Abstract
The peri‐infarct cortex (PIC) is the site of long‐term physiologic changes after ischemic stroke. Traditional methods for delineating the peri‐infarct gray matter (GM) have used a volumetric Euclidean distance metric to define its extent around the infarct. This metric has limitations in the case of cortical stroke, i.e., those where ischemia leads to infarction in the cortical GM, because the vascularization of the cerebral cortex follows the complex, folded topology of the cortical surface. Instead, we used a geodesic distance metric along the cortical surface to subdivide the PIC into equidistant rings emanating from the infarct border and compared this new approach to a Euclidean distance metric definition. This was done in 11 patients with [F‐18]‐Flumazenil ([18‐F]‐FMZ) positron emission tomography (PET) scans at 2 weeks post‐stroke and at 6 month follow‐up. FMZ is a PET radiotracer with specific binding to the alpha subunits of the type A γ‐aminobutyric acid (GABAA) receptor. Additionally, we used partial‐volume correction (PVC) of the PET images to compensate for potential cortical thinning and long‐term neuronal loss in follow‐up images. The difference in non‐displaceable binding potential (BPND) between the stroke unaffected and affected hemispheres was 35% larger in the geodesic versus the Euclidean peri‐infarct models in initial PET images and 48% larger in follow‐up PET images. The inter‐hemispheric BPND difference was approximately 17–20% larger after PVC when compared to uncorrected PET images. PET studies of peri‐infarct GM in cortical strokes should use a geodesic model and include PVC as a preprocessing step. Hum Brain Mapp 38:326–338, 2017. © 2016 Wiley Periodicals, Inc.
Keywords: PET, partial‐volume effects, receptor mapping, ischemic stroke, neuronal density
Abbreviations
- GABAA
γ‐Aminobutyric acid
- GM
Gray matter
- PET
Positron emission tomography
- PIC
Peri‐infarct cortex
- PVC
Partial‐volume correction
- PVE
Partial‐volume effects
- WM
White matter
INTRODUCTION
Long‐term physiologic changes occur after ischemic stroke in morphologically preserved gray matter (GM) surrounding the core of ischemic brain parenchyma, termed peri‐infarct cortex (PIC). This region can be defined along morphologic or physiologic criteria. When using a physiologic definition of the PIC, it is typically termed the “penumbra” and refers to cortex where cerebral blood flow is reduced below the level necessary for neuronal function, but above that needed for maintaining neuronal integrity [Astrup et al., 1981]. However, the PIC can also be defined as cortex that has undergone morphologic change resulting from the stroke.
Changes in the PIC are various, depending on the location of the infarct, time post‐stroke and the patient's use of rehabilitative therapy [Jones et al., 2009; Nudo, 2007; Nudo et al., 1996]. Whereas some of the physiologic changes in the PIC are adaptative, others are deleterious to the patient. An infarct in the somatosensory cortex results in an adaptative remapping of the body representation in this region through a decrease in the number of dendritic spines and an increase in axonal sprouting [Brown et al., 2009; Carmichael et al., 2001; Florence et al., 1998; Murphy and Corbett, 2009; Nudo and Milliken, 1996; Nudo et al., 1996; Stroemer et al., 1998; Ueno et al., 2012]. Similarly, gene expression related to axonal sprouting is up regulated in the PIC [Carmichael et al., 2005]. Other studies have demonstrated neuroinflammatory changes in the PIC post‐stroke [Gulyás et al., 2012; Price et al., 2006; Schroeter et al., 2009] and argued that this persistent neuroinflammation may be related to the development of post‐stroke cognitive impairment [Ceulemans et al., 2010; Thiel et al., 2014]. Changes in the PIC can develop over several months post‐stroke in both rat models and humans [Fink et al., 1993; Kim et al., 2014; Nudo, 2007; Zepper et al., 2012]. Previous studies have shown evidence of neuronal loss outside of the infarct core, both in post‐mortem studies [Nedergaard, 1987; Nedergaard et al., 1984, 1986; Torvik and Svindland, 1986] and in‐vivo [Guadagno et al., 2008; Nakagawara et al., 1997; Saur et al., 2006; Zepper et al., 2012].
The PIC may be useful for predicting patient outcomes, monitoring the evolution of the infarct, and may be an important site for post‐stroke therapy. Decreased FMZ in the PIC has been shown to correlate with decreased oxygen consumption and eventual infarction [Heiss et al., 1997]. An accurate measure of the extent of the PIC may be useful for predicting the patient's outcome and defining the region around the infarct that would benefit from therapeutic intervention. The size of penumbral PIC appears to be inversely correlated with functional outcome [Furlan et al., 1996]. For stroke therapy, electrical stimulation of PIC in nonhuman primates resulted in improved motor performance [Plautz et al., 2003].
When the PIC is taken as the region surrounding the infarct that is morphologically preserved after ischemia, it is typically defined by delineating a region surrounding the infarct using an Euclidean distance metric [Cramer et al., 2006; Heiss et al., 1992; Markus et al., 2003; Price et al., 2006; Sette et al., 1993; Zepper et al., 2012]. An Euclidean‐based PIC is limited when defining PIC in cortical infarcts, because the vascularization of the cerebral cortex follows the complex, folded topology of the cortical surface. That is, two points on neighbouring gyri may be close together in 3D space, but much further from one another when measuring the geodesic distance along the 2D cortical surface. We therefore propose a new method that uses the geodesic distance along a 2D representation of the cortical mantle to define the PIC. Our method allows the PIC to be segmented into rings based on the distance from the infarct and at the same time to study the effect of ischemia at regular intervals outwards from the infarct core.
In positron emission tomography (PET), regions that are less than approximately twice the resolution of the scanner are subject to partial‐volume effects (PVE) [Hoffman et al., 1979]. In regions of high radiotracer concentration, PVE result in a decrease in the measured radiotracer concentration because a portion of the signal “spills‐out” to neighboring regions. Cortical thickness is generally between 3–4 mm and thus tends to be less than or, in the case of high‐resolution PET scanners [Wienhard et al., 2002], just on the cusp of what the PET scanner can measure without spill‐out effects. Thus radiotracer concentrations in the cortical surface may be underestimated due to PVE resulting from cortical atrophy. PVE may also be increased by particularly low radiotracer binding in the infarct. The PIC may also be subject to important “spill‐in” PVE because reduced radiotracer binding in the PIC may lead an increased proportion in signal from adjacent healthy tissue spilling‐in.
