Abstract
Repetition suppression and enhancement refer to the reduction and increase in the neural responses for repeated rather than novel stimuli, respectively. This study provides a meta‐analysis of the effects of repetition suppression and enhancement, restricting the data used to that involving fMRI/PET, visual stimulus presentation, and healthy participants. The major findings were as follows. First, the global topography of the repetition suppression effects was strikingly similar to that of the “subsequent memory” effects, indicating that the mechanism for repetition suppression is the reduced engagement of an encoding system. The lateral frontal cortex effects involved the frontoparietal control network regions anteriorly and the dorsal attention network regions posteriorly. The left fusiform cortex effects predominantly involved the dorsal attention network regions, whereas the right fusiform cortex effects mainly involved the visual network regions. Second, the category‐specific meta‐analyses and their comparisons indicated that most parts of the alleged category‐specific regions showed repetition suppression for more than one stimulus category. In this regard, these regions may not be “dedicated cortical modules,” but are more likely parts of multiple overlapping large‐scale maps of simple features. Finally, the global topography of the repetition enhancement effects was similar to that of the “retrieval success” effects, suggesting that the mechanism for repetition enhancement is voluntary or involuntary explicit retrieval during an implicit memory task. Taken together, these results clarify the network affiliations of the regions showing reliable repetition suppression and enhancement effects and contribute to the theoretical interpretations of the local and global topography of these two effects. Hum Brain Mapp 38:1894–1913, 2017. © 2017 Wiley Periodicals, Inc.
Keywords: fMRI, memory, repetition priming, neural networks, meta‐analysis
INTRODUCTION
Repeated stimulus presentation typically results in reduced functional magnetic resonance imaging (fMRI)/positron emission tomography (PET) signals in some brain regions, relative to its first presentation (or the initial presentation of a matched but different stimulus). Although this phenomenon, typically termed repetition suppression, has prompted numerous neuroimaging studies during the last 20 years, its nature and theoretical interpretations remain controversial. The proposed explanations of repetition suppression include “sharpened” stimulus representation in the cortex [Wiggs and Martin, 1998], attentional modulating effects [Eger et al., 2004], reductions in top‐down prediction errors [Summerfield et al., 2008], attenuated novelty‐detection signals [Kumaran and Maguire, 2009], enhanced neural synchronization [Gotts et al., 2012], and neural fatigue or habituation [Turk‐Browne et al., 2008]. The most widely recognized behavioral correlate of repetition suppression is improved efficiency (i.e., greater accuracy and faster reaction times) in relatively simple perceptual/conceptual judgments involving the stimulus, leading to the hypothesis that repetition suppression underlies implicit memory retrieval [Schacter et al., 2007; Stevens et al., 2009]. Another, but less discussed, behavioral correlate concerns the finding that greater repetition suppression is associated with lower levels of subsequent memory [Wagner et al., 2000b; Xue et al., 2011], suggesting that repetition suppression may involve attenuated encoding activity.
Repeated stimulus presentation may also result in enhanced fMRI/PET signals relative to its initial presentation. Although, this phenomenon, typically termed repetition enhancement, has been reported less frequently, a given experiment may give rise to both repetition suppression and enhancement effects [Segaert et al., 2013], necessarily involving nonoverlapping regions. Some studies [Donaldson et al., 2001; Korsnes and Magnussen, 2014; Schott et al., 2005] suggested that repetition enhancement effects may reflect voluntary or involuntary explicit memory retrieval during an implicit memory task, noting that they tend to involve those regions that are typically activated during explicit memory retrieval. Another line of evidence [Henson et al., 2000; Müller et al., 2013] indicates that some brain regions that are typically associated with repetition suppression may instead show repetition enhancement when low‐visibility (degraded, unfamiliar, or masked) stimuli are repeated, purportedly reflecting the elaboration of stimulus representation.
A fundamental issue is the question of which brain regions show reliable repetition suppression and which ones show reliable repetition enhancement effects. Both of these effects have been associated with widely distributed regions in the cortex which, in the case of repetition suppression, most notably involved the ventral occipitotemporal cortex, lateral frontal cortex, and medial temporal lobe regions [O'Kane et al., 2005; Schacter et al., 2007]. However, specific findings have been highly variable across studies, reflecting, at least in part, the differences in the experimental parameters and analytic methods, including the stimulus type, task requirement, presence/absence of stimulus familiarization period, number of repetitions, length of repetition lag, block versus event‐related design, and statistical threshold [Henson, 2016; Segaert et al., 2013]. In this regard, a generalized regional topography of repetition suppression and enhancement effects, drawing on a compilation of study results, has yet to be clearly established. A related issue concerns whether and to what extent such a regional topography of repetition suppression or enhancement effects is similar or differential across different stimulus categories, such as words, faces, and scenes [Grill‐Spector and Weiner, 2014; Kanwisher, 2010]. To address these issues, the present study provides a meta‐analysis of repetition suppression and enhancement effects, with the data used being restricted to that involving visual stimulus presentation and healthy participants.
Resting‐state functional connectivity fMRI and other studies have provided strong evidence that the brain is composed of multiple large‐scale intrinsic networks [Power et al., 2011; Smith et al., 2009; Yeo et al., 2011]. Although the proposed numbers and exact boundaries of these intrinsic networks differ between studies, many networks show a similar topography across different analytic approaches, providing a critical basis for constraining the interpretations of the dispersed activation patterns across the cortex. However, only a few previous studies [Stevens et al., 2012; Vannini et al., 2013; Wig et al., 2009] have attempted to relate the regional topography of the repetition suppression or enhancement effects to the intrinsic network organization. In this regard, the aim of the present study includes clarifying the network affiliations of the regions that show reliable repetition suppression and enhancement. This study uses Yeo et al. [2011]'s 7‐network model, which is depicted in Figure 1, as a guide to determine the network membership of each region that shows reliable repetition suppression and enhancement. This model is based on a clustering analysis of resting‐state fMRI signals involving the correlation between the fMRI time series at each spatial location and 1,175 uniformly spaced cortical regions. The advantages of Yeo et al.'s model, relative to other similar models, include the fact that its modeling is based on a large data set (n = 1,000), it involves an extensive validation effort including a split‐half replication, it has a highly similar topography to that found in subtraction imaging studies, and its utility as a frame of reference was verified in several recent meta‐analysis studies [Benoit and Schacter, 2015; Fox et al., 2015; Kim, 2016].
Figure 1.
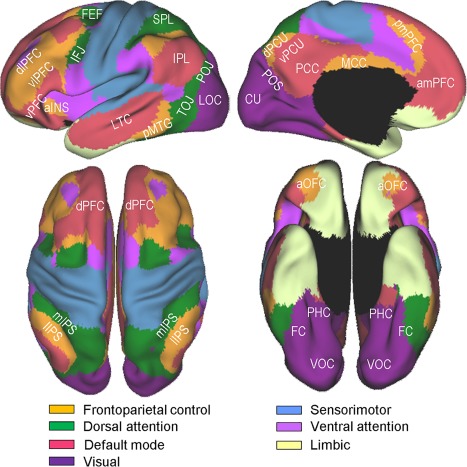
Yeo et al. [2011]'s 7‐network parcellation of the human cerebral cortex. The figures were adapted from Yeo et al. [2011] (Fig. 11) with permission from the American Physiological Society. Labeled are subregions within 4 networks, namely, the frontoparietal control, dorsal attention, visual, and default mode, which are more directly relevant to the present study. aINS, anterior insula; amPFC, anteromedial prefrontal cortex; aOFC, anterior orbitofrontal cortex; CU, cuneus; dlPFC, dorsolateral prefrontal cortex; dPCU, dorsal precuneus; dPFC, dorsal prefrontal cortex; FC, fusiform cortex; FEF, frontal eye fields; IFJ, inferior frontal junction; IPL, inferior parietal lobe; lIPS, lateral intraparietal sulcus; LOC, lateral occipital cortex; LTC, lateral temporal cortex; MCC, middle cingulate cortex; mIPS, medial intraparietal sulcus; PCC, posterior cingulate cortex; PHC, parahippocampal cortex; pmPFC, posteromedial prefrontal cortex; pMTG, posterior middle temporal gyrus; POJ parieto‐occipital junction; POS, parieto‐occipital sulcus; SPL, superior parietal lobe; TOJ, temporo‐occipital junction; vlPFC, ventrolateral prefrontal cortex; VOC, ventral occipital cortex; vPCU, ventral precuneus; vPFC, ventral prefrontal cortex. [Color figure can be viewed at http://wileyonlinelibrary.com]
A brief review of the relevant aspects of Yeo et al.'s model and related findings follows, because the subsequent discussion frequently refers to this model. Four networks, that is, the visual, frontoparietal control, dorsal attention, and default mode, are directly relevant to the results of the present study. First, the visual network includes the early and late visual processing areas and anteriorly includes the posterior parahippocampal cortex (PHC) (see violet regions in Fig. 1). Second, the frontoparietal control network comprises the lateral prefrontal cortex (PFC), posteromedial PFC, anterior orbitofrontal cortex, lateral intraparietal sulcus (IPS), middle cingulate cortex, and dorsal precuneus (orange regions). The observed increases in activity in this network are related to control demands, such as controlled retrieval, taxing working memory, interference resolution, and inhibiting habitual responses [Dosenbach et al., 2008; Vincent et al., 2008]. Third, the dorsal attention network involves the frontal eye fields, inferior frontal junction (IFJ), which is located at the intersection of the inferior frontal sulcus and the precentral sulcus, and the “C”‐shaped regions surrounding the posterior parietal and temporal lobes (green regions). This network, which is also referred to as “goal‐directed” or “top‐down” attention, embodies a mechanism for orienting attention to the external environment by sending top‐down biasing signals to selected sensory inputs [Corbetta and Shulman, 2002; Sestieri et al., 2012]. Finally, the default mode network, whose main components include the anteromedial PFC, posterior cingulate cortex, ventral precuneus, inferior parietal lobe, and lateral temporal cortex regions (pink regions), supports internally oriented cognition, such as recall, future thinking, mentalizing, and other types of spontaneous cognition [Andrews‐Hanna et al., 2014; Buckner et al., 2008].
The network compositions around the inferior frontal cortex and ventral temporal cortex are of particular interest, given the relatively strong associations of these regions with repetition suppression in previous studies [Schacter et al., 2007]. Large portions of the inferior frontal cortex are within the frontoparietal control network, whereas a relatively small area in its posterior extent, that is, the IFJ, is part of the dorsal attention network. The IFJ, which borders the premotor gyrus, middle frontal gyrus, and inferior frontal gyrus, is dissociable from its adjoining regions functionally [Derrfuss et al., 2012], connectionally [Muhle‐Karbe et al., 2016], and cytoarchitectonically [Amunts et al., 2004]. It has recently received a great deal of attention, due to its regular coactivations in an impressive array of cognitive control tasks, including task‐switching, Stroop, n‐back, Go/No‐GO, Stop signal, Posner‐cueing, top‐down attention, and oddball tasks [Brass et al., 2005; Derrfuss et al., 2005; Kim et al., 2012; Levy and Wagner, 2011; Zanto et al., 2010]. In this regard, it would be important to understand whether the repetition suppression effects around the inferior frontal cortex involve the frontoparietal control network subregions only or extend more posteriorly into the dorsal attention (IFJ) subregions.
