Abstract
Low frequency theta band oscillations (4–8 Hz) are thought to provide a timing mechanism for hippocampal place cell firing and to mediate the formation of spatial memory. In rodents, hippocampal theta has been shown to play an important role in encoding a new environment during spatial navigation, but a similar functional role of hippocampal theta in humans has not been firmly established. To investigate this question, we recorded healthy participants’ brain responses with a 160‐channel whole‐head MEG system as they performed two training sets of a virtual Morris water maze task. Environment layouts (except for platform locations) of the two sets were kept constant to measure theta activity during spatial learning in new and familiar environments. In line with previous findings, left hippocampal/parahippocampal theta showed more activation navigating to a hidden platform relative to random swimming. Consistent with our hypothesis, right hippocampal/parahippocampal theta was stronger during the first training set compared to the second one. Notably, theta in this region during the first training set correlated with spatial navigation performance across individuals in both training sets. These results strongly argue for the functional importance of right hippocampal theta in initial encoding of configural properties of an environment during spatial navigation. Our findings provide important evidence that right hippocampal/parahippocampal theta activity is associated with environmental encoding in the human brain. Hum Brain Mapp 38:1347–1361, 2017. © 2016 Wiley Periodicals, Inc.
Keywords: virtual reality, hippocampus, magnetoencephalography, spatial navigation, theta rhythm
Abbreviations
- HF
hippocampal formation
- iEEG
intracranial EEG
- MEG
magnetoencephalography
INTRODUCTION
The hippocampal formation (HF) represents an environment via the firing of “place cells” [Muller, 1996; O'Keefe & Nadel, 1978] and “grid cells” [Jacobs et al., 2013; Moser et al., 2008, 2015]. The HF is also thought to play a critical role in encoding new information into memory, via neurophysiological processes modulated by a slow sinusoidal rhythm—theta oscillations. The theta rhythm is a prominent mode of hippocampal activity and has been extensively characterized in studies of spatial navigation and memory with invasive electrophysiological recordings in animals [Agarwal et al., 2014; O'Keefe and Nadel, 1978; Wang et al., 2015; Zhang et al., 2016]. In rodents, hippocampal theta oscillations have been shown to play an important role in encoding a new environment during spatial navigation [Jeewajee et al., 2008; Penley et al., 2013]. In humans, previous fMRI studies [Doeller et al., 2008; Wolbers & Büchel, 2005] have linked activation of the hippocampus to environmental novelty processing and learning, but the electrophysiological mechanisms are yet to be fully understood [Park et al., 2014; Rutishauser et al., 2010; Staudigl & Hanslmayr, 2013; Suthana et al., 2012]. In this study, we investigated whether and how human hippocampal theta rhythm contributes to environment encoding.
Theta rhythms (∼4–8 Hz) associated with spatial navigation have been observed with intracranial EEG (iEEG) in epileptic patients [Caplan et al., 2001, 2003; Ekstrom et al., 2005; Vass et al., 2016], but the invasive nature of these methods has meant there have been limited opportunities to systematically explore cognitive correlates of human hippocampal theta oscillations. Non‐invasive magnetoencephalography (MEG) source imaging provides a window for examining the function of neuronal signals from deep brain structures, such as the hippocampus [Attal & Schwartz 2013; Backus et al., 2016; Cousijn et al., 2015; Cornwell et al., 2008a; Fuentemilla et al., 2014, 2010; Guitart‐Masip et al., 2013; Riggs et al, 2009; Tesche and Karhu, 2000], the amygdala [Cornwell et al., 2008b; Hung et al., 2010] and the thalamus [Attal & Schwartz, 2013] in both healthy and patient populations. Perhaps most compelling is the work [Dalal et al., 2013] showing that MEG‐reconstructed hippocampal activity highly correlates (i.e., zero phase delay) with simultaneous depth recordings of hippocampal electrical activity, allowing unprecedented validation of MEG deep source reconstruction. Using MEG deep source imaging techniques, Cornwell et al. [2008a] found greater theta activity in the left anterior hippocampus and parahippocampus during goal‐directed navigation relative to aimless movements in a virtual reality environment. In another recent MEG experiment, Kaplan et al. [2012] reported that hippocampal theta power increased during the self‐initiation of virtual movement, and that hippocampal theta oscillations during a pre‐retrieval planning phase predicted subsequent memory performance. Such experiments indicate that MEG source imaging can play a crucial role in bridging the gap between animal models and human research to determine what aspects of hippocampal function are common across species and what aspects are unique to the human brain.
In this study, we extended this work using MEG to address an important theoretical question, i.e., whether and how the human hippocampal theta rhythm contributes to environmental encoding, as has been reported in rodents [Jeewajee et al., 2008; Penley et al., 2013]. We recorded healthy male participants’ brain responses during navigation of a virtual Morris water maze, a computer‐simulated task modeled after one extensively used for testing hippocampal‐dependent spatial navigation in rodents [Morris, 1984]. The experimental design was adapted from that of Cornwell et al. [2008a], in which there were two conditions (hidden platform condition vs. random swimming condition). We expanded this design with an additional training set with the environmental layout constant across training sets to allow us to determine whether hippocampal theta rhythms are sensitive to environmental encoding as reported in animal literature (please refer to Fig. 1 for the overview of the experimental design). In the hidden platform condition, the environment consisted of a virtual pool with four visual cues (objects with abstract patterns) attached to the surrounding walls. In the random swimming condition, the virtual pool was the same as that in the hidden platform condition, except that there were no visual cues attached to the walls. The motivation for removing visual cues was to investigate whether hippocampal theta oscillations had a general role in environmental encoding; and if so, it should be modulated by novelty of both a cue‐rich environment as in the hidden platform condition and environment without cues as in the random swimming condition. In the hidden platform condition, participants needed to find the hidden platform as quickly as possible, while in the random swimming condition, the task was swimming aimlessly non‐stop. To avoid the possibility that the contrast between the first and second training set was confounded with learning of a specific location, we changed the hidden platform location in the second training set. This paradigm offered a way to replicate findings of Cornwell et al. [2008a] with a different whole‐head magnetometer (a KIT MEG system in the present experiment vs. a CTF MEG system in Cornwell et al., 2008a), while investigating a new potential cognitive function of right hippocampal theta oscillations.
Figure 1.
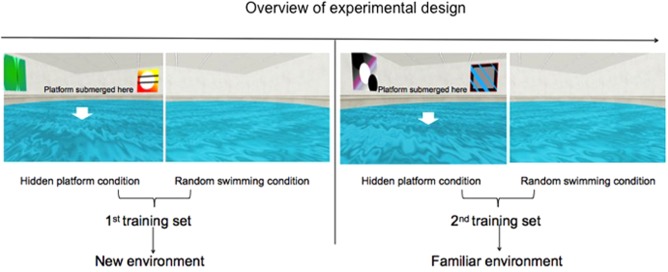
Overview of experimental design. There were two training sets in this task, each of which contained 20 hidden platform trials and 20 random swimming trials. Within each training set, the two conditions were alternatively presented (4 trials of hidden platform condition, 4 trials of random swimming condition, 4 trials of hidden platform condition, …). Hidden platform location differed between the two sets and was counterbalanced across participants. Environment layouts in each condition were kept constant so that the first training set was in a new environment and the second one was in a familiar environment. Participants were instructed to find the hidden platform as quickly as possible in the hidden platform condition and to swim non‐stop in the random swimming condition. [Color figure can be viewed at http://wileyonlinelibrary.com]
We made three predictions. First, left hippocampal theta activity should be greater in the hidden platform condition relative to the random swimming condition, as reported in Cornwell et al. [2008a], since the left hippocampus is thought to be a binding “device” [Kessels et al., 2004; Mitchell et al., 2000] instead of an environmental processor to form a cognitive map of the space [Burgess et al., 2002]. Second, right hippocampal theta activity should be greater in the new vs. familiar environment contrast in both conditions, as previous studies has shown that right hippocampal activation is associated with processing of a new configuration vs. a familiar one [Duzel et al., 2003] and with environmental novelty detection [Doeller et al., 2008]. Third, right hippocampal theta activity during encoding in the first training set should be significantly associated with behavioral performance in both training sets, because if right hippocampal theta is for environmental encoding, and since good formation of configural knowledge of an environment (i.e., formation of a cognitive map of the space) would facilitate participant to choose an efficient path to move to any place in that particular environment [Wolbers & Hegarty, 2010], we should expect the magnitude of environment encoding related right hippocampal theta correlated with navigation performance in both training sets where the environment was the same.
