Abstract
Diffusion tensor imaging (DTI) has often been used to examine white matter (WM) tract abnormalities in depressed subjects, but these studies have yielded inconsistent results, probably, due to gender composition or small sample size. In this study, we applied different analysis pipelines to a relatively large sample of individuals with depression to determine whether previous findings in depression can be replicated with these pipelines. We used a “standard” DTI algorithm and maps computed through a free‐water (FW) corrected DTI. This latter algorithm is able to identify and separate the effects of extracellular FW on DTI metrics. Additionally, skeletonized and WM voxel‐based analysis (VBA) methods were used. Using the skeletonized method, DTI maps showed lower fractional anisotropy (FA) in depressed subjects in the left brain hemisphere, including the anterior thalamic radiation (ATR L), cortical spinal tract (CST L), inferior fronto‐occipital fasciculus, inferior longitudinal fasciculus, and superior longitudinal fasciculus (SLF L). Differences in radial diffusivity (RD) were also found. For the VBA using RD, we found different results when we used FW uncorrected and corrected DTI metrics. Relative to the VBA approach, the skeletonized analysis was able to identify more clusters where WM integrity was altered in depressed individuals. Different significant correlations were found between RD and the Patient Health Questionnaire in the CST L, and SLF L. In conclusion, the skeletonized method revealed more clusters than the VBA and individuals with depression showed multiple WM abnormalities, some of which were correlated with disease severity Hum Brain Mapp 38:4690–4702, 2017. © 2017 Wiley Periodicals, Inc.
Keywords: diffusion tensor imaging, depression, free‐water DTI, voxel‐based analysis, Patient Health Questionnaire
INTRODUCTION
Major depressive disorder (MDD) is projected to account for an accumulated $16 trillion in lost productivity over the next 20 years and is among the most common and devastating mental health conditions worldwide [Whiteford et al., 2013]. This disorder is characterized by profound dysregulation of mood and additional symptoms including fatigue, insomnia, cognitive dysfunction, and appetite disturbance [APAD‐SMMD, 2000].
Diffusion tensor imaging (DTI) is probably the most popular noninvasive magnetic resonance imaging (MRI) techniques for assessing the integrity and orientation of white matter (WM) tracts. In recent years, DTI has been widely used to investigate differences in WM tracts between depressed and healthy control (HC) subjects. The most commonly used index in DTI is fractional anisotropy (FA). It has a scalar value between zero and one that describes the degree of anisotropy of a diffusion process. A value of zero means that diffusion is isotropic while a value of one means that diffusion occurs only along one axis and is fully restricted along all other directions. This index has often been used as a quantitative biomarker of WM integrity; however, equating FA to WM integrity is not strictly correct, because FA cannot disentangle the individual microscopic contributions of different non‐WM components [Jones and Cercignani, 2010; Vos et al., 2012]. Moreover, other DTI indices have been used in depression studies, such as axial (a.k.a. longitudinal or parallel) diffusivity, called or AD which is related to axonal damage, and radial (a.k.a. transverse or perpendicular) diffusivity, called or RD which correlates with myelin integrity, axonal diameter and density, and fiber coherence [Concha, 2014; Song et al., 2002]. Finally, mean diffusivity (MD) is an index often used in DTI studies, which comprises the average of the diffusion eigenvectors and quantifies the overall size of the tensor representing a rotationally invariant apparent diffusion coefficient (ADC) measure. It is important to emphasize that all DTI indices largely depend on the angle between two or more crossing fiber populations; therefore, DTI techniques cannot resolve different single‐fiber orientations within each voxel. This is a big issue, which is associated not only with the WM tractography but also with the DTI‐derived metrics.
The two common analysis methods in MDD studies include voxel‐based analysis (VBA) [Ashburner and Friston, 2000; Srivastava et al., 2016] and tract‐based spatial statistics (TBSS) [Guo et al., 2012a; Guo et al., 2012b; Han et al., 2014; Olvet et al., 2016; Zuo et al., 2012].
VBA is a fully automated approach that allows investigation of WM integrity in each voxel inside the whole brain. It involves the spatial normalization of high‐ and low‐resolution images from the subjects' native space to stereotactic space. The main issue with this method is related to the normalization and smoothing pipeline, requiring very reliable normalization between DTI data and the standard image. Moreover, pathologies and lesions can affect the results.
On the other hand, TBSS, introduced by Smith et al. [2006], is able to alleviate the alignment and smoothing problems related to VBA. It is a popular pipeline used to co‐register sets of DTI maps for performing voxel‐wise comparisons using a skeleton projection analysis. Therefore, it does not facilitate the study of whole WM tracts of the entire brain. In recent years, investigations [Zalesky, 2011] have questioned the reliability and interpretability of TBSS and various improvements over the original TBSS pipeline have been suggested [Schwarz et al., 2014].
The DTI methods described above have yielded conflicting results for individuals with depression. For instance, lower FA values in depressed individuals compared with HC have been reported in different WM locations [Guo et al., 2012a, 2012b; Versace et al., 2010; Wu et al., 2011; Zou et al., 2008]. In comparison, other studies failed to find significant differences in DTI metrics between depression and control groups [Choi et al., 2014; Olvet et al., 2016].
To date, the source of inconsistent findings in DTI investigations of individuals with depression has been unclear. It could stem from variability in extra‐experimental factors such as gender composition or be related to small sample size. Another important issue is that only few studies have used more than one analysis method to examine DTI indices [Choi et al., 2014; Olvet et al., 2016]. Furthermore, DTI metrics can be influenced by variability introduced by different brain tissue compartments, including cerebrospinal fluid and extracellular water [Pierpaoli et al., 1996]. For this reason, Pasternak et al. developed an algorithm for identifying and separating the effects of extracellular free water on DTI metrics to improve tissue specificity [Metzler‐Baddeley et al., 2012] and DTI‐based tract reconstruction [Pasternak et al., 2009]. This approach is able to remove the effects of extracellular free water on DTI metrics.
The main aim of this investigation was to apply different analysis pipelines to a relatively large sample of individuals with depression to determine whether previous findings in depression can be replicated with these pipelines. We investigated WM microstructural integrity in a cohort of individuals with depression compared to age‐matched HC subjects using FA, MD, AD, and RD indices to understand whether differences in DTI metrics can be found between HC and depressed groups. We examined these differences using two different methods: (1) a voxel‐wise statistical analysis, using DTI skeletonized maps, (skeletonized method) and (2) a WM‐VBA approach. For both analysis methods we used Advanced Normalization Tools (ANTs) with a symmetric image normalization (SyN) algorithm [Avants et al., 2008], instead of the standard TBSS pipeline for the skeletonized method, to coregister all DTI‐derived metrics to a common standard space. We decided to use two different analysis approaches to determine which method was more sensitive for detecting WM microstructural changes in depressed individuals and the location of these changes. The skeletonized approach is able to alleviate the alignment and smoothing problems related to VBA; therefore, our hypothesis was that it could be more sensitive in detecting alterations in WM tracts than the VBA method. In addition, a comparison between DTI‐derived “standard” indices (now called FA, RD, etc.) and maps computed by a FW‐corrected DTI matrix (now called FA‐FW, RD‐FW, etc.) was performed to verify results previously reported in another study [Bergamino et al., 2016].
