Abstract
Gait imagery and gait observation can boost the recovery of locomotion dysfunctions; yet, a neurologically justified rationale for their clinical application is lacking as much as a direct comparison of their neural correlates. Using functional magnetic resonance imaging, we measured the neural correlates of explicit motor imagery of gait during observation of in‐motion videos shot in a park with a steady cam (Virtual Walking task). In a 2 × 2 factorial design, we assessed the modulatory effect of gait observation and of foot movement execution on the neural correlates of the Virtual Walking task: in half of the trials, the participants were asked to mentally imitate a human model shown while walking along the same route (mental imitation condition); moreover, for half of all the trials, the participants also performed rhythmic ankle dorsiflexion as a proxy for stepping movements. We found that, beyond the areas associated with the execution of lower limb movements (the paracentral lobule, the supplementary motor area, and the cerebellum), gait imagery also recruited dorsal premotor and posterior parietal areas known to contribute to the adaptation of walking patterns to environmental cues. When compared with mental imitation, motor imagery recruited a more extensive network, including a brainstem area compatible with the human mesencephalic locomotor region (MLR). Reduced activation of the MLR in mental imitation indicates that this more visually guided task poses less demand on subcortical structures crucial for internally generated gait patterns. This finding may explain why patients with subcortical degeneration benefit from rehabilitation protocols based on gait observation. Hum Brain Mapp 38:5195–5216, 2017. © 2017 Wiley Periodicals, Inc.
Keywords: fMRI, gait control, motor imagery, action observation, imitation, elderly, brainstem
INTRODUCTION
Motor imagery and action observation share a common “motor representational space” with actual action execution (“common coding approach,” Prinz [1997]; “ideomotor theory,” Jeannerod [2001]). This tenet, supported by neurofunctional evidence (see Hétu et al. [2013] and Caspers et al. [2010] for reviews), has motivated the use of motor imagery and action observation as proxies to study and train higher level motor control in both healthy and disease conditions [Buccino et al., 2006; Caligiore et al., 2017; Di Rienzo et al., 2016; Mulder 2007; Ruffino et al., 2017; Small et al., 2013]. Few neurofunctional studies have directly compared the two processes during the execution of upper limb movements [Filimon et al., 2007; Macuga and Frey 2012], and none to date has examined movements of the lower limbs or gait behaviors. To fill this gap, we employed a novel naturalistic experimental setup to explore functional brain commonalities and differences between gait motor imagery and gait imagery coupled with gait observation. In addition, we wanted to provide a neurologically justified rationale for the clinical application of gait imagery and/or the same task complemented by gait observation in rehabilitation.
As gait imagery and gait observation are increasingly applied as complementary rehabilitation strategies to improve locomotion in neurological disorders or after prolonged immobilization [Bellelli et al., 2010; Buccino, 2014; Christakou et al., 2007; Malouin and Richards, 2010; Pelosin et al., 2010], comparing them from a neurofunctional perspective could have clinical implications for determining which of the two processes may be more appropriate in rehabilitation for different patient populations. This is particularly relevant for the elderly who experience difficulty in walking and undergo gait rehabilitation following peripheral (musculoskeletal) or central injury. Because of the importance of gait rehabilitation in later life, this study recruited participants in their seventh or eighth decade. It thus provides the needed neurophysiological reference point from which the motor control of gait can be studied in older adults with gait disorders.
Neural Processes Involved in Locomotion Control: Cortical and Subcortical Structures
In young adults, gait is a highly automatic behavior largely dependent on both subcortical and cortical control over spinal centers [Jahn et al., 2004, 2008a, 2008b; La Fougère et al., 2010; Takakusaki, 2013]. Central pattern generators (CPGs) located in the spinal cord [Grillner and Zangger, 1979; Grillner, 2006a] generate rhythmic and stereotyped patterns of muscle contractions, as shown by studies in nonhuman vertebrates [see Orlovskii et al., 1999; Grillner, 2006b for reviews]. The existence of spinal CPGs in humans was first demonstrated by evidence that locomotor movements can be produced in patients with total spinal transection [Dimitrijevic et al., 1998; Holmes, 1915; Kuhn and Macht, 1949] and complete paraplegia [Dietz et al., 1995; Harkema et al., 2011] if weight is partially unloaded. Similarly, human newborns generate locomotor‐like patterns when the feet are placed on a flat surface, even though they lack mature descending pathways [Forssberg, 1985; Yang et al., 1998; see also Dominici et al., 2011]. With maturation, these automated patterns are kept under supraspinal control by locomotor regions in the brainstem, cerebellum, and cortex, which seem to play a more prominent control over CPGs in humans than in most other mammals [Dietz, 2002]. As these regions are responsible for gait initiation, stepping modulation, and on‐line gait adaptation to environmental cues, they might be the likely or, at least, the desired targets of gait rehabilitation protocols.
Our knowledge of subcortical areas involved in gait control derives mainly from experiments on animals [Dubuc, 2009; Mori et al., 1999; Shik and Orlovsky, 1976; Whelan, 1996]. These areas include a mesencephalic locomotor region (MLR) and its descending projections to the ponto‐medullary reticular formation, a subthalamic locomotor region, and a cerebellar locomotor region. In humans, these regions have been functionally defined on the basis of the location of brainstem and cerebellar activation in gait‐related tasks [Jahn et al., 2008a,2008b; La Fougère et al., 2010]. For instance, the MLR is located in the pontomesencephalic tegmentum, in the vicinity of the pedunculopontine nucleus (PPN, Jenkinson et al. [2009]). Clinical reports suggest that a lesion in the PPN/MLR abolishes the ability to stand and generate stepping movements [Hathout and Bhidayasiri, 2005; Masdeu et al., 1994], and that deep‐brain stimulation in this region ameliorates gait disturbances in patients with Parkinson's disease [Plaha and Gill, 2005; Stefani et al., 2007]. More generally, owing to its deep connections with the basal ganglia (and subthalamic nucleus) and the spinal cord (via the ponto‐medullary reticular formation), the MLR may be a key region for the automatic regulation of rhythmic limb movements during locomotion [Takakusaki, 2013]. As a matter of fact, Weiss et al. [2015] have also shown that amelioration of gait disturbances in Parkinson's disease following subthalamic deep‐brain stimulation is mediated by modulation of the activity in the MLR.
A variety of imaging techniques have been applied to study cortical control of gait. Near‐infrared spectroscopy (NIRS) [Miyai et al., 2001], 18Fluorodeoxyglucose positron emission tomography (18F‐FDG PET), and 99mTc‐hexamethylpropyleneamineoxime single photon emission computed tomography (99Tc‐HMPAO SPECT) scanning studies [Fukuyama et al., 1997; Hanakawa et al., 1999a; La Fougère et al., 2010; Malouin et al., 2003] have shown widespread brain activations associated with locomotor‐like behaviors in premotor cortex (PM), supplementary motor area (SMA), primary motor and somatosensory cortices. Moreover, lesion studies also indicate that both the dorsal PM and parietal areas are involved in gait control, as poor adaptation of gait behaviors to environmental cues is observed after their lesion or disconnection (see Liston et al. [2003] and Nutt [2013] for reviews). Taken together, previous findings suggest that adaptive gait behaviors depend on fronto‐parietal cortical control over a complex subcortical network that, in turn, modulates activity in spinal CPGs. Also, it has been shown that the cortical component of gait control consolidates during early childhood and becomes more prominent with aging, possibly because of an impoverished automaticity of motor control in the later decades of life [Boisgontier et al., 2013; Ruffieux et al., 2015].
Neural Correlates of Gait Imagery and Gait Observation: Toward Neurologically Informed Strategies for Gait Rehabilitation
Gait behaviors typically occur without or with little explicit conscious control. A major challenge in gait rehabilitation protocols is to promote the explicit and voluntary mental rehearsal of gait‐related motor representations by re‐establishing the recruitment of the complex set of cortical‐subcortical regions responsible for locomotion control. This has been successfully attempted in clinical trials based on motor imagery or observation of gait [Bellelli et al., 2010; Buccino, 2014; Christakou et al., 2007; Malouin and Richards, 2010; Pelosin et al., 2010]. Although both processes involve the simulation of a motor act and recruit brain areas similar to those recruited during actual action execution [Jeannerod, 2001], they differ from each other in many ways. While motor imagery has been defined as a mental state in which real movements and the corresponding neural activity are internally evoked without an overt action [Jeannerod, 2001], action observation is considered motoric in nature because it evokes motor activations with the involvement of fronto‐parietal double‐duty “mirror” neurons [Di Pellegrino et al., 1992; Rizzolatti et al., 1996] that discharge both when a motor plan is actively retrieved by an agent to perform an action and when the same agent observes a similar action performed by a different person. Thus, one striking difference between motor imagery and action observation is that while the former implies that motor plans are internally generated, in the latter motor activations are triggered by a specific kind of visual stimulation. As a matter of fact, while fronto‐parietal areas seem equally recruited by motor imagery and action observation [Macuga and Frey, 2012], the supplementary motor area is more often found in motor imagery than in action observation studies on upper limb movements (see for instance Filimon et al. [2007] and Macuga and Frey [2012]).
With regard to gait, are the two approaches equivalent in terms of the brain processes involved or they show differences in neurophysiological underpinnings that might impact on their potential clinical application?
The short answer is that gait motor imagery and gait observation do not seem to be equivalent in the degree of involvement of subcortical locomotor centers, although these differences remain to be fully elucidated.
In particular, functional magnetic resonance imaging (fMRI) studies on motor imagery of gait have succeeded in detecting brain activity in the cortical and subcortical components of the locomotor circuit during explicit imagery tasks performed with eyes closed [Jahn et al., 2004, 2008a, 2008b]. La Fougère et al. [2010], in a combined 18F‐FDG PET and fMRI study, showed the activation of a core locomotion network in the frontal cortex, cerebellum, pontomesencephalic tegmentum, parahippocampal gyrus, fusiform, and occipital gyri, together with deactivations in multisensory vestibular cortices (e.g., in the superior temporal gyrus and inferior parietal lobule) during both real locomotion (with 18F‐FDG PET) and imagined locomotion (with fMRI), confirming the existence of a substantial neurofunctional overlap between the two.
Differently, fMRI studies on gait observation mainly showed task‐related activations restricted to the cortex. Iseki et al. [2008] investigated the neural correlates of passive observation of gait in young healthy volunteers shown videos of a walking model from a third‐person perspective. This task recruited brain activity in the SMA and PM cortices, in addition to more posterior visual areas; yet, no activity in cerebellar or brainstem structures was recorded. The lack of subcortical activations was perhaps due to the fact that the participants were not instructed to actively mentally imitate the observed behavior, which was also presented in a perspective incompatible with that of the participants. Yet, in their study, Wang et al. [2008a,2008b] used first‐person visual stimuli and asked participants to actively identify themselves with a model and to mentally imitate the observed gait‐related movements: they found activations in the SMA and dorsal PM, with additional recruitment of the inferior and middle frontal regions, parietal regions, occipital areas including MT/V5, and the cerebellum, but no brainstem activations. Finally, Dalla Volta et al. [2015] asked participants to step on a rolling cylinder while lying in the fMRI scanner and passively observe the same action performed by another person. They found a neurofunctional overlap between the observation and execution tasks in the SMA, PM, superior and inferior parietal areas, and the cerebellum but, again, no brainstem activations.
To summarize, unlike studies on motor imagery of gait, previous studies on gait observation have produced inconsistent evidence for the involvement of subcortical brainstem centers, possibly because the task requirements and stimuli varied across experiments. To date, gait imagery and gait observation have never been directly compared using a unitary procedure, something that we attempted with our study. In particular, using a strict subtraction strategy, our experimental design allowed us comparing pure gait motor imagery with motor imagery facilitated by the observation of a walking model, that is, a condition that we named “mental imitation.”
Our Virtual Walking Task
The purpose of this study was to directly compare the neural correlates of motor imagery and observation of gait behaviors using well‐matched visual stimuli apt to evoke the sensation of walking in elderly participants who may find it particularly difficult to voluntarily evoke motor representations of walking behaviors [Allali et al., 2014; Zwergal et al., 2012]. To evoke motor imagery of walking, we used a virtual walking task [Iseki et al., 2008, see also Buekers et al., 1999; Fung et al., 2006; Hollman et al., 2006] based on naturalistic in‐motion stimuli of viewing a path in a park from the first‐person perspective. The participants were asked to imagine walking along the path as if the camera were their own eyes (see Supporting Information). Matched visual stimuli were used to directly test the impact of action observation on the imagery task. The participants watched similar virtual‐walking videos either with (mental imitation condition) or without (imagery condition) a walker seen from behind, as if the walker preceded the participant walking along the path. This perspective, compatible with the participant's one (Fig. 1), was selected to minimize the need for visuo‐motor transformations during mental imitation and thus facilitate the recruitment of motor processes during the task [Jackson et al., 2006; Watanabe et al., 2011; Watanabe and Higuchi, 2016].
Figure 1.