METHODS
Participants
Eighteen right‐handed healthy male participants (mean age = 29 years; range = 18–39 years) participated in the present experiment and were included in the final data analyses. Two additional participants were excluded from the final data analyses due to excessive head movement during the MEG recording session. All participants had normal or corrected‐to‐normal vision. Inclusion criteria were: (1) no past or current psychiatric disorders; (2) no current use of psychoactive medications by self‐report. Participants were also screened for dental work, metallic implants, a cardiac pacemaker, metal rods, and other magnetic material permanently fixed to their body. All procedures were approved by the Human Research Ethics Committee of Macquarie University.
Virtual Morris Water Maze
This task was adapted from Cornwell et al. [2008a] (Fig. 1). PsychoPy software [Peirce, 2007, 2008] was used to present a first person perspective viewpoint of two virtual circular pools filled with opaque water. The two pools had the same size and geometry, with the diameter of the pools being 80 virtual units. One pool contained four visual cues fixed to the walls of the square room surrounding the pool, and the other had no visual cues. The pool with visual cues contained a hidden platform and the participants’ task was to navigate to the hidden platform as quickly as possible. If the hidden platform was not found within 25s, it became visible and participants were instructed to swim to it to finish the trial. If the pool with no cue objects (random swimming condition) was presented, the task was to swim aimlessly non‐stop for 15s. Environment layouts in each condition were the same for both training sets. Images were projected (InFocus Model IN5108; InFocus, Portland) onto a screen at a viewing distance of about 1 m.
Trial Structure
There were two training sets in the experiment, each containing 40 trials (20 hidden platform trials, 20 aimless swimming control trials). Four trials were grouped as a block to be presented, so that in each training set, there were five blocks of hidden platform condition and five blocks of random swimming condition. Blocks of the two conditions were alternately presented (i.e., block 1 of hidden platform condition preceded block 1 of random swimming condition, followed by block 2 of hidden platform condition, which came before block 2 of random swimming condition,…, followed by block 5 of hidden platform condition, which went before block 5 of random swimming condition). Within each training set, the position of the hidden platform across blocks was fixed, but different between the two training sets and was counterbalanced across participants to avoid learning effect of a specific location between training sets. Environment layout was the same in the two training sets in each condition. During the inter‐trial interval of 4.5–5.5 s duration (randomly jittered) participants viewed a blank gray screen. There was a 3 minute break between the two training sets.
Task
Participants used a button box with three fingers (index, middle, ring fingers) of their right hand to move forward or to turn left or right in the pools. They used the visual environment of the pool (wall cues or no cues) to determine whether they needed to search for a hidden platform or swim randomly. They were instructed to try their best to find the hidden platform as quickly as possible in the hidden platform condition. Thus, in the hidden platform, they would learn the hidden platform location trial by trial and would gradually take an optimal path to reach it. They were also told to look at the projected screen at all times and to swim non‐stop until the trial finished. Participants began each trial facing the wall of the pool at one of four starting points (North, South, East, and West in pseudo‐random order), and were observed throughout the experiment on a computer monitor outside the shielded room. Participants were monitored to ensure that they did not stop swimming for more than 1s at a time, and were attending to the visual display at all times. Path lengths to reach the platform from starting position were recorded for each trial. Before the start of the second training set, participants were told that the hidden platform was in a new position.
Data Acquisition
Before MEG recordings, fiducial positions, marker coil positions and head shape were measured with a pen digitizer (Polhemus Fastrack, Colchester, VT). Neuromagnetic data were measured using a whole‐head MEG system (Model PQ1160R‐N2, KIT, Kanazawa, Japan) in a magnetically shielded room (Fujihara Co. Ltd., Tokyo, Japan) with participants in a supine position. The MEG system consisted of 160 coaxial first‐order gradiometers with a 50 mm baseline [Kado et al., 1999]. Continuous MEG data were acquired during each training set at a sampling rate of 1000 Hz. Head positions were obtained from five head marker coils attached to an elasticized cap placed on each participant's head, and were measured before and after each recording. Maximum head movement tolerance was 4 mm in any direction.
High resolution T1‐weighted anatomical brain images were acquired on a 3T Siemens Magnetom Verio scanner with a 12‐channel head coil at Macquarie University Hospital. Those anatomical images were obtained using 3D GR\IR scanning sequence with the following parameters: repetition time, 2000 ms; echo time, 3.94 ms; flip angle, 9 degrees; slice thickness, 0.93 mm; field of view, 240 mm; image dimensions, 512 × 512 × 208.
Data Analyses
Data analyses included two steps: (1) localization of brain activity using the synthetic aperture magnetometry (SAM) beamformer implemented in the BrainWave Matlab toolbox (Version 3.0; http://cheynelab.utoronto.ca/brainwave) and (2) group analyses of volumetric beamformer images using Analysis of Functional NeuroImages (AFNI; Cox, 1996; http://afni.nimh.nih.gov).
Source Analysis
SAM [Robinson & Vrba, 1999] was used to estimate source activity in the theta frequency band (4–8 Hz). SAM estimates source signals at each brain voxel while suppressing signals from other locations by calculating optimum spatial filters or beamformer weights at the location of interest using the signal covariance matrix from the sensor array [Hillebrand et al., 2005].
In the current experiment, raw MEG data were epoched into 5 s windows including a 1s pretrial baseline period and 4 s following the onset of each trial (4 s was the fastest time from the starting point to the hidden platform, among all trials and participants). Magnetic fields were modeled with a single sphere head model derived from each participant's structural MRI to fit the inner skull surface of each participant's MRI [Sarvas, 1987]. Covariance matrices were calculated from unaveraged 1 s active time windows locked to trial onset and 1 s pretrial baseline windows for each condition separately within a training set after applying a 4–8 Hz bandpass filter. Total covariance window length was 40 s for each condition (20 trials × 2 s). The source space was sampled into a three‐dimensional grid of 4 mm3 voxels with an equivalent current dipole source at each location.
Since beamformer weights increase with depth, and the sensor level noise remains constant throughout the volume, the raw source power at each voxel of the brain must be normalized [Cheyne et al., 2007]. In our analyses, beamformer outputs were normalized by the dual‐state imaging method [Hillebrand et al., 2005], which is a standard way of beamformer analyses. In this method, normalization is carried out using real brain noise, a so‐called control state and the state being normalized is called the active state, so that the resulting brain volumes represent the voxel‐wise relative power difference between the two states. In the current study, we used pseudo‐F SAM images to represent the percentage change of the brain signal between the two states (active state and control state). In the case of event‐related synchronization, the pseudo‐F value is derived from the formula A/C −1, in which A is the source power in the active state (in our study, active state was post trial onset window) and C is the source power in the control state (in our study, the control state was pre‐trial window). For event‐related desychronization, the formula is 1 − 1/(A/C) = 1 − C/A. Therefore, the pseudo‐F SAM volumes for each participant contain a power ratio value in each voxel across the whole brain. The term “pseudo‐F” is used because the ratio of source power of active state over control state resembles the F‐ratio. But the estimates of variance in the calculation are based on sensor noise level instead of between‐state (active and control states) variability. Thus, it does not conform to the true F distribution.
In the current study, the 1s active windows were advanced in 250 ms increments (one lower bound theta cycle) with 75% overlap up to the 4 s (e.g., 0–1 s, 0.25–1.25 s, 0.5–1.5 s, etc.), which was the fastest time from the starting point to the hidden platform among all trials and participants. The sliding window method (as opposed to simply analyzing the average power change of the entire post trial onset window of 4 s) increases the detectability of theta power changes, given their transient nature [< 500 ms in some cases; Arai et al., 2014; Foster et al., 2013; Kaplan et al., 2012; Sakimoto et al., 2013; Wyble et al., 2004]. This analysis produced pseudo‐F SAM volumetric images to represent the percentage change of the theta power between the active window and baseline window (i.e. pseudo‐F values) for each condition.
