Abstract
Non‐invasive transcranial direct current stimulation (tDCS) can enhance recovery after stroke. However, fundamental knowledge about how tDCS impacts neural processing in the lesioned human brain is currently lacking. In the present study, it was investigated how tDCS modulates brain function in patients with post‐stroke language impairment (aphasia). In a cross‐over, randomized trial, patients named pictures of common objects during functional magnetic resonance imaging (fMRI). Concurrently, excitatory (anodal‐) or sham‐tDCS (1 mA, 20 min, or 30 s, respectively) was administered to the left primary motor cortex, a montage with demonstrated potential to improve aphasic language. By choosing stimuli that could reliable be named by the patients, the authors aimed to derive a pure measure of stimulation effects that was independent of treatment or performance effects and to assess how tDCS interacts with the patients' residual language network. Univariate fMRI data analysis revealed reduced activity in domain‐general regions mediating high‐level cognitive control during anodal‐tDCS. Independent component functional network analysis demonstrated selectively increased language network activity and an inter‐correlated shift from higher to lower frequency bands, indicative of increased within‐network communication. Compared with healthy controls, anodal‐tDCS resulted in overall “normalization” of brain function in the patients. These results demonstrate for the first time how tDCS modulates neural processing in stroke patients. Such information is crucial to assure that behavioral treatments targeting specific neural circuits overlap with regions that are modulated by tDCS, thereby maximizing stimulation effects during therapy. Hum Brain Mapp 38:1518–1531, 2017. © 2016 Wiley Periodicals, Inc.
Keywords: aphasia, stroke, language, brain stimulation, functional magnetic resonance imaging
INTRODUCTION
Transcranial direct current stimulation (tDCS) of the brain involves a weak electrical current that is administered via scalp attached electrodes. TDCS has successfully been used to modulate neural excitability and consequently behavioral performance in health and disease [Flöel, 2014; Miniussi et al., 2013; Perceval et al., 2016]. Acute behavioral effects of tDCS, as observed during or immediately after the end of the stimulation, are thought to be mediated by polarity specific modulation of the neural resting‐membrane potential, resulting in enhanced or reduced excitability [Stagg and Nitsche, 2011]. Moreover, previous studies have demonstrated that behavioral improvements due to multi‐session tDCS may be maintained for longer time periods after the end of the stimulation, with follow‐up periods ranging from 1 week to 12 months [Cohen Kadosh et al., 2010; Dockery et al., 2009; Meinzer et al., 2014a; Reis et al., 2009]. These long‐term effects of tDCS and its excellent safety profile [Bikson et al., 2016] have encouraged the use of tDCS to enhance the recovery potential in stroke patients suffering from language impairment (aphasia) by facilitating neural circuits engaged during treatment [Holland and Crinion, 2012].
Recent reviews and meta‐analyses have emphasized the potential of tDCS to enhance treatment outcome in aphasia, but also highlighted variable stimulation effects within and between studies [De Aguiar et al., 2015; Elsner et al., 2015; Holland and Crinion, 2012; Monti et al., 2013]. This is likely explained by the limited understanding of how tDCS modulates brain functions in stroke patients and whether stimulation effects of specific montages overlapped with the neural networks engaged during treatment. Indeed, while a number of studies have demonstrated that tDCS can enhance treatment‐induced motor recovery and neural plasticity in stroke patients [Allman et al., 2016; Lefebvre et al., 2015; Lindenberg et al., 2010; Stagg et al., 2012], only one study investigated the underlying mechanisms of superior treatment effects due to tDCS in aphasia [Marangolo et al., 2016]. In this study, the combination of language therapy and bi‐frontal tDCS resulted in enhanced connectivity within the lesioned left hemisphere compared with treatment and sham‐tDCS.
However, these studies only addressed the combined effects of treatment and tDCS, which does not allow to obtain a direct measure of how the stimulation itself impacts on brain function (i.e., the state patients are in during treatment). Thus, the presumed mechanisms by which tDCS provided “better working conditions” during treatment remained elusive. Such knowledge is imperative to assure that behavioral treatments targeting specific neural circuits in the lesioned brain are modulated by a given tDCS montage, thereby maximizing stimulation effects during therapy. Direct information about how tDCS impacts on brain function can be derived from studies that combine functional magnetic resonance imaging (fMRI) with simultaneous intrascanner tDCS [Meinzer et al., 2014b]. Previous studies that used this technique in healthy individuals and patient populations have demonstrated that tDCS not only modulates local task‐related brain activity at the stimulation site but also functionally connected distant brain regions [Antal et al., 2011; Holland et al., 2011; Lindenberg et al., 2013, 2016; Meinzer et al., 2012a, 2013b; Polania et al., 2011; Ulm et al., 2015].
In the present study, we used this technique to investigate how tDCS interacts with the residual language network in a group of patients with post‐stroke aphasia, thereby exploring the neural effects of acute tDCS effects as they would be present during concurrent therapy. We employed a cross‐over, sham‐tDCS controlled design and the patients named pictures of common objects during fMRI. This task was chosen as naming impairment is among the most common symptoms of aphasia [Lazar and Antoniello, 2008] which has frequently been targeted in previous tDCS trials in aphasia (for review see De Aguiar et al. [2015]). To assure that potential changes in neural processing due to tDCS were not confounded by performance [Fridriksson and Morrow, 2005; Price et al., 2006] or treatment effects [Lefebvre et al., 2015; Lindenberg et al., 2010; Marangolo et al., 2016; Stagg et al., 2012], we only included object pictures that could be named correctly by individual patients during repeated baseline assessments. Intrascanner tDCS targeted the left primary motor cortex (M1), a montage that improved short‐ and long‐term naming treatment outcome and everyday communication compared with treatment with sham‐tDCS in a previous clinical trial of our group [Meinzer et al., 2016].
The goals of this study were twofold: First, the within‐group comparison of patients scanned during active‐ versus sham‐tDCS explored for the first time in post‐stroke aphasia potential stimulation effects on functional brain activity (standard univariate approach; [Meinzer et al., 2013a]) and also large‐scale functional network modulations (using independent component analysis, ICA; [Carter et al., 2012]). Second, to further qualify the functional relevance of potential tDCS effects, we also compared the patients' data acquired during both stimulation conditions with those of matched healthy controls group that were scanned without stimulation. This analysis assessed whether tDCS‐induced changes in brain function would result in more “normal” patterns of brain activity and connectivity in language‐related regions in the patients, as previously shown in healthy older individuals and patients with Mild Cognitive Impairment [Meinzer et al., 2013b, 2015a].
MATERIALS AND METHODS
Sample Characteristics
Clinical and demographic information of the patients (6 women, 10 men; mean ± SD 56.7 ± 10.1 years of age) are detailed in Table 1. All patients were in the chronic stage, defined as at least 12 months post‐stroke (mean ± SD 54.3 ± 45.3 months post stroke) and had varying lesion sites and sizes (Fig. 1). None of the patients had previously participated in a tDCS study. Only patients without lesions affecting the hand representation of the left motor cortex and underlying white matter were included (i.e., the stimulation site). Patients with contraindications for tDCS and fMRI (e.g., cardiac pacemaker, history of seizures), a history of alcohol or drug abuse, other severe neurological, psychiatric or medical conditions or taking medication that may interfere with tDCS effects (e.g., antidepressants, anxiolytics) were excluded. After an initial screening for exclusion criteria, eligible patients were tested with the standardized German aphasia test battery (Aachen Aphasia Test [AAT]) to determine the degree and severity of language impairment. Because the main goal of the present study was to investigate how tDCS interacts with the reorganized naming network after stroke (i.e., in the presence of a structural lesion), we only recruited patients with mild aphasia to assure that the patients could correctly name enough stimuli to be included during the cross‐over phase (for details please see below). While all the patients in the present study had beneficial recovery in the chronic stage (i.e., when they were tested), clinical case reports obtained through treating neurologists and speech therapists indicated that all patients had moderate‐severe aphasia in the sub‐acute stage after stroke. Please note, only residual naming impairment was observed in the AAT at the time of testing. However, all patients reported subjective naming impairment during more challenging everyday situations. Moreover, naming latencies during baseline testing were above age‐corrected norms [Kargel et al., 2015], which confirmed those subjective reports. Sixteen age‐matched healthy individuals served as control group and were scanned with the same fMRI design (8 women, 8 men, 58.9 ± 15.9 years of age), but without stimulation.
