Abstract
Magnetic resonance imaging of subcortical gray matter structures, which mediate behavior, cognition and the pathophysiology of several diseases, is crucial for establishing typical maturation patterns across the human lifespan. This single site study examines T1‐weighted MPRAGE images of 3 healthy cohorts: (i) a cross‐sectional cohort of 406 subjects aged 5–83 years; (ii) a longitudinal neurodevelopment cohort of 84 subjects scanned twice approximately 4 years apart, aged 5–27 years at first scan; and (iii) a longitudinal aging cohort of 55 subjects scanned twice approximately 3 years apart, aged 46–83 years at first scan. First scans from longitudinal subjects were included in the cross‐sectional analysis. Age‐dependent changes in thalamus, caudate, putamen, globus pallidus, nucleus accumbens, hippocampus, and amygdala volumes were tested with Poisson, quadratic, and linear models in the cross‐sectional cohort, and quadratic and linear models in the longitudinal cohorts. Most deep gray matter structures best fit to Poisson regressions in the cross‐sectional cohort and quadratic curves in the young longitudinal cohort, whereas the volume of all structures except the caudate and globus pallidus decreased linearly in the longitudinal aging cohort. Males had larger volumes than females for all subcortical structures, but sex differences in trajectories of change with age were not significant. Within subject analysis showed that 65%–80% of 13–17 year olds underwent a longitudinal decrease in volume between scans (∼4 years apart) for the putamen, globus pallidus, and hippocampus, suggesting unique developmental processes during adolescence. This lifespan study of healthy participants will form a basis for comparison to neurological and psychiatric disorders. Hum Brain Mapp 38:3771–3790, 2017. © 2017 Wiley Periodicals, Inc.
Keywords: subcortical gray matter, thalamus, limbic structures, basal ganglia, FreeSurfer, Poisson curve, neurodevelopment, aging
INTRODUCTION
Magnetic resonance imaging (MRI) has enabled investigation of structural brain maturation across the normal human lifespan [Courchesne et al., 2000; Jernigan et al., 2001; Ostby et al., 2009; Sullivan et al., 2004; Tamnes et al., 2013]. This body of work collectively suggests that cortical gray matter volume expands rapidly during the first few years of life [Gilmore et al., 2012; Knickmeyer et al., 2008], peaks early in childhood and decreases thereafter with unique regional trajectories [Gogtay et al., 2004; Good et al., 2001; Lebel et al., 2012; Shaw et al., 2008], whereas white matter follows a protracted course of volume increase until around 40–50 years of age followed by a steady decline [Lebel et al., 2012; Westlye et al., 2010]. Although many studies have confirmed these global morphological changes, fewer have examined healthy developmental trajectories of individual subcortical gray matter structure volumes, with most only probing a subset of the human lifespan.
Subcortical gray matter structures mediate many aspects of motor and cognitive abilities (via the basal ganglia), integration of sensorimotor systems (via the thalamus) [Herrero et al., 2002] as well as memory, behavior and emotion (via the limbic system) [Catani et al., 2013]. Abnormalities in several of these subcortical structures have been implicated in developmental disorders, including enlarged volumes of the caudate and putamen in attention‐deficit hyperactivity disorder [Moreno‐Alcazar et al., 2016], reductions of the hippocampus and/or amygdala volumes in autism spectrum disorders [Osipowicz et al., 2015] and epilepsy [King et al., 1995], reduced thalamus volumes in schizophrenia [Van Erp et al., 2016] and reductions of deep gray matter in fetal alcohol spectrum disorders [Nardelli et al., 2011]. In older populations, reduced volumes of the hippocampus, amygdala and/or striatum have been linked to dementia and Alzheimer's disease [Teipel et al., 2013; Yi et al., 2016], late‐onset depressive symptoms [Van Uden et al., 2011], and Parkinson's disease [Bouchard et al., 2008]. Examination of the expected patterns of growth and decline in healthy individuals will further the understanding of deviations in deep gray matter development and degeneration associated with various disorders across the lifespan.
During the first 3 months of life, longitudinal studies of healthy development show deep gray matter volume increases of 47% (hippocampus) to 66% (caudate nucleus) [Holland et al., 2014] reaching 115% (hippocampus) to 145% (globus pallidus) of volume at birth by age 2 years [Gilmore et al., 2012]. During childhood, the trajectories of individual structures begin to diverge further. The thalamus has been shown to follow a quadratic trajectory of growth over ages 4–18 years (n = 325) [Brain Development Cooperative Group, 2012], followed by a linear decrease with age from 20 to 88 years (n = 73) [Walhovd et al., 2005]. The basal ganglia (putamen, caudate, globus pallidus and nucleus accumbens) generally follows linear (sometimes non‐linear) decreases of volume in cross‐sectional studies of subjects aged 4–18 years (n = 325) [Brain Development Cooperative Group, 2012], 8–30 years (n = 171) [Ostby et al., 2009], 18–94 years (n = 1,100) [Fjell et al., 2013], as well as in a longitudinal study of 85 subjects aged 8–22 years [Tamnes et al., 2013]. A longitudinal study of 711 scans in 275 individuals over 7–19 years showed unique trajectories for 6 deep gray matter structures; notably the caudate and putamen increased in volume at the early ages and then decreased thereafter, the accumbens and globus pallidus decreased and the amygdala and hippocampus increased across this age span [Goddings et al., 2014]. In another longitudinal study, the globus pallidus, striatum, and thalamus were shown to undergo increases and subsequent decreases of volume during development, with peak ages of 8–10, 12–15, and 14–17 years of age, respectively [Raznahan et al., 2014]. Other studies of the hippocampus and amygdala indicate a non‐linear (inverted U exponential) volumetric trajectory from childhood to puberty, peaking at a later age than the basal ganglia [Ostby et al., 2009; Wierenga et al., 2014], and then decreasing thereafter even after only 1 year between scans in the elderly from 60 to 91 years [Fjell et al., 2009a]. Longitudinal studies of healthy older subjects (over 60 years of age) report a general reduction in all subcortical volumes (except for the ventricles which increase with age), similar to reports in cross‐sectional studies of this same age span [Pfefferbaum et al., 2013; Raz et al., 2003; Tamnes et al., 2013]. Hippocampus and amygdala volumes have been reported to decrease nonlinearly with steep acceleration of hippocampal atrophy at older ages [Pfefferbaum et al., 2013].
Sex differences in brain structure have been well established in healthy populations, and may partially underlie sexual dimorphism of neurodevelopmental and neurodegenerative diseases [Cahill, 2006; Cosgrove et al., 2007]. Many previous studies have demonstrated that males tend to have 8%–10% larger brain volumes than females across most cortical and subcortical structures [e.g., Goldstein et al., 2001; Sowell et al., 2002], and some studies suggest later age‐at‐peak volumes for several structures in males [Giedd et al., 2012; Lenroot et al., 2007; Raznahan et al., 2014], whereas others show no sex difference [Wierenga et al., 2014].
The objective of this study is to describe age‐related volumetric growth and/or decline of key subcortical gray matter structures (thalamus, caudate, putamen, globus pallidus, nucleus accumbens, hippocampus and amygdala), as well as sex differences in these trajectories across the human “lifespan,” in one large cross‐sectional (n = 406, 5–85 years), and two longitudinal cohorts of younger (n = 84, 5–27 years) and older subjects (n = 55, 46–83 years), scanned twice each approximately 3–4 years apart. In addition to testing linear and quadratic regressions, Poisson fits were tested in the cross sectional cohort to allow differential slopes on either side of a “peak,” given the demonstrated utility of this model for describing lifespan changes in human white matter diffusion parameters [Lebel et al., 2012; Yeatman et al., 2014] and magnetic susceptibility of the brain [Li et al., 2014]. Analysis of longitudinal cohorts included linear and quadratic mixed models with a focus on within‐subject changes of volume in these subcortical brain regions over several years between scans.
METHODS
Subject Characteristics
This study was approved by the Health Research Ethics Board of the University of Alberta. All subjects gave written informed consent; child assent and parent/guardian consent was obtained for volunteers under 18 years prior to the start of all study procedures. Subjects were screened for contraindications to MRI, and had no self‐reported or parent‐reported history of neurological or psychiatric disease or brain injury. The study was composed of 3 cohorts: a cross‐sectional cohort of 406 subjects; a longitudinal neurodevelopmental cohort of 84 subjects (168 scans); and a longitudinal aging cohort of 55 subjects (110 scans) (Table 1).
Table 1.
Summary of cross‐sectional and longitudinal cohort characteristics
| Cohort | Subjects | Total scans | Min age (years) |
Max age (years) |
Mean age gap between 2 scan (±SD) (years) |
Male:female |
|---|---|---|---|---|---|---|
| Cross‐sectional | 406 | 406 | 5.6 | 83.3 | – | 191:215 |
| Longitudinal neurodevelopment | 84 | 168 | 5.6 | 29.7 | 4.0 ± 0.4 | 42:42 |
| Longitudinal aging | 55 | 110 | 46.1 | 86.6 | 3.1 ± 0.3 | 31:24 |
Cross sectional cohort
About 406 healthy volunteers (191 males) aged 5.6 to 83.3 years (mean ± SD: 31.3 ± 20.7) were included in this study (Table 1). This cross‐sectional cohort includes subjects with single MRI scans, as well as the first scans of subjects in both longitudinal cohorts, and overlaps with the subjects presented in our previous cross‐sectional diffusion tensor imaging (DTI) study of white matter development from 5 to 83 years, which also reports data on volumes of white matter, total gray matter, cerebrospinal fluid and total brain volume [Lebel et al., 2012], but not deep gray matter volumes.
Longitudinal cohort: neurodevelopment
Subjects included 84 healthy volunteers (43 males), aged 5.6–27.1 years (mean 15.4 ± 5.7 years) at first scan (Table 1). A total of 175 scans from 84 subjects were obtained, although only the first and last scans for each subject were included in this study (middle scans were excluded from 5 subjects with 3 scans and 1 subject with 4 scans), for a total of 168 scans. We chose to eliminate the middle scans in order to ensure an even number of time points and more closely matched inter‐scan intervals between all longitudinal subjects, as well as to facilitate non‐parametric analysis of change between scans (described below). The mean time between the first and last scans was 4.1 ± 0.9 years (Fig. 1). These subjects were included in our previous study of longitudinal DTI changes, which also reported overall white matter, gray matter and total brain volumes, but not individual deep gray matter structures [Lebel and Beaulieu, 2011]. The first scans of subjects in this longitudinal cohort are also included in the cross sectional cohort described above. Although this produces subject overlap between cohorts, conclusions about longitudinal change remain independent of this overlap.
Figure 1.
