Abstract
Large‐scale brain networks play a prominent role in cognitive abilities and their activity is impaired in psychiatric disorders, such as schizophrenia. Patients with 22q11.2 deletion syndrome (22q11DS) are at high risk of developing schizophrenia and present similar cognitive impairments, including executive functions deficits. Thus, 22q11DS represents a model for the study of neural biomarkers associated with schizophrenia. In this study, we investigated structural and functional connectivity within and between the Default Mode (DMN), the Central Executive (CEN), and the Saliency network (SN) in 22q11DS using resting‐state fMRI and DTI. Furthermore, we investigated if triple network impairments were related to executive dysfunctions or the presence of psychotic symptoms. Sixty‐three patients with 22q11DS and sixty‐eighty controls (age 6–33 years) were included in the study. Structural connectivity between main nodes of DMN, CEN, and SN was computed using probabilistic tractography. Functional connectivity was computed as the partial correlation between the time courses extracted from each node. Structural and functional connectivity measures were then correlated to executive functions and psychotic symptom scores. Our results showed mainly reduced structural connectivity within the CEN, DMN, and SN, in patients with 22q11DS compared with controls as well as reduced between‐network connectivity. Functional connectivity appeared to be more preserved, with impairments being evident only within the DMN. Structural connectivity impairments were also related to executive dysfunctions. These findings show an association between triple network structural alterations and executive deficits in patients with the microdeletion, suggesting that 22q11DS and schizophrenia share common psychopathological mechanisms. Hum Brain Mapp 38:2177–2189, 2017. © 2017 Wiley Periodicals, Inc.
Keywords: large‐scale networks, cognitive functions, psychotic symptoms, schizophrenia, resting‐state fMRI, DTI
INTRODUCTION
Cognitive impairments are an important feature of 22q11.2 deletion syndrome (22q11DS), a genetic disease caused by a microdeletion on the long arm of chromosome 22. In particular, borderline IQ levels, altered visuo‐spatial processing and impaired social cognition are part of the 22q11DS cognitive phenotype [Antshel et al., 2008; Campbell et al., 2015; Niklasson and Gillberg, 2010]. Executive dysfunctions have also been reported in patients with 22q11DS [Azuma et al., 2009; Campbell et al., 2010; Chow et al., 2006; Lewandowski et al., 2007; Maeder et al., 2016; McCabe et al., 2014; Shapiro et al., 2013, 2014]. Specifically, impaired inhibition [McCabe et al., 2014; Shapiro et al., 2013] and working memory [Azuma et al., 2009] have been shown in these patients. Moreover, a previous longitudinal study conducted by our group reported altered trajectories of executive functions development in patients with 22q11DS from childhood to early adulthood [Maeder et al., 2016].
Together with the cognitive impairments, the early manifestation of attenuated psychotic symptoms is another characteristic of patients with 22q11DS [Gothelf et al., 2013; Schneider et al., 2014a]. In particular, 50% of the adolescents with the syndrome manifest positive psychotic symptoms [Baker and Skuse, 2005; Debbané et al., 2006; Schneider et al., 2014b], while the prevalence of negative symptoms is even higher [Schneider et al., 2012, 2014c; Stoddard et al., 2010]. Furthermore, about 30%–40% of patients with 22q11DS develop schizophrenia [Murphy et al., 1999; Schneider et al., 2014a], thus making this syndrome a neurogenetic model of the disease [Bassett and Chow, 1999].
Little is known about the neural correlates of altered executive functions and psychotic symptoms in patients with 22q11DS. However, few studies showed white matter impairments [Radoeva et al., 2012] or aberrant functional activation [Gothelf et al., 2007a; Montojo et al., 2014] associated with executive dysfunctions in patients with the syndrome. On the opposite, in a recent study investigating functional connectivity within resting‐state networks [Mattiaccio et al., 2016], the authors found no association between altered connectivity and executive functions measures. A number of studies (reviewed in [Scariati et al., 2016b]) further reported an association between impairments in long‐range connections and psychotic symptoms in 22q11DS. Nevertheless, it is not yet clear how brain networks integrity is related to executive dysfunctions and psychotic symptoms in patients with 22q11DS.
The triple network model of psychopathology proposed by Menon in 2011 [Menon, 2011] represents a useful framework for understanding the relationship between brain networks activation, cognition and psychotic phenotype. This model proposes that three brain networks are specifically related to cognitive abilities (including executive functions) and to the manifestation of psychotic symptoms in the general population [Menon, 2011]. These core cognitive networks are: the default mode network (DMN, [Buckner et al., 2008; Fox et al., 2005; Greicius et al., 2003; Greicius and Menon, 2004; Raichle et al., 2001]), the central executive network (CEN, [Corbetta and Shulman, 2002; Seeley et al., 2007]) and the saliency network (SN, [Palaniyappan, 2012; Singer et al., 2009]).
These three networks have a complementary and coordinated activity [Fox et al., 2005; Menon, 2011]. The DMN and the CEN have an antagonist function, and thus appear to be anti‐correlated. The DMN is mostly active when the attention is oriented to inner processes, while the activity is switched in favor of the CEN during external‐directed attention. In this context, the SN, and in particular the insular cortex, seems to play a prominent role in switching between DMN and CEN activity ([Menon and Uddin, 2010; Sridharan et al., 2008]. It has been proposed that impairments in the coordinated activity and interaction between DMN, CEN and SN is common of several psychiatric disorders, such as autism, depression and schizophrenia and is responsible for cognitive impairments and psychotic symptoms [Menon, 2011].
Impairments within the DMN, CEN, and SN have also been showed in patients with 22q11DS. For instance, a previous study by our group [Debbané et al., 2012] showed impaired DMN activation in a sample of adolescents. This finding was further confirmed by a multimodal investigation [Padula et al., 2015] showing reduced structural and functional connectivity between medial DMN nodes. Moreover, Mattiaccio et al. [2016] further showed reduced activation of the CEN and the SN. However, no study to date directly investigated connectivity between the three networks in patients with 22q11DS. Nevertheless, in a recent study from our group [Scariati et al., 2016a], we reported altered connectivity of the ACC with the DMN and the DLPFC, as well as impaired maturation of the DLPFC connectivity with age. Furthermore, two studies using resting‐state electroencephalography (EEG) suggested that the shifting between CEN and SN activation is impaired in 22q11DS [Tomescu et al., 2014, 2015]. These authors also found that increased activation of the SN was related to the presence of hallucinations in patients with 22q11DS, thus pointing to similar psychopathological mechanisms in 22q11DS and schizophrenia. However, no study to date investigated triple network connectivity in patients with 22q11DS using MRI.
The present study has two main aims: (1) to investigate if triple network impairments are present in patients with 22q11DS, as reported in patients with schizophrenia; (2) to assess if triple network impairments are responsible for the impaired executive functions and the presence of psychotic symptoms in this specific population. To address these aims, specific nodes were selected for each network in order to measure connectivity between nodes belonging to the same network (within‐network connectivity), as well as between nodes belonging to different networks (between‐network connectivity). Furthermore, in line with the assumption that functional connectivity impairments would be accompanied by alterations in underlying white matter tracts [Rykhlevskaia et al., 2008], a multimodal design was chosen. Functional connectivity (using resting‐state fMRI) and structural connectivity (using DTI) were hence examined simultaneously in the same group of participants.
