Abstract
Research in amyotrophic lateral sclerosis (ALS) suggests that executive dysfunction, a prevalent cognitive feature of the disease, is associated with abnormal structural connectivity and white matter integrity. In this exploratory study, we investigated the white matter constructs of executive dysfunction, and attempted to detect structural abnormalities specific to cognitively impaired ALS patients. Eighteen ALS patients and 22 age and education matched healthy controls underwent magnetic resonance imaging on a 4.7 Tesla scanner and completed neuropsychometric testing. ALS patients were categorized into ALS cognitively impaired (ALSci, n = 9) and ALS cognitively competent (ALScc, n = 5) groups. Tract‐based spatial statistics and connectomics were used to compare white matter integrity and structural connectivity of ALSci and ALScc patients. Executive function performance was correlated with white matter FA and network metrics within the ALS group. Executive function performance in the ALS group correlated with global and local network properties, as well as FA, in regions throughout the brain, with a high predilection for the frontal lobe. ALSci patients displayed altered local connectivity and structural integrity in these same frontal regions that correlated with executive dysfunction. Our results suggest that executive dysfunction in ALS is related to frontal network disconnectivity, which potentially mediates domain‐specific, or generalized cognitive impairment, depending on the degree of global network disruption. Furthermore, reported co‐localization of decreased network connectivity and diminished white matter integrity suggests white matter pathology underlies this topological disruption. We conclude that executive dysfunction in ALSci is associated with frontal and global network disconnectivity, underlined by diminished white matter integrity. Hum Brain Mapp 38:1249–1268, 2017. © 2016 Wiley Periodicals, Inc.
Keywords: motor neuron disease, amyotrophic lateral sclerosis, white matter integrity, graph theory, tract‐based spatial statistics, executive function, structural connectivity
INTRODUCTION
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disorder characterized by degeneration of both upper motor neurons of the corticospinal and corticobulbar tracts, as well as lower motor neurons of the peripheral nervous system. While symptoms, such as muscular weakness, spasticity, and hyperreflexia, are initially manageable, progressive loss of respiratory muscle innervation leads to respiratory failure, which typically occurs within 2–4 years of symptom onset [Kiernan et al., 2011]. In addition to motor system pathology, it is now widely accepted that ALS is a multisystem disorder frequently accompanied by cognitive deficits and behavioral changes. Cognitive and behavioral impairment in ALS is multifaceted, including deficits in language, memory, social cognition, emotional processing, and theory of mind [Goldstein and Abrahams, 2013]. These deficits are related to syndromes of frontotemporal dysfunction, underlined by frontotemporal lobar degeneration (FTLD) [Woolley and Strong, 2015]. While frontotemporal dysfunction in ALS may present as a behavioral impairment (ALSbi) or as frontotemporal dementia (ALS‐FTD), the cognitively impaired (ALSci) syndrome is the most prevalent, with 35%–50% of patients exhibiting mild to moderate cognitive impairment [Strong, 2008]. Executive dysfunction, the most prevalent manifestation of cognitive impairment in ALS, is present in more than 40% of non‐demented ALS patients [Lonergan et al., 2014], and has been shown to be an indicator of poor prognosis, independent of a diagnosis of FTD [Elamin et al., 2011].
Executive dysfunction typically presents itself as a deficit in verbal fluency, set shifting, cognitive inhibition, selective attention, or memory [Lonergan et al., 2014], and has been shown through lesion and functional imaging studies to be related to frontal cortex damage [Alvarez and Emory, 2006]. A common finding of white matter studies in ALS utilizing diffusion tensor imaging (DTI) tractography [Sarro et al., 2011] and tract‐based spatial statistics (TBSS) [Ciccarelli et al., 2009; Prudlo et al., 2012; Zhang et al., 2014] is a reduction in fractional anisotropy (FA) within motor, as well as frontal and prefrontal regions. FA has been demonstrated to be a particularly sensitive DTI metric for detection of white matter degeneration in ALS [Tang et al., 2015]. FA has also been suggested to be a reliable metric in the prediction of ALS diagnosis [Tang et al., 2015] and disease progression rate [Agosta et al., 2010; Menke et al., 2012]. A similar observation in ALS is made when investigating neuronal connectomics by application of graph theory [Verstraete et al., 2011, 2014], a method for reconstruction and assessment of brain connectivity networks through representation of parcellated cortical and subcortical regions as nodes, and interconnecting white matter tracts as edges on a mathematical graph [Bullmore and Sporns, 2009; Minati et al., 2013]. Studies have found that the topological networks of ALS patients exhibit reduced local connectivity that expands beyond motor cortices, primarily propagating to frontal and parietal regions [Verstraete et al., 2011, 2014]. Regions of decreased structural network connectivity in ALS have also been shown to co‐localize with regions of reduced FA, suggesting that diminished white matter tract structural integrity may underlie network disconnectivity [Buchanan et al., 2015].
While predominantly frontally mediated, the multimodal nature of executive function, encompassing cognitive domains such as working memory, verbal reasoning, and problem solving, is suggestive of its dependence on various neuronal regions and their connecting white matter tracts. Previous studies utilizing graph theory and TBSS in neurodegenerative disorders such as Alzheimer's disease (AD) [Lo et al., 2010], cerebral amyloid angiopathy (CAA) [Reijmer et al., 2015], and Parkinson's disease (PD) [Rae et al., 2012], provide support for this notion in reporting associations between executive function performance and local connectivity and white matter integrity of regions throughout the frontal, temporal, parietal and occipital cortices. Within ALS, correlations between poorer executive function and decreased white matter integrity of fronto‐temporal association fibers [Sarro et al., 2011], as well as white matter in the superior and inferior frontal gyrus, corpus callosum, and hippocampal portion of the cingulum [Pettit et al., 2013], provide further support of a relationship between a global structural integrity deficit and executive dysfunction.
While these studies [Lo et al., 2010; Rae et al., 2012; Reijmer et al., 2015] suggest that neurodegeneration of inter‐cortical white matter tracts, and disconnectivity of the topological network may underlie executive dysfunction in neurodegenerative disorders, few studies have specifically investigated this relationship within ALS. Furthermore, despite accumulating evidence of frontal white matter abnormalities [Ciccarelli et al., 2009; Pettit et al., 2013; Prudlo et al., 2012; Sarro et al., 2011; Zhang et al., 2014], disrupted topological networks [Buchanan et al., 2015; Verstraete et al., 2011, 2014] and a high prevalence of executive dysfunction in ALS [Lonergan et al., 2014], only one study to date has attempted to determine if extra‐motor white matter abnormalities are specific to cognitively impaired, as compared with cognitively competent, ALS patients [Sarro et al., 2011]. While it was shown by Sarro et al., [2011] that ALS cognitively impaired (ALSci) patients display more extra‐motor reductions in structural integrity relative to controls, DTI tractography failed to identify differences in white matter microstructure in a direct comparison between ALSci and ALS cognitively competent (ALScc) patients.
In this exploratory study we aimed to elucidate the relationship between executive dysfunction in ALS and underlying abnormalities in structural connectivity and white matter integrity, through comparison of graph theory metrics and TBSS derived FA values between ALSci, ALScc, and healthy controls. We hypothesized that there are structural connectivity and white matter integrity abnormalities specific to ALSci patients. We further hypothesized that these abnormalities would be associated with executive dysfunction.
METHODOLOGY
Participants and Cognitive Testing
Our subject group consisted of 18 ALS patients recruited from the University of Alberta ALS clinic, and 22 age and education matched healthy control subjects (Table 1). All ALS subjects met the revised El Escorial criteria [Brooks et al., 2000] for probable or definite ALS, and had no family history of ALS or FTD. Subjects were included if they did not have other neurological or psychiatric diseases, and had no contraindications to MRI. To avoid confounding effects on cognition from respiratory insufficiency, subjects also had to have a forced vital capacity over 60% to be included in the study. All subjects gave written informed consent, and the study was approved by the Health Research Ethics Board at the University of Alberta. Exclusion criteria included a previous history of neurological or psychiatric disorders (including depression), cerebrovascular disease, brain trauma or stroke, and current use of psychotropic medication. Tasks of executive function included verbal fluency F (VFF), digit span forward (DSF), digit span backward (DSB), and digit ordering maximal span (DOM). DOM is a score derived from the digit ordering test [Hoppe et al., 2000], and is calculated as the maximal span of numbers recalled in ascending order with 0.5 points deducted if only one list was successfully recalled in two trials consisting of the same number of items. ALS patients were categorized into a cognitively impaired and cognitively competent group according to established methodology [Sarro et al., 2011]. For each assessment, the control subject scores were pooled to generate a normal distribution curve and the value corresponding to the 5th percentile was used as the cut‐off score. These cut‐off scores (6.05 for VFF, 6.00 for DSF, 4.05 for DSB, and 4.50 for DOM) were then applied to the ALS patients. ALS patients who were below the 5th percentile on one or more executive function assessments were considered cognitively impaired. In accordance with this differentiation scheme, the patient group was divided into nine ALSci and five ALScc patients. For the remaining four ALS patients only VFF data was available. Since their performance on this task was within the normal range, absence of data on other cognitive assessments made them indistinguishable as either ALSci or ALScc. These participants were therefore excluded from ALSci/ALScc group comparisons, though they were included in group comparisons between ALS patients and healthy controls.
Table 1.