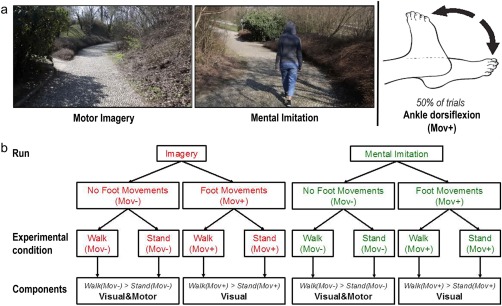
The figure shows (a) two snapshots taken from the in‐motion video clips used in the Imagery (left) and Mental Imitation (right) condition and (b) the structure of the second‐level full‐factorial ANOVA of the Virtual Walking analysis. As shown in (a), in 50% of the trials, the participants were also required to perform ankle dorsiflexion while they imagined standing or walking along the path. Walk indicates the conditions when the participants imagined or mentally imitated walking. Stand indicates the conditions when the participants imagined or mentally imitated standing on the spot. Mov+ indicates the conditions in which the participants also performed ankle dorsiflexion. Mov− indicates the conditions in which no foot movement was performed. Importantly, the “Walk(Mov+) > Stand(Mov+)” contrasts, reporting the voxel‐by‐voxel difference between the “Walk(Mov+)” and “Stand(Mov+)” experimental conditions, only inform about the visual processes involved in the Virtual Walking task because motor activations are cancelled out in the “Walk(Mov+)” condition by the presence of foot movements in the “Stand(Mov+)” baseline. This rationale holds both for condition when walk is internally imagined (Imagery run) and imagined by imitation (Mental Imitation run). As a result, the contrast “visual&motor > visual” performed in the second‐level analysis isolates the motor components of motor imagery/mental imitation of gait. Differently, a main effect of all contrast images identifies both visual and motor components of the Virtual Walking task. [Color figure can be viewed at http://wileyonlinelibrary.com]
We named mental imitation the experimental condition that combined both motor imagery and action observation to highlight that the act of watching the walking model was not passive; rather, the participants were explicitly instructed to “imagine imitating” the walker's behavior. Importantly, this condition allowed us to compare motor imagery during virtual walking with an equally “active” motor task in which gait‐related motor programs are voluntary recruited by participants and not just passively triggered by the observation of biological motion. What differentiates our motor imagery and “mental imitation” condition is the way in which the rhythm of the imagined gait is maintained throughout the task: while gait rhythm is internally generated in the motor imagery condition, observation of the walking model should contribute to gait rhythm generation during mental imitation.
As baseline for each experimental condition, the participants viewed stationary videos in which no action took place and were asked to imagine standing on the spot in the park (in the mental imitation condition, they did so while watching the person in the video who was also standing on the spot). Finally, in 50% of the trials, including 50% of the “standing” baselines, the participants were asked to move their feet and perform ankle dorsiflexion while watching the video clips [Dobkin, 2004]. This latter manipulation allowed us to compare the activation patterns associated with motor imagery of gait during Virtual Walking with the patterns associated with the rhythmic execution of lower limb movements, which are compatible, to the extent the fMRI environment so allows, with those implied in gait execution [Dobkin, 2004]. Briefly, this 2 × 2 × 2 full‐factorial design enabled us to directly explore (i) the neural correlates of locomotion control measured during the Virtual Walking task by comparing “imagery of gait” with “imagery of standing,” (ii) their relation with the neural correlates of execution of rhythmic foot movements, and (iii) the modulatory effect of imagined imitation over such activations.
MATERIALS AND METHODS
Participants and Neuropsychological Assessment
The study sample included 24 healthy adults (10 women, age 66.79 ±6.97 years, formal education 13.54 ± 4.14 years). All were right‐handed as determined by the Edinburgh Handedness Inventory [Oldfield, 1971] and reported normal or corrected‐to‐normal vision and no orthopedic pathologies. None had a medical history of neurological or psychiatric disorders or presented age‐related cognitive deficits, as measured with the Mini‐Mental State Examination (MMSE, Folstein et al. [1975]) (all scores >24) and confirmed by Raven's Colored Progressive Matrices [Basso et al., 1987]; participants were also screened for long‐term and short‐term verbal memory [Novelli et al., 1986] and executive functions deficits [Appollonio et al., 2005]. All subjects had an overall normal performance (Supporting Information, Table S1). On the Vividness of Movement Imagery Questionnaire (VMIQ) [Isaac et al., 1986], a self‐report questionnaire on explicit motor imagery abilities, subjects scored on average 46.04 ± 16.44 for motor imagery and 44.71 ± 16.41 for visual imagery, indicating good self‐reported motor imagery abilities [Isaac et al., 1986].
The experimental protocol was approved by the Local Ethics Committee (Comitato Etico Azienda Sanitaria Locale Città di Milano) and carried out in accordance with the ethical standards of the 1964 Declaration of Helsinki, with later revisions. All participants provided written informed consent to take part in the fMRI study.
Motor Performance and Chronometry Ability
To exclude gait disturbances and to obtain an additional measure of motor imagery abilities, the participants performed a version of the Timed Up and Go (TUG) test developed by Beauchet et al. [2010]. Subjects were seated, allowed to use the armrests to stand up and instructed to walk 3 m, turn around, walk back to the chair and sit down saying “stop”. Times for each condition were recorded with a stopwatch to the nearest 0.01 s. The stopwatch was started on the command “ready–set–go” and stopped as the subject sat down and said “stop.” For the imagined condition, subjects sat in the chair and were instructed to imagine performing the TUG (iTUG) with their eyes closed and to say “stop” when they were finished. Participants performed both the TUG and the iTUG twice and we averaged the times of the two trials. To assess mental chronometry abilities (CA) we calculated the time difference between the TUG and the iTUG with the following formula [Allali et al., 2014]: CA = (TUG − iTUG)/[(TUG + iTUG)/2]. CA was separately calculated per trial and the results were averaged to obtain one outcome measure. The lower the CA score, the smaller the difference between times recorded during the TUG and the iTUG, which would be an index of high motor imagery abilities.
fMRI Experiment
The experiment, at the first level of statistical analysis, conforms to a 2 × 2 × 2 repeated‐measures design with the within‐subject factors: (i) task (Motor Imagery or Mental Imitation), (ii) posture (imagery of Walking vs Standing), and (iii) foot movement (present or absent) (Fig. 1).
Stimuli and Task
During the fMRI session, the participants watched 15 s naturalistic videos of a path leading through a park in two conditions: (1) in‐motion, “virtual walking” condition (Walk), or stationary, standing condition (Stand) that served as baseline. Throughout the experiment, participants were asked to imagine themselves standing (Stand) or walking along the path (Walk) as if the camera were “their own eyes.” In the latter condition, the scene proceeded at a speed compatible with slow human walking rhythm (≈1.1 m/s). Naturalistic scenarios depicted two different paths that were shown as either ascending or descending (see Fig. 1 and Supporting Information). At the beginning of each video, a written prompt with either the instructions “no foot movement” or “move your feet” was displayed for 3 s. In the “move your feet” condition (50% of the trials), the participants executed alternate ankle dorsiflexion [Dobkin, 2004] in‐step with the rhythm of their imagined gait pattern (in the Walk condition). The participants were instructed to maintain this rhythm throughout the experiment whenever cued to make foot movements (i.e., also in the Stand condition): when the Stand condition was combined with foot movements, participants were told that a daily life analogue of the task would have been stepping on the spot. The experimenter monitored the participants' foot movements during the entire session to ensure they followed the instructions. Scans in which a participant failed to respond to the prompt, that is, did not move the feet when cued to “move your feet,” or vice‐versa, were discarded from the analysis (two videos in one participant and four videos in another one). The mean frequency of ankle dorsiflexion was 1.12 ± 0.26 movements per second. Importantly, the rhythm did not differ between conditions (all P corr > 0.1). Finally, eight times per run (one per video type), the participants were asked whether the path that they had just seen ascended or descended. The purpose of these questions was simply to keep their attention focused on the videos; the related fMRI brain volumes were not analyzed.
The aforementioned conditions were presented in two separate runs: in the Imagery run, the videos only depicted the paths seen from the first‐person perspective, whereas in the Mental Imitation run a walker was also shown from behind as if preceding the participant along the path (see Supporting Information and Fig. 1). The Mental Imitation videos presented four different walkers (two females, two males) who walked in‐step with the camera. In the Mental Imitation run, the participants were explicitly instructed to “imagine imitating” the walker's behavior. Crucially, instructions were thus similar between the Imagery and Mental Imitation condition, which both required imagine standing (Stand) or walking along the path (Walk) as if the camera were participants' “own eyes”: however, while in the Imagery condition the walking rhythm was internally generated, in the Mental Imitation condition, participants were required to imagine adopting the rhythm shown by the model. At the end of the experimental section, participants were also asked to rate on a Visual Analogue Scale ranging for 0–100 “how easy” it was for them to identify with the walking rhythm of the models: the mean rate was 90.8 ± 14.5, suggesting identification during Mental Imitation was quite an easy task for participants.
Each run lasted 11.5 min and 230 scans were acquired. The first 10 scans, corresponding to visualization of task instructions, were discarded from the analysis. In each run, thirty‐two 15 s videos were shown, that is, eight per experimental condition, presented in counterbalanced order, for a total of 40 scans acquired per experimental condition. The order of the Imagery/Imitation runs was counterbalanced between participants.
Before starting the fMRI session, the participants practiced the task outside the scanner tube for about 10 min, so that they could familiarize themselves with the videos and learn to correctly execute ankle dorsiflexion when prompted.
Foam padding was applied around the head to minimize head movements; a semicircular cushion supporting the legs was provided, so that the participants could freely move their ankles without bending their knees.
Stimuli presentation was controlled by Cogent 2000 MATLAB Toolbox (MathWorks). Visual stimuli were delivered using VisuaStim fiber‐optic goggles (600 × 800 pixel resolution). Responses were recorded through a response box placed under the right hand (Resonance Technology Inc., Northridge, CA, USA).
fMRI Data Acquisition and Analysis
Data acquisition
MRI scans were acquired using a Siemens Magnetom Avanto 1.5 T scanner, equipped with gradient‐echo echo‐planar imaging (flip angle 90°, TE 60 ms, TR 3000 ms, FOV 280 × 210 mm, matrix 96 × 64, slice thickness 4 mm) (Siemens AG, Erlangen, Germany). The first two brain images (TR periods) from each functional run were removed to allow for steady‐state tissue magnetization; an additional 10 volumes (corresponding to the task's instructions) were discarded from the analyses. MPRAGE high‐resolution T1‐weighted structural images were also acquired (flip angle 35°, TE 5 ms, TR 21 ms, FOV 256 × 192 mm, matrix 256 × 256, TI 768, for a total of 160 axial slices with 1 × 1 × 1 mm voxels).
Preprocessing
After image reconstruction, raw data visualization and conversion from the DICOM to the NIfTI format were performed with MRIcron (http://www.mricro.com) software. All subsequent data analyses were performed in MATLAB R2014b (Math Works) using Statistical Parametric Mapping software (SPM12, Wellcome Department of Imaging Neuroscience, London, UK). First, the fMRI scans were realigned and unwarped to account for any movement during the experiment; the unwarped images were co‐registered with the T1 structural image of each participant, which was then segmented and stereotactically normalized into the SPM12 template (tmp.nii) to allow for group analyses of the data [Ashburner and Friston, 2005]. Deformation fields used for T1 segmentation were then applied to the unwarped functional scans. At this stage, the data matrix was interpolated to produce voxels 2 × 2 × 2 mm in dimension. The stereotactically normalized scans were smoothed using a Gaussian filter of 10 × 10 × 10 mm to improve the signal‐to‐noise ratio.
Artifact Detection Tools (ART, Whitfield‐Gabrieli, http://www.nitrc.org/projects/artifact_detect) was used to identify outlier scans in global signal and movement for each participant. Timepoints were marked as outliers when the scan‐to‐scan global signal difference exceeded three standard deviations of the mean, and when the compounded measure of movement parameters exceeded 1 mm scan‐to‐scan movement (on average, excluded volumes were 13.52 ± 8.47 and 9.14 ± 8.41 in the Imagery and Mental Imitation runs, respectively, equal to 6.15% and 4.15% of total scans). Participants with more than 20% of outlier timepoints in a run were excluded from the analyses (two were excluded according to this criterion and one because of artifacts in the signal; the final sample was 21 participants).
Statistical analyses of the fMRI data
A two‐step statistical analysis, based on the general linear model (GLM), was performed. The blood oxygen level‐dependent (BOLD) signal associated with each experimental condition was analyzed by a convolution with a canonical hemodynamic response function [Worsley and Friston, 1995]. Global differences in the fMRI signal were removed by proportional scaling from all voxels. High‐pass filtering (128 s) was used to remove artefactual contributions to the fMRI signal, such as noise from cardiac and respiratory cycles. Realigning parameters calculated in the preprocessing step were added to the GLM as regressors of no interest. This first step implied a fixed‐effect analysis, in which condition‐specific effects were calculated. This was followed by second level random effect analyses, in which the original 2 × 2 × 2 design was collapsed into two orthogonal 2 × 2 ANOVAs.