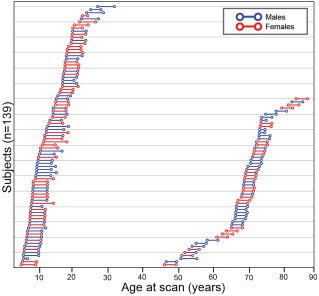
Schematic diagram representing 139 subject ages at first and last scans for both neurodevelopmental (n = 84) and aging (n = 55) longitudinal cohorts. The younger cohort had ages‐at‐first scan of 5.6–27.1 years, while the older cohort had 46.1–83.3 years. Lines connecting the circles represent the interval (neurodevelopmental cohort: 4.1 ± 0.9 years; aging cohort: 3.1 ± 0.3) between scans for each individual subject. [Color figure can be viewed at http://wileyonlinelibrary.com]
Longitudinal cohort: aging
Subjects included 55 healthy volunteers, aged 46.1–83.3 years at first scan (mean 68.9 ± 8.4 years) (Table 1). Although most subjects were scanned three times, only the first and last scans were used in this study (i.e., 2 scans per subject) for a total of 110 scans, again in order to ensure the same number of time points and closer inter‐scan intervals across longitudinal subjects and to facilitate calculation of volume change over time. The mean age gap between the first and last scans was 3.1 ± 0.3 years (Fig. 1). The first scans of these subjects were included in the cross‐sectional cohort described above, and were also included as controls for previous studies on Parkinson's Disease, although deep gray matter structure volumes were not examined [Camicioli et al., 2011; Martin et al., 2009].
Image Acquisition
Data in this study were pooled from three previously recruited cohorts, all collected on the same 1.5T Siemens Sonata MRI scanner in the Peter S. Allen MRI Research Centre at the University of Alberta. T1‐weighted 3D Magnetization Prepared Rapid Acquisition Gradient Echo (MPRAGE) was acquired using: (i) 1 × 1 × 1 mm3 voxels interpolated to 0.5 × 0.5 × 1 mm3, 144 slices with TE = 4.38 ms, TR = 1,870 ms, TI = 1,100 ms, and scan time of 4:29 minutes for 351 subjects in the neurodevelopmental and cross‐sectional cohorts [Lebel et al., 2012]; (ii) 1.0 × 1.0 × 1.5 mm3 voxels interpolated to 0.5 × 0.5 × 1.5 mm3, 128 slices with TE = 3.82 ms, TR = 1,800 ms, TI = 1,100 ms, and scan time of 5:53 minutes for 40 subjects of the aging cohort [Camicioli et al., 2011]; and (iii) 0.9 × 0.9 × 1.0 mm3 voxels interpolated to 0.45 × 0.45 × 1.0 mm3, 176 slices, with TE = 3.9 ms, TR = 2,120 ms, TI = 1,100 ms, two averages, and scan time of 16 minutes for 15 subjects in the aging cohort [Martin et al., 2009]. All scans from these original cohorts were previously visually inspected for subject motion, image artifacts and incidental findings as part of inclusion in previous studies [Camicioli et al., 2011; Lebel et al., 2012; Martin et al., 2009]; only scans that passed this quality control in those studies were included in this pooled dataset.
Image Analysis
All T1‐weighted MPRAGE images were segmented, labeled and analyzed on the exact same workstation using FreeSurfer v.4.1 to extract bilateral volumes of the hippocampus, amygdala, thalamus, caudate, putamen, globus pallidus, and nucleus accumbens, as well as total brain (excluding cerebellum, CSF, and ventricles), white matter, cortical gray matter, subcortical gray matter, and ventricles. All images were visually inspected after the automated analyses to assess appropriate segmentation and labeling by a single user, and all scans passed this quality control. No manual correction or segmentation was performed.
Inter‐Scan Reliability
A within‐subject inter‐scan sub study was performed to determine whether observed within‐subject increases/decreases between scans in the longitudinal datasets were greater than the magnitude of expected scanner variability for repeated measurements. As described in Treit et al [2013], five healthy adult subjects were scanned twice per day for 5 consecutive days (10 scans per subject; 50 scans total) using the same MPRAGE protocol as the neurodevelopmental cohorts here and on the same MRI scanner as all subjects in this study. Data were visually inspected for image quality and then analyzed using the same FreeSurfer pipeline as the main study. Within‐subject standard deviations (SD) across all ten scans per subject were calculated for each subcortical brain structure, and then averaged across the five subjects to produce a mean SD for each structure (Table 2). This average SD of each structure was used as the threshold for change between scans in the main subject data: volume changes between longitudinal scans were categorized as increases/decreases only if the absolute volume change exceeded this ±1 SD cutoff calculated from the reliability dataset, and were otherwise categorized as no change. The percentage of subjects with increased, decreased or unchanged volumes were then reported for each of 6 age bins in the longitudinal cohorts with age at first scan of: 5–8 years (n = 27); 9–12 years (n = 19); 13–18 years (n = 20); 18–29 (n = 18); 46–68 years (n = 29); and 69–83 years (n = 26).
Table 2.
Summary of inter‐scan variability of subcortical gray matter structures of 5 healthy volunteers scanned 10 times each in a week and the resultant ±1 SD change cut‐offs
| Structure | % Variability | Change cut‐off (mm3) |
|---|---|---|
| Nucleus accumbens | 6.7 | 79 |
| Globus pallidus | 3.7 | 135 |
| Amygdala | 3.3 | 113 |
| Putamen | 2.5 | 315 |
| Thalamus | 2.5 | 411 |
| Caudate | 2.2 | 177 |
| Hippocampus | 1.4 | 126 |
Curve Fitting and Statistical Analyses
All statistical analyses for the cross‐sectional cohorts were performed with Matlab R2014a (Mathworks, Massachusetts) using the Curve Fitting toolbox, while statistical analyses for longitudinal cohorts were processed with SPSS 17.0 (IBM, Armonk, New York). In the interest of reducing multiple comparisons, the absolute hemispheric volumes were summed into one bilateral volume variable per structure prior to statistical analysis.
Cross‐sectional cohort
Both linear and non‐linear regressions were used to estimate the effects of age and sex on the total bilateral volume of each structure. Starting from the most complex model that included the fixed effects of age, sex and the interaction of age and sex, subcortical volumes were fitted to (i) Poisson: Volume (cm3) = Intercept + A(Age) × e (−B × Age) + C(Sex) + D(Age × Sex) + residual error; (ii) Quadratic: Volume (cm3) = Intercept + A(Age) + B(Age2) + C(Sex) + D(Age × Sex) + E(Age2 × Sex) + residual error; or (iii) Linear: Volume (cm3) = Intercept + A(Age) + B(Sex) + C(Age × Sex) + residual error. Subsequently, each structure was then re‐fit with only main effect terms (without interaction terms). A total of 6 fits per structure were obtained and included in model selection. For structures with a significant effect of sex in the final model, regression lines were plotted separately in males and females for visualization purposes, but slopes and age at peak were not calculated or compared unless age‐by‐sex terms were significant. P‐values were FDR corrected for multiple comparisons at a corrected significance set to P < 0.05.
Longitudinal cohorts
Mixed models were used to estimate the effects of age and sex on the total bilateral volume of each structure, while accounting for repeated scans and varying scan intervals. Initially, each structure was fit with (i) Quadratic: Volume = Intercept + A(Age) + B(Age2) + C(Sex) + D(Age × Sex) + E(Age2×Sex) + residual error and (ii) Linear: Volume (cm3) = Intercept + A(Age) + B(Sex) + C(Age × Sex) + residual error. Subsequently, structures were then fit with only main effects without interaction terms, thereby producing a total of 4 fits per cohort, per structure. In structures with a significant main effect of sex, regression lines were plotted separately in males and females for better visualization, but regression analysis was not repeated and comparisons of slopes or peaks was not performed unless sex‐by‐group interactions were significant. All p‐values were FDR corrected for multiple comparisons, at a corrected significance threshold of p < 0.05. Poisson fitting was not carried out in the longitudinal cohorts due to their restricted age ranges.
Model Selection
Akaike Information Criterion (AIC) was used for both cross‐sectional and longitudinal studies to compare models and select the most probable age trajectory from multiple fits within the same structure [Tamnes et al., 2013; Wagenmakers and Farrell, 2004]. AIC takes into consideration the likelihood of the model and the number of parameters being fitted for the dataset, while penalizing models with more parameters. However, relative AIC values alone are not as reliable for studies with smaller sample sizes, specifically when the ratio of the number of subjects, n, to the number of parameters K is less than 40 [Wagenmakers and Farrell, 2004]. For this reason we transformed AIC values into second‐order AIC (AICc) values to account for the number of subjects in the study and the number of parameters in the fitted equation. The fit with the lowest AICc was chosen as the best fit model for each structure.
Timing and Magnitude of Changes
After model selection, age‐at‐peak was calculated for each structure from the first derivative of the Poisson or quadratic equations. Age‐at‐peak was not calculated for structures with linear fits. To quantify the magnitude of change for each structure in the cross‐sectional cohort, the slope of the best‐fit regression curve were calculated as a measure of the average percent volume change per annum before and after the peak. Similarly, an average longitudinal annual rate of change was calculated for each subject within an age bin and then averaged across subjects in order to quantify the rate at which each structure changes in each age group.
RESULTS
Cross‐Sectional and Longitudinal Brain Volume Changes with Age
Cross‐sectional cohort
Total brain, white matter, gray matter and ventricle volumes were all best fit to Poisson curves (Fig. 2), indicating an increase and subsequent gradual decline of total brain volume, non‐linear increase of ventricles, non‐linear decreases of cortical gray matter, and a sharp increase with a mid‐life plateau and subsequent decrease of white matter volume. Males had larger volumes than females for all structures by 10% for total brain, 20% for white matter, and 9% for total gray matter (Table 3), but age‐by‐sex interactions were only significant for ventricle volume, which showed acceleration of volume increase earlier in males than females.
Figure 2.
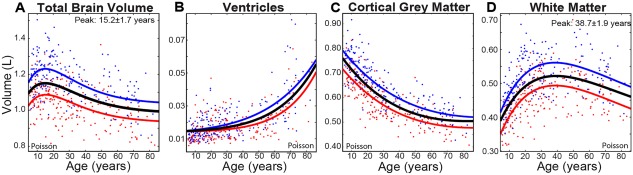
Cross‐sectional cohort trajectories of (A) total brain, (B) ventricles, (C) cortical gray, and (D) white matter volume change with age (n = 406). Males (blue lines) on average show greater volumes over all ages than females (red lines), but both sexes show very similar trajectories, with the exception of the ventricles with an age‐by‐sex interaction indicating earlier increases of volume in males than females. [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 3.
Volume differences of cortical and subcortical brain structures between sexes, ordered by largest to smallest in the cross‐sectional cohort
| Structure | Difference in volume (male–female, %) | ||
|---|---|---|---|
| Cross‐sectional (N = 406) | Longitudinal neurodevelopment (N=84) | Longitudinal aging (N = 55) | |
| Total white matter | 20.2 | 18.4 | 12.5 |
| Globus pallidus | 13.3 | 14.8 | 12.8 |
| Amygdala | 12.8 | 13.1 | 11.2 |
| Thalamus | 12.7 | 9.0 | 10.4 |
| Putamen | 11.3 | 8.9 | 11.7 |
| Total brain volume | 10.1 | 12.4 | 10.1 |
| Caudate | 9.6 | 9.4 | 13.2 |
| Nucleus accumbens | 9.5 | 11.7 | 11.2 |
| Cortical gray matter | 8.5 | 9.4 | 7.8 |
| Hippocampus | 7.9 | 9.7 | 5.5 |
Poisson‐curves were best fit to 5 of 7 subcortical volumes (thalamus, caudate, putamen, globus pallidus, and amygdala) whereas the hippocampus and nucleus accumbens were best fit to quadratic and linear regressions, respectively (Table 4, Fig. 3). For Poisson and quadratic fits, volumes increased early in childhood, reached a peak and then declined, with age at peak ranging from approximately 12 years for the putamen to approximately 23 years for the hippocampus. In general, basal ganglia structures tended to peak earlier (∼12–14 years), followed by the thalamus (∼19 years), and then by both limbic structures (∼23 years) (Figs. 3 and 4). Nucleus accumbens volume decreased steadily across the lifespan. Volume differences between males and females ranged from 8% in the hippocampus to 13% in the globus pallidus (Table 3), but age‐by‐sex interactions were not significant for any deep gray matter structure volume.