Table 1.
Details of demographic and clinical characteristics of patients
| Demographic and clinical information | Aachen Aphasia Test | Baseline naming* | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
tDCSorder |
Age (years) |
Sex |
TSS (months) |
Education (years) |
Handedness |
Lesion size (ccm) |
Lesion location (left hemisphere) |
TT (50) |
Rep (150) |
WL (90) |
Na (120) |
Co (120) |
% correct |
| S‐A | 65 | F | 169 | 10 | +1.0 | 39.4 | Ins, CN/PT, Thal, STG, ROC | 0 | 146 | 87 | 100 | 101 | 80.6 |
| A‐S | 53 | M | 37 | 12 | +1.0 | 149.2 | IFG, Ins, I/M/STG, IPL, IC | 5 | 98 | 80 | 85 | 99 | 88.8 |
| S‐A | 59 | M | 48 | 16 | +1.0 | 101.1 | IFG, Ins, PT, Thal, STG, IPG, MOG, IC | 5 | 148 | 79 | 100 | 87 | 86.3 |
| A‐S | 55 | M | 46 | 18 | +1.0 | 155.7 | IFG, Ins, PT, M/STG, ROC, MOG | 5 | 109 | 80 | 79 | 99 | 83.8 |
| A‐S | 59 | F | 84 | 12 | +1.0 | 94.9 | Ins, Put, Pre/PostCG, ROC, M/SOG | 1 | 143 | 81 | 100 | 118 | 95.3 |
| S‐A | 65 | M | 87 | 12 | +1.0 | 9.7 | Ins, PT | 3 | 142 | 82 | 102 | 110 | 88.1 |
| S‐A | 50 | M | 60 | 11 | +1.0 | 74.5 | IFG, Ins, PreCG, STG, ROC, IC | 5 | 146 | 81 | 112 | 113 | 80 |
| A‐S | 55 | F | 61 | 8 | +1.0 | 81.6 | IFG, Ins, PT, Pre/PostCG, IC | 1 | 148 | 80 | 99 | 94 | 87.3 |
| S‐A | 66 | M | 13 | 12 | +1.0 | 10.7 | M/STG | 2 | 133 | 84 | 99 | 110 | 90.6 |
| S‐A | 43 | F | 14 | 14 | +1.0 | 10.5 | STG, SMG, PostCG, ROC | 0 | 150 | 89 | 118 | 117 | 98.8 |
| A‐S | 51 | F | 13 | 11 | +1.0 | 37.5 | I/MFG, PreCG, ROC, STG | 0 | 148 | 88 | 108 | 102 | 96.3 |
| A‐S | 38 | F | 38 | 15 | +0.9 | 165.1 | IFG, Ins, CN/PT, PreCG, M/STG, IPG | 2 | 145 | 87 | 105 | 113 | 89.4 |
| S‐A | 70 | M | 12 | 14 | +1.0 | 35.0 | IFG, Ins, PreCG, IPG | 3 | 132 | 81 | 104 | 111 | 95 |
| S‐A | 65 | M | 134 | 12 | +0.9 | 85.5 | IFG, INS, CN/PT, STG | 4 | 148 | 87 | 105 | 100 | 85.8 |
| A‐S | 42 | M | 17 | 15 | +1.0 | 14.1 | IFG, INS, PT | 1 | 148 | 89 | 108 | 116 | 93.9 |
| A‐S | 71 | M | 35 | 13 | +1.0 | 94.6 | IFG, Ins, STG, Pre/PostCG, IC | 1 | 133 | 80 | 104 | 103 | 93.8 |
S, sham‐tDCS; A, anodal‐tDCS; M, male; F, female; Handedness, range 1 to −1 (positive score indicates strong right‐handedness); TSS, time since stroke; Lesion location: INS, Insula; CN, Caudate Nucleus; PT, Putamen; Thal, Thalamus; ROC, Rolandic Operculum; I/MFG, Inferior/Middle Frontal Gyrus; I/M/STG, Inferior/Middle/Superior Temporal Gyrus; I/M/SOG, Inferior/Middle/Superior Occipital Gyrus; Pre/PostCG, Pre/Postcentral Gyrus; IPG, Inferior Parietal Gyrus; SMG, Supramarginal Gyrus; IC, Internal Capsule; Aachen Aphasia Test subtests; in brackets (maximum score): TT, Token Test (error score); Rep, repetition; WL, written language; Na, naming; Co, comprehension; * score represents the mean number of correct responses during the two baseline assessments (N = 344 items).
Figure 1.
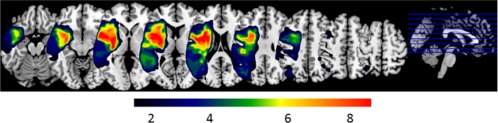
Lesion overlay plot: Colors indicate the number of patients with lesions in a given area. [Color figure can be viewed at http://wileyonlinelibrary.com]
The study was approved by the local ethics committee of The Charité University Hospital (Berlin, Germany), conducted in accordance with the Helsinki declaration and registered with clinicalTrials.gov (NCT01845129). Prior to study inclusion the patients provided written informed consent and were paid €150 for their participation.
Baseline Naming Assessments and Stimulus Selection
Given that it has been suggested that the impact of different interventions (e.g., tDCS) on brain function in aphasia “should be assessed using tasks that the patients can perform” [Price et al., 2006], the naming task only included pictures that could be named correctly during repeated behavioral baseline assessments. Moreover, as performance levels have been shown to impact on functional imaging parameters during language tasks in healthy individuals and patients with aphasia [Antonenko et al., 2012; Fridriksson and Morrow, 2005; Meinzer et al., 2012b], this strategy also aimed to minimize potential confounds induced by variable and erroneous naming performance in patients with aphasia and to assure stable imaging results in both stimulation sessions. All patients completed two behavioral baseline naming assessments. During each session, 344 line drawings of common object pictures from the Snodgrass [Snodgrass and Vanderwart, 1980] and International Picture Naming Project [Szekely et al., 2004] corpora were presented in random order using a laptop computer. Details of this standardised set have been described in previous studies of our group [e.g., Flöel et al., 2011; Meinzer et al., 2010, 2016; Menke et al., 2009]. Pictures were presented in eight sets of pictures (N = 43/set) with short breaks in between sets. Each picture was preceded by a brief auditory stimulus and presented for 3 s followed by a blank screen (max. 27 s). The patients were required to respond by naming each picture aloud as quickly and accurately as possible. After the first naming attempt, the investigator proceeded to the next item by pressing the mouse button. Verbal responses were digitally recorded and subsequently transcribed. Response latencies for correctly named pictures were determined using Adobe Audition© software by calculating the difference between picture onset (identified by the auditory stimulus) and the onset of naming responses were calculated. Pictures that could be named correctly during both baseline assessments were identified on an individual basis and the 160 responses with the fastest mean latency were selected. Those items were divided into two sets (80/set) that were matched for baseline response latency (mean ± SD ms Set1 1432.07 ± 711.79 vs. Set2 1420.84 ± 717.01; P = 1.00) and linguistic variables (word length 6.67 ± 0.38 vs. 6.71 ± 0.29; frequency 145.15 ± 23.82 vs. 144.5 ± 26.18; both P > 0.77). In addition, sets were matched for semantic categories (# manipulable objects: 19.00 ± 3.12 vs. 19.53 ± 2.31; # manmade objects: 24.39 ± 3.85 vs. 24.46 ± 2.71; both P > 0.44). The sets were randomly assigned to the two fMRI sessions. Controls were scanned with the same picture sets used in the patient group (i.e., one set from each patient was chosen and randomly assigned to participants of the control group).