PVC is of particular concern in the case of acute focal ischemia, because this has been shown to lead to selective neuronal loss [Baron et al., 2014; Carrera et al., 2011; Guadagno et al., 2008; Hatazawa et al., 1995; Nedergaard, 1987; Nedergaard et al., 1984; Sasaki et al., 1997; Saur et al., 2006; Sette et al., 1993] and may result in decreased cortical thickness [Li et al., 2014]. Using a GM representation extracted from MRI it is possible to perform PVC for potential spill‐out from the cortical GM.
It is the aim of this study to test the hypothesis that geodesic distances, which are based on more theoretically sound assumptions of cortical vasculature, provide significantly different results than obtained with an Euclidean distance metric for defining the PIC. Distance profiles of BPND of [18‐F]‐FMZ were created on PET images from patients with ischemic stroke using both metrics. [18‐F]‐FMZ was used because it binds to γ‐aminobutyric acid (GABAA) receptors that are found in large and uniform quantities in the cortical GM and thus serves as a surrogate marker for neuronal loss [Heiss et al., 1997]. To assess if there was any difference between the BPND distance profiles, we compared the sensitivity of each method to detect a difference between the stroke affected and unaffected hemispheres. The BPND distance profiles produced with both metrics were also compared directly to see if they were significantly different from one another. Additionally, we evaluated the effect of PVC on the BPND measured within the PIC using anatomic information extracted from MRI. Cortical thickness maps were calculated to determine if there was significant cortical atrophy between initial and follow‐up images and evaluate its effect on PVC.
METHOD
Subjects
All participants were prospectively recruited from the Jewish General Hospital and Montreal General Hospital acute stroke units between March 2012 to October 2014. This study was approved by McGill Faculty of Medicine Institutional Review Board. All participants were enrolled after informed consent in accordance with regulations for human participant's studies.
Eleven participants were enrolled if they presented with first ever anterior circulation ischemic stroke within 31 days post stroke onset, had a neurological deficit defined on National Institutes of Health Stroke Scale (NIHSS) score ≤5 upon entry to the study, were between 40 and 90 years of age and able to speak either English or French (Table 1). Patients were excluded if there was evidence of alcoholism or psychoactive drug use, benzodiazepine use ≤24 hour prior to the PET study, if they were unable to complete the initial MRI or PET studies or had a history of either epilepsy, psychiatric or neurodegenerative diseases. One participant withdrew from the study after the initial scan and was excluded from analysis when initial and follow‐up PET images were compared directly.
Table 1.
Demographic and clinical information for enrolled patients
| Patient | Age | Sex | Affected vascular territory | Initial scan time (Days) | F/U scan time (Months) | Initial infarct volume (cm3) | F/U infarct volume (cm3) |
|---|---|---|---|---|---|---|---|
| 1 | 66 | M | MCA | 21 | 5.53 | 65 | 75 |
| 2 | 87 | M | MCA | 13 | 5.63 | 0.46 | 0.36 |
| 3 | 71 | M | MCA | 18 | 5.93 | 4.6 | 4 |
| 4 | 63 | F | MCA | 14 | 6.16 | 3.7 | 3.8 |
| 5 | 54 | M | ACA | 10 | 5.93 | 109 | 104 |
| 6 | 62 | M | MCA | 21 | 5.93 | 5 | 2.3 |
| 7 | 53 | M | MCA | 27 | 6.83 | 2.5 | 1.4 |
| 8 | 50 | M | MCA | 15 | 6.03 | 3.8 | 3 |
| 9 | 69 | F | MCA | 27 | 5.93 | 99.6 | 82.5 |
| 10 | 72 | F | MCA | 17 | NA | 20.2 | NA |
| 11 | 55 | F | MCA | 31 | 7 | 6.5 | 5.5 |
Enrolled patients were evaluated in the sub‐acute phase of their stroke and on 6 months follow‐up images using a clinical assessment battery for motor and cognitive recovery, and with both MRI and PET scans to measure the peri‐infarct cortical thickness and estimate the neuronal cell density changes, respectively.
T1 and Flair MR Acquisition
All MR images were acquired on a SIEMENS MAGNETOM TrioTim syngo MR B17. The Fluid attenuated inversion recovery (FLAIR)—a T2 weighted MR image with attenuated CSF signal‐sequence used a TR of 9000 ms, TE of 75 ms and TI of 2500 ms with a flip angle of 150 degrees to acquire 60 transaxial slices with thickness of 2 mm and a base resolution of 192 × 192 voxels in plane. The T1‐weighted data set for surface extraction was acquired using a MPRAGE sequence with TR 2300 ms, TE 2.98 ms, TI 9 ms and 9 degree flip angle comprising 160 sagittal slices with isotropic 1mm voxels and 256 × 256 voxels in plane resolution.
The FLAIR image was upsampled to the resolution of the T1 image using linear interpolation and blurred with a 2 mm FWHM Gaussian kernel to attenuate noise.
CIVET
CIVET is an image processing pipeline that generates mesh representations of the cortical GM from T1 MR images. CIVET uses the non‐parametric N3 method to correct MR field non‐uniformity [Sled et al., 1998]. The MRI is then transformed to MNI stereotaxic space of the ICBM 152 6th generation non‐linear brain atlas (Mazziotta et al., 2001), using a 12 parameter affine transformation [Collins et al., 1994]. Spatially normalized images are then segmented into three tissue classes: GM, WM and cerebral spinal fluid using INSECT [Zijdenbos et al., 1998], a discrete classifier, as well as a probabilistic classifier [Tohka et al., 2004]. The Constrained Laplacian Anatomic Segmentation using Proximity algorithm generates a mesh representation of the cortical GM using two deformable mesh models consisting of 81924 vertices connected to form triangles. Cortical thickness is measured by taking the distance between the mesh fitted to the WM‐GM boundary and that fitted to the GM‐pia matter boundary.
Volumetric cortical GM binary images were created from the WM‐GM and GM‐Pia mater surface meshes using a ray‐tracing algorithm to identify the voxels that lie between the two surface meshes [Funck et al., 2014]. The GM masks and WM were transformed from MNI stereotaxic space to T1 native using nearest‐neighbor interpolation.
