Abstract
The default mode network (DMN) has been identified reliably during rest, as well as during the performance of tasks such as episodic retrieval and future imagining. It remains unclear why this network is engaged across these seemingly distinct conditions, though many hypotheses have been proposed to account for these effects. Prior to generating hypotheses explaining common DMN involvement, the degree of commonality in the DMN across these conditions, within individuals, must be statistically determined to test whether or not the DMN is truly a unitary network, equally engaged across rest, retrieval and future imagining. To provide such a test, we used comparable paradigms (self‐directed, uninterrupted thought of equal duration) across the three conditions (rest, retrieval, and future imagining) in a within‐participant design. We found lower than expected pattern similarity in DMN functional connectivity across the three conditions. Similarity in connectivity accounted for only 40–50% of the total variance. Partial Least Squares (PLS) analyses revealed the medial temporal regions of the DMN were preferentially coupled with one another during episodic retrieval and future imagining, whereas the non‐medial temporal regions of the DMN (e.g., medial prefrontal cortex, lateral temporal cortex, and temporal pole) were preferentially coupled during rest. These results suggest that DMN connectivity may be more flexible than previously considered. Our findings are in line with emerging evidence that the DMN is not a static network engaged commonly across distinct cognitive processes, but is instead a dynamic system, topographically changing in relation to ongoing cognitive demands. Hum Brain Mapp 38:1155–1171, 2017. © 2016 Wiley Periodicals, Inc.
Keywords: episodic memory, future imagining, resting state, default mode network, functional connectivity
INTRODUCTION
Since its original description [Mazoyer et al., 2001; Raichle et al., 2001; Shulman et al., 1997], the default mode network (DMN) has been intensely investigated [for reviews, see Andrews‐Hanna et al., 2014b; Buckner et al., 2008; Buckner, 2012; Raichle and Snyder, 2007]. Originally, the DMN was considered to be a task‐negative network due to its reliable reduction in activity during task performance and robust functional connectivity during resting state scans [Raichle et al., 2001; Grady et al., 2010]. Recent work, however, has demonstrated reliable engagement of the DMN during the active performance of tasks, such as episodic memory retrieval and imagining future events [Benoit and Schacter, 2015; Kim, 2012; Spreng and Grady, 2010; Spreng and Grady, 2009], among others [see Mars et al., 2012; Schilbach et al., 2012]. This suggests that the DMN operates in a more complex manner, exhibiting positive or negative fluctuations of activity depending on the current task requirements [Andrews‐Hanna et al., 2014b; Spreng, 2012; Leech et al., 2014].
The involvement of the DMN in episodic memory retrieval, future imagining and undirected rest raises an important question: how does this seemingly unitary system functionally support phenomenologically separable cognitive conditions? The present manuscript addresses this question by directly comparing the similarities and differences in the functional coupling between regions of the DMN across these conditions, within subjects.
Two responses have been offered to our question. One is that the DMN supports specific component processes that underlie more complex cognitive functions, such as episodic retrieval and future imagining, thus accounting for the neural overlap between execution of these functions and activity of the DMN [Andrews‐Hanna et al., 2014b; Binder et al., 2009; Buckner and Carroll, 2007; Cabeza and Moscovitch, 2013; Hassabis et al., 2007; Schacter, et al., 2012]. There has been considerable work demonstrating the neural overlap between episodic retrieval and future imagining, often centered around regions of the hippocampus and medial temporal lobes [Addis et al., 2007; Schacter et al., 2012] which are also part of the DMN [Andrews‐Hanna et al., 2010; Campbell et al., 2013]. Some processes attributed to these regions include scene and event construction [Maguire and Mullally, 2013], detail generation [Rosenbaum et al., 2009], and binding of details into coherent representations, all of which are necessary regardless of whether in the context of the past or future [Schacter et al., 2012]. Furthermore, midline regions of the DMN, such as the posterior cingulate (PCC) cortex and medial prefrontal cortices, often associated with mentalizing and processing of self‐referential information [D'Argembeau et al., 2005; Murray et al., 2014; Foster et al., 2015; Qin and Northoff, 2011], are reliably reported during rest [Buckner and Carroll, 2007; Christoff et al., 2009; Foster et al., 2015], future imagining [Benoit and Schacter, 2015], episodic retrieval [Kim, 2012; Martinelli et al., 2013; Sajonz et al., 2010; Foster et al., 2015] and other contexts where there is a focus on internal mentation rather than on externally oriented sensation [Christoff et al., 2009; Van Calster et al., 2016; for review, see Dixon et al., 2014]. Presence of the DMN during rest could reflect the engagement of similar cognitive or component processes (e.g., scene construction or self‐related cognition) during mind wandering, which itself can entail thinking about events from the past, imagining events that will occur in the future or more generally constructing situation models [Binder et al., 1999; Binder et al., 2009; Buckner and Carroll, 2007; Gorgolewski et al., 2014; Mason et al., 2007; Ranganath and Ritchey, 2012; Schacter et al., 2012; Schilbach et al., 2008]. A recent meta‐analysis by Benoit and Schacter [2015] investigated the neural overlap between imagining future events and episodic memory in comparison to a previously defined DMN and arrived at a similar conclusion, suggesting that the spatial overlap was due to the recruitment of core construction processes underlying imagining, remembering and the contents of mind wandering (i.e., cognition during rest). This ‘component process’ account, suggesting that the comparable neural pattern depends primarily on the recruitment of overlapping component processes, has much empirical support and remains a prominent conception of DMN function.
A component process approach, however, cannot provide a complete account of the current experimental data. DMN connectivity has been reported during anaesthesia when component processes deemed central to DMN activity cannot be engaged [Greicius et al., 2008; Vincent et al., 2007]. Similarly, connectivity within the DMN, specifically its posterior components, does not differ significantly between deep sleep and wakefulness [Horovitz et al., 2009]. Therefore, it is unlikely that the overlap of component cognitive processes alone can fully account for the neural similarity across episodic retrieval, future imagining and undirected rest.
As an alternative, or complement, to the component process account, investigators proposed that task‐unrelated intrinsic neurophysiological connectivity, which is relatively better measured in functional connectivity during states such as anaesthesia than during wakefulness [Barttfeld et al., 2014], may largely account for the observed functional homogeneity of the DMN. Regions of the DMN exhibit especially high consistency between functional and structural connectivity [Ghosh et al., 2008; Greicius et al., 2009; Hagmann et al., 2008; Horn et al., 2014], and the core nodes of the DMN show strong functional connectivity with many other intrinsic brain networks [Braga et al., 2013; Braga and Leech, 2015; de Pasquale et al., 2012; cf. Mattar et al., 2015]. This kind of connectivity profile may provide the necessary neural architecture for complex cognitive processes that require the integration of many different kinds of information across many distinct functional networks, such as episodic retrieval, future imagining, and mind wandering. This “intrinsic connectivity” driven account of inter‐task DMN overlap [Raichle and Snyder, 2007] is, therefore, complementary to the component process model.
The above two accounts, however intriguing, are predicated on the assumption that anatomically comparable parts of the DMN are engaged across these three processes, yet this assumption has not been rigorously tested. Meta‐analyses have provided support for common recruitment of the resting DMN during episodic retrieval [Andrews‐Hanna et al., 2014a; Kim, 2012] and future imagining [Spreng et al., 2009]. Neural overlap derived from these kinds of meta‐analyses, however, may not truly reflect within‐subject commonality, as the averaged neural patterns associated with each cognitive process of interest are often derived from separate samples. Few studies have explored DMN connectivity within individuals, specifically pertaining to its presence during retrieval, future imagining and rest, despite the prevalence of theoretical speculations about their neural overlap. To account for this, a recent meta‐analysis by Benoit and Schacter [2015] only included data from participants in which episodic retrieval and future imagining (referred to as episodic simulation) were both collected. Regions associated with both imagining future events and the retrieval of episodic memories were highly comparable within‐subjects and largely fell within the boundaries of the DMN as defined by Yeo et al. [2011].
Although the overlap is impressive, closer inspection indicates the presence of voxels in the medial temporal lobes and near the retrosplenial cortex during both imagining and episodic retrieval, but not for the resting DMN. This would suggest a spatial distinction between regions involved in episodic processes and the canonical DMN at rest [also, see Dastjerdi et al., 2011]. This discrepancy could emerge due to a meaningful difference in the processing capacity of these regions, or due to a fundamental difference in how measurements are taken during rest relative to episodic retrieval and imagining. For example, experimental studies examining the DMN during rest use very different paradigms from studies examining retrieval or future imagining. Resting state blocks are largely unconstrained, requiring the participants to keep their eyes‐closed or fixated on a stimulus, such as a “+,” for periods lasting minutes. Retrieval and future imagining tasks are often more constrained, comprised of cued trials where a word or picture is presented and the participant must remember or imagine an event associated with it [for review, see Schacter et al., 2012], an activity that lasts seconds at most. Moreover, whereas stimulus presentation and retrieval are determined entirely by the experimenter, no such control is exerted in the rest condition. Better equating of task structure across retrieval and future imagining tasks with rest is a necessary step to obtain a more accurate comparison of the DMN across conditions.