Transcranial Direct Current Stimulation
We used an established intrascanner tDCS set‐up that has successfully been used by our group to assess neurofunctional changes due to tDCS in healthy individuals and patient populations [Lindenberg et al., 2013, 2016; Meinzer et al., 2012a, 2013b, 2014c]. Details of this method have previously been reported [Meinzer et al., 2014b]. In short, tDCS was administered using an MRI‐compatible direct current stimulator (DC‐Stimulator Plus‐MR®, NeuroConn, Illmenau). The anode (5 × 7 cm2) was attached over the left representation of the hand M1 using two elastic rubber bands as in previous studies of our group (C3 of the 10–20 EEG system, [Lindenberg et al., 2013, 2016; Meinzer et al., 2014c, 2016 2016]). The return electrode (10 × 10 cm2) was positioned over the right supraorbital region. The larger size of this electrode renders the stimulation functionally inert at this site (i.e., there is no direct cathodal effect underneath the return electrode) without compromising the effects underneath the anode [Nitsche et al., 2007]. A constant direct current of 1 mA was administered and ramped‐up over 10 s prior to the start of the picture naming task and remained stable for 20 minutes (anodal‐tDCS) or was turned off after 30 s (sham‐tDCS). After each session the patients completed a brief adverse effects questionnaire (adapted from Brunoni et al. [2011]); after each session patients were also asked to guess whether they thought active‐ or sham‐tDCS was administered.
Functional Imaging Data Acquisition
Functional and structural images of the patients were acquired using a 3‐Tesla Siemens Trio MRI system at the Berlin Centre for Advanced Imaging of the Charité University Hospital with approximately one week in between sessions. Healthy controls were scanned only once using the same sequences. The overt picture naming task employed an event‐related design and a T2*‐weighted echo‐planar imaging (EPI) sequence (TR = 10 s, TA = 2 s, 8 s delay of TA; echo time 30 ms, matrix 64 × 64, 32 × 3 mm slices, 0.75 mm gap, flip angle 90°, in‐plane resolution 3 × 3 × 3 mm3). Two runs were acquired with a total of 40 naming and 10 baseline trials (the smaller number of baseline trials was chosen to reduce the time the patients had to spend in the scanner) using a sparse acquisition design [e.g., Meinzer et al., 2012a, 2013b]. This allows assessing overt verbal responses during a scanner off phase to avoid articulation related artifacts. Picture stimuli were presented via a projector and a system of mirrors. Trials were jittered and each trial commenced with a blank screen that was displayed for either 1–4 s (mean: 1.6 s) followed by an object picture (always 3 s). Subsequently, a blank screen was displayed for another 3–6 s, depending on the picture onset. The patients were instructed to name each picture aloud as fast as possible during this time. A single whole brain volume (2 s) was acquired at the end of each 10 s trial (i.e., 1–4 s after picture offset; sparse sampling). Individually selected pictures were presented in random order with interspersed fixation cross baseline trials (no response was required during the baseline trials). Naming responses were recorded using an MRI‐compatible microphone, recorded and transcribed for subsequent analysis. Accuracy and response latency of correct responses was determined using the recorded responses. After the end of the functional runs, standard high resolution 3D T1‐weighted and fluid attenuation inversion recovery (FLAIR) images were acquired for lesion identification and to facilitate normalization of the functional images. Prior to scanning, a short training session was conducted outside of the scanner using a different set of pictures. Controls were scanned with the same fMRI paradigms.
fMRI Data Analysis Pre‐Processing
Statistical Parametric Mapping (SPM8, Wellcome Department of Imaging Neuroscience, London) was used for data analysis. Pre‐processing comprised slice‐timing, image re‐alignment to the first image of the time series, co‐registration with the T1‐weighted structural image. The high‐resolution T1‐weighted image was warped into standard space using unified segmentation and cost function masking [Meinzer et al., 2013a]. The resulting normalization parameters were then applied to the co‐registered functional images. Afterward, data were spatially smoothed with an 8 × 8 × 8 mm3 Gaussian kernel. Data pre‐processing was identical for healthy controls (except that there was no cost function masking).
Univariate Statistical Analysis
Data were analyzed in the context of the general linear model (GLM). The statistical design matrix comprised covariates‐of‐interest (i.e., correct picture naming and baseline trials for both sessions). Movement parameters obtained during image re‐alignment were also included to improve the overall model fit. Afterward, a high‐pass filter (128 s) was applied, the data were modeled with a finite impulse response (FIR) and the contrasts‐of‐interest were estimated for each patient (picture naming vs. baseline trials for each session). For the group analysis, a whole brain paired t‐test assessed differences in functional task‐related activity between the two sessions (anodal‐ > sham‐tDCS and vice versa). The resulting contrast images were thresholded at a voxel threshold of P < 0.001. Only voxels surviving a corrected cluster threshold of P < 0.05 family‐wise error (FWE) corrected are reported. Again, statistical analysis was identical for the healthy control group (except that only one session was analyzed). Areas showing differences between the two stimulation sessions in the whole brain paired t‐test in the patients (please see below for details) were saved as regions‐of‐interest (ROIs) for comparison with the data of the healthy control group. For this comparison, mean beta values were extracted from each ROI and compared between the patients and healthy controls using unpaired t‐tests.
Independent Component Analysis
In a second step, we explored potential stimulation effects at the functional network level using ICA. This approach has successfully been used to identify changes in network structure due to recovery or intervention in stroke patients [Carter et al., 2012; Ulm et al., 2016]. ICA is well suited even for sparse sampling data, as long as the samples are evenly spaced with regard to the TR (which was the case in our study). Moreover, ICA based on sparse sampling and continuously acquired data yielded highly consistent networks in a previous study [Yakunina et al., 2016]. Importantly, while univariate data analysis approaches are biased to detect effects in non‐lesioned regions (due to reduced statistical power in regions lesion overlap; [Meinzer et al., 2013a; Price et al., 2006]), ICA offers a number of options to overcome this bias. For example, after identification of spatially independent network components across groups of patients, mean activity values or frequency distributions can be calculated within each network of interest and intervention effects in different parts within those networks in individual patients contribute to an overall change score. Thus, this approach is more resilient to the impact of different structural lesions in groups of patients.
In the present study, ICA was performed using the GIFT toolbox (version 4.0a, http://icatb.sourceforge.net). Pre‐processed images from the univariate SPM8 analysis were entered into the ICA. A full description of the ICA algorithm and theoretical justification has been outlined previously [Calhoun et al., 2001; Jafri et al., 2008]. Briefly, using a modified minimum description length (MDL) algorithm [Li et al., 2007] and two principal component analysis (PCA) steps, the individual fMRI datasets were reduced into 18 spatially independent components. A group spatial ICA was then performed using the infomax algorithm [Bell and Sejnowski, 1995] resulting in independent spatial maps and time courses for every component, subject, and session. The infomax algorithm was then repeated 10 times using ICASSO [Himberg and Hyvarinen, 2003] to improve the reliability of the decomposition. The spatial ICs were then back reconstructed onto each individual. Components that were deemed artifacts due to prominent white‐matter or cerebrospinal fluid involvement were removed from consideration. This resulted in 16 components taken into the task‐dependent analysis and a Bonferroni corrected significance level of P < 0.003 (0.05/16). In order to identify components that were associated with the picture naming task, a regression analysis was performed on the ICA time courses with the design matrix from the univariate analysis. This results in a set of beta weights for each regressor, subject and component. The resulting beta weights represent the degree to which the component was modulated by the task relative to the baseline. This is analogous to the GLM fit at each voxel in univariate analyses, however, here it is on the ICA time course. Higher beta weights represent a larger task‐related modulation of a component for a particular regressor.
Initially, beta weights associated with picture naming across all patients and sessions were used to determine components associated with the task. Three spatial components were identified to be positively associated with the picture‐naming task with positive beta weights during the naming task and negative beta weights for the baseline using a one‐sample t‐test (Bonferroni‐corrected, P < 0.003) within the GIFT toolbox. The spatial extent of the components in the two stimulation conditions was compared in SPM (P < 0.05 FWE corrected). The components were labeled the language, motor, and visual components for ease of understanding based on peak activity in the respective components (for details please see below).