Group Statistics
Individual SAM images were normalized to a Talairach brain template in AFNI to allow for group analysis in a standardized space. Normalized SAM images of 4–8 Hz theta power (pseudo‐F values) were analyzed with 2 (conditions: hidden platform condition vs. random swimming condition) × 2 (training sets: first vs. second) repeated measures ANOVAs. We chose three time windows of interest (1–2 s, 1.25–2.25 s, 1.5–2.5 s) based on the reported latencies of increased theta power in the study of Cornwell et al. [2008a]. Additional time windows (0–1 s, 0.25–1.25 s, …, 0.75–1.75 s, 1.75–2.75 s, …, 2.75–3.75 s) were explored in post‐hoc analyses.
Given our a priori hypothesis that the hippocampus and parahippocampal cortices would generate theta oscillations during spatial navigation and environmental learning [Cornwell et al., 2008a; Park et al., 2014], a small‐volume correction was performed over a mask containing both left and right hippocampi and parahippocampal cortices based on an automated Talairach atlas in AFNI. A cluster alpha of 0.05 was set as the threshold for statistical significance. A cluster size criterion was determined by Monte Carlo simulations conducted in the AFNI program 3dClustSim, an adaptation of the program AlphaSim. This correction method has been employed by previous MEG beamformer studies [Keil et al., 2012, 2015; Muller et al., 2012, 2013; Meltzer et al., 2013]. Briefly, this correction method determines a minimum cluster size (i.e. minimum number of continuous voxels) given a certain threshold that is required for significance (for a full description, refer to http://afni.nimh.nih.gov/pub/dist/doc/program_help/3dClustSim.html ). In the present study, for our a priori comparison in the three time windows of interest, Monte Carlo simulations were iterated 10,000 times, with the voxel threshold being set at p < 0.01 and adjusted alpha threshold set at 0.05/3 = 0.017 (to correct for multiple comparisons across three time windows of interest). This requires a minimum of 21 continuous voxels in bilateral hippocampi and parahippocampi to be significant. For post‐hoc comparisons, the voxel threshold was set at p < 0.01 and the adjusted alpha threshold at 0.05/9 = 0.006 (another six time windows except the primary three time windows of interest), requiring at least 30 continuous voxels to achieve significance.
Time Frequency Representations (TFRs)
TFRs were constructed from source waveforms at the peak location of environmental encoding related right hippocampus determined by the ANOVA results on the pseudo‐F images to show right hippocampal theta power change relative to the baseline window across the 4 s post trial onset window. This was accomplished using a five‐cycle Morlet wavelet transformation [Tallon‐Baudry et al., 1997] of single trial source activity over a frequency range of 3–50 Hz in 1 Hz steps using the formula:
Wavelets were normalized so that the total energy was 1, with the normalization factor A being equal to:
To be specific, we reconstructed the activity of the peak voxel we specified, with beamformer covariance matrices being computed from −1–4 s (the whole epoch). A convolution of the complex wavelet with the beamformed MEG signal of each trial was derived and then averaged across all the trials. The magnitude of this convolution was converted to percentage change in power relative to the pre‐trial baseline [Isabella et al., 2015]. We did not show TF plots of the peak voxel of goal‐oriented navigation related left hippocampus, because Cornwell et al. [2008a] has shown the evolution of goal‐oriented navigation related theta power change across time.
Post‐Hoc Analyses
To confirm the robustness of our results, we did a cross validation analysis. Participants were randomly split into two subsamples and separate 2 (conditions: hidden platform vs. random swimming) × 2 (training sets: first vs. second) within subject ANOVAs were performed with the beamformer volumetric images for each subsample for each time window. Moreover, in light of evidence that human hippocampal theta may extend below 4 Hz [Jacobs, 2014], we carried out secondary beamformer analyses using a 1–4 Hz bandpass filter to determine whether slower oscillatory power showed a similar pattern across conditions. We also performed an analysis encompassing a broad frequency range of 1–8 Hz. All other analytic steps were the same as above for these alternative frequency windows.
Correlation Analysis
For those regions showing significant effects of environmental encoding in the group‐level contrasts, individual cluster means of theta power (4–8 Hz, pseudo‐F values) in the hidden platform condition in the first training set were extracted and correlated, respectively, with individual spatial navigation performance indexed by average path length in the hidden platform condition in each training set, using Pearson correlation implemented in IBM SPSS software (version 22) to test our third hypothesis.
Post‐Hoc Correlation Analyses
To explore whether in the familiar environment, right hippocampal theta correlated with navigation performance, we extracted cluster means of theta power (pseudo‐F values) in the hidden platform condition in the second training set and correlated, respectively, with individual average path length in the hidden platform condition in each training set. The previous work [Cornwell et al., 2008a] found no significant association between theta elicited by goal‐oriented spatial navigation related left anterior hippocampus and behavioural performance [Cornwell et al., 2008a,b]. To confirm this, we also extracted individual cluster means of theta power (pseudo‐F values) in the hidden platform condition in each training set from the anterior hippocampal/parahippocampal region showing significant activation in the hidden platform vs. random swimming condition contrast, and computed correlations with average path length in the corresponding training set.
Finally, we performed a voxel‐wise correlation between the theta power in both training sets and the average path length in training set one and two, respectively, across the whole brain to investigate whether the correlation was lateralized to right hippocampus/parahippocampus, and whether the correlation was only restricted to the first training set in the initial stage of learning. The threshold was set at p < 0.005 (uncorrected).
RESULTS
Spatial Navigation Performance
Path length from the starting point to the hidden platform location was measured as an index of spatial navigation performance. One‐way repeated measures ANOVA showed that path lengths were significantly different across five blocks (four trials per block) in each training set: F(4, 68) = 12.601, p < 0.001, η 2 = 0.825 (run one); F(4, 68) = 10.949, p < 0.001, η 2 = 0.784 (run two). The decrease in path length over training (Fig. 2A) demonstrates a clear spatial learning effect, consistent with previous studies using the virtual Morris water maze task [Cornwell et al., 2008a, 2010]. Collapsed across blocks, average path length was significantly shorter in the second training set (run two) than the first (run one) [t (17) = 2.329, p = 0.032, Cohen's d = 0.29] (Fig. 2B).
Figure 2.
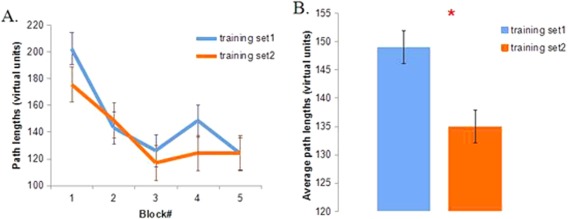
A. Average path length from the starting point to the hidden platform across five blocks (four trials per block) in training set one and two. B. Average path length in the two training sets. The diameter of the virtual pool was 80 virtual units. Error bar represents standard errors. * represents p < 0.05. [Color figure can be viewed at http://wileyonlinelibrary.com]
Theta Rhythm Associated With Environmental Encoding
A 2 (conditions: hidden platform condition vs. random swimming condition) × 2 (training sets: first vs. second) repeated measures ANOVA was performed for theta power at the source level in each of our time windows of interest.
In two of our main time windows of interest (1–2 s, 1.25–2.25 s), we found main effects of condition in the anterior hippocampus and parahippocampus (1 ‐ 2 s: F =8.4, p < 0.05, small volume corrected across time, 43 voxels, η2 = 0.506, peak voxel at left parahippocampus x = −26 y = −13 z = −20; 1.25–2.25 s: F = 8.4, p < 0.05, small volume corrected across time, 29 voxels, η2 = 0.392, peak voxel at left parahippocampus x = −22 y = −17 z = −24 (Fig. 3A,B). In the time window of 1.5–2.5s, we also found activation in the anterior left hippocampus (peak voxel at left hippocampus x = −30, y = −13, Z = −16), but it could not survive multiple comparison correction across time. These results are highly consistent with the findings by Cornwell et al. [2008a], showing that anterior left hippocampal/parahippocampal theta was stronger during navigation to the hidden platform relative to swimming aimlessly in the virtual pool. There was a slight difference in the timing between the present study and Cornwell et al. [2008a], where the peak difference of the similar comparison was during 1–2s and 1.5–2.5s.
Figure 3.