Table 4.
Fitting parameters for deep gray matter structures volume versus age within each cohort
| Structure | Cohort | Best fit model | Parameter (S.E.) | |||||
|---|---|---|---|---|---|---|---|---|
| Intercepta | Age (linear) | Age (exponential) | Sex | Age2 | Interaction (age * sex or age2 * sex) | |||
| Thalamus | Cross‐Sectional | Poisson | 13.44 (0.30) | 0.48 (0.04) | 0.05 (<0.01) | −1.61 (0.14) | – | n.s. |
| Long.—Young | None | – | – | – | – | – | – | |
| Long.—Aging | Linear | 17.51 (1.20) | −0.07 (0.02) | – | n.s. | – | n.s. | |
| Caudate | Cross‐Sectional | Poisson | 7.22 (0.15) | 0.23 (0.03) | 0.07 (0.01) | −0.66 (0.1) | – | n.s. |
| Long.—Young | None | – | – | – | – | – | – | |
| Long.—Aging | None | – | – | – | – | – | – | |
| Putamen | Cross‐Sectional | Poisson | 10.47 (0.15) | 0.65 (0.04) | 0.08 (<0.01) | −1.12 (0.11) | – | n.s. |
| Long.—Young | Quadratic | 11.51 (0.37) | 0.11 (0.04) | – | −1.1 (0.27) | −0.005 (0.001) | n.s. | |
| Long.—Aging | Linear | 12.18 (0.85) | −0.04 (0.01) | – | −1.51 (0.29) | – | n.s. | |
| Globus pallidus | Cross‐Sectional | Poisson | 3.3 (0.06) | 0.17 (0.01) | 0.08 (0.01) | −0.41 (0.04) | – | n.s. |
| Long.—Young | Quadratic | 3.19 (0.18) | 0.07 (0.02) | – | −0.52 (0.09) | −0.003 (0.001) | n.s. | |
| Long.—Aging | None | – | – | – | – | – | – | |
| Hippocampus | Cross‐Sectional | Quadratic | 9.1 (0.13) | 0.03 (0.01) | – | −0.69 (0.08) | −0.001 (<0.01) | n.s. |
| Long.—Young | Quadratic | 8.17 (0.22) | −0.02 (0.01) | – | −0.86 (0.17) | −0.003 (0.001) | n.s. | |
| Long.—Aging | Linear | 12.17 (0.6) | −0.07 (0.01) | – | −0.69 (0.17) | – | n.s. | |
| Amygdala | Cross‐Sectional | Poisson | 2.85 (0.08) | 0.08 (0.01) | 0.04 (<0.01) | −0.34 (0.03) | – | n.s. |
| Long.—Young | Quadratic | 2.57 (0.11) | 0.06 (0.01) | – | −0.37 (0.07) | −0.002 (<0.01) | n.s. | |
| Long.—Aging | Linear | 4.25 (0.32) | −0.02 (0) | – | −0.5 (0.5) | – | n.s. | |
| Nucleus accumbens | Cross‐Sectional | Linear | 1.42 (0.02) | −0.01 (0) | – | −0.13 (0.02) | – | n.s. |
| Long.—Young | Linear | 1.3 (0.05) | −0.01 (0) | – | −0.16 (0.04) | – | n.s. | |
| Long.—Aging | Linear | 1.41 (0.12) | −0.01 (0) | – | −0.17 (0.03) | – | n.s. | |
Intercept is the extrapolated volume at age zero.
– = not applicable; n.s. = non‐significant.
Figure 3.
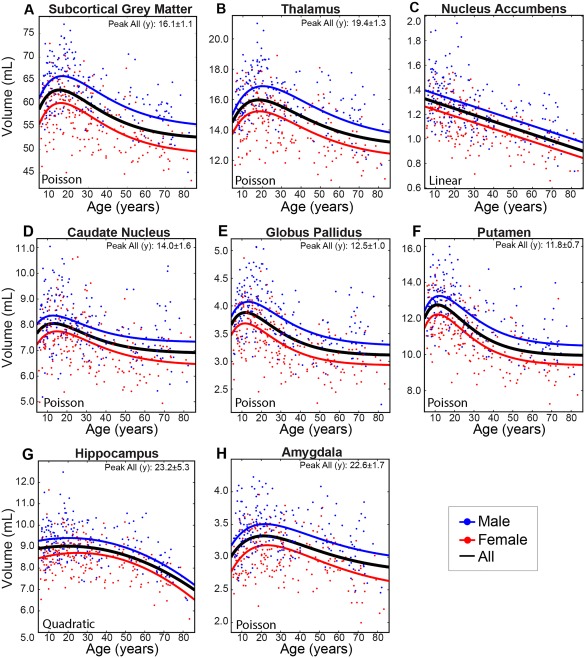
Cross‐sectional cohort trajectories of (A) total subcortical gray matter, (B) thalamus, (C) nucleus accumbens, (D) caudate nucleus, (E) globus pallidus, (F) putamen, (G) hippocampus, and (H) amygdala volumes with age (n = 406). The basal ganglia (caudate, globus pallidus, and putamen) peaked at approximately 12–14 years while the thalamus and limbic system structures (hippocampus and amygdala) peaked later at approximately 19–23 years. The nucleus accumbens (C) shows a persistent linear decrease of volume from 5 years onwards. The hippocampus, which follows a quadratic trajectory, is the only structure found to undergo accelerated declines in volume with increasing age in the latter portion of the lifespan. Note that males and females showed very similar age trajectories with no significant age‐by‐sex interactions, although males had significantly larger volumes across the age span (all p < 0.001). [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 4.
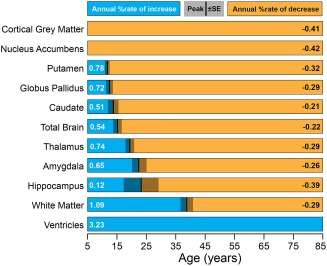
Relative timing and magnitude of volumetric changes within the cross‐sectional dataset. The magnitude of the annual increase or decrease in percentage volume is shown for each structure. Blue bars denote increases of volume before peak age, while orange bars indicate decreases. The age at peak volume and its standard error, if applicable, are represented by the vertical bar and gray panel. Structures are arranged by increasing age at peak volume. With the exception of the gray matter and nucleus accumbens volumes which decrease consistently across the lifespan, subcortical gray matter structures reach peak volume at different ages, with the striatum (putamen, globus pallidus, and caudate) peaking earlier than the limbic system (hippocampus and amygdala). Generally, white matter volume does not stop increasing until mid‐adulthood. The ventricle volume increases continuously throughout this age range. [Color figure can be viewed at http://wileyonlinelibrary.com]
Total brain volume increased by about 0.5% annually until approximately 15 years of age and decreased by 0.2% per year thereafter, white matter increased sharply by 1.1% per year until approximately 39 years of age and then decreased thereafter (though at a slower rate of 0.3% per year), and cortical gray matter decreased continuously until around approximately 60 years, with a lifespan average of −0.4% per year (Fig. 4). Ventricle volume increased gradually until about 40–50 years after which it increased sharply, averaging at an annual increase of 3.2% per year across the lifespan.
Annual percentage increases of subcortical gray matter volume prior to peak age ranged from 0.1% per year in the hippocampus to 0.8% per year for the putamen (Fig. 4). The amygdala and globus pallidus both grew at a rate of 0.7%/year, while the caudate increased by 0.5%/year. The subsequent period after the peak age show a reversal of the observed trend—structures of the basal ganglia tended to decline slower (e.g., 0.2% per year in the caudate) than either limbic structure (e.g., 0.4% per year in the hippocampus) or thalamus (0.7% per year). The nucleus accumbens atrophied at a rate of approximately 0.4% per year across the lifespan. With the exception of the hippocampus, all structures with a peak showed a faster rate of growth than rate of subsequent decline (Fig. 4).
Longitudinal cohort: Neurodevelopment
Best‐fit models indicated linear increases of white matter and ventricular volume, decreases of cortical gray matter volume and no significant change in total brain volume over 5–31 years (Fig. 5). Four of 7 deep gray matter structures were best fit to inverted U‐shaped quadratic curves (reaching maximal volume at ∼10–13 years for the putamen, globus pallidus, and hippocampus and ∼19 years for the amygdala), whereas the nucleus accumbens decreased linearly and the caudate and thalamus did not change significantly in this age range (Table 4, Figs. 6 and 7). Males had larger volumes than females for all structures, with differences ranging from 9% in the putamen to 18% in cortical white matter (Figs. 5, 6, 7; Table 3). No age‐by‐sex interactions were observed in this cohort.
Figure 5.
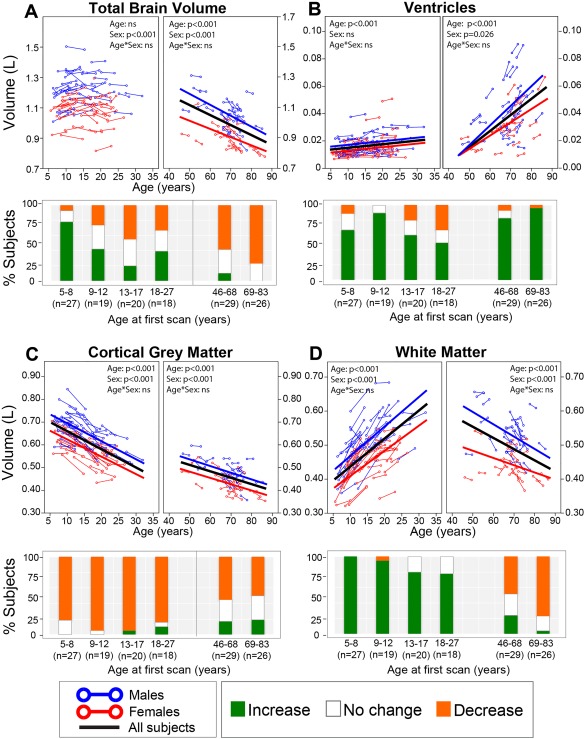
Longitudinal trajectories of (A) total brain, (B) ventricular, (C) cortical gray, and (D) white matter volumes with age with associated subject histograms denoting significant (greater than ±1SD) volume changes between the two scans approximately 3–4 years apart on average. The histograms reflect most of these same observations in both neurodevelopment and aging: the majority of the subjects experienced a decrease in total brain volume during adulthood—although the youngest age bin in childhood shows mostly increases, increase in volume of the ventricles at all ages, increases of white matter till young adulthood and then decreases in older subjects, and a decrease in volume of the cortical gray matter throughout all ages. [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 6.