First‐level within‐subject analyses
At the first level, separately for each run (Imagery and Mental Imitation) and each participant, we characterized the specific effects associated with the imagery of walking (Walk) and the imagery of standing (Stand) when the participants moved their feet, Walk(Mov+) and Stand(Mov+), or kept them still Walk(Mov−) and Stand(Mov−) 1. These four effects were then compared to generate contrast images that were entered into two separate second‐level full‐factorial ANOVAs that conformed to random effect analyses [Holmes and Friston, 1998; Penny and Holmes, 2004]: ANOVA #1, Virtual Walking Analysis; ANOVA #2, Foot Movement Analysis.
ANOVA #1, Virtual Walking analysis
We investigated the neural correlates of motor imagery of gait during Virtual Walking by comparing the brain responses during the in‐motion videos (Walk videos) with those collected while the participants imagined themselves standing on the spot (Stand videos that served as baseline); this was done separately for the Imagery and the Mental Imitation (henceforth Imitation) runs. We also separately analyzed the trials where explicit ankle dorsiflexion was present (Mov+ videos) or absent (Mov− videos) and calculated the following contrasts:
Walk(Mov−) > Stand(Mov−) in the Imagery run
Walk(Mov+) > Stand(Mov+) in the Imagery run
Walk(Mov−) > Stand(Mov−) in the Imitation run
Walk(Mov+) > Stand(Mov+) in the Imitation run
The contrasts “Walk(Mov−) > Stand(Mov−)” describe activations associated with both visual and (implicit) motor processes involved in the Virtual Walking imagery task (“visual&motor” effects), whereas in the contrasts “Walk(Mov+) > Stand(Mov+)” the neural activations associated with explicit foot movements were cancelled out by the baselines: these contrasts identify the activations related to the visual processing of in‐motion stimuli applied in the Virtual Walking tasks without the motoric components, and for this reason they are defined as “visual” effects in the description given below (Fig. 1).
Thus, the [Walk(Mov−) > Stand(Mov−)] contrast in the Imagery run corresponds to the Imagery (visual&motor) condition, the [Walk(Mov−) > Stand(Mov−)] contrast in the Imitation run corresponds to the Imitation (visual&motor) condition, the [Walk(Mov+) > Stand(Mov+)] contrast in the Imagery run corresponds to the Imagery (visual) condition, and the [Walk(Mov+) > Stand(Mov+)] contrast in the Imitation run corresponds to the Imitation (visual) condition.
The four contrast images from each participant were entered into a second‐level full‐factorial ANOVA with two within‐subject factors: Components (visual&motor vs visual) and Task (Imagery vs Imitation). The design matrix was organized as follows:
Imagery (visual&motor); Imitation (visual&motor); Imagery (visual); Imitation (visual)2.
We thus calculated the following linear contrasts to generate SPM[t] maps:
Main effect of the motor component of Virtual Walking (visual&motor > visual): contrast 1 1 −1 −1; this contrast describes the main effect of the task while controlling for visual stimulation;
Motor component of Imagery: Imagery(visual& motor) > Imagery(visual): contrast 1 0 −1 0; this contrast describes the neural correlates of the Imagery task while controlling for visual stimulation;
-
Motor component of mental Imitation: Imitation(visual&motor) > Imitation(visual), contrast 0 1 0 −1; this contrast describes the neural correlates of the Mental Imitation task while controlling for visual stimulation;
Second, we directly compared such motor‐related activations between the Imagery and Mental Imitation task and described their differences and commonalities. We thus calculated the following linear contrasts to generate SPM[t] maps:
Conjunction analysis reporting motor components shared by the Imagery and Imitation tasks, which identifies the voxels significantly recruited in both effects (contrast 1 0 −1 0 ∩ 0 1 0 −1, [“Imagery(visual&motor) > Imagery(visual)”] ∩ [Imitation(visual&motor) > Imitation(visual)]; this contrast describes the motor‐related activations in common between the Imagery and Mental Imitation condition that are independent from differences in the visual features of the stimuli used in the two tasks (as these differences are cancelled out by the “visual” baselines);
-
Interaction effects between the Component (visual&motor vs visual) and Task (Imitation vs Imagery) factors:
[Imagery(visual&motor) > Imagery(visual)] > [Imitation (visual&motor) > Imitation(visual)] (contrast 1 −1 −1 1), and [Imitation(visual&motor) > Imitation(visual)] > [Imagery(visual&motor) > Imagery(visual)] (contrast −1 1 1 −1); these contrasts describe the motor‐related activations that are stronger during the Imagery that during the Mental Imitation condition (or vice versa) while controlling for differences in the visual features of the stimuli used in the two tasks (as these differences are cancelled out by the “visual” baselines).
Contrasts (a), (b), and (c) were masked with the respective simple effects of each condition (thresholded at P < 0.001) to interrogate only the voxels showing a positive effect during the conditions of interest. For the sake of clarity, the simple effect of each condition is reported in the Supporting Information, Tables S2 and S3, as well as the direct comparisons between activations in the Imagery and Mental Imitation runs (Supporting Information, Table S4). Contrarily to the analyses reported in the main text, these direct comparisons are not controlled for differences in the visual stimulation used, and thus report task‐induced modulations in visual‐related rather than motor‐related areas. All analyses are conducted at the whole‐brain level, thresholded at P < 0.001uncorr, and only peaks in clusters significant at P < 0.05 for their spatial extent are reported. The regional effects that survived a voxel‐wise or a cluster‐wise family‐wise error (FWE) correction are indicated in the tables.
ANOVA #2, Foot Movement analysis
In this control analysis, we mapped the motor network involved in foot movement execution and the possible interaction effect between foot movement execution and the Virtual Walking motor imagery or imitation tasks. The contrast images for the evaluation were derived from the following linear contrasts calculated in the first level fixed‐effect analysis:
-
Effect of Foot Movements during Imagery or Imitation of Standing
“FootMov_Stand_Imag”=Stand(Mov+) > Stand(Mov−) in the Imagery run
“FootMov_Stand_Imit”=Stand(Mov+) > Stand(Mov−) in the Imitation run
-
Effect of Foot Movements during Imagery or Imitation of Walking
“FootMov_Walk_Imag”=Walk(Mov+) > Walk(Mov−) in the Imagery run
“FootMov_Walk_Imit”=Walk(Mov+) > Walk(Mov−) in the Imitation run
The ensuing four contrast images from each participant were then entered into the second level ANOVA, with Posture (Walk vs Stand) and Task (Imagery vs Imitation) as within‐subject factors.
First, we mapped the overall main effect of Foot Movements (linear contrast: 1 1 1 1) to identify the brain regions associated with the execution of lower limb movements compatible with gait behaviors (i.e., ankle dorsiflexion, see Dobkin [2004]). These results were then used to compare the motor network controlling Foot Movements with the network recruited during motor imagery of walking (Results; Fig. 2). We also tested the main effects and interactions of the second‐level ANOVA to determine whether motor imagery and imitation of walking had a modulatory effect on the neural correlates of explicit foot movements.
Figure 2.
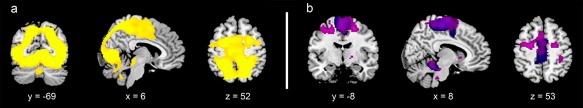
The figure shows the activations associated with (a) the main effect of task and (b) the motor components of Virtual Walking (“visual&motor > visual” contrast, purple), which have been superimposed on the main effect of ankle dorsiflexion (blue). As shown by the data illustrated in (b), the network recruited by the Virtual Walking task exceeded that involved in mere foot movement execution, and included posterior parietal and dorsal premotor regions. In the “visual&motor > visual” contrast, the “visual” control condition was obtained by contrasting the “gait imagery” with the “imagery of standing” trials, both associated with lower limb movement execution “Walk(Mov+) > Stand(Mov+)” contrasts (Fig. 1). Although one might hypothesize that execution of lower limb movements compatible with walking behaviors could facilitate the motor imagery task, our Foot Movement analysis showed this was not the case (see Results), because the neurofunctional patterns of gait imagery associated with lower limb movements did not differ from that of imagery of standing when associated with lower limb movements. When the activations associated with foot movement execution are cancelled out in the first level analysis, what the “(Walk(Mov+) > Stand(Mov+)” contrasts describe are the activations associated with visual processing of the stimuli. The coordinates of the activation maps shown in this figure match those listed in (a) Table 1 and (b) Table 2 (purple) and Table 3 (blue). Data are reported at the same threshold discussed in the main text and reported in the tables (P < 0.001uncorr), and only significant clusters are shown. [Color figure can be viewed at http://wileyonlinelibrary.com]
RESULTS
Motor Performance and Chronometry Ability
Mean times for TUG execution were 8.72 ± 1.69 s, range 11.47–5.14 s, which is within the normal range for elderly population [Bohannon, 2006]. We could thus exclude the presence of gait disturbances in our sample. The mean CA was also rather low (CA = 0.19 ± 0.24) as compared to previous studies on elderly participants (see for instance, Allali et al. [2014]) indicating good motor imagery abilities in our sample.
ANOVA #1. Virtual Walking Analysis
Motor component of Virtual Walking
Activations for the Virtual Walking task (overall main effect of the task, 1 1 1 1 contrast) are reported in Table 1 (Fig. 2a). Overall, the task recruited a widespread posterior network including the inferior parietal lobule and precuneus, the superior and middle occipital gyri, and the superior temporal gyrus, in addition to the right inferior frontal orbital gyrus, thalamus, putamen, and cerebellum, as expected, given the complexity intrinsic to virtual walking stimuli. However, when we controlled for visual stimulation by calculating the related contrast (visual&motor > visual), the results showed more frankly motor‐related activations in a bilateral fronto‐temporo‐parietal and cerebellar network, including the inferior, superior and middle frontal cortices, the SMA, the superior parietal and supramarginal gyri, and the superior and middle temporal gyri (mainly on the left). The large cerebellar cluster included activations in the anterior vermis, paravermal cerebellar cortices, and lateral cerebellar lobules, and extended into the brainstem via the cerebellar peduncle (Fig. 2b). Significant voxels that emerged from the contrast are reported in Table 2. Figure 2b also shows the qualitative overlap between the significant activations that emerged from the analysis of the motor component of the Virtual Walking task and the significant activations associated with execution of foot movements (see below and Table 3).
Table 1.
Main effect of the Virtual Walking task (Virtual Walking analysis, contrast 1 1 1 1)
| Left Hemisphere | Right Hemisphere | |||||||
|---|---|---|---|---|---|---|---|---|
| Brain area (BA) | X | Y | Z | Z‐score | X | Y | Z | Z‐score |
| Frontal lobe | ||||||||
| Inf. frontal orb. gyrus (47) | 30 | 26 | −4 | 4.2 | ||||
| 34 | 26 | −6 | 4.1 | |||||
| Parietal lobe | ||||||||
| Inf. parietal lobule (7) | 26 | −48 | 52 | >8a,b | ||||
| Occipital lobe | ||||||||
| Sup. occipital gyrus (19) | −20 | −76 | 36 | >8a,b | 20 | −86 | 22 | >8a,b |
| 24 | −80 | 30 | >8a,b | |||||
| Sup. occipital gyrus (18) | −18 | −88 | 22 | >8a,b | ||||
| Mid. occipital gyrus (18) | −20 | −88 | 0 | >8a,b | 26 | −86 | 12 | >8a,b |
| Mid. occipital gyrus (19) | −42 | −74 | 2 | >8a,b | ||||
| Mid. occipital gyrus (39) | 40 | −76 | 20 | >8a,b | ||||
| Precuneus | 12 | −42 | 52 | >8a,b | ||||
| Precuneus (5) | −14 | −58 | 60 | >8a,b | 12 | −54 | 58 | >8a,b |
| Cuneus (19) | 18 | −80 | 42 | >8a,b | ||||
| Lingual gyrus (18) | −14 | −76 | −8 | >8a,b | 10 | −70 | −2 | >8a,b |
| Temporal lobe | ||||||||
| Sup. temporal gyrus (41) | −46 | −34 | 18 | 4.3b | ||||
| Sup. temporal gyrus (42) | −62 | −32 | 18 | 3.4b | 62 | −36 | 20 | 4.7a,b |
| −66 | −30 | 18 | 3.2b | 60 | −36 | 16 | 4.7a,b | |
| Mid. temporal gyrus (37) | 46 | −64 | 4 | >8a,b | ||||
| Subcortical structures | ||||||||
| Putamen | −26 | −10 | 12 | 4.0b | 28 | −12 | 10 | 4.3b |
| −26 | −4 | 20 | 3.6b | 24 | 8 | 8 | 3.6b | |
| 26 | −2 | 12 | 3.5b | |||||
| Thalamus | −24 | −30 | 6 | 4.2b | 20 | −12 | 8 | 3.9b |
| −12 | −18 | 6 | 3.8b | 18 | −12 | 4 | 3.7b | |
| −18 | −10 | 2 | 3.6b | 8 | −16 | 2 | 3.2 | |
| Cerebellum and brainstem | ||||||||
| Cerebellum – IX lobule | −16 | −44 | −50 | 5.5a | ||||
| Brainstem | −8 | −26 | −6 | 3.6b | ||||
x, y, and z are the stereotactic coordinates of the activations in the Montreal Neurological Institute (MNI) space.