Between session differences (anodal‐ vs. sham‐tDCS) were determined by a second‐level analysis of the ICA results. There were no significant differences in spatial maps between anodal and sham stimulation conditions. Mean activity differences between the stimulation conditions within each component were tested using paired t‐tests. In addition, frequency distribution of the component time courses were evaluated by computing the power spectral density for all components of interest across stimulation conditions [Balsters et al., 2013; Garrity et al., 2007; Salvador et al., 2005]. The spectral power of each component time course was combined into three equally spaced data driven frequency bins (0–0.0167, 0.0168–0.0334, and 0.0335–0.050 Hz). Please note, the spectral resolution is a function of the TR of the study (here TR = 10). In our study, the Nyquist frequency (i.e., the minimum rate a signal can be sampled without introducing errors, which is twice the highest frequency present in the signal) is 0.05 Hz, which corresponds to 1 cycle every 20 s. This is relatively low frequency, but still includes a large portion of the typical BOLD fMRI spectrum. Importantly, enhanced lower frequency fluctuations are indicative of enhanced functional network integrity, connectivity, and performance, while higher frequencies may be associated with reduced connectivity and brain pathology [Balsters et al., 2013; Garrity et al., 2007; Malinen et al., 2010]. Differences between stimulation conditions were computed for each bin using paired t‐tests.
ICA Comparison with Healthy Controls
Following the initial ICA in the patients, we wanted to assess whether active stimulation resulted in network properties with closer resemblance to those in the healthy control group. Therefore, we needed to identify comparable networks in the HC group as those identified in the initial ICA in the patients. In order to constrain the components we used the Multivariate Objective Optimization Independent Component Analysis with Reference (MOO‐ICAR) method [for a detailed description of the method see Du and Fan, 2013], implemented in the GIFT toolbox. Briefly, following identical pre‐processing steps as in the patients, the fMRI data from the HCs was reduced into components using an ICA approach, but constrained using the networks identified in the initial patient ICA. Mean beta‐weights were then calculated for each of the three components in the HCs [Du and Fan, 2013]. Please note, the three components were also positively associated with the task in the control group. Frequency distributions of the component time courses were also evaluated by computing the power spectral density for the three components in healthy controls using the same time bins as in the patients. Differences in mean activity and spectral power between healthy controls and patients (separately for both stimulation conditions) were calculated for each component using independent t‐tests.
RESULTS
Details of demographic and clinical characteristics of the patients are reported in Table 1. All patients tolerated the stimulation well and only mild adverse effects were reported during both stimulation conditions (anodal‐/sham‐tDCS tingling: N = 3/2 patients; itching: 1/3; mild burning: 1/1; all P > 0.16). 9 of 16 patients guessed the stimulation condition correctly during the first session, 7/16 during the second session. The proportion of correct guesses were not different from chance level (binomial tests P = 0.804). Thus, the patients were unable to reliably distinguish between anodal‐ or sham‐tDCS.
Naming Performance During the Cross‐Over Phase
Two matched picture sets were used in the cross‐over imaging phase that had been pre‐selected based on performance during two baseline assessments. Those pictures only comprised correctly named pictures with the fastest response latencies to minimize performance effects. Accordingly, patients performed close to ceiling levels during both imaging sessions and were able to name greater than 90% of the pictures correctly in each session. No significant performance differences between the stimulation conditions were found with regard to response accuracy (mean ± SD % anodal‐tDCS: 90.70 ± 7.07, sham‐tDCS: 90.47 ± 6.15, t(15) = 0.19, P = 0.84) or response latency (anodal‐tDCS: 1.252 ± 288 ms, sham‐tDCS 1.253 ± 281 ms, t(15) = 0.04, P = 0.96). Thus, neural tDCS‐effects in this study (see below) were independent of performance effects.
Task‐Related Functional Activity during the Cross‐over Phase (Univariate Analysis)
During both imaging sessions, similar patterns of functional task‐related activity were found in the patients. As expected for a naming task, activity patterns comprised bilateral visual and motor related areas, but also fronto‐temporal language‐related regions (see Fig. 2 and Supporting Information Table 1, the latter details the statistics for the respective contrasts). However, the within group comparison of activity patterns during anodal‐ and sham‐tDCS in the patients also revealed that three regions showed a selective decrease during anodal‐ compared with sham‐tDCS. Those were located mainly in regions mediating high levels of cognitive control (i.e., bilaterally in the anterior cingulate cortex; ACC, Brodmann Area, BA 32, cluster size k = 92, Z = 4.75, peak MNI coordinates x/y/z = −9/27/24; left insula, BA 13, k = 73, Z = 3.73, −42/0/3 [Menon and Uddin, 2010]) and the right lingual gyrus (BA 19, k = 118, Z = 4.63, 27/−67/−1, Fig. 3A). No differences were found for the inverse contrast (anodal‐ > sham‐tDCS).
Figure 2.
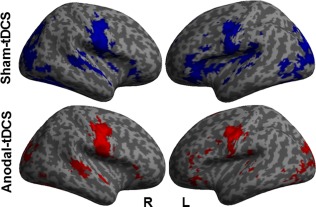
Univariate analysis: Illustrates activity patterns associated with correct naming trials during sham‐tDCS (blue) and anodal‐tDCS (red). Left (L) and right (R) hemisphere (voxel threshold P < 0.001; cluster P < 0.05 FWE‐corrected). [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 3.
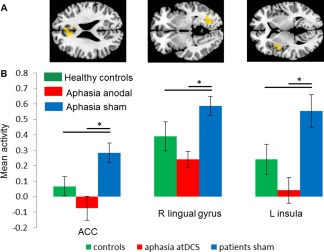
Univariate analysis: (A) Regions showing significant activity reductions in the patients during anodal‐ versus sham‐tDCS. Those regions were used for comparison with data of the healthy control group. (B) Illustrates activity in the three ROIs during anodal‐tDCS and sham‐tDCS in the patients relative to activity of healthy controls that was acquired during the same task but without stimulation. * = significant differences within and between groups. Means and SEMs are reported. [Color figure can be viewed at http://wileyonlinelibrary.com]
A subsequent ROI analysis that compared activity in the above regions during the two stimulation conditions in the patients with that of healthy controls revealed: (1) higher activity in the patients during sham‐tDCS in the anterior cingulate cortex (t(30) = 2.40, P = 0.023) and the left insula (t(30) = 2.19, P = 0.036). Activity in the right lingual gyrus tended to be higher in patients compared with controls (t(30) = 1.76, P = 0.09). (2) During anodal‐tDCS, the observed activity decreases in the patients (all ROIs t(15)>4.13, P < 0.0009) resulted in a more “normal” activity in all ROIs and no significant differences were found between patients and healthy controls (all t(30) = 1.44–1.57, all P = 0.12–0.17, see Fig. 3B).
Independent Component Functional Network Analysis
Three spatial components were identified that were positively associated with the picture naming task in the patients (i.e., positive beta weights during the naming task and negative beta weights for the baseline condition, Fig. 4 and Supporting Information Table 2). Those were named as “language,” “motor,” or “visual” components. The within group comparison between the stimulation conditions in the patients demonstrated no spatial differences in any of the three task‐related components. However, the paired t‐test on mean activity (i.e., beta weights) in the respective components demonstrated significantly greater activity during anodal‐ compared with sham‐tDCS in the language component in the patients (t(15) = 2.904, P = 0.011). No significant tDCS‐effects were found for either the visual (t(15) = 0.448, P = 0.660) or the motor component (t(15 = 0.224, P = 0.826, Fig. 5, Supporting Information Table 3). Analysis of the frequency distribution of each components time course in the patients identified a significant reduction in the highest frequency bin (0.0335–0.0500 Hz) during anodal‐ compared with sham‐tDCS in the language component (t(15) = −2.435, P = 0.028), coupled with a significant increase in the lowest frequency bin (0–0.0167Hz) during anodal‐ compared with sham‐tDCS (t(15) = 2.254, P = 0.040; correlation r = −0.74, P = 0.0012). No significant tDCS‐effects were found in either the motor or visual components (Fig. 6, Supporting Information Table 4). Given that enhanced lower versus higher frequency fluctuations have been linked to enhanced within network integrity, connectivity and performance [Balsters et al., 2013; Garrity et al., 2007; Malinen et al., 2010], this analysis suggests a beneficial effects of tDCS on functional network structure in the patients.
Figure 4.