The present study contributes to this line of inquiry by explicitly assessing the similarities and differences between the functional connectivity of the DMN across episodic retrieval, future imagining and undirected rest using a within‐subject design in which the time spent in each of these activities (conditions) is equated, and memory retrieval and future imagining are left almost entirely to the discretion of the participant rather than determined by the investigator. Instead of characterizing the similarities across condition by spatial overlap, we do so more precisely by using a pattern similarity analysis on DMN functional connectivity maps across the three conditions, whereas the differences were determined using a Partial Least Squares (PLS) multivariate analysis [McIntosh and Lobaugh, 2004]. Critically, we designed our rest, retrieval and future imagining conditions to be unconstrained and thus truly comparable within subjects. Finally, as future imagining and episodic retrieval processes rely on structures within the medial temporal lobes such as the hippocampus [for review, see Schacter et al., 2012], as well as other regions extending outside the canonical DMN [see Benoit and Schacter, 2015], it was important that we further probe the connectivity between the hippocampus and the rest of the brain across conditions. To do so, we performed a whole brain seed‐to‐voxel PLS analysis [McIntosh and Lobaugh, 2004].
METHODS
Participants
Twenty young adults (12 females), between 18 and 22 years of age (Mean = 21.3, SD = 1.9), were recruited from the University of Toronto's St. George campus. All were right‐handed, native English speakers, and free of any psychiatric or neurological conditions. The participants were paid $76 and gave their informed consent. The study was approved by the Research Ethics Board at Baycrest Health Sciences Centre (University of Toronto).
fMRI Task Procedure
After an anatomical MRI scan, participants first went through a standard 6‐minute resting scan in which they were asked to keep their eyes closed and remain still, relaxed, and awake. They were also allowed to think freely, without any explicit constraints. Then, participants performed an associative memory encoding task and face/house localizer task that were not relevant to the current study [for details, Liu, Grady & Moscovitch, 2016]. Next, participants went through two directed scans: the ThinkBack scan in which participants were asked to continually re‐experience past events for the entire 6 minutes, and the ThinkAhead scan in which they were asked to continually imagine future events for the entire 6 minutes. Stimulus‐free paradigms have been used previously to explore DMN connectivity across various task demands [Preminger et al., 2011], however they have not yet been used to compare episodic retrieval and future imagining against typical resting DMN connectivity. For both directed scans, participants were instructed similarly to keep eyes closed and remain still, relaxed, and awake. They were also asked to re‐experience past (in the ThinkBack condition) and imagine (in the ThinkAhead condition) future events as vividly as possible. After they finished remembering/imagining one event, they were instructed to continue to recall or imagine other events until the 6 minutes ended. Instructions were explained in detail prior to entering the scanner, in addition to a reminder at the beginning of each condition, to ensure full comprehension and compliance with the task instructions (for the instructions themselves, see Supporting Information). The order of the last two directed scans (i.e., ThinkBack and ThinkAhead) were counterbalanced across participants, but the first standard resting scan was always administered at the beginning of the experiment to avoid potential contaminations from other tasks.
Participants were also given two additional 6‐minute rests in between and after the memory encoding task runs. This reduced the potential carry‐over effects from the memory task to ThinkBack and ThinkAhead scans. Face/house localizer scans, also administered between the memory task and directed scans, were not demanding and contained stimuli such as faces, objects, and buildings that were already familiar to participants. As our initial rest run preceded the encoding and localizer blocks and our directed scans were performed afterwards, it remains possible that any differences in connectivity between them may be due to post‐encoding changes in functional connectivity. To confirm the current findings were not caused by post‐encoding changes in connectivity we also performed a control analysis using the resting scan that immediately followed the memory encoding task to replace the standard (i.e., the first) resting scan. This ensured that encoding preceded each of our conditions of interest, and thus any post‐encoding changes in functional connectivity should be present in all of our conditions. Our main findings were replicated in this control analysis, suggesting that encoding‐related changes in connectivity cannot explain our results (for details, see Supporting Information).
During each scanned task, the experimenter and MRI technologist carefully monitored each participant's online physiological signals (i.e., breathing and heart rate), body movement via MRI‐compatible camera, and lastly asked for general feedback after each run. These steps were taken to best ensure our participants' were awake and in compliance with our task instructions. A post‐experiment interview was conducted, during which we also asked participants to estimate how much time (in a percentage) they spent thinking about the future, remembering the past, or mind‐wandering per run. They were also asked to estimate the number of events they thought about during the directed scans, and how vivid and emotional the events were during all scans (7‐point scale). We did not find evidence that any participants had fallen asleep during the scans of interest, and none reported doing so.
Image Acquisition
Participants were tested using a Siemens Trio 3‐T scanner at the Rotman Research Institute at the Baycrest Centre for Geriatric Care. For the T1‐weighted structural scans a 3D MP‐RAGE pulse sequence was used (TR = 2000 ms, TE = 2.63 ms, flip angle = 9°, FOV = 25.6 cm2, 192 × 256 matrix, 160 slices of 1 mm thickness). Within a larger experimental procedure, three six‐minute runs of eyes‐closed resting scans were collected from each participant, using a T2‐weighted EPI pulse sequence (180 volumes, TR = 2000 ms, TE = 24 ms, flip angle = 70°, FOV = 20 cm2, 64 × 64 matrix, 30 slices of 3.5 mm thickness, gap = 0.5 mm). Images were acquired using an oblique orientation (30° clockwise to the anterior–posterior commissure axis) to correct for signal dropout observed in the ventral medial prefrontal cortex. E‐Prime software (v2, Psychology Software Tools, Inc.) was used to present the instructions for the functional runs, projected on a screen in the bore of the scanner viewed by the participants through a mirror mounted inside of the head coil. Cushions were placed in the head coil, comfortably stabilizing the participant's head and reducing head motion. Heart and breathing rates were also measured during the scanning session.
fMRI Data Preprocessing
Data were first preprocessed using the Statistical Parametric Mapping 8 (SPM8; Welcome Trust Center for Neuroimaging, University College London, UK; http://www.fil.ion.ucl.ac.uk/spm/, version 4661) in MATLAB (MathWorks, Natrick, MA). Specifically, after slice time correction using sync‐interpolation (with the midpoint slice as the reference), we performed a 6‐parameter rigid body motion correction through SPM realignment and coregistration procedure. Then, we normalized individuals' anatomical and functional images to MNI space using SPM8's two‐step procedure (tissue segmentation and normalization). Finally, functional images were spatially smoothed using an 8 mm Gaussian kernel and resampled at 2 mm × 2 mm × 2 mm resolution.
These spatially preprocessed images were then preprocessed temporally using the CONN Functional Connectivity Toolbox v.13 [Whitfield‐Gabrieli and Nieto‐Castanon, 2012]. First, signals from the white matter (WM) and cerebrospinal fluid (CSF) were used to regress out non‐specific variance from the fMRI time series using a principal component based noise correction method [Behzadi et al., 2007]. Five principal components were used for WM/CSF regressors. The six motion parameters obtained from the motion correction procedure were also used to regress out potential head motion effects. To reduce the impact of low frequency drift and high frequency physiological noise on the BOLD signal, the processed output was band‐pass filtered between 0.008 – 0.1 Hz. The data were then despiked using a hypobolic tangent function in CONN, reducing the impact of any potential outliers in the time‐series. This preprocessing pipeline was performed on the data from all three resting scans.
fMRI Data Analysis
ROI definition
Twenty widely used DMN nodes (10 in each hemisphere) were selected based on a previous study [Andrews‐Hanna et al., 2010]. To ensure the results were not biased by overrepresentation of nodes in one hemisphere, we made sure that all nodes were represented bilaterally. Any seed showing laterality was selected alongside its contralateral equivalent (i.e., we inverted the X coordinates to include the contralateral region; for a similar approach, see McCormick et al., 2014). This procedure produced 20 ROIs restricted to the DMN (for coordinates, see Table 1). Then, 8‐mm spheres surrounding these DMN nodes were used to produce ROI masks for functional connectivity analyses.
Table 1.
Regions of interest and MNI coordinates
| Region | Abbreviation | x | y | z | |
|---|---|---|---|---|---|
| Left | Hippocampal formation | lHF | −22 | −20 | −26 |
| Right | rHF | 22 | −20 | −26 | |
| Left | Parahippocampal cortex | lPHC | −28 | −40 | −12 |
| Right | rPHC | 28 | −40 | −12 | |
| Left | Retrosplenial cortex | lRsp | −14 | −52 | 8 |
| Right | rRsp | 14 | −52 | 8 | |
| Midline | Ventromedial prefrontal cortex | vmPFC | 0 | 26 | −18 |
| Midline | Dorsomedial prefrontal cortex | dmPFC | 0 | 52 | 26 |
| Left | Anterior medial prefrontal cortex | lamPFC | −6 | 52 | −2 |
| Right | ramPFC | 6 | 52 | −2 | |
| Left | Temporal pole | lTempP | −50 | 14 | −40 |
| Right | rTempP | 50 | 14 | −40 | |
| Left | Lateral temporal cortex | lLTC | −60 | −24 | −18 |
| Right | rLTC | 60 | −24 | −18 | |
| Left | Temporoparietal junction | lTPJ | −54 | −54 | 28 |
| Right | rTPJ | 54 | −54 | 28 | |
| Left | Posterior cingulate cortex | lPCC | −8 | −56 | 26 |
| Right | rPCC | 8 | −56 | 26 | |
| Left | Posterior inferior parietal lobule | lpIPL | −44 | −74 | 32 |
| Right | rpIPL | 44 | 74 | 32 |
Functional connectivity
Functional connectivity analyses were conducted using the CONN toolbox v.13 [Whitfield‐Gabrieli and Nieto‐Castanon, 2012] and custom Matlab scripts. First, BOLD timecourses were extracted from all voxels within an ROI and averaged to produce a mean timecourse associated with each ROI. Then Pearson correlations (r) among these ROI signals were calculated and transformed to Fisher's Z scores [0.5 * (ln(r + 1)/ln(r − 1))], which produced a DMN connectivity matrix for each participant. In total, there were 190 unique connectivity values among the 20 ROIs [20 × (20‐1)/2]. Seed‐to‐voxel functional connectivity was also calculated for bilateral PCC seeds. As the PCC is known to be a common seed region used to capture DMN connectivity, we used this analysis only to provide descriptive evidence of the visual similarity in DMN connectivity across Rest, ThinkBack and ThinkAhead. Using CONN toolbox, BOLD timecourses were extracted from all voxels within the bilateral PCC ROIs and averaged to produce a mean timecourse associated with the PCC, which was then correlated with the individual timecourses of all other voxels in the brain (P < 0.001, uncorrected). Results were visualized using BrainNet Viewer [Xia et al., 2013, http://www.nitrc.org/projects/bnv/).
