Abstract
Prenatal alcohol exposure (PAE) can cause central nervous system dysfunction and widespread structural anomalies as detected by magnetic resonance imaging (MRI). This study focused on diffusion tensor imaging (DTI) of white matter in a large sample of PAE participants that allowed us to examine correlations with behavioral outcomes. Participants were confirmed PAE (n = 69, mean age = 12.5 ± 3.2 years) or typically developing control children (n = 67, mean age = 12.1 ± 3.2 years) who underwent brain MRI, eye movement tasks, and psychometric tests. A semi‐automated tractography method extracted fractional anisotropy (FA) and mean diffusivity (MD) values from 15 white matter tracts. The PAE group displayed decreased FA compared with controls in multiple tracts including 3 corpus callosum regions, right corticospinal tract, and 3 left hemisphere tracts connecting to the frontal lobe (cingulum, uncinate fasciculus, and superior longitudinal fasciculus). Significant group by sex interactions were found for the genu, left superior longitudinal fasciculus, and the left uncinate, with females in the PAE group exhibiting lower FA compared with control females. Correlations were found between DTI and eye movement measures in the control group, but these same relationships were absent in the PAE group. In contrast, no correlations were found between DTI and any of the psychometric tests used in this study. These findings support the hypothesis that measures of eye movement control may be valuable functional biomarkers of the brain injury induced by PAE as these tasks reveal group differences that appear to be linked to deficits in white matter integrity in the brain. Hum Brain Mapp 38:444–456, 2017. © 2016 Wiley Periodicals, Inc.
Keywords: fetal alcohol spectrum disorder, eye tracking, sex effects, FASD, PAE, psychometric testing
INTRODUCTION
Prenatal alcohol exposure (PAE) can cause growth deficiency, facial dysmorphology, and mild to severe central nervous system dysfunction. The damage to the developing brain can lead to a variety of deficits in both primary functions, such as executive functions, visuospatial processing, motor skills, and language, as well as secondary disabilities, such as neuropsychiatric conditions and problems with adaptive skills [Jacobson et al., 2011; Kodituwakku, 2009; Mattson et al., 2011; Rasmussen, 2005; Rasmussen et al., 2008]. The spectrum of adverse effects induced by PAE is collectively referred to as Fetal Alcohol Spectrum Disorder (FASD) [Chudley et al., 2005]. One of the most significant problems associated with FASD diagnosis is that in the absence of facial dysmorphology, identification of children with FASD becomes increasingly difficult, especially if alcohol exposure cannot be confirmed. There is, therefore, a need to identify functional biomarkers of PAE that could be used to screen children at risk and enable earlier intervention. Magnetic resonance imaging (MRI) of the brain and eye movement control tasks may hold promise in this regard.
Structural MRI studies have demonstrated robust abnormalities of volume throughout the brain of PAE individuals [Autti‐Ramo et al., 2002; Riley et al., 1995; Swayze et al., 1997]. In addition to the decreased size of brain structures, connectivity via white matter tracts is also compromised. The first diffusion tensor imaging (DTI) study examined the corpus callosum in young adults (18–25 years old; n = 9) with fetal alcohol syndrome (FAS) and found decreased fractional anisotropy (FA) and increased mean diffusivity (MD) in the genu and splenium [Ma et al., 2005]. Since then, most DTI studies have focused on children and adolescents with PAE, with all showing diffusion parameter alterations in the corpus callosum [Fan et al., 2016; Fryer et al., 2009; Wozniak et al., 2009]. Additionally, a number of other key tracts including the cingulum, uncinate fasciculus, corticospinal tract, cerebellum, medial frontal, and occipital lobes have also been shown to be altered in children with PAE [Fryer et al., 2009; Lebel et al., 2008; Meintjes et al., 2014]. However, nearly all studies collapse participants across age and do not examine relationships with age despite the known increases of FA and decreases of MD with increasing age during healthy development [Lebel et al., 2012]. In addition, most previous DTI studies combined data between males and females; therefore, potential sex differences in diffusion parameters in both typical development and after PAE have not been explored.
The measurement of saccades is an accessible, portable method for probing brain function. The first eye movement control study in FASD examined performance of the prosaccade and antisaccade tasks using electrooculography in a small sample (n = 10) of children with FASD (8–12 years old), and found increased reaction time and errors in the tasks [Green et al., 2007]. Since then, studies have searched for additional differences between PAE and controls as well as combining eye tracking with magnetoencephalography/electroencephalography [Coffman et al., 2013; Hemington and Reynolds, 2014; Stephen et al., 2013]. Recently, two articles have linked eye tracking with DTI measures and found specific correlations between the corpus callosum, cerebellum, and longitudinal fasciculus [Green et al., 2013; Paolozza et al., 2014c]. As part of the NeuroDevNet multisite FASD study [Reynolds et al., 2011], children with PAE (n = 27) showed a greater number of additional saccades to reach the target and decreased accuracy of the saccade endpoint [Paolozza et al., 2013]. Additionally, in a different study, measures including increased reaction time and decreased saccade amplitude were found to be sex‐dependent in children with FASD (n = 71) [Paolozza et al., 2015].
In this multisite study, DTI measures of cerebral white matter and eye tracking measures were compared between children with a history of PAE (n = 69) and typically developing controls (n = 67) over a broad age range of 5–18 years. The relatively large sample size (for PAE studies) enabled the investigation of changes of diffusion parameters with age and sex, as well as more robust correlation analyses of DTI with eye tracking.
METHODS
Participants
Participants aged 5–18 years were recruited from Kingston, ON, Ottawa, ON, Edmonton, AB, Cold Lake, AB, Winnipeg, MB, and Vancouver, BC as part of a larger study funded by NeuroDevNet [Reynolds et al., 2011]. Children with PAE were recruited through diagnostic clinics and were previously diagnosed according to the Canadian Guidelines for FASD diagnosis (n = 62) [Chudley et al., 2005] or had confirmed PAE from a credible source but did not have a diagnosis of FASD because at the time of assessment they did not meet all of the diagnostic criteria (n = 19). Typically developing control children (n = 78) were recruited from the same geographic areas and were excluded if they had any neurological or psychiatric disorder, visual disturbance (other than requiring corrective lenses), or contraindications to MRI. All experimental procedures were reviewed and approved by the Health Research Ethics Boards at Queen's University, University of Alberta, Children's Hospital of Eastern Ontario, University of Manitoba, and the University of British Columbia. Written informed consent was obtained from a parent or legal guardian and assent was obtained from each child before study participation. Each child completed eye tracking, psychometric testing, and an MRI session, totaling a 3‐hour time commitment completed over 1 or 2 days depending on the child and parent's preference. Due to quality control measures (movement, braces, etc.), 12 participants with PAE were excluded from analysis, leaving 69 PAE participants with adequate DTI data, scanned in either Kingston (n = 22; mean age = 12.7 ± 3.3; 11 males), Edmonton (n = 22; mean age = 11.7 ± 3.1; 11 males), Winnipeg (n = 10; mean age = 14.1 ± 1.3; 5 males), or Vancouver (n = 15; mean age = 12.1 ± 4.0; 6 males). From the typically developing control children 11 were excluded due to either a pre‐existing disorder that was not disclosed at recruitment or failing quality control measures, leaving 67 control participants scanned in Kingston (n = 20; mean age = 12.4 ± 4.2; 10 males), Edmonton (n = 19; mean age = 11.0 ± 2.3; 9 males), Winnipeg (n = 10; mean age = 10.8 ± 2.6; 3 males), or Vancouver (n = 18; mean age = 13.5 ± 2.3; 5 males). Participant information is summarized in Table 1. PAE participants taking stimulant medication were requested to withhold administration of that drug on the day of testing in order to remove any confounding effects of stimulant drugs, which have relatively short half‐lives. Socioeconomic status (SES) was calculated using Hollingshead's Four‐factor Index of Social Status [Hollingshead, 2011]. Behavioural and cognitive data were collected and managed using RedCap software [Harris et al., 2009] and imaging data were collected and managed using the LORIS imaging database [Das et al., 2011].