Large portions of the ventral temporal cortex are within the boundaries of the visual network, whereas its lateral extent involving the fusiform cortex is part of the dorsal attention network. In line with this parcellation, recent studies [Caspers et al., 2013; Lorenz et al., 2011] confirmed the existence of a medial‐to‐lateral cytoarchitectonic transition within the fusiform cortex. A related observation is that the fusiform cortex component of the dorsal attention network is nontrivially more extensive in the left than in the right hemisphere. This asymmetry is not peculiar to Yeo et al.'s model, because other seed‐based connectivity studies [Caspers et al., 2014; Stevens et al., 2012; Zhang et al., 2016] have found similar asymmetry. A consequence of this network configuration is that some homologous ventral temporal cortex regions are part of the dorsal attention network in the left hemisphere, whereas they belong to the visual network in the right hemisphere. In this regard, it is of vital importance to ascertain whether and to what extent the repetition suppression effects in the left and right ventral temporal cortexes predominantly involve the dorsal attention and visual network subregions, respectively.
This study also addresses the question of to what extent the regional repetition suppression effects are category‐specific or more generalized. Some studies indicated that the dissociable ventral occipitotemporal regions show preferential responses to specific stimulus categories, for example the “parahippocampal place area” to scenes [Epstein et al., 1999], “fusiform face area” to faces [Kanwisher et al., 1997], and “visual word form area” to written words [Cohen et al., 2000]. It is well established that these regions show similar categorical specificity in their repetition suppression effects [Dehaene et al., 2001; Henson et al., 2002]. However, it remains a matter of debate as to what extent these regions are distinct category‐specific modules, because each of them typically responds significantly (albeit to a lesser degree) to objects that are not in the preferred class [Aminoff and Tarr, 2015; Grill‐Spector and Weiner, 2014; Kanwisher, 2010]. For example, the putative visual word form area shows high activity when processing line drawings, numbers, letter strings, and gratings [Kherif et al., 2011; Price and Devlin, 2003; Vogel et al., 2012]. A related, but underexplored, hypothesis concerns whether any regions outside of the ventral occipitotemporal cortex show preferential responses to a specific category [Bunzeck et al., 2006]. To address these issues, this study examines the overlaps and separations between the repetition suppression effects for different classes of stimuli in the ventral occipitotemporal and other brain regions. To conform to the analyses of the category effects and reduce the variance and increase the homogeneity within the whole sample, this study restricted the data used to that involving the word, scene, face, and object categories. Few other categories were available that would have ensured a meaningful analysis. Although the object category is a superordinate, not basic, one, it was included, because it has been extensively used in previous studies and is visually distinct from the other categories in the study.
MATERIAL AND METHODS
Data Collection
Candidate studies for the meta‐analysis were identified through multiple searches of the Pubmed and Google Scholar databases. The query terms included “repetition suppression,” “repetition enhancement,” “priming,” “fMRI adaptation,” “implicit memory,” “novelty,” “fMRI,” and “PET.” The inclusion/exclusion criteria for the meta‐analysis were as follows:
Only studies that presented stimuli through some visual modality were included.
Only studies that presented the same physical stimuli in the first and repeated conditions (i.e., identity priming) were included.
Studies that presented words, scenes, faces, or objects (or a combination of these 4 types; e.g., face‐name pairs) as stimuli were included, whereas those presenting other stimulus types such as pseudowords, sentences, nonreal objects, geometric shapes, video clips, audiovisual stimuli, or virtual environment were excluded (see the Introduction for the rationale behind this criterion).
Studies involving relatively simple and commonly employed perceptual, linguistic, or cognitive tasks or passive viewing were included, whereas those using unusually complex cognitive (e.g., the application of abstract rules), motor learning, or explicit memory retrieval tasks were excluded. A majority of the included tasks served to measure behavioral priming, but a minority (e.g., target detection) served only to ensure that the participant's attention was directed to the stimuli.
Given that relevant studies vary widely not only in terms of the stimulus/task type, but also in terms of numerous other experimental parameters, including pre‐familiarization, the number of repetitions, lag between presentations, stimulus masking, manipulation of attention or expectation, stimulus duration, and response modality [Weigelt et al., 2008], studies were broadly included irrespective of these parameters to ensure the relatively high generalizability of the results.
Only studies that performed whole‐brain analyses and reported peak activation foci in the standard reference space (i.e., Talairach or Montreal Neurological Institute [MNI] coordinates) were included, whereas those involving region of interest [ROI] analyses were excluded. Note that excluding ROI data is a mandatory practice in neuroimaging meta‐analysis, to accommodate the notion that the aim is to provide unbiased estimates of convergence across many independent studies. Given that repetition priming studies in the context of vision research tend to use ROI analyses more frequently than ones in the context of other topics (e.g., memory), relevant data from the vision literature are likely to be less represented in the meta‐analysis. In principle, this representation bias could interfere with unbiased estimates of convergence across studies. However, any such interference may be relatively minimal, because whole‐brain findings should be similar whether the aim of the study is to investigate vision or some other topic.
Only studies that tested healthy participants were included.
When studies reported more than one relevant experiment (contrast), if they involved completely different sets of trials, all of the experiments were included; otherwise, only one experiment was selected for inclusion to prevent this particular study from overly influencing the results. The one that involved the largest number of peak activation foci was selected, because this selection was thought to increase the sensitivity of the meta‐analysis.
Sample Characteristics
A total of 85 individual studies met the inclusion criteria (see Supporting Information available online for a list of studies included in the meta‐analyses). The imaging modality was PET in 4 studies and fMRI in the remainder. Twenty‐four studies provided both repetition suppression and repetition enhancement experiments, 59, repetition suppression only, and 2, repetition enhancement only.
The repetition suppression sample collectively involved 83 studies, 106 experiments, 1,020 peak foci, and 1,454 participants. The stimuli were words in 27 experiments, scenes in 16, faces in 22, objects in 32, and mixed types in 9. Table 1 lists the number of experiments and peak foci for each stimulus category subgroup. The word stimuli were common words in 23 experiments, words presented in mirror‐image orientation in 3, and word stems in 1. The scene stimuli were indoor or outdoor scenes, and the face stimuli were famous or unknown faces. The object stimuli were exemplars of assorted categories in 28 experiments and some specific categories (e.g., birds) in 4. The mixed‐type stimuli were images involving 2 stimulus types (e.g., scene and object) in 5 experiments and International Affective Picture System images in 4. The relatively common (n > 1) behavioral tasks were (a) within the word subgroup, abstract/concrete judgement, italic/upright shape judgement, man‐made/natural judgment, silent reading, mirror‐image word reading, and size judgement; (b) within the scene subgroup, indoor/outdoor judgement, passive viewing, and target detection; (c) within the face subgroup, gender judgement, passive viewing, face/house judgement, fame judgment, and target detection; (d) within the object subgroup, naming, real/unreal judgement, size judgement, category classification, living/nonliving judgement, man‐made/natural judgement, and target detection; and (e) within the mixture subgroup, fit/unfit judgment (e.g., “Is the name a good fit for the face or not?”), expectancy rating, and passive viewing.
Table 1.
Number of experiments and peak foci used in each effect/stimulus category subgroup
| Effect | Stimulus category | Experiments | Peak Foci |
|---|---|---|---|
| RS | Word | 27 | 202 |
| Scene | 16 | 233 | |
| Face | 22 | 157 | |
| Object | 32 | 292 | |
| Mixed | 9 | 136 | |
| RE | Word | 12 | 103 |
| Scene | 2 | 9 | |
| Face | 4 | 37 | |
| Object | 7 | 19 | |
| Mixed | 6 | 47 |
RS, repetition suppression; RE, repetition enhancement.
The repetition enhancement sample collectively involved 26 studies, 31 experiments, 215 peak foci, and 424 participants. The stimuli were words in 12 experiments, scenes in 2, faces in 4, objects in 7, and mixed types in 6 (see Table 1). The relatively common behavioral tasks were (a) within the word subgroup, abstract/concrete judgement, italic/upright shape judgement, meaningful/meaningless judgement, and target detection; (b) within the scene subgroup, indoor/outdoor judgement; (c) within the face subgroup, fame judgment; (d) within the object subgroup, naming; and (e) within the mixture subgroup, fit/unfit judgement (see the Supporting Information available online for a detailed description of the repetition suppression and enhancement experiments included in the meta‐analysis).
Data Analysis
Four sets of meta‐analyses were performed. The first meta‐analysis analyzed all of the repetition suppression experiments (n = 106) together to identify the regions generally associated with repetition suppression. The second was a series of subgroup meta‐analyses that separately evaluated the repetition suppression effects for four stimulus categories, that is, word, scene, face, and object. The third was a series of subtraction and conjunction analyses between repetition suppression effects for one subgroup (e.g., word) and repetition suppression effects collapsed over the other three subgroups (e.g., scene, face, and object). Four separate conjunction and subtraction analyses were performed, involving word‐ and nonword‐, scene‐ and nonscene‐, face‐ and nonface‐, and object‐ and nonobject‐based repetition suppression effects. The final meta‐analysis analyzed all of the repetition enhancement experiments (n = 31) together with the aim of determining those regions generally associated with repetition enhancement. The repetition enhancement sample could not accommodate a separate analysis of each stimulus category, due to the limited number of available experiments.
The length of the lag between priming and primed presentations is known to modulate repetition suppression effects [e.g., Henson et al., 2004; Wagner et al., 2000b]. The proportion of experiments involving a relatively short lag, defined here as “no intervening items,” nontrivially differed between the category subgroups, 33.3% in word, 18.8% in scene, 31.8% in face, and 18.8% in object. To control for this factor, the second and third meta‐analyses described above involved subgroups with a more balanced proportion of the variable formed by pseudorandomly subsampling the experiments. After this control, the word, scene, face, and object subgroups involved 20, 16, 18, and 29 experiments, involving 10.0, 18.8, 16.7, and 10.3% proportions of the short lag experiments, respectively. Although some experimental parameters other than the length of lag could potentially differ between subgroups and affect the results, it was impractical to control for additional factors. Thus, the control of repetition lag was intended merely as a preliminary effort to perform more balanced comparisons between the category subgroups.
Meta‐Analysis Techniques
All of the meta‐analyses were accomplished using the revised activation likelihood estimation (ALE) algorithm implemented in GingerALE 2.3.3 (http://www.brainmap.org). ALE meta‐analysis is a coordinate‐based method that can be used to determine the brain regions showing an above‐chance level of activation convergence across a set of independent studies [Eickhoff et al., 2009; Turkeltaub et al., 2012]. The ALE algorithm was applied to the analysis of the present data in the following steps (similar steps were previously described in Kim [2016]). The spatial normalization space was determined for each study and all activation foci reported in MNI coordinates were converted into Talairach coordinates by using the icbm2tal transform [Lancaster et al., 2007]. Each activation focus was modeled as a center of the 3 dimensional Gaussian probability distribution. The width of the Gaussian distribution was calculated using an extended ALE algorithm accounting for the between‐subject variability introduced by the different sample sizes and the between‐template variability introduced by the different spatial normalization techniques. A tighter, taller Gaussian was assigned to larger sample sizes to reflect the notion that the spatial uncertainty is less for these samples. To give a specific example, the meta‐analysis involving all of the repetition suppression experiments had minimum, median, and maximum values of the full‐width at half‐maximum [FWHM] of 8.94, 9.44, and 10.61 mm, respectively. Given this order of kernel sizes, it should be acknowledged that meta‐analyses can only detect relatively large‐scale repetition effects, but may miss smaller‐scale ones that involve a local area or voxel.