Finally, we calculated voxel‐based correlations between DTI indices and the Patient Health Questionnaire (PHQ‐9), a self‐reported depression scale, to better understand if the WM changes in depressed individuals may be related to illness severity, with the expectation that increased abnormality would be associated with increased disease severity.
METHODS
Subjects
This study was approved by the Western Institutional Review Board (http://www.wirb.com), and all participants signed informed consent prior to study participation. The current study included 131 depressed (90 females; mean age: 35.7 ± 11.2 years) and 40 HC (22 females; 33.4 ± 11.9 years) participants. All participants were recruited from the Tulsa, Oklahoma (USA) area. Subject characteristics are summarized in Table 1. The inclusion criteria for depressed subjects were as follows: (1) age between 18 and 55 years; (2) PHQ‐9 score ≥ 5; (3) body mass index between 17 to 38 kg/m2; and (4) sufficient proficiency in written and spoken English to understand and complete interviews, questionnaires, and all other study procedures. The exclusion criteria were as follows: (1) a reported history of brain injury or other neurocognitive disorder; (2) a positive test for drugs of abuse, including alcohol; (3) active suicidal ideation with intent or a plan; (4) any change in the dose or prescription of a medication that could affect brain functioning within 6 weeks before enrolling in the study; (5) moderate to severe traumatic brain injury or other neurocognitive disorder with evidence of neurological deficits, neurological disorders, or severe or unstable medical conditions; and (6) any of the following DSM‐IV disorders: schizophrenia spectrum and other psychotic disorders, bipolar and related disorders, and obsessive‐compulsive and related disorders.
Table 1.
Number of females and males, means with (standard deviations) for age, body mass index (BMI), education, number of depression episodes (# Episodes), and PHQ‐9 scores for HC and depressed subjects
| Group | Female | Male | Age (years) | BMI | Education | # Episodes | PHQ‐9 |
|---|---|---|---|---|---|---|---|
| HC | 22 | 18 | 33.4 (11.9) | 28.1 (5.9) | 6.6 (1.7) | 0 | 0.75 (1.13) |
| MDD | 90 | 41 | 35.7 (11.2) | 28.8 (5.3) | 6.5 (1.7) | 6.3 (9.4) | 13.33 (4.60) |
| Levels of depression severity | |||||||
| # (F) | PHQ‐9 scale | ||||||
| Mild dep. | 24 (17) | 5–9 | |||||
| Moderate dep. | 62 (45) | 10–14 | |||||
| Moderately severe dep. | 29 (18) | 15–19 | |||||
| Severe dep. | 16 (10) | 20–27 | |||||
For education: 1 = lowest education and 11 = highest education. Number of depressed subjects with different levels of depression severity. In parenthesis, the number of female participants.
All participants completed the PHQ‐9, a self‐administered diagnostic instrument for depression. The PHQ‐9 scores each of the 9 DSM‐IV criteria for depression as “0” (not at all) to “3” (nearly every day). Scores of 5–9 are considered mild depression, 10–14 moderate depression, 15–19 moderately severe depression, and 20–27 severe depression [Kroenke et al., 2001].
MRI Protocol
MRI data were acquired using a 3 T scanner (GE Discovery MR750) with a brain‐dedicated receive‐only 32‐element coil array optimized for parallel imaging (Nova Medical, Inc.). DTI was performed using 60 diffusion‐encoding directions (b value = 1000 s/mm2, TR/TE = 9000/83.6 ms, with acquisition and reconstruction matrix = 128 × 128, field of view (FOV) = 25.6 × 25.6 cm, slice thickness = 2 mm, without interslice spacing, 73 axial slices, acceleration factor R = 2 in the phase encoding direction) and 8 non‐diffusion‐weighted images (b0 images). Total acquisition time for the DTI sequence was 10 min and 50 s.
Data Preprocessing
DTI DICOM images were converted to NIFTI format and preprocessed using the functional magnetic resonance imaging of the brain (FMRIB) Software Library tool (FSL, version 5.0.4) [Smith et al., 2004]. Following translation and rotation estimation, the raw DTI images were corrected for eddy currents and motion; the relative‐motion parameters were estimated from the transformation matrices for each subject [Ling et al., 2012]. Data with translational or rotational motion estimates greater than three standard deviations (SDs) from the mean were excluded. To account for the rotational component of registration, after motion correction the gradient orientations were compensated prior to calculating b matrices. FSL FUGUE was used to reduce the distortions caused by B0 inhomogeneity during the DWI acquisition and a brain mask was defined for each subject by applying the Brain Extraction Toolbox (BET) [Smith, 2002] to the average of the b0 images. DTI “standard” maps were created by using dtifit tool included in FMRIB's Diffusion Toolbox.
DTI free‐water‐corrected maps were calculated using an in‐house MATLAB script in the subjects' native space. The free‐water maps were computed by fitting each voxel to the following model [Pasternak et al., 2009]:
| (1) |
in which A q is the modeled attenuated signal (normalized by b0) for the applied diffusion gradient q and b is the b value (1000 s/mm2). The first term in A q reflects the tissue compartment, D is the diffusion tensor of this compartment, and f is the fractional volume of the compartment. The second term reflects an isotropic free‐water compartment with a fractional volume of (1 − f); d water is the diffusion coefficient, set to the diffusivity of water at body temperature (3 × 10−3 mm2/s).
For both analysis methods, all DTI‐derived metrics were co‐registered to the Illinois Institute of Technology (IIT) Human Brain Atlas (v.4.1), which contains both anatomical and DTI brain templates in International Consortium for Brain Mapping (ICBM)‐152 space (Varentsova et al., 2014). For this purpose, we averaged the b0 images of each subject and then we created a b0 group template image using buildtemplateparallel.sh included in ANTs. The b0 group template image was subsequently normalized to the IITmean_b0 image by ANTs SyN coregistration algorithm. The warp fields obtained from this normalization and from the b0 group template creation were used to transfer all DTI maps to the IIT Human Brain standard space.