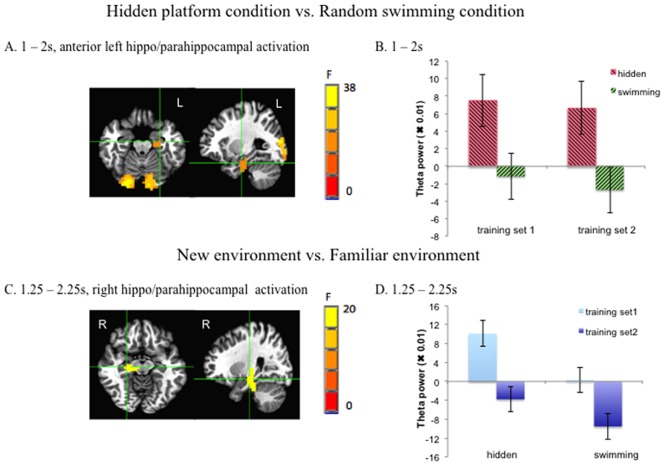
A. The whole brain images of main effect of condition in the time window of 1–2s as an example. The local maximum was in left parahippocampal gyrus (Talairach coordinates x = −26 y = −13 z = −20) (small volume corrected). B. Cluster mean of theta power (i.e. pseudo‐F values: the percentage change of theta power in the active window relative to the baseline window) of anterior left hippocampus/parahippocampus showing main effect of condition in each condition and each training set in the time window of 1–2 s. C. The whole brain images of main effect of training set in the time window of 1.25–2.25s. The local maximum was in the right hippocampus (Talairach coordinates x = 18 y = −21 z = −8) (small volume corrected). D. Cluster mean of theta power (i.e. pseudo‐F values: theta power percentage change relative to the baseline) of right hippocampal/parahippocampal activation region showing main effect of training set in each condition and each training set in the time window of 1.25–2.25 s. Error bar represents standard errors. [Color figure can be viewed at http://wileyonlinelibrary.com]
No main effect of condition was found in other regions of the hippocampus or parahippocampus.
We also found that in the 1.25–2.25 s time window, there was a main effect of training set with the peak in the right hippocampus (F = 8.4, p < 0.05, small volume corrected across time, 31 voxels, η2 = 0.453, peak voxel at right hippocampus: x = 18 y = −21 z = −8) (Fig. 3C,D). This suggests that right hippocampal theta power decreased as participants became familiar with the structure of the environment, in line with previous work showing that hippocampal activation was most prominent during the initial learning phase and decayed after performance had approached ceiling level [Wolbers & Büchel, 2005]. Time frequency plots also confirmed that during 1.25–2.25 s, there was a transient decrease in the second training set (Fig. 4). This transience is in line with the idea that theta power change is transient [Kaplan et al., 2012]. No other parts of the hippocampus/parahippocampus were found to show significant main effect of training set.
Figure 4.
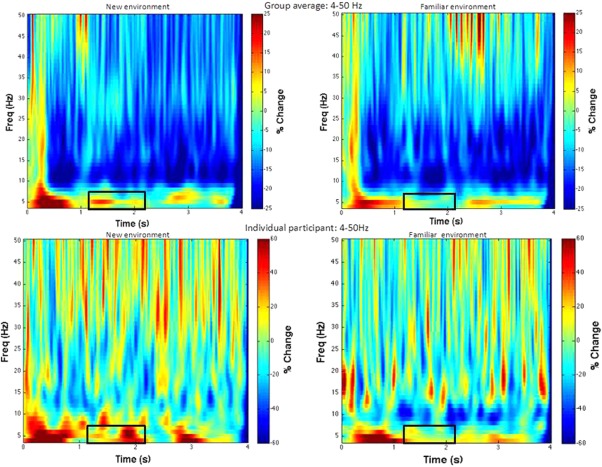
Time frequency plots (4–50 Hz) of the peak voxel of the environmental encoding related right hippocampus. The upper panel presents time frequency plots of the group average in the first (new environment) and the second (familiar environment) training set. The lower panel presents time frequency plots of one individual participant in the first (new environment) and the second (familiar environment) training set. The black rectangular shows the time window showing a decrease of theta band in the familiar environment relative to the new one revealed by SAM beamformer analysis (1.25–2.25s, 4–8 Hz). [Color figure can be viewed at http://wileyonlinelibrary.com]
No significant interactions between condition and training set were found. Exploratory post‐hoc tests of other time windows apart from the primary windows of interest (1–2 s, 1.25–2.25 s, 1.5–2.5 s) showed no statistically significant results.
For other activated brain regions, we only presented those in the new vs. familiar environment contrast, because the hidden platform vs. random swimming contrast was our replication result, and Cornwell et al. [2008a] has already reported those, which were similar to those in the present study. For new vs. familiar environment contrast, when the original voxel threshold was p < 0.005 (uncorrected), there were right hippocampus, left middle cingulate gyrus (peak voxel: x = −2, y = −25, z = 44). When the voxel threshold was set at p < 0.001 (uncorrected), only right hippocampus survived.
Split half analyses (cross validation) of the data are shown in Figure 5. The results were similar in both subsamples and also similar to the results of the overall analysis reported above. The local maxima of main effect of condition were in the anterior left hippocampus/parahippocampus (p < 0.05, small volume corrected). The local maximum of the main effect of training set was in the right hippocampus (p < 0.05, small volume corrected). These results confirm the robustness of our main results.
Figure 5.
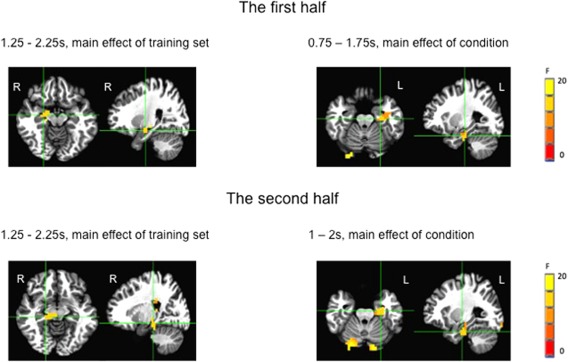
Whole brain images of split half analyses (p < 0.05, small volume corrected). The upper panel showed the results of the first half of the participants. There was a main effect of condition in 1.25–2.25s and main effects of condition in 0.5–1.5s and 0.75–1.75s (only images in 0.75–1.75 s are shown). The lower panel shows the results of the second half of the participants. There was a main effect of condition in 1.25–2.25s and main effects of condition in 1–2s, 1.25 −2.25s and 1.5–2.5s (only images in 1–2 s are shown). [Color figure can be viewed at http://wileyonlinelibrary.com]
The post hoc analyses for the frequency range of 1–4 Hz and 1–8 Hz did not show significant results.
Correlations Between Behavioral Performance and Theta Source Power
To test the third hypothesis, the cluster mean of pseudo‐F values of environment encoding‐related right hippocampal theta in the first training set in the time window of the significant main effect of training set (1.25–2.25 s) was correlated with average path lengths in training set one and two, respectively. We found a negative correlation between average path length in training set one and the pseudo‐F value of right hippocampus in the same training set (r = −0.5, p = 0.035) (6A). We also found that path length in the second training set correlated significantly with pseudo‐F value of right hippocampus in the first training set (r = −0.57, p = 0.014) as well (Fig. 6B). This result suggests that stronger right hippocampal theta during initial encoding of an environment is associated with better navigation performance (indexed by shorter average path length) in an environment both initially when it is new and subsequently when it is familiar. This finding is in line with previous reports that higher theta is associated with better performance [Kaplan et al., 2012; Staudigl & Hanslmayr, 2013].
Figure 6.