The probability values of all foci in a given study were combined for each voxel and subsequently summed across all of the included studies, generating voxelwise ALE scores reflecting the convergence of the results across all of the studies. These ALE scores so obtained were compared with those from an estimated null distribution assuming a random spatial association between studies. This comparison resulted in nonparametric P‐value maps, which were thresholded at a cluster level familywise error (FWE) corrected threshold of P < 0.05 (cluster‐forming threshold at the voxel level P < 0.005). The differences between each pair of ALE maps were assessed based on the ALE subtraction analysis algorithm described in Eickhoff et al. [2011]. All studies contributing to either map were pooled and randomly divided into two sets of studies having the same size as the original two groups. Subsequently, the ALE scores for the two randomly constructed groups were calculated and the difference between these ALE scores was recorded for each voxel. Repeating this process 10,000 times provided an estimated null distribution reflecting the chance differences in the ALE scores between the two ALE maps. The actual “observed” difference in ALE scores were then tested against this estimated null distribution. This comparison resulted in voxelwise P‐value maps, which were thresholded at a voxel level threshold of P < 0.005, and inclusively masked by the respective main effects, which were thresholded at a cluster‐level FWE corrected threshold of P < 0.05. To assess the similarity between the two ALE maps, a conjunction analysis was performed based on the intersection between the two thresholded maps. Therefore, any voxel determined to be significant in a conjunction analysis constituted one that survived a cluster‐level FWE corrected threshold of P < 0.05 in both ALE analyses. A spatial extent threshold exceeding 500 mm3 was applied to the results of all of the analyses to further reduce the likelihood of false‐positive findings.
To visualize the results of the meta‐analysis, the thresholded ALE maps were projected onto an inflated population‐average landmark surface (PALS) by using CARET [Van Essen, 2005]. The boundaries of the dorsal attention, frontoparietal cognitive control, visual and default‐mode networks, as estimated by Yeo et al. [2011; see Fig. 1], were also projected onto the PALS as a flexible guideline to evaluate whether a convergence cluster is located within or outside of the networks. The thresholded maps were also overlaid onto an International Consortium for Brain Mapping template using MANGO (http://ric.uthscsa.edu/mango), mainly to observe hippocampal activation clusters not visible on the PALS.
RESULTS
Repetition Suppression: A General Analysis
An ALE meta‐analysis was conducted including all of the repetition suppression experiments (n = 106) and the results are shown in Table 2 and Figure 2, indicating that the repetition suppression effects were most extensively associated with two regions, the inferior frontal cortex and ventral occipitotemporal cortex. Effects around the inferior frontal cortex were predominantly left‐lateralized and included the ventrolateral PFC (inferior frontal gyrus/sulcus) component of the frontoparietal control network anteriorly and the IFJ component of the dorsal attention network posteriorly.
Table 2.
Results from an ALE meta‐analysis of all repetition suppression experiments
| Talairach | |||||
|---|---|---|---|---|---|
| Volume (mm3) | x | Y | z | H | Region |
| 33,792 | −42 | −54 | −12 | L | Ventral occipitotemporal cortex, LOC, hippocampus, amygdala, lentiform nucleus |
| 33,096 | 28 | −38 | −14 | R | Ventral occipitotemporal cortex, LOC, POS, hippocampus, amygdala, lentiform nucleus |
| 20,256 | −40 | 2 | 30 | L | Ventrolateral PFC, IFJ, anterior OFC, anterior INS |
| 4,368 | −2 | 12 | 48 | B | Posteromedial PFC |
| 3,496 | 44 | 4 | 32 | R | IFJ |
| 2,880 | 32 | 20 | 6 | R | Anterior OFC, anterior INS |
| 2,800 | 42 | 28 | 18 | R | Ventrolateral PFC |
| 2,040 | 24 | −68 | 38 | R | Posterior IPS |
| 1,264 | −24 | −66 | 36 | L | Posterior IPS |
| 824 | 12 | 6 | 6 | R | Caudate nucleus |
ALE, activation likelihood estimation; B, bilateral; BA, Brodmann area; H, hemisphere; L, left; R, right. For other abbreviations, see Figure 1.
Figure 2.
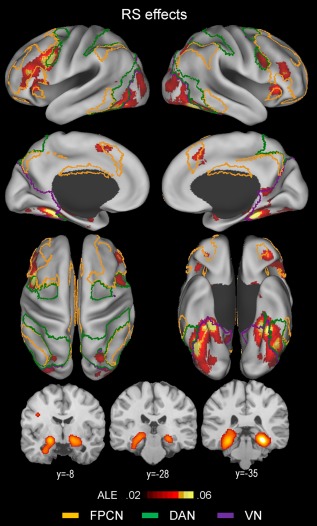
Above‐threshold brain regions in an ALE meta‐analysis of all repetition suppression (RS; novel > repeated) experiments (n = 106). The yellow‐, green‐, and violet‐colored borderlines mark estimates of the frontoparietal control, dorsal attention, and visual attention networks, respectively. The boundaries were drawn from Yeo et al.'s [2011] 7‐network parcellation data (see Fig. 1). [Color figure can be viewed at http://wileyonlinelibrary.com]
Effects around the ventral occipitotemporal cortex were more symmetric across the two hemispheres and mainly included the posterior PHC, fusiform, and ventral occipital cortex regions bilaterally. A striking observation was that left fusiform cortex effects predominantly involved the dorsal attention network regions, whereas right fusiform cortex effects were mainly associated with the visual network regions.
Other less extensive cortical effects included, within the frontoparietal control network, the posteromedial PFC, anterior orbitofrontal, and anterior insula regions bilaterally, within the dorsal attention network, the bilateral posterior IPS regions, and within the visual network, the bilateral lateral occipital regions. Subcortical effects included the bilateral hippocampal, striatal, and right cerebellar regions. Hippocampal effects included both the anterior and posterior segments.
Repetition Suppression: Category‐Specific Analyses
Table 3 and Figure 3 show the results of separate ALE meta‐analyses of word‐ (n = 20), scene‐ (n = 16), face‐ (n = 18), and object‐based (n = 29) repetition suppression effects. Table 4 and Figure 4 show the results of separate conjunction and subtraction analyses between word‐ and nonword‐, scene‐ and nonscene‐, face‐ and nonface‐, and object‐ and nonobject‐based repetition suppression effects.
Table 3.
Results of separate ALE meta‐analyses of word‐ (A), scene‐ (B), face‐ (C), and object‐based (D) repetition suppression experiments
| Talairach | |||||
|---|---|---|---|---|---|
| Volume (mm3) | x | Y | z | H | Region |
| A. Word RS | |||||
| 10,968 | −42 | 4 | 26 | L | Ventrolateral PFC, IFJ, anterior OFC |
| 6,208 | −42 | −52 | −14 | L | FC |
| 1,072 | 4 | −74 | −24 | R | Cerebellum |
| 720 | −32 | −90 | −2 | L | LOC |
| 608 | 38 | −42 | −12 | R | FC |
| 520 | −50 | −40 | 2 | L | Superior temporal cortex |
| 504 | 34 | 20 | 8 | R | Anterior INS |
| 432 | 42 | −66 | −10 | R | FC |
| B. Scene RS | |||||
| 9,192 | 28 | −36 | −14 | R | Posterior PHC, medial FC, hippocampus |
| 6,240 | −26 | −42 | −12 | L | Posterior PHC, media FC, hippocampus |
| 2,792 | −34 | −82 | 10 | L | LOC |
| 2,496 | 34 | −76 | 12 | R | LOC |
| 1,256 | −12 | −54 | 6 | B | POS |
| 1,136 | 0 | 10 | 46 | B | Posteromedial PFC |
| 1,040 | −44 | 6 | 28 | L | IFJ, ventrolateral PFC |
| 1,032 | −42 | 26 | 10 | L | Ventral PFC |
| 856 | −22 | −18 | −14 | L | Hippocampus |
| 736 | −38 | −6 | 16 | L | Middle INS |
| 656 | −30 | −8 | −30 | L | Amygdala |
| C. Face RS | |||||
| 11,976 | 38 | −58 | −14 | R | FC, ventral occipital cortex |
| 7,504 | −40 | −56 | −14 | L | FC |
| 1,680 | −52 | 18 | 6 | L | Ventral PFC |
| 800 | −18 | −6 | −10 | L | Lentiform nucleus |
| 672 | 30 | −86 | 6 | R | LOC |
| 504 | 14 | −6 | −8 | R | Lentiform nucleus |
| D. Object RS | |||||
| 13,896 | 34 | −48 | −14 | R | FC, posterior PHC, LOC |
| 11,976 | −42 | −58 | −8 | L | FC, posterior PHC, LOC |
| 5,272 | −44 | 30 | 18 | L | Ventrolateral PFC |
| 3,976 | −40 | 2 | 30 | L | IFJ |
| 2,592 | 38 | 2 | 32 | R | IFJ |
| 2,072 | 4 | 20 | 40 | B | Posteromedial PFC |
| 1,720 | 42 | 30 | 18 | R | Ventrolateral PFC |
| 1,128 | 34 | 18 | 6 | R | Anterior INS |
| 896 | 24 | −68 | 36 | R | Posterior IPS |
| 816 | −34 | −84 | 4 | L | LOC |
| 728 | 18 | −2 | −12 | R | Amygdala, lentiform nucleus |
| 704 | 20 | −62 | 46 | R | Posterior IPS |
| 648 | −24 | −66 | 34 | L | Posterior IPS |
| 600 | −20 | −2 | −12 | L | Amygdala, lentiform nucleus |
Figure 3.
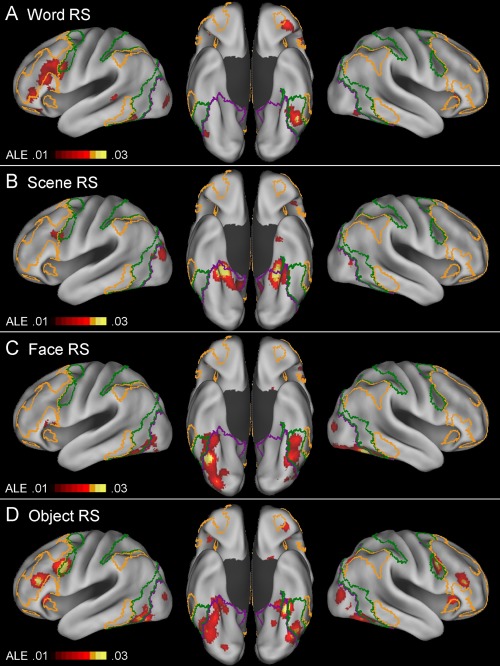
Above‐threshold brain regions in an ALE meta‐analysis of word‐based (A; n = 20), scene‐based (B; n = 16), face‐based (C; n = 18), and object‐based (D; n = 29) repetition suppression experiments. For an explanation of the yellow‐, green‐, and violet‐colored borderlines, see Figure 2. [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 4.