For the skeletonized analysis method, the mean of FA maps were created and thinned to produce a mean FA skeleton using a threshold value of 0.20 to exclusively select WM. Each subject's aligned FA data were projected onto this skeleton by assigning the maximum FA value found in a direction perpendicular to each tract to the skeleton voxel. DTI corrected and uncorrected maps were projected onto the group template skeleton for further analysis. For the VBA method, we analyzed all voxels inside of the WM using an FA threshold value of 0.20. Figure 1 shows the workflow of the images normalization and the different analysis pipelines.
Figure 1.
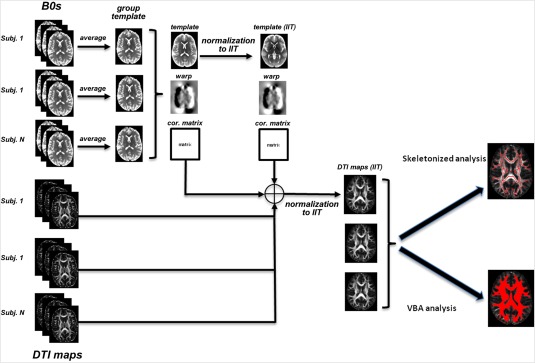
This diagram shows the workflow of the images normalization and the different analysis pipelines used in this study. [Color figure can be viewed at http://wileyonlinelibrary.com]
Statistical Analysis
We used FSL's randomise [Winkler et al., 2014] for per‐voxel statistical comparisons with threshold‐free cluster enhancement (TFCE) [Smith and Nichols, 2009] and with correction for multiple comparisons via Family‐Wise Error (FWE) Rate [Keihaninejad et al., 2012]. The statistical analysis was the same for both methods, except that for the skeletonized analysis we used the option “–T2” to generate the 2D WM skeleton and the mean of the WM FA skeleton binarized image as the mask. For VBA, we used option “−T” for normalized 3D data and the white matter image as the mask. The number of randomized permutations was set at 10,000 for both methods. We used age, gender, BMI, education, and number of depressive episodes as covariates. The WM tract each cluster belonged to was identified through labeling of the Johns Hopkins University White Matter Tractography Atlas. We report all results with a significance threshold of P value < 0.01, corrected for FWE.
Differences in age, BMI, education, and PHQ‐9 scores between the two groups were evaluated using two‐sample t tests. Correlations between DTI indices and PHQ‐9 scores were assessed by the Pearson correlation coefficient; the P values were corrected for multiple comparisons through the Bonferroni procedure.
RESULTS
Data from five depressed participants and one HC were excluded from the analyses due to excessive motion. Therefore, data from 128 MDD and 39 HC subjects were analyzed. MDD and HC groups did not differ significantly in age (P = 0.26), BMI (P = 0.50), or education (P = 0.75), but they did differ with respect to PHQ‐9 score (P < 0.001).
The skeletonized approach identified several clusters with lower values of FA‐FW and FA in subjects with depression. Greater volumes of these clusters were found when we used the FW uncorrected FA index. In addition, differences between these two groups were found in different WM tracts when VBA analysis and FA index were used. In comparison, only one cluster, in the left anterior thalamic radiation (ATR L), was found when FA‐FW was used (Fig. 2).
Figure 2.
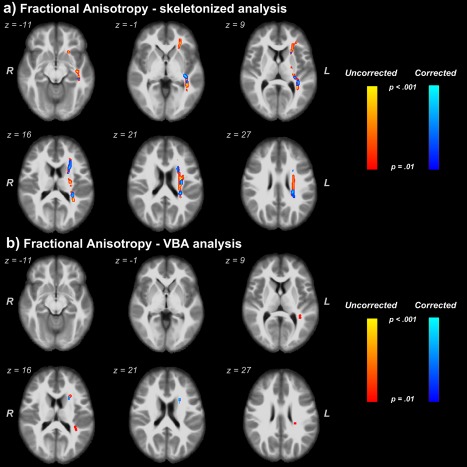
Clusters where lower values of free‐water corrected and uncorrected FA were found in depressed individuals. (a) Comparison of FA and FA‐FW through the skeletonized analysis method. (b) Comparison of FA and FA‐FW through the VBA analysis method. We report clusters at a significance threshold of P value < 0.01 corrected for FWE. Regions showing reduced FA values are thickened using the TBSS fill script for emphasis. The results are overlaid onto the IITmean_t1 template. [Color figure can be viewed at http://wileyonlinelibrary.com]
Skeletonized analysis on RD and RD‐FW metrics found clusters with higher RD values in depressed subjects in similar WM tracts already found by the FA index. The VBA approach found differences between groups in only 2 clusters, in cortical spinal tract (CST L) and in SLF L, when the FW uncorrected RD index was used. No differences were found by the RD‐FW metric (Fig. 3).
Figure 3.
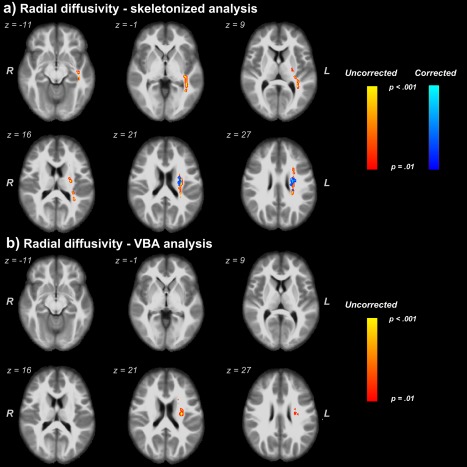
Clusters with higher values of free‐water corrected and uncorrected RD in depressed subjects. (a) Comparison of RD and RD‐FW through the skeletonized analysis method. (b) WM regions found by VBA analysis for only the FW‐uncorrected RD. We report clusters at a significance threshold of P value < 0.01 corrected for FWE. Regions showing increased RD values are thickened using the TBSS fill script for emphasis. The results are overlaid onto the IITmean_t1 template. [Color figure can be viewed at http://wileyonlinelibrary.com]
For both analysis pipelines, Table 2 shows the complete WM tracts and the mean of the DTI metric values (with SD) found inside the clusters shown in Figures 2 and 3. Figure 4 shows the differences in volume of the clusters found by both analysis approaches and by FW‐corrected and uncorrected metrics.
Table 2.
