Abstract
Sport concussion is associated with disturbances in brain function in the absence of gross anatomical lesions, and may have long‐term health consequences. Diffusion‐weighted magnetic resonance imaging (MRI) methods provide a powerful tool for investigating alterations in white matter microstructure reflecting the long‐term effects of concussion. In a previous study, diffusion tensor imaging (DTI) showed that athletes with a history of concussion had elevated fractional anisotropy (FA) and reduced mean diffusivity (MD) parameters. To better understand these effects, this study compared DTI results to neurite orientation dispersion and density imaging (NODDI), which was used to estimate the intracellular volume fraction (V IC) and orientation dispersion index (ODI). Sixty‐eight (68) varsity athletes were recruited, including 37 without a history of concussion and 31 with concussion >6 months prior to imaging. Univariate analyses showed elevated FA and decreased MD for concussed athletes, along with increased V IC and reduced ODI, indicating greater neurite density and coherence of neurite orientation within white matter. Multivariate analyses also showed that for athletes with a history of concussion, white matter regions with increased FA had increased V IC and decreased ODI, with greater effects among athletes who were imaged a longer time since their last concussion. These findings enhance our understanding of the relationship between the biophysics of water diffusion and concussion neurobiology for young, healthy adults. Hum Brain Mapp 38:4201–4211, 2017. © 2017 Wiley Periodicals, Inc.
Keywords: concussion, white matter, diffusion tensor imaging, NODDI
INTRODUCTION
Concussion is a clinical syndrome in which biomechanical injury leads to altered brain function, with impairments in physical function, cognition, and emotion regulation [McCrory et al., 2013]. Although these symptoms resolve within 7–10 days for most adults, there is mounting evidence of potential long‐term consequences of concussion. In the sport context, athletes with a history of concussion are at elevated risk of reinjury [Guskiewicz et al., 2003], along with increased long‐term risk of depression, memory problems and mild cognitive impairment [Guskiewicz et al., 2005, 2001]. An emerging body of neuroimaging research also shows differences in the brains of athletes with a history of concussion compared to those without prior injury, although detailed characterization of long‐term neurobiological alterations remains at an early stage.
Concussion is characterized by altered brain function in the absence of gross anatomical lesions. Nonetheless, advanced magnetic resonance imaging (MRI) has revealed subtle differences in neural microstructure associated with concussion and mild traumatic brain injury (TBI). The properties of water diffusion in brain tissue lend themselves to the detection of disrupted microstructure using the method of diffusion tensor imaging (DTI) [Le Bihan et al., 2001], particularly in white matter tracts where fiber bundles lead to highly restricted and anisotropic water diffusion. Alterations in diffusion parameters estimated using DTI have been well‐described in TBI. Compared to healthy individuals, the white matter tracts of TBI patients typically show decreased fractional anisotropy (FA; reflecting directionality of water diffusion) and increased mean diffusivity (MD; quantifying the total amount of water diffusion, independent of direction) [Arfanakis et al., 2002; Inglese et al., 2005; Nakayama et al., 2006; Newcombe et al., 2007]. These effects are often interpreted as indicators of axonal degeneration and demyelination following neuronal injury [Johnson et al., 2013].
In comparison, less consistent DTI effects have been reported across studies of concussion. Results obtained at the symptomatic and early asymptomatic stages of concussion have been highly variable, with reports of altered DTI parameters [Murugavel et al., 2014], limited effects [Zhu et al., 2015] or an absence of significant effects [Zhang et al., 2010]. The majority of studies examining the long‐term effects of concussion have focused on former athletes with a significant history of concussion and sub‐concussive impact, including hockey, football, and boxing. Among these cohorts, DTI has shown decreased FA and increased MD, which are typically interpreted as markers of white matter damage or tissue loss [Chappell et al., 2006; Tremblay et al., 2014], similar to changes seen in moderate to severe TBI. Nevertheless, for young athletes with a history of concussion and no persistent symptoms or cognitive problems, recent studies have shown the opposite effects, with elevated FA and decreased MD reported throughout white matter tracts [Churchill et al., 2016; Henry et al., 2011; Sasaki et al., 2014]. These findings suggest that long‐term effects of concussion on white matter microstructure for young athletes are distinct from the outcome of more severe and/or repetitive brain injury.
In Churchill et al. [2016], it was recently hypothesized that the increased FA and decreased MD associated with a history of concussion may reflect neural growth processes in response to injury, for example, axonal budding [Sidaros et al., 2008] or gliosis [Budde et al., 2011]. Alternatively, these effects may simply reflect change in the spatial organization of neurites. Standard DTI methods lack the ability to distinguish these effects; however, as they are based on a simplified model of brain tissue microstructure involving a single compartment with anisotropic, apparent Gaussian diffusion of water molecules. Thus, voxel‐level changes in FA and MD can arise from multiple mechanisms, including changes in cell morphology and packing density, or changes in white matter fiber orientation. In addition, the FA and MD parameter estimates can be confounded by partial volume effects of cerebrospinal fluid (CSF).
This study addresses these limitations by applying the advanced diffusion MRI method known as neurite orientation dispersion and density imaging (NODDI) [Zhang et al., 2012]. This method acquires imaging data at multiple different diffusion weightings, instead of one as in standard DTI, with each sampling many different spatial orientations at high angular resolution. These data, along with a three‐compartment geometric model of diffusion, are used to estimate fractional water contributions of different tissue types within each voxel. Moreover, this model computes the orientation dispersion index (ODI), which measures the amount of angular variation among neurites in a given voxel. Although initially developed for the assessment of healthy adult brains, NODDI has shown significant promise in the evaluation of stroke [Adluru et al., 2014], Alzheimer's disease [Colgan et al., 2016] and various other patient populations [Caverzasi et al., 2016]. A recent prospective study by [Mayer et al., 2016] also showed that NODDI is sensitive to the effects of repetitive head impacts among mixed‐martial artists, where decreased FA was correlated with increased ODI.