Results of subtraction and conjunction meta‐analyses between word‐ versus nonword‐ (A), scene‐ versus nonscene‐ (B), face‐ versus nonface‐ (C), and object‐ versus nonobject‐based (D) repetition suppression effects
| Talairach | ||||||
|---|---|---|---|---|---|---|
| Effect | Volume (mm3) | x | y | z | H | Region |
| A. Word versus nonword RS | ||||||
| W>NW | 1,072 | 10 | −76 | −35 | R | Cerebellum |
| 584 | −46 | 32 | −2 | L | Ventrolateral PFC | |
| NW>W | 1,832 | −39 | −73 | 2 | L | LOC |
| 1,168 | 32 | −40 | −22 | R | Posterior PHC | |
| 976 | −21 | −41 | −19 | L | Posterior PHC | |
| 824 | 22 | −70 | −6 | R | Ventral occipital cortex | |
| W∩NW | 4,016 | −42 | −54 | −14 | L | FC |
| 2,128 | −42 | 4 | 26 | L | IFJ | |
| 1,856 | −44 | 22 | 16 | L | Ventrolateral PFC | |
| 592 | 38 | −42 | −12 | R | FC | |
| B. Scene versus nonscene RS | ||||||
| S>NS | 4,744 | 25 | −35 | −11 | R | Posterior PHC, POS, hippocampus |
| 2,696 | −24 | −44 | −10 | L | Posterior PHC | |
| 1,216 | −36 | −81 | 14 | L | LOC | |
| 912 | −17 | −56 | 11 | L | POS | |
| 704 | −36 | −9 | 13 | L | Middle INS | |
| 512 | −21 | −15 | −16 | L | Hippocampus | |
| NS>S | 1,456 | −43 | −54 | −23 | L | FC |
| S∩NS | 3,064 | −30 | −42 | −14 | L | Posterior PHC, medial FC |
| 2,328 | 30 | −38 | −18 | R | Posterior PHC, medial FC | |
| 1,040 | −44 | 6 | 28 | L | IFJ | |
| 1,000 | −42 | 26 | 10 | L | Ventrolateral PFC | |
| C. Face versus nonface RS | ||||||
| F>NF | 1,104 | 36 | −78 | −18 | R | Ventral occipital cortex |
| NF>F | 8,944 | −43 | 6 | 30 | L | Ventrolateral PFC, IFJ |
| 3,056 | −22 | −36 | −13 | L | Posterior PHC | |
| 848 | 27 | −39 | −2 | R | Posterior PHC | |
| F∩NF | 5,784 | 38 | −58 | −12 | R | FC |
| 4,920 | −40 | −56 | −14 | L | FC | |
| D. Object versus nonobject RS | ||||||
| O>NO | 1,336 | 43 | −1 | 29 | R | IFJ |
| 944 | −47 | −2 | 34 | L | IFJ | |
| O∩NO | 8,392 | −42 | −56 | −10 | L | FC, posterior PHC |
| 7,704 | 36 | −48 | −14 | R | FC, posterior PHC | |
| 1,768 | −42 | 4 | 28 | L | IFJ | |
| 1,520 | −42 | 24 | 18 | L | Ventrolateral PFC | |
| 552 | 34 | 20 | 8 | R | Anterior INS | |
| 504 | −34 | 32 | −6 | L | Ventral PFC | |
Figure 4.
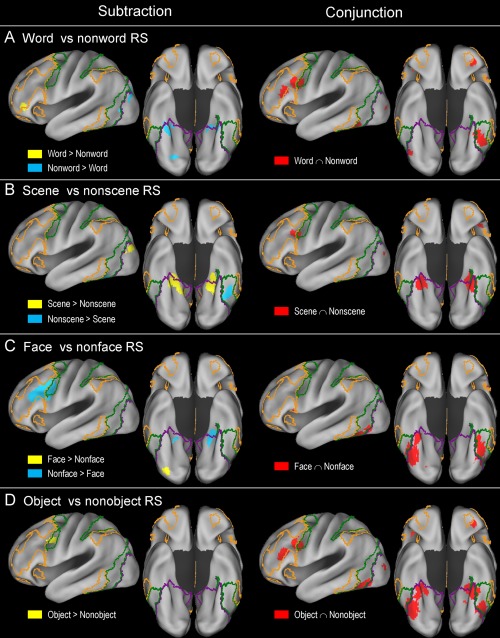
Results of subtraction and conjunction analysis between word‐ versus nonword‐ (A), scene‐ versus nonscene‐ (B), face‐ versus nonface‐ (C), and object‐ versus nonobject‐based (D) repetition suppression effects. For an explanation of the yellow‐, green‐, and violet‐colored borderlines, see Figure 2. [Color figure can be viewed at http://wileyonlinelibrary.com]
Word‐based repetition suppression
Word‐based repetition suppression effects were mainly associated with the left ventrolateral PFC, IFJ, anterior orbitofrontal, fusiform cortex, and right cerebellar regions (Fig. 3A). Left fusiform cortex effects predominantly involved the dorsal attention network regions. A conjunction analysis between word‐ and nonword‐based (scene, face, and object) effects indicated the existence of relatively extensive common regions, including the left ventrolateral PFC, IFJ, and fusiform cortex regions (red regions in Fig. 4A). The regions that were associated more with word‐ than nonword‐based effects included the left ventrolateral PFC and right cerebellar regions (yellow). The reverse effects—the regions that were associated more with nonword‐ than word‐based effects—included the bilateral posterior PHC, left lateral occipital, and right ventral occipital regions (cyan).
Scene‐based repetition suppression
Scene‐based repetition suppression effects were mainly associated with the bilateral posterior PHC/medial fusiform cortex/hippocampus, lateral occipital, parieto‐occipital sulcus, posteromedial PFC, and left IFJ regions (Fig. 3B). A conjunction analysis between scene‐ and nonscene‐based (word, face, and object) effects indicated that both involved the bilateral posterior PHC/medial fusiform cortex and left IFJ regions (red regions in Fig. 4B). The regions that were associated more with scene‐ than nonscene‐based effects mainly involved the bilateral posterior PHC/hippocampus, parieto‐occipital sulcus, and left lateral occipital cortex regions (yellow). The regions that were associated more with nonscene‐ than scene‐based effects involved only the left fusiform cortex regions (cyan).
Face‐based repetition suppression
Face‐based repetition suppression effects were mainly associated with the bilateral fusiform cortex, bilateral ventral occipital, and left ventral PFC regions (Fig. 3C). Left fusiform effects predominantly involved the dorsal attention network regions, whereas right fusiform effects were mainly associated with the visual network regions. A conjunction analysis between face‐ and nonface‐based (word, scene, and object) effects indicated that there was a broad range of common regions involving the bilateral ventral occipitotemporal cortex (red regions in Fig. 4C). The areas that were associated more with face‐ than nonface‐based effects involved only the right ventral occipital regions (yellow). The regions that were associated more with nonface‐ than face‐based effects involved large portions of the left ventrolateral PFC/IFJ and relatively small portions of the bilateral posterior PHC (cyan).
Object‐based repetition suppression
The meta‐analysis of object‐based repetition suppression effects yielded results that were largely comparable in topography to those observed in the meta‐analysis including all of the repetition suppression experiments, except that most of the effects were less extensive (compare Figs. 2 and 3D). Left fusiform effects predominantly involved the dorsal attention network regions, whereas right fusiform effects were mainly associated with the visual network regions. A conjunction analysis between object‐ and nonobject‐based (word, scene, and face) effects indicated the existence of relatively extensive common areas, including the bilateral posterior PHC, bilateral fusiform, left IFJ, and left ventrolateral PFC regions (red regions in Fig. 4D). The areas that were associated more with object‐ than nonobject‐based effects involved only the bilateral IFJ regions (yellow). No regions were associated more with nonobject‐ than object‐based effects.
Repetition Enhancement: A General Analysis
An ALE meta‐analysis was conducted including all of the repetition enhancement experiments (n = 31) and the results are shown in Table 5 and Figure 5, indicating that the repetition enhancement effects were mainly associated with the subregions of the frontoparietal control and default mode networks. Within the frontoparietal control network, these effects were mainly associated with the dorsolateral PFC (middle frontal gyrus), dorsal precuneus, and lateral IPS regions bilaterally, and within the default mode network, the ventral precuneus, anterior extent of the posterior cingulate cortex, and inferior parietal lobe regions bilaterally.
Table 5.
Results from an ALE meta‐analysis of all repetition enhancement experiments
| Talairach | |||||
|---|---|---|---|---|---|
| Volume (mm3) | x | y | z | H | Region |
| 6,152 | 8 | −66 | 28 | B | Dorsal/ventral precuneus |
| 3,360 | 44 | −56 | 38 | R | Lateral IPS, IPL |
| 3,000 | −36 | 48 | 12 | L | Dorsolateral PFC |
| 2,696 | 36 | 30 | 32 | R | Dorsolateral PFC |
| 1,552 | 24 | 54 | 22 | R | Dorsolateral PFC |
| 1,376 | −44 | −56 | 36 | L | Lateral IPS, IPL |
| 1,328 | 58 | −34 | 0 | R | Superior temporal cortex |
| 1,208 | 34 | 10 | 48 | R | Dorsolateral PFC |
| 856 | 2 | −32 | 38 | B | Anterior PCC |
| 800 | −40 | 24 | 42 | L | Dorsolateral PFC |
| 656 | 4 | −54 | 38 | R | Dorsal precuneus |
| 624 | 34 | 48 | 10 | R | Dorsolateral PFC |
Figure 5.
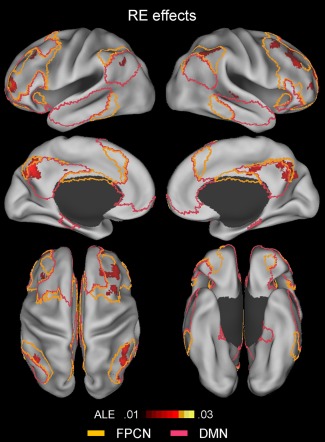
Above‐threshold brain regions in an ALE meta‐analysis of all repetition enhancement (RE; repeated > novel) experiments (n = 31). The yellow‐ and pink‐colored borderlines mark estimates of the frontoparietal control and default‐mode networks, respectively. The boundaries were drawn from Yeo et al.'s [2011] 7‐network parcellation data (see Fig. 1). [Color figure can be viewed at http://wileyonlinelibrary.com]
A direct comparison of the repetition suppression and enhancement effects provides some double dissociations involving the lateral PFC component of the frontoparietal control network, as may be seen in Figure 6. That is, in both hemispheres, the repetition suppression effects involved relatively extensive ventrolateral PFC regions, but few dorsolateral PFC ones (orange regions in Fig. 6), whereas the repetition enhancement effects involved relatively extensive dorsolateral PFC regions, but essentially no ventrolateral PFC ones (cyan regions).
Figure 6.
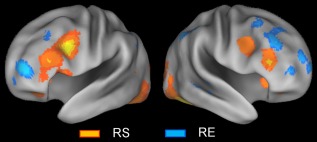
Above‐threshold brain regions in an ALE meta‐analysis of all repetition suppression (RS) experiments (orange regions) and all repetition enhancement (RE) experiments (cyan regions). [Color figure can be viewed at http://wileyonlinelibrary.com]
DISCUSSION
Repetition Suppression: General Effects
These results revealed that the repetition suppression effects were widely dispersed across the cortex and associated mainly with subregions of three intrinsic networks, namely, the frontoparietal control, dorsal attention, and visual networks. This multiple network configuration supports the hypothesis that the repetition suppression effects observed across the diverse cortical regions reflect multiple distinct mechanisms operating somewhat independently rather than as a single unitary process [Henson, 2016; Schacter et al., 2007]. The inferior frontal cortex showed one of the most extensive repetition suppression effects. A critical finding here was that the effects encompassed both the ventrolateral PFC component of the frontoparietal control network and the IFJ component of the dorsal attention network. While a single large cluster included both the left ventrolateral PFC and IFJ regions, making it difficult to discern whether the effects in these two regions were dissociable, two similar effects appeared as separate clusters in the right frontal cortex, indicating their distinct origins and network memberships. The ventrolateral PFC, especially the left part, plays a critical role in controlled semantic/conceptual processing [Badre and Wagner, 2007; Devlin et al., 2003; Noonan et al., 2013]. The magnitude of the repetition suppression in the PFC regions shows significant across‐subject correlations with behavioral priming [Bunzeck et al., 2006; Dobbins et al., 2004; Maccotta and Buckner, 2004; Soldan et al., 2010; Turk‐Browne et al., 2006]. In this regard, previous studies [Gold et al., 2005; Schacter et al., 2007; Wagner et al., 2000a] have typically ascribed reductions in ventrolateral PFC activity to facilitated semantic/conceptual processing of previously processed stimuli.