White matter tracts where differences between depressed and HC groups were found using the skeletonized and VBA analyses
| Skeleton—FA | Skeleton—FA‐FW | VBA—FA | VBA—FA‐FW | |||||
|---|---|---|---|---|---|---|---|---|
| WM Tract | HC | MDD | HC | MDD | HC | MDD | HC | MDD |
| ATR L | 0.438 (0.026) | 0.413 (0.024) | 0.560 (0.044) | 0.519 (0.033) | 0.514 (0.038) | 0.469 (0.047) | 0.519 (0.057) | 0.467 (0.048) |
| CST L | 0.608 (0.033) | 0.583 (0.030) | 0.740 (0.043) | 0.713 (0.043) | ‐ | ‐ | ‐ | ‐ |
| IFOF L | 0.567 (0.034) | 0.537 (0.025) | 0.714 (0.030) | 0.683 (0.034) | 0.572 (0.044) | 0.522 (0.034) | ‐ | ‐ |
| ILF L | 0.605 (0.036) | 0.572 (0.031) | 0.738 (0.032) | 0.711 (0.037) | 0.573 (0.058) | 0.517 (0.052) | ‐ | ‐ |
| SLF L | 0.506 (0.036) | 0.482 (0.030) | 0.632 (0.047) | 0.601 (0.046) | 0.496 (0.055) | 0.450 (0.046) | ‐ | ‐ |
| UF L | 0.475 (0.038) | 0.451 (0.032) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Skeleton—RD | Skeleton—RD‐FW | VBA—RD | VBA—RD‐FW | |||||
|---|---|---|---|---|---|---|---|---|
| WM Tract | HC | MDD | HC | MDD | HC | MDD | HC | MDD |
| ATR L | 4.90 (0.34) | 5.17 (0.37) | 4.01 (0.26) | 4.19 (0.27) | ‐ | ‐ | ‐ | ‐ |
| CST L | 4.59 (0.24) | 4.76 (0.23) | 3.19 (0.33) | 3.33 (0.30) | 4.85 (0.24) | 5.11 (0.29) | ‐ | ‐ |
| IFOF L | 4.48 (0.47) | 4.84 (0.33) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| ILF L | 4.68 (0.44) | 5.00 (0.35) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| SLF L | 4.88 (0.27) | 5.07 (0.23) | 3.75 (0.26) | 3.91 (0.26) | 4.86 (0.30) | 5.13 (0.32) | ‐ | ‐ |
ATR L = left anterior thalamic radiation; CST L = left cortical spinal tract; IFOF L = left inferior fronto‐occipital fasciculus; ILF L = left inferior longitudinal fasciculus; SLF L = left superior longitudinal fasciculus; UF L = left uncinate fasciculus.
Figure 4.
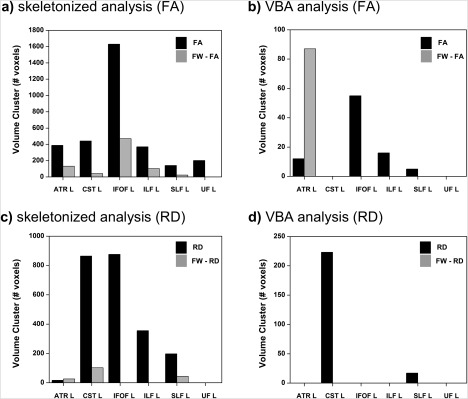
Comparison of cluster volumes with differences in DTI metrics between groups: (a) skeletonized method for FA and FA‐FW; (b) VBA method for FA and FA‐FW; (c) skeletonized method for RD and RD‐FW; (d) VBA method for RD and RD‐FW. ATR L = left anterior thalamic radiation; CST L = left cortical spinal tract; IFOF L = left inferior fronto‐occipital fasciculus; ILF L = left inferior longitudinal fasciculus; SLF L = left superior longitudinal fasciculus; UF L = left uncinate fasciculus.
No differences were detected in AD and MD between these two groups and there were no clusters indicating lower FA and higher RD in HC than the depression group.
No significant correlations were found between FA/FA‐FW metrics and PHQ‐9 scores. However, using the skeletonized approach we found significant correlations between RD and PHQ‐9 in the left cortical spinal tract (CST L; P < 0.01) and in the SLF L (P < 0.05), whereas the VBA analysis yielded correlations between PHQ‐9 and CST L (P < 0.05). Finally, using the RD‐FW we found one correlation only inside the CST L (P < 0.05) when the skeletonized approach was used (Fig. 5 and Table 3).
Figure 5.
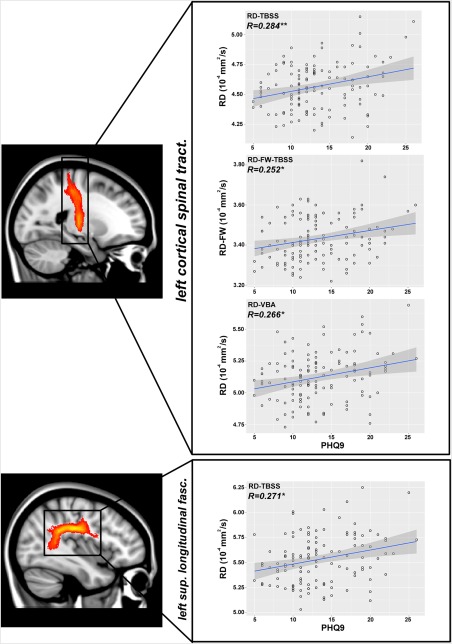
Pearson's correlation coefficient (R) between RD index and PHQ‐9 in the left cortical spinal tract and in the left superior longitudinal fasciculus. **P < 0.01 (Bonferroni corrected); *P < 0.05 (Bonferroni corrected). [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 3.
Pearson's correlation coefficients between PHQ‐9 scores and DTI metrics in white matter tracts where differences were found between groups
| WM Tract | skeleton – FA | skeleton – FA‐FW | VBA – FA | VBA – FA‐FW |
|---|---|---|---|---|
| ATR L | R = −0.133 | R = −0.013 | R = −0.111 | R = 0.025 |
| CST L | R = −0.150 | R = −0.035 | R = −0.138 | R = −0.037 |
| IFOF L | R = −0.054 | R = 0.022 | R = −0.075 | R = −0.005 |
| ILF L | R = −0.065 | R = −0.008 | R = −0.100 | R = −0.051 |
| SLF L | R = −0.133 | R = −0.089 | R = −0.136 | R = −0.080 |
| UF L | R = 0.004 | R = 0.059 | R = −0.019 | R = −0.052 |
| WM Tract | skeleton – RD | skeleton – RD‐FW | VBA – RD | VBA – RD‐FW |
|---|---|---|---|---|
| ATR L | R = 0.154 | R = 0.073 | R = 0.171 | R = −0.023 |
| CST L | R = 0.284b | R = 0.252a | R =0.266a | R = 0.008 |
| IFOF L | R = 0.092 | R = −0.051 | R = 0.096 | R = −0.006 |
| ILF L | R = 0.077 | R = −0.035 | R = 0.105 | R = 0.005 |
| SLF L | R = 0.271a | R = 0.032 | R = 0.172 | R = 0.053 |
| UF L | R = 0.046 | R = 0.088 | R = 0.072 | R = −0.037 |
p < .05 (Bonferroni corrected).
p < .01 (Bonferroni corrected).