Statistical threshold P < 0.001uncorr. Only voxels included in significant clusters are reported.
Z‐scores statistically significant also after family‐wise error (FWE) correction.
Voxels included in clusters surviving the FWE correction at the cluster level.
To improve readability, in each structure, a maximum of three stereotactic coordinates per hemisphere are reported.
Table 2.
Motor component of the Virtual Walking task, that is, “visual&motor > visual” contrast (Virtual Walking analysis, contrast 1 1 −1 −1)
| Left hemisphere | Right hemisphere | |||||||
|---|---|---|---|---|---|---|---|---|
| Brain area (BA) | X | Y | Z | Z‐score | X | Y | Z | Z‐score |
| Frontal lobe | ||||||||
| SMA (6) | −4 | −4 | 66 | 5.5ab | 6 | −10 | 74 | 5.0ab |
| −2 | 8 | 56 | 4.1b | |||||
| −8 | 2 | 52 | 4.0b | |||||
| Precentral gyrus (6) | −32 | −8 | 52 | 4.7ab | ||||
| Precentral gyrus (4) | 12 | −24 | 78 | 4.3b | ||||
| Paracentral lobule (4) | −4 | −24 | 60 | 5.2ab | 2 | −42 | 68 | 4.5b |
| −8 | −30 | 76 | 4.3b | |||||
| Sup. frontal gyrus (6) | −22 | −12 | 54 | 5.2ab | 22 | −10 | 56 | 5.7ab |
| −28 | 0 | 68 | 4.2b | |||||
| Mid. frontal gyrus (46) | −34 | 38 | 34 | 4.0 | ||||
| −36 | 40 | 24 | 3.6 | |||||
| −30 | 38 | 28 | 3.4 | |||||
| Middle frontal gyrus/insula | −30 | 30 | 16 | 4.4 | ||||
| Inf. frontal orb. gyrus (38) | −36 | 22 | −14 | 3.5 | 46 | 18 | −12 | 3.2b |
| Inf. frontal op. gyrus | −58 | 14 | 2 | 4.0 | 56 | 14 | 0 | 5.4ab |
| Insula | −30 | 20 | −12 | 3.5 | ||||
| Parietal lobe | ||||||||
| Postcentral gyrus (1/3) (area S1) | −28 | −40 | 70 | 4.6b | 32 | −28 | 44 | 4.1b |
| 32 | −36 | 68 | 3.2b | |||||
| Rolandic operculum (area S2) | 54 | −26 | 24 | 4.4b | ||||
| Postcentral/supramarginal gyrus (3/40) | 34 | −32 | 42 | 4.2b | ||||
| Supramarginal gyrus (40) | −48 | −44 | 28 | 4.2b | 36 | −34 | 38 | 4.1b |
| 66 | −24 | 20 | 3.6b | |||||
| Sup. parietal lobule (40) | 40 | −44 | 62 | 4.2b | ||||
| Precuneus (5) | −8 | −40 | 66 | 4.8ab | 12 | −52 | 70 | 4.1b |
| Temporal lobe | ||||||||
| Sup. temporal gyrus (42) | −54 | −24 | 14 | 4.7a | ||||
| −50 | −44 | 18 | 4.1b | |||||
| Temporal pole (38) | −44 | 20 | −16 | 3.8 | ||||
| −54 | 16 | −8 | 3.5 | |||||
| Mid. temporal gyrus (41/37/21) | −38 | −50 | 6 | 4.2b | ||||
| −42 | −46 | 16 | 3.8b | |||||
| −46 | −42 | 12 | 3.4 | |||||
| Subcortical structures | ||||||||
| Caudate | 8 | 12 | −2 | 3.7 | ||||
| 12 | 14 | −2 | 3.7 | |||||
| Pallidum | 18 | 2 | 4 | 4.4 | ||||
| Thalamus | 18 | −10 | 0 | 4.0 | ||||
| 16 | −4 | 0 | 3.8 | |||||
| Cerebellum and brainstem | ||||||||
| Cerebellum – vermis | 0 | −48 | −10 | 5.5ab | ||||
| Cerebellum – III lobule | 16 | −32 | −24 | 6.4ab | ||||
| 10 | −42 | −18 | 4.4b | |||||
| Cerebellum – IV–V lobule | −16 | −34 | −24 | 6.2ab | ||||
| −4 | −46 | −14 | 5.6ab | |||||
| −24 | −40 | −28 | 5.0ab | |||||
| Cerebellum – VI lobule | −28 | −44 | −30 | 5.0ab | 28 | −48 | −30 | 5.5ab |
| −26 | −56 | −24 | 4.5b | |||||
| Cerebellum – VI lobule | −8 | −60 | −22 | 3.6b | ||||
| Cerebellum – IX lobule | −20 | −42 | −48 | 3.8b | ||||
| Cerebellum – X lobule | −20 | −34 | −40 | 3.7b | ||||
| Cerebellum/middle cerebellar peduncle | −22 | −38 | −36 | 3.7b | ||||
| Brainstem | 8 | −32 | −40 | 3.5b | ||||
| Corpus callosum | −14 | 32 | 2 | 4.7a | ||||
| Corpus callosum | −20 | 34 | 2 | 4.8a | ||||
x, y, and z are the stereotactic coordinates of the activations in the Montreal Neurological Institute (MNI) space.
Statistical threshold P < 0.001uncorr. Only voxels included in significant clusters are reported.
Z‐scores statistically significant also after family‐wise error (FWE) correction.
Voxels included in clusters surviving the FWE correction at the cluster level.
To improve readability, for each structure, a maximum of three stereotactic coordinates per hemisphere are reported.
Table 3.
Main effect of Foot Movements (Foot Movement analysis, 1 1 1 1 contrast)
| Left hemisphere | Right hemisphere | |||||||
|---|---|---|---|---|---|---|---|---|
| Brain area (BA) | X | Y | Z | Z‐score | X | Y | Z | Z‐score |
| Frontal lobe | ||||||||
| Mid. cingulum (24) | −8 | 2 | 38 | 3.6b | ||||
| Mid. cingulum/SMA (24/6) | 6 | −2 | 48 | 4.4b | ||||
| SMA/paracentral lobule (6/4) | 8 | −18 | 64 | >8ab | ||||
| Paracentral lobule (4) | −6 | −20 | 68 | >8ab | 6 | −28 | 68 | >8ab |
| −6 | −30 | 68 | >8ab | |||||
| Parietal lobe | ||||||||
| Rolandic operculum | 52 | −26 | 20 | 3.8b | ||||
| 50 | −28 | 22 | 3.7b | |||||
| Temporal lobe | ||||||||
| Sup. temporal gyrus (42) | 56 | −24 | 16 | 3.8b | ||||
| Subcortical structures | ||||||||
| Putamen | 30 | −18 | 12 | 4.0 | ||||
| Thalamus | 22 | −16 | 16 | 3.4 | ||||
| Cerebellum | ||||||||
| Cerebellum – vermis | 0 | −46 | −12 | >8ab | ||||
| Cerebellum – IV–V lobule | −14 | −36 | −24 | >8ab | 18 | −36 | −24 | >8ab |
| Cerebellum – VI lobule | −30 | −46 | −28 | 3.7b | ||||
x, y, and z are the stereotactic coordinates of the activations in the Montreal Neurological Institute (MNI) space.
Statistical threshold P < 0.001uncorr. Only voxels included in significant clusters are reported.
Z‐scores statistically significant also after family‐wise error (FWE) correction.
Voxels included in clusters surviving the FWE correction at the cluster level.
The opposite contrast (visual > visual&motor) showed no significant activation (P < 0.001uncorr, even when no restriction at the cluster level was considered). This was further confirmed by the results of the Foot Movement analysis (see below) that showed that the activations associated with foot movements did not interact with the imagery task, as they were equal whether the participants imagined standing or walking. This also indicates that the “Walk(Mov+) > Stand(Mov+)” contrast images only inform about the visual processes involved in the Virtual Walking task, because motor activations are cancelled out in the “Walk(Mov+)” condition by the presence of foot movements in the “Stand(Mov+)” baseline (see Methods).
Motor component of Virtual Walking during imagery and mental imitation conditions
We then evaluated the effect of gait‐related motor activations (visual&motor > visual) separately for the Imagery and Mental Imitation conditions (Fig. 3). In the Imagery condition, the contrast showed significant activations in the right inferior frontal orbital gyrus, bilaterally in the insula, in the superior frontal gyrus and the SMA, and in the left paracentral lobule. Significant clusters were also shown in the left superior temporal pole, and in the right rolandic operculum and superior parietal lobule, thalamus, and pallidum; finally, the contrast showed activations in the vermal and paravermal cerebellum extending via the superior cerebellar peduncle bilaterally into the midbrain. Significant voxels are reported in Table 4A. The Imitation condition recruited a much narrower network, including the right precentral gyrus and paracentral lobule, the cerebellar vermis, and the left anterior cerebellar lobe (Table 4B). As a matter of fact, the conjunction analysis revealed common activations between the Imagery and Mental Imitation conditions only in the cerebellar vermis and the left anterior cerebellar lobe (Table 4C).
Figure 3.
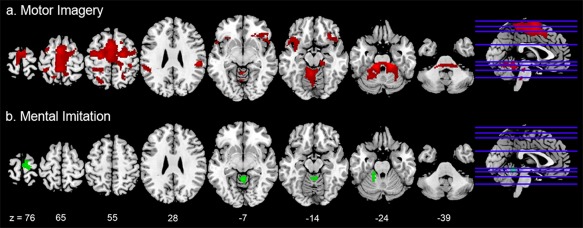
The figure shows the activations associated with (a) the motor components of Virtual Walking in the Imagery condition (“visual&motor > visual” contrast, red), and (b) the motor components of Virtual Walking in the Mental Imitation condition (“visual&motor > visual” contrast, green). The Mental Imitation condition recruited a more restricted motor network including the paracentral lobule, SMA, and cerebellum. A conjunction analysis also showed that the only areas equally recruited by the Imagery and the Mental Imitation conditions were two clusters in the cerebellar vermis and left hemisphere. The coordinates of the activation maps shown in this figure match those listed in Table 4. Data are reported at the same threshold discussed in the main text and reported in the tables (P < 0.001uncorr), and only significant clusters are shown. [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 4.