PET Acquisition
[18‐F]‐FMZ scans were obtained for all participants with an ECAT HRRT PET scanner in list mode (Siemens Medical Solutions, Knoxville, TN, USA) [Wienhard et al., 2002]. The ECAT HRRT is a dedicated full 3D high resolution brain scanner, with a field view of 25.2 cm (axially) and 31.2 cm (diameter), and has a spatial resolution of between 2.3 mm and 2.8 mm FWHM and enables data acquisition with high spatial resolution combined with high sensitivity. In addition, the use of two crystal layers (LSO/LYSO) permits photon detection with depth‐of‐interaction information. After a transmission scan for attenuation correction (137Cs‐source), approximately 370 MBq [18‐F]‐FMZ were injected intravenously as a slow bolus over 60 s. The list mode data were acquired for 60 min after injection and were subsequently binned into 2209 sinograms (each of size 256 radial bins × 288 azimuthal bins) using span 9 compression for a total of 17 time frames (40 s, 20 s, 2 × 30 s, 360 s, 4 × 50 s, 3 × 300 s, and 3 × 600 s), resulting in images with a voxel size of 1.22 × 1.22 × 1.22 mm3. Fully 3D FBP by 3D reprojection (3D RP) was carried out with a Hamming windowed Colsher filter (alpha = 0.5, cut off at the Nyquist frequency). The PET image was co‐registered and resampled using linear interpolation to the native T1 using a six parameter rigid transformation.
Partial‐Volume Correction
PET images were partial‐volume corrected using the idSURF algorithm [Funck et al., 2014]. IdSURF is an iterative algorithm based on Lucy‐Richardson deconvolution and attempts to make subsequently better guesses of the true tracer‐distribution [Lucy, 1974; Richardson, 1972]. The algorithm proceeds by filtering an estimate of the true tracer‐distribution, initially the PET image itself, with a model of the PET scanner point‐spread function; in this case a Gaussian filter (FWHM = 2.5). The difference between the original PET image and the filtered test image is used to update the estimate of the true tracer distribution. Anatomically constrained filtering is performed by taking the local mean of voxels within predefined anatomic regions. Whereas traditional linear filtering, e.g., Gaussian smoothing, are effective at removing noise, they also smooth between anatomic regions and thus increase PVE. By constraining the filtering to within anatomic regions, idSURF controls for noise while preserving edges between these regions. The infarct core, GM and WM were treated as separate anatomic regions for this analysis. Both the uncorrected PET and idSURF corrected images were compared to evaluate the effect of PVC on measured radiotracer concentrations in the PIC.
Tracer Kinetic Analysis
Tracer kinetic analysis was used to quantify specific tracer binding across patients. We computed the BPND maps from the PET images. BPND of a reversibly binding radioligand is related to the maximum available concentration of its receptor (Bmax) accounting for the binding affinity of the tracer and the fraction of non‐displaceable binding (i.e., tracer irreversibly bound to other molecules than the receptor) in the tissue. Parametric images of BPND were created using the Logan plot method [Logan et al., 1996], with a white matter (WM) reference region and a start time of 300 seconds for the linear regression. To avoid any potential spill‐over signal between the WM and GM, the WM reference region was eroded by 4 voxels, i.e., 4 mm.
Surface Infarct ROI
Volumetric ROIs were drawn on the FLAIR MR images acquired for each participant for both initial and follow‐up scans and independently verified by two stroke neurologists (Fig. 1). Initial surface infarct masks were produced by nearest neighbour interpolation of the volumetric infarct ROI with the mid‐surface produced using CIVET (Fig. 2.1C). The surface ROIs were then defragmented to remove isolated points outside, as well as holes within, the main infarct. All regions smaller than 25% of the largest ROI were removed.
Figure 1.
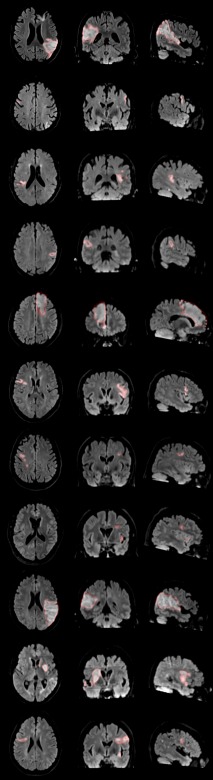
The infarcts were manually identified and drawn independently by two stroke neurologists on the FLAIR images. [Color figure can be viewed at http://wileyonlinelibrary.com.]
Figure 2.

(A) Volumetric masks were drawn by hand on flair MRI. (B) FMZ BPND parametric images show GABAA receptor density. (C) Infarct masks were intersected with surface representations of the cortical GM. (D) Distance maps were produced by calculating geodesic distances from the border of the infarct. (E) Illustrative comparison of distance maps. The Euclidean distance maps were calculated volumetrically, but interpolated onto the participant's cortical surface representation. Both B1/2 and C1/2 show that the Euclidean distance metric produces an inconsistent distance measure that does not increase monotonically. A1/2 show that the Euclidean distance metric underestimates distances compared to the geodesic metric.
Geodesic Peri‐Infarct Rings
Geodesic distances were calculated from the border of the infarct ROI. This produces a surface map where the value at each surface vertex is the minimum distance from the vertex to the border of the infarct (Fig. 2.1D). Distance maps were created using in‐house software that calculates the minimum distances by expanding a closed region outwards along the edges of a surface mesh. The distance maps were segmented into five rings of 3 mm, from 0 to 15 mm from the infarct. The rings were named such that the 3 mm contains vertices from 0 to 3 mm and the 6 mm ring contains vertices from 3 mm to 6 mm from the infarct core, and so forth for the other rings.
The PIC ring width of 3 mm was chosen because this approaches the spatial resolution of 2.5 mm FWHM of the HRRT [Wienhard et al., 2002]. Histological studies have shown decreased neuronal density up to 10 mm from the infarct core [Torvik and Svindland, 1986]. Hence the 9–12 mm PIC ring was the maximum distance at which BPND was expected to be reduced in the affected hemisphere. The maximum distance of 15 mm was therefore chosen to include a 12–15 mm PIC ring that would, presumably, include healthy tissue.
Geodesic PIC rings for the contralateral hemisphere were produced by reflecting the surface mesh along the midline of the x axis. The reflected surface mesh was then intersected with the infarct ROI to produce a surface ROI of the infarct in the contralateral hemisphere. Geodesic distances were calculated from the infarct reflected onto the unaffected hemisphere ROI and segmented to produce contralateral PIC rings.
Euclidean Peri‐Infarct Rings
Whereas the geodesic PIC rings were defined on a surface, the Euclidean PIC rings were volumetric and produced in a similar manner as [Zepper et al., 2012]. The first step was to intersect the infarct and GM ROI. Euclidean distance maps from the GM infarct were than calculated. As with the geodesic peri‐infarct rings, the Euclidean distance maps were segmented into five rings of 3 mm, from 0 to 15 mm from the infarct. These rings were then intersected with a binary GM image to eliminate voxels outside of the GM. Euclidean PIC rings for homologous regions in the unaffected hemisphere were created by reflecting the infarct ROI along the x‐axis and intersecting it with a volumetric binary image of the GM. Euclidean distances were then calculated in the unaffected hemisphere and segmented into Euclidean PIC rings.