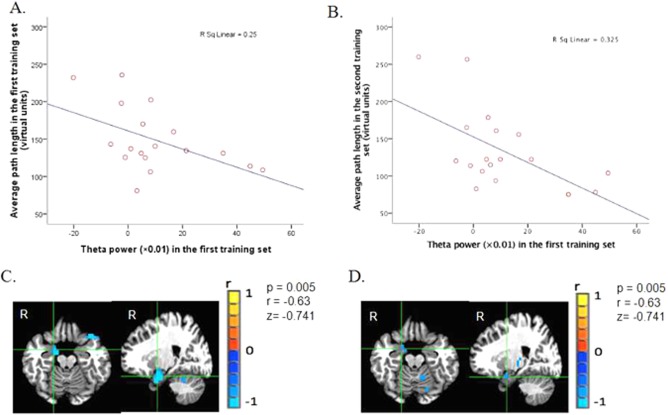
A. Cluster mean of theta power (i.e. pseudo‐F values: theta power percentage change relative to the baseline) of environmental encoding related right hippocampal theta for each participant in the hidden platform condition in the first training set plotted against his average path length in the same condition in the same training set. B. Cluster mean of theta power (pseudo‐F values) of environment layout encoding related right hippocampal theta for each participant in the hidden platform condition in training set one plotted against his average path length in the same condition in the second training set. C. Whole brain images of the correlation between theta power in the first training set in the hidden platform in the time window of 1.25–2.25s and average path length in the first training set, with the local maximum being in the right parahipp/hippocampus (Talairach coordinates x = 14, y = −5, z = −16). D. Whole brain images of the correlation between theta power in the first training set in the hidden platform in the time window of 1.25–2.25s and average path length in the second training set, with the local maximum being in the right parahippocampus/hippocampus (Talairach coordinates x = 14, y = −5, z = −16). [Color figure can be viewed at http://wileyonlinelibrary.com]
For the post hoc analyses, we did not find significant correlations between right hippocampal theta in the second training set with average path length in either training set. These results suggest that hippocampus may function prominently only at the early stage of learning [Wolbers & Büchel, 2005]. For the correlation between theta power in the hidden platform condition in the goal‐oriented navigation related anterior left hippocampus/parahippocampus and average path length, no significant correlation was found as well, in line with previous findings from Cornwell et al. [2008a].
Finally, to investigate whether the correlation was only lateralized to the right hippocampus/parahippocampus and whether the correlation was only restricted to the first training set in the initial stage of learning, we then did a voxel‐wise correlation between theta power in both training set with average path length in both training set, respectively, across the whole brain. We found there right hippocampal/parahippocampal theta in the first training set correlated with path length in both training sets (p < 0.005, uncorrected, local maxima were in the right hippocampus/parhippocampus: x = 14, y = −5, z = −16 and x = 14, y = −5, z = −16 for correlation between theta power in the first training set and average path length in the first training set and for correlation between theta power in the first training set and average path length in the second training set, respectively) (Fig. 6C,D). No correlation between theta from other parts of the hippocampus and parahippocampus and path length were found under the threshold of p < 0.005. For the correlation between theta power in the second training set and path length in both training sets, when p < 0.005, no single voxel in the bilateral hippocampi and parahippocampi was found to show correlation. The whole brain voxel‐wise correlation confirmed that the correlation was only lateralized to right hippocampus and only occurred between right hippocampal theta in the initial encoding phase and navigation performance in both new and familiar environments.
DISCUSSION
We investigated whether human hippocampal theta oscillations have a functional role in environmental encoding during spatial navigation in a virtual Morris water maze task. First, consistent with previous findings, we found that left anterior hippocampal/parahippocampal theta was stronger while navigating to the hidden platform relative to swimming randomly in a virtual pool. Second, in line with our hypotheses, we found evidence that right hippocampal/parahippocampal theta was stronger in the new relative to the familiar environment and the magnitude of right hippocampal/parahippocampal theta elicited during navigation in the new environment correlated with navigation performance in both the new and familiar environments.
The finding that anterior left hippocampal/parahippocampal theta was stronger in the hidden platform condition relative to the random swimming condition is consistent with the results of Cornwell et al. [2008a] who used a very similar experimental paradigm. These results confirm the robustness and specificity of anterior left hippocampal theta oscillations during goal‐oriented spatial navigation in the human brain. The striking consistency of results between these two studies using different MEG systems with two independent cohorts of participants provides support for the contention that non‐invasive MEG recordings of hippocampal theta are robust and reliable. Taken together with a small but growing body of MEG studies of the hippocampal theta rhythm [Backus et al., 2016; Fuentemilla et al., 2014, 2010; Poch et al., 2011; Riggs et al., 2009], our results also provide important confirmation of the hypothesis that the MEG‐recorded theta rhythm indexes neurophysiological mechanisms that are functionally comparable to those previously measured with invasive recordings in the hippocampus of humans [Caplan et al., 2001, 2003; Ekstrom et al., 2003, 2005; Jacobs et al., 2007; Kahana et al., 1999; Vass et al., 2016] and animals [Agarwal et al., 2014; O'Keefe & Dostrovsky, 1971; Harris et al., 2002; Mehta et al., 2002; Zhang et al., 2016]. Importantly, the virtual Morris water maze task used in our experiment and that of Cornwell et al. [2008a] provided a behavioral context for spatial navigation that is highly comparable to the Morris water maze used to elicit and study theta oscillations in the extensively characterized rodent model [Kelemen et al., 2005; Olvera‐Cortes et al., 2004, 2012]. The capability to reliably and non‐invasively measure hippocampal theta in humans now allows us to bridge the gap between animal and human models of hippocampal function, by systematically and rigorously characterizing the cognitive functions of the human theta rhythm in routine experimentation that does not rely on limited opportunities to invasively study human patients.
Our new finding that right hippocampal theta activation was greater in the first training set than in the second one in both hidden platform condition (cue rich environment) and random swimming condition (environment without cues), suggests an important role for the right hippocampus in encoding an environment in general. Since environmental layout was constant across training sets, we show that right hippocampal theta power was strongest when the requirement for environmental encoding was strongest (in the first training set), in line with the idea that hippocampal activation was prominent in the initial learning phase and decreased when performance improved [Wolbers & Buchel, 2005] and with the finding that right hippocampus was more active in processing new configuration/environment relative to familiar configuration/environment [Doeller et al., 2008; Duzel et al., 2003]. This function of human right hippocampal theta in new environmental encoding is consistent with results from animal research [Jeewajee et al., 2008; Penley et al., 2013; see Burgess & O'Keefe, 2011 for a review] and provides a direct link between the human and animal studies. Our MEG results are also consistent with the fMRI results of Igloi et al. [2010], showing a time‐dependent decrease in right hippocampal activity during learning in a spatial navigation task. In addition, Kaplan et al. [2012] reported MEG theta changes (at the sensor level) when participants encoded a new environment during spatial navigation.
Notably, we found that stronger right hippocampal theta power during the first training set was correlated with better navigation performance in both the first and second training sets. These associations bolster the conclusion that right hippocampal theta plays a functional role in encoding configural properties of an environment. Those who exhibited relatively greater right hippocampal theta power during the first training set took relatively shorter paths to the hidden platform. This was true for performance in the first training set as well as the second one when a novel platform location was introduced in the same environment. This observation demonstrates that the correlation between right hippocampal theta during encoding and spatial navigation performance is not contingent on learning a specific location and therefore strongly argues for the functional role of right hippocampal theta is about encoding the whole environment. Robust encoding of the configuration of the environment to form a cognitive map of the space confers the flexibility to navigate efficiently to any location in that particular environment [Wolbers & Hegarty, 2010]. This association is also consistent with previous studies linking the right hippocampus, more generally, to spatial navigation performance [Abrahams et al., 1997; Burgess et al., 2002; Nedelska et al., 2012; Spiers et al., 2001] and with previous reports that increased theta power was associated with successful/better memory formation in other experimental paradigms [Hanslmayr et al., 2011; Osipova et al., 2006; Staudigl & Hanslmayr, 2013; Sederberg et al., 2007]. However, the result that no correlation was found between right hippocampal theta in the familiar environment and path length, in conjunction with the result that in the familiar environment, there was an attenuation of right hippocampal theta power, indicates that the hippocampus might function prominently during the early stages of cognitive mapping [see Wolbers & Wiener, 2014 for a review].
Our results converge with a body of evidence that the right hippocampus is important in spatial navigation [Bohbot et al., 1998; Ekstrom et al., 2003; Grön et al., 2000; Maguire et al., 1997, 2000], and further indicate that this may reflect a role in encoding an environment to facilitate navigation performance. There is some evidence that impairment of the right hippocampus is associated with impaired navigation performance. Cornwell et al. [2010] reported that depressed patients exhibited impaired performance in a virtual Morris water maze task and this impairment was related to reduced right hippocampal theta oscillations compared to healthy controls.