Comparison of group characteristics and executive function scores between subject groups
| ALS vs. Controls | ALSci vs. ALScc | |||||||
|---|---|---|---|---|---|---|---|---|
| ALS (n = 18) | Controls (n = 22) | ALSci (n = 9) | ALScc (n = 5) | |||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Gender (M:F) | 11:7 | 10:12 | 7:2 | 2:3 | ||||
| Age | 57.3 | 11.1 | 55.0 | 8.7 | 57.0 | 11.2 | 54.8 | 9.6 |
| Years of education | 14.7 | 3.0 | 15.3 | 2.3 | 14.2 | 1.8 | 16.2 | 3.4 |
| ALSFRS‐R | 38.0 | 8.6 | – | – | 38.0 | 4.8* | 44.0 | 2.7 |
| Disease duration (months) | 19.8 | 19.1 | – | – | 22.7 | 24.8 | 18.6 | 11.9 |
| Site of onset (limb:bulbar) | 13:5 | – | 6:3 | 4:1 | ||||
| BDI | 9.3 | 9.0 | 5.6 | 4.5 | 10.5 | 9.9 | 7.4 | 8.1 |
| VFF | 13.2 | 4.1 | 13.7 | 5.3 | 11.3 | 3.7* | 15.4 | 2.0 |
| DSF | 7.9 | 2.2* | 9.6 | 1.9 | 7.2 | 2.4 | 9.2 | 1.3 |
| DSB | 7.2 | 1.9 | 7.6 | 2.0 | 6.7 | 1.8 | 8.2 | 1.9 |
| DOM | 5.3 | 1.5 | 5.9 | 1.3 | 4.7 | 1.5* | 6.3 | 1.2 |
Abbreviations: ALScc, ALS cognitively competent; ALSci, ALS cognitively impaired; ALSFRS‐R, ALS Functional Rating Score Revised; BDI, Beck Depression Inventory; VFF, Verbal Fluency F; DSF, Digit Span Forward; DSB, Digit Span Backward; DOM, Digit Ordering Maximal Span; SD, Standard Deviation. * indicates significant between group difference (uncorrected p < 0.05).
MRI Image Acquisition
MRI imaging was performed on a high field 4.7 Tesla Varian Unity Inova scanner at the Peter S. Allen MR Research Centre at the University of Alberta. DTI images were acquired using a 2D spin‐echo, single‐shot EPI sequence (GRAPPA R = 2). Image acquisition parameters were as follows: TR = 6,500 ms, TE = 52.31 ms, resolution = 2 × 2 mm2, slice thickness = 2 mm, number of slices = 70, number of non‐diffusion weighted images = 5, number of diffusion weighted directions = 30, and b‐value = 1,000 s/mm2.
DTI Processing and Network Reconstruction
Preprocessing of DTI data, including correction of subject motion and eddy current distortions, was conducted using ExploreDTI [Leemans et al., 2009]. Whole brain white matter tracts were constructed for each individual dataset using a deterministic streamline fiber tractography approach in ExploreDTI. Fiber tracts were terminated if they entered a voxel with an FA value lower than 0.2, or if they exceeded an angular threshold of 30°. Tractography was conducted at a step size of 1 mm, and only those fibers with a length of 50–500 mm were retained. Subsequently, application of the automated anatomical labeling atlas (AAL) to the whole brain tractography reconstructions was utilized to parcellate the brain networks into 90 nodes corresponding to regions of interest on the AAL atlas, and interconnecting edges representing the reconstructed white matter tracts. Two AAL node regions were considered to be connected if a fiber bundle either passed through or terminated in each nodal region of interest. Structural connectivity between parcellated regions was assessed using the average FA value of tracts connecting any two nodes on the AAL template. This procedure was used to generate an FA weighted 90 × 90 connectivity matrix for each subject. Justification for the use of fractional anisotropy for connectivity matrix weighting was twofold. Firstly, FA has previously been shown to be a sensitive marker to white matter pathology in ALS [Ciccarelli et al., 2009; Prudlo et al., 2012; Sarro et al., 2011; Zhang et al., 2014], and is a common metric in the assessment of white matter connectivity [Van den Heuvel and Sporns, 2011]. Secondly, the consistent use of FA facilitated a comparison of graph theory and TBSS results.
Network Metrics
Brain Connectivity Toolbox (BCT) [Rubinov and Sporns, 2010] was used to calculate global (i.e., global efficiency, transitivity, and characteristic path length) and local (i.e., local efficiency, clustering coefficient, and strength) network connectivity metrics. These metrics were selected based on previously reported associations in ALS [Buchanan et al., 2015], and studies suggesting these metrics to be the most applicable to the study of network correlates of cognition in neurodegenerative disorders [Lo et al., 2010; Reijmer et al., 2013]. Characteristic path length, a measure of global network connectivity, is defined as the average number of connections that must be traversed in connecting any two nodes along their shortest path. This metric is inversely related to network global efficiency, which reflects the ease with which a network is able to transfer information between brain regions. Local efficiency can be considered a local homolog of global efficiency, quantifying the ease of connectivity within local networks. In structural MRI, these network metrics are used to quantify the potential of a structural network to support functional integration of information across distributed brain regions [Rubinov and Sporns, 2010]. Transitivity and clustering coefficient, on the other hand, are quantifications of network interconnectivity on a global, or local scale, respectively. These metrics provide insight into the extent to which a network is structurally segregated into densely interconnected units; presumably for specialized information processing [Rubinov and Sporns, 2010]. Subject local network topology was additionally assessed via nodal strength, the FA‐weighted sum of a node's edges, which reflects the cumulative structural integrity and number of a node's connections. Strength is a metric of degree or centrality that is utilized to assess the extent of connections and significance of a node in a network [Crossley et al., 2014; Hwang et al., 2013]. Brain network topology has been found to demonstrate small‐world architecture, which is characterized by the presence of hub nodes with a high degree of connectivity that serve as relay stations, transferring information across structurally segregated brain modules [Bullmore and Sporns, 2012; Sporns et al., 2007]. A highly connected highly efficient small‐world network, is therefore one that displays a decreased characteristic path length, and increased global and local efficiency, transitivity, clustering coefficients, and nodal strength [Bullmore and Sporns, 2009, 2012; Sporns et al., 2007].
Tract‐Based Spatial Statistics
Registration and spatial normalization of subject DTI volumes was conducted using DTI Tool Kit (DTI‐TK) [Zhang et al., 2006], in accordance with a recent publication [Bach et al., 2014] recommending the use of DTI‐TK to enhance TBSS specificity. In brief, this involved bootstrapping subject DTI volumes to an initial aging template, followed by affine and diffeomorphic alignment to create a population specific template space [Zhang et al., 2007]. The generated template included the use of four additional subjects (three controls and one ALS patient) that were included in the group comparison of ALS patients and healthy controls, but were excluded from all other analyses due to incomplete neurocognitive data. Next TBSS was used to generate the white matter skeleton from a 1 mm3 resolution mean FA map registered onto a corresponding population specific template, by identifying the voxel with the highest FA value perpendicular to the tract direction as lying on the center of the white matter skeleton [Smith et al., 2006]. A threshold of 0.2 was applied to the mean FA skeleton in order to eliminate voxels with a low FA value, after which aligned subject FA images were projected onto the mean FA skeleton.
Statistical Analysis
Group characteristics were compared using Mann–Whitney U test in IBM SPSS Statistics (version 21.0) [IBM Corp., 2012]. Group comparison of network metrics was similarly conducted using a Mann–Whitney U test, while correlations of executive function scores with network metrics within the ALS patient group was conducted using Spearman's correlation. For TBSS, statistical analysis, including group wise comparison and correlations of executive function scores, was carried out using a threshold‐free clustering enhancement nonparametric test with 5,000 permutations, and family‐wise error (FWE) correction (p < 0.05) for multiple comparison, via the “randomize” function in FSL. In all other statistical analyses, false discovery rate (FDR) was used to correct for multiple comparisons. Due to poor survival rate however, significance is reported as the uncorrected p‐values (p < 0.05) unless specified otherwise.
RESULTS
Group Characteristics
Comparison between ALS patients and healthy controls revealed that there were no significant differences in age, years of education, BDI score, disease duration, and all executive function tasks except for DSF, in which the ALS group was significantly impaired as compared with the healthy control group (Table 1). With regards to the ALSci and ALScc groups, ALSci patients had significantly reduced ALSFRS‐R, VFF, and DOM, as compared with the ALScc group.
Structural Comparison
ALS versus controls
In comparing global network properties of the healthy control and ALS group brain networks, characteristic path length was significantly increased, while transitivity was significantly reduced in the ALS group (Table 2). Global efficiency was not significantly different between the two groups. When assessing the connectivity properties of individual nodes, local clustering coefficients, local efficiency, and nodal strength were significantly reduced in the ALS group in regions throughout the brain, primarily in the frontal and parietal cortices (Fig. 1, Supporting Information Table I). FA was reduced in the ALS group in voxels distributed throughout the brain in bilateral frontal, parietal, occipital and temporal regions (Fig. 2).
Table 2.
Comparison of global network properties in healthy control and ALS subject groups
| Controls (n = 22) | ALS (n = 18) | ||||
|---|---|---|---|---|---|
| Network metric | Mean | SD | Mean | SD | |
| Characteristic path length | 3.621 | 0.234 | 3.801 | 0.247* | |
| Transitivity | 0.291 | 0.017 | 0.280 | 0.013* | |
| Global efficiency | 0.294 | 0.034 | 0.287 | 0.032 | |
Abbreviations: SD, Standard Deviation. * indicates significant between group difference (uncorrected p < 0.05).
Figure 1.

Comparison of control and ALS group local network metrics. Between group comparison showing nodal brain regions in which the ALS group displayed reduced clustering coefficient (left), local efficiency (center), and strength (right), relative to control subjects, in axial (top), sagittal (middle), and coronal (bottom) view (uncorrected p < 0.05). [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 2.
