Abstract
Based on higher prevalence rates of several mental disorders for city dwellers, psychosocial stress effects of urban living have been proposed as an environmental risk factor contributing to the development of mental disorders. Recently, it was shown that amygdala activation differs between city dwellers and rural residents in response to a cognitive‐social stressor. Besides its influence on the amygdala, chronic stress also affects mesocorticolimbic brain regions involved in reward processing, and stress‐related dysregulation of the mesocorticolimbic dopamine system is thought to contribute to onset and manifestation of psychiatric disorders. Here, we investigated differences in reward systems functioning in 147 healthy subjects living either in cities or in less urban areas by means of functional magnetic resonance imaging during performance of the desire‐reason‐dilemma paradigm, which permits a targeted investigation of bottom–up activation and top–down regulation of the reward circuit. Compared with subjects from less urban areas, city dwellers showed an altered activation and modulation capability of the midbrain (VTA) dopamine system. City dwellers also revealed increased responses in other brain regions involved in reward processing and in the regulation of stress and emotions, such as amygdala, orbitofrontal, and pregenual anterior cingulate cortex. These results provide further evidence for effects of an urban environment on the mesolimbic dopamine system and the limbic system which may increase the risk to develop mental disorders. Hum Brain Mapp 38:3444–3453, 2017. © 2017 Wiley Periodicals, Inc.
Keywords: neuroimaging, stress, schizophrenic disorders, affective disorders, environmental factors
HIGHLIGHTS
-
City living affects human brain function in cortical and subcortical regions involved in stress and reward processing:
Altered regulation of midbrain dopamine system
Increased neural responses in the limbic system.
Urban environment may increase the risk to develop mental disorders via dysregulation of the mesolimbic dopamine system and the limbic system.
INTRODUCTION
Mental and behavioral disorders affect approximately 20–25% of all people at some time during their lives and have a large impact on individuals, families, and communities [World Health Organization et al., 2001]. Diathesis‐stress models [Ingram and Luxton, 2005] suggest that genetic factors, developmental hazards, and environmental risk factors create a vulnerability that contributes to the development and maintenance of mental and behavioral disorders. Negative effects of urbanization are seen as an environmental risk factor. This is corroborated by recent meta‐analyses which revealed a higher risk for schizophrenia [Vassos et al., 2012] and higher prevalence rates for mood and anxiety disorders [Peen et al., 2010] for people living in cities. Also the latest German mental health survey showed significantly increased prevalence rates of affective disorders (13.9% vs 7.8%) and psychotic disorders (5.2% vs 2.5%) for people living in cities with more than 500k inhabitants compared to people in rural areas with <20k inhabitants [Jacobi et al., 2014].
Urban life has benefits, for example, employment opportunities, better health care, but has also negative effects on the health of city residents. Higher air pollution may lead to the development and exacerbation of asthmatic symptoms [Cyril et al., 2013]. An analysis of four European cohorts also found relationships between air pollution and depressed mood [Zijlema et al., 2016]. Higher environmental noise levels have also been reported to lead to health problems in individuals exposed to [Niemann et al., 2014]. A possible influence of traffic noise on mental health seems to depend on individual sleep quality [Sygna et al., 2014]. Social isolation and marginalization are concentrated in cities [Godfrey and Julien, 2005]. These and several other factors constitute an environment which could foster the development of mental disorders.
In contrast to that, rural areas entail features which could promote mental health. For example, easy access to green space facilitates physical activity [Lee and Maheswaran, 2011]. This is known to be beneficial for patients [Stanton and Happell, 2014], and it may reduce the risk of developing mental disorders [Mammen and Faulkner, 2013].
The effect of stress associated with urban living seems to be a driving force on the neurobiological level. Air pollution has been associated with increases in perceived stress, which might be mediated by brain inflammation and glucocorticoid activity [Mehta et al., 2015]. Higher levels of environmental noise [Spreng, 2000; Stansfeld and Clark, 2015] and social isolation [Cacioppo et al., 2015] are other factors contributing to psychosocial stress associated with urban living [Kaplan, 1983]. Living in a big city is seen as a proxy for social stress [Lederbogen et al., 2013], although the exact neurobiological mechanisms that may lead to increased prevalence rates of mental disorders in urban environments remain largely unclear.
Psychological stress stimulates the hypothalamus–pituitary–adrenal (HPA) axis via amygdala and hypothalamus leading to cortisol release (see Ulrich‐Lai and Herman [2009] for a review). Increased cortisol levels further stimulate the amygdala, which provides positive feedback to the HPA axis. Cortisol stimulation of the hippocampus inhibits the HPA axis limiting the HPA axis activity. Additionally, the dorsal medial prefrontal cortex (DMPFC) exerts negative feedback over the HPA axis enabling emotional self‐regulation of the stress response.
Psychosocial stress acting on the HPA axis is seen as one of the factors involved in the development and manifestation of schizophrenic and affective disorders. For example, patients with schizophrenia exhibit higher cortisol levels and reductions in volume of the hippocampus [Walker et al., 2008]. Altered hippocampal activity could contribute to dysregulation of stress processing [Ulrich‐Lai and Herman, 2009] and could affect the mesolimbic dopamine system because the ventral subiculum (vSub) of the hippocampus controls the burst firing of ventral tegmental area (VTA) dopamine (DA) neurons via nucleus accumbens (NAc) and ventral pallidum pathways [Grace, 2010]. A similar mechanism could contribute to the development and manifestation of depression because altered HPA axis activity [Marques et al., 2009] and attenuated mesolimbic DA system were also observed in patients with depression [Price and Drevets, 2009].