The principal aim of this study was to conduct DTI and NODDI analyses, to determine whether the increased FA and decreased MD seen among athletes with a history of concussion compared to controls is associated with (a) increased intracellular volume fraction (V IC), reflecting an increase in neurite density and potential axonal growth processes, (b) decreased orientation dispersion index (ODI), reflecting increased coherence in the spatial organization of neurites, or (c) both. This was evaluated using univariate voxel‐wise analyses, along with multivariate N‐way partial least squares (NPLS) to examine spatially distributed brain regions showing covariation between DTI and NODDI parameters. A secondary analysis used NPLS to examine how DTI and NODDI parameter estimates are related to clinical history, including number of previous concussions, recovery time from last injury and time since last injury. The long‐term effects of concussion are an important area of study using NODDI, as acute concussion effects associated with edema (e.g., altered membrane permeability, ionic shifts and global perfusion deficits) are expected to have resolved [Giza and Hovda, 2014; Pasco et al., 2007] and any differences among athletes with concussion compared to those without concussion are presumed to reflect chronic microstructural changes. The results of this work are also relevant to understanding the long‐term neurobiological effects of concussion, which in cases of more severe, repetitive impact may potentially lead to long‐term impairments in cognition and mood [Guskiewicz et al., 2005, 2007a].
METHODS
Study Participants
Sixty‐eight (68) athletes were recruited from seven varsity teams (volleyball, hockey, soccer, football, rugby, basketball, and lacrosse) at the University of Toronto, via the university Sport Medicine Clinic. This included 37 athletes without prior history of concussion and 31 with a history of concussion, imaged a minimum of 6 months since their last concussion. Demographic information for both cohorts is reported in Table 1. All athletes were recruited and imaged at the start of their respective seasons. Pre‐season assessments were conducted in‐clinic using the Sport Concussion Assessment Tool 3 (SCAT3) [Guskiewicz et al., 2013] to evaluate symptoms, cognitive function, and balance, ensuring that there were no significant deficits at the time of imaging. The study procedures were approved by institutional review boards at the University of Toronto and St. Michael's Hospital in Toronto, and all athletes gave written informed consent prior to study participation.
Table 1.
Subject demographics clinical and demographic measures for participants, including test scores for the Sport Concussion Assessment Tool 3 (SCAT3) and subscales, including the Balance Error Scoring System (BESS)
| Controls | Concussed | |
|---|---|---|
| Age | 20.1 ± 1.7 | 21.0 ± 1.7 |
| Female | 20/37 | 16/31 |
| Prior concussions | – | 2 [1, 7] |
| Recovery time (days) | – | 23 [1, 180] |
| Since last concussion (months) | – | 24 [9, 120] |
| Symptom severity | 1 [0, 16] | 2 [0, 13] |
| Total symptoms | 1 [0, 29] | 3 [0, 20] |
| Orientation | 5 [4, 5] | 5 [4, 5] |
| Immediate memory | 15 [9, 15] | 15 [13, 15] |
| Concentration | 3 [1, 5] | 4 [1, 5] |
| Delayed recall | 4 [0, 5] | 4 [1, 5] |
| Total BESS score | 3 [0, 10] | 1 [0, 10] |
Age is reported as mean ± SD, while all clinical history and SCAT3 scores are reported as median [min, max] of the athlete cohorts.
Magnetic Resonance Imaging
Participants were imaged at St. Michael's Hospital using an MRI system operating at 3 T (Magnetom Skyra, Siemens, Erlangen, Germany) and standard 20‐channel head receiver coil. To assess for structural lesions, 3D T1‐weighted Magnetization Prepared Rapid Acquisition Gradient Echo (MPRAGE) images were acquired with field‐of‐view (FOV)=24 × 24 cm, 240 × 240 × 192 acquisition matrix, 0.9 mm isotropic voxels, bandwidth = 250 Hz/Pixel, inversion time (TI)/echo time (TE)/repetition time (TR)=850/2.63/2000 ms, and flip angle (FA) = 8°. Fluid‐attenuated inversion recovery imaging (FLAIR) was also obtained with FOV = 22 × 18.6 cm, 256 × 196 acquisition matrix, 1.1 × 0.9 × 3.0 mm voxels, TI/TE/TR = 2200/96/9000 ms. In addition, to assess for potential vascular abnormalities, susceptibility‐weighted imaging (SWI) was performed with FOV = 22 × 19.2 cm, 0.6 × 0.6 × 1.2 mm voxels with encoding gap of 0.2 mm, TR/TE = 28/20 ms, FA = 15°, and 384 × 307 acquisition matrix. Structural scans were reviewed in a two‐step procedure: (1) images were visually inspected by an MRI technologist and subsequently reviewed by a neuroradiologist and reported, if abnormalities were identified; (2) statistical testing for was performed, by obtaining global statistics on masked brain images (mean, variance, and skew), generating a Z‐score per concussed athlete relative to the control distribution, and identifying individuals significantly different at P < 0.05.
A two‐shell protocol was used for diffusion MRI to enable standard estimation of DTI parameters and NODDI parameters (FOV = 24 × 24 cm, 120 × 120 acquisition matrix, 66 slices, 2 × 2 × 2 mm voxels, bandwidth 1736 Hz/Px). The first shell was used for both DTI and NODDI analysis and consisted of 30 diffusion‐weighting directions acquired at TR/TE = 7800/83 ms and b = 700 s/mm2, along with 9 b0 scans, while the second shell was only used for NODDI analysis and consisted of 64 diffusion‐weighting directions acquired at TR/TE = 12300/91 ms and b = 2,000 s/mm2, along with 1 b0 scan.
Preprocessing
For all diffusion‐weighted MRI data, the FMRIB Software Library (FSL; http://www.fmrib.ox.ac.uk/fsl) eddy protocol was used to perform simultaneous correction of eddy current distortions and rigid‐body head motion, and FSL bet was used to mask out nonbrain voxels, based on subject b0 images. The data were analyzed to extract voxel‐wise standard DTI metrics (FA, MD) using FSL dtifit for the 30‐direction shell, avoiding signal attenuation (with lower signal‐to‐noise ratio, SNR) and restricted diffusion effects present in the 64‐direction shell acquired at the higher b value. Because the two diffusion imaging shells had different TR/TE values, they were normalized prior to NODDI analyses using the approach implemented in [Chang et al., 2015; Owen et al., 2014]: within each shell, diffusion‐weighted voxel values were divided by the corresponding voxel value of the initial b0 scan. The NODDI analyses were then conducted using the open‐source Matlab toolbox (http://nitrc.org/projects/noddi_toolbox) with the “WatsonSHStickTortIsoV_B0” parameterization.