Similarities
To investigate the pattern similarity of DMN connectivity across Rest, ThinkBack and ThinkAhead, we first vectorized the DMN connectivity matrix obtained using the above‐mentioned method and then calculated Pearson correlation r between DMN connectivity patterns (i.e., vectors) of different conditions within subjects. This produced three correlation coefficients (rs) for each subject (i.e., between: Rest‐ThinkBack, Rest‐ThinkAhead, and ThinkBack‐ThinkAhead). We chose to derive these correlation coefficients within subjects first, prior to averaging across subjects, to ensure that any similarities we observed were in fact present in our individual subjects rather than derived only from a group average (for discussion of differences between within‐subject vs. group‐averaged approaches, see Supporting Information; and Roberts, Hach, Tippett & Addis, 2016]. These correlation coefficients were then averaged across subjects to quantify group‐level pattern similarity between the three conditions, since the square of this similarity measure (r) reflects proportion of shared variances between two conditions. Importantly, the resting condition was always performed prior to the two directed runs; thus one could expect the overall similarity between the directed runs to be higher in part due to temporal proximity (i.e., the directed runs were administered closer in time) or any other progressive change in our participants' cognitive states over time (e.g., fatigue). If temporal proximity was to influence our results, one would expect a generalized increased similarity between the directed conditions, with no particular spatial specificity. As our pattern similarity analysis produces a similarity estimate while collapsing over the entire DMN, it is susceptible to this confound and not optimal to explore differences across conditions. Thus, we focus on the averaged r 2 only, and do not interpret differences in similarity scores across conditions using this specific analysis. A more fine‐grained analysis, able to examine the specific regions of the DMN with functional connectivity patterns that reliably differentiate our task conditions, would have the spatial specificity necessary to circumvent this issue (e.g., Partial Least Squares).
Differences
Although the pattern similarity analysis mentioned above can, to some extent, reflect the dissimilarity of the DMN connectivity pattern between different conditions (e.g., 1 − r 2 reflecting unshared variance between different connectivity patterns), this test is not optimal for revealing the specific ROI‐ROI coupling patterns that reliably contribute to the overall condition‐related differences in functional connectivity. To this end, we conducted PLS analysis [McIntosh and Lobaugh, 2004; Krishnan et al., 2011]. PLS is a data‐driven multivariate analysis that uses singular value decomposition (SVD) to decompose the dot product of a task design contrast matrix and a brain data matrix. This produces latent variables (LVs) derived from both the task design contrast and brain data simultaneously, maximizing the amount of covariance accounted for across both datasets. We chose to use mean‐centered task PLS analysis to examine how connectivity between our DMN ROIs differed among the three conditions. Specifically, we identified the difference in connectivity values between the Rest, ThinkBack and ThinkAhead conditions that accounted for the most covariance with our measures of within‐subject DMN connectivity. A thorough technical description of the PLS method can be found elsewhere [see Krishnan et al., 2011]. An alternative approach consisted of running pairwise t‐tests for all possible ROI‐ROI correlations across conditions. This approach, however, would entail 190 t‐tests per condition comparison and, when considering corrections for multiple comparisons, the test would lack sensitivity. PLS is multivariate, such that all conditions are modelled simultaneously and thus, differences between them are conducted in one test, providing considerably more statistical power
Briefly, in this mean‐centered PLS analysis, we created a cross‐block covariance matrix between DMN correlations across conditions (i.e., 190 unique Fisher‐Z transformed Pearson correlation coefficients, derived from our cross‐correlations between 20 DMN ROIs) and the task design contrast (i.e., a dummy coded contrast matrix representing our conditions: Rest, ThinkBack and ThinkAhead). A SVD was then conducted on this combined matrix resulting in a set of LVs that identified how patterns of DMN connectivity varied across the conditions. Each LV accounted for a proportion of the covariance between conditions and DMN connectivity, with the first LV accounting for the largest part. LVs calculated using PLS are orthogonal to one another, and each consist of clearly dissociable positive and negative dimensions. In our analysis, because our brain data matrix was comprised of connectivity values, these dimensions reflect two distributions of functional connections (i.e., ROI‐ROI correlations) that covary with the task conditions in a separable way. The positive and negative attributions themselves are arbitrary and do not mean positive or negative functional connections. Instead, they indicate that the relationship between these distributions of functional connectivity and the task design matrix is separable via the initial SVD. To test whether the latent variables obtained from the PLS analysis were statistically significant, we used a permutation test in which the correspondence between task contrast matrix and brain data matrix was reshuffled 500 times to produce a null distribution for singular values. The probability of the original true singular values was calculated using this distribution. The LVs with P < 0.05 (no need of multiple testing correction, due to the single analytic step) were considered as statistically significant.
Furthermore, each functional connection has a weight, known as salience, which in this case was proportional to the covariance between functional connectivity and the task design contrast for each LV. To test stability of the contribution (or salience) of individual functional connections to each connectivity latent variable, we used bootstrapping resampling method in which participants were resampled 500 times with replacement to produce a null distribution of saliences. Then, bootstrap ratio (BSR) scores were calculated by dividing the salience value by the bootstrap standard error for each functional connection to reflect how robustly that ROI‐ROI connection contributed to the corresponding connectivity latent variable. In the current study, BSRs with a magnitude of >1.96 (equivalent to a P‐value of 0.05) were considered to be robust. This value may be lower than previous whole‐brain PLS analyses, although, in the present experiment, there are far fewer comparisons relative to the typical whole‐brain voxelwise datasets on which PLS normally would operate. As such, a less conservative threshold is warranted. Finally, to obtain summary measures of each participant's contribution to the significant LV, we multiplied the salience for each functional connection by its Fisher‐Z transformed correlation and then summed across all 190 connections to create a “brain score” for each participant, per condition. In PLS, the group‐averaged brain scores correspond to the condition contrast, reflecting how a specific LV of neural data differentiates the conditions. Following PLS conventions [Grady et al., 2010], we plot the differential neural patterns (i.e., LVs) using a warm‐cool color scheme (see Fig. 3A). In this type of plot, the warm coloured neural data (i.e., functional connections) show stronger connectivity in conditions with positive group‐averaged brain scores and cool coloured functional connections show stronger connectivity in conditions with negative group‐averaged brain scores. Confidence intervals (95% CIs) were also built around the group‐averaged brain scores using the bootstrap resampling procedure. Group‐averaged brain scores with 95% CIs were plotted in Figure 3B. All computations were conducted using the command line version of PLS software [McIntosh and Lobaugh, 2004].
Figure 3.
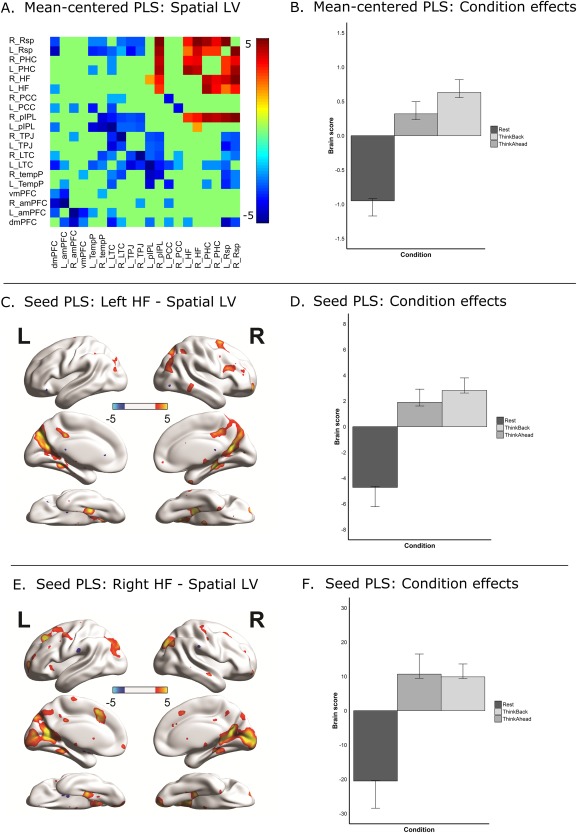
Results from Partial Least Squares (PLS) analyses. (A) Connectivity matrix depicting the spatial distribution of connectivity differences contributing to the significant LV in mean‐centered PLS (BSRs thresholded at z = 1.96, P = 0.05, two‐tailed). Warm coloured functional connections reflect significant greater coupling during ThinkBack and ThinkAhead (i.e., conditions with positive values in 3B). Cool coloured functional connections reflect significant greater coupling during Rest (i.e., conditions with negative values in 3B). (B) Bar graph presents the brain scores averaged across‐subjects per condition. Conditions with positive values are positively related with coupling amongst warm coloured functional connections in 3A. Conditions with negative values are positively related with coupling amongst cool coloured functional connections in 3A. (C) Spatial distribution of voxels showing difference in connectivity with the left hippocampal formation (HF) seed, from significant LV in seed PLS analysis (BSRs thresholded at z = 1.96, P = 0.05, two‐tailed). (D) Bar graph presents the brain scores averaged across‐subjects per condition for seed PLS analysis. Conditions with positive values are positively related with coupling between the left HF and warm coloured regions in 3C. Conditions with negative values are positively related with coupling between the left HF and cool coloured regions in 3C. (E) Spatial distribution of voxels showing difference in connectivity with the right HF seed, from significant LV in seed PLS analysis (BSRs thresholded at z = 1.96, P = 0.05, two‐tailed). (F) Bar graph presents the brain scores averaged across‐subjects per condition for seed PLS analysis. Conditions with positive values are positively related with coupling between the right HF and warm coloured regions in 3E. Conditions with negative values are positively related with coupling between the right HF and cool coloured regions in 3E. Note: All error bars in Figure 3 represent 95% confidence intervals. [Color figure can be viewed at http://wileyonlinelibrary.com]
Lastly, a seed‐PLS analysis was conducted [Krishnan et al., 2011; McIntosh and Lobaugh, 2004], where connectivity between our bilateral hippocampal seeds and all voxels in the brain was explored. Seed‐PLS is a similar procedure to the previously described mean‐centered PLS, but instead of entering our 190 DMN cross‐correlation values for each condition, we input the correlations between the timecourse of every voxel in the brain and the mean timecourse of our hippocampal seed (each hemisphere was analyzed separately) per condition. Results were visualized using BrainNet Viewer [Xia et al., 2013, http://www.nitrc.org/projects/bnv/).