Using functional magnetic resonance imaging (fMRI), it has already been shown that urban environment may lead to altered stress responses in the brain. Subjects living in a more urban environment exhibited higher amygdala activation in response to a cognitive‐social stress task, and subjects grown up in the city showed increased activation of the pregenual anterior cingulate cortex (pgACC) in the same task. Interestingly, such effects of city living were not observed when the participants performed a working memory or an emotional face matching task [Lederbogen et al., 2011].
Given the central role that stress‐related dysregulation of the mesocorticolimbic dopamine system may play in the development of major mental disorders, here we aimed to investigate effects of urban living on the mesolimbic dopamine system and interconnected brain areas, such as specific subregions of the prefrontal cortex (PFC). For this purpose, we used the Desire–Reason–Dilemma (DRD) paradigm [Diekhof et al., 2012b; Diekhof and Gruber, 2010] as an experimental task that reliably activates the reward circuit and interacting prefrontal areas.
This paradigm includes (i) conditioned reward stimuli that automatically elicit reward signals in the VTA and the NAc and (ii) a superordinate goal that, in some experimental situations, produces a conflict with the immediate desire for the reward stimulus, and that leads to top–down–suppression of the reward signals. Using this task, we investigated differences in reward systems functioning in 147 healthy subjects living either in cities or in less urban areas.
METHODS
Subjects
One hundred and forty‐seven Caucasian right‐handed healthy volunteers (91 females), mean age 24 (−5, +7), provided current and early life urbanicity information and were included in the study. Subjects were university students with similar socioeconomic status. During a psychiatric interview, they were screened to exclude substance dependence, medication, any history or presence of psychiatric or neurological disorders, and any history or presence of psychiatric or neurological disorders in first‐degree relatives. The study was approved by the ethics committee from Göttingen University Medical Center and written informed consent was obtained before participation.
Urbanicity Scores
The German Federal Institute for Research on Building, Urban Affairs and Spatial Development distinguishes three types of municipal corporations according to the number of inhabitants: big cities (>100k), middle town (>20k <100k), and small town or community (<20k) [Milbert, 2013]. Following this definition, the current urbanicity (CU) of each participant was scored according to their residency (1: <20k; 2: between 20k and 100k; 3: >100k). Also the early life urbanicity (ELU) scores were calculated for the first 15 years of life. Every year of residence was multiplied with the value of the related residency (see Supporting Information, Table S1). For the factorial analysis, participants with score 1 or 2 were assigned to the low CU group (15), and with score 3 to the high CU group (132).
To avoid that the results were confounded by urban effects during development, we defined a second sample with participants matched for their early life urbanicity score, sex, and age. For this matched sample, participant numbers were equal for each urbanicity group (15 with low and 15 with high CU score). Additionally, this allowed us to assess the influence of unequal sample size between groups.
Task
For the experiment, we used the previously introduced DRD paradigm [Diekhof et al., 2012b]. Experimental stimuli consisted of colored squares. Before scanning a contingency between two colors, a reward was established. Therefore, all colored squares used in the experiment were presented and participants were instructed to accept or reject them. They learned that acceptance and rejection of all colors except green and red yielded 0 points. The acceptance of red and green yielded 10 and their rejection, 0 points.
The training session before scanning included a written and a verbal instruction. The task consisted of two different block types. At the beginning of a “Z” block (“Z” for “Ziel” meaning “goal” in German), two out of six target colors were presented. Subjects had to accept all stimuli/colors that matched the target colors, and to reject all other stimuli (Fig. 1, bottom row). Successful performance of this task was the superordinate task goal to gain 50 points per block. This rule was also valid for the other block type the “B” block too (“B” for “Bonus”). But during a “B” block participants, we allowed to additionally accept conditioned stimuli (red and green squares) to achieve an additional bonus of 10 points even in situations in which these colors were not included in the target set (Fig. 1, top row). The “B” block represented the desire context (DC) and the “Z” block, the reason context (RC). Erroneous (i) acceptance of nontarget colors, (ii) rejection of target colors, and (iii) acceptance of the bonus colors in RC ended the block and all points for the current block were lost. As a part of the instruction, participants were informed that they can increase their monetary compensation (20€) paid for the participation with an additional bonus of up to 20€ by increasing the total of points they did accumulate at the end of the experiment.
Figure 1.
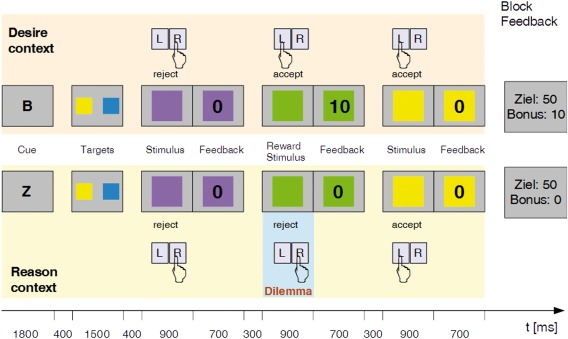
The desire‐reason dilemma paradigm. Participants performed the task with two target colors presented at the beginning of each block. Successful performance of this task was the superordinate goal to gain 50 points per block. In the Desire Context (DC: top row), participants were allowed to also accept reward colors (e.g., green) to gain additional 10 bonus points. The contingency between color and reward was established before the experiment. By contrast, in the Reason Context (RC: bottom row), these reward stimuli had to be rejected if they did not match one of the target colors. The error to accept conditioned reward stimuli during the RC block led to the loss of the 50 points. [Color figure can be viewed at http://wileyonlinelibrary.com]
After a training session, the participants performed two task sessions inside the scanner. Two blocks of one type were followed by two blocks of the other type, where one type of block had the length of 4 and the other one, the length of 8 (i.e., probe stimuli). The sequence between blocks with 8 and 4 trials varied pseudorandomly. Each trial lasted 1900 ms and the interval between the stimuli was 300ms. If the subject did not respond within 900 ms, the trial timed out and the block ended. Detailed trial timing is shown in Figure 1.