Based on the previous findings in patients with 22q11DS, we expected to observe within‐ and between‐network connectivity impairments. We also hypothesized that impaired functional connectivity would be accompanied by a concomitant alteration of the underlying white matter connections. Finally, we speculated that triple network impairments would be responsible for executive dysfunctions and psychotic symptoms in 22q11DS.
MATERIALS AND METHODS
Participants
Participants were recruited through announcement in regional parents' associations in the context of a longitudinal study on 22q11DS conducted in Geneva [Maeder et al., 2016; Schaer et al., 2009; Schneider et al., 2014b]. From the 212 available subjects (90 controls and 122 patients), 81 were excluded (22 controls and 59 patients) because of bad quality MRI data (see Supporting Information File 1 for further information). The final group of patients with 22q11DS comprised 63 subjects aged between 8 and 33 years old. Demographic details are presented in Table 1. The presence of psychiatric disorders was assessed with the Diagnostic Interview for Children and Adolescents Revised (DICA‐R, [Reich, 2000]), the psychosis supplement from the Kiddie‐Schedule for Affective Disorders and Schizophrenia Present and Lifetime version (K‐SADS‐PL, [Kaufman et al., 1997]) and the Structured Clinical Interview for DSM‐IV Axis I Disorders (SCID‐I, [First et al., 1996]). Information about psychiatric diagnosis and medication is reported in Table 1.
Table 1.
Demographic information
| Controls | 22q11DS | P value | |
|---|---|---|---|
| No. of subjects (females) | 68 (34) | 63 (35) | 0.53 |
| Mean age (range) |
16.7 ± 6 y.o. (6.3–30) |
17.5 ± 5.6 y.o. (8.1–33.8) |
0.46 |
| Right handed (%)a | 79.4% | 79.4% | 0.8 |
| Mean IQb | 110 ± 15.6 | 68.5 ± 11.5 | <0.001 |
| No. of subjects meeting criteria for psychiatric diagnosis | N/A | 40 (63%) | |
| Anxiety disorder | N/A | 10 | |
| Attention deficit hyperactivity disorder | N/A | 4 | |
| Mood disorder | N/A | 4 | |
| Schizophrenia | N/A | 3 | |
| More than one psychiatric disorder | N/A | 19 | |
| No. of subjects medicated | 0 | 20 (32%) | |
| Methylphenidate | 0 | 9 | |
| Antidepressants | 0 | 2 | |
| Antipsychotics | 0 | 3 | |
| Anticonvulsants | 0 | 1 | |
| More than one class of medication | 0 | 5 |
Handedness was measured using the Edinburgh laterality quotient.
IQ was measured using the Wechsler Intelligence Scale for Children–III or the Wechsler Adult Intelligence Scale–III for adults [Wechsler, 1997].
The control group included 68 participants aged between 6 and 30 years old (Table 1) recruited among healthy siblings of the patients (N = 37, 54%) and from the Geneva state school system (N = 31, 46%). None of the healthy participants had a past or present history of neurological or psychiatric disorders. Written informed consent was received from all participants or their parents under protocols approved by a local ethical review board.
Different executive functions domains (working memory, inhibition, and verbal fluency) were evaluated as described in our previous work [Maeder et al., 2016]. Briefly, we used the Wechsler Digit Span subtest to assess working memory, the Continuous Performance Test (CPT, [Conners and Staff, 2000]) for motor inhibition, the Stroop task [Stroop, 1935] for cognitive inhibition and the semantic verbal fluency test, animal category for verbal fluency. In particular, the following scores for each test were used: digit span raw scores, commission errors, omission errors, and reaction time of the CPT, the interference index of the Stroop and the number of words produced in the verbal fluency test.
The presence of positive and negative psychotic symptoms in patients older than 12 years old was further assessed using the Structured Interview for Prodromal Syndromes (SIPS, [Miller et al., 2002]). Specifically, five positive subscales (unusual thoughts/delusional ideas, suspiciousness/persecutory ideas, grandiose ideas, hallucinations, disorganized communication) and six negative subscales (social anhedonia, avolition, expression of emotion, experience of emotion and self, ideational richness, occupational functioning) were used.
Images Acquisition
T1‐weighted, diffusion‐weighted and resting‐state fMRI images were acquired using a Siemens Prisma (N = 111) or a Siemens Trio (N = 20) 3 Tesla MRI machine at the Center for Biomedical Imaging (CIBM) in Geneva. The T1‐weighted sequence was acquired with a 3D volumetric pulse and the following parameters: TR = 2,500 ms, TE = 3 ms, flip angle = 8°, acquisition matrix = 256 × 256, field of view = 23.5 cm, slice thickness = 3.2 mm, 192 slices. Parameters for the DTI images were the following: number of directions = 30, b = 1,000 s/mm2, TR = 8800 ms, TE = 84 ms, flip angle = 90°, acquisition matrix = 128 × 128, field of view = 25.6 cm, 64 axial slices, slice thickness = 2 mm. Resting‐state fMRI scans were acquired over 8 minutes asking the participants to fix a cross on the screen and to let their thoughts go. The sequence consisted of 200 blood‐oxygenation‐level‐dependent (BOLD) images with TR = 2,400 ms, TE = 30 ms, 38 axial slices, slice thickness = 3.2 mm, flip angle = 85°, acquisition matrix = 94 × 128, field of view = 96 × 128.
Definition of Networks' Nodes
In order to investigate connectivity between nodes belonging to each network, regions of interest (ROIs) were selected from a resting‐state networks atlas (http://findlab.stanford.edu/functional_ROIs.html). For the DMN, six masks were selected, corresponding to the ventromedial prefrontal cortex (anterior DMN), the posterior cingulate cortex/precuneus (posterior DMN), the bilateral medial temporal cortex (MTL) and the bilateral angular gyrus. For the CEN we selected four masks corresponding to the bilateral dorso‐lateral prefrontal cortex (DLPF) and the bilateral parietal cortex. For the SN, we selected masks corresponding to the dorsal anterior cingulate cortex (dorsal ACC) and the bilateral insular cortex. These masks were warped in the participants' native space using normalization parameters estimated with Diffeomorphic Anatomical Registration using Exponentiated Lie Algebra (DARTEL [Ashburner, 2007]) and used as seeds in the structural connectivity analysis. For the functional connectivity analysis, 6‐mm spheres were constructed starting from the center of each mask and warped in the participants' space. One control participant and three patients with 22q11DS were excluded from the functional connectivity analysis because of normalization errors.
Resting‐State fMRI Analysis
Functional scans were preprocessed using the DPARSF toolbox (http://rfmri.org/DPARSF). Preprocessing of the functional images included: removal of the first five volumes and realignment to the mean image. T1‐weighted images were segmented and co‐registered to the mean functional image. Images were then spatially smoothed using an isotropic Gaussian smoothing kernel with a full width at half maximum (FWHM) of 4 mm. The signal was then linearly detrended and white matter and cerebro‐spinal fluid signals were regressed out. The Friston 24‐parameter model was used to correct for motion artifacts. Finally, the signal was bandpass‐filtered (0.01–0.1 Hz). Mean translational and rotational movement as well as the mean framewise displacement for control and 22q11DS participants are reported in Table 2. The six motion parameters did not significantly differ between controls and patients with 22q11DS, nonetheless the mean framewise displacement was significantly higher in the group of patients.