Statistical Methods
The effect of the ischemic insult on the PIC was measured by subtracting BPND in the unaffected hemisphere by that of the affected hemisphere to give the inter‐hemispheric difference of BPND (IHDBP =BPND Unaffected H.−BPND Affected H). In all but one of the two‐way repeated measures (2RM) ANOVA described below, the geodesic PIC rings were used as one of the two factors.
The following tests were performed on initial and follow‐up images separately. The effect on BPND of (1) PIC ring and (2) cerebral hemisphere (i.e., unaffected vs ischemia affected) was tested using a 2RM ANOVA. For the purpose of comparison, this same test was performed with the Euclidean peri‐infarct rings. To determine that the two distance metrics produced different results, the 2RM ANOVA used (1) PIC ring and (2) distance metric (i.e., Euclidean vs. geodesic) as factors. Lastly, to evaluate the effect of PVC on IHDBP we performed a 2RM ANOVA with (1) PIC ring and (2) PVC method (i.e., uncorrected vs idSURF) as factors.
To determine if there was a change in the IHDBP between initial and follow‐up images, a 2RM ANOVA was performed with (1) PIC ring and (2) scanning session as factors. This same test was also performed for the cortical thickness values extracted from the initial and follow‐up MRI.
If the interaction effect of the 2RM ANOVA reached significance at the 5% level, post‐hoc tests were performed on each PIC ring to test if there was a significant difference in the measured response variable between the two‐levels of the factor. The purpose of the post‐hoc testing was to determine which PIC rings contributed to the significant interaction effect of the ANOVA. This was done using Wilcoxon signed‐rank [Wilcoxon, 1945], with P‐values adjusted for multiple comparisons with Hochberg's method [Hochberg, 1988]. The correction for multiple comparisons was performed independently for each set of ANOVA, because these tests asking conceptually independent questions about the data and do not use the same set of response variables.
RESULTS
Surface Geodesic Distances Show Decreased BPND Closer to Infarct
The results of the surface PIC analysis indicate first that BPND is lower in the affected hemisphere compared to the unaffected hemisphere in both the initial and follow‐up images. The difference in BPND between hemispheres was significant both in the main effect of hemisphere (Initial: P < 0.01, F = 16.15; Follow‐Up: P < 0.001; F = 20.98) and in the interaction between hemisphere and PIC ring in both initial and follow‐up images (Initial: P < 0.001, F = 18.78; Follow‐Up: P < 0.001; F = 20.34). Post‐hoc testing revealed that the difference in BPND between hemispheres is significant from 0 mm to 6 mm in the initial images and 0 mm to 15 mm in the follow‐up images (Fig. 3).
Figure 3.
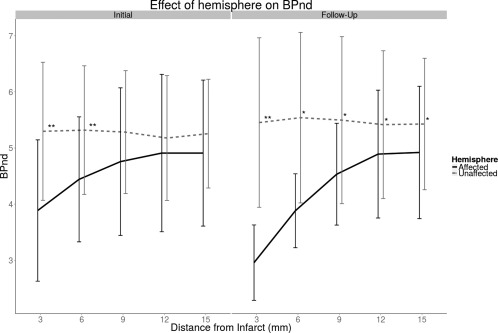
Average BPND measured in affected and unaffected hemispheres for initial and follow‐up images. BPND is reduced closer to the infarct and gradually increases to close to normal levels further away. ‘*’: P < 0.05; ‘**’: P < 0.01.
There appears to have been a significant increase in IHDBP from initial PET images to follow‐up. The 2RM ANOVA showed an overall significant difference in IHDBP in the PIC rings between initial and follow‐up images (P < 0.01, F = 4.88). However, using the Wilcoxon signed‐rank test for post‐hoc testing, none of the individual PIC were significantly different in IHDBP between initial and follow‐up images.
Geodesic and Euclidean Distances Produce Different Results
The distance profiles produced with the Euclidean PIC rings do not show the gradual increase in BPND in the stroke affected hemisphere that was observed with the geodesic profiles (Fig. 4). In the main effect of hemisphere, the difference in BPND between hemispheres was trending towards significant in the initial scanning session and significant in the follow‐up session (Initial: P < 0.1, F = 4.59; Follow‐Up: P < 0.01; F = 11.7). There was a significant interaction effect between hemisphere and PIC ring in both initial and follow‐up images (Initial: P < 0.001, F = 15.06; Follow‐Up: P < 0.001; F = 19.95). In post‐hoc testing, however, the difference between hemispheres was only significant in the 0–3 mm PIC rings.
Figure 4.
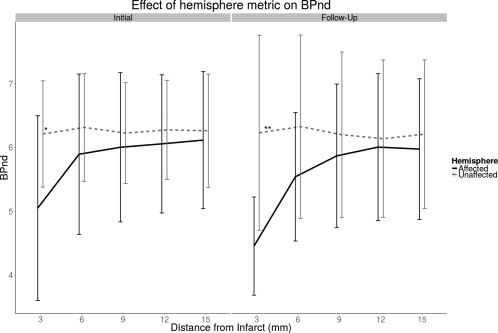
Using an Euclidean distance metric, BPND in the affected hemisphere is generally lower than in the unaffected hemisphere. However only in the 3 mm ring is there a statistically significant difference between hemispheres. '*': P < 0.05; ''**': P < 0.01.
We compared the Euclidean IHDBP profiles to the IHDBP profiles produced with a geodesic distance metric (Fig. 5, see Fig. 2 for visual example). In the initial and follow‐up scans, there was a significant difference in IHDBP due to the main effect of distance metric (Initial: P < 0.05, F = 6.45; P < 0.01, F = 13.09), but no significant interaction effect between distance metric and peri‐infarct ring. The area under the geodesic IHDBP profile was 35% larger than that under the Euclidean IHDBP profile in the initial images and 48% larger in follow‐up images.
Figure 5.
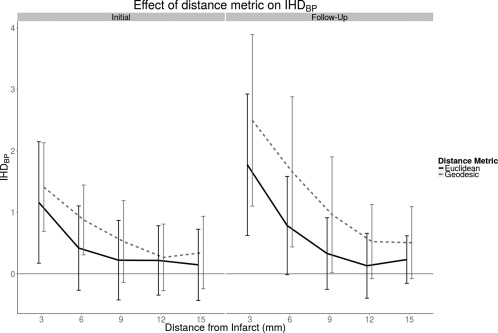
IHDBP is consistently larger with the geodesic masks, particularly in follow‐up images.