Motor components of the Virtual Walking task (Virtual Walking analysis)
| A. In the Imagery condition, i.e., “Imagery(visual&motor) > Imagery(visual)” contrast | ||||||||
|---|---|---|---|---|---|---|---|---|
| Left hemisphere | Right hemisphere | |||||||
| Brain area (BA) | X | Y | Z | Z‐score | X | Y | Z | Z‐score |
| Frontal lobe | ||||||||
| SMA (6) | −4 | −2 | 64 | 6.0ab | 4 | 4 | 58 | 5.0ab |
| −2 | 6 | 52 | 5.3ab | 8 | −22 | 62 | 4.8ab | |
| −6 | −18 | 58 | 5.3ab | |||||
| Precentral gyrus (6) | −32 | −10 | 52 | 5.1ab | ||||
| Paracentral lobule (4) | −4 | −26 | 62 | 5.0ab | ||||
| −4 | −32 | 64 | 4.8ab | |||||
| Sup. frontal gyrus (6) | −22 | −10 | 52 | 5.7ab | 22 | −10 | 56 | 6.1ab |
| Superior orb. gyrus | 20 | 30 | −10 | 4.0b | ||||
| Inf. frontal orb. gyrus (47) | 30 | 26 | −14 | 4.8ab | ||||
| 38 | 30 | −8 | 4.0b | |||||
| 50 | 30 | −10 | 3.7b | |||||
| Inf. frontal op. gyrus | 56 | 14 | 0 | 5.5ab | ||||
| Rolandic operculum | 58 | 2 | 8 | 3.9b | ||||
| 58 | 10 | 10 | 3.9b | |||||
| 48 | 6 | 12 | 3.2b | |||||
| Insula | −30 | 20 | −12 | 4.0b | 44 | 18 | −12 | 4.0b |
| −36 | 2 | −14 | 3.8b | |||||
| Parietal lobe | ||||||||
| Postcentral gyrus | 30 | −28 | 42 | 4.8ab | ||||
| Sup. parietal lobule (40) | 40 | −44 | 62 | 5.1ab | ||||
| Rolandic operculum (area S2) | 54 | −26 | 24 | 6.1ab | ||||
| Occipital lobe | ||||||||
| Precuneus | −2 | −38 | 64 | 5.1ab | ||||
| Temporal lobe | ||||||||
| Sup. temporal gyrus | −52 | −26 | 12 | 4.4 | ||||
| Temporal pole (38) | −44 | 20 | −16 | 4.6b | ||||
| −52 | 14 | −14 | 4.1b | |||||
| Subcortical structures | ||||||||
| Pallidum | 16 | 4 | 2 | 4.3 | ||||
| 14 | −2 | 0 | 4.1 | |||||
| 18 | 10 | 0 | 3.7 | |||||
| Thalamus | 16 | −10 | 4 | 3.7 | ||||
| 18 | −18 | 0 | 3.6 | |||||
| 18 | −16 | 4 | 3.4 | |||||
| Cerebellum and brainstem | ||||||||
| Cerebellum ‐ Vermis | 0 | −46 | −6 | 4.4b | ||||
| Cerebellum – III lobule | −12 | −34 | −24 | 6.5ab | 16 | −32 | −24 | 6.8ab |
| Cerebellum – IV‐V lobule | −6 | −50 | −16 | 5.8ab | 10 | −42 | −18 | 4.6b |
| Cerebellum – VI lobule | −28 | −44 | −30 | 4.8ab | 28 | −44 | −30 | 5.6ab |
| −26 | −54 | −26 | 4.8ab | 26 | −58 | −28 | 4.7ab | |
| Cerebellum – X lobule | −18 | −34 | −40 | 4.7ab | ||||
| Cerebellum – IX lobule | 16 | −48 | −52 | 4.5b | ||||
| 10 | −54 | −52 | 3.7b | |||||
| Cerebellum – VIII lobule | 20 | −46 | −50 | 4.1b | ||||
| 24 | −40 | −46 | 3.9b | |||||
| Brainstem | 2 | −26 | −24 | 4.4b | ||||
| 8 | −30 | −40 | 4.2b | |||||
| B. In the Mental Imitation condition, i.e., “Imitation(visual&motor) > Imitation(visual)” contrast | ||||||||
|---|---|---|---|---|---|---|---|---|
| Left hemisphere | Right hemisphere | |||||||
| Area (BA) | X | Y | Z | z‐score | X | Y | Z | z‐score |
| Frontal lobe | ||||||||
| Precentral gyrus (6/4) | 16 | −22 | 76 | 3.7 | ||||
| Paracentral lobule (4) | 10 | −20 | 76 | 3.6 | ||||
| Cerebellum | ||||||||
| Cerebellum ‐ vermis | 0 | −48 | −10 | 4.6 | ||||
| 2 | −56 | −10 | 3.6 | |||||
| Cerebellum – IV–V lobule | −18 | −44 | −26 | 4.1 | ||||
| −16 | −36 | −26 | 4.1 | |||||
| −24 | −40 | −28 | 3.8 | |||||
| C. In common between the Imagery and the Mental Imitation conditions, i.e., conjunction analysis “Imagery(visual&motor) > Imagery(visual)” ∩ “Imitation(visual&motor) > Imitation(visual)” | ||||||||
|---|---|---|---|---|---|---|---|---|
| Left hemisphere | Right hemisphere | |||||||
| Brain area (BA) | X | Y | Z | z‐score | X | Y | Z | z‐score |
| Cerebellum – vermis | −2 | −48 | −8 | 4.2 | 0 | −48 | −12 | 4.2 |
| Cerebellum – IV–V lobule | −16 | −36 | −26 | 4.1 | ||||
| −24 | −40 | −28 | 3.8 | |||||
| −18 | −50 | −24 | 3.4 | |||||
x, y, and z are the stereotactic coordinates of the activations in the Montreal Neurological Institute (MNI) space.
Statistical threshold P < 0.001uncorr. Only voxels included in significant clusters are reported.
Z‐scores statistically significant also after family‐wise error (FWE) correction.
Voxels included in clusters surviving the FWE correction at the cluster level.
To improve readability, for each structure, a maximum of three stereotactic coordinates per hemisphere are reported.
Interaction effects between the imagery and mental imitation conditions
The interaction effects between Task (Imagery vs Imitation) and Components (visual&motor vs visual) directly highlighted whether gait‐related motor activations (identified by the “visual&motor > visual” contrast) were modulated when the walker was present in the video during the Virtual Walking task. The analysis revealed that the motor component of the Imagery condition recruited significantly higher activations than the motor component of the Mental Imitation condition in a right parietal cluster extending from the parietal operculum to the supramarginal gyrus; moreover, it recruited a small cluster in the left exterior cerebellum and midbrain, in a region compatible with the human mesencephalic locomotor region (MLR, Jahn et al., 2008b; see Fig. 4 and Table 5). Although these effects do not survive a formal FWE voxel level correction, their p‐values were well below the conventional P = 0.001 threshold (P = 0.00001 for the supramarginal gyrus and P = 0.00007 for the MLR).
Figure 4.
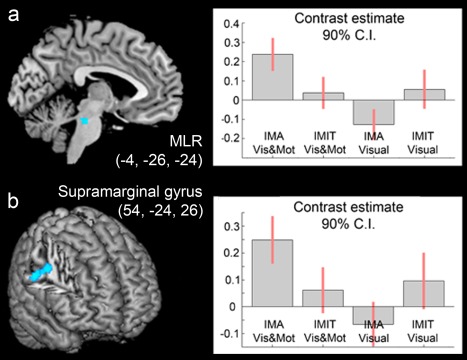
The figure shows the results that emerged from the interaction analysis (“Imagery > Mental Imitation” × “visual&motor > visual”). As shown by the plot of the parameter estimate, both local maxima in the (a) brainstem and (b) supramarginal gyrus were more active in the visual&motor condition during the Imagery than the Mental Imitation task. The coordinates of the activation maps shown in this figure match those listed in Table 5. Data are reported at the same threshold discussed in the main text and reported in the tables (P < 0.001uncorr), and only significant clusters are shown. [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 5.
Interaction analysis: (a) “Imagery(visual&motor) > Imagery(visual)” > “Imitation(visual&motor) > Imitation(visual)” contrast; (b) “Imitation(visual&motor) > Imitation(visual)” > “Imagery(visual&motor) > Imagery(visual)” contrast. Virtual Walking analysis
| Left hemisphere | Right hemisphere | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cluster size | Brain area (BA) | X | Y | Z | Z‐score | X | Y | Z | Z‐score |
| A. Imagery > Imitation | |||||||||
| N = 136 | Supramarginal gyrus | 54 | −24 | 26 | 4.3 | ||||
| Postcentral gyrus | 42 | −16 | 30 | 3.8 | |||||
| 38 | −20 | 36 | 3.2 | ||||||
| N = 92 | Cerebellum – III lobule | −10 | −36 | −20 | 3.5 | ||||
| Cerebellum – IV–V lobule | −14 | −38 | −18 | 3.3 | |||||
| Brainstem | −4 | −26 | −24 | 3.8 | 2 | −26 | −24 | 3.6 | |
| −6 | −32 | −24 | 3.7 | 6 | −24 | −22 | 3.1 | ||
| −8 | −28 | −24 | 3.7 | ||||||
| B. Imitation > Imagery | |||||||||
| No significant results | |||||||||
x, y, and z are the stereotactic coordinates of the activations in the Montreal Neurological Institute (MNI) space.
Statistical threshold P < 0.001uncorr. Only voxels included in significant clusters are reported.
Exploration of the interaction effect in the opposite direction, that is, the effect [Imitation(visual&motor) > Imitation(visual)] > [Imagery(visual&motor) > Imagery(visual)], showed no significant clusters.
Brainstem analysis of minimally smoothed data
Previous fMRI studies have revealed significant activations in the MLR during motor imagery of gait by applying a whole‐brain analysis like the present one [Jahn et al., 2008b; Snijders et al., 2011], and whole‐brain normalization procedures with SPM [Ashburner and Friston, 2005] have produced good results with regard to brainstem structures [Beissner et al., 2011]. These findings provide ground for reliable interpretation of the signals seen in the brainstem; however, in order to rule out the possibility that our brainstem results emerged merely as an artifact of the smoothing involved in standard spatial preprocessing, we performed an additional analysis on the brainstem data after a minimal amount of smoothing (2 × 2 × 2 mm, see Beissner [2015]). This second‐level full‐factorial ANOVA was explicitly masked with the brainstem mask proposed by Beissner et al. [2014]. The results from the interaction analysis ([Imagery(visual&motor) > Imagery(visual)] > [Imitation(visual&motor) > Imitation(visual)]) showed a local maximum (x = −4, y = −26, z = −22; z‐score = 3.30) in virtually the same location as that identified by the whole‐brain analysis (x = −4, y = −26, z = −24; Table 5) based on a 10 × 10 × 10 smoothing. This confirms the spatial specificity of our findings regarding activations in the brainstem, and suggests that a cluster compatible with the MLR was, indeed, more active during motor imagery of gait than during its mental imitation.
ANOVA #2. Foot Movement Analysis
Activations associated with the main effect of foot movements (overall main effect, 1 1 1 1 contrast) are reported in Table 3. Foot movements recruited the SMA and the paracentral lobule, the Rolandic operculum, the thalamus and putamen, and the cerebellum (Fig. 2b). Direct comparison between conditions when foot movements were associated with gait imagery versus imagery of standing (“FootMov_Walk > FootMov_Stand” contrasts) yielded no significant results when assessed as a main effect or tested separately in the Imagery and Mental Imitation conditions. The analysis of interaction effects gave no significant results.
DISCUSSION
The existence of a common motor representational space for motor imagery and action observation is a widely accepted notion [Jeannerod, 2001; Prinz, 1997]; however, there is no clear‐cut evidence for the degree of neurofunctional overlap between the two tasks with regard to gait behaviors. With this study, we wanted to directly compare the neural correlates of motor imagery (Imagery condition) and observation of gait behaviors (Mental Imitation condition) using similar visual scenarios that differed only for the presence/absence of a person in the video showing the to‐be‐imagined walking behaviors. We defined Mental Imitation the experimental condition that combined both motor imagery and action observation to highlight that the participants were explicitly instructed to “imagine imitating” the walker's behavior. Investigating whether the neural correlates of gait motor imagery are modulated by action observation allows to tease apart the ideal targets of rehabilitation protocols based on either the former or the latter process, and to eventually suggest which of the two processes might be more appropriate in different clinical situations. This study also provided evidence for the potentials of the Virtual Walking task for exploring the neural correlates of gait motor control in elderly participants and in clinical populations who tend to be older, for example, neurological and orthopedic patients.
We compared the neural correlates of motor imagery and mental imitation of gait elicited by naturalistic, in‐motion visual stimuli (Virtual Walking task, see also Iseki et al., 2008) in a sample of healthy elderly participants. We assessed (i) whether virtual walking stimuli, independent of the presence of a person shown in the video, engage the whole set of cortico‐subcortical brain regions responsible for gait control and (ii) the possible neurofunctional modulation elicited when the motor imagery task is combined with the observation of a person performing the to‐be‐imagined walking behaviors. As in 50% of the trials the participants also performed explicit ankle dorsiflexion while observing the—either stationary or in‐motion—clips, we were also able to evaluate the extent to which the motor network associated with gait imagery overlaps that associated with explicit lower limb movements.
With regard to the latter manipulation, it is worth noting that although ankle dorsiflexion represents only a proxy of explicit locomotion, adopted here because of the obvious constraints of the fMRI technique, motor activations during ankle dorsiflexion have face validity in that they correlate with clinical performance in locomotion tasks outside the scanner [Dobkin, 2004]. Our findings of the neural correlates of ankle dorsiflexion (see Foot Movement analysis) were strikingly similar to those measured with the 18F‐FDG technique in participants scanned after explicit walking performed during the accumulation of the radiotracer prior to actual scanning [La Fougère et al., 2010]. In line with previous work in this area, we considered the neural correlates of ankle dorsiflexion as a valid proxy for the neural correlates of execution of stepping movements, and compared them with the activations associated with motor imagery and mental imitation of gait.
Our results show that, overall, the neural resources recruited during gait imagery elicited by virtual walking stimuli comprise a wide cortico‐subcortical network: these activations were not restricted to the set of regions implicated in ankle dorsiflexion, which were limited to the motor cortices of the medial wall (SMA and paracentral lobule), the basal ganglia, and the cerebellum; rather, they also included lateral pre‐motor and dorsal parietal cortices (Fig. 2). Also, we found that motor imagery and mental imitation, although sharing similar cerebellar activations, differed in both quantitative and qualitative terms: motor imagery recruited a more extensive cortico‐subcortical network comprising two crucial clusters not involved in gait mental imitation, which were located in the right supramarginal gyrus and in the brainstem in a region compatible with the location of the MLR.
We will now discuss these results in light of previous evidence for normal and pathological control of locomotion to highlight their relevance for the development of neurologically sound gait rehabilitation strategies.