Table 2.
FMRI data motion parameters
| Controls | 22q11DS | P value | ||
|---|---|---|---|---|
| Mean translation | x | 0.1343 ± 0.14 | 0.1156 ± 0.12 | 0.42 |
| y | 0.2036 ± 0.2 | 0.1603 ± 0.19 | 0.22 | |
| z | 0.2692 ± 0.26 | 0.354 ± 0.27 | 0.07 | |
| Mean rotation | x (pitch) | 0.006 ± 0.0043 | 0.0074 ± 0.0056 | 0.1 |
| y (roll) | 0.0036 ± 0.0032 | 0.0033 ± 0.0022 | 0.54 | |
| z (yaw) | 0.0026 ± 0.0025 | 0.0037 ± 0.0043 | 0.078 | |
| Mean framewise displacement | 0.0915 ± 0.04 | 0.136 ± 0.76 | <0.001 |
Regions of interest for the functional connectivity analysis were extracted as described in paragraph “Definition of Networks' Nodes.” Functional connectivity was computed using partial correlation between the time courses extracted from each pairs of nodes. R scores where then transformed to Z scores using Fisher's transformation and statistical analysis was conducted using ANCOVA with age and gender as covariates. Given the low number of connections in the within‐network analysis a Bonferroni correction for multiple comparisons was used. False discovery rate (FDR) was instead used to correct for multiple comparisons in the between‐network analysis.
DTI Analysis
Analysis of intra‐ and inter‐network structural connectivity was performed using the FSL Diffusion Toolbox (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/). Preprocessing included: skull and non‐brain tissue stripping using the Brain Extraction Tool (BET, [Smith, 2002]), motion and eddy currents distortion correction, registration of the T1‐weighted images on the set of diffusion images with the FMRIB's Linear Image Registration Tool (FLIRT, [Jenkinson et al., 2002; Jenkinson and Smith, 2001]). DTI motion parameters were extracted using the software TRACULA (https://surfer.nmr.mgh.harvard.edu/fswiki/Tracula, [Yendiki et al., 2014]) and are reported in Table 3. No significant difference in mean motion scores was observed between the two groups. However, four additional patients were excluded from the DTI analysis because they had excessive motion values compared with the remaining participants (>2 standard deviations above the mean of the group).
Table 3.
DTI data motion parameters
| Controls | 22q11DS | P value | |
|---|---|---|---|
| Translation (mm) | 0.867 ± 0.2341 | 0.882 ± 0.32 | 0.76 |
| Rotation (degrees) | 0.0067 ± 0.002 | 0.007 ± 0.004 | 0.32 |
| Slice with drop‐out (%) | 0.16% | 2.0% | 0.057 |
| Drop out score | 1.002 ± 0.018 | 1.015 ± 0.07 | 0.16 |
Structural connectivity analysis was conducted with the Probtrackx probabilistic tractography software from FSL [Behrens et al., 2007], starting from the seed masks described in paragraph “Definition of Networks' Nodes.” The output was a connectivity matrix reporting, for each pair of regions, the number of streamlines generated from region i to region j and from region j to region i. We then computed an average connectivity matrix containing the mean number of streamlines connecting each pair of nodes. Connections (N = 13) that were not present in all the subjects and with an average of less than 50 reconstructed streamlines were not considered for further analysis (see Supporting Information File 1). Each connection was then normalized by the total number of connections in the connectivity matrix. Given the non‐normal distribution of the structural connectivity data, statistical analysis was conducted using the Wilcoxon rank sum test (or Mann–Whitney U‐test) covarying for age and gender. As for the functional connectivity analysis, correction for multiple comparisons was performed using Bonferroni correction for the within network connectivity, and FDR for the between network connectivity.
Correlation Between Connectivity Measures, Executive Functions, and Psychotic Symptoms
In line with the second aim of this study, we investigated if impairments in the within‐ or between‐networks connectivity were related to cognitive dysfunctions and/or psychotic symptoms in 22q11DS. To do so, we computed Spearmann correlations coefficient between the connections that were significantly different between the two groups and both the clinical and cognitive scores mentioned in section “Participants.” Age and gender were used as nuisance covariates in the correlation analysis.
RESULTS
Functional Connectivity
Functional connectivity was significantly reduced in patients with 22q11DS between the anterior and posterior node of the DMN (P uncorrected < 0.001, Bonferroni threshold 0.003). There were no other significant differences in functional connectivity between patients with 22q11DS and controls.
Structural Connectivity
Intra‐network connectivity
DMN structural connectivity was significantly reduced in patients with 22q11DS between the left angular gyrus and the left MTL, while it was significantly increased between the posterior node of the DMN and the right MTL (Fig. 1A, Table 4). However, it should be noted that this increase is relative, as the number of connections is normalized by the overall sum of connections in the connectivity matrix.
Figure 1.
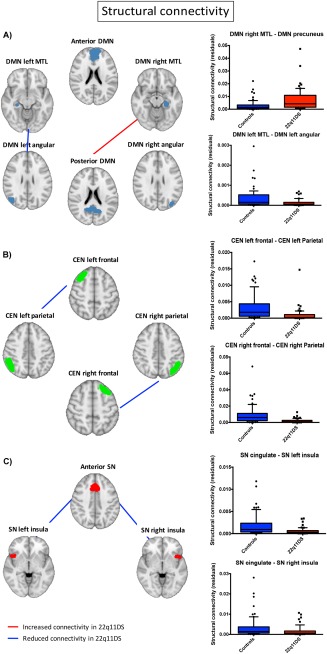
Group differences in within‐network structural connectivity. The figure shows the connections within DMN (A), CEN (B), and SN (C) that significantly differed between patients and controls. [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 4.
Structural connections significantly different between controls and patients with 22q11DS
| Structural connectivity | |||
|---|---|---|---|
| Intra‐network connectivity | |||
| Direction | P uncorrected | Bonferroni threshold | |
| CEN left frontal–CEN left parietal | controls>22q11DS | <0.001 | 0.008 |
| CEN right frontal–CEN right parietal | controls>22q11DS | <0.001 | 0.008 |
| DMN left angular–DMN left MTL | controls>22q11DS | 0.001 | 0.003 |
| DMN posterior–DMN right MTL | 22q11DS>controls | <0.001 | 0.003 |
| SN anterior–SN left insula | controls>22q11DS | <0.001 | 0.02 |
| SN anterior–SN right insula | controls>22q11DS | 0.02 | 0.02 |
| Inter‐networks connectivity | |||
| Direction | P uncorrected | P corrected | |
| DMN anterior–CEN left parietal | controls>22q11DS | <0.001 | <0.001 |
| DMN left angular–CEN left frontal | controls>22q11DS | 0.002 | 0.01 |
| DMN left MTL–CEN left parietal | controls>22q11DS | 0.003 | 0.01 |
| DMN posterior–CEN right frontal | controls>22q11DS | <0.001 | 0.005 |
| DMN right angular–CEN right frontal | controls>22q11DS | <0.001 | <0.001 |
| SN anterior–CEN left parietal | 22q11DS>controls | <0.001 | <0.001 |
| SN anterior–CEN right parietal | controls>22q11DS | <0.001 | <0.001 |
| SN anterior–DMN left angular | controls>22q11DS | <0.001 | 0.002 |
| SN left insula–CEN left parietal | controls>22q11DS | <0.001 | <0.001 |
| SN anterior–DMN right angular | controls>22q11DS | 0.006 | 0.02 |
Structural connectivity within the CEN was also reduced in patients with 22q11DS, in the bilateral connections between frontal and parietal nodes (Fig. 1B, Table 4).