Table 1.
Study population characteristics
| Diagnostic subtype, n (%): | Control (n = 67) | PAE (n = 69) | |
|---|---|---|---|
| FAS | – | 4 (6) | |
| pFAS | – | 12 (17) | |
| ARND | – | 36 (52) | |
| PAE | – | 17 (25) | |
| Demographics: | t‐test P‐value | ||
| Mean age ± SD (range) | 12.1 ± 3.2 (6–18) | 12.5 ± 3.2 (5–18) | 0.456 |
| Males n (%) | 27 (40) | 33 (48) | 0.319 |
| Socioeconomic status | 46.5 | 42.1 | 0.054 |
| Ethnicity: | Chi‐squared P‐value | ||
| Caucasian n (%) | 64 (95) | 26 (38) | <0.0001 |
| First Nations n (%) | 1 (2) | 34 (49) | <0.0001 |
| Other Ethnicity n (%) | 2 (3) | 9 (13) | 0.017 |
| Comorbidities n (%): | Chi‐squared P‐value | ||
| ADHD | 0 (0) | 37 (54) | <0.0001 |
| ODD | 0 (0) | 5 (7) | 0.007 |
| Anxiety | 0 (0) | 9 (13) | 0.0002 |
| Depression | 0 (0) | 6 (9) | 0.002 |
| Other | 0 (0) | 24 (35) | <0.0001 |
| Medications n (%): | Chi‐squared P‐value | ||
| Stimulants | 0 (0) | 28 (41) | <0.0001 |
| Antipsychotics | 0 (0) | 11 (16) | <0.0001 |
| Antidepressant | 0 (0) | 5 (7) | 0.007 |
| Other | 0 (0) | 2 (3) | 0.081 |
PAE, prenatal alcohol exposure; FAS, Fetal Alcohol Syndrome; Pfas, Partial Fetal Alcohol Syndrome; SD, standard deviation; ARND, Alcohol Related Neurodevelopment Disorder; n, number; ADHD, Attention Deficit Hyperactivity Disorder; ODD, Oppositional Defiant Disorder.
Image Acquisition
MRI data were collected at 4 sites (Edmonton, AB, 1.5T Siemens Sonata; Kingston, ON, and Winnipeg, MB, both 3T Siemens Trio, Vancouver, BC, 3T Philips Achieva), including DTI, T1‐weighted (not used here), T2‐weighted (not used here), fluid‐attenuated inversion recovery (FLAIR; not used here), and resting state functional MRI (not used here) scans for a total scan time of approximately 20 min. All DTI was acquired using a dual spin‐echo echo planar imaging sequence with: 2.2 mm isotropic voxels, 50 axial‐oblique slices with no interslice gap; 30 non‐collinear diffusion sensitizing gradient directions with b = 1,000 s/mm2; 5 (Edmonton) or 1 (Kingston, Winnipeg and Vancouver) b = 0 s/mm2; 1 average; FOV = 220 × 220 mm2; matrix of 96 × 96; TE = 94 ms (Kingston, Winnipeg, and Edmonton) or TE = 69 ms (Vancouver); TR = 6,600 ms (Kingston and Winnipeg) or TR = 7,700 ms (Edmonton) or TR = 6,828 ms (Vancouver); acquisition time 3:45 (Vancouver, Kingston, Winnipeg) or 4:39 minutes (Edmonton).
DTI Tractography
All data were first inspected via visual evaluation, then motion/distortion corrected using ExploreDTIv8.3. A semi‐automated tractography method was performed using previously described methods [Lebel et al., 2008] to extract FA and MD values from 15 major white matter tracts (the genu, body, and splenium of the corpus callosum, left and right inferior and superior longitudinal fasciculi (ILF and SLF, respectively), inferior fronto‐occipital fasciculus (IFO), cingulum, uncinate fasciculus, and corticospinal tracts (CST)). In short, tensor maps were normalized to the ICBM‐DTI81 template in DTI‐TK using a deformable tensor‐based registration algorithm [Zhang et al., 2006; http://dti-tk.sourceforge.net]. Regions of interest (ROIs) for each tract were then manually drawn on the template, using seed, target, and exclusion regions based on a priori information about tract location [Wakana et al., 2004]. The inverse of normalization parameters were used to warp ROIs back into native space for tracking, which was carried out using a minimum FA threshold of 0.25 to initiate and continue tracking, and an angle threshold of 60° for the uncinate and SLF, or 30° for all other tracts. FA and MD for all tracts were calculated by averaging across all voxels in a given tract. Each tract was manually examined in native space using ExploreDTIv8.3 by a single operator (AEP), blinded to participant group, age, and sex. Additional “Not” regions were used to exclude any spurious tracts. With the exception of the corpus callosum, left and right tracts were measured and analyzed separately.
Site Correction and DTI Group and Age Analysis
Since the MRI data were collected on different scanners throughout Canada, we performed a reliability study using “travelling phantoms” (adults tested at each site to evaluate scanner consistency). In this study 8 adult participants travelled to all sites and were scanned on each scanner twice using the same protocol (8 scans per person, 64 scans total, mean of 120 days from first to last scan). Within and between‐subject coefficient of variation (CV) and intra‐class correlation coefficients (ICC) were calculated for each outcome variable (FA and MD), and two‐way repeated‐measures ANOVA was used to test for significant effects of site, scan, and site‐by‐scan interactions. A correction factor was applied to all variables with a significant omnibus effect of site at P < 0.05. First, a difference value (Δ) was calculated for each site by subtracting the site average from the total mean value across participants and sites. The inverse of this Δ value was then applied to each raw data point for each participant within each site. Pearson's linear correlations of the corrected FA and MD values versus age were assessed per tract for FASD and control groups separately. To control for the effects of age, residual scores were calculated for each individual tract from the linear regression of DTI and age as previously described [Paolozza et al., 2014c]. These age‐corrected values were used in the correlational analyses with age‐corrected scores obtained from the eye movement and psychometric test batteries. In addition, group differences of FA and MD were compared using a two‐way ANCOVA including group and sex, with age as a covariate and Sidak's correction for multiple comparisons for all 15 tracts. The P‐values reported are corrected for multiple comparisons.