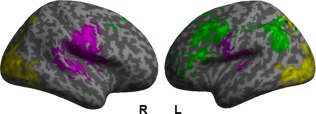
Independent component analysis: Illustrates the three networks that were associated with the naming task as identified by independent components analysis. Green = language network; purple = motor network; yellow = visual network. Left (L) and right (R) hemisphere (Bonferroni corrected, P < 0.003). [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 5.
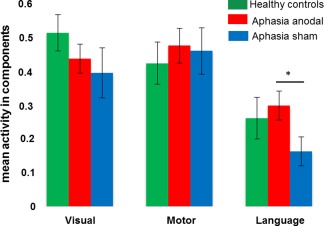
Independent component analysis: Shows selective modulation of mean activity in the language network in the patients during anodal‐ versus sham‐tDCS. * = significant difference between sham‐ and anodal‐tDCS in the patients. Means and SEMs are reported. [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 6.
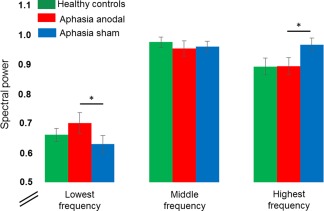
Independent component analysis: Illustrates the inter‐correlated shift of enhanced lower frequency oscillations and reduced higher frequency oscillations in the patients during anodal‐ versus sham‐tDCS in the language component. * = significant difference between sham‐ and anodal‐tDCS in the patients. Means and SEMs are reported. [Color figure can be viewed at http://wileyonlinelibrary.com]
The between group comparison revealed that mean activity in the language component was numerically lower in the patients compared with healthy controls during sham‐tDCS and higher in the patients compared with controls during anodal‐tDCS (please see Fig. 5). However, the statistical comparison of mean activity between patients (either during anodal‐ or sham‐tDCS) and controls revealed no significant differences in any of the three components (all P > 0.19, Supporting Information Table 3). For the frequency analysis, no differences were found between controls and patients except for enhanced power in the middle frequency bin in the patients for the visual component during sham‐tDCS (t(30) = 6.22, P < 0.001). This difference was no longer significant during anodal‐tDCS (t(30) = 1.37, P = 0.18; Supporting Information Table 3).
DISCUSSION
This study investigated for the first time how tDCS modulates local brain activity and functional network characteristics in patients with chronic post‐stroke aphasia. We employed an M1‐tDCS montage that enhanced naming treatment outcome in a previous clinical trial of our group [Meinzer et al., 2016] and the stimulation was administered during simultaneous fMRI while the patients performed a naming task. This allowed investigating how tDCS interacted with the residual language network in patients, thereby exploring the neural effects of acute tDCS effects as they would be present during concurrent therapy. By choosing stimuli that could reliably be named by the patients, we aimed to derive a “pure” measure of stimulation effects that is independent of treatment or performance effects.
In line with previous intrascanner tDCS studies in other populations [Antal et al., 2011; Holland et al., 2011; Lindenberg et al., 2013, 2016; Meinzer et al., 2012a, 2013b; Polania et al., 2011], our results demonstrate that tDCS modulated neural processing on multiple levels: First, the univariate data analysis revealed that anodal‐ vs. sham‐tDCS resulted mainly in reduced activity in domain general brain regions associated with high‐level cognitive control [Menon and Uddin, 2010]. (2) The ICA demonstrated that anodal‐tDCS selectively enhanced activity in a larger language‐related network but not in other task‐related components. (3) The spectral frequency analysis demonstrated a shift from higher to lower frequency oscillation during anodal‐tDCS, which indicates higher within‐network connectivity [Balsters et al., 2013; Garrity et al., 2007; Malinen et al., 2010]. Importantly, these effects were confined to the language network. (4) The comparison of data acquired in the patients with those of healthy controls showed that anodal‐tDCS resulted in an overall “normalization” of task‐related activity and network characteristics. (5) Neural facilitation in the present study was evident in the absence of confounding treatment or performance effects, thus reflecting the direct effects of tDCS on brain function. (6) Lastly, M1‐tDCS did not affect the motor network. This suggests that stimulation effects are not primarily determined by the stimulation site but rather depend on the task that is performed during tDCS. We will discuss those findings in more detail below.
Univariate Task‐Related Activity Analysis
This analysis explored tDCS‐induced local changes in task‐related activity and demonstrated that anodal‐ versus sham‐tDCS resulted in reduced activity in the ACC, the left insula and the right lingual gyrus. Moreover, while the patients exhibited enhanced activity in those regions compared with healthy controls during sham‐tDCS, activity levels were no longer different in the two groups during anodal‐tDCS. Reduced task‐related activity due to anodal‐tDCS in previous intrascanner tDCS studies [Antal et al., 2011; Holland et al., 2011; Meinzer et al., 2012a, 2013b, 2015a] has been linked to enhanced neural efficiency or less effortful processing [Kar and Wright, 2014]. In the present study, such an interpretation is supported by the function those regions subserve: ACC and left insula are part of the domain general salience network that facilitates access to working memory and attentional resources, rapid access to the motor system and behavioral response selection [Menon and Uddin, 2010]. Activity in these regions is up‐regulated during task conditions that require more controlled processing [Dosenbach et al., 2006; Erb and Obleser, 2013; Harris et al., 2009], including word retrieval tasks [Meinzer et al., 2012b; Persson et al., 2004]. Moreover, anodal‐tDCS reduced activity in these areas compared with sham‐tDCS in healthy older adults during semantic word‐retrieval [Meinzer et al., 2013b]. Similarly, right lingual gyrus is not part of the classical language network but has been shown to be up‐regulated during more effortful retrieval [Cho et al., 2012] and modulated by semantic priming during naming tasks in healthy individuals and patients with aphasia [Heath et al., 2012a, 2012b]. Thus, in the context of enhanced language network activity and connectivity during anodal‐tDCS (ICA, see below), the results of the univariate analysis may reflect overall reduced retrieval demands in the patients during naming and that identical performance was achieved more efficiently at the neural level [Price et al., 2006].
Independent Component Analysis
In recent years it has been recognized that the effects of circumscribed brain pathology, such as a stroke, may affect large‐scale functional network structure [Carter et al., 2012]. Moreover, tDCS effects are not limited to the stimulation site but extend to distant brain regions and even interconnected networks [Lindenberg et al., 2013, 2016; Meinzer et al., 2012a, 2013b; Polania et al., 2011]. Such effects cannot be captured with a univariate approach [Meinzer et al., 2013a] that also lacks power to detect potential effects in regions showing substantial lesion overlap in stroke patients [Meinzer et al., 2013a; Price et al., 2006]. The ICA approach employed in this study addressed both problems: First, it allowed assessing for the first time tDCS effects on larger task‐related functional networks in aphasia. Second, mean activity values or frequency distributions within identified networks could be calculated, and tDCS effects in different parts of a given network in individual patients contributed to an overall change score, rendering this approach more resilient to the impact of variable structural lesions.
This illustrated by the fact that we were able to show a specific increase of overall activity in the language network in the patients during anodal‐ compared with sham‐tDCS, which was not evident in the univariate analysis. Indeed, the strongest effects in the latter analysis were found in regions that were unaffected by the structural lesion. The different results in the two analyses also suggest that, depending on lesion site and language reorganization in individual patients, different regions contributed to this overall activity increase. Such an assumption would be in line with previous imaging studies in aphasia showing that different parts of the residual language network can subserve recovery or treatment effects (for recent reviews see [Crinion and Leff, 2015; Meinzer et al., 2015b]). Previous studies found differences in functional network structure in language, motor and attention networks in stroke patients compared with healthy controls [Carter et al., 2010; Warren et al., 2009]. In the present study, mean activity levels were lower in the patients compared with controls during sham‐tDCS, but the direct comparison did not reach significance. This is likely explained by the fact that only patients with mild residual aphasia were included and performance levels during the naming task were close to ceiling levels. Nonetheless, significantly enhanced language network activity during anodal‐tDCS resulted in further normalization of language network activity compared with healthy controls. Similarly, the spectral frequency analysis revealed that active stimulation resulted in an inter‐correlated shift from higher to lower frequency bands, selectively within the language network. Such a shift has previously been linked to enhanced within‐network connectivity and more efficient processing in other populations [Balsters et al., 2013; Garrity et al., 2007; Malinen et al., 2010]. Frequency distributions were also more similar to those of healthy controls during anodal‐ compared with sham‐tDCS. Thus, the pattern of network modulations was highly consistent with that of the univariate analysis.