Similarly, SN structural connectivity was significantly reduced between the anterior node and the bilateral insular cortex in patients with 22q11DS (Fig. 1C, Table 4).
Inter‐network connectivity
After correction for multiple comparisons, between‐networks structural connectivity was significantly reduced in patients with 22q11DS between CEN and SN (CEN right parietal‐SN cingulate, CEN left parietal‐SN left insula), between the CEN and the DMN (CEN left parietal‐DMN anterior, CEN left frontal‐DMN left angular, CEN left parietal‐DMN left MTL, CEN right frontal‐DMN posterior, CED right frontal‐DMN posterior), and between the DMN and the SN (Anterior SN and bilateral DMN angular gyrus) (Fig. 2 and Table 4). Only one connection between the CEN and the SN was significantly increased in the group of patients after multiple comparison correction, while no connections were significantly increased between DMN‐CEN and DMN‐SN. Our findings suggest widespread and complex patterns of white matter dysconnectivity between the three networks in patients with 22q11DS. It should also be noted that two main nodes were specifically dysconnected, as they presented reduced or increased connectivity with several other nodes: the left parietal node of the CEN and the anterior node of the SN.
Figure 2.
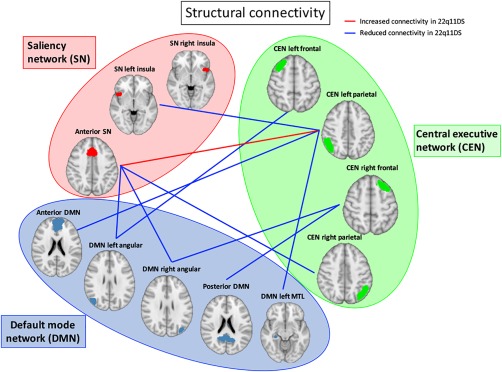
Between network structural connectivity. The figure indicates the structural connections that significantly differed between patients and controls after multiple comparisons correction. [Color figure can be viewed at http://wileyonlinelibrary.com]
Correlation Between Connectivity, Executive Functions, and Psychotic Symptoms Scores
For the connections that were significantly different after multiple comparisons correction, we computed the Spearmann correlations with executive functions and the psychotic symptoms scores.
Only structural connectivity showed significant associations with cognitive scores. As shown in Figure 3A–F, six connections were significantly correlated to the CPT scores. In particular, we observed a positive correlation between reduced structural connectivity and the number of commission errors during the CPT in two connections (CEN left frontal‐CEN left parietal, CEN left frontal‐DMN left angular), and a negative correlation between reduced structural connectivity and reaction time during the CPT in the same two connections as well as in the connections between CEN right parietal‐CEN right frontal, CEN left parietal‐DMN left MTL, DMN left MTL‐DMN left angular.
Figure 3.
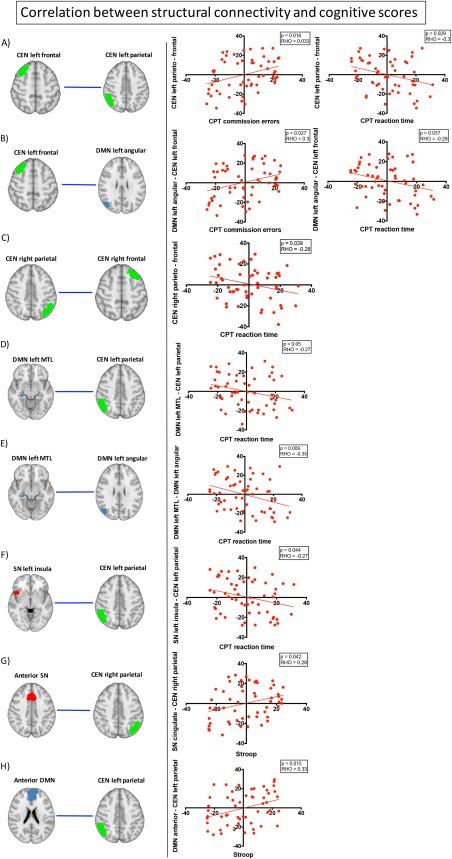
Correlation between structural connectivity and cognitive scores. The figures in the left column show the within‐ or between‐network connections that significantly correlated with the executive functions scores in the group of patients with 22q11DS. The plots show the Spearmann correlation between the structural connectivity measures and the specific scores. [Color figure can be viewed at http://wileyonlinelibrary.com]
Furthermore, reduced structural connectivity in two networks (SN anterior–CEN right parietal and DMN anterior–CEN left parietal) was related to reduced interference index of the Stroop task, which is an indicator of inhibition difficulties (Fig. 3G, H).
No significant correlation was observed between connectivity measures and either positive or negative symptoms scores in the group of patients with 22q11DS.
DISCUSSION
In this work, we showed impaired structural connectivity within the DMN, CEN, and SN in patients with 22q11DS, as well as impaired connections between the three networks. The structural impairment was further related to specific aspects of executive functions, namely impaired motor and cognitive inhibition. At the functional level, we found that long‐range connections within the DMN were impaired in patients with 22q11DS, while connectivity within the other networks was preserved. In the following paragraphs, we will discuss the findings of functional and structural connectivity within each network in the context of the existing literature in 22q11DS. We will further discuss our novel findings regarding between‐networks connectivity and the dissociation between structural and functional connectivity alterations. We will next discuss how impaired structural connectivity within and between the three networks is related to impaired executive functions in patients with 22q11DS. Lastly, we will discuss the findings in 22q11DS in the context of the triple network model of psychopathology, with a specific focus on the common impairments in patients with 22q11DS and schizophrenia.
Impaired within‐ and between‐network connectivity in patients with 22q11DS
Impairments in the within‐network and between‐networks connectivity in patients with 22q11DS mostly involved a reduction of structural connections, while functional connectivity seemed to be preserved.
Our results reporting reduced functional connectivity between the anterior and posterior node of the DMN are in line with previous studies [Padula et al., 2015; Schreiner et al., 2014] and confirm the presence of impaired long‐range connections between medial DMN regions. We further showed significantly increased structural connectivity between the posterior DMN node and the right MTL. Together, these findings suggest that patients with 22q11DS have a different pattern of within DMN connectivity compared with controls, with some connections being reduced and other increased.