In the NODDI model, tissue water signal is partitioned into three compartments, with volume fractions consisting of (1) free water associated with partial volume effects of CSF, with isotropic Gaussian diffusion (V ISO); (2) intracellular water in neurites, with anisotropic restricted diffusion (V IC); and (3) extracellular water, with anisotropic hindered (Gaussian) diffusion (V EC). The intracellular signal is modelled as a Watson distribution over cylinders of zero radius, which has a mean orientation vector and a concentration parameter indicating how much the distribution tends to spread out around . The concentration parameter is transformed into the orientation dispersion index , which gives a bounded value ranging from 0 (completely parallel neurites) to 1 (completely random neurite orientation). This study focused on the two NODDI parameters relevant to stated hypotheses, that is, increasing FA and decreasing MD leading to either neuronal growth or changes in neurite spatial organization: (1) volume fraction of anisotropic intracellular water (V IC) as a measure of regional neurite density and (2) the orientation dispersion index (ODI) as a measure of neurite spatial organization.
To enable comparisons of the diffusion MRI parameters between athletes, data were co‐registered to the FMRIB58 FA template and a study‐specific template generated using an iterative procedure adapted from the FMRIB Diffusion Toolbox protocol (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FDT). After eroding subject FA maps by 1 voxel width at brain edges (1), an initial coarse estimate of the template was generated by affine warping of subject FA maps to FMRIB58 using the FSL flirt algorithm, and averaging the transformed images. An improved template was then generated via nonlinear warping of subject FA maps to the previously estimated affine template using the FSL fnirt algorithm (with transformations initialized using the affine transform matrix), and again averaging over the transformed images. Finally, the subject FA maps were registered to the new nonlinear template via FSL fnirt. During the final registration step, images were resampled to 2 × 2 × 2 mm isotropic resolution. This same set of transforms was also applied to the MD, V IC, and ODI maps.
For analyses of DTI and NODDI parameter maps, a mask of voxels with mean FA > 0.25 was selected. To reduce the impact of fine‐grained local variation in anatomy between individuals, voxels within the mask region were then convolved with a 6 mm FWHM 3D Gaussian smoothing kernel, with regions outside the mask given a weight of zero during convolution (and the convolution kernel rescaled to ensure that it summed to unity), to reduce confounds due to non‐white‐matter tissue. In addition, the brainstem and cerebellum were manually segmented and removed prior to analyses, to avoid spatial registration errors caused by substantial magnetic field inhomogeneity in these regions.
Analyses
The diffusion MRI data were examined using both univariate and multivariate methods, within a bootstrapped resampling framework. This nonparametric approach avoids assumptions about the statistical distribution of MRI data across athletes, and is robust to the influence of outliers.
Univariate analysis
Each DTI and NODDI parameter estimate (FA, MD, V IC, and ODI) was tested for a mean effect of concussion history at the voxel level. Mean differences in voxel‐wise MRI values for athletes with a history of concussion were computed relative to the control cohort. Significance was evaluated by bootstrap resampling on the mean difference (1000 resampling iterations) and reporting brain regions where the bootstrapped 95% confidence intervals (CIs) did not include zero effect. Adjustment for multiple comparisons was undertaken in a three‐step procedure: (1) voxels were thresholded to include all locations where the 99.5% CIs did not include zero (corresponding to P = 0.005 two‐tailed significance); (2) cluster‐size thresholding was performed at P = 0.05 using the Analysis of Functional NeuroImages (AFNI) 3dFWHMx algorithm to estimate spatial smoothness of each dataset; and (3) the output of 3dFWHMx was input to AFNI 3dClustSim to estimate the minimum cluster‐size threshold. For significant brain voxels, the effect size was reported as the bootstrap ratio (bootstrapped mean/standard error).
An additional post‐hoc test was performed to verify that univariate results were not artifacts of spatial smoothing or voxels near the edge of white matter tracts, which may include partial contributions of grey matter signal. For each significant cluster identified in univariate analyses, the subset of voxels overlapping with the John Hopkins University (JHU) ICBM‐DTI‐81 white‐matter atlas (http://loni.usc.edu/ICBM/Downloads/Downloads_DTI-81.shtml) were retained, and mean signal was calculated within the overlapped regions, using the unsmoothed DTI or NODDI parameter map. Bootstrap analyses were then conducted to test for significant differences between athletes with and without a history of concussion, for these conservative, unsmoothed regions of interest (ROIs).
Multivariate analysis: effects of concussion
The univariate approach described above is representative of the approach used in many DTI studies, but does not identify white matter regions that show consistent effect of concussion across multiple diffusion MRI parameters, or assess covariation between these parameters. This issue was addressed in this work using N‐way Partial Least Squares (NPLS) [Bro, 1996]. This is an extension of bilinear PLS [Krishnan et al., 2011], which identifies latent covariance relationships between brain measures and behavioral or clinical data. Whereas PLS quantifies two‐way relationships (e.g., between voxel values and behavioral variables, for a single MRI metric), NPLS measures higher order relationships in the input data (e.g., between voxel values, behavioral variables, and multiple MRI metrics) [Bro, 1996]. In this case, NPLS was used to examine covariation between voxels, diffusion MRI measures, and concussion history. Each athlete n = 1 … N has a set of DWI brain images, stacked as columns in matrix with dimensions , and a binary scalar value , where 0 = no prior concussion and 1 = history of concussion. NPLS obtains paired vectors of “voxel saliences” a k and “diffusion parameter saliences” b k , which transform athlete data matrices into “brain scores” that have maximized covariance with the concussion history variable ; this allows iterative estimation of k = 1 … K component pairs, where K ≤ min (V, M). Pairs of components and are orthogonal to components 1 to k − 1 and ordered by decreasing amount of explained data covariance.