Partial‐Volume Correction Increases BPIDH
PVC produces larger IHDBP than observed in uncorrected PET images (Table 2). However, only in follow‐up images was there a significant interaction effect between PIC ring and PVC. In post‐hoc testing, the only significant difference between PVC and uncorrected images was in follow‐up images in the 3 mm PIC ring, but this did not survive correction for multiple comparisons (Fig. 6). PVC IHDBP was found to be 17% larger than uncorrected IHDBP in the initial images and 20% larger in the follow‐up images.
Table 2.
2W‐RM ANOVA testing the effect of PVC on BPND
| Response Variable | Main effect 1 | Main effect 2 | Interaction effect | |
|---|---|---|---|---|
| Scanning Session | PIC ring | PVC | PIC ring × PVC | |
| Initial | BPND | P <0.001, F=19.75 | P <0.05, F = 10.35 | N.S. (P <0.1), F = 2.28 |
| Follow‐up | BPND | P <0.001, F = 26.01 | P <0.05, F = 5.45 | P <0.01, F = 4.61 |
“N.S.”: Not significant.
Figure 6.

IHDBP for PVC and uncorrected PET at initial and follow‐up images. Without PVC, the inter‐hemispheric difference in BPND appears to be underestimated.
Cortical Thickness Is Reduced in Follow‐up Scans
The difference in cortical thickness between affected and unaffected hemispheres was only trending towards significance in the initial cortical thickness maps and was found to be significant in the follow‐up maps (Table 3). The interaction effect between PIC rings and hemisphere was significant in the follow‐up images, but this was not attributable to any specific PIC ring.
Table 3.
2W‐RM ANOVA testing effect of scanning session on cortical thickness (mm)
| Response variable | Main effect 1 | Main effect 2 | Interaction effect | |
|---|---|---|---|---|
| Scanning Session | PIC ring | Hemisphere | PIC ring × Hemisphere | |
| Initial | Cortical thickness (mm) | N.S., F = 1.12 | N.S. (P <0.1), F = 4.86 | N.S., F = 0.63 |
| Follow‐up | Cortical thickness (mm) | P <0.05, F = 2.99 | P <0.05, F = 7.32 | N.S., F = 1.57 |
“N.S.”: Not significant.
DISCUSSION
The PIC is the site of important physiologic changes after ischemic stroke in initially viable cortex and is often defined using a Euclidean distance metric to measure distances from the infarct. We developed a new PIC model that uses geodesic distances measured along a surface model of the cortical GM and used it to analyze [18‐F]‐FMZ PET images of stroke patients. Using the geodesic method results in IHDBP that is 35–48% larger than that obtained with a traditional Euclidean method and is 17–20% larger with PVC.
IHDBP Measured with Geodesic PIC Rings Show Long‐Term Neuronal Cell Loss
Distances measured relative to the cortical vasculature are better modeled with a geodesic distance metric than an Euclidean metric, because the former does not cut across the folds of the cortical surface. Defining the PIC using a geodesic distance metric allows for a more anatomically realistic measurement of changes in BPND along the cortical surface. There was a significant reduction of BPND in the PIC in the affected hemisphere compared to the unaffected hemisphere and this difference was larger closer to the infarct core. This suggests that the effect of the stroke on viable cortex does not have a sharp boundary and should be studied at regular distance intervals from the infarct border rather than in a single region.
IHDBP was significantly larger at follow‐up than in the initial images. [18‐F]‐FMZ binds to GABAA receptors on cortical neurons and is used as a surrogate marker of neuronal density [Heiss et al., 1998; la Fougère et al., 2011]. The increase in IHDBP between initial and follow‐up is consistent with long‐term neuronal cell death in the peri‐infarct grey matter after ischemic stroke. This interpretation is consistent with findings from post‐mortem histological studies of humans with ischemic infarcts [Nedergaard et al., 1984, 1986; Torvik and Svindland, 1986]. In these participants, a transition zone of decreased neuronal density from 0–5 mm (and up to 10 mm in some patients) around the infarct border was observed. It should be noted that these histological studies measured distance from the infarct by projecting distances along the cortical surface onto the outside surface of the brain, excluding the sulcal depths. This distance metric will produce uniformly larger distances than the geodesic distance metric. In the initial PET images we found a decrease in BPND in the affected hemisphere from 0 mm to 6 mm, which was consistent with previous results from histological studies. In the follow‐up images, however, the decrease in BPND in the affected hemisphere extended up to 15 mm, suggesting a larger and more gradual transition zone between infarcted and healthy tissue.
In vivo evidence has also been found for selective neuronal loss in the non‐infarcted penumbra both in FMZ PET [Carrera et al., 2013; Guadagno et al., 2008; Zepper et al., 2012] and [123‐I]‐Iomazenil SPECT [Nakagawara et al., 1997; Saur et al., 2006]; for a review, see [Baron et al., 2014]. The limitation of these methods is that they define the PIC as a single, uniform region and do not reveal changes in BPND within the PIC. The current findings suggest that the PIC is not homogeneous and may vary as a function of the distance from the infarct core.
Previous studies of the effect of stroke on cortical thickness have also reported changes in cortical thickness. Some studies have also reported increases in cortical thickness in regions associated with functional remapping in both acute [Schaechter et al., 2006] and chronic stroke [Brodtmann et al., 2012]. A study of sub‐cortical infarcts from cerebral small vessel disease found long‐term cortical thinning in GM regions connected via WM tracts to the infarct [Duering et al., 2012].
Euclidean PIC Rings Are Less Sensitive to IHDBP
The PIC can be defined morphologically by measuring the distance from the border of the infarct to the surrounding tissue. Traditional approaches have delineated the PIC using a Euclidean distance metric [Cramer et al., 2006; Fink et al., 1993; Heiss et al., 1992; Markus et al., 2003; Price et al., 2006]. These methods start by defining a volumetric ROI of the infarct. The PIC is then defined by identifying points that are within a given distance from the infarct, where the distance is measured as a straight line in 3D space. This is typically done by performing a pixel‐ or voxel‐wise dilation of the infarct to produce PIC rings or spheres. Dilations of the infarct implicitly use a Euclidean metric by assuming that all voxels contained within a dilation are equally far from the infarct.
The geodesic and Euclidean PIC rings produced different BPND profiles. With the Euclidean peri‐infarct rings, unlike the geodesic IHDBP profiles, there was little difference between the stroke affected and unaffected hemispheres beyond the 3 mm ring. The difference between geodesic and Euclidean IHDBP was larger in follow‐up images. The reason for this appears to be that the BPND profiles produced with Euclidean and geodesic PIC rings differ in how they relate to the topology of the cortical surface and its vasculature.