We observed no difference in functional connectivity within the CEN and the SN. This result is in contrast with recent findings in 22q11DS [Mattiaccio et al., 2016], but is in line with previous results from our group [Debbané et al., 2012]. This discrepancy in the results may be due to the use of different acquisition, processing and analysis techniques as well as to different sample characteristics. For instance, we computed the correlation between functional time courses extracted from specific CEN and SN nodes. Contrarily, Mattiaccio et al. [2016] compared the activation of spatial CEN and SN maps obtained from ICA. Furthermore, in this study we included patients from 8 to 33 years old, while Mattiaccio et al. [2016] mostly included adolescents and young adults (17–26 years old). Given the presence of impaired developmental trajectories in patients with 22q11DS [Gothelf et al., 2007b; Schaer et al., 2009], it is possible that adult patients present a greater pattern of connectivity impairment compared with children.
Despite the absence of functional connectivity impairments, we showed a significant reduction in structural connections within the CEN and the SN. Structural connectivity impairments have been largely described in 22q11DS (reviewed in [Scariati et al., 2016b]). In particular, most studies point to alterations in long range‐tracts including the inferior longitudinal fasciculus (ILF), the inferior fronto‐occipital fasciculus and the uncinate fasciculus [Scariati et al., 2016b], which connect the frontal lobe to parietal and/or temporal cortices. According to the previous findings, our results show reduced connections between the DLPFC and the posterior parietal cortex of the CEN as well as reduced connections between the dorsal ACC and the bilateral insular cortex of the SN, confirming the presence of reduced integrity of long‐range connections in patients with 22q11DS.
For the between‐network connectivity, widespread alterations in structural connections were evident in patients with 22q11DS compared with controls, again confirming the impairment in long‐range white matter tracts observed in this population [Scariati et al., 2016b]. However, no significant functional impairments were evident after correction for multiple comparisons.
Despite the finding of preserved resting‐state functional connectivity between DMN, CEN, and SN in 22q11DS, the impaired shifting between networks' activation may become evident during the transition from resting‐state to goal‐directed conditions. Indeed, studies investigating the switching between resting‐state to goal‐directed activities in patients with schizophrenia showed abnormal patterns of activation/de‐activation of DMN and CEN, which was associated with decreased behavioral performance [Hasenkamp et al., 2011; Pomarol‐Clotet et al., 2008].
Another possible explanation for the observed dissociation between structural and functional connectivity is that functional connectivity compensates the reduced integrity of structural connections [Fornito et al., 2015; Honey et al., 2009], at least during the early stages of the disease. Indeed, while preserved functional connectivity was evident in the present study, which mostly included patients with 22q11DS at‐risk but not diagnosed with schizophrenia, this is not the case in schizophrenic patients [Chang et al., 2014; Iwabuchi et al., 2015; Lefebvre et al., 2016; Manoliu et al., 2014; Moran et al., 2013; Orliac et al., 2013; Palaniyappan et al., 2013]. This may suggest that the structure‐function relationship may change at different stages of the disease, and that functional connectivity impairments would correspond to later, more severe stages.
Relationship between altered triple network connectivity and executive functions in patients with 22q11DS
Within‐ and between‐networks alterations were related to altered motor and cognitive inhibition in patients with 22q11DS. Reduced connectivity within the CEN and between CEN‐SN and CEN‐DMN was related to increased reaction time in the CPT task and/or to reduced commission errors (Fig. 3A–F). These results are in line with previous studies that reported impaired reaction time in patients with 22q11DS during different cognitive tasks [Franchini et al., 2016; Howley et al., 2012]. In this study, we further observed that the slower performance of patients with 22q11DS on the CPT task was related to impaired within and between CEN connectivity. However, the same impairment in the CEN structural connectivity was associated to reduced number of commission errors. The number of commission errors and the reaction time were negatively correlated in our group of patients with 22q11DS (P = 0.02, RHO = −0.32), indicating that higher reaction time was related to fewer commission errors. The combination of fast responses with increased errors is an index of impulsivity [Conners and Staff, 2000]. On the opposite, our results suggest that the lower connectivity within and between the CEN in patients with 22q11DS is rather related to an increased time of response, which is in turn associated with reduced number of errors.
Reduced connectivity between the right parietal node of the CEN and both the anterior SN and the anterior DMN nodes was related to impaired cognitive inhibition, as measured with the Stroop task, in patients with 22q11DS. Both the anterior SN and the anterior DMN nodes comprise the ACC, that plays a prominent role in cognitive inhibition [Botvinick et al., 2001; Bush et al., 2000; Petersen and Posner, 2012]. Furthermore, in patients with schizophrenia, inhibition responses have been related to reduced ACC activity [Minzenberg et al., 2009; Rubia et al., 2001]. Similarly, in our group of patients with 22q11DS, inhibition deficits appear to be related to ACC dysconnectivity.
These results suggest that alterations in executive functions are related to underlying impairments in core cognitive networks in patients with 22q11DS. Findings reporting a similar relationship between impaired triple network connectivity and cognitive dysfunctions in schizophrenia will be further discussed in the following paragraph.
Triple network model of psychopathology: common impairments in patients with 22q11DS and schizophrenia
In this study, we showed that the triple network model of psychopathology, defined in psychiatric disorders such as autism, depression and schizophrenia [Menon, 2011], can be applied to patients with 22q11DS. This model suggests that impairments in the within‐ and between‐network connectivity are responsible for impaired cognitive functions. In our group of patients with 22q11DS, we observed structural connectivity alterations within and between SN, DMN, and CEN and their association with impairments in executive functions.
Studies conducted in patients with schizophrenia mainly showed functional impairments between the three networks [Iwabuchi et al., 2015; Lefebvre et al., 2016; Manoliu et al., 2014; Moran et al., 2013; Palaniyappan et al., 2013], however altered structural connectivity between CEN and SN [Chen et al., 2016; Iwabuchi et al., 2015] has also been reported, in line with our results in patients with 22q11DS.
Furthermore, triple network impairments in patients with schizophrenia have mostly been related to psychotic symptoms [Lefebvre et al., 2016; Manoliu et al., 2014]. However, we did not observe a relationship between altered triple network connectivity and psychotic symptoms in patients with 22q11DS. Of note, the studies conducted in schizophrenic patients reported functional connectivity impairments related to psychotic symptoms [Lefebvre et al., 2016; Manoliu et al., 2014], while no evidence exists of a relationship between triple network structural dysconnectivity and the symptomatic profile [Chen et al., 2016; Iwabuchi et al., 2015]. As discussed in the previous paragraphs, functional connectivity seems to be preserved in patients with 22q11DS, and this might explain the lack of correlations with psychotic symptoms in this population. The inclusion of older, more symptomatic subjects would maybe reveal similar relationships between triple network functional dysconnectivity and psychotic symptoms in patients with 22q11DS and schizophrenia. Nevertheless, several findings in schizophrenia indicate a relationship between cognitive impairments and impaired connectivity in CEN and SN regions (e.g., [Meda et al., 2009; Meyer‐Lindenberg et al., 2001; Sheffield et al., 2015]. Furthermore, Moran et al. [2013] found that abnormal interactions between SN–DMN and SN–CEN were related to impairments in sustained attention and working memory in schizophrenia. Similar findings have been shown in patients at high risk for psychosis, where increased connectivity between DMN and CEN was related to working memory impairments [Wotruba et al., 2014]. These findings are in line with our results in patients with 22q11DS, where impaired CEN, CEN‐DMN, and CEN‐SN connectivity was related to executive dysfunctions.