fMRI Data Acquisition
The experiment was performed on a 3 T MRI scanner (Siemens TRIO) equipped with an eight‐channel head coil. Head motion was restricted by small cushions. A high‐resolution T1‐weighted anatomical scan (3D‐MPRAGE, voxel size 1 × 1 × 1 mm³) was obtained for each subject. Functional images were acquired using a T2*‐sensitive echo planar imaging (EPI) sequence (voxel size, 3 × 3 × 3mm3; gap, 20%; interscan interval, 1.9 s; echo time, 30 ms; flip angle, 70°; field of view, 192 mm) parallel to the anterior commissure–posterior commissure plane in ascending direction. During the two sessions, a total of 370 image volumes were acquired.
Behavioral Analysis
Statistical analyses of the behavioral data were done with SPSS for Windows (version 23.0; IBM). An ANOVA was used to compare the response times of the experimental conditions: (i) acceptance of reward in DC, (ii) rejection of reward in RC, (iii) the number of correctly accepted reward stimuli in DC, (iv) the number of correctly rejected reward stimuli in RC, and (v) the number of aborted blocks (errors) between the high and low urbanicity group.
fMRI Analysis
Functional imaging data preprocessing and analysis were performed with Statistical Parametric Mapping SPM12 (Wellcome Trust Centre for Neuroimaging, London, UK). The realigned and unwarped functional images were slice time corrected, normalized to MNI space, and saved with a spatial resolution of 2 × 2 × 2 mm3. Smoothing utilized a 6‐mm full‐width at half maximum (FWHM) isotropic Gaussian kernel. The high‐resolution anatomical image was segmented and co‐registered with the mean EPI image.
Statistical analyses used a general linear model (GLM), which comprised six regressors (i.e., target‐stimuli, non‐target‐stimuli, and conditioned reward stimuli for both the DC and the RC blocks). The block cues, the target cues, and the block feedback for either successful goal completion or overall goal failure were also modeled as independent regressors, which resulted in a total of 11 onset regressors. Incorrect trials and trials in which the conditioned reward stimulus was not collected in the DC were excluded from the analyses. A vector representing the temporal onset of stimulus presentation was convolved with a canonical hemodynamic response function (hrf) to produce a predicted hemodynamic response to each experimental condition. Linear t‐contrasts were defined for assessing differential effects elicited by the experimental conditions. All statistical analyses of the single subject data included a high‐pass filter with 128 s cut‐off and an autoregressive AR(1) model to account for serial correlations in fMRI time series.
The effects of urban living on reward processing were assessed by a second level 2 × 2 full‐factorial analysis. The model included the first‐level contrast images of the subjects with high and low urbanicity score (factor Urbanicity) for the conditions when reward stimuli were accepted in DC and rejected in RC (factor Task). To account for ELU and group size effects, we performed an additional full‐factorial analysis with a sample matched for sex, age, and ELU score. Post‐hoc t‐tests were applied to determine statistical differences between the high and the low urbanicity groups.
Statistically significant effects were determined at a level of P < 0.05 family‐wise error corrected (FWE) for the whole brain. Small volume corrections were applied for regions with a priori hypotheses derived from the literature. The significance threshold for the small volume correction was P < 0.05 (svc) [Worsley et al., 1996]. For the small volume correction, we used MNI coordinates for brain regions previously associated with the processing of stress and rewards. For the amygdala, we used [−20, −2, −16] for the left and [20, −2, −20] for the right hemisphere. Both amygdala coordinates have been reported for an interaction of stress and glucocorticoid receptors and stress and amygdala activity [Geuze et al., 2012]. The coordinate for the pgACC [0, 50, 4] was reported for the interaction of stress and reward [Treadway et al., 2013] and the one for the left VTA [−4, −16, −14] derived from a meta‐analysis of reward processing [Diekhof et al., 2012a]. The search region for svc was a sphere with 4 mm radius for the VTA and with 5 mm radius for all other areas. The beta values for the experimental conditions were extracted from 2 mm boxes at the point of maximal activation using marsbar [Brett et al., 2002].
RESULTS
Behavioral Results
There were no significant differences in response time, number of accepted rewards in DC, number of rejected rewards in RC, and errors between the two groups. Mean values are shown in Supporting Information, Figure 1.
fMRI Results
The factorial analysis based on the full sample (N = 147) and on the matched sample (N = 30) revealed a brain wide significant main effect of urbanicity on BOLD response in the left medial orbital gyrus and the left lingual gyrus. There was also a small volume corrected significant effect in the right amygdala and the right pgACC for the full sample (N = 147) as well as the matched sample (N = 30). The effect of urbanicity in the left VTA was significant using small volume correction (svc), but only for the full sample. In the sample matched for sex, age, and ELU scores, this effect did not reach the level of significance (P = 0.07 svc), probably due to the small sample size.
A significant main effect of task, whole brain corrected, was found in left and right NAc and the left VTA for the whole sample. The same effect was found for the matched sample applying the svc. A full listing of the main effects can be found in Supporting Information, Tables S2 and S3.
