of anxiety and postural control. Semin Neurol 33:297–306. [DOI] [PubMed] [Google Scholar]
- Staab JP, Rohe DE, Eggers SD, Shepard NT (2014): Anxious, introverted personality traits in patients with chronic subjective dizziness. J Psychosom Res 76:80–83. [DOI] [PubMed] [Google Scholar]
- Sylvester CM, Corbetta M, Raichle ME, Rodebaugh TL, Schlaggar BL, Sheline YI, Zorumski CF, Lenze EJ (2012): Functional network dysfunction in anxiety and anxiety disorders. Trends Neurosci 35:527–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trull TJ, Sher KJ (1994): Relationship between the five‐factor model of personality and Axis I disorders in a nonclinical sample. J Abnorm Psychol 103:350–360. [DOI] [PubMed] [Google Scholar]
- Tschan R, Best C, Beutel ME, Knebel A, Wiltink J, Dieterich M, Eckhardt‐Henn A (2011): Patients' psychological well‐being and resilient coping protect from secondary somatoform vertigo and dizziness (SVD) 1 year after vestibular disease. J Neurol 258:104–112. [DOI] [PubMed] [Google Scholar]
- Viaud‐Delmon I, Berthoz A, Jouvent R (2002): Multisensory integration for spatial orientation in trait anxiety subjects: Absence of visual dependence. Eur Psychiatry 17:194–199. [DOI] [PubMed] [Google Scholar]
- Viaud‐Delmon I, Venault P, Chapouthier G (2011): Behavioral models for anxiety and multisensory integration in animals and humans. Prog Neuropsychopharmacol Biol Psychiatry 35:1391–1399. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Friston KJ (1995): Analysis of fMRI time‐series revisited—again. Neuroimage 2:173–181. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC (1996): A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp 4:58–73. [DOI] [PubMed] [Google Scholar]
- zu Eulenburg P, Caspers S, Roski C, Eickhoff SB (2012): Meta‐analytical definition and functional connectivity of the human vestibular cortex. Neuroimage 60:162–169. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