The Euclidean metric measures distances in the PIC as a straight line to the border of the infarct. The limitation of this method is that it cuts across folds in the cortical surface (Fig. 2.1.E and 2.2.E), and thus underestimates the true distance traveled by the vasculature (Fig. 2.1.D and 2.2.D). The result of this underestimation is that beyond 3 mm, where the geodesic and Euclidean rings sample approximately the same voxels, the Euclidean PIC rings include regions that are relatively unaffected by the stroke and thus have normal BPND. Hence, beyond the 3 mm PIC rings, the Euclidean PIC rings exhibited no difference in BPND between the unaffected and stroke affected hemispheres in both initial and follow‐up images.
The surface‐fitting used by CIVET depends on a sharp WM‐GM gradient, which may be eroded or eliminated by the infarction caused by acute ischemia. Visual inspection of the CIVET surfaces revealed that they closely followed the cortical mantle outside of the infarct where the WM‐GM border was preserved. The surfaces did not follow the cortical mantle inside of the infarct, but this region was not used in the present analysis.
Partial‐Volume Correction Results in Higher Sensitivity to Long‐Term Neuronal Cell Loss
In the initial and follow‐up PET images there was significant difference between uncorrected and PVC IHDBP. However, at follow‐up there was a significant interaction between PVC and PIC ring. This suggests that PVEs are a more important confounds in PET‐images acquired 6 months after the stroke.
Despite these concerns, PVC has not been widely implemented in studies involving the PIC and may be a source of bias in results. For example, several PET and SPECT studies have reported decreased binding to GABAA receptors [Carrera et al., 2013; Kim et al., 2014; Nakagawara et al., 1997; Saur et al., 2006; Sette et al., 1993] at several weeks to months post‐stroke. Without PVC it is difficult to conclude whether their results are consistent with those reported here, because it remains possible that their reported decrease in radiotracer binding to GABAA was the result of a change in cortical thickness and not a change in the availability of GABAA receptors.
While PVC is not widely used in studies of the PIC, some studies have used simple PVC algorithms that compensate for spill‐out effects [Guadagno et al., 2008; Zepper et al., 2012]. In the present study we found that spill‐in PVE were particularly important because small but significant changes in radiotracer binding within a region can be obscured by spill‐in radiotracer concentration from adjacent regions.
There are a multitude of factors that come into play when determining whether or not PVC be important in PET images of stroke patients: size of the infarct, presence of cortical thinning, the gradient of radiotracer concentration between the infarct and healthy tissue. It is difficult to know a priori how these will come into play. The use of PVC methods that correct for both spill‐in and spill‐out effects in the analysis may thus reduce the risk of overlooking significant results due to PVE and should be considered in standard data analysis procedures.
The aforementioned studies of GABAA with PET and SPECT use mismatch between an image of the initial hypoperfusion, e.g., from perfusion CT, with the an image of the final infarct, e.g., from FLAIR MRI, and thereby define one large peri‐infarct region [Carrera et al., 2013; Guadagno et al., 2008; Kim et al., 2014; Nakagawara et al., 1997; Saur et al., 2006; Sette et al., 1993]. Our geodesic PIC method is compatible with this approach because the image of the initial hypoperfusion can be mapped onto a surface representation of the cortical GM and used to constrain the spatial extent of the PIC rings. This would ensure that the PIC rings only sample from tissue that is known to be hypoperfused and improve the signal‐to‐noise ratio of the BPND distance profiles.
Limitations
One limitation of the present study was the use of he contra‐lateral hemispheres instead as comparator. Kim et al. [2014] have reported both widespread bilateral decrease in GABAA receptor density in human stroke patients as well as some localized increase in GABAA receptor density in the contralateral peri‐infarct. A global decrease however should not affect the results presented here, because the difference between the hemispheres remains the same given a global reduction in both hemispheres. Moreover, if the peri‐infarct rings of the unaffected hemisphere in this study do include regions with increased GABAA receptor density, then the results would only tend to underestimate the true difference in BPIHD and would only introduce a bias into the conservative direction.
Infarction causes a local reduction in the gradient of the MRI signal between the WM and GM and and thus interferes with the definition of a WM‐GM surface mesh. Without a reliable WM‐GM surface mesh, it is impossible to accurately measure cortical thickness. Visual inspection of the surface meshes revealed that the distortion of the mesh is limited to the infarct and thus had no visible effect on the surrounding PIC.
The most important limitation is the high variability of the size, shape and location of the stroke volumes. The only way to account for this variability would be to use the size or location of the stroke as an additional factor in the statistical analysis. A complex statistical analysis such as this would require additional participants and was not possible in the present study.
Conclusions
In cortical strokes, a geodesic distance metric provides an anatomically realistic method for modeling the PIC because it conforms more closely to the anatomy of the vasculature than traditional, Euclidean distance metric. PVC may be important in the context of cortical ischemic stroke because it gives a more anatomically realistic estimate of BPND and controls for long‐term changes in cortical thickness due to the ischemic insult.
ACKNOWLEDGMENTS
This study was funded by the Canadian Institutes for Health Research (CIHR) grant MOP‐115107 to AT and support from Fonds de Recherche du Québec‐Santé (FRQ‐S) to AT.