LIMITATIONS AND CONCLUSION
One limitation of this study is related to the excessive head motion during the resting‐state fMRI acquisition in patients with 22q11DS. In order to remove the effect of motion from the resting‐state fMRI data, we used previously adopted state of the art methodologies [Padula et al., 2015; Scariati et al., 2014], based on the regression of motion parameters. An additional criterion was the exclusion of subjects with movement parameters higher that 3 mm in translation and 3° in rotation. Despite these precautions, we cannot completely exclude the effect of motion on our results. Another limitation is related to our DTI sequence, which do not allow a precise reconstruction of crossing fibers. This drawback was partially solved by the use of a probabilistic tractography algorithm for fibers' tracking, but future studies should further address this issue by the use of DSI acquisitions. An additional confounding factor is the presence of a significantly reduced QI in patients with 22q11DS compared with controls. However, we decided not to use the IQ as covariate in the analysis, as reduced IQ is one of the several phenotypic characteristics of patients with 22q11DS. Only the use of a group of controls matched for IQ would allow to remove this confounding effect. Finally, although our results point to altered triple network connectivity associated to impaired executive functions in patients with 22q11DS, these results may not be exclusive, and impairments in other networks may contribute to the cognitive difficulties observed in these patients.
To conclude, the present study shows that triple network impairments are related to cognitive dysfunctions in patients with 22q11DS, similarly to findings in schizophrenia. These results suggest the presence of common mechanisms responsible for cognitive impairments in both diseases. However, future studies are needed to assess the patterns of activation/de‐activation of the three networks during the switching between resting‐state and goal‐directed tasks in patients with 22q11DS. Furthermore, longitudinal studies will further clarify the relationship between the development of connectivity within and between SN, DMN, and CEN and executive functions.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
We would like to acknowledge the families that participated to our study as well as the group of the CIBM (and in particular François Lazeyras) for their help in the scanning acquisitions. We further thank Sarah Menghetti and Léa Chambaz for their involvement with the families and Frédérique Bena Sloan for the genetic analyses.
The authors have no conflict of interest to declare.
REFERENCES
- Antshel KM, Fremont W, Kates WR (2008): The neurocognitive phenotype in velo‐cardio‐facial syndrome: A developmental perspective. Dev Disabil Res Rev 14:43–51. [DOI] [PubMed] [Google Scholar]
- Ashburner J (2007): A fast diffeomorphic image registration algorithm. NeuroImage 38:95–113. [DOI] [PubMed] [Google Scholar]
- Azuma R, Daly EM, Campbell LE, Stevens AF, Deeley Q, Giampietro V, Brammer MJ, Glaser B, Ambery FZ, Morris RG, Williams SCR, Owen MJ, Murphy DGM, Murphy KC (2009): Visuospatial working memory in children and adolescents with 22q11.2 deletion syndrome; an fMRI study. J Neurodev Disord 1:46–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KD, Skuse DH (2005): Adolescents and young adults with 22q11 deletion syndrome: Psychopathology in an at‐risk group. Br J Psychiatry J Ment Sci 186:115–120. [DOI] [PubMed] [Google Scholar]
- Bassett AS, Chow EW (1999): 22q11 deletion syndrome: A genetic subtype of schizophrenia. Biol Psychiatry 46:882–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TEJ, Berg HJ, Jbabdi S, Rushworth MFS, Woolrich MW (2007): Probabilistic diffusion tractography with multiple fibre orientations: What can we gain?. NeuroImage 34:144–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD (2001): Conflict monitoring and cognitive control. Psychol Rev 108:624–652. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews‐Hanna JR, Schacter DL (2008): The brain's default network: Anatomy, function, and relevance to disease. Ann N Y Acad Sci 1124:1–38. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI (2000): Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci 4:215–222. [DOI] [PubMed] [Google Scholar]
- Campbell LE, Azuma R, Ambery F, Stevens A, Smith A, Morris RG, Murphy DGM, Murphy KC (2010): Executive functions and memory abilities in children with 22q11.2 deletion syndrome. Aust N Z J Psychiatry 44:364–371. [DOI] [PubMed] [Google Scholar]
- Campbell LE, McCabe KL, Melville JL, Strutt PA, Schall U (2015): Social cognition dysfunction in adolescents with 22q11.2 deletion syndrome (velo‐cardio‐facial syndrome): relationship with executive functioning and social competence/functioning. J Intellect Disabil Res 59:845–859. [DOI] [PubMed] [Google Scholar]
- Chang X, Shen H, Wang L, Liu Z, Xin W, Hu D, Miao D (2014): Altered default mode and fronto‐parietal network subsystems in patients with schizophrenia and their unaffected siblings. Brain Res 1562:87–99. [DOI] [PubMed] [Google Scholar]
- Chen Q, He X, Chen X, Wang L, Wang K, Qiuac B (2016): Aberrant structural and functional connectivity in the salience network and central executive network circuit in schizophrenia. Neurosci Lett 627:178–184. [DOI] [PubMed] [Google Scholar]
- Chow EWC, Watson M, Young DA, Bassett AS (2006): Neurocognitive profile in 22q11 deletion syndrome and schizophrenia. Schizophr Res 87:270–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners C, Staff M (2000): Conner's Continuous Performance Test II: Computer Program for Windows Technical Guide and Software Manual. North Tonawanda: Multi‐Health Systems. [Google Scholar]
- Corbetta M, Shulman GL (2002): Control of goal‐directed and stimulus‐driven attention in the brain. Nat Rev Neurosci 3:201–215. [DOI] [PubMed] [Google Scholar]
- Debbané M, Glaser B, David MK, Feinstein C, Eliez S (2006): Psychotic symptoms in children and adolescents with 22q11.2 deletion syndrome: Neuropsychological and behavioral implications. Schizophr Res 84:187–193. [DOI] [PubMed] [Google Scholar]
- Debbané M, Lazouret M, Lagioia A, Schneider M, Van De Ville D, Eliez S (2012): Resting‐state networks in adolescents with 22q11.2 deletion syndrome: Associations with prodromal symptoms and executive functions. Schizophr Res 139:33–39. [DOI] [PubMed] [Google Scholar]
- First M, Gibbon M, Spitzer R, Williams J, Benjamin L (1996): Structured Clinical Interview for the DSM‐IV Axis I Disorders (SCID‐I). Washington, DC: American Psychiatric Association. [Google Scholar]
- Fornito A, Zalesky A, Breakspear M (2015): The connectomics of brain disorders. Nat Rev Neurosci 16:159–172. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME (2005): The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A 102:9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchini M, Schaer M, Glaser B, Kott‐Radecka M, Debanné M, Schneider M, Menghetti S, Sander D, Eliez S (2016): Visual processing of emotional dynamic faces in 22q11.2 deletion syndrome. J Intellect Disabil Res 60:464–477. [DOI] [PubMed] [Google Scholar]