In sum, these findings suggest that anodal‐tDCS resulted in more efficient recruitment and connectivity specifically within the patients' language network. However, because we only included patients with mild naming impairment, it remains to be determined in future studies whether similar effects may have contributed to enhanced treatment effects in a previous clinical trial that used the same montage during intensive naming therapy in more severely affected patients [Meinzer et al., 2016]. Moreover, we successfully eliminated the confounding effects of performance on functional brain activity patterns and patients performed equally well and close to ceiling levels during both stimulation conditions; consequently, our results reflect direct effects of anodal‐tDCS on neural processing. However, while there is substantial overlap between brain regions recruited during easy and more difficult naming conditions or errors, the latter are typically associated with enhanced activity in core language regions or neural systems supporting attentional processes and working memory [e.g., Fridriksson and Morrow, 2005; Meinzer et al., 2006; Postman‐Caucheteux et al., 2010]. Thus, it cannot be fully determined whether the changes seen in this study are identical to those responsible for behavioral effects seen during treatment.
Language Versus Motor Network Modulation
Previous behavioral studies demonstrated that pre‐activation of the motor system, which is tightly connected to the language system [Pulvermuller and Fadiga, 2010; Willems and Hagoort, 2007], can facilitate language processing in healthy individuals [Dick et al., 2009; Hadar et al., 1998; Holle and Gunter, 2007; Meinzer et al., 2014c] and patients with aphasia [Harnish et al., 2014; Hesse et al., 2007; Meinzer et al., 2011]. However, the neural mechanisms underlying those beneficial behavioral effects remained largely elusive. Interestingly, while previous intrascanner M1‐tDCS studies demonstrated modulation of activity in primary and secondary motor cortices during motor tasks [Antal et al., 2011; Lindenberg et al., 2013, 2016; Sehm et al., 2012], neither activity nor frequency distributions in the motor network were affected by the stimulation in the present study. The latter is in line with a previous study that administered M1‐tDCS during a word‐retrieval task in healthy individuals [Meinzer et al., 2014c]. In this study, stimulation effects were found in bilateral prefrontal but not motor regions. Importantly, previous intrascanner tDCS studies that used resting‐state paradigms [Lindenberg et al., 2013, 2016; Sehm et al., 2012] demonstrated that M1 stimulation also modulates connectivity of bilateral frontal and parietal cortices, the cerebellum and subcortical areas. This suggests that neural stimulation effects may largely depend on the task that is performed during tDCS and can occur in a number of different brain regions and networks that are influenced by the current. Therefore, our results are likely specific to the naming task and stimulation site that were used in the present study. Moreover, modeling studies have demonstrated that the conventional (vs. high‐definition) tDCS set‐up used in the present study does not result in selective modulation of M1, and that other regions in between the two electrodes may be affected by the current [Bortoletto et al., 2016; Kuo et al., 2013]. In the future, simultaneous fMRI and high‐definition tDCS [Gbadeyan et al., 2016] that allows administration of the current with high spatial precision may allow to scrutinize the exact source of the neural modulation found in the present study.
Future Directions
We only included patients with mild aphasia that were able to name a sufficient number of pictures to elicit reliable activity patterns during fMRI. Future studies are required to explore tDCS effects in more severely affected patients. However, variable and low naming performance in these patients may require different activation paradigms (e.g., language comprehension tasks) to allow exploring the interaction between residual language network activation and tDCS. Moreover, the intrascanner paradigm also allows assessing the neural mechanisms of improved language performance due to tDCS, as previously shown in other populations [Holland et al., 2011; Meinzer et al., 2012a, 2013b, 2015a]. This would allow teasing apart the relative contribution of performance and stimulation effects. In addition, the present study aimed at elucidating the neural mechanisms by which M1‐tDCS impacts on naming network activity, inclusion of resting‐state fMRI conditions would allow identifying the network(s) affected by M1‐tDCS independent of tasks. Finally, the flexibility of the intrascanner paradigm will also allow exploring how other effective montages modulate neural processing in aphasia.
CONCLUSIONS
Our results demonstrate that M1‐tDCS selectively enhanced activity and connectivity within a larger naming network which was accompanied by reduced activity in regions associated with higher order cognitive control. Both phenomena may have contributed to superior outcome in a previous study that combined naming treatment was with M1‐tDCS [Meinzer et al., 2016].
COMPETING INTERESTS
The authors report no conflicts of interest
Supporting information
Supporting Table 1
Supporting Table 2
Supporting Table 3
Supporting Table 4
ACKNOWLEDGMENTS
The funding bodies had no influence on the design, collection, analysis and interpretation of the data and in writing the manuscript.
Agnes Flöel and Marcus Meinzer contributed equally to this work.
REFERENCES
- Allman C, Amadi U, Winkler AM, Wilkins L, Filippini N, Kischka U, Stagg CJ, Johansen‐Berg H (2016): Ipsilesional anodal tDCS enhances the functional benefits of rehabilitation in patients after stroke. Sci Tranl Med 8:e330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antal A, Polania R, Schmidt‐Samoa C, Dechent P, Paulus W (2011): Transcranial direct current stimulation over the primary motor cortex during fMRI. NeuroImage 55:590–596. [DOI] [PubMed] [Google Scholar]
- Antonenko D, Meinzer M, Lindenberg R, Witte AV, Floel A (2012): Grammar learning in older adults is linked to white matter microstructure and functional connectivity. NeuroImage 62:1667–1674. [DOI] [PubMed] [Google Scholar]
- Balsters JH, Robertson IH, Calhoun VD (2013): BOLD frequency power indexes working memory performance. Front Human Neurosci 7:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell AJ, Sejnowski TJ (1995): An information‐maximization approach to blind separation and blind deconvolution. Neural Comput 7:1129–1159. [DOI] [PubMed] [Google Scholar]
- Bikson M, Grossman P, Thomas C, Zannou AL, Jiang J, Adnan T, Mourdoukoutas AP, Kronberg G, Truong D, Boggio P, Brunoni AR, Charvet L, Fregni F, Fritsch B, Gillick B, Hamilton RH, Hampstead BM, Jankord R, Kirton A, Knotkova H, Liebetanz D, Liu A, Loo C, Nitsche MA, Reis J, Richardson JD, Rotenberg A, Turkeltaub PE, Woods AJ (2016): Safety of transcranial direct current stimulation: Evidence based update 2016. Brain Stim 9:641–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortoletto M, Rodella C, Salvador R, Miranda PC, Miniussi C (2016): Reduced current spread by concentric electrodes in transcranial electrical stimulation (tES). Brain Stim 9:525–528. [DOI] [PubMed] [Google Scholar]
- Brunoni AR, Amadera J, Berbel B, Volz MS, Rizzerio BG, Fregni F (2011): A systematic review on reporting and assessment of adverse effects associated with transcranial direct current stimulation. Int J Neuropsychopharmacol 14:1133–1145. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pearlson GD, Pekar JJ (2001): A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp 14:140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter AR, Astafiev SV, Lang CE, Connor LT, Rengachary J, Strube MJ, Pope DL, Shulman GL, Corbetta M (2010): Resting interhemispheric functional magnetic resonance imaging connectivity predicts performance after stroke. Ann Neurol 67:365–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter AR, Shulman GL, Corbetta M (2012): Why use a connectivity‐based approach to study stroke and recovery of function?. NeuroImage 62:2271–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S, Metcalfe AW, Young CB, Ryali S, Geary DC, Menon V (2012): Hippocampal‐prefrontal engagement and dynamic causal interactions in the maturation of children's fact retrieval. J Cogn Neurosci 24:1849–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen Kadosh R, Soskic S, Iuculano T, Kanai R, Walsh V (2010): Modulating neuronal activity produces specific and long‐lasting changes in numerical competence. Curr Biol 20:2016–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crinion J, Leff AP (2015): Using functional imaging to understand therapeutic effects in poststroke aphasia. Curr Opin Neurol 28:330–337. [DOI] [PubMed] [Google Scholar]