Taken together, our results indicate that left and right hippocampi may have different functional roles [Burgess et al., 2002], with right hippocampus playing a role in encoding an environment to form a cognitive map of the space and left hippocampus being involved in navigating to a specific location but not in environmental processing. The left hippocampus is thought to play a role in associative processing [Igloi et al., 2010] and to mediate specific component processes of spatial navigation, such as binding the platform to its spatial location [Cornwell et al., 2008a; Kessels et al., 2004; Mitchell et al., 2000]. Consistent with this hypothesis, our results show that left hippocampal theta was elicited with comparable magnitude in both training sets with the hidden platform presented in two different locations, and was not modulated by environmental novelty, adding another piece of evidence that left hippocampus was not sensitive to environment. Further work is required to nail down the potential functional dissociation of left and right hippocampi.
We note that all the effects observed in the current study were localized to the anterior portion of the hippocampus/parahippocampal cortices. Some authors have argued for functional specialization along the longitudinal axis of the hippocampus in both rodents [see Fanselow & Dong, 2010 for a review] and humans [see Poppenk et al., 2013 for a review]. No definitive conclusion on the specific functional differentiation between anterior and posterior portion of human hippocampus has been drawn though, our results that right anterior hippocampal theta encodes environmental layout fits with one idea that anterior hippocampus may predominantly encode coarse, global representations and that encoding is more linked to anterior portion of the hippocampus [Poppenk et al., 2013].
Our results stand in contrast to those of a recent iEEG study [Park et al., 2014] which reported bilateral hippocampal involvement during encoding of a new environment; further, these researchers reported that hippocampal theta power increased with increasing familiarity with the environment. There are two possible reasons for the discrepancies. First, Long et al. [2014] reported there existed temporal dynamics of the subsequent memory effect, with theta power increasing in the early encoding phase and decreasing in the late encoding phase. Different hippocampal theta power change patterns found in the current study and Park et al. [2014] might be due to difference in length of encoding phases. In Park et al., [2014], new environment was defined as the first trial in the learning phase, which can be regarded as very early encoding phase. In the current experiment, encoding effect was the average of the learning effect in the first training set, which contains 20 trials in hidden platform condition and random swimming condition, respectively. Thus, the encoding effect might reflect the average effect of very early encoding phase and later encoding phase. Second, in Park et al. [2014]'s study, target locations were constant in the new and familiar environments, so that the environment encoding was confounded with encoding a specific location within the environment. In our study, target location was dissociated from environmental familiarity in the first and second training sets. Thus, bilateral hippocampal activation found in Park et al. [2014] might reflect both encoding of new environment and location within that environment.
One may query whether the right hippocampal theta that was observed is related to general novelty processing instead of environmental novelty processing. However, the correlation between right hippocampal theta power in the first training set and spatial navigation performance in both training sets argues against this possibility and suggests a more specific association with learning the environment. If the right hippocampal theta was only for general novelty processing and had nothing to do with spatial processing, the chance of being able to observe a correlation with behavioral measures of spatial cognition would be extremely slim. To yield a more definitive conclusion in this regard, a third training set in a new environment is needed, in which we would predict a rebound in right hippocampal theta.
Co‐registration errors and head movement introduce spatial uncertainty of hippocampal estimates and the peak theta power at the individual level. Although group‐level statistics should average out these differences, since co‐registration errors and head movement across participants are unlikely to be systematic in direction, the ability of MEG to differentiate source signals from the hippocampus versus parahippocampus is questionable, and the clusters of differential power observed here generally spanned both structures. This is an important limitation given evidence that hippocampus and parahippocampal cortices mediate distinct functions. For instance, Ekstrom and Bookheimer [2007] and Ekstrom et al., [2011] documented that the hippocampus and parahippocampus responded differentially to spatial and temporal order source retrieval. Aggleton and Brown [2006] argued that the role of parahippocampus relied on an item‐based familiarity discrimination mechanism, while the function of the hippocampus concerns novel spatial arrangement of stimuli and associative and contextual aspects of memory. Future development of higher resolution source reconstruction techniques will facilitate the investigation of functional differentiation of hippocampal and parahippocampal theta oscillations.
CONCLUSION
In the past several decades, numerous studies have attempted to determine the precise function and behavioral correlates of hippocampal theta oscillations [Ekstrom and Watrous, 2014]. Our study contributes to this literature by presenting evidence for the function of right hippocampal theta rhythm in environment encoding during navigation in humans, directly linking results from invasive studies in animals with results from non‐invasive measurements in healthy humans. Most importantly, these results demonstrate a robust relationship between hippocampal theta rhythm and behavioral measures of spatial navigation performance.
ACKNOWLEDGMENTS
The authors thank Mr. Steven Saunders for help with the implementation of the virtual Morris water maze programme, Dr. Jie Yang and Dr. Xiangzhen Kong for discussion and comments on an early draft of the manuscript. We also express our appreciations to China Scholarship Council for financial support.
REFERENCES
- Arai M, Brandt V, Dabaghian Y (2014): The effects of theta precession on spatial learning and simplicial complex dynamics in a topological model of the hippocampal spatial map. PLoS Comput Biol 10:e1003651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahams S, Pickering A, Polkey CE, Morris RG (1997): Spatial memory deficits in patients with unilateral damage to the right hippocampal formation. Neuropsychologia 35:11–24. [DOI] [PubMed] [Google Scholar]
- Agarwal G, Stevenson IH, Berenyi A, Mizuseki K, Buzsaki G, Sommer FT (2014): Spatially distributed local fields in the hippocampus encode rat position. Science 344:626–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggleton JP, Brown MW (2006): Interleaving brain systems for episodic and recognition memory. Trends Cogn Sci 10:455–463. [DOI] [PubMed] [Google Scholar]
- Attal Y, Schwartz D (2013): Assessment of subcortical source localization using deep brain activity imaging model with minimum norm operators: A MEG Study. PLoS ONE 8:e59856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backus AR, Schoffelen JM, Szebenyi S, Hanslmayr S, Doeller CF (2016): Hippocampal‐prefrontal theta oscillations support memory integration. Curr Biol 26:450–457. [DOI] [PubMed] [Google Scholar]
- Bohbot VD, Kalina M, Stepankova K, Spackova N, Petrides M, Nadel L (1998): Spatial memory deficits in patients with lesions to the right hippocampus and to the right parahippocampal cortex. Neuropsychologia 36:1217–1238. [DOI] [PubMed] [Google Scholar]
- Burgess N, O'Keefe J (2011): Models of place and grid cell firing and theta rhythmicity. Curr Opin Neurol 21:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, O'Keefe J (2002): The human hippocampus and spatial and episodic memory. Neuron 35:625–641. [DOI] [PubMed] [Google Scholar]
- Buzsaki G (2005): Theta rhythm of navigation: Link between path integration and landmark navigation, episodic and semantic memory. Hippocampus 15:827–840. [DOI] [PubMed] [Google Scholar]
- Buzsaki G (2006): Rhythms of the Brain. Oxford: Oxford University Press. [Google Scholar]
- Caplan JB, Madsen JR, Raghavachari S, Kahana MJ (2001): Distinct patterns of brain oscillations underlie two basic parameters of human maze learning. J Neurophysiol 86:368–380. [DOI] [PubMed] [Google Scholar]