The voxel saliences a k and diffusion parameter saliences b k reflect the relative importance of specific brain regions and DTI/NODDI parameter estimates, respectively, in the identified covariance relationship with history of concussion. The reliability of these saliences was subsequently assessed by bootstrap resampling on subjects (1000 iterations). As with univariate analyses, the voxel saliences (spatial brain maps) were identified where the bootstrapped 95% CIs did not include zero effect after cluster‐size correction. The diffusion parameter saliences (weights on individual DTI/NODDI parameters) were also identified where the bootstrapped 95% CIs did not include zero; these values were corrected for multiple comparisons at a false‐discovery rate (FDR) of 0.05.
Multivariate analysis: effects of clinical history
Given the substantial group effects observed for univariate and multivariate analyses (see Results), it is reasonable to conduct further analyses within the concussed cohort using NPLS, to examine how concussion history affects DTI and NODDI parameter estimates. For this section, the NPLS approach was applied only to subjects with a history of concussion, and three separate NPLS analyses were conducted, by covarying imaging data with continuous variables including (1) total number of concussions, (2) recovery time for last concussion, and (3) time since last concussion. To ensure robust covariance estimates, clinical variables were rank‐normalized prior to NPLS analysis, as heavy distribution tails in the behavioral values may dramatically affect bootstrap estimates (kurtosis = 6.8 for number of concussions (P < 0.001), 4.4 for recovery time (P = 0.03), 3.4 for time since last concussion (P = 0.16), indicating the first two variables are highly leptokurtic). For current results, MRI data were not transformed, as rank‐normalization showed no significant effect on NPLS saliences; this is expected, as all modalities had median kurtosis ≤3.7 (P = 0.10). As in the previous section, voxel saliences were identified where bootstrapped 95% CIs did not include zero effect after cluster‐size correction. Diffusion parameter saliences were also identified where the bootstrapped 95% CIs did not include zero, correcting for multiple comparisons at an FDR of 0.05.
RESULTS
Subject Demographics
The demographic and clinical measures for both study cohorts are summarized in Table 1. The concussed cohort had a small but significant increase in age (mean difference, 0.89 [95% CI, 0.13 1.71] yr; P = 0.038). Both cohorts were balanced on sex (54% controls and 52% concussed). Athletes with prior concussions had a median of 2 prior injuries, with time to medical clearance occurring a median of 23 days for their last concussion, although recovery times were reported in a wide range, from 1 to 180 days. Similarly, athletes were imaged a median of 2 years since last concussion, but ranging from 9 months up to 10 years. Examining the different metrics of symptoms, cognition, and balance, there were no significant differences between cohorts (P > 0.21, all tests). Based on structural imaging, no abnormalities (e.g., white matter hyperintensities, contusions and microhemorrhage, or statistical outliers) were detected for concussed athletes in this study.
Univariate Analysis
Figure 1 (top row) shows the mean voxel‐wise pattern of each DTI and NODDI parameter estimate across both cohorts of athletes. Highest FA is observed within white matter tracts in comparison to gray matter regions, whereas MD is lower in white matter and highest near regions of unrestricted water diffusion, for example, near the ventricles (which are masked out), likely reflecting partial volume effects. The V IC map quantifies intracellular water content and is similar to FA, with highest values within areas of densely packed white matter fibers, including the body of the corpus callosum and internal capsule. The ODI map shows a spatial pattern that is inverted relative to FA, with the lowest dispersion indices in the center of white matter tracts, indicating highly coherent neurite orientation in these regions. These findings are highly consistent with the established NODDI literature [Zhang et al., 2012].
Figure 1.
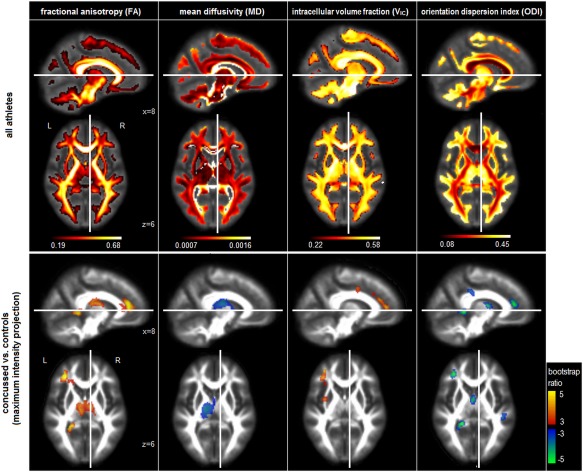
Top row: Voxel‐wise DTI and NODDI diffusion parameter estimates averaged over both groups of athletes. DTI parameters include fractional anisotropy (FA) and mean diffusivity (MD). NODDI parameters include volume fractions of intracellular water (VIC) and orientation dispersion index (ODI). Bottom row: Plots of reliable group differences in the same voxel‐wise DTI and NODDI diffusion parameter estimates, for athletes with a history of concussion, compared to those without. Effect sizes are reported in terms of bootstrap ratios, with cluster‐size correction at P = 0.05. Brain maps in the bottom row are shown as maximum intensity projections (MIPs) in sagittal and axial planes, and all images are centered on the MNI coordinates (x = 8; z = 6). [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 1 (bottom row) shows the regions of significant mean difference in DTI and NODDI diffusion parameters between athletes with and without a history of concussion. For concussed athletes, FA is significantly increased, with clusters in the fornix and body of the corpus callosum (cluster volume: 2368 mm3, center of mass (CoM) in MNI coordinates: −2, −14, 18), left anterior corona radiata (volume: 1920 mm3, CoM: −32, 36, 14), along with the splenium of the corpus callosum (volume: 592 mm3, CoM: −22, −44, 4). MD is significantly decreased in a single cluster, centered on the left posterior limb of the internal capsule (volume: 2832 mm3, CoM: −18, −16, 16), which extends into the fornix and body of the corpus callosum. Examining NODDI parameters, V IC is significantly elevated for concussed athletes, with clusters in the left anterior corona radiata (volume: 1232 mm3, CoM: −32, 34, 20) and proximal to the left superior corona radiata (volume: 624 mm3, CoM: −30, −2, 38). In contrast, ODI shows more spatially extensive decreases for concussed athletes, with clusters in the left anterior corona radiata (volume: 768 mm3, CoM: −32, 36, 16), splenium of the corpus callosum (volume: 688 mm3, CoM: −22, −44, 6), fornix extending into the body of the corpus callosum (volume: 576 mm3, CoM: 0, −4, 12) and right superior longitudinal fasciculus (volume: 536 mm3, CoM: 42, −30, 34). Post‐hoc analyses of mean unsmoothed parameter values, within cluster regions that overlapped with the ICBM‐DTI‐81 atlas, showed that all the regions remained significant with bootstrap ratio values consistently >2.71 (P < 0.006, two‐tailed).