- Gothelf D, Hoeft F, Hinard C, Hallmayer JF, Stoecker JVD, Antonarakis SE, Morris MA, Reiss AL (2007a): Abnormal cortical activation during response inhibition in 22q11.2 deletion syndrome. Hum Brain Mapp 28:533–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothelf D, Penniman L, Gu E, Eliez S, Reiss A (2007b): Developmental trajectories of brain structure in adolescents with 22q11.2 deletion syndrome: A longitudinal study. Schizophr Res 96:72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothelf D, Schneider M, Green T, Debbané M, Frisch A, Glaser B, Zilkha H, Schaer M, Weizman A, Eliez S (2013): Risk factors and the evolution of psychosis in 22q11.2 deletion syndrome: A longitudinal 2‐site study. J Am Acad Child Adolesc Psychiatry 52:1192–1203.e3. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Menon V (2004): Default‐mode activity during a passive sensory task: Uncoupled from deactivation but impacting activation. J Cogn Neurosci 16:1484–1492. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V (2003): Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc Natl Acad Sci 100:253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenkamp W, James GA, Boshoven W, Duncan E (2011): Altered engagement of attention and default networks during target detection in schizophrenia. Schizophr Res 125:169–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey CJ, Sporns O, Cammoun L, Gigandet X, Thiran J‐P, Meuli R, Hagmann P (2009): Predicting human resting‐state functional connectivity from structural connectivity. Proc Natl Acad Sci 106:2035–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howley SA, Prasad SE, Pender NP, Murphy KC (2012): Relationship between reaction time, fine motor control, and visual–spatial perception on vigilance and visual‐motor tasks in 22q11.2 Deletion Syndrome. Res Dev Disabil 33:1495–1502. [DOI] [PubMed] [Google Scholar]
- Iwabuchi SJ, Liddle PF, Palaniyappan L (2015): Structural connectivity of the salience‐executive loop in schizophrenia. Eur Arch Psychiatry Clin Neurosci 265:163–166. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S (2001): A global optimisation method for robust affine registration of brain images. Med Image Anal 5:143–156. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S (2002): Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage 17:825–841. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N (1997): Schedule for affective disorders and schizophrenia for school‐age children‐present and lifetime version (K‐SADS‐PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 36:980–988. [DOI] [PubMed] [Google Scholar]
- Lefebvre S, Demeulemeester M, Leroy A, Delmaire C, Lopes R, Pins D, Thomas P, Jardri R (2016): Network dynamics during the different stages of hallucinations in schizophrenia. Hum Brain Mapp 37:2571–2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandowski KE, Shashi V, Berry PM, Kwapil TR (2007): Schizophrenic‐like neurocognitive deficits in children and adolescents with 22q11 deletion syndrome. Am J Med Genet Part B Neuropsychiatr Genet off Publ Int Soc Psychiatr Genet 144B:27–36. [DOI] [PubMed] [Google Scholar]
- Maeder J, Schneider M, Bostelmann M, Debbané M, Glaser B, Menghetti S, Schaer M, Eliez S (2016): Developmental trajectories of executive functions in 22q11.2 deletion syndrome. J Neurodev Disord 8:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoliu A, Riedl V, Zherdin A, Muhlau M, Schwerthoffer D, Scherr M, Peters H, Zimmer C, Forstl H, Bauml J, Wohlschlager AM, Sorg C (2014): Aberrant dependence of default mode/central executive network interactions on anterior insular salience network activity in schizophrenia. Schizophr Bull 40:428–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattiaccio LM, Coman IL, Schreiner MJ, Antshel KM, Fremont WP, Bearden CE, Kates WR (2016): Atypical functional connectivity in resting‐state networks of individuals with 22q11.2 deletion syndrome: Associations with neurocognitive and psychiatric functioning. J Neurodev Disord 8:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe KL, Atkinson RJ, Cooper G, Melville JL, Harris J, Schall U, Loughland CM, Thienel R, Campbell LE (2014): Pre‐pulse inhibition and antisaccade performance indicate impaired attention modulation of cognitive inhibition in 22q11.2 deletion syndrome (22q11DS). J Neurodev Disord 6:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meda SA, Stevens MC, Folley BS, Calhoun VD, Pearlson GD (2009): Evidence for anomalous network connectivity during working memory encoding in schizophrenia: An ICA based analysis. PloS One 4:e7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V (2011): Large‐scale brain networks and psychopathology: A unifying triple network model. Trends Cogn Sci 15:483–506. [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin LQ (2010): Saliency, switching, attention and control: A network model of insula function. Brain Struct Funct 214:655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer‐Lindenberg A, Poline JB, Kohn PD, Holt JL, Egan MF, Weinberger DR, Berman KF (2001): Evidence for abnormal cortical functional connectivity during working memory in schizophrenia. Am J Psychiatry 158:1809–1817. [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, Somjee L, Markovich PJ, Stein K, Woods SW (2002): Prospective diagnosis of the initial prodrome for schizophrenia based on the Structured Interview for Prodromal Syndromes: Preliminary evidence of interrater reliability and predictive validity. Am J Psychiatry 159:863–865. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC (2009): MEta‐analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry 66:811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montojo CA, Ibrahim A, Karlsgodt KH, Chow C, Hilton AE, Jonas RK, Vesagas TK, Bearden CE (2014): Disrupted working memory circuitry and psychotic symptoms in 22q11.2 deletion syndrome. NeuroImage Clin 4:392–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran LV, Tagamets MA, Sampath H, O'Donnell A, Stein EA, Kochunov P, Hong LE (2013): Disruption of anterior insula modulation of large‐scale brain networks in schizophrenia. Biol Psychiatry 74:467–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KC, Jones LA, Owen MJ (1999): High rates of schizophrenia in adults with velo‐cardio‐facial syndrome. Arch Gen Psychiatry 56:940–945. [DOI] [PubMed] [Google Scholar]
- Niklasson L, Gillberg C (2010): The neuropsychology of 22q11 deletion syndrome. A neuropsychiatric study of 100 individuals. Res Dev Disabil 31:185–194. [DOI] [PubMed] [Google Scholar]
- Orliac F, Naveau M, Joliot M, Delcroix N, Razafimandimby A, Brazo P, Dollfus S, Delamillieure P (2013): Links among resting‐state default‐mode network, salience network, and symptomatology in schizophrenia. Schizophr Res 148:74–80. [DOI] [PubMed] [Google Scholar]