Correlations were computed only within the MDD group. Bonferroni correction was used to correct the P values for multiple comparisons. Significant correlations were found for RD index inside the CST L and SLF L for the skeletonized analysis and inside the CST L for the VBA analysis. For RD‐FW only inside the CST L when the skeletonized approach was used. The names of the tracts are the same of Table 2.
DISCUSSION
This study was aimed to compare different analysis pipelines (skeletonized method and a white matter VBA approach) and measures (FW corrected and uncorrected DTI metrics) in a large group of individuals with depression to compare which measurement approach would yield the most robust findings.
Lower values of FA in depressed subjects were found in ATR L, CST L, left inferior fronto‐occipital fasciculus (IFOF L), left inferior longitudinal fasciculus (ILF L), superior longitudinal fasciculus (SLF L), and left uncinate fasciculus (UF L) by skeletonized analysis. Similar regions were also found when FA‐FW maps were analyzed. In the last case, only the UF L did not reveal differences between groups. In addition, with the FA maps we obtained higher cluster volumes than with the FA‐FW maps. By using the VBA analysis, we found differences between groups in the FA index in ATR L, IFOF L, ILF L, and SLF L. Only one cluster inside the ATR L was found to have lower FA values in depressed participants when the FA‐FW index was used.
Differences between groups were also detected for RD. Skeletonized analysis showed higher RD values in depressed subjects in ATR L, CST L, IFOF L, ILF L, and SLF L. Fewer clusters were found when RD‐FW maps were used. Additionally, with RD maps we found higher cluster volumes than with RD‐FW maps. By VBA analysis, we found differences in the RD index between groups in only one cluster inside the CST L. On the other hand, no cluster was found when RD‐FW maps were used.
These findings support the notion that relative to the VBA approach, the skeletonized analysis is more sensitive in detecting altered depression‐related integrity in WM areas. For the FA index, we did not find large differences when the FW uncorrected and corrected maps were used (we did not find any difference between group only in UF L when free‐water corrected maps were analyzed). However, relevant differences were found when the comparison between free‐water corrected and uncorrected maps was evaluated for FA index in the VBA approach and for the RD metric (Table 2). It is recognized that the skeletonized method is less sensitive to the partial volume effects than VBA [Van Hecke et al., 2016]; therefore, the differences between corrected and uncorrected DTI metrics found for VBA analysis might be associated to its sensitivity to partial volume effects.
Significant Pearson's correlations between the RD index and PHQ‐9 scores were found in CST L in in the SLF L when the skeletonized analysis was employed, which might suggest that more severe depression is a predictor of WM abnormalities in these tracts. Similar correlations were also found inside the CST L for RD through the VBA analysis and for RD‐FW through the skeletonized approach.
To date, many DTI studies in depressed subjects can be found in the extant literature, but conflicting results are often reported. The small sample size of depressed participants and gender composition may lead to spurious results. Additionally, the findings can also be related to the DTI method and acquisition used for the analysis. Some studies on depression have been performed using <30 directions of DTI acquisition [Guo et al., 2012a, 2012b; Han et al., 2014; Li et al., 2007; Zhu et al., 2011]. To achieve robust anisotropy estimation, at least 20 unique sampling orientations and at least 30 tensor orientations are required. Otherwise, schemes with a lower number of sampling orientations may introduce bias and spurious correlations between tensor orientation and apparent diffusion characteristics [Jones, 2004].
Additionally, it is important to consider that the standard DTI metrics can be influenced by contributions of different brain tissue compartments. To overcome this problem, Bergamino et al. [2016] compared standard DTI metrics and FW‐corrected DTI indices in a group of 17 MDD females finding that applying a free‐water correction to DTI data may increase the sensitivity of DTI‐based metrics to detect clinical effects in MDD.
For the skeletonized analysis method, we did not use the standard TBSS pipeline. In the past years, several studies have evaluated and updated the TBSS processing pipeline with contemporary advancements in registration techniques. The TBSS registration process can be improved by using, for instance, a single registration step, where DTI metrics are constrained to the skeleton, instead of a standard TBSS's two‐step registration–projection pipeline [de Groot et al., 2013], or by using group‐wise registration for co‐registering FA images to a custom‐generated template, rather than co‐registering all images to a standard template [Keihaninejad et al., 2012]. Therefore, in our study we decided to use the ANTs SyN algorithm and a group‐wise b0 template, by using the subjects' b0 images, to improve the DTI maps co‐registration to a standard space.
Klein et al. [2009] evaluated ANTs coregistration software, finding that this algorithm provides superior registration performance in T1‐weighted MR registration tasks and metrics when compared to FNIRT and 13 other coregistration tools. In addition, Tustison et al. [2014] found that ANTs algorithm is superior to FNIRT specifically for FA coregistration.
Clusters in which WM integrity was altered in the depressed subjects were located in the ATR L, CST L, IFOF L, ILF L, SLF L, and in UF L. This indicates that our results are in line with other previous DTI studies in depression. Huang et al. examined the WM integrity in 15 depressed and 15 non‐depressed patients with Parkinson's disease. Interesting, they found decreased FA in the left UF, SLF, ATR, and in the ILF. Moreover, they also found other WM differences between these two groups in the forceps minor [Huang et al., 2014]. Liu et al. found similar WM locations. They evaluated the relationship between WM integrity during a first depressive episode in drug‐naive patients with MDD using TBSS method. Also in this case, the MDD group was found to have significantly reduced FA values in the IFOF, UF and ATR [Liu et al., 2016]. Zou et al., by using a voxel‐based analysis, investigated the difference in FA in a group of 45 patients with MDD compared with 45 healthy controls. Different WM locations in the left hemisphere, including the SLF, were found to have decreased value of FA in MDD patients [Zou et al., 2008]. Similar to our results, Lai and Wu in 2016 found low FA values in depressed subjects in SLF, ILF, IFOF, and in the corpus callosum. Ota et al. [2015] studied 21 individuals with depression found significant decreases of FA and increases of MD in patients with MDD compared with HC in the corpus callosum, IFOF, and left SLF.