The involvement of the IFJ in repetition suppression effects has prompted relatively little specific theoretical discussion, because activity in this region has often been ascribed to the most adjoining classical areas, such as the posterior inferior frontal gyrus and the premotor cortex. An original functional hypothesis for the IFJ, based on its involvement in a broad spectrum of cognitive control tasks [Derrfuss et al., 2004], emphasized its role in the maintenance and manipulations of “task representations,” which roughly correspond to abstract representations of stimulus‐response mapping rules [Brass et al., 2005]. A related line of research highlighted its critical role in top‐down attentional control [Gazzaley and Nobre, 2012; Zanto et al., 2010]. In this regard, an rTMS study [Zanto et al., 2011] showed that disruption of IFJ activity diminishes the top‐down modulation of activity in visual regions. Recent meta‐analysis studies [Kim, 2014; Levy and Wagner, 2011] showed the reliable association of IFJ activity with oddball stimulus processing, indicating its role in the detection of salient, environmental changes. In this regard, repetition suppression in IFJ regions may have many nuances, including automatized stimulus‐response mapping [Dobbins et al., 2004; Henson et al., 2014], less demand for top‐down enhancement [Gazzaley et al., 2005; Vuilleumier et al., 2005], and reductions in novelty (or expectation)‐related signals [Summerfield et al., 2008].
The ventral occipitotemporal cortex also showed highly extensive repetition suppression effects, including large portions of the bilateral posterior PHC, fusiform cortex, and ventral occipital cortex. Repetition suppression effects in ventral occipitotemporal regions are largely specific to the visual modality [Andics et al., 2013; Bergerbest et al., 2004] and some studies [Soldan et al., 2010; Turk‐Browne et al., 2006] showed a significant across‐subject correlation between the magnitude of repetition suppression in these regions and behavioral priming, although the effects tended to be less consistent than those in the PFC regions [Maccotta and Buckner, 2004; Sayres and Grill‐Spector, 2006]. In this regard, repetition suppression effects in the ventral occipitotemporal regions may reflect facilitated sensory/perceptual processing of previously processed stimuli [Schacter et al., 2007; Wiggs and Martin, 1998]. However, repetition suppression effects in these and other regions are not automatic but, rather, depend to a large extent on selective attention to an item, as indicated by the absence of, or diminished repetition suppression for, ignored stimuli at the time of initial and/or repeated presentation [Eger et al., 2004; Henson and Mouchlianitis, 2007; Moore et al., 2013; Vuilleumier et al., 2005; Yi and Chun, 2005]. Thus, repetition suppression effects in the ventral occipitotemporal and other regions may also reflect a lower demand for selective attention in processing repeated versus novel stimuli. Consistent with this hypothesis, the results of this study indicated the prominent contribution of the dorsal attention network subregions to repetition suppression effects.
A related and critical finding here was that left fusiform effects predominantly involved the dorsal attention network subregions, whereas right fusiform effects were mainly associated with the visual network subregions. Various studies [Eger et al., 2005; Koutstaal et al., 2001; Pourtois et al., 2005; Simons et al., 2003; Stevens et al., 2012; Vuilleumier et al., 2002] reported consistent hemispheric asymmetry, indicating the existence of relatively more form‐abstract versus more prominent form‐specific repetition suppression in the left and right ventral temporal cortexes, respectively. For example, one study [Vuilleumier et al., 2002] indicated that the left fusiform cortex showed reductions in repetition activity for both the same and different views of an object (albeit to a lesser degree for a different view), whereas the right fusiform cortex showed reductions only for repetitions of the same view. Similar findings have been reported for face [Eger et al., 2005; Pourtois et al., 2005] and scene stimuli [Stevens et al., 2012]. A related line of studies showed that the left fusiform cortex showed decreased repetition activity for both the same and different exemplars of an object, whereas the right fusiform cortex only showed decreased repetition for the same exemplar [Koutstaal et al., 2001; Simons et al., 2003]. These hemispheric asymmetries may be underpinned by more prominent associations of the left versus right ventral temporal cortex with the dorsal attention and visual networks, respectively.
Some studies [de Gardelle et al., 2013; Larsson and Smith, 2012; Mayrhauser et al., 2014; Summerfield et al., 2008] showed that repetition suppression is attenuated in low versus high stimulus repetition probability contexts, suggesting that repetition suppression reflects “fulfilled expectations” or reductions in top‐down prediction errors. The dorsal attention network may also play a critical role in such modulating effects of stimulus expectation, because an expected versus an unexpected item tends to differ in its demand for selective attention. In line with this hypothesis, one study [Larsson and Smith, 2012] found that the modulating effects of stimulus expectation are significant for attended stimuli, but not for ignored stimuli. Given the greater representation of the dorsal network subregions in the left than in the right ventral temporal cortex, one prediction for this hypothesis is that the expectation‐related modulation of repetition suppression would be more robust in the left than in the right ventral temporal cortex. Consistent with this prediction, a recent study [Mayrhauser et al., 2014] indicated that the probability of stimulus repetition modulates repetition suppression in the left, but not in the right, ventral occipitotemporal regions.
Other less extensive repetition suppression effects included, within the frontoparietal control network, the posteromedial PFC, anterior orbitofrontal, and anterior insula regions, within the dorsal attention network, the posterior IPS regions, within the visual network, the lateral occipital regions, and within the subcortex, the hippocampal, striatal, and cerebellar regions. Although detailed functional discussions of each of these regions are beyond the scope of this paper, the involvement of the hippocampus deserves some commentary. Partly due to the strong neuropsychological evidence that the hippocampus plays a minor role in implicit memory, studies [Gonsalves et al., 2005; Kumaran and Maguire, 2009; Maass et al., 2014; Nyberg, 2005; O'Kane et al., 2005] have typically related hippocampal repetition suppression to factors other than implicit memory, most frequently to reduced novelty responses. Novelty detection and encoding are highly intertwined, because an encoding system is biased toward the registration of novel rather than familiar information [Ben‐Yakov et al., 2014; Kirchhoff et al., 2000; Law et al., 2005; Tulving and Kroll, 1995]. In this regard, alleged reductions in novelty responses in the hippocampus and other regions may trigger relatively less engagement of an encoding system. The enhanced deployment of selective attention to novel relative to familiar stimuli may act as a bridge between novelty/familiarity detection and subsequent increased/decreased engagement of a given encoding system.
The most extensively adopted paradigm when using neuroimaging in the analysis of memory encoding is to compare neural activity for the stimuli later remembered with that for the stimuli later forgotten [Wagner et al., 1998b], typically termed “subsequent memory” effects. Supporting the hypothesis that a mechanism for repetition suppression is less engagement of an encoding system, the global topography of repetition suppression effects was strikingly similar to that observed in a recent meta‐analysis of subsequent memory effects (compare Fig. 2 with Fig. 1 of Kim [2011]), with both the present and previous studies mainly involving the ventrolateral PFC, IFJ, hippocampal, ventral occipitotemporal, lateral occipital, anterior insula, posteromedial PFC, and posterior IPS regions. Also consistent with this hypothesis, some studies [Callan and Schweighofer, 2010; Wagner et al., 2000b; Xue et al., 2011; Zhao et al., 2015] showed that stronger repetition suppression is associated with lower levels of subsequent memory. As mentioned previously, there is evidence that repetition suppression in many regions reflects facilitates conceptual/perceptual processing of previously processed stimuli and, thus, implicit memory retrieval. In this regard, repetition suppression effects in many regions may have dual nuances, involving, on the one hand, more efficient reprocessing of previously processed stimuli and, on the other hand, relatively reduced encoding activity for repeated than for novel stimuli. It remains an open question as to whether and to what extent these two mechanisms are two sides of the same coin or dissociable processes [Bunzeck and Thiel, 2016; Habib, 2001].
Repetition Suppression: Category‐Specific and Common Effects
Separate meta‐analyses of word‐, scene‐, face, and object‐based repetition suppression effects indicated a relatively distinct global topography for each category. Word‐based repetition suppression effects most pronouncedly involved the left inferior frontal and left fusiform cortex regions (most likely corresponding to the visual word form area), while scene‐based ones were mainly associated with the bilateral posterior parahippocampal/medial fusiform regions (most likely corresponding to the parahippocampal place area), face‐based with a broad range of bilateral ventral occipitotemporal regions (including fusiform face area), and object‐based with the bilateral inferior frontal cortex and a range of bilateral ventral occipitotemporal regions. However, direct comparisons between word‐ and nonword‐, scene‐ and nonscene‐, face‐ and nonface‐, and object‐ and nonobject‐based repetition suppression effects indicated that most regions observed in separate meta‐analyses of each category showed significant repetition suppression for other categories as well.
The results within the ventral occipitotemporal cortex generally indicated the existence of relatively large overlapping regions, but few or no regions that were more strongly associated with a specific category. Exceptions to this rule included the parahippocampal/medial fusiform cortex regions that showed a medial‐to‐lateral gradient of scene‐specific and more generalized effects. The observation of largely overlapping regions is consistent with reports that the putative category‐selective regions respond significantly to stimuli that are not in the preferred class [Aminoff and Tarr, 2015; de Beeck et al., 2010; Price and Devlin, 2003; Vogel et al., 2012]. In this regard, some studies [de Beeck et al., 2008; Grill‐Spector and Weiner, 2014; Haxby et al., 2011] suggested that these regions are not “dedicated cortical modules,” but most likely parts of many overlapping large‐scale cortical maps of simple features that give rise to broader categorical distinctions, such as an eccentricity map [Hasson et al., 2002], a real‐world object size map [Konkle and Oliva, 2012], an animate/inanimate map [Haxby et al., 2011], and a functional connectivity map [Stevens et al., 2015]. To give a concrete example, one study [Konkle and Oliva, 2012] indicated a medial‐to‐lateral gradient of big and small object preferences across the ventral surface of the temporal cortex. This map may account for the preferential association of scene‐based effects with the parahippocampal regions, that of face‐ and word‐based effects with the more laterally located, fusiform regions, and that of object‐based effects with both the parahippocampal and fusiform regions. In another example, the connectivity map may account for the left‐versus right‐lateralization of word‐ and face‐based effects.
The results obtained within the lateral frontal cortex regions also generally indicated the existence of relatively more extensive overlapping regions. Exceptions to this rule included a small anterior extent of the left ventrolateral PFC that was more strongly associated with word‐ than nonword‐based effects, likely reflecting its more distinct role in verbal processing [Golby et al., 2001; Wagner et al., 1998a]. The bilateral IFJ regions were more strongly associated with object‐ than nonobject‐based effects, likely reflecting a greater demand on IFJ control functions by dual‐mode (verbal and pictorial) versus single‐mode processing. Large portions of the left ventrolateral PFC/IFJ regions were more strongly associated with nonface‐ than face‐based effects, likely reflecting their relatively limited role in nonverbal processing [Golby et al., 2001; Wagner et al., 1998a]. Perhaps not surprisingly, these results indicate that the frontal cortex regions are not organized by stimulus category, but by more abstract features, such as verbal versus nonverbal and types of controlled processing [Badre and D'Esposito, 2009; Levy and Wagner, 2011; Race et al., 2009].