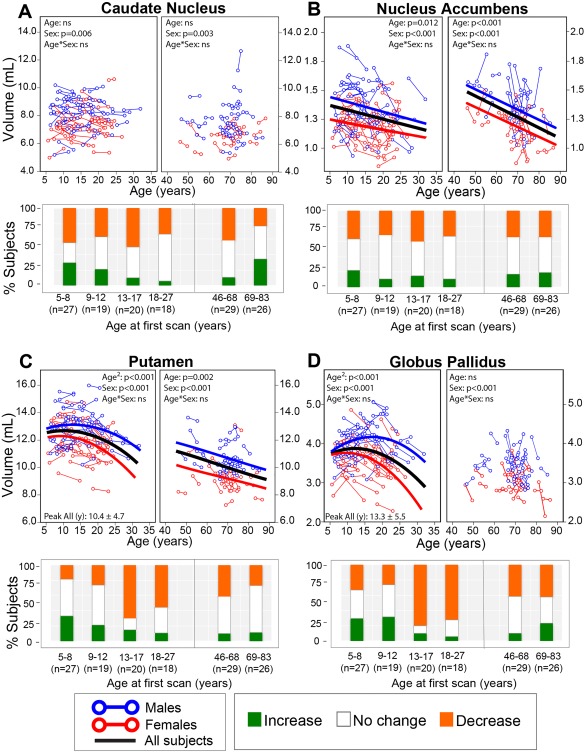
Longitudinal trajectories of (A) caudate nucleus, (B) nucleus accumbens, (C) putamen, and (D) globus pallidus volumes with age with associated subject histograms denoting significant (greater than ±1 SD) volume changes between the two scans approximately 3–4 years apart on average. Panels with no best‐fit curves indicate non‐significant age effects in that cohort. The putamen and globus pallidus follow an inverted U‐shaped trajectory in the younger cohort, whereas putamen volume gradually declined in the aging cohort while globus pallidus volume did not change with age in the aging cohort. Nucleus accumbens volume decreased linearly with age over both groups. The caudate did not change significantly with age in either young or aging cohorts. The histograms show that the two youngest age bins show a mix of volume changes, but by age 13–17 years a large number of subjects are showing a reduction of volume between scans for the putamen and globus pallidus. In addition the two oldest age bins (after 46 years) show a mix with many showing no volume changes. [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 7.
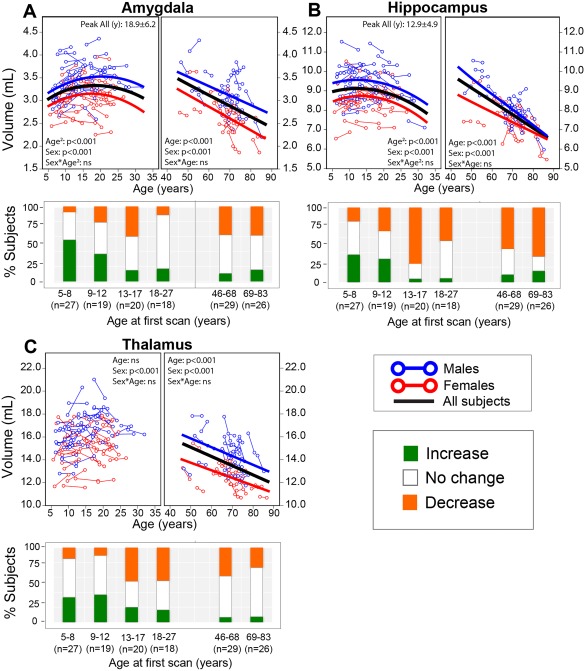
Longitudinal trajectories of (A) amygdala, (B) hippocampus, and (C) thalamus volumes with age and their associated subject histograms denoting significant (greater than ±1 SD) volume changes between the two scans approximately 3–4 years apart on average. The amygdala and hippocampus follow an inverted U‐shaped trajectory peaking in volume in adolescence, and experience the steepest declines in volume among the 7 subcortical structures after 46 years of age. The thalamus did not change significantly with age in the neurodevelopmental cohort, but declined in volume in the aging cohort. The individual subject histograms shows growth of the amygdala in half of the subjects in the 5–8 year old bin, but is variable with increases, decreases, and no change for other structures over all age bins. An exception is the hippocampus that shows about 50%–75% of the subjects decreasing in volume in the four oldest bins, 13 years and up. [Color figure can be viewed at http://wileyonlinelibrary.com]
The annual rates of volume change averaged over the longitudinal subjects in each of the 6 age bins (including those who were deemed to undergo “no change”) are shown for the overall brain structures (Fig. 8A) and the 7 subcortical gray matter structures (Fig. 8B). The four youngest age bins covering 5–27 years all show increases of white matter (∼1% to 3%/year), decreases of cortical gray matter (∼−1% to −1.5%/year), and increases of ventricle volume (∼1.5% to 2%/year); however, the total subcortical gray matter shows no change in the 5–8 and 9–12 year old bins, but decreases by approximately 0.8%/year in the 13–17 and 18–27 year old age bins. The thalamus grew 0.4%–0.5%/year in age bins spanning 5 to 12 years, followed by a reversal starting at age bin 13–17 years to yield a mean 0.5% annual decline until adulthood. Caudate volume declined by 0.1%–0.5%/year across all age bins of the neurodevelopment cohort. The putamen, globus pallidus and hippocampus experienced a similar rate of growth of less than 0.5% per year in the 5–8 year old bin, while the globus pallidus and putamen declined faster (at 1.5%/year and 1.2%/year, respectively) than the hippocampus (0.8%/year) in the age bin spanning 13–17 years. Among all structures, the amygdala grew fastest (1.1%/year) in the age bin spanning 5–8 years, with a modest slowdown of this growth in the 9–12 year old bin and followed by a decrease of volume by 0.5%/year in the 13–17 year old bin. The nucleus accumbens experienced varying rates of decline in volume across neurodevelopment.
Figure 8.
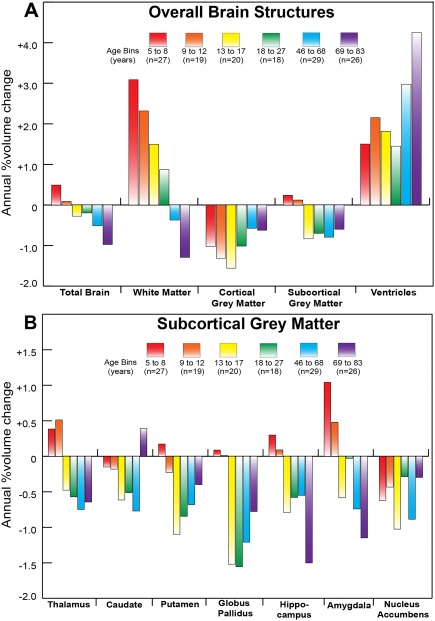
Average annual percent volume change per age bin of (A) overall brain structures and (B) subcortical gray matter for the longitudinal neurodevelopmental and aging cohorts. This figure reveals the magnitude of annual volume change (in percent) experienced on average at each age bin as a complement to the histograms in Figures 5–7 which show the number of subjects experiencing significant changes in a brain structure between two scans separated by approximately 3–4 years on average. (A) The white matter increases lessen in magnitude with age and then reverse to decreases in older age. The cortical gray matter decreases at all ages and the subcortical gray matter only decreases above 13 years. The ventricle volume shows the greatest proportion of increase at the oldest age bin. (B) Structures that tend to peak later (thalamus and amygdala) experience positive and higher magnitudes of annual change in the two youngest age bins compared with structures of the basal ganglia. The reduction of subcortical gray matter volume is evident for all bins above 13 years. The largest percent annual reductions are in the 13–27 year range for globus pallidus and the 69–83 year range for hippocampus. [Color figure can be viewed at http://wileyonlinelibrary.com]
Longitudinal cohort: Aging
In contrast to the neurodevelopment cohort, total brain, cortical gray and white matter volume decreased linearly as a function of age, accompanied by corresponding linear increases of bilateral ventricular volumes from 46 to 86 years (Fig. 5). Five of the seven subcortical structures showed linear decreases of volume, while the caudate and globus pallidus volume did not change significantly with age (Table 4; Figs. 6 and 7). Males had larger raw overall cortical and subcortical volumes for all structures, by 5% in the hippocampus to 13% in the caudate (Table 3). There were no significant age‐by‐sex interactions for any structure in this cohort.
All overall brain structures in the longitudinal aging cohort showed marked reductions (−0.5% to −1.5%/year) whereas the ventricle volume increased by 3%–4%/year (Fig. 8A). Between ages 46–68 years, annual rates of decline ranged from 0.6%/year in the hippocampus to 0.9%/year in the nucleus accumbens (Fig. 8B). By age bin 69–83 years, while the hippocampus and amygdala experienced an abrupt increase in the rate of volumetric decline (1.5%/year and 1.2%/year, respectively), the caudate had a modest positive rate of growth (0.4%/year), though note that caudate volume was not found to change with age when these two age bins were pooled in trajectory analysis. The other four structures experienced rates of decline from 0.3%/year in the nucleus accumbens to 0.8%/year in the globus pallidus in this oldest age bin (Fig. 8B).
Proportions of Longitudinal Subjects That Change in Volume for Different Age Groups
Total brain volume increased in 75% of 5–8 year old subjects and 40% of the 9–12 year olds, with greater variability in adolescence and young adult age bins and more than 50% of subjects in the 46+ age group undergoing volume decreases (Fig. 5). Cortical gray matter showed consistent volume decreases in 75%–100% of subjects at each age bin in the younger cohort and about 50% of subjects in each bin of the aging cohort. Conversely, 75%–100% of subjects underwent increases of white matter volume across all neurodevelopment age bins, while 50%–70% of subjects underwent decreases in the older age bins. The majority of subjects in all age bins showed increases of ventricular volume, although the magnitude of volume change was far greater at the older ages. This longitudinal data fits with the cross‐sectional curves (Fig. 2).
Overall, there was much less consistency in the proportion of subjects changing in each age bin among deep gray matter structures than for global measures. The caudate showed a smaller proportion of subjects experiencing volumetric growth with each increasing age bin, in contrast to a fairly stable proportion experiencing decreases (40%–50%) during the same period (Fig. 6A). Between ages 69–83 years, about 40% of subjects experience volumetric growth in the caudate, which was not observed in other subcortical structures (Fig. 6A). About 10%–20% of subjects in both neurodevelopmental and aging cohorts experienced increases in nucleus accumbens volume, with approximately 40%–50% showing no change and 30%–40% showing volume decreases (Fig. 6B). The putamen and globus pallidus had similar histogram proportion patterns with each other across all age bins: 20%–30% experienced increases and 20%–30% experienced decreases in volume between age bins 5–8 and 9–12 years, 60%–85% of subjects experience decreases in volume during adolescence and early adulthood (13–27 years), and 30%–40% experience decreases for 46 years and up bins (Figs. 6C, D). The amygdala had 50% of 5–8 year olds with growth, but there was a mix of changes at all older age bins (Fig. 7A). The hippocampus had mixed changes at the two youngest age bins, but 75% showed reductions in the 13–17 year old age bin followed by approximately 50%–70% of individuals with reductions in the three older age bins (Fig. 7B). Thalamus volume had a mix of changes, but decreased in approximately 50% of subjects in the 13–17 and 18–27 year old bins (Fig. 7C).
Agreements and Differences Between Cross‐Sectional and Longitudinal Data
Total brain volume increased until 15 years of age in the cross‐sectional cohort (Fig. 2A), whereas no significant change with age was observed between ages 5–31 years in the longitudinal neurodevelopment cohort (Fig. 5A). Both cross‐sectional and longitudinal data support larger volumes in males than females and increases in ventricle volume across the lifespan with greater changes at older ages (Figs. 2B and 5B), but only the cross‐sectional cohort suggested an earlier acceleration of this process in males. In addition, both datasets support a faster decline of cortical gray matter volume earlier in the lifespan, as compared with a slower decline in late adulthood (Figs. 2C and 5C) as well as increasing white matter volume at younger ages and decreasing at older ages (Figs. 2D and 5D).