Saccadic Eye Movement Recordings
Participants were seated comfortably in a dark, quiet room on a stable chair and instructions for each trial were given verbally, and repeated before each task started. Eye position was recorded using the Eyelink 1000 (SR Research, Kanata, ON), using previously described methods [Paolozza et al., 2013, 2015]. In the prosaccade task, each trial started with the illumination of a central fixation point, which then disappeared and, after a delay of 200 ms (gap period), a peripheral target appeared randomly at 10° to the left or right of the central fixation point. Participants were instructed to complete a saccade to the peripheral target. No error feedback was given. One block of 60 trials was obtained from each participant.
Quality Control of Eye Tracking Measures
Data were analyzed using custom software developed in MATLAB (R2009b, The Mathworks, Inc, Natick, Massachusetts). Saccades were defined as having a speed of greater than 2.5 times the standard deviation of the background noise (measured during fixation) for at least five continuous sample points in time. The only trials used were those for which the participant was fixating on the fixation point at the appropriate time. If the participant broke fixation inappropriately (i.e., not to a target location or away from the screen) the trial was discarded from analysis. Any trials where eye tracking was lost were removed from the analysis, and to be included each participant had to achieve greater than 50% viable trials in the task; under these conditions no participants were excluded.
Saccade Outcome Measures
Saccade performance for all viable trials was assessed by calculating the saccadic reaction time (SRT; the time from the appearance of the peripheral target to the initiation of the first saccade during a correct trial), anticipatory saccades (saccades made before 90 ms after the target disappeared), the deviation of the saccade endpoint (the angular distance between the ideal path from fixation to target and the trajectory of the first saccade toward the goal by drawing a straight line from the beginning to the end of the saccade [see Paolozza et al., 2013 for additional description of these measures)], amplitude (the distance of the saccade in degrees), and the percentage of trials with additional saccades (more than one saccade generated in the direction toward the target).
Eye movement measures were age‐corrected using the entire NeuroDevNet control cohort (n = 114, mean age 10.4 years) to calculate a standardized t‐score equation for each age [Paolozza et al., 2014a, b, 2015]. Standard scores for the control and FASD groups in the current study were then calculated using the age‐dependent t‐score equation obtained from the larger control group. These scores were used to run the correlations.
Psychometric Assessment
A battery of psychometric assessments were performed on each child as part of the larger study. Executive functioning was assessed using the Neuropsychological Assessment‐II (NEPSY‐II) subtests of Auditory Attention (ages 5–16), Response Set (ages 7–16), Inhibition (ages 5–16), Animal Sorting (ages 7–16), and Arrows (ages 5–16) [Korkman et al., 2007]. Working memory was assessed using the Working Memory Test Battery for Children (WMTB‐C) subtests of Digit Recall and Block Recall (both ages 5–15) [Gathercole and Pickering, 2001]. All subtests were scored using the provided scoring software, and standard/scaled scores were generated to correct for age.
Correlation Analysis
Hypothesis‐driven partial correlations (correcting for age) were used to identify whether the measures from the prosaccade task (SRT, anticipatory saccades, amplitude, additional saccades, and saccade endpoint) were correlated with FA or MD of specific tracts, with Holm–Sidak correction for multiple comparisons. The particular tracts were chosen based on a literature search for those related to eye movements, namely corpus callosum (body, genu, and splenium), cingulum, and uncinate [Colby et al., 2005; Gross et al., 1977; Holtzman, 1984; Muri and Nyffele, 2008; Noda et al., 1992; Raabe et al., 2013]. Additionally, previous analyses within this same cohort has found group and sex differences between PAE and control participants on SRT, amplitude, additional saccades, and saccade endpoint [Paolozza et al., 2014a, 2014b]. Therefore, correlations of FA and MD of the corpus callosum (body, genu, and splenium), cingulum, and uncinate were assessed versus the SRT, anticipatory saccades, amplitude, additional saccades, and saccade endpoint. Previous studies on this cohort reported group differences between children with PAE and controls on several psychometric tests including Animal Sorting, Auditory Attention, Response Set, Inhibition, Arrows, Digit Recall and Block Recall standard scores [Paolozza et al., 2014b, 2014c]. Therefore, partial correlations (correcting for age) were also conducted for FA and MD of all nine tracts (left and right separately) versus Animal Sorting, Auditory Attention, Response Set, Inhibition, Arrows, Digit Recall, and Block Recall standard scores.
RESULTS
DTI Group Comparisons and Interactions with Age and Sex
Of the 15 tracts examined, 7 exhibited statistically significant differences in FA between the control and PAE groups after multiple comparison corrections (adjusted P‐values are presented in text and in Fig. 1A). Main effects of group were found in the genu (F(1,134) = 4.29, P = 0.04), body (F(1, 123) = 6.80, P = 0.01) and splenium (F(1,129) = 4.47, P = 0.036) regions of the corpus callosum, left cingulum (F(1,130) = 4.84, P = 0.03), right corticospinal tract (F(1,131) = 4.92, P = 0.028), left superior longitudinal fasciculus (F(1,128) = 5.28, P = 0.015), and left uncinate fasciculus (F(1,128) = 5.28, P = 0.015) with the PAE group exhibiting lower FA compared with the control group in all cases.
Figure 1.
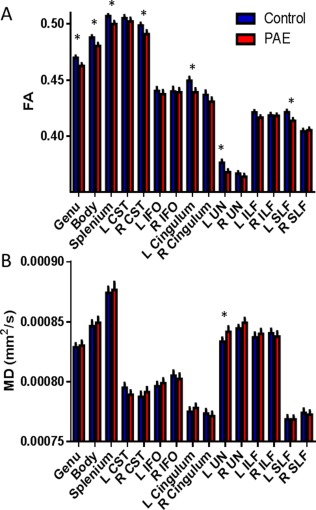
Group differences for FA and MD (mean ± SEM) are presented for the control (shown in blue; n = 78) and PAE (shown in red; n = 62) groups. The PAE group displayed decreased FA in 7 of the 15 tracts and increased MD in 1 of the 15 tracts examined. * indicates significant differences between groups with P < 0.05 after multiple comparisons. [Color figure can be viewed at http://wileyonlinelibrary.com.]
In addition to these group differences, five tracts exhibited effects of sex for FA either within and/or between groups (Table 2). An interaction between group and sex (F(1,134) = 4.11, P = 0.049) was found for FA of the genu of the corpus callosum. Post‐hoc analysis revealed that the control females had higher FA compared with the females with PAE (P = 0.009; Fig. 2A). There was a significant overall main effect of sex for FA of the left corticospinal tract (F(1,131) = 4.13, P = 0.044), with the males showing higher FA compared with females. A main effect of sex (F(1,135) = 5.26, P = 0.023) and an interaction between group and sex (F(1,135) = 4.15, P = 0.044) were found for the right inferior longitudinal fasciculus. Post‐hoc analysis revealed that the control males had lower FA compared with control females (P = 0.016). An interaction between group and sex (F(1,128) = 6.13, P = 0.043) was found for the left superior longitudinal fasciculus. Post‐hoc analysis revealed that the control females had greater FA compared with the females with PAE (P = 0.003; Fig. 2B). Similarly, an interaction between group and sex (F(1,135) = 5.65, P = 0.039) was found for FA of the left uncinate fasciculus. Post‐hoc analysis revealed that again control females had higher FA than females with PAE (P = 0.005; Fig. 2C)
Table 2.