Repetition Enhancement: General Effects
Repetition enhancement effects were mainly associated with subregions of the frontoparietal control and default mode networks. The involvement of the frontoparietal control network included the bilateral dorsolateral PFC, lateral IPS, and dorsal precuneus regions, while that of the default mode network included the bilateral ventral precuneus, anterior extent of posterior cingulate cortex, and inferior parietal lobe regions. Although repetition enhancement effects were less extensive than repetition suppression effects, it is relevant that they were revealed with a lower statistical power. Some studies [Blondin and Lepage, 2005; Donaldson et al., 2001; Henson et al., 2002; Korsnes and Magnussen, 2014; Koutstaal et al., 2001; Schott et al., 2005] hypothesized that repetition enhancement effects reflect voluntary or involuntary explicit retrieval during an implicit retrieval task. The most extensively adopted paradigm in using neuroimaging in the analysis of explicit retrieval is to compare the correct recognition of studied items (hit) with the correct nonrecognition of novel items (correct rejection), typically termed “retrieval success” effects. Supporting the explicit “contamination” hypothesis, the global topography of repetition enhancement effects was similar to that observed in a recent meta‐analysis of retrieval success effects (compare Fig. 5 with Fig. 1 of Kim [2013]), with both effects mainly involving the dorsolateral PFC, lateral IPS, inferior parietal lobe, dorsal/ventral precuneus, and anterior extent of posterior cingulate cortex regions. However, each of these clusters was more significantly associated with retrial success than with repetition enhancement effects, which is perhaps not surprising given that explicit retrieval is required in recognition memory tasks, but not in neural priming ones.
A direct comparison between repetition suppression and enhancement effects indicated some double dissociation. That is, repetition suppression involved relatively extensive areas of the ventrolateral PFC, but few dorsolateral PFC regions, whereas repetition enhancement involved relatively extensive dorsolateral areas of the PFC, but essentially no ventrolateral PFC regions. This dissociation cannot be easily accommodated by traditional models of lateral PFC organization, which typically emphasize organization by material, such as spatial versus nonspatial [Wilson et al., 1993], or organization by process, such as maintenance versus manipulation [D'Esposito et al., 2000]. An alternative hypothesis proposed here is that the ventrolateral PFC contributes more to the modulation of externalized cognition (e.g., stimulus processing) than to internalized cognition (e.g., recall), whereas the dorsolateral PFC contributes more to the modulation of internalized cognition than to externalized cognition. In line with this hypothesis, repetition suppression effects showed coactivation of the ventrolateral PFC regions with subregions of the dorsal attention network, whereas repetition enhancement effects showed coactivation of the dorsolateral PFC regions with subregions of the default mode network. Supporting the “externalized cognition” part of this hypothesis, recent meta‐analyses of subsequent memory effects [Kim, 2011] and external mode tasks [Duncan, 2013; Hugdahl et al., 2015] indicated the involvement of the lateral PFC mainly within its ventral extent. On the contrary, supporting the “internalized cognition” part of the hypothesis, recent meta‐analyses of retrieval success effects [Kim, 2013] and encoding failure effects [Kim, 2011] indicated the involvement of more dorsal PFC regions. Supporting both parts of this hypothesis, a direct meta‐analytic comparison between subsequent memory and retrieval success effects [Spaniol et al., 2009] indicated the association of the ventrolateral PFC regions mainly with preferential encoding activity and that of the dorsolateral PFC regions mainly with preferential retrieval activity.
Limitations, Future Directions, and Summary
The scope of this meta‐analysis was limited to studies presenting the same physical stimuli between the initial and repeated presentations (i.e., identity priming). However, some important advances in our understanding of the repetition suppression phenomenon have come from studies that systematically changed the stimuli between the two presentations along some dimensions, such as size, position, illumination, and rotation, with the aim of elucidating the properties of neural representations in the visual cortex and other regions [Grill‐Spector and Malach, 2001; Kourtzi and Grill‐Spector, 2005; Malach, 2012]. One of the first studies of this type [Grill‐Spector et al., 1999] demonstrated that the extent of object‐based repetition suppression in the fusiform cortex regions was largely invariant to changes in size and position, suggesting the involvement of neural representations invariant to these dimensions, whereas the more posterior visual regions showed repetition effects that were sensitive to these and other stimulus changes, suggesting the involvement of neural representations more selective to specific stimulus properties. In this regard, future studies should investigate more fine‐grained topographies of repetition suppression effects that may show invariance/selectivity to manipulations of multiple stimulus dimensions.
Another important issue in repetition suppression, but which is also beyond the scope of this study, concerns its neural mechanisms. Briefly, the proposed models of these mechanisms include the hypothesis that repetition leads to activation of fewer neurons (sharpening), repetition results in faster neural responses (facilitation), and repetition increases both local and global neural synchronization (synchrony) [Gotts et al., 2012; Grill‐Spector et al., 2006]. These and other “bottom‐up” models are important, because they complement, or provide neural underpinning of, more “top‐down” models involving functional constructs, such as attention, expectation, learning, and implicit memory. A related question for future studies is whether a single neural mechanism universally applies for all repetition suppression effects or whether different neural mechanisms hold for different brain regions [Grill‐Spector et al., 2006; Weiner et al., 2010].
This study considered repetition suppression/enhancement effects in the context of the 7‐network cortical architecture reported by Yeo et al [2011]. A related finding was that those repetition suppression effects involving left versus right fusiform cortex regions were predominantly associated with the dorsal attention and visual network regions, respectively. Because this finding is tied to the asymmetric parcellation of the ventral temporal regions in Yeo et al.'s model, it should be treated with some caution and further validated in the context of other parcellation models. Having said that, some recent connectivity studies have reported results that are well in line with Yeo et al.'s model. Using the BrainMap database [Fox et al., 2005], Caspers et al. [2014] performed a meta‐analytic connectivity modeling of left and right lateral fusiform regions of interest with no interhemispheric differences in cytoarchitecture, cortical volume, or relative stereotaxic location. The results indicated the differentially stronger connectivity of the left and right fusiform regions with higher‐order fronto‐parietal regions versus posterior visual processing regions, respectively. Zhang et al. [2016] performed a resting‐state connectivity analysis of similar left and right fusiform regions and found largely congruent interhemispheric differences. Interestingly, another resting‐state connectivity analysis [Stevens et al., 2012] indicated comparable asymmetric connectivity profiles for the left versus right parahippocampal place area. In view of the existence of more form‐abstract versus more prominent form‐specific repetition suppression in the left and right ventral temporal cortexes, respectively, as discussed previously, these findings support “the notion that the functional behavior of a particular cortical location is determined by both, its microstructural organization and its connectivity” [Caspers et al., 2014, p. 2764].
To summarize, the major findings were as follows. First, with regard to repetition suppression, the lateral frontal cortex effects involved the frontoparietal control network regions anteriorly and the dorsal attention network regions posteriorly. The left fusiform cortex effects predominantly involved the dorsal attention network regions, whereas the right fusiform cortex effects mainly involved the visual network regions. The global topography of the repetition suppression effects was strikingly similar to that of the “subsequent memory” effects, indicating that the mechanism for repetition suppression is the reduced engagement of an encoding system. It remains an open question as to whether and to what extent this mechanism and the one that supports implicit memory retrieval are two sides of the same coin or independent processes. Second, the category‐specific meta‐analyses and their comparisons indicated that most parts of the alleged category‐specific regions showed repetition suppression for more than one stimulus category. In this regard, these regions may not be “dedicated cortical modules,” but are most likely parts of multiple overlapping large‐scale maps of simple features that give rise to broader categorical distinctions. Third, the global topography of the repetition enhancement effects was similar to that of the “retrieval success” effects, suggesting that the mechanism for repetition enhancement is voluntary or involuntary explicit retrieval during an implicit memory task. Finally, the dorsolateral PFC regions were predominantly associated with repetition enhancement, whereas the ventrolateral PFC regions were mainly associated with repetition suppression, likely reflecting the greater contribution of the dorsolateral PFC to the modulation of internalized cognition and that of the ventrolateral PFC to the modulation of externalized cognition. Taken together, these results clarify the network affiliations of brain regions showing reliable repetition suppression and enhancement effects and contribute to theoretical interpretations of the local and global topography of these two effects.
Supporting information
Supporting Information
This research was supported by a Daegu University Research Grant, 2015.