In those structures that had non‐linear fits in both cross‐sectional (Fig. 3) and longitudinal (neurodevelopment) data sets (Figs. 6 and 7), the peak ages were similar between cross sectional and longitudinal cohorts for putamen (12 versus 10 years), globus pallidus (13 versus 13 years) and amygdala (19 versus 23 years), whereas hippocampal volume peaked at 23 years in the cross‐sectional cohort and 13 years in the longitudinal cohort, although in both cases the quadratic curve “peak” for the hippocampus is quite flat with a wide standard deviation. The nucleus accumbens decreased linearly in all 3 cohorts (cross sectional and both longitudinal). Both the caudate and thalamus volume did not change significantly with age in the longitudinal neurodevelopment cohort as opposed to peaking at about 14 and 19 years, respectively, in the cross‐sectional data.
In the longitudinal aging cohort, all subcortical gray matter structures showed significant linear volume reductions with the exception of the caudate and globus pallidus; in these two latter cases this was in keeping with the flattened trajectory of the cross‐sectional Poisson curves during this age. However, over 25% of the subjects in the oldest longitudinal age bin (69–83 years of age) were found to have increases in caudate volume. An accelerated decline in the hippocampus during late adulthood was observed in both datasets. The longitudinal aging volume reductions in the amygdala and thalamus are mirrored in the cross‐sectional data, but the putamen shows a rather flat cross‐sectional curve at older ages despite a significant decrease in the longitudinal aging cohort.
DISCUSSION
Cross‐Sectional Cohort
Several cross‐sectional studies with large sample sizes of healthy “typical” participants have examined age‐dependent trajectories of subcortical gray matter volume change during childhood and adolescence, as well as within adulthood and aging. However, these studies have not had the age span needed to capture volume changes in both development and aging across multiple subcortical gray matter structures concurrently in the same sample. In this lifespan cross‐sectional study of 406, 5–83 year olds, we find non‐linear trajectories of change in all deep gray matter structures except the nucleus accumbens, which is shown to decrease linearly. Five of six deep gray matter structures with non‐linear trajectories were best fit to Poisson curves, indicating unique patterns of growth and decline before and after “peak” volume was reached, which may better model the unique anatomical mechanisms underlying morphological change during development versus aging. Maximal volumes were reached between ages 11–23 years (Fig. 3), with basal ganglia structures peaking earlier at 12–14 years of age, followed by the thalamus (∼19 years), and then limbic regions (∼23 years).
When examining deep gray matter structures, some studies report volumes that are corrected or normalized for intracranial volume (ICV) [Fjell et al., 2013; Jernigan and Gamst, 2005; Ostby et al., 2009; Walhovd et al., 2005; Walhovd et al., 2011], while others report change with age for raw volumes [Brain Development Cooperative Group, 2012; Brown et al., 2014; Giedd et al., 1996; Uematsu et al., 2012; Ziegler et al., 2012] as we did here. This is an important detail to consider while comparing results across these studies, particularly in portions of the lifespan (i.e., young and old ages) when changes in ICV or total brain volume are expected. While normalized volumes reduce inter‐subject variability, they also represent the relative slopes of these two processes (i.e., the rate at which a subcortical volume is changing relative to the rate at which total brain volume/ICV is changing), which follow unique developmental trajectories that can impact interpretation [Mills et al., 2016].
In contrast to our findings, previous cross‐sectional studies spanning childhood to late adolescence or young adulthood (i.e., overlapping with approximately the first one third of our age span) have not identified consistent upswings across all structures during the developmental period. Rather, several studies have identified linear decreases of volume in the caudate [Giedd et al., 1996, 4–18 years; Ostby et al., 2009, 8–30 years], globus pallidus [Ostby et al., 2009] and putamen [Brain Development Cooperative Group, 2012, 4–18 years; Giedd et al., 1996; Ostby et al., 2009]. Nonetheless, linear [Ostby et al., 2009] or non‐linear volume increases of volume have been identified for the thalamus [Brain Development Cooperative Group, 2012; Brown et al., 2012, 3–21 years; Giedd et al., 1996], amygdala [Ostby et al., 2009], and hippocampus [Brown et al., 2012; Ostby et al., 2009] in these same cohorts. Although somewhat inconsistent with our findings, linear decreases of volume may nonetheless suggest earlier development in basal ganglia structures (that have maximized in size at younger ages) relative to the thalamus, hippocampus, and amygdala, in keeping with the order of maturation we observe.
In the latter portion of the lifespan, Poisson curves model a gradual decline in volume that levels off in late adulthood, with the exception of the nucleus accumbens (decreasing linearly) and the hippocampus, which was fit to a quadratic trajectory indicating acceleration of volume loss starting at approximately 50–60 years of age. Previous cross‐sectional studies of adults (i.e., overlapping with the latter one half to two thirds of our age span) have consistently identified linear decreases in the nucleus accumbens and thalamus [Fjell et al., 2013; Walhovd et al., 2005; Walhovd et al., 2011], and non‐linear decreasing trajectories in the hippocampus [Fjell et al., 2013; Walhovd et al., 2005; Walhovd et al., 2011; Ziegler et al., 2012]. Conversely, these studies yield a mix of results for the remaining structures, with some studies finding linear decreases while others find non‐linear decreases (amygdala, caudate, putamen) or no change (globus pallidus) in subjects spanning approximately 20–90 years of age [Fjell et al., 2013; Walhovd et al., 2005; Walhovd et al., 2011; Ziegler et al., 2012].
Although it is tempting to make comparisons of the age at which each structure reaches “peak” volume across studies (for those that use model fits that yield peaks), this must be done with caution. Quadratic fits of cross sectional data can be substantially influenced by characteristics of the sample such as age‐range [Fjell et al., 2010]. This is demonstrated in the cross‐sectional literature in which the hippocampus has been shown to reach a peak volume anywhere from approximately 10 to 11 years [Uematsu et al., 2012; subjects spanning 1 month to 25 years of age], approximately 14 years [Brown et al., 2012, 3–21 years], 23–26 years [this study, 5–85 years of age; Jernigan and Gamst, 2005, 10–90 years of age] or approximately 45 years of age [Walhovd et al., 2005, 20–88 years]. It is evident that “peak” ages are not only influenced by the total age span, but also the starting age, which makes sense given that very large gains in volume are made very early in the lifespan (<2 years of age) and likewise with very large decreases late in the lifespan, which can overshadow the more subtle changes observed across the remaining years. Nonetheless, even with these limitations, non‐linear models can be useful for comparing the relative timing of maturation across structures within a single study, where the age range and sample characteristics are controlled. This also further demonstrates the value of longitudinal datasets (discussed next), which allow for assessment of change within subjects to complement model fitting.
Longitudinal Neurodevelopment Cohort
As with cross‐sectional studies, some longitudinal studies examining deep gray matter structures report values that are corrected/controlled for ICV [Fjell et al., 2009a; Pfefferbaum et al., 2013], while other report raw values [Goddings et al., 2014; Lenroot et al., 2007; Raznahan et al., 2014; Tamnes et al., 2013; Wierenga et al., 2014], as in this study. In our longitudinal neurodevelopment cohort of 84 subjects scanned twice each approximately 3–4 years apart (5–27 years at first scan), four of the seven subcortical gray matter structures were found to significantly fit a quadratic trajectory in the longitudinal neurodevelopment cohort, indicating an upswing followed by a plateau and subsequent decrease of volume, namely for the globus pallidus, putamen, hippocampus and amygdala. For all four of these structures, 30%–50% of subjects in the youngest two age bins underwent increases of volume, followed by a high proportion of subjects (up to 80% for the globus pallidus) who underwent decreases of volume between scans in the 13–17 year age bin, confirming that this adolescent period is when an inflection in subcortical volume is observed. This supports the Poisson fit found in the cross sectional data for the globus pallidus, putamen and amygdala, and might suggest that the quadratic fit for the hippocampus in the cross sectional data is underestimating an inflection during childhood, potentially as a result of the influence of steep declines late in life.
Previous longitudinal studies with inter‐scan intervals of approximately 2–3 years in healthy subjects covering this age range have likewise uncovered non‐linear trajectories for the globus pallidus [Raznahan et al., 2014; Wierenga et al., 2014], hippocampus and amygdala [Goddings et al., 2014; Wierenga et al., 2014]. Previous studies of the putamen have been less consistent, with some indicating non‐linear changes similar in shape to the trajectory we observe here [Goddings et al., 2014], while others find linear decreases in volume [Tamnes et al., 2013; Wierenga et al., 2014]. The age at which volume levels off varies between these studies (e.g., as early as 8–10 years [Raznahan et al., 2014] or 17–18 years [Wierenga et al., 2014] for the globus pallidus), likely stemming from differences in sample and fitting parameters (e.g., cubic vs. quadratic), or the interval between scans. Puberty (not measured here) is another key element to consider in studies of neurodevelopment, and may partially contribute to variability in peak ages. In a very large longitudinal study of 275 individuals, ages 7–20 years, puberty stage was found to independently influence the volume of the amygdala, hippocampus and putamen in both males and females [Goddings et al., 2014]. This finding may account for some inter‐subject variability in longitudinal measurements of change with age, given inter‐individual and sex differences in puberty onset.
The caudate and thalamus did not undergo significant change with age in this longitudinal neurodevelopment cohort. For the caudate, there were a roughly equal proportion of subjects undergoing increases and decreases of volume in the youngest two age bins, though the greater proportion of subjects who underwent volume decreases in the 13–17 year old age bin suggesting a unique period of development in adolescence that may be ubiquitous to all structures. In the thalamus, over 50% of subjects in the first two age bins were found to undergo no change, though an increase in the number of subjects undergoing volume reduction was again seen starting in the 13–17 year old age bin. For both structures, a Poisson curve was fit to the cross sectional data (albeit a shallow curve for the caudate), which these longitudinal results may suggest would have overestimated an upswing in childhood. Previous longitudinal work has uncovered a mix of results, including linear decreases [Tamnes et al., 2013; Wierenga et al., 2014] or non‐linear increases in the caudate [Lenroot et al., 2007] and thalamus [Raznahan et al., 2014; Tamnes et al., 2013; Wierenga et al., 2014] in the neurodevelopment age range.
Lastly, the nucleus accumbens was fit to a linear trajectory here, decreasing with age. Likewise the proportion of subjects undergoing increases, decreases or no change remained consistent across each age bin, in keeping with a linear fit. These longitudinal findings in the nucleus accumbens also match the linear fit in our cross‐sectional sample, as well as in previous longitudinal samples finding linear decreases of volume across similar age ranges [Goddings et al., 2014; Tamnes et al., 2013; Wierenga et al., 2014].
Longitudinal Aging Cohort
The 55‐subject longitudinal aging cohort (ages 46–83 years) showed linear decreases of volume over 3 years in total brain, cortical gray, white matter, and 5 of 7 subcortical gray matter structures, along with concomitant increases of ventricle volume. This overall pattern is in keeping with previous reports of volumetric atrophy of subcortical brain structures that starts in late adulthood, continuing into old age [Fjell et al., 2009a; Pfefferbaum et al., 2013].