P values for group (PAE vs. controls), sex differences, and group by sex interactions of FA and MD
| TRACT | GROUP | SEX | GROUP by SEX | |
|---|---|---|---|---|
| Genu of the corpus callosum | FA | 0.040* | 0.407 | 0.049* |
| MD | 0.699 | 1.000 | 0.822 | |
| Body of the corpus callosum | FA | 0.010* | 0.744 | 0.387 |
| MD | 0.615 | 0.532 | 0.333 | |
| Splenium of the corpus callosum | FA | 0.036* | 0.352 | 0.367 |
| MD | 0.755 | 0.119 | 0.858 | |
| L Corticospinal | FA | 0.183 | 0.040* | 0.626 |
| MD | 0.441 | 0.496 | 1.000 | |
| R Corticospinal | FA | 0.028* | 0.138 | 0.886 |
| MD | 0.283 | 0.623 | 0.390 | |
| L Inferior fronto‐occipital fasciculus | FA | 0.365 | 0.058 | 0.864 |
| MD | 0.521 | 0.379 | 0.464 | |
| R Inferior fronto‐occipital fasciculus | FA | 0.864 | 0.054 | 0.403 |
| MD | 0.719 | 0.999 | 0.708 | |
| L Cingulum | FA | 0.030* | 0.708 | 0.413 |
| MD | 0.347 | 0.455 | 0.282 | |
| R Cingulum | FA | 0.310 | 0.508 | 0.073 |
| MD | 0.735 | 1.000 | 0.066 | |
| L Uncinate fasciculus | FA | 0.019* | 0.654 | 0.039* |
| MD | 0.046* | 0.517 | 0.743 | |
| R Uncinate fasciculus | FA | 0.308 | 0.816 | 0.079 |
| MD | 0.172 | 0.173 | 0.204 | |
| L Inferior longitudinal fasciculus | FA | 0.059 | 0.412 | 0.162 |
| MD | 0.374 | 0.776 | 1.000 | |
| R Inferior longitudinal fasciculus | FA | 0.938 | 0.023* | 0.044* |
| MD | 0.639 | 0.168 | 0.233 | |
| L Superior longitudinal fasciculus | FA | 0.015* | 0.530 | 0.043* |
| MD | 0.999 | 0.375 | 0.152 | |
| R Superior longitudinal fasciculus | FA | 0.721 | 0.403 | 0.111 |
| MD | 0.775 | 0.477 | 0.033* |
FA, fractional anisotropy; MD, mean diffusivity; L, left; R, right; * indicates significance after multiple comparisons.
Figure 2.
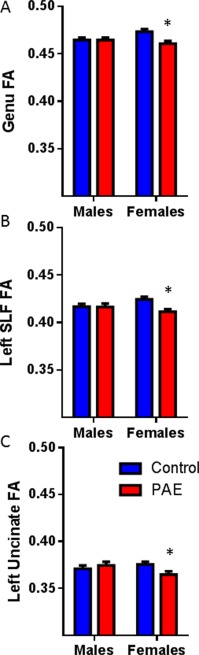
White matter tract FA group and sex interactions. Group differences for FA is presented for the genu (A), left superior longitudinal fasciculus (SLF; B) and the left uncinate fasciculus (UF; C). Females with PAE had significantly lower FA compared with control females, with no differences between males with PAE and control males. All tracts had overall group differences and significant interactions between sex and group. * indicates significant differences between groups with P < 0.05 after multiple comparisons. [Color figure can be viewed at http://wileyonlinelibrary.com.]
In contrast, only one tract exhibited a main group difference of MD (Fig. 1B), namely the left uncinate fasciculus (F(1,135) = 4.07, P = 0.045) with the PAE group showing increased MD compared with controls. There was a significant interaction between group and sex (F(1,132) = 4.63, P = 0.033) for the right superior longitudinal fasciculus MD, but no main effects of group or sex.
As shown in Table 3, FA increased with age for the majority of the tracts in both groups, 73% (11/15 tracts) in controls and 93% (14/15 tracts) in PAE. The same proportions of tracts showed reductions of MD with age.
Table 3.
Age correlations for the FA and MD of all tracts
| TRACT | CON FA vs. age | PAE FA vs. age | CON MD vs. Age | PAE MD vs. age |
|---|---|---|---|---|
| (r, P) | (r, P) | (r, P) | (r, P) | |
| Genu of the corpus callosum | 0.10, 0.417 | 0.28, 0.019* | −0.14, 0.281 | −0.38, 0.001* |
| Body of the corpus callosum | 0.28, 0.0288* | 0.42, 0.0006* | −0.26, 0.046* | −0.43, <0.0001* |
| Splenium of the corpus callosum | 0.12, 0.356 | 0.052, 0.672 | −0.057, 0.651 | −0.41, 0.001* |
| L Corticospinal | 0.39, 0.0014* | 0.43, 0.0003* | −0.29, 0.017* | −0.43, <0.0001* |
| R Corticospinal | 0.070, 0.587 | 0.41, 0.0006* | −0.58, <0.0001* | −0.26, 0.035* |
| L Inferior fronto‐occipital fasciculus | 0.30, 0.014* | 0.46, <0.0001* | −0.10, 0.438 | −0.11, 0.351 |
| R Inferior fronto‐occipital fasciculus | 0.30, 0.015* | 0.43, 0.0002* | −0.12, 0.351 | −0.26, 0.046* |
| L Cingulum | 0.19, 0.356 | 0.31, 0.010* | −0.43, <0.0001* | −0.40, 0.001* |
| R Cingulum | 0.28, 0.034* | 0.24, 0.049* | −0.39, 0.001* | −0.43, <0.0001* |
| L Uncinate fasciculus | 0.40, 0.0009* | 0.38, 0.0014* | −0.42, <0.0001* | −0.47, <0.0001* |
| R Uncinate fasciculus | 0.51, <0.0001* | 0.40, 0.0006* | −0.52, <0.0001* | −0.50, <0.0001* |
| L Inferior longitudinal fasciculus | 0.38, 0.0019* | 0.35, 0.0032* | −0.46, <0.0001* | −0.39, 0.001* |
| R Inferior longitudinal fasciculus | 0.27, 0.027* | 0.33, 0.0069* | −0.41, 0.001* | −0.47, <0.0001* |
| L Superior longitudinal fasciculus | 0.25, 0.048* | 0.46, <0.0001* | −0.63, <0.0001* | −0.48, <0.0001* |
| R Superior longitudinal fasciculus | 0.31, 0.014* | 0.36, 0.0027* | −0.45, <0.0001* | −0.43, <0.0001* |
Con, control; PAE, prenatal alcohol exposure; FA, fractional anisotropy; MD, mean diffusivity; L, left; R, right; * indicates significant correlation after multiple comparisons.