REFERENCES
- Aminoff EM, Tarr MJ (2015): Associative processing is inherent in scene perception. PLoS One 10:e0128840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amunts K, Palomero‐Gallagher N, Brass M, Derrfuss J, Zilles K, von Cramon D (2004): A receptor‐and cytoarchitectonic correlate of the functionally defined inferior‐frontal junction area. NeuroImage 22, Suppl 1:50. [Google Scholar]
- Andics A, Gál V, Vicsi K, Rudas G, Vidnyánszky Z (2013): FMRI repetition suppression for voices is modulated by stimulus expectations. NeuroImage 69:277–283. [DOI] [PubMed] [Google Scholar]
- Andrews‐Hanna JR, Smallwood J, Spreng RN (2014): The default network and self‐generated thought: Component processes, dynamic control, and clinical relevance. Ann N Y Acad Sci 1316:29–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badre D, D'Esposito M (2009): Is the rostro‐caudal axis of the frontal lobe hierarchical? Nat Rev Neurosci 10:659–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badre D, Wagner A (2007): Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia 45:2883–2901. [DOI] [PubMed] [Google Scholar]
- Ben‐Yakov A, Rubinson M, Dudai Y (2014): Shifting gears in hippocampus: Temporal dissociation between familiarity and novelty signatures in a single event. J Neurosci 34:12973–12981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit RG, Schacter DL (2015): Specifying the core network supporting episodic simulation and episodic memory by activation likelihood estimation. Neuropsychologia 75:450–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergerbest D, Ghahremani DG, Gabrieli JD (2004): Neural correlates of auditory repetition priming: Reduced fMRI activation in the auditory cortex. J Cogn Neurosci 16:966–977. [DOI] [PubMed] [Google Scholar]
- Blondin F, Lepage M (2005): Decrease and increase in brain activity during visual perceptual priming: An fMRI study on similar but perceptually different complex visual scenes. Neuropsychologia 43:1887–1900. [DOI] [PubMed] [Google Scholar]
- Brass M, Derrfuss J, Forstmann B, Cramon D (2005): The role of the inferior frontal junction area in cognitive control. Trends Cogn Sci 9:314–316. [DOI] [PubMed] [Google Scholar]
- Buckner R, Andrews‐Hanna J, Schacter D (2008): The brain's default network: Anatomy, function, relevance to disease. Ann N Y Acad Sci 1124:1–38. [DOI] [PubMed] [Google Scholar]
- Bunzeck N, Thiel C (2016): Neurochemical modulation of repetition suppression and novelty signals in the human brain. Cortex 80:161–173. [DOI] [PubMed] [Google Scholar]
- Bunzeck N, Schütze H, Düzel E (2006): Category‐specific organization of prefrontal response‐facilitation during priming. Neuropsychologia 44:1765–1776. [DOI] [PubMed] [Google Scholar]
- Callan DE, Schweighofer N (2010): Neural correlates of the spacing effect in explicit verbal semantic encoding support the deficient‐processing theory. Hum Brain Mapp 31:645–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspers J, Zilles K, Eickhoff SB, Schleicher A, Mohlber H, Amunts K (2013): Cytoarchitectonical analysis and probabilistic mapping of two extrastriate areas of the human posterior fusiform gyrus. Brain Struct Funct 218:511–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspers J, Zilles K, Amunts K, Laird AR, Fox PT, Eickhoff SB (2014): Functional characterization and differential coactivation patterns of two cytoarchitectonic visual areas on the human posterior fusiform gyrus. Hum Brain Mapp 35:2754–2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L, Dehaene S, Naccache L, Lehéricy S, Dehaene‐Lambertz G, Hénaff M‐A, Michel F (2000): The visual word form area. Brain 123:291–307. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman G (2002): Control of goal‐directed and stimulus‐driven attention in the brain. Nat Rev Neurosci 3:201–215. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Postle BR, Rypma B (2000): Prefrontal cortical contributions to working memory: Evidence from event‐related fMRI studies. Exp Brain Res 133:3–11. [DOI] [PubMed] [Google Scholar]
- de Beeck HPO, Haushofer J, Kanwisher NG (2008): Interpreting fMRI data: Maps, modules and dimensions. Nat Rev Neurosci 9:123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Beeck HPO, Brants M, Baeck A, Wagemans J (2010): Distributed subordinate specificity for bodies, faces, and buildings in human ventral visual cortex. NeuroImage 49:3414–3425. [DOI] [PubMed] [Google Scholar]
- de Gardelle V, Stokes MG, Johnen VM, Wyart V, Summerfield C (2013): Overlapping multivoxel patterns for two levels of visual expectation. Front Hum Neurosci 7:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S, Naccache L, Cohen L, Le Bihan D, Mangin J‐F, Poline J‐B, Rivière D (2001): Cerebral mechanisms of word masking and unconscious repetition priming. Nat Neurosci 4:752–758. [DOI] [PubMed] [Google Scholar]
- Derrfuss J, Brass M, von Cramon DY (2004): Cognitive control in the posterior frontolateral cortex: Evidence from common activations in task coordination, interference control, and working memory. NeuroImage 23:604–612. [DOI] [PubMed] [Google Scholar]
- Derrfuss J, Brass M, Neumann J, Von Cramon D (2005): Involvement of the inferior frontal junction in cognitive control: Meta‐analyses of switching and Stroop studies. Hum Brain Mapp 25:22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrfuss J, Vogt V, Fiebach CJ, von Cramon DY, Tittgemeyer M (2012): Functional organization of the left inferior precentral sulcus: Dissociating the inferior frontal eye field and the inferior frontal junction. NeuroImage 59:3829–3837. [DOI] [PubMed] [Google Scholar]
- Devlin JT, Matthews PM, Rushworth MFS (2003): Semantic processing in the left inferior prefrontal cortex: A combined functional magnetic resonance imaging and transcranial magnetic stimulation study. J Cogn Neurosci 15:71–84. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Schnyer DM, Verfaellie M, Schacter DL (2004): Cortical activity reductions during repetition priming can result from rapid response learning. Nature 428:316–319. [DOI] [PubMed] [Google Scholar]
- Donaldson DI, Petersen SE, Buckner RL (2001): Dissociating memory retrieval processes using fMRI: Evidence that priming does not support recognition memory. Neuron 31:1047–1059. [DOI] [PubMed] [Google Scholar]
- Dosenbach N, Fair D, Cohen A, Schlaggar B, Petersen S (2008): A dual‐networks architecture of top‐down control. Trends Cogn Sci 12:99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J (2013): The structure of cognition: Attentional episodes in mind and brain. Neuron 80:35–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eger E, Henson R, Driver J, Dolan RJ (2004): BOLD repetition decreases in object‐responsive ventral visual areas depend on spatial attention. J Neurophysiol 92:1241–1247. [DOI] [PubMed] [Google Scholar]
- Eger E, Schweinberger S, Dolan R, Henson R (2005): Familiarity enhances invariance of face representations in human ventral visual cortex: fMRI evidence. NeuroImage 26:1128–1139. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT (2009): Coordinate‐based activation likelihood estimation meta‐analysis of neuroimaging data: A random‐effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp 30:2907–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, Roski C, Caspers S, Zilles K, Fox PT (2011): Co‐activation patterns distinguish cortical modules, their connectivity and functional differentiation. NeuroImage 57:938–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein R, Harris A, Stanley D, Kanwisher N (1999): The parahippocampal place area: Recognition, navigation, or encoding? Neuron 23:115–125. [DOI] [PubMed] [Google Scholar]
- Fox PT, Laird AR, Fox SP, Fox PM, Uecker AM, Crank M, Koenig SF, Lancaster JL (2005): BrainMap taxonomy of experimental design: Description and evaluation. Hum Brain Mapp 25:185–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox KCR, Spreng RN, Ellamil M, Andrews‐Hanna JR, Christoff K (2015): The wandering brain: Meta‐analysis of functional neuroimaging studies of mind‐wandering and related spontaneous thought processes. NeuroImage 111:611–621. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Nobre AC (2012): Top‐down modulation: Bridging selective attention and working memory. Trends Cogn Sci 16:129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaley A, Cooney JW, McEvoy K, Knight RT, D'Esposito M (2005): Top‐down enhancement and suppression of the magnitude and speed of neural activity. J Cogn Neurosci 17:507–517. [DOI] [PubMed] [Google Scholar]
- Golby A, Poldrack R, Brewer J, Spencer D, Desmond J, Aron A, Gabrieli J (2001): Material‐specific lateralization in the medial temporal lobe and prefrontal cortex during memory encoding. Brain 124:1841–1854. [DOI] [PubMed] [Google Scholar]
- Gold BT, Balota DA, Kirchhoff BA, Buckner RL (2005): Common and dissociable activation patterns associated with controlled semantic and phonological processing: Evidence from fMRI adaptation. Cereb Cortex 15:1438–1450. [DOI] [PubMed] [Google Scholar]
- Gonsalves B, Kahn I, Curran T, Norman K, Wagner A (2005): Memory strength and repetition suppression: Multimodal imaging of medial temporal cortical contributions to recognition. Neuron 47:751–761. [DOI] [PubMed] [Google Scholar]
- Gotts SJ, Chow CC, Martin A (2012): Repetition priming and repetition suppression: A case for enhanced efficiency through neural synchronization. Cogn Neurosci 3:227–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill‐Spector K, Malach R (2001): fMR‐adaptation: A tool for studying the functional properties of human cortical neurons. Acta Psychol 107:293–321. [DOI] [PubMed] [Google Scholar]
- Grill‐Spector K, Weiner KS (2014): The functional architecture of the ventral temporal cortex and its role in categorization. Nat Rev Neurosci 15:536–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill‐Spector K, Kushnir T, Edelman S, Avidan G, Itzchak Y, Malach R (1999): Differential processing of objects under various viewing conditions in the human lateral occipital complex. Neuron 24:187–203. [DOI] [PubMed] [Google Scholar]
- Grill‐Spector K, Henson R, Martin A (2006): Repetition and the brain: Neural models of stimulus‐specific effects. Trends Cogn Sci 10:14–23. [DOI] [PubMed] [Google Scholar]
- Habib R (2001): On the relation between conceptual priming, neural priming, and novelty assessment. Scand J Psychol 42:187–195. [DOI] [PubMed] [Google Scholar]
- Hasson U, Levy I, Behrmann M, Hendler T, Malach R (2002): Eccentricity bias as an organizing principle for human high‐order object areas. Neuron 34:479–490. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Guntupalli JS, Connolly AC, Halchenko YO, Conroy BR, Gobbini MI, Hanke M, Ramadge PJ (2011): A common, high‐dimensional model of the representational space in human ventral temporal cortex. Neuron 72:404–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson RN (2016): Repetition suppression to faces in the fusiform face area: A personal and dynamic journey. Cortex 80:174–184. [DOI] [PubMed] [Google Scholar]
- Henson R, Shallice T, Dolan R (2000): Neuroimaging evidence for dissociable forms of repetition priming. Science 287:1269–1272. [DOI] [PubMed] [Google Scholar]
- Henson R, Shallice T, Gorno‐Tempini M, Dolan R (2002): Face repetition effects in implicit and explicit memory tests as measured by fMRI. Cereb Cortex 12:178–186. [DOI] [PubMed] [Google Scholar]
- Henson R, Rylands A, Ross E, Vuilleumeir P, Rugg M (2004): The effect of repetition lag on electrophysiological and haemodynamic correlates of visual object priming. NeuroImage 21:1674–1689. [DOI] [PubMed] [Google Scholar]
- Henson RN, Mouchlianitis E (2007): Effect of spatial attention on stimulus‐specific haemodynamic repetition effects. NeuroImage 35:1317–1329. [DOI] [PubMed] [Google Scholar]
- Henson RN, Eckstein D, Waszak F, Frings C, Horner AJ (2014): Stimulus–response bindings in priming. Trends Cogn Sci 18:376–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugdahl K, Raichle ME, Mitra A, Specht K (2015): On the existence of a generalized non‐specific task‐dependent network. Front Hum Neurosci 9:430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N (2010): Functional specificity in the human brain: A window into the functional architecture of the mind. Proc Natl Acad Sci USA 107:11163–11170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM (1997): The fusiform face area: A module in human extrastriate cortex specialized for face perception. J Neurosci 17:4302–4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kherif F, Josse G, Price CJ (2011): Automatic top‐down processing explains common left occipito‐temporal responses to visual words and objects. Cereb Cortex 21:103–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Cilles SE, Johnson NF, Gold BT (2012): Domain general and domain preferential brain regions associated with different types of task switching: A meta‐analysis. Hum Brain Mapp 33:130–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H (2011): Neural activity that predicts subsequent memory and forgetting: A meta‐analysis of 74 fMRI studies. NeuroImage 54:2446–2461. [DOI] [PubMed] [Google Scholar]
- Kim H (2013): Differential neural activity in the recognition of old versus new events: An activation likelihood estimation meta‐analysis. Hum Brain Mapp 34:814–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H (2014): Involvement of the dorsal and ventral attention networks in oddball stimulus processing: A meta‐analysis. Hum Brain Mapp 35:2265–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H (2016): Default network activation during episodic and semantic memory retrieval: A selective meta‐analytic comparison. Neuropsychologia 80:35–46. [DOI] [PubMed] [Google Scholar]