RESULTS
Self‐Report Behaviour
To determine whether participants followed the task instructions, we calculated the mean percentage of time that participants reported thinking about the past, future, or mind wandering during all three scans (for means, see Fig. 1). Paired t‐tests showed that participants spent more time thinking about past events during ThinkBack than during Rest and the ThinkAhead condition [t(19) = 6.00 and 22.88, respectively, both P < 0.001], and spent more time thinking about future events in ThinkAhead than Rest and ThinkBack condition [t(19) = 21.11, and 11.99, respectively, both P < 0.001]. This suggests that our manipulation worked and the ThinkBack condition primarily reflects retrieval processes, ThinkAhead condition reflects future imagining, and Rest includes an amalgam of both, in addition to mind wandering and/or other types of unrelated thought.
Figure 1.

Percentage of time participants reported thinking about past events, imagining future events, or mind wandering during the ThinkBack, ThinkAhead, and Rest conditions.
To provide further description of thought content during our task, we asked our participants to rate their thought content for each scan, on both vividness and emotionality, using a 7‐point scale (1 = lowest, 7 = highest). Ratings were not collected for two of our participants, thus the following analyses are conducted on the remaining 18 participants. A one‐way ANOVA indicated a main effect of scan on vividness ratings [F(2, 54) = 5.13 P = 0.009, η2 = 0.16]. Vividness was highest during ThinkBack (M = 5.8, SD = 0.85), followed by ThinkAhead (M = 5.3, SD = 1.22) and Rest (M = 4.38, SD = 1.78). Bonferroni corrected post hoc comparisons indicated a significant difference in vividness ratings between Rest and ThinkBack (P = 0.008), but no significant differences between Rest and ThinkAhead (P = 0.115) or ThinkBack and ThinkAhead (P = 0.847). A one‐way ANOVA also indicated a main effect of scan on emotionality ratings [F(2, 54) = 6.79, P = 0.002, η2 = 0.2]. Emotionality was highest during ThinkBack (M = 5.0, SD = 1.4), followed by Rest (M = 3.8, SD = 1.74) and ThinkAhead (M = 3.2, SD = 1.5). Bonferroni corrected pairwise post hoc comparisons indicated a significant difference in emotionality ratings between ThinkBack and ThinkAhead (P = 0.002), but no significant differences between Rest and ThinkAhead (P = 0.641) or Rest and ThinkBack (P = 0.089). Lastly, we also asked our participants to estimate the number of events they thought of during the directed conditions. A paired t‐test revealed no significant difference between ThinkBack and ThinkAhead [t(16) = −2.049, P = 0.057], though ThinkAhead was associated with numerically more events on average (M = 9.7, SD = 6.9) than ThinkBack (M = 5.4, SD = 4.7).
Pattern Similarity
Before we present the results of our main analyses, we first show the whole‐brain functional connectivity of the PCC, a commonly used DMN seed, for each of the three conditions (P < 0.001, uncorrected). We did this to illustrate and confirm that the DMN would be present in each condition, at least in terms of visual comparison, as suggested by previous research. As can be seen in Figure 2A, all three conditions showed typical resting state connectivity patterns in that the PCC was connected with medial prefrontal cortex, medial and lateral temporal lobe regions, and medial and lateral parietal regions [Andrews‐Hanna et al., 2014a,b; Fox et al., 2005; Campbell et al., 2013; Raichle et al., 2001; Raichle and Snyder, 2007; Spreng et al., 2009; Yeo et al., 2011].
Figure 2.

(A) Posterior cingulate seed‐to‐voxel connectivity in the ThinkBack, ThinkAhead, and Rest conditions, depicting a typical DMN pattern in all three conditions (P <.001, uncorrected). Warm colored voxels were associated with significant positive correlations with the timecourse of our bilateral PCC seed. Cool colored voxels were associated with significant negative correlations. Only the lateral and medial surfaces of the left hemisphere are presented, though a similar pattern was present bilaterally. (B) Similarity in DMN functional connectivity (r 2) between ThinkBack, ThinkAhead, and Rest conditions. The color matrices depict the DMN connectivity (Fisher Z transformed) in each condition, for each subject separately. Similarity correlation coefficients were calculated per subject before their group average was computed. Abbreviations: Left hemisphere (L), right hemisphere (R); retrosplenial cortex (Rsp), parahippocampal cortex (PHC), hippocampal formation (HF), posterior cingulate cortex (PCC), posterior inferior parietal lobule (pIPL), temporoparietal junction (TPJ), lateral temporal cortex (LTC), temporal pole (TempP), ventromedial prefrontal cortex (vmPFC), anterior medial prefrontal cortex (amPFC), and dorsomedial prefrontal cortex (dmPFC). (C) Distribution of individual subject similarity coefficients (r) between conditions plotted as points around their respective averages, represented by bars. [Color figure can be viewed at http://wileyonlinelibrary.com]
Next, we focused on our main question: the similarity of the connectivity among the 20 DMN seeds for the three conditions. The within‐subject design of the current study allowed us to examine, at the individual level, the degree of similarity in functional connectivity across rest, retrieval and future imagining. The mean correlation among the three conditions ranged between 0.63 to 0.71, indicating that less than 50% of connectivity variance was shared between any two conditions (averaged r 2 = 0.45, or 45% of variance, see Fig. 2B). The individual correlations per subject, per condition, are presented in Figure 2C, demonstrating relatively comparable similarity estimates within our sample.
Connectivity Differences
Our PLS analysis revealed one significant LV (P < 0.002; explaining 83.11% of the summed squared covariance), depicting a data‐driven difference in connectivity patterns between the directed conditions (i.e., ThinkBack and ThinkAhead) and Rest. Brain scores, i.e., the specific group‐averaged brain connectivity pattern corresponding with the task contrast, are presented in Figure 3B. Figure 3A shows the corresponding connectivity difference pattern (BSRs thresholded at z = 1.96, P = 0.05, two‐tailed). In this figure, the warm coloured connectivity pattern corresponded to the positive contrast values (i.e., ThinkBack and ThinkAhead). The cool coloured connectivity pattern corresponded to the negative contrast values, (i.e., Rest). As can be seen from this figure, this analysis showed that the connectivity within medial temporal seeds of the DMN, such as the HF and PHC, was stronger in ThinkBack and ThinkAhead, compared to the Rest condition, whereas connectivity within non‐medial temporal seeds of the DMN, such as dorsomedial prefrontal cortex (dmPFC), amPFC, TempP and lateral temporal cortex (LTC), was stronger during Rest relative to the ThinkBack and ThinkAhead conditions. Despite both ThinkBack and ThinkAhead conditions contributing to the positive dimension of the LV, the 95% bootstrapped CIs are non‐overlapping, indicating that the ThinkBack condition contributes quantitatively more than ThinkAhead. It is important to emphasize that these results were all data‐driven, and not derived from a specified contrast.
Finally, as the hippocampus is a particularly critical structure for episodic retrieval and future imagining (for review, see Schacter et al., 2012], we explored whole brain seed‐to‐voxel connectivity with bilateral hippocampal seeds across Rest, ThinkBack and ThinkAhead. We conducted this analysis to examine how hippocampal connectivity with brain regions extending beyond the DMN differed across our three task conditions. Similar to the PLS analysis within the DMN, we found a significant connectivity difference pattern for both the left and right hippocampal connectivity (ps < 0.002) again reflecting the difference between the directed conditions (i.e., the ThinkBack and ThinkAhead) and Rest. Figure 3C–F illustrates both brain scores and hippocampal connectivity differences (BSR thresholded at 1.96, P = 0.05, two‐tailed). As can be seen from Figure 3C,E, the hippocampal ROI was connected more strongly with posterior medial and ventral temporo‐occipital regions, as well as lateral parietal and occipital regions, during the directed conditions. This result is consistent with the hypothesis that ThinkBack and ThinkAhead tasks involved episodic retrieval and construction processes to a larger extent than compared to Rest. Unlike in the mean centered analysis, no quantitative differences were observed between ThinkBack and ThinkAhead, as the 95% bootstrapped CIs were overlapping across conditions for both hippocampal seeds (see Fig. 3D,F).