REFERENCES
- Astrup J, Siesjo B, Symon L (1981): Thresholds in cerebral ischemia. Stroke 12:723–725. [DOI] [PubMed] [Google Scholar]
- Baron JC, Yamauchi H, Fujioka M, Endres M (2014): Selective neuronal loss in ischemic stroke and cerebrovascular disease. J Cereb Blood Flow Metab 34:2–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodtmann A, Pardoe H, Li Q, Lichter R, Ostergaard L, Cumming T (2012): Changes in regional brain volume three months after stroke. J Neurol Sci 322:122–128. [DOI] [PubMed] [Google Scholar]
- Brown CE, Aminoltejari K, Erb H, Winship IR, Murphy TH (2009): In vivo voltage‐sensitive dye imaging in adult mice reveals that somatosensory maps lost to stroke are replaced over weeks by new structural and functional circuits with prolonged modes of activation within both the peri‐infarct zone and distant sites. J Neurosci 29:1719–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael ST, Wei L, Rovainen CM, Woolsey TA (2001): New patterns of intracortical projections after focal cortical stroke. Neurobiol Dis 8:910–922. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Archibeque I, Luke L, Nolan T, Momiy J, Li S (2005): Growth‐associated gene expression after stroke: Evidence for a growth‐promoting region in peri‐infarct cortex. Exp Neurol 193:291–311. [DOI] [PubMed] [Google Scholar]
- Carrera E, Jones PS, Iglesias S, Guadagno JV, Warburton EA, Fryer TD, Aigbirhio FI, Baron JC (2011): The vascular mean transit time: A surrogate for the penumbra flow threshold? J Cereb Blood Flow Metab 31:1027–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrera E, Jones PS, Morris RS, Alawneh J, Hong YT, Aigbirhio FI, Fryer TD, Adrian Carpenter T, Warburton E. a, Baron JC (2013): Is neural activation within the rescued penumbra impeded by selective neuronal loss? Brain 136:1816–1829. [DOI] [PubMed] [Google Scholar]
- Ceulemans AG, Zgavc T, Kooijman R, Hachimi‐Idrissi S, Sarre S, Michotte Y (2010): The dual role of the neuroinflammatory response after ischemic stroke: Modulatory effects of hypothermia. J Neuroinflammation 7:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC (1994): Automatic 3D intersuject registration fo MR volumetric data in standardized Talairach space. J Comput Assist Tomogr 10:192–205. [PubMed] [Google Scholar]
- Cramer SC, Shah R, Juranek J, Crafton KR, Le V (2006): Activity in the peri‐infarct rim in relation to recovery from stroke. Stroke 37:111–115. [DOI] [PubMed] [Google Scholar]
- Duering M, Righart R, Csanadi E, Jouvent E, Herve D, Chabriat H, Dichgans M (2012): Incident subcortical infarcts induce focal thinning in connected cortical regions. Neurology 79:2025–2028. [DOI] [PubMed] [Google Scholar]
- Fink GR, Herholz K, Pietrzyk U, Huber M, Heiss WD (1993): Peri‐Infarct perfusion in human ischemia: Its relation to tissue metabolism, morphology, and clinical outcome. J Stroke Cerebrovasc Dis 3:123–131. [DOI] [PubMed] [Google Scholar]
- Florence SL, Taub HB, Kaas JH (1998): Large‐scale sprouting of cortical connections after peripheral injury in adult macaque monkeys. Science 282:1117–1121. [DOI] [PubMed] [Google Scholar]
- Funck T, Paquette C, Evans A, Thiel A (2014): Surface‐based partial‐volume correction for high‐resolution PET. Neuroimage 102:674–687. [DOI] [PubMed] [Google Scholar]
- Furlan M, Marchal G, Viader F, Derlon JM, Baron JC (1996): Spontaneous neurological recovery after stroke and the fate of the ischemic penumbra. Ann Neurol 40:216–226. [DOI] [PubMed] [Google Scholar]
- Guadagno JV, Jones PS, Aigbirhio FI, Wang D, Fryer TD, Day DJ, Antoun N, Nimmo‐Smith I, Warburton EA, Baron JC (2008): Selective neuronal loss in rescued penumbra relates to initial hypoperfusion. Brain 131:2666–2678. [DOI] [PubMed] [Google Scholar]
- Gulyás B, Tóth M, Schain M, Airaksinen A, Vas Á, Kostulas K, Lindström P, Hillert J, Halldin C (2012): Evolution of microglial activation in ischaemic core and peri‐infarct regions after stroke: A PET study with the TSPO molecular imaging biomarker [11C]vinpocetine. J Neurol Sci 320:110–117. [DOI] [PubMed] [Google Scholar]
- Hatazawa J, Satoh T, Shimosegawa E, Okudera T, Inugami a, Ogawa T, Fujita H, Noguchi K, Kanno I, Miura S (1995): Evaluation of cerebral infarction with iodine 123‐iomazenil SPECT. J Nucl Med 36:2154–2161. [PubMed] [Google Scholar]
- Heiss WD, Huber M, Fink GR, Herholz K, Pietrzyk U, Wagner R, Wienhard K (1992): Progressive derangement of periinfarct viable tissue in ischemic stroke. J Cereb Blood Flow Metab 12:193–203. [DOI] [PubMed] [Google Scholar]
- Heiss WD, Graf R, Fujita T, Ohta K, Bauer B, Lottgen J, Wienhard K (1997): Early detection of irreversibly damaged ischemic tissue by flumazenil positron emission tomography in cats. Stroke 28:2045–2052. [DOI] [PubMed] [Google Scholar]
- Heiss WD, Grond M, Thiel a, Ghaemi M, Sobesky J, Rudolf J, Bauer B, Wienhard K (1998): Permanent cortical damage detected by flumazenil positron emission tomography in acute stroke. Stroke 29:454–461. [DOI] [PubMed] [Google Scholar]
- Hochberg Y (1988): A sharper Bonferroni procedure for multiple tests of significance. Biometrika 75:800–802. [Google Scholar]
- Hoffman EJ, Huang SC, Phelps ME (1979): Quantitation in positron emission computed tomography: 1. Effect of object size. J Comput Assist Tomogr 3:299–308. [DOI] [PubMed] [Google Scholar]
- Jones TA, Allred RP, Adkins DL, Hsu JE, O'Bryant A, Maldonado MA (2009): Remodeling the brain with behavioral experience after stroke. Stroke 40:S136–S138. S139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YK, Yang EJ, Cho K, Lim JY, Paik NJ (2014): Functional recovery after ischemic stroke is associated with reduced GABAergic inhibition in the cerebral cortex: A GABA PET study. Neurorehabil Neural Repair 28:576–583. [DOI] [PubMed] [Google Scholar]
- la Fougère C, Grant S, Kostikov A, Schirrmacher R, Gravel P, Schipper HM, Reader A, Evans A, Thiel A (2011): Where in‐vivo imaging meets cytoarchitectonics: The relationship between cortical thickness and neuronal density measured with high‐resolution [18F]flumazenil‐PET. Neuroimage 56:951–960. [DOI] [PubMed] [Google Scholar]
- Li Q, Pardoe H, Lichter R, Werden E, Raffelt A, Cumming T, Brodtmann A (2014): Cortical thickness estimation in longitudinal stroke studies: A comparison of 3 measurement methods. NeuroImage Clin 8:526–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL (1996): Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab 16:834–840. [DOI] [PubMed] [Google Scholar]