- Kirchhoff BA, Wagner AD, Maril A, Stern CE (2000): Prefrontal‐temporal circuitry for episodic encoding and subsequent memory. J Neurosci 20:6173–6180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konkle T, Oliva A (2012): A real‐world size organization of object responses in occipitotemporal cortex. Neuron 74:1114–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korsnes M, Magnussen S (2014): fMRI evidence for dissociation between priming and conscious recognition. J Integr Neurosci 13:509–517. [DOI] [PubMed] [Google Scholar]
- Kourtzi Z, Grill‐Spector K (2005): fMRI adaptation: A tool for studying visual representations in the primate brain In: Clifford C, Rhodes G, editors. Fitting the Mind to the World: Adaptation and After‐Effects in High‐Level Vision. New York: Oxford University Press; pp 173–188. [Google Scholar]
- Koutstaal W, Wagner A, Rotte M, Maril A, Buckner R, Schacter D (2001): Perceptual specificity in visual object priming: Functional magnetic resonance imaging evidence for a laterality difference in fusiform cortex. Neuropsychologia 39:184–199. [DOI] [PubMed] [Google Scholar]
- Kumaran D, Maguire EA (2009): Novelty signals: A window into hippocampal information processing. Trends Cogn Sci 13:47–54. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Tordesillas‐Gutiérrez D, Martinez M, Salinas F, Evans A, Zilles K, Mazziotta JC, Fox PT (2007): Bias between MNI and Talairach coordinates analyzed using the ICBM‐152 brain template. Hum Brain Mapp 28:1194–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson J, Smith AT (2012): fMRI repetition suppression: Neuronal adaptation or stimulus expectation? Cereb Cortex 22:567–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law J, Flanery M, Wirth S, Yanike M, Smith A, Frank L, Suzuki W, Brown E, Stark C (2005): Functional magnetic resonance imaging activity during the gradual acquisition and expression of paired‐associate memory. J Neurosci 25:5720–5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy BJ, Wagner AD (2011): Cognitive control and right ventrolateral prefrontal cortex: Reflexive reorienting, motor inhibition, and action updating. Ann N Y Acad Sci 1224:40–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz S, Weiner KS, Caspers J, Mohlberg H, Schleicher A, Bludau S, Eickhoff SB, Grill‐Spector K, Zilles K, Amunts K (2011): Two new cytoarchitectonic areas on the human mid‐fusiform gyrus. Cereb Cortex 13. pii: bhv225. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maass A, Schütze H, Speck O, Yonelinas A, Tempelmann C, Heinze H‐J, Berron D, Cardenas‐Blanco A, Brodersen KH, Stephan KE (2014): Laminar activity in the hippocampus and entorhinal cortex related to novelty and episodic encoding. Nat Commun 5:5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccotta L, Buckner RL (2004): Evidence for neural effects of repetition that directly correlate with behavioral priming. J Cogn Neurosci 16:1625–1632. [DOI] [PubMed] [Google Scholar]
- Malach R (2012): Targeting the functional properties of cortical neurons using fMR‐adaptation. NeuroImage 62:1163–1169. [DOI] [PubMed] [Google Scholar]
- Mayrhauser L, Bergmann J, Crone J, Kronbichler M (2014): Neural repetition suppression: Evidence for perceptual expectation in object‐selective regions. Front Hum Neurosci 8:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore KS, Yi D‐J, Chun M (2013): The effect of attention on repetition suppression and multivoxel pattern similarity. J Cogn Neurosci 25:1305–1314. [DOI] [PubMed] [Google Scholar]
- Muhle‐Karbe PS, Derrfuss J, Lynn MT, Neubert FX, Fox PT, Brass M, Eickhoff SB (2016): Co‐activation‐based parcellation of the lateral prefrontal cortex delineates the inferior frontal junction area. Cereb Cortex 26:2225–2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller NG, Strumpf H, Scholz M, Baier B, Melloni L (2013): Repetition suppression versus enhancement—It's quantity that matters. Cereb Cortex 23:315–322. [DOI] [PubMed] [Google Scholar]
- Noonan KA, Jefferies E, Visser M, Lambon Ralph MA (2013): Going beyond inferior prefrontal involvement in semantic control: Evidence for the additional contribution of dorsal angular gyrus and posterior middle temporal cortex. J Cogn Neurosci 25:1824–1850. [DOI] [PubMed] [Google Scholar]
- Nyberg L (2005): Any novelty in hippocampal formation and memory? Curr Opin Neurol 18:424–428. [DOI] [PubMed] [Google Scholar]
- O'Kane G, Insler R, Wagner A (2005): Conceptual and perceptual novelty effects in human medial temporal cortex. Hippocampus 15:326–332. [DOI] [PubMed] [Google Scholar]
- Pourtois G, Schwartz S, Seghier ML, Lazeyras F, Vuilleumier P (2005): Portraits or people? Distinct representations of face identity in the human visual cortex. J Cogn Neurosci 17:1043–1057. [DOI] [PubMed] [Google Scholar]
- Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, Vogel AC, Laumann TO, Miezin FM, Schlaggar BL (2011): Functional network organization of the human brain. Neuron 72:665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price C, Devlin J (2003): The myth of the visual word form area. NeuroImage 19:473–481. [DOI] [PubMed] [Google Scholar]
- Race EA, Shanker S, Wagner AD (2009): Neural priming in human frontal cortex: Multiple forms of learning reduce demands on the prefrontal executive system. J Cogn Neurosci 21:1766–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayres R, Grill‐Spector K (2006): Object‐selective cortex exhibits performance‐independent repetition suppression. J Neurophysiol 95:995–1007. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Wig GS, Stevens WD (2007): Reductions in cortical activity during priming. Curr Opin Neurobiol 17:171–176. [DOI] [PubMed] [Google Scholar]
- Schott BH, Henson RN, Richardson‐Klavehn A, Becker C, Thoma V, Heinze H‐J, Düzel E (2005): Redefining implicit and explicit memory: The functional neuroanatomy of priming, remembering, and control of retrieval. Proc Natl Acad Sci USA 102:1257–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segaert K, Weber K, de Lange FP, Petersson KM, Hagoort P (2013): The suppression of repetition enhancement: A review of fMRI studies. Neuropsychologia 51:59–66. [DOI] [PubMed] [Google Scholar]
- Sestieri C, Shulman GL, Corbetta M (2012): Orienting to the environment: Separate contributions of dorsal and ventral frontoparietal attention networks In: Mangun GR, editor. The Neuroscience of Attention: Attentional Control and Selection. New York: Oxford University Press; pp 100–130. [Google Scholar]
- Simons JS, Koutstaal W, Prince S, Wagner AD, Schacter DL (2003): Neural mechanisms of visual object priming: Evidence for perceptual and semantic distinctions in fusiform cortex. NeuroImage 19:613–626. [DOI] [PubMed] [Google Scholar]
- Smith S, Fox P, Miller K, Glahn D, Fox P, Mackay C, Filippini N, Watkins K, Toro R, Laird A (2009): Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci USA 106:13040–13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldan A, Habeck C, Gazes Y, Stern Y (2010): Neural mechanisms of repetition priming of familiar and globally unfamiliar visual objects. Brain Res 1343:122–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaniol J, Davidson P, Kim A, Han H, Moscovitch M, Grady C (2009): Event‐related fMRI studies of episodic encoding and retrieval: Meta‐analyses using activation likelihood estimation. Neuropsychologia 47:1765–1779. [DOI] [PubMed] [Google Scholar]
- Stevens W, Wig G, Schacter D (2009): Implicit memory and priming In: Byrne JH, editor. Concise Learning and Memory: The Editor's Selection. London: Academic Press; pp 65–86. [Google Scholar]
- Stevens WD, Kahn I, Wig GS, Schacter DL (2012): Hemispheric asymmetry of visual scene processing in the human brain: Evidence from repetition priming and intrinsic activity. Cereb Cortex 22:1935–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens WD, Tessler MH, Peng CS, Martin A (2015): Functional connectivity constrains the category‐related organization of human ventral occipitotemporal cortex. Hum Brain Mapp 36:2187–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerfield C, Trittschuh EH, Monti JM, Mesulam M‐M, Egner T (2008): Neural repetition suppression reflects fulfilled perceptual expectations. Nat Neurosci 11:1004–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E, Kroll N (1995): Novelty assessment in the brain and long‐term memory encoding. Psychon Bull Rev 2:387–390. [DOI] [PubMed] [Google Scholar]
- Turk‐Browne NB, Yi D‐J, Chun MM (2006): Linking implicit and explicit memory: Common encoding factors and shared representations. Neuron 49:917–927. [DOI] [PubMed] [Google Scholar]
- Turk‐Browne NB, Scholl BJ, Chun MM (2008): Babies and brains: Habituation in infant cognition and functional neuroimaging. Front Hum Neurosci 2:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub PE, Eickhoff SB, Laird AR, Fox M, Wiener M, Fox P (2012): Minimizing within experiment and within group effects in activation likelihood estimation meta‐analyses. Hum Brain Mapp 33:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen D (2005): A population‐average, landmark‐ and surface‐based (PALS) atlas of human cerebral cortex. NeuroImage 28:635–662. [DOI] [PubMed] [Google Scholar]
- Vannini P, Hedden T, Sullivan C, Sperling RA (2013): Differential functional response in the posteromedial cortices and hippocampus to stimulus repetition during successful memory encoding. Hum Brain Mapp 34:1568–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent J, Kahn I, Snyder A, Raichle M, Buckner R (2008): Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol 100:3328–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel AC, Petersen SE, Schlaggar BL (2012): The left occipitotemporal cortex does not show preferential activity for words. Cereb Cortex 22:2715–2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P, Henson R, Driver J, Dolan R (2002): Multiple levels of visual object constancy revealed by event‐related fMRI of repetition priming. Nat Neurosci 5:491–499. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Schwartz S, Duhoux S, Dolan RJ, Driver J (2005): Selective attention modulates neural substrates of repetition priming and “implicit” visual memory: Suppressions and enhancements revealed by fMRI. J Cogn Neurosci 17:1245–1260. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Poldrack R, Eldridge L, Desmond J, Glover G (1998a): Material‐specific lateralization of prefrontal activation during episodic encoding and retrieval. NeuroReport 9:3711–3717. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Schacter DL, Rotte M, Koutstaal W, Maril A, Dale AM, Rosen BR, Buckner RL (1998b): Building memories: Remembering and forgetting of verbal experiences as predicted by brain activity. Science 281:1188–1191. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Koutstaal W, Maril A, Schacter DL, Buckner RL (2000a): Task‐specific repetition priming in left inferior prefrontal cortex. Cereb Cortex 10:1176–1184. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Maril A, Schacter DL (2000b): Interactions between forms of memory: When priming hinders new episodic learning. J Cogn Neurosci 12, Suppl 2:52–60. [DOI] [PubMed] [Google Scholar]
- Weigelt S, Muckli L, Kohler A (2008): Functional magnetic resonance adaptation in visual neuroscience. Rev Neurosci 19:363–380. [DOI] [PubMed] [Google Scholar]
- Weiner KS, Sayres R, Vinberg J, Grill‐Spector K (2010): fMRI‐adaptation and category selectivity in human ventral temporal cortex: Regional differences across time scales. J Neurophysiol 103:3349–3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wig GS, Buckner RL, Schacter DL (2009): Repetition priming influences distinct brain systems: Evidence from task‐evoked data and resting‐state correlations. J Neurophysiol 101:2632–2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggs CL, Martin A (1998): Properties and mechanisms of perceptual priming. Curr Opin Neurobiol 8:227–233. [DOI] [PubMed] [Google Scholar]
- Wilson FA, Scalaidhe SP, Goldman‐Rakic PS (1993): Dissociation of object and spatial processing domains in primate prefrontal cortex. Science 260:1955–1958. [DOI] [PubMed] [Google Scholar]
- Xue G, Mei L, Chen C, Lu Z‐L, Poldrack R, Dong Q (2011): Spaced learning enhances subsequent recognition memory by reducing neural repetition suppression. J Cogn Neurosci 23:1624–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BTT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zöllei L, Polimeni JR (2011): The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol 106:1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi D‐J, Chun MM (2005): Attentional modulation of learning‐related repetition attenuation effects in human parahippocampal cortex. J Neurosci 25:3593–3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanto TP, Rubens MT, Bollinger J, Gazzaley A (2010): Top‐down modulation of visual feature processing: The role of the inferior frontal junction. NeuroImage 53:736–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanto TP, Rubens MT, Thangavel A, Gazzaley A (2011): Causal role of the prefrontal cortex in top‐down modulation of visual processing and working memory. Nat Neurosci 14:656–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Wang J, Fan L, Zhang Y, Fox PT, Eickhoff SB, Yu C, Jiang T (2016): Functional organization of the fusiform gyrus revealed with connectivity profiles. Hum Brain Mapp 37:3003–3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Wang C, Liu Q, Xiao X, Jiang T, Chen C, Xue G (2015): Neural mechanisms of the spacing effect in episodic memory: A parallel EEG and fMRI study. Cortex 69:76–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