Differing results have been found by other authors. Choi et al. [2014] published a study in 2014 where 134 MDD patients were compared to HCs using voxel‐based morphometry and (TBSS) approaches. In that study, MDD patients, compared with a HC group, showed no significant differences in FA, RD, MD, and AD with either the VBM or the TBSS methods. Similar results were also found by Olvet et al. [2016], where 139 participants with MDD were studied using ROI, TBSS, and diffusion tractography methods. Some differences can be found between these two studies and our work. For instance, in Ovelt at al., only unmedicated patients were analyzed. Moreover, in both studies, the standard TBSS pipeline, with FLIRT and FNIRT coregistration algorithms, was used, while in our study, we replaced these algorithms with ANTs SyN coregistration (and a group template), which may lead to superior registration performance than the algorithms included in FSL. Another important difference was that they used only age [Choi et al., 2014] and age and sex [Olvet et al., 2016] as coveriates in the statistical analysis, while in our study, we also used BMI, education, and number of depressive episodes.
It is important to observe that our results also differ from findings reported in a previous study where FW‐corrected indices were found to be more sensitive than the metrics computed by the standard DTI [Bergamino et al., 2016]. This may be due to the difference in the sample size.
Fibers running through the UF connect the orbitofrontal cortex with the temporopolar region, the rostral parahippocampal gyrus (entorhinal/perirhinal region), and the amygdala. Therefore, alterations in the UF integrity may indicate that information processing between these regions is less well integrated in MDD patients.
CST may be related to a range of depression‐related functioning, such as somatosensory, affective, and cognitive functions. In some case, higher values of FA inside CST in depressed subjects have been found [Sacchet et al., 2014]. Moreover, other investigators have proposed that increased FA in this tract can be connected to decreased FA in SLF [Douaud et al., 2011].
SLF is a major bidirectional association tract connecting large parts of the frontal cortex with the parietal, temporal, and occipital lobes. The SLF facilitates the formation of a bidirectional neural network that is necessary for core processes such as attention, memory, emotions, and language [Mesulam, 1998; Petrides and Pandya, 2002]. Consequently, structural damage of the SFL would have detrimental effects on mood regulation [Alexander et al., 1986; Clark et al., 2009]. SLF damage and depression severity have also been found [Murphy and Frodl, 2011] with the suggestion that WM changes in this tract may precede the onset of depressive illness [Huang et al., 2011].
IFOF connects the inferior and lateral margins of the occipital lobe to the inferolateral and dorsolateral regions of the frontal lobe. Emotional visual function is connected to this tract [Catani et al., 2002]. Frodi et al. [2012] found that unaffected first‐degree healthy relatives of patients with MDD showed greater FA than controls in this tract, other studies have shown that lower FA index in MDD patients may be associated to alterations in emotional visual perception [Phillips et al., 2003].
Similar to the IFOF, the ILF fiber bundle connects the occipital lobe with the anterior part of the temporal lobe, running laterally and inferiorly above optic radiation fibers. This tract is involved in face recognition [Fox et al., 2008], visual perception [Ffytche, 2008], reading [Epelbaum et al., 2008], visual memory [Ross, 2008], and other functions related to language [Catani and Mesulam, 2008]. Ortibus et al. [2012] noted correlations between this DTI index in ILF and impaired object recognition in children with visual–perceptual impairment.
Frequently, differences or changes in DTI‐derived indices are interpreted as changes in the integrity of the WM microstructure (or, in the opposite way, as structural damage, decline or degeneration). This suggests that some aspect of the WM microstructure is damaged [Jones et al., 2013]. However, this interpretation is not completely correct. For instance, the anisotropy in a brain region may also be lower due to larger axon diameters, lower packing density, or increased membrane permeability [Takahashi et al., 2002]. Therefore, even if we found lower values of FA and higher values of RD in our depressed group compared with the HCs, we cannot affirm that this variation in the diffusion metrics is totally connected to integrity and/or damage of the WM.
This study has several strengths: (1) combining the strengths of both “standard” DTI and FW‐corrected DTI metrics, which may improve the sensitivity of detecting WM alterations in depression; (2) the comparison of two DTI analyses pipelines; (3) a large cohort of depressed subjects that includes both males and females. This work has also some limitations. The first limitation is related to the FW algorithm that we used for our DTI. The MRI data were acquired with a single b‐value shell. This means that the algorithm used to fit the FW imaging model involved spatial regularization of the data [Pasternak et al., 2009]. This decreases intra‐group variability and may thus obscure subtle spatial features. However, there are other FW algorithms able to fit multi shell DTI data that may yield better accuracy of the FW model [Hoy et al., 2014]. Moreover, often patients suffering from depression show related disorders, which were not controlled for here. For example, depression is often accompanied by alterations in sleep patterns, which are also related to WM integrity [Rosenberg et al., 2014]. Additionally, as this is a cross‐sectional study, we have only demonstrated a correlation between DTI metrics and depression, but cannot infer which occurred first.
In conclusion, for both analysis methods, differences in FA and RD indices were observed in WM tracts previously reported in other depression studies. In addition, the skeletonized approach was able to find more clusters than the VBA analysis method. This difference might be related to the reduction of the co‐registration errors and partial volume effects for the skeletonized method.
Even though we revealed better sensitivity with the FW uncorrected DTI maps than the FW corrected maps, we cannot affirm if this sensitivity is related only to the pathology of depression or, also, connected to the partial volume effects that influence the DTI‐uncorrected indices. However, for our dataset, FW corrected DTI metrics do not seem to improve the sensitivity to detect WM tract abnormalities in depressed individuals.