The only two subcortical structures not observed to undergo longitudinal linear decreases of volume over 46–83 years were the caudate and globus pallidus, which did not change significantly with age here in keeping with the flattened trajectory of change found across these older ages in our cross‐sectional cohort. Nonetheless, nearly half of the subjects in the 46–68 year old age bin underwent decreases of volume between scans for both the caudate and globus pallidus, suggesting some degree of atrophy. In contrast, 60%–70% of subjects underwent a decrease in volume of the hippocampus in the oldest two age bins, in keeping our cross‐sectional results as well as previous cross‐sectional and longitudinal studies finding acceleration of hippocampal atrophy late in life [Pfefferbaum et al., 2013; Walhovd et al., 2005; Ziegler et al., 2012].
These longitudinal findings are somewhat incongruent with the cross sectional findings for the thalamus, putamen and amygdala, which are fit to Poisson trajectories and model a relative leveling off of volume change in the oldest ages, potentially underestimating change in this segment of the lifespan.
Sex Differences
This study observed larger absolute brain volumes in males than females in both cross‐sectional and longitudinal cohorts, with differences ranging from 8% to 20% across the lifespan, in keeping with previous work [Jancke et al., 2015; Lenroot and Giedd, 2006]. However, no age‐by‐sex interactions were found to be significant for any deep gray matter structure, suggesting similar trajectories of change with age between males and females across the lifespan. Although some studies suggest differences in age‐to‐peak between males and females [e.g., Goddings et al., 2014; Lenroot et al., 2007; Raznahan et al., 2014; Uematsu et al., 2012], or differences in the proportion of increases/decreases between scans for males versus females [Dennison et al., 2013], others have shown similar peak ages of deep gray matter volumes between sexes [Wierenga et al., 2014] and lack of age‐by‐sex interactions [Fjell et al., 2009b]. Although there is evidence that factors such as puberty stage may influence deep gray matter volume trajectories [Goddings et al., 2014], which may partially mediate sex difference observed in some studies, many other factors, for example, genetics, may play a more dominant role than sex or hormones alone [Hibar et al., 2015].
Biological Mechanisms
Our results generally suggest heterogeneous non‐linear development during childhood and adolescence that follows a more complex pattern than the generally consistent linear decline seen in late adulthood. There is also some evidence to suggest earlier development of basal ganglia structures relative to the thalamus and limbic structures. Early maturation of the basal ganglia may relate to its more basic, unconscious processes such as motor initiation, learning and reward‐seeking behavior required for early development [Leisman et al., 2014]. As children age, more complex acquired experiences require concurrent and integrated sensory and motor processes, necessitating a mature thalamus to parse and prioritize information to different brain regions for integrated responses [Muftuler et al., 2011]. Late maturation of the amygdala and hippocampus may be explained by their involvement in higher‐order functions including emotional processing, memory consolidation and behavior [Catani et al., 2013], or by working in tandem with cortical areas involved in higher‐order executive function such as the temporal and frontal lobes, which also mature later relative to other cortical areas [Gogtay et al., 2004; Shaw et al., 2008].
For all deep gray matter structures, approximately 45%–85% of subjects experienced significant volume reduction during the 13–17 year old period. In the amygdala and hippocampus, a higher proportion of 13–17 year old subjects underwent a volume decrease between scans (∼45% for the amygdala; 75% for the hippocampus) than in the 18–27 year old bin (∼10% for the amygdala; 45% for the hippocampus), suggesting widespread volume reductions in the subcortical brain that may accelerate during adolescence, particularly in limbic structures. The globus pallidus showed the greatest proportions (85%) of 13–17 year olds undergoing volume decreases over 3–4 years, suggesting its relevance to changes in motor, cognitive, and emotional development during this period, although these were not measured in this study. Deep grey matter volume loss during adolescence fits with an acceleration of cortical thinning seen in the cortex during this time [Zhou et al., 2015] and coincides with the period of gradual synaptic pruning observed during adolescence in human histology studies [Huttenlocher, 1979] and within subcortical structures such as the hippocampus in animal models [Afroz et al., 2016]. Widespread longitudinal deep gray matter volume reductions during adolescence are intriguing given a recent large population (n = 4568) multi‐site ENIGMA study showing that 5/7 deep gray matter structures were altered in schizophrenia and that globus pallidus volume increases (perhaps due to lack of appropriate pruning) correlated with duration of illness [Van Erp et al., 2016].
At older ages, trajectories of volume change appear to converge, with widespread linear decreases seen for nearly all subcortical structures. Despite concurrent morphological change, the mechanisms driving these volume reductions may nonetheless be specific to each structure. For example, decline in serum BDNF levels have been shown to predict hippocampal but not caudate volume decline in older adults [Erickson et al., 2010]. Likewise, brain iron accumulation has been shown to predict putamen but not caudate volume decline in late life [Daugherty and Raz, 2016], again suggesting that volume decline in deep gray matter may be influenced by unique physiological mechanisms.
Across both cross sectional and longitudinal cohorts, we see pronounced decline of hippocampal volume in late life, in keeping with previous reports [e.g., Pfefferbaum et al., 2013] and suggesting a unique vulnerability of this structure to aging. Indeed, hippocampal atrophy at older ages (65–80 years) but not middle ages (55–60 years) has been correlated with episodic memory decline in healthy adults [Gorbach et al., 2017], and may stem from a confluence of cellular mechanisms including reduced hippocampal neurogenesis secondary to reduced stem cell proliferation [Kempermann et al., 1998] and increased apoptosis [Sun et al., 2004].
Limitations
The cross‐sectional data spanned nearly the entire human lifespan with the exception of perinatal and early childhood years (<5 years), while our longitudinal cohort did not contain these younger subjects nor subjects from ages 31–45 years of age. The lack of longitudinal data in this latter time span makes it difficult to compare the best fit curves with our cross‐sectional cohort. For both longitudinal cohorts, models were only tested for linear and quadratic fits because of the age span limitation in the longitudinal subject population, in contrast to the 8‐decade age range available in the cross‐sectional cohort allowing more complex Poisson curves. Both left and right hemispheres were combined into a single absolute volume per structure, although significant volume asymmetry of deep gray matter structures, albeit rather small, has been reported in 15,847 scans from 52 datasets [Guadalupe et al., 2016].
Given that our sample represents a pooled dataset from 3 studies, the MPRAGE protocols used for each varied slightly, which could have the potential to impact age‐related change in the cross‐sectional cohort. However, contrast‐to‐noise ratios (measured in caudate versus frontal white matter) in a random sampling of 5 subjects from each protocol (15 scans total) yielded values of 12.5 ± 1.1, 8.1 ± 2.1, and 11.3 ± 2.2 for the 3 protocols listed in the methods, respectively, confirming similar image contrast between acquisitions. Moreover, all subjects were scanned using the same scanner and analyzed using the exact same version of FreeSurfer on the same computer, and longitudinal subject follow up scans were acquired with identical parameters as scan 1 data, thus eliminating any influence of protocol on longitudinal change measures.
In addition, data in this study were processed with Freesurfer v4.1 rather than the latest version available at the time of publication. Post‐hoc analysis of 19 longitudinal subjects re‐processed with the longitudinal pipeline in version 5.3 revealed small differences in the absolute value of volumes measured in keeping with previous reports [Gronenschild et al., 2012], but no difference in change with age between versions, suggesting that using the newest version would not have changed any of the conclusions of this article. Nonetheless, continued refinement of the segmentation algorithm in subsequent versions and in particular use of the longitudinal pipeline (which aims to reduce variability) may have advantages that cannot be appreciated in this relatively small (n = 19) subsample.
CONCLUSIONS
This large sample cross‐sectional and longitudinal study showed significant age‐dependent maturational patterns that differed among deep gray matter structures across the typical human lifespan. The cross‐sectional results fit well with a Poisson model of differential up and down swings of volume with age. Basal ganglia structure volumes peaked earlier than the thalamus, which in turn peaked earlier than the hippocampus and amygdala. One of the most striking observations from the longitudinal cohort was the large proportion of individuals with volume reduction of many deep gray matter structures between scans approximately 3–4 years apart in the 13–17 year age range that persisted into young adulthood. Although sex differences in absolute brain volumes were noted in all structures, no age‐by‐sex interactions were found for deep gray matter structures across the lifespan, suggesting sexual dimorphism of absolute brain volumes but not in trajectories of change with age. Given the crucial roles that these subcortical structures play in several behavioral and cognitive functions, it is imperative that a robust spatiotemporal understanding of their morphometric growth and decline is established across the lifespan.
ACKNOWLEDGMENTS
We thank Lindsay Walker, Catherine Lebel, Marguerite Wieler, and Myrlene Gee for MRI acquisition of many of the participants. Salary awards were provided by Alberta Innovates Health Solutions (AIHS) to co‐authors CB, ST, and KN and by the Canada Research Chairs program to CB.
Karl Narvacan and Sarah Treit are co‐first authors.