- Caplan JB, Madsen JR, Schulze‐Bonhage A, Aschenbrenner‐Scheibe R, Kahana MJ (2003): Human theta oscillations related to sensorimotor integration and spatial learning. J Neurosci 23:4726–4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheyne D, Bostan AC, Gaetz W, Pang EW (2007): Event‐related beamforming: A robust method for presurgical functional mapping using MEG. Clin Neurophysiol 118:1691–1704. [DOI] [PubMed] [Google Scholar]
- Cornwell BR, Johnson LL, Holroyd T, Carver FW, Grillon C (2008a): Human hippocampal and parahippocampal theta during goal‐directed spatial navigation predicts performance on a virtual Morris water maze. J Neurosci 23:5983–5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwell BR, Carver FW, Coppola R, Johnson L, Alvarez R, Coppola R, Manji H, Zarate CA Jr, Grillon C (2008b): Evoked amygdala responses to negative faces revealed by adaptive MEG beamformers. Brain Res 1244:103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwell BR, Salvadore G, Colon‐Rosario V, Latov DR, Holroyd T, Carver FW, Grillon C (2010): Abnormal hippocampal functioning and impaired spatial navigation in depressed individuals: Evidence from whole‐head magnetoencephalography. Am J Psychiatry 167:836–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwell BR, Arkin N, Overstreet C, Carver FW, Grillon C (2012): Distinct contributions of human hippocampal theta to spatial cognition and anxiety. Hippocampus 22:1848–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousijn H, Tunbridge EM, Rolinski M, Wallis G, Colclough GL, Woolrich MW, … Harrison PJ (2015): Modulation of hippocampal theta and hippocampal‐prefrontal cortex function by a schizophrenia risk gene. Hum Brain Mapp 36:2387–2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW (1996): AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29:162–173. [DOI] [PubMed] [Google Scholar]
- Dalal S, Jerbi K, Bertrand O, Adam C, Ducorps A, Schwarts D, Martinerie J, Lachaux J (2013): Simultaneous MEG‐intracranial EEG: New insights into the ability of MEG to capture oscillatory modulations in the neocortex and the hippocampus. Epilepsy Behav 28:310–311. [Google Scholar]
- Doeller CF, King JA, Burgess N (2008): Parallel striatal and hippocampal systems for landmarks and boundaries in spatial memory. Proc Natl Acad Sci USA 105:5915–5920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duzel E, Habib R, Rotte M, Guderian S, Tulving E, Heinze H (2003): Human hippocampal and parahippocampal activity during visual associative recognition memory for spatial and nonspatial stimulus configurations. J Neurosci 23:9439–9444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom AD, Bookheimer SY (2007): Spatial and temporal episodic memory retrieval recruit dissociable functional networks in the human brain. Learn Mem 14:645–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom AD, Watrous AJ (2014): Multifaceted roles for low frequency oscillations in bottom‐up and top‐down processing during spatial navigation and memory. Neuroimage 85:667–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom AD, Caplan JB, Fields TA, Isham EA, Newman EL, Kahana MJ, Fried I (2003): Cellular networks underlying human spatial navigation. Nature 425:184–188. [DOI] [PubMed] [Google Scholar]
- Ekstrom AD, Caplan JB, Ho E, Shattuck K, Fried I, Kahana MJ (2005): Human hippocampal theta activity during virtual navigation. Hippocampus 15:881–889. [DOI] [PubMed] [Google Scholar]
- Ekstrom AD, Copara MS, Isham EA, Wang WC, Yonelinas AP (2011): Dissociable networks involved in spatial and temporal order source retrieval. Neuroimage 56:1803–1813. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Dong HW (2010): Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 65:7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster BL, Kaveh A, Dastjerdi M, Miller KJ, Parvizi J (2013): Human retrosplenial cortex displays transient theta phase locking with medial temporal cortex prior to activation during autobiographical memory retrieval. J Neurosci 33:10439–10446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentemilla L, Barnes GR, Duzel E, Levine B (2014): Theta oscillations orchestrate medial temporal lobe and neocortex in remembering autobiographical memories. Neuroimage 85 Pt 2:730–737. [DOI] [PubMed] [Google Scholar]
- Fuentemilla L, Penny WD, Cashdollar N, Bunzeck N, Duzel E (2010): Theta‐coupled periodic replay in working memory. Curr Biol 20:606–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guitart‐Masip M, Barnes GR, Horner A, Bauer M, Dolan RJ, Duzel E (2013): Synchronization of medial temporal lobe and prefrontal rhythms in human decision making. J Neurosci 33:442–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grön G, Wunderlich AP, Spitzer M, Tomczak R, Riepe MW (2000): Brain activation during human navigation: Gender‐different neural networks as substrate of performance. Nat Neurosci 3:404–408. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S, Volberg G, Wimber M, Raabe M, Greenlee MW, Bauml KH (2011): The relationship between brain oscillations and BOLD signal during memory formation: A combined EEG‐fMRI study. J Neurosci 31:15674–15680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KD, Henze DA, Hirase H, Leinekugel X, Dragoi G, Czurko A, Buzsaki G (2002): Spike train dynamics predicts theta‐related phase precession in hippocampal pyramidal cells. Nature 417:738–741. [DOI] [PubMed] [Google Scholar]
- Hillebrand A, Singh KD, Holliday IE, Furlong PL, Barnes GR (2005): A new approach to neuroimaging with magnetoencephalography. Hum Brain Mapp 25:199–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung Y, Smith ML, Bayle DJ, Mills T, Cheyne D, Taylor MJ (2010): Unattended emotional faces elicit early lateralized amygdala–frontal and fusiform activations. Neuroimage 50:727–733. [DOI] [PubMed] [Google Scholar]
- Igloi K, Doeller CF, Berthoz A, Rondi‐Reig L, Burgess N (2010): Lateralized human hippocampal activity predicts navigation based on sequence or place memory. Proc Natl Acad Sci USA 107:14466–14471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isabella S, Ferrari P, Jobst C, Cheyne JA, Cheyne D (2015): Complementary roles of cortical oscillations in automatic and controlled processing during rapid serial tasks. Neuroimage 118:268–281. [DOI] [PubMed] [Google Scholar]
- Jacobs J (2014): Hippocampal theta oscillations are slower in humans than in rodents: Implications for models of spatial navigation and memory. Philos Trans R Soc Lond B Biol Sci 369:20130304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J, Kahana MJ, Ekstrom AD, Fried I (2007): Brain oscillations control timing of single‐neuron activity in humans. J Neurosci 27:3839–3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J, Weidemann CT, Miller JF, Solway A, Burke JF, Wei XX, … Kahana MJ (2013): Direct recordings of grid‐like neuronal activity in human spatial navigation. Nat Neurosci 16:1188–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeewajee A, Lever C, Burton S, O'Keefe J, Burgess N (2008): Environmental novelty is signaled by reduction of the hippocampal theta frequency. Hippocampus 18:340–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kado H, Higuchi M, Shimogawara M, Haruta Y, Adachi Y, Kawai J, Uehara G (1999): Magnetoencephalogram systems developed at KIT. IEEE Trans Appl Supercond 9:4057–4062. [Google Scholar]
- Kahana MJ, Sekuler R, Caplan JB, Kirschen M, Madsen JR (1999): Human theta oscillations exhibit task dependence during virtual maze navigation. Nature 399:781–784. [DOI] [PubMed] [Google Scholar]
- Kaplan R, Doeller CF, Barnes GR, Litvak V, Duzel E, Bandettini PA, Burgess N (2012): Movement‐related theta rhythm in humans: Coordinating self‐directed hippocampal learning. PLoS Biol 10:e1001267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil J, Muller N, Ihssen N, Weisz N (2012): On the variability of the McGurk effect: Audiovisual integration depends on prestimulus brain states. Cereb Cortex 22:221–231. [DOI] [PubMed] [Google Scholar]
- Keil J, Pomper U, Senkowski D (2015): Distinct patterns of local oscillatory activity and functional connectivity underlie intersensory attention and temporal prediction. Cortex 74:277–288. [DOI] [PubMed] [Google Scholar]
- Kelemen E, Moron I, Fenton AA (2005): Is the hippocampal theta rhythm related to cognition in a non‐locomotor place recognition task?. Hippocampus 15:472–479. [DOI] [PubMed] [Google Scholar]
- Kessels RP, Hendriks MP, Schouten J, Asselen MV, Postma A (2004): Spatial memory deficits in patients after unilateral selective amygdalohippocampectomy. J Int Neuropsychol Soc 10:907–912. [DOI] [PubMed] [Google Scholar]