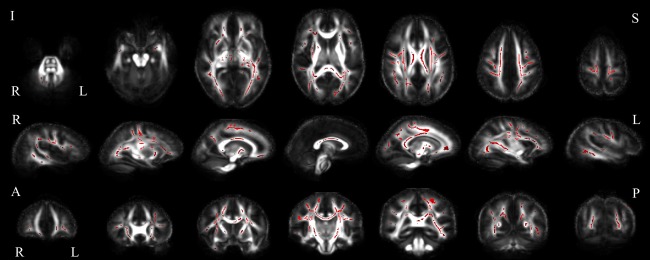
Comparison of control and ALS group FA. Between group comparison showing white matter voxels in which the ALS group displayed decreased FA relative to control subjects, in axial (top), sagittal (middle) and coronal (bottom) slices (uncorrected p < 0.05). Abbreviations: I, inferior; S, superior; R, right; L, left; A, anterior; P, posterior. [Color figure can be viewed at http://wileyonlinelibrary.com]
ALSci versus ALScc
In comparing the ALSci to ALScc groups, clustering coefficients were increased, while strength was decreased in the ALSci group in bilateral frontal and temporal cortices (Fig. 3, Supporting Information Table II). No differences were observed in local efficiency or global network metrics. In terms of white matter integrity, the ALSci group had reduced FA predominantly in clusters in the frontal lobe bilaterally, and the right posterior cingulum (Fig. 4).
Figure 3.

Comparison of ALSci and ALScc group local network metrics. Between group comparison showing nodal brain regions in which the ALSci group displayed increased clustering coefficients (left), no difference in local efficiency (center), and decreased strength (right), relative to ALScc subjects, in axial (top), sagittal (middle), and coronal (bottom) view (uncorrected p < 0.05). [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 4.
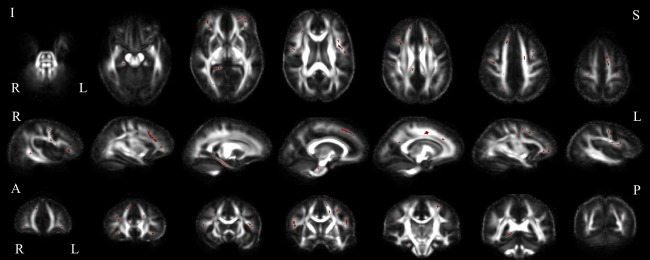
Comparison of ALSci and ALScc group FA. Between group comparison showing white matter voxels in which the ALSci group displayed decreased FA relative to ALScc subjects, in axial (top), sagittal (middle) and coronal (bottom) slices (uncorrected p < 0.05). Abbreviations: I, inferior; S, superior; R, right; L, left; A, anterior; P, posterior. [Color figure can be viewed at http://wileyonlinelibrary.com]
Correlations with Executive Functioning
Verbal fluency F
Global network metrics significantly correlated with performance on the VFF task (Table 3). VFF correlated with all global and local network measures except transitivity. Positive correlations with clustering coefficients were primarily in the right frontal and temporal lobes. A negative correlation with clustering coefficient was observed at one node, the right frontal superior medial gyrus. Positive correlations with nodal strength were observed predominantly in the right temporal and occipital lobes; here, correlations in the right hippocampus, right fusiform and superior occipital gyri, and left lingual cortex were significant after FDR correction (p < 0.05) (Fig. 5, Supporting Information Table III). Similarly, local efficiency also positively correlated with VFF in temporal and occipital regions. In addition, VFF scores correlated positively with FA throughout the brain, including bilateral frontal, parietal and occipital regions, the posterior cingulum, subcortical nuclei, brain stem, and cerebellum (Fig. 6).
Table 3.
Global network metrics correlated with executive function scores of the ALS patient group
| Verbal fluency F | Digit span forward | Digit span backward | Digit ordering maximal span | |
|---|---|---|---|---|
| Network metric | r‐value | r‐value | r‐value | r‐value |
| Transitivity | 0.339 | −0.088 | 0.256 | 0.590* |
| Characteristic path length | −0.470* | 0.126 | −0.312 | −0.625* |
| Global efficiency | 0.492* | −0.097 | 0.366 | 0.641* |
indicates significant between group difference (uncorrected p < 0.05).
Figure 5.
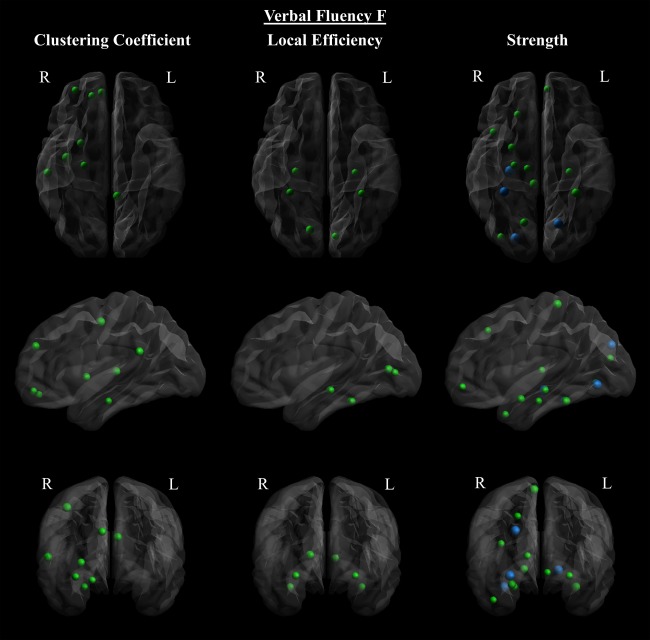
Verbal fluency F correlated with local network metrics in the ALS patient group. Verbal fluency F of ALS patients correlated positively with clustering coefficients (left), local efficiency (center), and strength (right), in axial (top), sagittal (middle), and coronal (bottom) view. A negative correlation with clustering coefficient was observed at one node, the right frontal superior medial gyrus. The size of the nodes is proportional to the absolute value of Spearman's correlation coefficient. The colors of the nodes indicate: blue = FDR corrected (p < 0.05), and green = uncorrected (p < 0.05). [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 6.
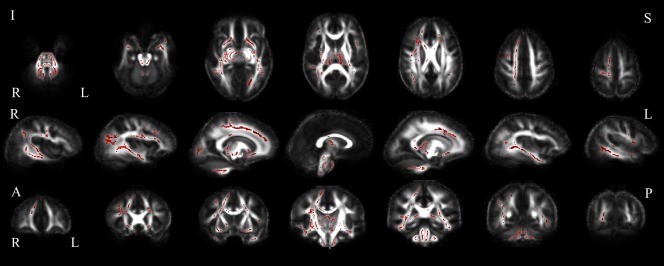
Verbal fluency F correlated with FA in the ALS patient group. Positive correlations of ALS patient verbal fluency F with white matter FA in axial (top), sagittal (middle) and coronal (bottom) slices (uncorrected p < 0.05). Abbreviations: I, inferior; S, superior; R, right; L, left; A, anterior; P, posterior. [Color figure can be viewed at http://wileyonlinelibrary.com]
Digit span forward
DSF performance correlated inversely with clustering coefficients in the left superior frontal gyrus, right middle temporal pole, and left fusiform gyri (Supporting Information Table IV). Nodal strength was inversely correlated with DSF performance bilaterally within the basal ganglia (putamen and pallidum), posterior cingulum, and temporal lobes. Local efficiency was inversely correlated with DSF performance at one node, the right supplementary motor area. Digit span forward performance was positively correlated with FA values bilaterally in superior frontal regions, as well as the right pre and post central gyri (Supporting Information Fig. 1). Inverse correlations were observed in the brain stem and temporal lobes bilaterally, as well as the left thalamus and left internal capsule (Supporting Information Fig. 2).
Digit span backward
Clustering coefficients correlated positively with DSB performance at nodes located in the frontal, and temporal lobes, as well as the insula, putamen, and pallidum (Fig. 7 and Supporting Information Table V). Negative correlations were observed in the left superior frontal and fusiform gyri, as well as the right paracentral lobule. Nodal strength correlated positively with nodes located dispersedly throughout the brain, including regions of the frontal, parietal, temporal and occipital lobes. Negative correlations were observed in the bilateral pallidum and right posterior cingulum. DSB performance correlated inversely with local efficiency at the right supplementary motor area, and positively at the left calcarine sulcus. DSB score positively correlated with FA bilaterally in white matter below the precentral gyri, superior frontal cortex, subcortical nuclei, and the anterior cingulum. Additional positive correlations were observed in the right post central gyri, parietal and temporal regions, as well as the posterior and medial cingulum (Fig. 8). FA inversely correlated with DSB performance in two small clusters in the right crus cerebri and temporal lobe (data not shown).
Figure 7.

Digit span backward correlated with local network metrics in the ALS patient group. Digit span backward of ALS patients correlated with clustering coefficients (left), local efficiency (center), and strength (right), in axial (top), sagittal (middle), and coronal (bottom) view. The size of the nodes is proportional to the absolute value of Spearman's correlation coefficient (uncorrected p < 0.05). [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 8.
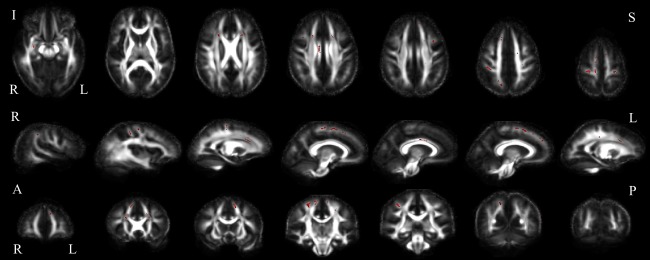
Digit span backward correlated with FA in the ALS patient group. Positive correlations of ALS patient digit span backward with white matter FA in axial (top), sagittal (middle) and coronal (bottom) slices (uncorrected p < 0.05). Abbreviations: I, inferior; S, superior; R, right; L, left; A, anterior; P, posterior. [Color figure can be viewed at http://wileyonlinelibrary.com]
Digit ordering maximal span
All global network metrics correlated with DOM performance, with a positive correlation in the case of transitivity and global efficiency, and a negative correlation with characteristic path length (Table 3). DOM performance positively correlated with local clustering coefficients in the frontal, parietal and temporal lobes (Fig. 9 and Supporting Information Table VI). Nodal strength was similarly positively correlated with nodes in these regions, as well as within the occipital lobe. Local efficiency correlated positively with DOM performance in two regions within the left temporal lobe. DOM performance positively correlated with tract FA values in the ALS group dispersedly throughout the brain, including bilateral frontal, temporal, occipital and parietal regions, the corpus callosum, cingulum, and subcortical nuclei (Fig. 10). Correlations within the bilateral anterior corpus callosum survived FDR correction for multiple comparisons, at p < 0.05.