For the full sample, subsequent Post‐hoc t‐tests showed that subjects living in the city (high CU) presented reduced activation of the left VTA (t 290 = 3.26, P < 0.05 svc) during acceptance of additional reward in the DC (Fig. 2). Overall, these findings suggest a significantly reduced modulation capability of the VTA in subjects living in the city (Fig. 3).
Figure 2.
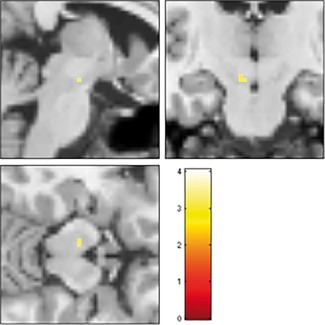
Relationship between city living and VTA activation by rewards (full sample, N = 147). Reduced bottom–up activation of left VTA by conditioned reward stimuli in low vs high urbanicity subjects [−4; −16; −14], t 290 = 3.26, P = 0.009 svc in DC; image shown at P < 0.001. [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 3.
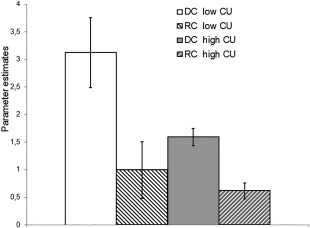
Reduced modulation capability of left VTA in participants with high current urbanicity (CU) scores (full sample, N = 147). Compared to participants living in small town/rural areas, the ones living in cities (high CU) showed both decreased bottom–up activation and reduced top–down suppression of reward‐related activity in the left VTA. Bars show the parameter estimates of the left VTA when subjects with low CU (left bars) and high CU (right bars) accepted the conditioned reward stimuli in the DC and rejected it in the RC; error bars denote standard error.
Also in the full sample, subjects with high CU scores (in comparison to subjects with low CU scores) showed increased activations in brain regions with dense connections to the human reward system, such as amygdala, orbitofrontal, and pregenual anterior cingulate cortex. Amygdala activation was higher in people living in cities when the reward was accepted in DC (left: t 290 = 2.76, P < 0.05 svc; right t 290 = 3.68, P < 0.05 svc) (Fig. 4), reflecting the significant main effect reported above. Additionally, subjects with high CU as compared to subjects with low CU revealed higher activations of the left medial orbital gyrus (DC: t 290 = 4.92, P FWE < 0.05, RC: t 290 = 3.41, P < 0.05 svc) and the right pgACC (DC: t 290 = 2.67, P < 0.05 svc, RC: t 290 = 3.92, P < 0.005 svc).
Figure 4.
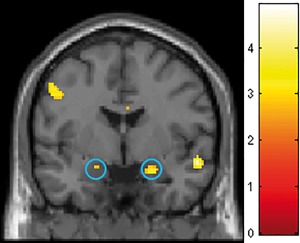
Effects of city living on amygdala activity in the desire context (DC) (full sample, N = 147). T‐map shows the result of the post‐hoc comparison between subjects with high versus low urbanicity score when they accepted the additional reward in desire context. Significantly higher amygdala activation (blue circles, left [−20; −2; −16] t 290 = 2.76, P = 0.032 svc; right [22; −2; −18], t 290 = 3.64, P = 0.002 svc) was observed for subjects with high urbanicity scores. Crosshair at 0 −2 −18; for display purposes, the image is shown at P < 0.005 uncorrected. [Color figure can be viewed at http://wileyonlinelibrary.com]
In the smaller sample that was matched for sex, age, and ELU scores, the factorial analysis confirmed most findings of the full sample analysis and, overall, indicated that the effects observed in the full sample (at a level of statistical significance) were not driven by differences in early life urbanicity. In comparison to the full sample analysis, the main effects of urbanicity and task were smaller, and less regions survived the P < 0.05 correction for the whole brain. However, the activations in regions of interest were also significant in the matched sample when corrected for small volume (see Supporting Information, Tables S2 and S3). This was also observed for the post‐hoc t‐test. The significantly reduced modulation of VTA activity (Supporting Information, Fig. S2) in subjects living in the city numerically showed the same extent also in the matched sample, although in this case it did not survive small volume correction (P = 0.07 svc), most probably due to the decreased statistical power. Significant differences in reward‐related brain activations between participants with high and low urbanicity scores for the full and the matched sample are depicted in Table 1.
Table 1.
Significant differences in reward‐related brain activations between subjects with high (H) and low (L) urbanicity scores listed for the full and the matched sample
| DC H > L | RC H > L | |||||||
|---|---|---|---|---|---|---|---|---|
| Sample | Full | Matched | Full | Matched | ||||
| Region | MNI | t‐val. | MNI | t‐val. | MNI | t‐val. | MNI | t‐val. |
| L Amygdala | −20; −2; −16 | 2.76* | ||||||
| R Amygdala | 22; −2; −18 | 3.68* | 20; −2; −18 | 3.19* | ||||
| R Pregenual cingulate gyrus | 2; 52; 4 | 2.67* | 2; 48; 6 | 3.92* | 2; 50; 6 | 3.44* | ||
| L Medial orbital gyrus | −12; 52; −18 | 4.92 | −10; 52; −18 | 4.92* | 12; 52; −18 | 3.40* | −8; 54; −18 | 4.11* |
DC, desire context; RC, reason context. Statistical effects presented at P < 0.05; FWE corrected for whole brain; * FWE corrected for small volume P < 0.05.