Multivariate Analysis: effects of Concussion
Figure 2 shows the NPLS analysis results comparing athletes with and without history of concussion. Only one pair of components (i.e., both voxel and diffusion parameter saliences) showed significant effects after multiple comparison correction. Consistent with the univariate analyses, four white matter clusters were identified (Fig. 2A), with the first cluster centered on the body of the corpus callosum, extending into the fornix and anterior limb of right internal capsule (volume: 4616 mm3, CoM: −4, −8, 20), the second cluster centered on the left anterior corona radiata, extending into the genu of the corpus callosum (volume: 3680 mm3, CoM: −24, 34, 12), the third located in the left posterior limb of the internal capsule (volume: 976 mm3, CoM: −24, −28, 12), and the fourth in the splenium of the corpus callosum (volume: 864 mm3, CoM: −20, −44, 4). These brain regions exhibited a concurrent increase in FA, increase in V IC, and a decrease in ODI (Fig. 2B), whereas MD effects were nonsignificant. The brain scores associated with these saliences were significantly higher for athletes with a history of concussion compared to those without (t‐statistic: 3.79, P < 0.001, two‐tailed) (Fig. 2C).
Figure 2.
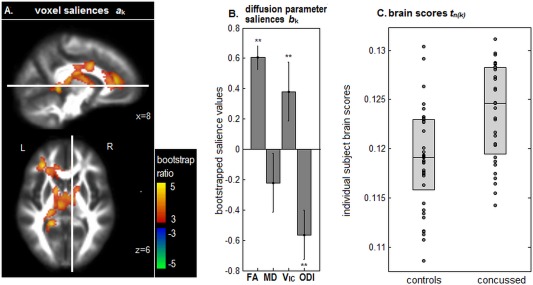
NPLS results showing differences in diffusion‐weighted imaging parameter estimates between athletes with and without a history of concussion. (A) Significant clusters of voxel saliences with effect sizes reported in terms of bootstrap ratios. (B) Diffusion parameter saliences that distinguish the two cohorts in these brain regions. Significant saliences are denoted by “**.” (C) Individual subject brain scores, indicating how much each subject expresses this salience pattern, plotted for athletes with and without history of concussion. Box plots denote the 25th, 50th, and 75th percentiles. The thresholded brain map is shown as a maximum intensity projection (MIP) in sagittal and axial planes, centered on MNI coordinates (x = 8; z = 6). [Color figure can be viewed at http://wileyonlinelibrary.com]
Multivariate Analysis: effects of Clinical History
Among the three clinical history variables analyzed in this study (total number of concussions, recovery time for last concussion, and time since last concussion), only time since last concussion showed significant voxel and diffusion parameter saliences, after correcting for multiple comparisons. Figure 3 depicts the NPLS analysis results, representing covariation between diffusion MRI parameters and time since injury. An extensive set of 12 significant clusters were identified (Fig. 3A), located in the superior longitudinal fasciculus (volume: 2840 mm3, CoM: 30, −36, 38; volume: 976 mm3, CoM: −36, −8, 32; volume: 920 mm3, CoM: 36, −6, 26; volume: 536 mm3, CoM: −42, 2, 20), body of the corpus callosum (volume: 7048 mm3, CoM: 10, 8, 32; volume: 984 mm3, CoM: −14, 16, 22), posterior corona radiata (volume: 4976 mm3, CoM: −32, −52, 14; volume: 760 mm3, CoM: −24, −40, 44), internal capsule (volume: 1136 mm3; CoM: 36, −26, 0; volume: 520 mm3, CoM: −26, 10, 14), anterior corona radiata (volume: 712 mm3, CoM: −14, 22, −6), and superior corona radiata (volume: 632 mm3, CoM: −20, −28, 54).
Figure 3.
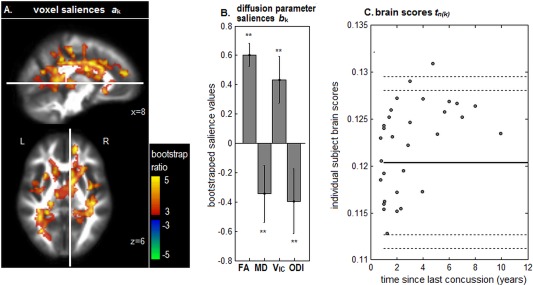
NPLS results showing covariation between diffusion‐weighted imaging parameters and time since last concussion, for concussed athletes. (A) Significant clusters of voxel saliences with effect sizes reported in terms of bootstrap ratios. (B) Diffusion parameter saliences for these brain regions. Significant parameters are denoted by “**.” (C) Individual subject brain scores, indicating how much each athlete expresses the salience pattern, plotted against time since last injury. Solid horizontal line = mean of control brain scores, along with 90% and 95% confidence bounds (dashed lines), for comparison. The thresholded brain map is shown as a maximum intensity projection (MIP) in sagittal and axial planes, centered on MNI coordinates (x = 8; z = 6). [Color figure can be viewed at http://wileyonlinelibrary.com]
As in the previous NPLS analysis, these brain regions were associated with a concurrent increase in FA, increase in V IC, and decrease in ODI, along with a significant decrease in MD (Fig. 3B). As shown by subject brain scores (Fig. 3C), these effects were positively correlated with recovery time (Pearson correlation: 0.36, P = 0.003, two‐tailed). Thus, athletes scanned at a longer time interval since their last concussion had more elevated FA and V IC, along with reduced MD and ODI values. Although recovery time was reliably associated with diffusion parameter values in the concussed athlete cohort, the range of brain variability was generally comparable to the uninjured controls, with only a single subject above the 95% confidence bound on normal controls (outer dashed lines).