DISCUSSION
The present study shows that despite the apparent similarity between the DMN as observed during rest, episodic retrieval, and future imagining (see Fig. 2A for PCC seed connectivity maps across conditions), there are substantial differences in functional connectivity across these three task states within the DMN and beyond. These differences are apparent despite our attempts to equate, as much as possible, the memory retrieval and future thinking conditions to the rest condition. Importantly, these differences have implications for theories regarding contributions of the DMN to these cognitive processes, and cognition more generally.
Firstly, when quantified, similarity in DMN connectivity accounted for less than half the total variance and was, therefore, not as comparable across these three processes as has been suggested in the literature [Buckner and Carroll, 2007; Hassabis et al., 2007; Schacter et al., 2012]. When we directly tested for differences across these conditions, DMN connectivity at rest was found to be highly separable from connectivity during episodic retrieval and future imagining, which did not show statistically separable connectivity patterns from one another in any subsequent LV. Specifically, we observed strong MTL connectivity during retrieval and future imagining relative to rest. Conversely, dorsomedial prefrontal cortex, lateral temporal cortex, temporal poles and other non‐MTL regions of the DMN were highly connected during rest relative to retrieval and future imagining. Probing specific seed‐to‐voxel connectivity patterns revealed further dissociations again between rest relative to both retrieval and imagining the future in the whole‐brain connectivity of the hippocampus, a critical hub related to episodic retrieval and future imagining,
Similarities Within the Default Mode Network
As rest, episodic retrieval and future imagining are separable at the phenomenological level [D'Argembeau and Van der Linden, 2004, 2006], one would also expect a difference in their neural substrates (for a discussion, see “functional neural isomorphism” in Moscovitch, Cabeza, Winocur & Nade, 2016). Previous work, however, has been heavily focused on the similarities rather than differences in the DMN across these conditions. The reliable presence and ostensibly striking overlap of the DMN during these disparate task conditions has been used as evidence for a common role of the DMN across all three conditions (e.g., component process and intrinsic connectivity accounts). This emphasis on commonality rather than differences is not only relevant to the study of the DMN, but may reflect a fundamental aspect of the brain's architecture, potentially affording the ability to support disparate cognitive processes with only minor metabolically efficient changes in functional network topology [Cole et al., 2014; Krienen et al., 2014]. For example, Cole et al. [2014] had participants perform a complex task in which variable instructions preceded target word pairs, and depending on the instructions specific to each trial, different cognitive processes (or “task states”) were required for successful performance. This clever design allowed them to correlate functional connectivity matrices for the multitask paradigm against functional connectivity during rest, where they found a correlation of r = 0.90. This high correlation value was used to support their hypothesis that a core architecture underlies functional connectivity across divergent task states and rest alike. Though the Cole et al. study did not investigate the DMN in isolation, the notion of an underlying architecture that supports processing in a variety of domains is an important tenet of “intrinsic connectivity”‐based explanations of why the DMN would be reliably engaged across rest, episodic retrieval and future imagining.
In the present experiment, we observed correlations ranging between 0.63 and 0.71, also supporting the idea that there are common aspects of the DMN supporting cognition during rest, episodic retrieval and future imagining (for a discussion of why our estimates are lower than those of Cole et al., 2014, see Supporting Information). Importantly, however, an averaged r 2 = 0.45 suggests that commonality in DMN connectivity across conditions only accounts for slightly less than half the total variance in connectivity. These data emphasize that the DMN cannot be considered to be a static network, unchanging in its connectivity during these different cognitive states, but instead a dynamic network that flexibly reorients its connectivity based on the current task demands around a common core connectivity structure [Andrews‐Hanna et al., 2014a,b; Bressler and McIntosh, 2007; Bressler and Menon, 2010; Dastjerdi et al., 2011; Leech et al., 2014; Robin et al., 2015; McIntosh, 2000; Spreng and Grady, 2010; Spreng et al., 2015], in accord with Cabeza and Moscovitch's [2013]; Moscovitch et al. [2016] proposal of Process Specific Alliances, to account for the shifting nodes of activation within a network.
Heterogeneity Within the Default Mode Network
Recent work has demonstrated that the DMN is not a unitary system, but is instead composed of subnetworks that are separable both on the basis on resting functional connectivity and the cognitive tasks that recruit them [Andrews‐Hanna et al., 2010, 2014a,b; Campbell et al., 2013; Laird et al., 2009; Leech et al., 2014; Robin et al., 2015; Spreng et al., 2015]. Using a graph‐theoretic approach on resting functional connectivity data, Andrews‐Hanna et al. [2010] parsed the DMN into three separable systems: (1) the midline core, consisting of the anterior medial prefrontal cortex (amPFC) and the PCC, (2) the dorsomedial prefrontal subsystem, consisting of the dmPFC, LTC, temporal pole (TempP), and temporoparietal junction (TPJ), and (3) the medial temporal (MTL) subsystem, consisting of the hippocampal formation (HF), parahippocampal cortex (PHC), retrosplenial cortex (Rsp), and posterior inferior parietal lobule/angular gyrus (pIPL). During rest, the dmPFC and MTL subsystems were shown to have strong connectivity within their constituent nodes and with the midline core, but did not show reliable connectivity between one another. Furthermore, in a separate experiment, they were able to demonstrate that the dmPFC subsystem responded to self‐referential judgements based in the present, the MTL subsystem responded to self‐referential episodic judgements based in the future, and the midline core responded to judgements both about the present and future, as long as they were self‐referential [Andrews‐Hanna et al., 2010]. Many studies have since corroborated these findings, providing evidence in support of the role of the MTL subsystem of the DMN in the retrieval of episodic memories [for review, see Rugg and Vilberg, 2013], imagining future events [for review, see Schacter et al., 2012], and the dmPFC subsystems in self‐referential processing and mentalizing about others [Spreng and Andrews‐Hanna, 2015; Mars et al., 2012; Schilbach et al., 2008; Uddin et al., 2007].
Using a data‐driven PLS analysis, we found highly similar dissociable DMN subsystems in relation to our task conditions, specifically separating DMN connectivity during rest from episodic retrieval and future imagining. Our analysis revealed two patterns within the DMN: regions of the MTL subsystem showed better connectivity during the episodic and future imagining conditions, while the midline core and the dmPFC subsystem showed better connectivity during rest. Note that these conditions were identical to the uninterrupted eyes‐closed resting run but varied only in terms of the instructions provided at the start of the scan, biasing thought content towards memories or future thoughts. Thus, our data support the relationship between the MTL subsystem of the DMN and processes related to future imagining and episodic retrieval. Interestingly, the dmPFC subsystem, often associated with self‐referential processes and mentalizing, showed preferential association with rest. The stronger involvement of the dmPFC subnetwork in our passive rest run may reflect self‐referential contents of our participants' thoughts during rest [Buckner and Carroll, 2007; Schilbach et al., 2008; Qin & Northoff, 2011] and/or the dmPFC subsystem may preferentially support the kind of unconstrained cognition typical of a resting scan. Our data add to the growing body of literature supporting the dissociable subsystems of the DMN and further suggest that the DMN undergoes meaningful changes in connectivity from rest to episodic retrieval and future imagination.
Hippocampal Interactions Outside the Default Mode Network
In addition to changes in connectivity within the DMN, changes in functional connectivity between nodes of the DMN and the rest of the brain can also be informative regarding the neural dynamics underlying rest, retrieval and future imagining. Due to the specific importance of the hippocampus in retrieving past events from memory and imagining future ones [for review, see Schacter et al., 2012], we used seed‐PLS to highlight functional coupling between the hippocampus and all voxels in the brain across our resting conditions. Critically, a consistent distinction emerged, in which hippocampal‐whole brain connectivity during retrieval and future imagining were distinguished from rest. Specifically, the hippocampus revealed markedly increased connectivity with regions of visual cortex during retrieval and future imagining (see Fig. 3C,E). This was especially prominent with the right hippocampal seed, though higher order visual cortices were still present in our analysis of the left seed. We speculate that this MTL‐visual cortex connectivity may possibly support the generation of rich contextual visual details during the construction of past and imagined future events [Addis et al., 2009; Bosch et al., 2014; McCormick et al., 2013; Ritchey et al., 2015; Sheldon and Levine, 2016; Wing et al., 2015]. The cortical reinstantiation of areas of the brain engaged during encoding has been reliably reported during episodic processes [Danker and Anderson, 2010; Leiker and Johnson, 2014; Gordon, Rissman et al., 2014; Ritchey et al., 2012; St‐Laurent et al., 2015; Staresina et al., 2012; Wheeler et al., 2000; Wing et al., 2015] and has been thought to be coordinated by coupling between the hippocampus and neocortex [Fuentemilla et al., 2014; for discussion, see Moscovitch, 1992]. This kind of intentional retrieval of previously experienced visual details in order to recreate coherent scenes is likely not required to the same degree during unconstrained rest relative to future imagining and episodic retrieval, and, as expected, hippocampal‐whole brain connectivity preferential to rest did not show any relationship to visual cortices. It is also noticeable that neither hippocampal seed showed a robust pattern of connectivity preferential to the resting condition anywhere else in the brain. This finding, in combination with the within‐DMN PLS results indicating distinct intra‐MTL connectivity present during retrieval and future imagining, demonstrates that hippocampal connectivity, within the DMN and beyond, is largely task specific. This conclusion is in line with previous work suggesting hippocampal connectivity with the DMN supports the retrieval and maintenance of episodic information over a delay [Chen et al., 2016]. This kind of task‐specific coupling further emphasizes the heterogeneity within the DMN and the reliability of a functionally distinct MTL‐subsystem as identified by Andrews‐Hanna et al. [2010] and others [see also, Vincent et al., 2006; Rugg and Vilberg, 2013].