With regard to the NAc, we did not find urbanicity‐related effects neither in the full nor in the matched sample. There was no difference between the two groups (high vs low CU) in that region even when lowering the threshold to P < 0.05 uncorrected.
DISCUSSION
The objective of this study was to investigate whether living in big cities is associated with variation of brain function in the mesolimbic reward system and densely connected cortical and subcortical structures. The DRD paradigm has been established as an instrument to assess the prefrontal modulation of the reward system when humans have to choose between immediate and long‐term rewards [Diekhof et al., 2012b; Diekhof and Gruber, 2010]. When applied to elucidate the effects of city living, we found that subjects living in cities showed a reduced activation and modulation capability of the midbrain (VTA) dopamine system. Moreover, activation was increased in brain regions involved in stress and reward processing, such as the amygdala, medial orbitofrontal, and pregenual anterior cingulate cortex, comparing participants living in cities to those living in rural areas.
These findings are in good accordance with the extensive literature about the influence of corticosubcortical networks on the VTA. The VTA receives a multitude of afferents originating from MPFC, amygdala, hypothalamus, and other brain regions involved in the regulation of the stress response [Ulrich‐Lai and Herman, 2009; Yetnikoff et al., 2014]. Furthermore, it is known that VTA dopamine neurons respond to several forms of stress [Marinelli and McCutcheon, 2014]. Finally, recent research with rodents found that chronic mild stress attenuates VTA dopamine activity via an amygdala–ventral pallidum pathway [Chang and Grace, 2014].
In addition to the findings in the VTA, city dwellers also exhibited increased amygdala responses to conditioned reward stimuli when compared with subjects living in more rural areas. A similar effect was previously reported by Lederbogen et al. [2011] using a task which induces social stress during arithmetical calculations, the Montreal Stress Imaging Task (MIST [Dedovic et al., 2005]). These findings are supported by human and animal research. For example, experiments with rodents have demonstrated that chronic stress alters amygdala's neuronal properties [Liu et al., 2014; Rosenkranz et al., 2010] and its morphology [Joëls et al., 2007]. Furthermore, a neuroimaging study in humans reported an association between peripheral glucocorticoid receptor number and amygdala activity [Geuze et al., 2012] that may be mechanistically involved in increased vulnerability to stress‐related disorders.
Furthermore, we also observed that activity in two regions of the ventral–medial prefrontal cortex (vmPFC), that is, the medial orbitofrontal and the pregenual anterior cingulate cortex (pgACC), was associated with differences in urbanicity scores. According to a recent review [Roy et al., 2012], the vmPFC is involved in the integration of memory, social cognition, emotion, reward, and other functions. One of the two vmPFC subregions showing a very strong difference in activation between city dwellers and small town/rural residents was the medial–orbital prefrontal cortex (mOFC) which also plays a role in reward evaluation [Diekhof et al., 2012a]. The other subregion of the vmPFC, the pgACC, has previously been shown to contribute to the regulation of emotional conflict [Etkin et al., 2011] as well as to the inhibition of HPA responses to psychogenic stressors [Ulrich‐Lai and Herman, 2009].
Living in cities has been shown to be a risk factor contributing to the development of psychiatric diseases [Peen et al., 2010; Vassos et al., 2012]. With the current research, we show an association of city living with differences in activation and modulation of the mesolimbic dopamine system. Several reviews have discussed how alterations in the dopamine system may contribute to development of mental disorders [Heinz et al., 1994; Howes and Kapur, 2009]. In particular, it has been proposed that stress may influence afferent structures of the dopamine system, such as the hippocampus and the amygdala, and that this may lead to dysregulation of the dopamine system [Belujon and Grace, 2015].
Furthermore, empirical evidence for dysregulations of the dopamine system in mental disorders has been addressed by two recent reviews that describe the central role of dopamine in the pathogenesis and pathophysiology of schizophrenia [Howes and Murray, 2014] and depression [Pizzagalli, 2014]. The first review describes an integrated social developmental model of schizophrenia proposing that genetic liability, developmental factors, and subsequent stressors on the dopamine system determine the trajectory towards psychosis [Howes and Murray, 2014]. In support of this model, the authors summarize studies showing how gene variation, neurodevelopmental hazards such as obstetric complications, childhood adversity, and social stress influence the stress response, disrupt the development of and sensitize the dopamine system. In the second step, social adversity and subsequent stress may lead to dysregulations of the dopamine system and to the manifestation of schizophrenic disorders. The second review addressed the role of anhedonia, dopamine and stress in depression. On the basis of empirical evidence, the authors argue that acute and chronic stress may lead to dysfunctions within the mesolimbic dopaminergic pathways that may be responsible for disrupted reinforcement learning and lack of reactivity to pleasurable stimuli seen in depression [Pizzagalli, 2014].
As every study, the present one also has several limitations. First of all, it is important to note that our findings merely indicate an association of city living with differences in activation and modulation of activity in brain regions of the mesolimbic reward system and interconnected regions, such as amygdala, orbitofrontal, and pregenual anterior cingulate cortex. From this, we cannot draw any conclusions about the factors and mechanisms that mediate this association, for example social stress. There may be other relevant (environmental) factors possibly mediating the observed association that need to be discussed briefly. For example, it has been reported that when urban residents moved to an area with more green space their mental health improved. However, no decline in the mental health of urban residents was observed when they moved to areas with less green space [Alcock et al., 2014]. Also, actual data do not support the assumption that the availability of green space outside the city fosters physical activity. No significant differences in physical activity were found between rural and urban residents by a Swiss survey [Lamprecht et al., 2014].