- Long NM, Burke JF, Kahana MJ (2014): Subsequent memory effect in intracranial and scalp EEG. Neuroimage 84:488–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Frackowiak RS, Frith CD (1997): Recalling routes around London: Activation of the right Hippocampus in taxi drivers. J Neurosci 17:7103–7110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Burgess N, O'Keefe J (1999): Human spatial navigation: Cognitive maps, sexual dimorphism, and neural substrates. Curr Opin Neurobiol 9:171–177. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Gadian DG, Johnsrude IS, Good CD, Ashburner J, Frackowiak RS, Frith CD (2000): Navigation‐related structural change in the hippocampi of taxi drivers. Proc Natl Acad Sci USA 97:4398–4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta MR, Lee AK, Wilson MA (2002): Role of experience and oscillations in transforming a rate code into a temporal code. Nature 417:741–746. [DOI] [PubMed] [Google Scholar]
- Meltzer JA, Wagage S, Ryder J, Solomon B, Braun AR (2013): Adaptive significance of right hemisphere activation in aphasic language comprehension. Neuropsychologia 51:1248–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell KJ, Johnson MK, Raye CL, D'Esposito M (2000): fMRI evidence of age‐related hippocampal dysfunction in feature binding in working memory. Brain Res Cogn Brain Res 10:197–206. [DOI] [PubMed] [Google Scholar]
- Morris R (1984): Developments of a water‐maze procedure for studying spatial learning in the rat. J Neurosci Methods 11:47–60. [DOI] [PubMed] [Google Scholar]
- Moser EI, Kropff E, Moser MB (2008): Place cells, grid cells, and the brain's spatial representation system. Annu Rev Neurosci 31:69–89. [DOI] [PubMed] [Google Scholar]
- Moser MB, Rowland DC, Moser EI (2015): Place cells, grid cells, and memory. Cold Spring Harb Perspect Biol 7:a021808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller R (1996): A quarter of a century of place cell. Neuron 17:813–822. [DOI] [PubMed] [Google Scholar]
- Nedeska Z, Andel R, Laczo J, Vlcek K, Horinek D, Lisy J, & Hort J (2012): Spatial navigation impairment is proportional to right hippocampal volume. Proc Natl Acad Sci USA 109:2590–2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller N, Weisz N (2012): Lateralized auditory cortical alpha band activity and interregional connectivity pattern reflect anticipation of target sounds. Cereb Cortex 22:1604–1613. [DOI] [PubMed] [Google Scholar]
- Muller N, Keil J, Obleser J, Schulz H, Grunwald T, Bernays RL, … Weisz N (2013): You can't stop the music: Reduced auditory alpha power and coupling between auditory and memory regions facilitate the illusory perception of music during noise. Neuroimage 79:383–393. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Dostrovsky J (1971): The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely‐moving rat. Brain Res 34:171–175. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Nadel L (1978): The hippocampus as a cognitive map. Hippocampus 9:352–364. [Google Scholar]
- Olvera‐Cortes E, Guevara MA, Gonzalez‐Burgos I (2004): Increase of the hippocampal theta activity in the Morris water maze reflects learning rather than motor activity. Brain Res Bull 62:379–384. [DOI] [PubMed] [Google Scholar]
- Olvera‐Cortes ME, Garcia‐Alcantar I, Gutierrez‐Guzman B, Hernandez‐Perez JJ, Lopez‐Vazquez MA, Cervantes M (2012): Differential learning‐related changes in theta activity during place learning in young and old rats. Behav Brain Res 226:555–562. [DOI] [PubMed] [Google Scholar]
- Osipova D, Takashima A, Oostenveld R, Fernandez G, Maris E, Jensen O (2006): Theta and gamma oscillations predict encoding and retrieval of declarative memory. J Neurosci 26:7523–7531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Lee H, Kim T, Park GY, Lee EM, Baek S, Ku J, Kim IY, Jang DP, Kang JK (2014): Role of low‐ and high‐frequency oscillations in the human hippocampus for encoding environmental novelty during a spatial navigation task. Hippocampus 24:1314–1352. [DOI] [PubMed] [Google Scholar]
- Peirce JW (2007): PsychoPy—psychophysics software in Python. J Neurosci Methods 162:8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirce JW (2008): Generating stimuli for neuroscience using PsychoPy. Front Neuroinformat 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penley SC, Hinman JR, Long LL, Markus EJ, Escabi MA, Chrobak JJ (2013): Novel space alters theta and gamma synchrony across the longitudinal axis of the hippocampus. Front Syst Neurosci 7:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poch C, Fuentemilla L, Barnes GR, Duzel E (2011): Hippocampal theta‐phase modulation of replay correlates with configural‐relational short‐term memory performance. J Neurosci 31:7038–7042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppenk J, Evensmoen HR, Moscovitch M, Nadel L (2013): Long‐axis specialization of the human hippocampus. Trends Cogn Sci 17:230–240. [DOI] [PubMed] [Google Scholar]
- Riggs L, Moses SN, Bardouille T, Herdman AT, Ross B, Ryan JD (2009): A complementary analytic approach to examining medial temporal lobe sources using magnetoencephalography. Neuroimage 45:627–642. [DOI] [PubMed] [Google Scholar]
- Robinson, S. E. , Vrba, J. (1999). Functional neuroimaging by synthetic aperture magnetometry (SAM) In: Yoshimoto T, Kotani M, Kuriki S, Karibe H, Nakasato N, editors. Recent Advances in Biomagnetism. Sendai, Japan: Tohoku UP; pp 302–305. [Google Scholar]
- Rutishauser U, Ross IB, Mamelak AN, Schuman EM (2010): Human memory strength is predicted by theta‐frequency phase‐locking of single neurons. Nature 464:903–907. [DOI] [PubMed] [Google Scholar]
- Sakimoto Y, Okada K, Takeda K, Sakata S (2013): Transient decline in hippocampal theta activity during the acquisition process of the negative patterning task. PLoS One 8:e70756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarvas J (1987): Basic mathematical and electromagnetic concepts of the biomagnetic inverse problem. Phys Med Biol 32:11–22. [DOI] [PubMed] [Google Scholar]
- Sederberg PB, Schulze‐Bonhage A, Madsen JR, Bromfield EB, McCarthy DC, Brandt A, … Kahana MJ (2007): Hippocampal and neocortical gamma oscillations predict memory formation in humans. Cereb Cortex 17:1190–1196. [DOI] [PubMed] [Google Scholar]
- Spiers HJ, Burgess N, Maguire EA, Baxendale SA, Hartley T, Thompson PJ, O'Keefe J (2001): Unilateral temporal lobectomy patients show lateralized topographical and episodic memory deficits in a virtual town. Brain 124:2476–2489. [DOI] [PubMed] [Google Scholar]
- Staudigl T, Hanslmayr S (2013): Theta oscillations at encoding mediate the context‐dependent nature of human episodic memory. Curr Biol 23:1101–1106. [DOI] [PubMed] [Google Scholar]
- Suthana N, Haneef Z, Stern J, Mukamel R, Behnke E, Knowlton B, Fried I (2012): Memory enhancement and deep‐brain stimulation of the entorhinal area. N Engl J Med 366:502–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallon‐Baudry C, Bertrand O, Delpuech C, Permier J (1997): Oscillatory gamma‐band (30‐70 Hz) activity induced by a visual search task in humans. J Neurosci 17:722–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesche CD, Karhu J (2000): Theta oscillations index human hippocampal activation during a working memory task. Proc Natl Acad Sci USA 97:9191–9924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vass LK, Copara MS, Seyal M, Shahlaie K, Farias ST, Shen PY, Ekstrom AD (2016): Oscillations go the distance: Low‐frequency human hippocampal oscillations code spatial distance in the absence of sensory cues during teleportation. Neuron 89:1180–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Romani S, Lustig B, Leonardo A, Pastalkova E (2015): Theta sequences are essential for internally generated hippocampal firing fields. Nat Neurosci 18:282–288. [DOI] [PubMed] [Google Scholar]
- Wolbers T, Büchel C (2005): Dissociable retrosplenial and hippocampal contributions to successful formation of survey representations. J Neurosci 25:3333–3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolbers T, Hegarty M (2010): What determines our navigational abilities? Trends Cogn Sci 14:138–146. [DOI] [PubMed] [Google Scholar]
- Wolbers T, Wiener JM (2014): Challenges for identifying the neural mechanisms that support spatial navigation: The impact of spatial scale. Front Hum Neurosci 8:571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyble BP, Hyman JM, Rossi CA, Hasselmo ME (2004): Analysis of theta power in hippocampal EEG during bar pressing and running behavior in rats during distinct behavioral contexts. Hippocampus 14:662–674. [DOI] [PubMed] [Google Scholar]
- Zhang L, Ma X, Chen G, Barkai E, Lin L (2016): Theta rhythmic clock‐like activity of single units in the mouse hippocampus. J Neurosci 36:4415–4420. [DOI] [PMC free article] [PubMed] [Google Scholar]