Theoretical Implications
The present study demonstrates that despite commonality in DMN functional connectivity across rest, episodic retrieval and future imagining, there are also robust and meaningful differences across task conditions. We suggest that the observed task‐related heterogeneity in the DMN should be taken into account when developing models of the DMN's contributions to cognition. First, it is important to acknowledge that the commonality accounts for only a subset of the total variance and not the network as a whole, thus limiting the extent of our inferences drawn from this commonality; second, by implication, it is important to include the heterogeneity of the DMN into our theoretical accounts [Andrews‐Hanna et al., 2014b).
For example, we can begin by considering the ‘component process’ and ‘intrinsic connectivity’ accounts of DMN function outlined in the introduction. As these accounts were derived from the common recruitment of the DMN across rest, episodic retrieval and future imagining, proponents of these accounts can only make inferences about the portion of variance associated with the commonality in connectivity across conditions, which, in our data, leaves more than half of the variance associated with DMN connectivity during these three conditions unexplained. In terms of differences, regions of the MTL have long been recognized as critical for processes underlying remembering past events and imagining future ones [Tulving, 1985; Moscovitch, 1992; Moscovitch, 2008; Preston and Eichenbaum, 2013; Maguire et al., 2015; Ranganath, 2010; Ranganath and Ritchey, 2012; Scoville and Milner, 1957; Schacter et al., 2012; Shimamura, 2010]. Therefore, it is not surprising that the MTL subsystem of the DMN shows a preferential association with these two conditions in the present experiment. The anatomical architecture of the MTL and its pattern of connectivity with other areas of cortex is optimally structured to support the binding of unique, non‐overlapping, mnemonic details into a coherent and meaningful context [Aggleton, 2012; Norman and O'Reilly, 2003] affording the unique information processing capabilities necessary for complex processes such as retrieving an episodic memory or imagining a plausible future event [Mišić et al., 2014]. These processes may not be engaged to the same degree during unconstrained rest, leading us to suggest that DMN connectivity undergoes flexible changes to incorporate regions/structures better suited to support the current task demands [Cabeza and Moscovitch, 2013; Moscovitch, 1992; Moscovitch et al, 2016]. This recruitment of process‐specific regions during relevant tasks applies equally to our resting condition, as it recruited the dmPFC subsystem of the DMN, often posited to support mentalizing and theory of mind‐related processing [Andrews‐Hanna et al., 2010; Andrews‐Hanna et al., 2014a,b]. The nature of what participants are truly doing at rest is unclear, but what is clear is that rest does not reflect an absence of cognitive processing nor can it be considered a pure measure of the brain's structure. For example, Krienen et al. found no benefit to using rest in predicting the common intrinsic architecture underlying the performance of 14 different cognitive tasks, suggesting that rest does not reflect the brain's intrinsic architecture any better than any other cognitive task [Krienen et al., 2014]. The resting state has its own unique processing demands, likely including a variable smorgasbord of different fleeting cognitive processes, including remembering past events, consolidating new ones, imagining future ones, mentalizing about the cognitive states of others, self‐related processing, externally‐oriented sensation, shallow sleep, and various other task‐irrelevant thoughts, all melded into an ever‐changing continuous stream lasting the duration of the scan [Allen et al., 2014; Gorgolewski et al., 2014; Spreng, 2012; Van Calster et al., 2016]. Perhaps, the nature of this kind of processing is better suited for the connectivity profile of the DMN's dmPFC subsystem, explaining its preferential association with rest during this experiment. Alternatively, as has been argued by certain groups, the resting state is primarily comprised of mentalizing and social cognitive processes [Schilbach et al., 2008], thus recruiting the dmPFC subsystem [Andrews‐Hanna et al., 2014a,b].
Limitations
The present study was designed to ensure that the task structures of the retrieval and future imagining conditions were as comparable to a typical resting scan as possible. This enabled a direct comparison across processes without including common methodological discrepancies such as the trial‐based design often used during retrieval/imagining paradigms, and the unconstrained paradigm used for rest. Also, it allowed our results to be related to the majority of the DMN literature, largely conducted on unconstrained resting scans.
By emphasizing similar task structure, however, we were limited in our ability to collect behavioural data to ensure compliance with our task. Despite this drawback, there are strong reasons to believe that participants did comply. Participants were given detailed instructions about the task prior to entering the scanner, in addition to reminders prior to each condition, to ensure compliance. Our participants' compliance was corroborated by our post‐scan questionnaire. Most importantly, the pattern of neural results observed supported the conclusion that there was a reliable difference in functional connectivity across conditions, despite the unconstrained nature of our paradigm. Considering the primary difference in these tasks was their instructions (e.g., unconstrained rest, think about the past, or think about the future), we contend that these neural differences reflect greater, on average, retrieval/future imagining during our directed scans as suggested by our post‐scan questionnaire (see Fig. 1). Future work using experience‐sampling with this kind of design could be used to better capture specific thought content during scans and the corresponding changes in ongoing DMN dynamics.
It is also important to consider that ThinkBack and ThinkAhead were directed conditions, while the initial resting condition had instructions, but was considerably less directed with fewer constraints (i.e., “think about anything,” relative to “think about the past”). Thus, one possibility is that the observed neural difference is due to how directed/constrained the conditions were, rather than tapping into the process of interest. The current study cannot completely rule out this explanation, which likely does contribute to some degree, though the pattern of results observed suggests that this explanation alone is unlikely to account for our findings. Increases in functional connectivity during retrieval and future imagining were restricted to the medial temporal lobes, as would be expected from a standard retrieval or imagining paradigm (for review, see Rugg and Vilberg, 2013; Schacter et al., 2012]. Furthermore, coupling between the hippocampus and visual cortex is a common signature of memory supported visual imagery [Bosch et al., 2014; Wing et al., 2015; for review, see Danker and Anderson, 2010], rather than a general effect of directedness. These observations suggest that our results cannot be wholly explained by the directedness of the tasks alone, but must also reflect some degree of the targeted cognitive processes.
A final limitation of our design is the introduction of potential order effects, as the Rest condition always appeared prior to ThinkBack and ThinkAhead. If participants progressively engaged in a reliably different set of cognitive processes earlier relative to later in the scan, one might expect higher similarity estimates for the directed conditions irrespective of the specific cognitive demands particular to these conditions. However, as noted above, we believe that the spatial specificity of our PLS findings to the medial temporal subsystem of the DMN are consistent with an interpretation that performance on the ThinkBack and ThinkAhead conditions required similar episodic construction processes [Schacter et al., 2012], and thus was accompanied by changes in DMN functional connectivity to accommodate task demands. The contributions of extraneous cognitive processes confounded with time, however, cannot be conclusively ruled out with the present design.
CONCLUSIONS
The present study provided a quantification of the similarities and differences in DMN connectivity across rest, episodic retrieval and future imagining using a within‐subject design and comparable unconstrained instructions across the three conditions. Though we observed commonality in the DMN connectivity across these conditions, there was also robust divergence, specifically between the rest and both the episodic retrieval and future imagining conditions. These results support previous work emphasizing the heterogeneity of the DMN, corroborating previously reported subsystems while using a novel within‐subject phenomenologically comparable task‐based design. Also, by focusing on ROI–ROI correlations, our analysis was able to add precision to the current literature by highlighting the specific coupling patterns distinguishing between conditions amongst nodes of the DMN. Furthermore, it emphasizes the flexible nature of the DMN, dynamically reorganizing to better integrate functionally separable subsystems depending on the current task demands. Taking this heterogeneity and flexibility into account is a necessary step for future theoretical models of DMN structure and function.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
The authors thank Jessica Robin and Vincent Man for many helpful discussions regarding this project. They also thank our fMRI technologist, Annette Weekes‐Holder, and the following people for their generosity in support of the imaging centre at Baycrest: Jack & Anne Weinbaum, Sam & Ida Ross, Joseph & Sandra Rotman.
Contributor Information
B. Bellana, Email: b.bellana@mail.utoronto.ca.
Z.‐X. Liu, Email: zhongxuliu@gmail.com.