REFERENCES
- Alexander GE, DeLong MR, Strick PL (1986): Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Ann Rev Neurosci 9:357–381. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ (2000): Voxel‐based morphometry—The methods. NeuroImage 11:805–821. [DOI] [PubMed] [Google Scholar]
- APAD‐SMMD (2000): American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington, DC: American Psychiatric Press. [Google Scholar]
- Avants BB, Epstein CL, Grossman M, Gee JC (2008): Symmetric diffeomorphic image registration with cross‐correlation: Evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal 12:26–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergamino M, Pasternak O, Farmer M, Shenton ME, Hamilton JP (2016): Applying a free‐water correction to diffusion imaging data uncovers stress‐related neural pathology in depression. Neuroimage Clin 10:336–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Howard RJ, Pajevic S, Jones DK (2002): Virtual in vivo interactive dissection of white matter fasciculi in the human brain. NeuroImage 17:77–94. [DOI] [PubMed] [Google Scholar]
- Catani M, Mesulam M (2008): The arcuate fasciculus and the disconnection theme in language and aphasia: History and current state. Cortex 44:953–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KS, Holtzheimer PE, Franco AR, Kelley ME, Dunlop BW, Hu XP, Mayberg HS (2014): Reconciling variable findings of white matter integrity in major depressive disorder. Neuropsychopharmacology 39:1332–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark L, Chamberlain SR, Sahakian BJ (2009): Neurocognitive mechanisms in depression: Implications for treatment. Ann Rev Neurosci 32:57–74. [DOI] [PubMed] [Google Scholar]
- Concha L (2014): A macroscopic view of microstructure: Using diffusion‐weighted images to infer damage, repair, and plasticity of white matter. Neuroscience 276:14–28. [DOI] [PubMed] [Google Scholar]
- de Groot M, Vernooij MW, Klein S, Ikram MA, Vos FM, Smith SM, Niessen WJ, Andersson JL (2013): Improving alignment in tract‐based spatial statistics: Evaluation and optimization of image registration. NeuroImage 76:400–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douaud G, Jbabdi S, Behrens TE, Menke RA, Gass A, Monsch AU, Rao A, Whitcher B, Kindlmann G, Matthews PM, Smith S (2011): DTI measures in crossing‐fibre areas: Increased diffusion anisotropy reveals early white matter alteration in MCI and mild Alzheimer's disease. NeuroImage 55:880–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epelbaum S, Pinel P, Gaillard R, Delmaire C, Perrin M, Dupont S, Dehaene S, Cohen L (2008): Pure alexia as a disconnection syndrome: New diffusion imaging evidence for an old concept. Cortex 44:962–974. [DOI] [PubMed] [Google Scholar]
- Ffytche DH (2008): The hodology of hallucinations. Cortex 44:1067–1083. [DOI] [PubMed] [Google Scholar]
- Fox CJ, Iaria G, Barton JJ (2008): Disconnection in prosopagnosia and face processing. Cortex 44:996–1009. [DOI] [PubMed] [Google Scholar]
- Frodl T, Carballedo A, Fagan AJ, Lisiecka D, Ferguson Y, Meaney JF (2012): Effects of early‐life adversity on white matter diffusivity changes in patients at risk for major depression. J Psychiatry Neurosci 37:37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo WB, Liu F, Chen JD, Xu XJ, Wu RR, Ma CQ, Gao K, Tan CL, Sun XL, Xiao CQ, Chen HF, Zhao JP (2012a): Altered white matter integrity of forebrain in treatment‐resistant depression: A diffusion tensor imaging study with tract‐based spatial statistics. Progr Neuropsychopharmacol Biol Psychiatry 38:201–206. [DOI] [PubMed] [Google Scholar]
- Guo WB, Liu F, Xue ZM, Gao K, Wu RR, Ma CQ, Liu ZN, Xiao CQ, Chen HF, Zhao JP (2012b): Altered white matter integrity in young adults with first‐episode, treatment‐naive, and treatment‐responsive depression. Neurosci Lett 522:139–144. [DOI] [PubMed] [Google Scholar]
- Han KM, Choi S, Jung J, Na KS, Yoon HK, Lee MS, Ham BJ (2014): Cortical thickness, cortical and subcortical volume, and white matter integrity in patients with their first episode of major depression. J Affect Disord 155:42–48. [DOI] [PubMed] [Google Scholar]
- Hoy AR, Koay CG, Kecskemeti SR, Alexander AL (2014): Optimization of a free water elimination two‐compartment model for diffusion tensor imaging. NeuroImage 103:323–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Fan X, Williamson DE, Rao U (2011): White matter changes in healthy adolescents at familial risk for unipolar depression: A diffusion tensor imaging study. Neuropsychopharmacology 36:684–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P, Xu X, Gu Q, Xuan M, Yu X, Luo W, Zhang M (2014): Disrupted white matter integrity in depressed versus non‐depressed Parkinson's disease patients: A tract‐based spatial statistics study. J Neurol Sci 346:145–148. [DOI] [PubMed] [Google Scholar]
- Jones DK (2004): The effect of gradient sampling schemes on measures derived from diffusion tensor MRI: A Monte Carlo study. Magn Reson Med 51:807–815. [DOI] [PubMed] [Google Scholar]
- Jones DK, Cercignani M (2010): Twenty‐five pitfalls in the analysis of diffusion MRI data. NMR Biomed 23:803–820. [DOI] [PubMed] [Google Scholar]
- Jones DK, Knosche TR, Turner R (2013): White matter integrity, fiber count, and other fallacies: The do's and don'ts of diffusion MRI. NeuroImage 73:239–254. [DOI] [PubMed] [Google Scholar]
- Keihaninejad S, Ryan NS, Malone IB, Modat M, Cash D, Ridgway GR, Zhang H, Fox NC, Ourselin S (2012): The importance of group‐wise registration in tract based spatial statistics study of neurodegeneration: A simulation study in Alzheimer's disease. PLoS One 7:e45996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A, Andersson J, Ardekani BA, Ashburner J, Avants B, Chiang MC, Christensen GE, Collins DL, Gee J, Hellier P, Song JH, Jenkinson M, Lepage C, Rueckert D, Thompson P, Vercauteren T, Woods RP, Mann JJ, Parsey RV (2009): Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. NeuroImage 46:786–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB (2001): The PHQ‐9: Validity of a brief depression severity measure. J Gen Intern Med 16:606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Ma N, Li Z, Tan L, Liu J, Gong G, Shu N, He Z, Jiang T, Xu L (2007): Prefrontal white matter abnormalities in young adult with major depressive disorder: A diffusion tensor imaging study. Brain Res 1168:124–128. [DOI] [PubMed] [Google Scholar]
- Ling J, Merideth F, Caprihan A, Pena A, Teshiba T, Mayer AR (2012): Head injury or head motion? Assessment and quantification of motion artifacts in diffusion tensor imaging studies. Hum Brain Mapp 33:50–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Watanabe K, Kakeda S, Yoshimura R, Abe O, Ide S, Hayashi K, Katsuki A, Umene‐Nakano W, Watanabe R, Ueda I, Nakamura J, Korogi Y (2016): Relationship between white matter integrity and serum cortisol levels in drug‐naive patients with major depressive disorder: Diffusion tensor imaging study using tract‐based spatial statistics. Br J Psychiatry 208:585–590. [DOI] [PubMed] [Google Scholar]
- Mesulam MM (1998): From sensation to cognition. Brain 121: 1013–1052. [DOI] [PubMed] [Google Scholar]
- Metzler‐Baddeley C, O'Sullivan MJ, Bells S, Pasternak O, Jones DK (2012): How and how not to correct for CSF‐contamination in diffusion MRI. NeuroImage 59:1394–1403. [DOI] [PubMed] [Google Scholar]