- Lucy LB (1974): An iterative technique for the rectification of observed distributions. Astron J 79:745. [Google Scholar]
- Markus R, Reutens DC, Kazui S, Read S, Wright P, Chambers BR, Sachinidis JI, Tochon‐Danguy HJ, Donnan GA (2003): Topography and temporal evolution of hypoxic viable tissue identified by 18F‐fluoromisonidazole positron emission tomography in humans after ischemic stroke. Stroke 34:2646–2652. [DOI] [PubMed] [Google Scholar]
- Mazziotta J, Toga A, Evans A, Fox P, Lancaster J, Zilles K, Woods R, Paus T, Simpson G, Pike B, Holmes C, Collins L, Thompson P, MacDonald D, Iacoboni M, Schormann T, Amunts K, Palomero‐Gallagher N, Geyer S, Parsons L, Narr K, Kabani N, Goualher GL, Boomsma D, Cannon T, Kawashima R, Mazoyer B (2001): A probabilistic atlas and reference system for the human brain: International Consortium for Brain Mapping (ICBM). Philos Trans R Soc B Biol Sci 356:1293–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TH, Corbett D (2009): Plasticity during stroke recovery: From synapse to behaviour. Nat Rev Neurosci 10:861–872. [DOI] [PubMed] [Google Scholar]
- Nakagawara J, Sperling B, Lassen NA (1997): Incomplete brain infarction of reperfused cortex may be quantitated with iomazenil. Stroke 28:124–132. [DOI] [PubMed] [Google Scholar]
- Nedergaard M (1987): Neuronal injury in the infarct border: A neuropathological study in the rat. Acta 267–274. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Astrup J, Klinken L (1984): Cell density and cortex thickness in the border zone surrounding old infarcts in the human brain. Stroke 15:1033–1039. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Vorstrup S, Astrup J (1986): Cell density in the border zone around old small human brain infarcts. Stroke 17:1129–1137. [DOI] [PubMed] [Google Scholar]
- Nudo RJ (2007): Postinfarct cortical plasticity and behavioral recovery. Stroke 38:840–845. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Milliken GW (1996): Reorganization of movement representations in primary motor cortex following focal ischemic infarcts in adult squirrel monkeys. J Neurophysiol 75:2144–2149. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Wise BM, SiFuentes F, Milliken GW (1996): Neural substrates for the effects of rehabilitative training on motor recovery after ischemic infarct. Science 272:1791–1794. [DOI] [PubMed] [Google Scholar]
- Plautz EJ, Barbay S, Frost SB, Friel KM, Dancause N, Zoubina EV, Stowe AM, Quaney BM, Nudo RJ (2003): Post‐infarct cortical plasticity and behavioral recovery using concurrent cortical stimulation and rehabilitative training: A feasibility study in primates. Neurol Res 25:801–810. [DOI] [PubMed] [Google Scholar]
- Price CJS, Wang D, Menon DK, Guadagno JV, Cleij M, Fryer T, Aigbirhio F, Baron JC, Warburton EA (2006): Intrinsic activated microglia map to the peri‐infarct zone in the subacute phase of ischemic stroke. Stroke 37:1749–1753. [DOI] [PubMed] [Google Scholar]
- Richardson WH (1972): Bayesian‐based iterative method of image restoration. J Opt Soc Am 62:55. [Google Scholar]
- Sasaki M, Ichiya Y, Kuwabara Y, Yoshida T, Fukumura T, Masuda K (1997): Benzodiazepine receptors in chronic cerebrovascular disease: Comparison with blood flow and metabolism. J Nucl Med 38:1693–1698. [PubMed] [Google Scholar]
- Saur D, Buchert R, Knab R, Weiller C, Röther J (2006): Iomazenil‐single‐photon emission computed tomography reveals selective neuronal loss in magnetic resonance‐defined mismatch areas. Stroke 37:2713–2719. [DOI] [PubMed] [Google Scholar]
- Schaechter JD, Moore CI, Connell BD, Rosen BR, Dijkhuizen RM (2006): Structural and functional plasticity in the somatosensory cortex of chronic stroke patients. Brain 129:2722–2733. [DOI] [PubMed] [Google Scholar]
- Schroeter M, Dennin M. a, Walberer M, Backes H, Neumaier B, Fink GR, Graf R (2009): Neuroinflammation extends brain tissue at risk to vital peri‐infarct tissue: A double tracer [11C]PK11195‐ and [18F]FDG‐PET study. J Cereb Blood Flow Metab 29:1216–1225. [DOI] [PubMed] [Google Scholar]
- Sette G, Baron JC, Young AR, Miyazawa H, Tillet I, Barré L, Travère JM, Derlon JM, MacKenzie ET (1993): In vivo mapping of brain benzodiazepine receptor changes by positron emission tomography after focal ischemia in the anesthetized baboon. Stroke 24:2046–2057; discussion 2057–8. [DOI] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC (1998): A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging 17:87–97. [DOI] [PubMed] [Google Scholar]
- Stroemer RP, Kent TA, Hulsebosch CE, Feeney DM (1998): Enhanced neocortical neural sprouting, synaptogenesis, and behavioural recovery with D‐amphetamine therapy after neocortical infarction in rats. Stroke 29:2381–2395. [DOI] [PubMed] [Google Scholar]
- Thiel A, Cechetto DF, Heiss WD, Hachinski V, Whitehead SN (2014): Amyloid burden, neuroinflammation, and links to cognitive decline after ischemic stroke. Stroke 2825–2829. [DOI] [PubMed] [Google Scholar]
- Tohka J, Zijdenbos A, Evans A (2004): Fast and robust parameter estimation for statistical partial volume models in brain MRI. Neuroimage 23:84–97. [DOI] [PubMed] [Google Scholar]
- Torvik A, Svindland A (1986): Is there a transitional zone between brain infarcts and the surrounding brain? A histological study. Acta Neurol Scand 74:365–370. [DOI] [PubMed] [Google Scholar]
- Ueno Y, Chopp M, Zhang L, Buller B, Liu Z, Lehman NL, Liu XS, Zhang Y, Roberts C, Zhang ZG (2012): Axonal outgrowth and dendritic plasticity in the cortical peri‐infarct area after experimental stroke. Stroke 43:2221–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wienhard K, Schmand M, Casey ME, Baker K, Bao J, Eriksson L, Jones WF, Knoess C, Lenox M, Lercher M, Luk P, Michel C, Reed JH, Richerzhagen N, Treffert J, Vollmar S, Young JW, Heiss WD, Nutt R (2002): The ECAT HRRT: Performance and first clinical application of the new high resolution research tomograph. IEEE Trans Nucl Sci 49:104–110. [Google Scholar]
- Wilcoxon F (1945): Individual comparisons by ranking methods. Biometrics Bull 1:80–83. [Google Scholar]
- Zepper P, Funck T, la Fougere C, Kostikov A, Schirrmacher R, Thiel A (2012): Imaging delayed cell death in subacute stroke with high‐resolution 18F‐Flumazenil. Soc Nucl Med Annu Meet Abstr 53:37. [Google Scholar]
- Zijdenbos AP, Forghani R, Evans AC. 1998. Automatic quantification of MS lesions in 3D MRI brain data sets: Validation of INSECT, in: MICCAI ′98 Proceedings of the First International Conference on Medical Image Computing and Computer‐Assisted Intervention. Springer‐Verlag, pp. 439–448. [Google Scholar]