Figure 9.
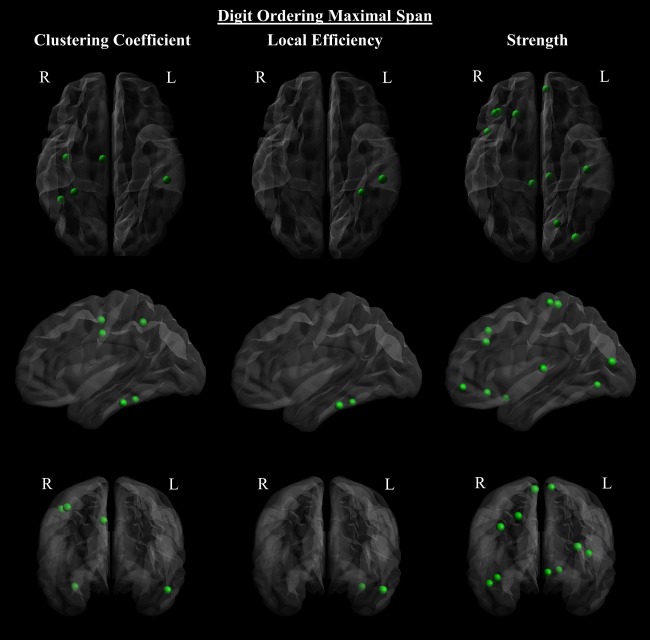
Digit ordering maximal span correlated with local network metrics in the ALS patient group. Digit ordering maximal span of ALS patients correlated positively with clustering coefficients (left), local efficiency (center), and strength (right), (top), sagittal (middle), and coronal (bottom) view. The size of the nodes is proportional to the absolute value of Spearman's correlation coefficient (uncorrected p < 0.05). [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 10.

Digit ordering maximal span correlated with FA in the ALS patient group. Positive correlations of ALS patient digit ordering maximal span with white matter FA in axial (top), sagittal (middle) and coronal (bottom) slices. The colors of the voxels indicate: green = FWE corrected (p < 0.05), and red = uncorrected (p < 0.05). Abbreviations: I, inferior; S, superior; R, right; L, left; A, anterior; P, posterior. [Color figure can be viewed at http://wileyonlinelibrary.com]
DISCUSSION
The current study aimed to further our understanding of white matter pathology in ALS and its impact on cognition by investigating the properties of the structural brain network in terms of regional network connectivity, and white matter integrity. Here we provide novel evidence of a dependence of executive function on the structural brain network, and insight into the biological constructs of executive dysfunction in ALS.
Evidence Suggestive of a Globally Disrupted Structural Network in ALS, Underlined by Diminished White Matter Integrity
We found reduced connectivity in ALS throughout the brain, within both primary and secondary motor cortices, as well as frontal and parietal regions. We further reported diminished structural integrity of white matter tracts, evident as reduced FA, occurring widely throughout the brain. These findings are consistent with those of previous investigations of topological white matter network connectivity [Schmidt et al., 2014; Verstraete et al., 2011, 2014] and tract structural integrity [Ciccarelli et al., 2009; Heimrath et al., 2014; Pettit et al., 2013; Prudlo et al., 2012; Zhang et al., 2014] in ALS, and provide supporting evidence of white matter pathology in ALS that extends beyond motor regions, primarily propagating to frontal and parietal areas. Importantly, we also noted that regions of reduced FA within both the ALS and ALSci groups (relative to healthy controls, and ALScc patients, respectively) overlapped neuroanatomically with nodes where structural connectivity was diminished; suggesting that network connectivity and structural white matter integrity are tightly coupled. Presumably, impairment of local network connectivity results from diminished structural integrity of the underlying white matter tracts [Buchanan et al., 2015]. This notion is further supported by our correlates of executive function, in which we observed strong co‐localization of FA and network metrics, particularly in those nodes that survived FDR correction, as in the case of VFF. In terms of global topology, we report for the first time a significant reduction in global structural network connectivity of ALS patients, as defined by an increased characteristic path length, and decreased transitivity. These findings, indicate that the topological network in ALS is less interconnected, which necessitates the utilization of longer structural pathways in transferring information across distal nodes [Rubinov and Sporns, 2010]. While the trend toward decreased global efficiency in the ALS group failed to reach significance, this conclusion is further supported by our finding of widespread reduction in local efficiency, indicating that local network communicative potential may be diminished throughout the brain. In considering reported reliance of cognition on the integration of information across multiple brain regions [Caeyenberghs et al., 2014; Li et al., 2009; Panigrahy et al., 2015; Sheffield et al., 2015; Van den Heuvel et al., 2009; Vlooswijk et al., 2011; Wen et al., 2011], this loss of short path network interconnectivity may hinder global integrative processing, and could be responsible for cognitive decline in ALS. Taken together, these results suggest that white matter pathology in ALS resulting in diminished tract integrity may be responsible for regionally specific decreases in local connectivity, which when sufficiently widespread, leads to a topological network that is globally disconnected. A proposed biological mechanism wherein expansion of motor region white matter pathology in ALS leads to a disrupted local, and subsequently global, topological network, is supported by recent evidence showing propagation of topological disconnectivity beyond motor areas, toward frontal and parietal regions [Verstraete et al., 2014]. In considering the frontal dependence of executive function [Alvarez and Emory, 2006], and that functional connectivity, necessary for executive processing [Agosta et al., 2013a; Sheffield et al., 2015], is dependent on the structural network [Damoiseaux and Greicius, 2009; Honey et al., 2009; Schmidt et al., 2014], the propagation of disconnected structural topology to frontal regions may explain extra‐motor symptoms in ALS.
Executive Dysfunction in ALS May Be Related to Local or Global Topological Disruption, Potentially Mediating Domain‐Specific or Generalized Cognitive Deficit
To the best of our knowledge, the current study is the first to correlate structural network properties as evaluated by graph theoretical analysis with executive function in ALS. Consistent with previous observations in healthy aging [Wen et al., 2011] as well as other neurological disorders [Caeyenberghs et al., 2014; Lo et al., 2010], we found executive function correlated with local network metrics throughout the brain (including frontal, temporal, occipital and parietal cortices, as well as subcortical nuclei), with a high predilection for the frontal and temporal lobes bilaterally. In addition, we found white matter structural integrity correlated with executive function performance in a similarly widespread fashion, results that are consistent with findings of previous tractography studies in ALS [Pettit et al., 2013; Sarro et al., 2011]. Notably, co‐localization of structural integrity and local connectivity correlates was apparent for most executive function tasks. Those executive function tasks (VFF and DOM) that presented the most widespread and overlapping association between decreased structural integrity and connectivity, and poorer task performance, also correlated with global network metrics: decreased global efficiency and increased characteristic path length were associated with poorer task performance. These results are consistent with previous structural topology studies in healthy [Wen et al., 2011] and pathological [Kim et al., 2014; Lo et al., 2010; Panigrahy et al., 2015; Reijmer et al., 2013] states, and suggest that overall executive function performance may be dependent on a globally efficient network characterized by short communicative paths, as well as dispersed local connectivity and underlying white matter tract integrity. Interestingly, the most striking overlap of local network correlates across executive function tasks was seen in positive correlations with strength at select nodes within frontal, temporal and occipital regions. Within these regions we also observed a strong overlap of positive strength and FA correlates within those tasks that appeared to be most globally dependent (DOM and VFF). This was especially true in white matter surrounding nodes (right superior occipital, right fusiform, and left lingual cortices) that survived FDR correction in VFF. In considering that the strength of a node is related to the number of connections it possesses [Crossley et al., 2014; Hwang et al., 2013], one possible explanation for the involvement of this fronto‐temporal‐occipital network across tasks is that nodes within this network serve as hubs, connecting distant executive function circuits to facilitate information integration and global cognitive processing [Bullmore and Sporns, 2012; Crossley et al., 2013; Sporns et al., 2007]. Within healthy populations [Hagmann et al., 2008], ALS [Ma et al., 2015], and other neurodegenerative disorders [Buckner et al., 2009; Caeyenberghs et al., 2014; Crossley et al., 2014; Lo et al., 2010; Rae et al., 2012], general network hubs are frequently identified within these frontal, temporal and occipital regions. The facilitation of global processing through network hubs has also been shown to be essential to cognition [Bertolero et al., 2015; Braun et al., 2015; Crossley et al., 2013; Sheffield et al., 2015], and disruption of hub connectivity is suggested to be associated with decreased cognitive performance [Crossley et al., 2013, 2014; Gleichgerrcht et al., 2015].