DTI Correlational Analysis
Hypothesis‐driven correlations were run between the prosaccade task and FA and MD of five specific white matter tracts. After correcting for multiple comparisons, several correlations were found with measures involved in saccade accuracy. Increased MD of the body of the corpus callosum was found to be significantly correlated to an increase in additional saccades in control participants (r = 0.465, P = 0.005; Fig. 3A). FA of the body of the corpus callosum was also trending toward significance in the controls with an increase in FA correlating to a decrease in additional saccades (r = −0.4413, P = 0.009), but this result did not pass multiple comparisons. In contrast, there was no correlation found in this tract in the PAE group (Fig. 3B). Similarly, a negative correlation was found between FA of the genu of the corpus callosum and additional saccades in the control group, with a higher FA correlating to less additional saccades (r = −0.484, P = 0.003; Fig. 3C). Again, no correlation was found for this tract in the PAE group (Fig. 3D). A negative correlation was also found in the control group for right uncinate FA and saccade endpoint angle of error (r = −0.561, P = 0.0006; Fig. 4A), where an increase in FA correlated to a decrease in the angle of error. This same correlation was not found in the PAE group (Fig. 4B). The only significant result found in the PAE group was a positive correlation between MD of the left cingulum and additional saccades (r = 0.386, P = 0.006; Fig. 4D), in which higher MD correlated with a greater frequency of additional saccades. This result was not found in the control group (Fig. 4C).
Figure 3.
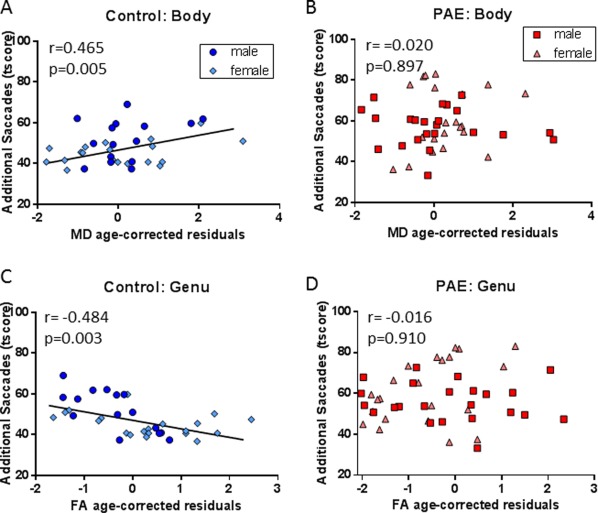
DTI and eye tracking correlations in the corpus callosum. Body MD residuals were positively correlated to additional saccades t‐score in the control group (A; n = 34) but not the PAE group (B; n = 45). Genu FA residuals were negatively correlated with additional saccades t‐score in the control group (C; n = 35) but not the PAE group (D; n = 51). All measures have been age corrected using residuals or t‐scores. [Color figure can be viewed at http://wileyonlinelibrary.com.]
Figure 4.
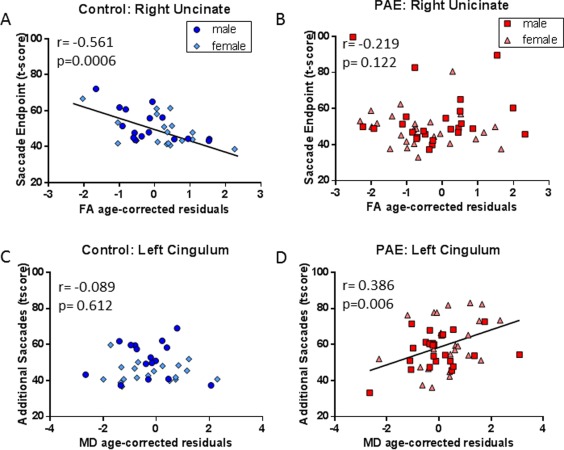
DTI and eye tracking correlations in two temporal‐frontal tracts. The age‐corrected right uncinate FA residuals were negatively correlated to the age‐corrected saccade endpoint t‐score in the control group (A; n = 34) but not the PAE group (B; n = 51). Left cingulum MD residuals were positively correlated with additional saccades t‐score in the PAE group (D; n = 35) but not the control group (C; n = 49). [Color figure can be viewed at http://wileyonlinelibrary.com.]
Psychometric testing did not correlate with DTI parameters in either group.
Study Population Findings
Differences in sex distribution between the two groups were calculated by performing Fisher's exact test for both groups and no significant differences were found. Age was analyzed via a t‐test and no significant differences were found, indicating these two groups were well matched for age and sex. PAE subtype was investigated by dividing the group into two subgroups (diagnosed with FASD and exposed but undiagnosed group) and comparing these subgroups to the control group on all eye tracking, psychometric, and DTI measures using a one‐way ANOVA. No significant differences were detected between the undiagnosed group compared with the FASD group, indicating the alcohol exposed but undiagnosed group performed similarly to the diagnosed FASD group. Due to the relatively low number of comorbidities in the PAE group, only attention deficit hyperactivity disorder (ADHD) could be properly investigated. This was accomplished by dividing the PAE group into those with ADHD and those without and comparing the two groups on all measures using a t‐test. No significant differences were found, indicating that a comorbidity of ADHD did not affect the data for this cohort. Next, SES was investigated using a t‐test to compare the two groups, with the PAE group showing a trend toward lower SES but no significant results were found. However, we ran correlations between SES score and each eye movement, psychometric, and DTI (FA and MD) score and no significant relationships were detected indicating that SES did not affect any scores in this cohort. Finally, ethnicity was examined in the same way as ADHD by dividing the PAE group into those identified as First Nations and those with any other ethnicity (primarily Caucasian). Using a t‐test, no significant differences were found between the two groups. Therefore, sex, diagnostic subgroup, comorbidities, SES, and ethnicity did not significantly influence the data for this cohort and did not need to be included as covariates.
DISCUSSION
This study investigated the integrity of multiple white matter tracts in the brain of children with PAE and how these measures relate to eye tracking measurements as a proxy for brain function. Most of the tracts had DTI measures that correlated linearly with age in both groups. However, children with PAE had significantly lower FA in 7, and higher MD in 1 white matter tracts compared with typically developing control children. There were significant effects of sex and interactions between group and sex for FA in multiple white matter tracts. In three of these tracts (genu, left superior longitudinal fasciculus, left uncinate), the interaction was driven by the females with PAE exhibiting lower FA than control females. When the outcome measures from the eye movement control task were correlated with the DTI measures several significant relationships were found in the control group that were absent in the PAE group. There were no correlations between DTI measures and psychometric test scores that passed the threshold for multiple comparison corrections.