REFERENCES
- Addis DR, Moscovitch M, McAndrews MP (2007): Consequences of hippocampal damage across the autobiographical memory network in left temporal lobe epilepsy. Brain 130(Pt 9):2327–2342. [DOI] [PubMed] [Google Scholar]
- Addis DR, Pan L, Vu MA, Laiser N, Schacter DL (2009): Constructive episodic simulation of the future and the past: Distinct subsystems of a core brain network mediate imagining and remembering. Neuropsychologia 47:2222–2238. [DOI] [PubMed] [Google Scholar]
- Aggleton JP (2012): Multiple anatomical systems embedded within the primate medial temporal lobe: Implications for hippocampal function. Neurosci Biobehav Rev 36:1579–1596. [DOI] [PubMed] [Google Scholar]
- Allen EA, Damaraju E, Plis SM, Erhardt EB, Eichele T, Calhoun VD (2014): Tracking whole‐brain connectivity dynamics in the resting state. Cereb Cortex 24:663–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews‐Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL (2010): Functional‐anatomic fractionation of the brain's default network. Neuron 65:550–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews‐Hanna JR, Saxe R, Yarkoni T (2014a): Contributions of episodic retrieval and mentalizing to autobiographical thought: Evidence from functional neuroimaging, resting‐state connectivity, and fMRI meta‐analyses. Neuroimage 91:324–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews‐Hanna JR, Smallwood J, Spreng RN (2014b): The default network and self‐generated thought: Component processes, dynamic control, and clinical relevance. Ann N Y Acad Sci 1316:29–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barttfeld P, Uhrig L, Sitt JD, Sigman M, Jarraya B, Dehaene S (2014): Signature of consciousness in the dynamics of resting‐state brain activity. Proc Natl Acad Sci U S A 112:887–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT (2007): A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 37:90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit RG, Schacter DL (2015): Specifying the CORE network supporting episodic simulation and episodic memory by activation likelihood estimation. Neuropsychologia 75:450–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder J, Frost J, Hammeke T, Bellgowan PSF, Rao SM, Cox RW (1999): Conceptual processing during the conscious resting state: A functional MRI study. J Cogn Neurosci 11:80–93. [DOI] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL (2009): Where is the semantic system? A critical review and meta‐analysis of 120 functional neuroimaging studies. Cereb Cortex 19:2767–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch SE, Jehee JFM, Fernández G, Doeller CF (2014): Reinstatement of associative memories in early visual cortex is signaled by the hippocampus. J Neurosci 34:7493–7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga RM, Leech R: Echoes of the brain: Local‐scale representation of whole‐brain functional networks within transmodal cortex. The Neuroscientist, 21:540–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga RM, Sharp DJ, Leeson C, Wise RJS, Leech R (2013): Echoes of the brain within default mode, association, and heteromodal cortices. J Neurosci 33:14031–14039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressler SL, Mcintosh AR (2007): The role of neural context in large‐scale neurocognitive network operations. Understanding Complex Syst 2007:403–419. [Google Scholar]
- Bressler SL, Menon V (2010): Large‐scale brain networks in cognition: emerging methods and principles. Trends Cogn Sci 14:277–290. [DOI] [PubMed] [Google Scholar]
- Buckner RL (2012): The serendipitous discovery of the brain's default network. Neuroimage 62:1137–1145. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Carroll DC (2007): Self‐projection and the brain. Trends Cogn Sci 11:49–57. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews‐Hanna JR, Schacter DL (2008): The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci 1124:1–38. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Moscovitch M (2013): Memory systems, processing modes, and components: Functional neuroimaging evidence. Perspect Psychol Sci 8:49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell K, Grigg O, Saverino C, Churchill N, Grady CL (2013): Age differences in the intrinsic functional connectivity of default network subsystems. Front Hum Neurosci 5: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Honey CJ, Simony E, Arcaro MJ, Norman KA, Hasson U: Accessing real‐life episodic information from minutes versus hours earlier modulates hippocampal and high‐order cortical dynamics. Cereb Cortex 26:3428–3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoff K, Gordon AM, Smallwood J, Smith R, Schooler JW (2009): Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proc Natl Acad Sci U S A 106:8719–8724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Bassett DS, Power JD, Braver TS, Petersen SE (2014): Intrinsic and task‐evoked network architectures of the human brain. Neuron 83:238–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Argembeau A, Van der Linden M (2004): Phenomenal characteristics associated with projecting oneself back into the past and forward into the future: Influence of valence and temporal distance. Conscious Cogn 13:844–858. [DOI] [PubMed] [Google Scholar]
- D'Argembeau A, Collette F, Van Der Linden M, Laureys S, Del Fiore G, Degueldre C, Luxen A, Salmon E (2005): Self‐referential reflective activity and its relationship with rest: A PET study. Neuroimage 25:616–624. [DOI] [PubMed] [Google Scholar]
- D'Argembeau A, Van der Linden M (2006): Individual differences in the phenomenology of mental time travel: The effect of vivid visual imagery and emotion regulation strategies. Conscious Cogn 15:342–350. [DOI] [PubMed] [Google Scholar]
- Danker JF, Anderson JR (2010): The ghosts of brain states past: remembering reactivates the brain regions engaged during encoding. Psychol Bull 136:87–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dastjerdi M, Foster BL, Nasrullah S, Rauschecker AM, Dougherty RF, Townsend JD, Chang C, Greicius MD, Menon V, Kennedy DP, Parvizi J (2011): Differential electrophysiological response during rest, self‐referential, and non‐self‐referential tasks in human posteromedial cortex. Proc Natl Acad Sci U S A 108:3023–3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Pasquale F, Della Penna S, Snyder AZ, Marzetti L, Pizzella V, Romani GL, Corbetta M (2012): A cortical core for dynamic integration of functional networks in the resting human brain. Neuron 74:753–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon ML, Fox KCR, Christoff K (2014): A framework for understanding the relationship between externally and internally directed cognition. Neuropsychologia 62:321–330. [DOI] [PubMed] [Google Scholar]
- Foster BL, Rangarajan V, Shirer WR, Parvizi J (2015): Intrinsic and task‐dependent coupling of neuronal population activity in human parietal cortex. Neuron 86:578–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME (2005): The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A 102:9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentemilla L, Barnes GR, Düzel E, Levine B (2014): Theta oscillations orchestrate medial temporal lobe and neocortex in remembering autobiographical memories. Neuroimage 85:730–737. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Rho Y, McIntosh AR, Kötter R, Jirsa VK (2008): Cortical network dynamics with time delays reveals functional connectivity in the resting brain. Cogn Neurodynamics 2:115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon AM, Rissman J, Kiani R, Wagner AD (2014): Cortical reinstatement mediates the relationship between content‐specific encoding activity and subsequent recollection decisions. Cereb Cortex 24:3350–3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgolewski KJ, Lurie D, Urchs S, Kipping JA, Craddock RC, Milham MP, Margulies DS, Smallwood J (2014): A Correspondence between individual differences in the brain's intrinsic functional architecture and the content and form of self‐generated thoughts. PloS One 9:e97176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, Protzner AB, Kovacevic N, Strother SC, Afshin‐pour B, Wojtowicz M, Anderson JAE, Churchill N, McIntosh AR (2010): A multivariate analysis of age‐related differences in default mode and task‐positive networks across multiple cognitive domains. Cereb Cortex 20:1432–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Kiviniemi V, Tervonen O, Vainionpää V, Alahuhta S, Reiss AL, Menon V (2008): Persistent default‐mode network connectivity during light sedation. Hum Brain Mapp 29:839–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Supekar K, Menon V, Dougherty RF (2009): Resting‐state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex 19:72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, Sporns O (2008): Mapping the structural core of human cerebral cortex. PLoS Biol 6:e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassabis D, Kumaran D, Maguire EA (2007): Using imagination to understand the neural basis of episodic memory. J Neurosci 27:14365–14374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn A, Ostwald D, Reisert M, Blankenburg F (2014): The structural‐functional connectome and the default mode network of the human brain. Neuroimage 102:142–151. [DOI] [PubMed] [Google Scholar]
- Horovitz SG, Braun AR, Carr WS, Picchioni D, Balkin TJ, Fukunaga M, Duyn JH (2009): Decoupling of the brain's default mode network during deep sleep. Proc Natl Acad Sci U S A 106:11376–11381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H (2012): A dual‐subsystem model of the brain's default network: self‐referential processing, memory retrieval processes, and autobiographical memory retrieval. Neuroimage 61:966–977. [DOI] [PubMed] [Google Scholar]
- Krienen F, Yeo BTT, Buckner R (2014): Reconfigurable task‐dependent functional coupling modes cluster around a core functional architecture. Philos Trans R Soc Lond B Biol Sci 369:20130526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan A, Williams LJ, McIntosh AR, Abdi H (2011): Partial Least Squares (PLS) methods for neuroimaging: A tutorial and review. Neuroimage 56:455–475. [DOI] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Li K, Robin DA, Glahn DC, Fox PT (2009): Investigating the functional heterogeneity of the default mode network using coordinate‐based meta‐analytic modeling. J Neurosci 29:14496–14505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R, Scott G, Carhart‐Harris R, Turkheimer F, Taylor‐Robinson SD, Sharp DJ (2014): Spatial dependencies between large‐scale brain networks. PloS One 9:e98500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiker EK, Johnson JD (2014): Neural reinstatement and the amount of information recollected. Brain Res 1582:125–138. [DOI] [PubMed] [Google Scholar]