Visuo‐Motor Cortical Control of Gait Imagery
What happens in our brain when we walk along a path in an open space? Though the constraints of functional imaging preclude experiencing the proprioceptive and gravitational feedbacks typical of real locomotion while lying in the scanner tube, the main effect of Virtual Walking, as compared with imagery of standing on the spot, may in part answer this question, thanks to the combination of an explicit motor imagery task with the congruous observation of a dynamic scenario shown in the first‐person perspective. Under these circumstances, we found significant activations in a widespread fronto‐temporo‐parietal network, including, it is worth recalling, premotor and motor cortices responsible for motor control of the lower limbs.
The widespread temporo‐occipito‐parietal activations that emerged from the main effect of the task largely exceeded the effect seen for mere ankle dorsiflexion. This difference might have been due to the complexity of the visual stimulation elicited by the virtual walking dynamic scenarios. However, these activations are also related to locomotion control. Indeed, the temporo‐parietal and occipital brain regions, and their connections to the frontal lobe, are thought to be involved in the modulation of gait patterns in response to environmental cues to achieve a dynamic and efficient integration of body movements in the outside world. Consistent with this interpretation, clinical data show that parietal white‐matter lesions correlate with impaired mobility [Benson et al., 2002; Moscufo et al., 2012]. The “active” contribution of the posterior multisensory regions might be particularly relevant in the elderly [Zwergal et al., 2012] to scaffold motor performance and cope with age‐related sensory system decline (for similar interpretations in different contexts, see Heuninckx et al. [2005, 2008], Venkatraman et al. [2010], and Zapparoli et al. [2013, 2016]).
When we applied the appropriate comparisons controlling for the effect of visual stimulation (“visual&motor > visual” contrast), a frankly motor network emerged that included somatotopically specific activations in the sensorimotor cortices and cerebellum, as well as in the lateral premotor cortices, SMA, and brainstem, confirming that our Virtual Walking motor imagery tasks engaged both the cortical and the subcortical areas implicated in the motor control of locomotion [Fukuyama et al., 1997; La Fougère et al., 2010; Miyai et al., 2001]. The SMA has been described as being the pivotal node in this network. As such, it is responsible for planning gait behaviors and their voluntary modulation through the regulation of brainstem centers (i.e., the subthalamic locomotor region and MLR) via the basal ganglia [La Fougère et al., 2010; Takakusaki, 2013]. In keeping with this role of the SMA in motor control of gait, lesion of the SMA is associated with walking deficits, especially in gait initiation, in patients who do not exhibit any primary motor or sensory deficit (“gait apraxia,” Della Sala et al. [2002], see Liston et al. [2003] for a review). The main effect defined by the “visual&motor > visual” contrast also revealed the recruitment of prefrontal areas, in line with what has already been reported by previous studies on motor imagery of gait in elderly (see for instance, Allali et al. [2014]). This pronounced activation of prefrontal regions in elderly subjects has been discussed as evidence for an age‐related increase of the need for executive control to support gait behaviors later in life [Boisgontier et al., 2013; Ruffieux et al., 2015].
Comparison of this overall pattern with the one derived from the separate analysis that isolated the correlates of explicit ankle dorsiflexion, all other components being subtracted out (Foot Movement analysis), also permitted us to tease apart the areas involved in gait motor planning from those responsible for the mere execution of foot movements. Indeed, the activations associated with the Virtual Walking tasks were not restricted to the set of regions implicated in ankle dorsiflexion, which were limited to the motor cortices of the medial wall and to the cerebellum; rather, they also included lateral pre‐motor and dorsal parietal cortices (Fig. 2). This suggests a functional differentiation between the areas more strictly responsible for the control of (executed or imagined) foot movements and those associated with the integration of environmental information gathered from the dynamic visual stimuli into motor plans. For instance, the lateral PM cortex (involved in gait imagery but not in ankle dorsiflexion) is responsible for visually guided gait control. Lesion to this area results in an impaired ability to adapt gait to turns or navigate narrow passages [Nutt et al., 1993], with poor responses to external cues (see Nutt [2013] and Liston et al. [2003] for reviews). Interestingly, it also plays a role in mediating visually guided ameliorations of gait initiation in patients with Parkinson's disease [Hanakawa et al., 1999b]. Overall, our results suggest that the Virtual Walking task goes beyond the mere imagination of lower limb movements and engages participants in active processes involved in adaptive gait behaviors. The pattern of these results, which is in line with previous studies on gait motor imagery (see for instance, Jahn et al. [2004, 2008a, 2008b], Iseki et al. [2008], and La Fougère et al. [2010]), also provides indirect evidence on participants' effective engagement in the motor imagery tasks during the fMRI sessions.
Action Observation and “Internally Generated” Imagined Gait Patterns
Action observation evokes motor activations (see Caspers et al. [2010] for a review) in a somatotopically organized fashion [Buccino et al., 2001; Sakreida et al., 2005]. These effects have been associated with the recruitment of fronto‐parietal “mirror” neurons [Di Pellegrino et al., 1992; Rizzolatti et al., 1996] that discharge when an individual performs a hand action and when he/she observes the same action performed by another agent (see also Chong et al. [2008], Kilner et al. [2009], and Mukamel et al. [2010] for evidence in humans). Such mechanisms have been largely investigated in the context of upper‐limb actions that are apt to favor the development of visuo‐motor associations [Heyes, 2010; Keysers and Gazzola, 2014]. Evidence for the lower limbs is more limited and partly inconsistent, as action observation has been shown to lead to motor activations only when coupled with motor imagery [Villiger et al., 2013; Mouthon et al., 2015; but see Orr et al., 2008]. As discussed, here we wanted to investigate whether watching a person walk (coupled with the prompt to imagine imitating the person in the video) could modulate the neural correlates of an explicit motor imagery task.
Our results clearly show reduced recruitment of specific brain regions during mental imitation than during pure motor imagery, ruling out the additional contribution of a dedicated network for gait imitation independent of the one involved in motor imagery. This suggests that if a facilitatory effect of mental imitation exists, it takes the form of a reduced activation of specific areas. Moreover, the direct comparison between the motor components of Imagery and Mental Imitation (assessed as an interaction effect) highlighted stronger recruitment of two areas in the supramarginal gyrus and the brainstem during the former task. Although these results did not survive a formal FWE correction, they had P values well below the canonical 0.001 threshold (see Results); the results on brainstem activations were also confirmed by the analysis on minimally smoothed data, ruling out the possibility that they emerged merely as an artifact of the smoothing involved in standard spatial preprocessing.
As for the supramarginal gyrus, studies on motor imagery of lower and upper limb movements have interpreted activations in this area as the neural correlates of the analysis of virtual sensory signals forwarded by the SMA during the willed generation of virtual motor commands [Hanakawa et al., 2008; Lui et al., 2008; Sauvage et al., 2013]. Berneiser et al. [2016] found increased activation of the right supramarginal gyrus after motor imagery training, which they associated with the assumption of a more frankly motor strategy to solve an imagery task. The right supramarginal gyrus might be responsible for manipulating imagined sensorimotor information during the voluntary mental rehearsal of the motor act, a process that might not take place during mental imitation.
The brainstem activations were found in a cluster compatible with the location of brainstem centers responsible for internally generated gait initiation such as the MLR [Jahn et al., 2008b]. In vertebrates, the MLR acts as a pacemaker that regulates locomotion rhythms and velocity [Ryczo, 2013; Shik et al., 1966]. In humans, it is more active during more demanding tasks (e.g., gait initiation and termination) than during stable gait [La Fougère et al., 2010], and atrophy in this region has been associated with balance deficits in elderly people with higher level gait disorders [Demain et al., 2014]. The MLR is also hyperactivated during gait motor imagery in patients with Parkinson's disease (PD) showing gait freezing as compared to those without freezing. This difference has been interpreted as the result of a possible compensatory attempt made by PD patients with gait freezing to solve the imagery task [Snijders et al., 2011]. Finally, clinical studies have reported a restoration of gait after deep brain stimulation in the MLR [Stefani et al., 2007]. Overall, the MLR in humans might functionally represent a cross‐point for motor information from the basal ganglia and cerebellar loops [La Fougère et al., 2010; Takakusaki, 2013]. Its recruitment in the virtual walking motor imagery condition suggests that this task is effective in recruiting the neural machinery needed for the proper rehearsal of gait motor programs, from the premotor cortices down to the supraspinal centers responsible for internally generated gait patterns.
In line with previous studies on gait observation [Dalla Volta et al., 2015; Wang et al., 2008a,2008b], our mental imitation task, which combined explicit motor imagery with the observation of a person walking, also showed “motor” activations in the paracentral lobule, SMA, and cerebellum. However, the less extensive activation patterns as compared to pure motor imagery, coupled with evidence for a lack of activations in the right parietal and brainstem regions, indicate that mental imitation during gait observation is a “less motorically engaging” and a possibly predominantly visual task.
This could be interpreted in two ways: the presence of the person in the video may have led the participants to focus more on the person's behavior rather than on the imagery of walking at their own pace; alternatively, mental imitation of walking might be intrinsically less reliant on internally driven motor programs, not requiring as much activation of subcortical pacemakers. The person shown in the video might have served as an external trigger that generates activation of gait‐related motor programs at the cortical and cerebellar level, bypassing the subcortical nodes of the locomotor network. Although we have no empirical data to decide which of these two hypotheses is stronger, the latter is consistent with clinical observations. Indeed, gait rehabilitation training programs based on gait observation have proven effective in patients with locomotion impairments due to poor functioning of subcortical locomotion centers, e.g., in PD patients with gait freezing [Agosta et al., 2017; Pelosin et al., 2010] who have a dysfunction of specific brainstem regions (see Grabli et al. [2012] for a review). We suggest that this might be the case because patients might anchor to (memory of) the visual stimulus depicting a model's gait behaviour to overcome freezing episodes and trigger their own motor programs, bypassing the (dysfunctional) subcortical nodes responsible for internally generated gait patterns, such as the MLR. Evidence that PD patients recruit additional extrastriate visual resources as a compensatory strategy to solve a MI task [Helmich et al., 2007] seems in favor of this speculation. Although one might expect rehabilitation protocols to train precisely the system that is impaired (like, for instance, subcortical gait centres in patients with subcortical dysfunctions), an alternative approach, largely applied in neuropsychology, is to implicitly “teach” the patients unconventional strategies to solve the task when the brain structures subserving the primary strategy are no longer functioning (see, for instance, strategies to augment communicative skills in aphasic patients, Russo et al. [2017] and Beukelman et al. [2015]). We suggest that mental imitation might be considered one of such alternative strategies to improve gait control in neurological disorders.
Of course, this is a speculative hypothesis as yet that should be tested in future clinical studies on different pathological populations.
CONCLUSIONS
This study was motivated by the need to gain a better understanding of the physiology of motor imagery and action observation made explicit during Virtual Walking tasks. The findings may contribute to a finer neurophysiological interpretation of the impact of gait rehabilitation strategies. Elderly adults seem capable of taking advantage of virtual walking stimuli and recruit a widespread yet specific cortical and subcortical network responsible for gait control. Furthermore, the presence of a human model in the virtual walking scenarios makes the imagery task more visually guided and less internally driven, with less need for the activation of subcortical areas such as the MLR. While clinical studies indicate that action observation is successful in defreezing patients with difficulties in gait initiation and maintenance [Agosta et al., 2017; Pelosin et al., 2010], our data suggest that full‐blown gait imagery recruits the gait motor network more extensively. Thus, while gait observation may best aid gait rehabilitation in patients with impaired functioning of brainstem centers, motor imagery could be particularly beneficial in those presenting no neurological dysfunction in subcortical areas, for instance, in patients with a functional limitation of locomotion of peripheral origin, as in orthopedic patients. These hypotheses remain to be tested in future studies, which might include physiological measures to monitor patients' motor imagery performance in the scanner (see for instance, Ionta et al. [2010]) and examine age‐related changes in the neurofunctional effects.
CONFLICT OF INTEREST
The authors declare no conflict of interest that could have direct or potential influence on the work.
All procedures performed in the study were in accordance with the ethical standards of the institutional research committee and the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all participants included in the study.
Supporting information
Supporting Information
Supporting Information Video 1
Supporting Information Video 2
ACKNOWLEDGMENTS
The study was funded by the Italian Ministry of Health, Grant N. RF‐2011‐02350666 ‐ Can mental training based on motor imagery speed up the rehabilitation of walking? Efficacy of a controlled procedure and neurofunctional bases of recovery in patients with total knee arthroplasty.
Footnotes
Specific regressors were also defined for two classes of events of no interest: (i) presentation of the written prompts (“no foot movement” or “move your feet,” lasting 3 s each), and (ii) presentation of the questions about the videos and the time‐window to record participants' response (8 s in total per question).
The aim of our study was to identify and compare the brain regions that were specifically recruited to perform the motor Imagery task, or the Mental Imitation task, while controlling for the complexity of the visual stimulation used: indeed, it is worth noting that the visual stimulation used in the Imagery and Imitation stimuli differed because of the actor in the Imitation ones. To compare the motor components of our tasks controlling for differences in visual stimulation, we first isolated the motor components of each task by subtracting each “visual” condition from the related “visual&motor” condition.