DISCUSSION
Diffusion‐weighted MRI provides a powerful tool for investigating changes in the microstructure of white matter that are associated with concussion and TBI. However, as demonstrated in this study, it is important to examine measures other than conventional DTI estimates of FA and MD, such as those provided by NODDI. The results of this study have confirmed that DTI findings associated with a history of concussion are robust across different diffusion imaging models, and NODDI was used to better understand the biophysical alterations that are associated with sport concussion. Moreover, the results were corroborated across different analytic methods, both univariate and multivariate, ensuring that the findings are not model‐dependent.
In this study, athletes with a history of concussion had elevated FA and lower MD compared to controls, indicating increasingly anisotropic, hindered water diffusion within white matter tracts. These results are consistent with prior DTI studies of athletes with a history of sport concussion, where increased FA and decreased MD were reported in the absence of significant symptoms or cognitive impairments, for standard clinical assessments [Churchill et al., 2016; Henry et al., 2011; Sasaki et al., 2014]. Based on these findings, the long‐term effects of concussion appear to differ from subacute concussion, where MD has been reported to be a more sensitive correlate of injury than FA [Cubon et al., 2011]. Importantly, the present findings also differ from studies of more severe TBI, wherein decreased FA and increased MD are generally observed and individuals are often clinically symptomatic [Arfanakis et al., 2002; Inglese et al., 2005; Nakayama et al., 2006; Newcombe et al., 2007]. This provides supporting evidence that persistent microstructural changes associated with concussion may be fundamentally distinct from those seen in more severe forms of TBI.
Examining NODDI parameters, concussed athletes had both increased V IC and decreased ODI, indicating that DTI results are due to increased density of neurites and greater coherency of fiber orientation in white matter tracts. Increased FA after brain injury has been previously interpreted as a marker of axonal regrowth [Sidaros et al., 2008; Voss et al., 2006], and the increased V IC and reduced ODI in this study supports this mechanism as a cause of the observed FA and MD differences among athletes with a history of concussion. Conversely, gliosis and the presence of glial scarring may contribute to increased FA following TBI [Budde et al., 2011], but this would reduce V IC in white matter due to increased extraneurite volume, which was not observed in this study. Similarly, it has been suggested that increased FA during the chronic phase of injury may be due to selective degeneration of crossing fibers [Henry et al., 2011]; however, elevated V IC among concussed athletes indicates that this is unlikely to be the primary mechanism of between‐group differences. The NODDI results, which are based on an alternative diffusion‐weighted imaging model, support the interpretation of DTI parameters as potential markers of neurite regrowth and spatial reorganization in response to prior brain injury, although further research is required to confirm the etiology and clinical significance of these effects. This includes the acquisition of NODDI at multiple time points in the concussion recovery timeline, along with animal and simulation models.
Univariate results suggest that ODI is a more sensitive marker of concussion history than V IC, with a greater number of significant clusters. However, multivariate NPLS analyses indicate that the effects of concussion history on V IC and ODI tend to co‐occur within a similar set of distributed brain regions. These NODDI parameters were also more reliably related to variations in FA than MD, as the latter had nonsignificant parameter salience values (Fig. 2B). The NPLS analyses of clinical history also established that variations in DTI and NODDI measures were significantly related to the time since last concussion. The latter results suggest that the observed increases in neurite density and coherence following concussion may reflect long‐term biological processes that evolve over multiple years postinjury. Interestingly, no reliable relationship was observed between diffusion MRI and other clinical measures, including the number of prior concussions and recovery time for their last injury, despite evidence that multiple concussions lead to increased neurobiological and clinical consequences [Guskiewicz et al., 2007b; Huh et al., 2007; Pellman et al., 2004]. These results are in alignment with prior DTI analyses conducted by Churchill et al. [2016], which found limited associations between DTI and these clinical measures. Overall, these results provide evidence that elevated FA and V IC, along with lower MD and ODI, may be primarily a marker of good long‐term biological and clinical recovery for athletes with a history of sport concussion.
Across all analyses, the corpus callosum was the region most reliably associated with concussion history, followed by anterior corona radiata. Multivariate analysis consistently showed “cross‐talk” between DTI and NODDI parameters in the corpus callosum, as athletes with history of concussion tended to modulate these markers simultaneously. The corpus callosum is a thick white matter tract crucial for interhemispheric communication. It is protected from direct impact against the skull; however, finite element analyses suggest that this area may experience the greatest strain during concussive impacts, due to trauma‐related shear forces [Viano et al., 2005]. In addition, the body of the corpus callosum has densely packed, highly coherent neurite fibers; hence, both DTI and NODDI methods are expected to be sensitive to microstructure changes in this region. The results of this study are consistent with prior TBI analyses, which have identified the corpus callosum as a sensitive neuroimaging marker of brain injury and neurocognitive sequelae [Arenth et al., 2014; Gale et al., 1995; Hasan et al., 2014; Shenton et al., 2012]. Moreover, a DTI meta‐analysis by [Eierud et al., 2014] showed that the body of the corpus callosum and anterior corona radiata were most consistently affected by diffuse injury following mild TBI. This work thus further supports the importance of these regions as concussion biomarkers, for both DTI and advanced imaging techniques such as NODDI.
This study has helped to extend our understanding of the effects of concussion and mild TBI on neural microstructure, improving our ability to interpret standard DTI findings among young, currently active athletes. Long‐term effects of sport concussion in this cohort are associated with increased density and coherency of neurites in white matter, which likely reflect adaptive response to injury [Voss et al., 2006], rather than reflecting neural pathology [Budde et al., 2011; Henry et al., 2011], particularly given the lack of significant clinical impairments among study participants. This work demonstrates the importance of using advanced diffusion MRI models such as NODDI to obtain greater insight into the neurobiological changes that are associated with concussion and TBI.