- Liu ZX, Grady C, Moscovitch M (2016): Effects of prior‐knowledge on brain activation and connectivity during associative memory encoding. Cerebral Cortex bhw047. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Mullally SL (2013): The hippocampus: A manifesto for change. J Exp Psychol Gen 142:1180–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Intraub H, Mullally SL (2015): Scenes, spaces, and memory traces: What does the hippocampus do? The Neuroscientist [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mars RB, Neubert F‐X, Noonan MP, Sallet J, Toni I, Rushworth MFS (2012): On the relationship between the “default mode network” and the “social brain”. Front Hum Neurosci 6: 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinelli P, Sperduti M, Piolino P (2013): Neural substrates of the self‐memory system: New insights from a meta‐analysis. Hum Brain Mapp 34:1515–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN (2007): Wandering minds: Stimulus‐independent thought. Science 315: 393–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattar MG, Cole MW, Thompson‐Schill S, Bassett DS (2015): A functional cartography of cognitive systems. PLoS Compl Biol 11:1–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazoyer B, Zago L, Mellet E, Bricogne S, Etard O, Houdé O, Crivello F, Joliot M, Petit L, Tzourio‐Mazoyer N (2001): Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain Res Bull 54:287–298. [DOI] [PubMed] [Google Scholar]
- McCormick C, St‐Laurent M, Ty A, Valiante T.A, McAndrews MP (2013): Functional and effective hippocampal‐neocortical connectivity during construction and elaboration of autobiographical memory retrieval. Cerebral Cortex http://doi.org/10.1093/cercor/bht324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick C, Protzner AB, Barnett AJ, Cohn M, Valiante TA, McAndrews MP (2014): Linking DMN connectivity to episodic memory capacity: What can we learn from patients with medial temporal lobe damage. NeuroImage: Clinical 5:188–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh AR (2000): Towards a network theory of cognition. Neural Netw 13:861–870. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Lobaugh NJ (2004): Partial least squares analysis of neuroimaging data: Applications and advances. Neuroimage 23 Suppl 1:S250–S263. [DOI] [PubMed] [Google Scholar]
- Mišić B, Goñi J, Betzel RF, Sporns O, McIntosh AR (2014): A network convergence zone in the hippocampus. PLoS Comp Biol 10:e1003982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovitch M (1992): Memory and working‐with‐memory: A component process model based on modules and central systems. J Cogn Neurosci 4:257–267. [DOI] [PubMed] [Google Scholar]
- Moscovitch M (2008): The hippocampus as a “stupid,” domain‐specific module: Implications for theories of recent and remote memory, and of imagination. Can J Exp Psychol 62:62–79. [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Cabeza R, Winocur G, Nadel L (2016): Episodic Memory and Beyond?: The Hippocampus and Neocortex in Transformation. Annual Review of Psychology, (September 2015), 1–30. http://doi.org/10.1146/annurev‐psych‐113011‐143733. [DOI] [PMC free article] [PubMed]
- Murray RJ, Debbané M, Fox PT, Bzdok D, Eickhoff SB (2014): Functional connectivity mapping of regions associated with self‐ and other‐processing. Hum Brain Mapp 36:1304–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman KA, O'Reilly RC (2003): Modeling hippocampal and neocortical contributions to recognition memory: a complementary‐learning‐systems approach. Psychol Rev 110:611–646. [DOI] [PubMed] [Google Scholar]
- Preminger S, Harmelech T, Malach R (2011): Stimulus‐free thoughts induce differential activation in the human default network. Neuroimage 54:1692–1702. [DOI] [PubMed] [Google Scholar]
- Preston AR, Eichenbaum H (2013): Interplay of hippocampus and prefrontal cortex in memory. Curr Biol 23:R764–R773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin P, Northoff G (2011): How is our self related to midline regions and the default‐mode network? Neuroimage 57:1221–1233. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL (2001): A default mode of brain function. Proc Natl Acad Sci U S A 98:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, Snyder AZ (2007): A default mode of brain function: a brief history of an evolving idea. Neuroimage 37:1083–1090. [DOI] [PubMed] [Google Scholar]
- Ranganath C (2010): A unified framework for the functional organization of the medial temporal lobes and the phenomenology of episodic memory. Hippocampus 20:1263–1290. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Ritchey M (2012): Two cortical systems for memory‐guided behaviour. Nat Rev Neurosci 13:713–726. [DOI] [PubMed] [Google Scholar]
- Ritchey M, Wing EA, LaBar KS, Cabeza R (2012): Neural similarity between encoding and retrieval is related to memory via hippocampal interactions. Cereb Cortex 23:2818–2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchey M, Libby LA, Ranganath C (2015): Cortico‐hippocampal systems involved in memory and cognition: the PMAT framework In: Progress in Brain Research. pp. 45–64. [DOI] [PubMed] [Google Scholar]
- Roberts RP, Hach S, Tippett LJ, Addis DR (2016): The Simpson's paradox and fMRI: Similarities and differences between functional connectivity measures derived from within‐subject and across‐subject correlations. NeuroImage 135: 1–15. [DOI] [PubMed] [Google Scholar]
- Robin J, Hirshhorn M, Rosenbaum RS, Winocur G, Moscovitch M, Grady CL (2015): Functional connectivity of hippocampal and prefrontal networks during episodic and spatial memory based on real‐world environments. Hippocampus 25:81–93. [DOI] [PubMed] [Google Scholar]
- Rosenbaum RS, Gilboa A, Levine B, Winocur G, Moscovitch M (2009): Amnesia as an impairment of detail generation and binding: Evidence from personal, fictional, and semantic narratives in K.C. Neuropsychologia 47:2181–2187. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Vilberg KL (2013): Brain networks underlying episodic memory retrieval. Curr Opin Neurobiol 23:255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajonz B, Kahnt T, Margulies DS, Park SQ, Wittmann A, Stoy M, Ströhle A, Heinz A, Northoff G, Bermpohl F (2010): Delineating self‐referential processing from episodic memory retrieval: common and dissociable networks. Neuroimage 50:1606–1617. [DOI] [PubMed] [Google Scholar]
- Salomon R, Levy DR, Malach R (2014): Deconstructing the default: Cortical subdivision of the default mode/intrinsic system during self‐related processing. Hum Brain Mapp 35:1491–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Hassabis D, Martin VC, Spreng RN, Szpunar KK (2012): The future of memory: Remembering, imagining, and the brain. Neuron 76:677–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilbach L, Eickhoff SB, Rotarska‐Jagiela A, Fink GR, Vogeley K (2008): Minds at rest? Social cognition as the default mode of cognizing and its putative relationship to the “default system” of the brain. Conscious Cogn 17:457–467. [DOI] [PubMed] [Google Scholar]
- Schilbach L, Bzdok D, Timmermans B, Fox PT, Laird AR, Vogeley K, Eickhoff SB (2012): Introspective minds: Using ALE meta‐analyses to study commonalities in the neural correlates of emotional processing, social & unconstrained cognition. PLoS ONE 7:e30920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoville WB, Milner B (1957): Loss of recent memory after bilateral hippocampal lesions. J Neurol, Neurosurg Psychiatry 20:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon S, Levine B (2016): The role of the hippocampus in memory and mental construction Ann N Y Acad Sci 1–17. [DOI] [PubMed] [Google Scholar]
- Shimamura AP (2010): Hierarchical relational binding in the medial temporal lobe: The strong get stronger. Hippocampus 20:1206–1216. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, Petersen SE (1997): Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. J Cogn Neurosci 9:648–663. [DOI] [PubMed] [Google Scholar]
- Spreng RN (2012): The fallacy of a “task‐negative” network. Front Psychol 3:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Grady CL (2010): Patterns of brain activity supporting autobiographical memory, prospection, and theory of mind, and their relationship to the default mode network. J Cogn Neurosci 22:1112–1123. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Andrews‐Hanna JR (2015): The default network and social cognition In: Brain Mapping: An Encyclopedic Reference, pp. 165–169. [Google Scholar]
- Spreng RN, Mar RA, Kim ASN (2009): The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta‐analysis. J Cogn Neurosci 21:489–510. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Gerlach KD, Turner GR, Schacter DL (2015): Autobiographical planning and the brain: Activation and its modulation by qualitative features. J Cogn Neurosci 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St‐Laurent M, Abdi H, Buchsbaum BR (2015): Distributed patterns of reactivation predict vividness of recollection. J Cogn Neurosci 1–19. [DOI] [PubMed] [Google Scholar]
- Staresina BP, Henson RNA, Kriegeskorte N, Alink A (2012): Episodic reinstatement in the medial temporal lobe. J Neurosci 32:18150–18156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving, E. (1985). Elements of episodic memory. Oxford, UK: Clarendon.
- Uddin LQ, Iacoboni M, Lange C, Keenan JP (2007): The self and social cognition: the role of cortical midline structures and mirror neurons. Trends Cogn Sci 11:153–157. [DOI] [PubMed] [Google Scholar]
- Van Calster L, D'Argembeau A, Salmon E, Peters F, Majerus S (2016): Fluctuations of attentional networks and default mode network during the resting state reflect variations in cognitive states: Evidence from a novel resting‐state experience sampling method. J Cogn Neurosci 26:1–19. 10.1162/jocn_a_01025 [DOI] [PubMed] [Google Scholar]
- Vincent JL, Snyder AZ, Fox MD, Shannon BJ, Andrews JR, Raichle ME, Buckner RL (2006): Coherent spontaneous activity identifies a hippocampal‐parietal memory network. J Neurophysiol 96:3517–3531. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, Zempel JM, Snyder LH, Corbetta M, Raichle ME (2007): Intrinsic functional architecture in the anaesthetized monkey brain. Nature 447: 83–86. [DOI] [PubMed] [Google Scholar]
- Wheeler ME, Petersen SE, Buckner RL (2000): Memory's echo: Vivid remembering reactivates sensory‐specific cortex. Proc Natl Acad Sci U S A 97:11125–11129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield‐Gabrieli S, Nieto‐Castanon A (2012): Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Conn 2:125–141. [DOI] [PubMed] [Google Scholar]
- Wing EA, Ritchey M, Cabeza R (2015): Reinstatement of individual past events revealed by the similarity of distributed activation patterns during encoding and retrieval. J Cogn Neurosci 27:679–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia M, Wang J, He Y (2013): BrainNet Viewer: A network visualization tool for human brain connectomics. PLoS ONE 8: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BTT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zöllei L, Polimeni JR, Fischl B, Liu H, Buckner RL (2011): The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol 106:1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