REFERENCES
- Agosta F, Gatti R, Sarasso E, Volonté MA, Canu E, Meani A, Sarro L, Copetti M, Cattrysse E, Kerckhofs E, Comi G, Falini A, Filippi M (2017): Brain plasticity in Parkinson's disease with freezing of gait induced by action observation training. J Neurol 264:88–101. [DOI] [PubMed] [Google Scholar]
- Allali G, Van Der Meulen M, Beauchet O, Rieger SW, Vuilleumier P, Assal F (2014): The neural basis of age‐related changes in motor imagery of gait: An fMRI study. J Gerontol A Biol Sci Med Sci 69:1389–1398. [DOI] [PubMed] [Google Scholar]
- Appollonio I, Leone M, Isella V, Piamarta F, Consoli T, Villa ML, Forapani E, Russo A, Nichelli P (2005): The Frontal Assessment Battery (FAB): Normative values in an Italian population sample. Neurol Sci 26:108–116. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ (2005): Unified segmentation. NeuroImage 26:839–851. [DOI] [PubMed] [Google Scholar]
- Basso A, Capitani E, Laiacona M (1987): Raven's coloured progressive matrices: Normative values on 305 adult normal controls. Funct Neurol 2:189–194. [PubMed] [Google Scholar]
- Beauchet O, Annweiler C, Assal F, Bridenbaugh S, Herrmann FR, Kressig RW, Allali G (2010): Imagined Timed Up & Go test: A new tool to assess higher‐level gait and balance disorders in older adults?. J Neurol Sci 294:102–106. [DOI] [PubMed] [Google Scholar]
- Beissner F, Deichmann R, Baudrexel S (2011): fMRI of the brainstem using dual‐echo EPI. NeuroImage 55:1593–1599. [DOI] [PubMed] [Google Scholar]
- Beissner F, Schumann A, Brunn F, Eisenträger D, Bär KJ (2014): Advances in functional magnetic resonance imaging of the human brainstem. NeuroImage 86:91–98. [DOI] [PubMed] [Google Scholar]
- Beissner F (2015): Functional MRI of the brainstem: Common problems and their solutions. Clin Neuroradiol 25: 251–257. [DOI] [PubMed] [Google Scholar]
- Berneiser J, Jahn G, Grothe M, Lotze M (2016): From visual to motor strategies: Training in mental rotation of hands. NeuroImage pii:S1053–S8119. 30247–6 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Benson RR, Guttmann CR, Wei X, Warfield SK, Hall C, Schmidt JA, Kikinis R, Wolfson LI (2002): Older people with impaired mobility have specific loci of periventricular abnormality on MRI. Neurology 58:48–55. [DOI] [PubMed] [Google Scholar]
- Bellelli G, Buccino G, Bernardini B, Padovani A, Trabucchi M (2010): Action observation treatment improves recovery of postsurgical orthopedic patients: Evidence for a top‐down effect?. Arch Phys Med Rehabil 91:1489–1494. [DOI] [PubMed] [Google Scholar]
- Beukelman DR, Hux K, Dietz A, McKelvey M, Weissling K (2015): Using visual scene displays as communication support options for people with chronic, severe aphasia: A summary of AAC research and future research directions. Augment Altern Commun 31:234–245. [DOI] [PubMed] [Google Scholar]
- Bohannon RW (2006): Reference values for the timed up and go test: A descriptive meta‐analysis. J Geriatr Phys Ther 29:64–68. [DOI] [PubMed] [Google Scholar]
- Boisgontier MP, Beets IA, Duysens J, Nieuwboer A, Krampe RT, Swinnen SP (2013): Age‐related differences in attentional cost associated with postural dual tasks: Increased recruitment of generic cognitive resources in older adults. Neurosci Biobehav Rev 37:1824–1837. [DOI] [PubMed] [Google Scholar]
- Buccino G (2014): Action observation treatment: A novel tool in neurorehabilitation. Phil Trans R Soc B 369:20130185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buccino G, Binkofski F, Fink GR, Fadiga L, Fogassi L, Gallese V, Freund HJ (2001): Action observation activates premotor and parietal areas in a somatotopic manner: An fMRI study. Eur J Neurosci 13:400–404. [PubMed] [Google Scholar]
- Buccino G, Solodkin A, Small SL (2006): Functions of the mirror neuron system: Implications for neurorehabilitation. Cogn Behav Neurol 19:55–63. [DOI] [PubMed] [Google Scholar]
- Buekers M, Montagne G, de Rugy A, Laurent M (1999): The regulation of externally paced human locomotion in virtual reality. Neurosci Lett 275:71–174. [DOI] [PubMed] [Google Scholar]
- Caligiore D, Mustile M, Spalletta G, Baldassarre G (2017): Action observation and motor imagery for rehabilitation in Parkinson's disease: A systematic review and an integrative hypothesis. Neurosci Biobehav Rev 72:210–222. [DOI] [PubMed] [Google Scholar]
- Caspers S, Zilles K, Laird AR, Eickhoff SB (2010): ALE meta‐analysis of action observation and imitation in the human brain. NeuroImage 50:1148–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christakou A, Zervas Y, Lavallee D (2007): The adjunctive role of imagery on the functional rehabilitation of a grade II ankle sprain. Hum Mov Sci 26:141–154. [DOI] [PubMed] [Google Scholar]
- Chong TTJ, Cunnington R, Williams MA, Kanwisher N, Mattingley JB (2008): fMRI adaptation reveals mirror neurons in human inferior parietal cortex. Curr Biol 18:1576–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla Volta R, Fasano F, Cerasa A, Mangone G, Quattrone A, Buccino G (2015): Walking indoors, walking outdoors: An fMRI study. Front Psychol 6:1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Sala S, Francescani A, Spinnler H (2002): Gait apraxia after bilateral supplementary motor area lesion. J Neurol Neurosurg Psychiatry 72:77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demain A, Westby GW, Fernandez‐Vidal S, Karachi C, Bonneville F, Do MC, Delmaire C, Dormont D, Bardinet E, Agid Y, Chastan N, Welter ML (2014): High‐level gait and balance disorders in the elderly: A midbrain disease? J Neurology 261:196–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pellegrino G, Fadiga L, Fogassi L, Gallese V, Rizzolatti G (1992): Understanding motor events: A neurophysiological study. Exp Brain Res 91:176–180. [DOI] [PubMed] [Google Scholar]
- Di Rienzo F, Debarnot U, Daligault S, Saruco E, Delpuech C, Doyon J, Collet C, et al. (2016): Online and offline performance gains following motor imagery practice: A comprehensive review of behavioral and neuroimaging studies. Front Hum Neurosci 10:315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz V (2002): Do human bipeds use quadrupedal coordination?. Trends Neurosci 25:462–467. [DOI] [PubMed] [Google Scholar]
- Dietz V, Colombo G, Jensen L, Baumgartner L (1995): Locomotor capacity of spinal cord in paraplegic patients. Ann Neurol 37:574–582. [DOI] [PubMed] [Google Scholar]
- Dimitrijevic MR, Gerasimenko Y, Pinter MM (1998): Evidence for a spinal central pattern generator in humans. Ann N Y Acad Sci 860:360–376. [DOI] [PubMed] [Google Scholar]
- Dobkin BH (2004): Ankle dorsiflexion as an fMRI paradigm to assay motor control for walking during rehabilitation. NeuroImage 23:370–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici N, Ivanenko YP, Cappellini G, d'Avella A, Mondi V, Cicchese M, Fabiano A, Silei T, Di Paolo A, Giannini C, Poppele RE, Lacquaniti F (2011): Locomotor primitives in newborn babies and their development. Science 334:997–999. [DOI] [PubMed] [Google Scholar]
- Dubuc R (2009): Locomotor regions in the midbrain (MLR) and diencephalon (DLR) In: Binder MD, Hirokawa N, Windhorst U, editors. Encyclopedia of Neuroscience. Berlin Heidelberg: Springer‐Verlag GmbH; pp 2167–2171. [Google Scholar]
- Filimon F, Nelson JD, Hagler DJ, Sereno MI (2007): Human cortical representations for reaching: Mirror neurons for execution, observation, and imagery. NeuroImage 37:1315–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR (1975): “Mini‐mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198. [DOI] [PubMed] [Google Scholar]
- Forssberg H (1985): Ontogeny of human locomotor control I. Infant stepping, supported locomotion and transition to independent locomotion. Exp Brain Res 57:480–493. [DOI] [PubMed] [Google Scholar]
- Fukuyama H, Ouchi Y, Matsuzaki S, Nagahama Y, Yamauchi H, Ogawa M, Kimura J, Shibasaki H (1997): Brain functional activity during gait in normal subjects: A SPECT study. Neurosci Lett 228:183–186. [DOI] [PubMed] [Google Scholar]
- Fung J, Richards CL, Malouin F, McFadyen BJ, Lamontagne A (2006): A treadmill and motion coupled virtual reality system for gait training post‐stroke. Cyberpsychol Behav 9:157–162. [DOI] [PubMed] [Google Scholar]
- Grabli D, Karachi C, Welter ML, Lau B, Hirsch EC, Vidailhet M, François C (2012): Normal and pathological gait: What we learn from Parkinson's disease. J Neurol Neurosurg Psychiatry 83:979–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S (2006a): Biological pattern generation: The cellular and computational logic of networks in motion. Neuron 52:751–766. [DOI] [PubMed] [Google Scholar]
- Grillner S (2006b): Neuronal networks in motion from ion channels to behaviour. An R Acad Nac Med (Madr) 123:297–298. [PubMed] [Google Scholar]
- Grillner S, Zangger P (1979): On the central generation of locomotion in the low spinal cat. Exp Brain Res 34:241–261. [DOI] [PubMed] [Google Scholar]
- Hanakawa T, Katsumi Y, Fukuyama H, Honda M, Hayashi T, Kimura J, Shibasaki H (1999a): Mechanisms underlying gait disturbance in Parkinson's disease: A single photon emission computed tomography study. Brain 122: 1271–1282. [DOI] [PubMed] [Google Scholar]
- Hanakawa T, Fukuyama H, Katsumi Y, Honda M, Shibasaki H (1999b): Enhanced lateral premotor activity during paradoxical gait in Parkinson's disease. Ann Neurol 45:329–336. [DOI] [PubMed] [Google Scholar]
- Hanakawa T, Dimyan MA, Hallett M (2008): Motor planning, imagery, and execution in the distributed motor network: A time‐course study with functional MRI. Cereb Cortex 18:2775–2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkema S, Gerasimenko Y, Hodes J, Burdick J, Angeli C, Chen Y, Ferreira C, Willhite A, Rejc E, Grossman RG, Edgerton VR (2011): Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: A case study. Lancet 377:1938–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hathout GM, Bhidayasiri R (2005): Midbrain ataxia: An introduction to the mesencephalic locomotor region and the pedunculopontine nucleus. AJR Am J Roentgenol 184:953–956. [DOI] [PubMed] [Google Scholar]
- Helmich RC, de Lange FP, Bloem BR, Toni I (2007): Cerebral compensation during motor imagery in Parkinson's disease. Neuropsychologia 45:2201–2215. [DOI] [PubMed] [Google Scholar]
- Hétu S, Grégoire M, Saimpont A, Coll MP, Eugène F, Michon PE, Jackson PL (2013): The neural network of motor imagery: An ALE meta‐analysis. Neurosci Biobehav Rev 37:930–949. [DOI] [PubMed] [Google Scholar]
- Heuninckx S, Wenderoth N, Debaere F, Peeters R, Swinnen SP (2005): Neural basis of aging: The penetration of cognition into action control. J Neurosci 25:6787–6796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuninckx S, Wenderoth N, Swinnen SP (2008): Systems neuroplasticity in the aging brain: Recruiting additional neural resources for successful motor performance in elderly persons. J Neurosci 28:91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyes C (2010): Where do mirror neurons come from? Neurosci Biobehav Rev 34:575–583. [DOI] [PubMed] [Google Scholar]
- Hollman JH, Brey RH, Robb RA, Bang TJ, Kaufman KR (2006): Spatiotemporal gait deviations in a virtual reality environment. Gait Posture 23:441–444. [DOI] [PubMed] [Google Scholar]
- Holmes G (1915): The Goulstonian lectures on spinal injuries of warfare: Delivered before the Royal College of Physicians of London. Br Med J 2:769–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Friston K (1998): Generalisability, random effects and population inference. NeuroImage 7:S754. [Google Scholar]
- Ionta S, Ferretti A, Merla A, Tartaro A, Romani GL (2010): Step‐by‐step: The effects of physical practice on the neural correlates of locomotion imagery revealed by fMRI. Hum Brain Mapp 31:694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac AR, Marks DF, Russell DG (1986): An instrument for assessing imagery of movement: The Vividness of Movement Imagery Questionnaire. J Mental Imag 10:23–30. [Google Scholar]