- Murphy ML, Frodl T (2011): Meta‐analysis of diffusion tensor imaging studies shows altered fractional anisotropy occurring in distinct brain areas in association with depression. Biol Mood Anxiety Disord 1:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olvet DM, Delaparte L, Yeh FC, DeLorenzo C, McGrath PJ, Weissman MM, Adams P, Fava M, Deckersbach T, McInnis MG, Carmody TJ, Cooper CM, Kurian BT, Lu H, Toups MS, Trivedi MH, Parsey RV (2016): A comprehensive examination of white matter tracts and connectometry in major depressive disorder. Depression Anxiety 33:56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortibus E, Verhoeven J, Sunaert S, Casteels I, de Cock P, Lagae L (2012): Integrity of the inferior longitudinal fasciculus and impaired object recognition in children: A diffusion tensor imaging study. Dev Med Child Neurol 54:38–43. [DOI] [PubMed] [Google Scholar]
- Ota M, Noda T, Sato N, Hattori K, Hori H, Sasayama D, Teraishi T, Nagashima A, Obu S, Higuchi T, Kunugi H (2015): White matter abnormalities in major depressive disorder with melancholic and atypical features: A diffusion tensor imaging study. Psychiatry Clin Neurosci 69:360–368. [DOI] [PubMed] [Google Scholar]
- Pasternak O, Sochen N, Gur Y, Intrator N, Assaf Y (2009): Free water elimination and mapping from diffusion MRI. Magn Reson Med 62:717–730. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN (2002): Comparative cytoarchitectonic analysis of the human and the macaque ventrolateral prefrontal cortex and corticocortical connection patterns in the monkey. Eur J Neurosci 16:291–310. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R (2003): Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biol Psychiatry 54:515–528. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di Chiro G (1996): Diffusion tensor MR imaging of the human brain. Radiology 201:637–648. [DOI] [PubMed] [Google Scholar]
- Rosenberg J, Maximov II, Reske M, Grinberg F, Shah NJ (2014): “Early to bed, early to rise”: Diffusion tensor imaging identifies chronotype‐specificity. NeuroImage 84:428–434. [DOI] [PubMed] [Google Scholar]
- Ross ED (2008): Sensory‐specific amnesia and hypoemotionality in humans and monkeys: Gateway for developing a hodology of memory. Cortex 44:1010–1022. [DOI] [PubMed] [Google Scholar]
- Sacchet MD, Prasad G, Foland‐Ross LC, Joshi SH, Hamilton JP, Thompson PM, Gotlib IH (2014): Structural abnormality of the corticospinal tract in major depressive disorder. Biol Mood Anxiety Disord 4:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz CG, Reid RI, Gunter JL, Senjem ML, Przybelski SA, Zuk SM, Whitwell JL, Vemuri P, Josephs KA, Kantarci K, Thompson PM, Petersen RC, Jack CR Jr., Alzheimer's Disease Neuroimaging, I (2014): Improved DTI registration allows voxel‐based analysis that outperforms tract‐based spatial statistics. NeuroImage 94:65–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM (2002): Fast robust automated brain extraction. Hum Brain Mapp 17:143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen‐Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE (2006): Tract‐based spatial statistics: Voxelwise analysis of multi‐subject diffusion data. NeuroImage 31:1487–1505. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen‐Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM (2004): Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 23 Suppl 1:S208–S219. [DOI] [PubMed] [Google Scholar]
- Smith SM, Nichols TE (2009): Threshold‐free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage 44:83–98. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH (2002): Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. NeuroImage 17:1429–1436. [DOI] [PubMed] [Google Scholar]
- Srivastava S, Bhatia MS, Bhargava SK, Kumari R, Chandra S (2016): A diffusion tensor imaging study using a voxel‐based analysis, region‐of‐interest method to analyze white matter abnormalities in first‐episode, treatment‐naive major depressive disorder. J Neuropsychiatry Clin Neurosci 28:131–137. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Hackney DB, Zhang G, Wehrli SL, Wright AC, O'Brien WT, Uematsu H, Wehrli FW, Selzer ME (2002): Magnetic resonance microimaging of intraaxonal water diffusion in live excised lamprey spinal cord. Proc Natl Acad Sci USA 99:16192–16196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tustison NJ, Avants BB, Cook PA, Kim J, Whyte J, Gee JC, Stone JR (2014): Logical circularity in voxel‐based analysis: Normalization strategy may induce statistical bias. Hum Brain Mapp 35:745–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hecke W, Emsell L, Sunaert S (2016): Diffusion Tensor Imaging: A Practical Handbook.
- Varentsova A, Zhang S, Arfanakis K (2014): Development of a high angular resolution diffusion imaging human brain template. Neuroimage 91:177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versace A, Almeida JR, Quevedo K, Thompson WK, Terwilliger RA, Hassel S, Kupfer DJ, Phillips ML (2010): Right orbitofrontal corticolimbic and left corticocortical white matter connectivity differentiate bipolar and unipolar depression. Biol Psychiatry 68:560–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos SB, Jones DK, Jeurissen B, Viergever MA, Leemans A (2012): The influence of complex white matter architecture on the mean diffusivity in diffusion tensor MRI of the human brain. NeuroImage 59:2208–2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, Charlson FJ, Norman RE, Flaxman AD, Johns N, Burstein R, Murray CJ, Vos T (2013): Global burden of disease attributable to mental and substance use disorders: Findings from the Global Burden of Disease Study 2010. Lancet 382:1575–1586. [DOI] [PubMed] [Google Scholar]
- Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE (2014): Permutation inference for the general linear model. NeuroImage 92:381–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F, Tang Y, Xu K, Kong L, Sun W, Wang F, Kong D, Li Y, Liu Y (2011): Whiter matter abnormalities in medication‐naive subjects with a single short‐duration episode of major depressive disorder. Psychiatry Res 191:80–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalesky A (2011): Moderating registration misalignment in voxelwise comparisons of DTI data: A performance evaluation of skeleton projection. Magn Reson Imag 29:111–125. [DOI] [PubMed] [Google Scholar]
- Zhu X, Wang X, Xiao J, Zhong M, Liao J, Yao S (2011): Altered white matter integrity in first‐episode, treatment‐naive young adults with major depressive disorder: A tract‐based spatial statistics study. Brain Res 1369:223–229. [DOI] [PubMed] [Google Scholar]
- Zou K, Huang X, Li T, Gong Q, Li Z, Ou‐yang L, Deng W, Chen Q, Li C, Ding Y, Sun X (2008): Alterations of white matter integrity in adults with major depressive disorder: A magnetic resonance imaging study. J Psychiatry Neurosci 33:525–530. [PMC free article] [PubMed] [Google Scholar]
- Zuo N, Fang J, Lv X, Zhou Y, Hong Y, Li T, Tong H, Wang X, Wang W, Jiang T (2012): White matter abnormalities in major depression: A tract‐based spatial statistics and rumination study. PLoS One 7:e37561. [DOI] [PMC free article] [PubMed] [Google Scholar]