- De Aguiar V, Paolazzi CL, Miceli G (2015): tDCS in post‐stroke aphasia: The role of stimulation parameters, behavioral treatment and patient characteristics. Cortex 63:296–316. [DOI] [PubMed] [Google Scholar]
- Dick AS, Goldin‐Meadow S, Hasson U, Skipper JI, Small SL (2009): Co‐speech gestures influence neural activity in brain regions associated with processing semantic information. Hum Brain Mapp 30:3509–3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockery CA, Hueckel‐Weng R, Birbaumer N, Plewnia C (2009): Enhancement of planning ability by transcranial direct current stimulation. J Neurosci 29:7271–7277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE (2006): A core system for the implementation of task sets. Neuron 50:799–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Fan Y (2013): Group information guided ICA for fMRI data analysis. NeuroImage 69:157–197. [DOI] [PubMed] [Google Scholar]
- Elsner B, Kugler J, Pohl M, Mehrholz J (2015): Transcranial direct current stimulation (tDCS) for improving aphasia in patients with aphasia after stroke. Cochrane Database Syst Rev 5:CD009760. [DOI] [PubMed] [Google Scholar]
- Erb J, Obleser J (2013): Upregulation of cognitive control networks in older adults' speech comprehension. Front Syst Neurosci 7:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flöel A (2014): tDCS‐enhanced motor and cognitive function in neurological diseases. NeuroImage 85:934–947. [DOI] [PubMed] [Google Scholar]
- Flöel A, Meinzer M, Kirstein R, Nijhof S, Deppe M, Knecht S, Breitenstein C (2011): Short‐term anomia training and electrical brain stimulation. Stroke 42:2065–2067. [DOI] [PubMed] [Google Scholar]
- Fridriksson J, Morrow L (2005): Cortical activation and language task difficulty in aphasia. Aphasiology 19:239–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrity AG, Pearlson GD, McKiernan K, Lloyd D, Kiehl KA, Calhoun VD (2007): Aberrant “default mode” functional connectivity in schizophrenia. Am J Psychiatry 164:450–457. [DOI] [PubMed] [Google Scholar]
- Gbadeyan O, Steinhauser M, McMahon K, Meinzer M (2016): Safety, tolerability, blinding efficacy and behavioural effects of a novel mri‐compatible, high‐definition tdcs set‐up. Brain Stim 9:545–552. [DOI] [PubMed] [Google Scholar]
- Hadar U, Wenkert‐Olenik D, Krauss R, Soroker N (1998): Gesture and the processing of speech: Neuropsychological evidence. Brain Lang 62:107–126. [DOI] [PubMed] [Google Scholar]
- Harnish S, Meinzer M, Trinastic J, Fitzgerald D, Page S (2014): Language changes coincide with motor and fMRI changes following upper extremity motor therapy for hemiparesis: A brief report. Brain Imaging Behav 8:370–377. [DOI] [PubMed] [Google Scholar]
- Harris KC, Dubno JR, Keren NI, Ahlstrom JB, Eckert MA (2009): Speech recognition in younger and older adults: A dependency on low‐level auditory cortex. J Neurosci 29:6078–6087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath S, McMahon K, Nickels L, Angwin A, Macdonald A, van Hees S, Johnson K, Copland D (2012a): The neural correlates of picture naming facilitated by auditory repetition. BMC Neurosci 13:e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath S, McMahon K, Nickels L, Angwin A, MacDonald A, van Hees S, Johnson K, Copland D (2012b): Priming picture naming with a semantic task: An fMRI investigation. PloS One 7:e32809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse S, Werner C, Schonhardt EM, Bardeleben A, Jenrich W, Kirker SG (2007): Combined transcranial direct current stimulation and robot‐assisted arm training in subacute stroke patients: A pilot study. Restor Neurol Neurosci 25:9–15. [PubMed] [Google Scholar]
- Himberg J, Hyvarinen A (2003): ICASSO: Software for investigating the reliability of ICA estimates by clustering and visualization. In Proceedings of the 2003 Ieee Xiii Workshop on Neural Networks for Signal Processing ‐ Nnsp'03; 2003. pp 259–268.
- Holland R, Crinion J (2012): Can tDCS enhance treatment of aphasia after stroke?. Aphasiology 26:1169–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland R, Leff AP, Josephs O, Galea JM, Desikan M, Price CJ, Rothwell JC, Crinion J (2011): Speech facilitation by left inferior frontal cortex stimulation. Curr Biol 21:1403–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holle H, Gunter TC (2007): The role of iconic gestures in speech disambiguation: ERP evidence. J Cogn Neurosci 19:1175–1192. [DOI] [PubMed] [Google Scholar]
- Jafri MJ, Pearlson GD, Stevens M, Calhoun VD (2008): A method for functional network connectivity among spatially independent resting‐state components in schizophrenia. NeuroImage 39:1666–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kar K, Wright J (2014): Probing the mechanisms underlying the mitigation of cognitive aging with anodal transcranial direct current stimulation. J Neurophysiol 111:1397–1399. [DOI] [PubMed] [Google Scholar]
- Kargel S, Stielow A, Merz M, Domhas U, Domhas F (2015): Latenzmessungen in der Diagnostik diskreter Benennstörungen. Logos 2:92–99. [Google Scholar]
- Kuo HI, Bikson M, Datta A, Minhas P, Paulus W, Kuo MF, Nitsche MA (2013): Comparing cortical plasticity induced by conventional and high‐definition 4 x 1 ring tDCS: A neurophysiological study. Brain Stim 6:644–648. [DOI] [PubMed] [Google Scholar]
- Lazar RM, Antoniello D (2008): Variability in recovery from aphasia. Curr Neurol Neurosci 8:497–502. [DOI] [PubMed] [Google Scholar]
- Lefebvre S, Dricot L, Laloux P, Gradkowski W, Desfontaines P, Evrard F, Peeters A, Jamart J, Vandermeeren Y (2015): Neural substrates underlying stimulation‐enhanced motor skill learning after stroke. Brain 138:149–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YO, Adali T, Calhoun VD (2007): Estimating the number of independent components for functional magnetic resonance imaging data. Hum Brain Mapp 28:1251–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenberg R, Nachtigall L, Meinzer M, Sieg MM, Flöel A (2013): Differential effects of dual and unihemispheric motor cortex stimulation in older adults. J Neurosci 33:9176–9183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenberg R, Renga V, Zhu LL, Nair D, Schlaug G (2010): Bihemispheric brain stimulation facilitates motor recovery in chronic stroke patients. Neurology 75:2176–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenberg R, Sieg MM, Meinzer M, Nachtigall L, Flöel A (2016): Neural correlates of unihemispheric and bihemispheric motor cortex stimulation in healthy young adults. NeuroImage 140:141–149. [DOI] [PubMed] [Google Scholar]
- Malinen S, Vartiainena N, Hlushchuk Y, Koskinen M, Ramkumar P, Forss N, Kalso E, Hari R (2010): Aberrant temporal and spatial brain activity during rest in patients with chronic pain. Proc Natl Acad Sci USA 107:6493–6497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marangolo P, Fiori V, Sabatini U, De Pasquale G, Razzano C, Caltagirone C, Gili T (2016): Bilateral transcranial direct current stimulation language treatment enhances functional connectivity in the left hemisphere: Preliminary data from aphasia. J Cogn Neurosci 16:724–739. [DOI] [PubMed] [Google Scholar]
- Meinzer M, Flaisch T, Obleser J, Assadollahi R, Djundja D, Barthel G, Rockstroh B (2006): Brain regions essential for improved lexical access in an aged aphasic patient: A case report. BMC Neurol 6:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinzer M, Mohammadi S, Kugel H, Schiffbauer H, Floel A, Albers J, Kramer K, Menke R, Baumgartner A, Knecht S, Breitenstein C, Deppe M (2010): Integrity of the hippocampus and surrounding white matter is correlated with language training success in aphasia. NeuroImage 53:283–290. [DOI] [PubMed] [Google Scholar]