- Iseki K, Hanakawa T, Shinozaki J, Nankaku M, Fukuyama H (2008): Neural mechanisms involved in mental imagery and observation of gait. NeuroImage 41:1021–1031. [DOI] [PubMed] [Google Scholar]
- Jackson PL, Metzoff A, Decety J (2006): Neural circuits involved in imitation and perspective‐taking. NeuroImage 31:429–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn K, Deutschländer A, Stephan T, Strupp M, Wiesmann M, Brandt T (2004): Brain activation patterns during imagined stance and locomotion in functional magnetic resonance imaging. NeuroImage 22:1722–1731. [DOI] [PubMed] [Google Scholar]
- Jahn K, Deutschländer A, Stephan T, Kalla R, Hüfner K, Wagner J, Strupp M, Brandt T (2008a): Supraspinal locomotor control in quadrupeds and humans. Prog Brain Res 171:353–362. [DOI] [PubMed] [Google Scholar]
- Jahn K, Deutschländer A, Stephan T, Kalla R, Wiesmann M, Strupp M, Brandt T (2008b): Imaging human supraspinal locomotor centers in brainstem and cerebellum. NeuroImage 39:786–792. [DOI] [PubMed] [Google Scholar]
- Jeannerod M (2001): Neural simulation of action: A unifying mechanism for motor cognition. NeuroImage 14:S103–S109. [DOI] [PubMed] [Google Scholar]
- Jenkinson N, Nandi D, Muthusamy K, Ray NJ, Gregory R, Stein JF, Aziz TZ (2009): Anatomy, physiology, and pathophysiology of the pedunculopontine nucleus. Mov Disord 24:319–328. [DOI] [PubMed] [Google Scholar]
- Keysers C, Gazzola V (2014): Hebbian learning and predictive mirror neurons for actions, sensations and emotions. Phil Trans R Soc B 369:20130175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilner JM, Neal A, Weiskopf N, Friston KJ, Frith CD (2009): Evidence of mirror neurons in human inferior frontal gyrus. J Neurosci 29:10153–10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn RA, Macht MB (1949): Some manifestations of reflex activity in spinal man with particular reference to the occurrence of extensor spasm. Bull Johns Hopkins Hosp 84:43–75. [PubMed] [Google Scholar]
- La Fougère C, Zwergal A, Rominger A, Förster S, Fesl G, Dieterich M, Brandt T, Strupp M, Bartenstein P, Jahn K (2010): Real versus imagined locomotion: A [18F]‐FDG PET‐fMRI comparison. NeuroImage 50:1589–1598. [DOI] [PubMed] [Google Scholar]
- Liston R, Mickelborough J, Bene J, Tallis R (2003): A new classification of higher level gait disorders in patients with cerebral multi‐infarct states. Age Ageing 32:252–258. [DOI] [PubMed] [Google Scholar]
- Lui F, Buccino G, Duzzi D, Benuzzi F, Crisi G, Baraldi P, Nichelli P, Porro CA, Rizzolatti G (2008): Neural substrates for observing and imagining non‐object‐directed actions. Soc Neurosci 3:261–275. [DOI] [PubMed] [Google Scholar]
- Macuga KL, Frey SH (2012): Neural representations involved in observed, imagined, and imitated actions are dissociable and hierarchically organized. NeuroImage 59:2798–2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malouin F, Richards CL (2010): Mental practice for relearning locomotor skills. Phys Therapy 90:240–251. [DOI] [PubMed] [Google Scholar]
- Malouin F, Richards CL, Jackson PL, Dumas F, Doyon J (2003): Brain activations during motor imagery of locomotor‐related tasks: A PET study. Hum Brain Mapp 19:47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masdeu JC, Alampur U, Cavaliere R, Tavoulareas G (1994): Astasia and gait failure with damage of the pontomesencephalic locomotor region. Ann Neurol 35:619–621. [DOI] [PubMed] [Google Scholar]
- Miyai I, Tanabe HC, Sase I, Eda H, Oda I, Konishi I, Tsunazawa Y, Suzuki T, Yanagida T, Kubota K (2001): Cortical mapping of gait in humans: A near‐infrared spectroscopic topography study. NeuroImage 14:1186–1192. [DOI] [PubMed] [Google Scholar]
- Mori S, Matsui T, Kuze B, Asanome M, Nakajima K, Matsuyama K (1999): Stimulation of a restricted region in the midline cerebellar white matter evokes coordinated quadrupedal locomotion in the decerebrate cat. J Neurophysiol 82:290–300. [DOI] [PubMed] [Google Scholar]
- Moscufo N, Wolfson L, Meier D, Liguori M, Hildenbrand PG, Wakefield D, Schmidt JA, Pearlson GD, Guttmann CR (2012): Mobility decline in the elderly relates to lesion accrual in the splenium of the corpus callosum. Age (Dordr) 34:405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouthon A, Ruffieux J, Wälchli M, Keller M, Taube W (2015): Task‐dependent changes of corticospinal excitability during observation and motor imagery of balance tasks. Neuroscience 303:535–543. [DOI] [PubMed] [Google Scholar]
- Mukamel R, Ekstrom AD, Kaplan J, Iacoboni M, Fried I (2010): Single‐neuron responses in humans during execution and observation of actions. Curr Biol 20:750–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder T (2007): Motor imagery and action observation: Cognitive tools for rehabilitation. J Neural Transm (Vienna) 114:265–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novelli G, Papagno C, Capitani E, Laiacona N, Vallar G, Cappa SF (1986): Tre test clinici di ricerca e produzione lessicale. Taratura su soggetti normali. Archivio di psicologia neurologia e Psichiatria 47:477–506. [Google Scholar]
- Nutt JG, Marsden CD, Thompson PD (1993): Human walking and higher‐level gait disorders, particularly in the elderly. Neurology 43:268–279. [DOI] [PubMed] [Google Scholar]
- Nutt JG (2013): Higher‐level gait disorders: An open frontier. Mov Disord 28:1560–1565. [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9:97–113. [DOI] [PubMed] [Google Scholar]
- Orlovskii GN, Deliagina TG, Grillner S (1999): Neuronal Control of Locomotion: From Mollusc to Man. New York: Oxford University Press. [Google Scholar]
- Orr EL, Lacourse MG, Cohen MJ, Cramer SC (2008): Cortical activation during executed, imagined, and observed foot movements. Neuroreport 19:625–630. [DOI] [PubMed] [Google Scholar]
- Pelosin E, Avanzino L, Bove M, Stramesi P, Nieuwboer A, Abbruzzese G (2010): Action observation improves freezing of gait in patients with Parkinson's disease. Neurorehabil Neural Repair 24:746–752. [DOI] [PubMed] [Google Scholar]
- Penny W, Holmes AP (2004): Random‐effects analysis In: Frackowiak RJS, et al., editors. Human Brain Function. San Diego: Elsevier; pp 843–850. [Google Scholar]
- Plaha P, Gill SS (2005): Bilateral deep brain stimulation of the pedunculopontine nucleus for Parkinson's disease. Neuroreport 16:1883–1887. [DOI] [PubMed] [Google Scholar]
- Prinz W (1997): Perception and action planning. Eur J Cogn Psychol 9:129–154. [Google Scholar]
- Rizzolatti G, Fadiga L, Gallese V, Fogassi L (1996): Premotor cortex and the recognition of motor actions. Brain Res Cogn Brain Res 3:131–141. [DOI] [PubMed] [Google Scholar]
- Ruffieux J, Keller M, Lauber B, Taube W (2015): Changes in standing and walking performance under dual‐task conditions across the lifespan. Sports Med 45:1739–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffino C, Papaxanthis C, Lebon F (2017): Neural plasticity during motor learning with motor imagery practice: Review and perspectives. Neuroscience 341:61–78. [DOI] [PubMed] [Google Scholar]
- Russo MJ, Prodan V, Meda NN, Carcavallo L, Muracioli A, Sabe L, Bonamico L, Allegri RF, Olmos L (2017): High‐technology augmentative communication for adults with post‐stroke aphasia: A systematic review. Expert Rev Med Devices 14:355–370. [DOI] [PubMed] [Google Scholar]
- Ryczo D (2013): The multifunctional mesencephalic locomotor region. Curr Pharm Des 19:4448–4470. [DOI] [PubMed] [Google Scholar]
- Sakreida K, Schubotz RI, Wolfensteller U, von Cramon DY (2005): Motion class dependency in observers' motor areas revealed by functional magnetic resonance imaging. J Neurosci 25:1335–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauvage C, Jissendi P, Seignan S, Manto M, Habas C (2013): Brain areas involved in the control of speed during a motor sequence of the foot: Real movement versus mental imagery. J Neuroradiol 40:267–280. [DOI] [PubMed] [Google Scholar]
- Shik ML, Orlovsky GN (1976): Neurophysiology of locomotor automatism. Physiol Rev 56:465–501. [DOI] [PubMed] [Google Scholar]
- Shik ML, Severin FV, Orlovsky GN (1966): Control of walking and running by means of stimulation of the mid‐brain. Biophysics 11:756–765. [Google Scholar]
- Small SL, Buccino G, Solodkin A (2013): Brain repair after stroke—a novel neurological model. Nat Rev Neurol 9:698–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijders AH, Leunissen I, Bakker M, Overeem S, Helmich RC, Bloem BR, Toni I (2011): Gait‐related cerebral alterations in patients with Parkinson's disease with freezing of gait. Brain 134:59–72. [DOI] [PubMed] [Google Scholar]
- Stefani A, Lozano AM, Peppe A, Stanzione P, Galati S, Tropepi D, Pierantozzi M, Brusa L, Scarnati E, Mazzone P (2007): Bilateral deep brain stimu‐ lation of the pedunculopontine and subthalamic nuclei in severe Parkinson's disease. Brain 13:1596–1607. [DOI] [PubMed] [Google Scholar]
- Takakusaki K (2013): Neurophysiology of gait: From the spinal cord to the frontal lobe. Mov Disord 28:1483–1491. [DOI] [PubMed] [Google Scholar]
- Venkatraman VK, Aizenstein H, Guralnik J, Newman AB, Glynn NW, Taylor C, Studenski S, Launer L, Pahor M, Williamson J, Rosano C (2010): Executive control function, brain activation and white matter hyperintensities in older adults. NeuroImage 49:3436–3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villiger M, Estévez N, Hepp‐Reymond MC, Kiper D, Kollias SS, Eng K, Hotz‐Boendermaker S (2013): Enhanced activation of motor execution networks using action observation combined with imagination of lower limb movements. PLoS One 8:e72403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Wai Y, Weng Y, Yu J, Wang J (2008a): The cortical modulation from the external cues during gait observation and imagination. Neurosci Lett 443:232–235. [DOI] [PubMed] [Google Scholar]
- Wang C, Wai Y, Kuo B, Yeh YY, Wang J (2008b): Cortical control of gait in healthy humans: An fMRI study. J Neural Transm (Vienna) 115:1149–1158. [DOI] [PubMed] [Google Scholar]
- Watanabe R, Higuchi T (2016): Behavioral advantages of the first‐person perspective model for imitation. Front Psychol 7:701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe R, Watanabe S, Kuruma H, Murakami Y, Seno A, Matsuda T (2011): Neural activation during imitation of movements presented from four different perspectives: A functional magnetic resonance imaging study. Neurosci Lett 503:100–104. [DOI] [PubMed] [Google Scholar]
- Weiss PH, Herzog J, Pötter‐Nerger MD, Falk D, Herzog H, Deuschl G, Volkmann J, Fink GR (2015): Subthalamic nucleus stimulation improves parkinsonian gait via brainstem locomotor centers. Mov Disord 30:1121–1125. [DOI] [PubMed] [Google Scholar]
- Whelan PJ (1996): Control of locomotion in the decerebrate cat. Prog Neurobiol 49:481–515. [DOI] [PubMed] [Google Scholar]
- Yang JF, Stephens MJ, Vishram R (1998): Infant stepping: A method to study the sensory control of human walking. J Physiol 507:927–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley K, Friston K (1995): Analysis of fMRI time‐series revisited— again. NeuroImage 2:173–181. [DOI] [PubMed] [Google Scholar]
- Zapparoli L, Invernizzi P, Gandola M, Verardi M, Berlingeri M, Sberna M, De Santis A, Zerbi A, Banfi G, Bottini G, Paulesu E (2013): Mental images across the adult lifespan: A behavioural and fMRI investigation of motor execution and motor imagery. Exp Brain Res 224:519–540. [DOI] [PubMed] [Google Scholar]
- Zapparoli L, Saetta G, De Santis C, Gandola M, Zerbi A, Banfi G, Paulesu E (2016): When I am (almost) 64: The effect of normal ageing on implicit motor imagery in young elderlies. Behav Brain Res 303:137–151. [DOI] [PubMed] [Google Scholar]
- Zwergal A, Linn J, Xiong G, Brandt T, Strupp M, Jahn K (2012): Aging of human supraspinal locomotor and postural control in fMRI. Neurobiol Aging 33:1073–1084. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information Video 1
Supporting Information Video 2