While these results support the notion that executive function as a whole is dependent on global connectivity between various neuronal regions [Dehaene and Changeux, 2011], variance in task specific correlations suggests a dependence of dissociable cognitive processes on local circuitry [Alvarez and Emory, 2006; Kim et al., 2014; Reijmer et al., 2013, 2015]. While overlap of structural network correlates was observable across some tasks, no single node had network metrics that correlated with all executive function assessments, and each assessment demonstrated a dependence on local task‐specific networks, primarily located within frontal and temporal regions. Within these task‐specific local networks, cognitive performance correlated with FA, nodal strength, and clustering coefficients more so than with local efficiency, suggesting a higher dependence on the tract integrity, connection strength, and clustering of local circuits, than on the presence of short connections between proximal regions. These findings, consistent with those in normal aging [Wen et al., 2011], suggest that at a local level, executive function depends on dissociable, structurally segmented regions of dense interconnectivity that facilitate information processing within specific cognitive domains [Panigrahy et al., 2015]. Taken together, these findings suggest that executive function in ALS may be dependent on both the interconnectivity of local frontal and temporal networks for domain specific cognitive processing, as well as the strength of fronto‐temporal‐occipital connections that facilitate integrative processing across multiple cognitive domains. This conclusion is consistent with previous findings of structural [Li et al., 2009; Reijmer et al., 2013, 2015; Wen et al., 2011] and functional [Bertolero et al., 2015; Sheffield et al., 2015; Van den Heuvel et al., 2009; Vlooswijk et al., 2011] network correlates of cognition, as well as current theories on higher‐order conscious processing [Dehaene and Changeux, 2011]. In considering the association between network connectivity and underlying white matter integrity [Buchanan et al., 2015], it follows that executive dysfunction in ALS may result from either localized frontal/temporal white matter pathology, or the targeting of larger tracts that connect these distant brain regions. In accordance with such a model we would predict that while destruction of local circuity may disrupt specific executive functions, loss of distant connections between global network hubs may impair patient ability to integrate across various cognitive domains, and lead to a more generalized executive dysfunction, accompanied by a globally disrupted topology.
Prevalence of Executive Dysfunction in ALS May Be Related to an Increased Susceptibility of Frontal‐Mediated and Globally Dependent Cognitive Domains to White Matter Pathology
Despite the wide range of potential cognitive and behavioral impairments associated with ALS [Goldstein and Abrahams, 2013], executive dysfunction remains the most prevalent [Lonergan et al., 2014; Strong, 2008; Tsermentseli et al., 2012; Woolley and Strong, 2015]. Studies have demonstrated a dependence of executive processing on frontal and temporal lobes [Alvarez and Emory, 2006]. Furthermore, white matter pathology within these regions has been shown to be associated with executive dysfunction in ALS [Pettit et al., 2013; Sarro et al., 2011], and the prevalence of its report has provided contributing evidence of a shared pathology in ALS and frontotemporal dementia [Kiernan et al., 2011; Neumann et al., 2006; Ringholz and Greene, 2006; Strong, 2008; Woolley and Strong, 2015]. In comparing the cognitive phenotype of ALSci and ALScc patients, we found that the ALSci group was only significantly impaired in those tasks (VFF and DOM) that appeared to demonstrate the highest dependence on local frontal lobe circuitry, as well as global network topology. We found that the majority of local network connectivity and white matter integrity abnormalities in the ALSci group were localized to the frontal lobe. These frontal lobe abnormalities correlated with decreased performance in the VFF and DOM tasks, after correction for multiple comparisons. These findings suggest that there may be white matter structural network abnormalities specific to ALSci patients, a notion that is supported by a previous DTI study that found more widespread extra‐motor white matter damage in ALSci patients relative to healthy controls [Sarro et al., 2011]. The high predilection of these abnormalities to the frontal lobe, and high specificity of overlap with VFF and DOM task local networks, suggests that frontal white matter pathology may mediate executive dysfunction in ALS, potentially through disruption of task‐specific local circuits. Interestingly however, decreased nodal strength at only a handful of the nodes on which these tasks were shown to rely appeared to be sufficient to impair cognitive performance. We found that nodes with decreased strength were localized to either frontal, or temporal–occipital regions, and that half of these nodes (left frontal medial orbital and left lingual gyri) were part of the fronto‐temporal‐occipital hub network proposed to be involved in facilitation of global processing [Bullmore and Sporns, 2012; Crossley et al., 2013; Sporns et al., 2007]. Taken together, these observations suggests that cognitive impairment in ALS may result from white matter pathology targeting tracts that interconnect clusters of nodes in frontal regions, or those connecting distant frontal and temporal hub regions, which presumably hinders domain specific [Wen et al., 2011], or integrative processing [Crossley et al., 2013; Panigrahy et al., 2015], respectively.
In considering evidence for the expansion of white matter pathology in ALS beyond motor areas [Verstraete et al., 2014] resulting in diminished frontal white matter integrity [Ciccarelli et al., 2009; Pettit et al., 2013; Prudlo et al., 2012; Zhang et al., 2014], and subsequent disruption of structural network topology [Schmidt et al., 2014; Verstraete et al., 2011, 2014], these findings provide insight into the biological mechanism of white matter pathology underlying executive dysfunction in ALS, and may help explain the variability of cognitive symptoms along the ALS‐FTD spectrum [Goldstein and Abrahams, 2013; Ringholz and Greene, 2006; Tsermentseli et al., 2012]. Our results suggest that the degree of executive dysfunction experienced by a patient may be dependent not only on the extent to which frontal region white matter pathology is present, but also on the extent to which pathology targets global, as opposed to local network connectivity. According to such a model, an ALS patient with more severe cognitive deficit would be expected to have a higher degree of extra motor white matter changes and greater pathological involvement of fronto‐temporal hub connectivity, whereas a patient with domain‐specific cognitive impairment may display only mild extra‐motor pathology, which selectively disrupts localized network interconnectivity. While this conclusion is supported by previous tractography studies reporting more widespread white matter damage in cognitively impaired ALS patients [Sarro et al., 2011], and association between local white matter damage and specific cognitive impairments [Pettit et al., 2013], the extent to which executive dysfunction in ALS is associated with selective targeting of network hubs is unclear. While it has been shown that hubs are common targets of neurodegenerative disorders [Crossley et al., 2014], and that greater structural hub loss is associated with poorer verbal fluency performance following stroke [Gleichgerrcht et al., 2015], the relationship between selective loss of structural hubs and cognitive outcome in ALS remains to be determined. Though further investigation is necessary to validate this model, our findings suggest that executive functions that are highly dependent on frontal network circuitry, and integrative global processing, may be more susceptible to white matter pathology in ALS. This may explain the prevalence of verbal fluency deficit amongst patients [Goldstein and Abrahams, 2013; Tsermentseli et al., 2012], and suggests that cognitive screening in ALS should be inclusive of tasks such as VFF and DOM that rely on integrative global processing across frontotemporal networks.
Frontal Region Pathology Underlying Executive Dysfunction in ALS May Be Facilitated by High Structural Interconnectivity
An unexpected finding of our study was that while the ALSci patients displayed decreased nodal strength within frontal, temporal and occipital regions, clustering coefficients were increased in three frontal (right superior frontal, right superior medial, left frontal medial orbital gyri), and one occipital (left lingual) region, suggesting ALSci patients had greater local interconnectivity in these regions relative to the ALScc group. Furthermore, an increased clustering coefficient at the right frontal superior medial cortex was associated with decreased VFF performance, suggesting that this increased frontal interconnectivity may be associated with executive dysfunction. Negative correlations between clustering coefficients and executive function performance were also observed in frontal and temporal regions on the DSB and DSF tasks. Importantly, the localization of these negative correlations were consistent across the two tasks. In an effort to explain this paradoxical association between increased network interconnectivity and decreased executive function performance, we propose two potential biological mechanisms.
The first possible explanation is that this increased frontal interconnectivity reflects a failed compensatory mechanism in ALSci, in which strengthening of local circuits in the presence of structurally compromised global connections reflects an unsuccessful attempt to restore cognitive function. Functional connectivity studies in ALS [Agosta et al., 2013a; Heimrath et al., 2014] provide evidence of increased functional connectivity between secondarily recruited cognitive circuits, suggesting the brain undergoes dynamic reconfiguration in an attempt to maintain cognition when primarily recruited circuits are dysfunctional. In considering that functional and structural connectivity changes have been shown to be tightly linked in ALS [Schmidt et al., 2014], it is possible that the increased frontal network interconnectivity in the ALSci group reflects underlying structural network changes to accommodate functional reconfiguration. In addition to negative correlations with clustering coefficients, we also reported co‐localized negative correlations with nodal strength and FA in the DSB and DSF task. These results support this conclusion, in that they suggest that increased tract integrity and structural strengthening within select regions may be associated with executive dysfunction in ALS.