- Meinzer M, Breitenstein C, Westerhoff U, Sommer J, Rosser N, Rodriguez AD, Harnish S, Knecht S, Floel A (2011): Motor cortex preactivation by standing facilitates word retrieval in aphasia. Neurorehabil Neural Rep 25:178–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinzer M, Antonenko D, Lindenberg R, Hetzer S, Ulm L, Avirame K, Flaisch T, Flöel A (2012a): Electrical brain stimulation improves cognitive performance by modulating functional connectivity and task‐specific activation. J Neurosci 32:1859–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinzer M, Flaisch T, Seeds L, Harnish S, Antonenko D, Witte V, Lindenberg R, Crosson B (2012b): Same modulation but different starting points: Performance modulates age differences in inferior frontal cortex activity during word‐retrieval. PloS One 7:e33631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinzer M, Beeson PM, Cappa S, Crinion J, Kiran S, Saur D, Parrish T, Crosson B, Thompson CK (2013a): Neuroimaging in aphasia treatment research: Consensus and practical guidelines for data analysis. NeuroImage 73:215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinzer M, Lindenberg R, Antonenko D, Flaisch T, Flöel A (2013b): Anodal transcranial direct current stimulation temporarily reverses age‐associated cognitive decline and functional brain activity changes. J Neurosci 33:12470–12478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinzer M, Jähnigen S, Copland DA, Darkow R, Grittner U, Avirame K, Rodriguez AD, Lindenberg R, Flöel A (2014a): Transcranial direct current stimulation over multiple days improves learning and maintenance of a novel vocabulary. Cortex 50:137–147. [DOI] [PubMed] [Google Scholar]
- Meinzer M, Lindenberg R, Darkow R, Ulm L, Copland D, Flöel A (2014b): Transcranial direct current stimulation and simultaneous functional magnetic resonance imaging. J Vis Exp 86:e51730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinzer M, Lindenberg R, Sieg MM, Nachtigall L, Ulm L, Flöel A (2014c): Transcranial direct current stimulation of the primary motor cortex improves word‐retrieval in older adults. Front Aging Neurosci 6:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinzer M, Lindenberg R, Phan MT, Ulm L, Volk C, Flöel A (2015a): Transcranial direct current stimulation in mild cognitive impairment: Behavioral effects and neural mechanisms. Alzheimers Dement 11:1032–1040. [DOI] [PubMed] [Google Scholar]
- Meinzer M, Ulm L, Lindenberg R (2015b): Biological markers of aphasia recovery In: Raymer A, Gonzalez Rothi LJ, editors. Oxford Handbook of Aphasia and Language Disorders. Oxford: Oxford University Press. [Google Scholar]
- Meinzer M, Darkow R, Lindenberg R, Flöel A (2016): Electrical stimulation of the motor cortex enhances treatment outcome in post‐stroke aphasia. Brain 139:1152–1163. [DOI] [PubMed] [Google Scholar]
- Menke R, Meinzer M, Kugel H, Deppe M, Baumgartner A, Schiffbauer H, Thomas M, Kramer K, Lohmann H, Floel A, Knecht S, Breitenstein C (2009): Imaging short‐ and long‐term training success in chronic aphasia. BMC Neurosci 10:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Uddin LQ (2010): Saliency, switching, attention and control: A network model of insula function. Brain Struct Funct 214:655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miniussi C, Harris JA, Ruzzoli M (2013): Modelling non‐invasive brain stimulation in cognitive neuroscience. Neurosci Biobehav Rev 37:1702–1712. [DOI] [PubMed] [Google Scholar]
- Monti A, Ferrucci R, Fumagalli M, Mameli F, Cogiamanian F, Ardolino G, Priori A (2013): Transcranial direct current stimulation (tDCS) and language. J Neurol Neurosurg Psychiatry 84:832–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Doemkes S, Karakose T, Antal A, Liebetanz D, Lang N, Tergau F, Paulus W (2007): Shaping the effects of transcranial direct current stimulation of the human motor cortex. J Neurophysiol 97:3109–3117. [DOI] [PubMed] [Google Scholar]
- Perceval G, Floel A, Meinzer M (2016): Can transcranial direct current stimulation counteract age‐associated functional impairment?. Neurosci Biobehav Rev 65:157–172. [DOI] [PubMed] [Google Scholar]
- Persson J, Sylvester CY, Nelson JK, Welsh KM, Jonides J, Reuter‐Lorenz PA (2004): Selection requirements during verb generation: Differential recruitment in older and younger adults. NeuroImage 23:1382–1390. [DOI] [PubMed] [Google Scholar]
- Polania R, Nitsche MA, Paulus W (2011): Modulating functional connectivity patterns and topological functional organization of the human brain with transcranial direct current stimulation. Hum Brain Mapp 32:1236–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postman‐Caucheteux WA, Birn RM, Pursley RH, Butman JA, Solomon JM, Picchioni D, McArdle J, Braun AR (2010): Single‐trial fMRI shows contralesional activity linked to overt naming errors in chronic aphasic patients. J Cogn Neurosci 22:1299–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Crinion J, Friston KJ (2006): Design and analysis of fMRI studies with neurologically impaired patients. J Magn Res Imaging 23:816–826. [DOI] [PubMed] [Google Scholar]
- Pulvermuller F, Fadiga L (2010): Active perception: Sensorimotor circuits as a cortical basis for language. Nat Rev Neurosci 11:351–360. [DOI] [PubMed] [Google Scholar]
- Reis J, Schambra HM, Cohen LG, Buch ER, Fritsch B, Zarahn E, Celnik PA, Krakauer JW (2009): Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc Natl Acad Sci U S A 106:1590–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvador R, Suckling J, Schwarzbauer C, Bullmore E (2005): Undirected graphs of frequency‐dependent functional connectivity in whole brain networks. Philos Trans R Soc Lond B Biol Sci 360:937–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehm B, Schafer A, Kipping J, Margulies D, Conde V, Taubert M, Villringer A, Ragert P (2012): Dynamic modulation of intrinsic functional connectivity by transcranial direct current stimulation. J Neurophysiol 108:3253–3263. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, Vanderwart M (1980): A standardized set of 260 pictures: Norms for name agreement, image agreement, familiarity, and visual complexity. J Exp Psychol Hum Learn 6:174–215. [DOI] [PubMed] [Google Scholar]
- Stagg CJ, Nitsche MA (2011): Physiological basis of transcranial direct current stimulation. Neuroscientist 17:37–53. [DOI] [PubMed] [Google Scholar]
- Stagg CJ, Bachtiar V, O'Shea J, Allman C, Bosnell RA, Kischka U, Matthews PM, Johansen‐Berg H (2012): Cortical activation changes underlying stimulation‐induced behavioural gains in chronic stroke. Brain 135:276–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szekely A, Jacobsen T, D'Amico S, Devescovi A, Andonova E, Herron D, Lu CC, Pechmann T, Pleh C, Wicha N, Federmeier K, Gerdjikova I, Gutierrez G, Hung D, Hsu J, Iyer G, Kohnert K, Mehotcheva T, Orozco‐Figueroa A, Tzeng A, Tzeng O, Arevalo A, Vargha A, Butler AC, Buffington R, Bates E (2004): A new on‐line resource for psycholinguistic studies. J Mem Lang 51:247–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulm L, McMahon K, Copland D, de Zubicaray GI, Meinzer M (2015): Neural mechanisms underlying perilesional transcranial direct current stimulation in aphasia: A feasibility study. Front Hum Neurosci 9:e550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulm L, Copland D, Meinzer M (2016): A new era of systems neuroscience in aphasia? Aphasiology doi: 10.1080/02687038.2016.1227425. [DOI] [Google Scholar]
- Warren JE, Crinion JT, Lambon Ralph MA, Wise RJ (2009): Anterior temporal lobe connectivity correlates with functional outcome after aphasic stroke. Brain 132:3428–3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems RM, Hagoort P (2007): Neural evidence for the interplay between language, gesture, and action: A review. Brain Lang 101:278–289. [DOI] [PubMed] [Google Scholar]
- Yakunina N, Kim TS, Tae WS, Kim SS, Nam EC (2016): Applicability of the sparse temporal acquisition technique in resting‐state brain network analysis. Am J Neuroradiol 37:515–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Table 1
Supporting Table 2
Supporting Table 3
Supporting Table 4