ACKNOWLEDGMENT
This study was approved by the Canadian Forces Surgeon General's Health Research Program.
REFERENCES
- Adluru G, Gur Y, Anderson JS, Richards LG, Adluru N, DiBella EV (2014): Assessment of white matter microstructure in stroke patients using NODDI. Conf Proc IEEE Eng Med Biol Soc 2014:742–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenth PM, Russell KC, Scanlon JM, Kessler LJ, Ricker JH (2014): Corpus callosum integrity and neuropsychological performance after traumatic brain injury: A diffusion tensor imaging study. J Head Trauma Rehab 29:E1–E10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arfanakis K, Haughton VM, Carew JD, Rogers BP, Dempsey RJ, Meyerand ME (2002): Diffusion tensor MR imaging in diffuse axonal injury. Am J Neuroradiol 23:794–802. [PMC free article] [PubMed] [Google Scholar]
- Bro R (1996): Multiway calibration. multilinear PLS. J Chemometrics 10. [Google Scholar]
- Budde MD, Janes L, Gold E, Turtzo LC, Frank JA (2011): The contribution of gliosis to diffusion tensor anisotropy and tractography following traumatic brain injury: Validation in the rat using Fourier analysis of stained tissue sections. Brain 134:2248–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caverzasi E, Papinutto N, Castellano A, Zhu AH, Scifo P, Riva M, Bello L, Falini A, Bharatha A, Henry RG (2016): Neurite orientation dispersion and density imaging color maps to characterize brain diffusion in neurologic disorders. J Neuroimag. [DOI] [PubMed] [Google Scholar]
- Chang YS, Owen JP, Pojman NJ, Thieu T, Bukshpun P, Wakahiro ML, Berman JI, Roberts TP, Nagarajan SS, Sherr EH (2015): White matter changes of neurite density and fiber orientation dispersion during human brain maturation. PLoS One 10:e0123656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell M, Uluǧ A, Zhang L, Heitger M, Jordan B, Zimmerman R, Watts R (2006): Distribution of microstructural damage in the brains of professional boxers: A diffusion MRI study. J Magn Reson Imag 24:537–542. [DOI] [PubMed] [Google Scholar]
- Churchill N, Hutchison M, Richards D, Leung G, Graham S, Schweizer T (2016): Brain structure and function associated with a history of sport concussion: A multi‐modal MRI study. J Neurotrauma. [DOI] [PubMed] [Google Scholar]
- Colgan N, Siow B, O'Callaghan J, Harrison I, Wells J, Holmes H, Ismail O, Richardson S, Alexander D, Collins E (2016): Application of neurite orientation dispersion and density imaging (NODDI) to a tau pathology model of Alzheimer's disease. NeuroImage 125:739–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubon V, Putukian M, Boyer C, Dettwiler A (2011): A diffusion tensor imaging study on the white matter skeleton in individuals with sports‐related concussion. J Neurotrauma 28:189–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eierud C, Craddock RC, Fletcher S, Aulakh M, King‐Casas B, Kuehl D, LaConte SM (2014): Neuroimaging after mild traumatic brain injury: Review and meta‐analysis. NeuroImage Clin 4:283–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale SD, Johnson SC, Bigler ED, Blatter DD (1995): Nonspecific white matter degeneration following traumatic brain injury. J Int Neuropsychol Soc 1:17–28. [DOI] [PubMed] [Google Scholar]
- Giza CC, Hovda DA (2014): The new neurometabolic cascade of concussion. Neurosurgery 75:S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guskiewicz K, Marshall S, Bailes J, McCrea M, Cantu R, Randolph C, Jordan B (2005): Association between recurrent concussion and late‐life cognitive impairment in retired professional football players. Neurosurgery 57:719–726. [DOI] [PubMed] [Google Scholar]
- Guskiewicz K, Marshall S, Bailes J, McCrea M, Harding H, Matthews A, Mihalik J, Cantu R (2007a): Recurrent concussion and risk of depression in retired professional football players. Med Sci Sports Exerc 39:903–909. [DOI] [PubMed] [Google Scholar]
- Guskiewicz K, McCrea M, Marshall S, Cantu R, Randolph C, Barr W, Kelly J (2003): Cumulative effects associated with recurrent concussion in collegiate football players: The NCAA Concussion Study. J Am Med Assoc 290:2549–2555. [DOI] [PubMed] [Google Scholar]
- Guskiewicz K, Register‐Mihalik J, McCrory P, McCrea M, Johnston K, Makdissi M, Meeuwisse W (2013): Evidence‐based approach to revising the SCAT2: Introducing the SCAT3. Br J Sports Med 47:289–293. [DOI] [PubMed] [Google Scholar]
- Guskiewicz K, Ross S, Marshall S (2001): Postural stability and neuropsychological deficits after concussion in collegiate athletes. J Athlet Train 36:263. [PMC free article] [PubMed] [Google Scholar]
- Guskiewicz KM, Mihalik JP, Shankar V, Marshall SW, Crowell DH, Oliaro SM, Ciocca MF, Hooker DN (2007b): Measurement of head impacts in collegiate football players: Relationship between head impact biomechanics and acute clinical outcome after concussion. Neurosurgery 61:1244–1253. [DOI] [PubMed] [Google Scholar]
- Hasan KM, Wilde EA, Miller ER, Kumar Patel V, Staewen TD, Frisby ML, Garza HM, McCarthy JJ, Hunter JV, Levin HS (2014): Serial atlas‐based diffusion tensor imaging study of uncomplicated mild traumatic brain injury in adults. J Neurotrauma 31:466–475. [DOI] [PubMed] [Google Scholar]
- Henry LC, Tremblay J, Tremblay S, Lee A, Brun C, Lepore N, Theoret H, Ellemberg D, Lassonde M (2011): Acute and chronic changes in diffusivity measures after sports concussion. J Neurotrauma 28:2049–2059. [DOI] [PubMed] [Google Scholar]