A second possible explanation for increased frontal interconnectivity in the ALSci group is that higher local interconnectivity reflects an increased susceptibility of these frontal regions to white matter pathology in ALS, potentially due to the concentration of hubs in densely interconnected local networks [Van den Heuvel and Sporns, 2011]. Previous studies [Buckner et al., 2009; Crossley et al., 2014] have shown that network hubs are frequent targets of neurodegenerative disorders, presumably due to increased biological demand [Liang et al., 2013; Tomasi et al., 2013], or increased connectivity to sources of pathology [Zhou et al., 2012]. Cortical hubs of the human cortex are said to be organized into “clubs” of rich interconnectivity amongst members [Van den Heuvel and Sporns, 2011]. With this in mind, the increased frontal network interconnectivity in the ALSci group may reflect a greater concentration of highly interconnected hubs in these regions, which infers a greater susceptibility to neurodegenerative pathology. This would explain why the left frontal medial orbital and left lingual gyri in the ALSci patient group displayed both decreased nodal strength, and increased clustering coefficients relative to ALScc patients. While there are several hypothesized mechanisms for the increased pathological susceptibility of hub regions [Zhou et al., 2012], one that is consistent with recent proteinopathy findings in ALS [Brettschneider et al., 2013; Schmidt et al., 2016] is the transneuronal spread model, in which a toxic agent spreads to regions of high connectivity by propagating along structural connections [Frost and Diamond, 2010; Pradat et al., 2015]. Within ALS, 43 kDA TAR DNA‐binding protein (TDP‐43), a hallmark proteinopathopy of ALS and FTLD [Brettschneider et al., 2014b; Neumann et al., 2006], has been suggested to propagate along structural network connections [Schmidt et al., 2016], expanding from primary motor cortices toward frontal, and subsequently temporal, regions [Brettschneider et al., 2013]. In consideration of this model, it's possible that the presence of increased frontal interconnectivity in the ALSci group may facilitate TDP‐43 spread to hubs, resulting in white matter pathology that disrupts global network connectivity, and leads to executive dysfunction. While the mechanism by which TDP‐43 aggregation might disrupt axonal tract integrity is unknown, evidence of oligodendroglia TDP‐43 proteinopathy in ALS [Brettschneide et al., 2014a], and co‐localized TDP‐43 pathology, neuronal loss, and microglia activation in frontal and temporal white matter [Brettschneider et al., 2012], is suggestive of demyelination and a microglia‐mediated inflammatory response [Komine and Yamanaka, 2015; Philips and Robberecht, 2011; Xu et al., 2015]. This mechanism, and the involvement of TDP‐43 proteinopathy in executive dysfunction in ALS, is supported by correlations of decreased executive function performance with increased TDP‐43 burden and microglia activation in frontal and temporal regions [Brettschneider et al., 2012]. In considering the shared TDP‐43 proteinopathy [Neumann et al., 2006] and functional connectivity abnormalities [Trojsi et al., 2015] seen in ALS and FTD, the susceptibility of frontal region hubs to FTLD pathology is further supported by evidence of selective loss of frontal hub regions in behavioral variant FTD (bvFTD) [Agosta et al., 2013b]. Consistent with our results that are suggestive of decreased nodal strength in ALSci group frontal and occipital regions, Agosta et al. [2013b] reported that the most significant decrease in functional connectivity occurred between frontal‐occipital regions and frontal, temporal and occipital areas. Furthermore, consistent with our proposal that destruction of inter‐regional hub connections may underlie general executive dysfunction, disruption of global network topology in bvFTD was associated with executive dysfunction [Agosta et al., 2013b]. Frontal region functional disconnectivity has also been reported in ALS [Agosta et al., 2013a]. While these studies [Agosta et al., 2013a, 2013b] focused on functional connectivity, evidence that structural and functional network topology is tightly linked [Damoiseaux and Greicius, 2009; Honey et al., 2009] suggests that structural network disruption may underlie functional disconnectivity. Within ALS, correlation between structural and functional network disconnectivity has also been reported [Schmidt et al., 2014]. Furthermore, consistent with evidence of a frontal‐propagating disconnected structural network [Verstraete et al., 2014], frontal and parietal areas with short path connectivity to motor areas have been shown to display the most prominent functional and structural network disruption [Schmidt et al., 2014]. Taken together, these observations suggest that frontal network disruption underlying executive dysfunction in ALS may be the result of pathological spread through highly interconnected circuits and the targeting of frontal hub regions. While the underlying biological mechanism of white matter pathological propagation and ensuing cognitive decline remains unclear, we speculate a potential role for TDP‐43 proteinopathy.
Methodological Limitations and Future Direction
Strengths of the current study include the use of high resolution DTI data acquired on a 4.7T MRI research scanner, as well as the use of TBSS and graph theoretical analysis, shown to be novel methods of investigating structural white matter networks with high sensitivity and specificity [Smith et al., 2006; Verstraete et al., 2014]. The study is, however, not without limitations. A significant limitation is that the small sample size introduces the possibility that subtle group differences might be masked, and that significant findings may not actually be true effects. Studies with low statistical power due to small sample size are prone to both type I and type II errors [Button et al., 2013], as well as exaggeration of the magnitude of true effects [Ioannidis, 2005, 2008]. While the co‐localization of FA reductions, network abnormalities, and correlates of executive dysfunction in the ALSci group suggests that there are true abnormalities specific to ALSci patients that underlie cognitive impairment, the small sample size of these exploratory analyses merits caution in interpreting results. Certainly, future studies incorporating more participants will be helpful to determine if there are in fact white matter abnormalities specific to ALSci patients. With respect to our study cohort, it is also worth noting that we reported a greater percentage of cognitively impaired patients than is commonly reported in the literature (35%–50%) [Strong, 2008]. This discrepancy, however, is likely due to the classification system utilized to define ALSci patients, rather than to a selection bias. So that our results would be comparable to their study, we utilized the ALSci definition used by Sarro et al. [2011]. While higher than that reported in the literature, the percentage of subjects in our study classified as ALSci according to this definition (64%) was comparable to that reported by Sarro et al. [2011] (69%), suggesting our cohort was still representative of the general population with ALS. Another potential limitation of our study is that we did not control for between group differences in white matter hyperintensities (WMHs). WMHs have been shown in small vessel disease to be associated with decreased network strength and efficiency, as well as with poorer cognitive functioning [Tuladhar et al., 2016]. However, visual inspection of fluid attenuated inversion recovery (FLAIR) images revealed an equal occurrence of WMHs across all subject groups, suggesting this was not a factor affecting group comparison results. Additionally, due to a lack of genetic testing, we were unable to control for group differences related to C9orf72 hexanucleotide expansions, which could potentially have influenced our results. C9orf72 repeat expansions, which have been extensively linked to both ALS and FTD [Rohrer et al., 2015], are associated with a greater incidence of cognitive impairment, as well as extra‐motor white matter abnormalities [Bede et al., 2013].
With regards to the utilized analytic tools, a common concern with the DTI model is its inability to resolve fiber orientation in regions of crossing and kissing fibers. Furthermore, deterministic tractography, utilized in constructing the connectivity matrices for our graph theoretical analyses, has been suggested to be particularly limited by the presence of crossing fibers, in that low FA values associated with regions containing crossing fibers may cause premature termination of fiber reconstruction, and hence loss of edges in the resulting connectivity matrices [Caeyenberghs et al., 2014]. In future studies, strategies such as the collection of high angular resolution diffusion imaging (HARDI) data, coupled with alternative diffusion models, such as the fiber orientation distribution (FOD) estimated by constrained spherical deconvolution (CSD) [Tournier et al., 2007], could be considered to combat this issue. In addition, while TBSS has been proposed to overcome a lot of the issues associated with the registration processes in whole brain voxel‐wise analysis [Smith et al., 2006], some question always remains as to if significant findings correspond to the same neuroanatomical regions across subjects. To combat this concern, we would recommend coupling TBSS analyses with that of network based statistics (NBS) to study the edges of the structural network, given that it does not require scan registration to a common space, and results of these two methods have been shown to strongly co‐localize within ALS [Buchanan et al., 2015]. Lastly, incorporation of a larger array of executive function tasks, as well as additional graph theory metrics, would have potentially provided more insight into the organization of local networks on which dissociable cognitive domains rely, and how these networks are integrated to support multi‐domain executive function tasks. Furthermore, inclusion of more specific metrics of centrality for assessment the importance of a node in global connectivity [Rubinov and Sporns, 2010] and robust identification of network hubs [Sporns et al., 2007], would potentially further support our claims related to global information integration and network susceptibility related to the presence of frontal region hubs.
CONCLUSION
In this exploratory analysis we report for the first time a globally disrupted structural network in ALS, and provide evidence suggestive of the existence of structural network abnormalities specific to cognitively impaired ALS patients. We found that these network abnormalities, predominantly located within frontal regions, are associated with decreased cognitive performance, suggesting that white matter pathology within these regions may underlie executive dysfunction in ALS. In addition, reported findings suggest the existence of dissociable cognitive impairments of executive function within ALS, that while individually reliant on the connectivity and underlying white matter integrity of local circuits, are collectively dependent on an efficiently connected global network. Our results suggest that executive dysfunction in ALS may result from the white matter pathology in frontal regions, resulting in disrupted local, and global topology, which mediate domain specific and generalized executive dysfunctions, respectively. We speculate that high interconnectivity amongst frontal regions may facilitate white matter pathology, which infers an increased susceptibility of frontal‐mediated cognitive domains to impairment, and may explain the prevalence of executive dysfunction in ALS.
Supporting information
Additional supporting information may be found in the online version of this article.
Supporting Information
ACKNOWLEDGMENTS
The funders had no role in study design, data collection, analysis or preparation of the manuscript. None of the authors reported any conflicts of interest.
Corrections added on 9 November 2016.