REFERENCES
- Afroz S, Parato J, Shen H, Smith SS (2016): Synaptic pruning in the female hippocampus is triggered at puberty by extrasynaptic GABA(A) receptors on dendritic spines. Elife 5: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard TP, Malykhin N, Martin WR, Hanstock CC, Emery DJ, Fisher NJ, Camicioli RM (2008): Age and dementia‐associated atrophy predominates in the hippocampal head and amygdala in Parkinson's disease. Neurobiol Aging 29:1027–1039. [DOI] [PubMed] [Google Scholar]
- Brain Development Cooperative Group (2012): Total and regional brain volumes in a population‐based normative sample from 4 to 18 years: The nih mri study of normal brain development. Cereb Cortex 22:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TT, Kuperman JM, Chung YH, Erhart M, McCabe C, Hagler DJ, Venkatraman VK, Akshoomoff N, Amaral DG, Bloss CS, Casey BJ, Chang L, Ernst TM, Frazier JA, Gruen JR, Kaufmann WE, Kenet T, Kennedy DN, Murray SS, Sowell ER, Jernigan TL, Dale AM (2012): Neuroanatomical assessment of biological maturity. Curr Biol 22:1693–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CJ, Miller SP, Booth BG, Andrews S, Chau V, Poskitt KJ, Hamarneh G (2014): Structural network analysis of brain development in young preterm neonates. Neuroimage 101:667–680. [DOI] [PubMed] [Google Scholar]
- Cahill L (2006): Why sex matters for neuroscience. Nat Rev Neurosci 7:477–484. [DOI] [PubMed] [Google Scholar]
- Camicioli R, Sabino J, Gee M, Bouchard T, Fisher N, Hanstock C, Emery D, Martin WRW (2011): Ventricular dilatation and brain atrophy in patients with parkinson's disease with incipient dementia. Mov Disord 26:1443–1450. [DOI] [PubMed] [Google Scholar]
- Catani M, Dell'Acqua F, Thiebaut de Schotten M (2013): A revised limbic system model for memory, emotion and behaviour. Neurosci Biobehav Rev 37:1724–1737. [DOI] [PubMed] [Google Scholar]
- Cosgrove KP, Mazure CM, Staley JK (2007): Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol Psychiatry 62:847–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E, Chisum HJ, Townsend J, Cowles A, Covington J, Egaas B, Harwood M, Hinds S, Press GA (2000): Normal brain development and aging: Quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology 216:672–682. [DOI] [PubMed] [Google Scholar]
- Daugherty AM, Raz N (2016): Accumulation of iron in the putamen predicts its shrinkage in healthy older adults: A multi‐occasion longitudinal study. Neuroimage 128:11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennison M, Whittle S, Yucel M, Vijayakumar N, Kline A, Simmons J, Allen NB (2013): Mapping subcortical brain maturation during adolescence: Evidence of hemisphere‐ and sex‐specific longitudinal changes. Dev Sci 16:772–791. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Prakash RS, Voss MW, Chaddock L, Heo S, McLaren M, Pence BD, Martin SA, Vieira VJ, Woods JA, McAuley E, Kramer AF (2010): Brain‐derived neurotrophic factor is associated with age‐related decline in hippocampal volume. J Neurosci 30:5368–5375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB, Fennema‐Notestine C, McEvoy LK, Hagler DJ, Holland D, Brewer JB, Dale AM (2009a): One‐year brain atrophy evident in healthy aging. J Neurosci 29:15223–15231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Amlien I, Espeseth T, Reinvang I, Raz N, Agartz I, Salat DH, Greve DN, Fischl B, Dale AM, Walhovd KB (2009b): Minute effects of sex on the aging brain: A multisample magnetic resonance imaging study of healthy aging and alzheimer's disease. J Neurosci 29:8774–8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB, Westlye LT, Ostby Y, Tamnes CK, Jernigan TL, Gamst A, Dale AM (2010): When does brain aging accelerate? Dangers of quadratic fits in cross‐sectional studies. Neuroimage 50:1376–1383. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Grydeland H, Amlien I, Espeseth T, Reinvang I, Raz N, Holland D, Dale AM, Walhovd KB, Alzheimer Dis Neuroimaging I (2013): Critical ages in the life course of the adult brain: Nonlinear subcortical aging. Neurobiol Aging 34:2239–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, Vaituzis AC, Vauss YC, Hamburger SD, Kaysen D, Rapoport JL (1996): Quantitative magnetic resonance imaging of human brain development: Ages 4‐18. Cereb Cortex 6:551–560. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Raznahan A, Mills KL, Lenroot RK (2012): Review: Magnetic resonance imaging of male/female differences in human adolescent brain anatomy. Biol Sex Differ 3:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore JH, Shi F, Woolson SL, Knickmeyer RC, Short SJ, Lin WL, Zhu HT, Hamer RM, Styner M, Shen DG (2012): Longitudinal development of cortical and subcortical gray matter from birth to 2 years. Cereb Cortex 22:2478–2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddings A‐L, Mills KL, Clasen LS, Giedd JN, Viner RM, Blakemore S‐J (2014): The influence of puberty on subcortical brain development. Neuroimage 88:242–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM (2004): Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A 101:8174–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, Horton NJ, Makris N, Kennedy DN, Caviness VS, Faraone SV, Tsuang MT (2001): Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cereb Cortex 11:490–497. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RNA, Friston KJ, Frackowiak RSJ (2001): A voxel‐based morphometric study of ageing in 465 normal adult human brains. Neuroimage 14:21–36. [DOI] [PubMed] [Google Scholar]
- Gorbach T, Pudas S, Lundquist A, Oradd G, Josefsson M, Salami A, de Luna X, Nyberg L (2017): Longitudinal association between hippocampus atrophy and episodic‐memory decline. Neurobiol Aging 51:167–176. [DOI] [PubMed] [Google Scholar]
- Gronenschild E, Habets P, Jacobs HIL, Mengelers R, Rozendaal N, van Os J, Marcelis M (2012): The effects of freesurfer version, workstation type, and macintosh operating system version on anatomical volume and cortical thickness measurements. PLoS One 7:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guadalupe T, Mathias SR, vanErp TG, Whelan CD, Zwiers MP, Abe Y, Abramovic L, Agartz I, Andreassen OA, Arias‐Vasquez A, Aribisala BS, Armstrong NJ, Arolt V, Artiges E, Ayesa‐Arriola R, Baboyan VG, Banaschewski T, Barker G, Bastin ME, Baune BT, Blangero J, Bokde AL, Boedhoe PS, Bose A, Brem S, Brodaty H, Bromberg U, Brooks S, Buchel C, Buitelaar J, Calhoun VD, Cannon DM, Cattrell A, Cheng Y, Conrod PJ, Conzelmann A, Corvin A, Crespo‐Facorro B, Crivello F, Dannlowski U, de Zubicaray GI, de Zwarte SM, Deary IJ, Desrivieres S, Doan NT, Donohoe G, Dorum ES, Ehrlich S, Espeseth T, Fernandez G, Flor H, Fouche JP, Frouin V, Fukunaga M, Gallinat J, Garavan H, Gill M, Suarez AG, Gowland P, Grabe HJ, Grotegerd D, Gruber O, Hagenaars S, Hashimoto R, Hauser TU, Heinz A, Hibar DP, Hoekstra PJ, Hoogman M, Howells FM, Hu H, Hulshoff Pol HE, Huyser C, Ittermann B, Jahanshad N, Jonsson EG, Jurk S, Kahn RS, Kelly S, Kraemer B, Kugel H, Kwon JS, Lemaitre H, Lesch KP, Lochner C, Luciano M, Marquand AF, Martin NG, Martinez‐Zalacain I, Martinot JL, Mataix‐Cols D, Mather K, McDonald C, McMahon KL, Medland SE, Menchon JM, Morris DW, Mothersill O, Maniega SM, Mwangi B, Nakamae T, Nakao T, Narayanaswaamy JC, Nees F, Nordvik JE, Onnink AM, Opel N, Ophoff R, Paillere Martinot ML, Papadopoulos Orfanos D, Pauli P, Paus T, Poustka L, Reddy JY, Renteria ME, Roiz‐Santianez R, Roos A, Royle NA, Sachdev P, Sanchez‐Juan P, Schmaal L, Schumann G, Shumskaya E, Smolka MN, Soares JC, Soriano‐Mas C, Stein DJ, Strike LT, Toro R, Turner JA, Tzourio‐Mazoyer N, Uhlmann A, Hernandez MV, van den Heuvel OA, van der Meer D, van Haren NE, Veltman DJ, Venkatasubramanian G, Vetter NC, Vuletic D, Walitza S, Walter H, Walton E, Wang Z, Wardlaw J, Wen W, Westlye LT, Whelan R, Wittfeld K, Wolfers T, Wright MJ, Xu J, Xu X, Yun JY, Zhao J, Franke B, Thompson PM, Glahn DC, Mazoyer B, Fisher SE, Francks C (2016): Human subcortical brain asymmetries in 15,847 people worldwide reveal effects of age and sex. Brain Imaging Behav 1:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero MT, Barcia C, Navarro JM (2002): Functional anatomy of thalamus and basal ganglia. Childs Nervous Syst 18:386–404. [DOI] [PubMed] [Google Scholar]
- Hibar DP, Stein JL, Renteria ME, Arias‐Vasquez A, Desrivieres S, Jahanshad N, Toro R, Wittfeld K, Abramovic L, Andersson M, Aribisala BS, Armstrong NJ, Bernard M, Bohlken MM, Boks MP, Bralten J, Brown AA, Chakravarty MM, Chen Q, Ching CRK, Cuellar‐Partida G, den Braber A, Giddaluru S, Goldman AL, Grimm O, Guadalupe T, Hass J, Woldehawariat G, Holmes AJ, Hoogman M, Janowitz D, Jia TY, Kim S, Klein M, Kraemer B, Lee PH, Loohuis LMO, Luciano M, Macare C, Mather KA, Mattheisen M, Milaneschi Y, Nho K, Papmeyer M, Ramasamy A, Risacher SL, Roiz‐Santianez R, Rose EJ, Salami A, Samann PG, Schmaal L, Schork AJ, Shin J, Strike LT, Teumer A, van Donkelaar MMJ, van Eijk KR, Walters RK, Westlye LT, Whelan CD, Winkler AM, Zwiers MP, Alhusaini S, Athanasiu L, Ehrlich S, Hakobjan MMH, Hartberg CB, Haukvik UK, Heister A, Hoehn D, Kasperaviciute D, Liewald DCM, Lopez LM, Makkinje RRR, Matarin M, Naber MAM, McKay DR, Needham M, Nugent AC, Putz B, Royle NA, Shen L, Sprooten E, Trabzuni D, van der Marel SSL, van Hulzen KJE, Walton E, Wolf C, Almasy L, Ames D, Arepalli S, Assareh AA, Bastin ME, Brodaty H, Bulayeva KB, Carless MA, Cichon S, Corvin A, Curran JE, Czisch M, de Zubicaray GI, Dillman A, Duggirala R, Dyer TD, Erk S, Fedko IO, Ferrucci L, Foroud TM, Fox PT, Fukunaga M, Gibbs JR, Goring HHH, Green RC, Guelfi S, Hansell NK, Hartman CA, Hegenscheid K, Heinz A, Hernandez DG, Heslenfeld DJ, Hoekstra PJ, Holsboer F, Homuth G, Hottenga JJ, Ikeda M, Jack CR, Jenkinson M, Johnson R, Kanai R, Keil M, Kent JW, Kochunov P, Kwok JB, Lawrie SM, Liu XM, Longo DL, McMahon KL, Meisenzah E, Melle I, Mahnke S, Montgomery GW, Mostert JC, Muhleisen TW, Nalls MA, Nichols TE, Nilsson LG, Nothen MM, Ohi K, Olvera RL, Perez‐Iglesias R, Pike GB, Potkin SG, Reinvang I, Reppermund S, Rietschel M, Romanczuk‐Seiferth N, Rosen GD, Rujescu D, Schnell K, Schofield PR, Smith C, Steen VM, Sussmann JE, Thalamuthu A, Toga AW, Traynor BJ, Troncoso J, Turner JA, Hernandez MCV, van't Ent D, van der Brug M, van der Wee NJA, van Tol MJ, Veltman DJ, Wassink TH, Westman E, Zielke RH, Zonderman AB, Ashbrook DG, Hager R, Lu L, McMahon FJ, Morris DW, Williams RW, Brunner HG, Buckner RL, Buitelaar JK, Cahn W, Calhoun VD, Cavalleri GL, Crespo‐Facorro B, Dale AM, Davies GE, Delanty N, Depondt C, Djurovic S, Drevets WC, Espeseth T, Gollub RL, Ho BC, Hoffman W, Hosten N, Kahn RS, L, Hellard S, Meyer‐Lindenberg A, Muller‐Myhsok B, Nauck M, Nyberg L, Pandolfo M, Penninx B, Roffman JL, Sisodiya SM, Smoller JW, van Bokhoven H, van Haren NEM, Volzke H, Walter H, Weiner MW, Wen W, White T, Agartz I, Andreassen OA, Blangero J, Boomsma DI, Brouwer RM, Cannon DM, Cookson MR, de Geus EJC, Deary IJ, Donohoe G, Fernandez G, Fisher SE, Francks C, Glahn DC, Grabe HJ, Gruber O, Hardy J, Hashimoto R, Pol HEH, Jonsson EG, Kloszewska I, Lovestone S, Mattay VS, Mecocci P, McDonald C, McIntosh AM, Ophoff RA, Paus T, Pausova Z, Ryten M, Sachdev PS, Saykin AJ, Simmons A, Singleton A, Soininen H, Wardlaw JM, Weale ME, Weinberger DR, Adams HHH, Launer LJ, Seiler S, Schmidt R, Chauhan G, Satizabal CL, Becker JT, Yanek L, van der Lee SJ, Ebling M, Fischl B, Longstreth WT, Greve D, Schmidt H, Nyquist P, Vinke LN, van Duijn CM, Xue LT, Mazoyer B, Bis JC, Gudnason V, Seshadri S, Ikram MA, Martin NG, Wright MJ, Schumann G, Franke B, Thompson PM, Medland SE, Alzheimers Dis N, Consortium C, Epigen Imagen Syst (2015): Common genetic variants influence human subcortical brain structures. Nature 520:224–U216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland D, Chang LD, Ernst TM, Curran M, Buchthal SD, Alicata D, Skranes J, Johansen H, Hernandez A, Yamakawa R, Kuperman JM, Dale AM (2014): Structural growth trajectories and rates of change in the first 3 months of infant brain development. Jama Neurol 71:1266–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher PR (1979): Synaptic density in human frontal‐cortex ‐ developmental‐changes and effects of aging. Brain Res 163:195–205. [DOI] [PubMed] [Google Scholar]