The linear increase of FA and decrease of MD with age is a common finding in the DTI literature during typical childhood and adolescence [Ashtari et al., 2007; Barnea‐Goraly et al., 2005; Ben et al., 2005; Lebel et al., 2012; Snook et al., 2007]. Interestingly, the PAE group had a greater number of tracts that changed linearly with age for both FA and MD (14 tracts vs. 11 in controls), suggesting that the PAE group may be catching up to the control group. A cross‐sectional study suggested that diffusion parameters change with age in PAE, although age was not a primary focus of the study and the analysis with age was limited due to small sample sizes [Lebel et al., 2008]. A longitudinal DTI study confirmed FA increases and MD decreases over several years between scans within individuals with PAE, with the degree of change related to specific cognitive changes. The study also found greater reductions of MD with age compared with controls [Treit et al., 2013] that were interpreted as delayed compensation of underlying cellular events such as myelination.
Several white matter tracts in the brain were found to have significantly different diffusion parameters between the children with PAE and controls. In agreement with previous studies, the genu, body, and splenium of the corpus callosum were found to have lower FA in the PAE group compared with controls [Fryer et al., 2009; Lebel et al., 2008; Ma et al., 2005; Wozniak et al., 2009]. Asymmetrical group differences were seen on several tracts including decreased FA in the left cingulum, left superior longitudinal fasciculus, left uncinate fasciculus, and right corticospinal tract. Asymmetrical DTI findings are not uncommon in PAE [Donald et al., 2015; Fan et al., 2016; Fryer et al., 2009; Lebel et al., 2008; Sowell et al., 2008; Spottiswoode et al., 2011]. There was only one group difference found for MD, with the left uncinate fasciculus showing increased MD in the PAE group. Lebel et al. [2008] found elevated MD in the right corticospinal tract, bilateral inferior fronto‐occipital fasciculus, and left inferior longitudinal fasciculus. Ma et al. [2005] found elevated MD in the corpus callosum. However, the specific tracts found to be different are not consistent between studies, which may be due to differences in age, diagnosis, disease severity, sample size, or methodology (different scanners, number of directions, analysis, etc.).
Prior DTI studies have combined male and female PAE participants (due to small sample sizes) and did not examine potential sex effects. Recently, sex differences were reported in a subset of this PAE cohort for eye movement scores on the prosaccade task. The results indicated that males with FASD had decreased accuracy while females with FASD showed selective deficits on several measures of saccade metrics such as velocity, deceleration, and duration [Paolozza et al., 2015]. Importantly, in the current study females with PAE were found to have lower FA in the genu, left corticospinal tract, left superior longitudinal fasciculus, and left uncinate compared with female controls. Many animal studies have also found both physiological and behavioral sex‐dependent differences when examining PAE [Halasz et al., 1993; Kelly et al., 2009]. For example, increased impairment in female animals was found for spatial working memory after PAE [Weinberg, 1992a, 1992b]. The mechanism of how this occurs is unknown but animal studies have suggested that PAE may be altering the gonadal‐adrenal interactions during fetal development [Carter et al., 2014; Weinberg et al., 2008].
A positive relationship was found between MD of the body of the corpus callosum and additional saccades in control participants only. Similarly, there was a negative correlation between FA of the genu of the corpus callosum and additional saccades found in the control but not PAE group. The corpus callosum has been found to be important for the transfer of visual information across hemispheres, which is essential for the coordinated movement of the eyes toward a visual target. Studies of split‐brain humans and monkeys have demonstrated the necessity of the corpus callosum for the integration of visual and visuomotor processes [Colby et al., 2005; Gross et al., 1977; Holtzman, 1984]. The PAE group did not exhibit these relationships, which is not surprising since a lower FA and increased variability was found in both of these tracts, indicating damage or delayed development. The PAE group did, however, show a positive correlation between MD of the left cingulum and additional saccades. This finding was surprising as previous research in healthy controls has found that the cingulum plays a role in higher cognitive functions during saccade tasks and but not in more automatic behaviors [Muri and Nyffeler, 2008; Raabe et al., 2013]. This suggests that children with PAE may have to engage higher order brain processes to successfully complete the prosaccade task. This conclusion is further supported by other brain imaging studies which also report differential activity in the PAE group compared with controls, including the recruitment of additional brain regions not used by controls [Diwadkar et al., 2012; Gautan et al., 2015; O'Hare et al., 2009].
A negative correlation was found in the control group for right uncinate FA and saccade endpoint angle of error, fitting with previous assertions that the uncinate conveys signals for the horizontal component of saccades [Noda et al., 1992]. Again, this relationship was not seen in the PAE group, which displayed significantly lower FA and higher MD of the left uncinate. Additionally, the PAE group had significantly increased endpoint angle of error and were therefore less accurate when looking to the target. Again, this would be consistent with damage to or delayed development of this pathway causing a larger error in saccade accuracy.
Finally, we also examined correlations with the psychometric scores, however, because of the large number of correlations none passed the correction for multiple comparisons. Other PAE studies have also attempted to find correlations between DTI measures and psychometric test scores without success [Lebel et al., 2008; Li et al., 2009; Ma et al., 2005], although a voxel‐based DTI analysis did find correlations of white matter FA with math ability in PAE [Lebel et al., 2012]. It is hypothesized that correlations are not being found because these tasks require distributed cortical networks, and most psychometric tests give one or two scores for each cognitive domain, which actually encompasses many different but related functions. Eye movement tasks may be a better behavioral measure of specific white matter structures as the pathways are well defined through a large body of literature, and specific measures of the task can be more readily extracted.
CONCLUSIONS
DTI measures of several white matter tracts and the performance of eye movement control tasks were investigated in children with PAE and typically developing controls. The majority of tracts showed developmental changes with age for both FA and MD in both groups, with more tracts changing in the PAE group. Significant group differences were found in eight tracts and interactions for group and sex were found in three tracts, with females in the PAE group exhibiting lower FA compared with control females. Several significant relationships were found between DTI and eye movement measures in the control group, but these same relationships were absent in the PAE group, suggesting that children with PAE may need to recruit additional brain structures to complete the automatic response of looking to a visual target. Although this study needs to be replicated in other more ethnically and clinically balanced populations, our results support the notion that outcome measures obtained using eye movement control tasks may be a valuable functional biomarker of the structural brain injury induced by PAE since they reveal group differences linked to deficits in white matter integrity in the brain.
ACKNOWLEDGMENTS
We thank the participants and their families for taking part in the study. This work was supported by NeuroDevNet, which is funded by the Networks of Centres of Excellence, a program of the federal government of Canada to advance science and technology. We also acknowledge the NeuroDevNet NeuroInformatics Core for data management system implementation and support, Donald Brien for his technical expertise in the analysis of the eye movement data, and Zhang Chen for normalization of the DTI data. This work was also supported by the Women's and Children's Health Research Institute (Edmonton, Alberta) for operating expenses and salary (ST). Salary support was also provided by Alberta Innovates Health Solutions (for co‐authors CB and ST). The authors declare no competing financial interests.