There is also growing evidence that environmental noise increases the risk for affective disorders although it seems not to be dependent on city living itself. Rather it appears to depend on the noise level and individual factors such as the socioeconomic status [Beutel et al., 2016; Orban et al., 2015]. Further evidence for the influence of socioeconomic status on the prevalence rates for mental disorders has been documented by the latest German mental health survey [Jacobi et al., 2014]. Other surveys reported such an influence on the physical activity [Bös and Federal Republic of Germany, 2009; Lamprecht et al., 2014].
Thus, socioeconomic status may also be a factor that may have mediated the effect of urbanicity on brain function observed in our study. Unfortunately, in our sample consisting of only university students, we were unable to strictly control for socioeconomic status because the occupational status of students is not listed in validated epidemiologic scales [Lampert et al., 2013], and because students have been explicitly excluded during the validation process for such scales [Cirino et al., 2002]. The reason for this may be the fact that students have the same educational level and that especially in Germany their mean monthly income varies only <5% when comparing the groups with low background family SES and with high background family SES [Middendorff et al., 2013]. This might result from monthly grants provided due to the German Federal Training Assistance Act. Given these facts, it seems reasonable to assume a very similar socioeconomic status (SES) for the subjects in our sample regarding education, occupation, and income. However, in particular, we cannot exclude that other individual status parameters such as subjective social rank, living with spouse, or living in shared flat may have influenced our results.
Another potential confound in our study may be influences during early life. For example, hazards during early development could contribute to neurofunctional changes [van Os et al., 2010; Schmitt et al., 2014]. All participants of our study were screened for any history of mental disorders, and the smaller sample was matched for their early life urbanicity scores ensuring a similar distribution of city living during the first 15 years of life. The results from the matched sample confirmed most of the findings from the full sample clearly suggesting that the observed effects of current urbanicity were not confounded by early life urbanicity.
The fact that our participants were students limits the generalizability of our findings to other populations. Further, the group sizes were unbalanced in the full sample because most participants lived in the city. To account for that, the analysis in the smaller sample matched for early life urbanicity was restricted to groups of equal size. The results of this analysis suggest stable effects of urban life on the function of the mesolimbic dopamine system also with balanced group sizes. Future studies of the effects of urbanicity on the mesolimbic reward system in humans should include a larger range of the population spectrum, a stricter matching for age and socioeconomic status, a larger and equal sample size for the rural and urban groups and participants from bigger cities >500k.
To sum up, using an established paradigm to investigate the reward circuit, we found urbanicity‐related differences in brain function not only within the reward circuit itself, but also in closely connected brain circuits involved in emotion and stress regulation. Neurofunctional changes in these networks play a major role in the pathogenesis and pathophysiology of mental disorders. There is substantial evidence that effects of urban living are associated with psychosocial stress. However, because of the lack of longitudinal studies examining the effects of chronic urban stress on the stress response, a direct link between increased prevalence rates of mental disorders and the urban environment is still missing. The results of this study provide further indirect evidence for effects of an urban environment on the functioning of the mesolimbic dopamine system and the limbic system which may increase the risk to develop mental disorders.
AUTHOR CONTRIBUTIONS
O.G. and B.K. designed the experiment; E.D. and O.G. designed the DRD‐paradigm; E.D. and M.K. conducted the experiment; and B.K. and O.G. analyzed the data and wrote the manuscript.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
We would like to thank Mohammad Al‐Bayati for collecting information on urbanicity scores, Maria Keil for assistance in MR data acquisition, and Claudia Wolf for her helpful remarks.
REFERENCES
- Alcock I, White MP, Wheeler BW, Fleming LE, Depledge MH (2014): Longitudinal effects on mental health of moving to greener and less green urban areas. Environ Sci Technol 48:1247–1255. [DOI] [PubMed] [Google Scholar]
- Belujon P, Grace AA (2015): Regulation of dopamine system responsivity and its adaptive and pathological response to stress. Proc R Soc Lond B Biol Sci 282:20142516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutel ME, Jünger C, Klein EM, Wild P, Lackner K, Blettner M, Binder H, Michal M, Wiltink J, Brähler E, Münzel T (2016): Noise annoyance is associated with depression and anxiety in the general population ‐ The contribution of aircraft noise. PLoS One 11:e0155357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bös K, Federal Republic of Germany eds. (2009): Motorik‐Modul: eine Studie zur motorischen Leistungsfähigkeit und körperlich‐sportlichen Aktivität von Kindern und Jugendlichen in Deutschland; Abschlussbericht zum Forschungsprojekt 1. Aufl., Stand: Januar 2009. Baden‐Baden: Nomos‐Verl. Forschungsreihe/Bundesministerium für Familie, Senioren, Frauen und Jugend 5.