REFERENCES
- Agosta F, Pagani E, Petrolini M, Caputo D, Perini M, Prelle A, Salvi F, Filippi M (2010): Assessment of white matter tract damage in patients with amyotrophic lateral sclerosis: A diffusion tensor MR imaging tractography study. AJNR Am J Neuroradiol 31:1457–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agosta F, Canu E, Valsasina P, Riva N, Prelle A, Comi G, Filippi M (2013a): Divergent brain network connectivity in amyotrophic lateral sclerosis. Neurobiol Aging 34:419–427. [DOI] [PubMed] [Google Scholar]
- Agosta F, Sala S, Valsasina P, Meani A, Canu E, Magnani G, Cappa SF, Scola E, Quatto P, Horsfield MA, Falini A, Comi G, Filippi M (2013b): Brain network connectivity assessed using graph theory in frontotemporal dementia. Neurology 81:134–143. [DOI] [PubMed] [Google Scholar]
- Alvarez JA, Emory E (2006): Executive function and the frontal lobes: A meta‐analytic review. Neuropsychol Rev 16:17–42. [DOI] [PubMed] [Google Scholar]
- Bach M, Laun FB, Leemans A, Tax CM, Biessels GJ, Stieltjes B, Maier‐Hein KH (2014): Methodological considerations on tract‐based spatial statistics (TBSS). Neuroimage 100:358–369. [DOI] [PubMed] [Google Scholar]
- Bede P, Bokde AL, Byrne S, Elamin M, McLaughlin RL, Kenna K, Fagan AJ, Pender N, Bradley DG, Hardiman O (2013): Multiparametric MRI study of ALS stratified for the C9orf72 genotype. Neurology 81:361–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolero MA, Yeo BT, D'Esposito M (2015): The modular and integrative functional architecture of the human brain. Proc Natl Acad Sci U S A 112:E6798–E6807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun U, Schäfer A, Walter H, Erk S, Romanczuk‐Seiferth N, Haddad L, Schweiger JI, Grimm O, Heinz A, Tost H, Meyer‐Lindenberg A, Bassett DS (2015): Dynamic reconfiguration of frontal brain networks during executive cognition in humans. Proc Natl Acad Sci U S A 112:11678–11683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brettschneider J, Libon DJ, Toledo JB, Xie SX, McCluskey L, Elman L, Geser F, Lee VM, Grossman M, Trojanowski JQ (2012): Microglial activation and TDP‐43 pathology correlate with executive dysfunction in amyotrophic lateral sclerosis. Acta Neuropathol 123:395–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brettschneider J, Del Tredici K, Toledo JB, Robinson JL, Irwin DJ, Grossman M, Suh E, Van Deerlin VM, Wood EM, Baek Y, Kwong L, Lee EB, Elman L, McCluskey L, Fang L, Feldengut S, Ludolph AC, Lee VM, Braak H, Trojanowski JQ (2013): Stages of pTDP‐43 pathology in amyotrophic lateral sclerosis. Ann Neurol 74:20–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brettschneider J, Arai K, Del Tredici K, Toledo JB, Robinson JL, Lee EB, Kuwabara S, Shibuya K, Irwin DJ, Fang L, Van Deerlin VM, Elman L, McCluskey L, Ludolph AC, Lee VM, Braak H, Trojanowski JQ (2014a): TDP‐43 pathology and neuronal loss in amyotrophic lateral sclerosis spinal cord. Acta Neuropathol 128:423–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brettschneider J, Del Tredici K, Irwin DJ, Grossman M, Robinson JL, Toledo JB, Lee EB, Fang L, Van Deerlin VM, Ludolph AC, Lee VM, Braak H, Trojanowski JQ (2014b): Sequential distribution of pTDP‐43 pathology in behavioral variant frontotemporal dementia (bvFTD). Acta Neuropathol 127:423–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks BR, Miller RG, Swash M, Munsat TL, W.F.O.N.R.G.O.M.N Diseases (2000): El Escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 1:293–299. [DOI] [PubMed] [Google Scholar]
- Buchanan CR, Pettit LD, Storkey AJ, Abrahams S, Bastin ME (2015): Reduced structural connectivity within a prefrontal‐motor‐subcortical network in amyotrophic lateral sclerosis. J Magn Reson Imaging 41:1342–1352. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, Andrews‐Hanna JR, Sperling RA, Johnson KA (2009): Cortical hubs revealed by intrinsic functional connectivity: Mapping, assessment of stability, and relation to Alzheimer's disease. J Neurosci 29:1860–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore E, Sporns O (2009): Complex brain networks: Graph theoretical analysis of structural and functional systems. Nat Rev Neurosci 10:186–198. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Sporns O (2012): The economy of brain network organization. Nat Rev Neurosci 13:336–349. [DOI] [PubMed] [Google Scholar]
- Button KS, Ioannidis JP, Mokrysz C, Nosek BA, Flint J, Robinson ES, Munafò MR (2013): Power failure: Why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci 14:365–376. [DOI] [PubMed] [Google Scholar]
- Caeyenberghs K, Leemans A, Leunissen I, Gooijers J, Michiels K, Sunaert S, Swinnen SP (2014): Altered structural networks and executive deficits in traumatic brain injury patients. Brain Struct Funct 219:193–209. [DOI] [PubMed] [Google Scholar]
- Ciccarelli O, Behrens TE, Johansen‐Berg H, Talbot K, Orrell RW, Howard RS, Nunes RG, Miller DH, Matthews PM, Thompson AJ, Smith SM (2009): Investigation of white matter pathology in ALS and PLS using tract‐based spatial statistics. Hum Brain Mapp 30:615–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossley NA, Mechelli A, Vértes PE, Winton‐Brown TT, Patel AX, Ginestet CE, McGuire P, Bullmore ET (2013): Cognitive relevance of the community structure of the human brain functional coactivation network. Proc Natl Acad Sci U S A 110:11583–11588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossley NA, Mechelli A, Scott J, Carletti F, Fox PT, McGuire P, Bullmore ET (2014): The hubs of the human connectome are generally implicated in the anatomy of brain disorders. Brain 137:2382–2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Greicius MD (2009): Greater than the sum of its parts: A review of studies combining structural connectivity and resting‐state functional connectivity. Brain Struct Funct 213:525–533. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Changeux JP (2011): Experimental and theoretical approaches to conscious processing. Neuron 70:200–227. [DOI] [PubMed] [Google Scholar]
- Elamin M, Phukan J, Bede P, Jordan N, Byrne S, Pender N, Hardiman O (2011): Executive dysfunction is a negative prognostic indicator in patients with ALS without dementia. Neurology 76:1263–1269. [DOI] [PubMed] [Google Scholar]
- Frost B, Diamond MI (2010): Prion‐like mechanisms in neurodegenerative diseases. Nat Rev Neurosci 11:155–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleichgerrcht E, Kocher M, Nesland T, Rorden C, Fridriksson J, Bonilha L (2015): Preservation of structural brain network hubs is associated with less severe post‐stroke aphasia. Restor Neurol Neurosci 34:19–28. [DOI] [PubMed] [Google Scholar]
- Goldstein LH, Abrahams S (2013): Changes in cognition and behaviour in amyotrophic lateral sclerosis: Nature of impairment and implications for assessment. Lancet Neurol 12:368–380. [DOI] [PubMed] [Google Scholar]
- Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, Sporns O (2008): Mapping the structural core of human cerebral cortex. PLoS Biol 6:e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimrath J, Gorges M, Kassubek J, Müller HP, Birbaumer N, Ludolph AC, Lulé D (2014): Additional resources and the default mode network: Evidence of increased connectivity and decreased white matter integrity in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener 15:537–545. [DOI] [PubMed] [Google Scholar]
- Honey CJ, Sporns O, Cammoun L, Gigandet X, Thiran JP, Meuli R, Hagmann P (2009): Predicting human resting‐state functional connectivity from structural connectivity. Proc Natl Acad Sci U S A 106:2035–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppe CD, Müller UD, Werheid KD, Thöne AD, von Cramon YD (2000): Digit Ordering Test: Clinical, psychometric, and experimental evaluation of a verbal working memory test. Clin Neuropsychol 14:38–55. [DOI] [PubMed] [Google Scholar]
- Hwang K, Hallquist MN, Luna B (2013): The development of hub architecture in the human functional brain network. Cereb Cortex 23:2380–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBM Corp. Released . (2012): IBM SPSS Statistics for Windows. Version 21.0. Armonk, NY: IBM Corp. [Google Scholar]
- Ioannidis JP (2005): Why most published research findings are false. PLoS Med 2:e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannidis JP (2008): Why most discovered true associations are inflated. Epidemiology 19:640–648. [DOI] [PubMed] [Google Scholar]
- Kiernan MC, Vucic S, Cheah BC, Turner MR, Eisen A, Hardiman O, Burrell JR, Zoing MC (2011): Amyotrophic lateral sclerosis. Lancet 377:942–955. [DOI] [PubMed] [Google Scholar]
- Kim J, Parker D, Whyte J, Hart T, Pluta J, Ingalhalikar M, Coslett HB, Verma R (2014): Disrupted structural connectome is associated with both psychometric and real‐world neuropsychological impairment in diffuse traumatic brain injury. J Int Neuropsychol Soc 20:887–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komine O, Yamanaka K (2015): Neuroinflammation in motor neuron disease. Nagoya J Med Sci 77:537–549. [PMC free article] [PubMed] [Google Scholar]
- Leemans A, Jeurissen B, Sijbers J, Jones DK (2009): ExploreDTI: A graphical toolbox for processing, analyzing, and visualizing diffusion MR data. In: 17th Annual Meeting of the International Society for Magnetic Resonance in Medicine. Hawaii, USA. p 3537.