REFERENCES
- Ashtari M, Cervellione KL, Hasan KM, Wu J, McIlree C, Kester H, Ardekani BA, Roofeh D, Szeszko PR, Kumra S (2007): White matter development during late adolescence in healthy males: A cross‐sectional diffusion tensor imaging study. Neuroimage 35:501–510. [DOI] [PubMed] [Google Scholar]
- Autti‐Ramo I, Autti T, Korkman M, Kettunen S, Salonen O, Valanne L (2002): MRI findings in children with school problems who had been exposed prenatally to alcohol. Dev Med Child Neurol 44:98–106. [DOI] [PubMed] [Google Scholar]
- Barnea‐Goraly N, Menon V, Eckert M, Tamm L, Bammer R, Karchemskiy A, Dant CC, Reiss AL (2005): White matter development during childhood and adolescence: A cross‐sectional diffusion tensor imaging study. Cereb Cortex 15:1848–1854. [DOI] [PubMed] [Google Scholar]
- Ben BD, Ben SL, Graif M, Pianka P, Hendler T, Cohen Y, Assaf Y (2005): Normal white matter development from infancy to adulthood: Comparing diffusion tensor and high b value diffusion weighted MR images. J Magn Reson Imaging 21:503–511. [DOI] [PubMed] [Google Scholar]
- Carter RC, Jacobson JL, Dodge NC, Granger DA, Jacobson SW (2014): Effects of prenatal alcohol exposure on testosterone and pubertal development. Alcohol Clin Exp Res 38:1671–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudley AE, Conry J, Cook JL, Loock C, Rosales T, LeBlanc N (2005): Fetal alcohol spectrum disorder: Canadian guidelines for diagnosis. CMAJ 172:S1–S21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman BA, Kodituwakku P, Kodituwakku EL, Romero L, Sharadamma NM, Stone D, Stepen JM (2013): Primary visual response (M100) delays in adolescents with FASD as measured with MEG. Hum Brain Mapp 34:2852–2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby CL, Berman RA, Heiser LM, Saunders RC (2005): Corollary discharge and spatial updating: When the brain is split is space still unified?. Prog Brain Res 149:187–205. [DOI] [PubMed] [Google Scholar]
- Das S, Zijdenbos AP, Harlap J, Vins D, Evans AC (2011): LORIS: A web‐based data management system for multi‐center studies. Front Neuroinform 5:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diwadkar VA, Meintjes EM, Goradia D, Dodge NC, Warton C, Molteno CD, Jacobson S, Jacobson JL (2012): Differences in cortico‐striatal‐cerebellar activation during working memory in syndromal and nonsyndromal children with prenatal alcohol exposure. Hum Brain Mapp 8:1931–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donald KA, Roos A, Fouche JP, Koen N, Howells FM, Woods RP, Zar HJ, Narr KL Stein DJ (2015): A study of the effects of prenatal alcohol exposure on white matter microstructural integrity at birth. Acta Neuropsychiatrica 27:197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Jacobson SW, Taylor PA, Molteno CD, Dodge NC, Stanton ME, Jacobson JL, Meintjes EM (2016): White matter deficits mediate effects of prenatal alcohol exposure on cognitive development in childhood. Hum Brain Mapp 37:2943–2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer SL, Schweinsburg BC, Bjorkquist OA, Frank LR, Mattson SN, Spadoni AD, Riley EP (2009): Characterization of white matter microstructure in fetal alcohol spectrum disorders. Alcohol Clin Exp Res 33:514–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautan P, Nunex SC, Narr KL, Mattson SN, May PA, Adnams CM, Riley EP, Jones KL, Kan EC, Sowell ER (2015): Developmental trajectories for visuo‐spatial attention are altered by prenatal alcohol exposure: A longitudinal fmri study. Cereb Cortex 12:4761–4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gathercole SE, Pickering SJ (2001): Working Memory Test Battery for Children. London, England: Psychological Corporation Europe. [Google Scholar]
- Green CR, Munoz DP, Nikkel SM, Reynolds JN (2007): Deficits in eye movement control in children with fetal alcohol spectrum disorders. Alcohol Clin Exp Res 31:500–511. [DOI] [PubMed] [Google Scholar]
- Gross CG, Bender DB, Mishkin M (1977): Contributions of the corpus callosum and the anterior commissure to visual activation of inferior temporal neurons. Brain Res 131:227–239. [DOI] [PubMed] [Google Scholar]
- Halasz I, Aird F, Li L, Prystowsky MB, Redei E (1993): Sexually dimorphic effects of alcohol exposure in utero on neuroendocrine and immune functions in chronic alcohol‐exposed adult rats. Mol Cell Neurosci 4:343–353. [DOI] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG (2009): Research electronic data capture (REDCap)–a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemington KS, Reynolds JN (2014): Electroencephalographic correlates of working memory deficits in children with Fetal Alcohol Spectrum Disorder using a single‐electrode pair recording device. Clin Neurophysiol 125:2364–2371. [DOI] [PubMed] [Google Scholar]
- Hollingshead A (2011): Four factor index of social status. Yale J Sociol 8:21–51. [Google Scholar]
- Holtzman JD (1984): Interactions between cortical and subcortical visual areas: Evidence from human commissurotomy patients. Vis Res 24:801–813. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Jacobson JL, Stanton ME, Meintjes EM, Molteno CD (2011): Biobehavioral markers of adverse effect in fetal alcohol spectrum disorders. Neuropsychol Rev 21:148–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly SJ, Leggett DC, Cronise K (2009): Sexually dimorphic effects of alcohol exposure during development on the processing of social cues. Alcohol Alcohol 44:555–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodituwakku PW (2009): Neurocognitive profile in children with fetal alcohol spectrum disorders. Dev Disabil Res Rev 15:218–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkman M, Kirk U, Kemp S (2007): NEPSY‐II: Clinical and Interpretation Manual. San Antonio TX: Harcourt Assessment. [Google Scholar]
- Lebel C, Rasmussen C, Wyper K, Walker L, Andrew G, Yager J, Beaulieu C (2008): Brain diffusion abnormalities in children with fetal alcohol spectrum disorder. Alcohol Clin Exp Res 32:1732–1740. [DOI] [PubMed] [Google Scholar]
- Lebel C, Gee M, Camicioli R, Wieler M, Martin W, Beaulieu C (2012): Diffusion tensor imaging of white matter tract evolution over the lifespan. Neuroimage 60:340–352. [DOI] [PubMed] [Google Scholar]