- Jancke L, Merillat S, Liem F, Hanggi J (2015): Brain size, sex, and the aging brain. Hum Brain Mapp 36:150–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL, Gamst AC (2005): Changes in volume with age ‐ consistency and interpretation of observed effects. Neurobiol Aging 26:1271–1274. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Fennema‐Notestine C, Gamst AC, Stout JC, Bonner J, Hesselink JR (2001): Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiol Aging 22:581–594. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH (1998): Experience‐induced neurogenesis in the senescent dentate gyrus. J Neurosci 18:3206–3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King D, Spencer SS, McCarthy G, Luby M, Spencer DD (1995): Bilateral hippocampal atrophy in medial temporal‐lobe epilepsy. Epilepsia 36:905–910. [DOI] [PubMed] [Google Scholar]
- Knickmeyer RC, Gouttard S, Kang CY, Evans D, Wilber K, Smith JK, Hamer RM, Lin W, Gerig G, Gilmore JH (2008): A structural mri study of human brain development from birth to 2 years. J Neurosci 28:12176–12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Beaulieu C (2011): Longitudinal development of human brain wiring continues from childhood into adulthood. J Neurosci 31:10937–10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Gee M, Camicioli R, Wieler M, Martin W, Beaulieu C (2012): Diffusion tensor imaging of white matter tract evolution over the lifespan. Neuroimage 60:340–352. [DOI] [PubMed] [Google Scholar]
- Leisman G, Braun‐Benjamin O, Melillo R (2014): Cognitive‐motor interactions of the basal ganglia in development. Front Syst Neurosci 8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN (2006): Brain development in children and adolescents: Insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev 30:718–729. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, Blumenthal JD, Lerch J, Zijdenbos AP, Evans AC, Thompson PM, Giedd JN (2007): Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage 36:1065–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Wu B, Batrachenko A, Bancroft‐Wu V, Morey RA, Shashi V, Langkammer C, De Bellis MD, Ropele S, Song AW, Liu C (2014): Differential developmental trajectories of magnetic susceptibility in human brain gray and white matter over the lifespan. Hum Brain Mapp 35:2698–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin WRW, Wieler M, Gee M, Camicioli R (2009): Temporal lobe changes in early, untreated Parkinson's disease. Mov Disord 24:1949–1954. [DOI] [PubMed] [Google Scholar]
- Mills KL, Goddings AL, Herting MM, Meuwese R, Blakemore SJ, Crone EA, Dahl RE, Guroglu B, Raznahan A, Sowell ER, Tamnes CK (2016): Structural brain development between childhood and adulthood: Convergence across four longitudinal samples. Neuroimage 141:273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno‐Alcazar A, Ramos‐Quiroga JA, Radua J, Salavert J, Palomar G, Bosch R, Salvador R, Blanch J, Casas M, McKenna PJ, Pomarol‐Clotet E (2016): Brain abnormalities in adults with Attention Deficit Hyperactivity Disorder revealed by voxel‐based morphometry. Psychiatry Res‐Neuroimaging 254:41–47. [DOI] [PubMed] [Google Scholar]
- Muftuler LT, Davis EP, Buss C, Head K, Hasso AN, Sandman CA (2011): Cortical and subcortical changes in typically developing preadolescent children. Brain Res 1399:15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardelli A, Lebel C, Rasmussen C, Andrew G, Beaulieu C (2011): Extensive deep gray matter volume reductions in children and adolescents with fetal alcohol spectrum disorders. Alcoholism 35:1404–1417. [DOI] [PubMed] [Google Scholar]
- Osipowicz K, Bosenbark DD, Patrick KE (2015): Cortical changes across the autism lifespan. Autism Res 8:379–385. [DOI] [PubMed] [Google Scholar]
- Ostby Y, Tamnes CK, Fjell AM, Westlye LT, Due‐Tonnessen P, Walhovd KB (2009): Heterogeneity in subcortical brain development: A structural magnetic resonance imaging study of brain maturation from 8 to 30 years. J Neurosci 29:11772–11782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Rohlfing T, Rosenbloom MJ, Chu WW, Colrain IM, Sullivan EV (2013): Variation in longitudinal trajectories of regional brain volumes of healthy men and women (ages 10 to 85 years) measured with atlas‐based parcellation of MRI. Neuroimage 65:176–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Kennedy KM, Head D, Gunning‐Dixon F, Acker JD (2003): Differential aging of the human striatum: Longitudinal evidence. Am J Neuroradiol 24:1849–1856. [PMC free article] [PubMed] [Google Scholar]
- Raznahan A, Shaw PW, Lerch JP, Clasen LS, Greenstein D, Berman R, Pipitone J, Chakravarty MM, Giedd JN (2014): Longitudinal four‐dimensional mapping of subcortical anatomy in human development. Proc Natl Acad Sci U S A 111:1592–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, Greenstein D, Clasen L, Evans A, Rapoport JL, Giedd JN, Wise SP (2008): Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci 28:3586–3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Trauner DA, Gamst A, Jernigan TL (2002): Development of cortical and subcortical brain structures in childhood and adolescence: A structural MRI study. Dev Med Child Neurol 44:4–16. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom M, Serventi KL, Pfefferbaum A (2004): Effects of age and sex on volumes of the thalamus, pons, and cortex. Neurobiol Aging 25:185–192. [DOI] [PubMed] [Google Scholar]
- Sun W, Winseck A, Vinsant S, Park OH, Kim H, Oppenheim RW (2004): Programmed cell death of adult‐generated hippocampal neurons is mediated by the proapoptotic gene Bax. J Neurosci 24:11205–11213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamnes CK, Walhovd KB, Dale AM, Ostby Y, Grydeland H, Richardson G, Westlye LT, Roddey JC, Hagler DJ, Due‐Tonnessen P, Holland D, Fjell AM, Alzheimers Dis Neuroimaging I (2013): Brain development and aging: Overlapping and unique patterns of change. Neuroimage 68:63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teipel SJ, Grothe M, Lista S, Toschi N, Garaci FG, Hampel H (2013): Relevance of magnetic resonance imaging for early detection and diagnosis of alzheimer disease. Med Clin N Am 97:399. [DOI] [PubMed] [Google Scholar]
- Treit S, Lebel C, Baugh L, Rasmussen C, Andrew G, Beaulieu C (2013): Longitudinal MRI reveals altered trajectory of brain development during childhood and adolescence in fetal alcohol spectrum disorders. J Neurosci 33:10098–10109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uematsu A, Matsui M, Tanaka C, Takahashi T, Noguchi K, Suzuki M, Nishijo H (2012): Developmental trajectories of amygdala and hippocampus from infancy to early adulthood in healthy individuals. PLoS One 7:e46970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Erp TGM, Hibar DP, Rasmussen JM, Glahn DC, Pearlson GD, Andreassen OA, Agartz I, Westlye LT, Haukvik UK, Dale AM, Melle I, Hartberg CB, Gruber O, Kraemer B, Zilles D, Donohoe G, Kelly S, McDonald C, Morris DW, Cannon DM, Corvin A, Machielsen MWJ, Koenders L, de Haan L, Veltman DJ, Satterthwaite TD, Wolf DH, Gur RC, Gur RE, Potkin SG, Mathalon DH, Mueller BA, Preda A, Macciardi F, Ehrlich S, Walton E, Hass J, Calhoun VD, Bockholt HJ, Sponheim SR, Shoemaker JM, van Haren NEM, Pol HEH, Ophoff RA, Kahn RS, Roiz‐Santianez R, Crespo‐Facorro B, Wang L, Alpert KI, Jonsson EG, Dimitrova R, Bois C, Whalley HC, McIntosh AM, Lawrie SM, Hashimoto R, Thompson PM, Turner JA, Grp ESW (2016): Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol Psychiatry 21:547–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Uden IWM, van Norden AGW, de Laat KF, van Oudheusden LJB, Gons RAR, Tendolkar I, Zwiers MP, de Leeuw FE (2011): Depressive symptoms and amygdala volume in elderly with cerebral small vessel disease: The ruN DMC study. J Aging Res 2011:647869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenmakers EJ, Farrell S (2004): AIC model selection using Akaike weights. Psychon Bull Rev 11:192–196. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Reinvang I, Lundervold A, Dale AM, Eilertsen DE, Quinn BT, Salat D, Makris N, Fischl B (2005): Effects of age on volumes of cortex, white matter and subcortical structures. Neurobiol Aging 26:1261–1270. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Westlye LT, Amlien I, Espeseth T, Reinvang I, Raz N, Agartz I, Salat DH, Greve DN, Fischl B, Dale AM, Fjell AM (2011): Consistent neuroanatomical age‐related volume differences across multiple samples. Neurobiol Aging 32:916–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westlye LT, Walhovd KB, Dale AM, Bjornerud A, Due‐Tonnessen P, Engvig A, Grydeland H, Tamnes CK, Ostby Y, Fjell AM (2010): Life‐span changes of the human brain white matter: Diffusion tensor imaging (dti) and volumetry. Cereb Cortex 20:2055–2068. [DOI] [PubMed] [Google Scholar]
- Wierenga L, Langen M, Ambrosino S, van Dijk S, Oranje B, Durston S (2014): Typical development of basal ganglia, hippocampus, amygdala and cerebellum from age 7 to 24. Neuroimage 96:67–72. [DOI] [PubMed] [Google Scholar]
- Yeatman JD, Wandell BA, Mezer AA (2014): Lifespan maturation and degeneration of human brain white matter. Nat Commun 5:5932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi HA, Moller C, Dieleman N, Bouwman FH, Barkhof F, Scheltens P, van der Flier WM, Vrenken H (2016): Relation between subcortical gray matter atrophy and conversion from mild cognitive impairment to Alzheimer's disease. J Neurol Neurosurg Psychiatry 87:425–432. [DOI] [PubMed] [Google Scholar]
- Zhou D, Lebel C, Treit S, Evans A, Beaulieu C (2015): Accelerated longitudinal cortical thinning in adolescence. Neuroimage 104:138–145. [DOI] [PubMed] [Google Scholar]
- Ziegler G, Dahnke R, Jancke L, Yotter RA, May A, Gaser C (2012): Brain structural trajectories over the adult lifespan. Hum Brain Mapp 33:2377–2389. [DOI] [PMC free article] [PubMed] [Google Scholar]