- Huh JW, Widing AG, Raghupathi R (2007): Repetitive mild non‐contusive brain trauma in immature rats exacerbates traumatic axonal injury and axonal calpain activation: A preliminary report. J Neurotrauma 24:15–27. [DOI] [PubMed] [Google Scholar]
- Inglese M, Makani S, Johnson G, Cohen BA, Silver JA, Gonen O, Grossman RI (2005): Diffuse axonal injury in mild traumatic brain injury: A diffusion tensor imaging study. J Neurosurg 103:298–303. [DOI] [PubMed] [Google Scholar]
- Johnson VE, Stewart W, Smith DH (2013): Axonal pathology in traumatic brain injury. Exp Neurol 246:35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan A, Williams L, McIntosh A, Abdi H (2011): Partial Least Squares (PLS) methods for neuroimaging: A tutorial and review. NeuroImage 56:455–475. [DOI] [PubMed] [Google Scholar]
- Le Bihan D, Mangin JF, Poupon C, Clark CA, Pappata S, Molko N, Chabriat H (2001): Diffusion tensor imaging: Concepts and applications. J Magn Reson Imag 13:534–546. [DOI] [PubMed] [Google Scholar]
- Mayer AR, Ling JM, Dodd AB, Meier TB, Hanlon FM, Klimaj SD (2016): A prospective microstructure imaging study in mixed‐martial artists using geometric measures and diffusion tensor imaging: Methods and findings. Brain Imag Behav 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrory P, Meeuwisse W, Aubry M, Cantu B, Dvořák J, Echemendia R, Sills A (2013): Consensus statement on concussion in sport: The 4th International Conference on Concussion in Sport held in Zurich, November 2012. Br J Sports Med 47:250–258. [DOI] [PubMed] [Google Scholar]
- Murugavel M, Cubon V, Putukian M, Echemendia R, Cabrera J, Osherson D, Dettwiler A (2014): A longitudinal diffusion tensor imaging study assessing white matter fiber tracts after sports‐related concussion. J Neurotrauma 31:1860–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama N, Okumura A, Shinoda J, Yasokawa Y, Miwa K, Yoshimura S, Iwama T (2006): Evidence for white matter disruption in traumatic brain injury without macroscopic lesions. J Neurol Neurosurg Psychiatry 77:850–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcombe V, Williams G, Nortje J, Bradley P, Harding S, Smielewski P, Coles J, Maiya B, Gillard J, Hutchinson P (2007): Analysis of acute traumatic axonal injury using diffusion tensor imaging. Br J Neurosurg. [DOI] [PubMed] [Google Scholar]
- Owen JP, Chang YS, Pojman NJ, Bukshpun P, Wakahiro ML, Marco EJ, Berman JI, Spiro JE, Chung WK, Buckner RL (2014): Aberrant white matter microstructure in children with 16p11. 2 deletions. J Neurosci 34:6214–6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasco A, Lemaire L, Franconi F, Lefur Y, Noury F, Saint‐André J‐P, Benoit J‐P, Cozzone PJ, Le Jeune J‐J (2007): Perfusional deficit and the dynamics of cerebral edemas in experimental traumatic brain injury using perfusion and diffusion‐weighted magnetic resonance imaging. J Neurotrauma 24:1321–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellman EJ, Viano DC, Casson IR, Tucker AM, Waeckerle JF, Powell JW, Feuer H (2004): Concussion in professional (American) football: Repeat injuries‐Part 4. Neurosurgery 55:860–876. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Pasternak O, Mayinger M, Muehlmann M, Savadjiev P, Bouix S, Kubicki M, Fredman E, Dahlben B, Helmer KG (2014): Hockey Concussion Education Project, Part 3. White matter microstructure in ice hockey players with a history of concussion: A diffusion tensor imaging study: Clinical article. J Neurosurg 120:882–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenton M, Hamoda H, Schneiderman J, Bouix S, Pasternak O, Rathi Y, Vu M‐A, Purohit M, Helmer K, Koerte I (2012): A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imag Behav 6:137–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidaros A, Engberg A, Sidaros K, Liptrot M, Herning M, Petersen P, Rostrup E (2008): Diffusion tensor imaging during recovery from severe traumatic brain injury and relation to clinical outcome: A longitudinal study. Brain 131:559–572. [DOI] [PubMed] [Google Scholar]
- Tremblay S, Henry L, Bedetti C, Larson‐Dupuis C, Gagnon J, Evans A, De Beaumont L (2014): Diffuse white matter tract abnormalities in clinically normal ageing retired athletes with a history of sports‐related concussions. Brain awu236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viano DC, Casson IR, Pellman EJ, Zhang L, King AI, Yang KH (2005): Concussion in professional football: Brain responses by finite element analysis: Part 9. Neurosurgery 57:891–916. [DOI] [PubMed] [Google Scholar]
- Voss HU, Uluç AM, Dyke JP, Watts R, Kobylarz EJ, McCandliss BD, Heier LA, Beattie BJ, Hamacher KA, Vallabhajosula S (2006): Possible axonal regrowth in late recovery from the minimally conscious state. J Clin Invest 116:2005–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Schneider T, Wheeler‐Kingshott C, Alexander D (2012): NODDI: Practical in vivo neurite orientation dispersion and density imaging of the human brain. NeuroImage 61:1000–1016. [DOI] [PubMed] [Google Scholar]
- Zhang K, Johnson B, Pennell D, Ray W, Sebastianelli W, Slobounov S (2010): Are functional deficits in concussed individuals consistent with white matter structural alterations: Combined FMRI & DTI study. Exp Brain Res 204:57–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D, Covassin T, Nogle S, Doyle S, Russell D, Pearson R, Kaufman D (2015): A potential biomarker in sports‐related concussion: Brain functional connectivity alteration of the default‐mode network measured with longitudinal resting‐state fMRI over thirty days. J Neurotrauma 32:327–341. [DOI] [PubMed] [Google Scholar]