- Leemans A, Jones DK (2009): The B‐matrix must be rotated when correcting for subject motion in DTI data. Magn Reson Med 61:1336–1349. [DOI] [PubMed] [Google Scholar]
- Li Y, Liu Y, Li J, Qin W, Li K, Yu C, Jiang T (2009): Brain anatomical network and intelligence. PLoS Comput Biol 5:e1000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Zou Q, He Y, Yang Y (2013): Coupling of functional connectivity and regional cerebral blood flow reveals a physiological basis for network hubs of the human brain. Proc Natl Acad Sci U S A 110:1929–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo CY, Wang PN, Chou KH, Wang J, He Y, Lin CP (2010): Diffusion tensor tractography reveals abnormal topological organization in structural cortical networks in Alzheimer's disease. J Neurosci 30:16876–16885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonergan R, Mistumoto H, Murray B (2014): Amyotrophic lateral sclerosis In: Katirji B, Kaminski H, Ruff R, editors. Neuromuscular Disorders in Clinical Practice. New York: Springer New York; pp. 395–423. [Google Scholar]
- Ma X, Zhang J, Zhang Y, Chen H, Li R, Wang J (2015): Altered cortical hubs in functional brain networks in amyotrophic lateral sclerosis. Neurol Sci 36:2097–2104. [DOI] [PubMed] [Google Scholar]
- Menke RA, Abraham I, Thiel CS, Filippini N, Knight S, Talbot K, Turner MR (2012): Fractional anisotropy in the posterior limb of the internal capsule and prognosis in amyotrophic lateral sclerosis. Arch Neurol 69:1493–1499. [DOI] [PubMed] [Google Scholar]
- Minati L, Varotto G, D'Incerti L, Panzica F, Chan D (2013): From brain topography to brain topology: Relevance of graph theory to functional neuroscience. Neuroreport 24:536–543. [DOI] [PubMed] [Google Scholar]
- Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, McCluskey LF, Miller BL, Masliah E, Mackenzie IR, Feldman H, Feiden W, Kretzschmar HA, Trojanowski JQ, Lee VM (2006): Ubiquitinated TDP‐43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314:130–133. [DOI] [PubMed] [Google Scholar]
- Panigrahy A, Schmithorst VJ, Wisnowski JL, Watson CG, Bellinger DC, Newburger JW, Rivkin MJ (2015): Relationship of white matter network topology and cognitive outcome in adolescents with d‐transposition of the great arteries. Neuroimage Clin 7:438–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettit LD, Bastin ME, Smith C, Bak TH, Gillingwater TH, Abrahams S (2013): Executive deficits, not processing speed relates to abnormalities in distinct prefrontal tracts in amyotrophic lateral sclerosis. Brain 136:3290–3304. [DOI] [PubMed] [Google Scholar]
- Philips T, Robberecht W (2011): Neuroinflammation in amyotrophic lateral sclerosis: Role of glial activation in motor neuron disease. Lancet Neurol 10:253–263. [DOI] [PubMed] [Google Scholar]
- Pradat PF, Kabashi E, Desnuelle C (2015): Deciphering spreading mechanisms in amyotrophic lateral sclerosis: Clinical evidence and potential molecular processes. Curr Opin Neurol 28:455–461. [DOI] [PubMed] [Google Scholar]
- Prudlo J, Bißbort C, Glass A, Grossmann A, Hauenstein K, Benecke R, Teipel SJ (2012): White matter pathology in ALS and lower motor neuron ALS variants: A diffusion tensor imaging study using tract‐based spatial statistics. J Neurol 259:1848–1859. [DOI] [PubMed] [Google Scholar]
- Rae CL, Correia MM, Altena E, Hughes LE, Barker RA, Rowe JB (2012): White matter pathology in Parkinson's disease: The effect of imaging protocol differences and relevance to executive function. Neuroimage 62:1675–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reijmer YD, Leemans A, Caeyenberghs K, Heringa SM, Koek HL, Biessels GJ , U.V.C.I.S Group (2013): Disruption of cerebral networks and cognitive impairment in Alzheimer disease. Neurology 80:1370–1377. [DOI] [PubMed] [Google Scholar]
- Reijmer YD, Fotiadis P, Martinez‐Ramirez S, Salat DH, Schultz A, Shoamanesh A, Ayres AM, Vashkevich A, Rosas D, Schwab K, Leemans A, Biessels GJ, Rosand J, Johnson KA, Viswanathan A, Gurol ME, Greenberg SM (2015): Structural network alterations and neurological dysfunction in cerebral amyloid angiopathy. Brain 138:179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringholz GM, Greene SR (2006): The relationship between amyotrophic lateral sclerosis and frontotemporal dementia. Curr Neurol Neurosci Rep 6:387–392. [DOI] [PubMed] [Google Scholar]
- Rohrer JD, Isaacs AM, Mizielinska S, Mead S, Lashley T, Wray S, Sidle K, Fratta P, Orrell RW, Hardy J, Holton J, Revesz T, Rossor MN, Warren JD (2015): C9orf72 expansions in frontotemporal dementia and amyotrophic lateral sclerosis. Lancet Neurol 14:291–301. [DOI] [PubMed] [Google Scholar]
- Rubinov M, Sporns O (2010): Complex network measures of brain connectivity: Uses and interpretations. Neuroimage 52:1059–1069. [DOI] [PubMed] [Google Scholar]
- Sarro L, Agosta F, Canu E, Riva N, Prelle A, Copetti M, Riccitelli G, Comi G, Filippi M (2011): Cognitive functions and white matter tract damage in amyotrophic lateral sclerosis: A diffusion tensor tractography study. AJNR Am J Neuroradiol 32:1866–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R, Verstraete E, de Reus MA, Veldink JH, van den Berg LH, van den Heuvel MP (2014): Correlation between structural and functional connectivity impairment in amyotrophic lateral sclerosis. Hum Brain Mapp 35:4386–4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R, de Reus MA, Scholtens LH, van den Berg LH, van den Heuvel MP (2016): Simulating disease propagation across white matter connectome reveals anatomical substrate for neuropathology staging in amyotrophic lateral sclerosis. Neuroimage 124:762–769. [DOI] [PubMed] [Google Scholar]
- Sheffield JM, Repovs G, Harms MP, Carter CS, Gold JM, MacDonald AW, Daniel Ragland J, Silverstein SM, Godwin D, Barch DM (2015): Fronto‐parietal and cingulo‐opercular network integrity and cognition in health and schizophrenia. Neuropsychologia 73:82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen‐Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE (2006): Tract‐based spatial statistics: Voxelwise analysis of multi‐subject diffusion data. Neuroimage 31:1487–1505. [DOI] [PubMed] [Google Scholar]
- Sporns O, Honey CJ, Kötter R (2007): Identification and classification of hubs in brain networks. PLoS One 2:e1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong MJ (2008): The syndromes of frontotemporal dysfunction in amyotrophic lateral sclerosis. Amyotroph Lateral Scler 9:323–338. [DOI] [PubMed] [Google Scholar]
- Tang M, Chen X, Zhou Q, Liu B, Liu Y, Liu S, Chen Z (2015): Quantitative assessment of amyotrophic lateral sclerosis with diffusion tensor imaging in 3.0T magnetic resonance. Int J Clin Exp Med 8:8295–8303. [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Wang GJ, Volkow ND (2013): Energetic cost of brain functional connectivity. Proc Natl Acad Sci U S A 110:13642–13647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournier JD, Calamante F, Connelly A (2007): Robust determination of the fibre orientation distribution in diffusion MRI: Non‐negativity constrained super‐resolved spherical deconvolution. Neuroimage 35:1459–1472. [DOI] [PubMed] [Google Scholar]
- Trojsi F, Esposito F, de Stefano M, Buonanno D, Conforti FL, Corbo D, Piccirillo G, Cirillo M, Monsurrò MR, Montella P, Tedeschi G (2015): Functional overlap and divergence between ALS and bvFTD. Neurobiol Aging 36:413–423. [DOI] [PubMed] [Google Scholar]
- Tsermentseli S, Leigh PN, Goldstein LH (2012): The anatomy of cognitive impairment in amyotrophic lateral sclerosis: More than frontal lobe dysfunction. Cortex 48:166–182. [DOI] [PubMed] [Google Scholar]
- Tuladhar AM, van Dijk E, Zwiers MP, van Norden AG, de Laat KF, Shumskaya E, Norris DG, de Leeuw FE (2016): Structural network connectivity and cognition in cerebral small vessel disease. Hum Brain Mapp 37:300–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Heuvel MP, Sporns O (2011): Rich‐club organization of the human connectome. J Neurosci 31:15775–15786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Heuvel MP, Stam CJ, Kahn RS, Hulshoff Pol HE (2009): Efficiency of functional brain networks and intellectual performance. J Neurosci 29:7619–7624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstraete E, Veldink JH, Mandl RC, van den Berg LH, van den Heuvel MP (2011): Impaired structural motor connectome in amyotrophic lateral sclerosis. PLoS One 6:e24239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstraete E, Veldink JH, van den Berg LH, van den Heuvel MP (2014): Structural brain network imaging shows expanding disconnection of the motor system in amyotrophic lateral sclerosis. Hum Brain Mapp 35:1351–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlooswijk MC, Vaessen MJ, Jansen JF, de Krom MC, Majoie HJ, Hofman PA, Aldenkamp AP, Backes WH (2011): Loss of network efficiency associated with cognitive decline in chronic epilepsy. Neurology 77:938–944. [DOI] [PubMed] [Google Scholar]
- Wen W, Zhu W, He Y, Kochan NA, Reppermund S, Slavin MJ, Brodaty H, Crawford J, Xia A, Sachdev P (2011): Discrete neuroanatomical networks are associated with specific cognitive abilities in old age. J Neurosci 31:1204–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley SC, Strong MJ (2015): Frontotemporal dysfunction and dementia in amyotrophic lateral sclerosis. Neurol Clin 33:787–805. [DOI] [PubMed] [Google Scholar]
- Xu L, He D, Bai Y (2015): Microglia‐mediated inflammation and neurodegenerative disease. Mol Neurobiol [DOI] [PubMed] [Google Scholar]
- Zhang H, Yushkevich PA, Alexander DC, Gee JC (2006): Deformable registration of diffusion tensor MR images with explicit orientation optimization. Med Image Anal 10:764–785. [DOI] [PubMed] [Google Scholar]
- Zhang H, Yushkevich PA, Rueckert D, Gee JC (2007): Unbiased white matter atlas construction using diffusion tensor images. Med Image Comput Comput Assist Interv 10:211–218. [DOI] [PubMed] [Google Scholar]
- Zhang J, Yin X, Zhao L, Evans AC, Song L, Xie B, Li H, Luo C, Wang J (2014): Regional alterations in cortical thickness and white matter integrity in amyotrophic lateral sclerosis. J Neurol 261:412–421. [DOI] [PubMed] [Google Scholar]
- Zhou J, Gennatas ED, Kramer JH, Miller BL, Seeley WW (2012): Predicting regional neurodegeneration from the healthy brain functional connectome. Neuron 73:1216–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional supporting information may be found in the online version of this article.
Supporting Information