- Padula MC, Schaer M, Scariati E, Schneider M, Van De Ville D, Debbané M, Eliez S (2015): Structural and functional connectivity in the default mode network in 22q11.2 deletion syndrome. J Neurodev Disord 7:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palaniyappan L (2012): Does the salience network play a cardinal role in psychosis? An emerging hypothesis of insular dysfunction. J Psychiatry Neurosci 37:17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palaniyappan L, Simmonite M, White TP, Liddle EB, Liddle PF (2013): Neural primacy of the salience processing system in schizophrenia. Neuron 79:814–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SE, Posner MI (2012): The attention system of the human brain: 20 years after. Annu Rev Neurosci 35:73–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomarol‐Clotet E, Salvador R, Sarró S, Gomar J, Vila F, Martínez Á, Guerrero A, Ortiz‐Gil J, Sans‐Sansa B, Capdevila A, Cebamanos JM, McKenna PJ (2008): Failure to deactivate in the prefrontal cortex in schizophrenia: Dysfunction of the default mode network?. Psychol Med 38:1185–1193. [DOI] [PubMed] [Google Scholar]
- Radoeva PD, Coman IL, Antshel KM, Fremont W, McCarthy CS, Kotkar A, Wang D, Shprintzen RJ, Kates WR (2012): Atlas‐based white matter analysis in individuals with velo‐cardio‐facial syndrome (22q11. 2 deletion syndrome) and unaffected siblings. Behav Brain Funct 8:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL (2001): A default mode of brain function. Proc Natl Acad Sci 98:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich W (2000): Diagnostic interview for children and adolescents (DICA). J Am Acad Child Adolesc Psychiatry 39:59–66. [DOI] [PubMed] [Google Scholar]
- Rubia K, Russell T, Bullmore ET, Soni W, Brammer MJ, Simmons A, Taylor E, Andrew C, Giampietro V, Sharma T (2001): An fMRI study of reduced left prefrontal activation in schizophrenia during normal inhibitory function. Schizophr Res 52:47–55. [DOI] [PubMed] [Google Scholar]
- Rykhlevskaia E, Gratton G, Fabiani M (2008): Combining structural and functional neuroimaging data for studying brain connectivity: A review. Psychophysiology 45:173–187. [DOI] [PubMed] [Google Scholar]
- Scariati E, Schaer M, Richiardi J, Schneider M, Debbané M, Van De Ville D, Eliez S (2014): Identifying 22q11.2 deletion syndrome and psychosis using resting‐state connectivity patterns. Brain Topogr 27:808–821. [DOI] [PubMed] [Google Scholar]
- Scariati E, Schaer M, Karahanoglu FI, Schneider M, Richiardi J, Debbané M, Van D, Ville D, Eliez S (2016a): Large‐scale functional network reorganization in 22q11.2 deletion syndrome revealed by modularity analysis. Cortex 82:86–99. [DOI] [PubMed] [Google Scholar]
- Scariati E, Padula MC, Schaer M, Eliez S (2016b): Long‐range dysconnectivity in frontal and midline structures is associated to psychosis in 22q11.2 deletion syndrome. J Neural Transm 123:823–839. [DOI] [PubMed] [Google Scholar]
- Schaer M, Debbané M, Bach Cuadra M, Ottet M‐C, Glaser B, Thiran J‐P, Eliez S (2009): Deviant trajectories of cortical maturation in 22q11.2 deletion syndrome (22q11DS): a cross‐sectional and longitudinal study. Schizophr Res 115:182–190. [DOI] [PubMed] [Google Scholar]
- Schneider M, Van der Linden M, Glaser B, Rizzi E, Dahoun SP, Hinard C, Bartoloni L, Antonarakis SE, Debbané M, Eliez S (2012): Preliminary structure and predictive value of attenuated negative symptoms in 22q11.2 deletion syndrome. Psychiatry Res 196:277–284. [DOI] [PubMed] [Google Scholar]
- Schneider M, Debbané M, Bassett AS, Chow EW, Fung WLA, van den Bree MB, Owen M, Murphy KC, Niarchou M, Kates WR, others (2014a): Psychiatric disorders from childhood to adulthood in 22q11. 2 deletion syndrome: Results from the International Consortium on Brain and Behavior in 22q11. 2 Deletion Syndrome. Am J Psychiatry 171:627–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M, Schaer M, Mutlu AK, Menghetti S, Glaser B, Debbané M, Eliez S (2014b): Clinical and cognitive risk factors for psychotic symptoms in 22q11.2 deletion syndrome: A transversal and longitudinal approach. Eur Child Adolesc Psychiatry 23:425–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M, Van der Linden M, Menghetti S, Glaser B, Debbané M, Eliez S (2014c): Predominant negative symptoms in 22q11.2 deletion syndrome and their associations with cognitive functioning and functional outcome. J Psychiatr Res 48:86–93. [DOI] [PubMed] [Google Scholar]
- Schreiner MJ, Karlsgodt KH, Uddin LQ, Chow C, Congdon E, Jalbrzikowski M, Bearden CE (2014): Default mode network connectivity and reciprocal social behavior in 22q11.2 deletion syndrome. Soc Cogn Affect Neurosci 9:1261–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD (2007): Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro HM, Wong LM, Simon TJ (2013): A cross‐sectional analysis of the development of response inhibition in children with chromosome 22q11.2 deletion syndrome. Front Psychiatry 4:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro HM, Tassone F, Choudhary NS, Simon TJ (2014): The development of cognitive control in children with chromosome 22q11.2 deletion syndrome. Front Psychol 5:566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield JM, Repovs G, Harms MP, Carter CS, Gold JM, MacDonald AW III, Daniel Ragland J, Silverstein SM, Godwin D, Barch DM (2015): Fronto‐parietal and cingulo‐opercular network integrity and cognition in health and schizophrenia. Neuropsychologia 73:82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T, Critchley HD, Preuschoff K (2009): A common role of insula in feelings, empathy and uncertainty. Trends Cogn Sci 13:334–340. [DOI] [PubMed] [Google Scholar]
- Smith SM (2002): Fast robust automated brain extraction. Hum Brain Mapp 17:143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V (2008): A critical role for the right fronto‐insular cortex in switching between central‐executive and default‐mode networks. Proc Natl Acad Sci U S A 105:12569–12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoddard J, Niendam T, Hendren R, Carter C, Simon TJ (2010): Attenuated positive symptoms of psychosis in adolescents with chromosome 22q11.2 deletion syndrome. Schizophr Res 118:118–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroop J (1935): Studies of interference in serial verbal reactions. J Exp Psychol 18:643–662. [Google Scholar]
- Tomescu MI, Rihs TA, Becker R, Britz J, Custo A, Grouiller F, Schneider M, Debbané M, Eliez S, Michel CM (2014): Deviant dynamics of EEG resting state pattern in 22q11.2 deletion syndrome adolescents: A vulnerability marker of schizophrenia?. Schizophr Res 157:175–181. [DOI] [PubMed] [Google Scholar]
- Tomescu MI, Rihs TA, Roinishvili M, Karahanoglu FI, Schneider M, Menghetti S, Van De Ville D, Brand A, Chkonia E, Eliez S, Herzog MH, Michel CM, Cappe C (2015): Schizophrenia patients and 22q11.2 deletion syndrome adolescents at risk express the same deviant patterns of resting state EEG microstates: A candidate endophenotype of schizophrenia. Schizophr Res Cogn 2:159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (1997): Wechsler Adult Intelligence Scale, 3rd ed. (WAIS‐3). San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Wotruba D, Michels L, Buechler R, Metzler S, Theodoridou A, Gerstenberg M, Walitza S, Kollias S, Rossler W, Heekeren K (2014): Aberrant coupling within and across the default mode, task‐positive, and salience network in subjects at risk for psychosis. Schizophr Bull 40:1095–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yendiki A, Koldewyn K, Kakunoori S, Kanwisher N, Fischl B (2014): Spurious group differences due to head motion in a diffusion MRI study. NeuroImage 88:79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