- Brett M, Anton J‐L, Valabregue R, Poline J‐B (2002): Region of interest analysis using the MarsBar toolbox for SPM 99. Neuroimage 16:S497. [Google Scholar]
- Cacioppo JT, Cacioppo S, Capitanio JP, Cole SW (2015): The Neuroendocrinology of Social Isolation. Annu Rev Psychol 66:733–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Grace AA (2014): Amygdala‐ventral pallidum pathway decreases dopamine activity after chronic mild stress in rats. Biol Psychiatry 76:223–230. Neurostimulation Treatments for Depression: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirino PT, Chin CE, Sevcik RA, Wolf M, Lovett M, Morris RD (2002): Measuring socioeconomic status: reliability and preliminary validity for different approaches. Assessment 9:145–155. [DOI] [PubMed] [Google Scholar]
- Cyril S, Oldroyd JC, Renzaho A (2013): Urbanisation, urbanicity, and health: a systematic review of the reliability and validity of urbanicity scales. BMC Pub Health 13:513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedovic K, Renwick R, Mahani NK, Engert V, Lupien SJ, Pruessner JC (2005): The Montreal Imaging Stress Task: using functional imaging to investigate the effects of perceiving and processing psychosocial stress in the human brain. J Psychiatry Neurosci 30:319–325. [PMC free article] [PubMed] [Google Scholar]
- Diekhof EK, Gruber O (2010): When desire collides with reason: Functional interactions between anteroventral prefrontal cortex and nucleus accumbens underlie the human ability to resist impulsive desires. J Neurosci 30:1488–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekhof EK, Kaps L, Falkai P, Gruber O (2012a): The role of the human ventral striatum and the medial orbitofrontal cortex in the representation of reward magnitude – An activation likelihood estimation meta‐analysis of neuroimaging studies of passive reward expectancy and outcome processing. Neuropsychologia 50:1252–1266. [DOI] [PubMed] [Google Scholar]
- Diekhof EK, Nerenberg L, Falkai P, Dechent P, Baudewig J, Gruber O (2012b): Impulsive personality and the ability to resist immediate reward: An fMRI study examining interindividual differences in the neural mechanisms underlying self‐control. Hum Brain Mapp 33:2768–2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R (2011): Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cognit Sci 15:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuze E, van Wingen GA, van Zuiden M, Rademaker AR, Vermetten E, Kavelaars A, Fernández G, Heijnen CJ (2012): Glucocorticoid receptor number predicts increase in amygdala activity after severe stress. Psychoneuroendocrinology 37:1837–1844. [DOI] [PubMed] [Google Scholar]
- Godfrey R, Julien M (2005): Urbanisation and health. Clin Med 5:137–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA (2010): Dopamine system dysregulation by the ventral subiculum as the common pathophysiological basis for schizophrenia psychosis, psychostimulant abuse, and stress. Neurotox Res 18:367–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz A, Schmidt LG, Reischies FM (1994): Anhedonia in schizophrenic, depressed, or alcohol‐dependent patients ‐ Neurobiological correlates. Pharmacopsychiatry 27:7–10. [DOI] [PubMed] [Google Scholar]
- Howes OD, Kapur S (2009): The dopamine hypothesis of schizophrenia: Version III—The final common pathway. Schizophr Bull 35:549–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, Murray RM (2014): Schizophrenia: an integrated sociodevelopmental‐cognitive model. Lancet 383:1677–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram RE, Luxton DD (2005): Vulnerability‐stress models In: Development of Psychopathology: A Vulnerability‐Stress Perspective, Chapter 2. Thousand Oaks, CA: SAGE Publications, Inc; pp 32–47. [Google Scholar]
- Jacobi F, Höfler M, Siegert J, Mack S, Gerschler A, Scholl L, Busch MA, Hapke U, Maske U, Seiffert I, Gaebel W, Maier W, Wagner M, Zielasek J, Wittchen H‐U (2014): Twelve‐month prevalence, comorbidity and correlates of mental disorders in Germany: the Mental Health Module of the German Health Interview and Examination Survey for Adults (DEGS1‐MH). Int J Methods Psychiatr Res 23:304–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joëls M, Karst H, Krugers HJ, Lucassen PJ (2007): Chronic stress: Implications for neuronal morphology, function and neurogenesis. Front Neuroendocrinol 28:72–96. [DOI] [PubMed] [Google Scholar]
- Kaplan HB, editor (1983): Psychosocial Stress: Trends in Theory and Research. New York: Academic Press. [Google Scholar]
- Lampert DT, Kroll L, Müters S, Stolzenberg H (2013): Messung des sozioökonomischen Status in der Studie zur Gesundheit Erwachsener in Deutschland (DEGS1). Bundesgesundheitsbl 56:631–636. [DOI] [PubMed] [Google Scholar]
- Lamprecht M, Fischer A, Stamm H (2014): Sport Schweiz 2014 Sportaktivität und Sportinteresse der Schweizer Bevölkerung. Bundesamt für Sport BASPO. http://www.baspo.admin.ch/internet/baspo/de/home/dokumentation.parsys.000106.downloadList.50622.DownloadFile.tmp/sportschweiz2014.pdf.
- Lederbogen F, Haddad L, Meyer‐Lindenberg A (2013): Urban social stress – Risk factor for mental disorders. The case of schizophrenia. Environmental Pollution 183. Selected Papers from Urban Environmental Pollution 2012:2–6. [DOI] [PubMed]
- Lederbogen F, Kirsch P, Haddad L, Streit F, Tost H, Schuch P, Wüst S, Pruessner JC, Rietschel M, Deuschle M, Meyer‐Lindenberg A (2011): City living and urban upbringing affect neural social stress processing in humans. Nature 474:498–501. [DOI] [PubMed] [Google Scholar]
- Lee ACK, Maheswaran R (2011): The health benefits of urban green spaces: a review of the evidence. J Public Health 33:212–222. [DOI] [PubMed] [Google Scholar]
- Liu Z‐P, Song C, Wang M, He Y, Xu X‐B, Pan H‐Q, Chen W‐B, Peng W‐J, Pan B‐X (2014): Chronic stress impairs GABAergic control of amygdala through suppressing the tonic GABAA receptor currents. Mol Brain 7:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammen G, Faulkner G (2013): Physical activity and the prevention of depression: A systematic review of prospective studies. Am J Prevent Med 45:649–657. [DOI] [PubMed] [Google Scholar]
- Marinelli M, McCutcheon JE (2014): Heterogeneity of dopamine neuron activity across traits and states. Neuroscience 282:The Ventral Tegmentum and Dopamine: A New Wave of Diversity:176–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques AH, Silverman MN, Sternberg EM (2009): Glucocorticoid dysregulations and their clinical correlates. Ann N Y Acad Sci 1179:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta AJ, Kubzansky LD, Coull BA, Kloog I, Koutrakis P, Sparrow D, Spiro A, Vokonas P, Schwartz J (2015): Associations between air pollution and perceived stress: the Veterans Administration Normative Aging Study. Environ Health 14:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middendorff E, Apolinarski B, Poskowsky J, Kandulla M, Netz N (2013): Die wirtschaftliche und soziale Lage der Studierenden in Deutschland 2012. Sozialerhebung des Deutschen Studentenwerks, durchgeführt durch das HIS‐Institut für Hochschulforschung Berlin. http://www.thm.de/planung/images/stories/DSW_20_Sozialerhebung_2012_A5.pdf.