- Li L, Coles CD, Lynch ME, Hu X (2009): Voxelwise and skeleton‐based region of interest analysis of fetal alcohol syndrome and fetal alcohol spectrum disorders in young adults. Hum Brain Mapp 30:3265–3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Coles CD, Lynch ME, Laconte SM, Zurkiya O, Wang D, Hu X (2005): Evaluation of corpus callosum anisotropy in young adults with fetal alcohol syndrome according to diffusion tensor imaging. Alcohol Clin Exp Res 29:1214–1222. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Crocker N, Nguyen TT (2011): Fetal alcohol spectrum disorders: Neuropsychological and behavioral features. Neuropsychol Rev 21:81–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meintjes EM, Narr KL, van der Kouwe AJ, Molteno CD, Pirnia T, Gutman B, Woods RP, Thompson PM, Jacobson JL, Jacobson SW (2014): A tensor‐based morphometry analysis of regional differences in brain volume in relation to prenatal alcohol exposure. Neuroimage Clin 5:152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muri RM, Nyffeler T (2008): Using transcranial magnetic stimulation to probe decision‐making and memory. Prog Brain Res 171:413–418. [DOI] [PubMed] [Google Scholar]
- Noda H, Sato H, Ikeda Y, Sugita S (1992): Fastigiofugal fibers encoding horizontal and vertical components of saccades as determined by microstimulation in monkeys. Neurosci Res 13:163–173. [DOI] [PubMed] [Google Scholar]
- O'Hare ED, Lu LH, Houston SM, Bookheimer SY, Mattson SN, O'Connor MJ, Sowell ER (2009): Altered frontal‐parietal functioning during verbal working memory in children and adolescents with heavy prenatal alcohol exposure. Hum Brain Mapp 10:3200–3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolozza A, Titman R, Brien D, Munoz DP, Reynolds JN (2013): Altered accuracy of saccadic eye movements in children with fetal alcohol spectrum disorder. Alcohol Clin Exp Res 37:1491–1498. [DOI] [PubMed] [Google Scholar]
- Paolozza A, Rasmussen C, Pei J, Hanlon‐Dearman A, Nikkel SM, Andrew G, McFarlane A, Samdu D, Reynolds JN (2014a): Deficits in response inhibition correlate with oculomotor control in children with fetal alcohol spectrum disorder and prenatal alcohol exposure. Behav Brain Res 259:97–105. [DOI] [PubMed] [Google Scholar]
- Paolozza A, Rasmussen C, Pei J, Hanlon‐Dearman A, Nikkel SM, Andrew G, McFarlane A, Samdu D, Reynolds JN (2014b): Working memory and visuospatial deficits correlate with oculomotor control in children with fetal alcohol spectrum disorder. Behav Brain Res 263:70–79. [DOI] [PubMed] [Google Scholar]
- Paolozza A, Treit S, Beaulieu C, Reynolds JN (2014c): Response inhibition deficits in children with Fetal Alcohol Spectrum Disorder: Relationship between diffusion tensor imaging of the corpus callosum and eye movement control. Neuroimage Clin 5:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolozza A, Munn R, Munoz DP, Reynolds JN (2015): Eye movements reveal sexually dimorphic deficits in children with fetal alcohol spectrum disorder. Front Neurosci 9:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raabe M, Fischer V, Bernhardt D, Greenlee MW (2013): Neural correlates of spatial working memory load in a delayed match‐to‐sample saccade task. Neuroimage 71:84–91. [DOI] [PubMed] [Google Scholar]
- Rasmussen C (2005): Executive functioning and working memory in fetal alcohol spectrum disorder. Alcohol Clin Exp Res 29:1359–1367. [DOI] [PubMed] [Google Scholar]
- Rasmussen C, Andrew G, Zwaigenbaum L, Tough S (2008): Neurobehavioural outcomes of children with fetal alcohol spectrum disorders: A Canadian perspective. Paediatr Child Health 13:185–191. [PMC free article] [PubMed] [Google Scholar]
- Reynolds JN, Weinberg J, Clarren S, Beaulieu C, Rasmussen C, Kobor M, Dube MP, Goldowitz D (2011): Fetal alcohol spectrum disorders: Gene‐environment interactions predictive biomarkers and the relationship between structural alterations in the brain and functional outcomes. Semin Pediatr Neurol 18:49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley EP, Mattson SN, Sowell ER, Jernigan TL, Sobel DF, Jones KL (1995): Abnormalities of the corpus callosum in children prenatally exposed to alcohol. Alcohol Clin Exp Res 19:1198–1202. [DOI] [PubMed] [Google Scholar]
- Snook L, Plewes C, Beaulieu C (2007): Voxel based versus region of interest analysis in diffusion tensor imaging of neurodevelopment. Neuroimage 34:243–252. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Johnson A, Kan E, Lu LH, Van Horn JD, Toga AW, O'Connor MJ, Bookheimer SY (2008): Mapping white matter integrity and neurobehavioral correlates in children with fetal alcohol spectrum disorders. J Neurosci 28:1313–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spottiswoode BS, Meintjes EM, Anderson AW, Molteno CD, Stanton ME, Dodge NC, Gore JC, Peterson BS, Jacobson JL, Jacobson SW (2011): Diffusion tensor imaging of the cerebellum and eyeblink conditioning in fetal alcohol spectrum disorder. Alcohol Clin Exp Res 35:2174–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephen JM, Coffman BA, Stone DB, Kodituwakku P (2013): Differences in MEG gamma oscillatory power during performance of a prosaccade task in adolescents with FASD. Front Hum Neurosci 7:900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swayze VW, Johnson VP, Hanson JW, Piven J, Sato Y, Giedd JN, Mosnik D, Andreasen NC (1997): Magnetic resonance imaging of brain anomalies in fetal alcohol syndrome. Pediatrics 99:232–240. [DOI] [PubMed] [Google Scholar]
- Treit S, Lebel C, Baugh L, Rasmussen C, Andrew G, Beaulieu C (2013): Longitudinal MRI reveals altered trajectory of brain development during childhood and adolescence in fetal alcohol spectrum disorders. J Neurosci 33:10098–10109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakana S, Jiang H, Nagae‐Poetscher LM, van Zijl PC, Mori S (2004): Fiber tract‐based atlas of human white matter anatomy. Radiology 230:77–87. [DOI] [PubMed] [Google Scholar]
- Weinberg J (1992a): Prenatal ethanol effects: Sex differences in offspring stress responsiveness. Alcohol 9:219–223. [DOI] [PubMed] [Google Scholar]
- Weinberg J (1992b): Prenatal ethanol exposure alters adrenocortical response to predictable and unpredictable stressors. Alcohol 9:427–432. [DOI] [PubMed] [Google Scholar]
- Weinberg J, Sliwowska JH, Lan N, Hellemans KG (2008): Prenatal alcohol exposure: Foetal programming the hypothalamic‐pituitary‐adrenal axis and sex differences in outcome. J Neuroendocrinol 20:470–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak JR, Muetzel RL, Mueller BA, McGee CL, Freerks MA, Ward EE, Nelson ML, Chang PN, Lim KO (2009): Microstructural corpus callosum anomalies in children with prenatal alcohol exposure: An extension of previous diffusion tensor imaging findings. Alcohol Clin Exp Res 33:1825–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Yushkevich PA, Alexander DC, Gee JC (2006): Deformable registration of diffusion tensor MR images with explicit orientation optimization. Medical Image Analysis ‐ Special Issue: The Eighth International Conference on Medical Imaging and Computer Assisted intervention ‐ MICCAI 2005. 10:764–785. [DOI] [PubMed]