- Milbert A (2013): BBSR Homepage ‐ Stadt‐ und Gemeindetyp. Stadt‐ und Gemeindetypen in Deutschland. http://www.bbsr.bund.de/BBSR/DE/Raumbeobachtung/Raumabgrenzungen/StadtGemeindetyp/StadtGemeindetyp_node.html.
- Niemann H, Hoebel J, Hammersen F, Lau\s smann D (2014): Lärmbelästigung–Ergebnisse der GEDA‐Studie 2012. http://edoc.rki.de/docviews/abstract.php?id=3723.
- Orban E, McDonald K, Sutcliffe R, Hoffmann B, Fuks KB, Dragano N, Viehmann A, Erbel R, Jöckel K‐H, Pundt N, Moebus S (2015): Residential road traffic noise and high depressive symptoms after five years of follow‐up: Results from the Heinz Nixdorf recall study. Environ Health Persp 124:http://ehp.niehs.nih.gov/14-09400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Os J, Kenis G, Rutten BPF (2010): The environment and schizophrenia. Nature 468:203–212. [DOI] [PubMed] [Google Scholar]
- Peen J, Schoevers RA, Beekman AT, Dekker J (2010): The current status of urban‐rural differences in psychiatric disorders. Acta Psychiatr Scand 121:84–93. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA (2014): Depression, stress, and anhedonia: Toward a synthesis and integrated model. Annu Rev Clin Psychol 10:393–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Drevets WC (2009): Neurocircuitry of mood disorders. Neuropsychopharmacology 35:192–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz JA, Venheim ER, Padival M (2010): Chronic stress causes amygdala hyperexcitability in rodents. Biol Psychiatry 67:1128–1136. Amygdala Activity and Anxiety: Stress Effects: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy M, Shohamy D, Wager TD (2012): Ventromedial prefrontal‐subcortical systems and the generation of affective meaning. Trends Cognit Sci 16:147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt A, Malchow B, Hasan A, Fallkai P (2014): The impact of environmental factors in severe psychiatric disorders. Front Neurosci 8:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng M (2000): Central nervous system activation by noise. Noise Health 2:49. [PubMed] [Google Scholar]
- Stansfeld S, Clark C (2015): Health effects of noise exposure in children. Curr Envir Health 2:171–178. Rpt [DOI] [PubMed] [Google Scholar]
- Stanton R, Happell B (2014): A systematic review of the aerobic exercise program variables for people with Schizophrenia. Curr Sports Med Rep 13:260–266. [DOI] [PubMed] [Google Scholar]
- Sygna K, Aasvang GM, Aamodt G, Oftedal B, Krog NH (2014): Road traffic noise, sleep and mental health. Environ Res 131:17–24. [DOI] [PubMed] [Google Scholar]
- Treadway MT, Buckholtz JW, Zald D (2013): Perceived stress predicts altered reward and loss feedback processing in medial prefrontal cortex. Front Hum Neurosci 7:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich‐Lai YM, Herman JP (2009): Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci 10:397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassos E, Pedersen CB, Murray RM, Collier DA, Lewis CM (2012): Meta‐analysis of the association of urbanicity with schizophrenia. Schizophr Bull 38:1118–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker E, Mittal V, Tessner K (2008): Stress and the hypothalamic pituitary adrenal axis in the developmental course of schizophrenia. Annu Rev Clin Psychol 4:189–216. [DOI] [PubMed] [Google Scholar]
- World Health Organization, Organisation Mondiale de la Santé, World Health Organization (2001): The World Health Report 2001, Mental Health: New Understanding, New Hope. Genève: World Health Organization.
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC (1996): A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp 4:58–73. [DOI] [PubMed] [Google Scholar]
- Yetnikoff L, Lavezzi HN, Reichard RA, Zahm DS (2014): An update on the connections of the ventral mesencephalic dopaminergic complex. Neuroscience 282:23–48. The Ventral Tegmentum and Dopamine: A New Wave of Diversity: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlema WL, Wolf K, Emeny R, Ladwig KH, Peters A, Kongsgård H, Hveem K, Kvaløy K, Yli‐Tuomi T, Partonen T, Lanki T, Eeftens M, de Hoogh K, Brunekreef B, Stolk RP, Rosmalen JGM (2016): The association of air pollution and depressed mood in 70,928 individuals from four European cohorts. Int J Hygiene Environ Health 219:212–219. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


