Abstract
Memories associated with the self are remembered more accurately than those associated with others. The memory enhancement related to the self is known as the self‐reference effect (SRE). However, little is known regarding the neural mechanisms underlying the SRE in a social context modulated by social relationships. In the present fMRI study, we investigated encoding‐related activation of face memories encoded with the self‐referential process in a social context that was manipulated by imagining a person‐to‐person relationship. Healthy young adults participated in the present study. During encoding, participants encoded unfamiliar target faces by imagining a future friendship with themselves (Self), their friends (Friend), or strangers (Other). During retrieval, participants were presented with target and distracter faces one by one, and they judged whether each face had been previously learned. In the behavioral results, target faces encoded in the Self condition were remembered more accurately than those encoded in the Other condition. fMRI results demonstrated that encoding‐related activation in the medial prefrontal cortex (mPFC) was significantly greater in the Self condition than in the Friend or Other conditions. In addition, the generalized psycho‐physiological interaction (gPPI) analysis showed that functional connectivity between activation in the hippocampus and the cortical midline structures (CMSs), including the mPFC and precuneus, was significant in the Self but not in the Other condition. These findings suggest that the SRE in a social context could be involved in the interaction between the CMS regions, which are related to the self‐referential process, and the hippocampus related to the memory process. Hum Brain Mapp 38:4256–4269, 2017. © 2017 Wiley Periodicals, Inc.
Keywords: fMRI, encoding, self‐reference effect, medial prefrontal cortex, cortical midline structure, hippocampus, social context
INTRODUCTION
Memories associated with selves are remembered more accurately than those with others. Previous psychological studies have reported that self‐related memories are significantly enhanced compared to other‐related memories or memories encoded with the semantic process [Rogers et al., 1977; Symons and Johnson, 1997]. The enhancement of remembering memories involving self‐related information is known as the self‐reference effect (SRE) [Cunningham et al., 2008, 2013; Symons and Johnson, 1997]. Given that compared to healthy individuals, individuals with autism showed the decreasing enhancement of self‐performed memories [Millward et al., 2000] and the reduced SRE [Toichi et al., 2002], the impairment of social interaction in autism may be associated with the SRE deficit in a social context. However, approximately 70% of the studies showing a significant SRE have employed a laboratory‐based paradigm in which participants were required to encode adjective words describing personality traits by judging whether the words were appropriate to express their own personalities [Symons and Johnson, 1997], and hence, little is known regarding the neural mechanisms underlying the SRE in a social context. The present functional MRI (fMRI) study investigated the neural mechanisms associated with the SRE in a social context that was manipulated by imagining the social relationship with others.
Functional neuroimaging studies have consistently demonstrated the importance of the medial prefrontal cortex (mPFC) in the self‐referential process [Craik et al., 1999; Feyers et al., 2010; Gusnard et al., 2001; Heatherton et al., 2006; Johnson et al., 2002; Kelley et al., 2002; Zhu et al., 2007]. For example, one fMRI study reported that the mPFC region showed greater activation when judging whether adjectives satisfactorily described the personality traits of the self than when judging whether adjectives accurately described the personality traits of friends or judging whether adjectives were presented in capitalized letters [Heatherton et al., 2006]. There is also functional neuroimaging evidence that the cortical midline structures (CMSs), including the mPFC or other medial structures, contribute to the self‐referential process [Kim, 2012; Martinelli et al., 2013; Northoff et al., 2006]. For example, one fMRI study found that activation in the mPFC and posterior cingulate gyrus/precuneus was greater when judging whether adjectives appropriately expressed the personality traits of selves than when judging the semantics of adjectives [Saxe et al., 2006]. In addition, another fMRI study identified that activation within the mPFC was dissociable between processes related to selves and to self–other interactions and that activation in the posterior cingulate gyrus reflected an interaction between these processes [Mano et al., 2011]. Thus, the CMS regions including the mPFC and posterior cingulate gyrus could be involved not only in the simple association between selves and adjectives related to personality traits but also in the reference processes for self‐related information in a social context.
Previous studies have reported enhanced retrieval of memories encoded by the self‐referential process, or the SRE [Bower and Gilligan, 1979; Cunningham et al., 2014, 2008, 2013; D'Argembeau et al., 2005; Gutchess et al., 2007b; Kesebir and Oishi, 2010; Kuiper and Rogers, 1979; Leshikar et al., 2015; Pullyblank et al., 1985; Rogers et al., 1977; Serbun et al., 2011; Sui and Zhu, 2005; Turk et al., 2013, 2008; Yang et al., 2012; Zhang et al., 2014]. Functional neuroimaging studies investigating the SRE have demonstrated that activation in the mPFC region is significantly greater during the encoding of self‐related events than that of events unrelated to the self [Gusnard et al., 2001; Gutchess et al., 2007a; Leshikar and Duarte, 2012] and that mPFC activation related to the SRE is also identified during the retrieval of self‐related events [Cabeza et al., 2004; Lou et al., 2004]. In addition, another fMRI study revealed that the mPFC, hippocampus, insula, and lateral temporal cortex showed significant encoding‐related activation associated with the SRE and that functional connectivity patterns between the posterior cingulate gyrus and mPFC and between the hippocampus and posterior cingulate gyrus were significant in the SRE [Morel et al., 2014]. Given that the medial temporal lobe (MTL) region, including the hippocampus and parahippocampal gyrus, reflected the successful encoding of episodic memories [Davachi, 2006; Paller and Wagner, 2002], the interaction of the CMS regions including the mPFC or other medial structures, which are related to the processing of self‐related information, with the MTL, which reflects successful encoding, could contribute to the SRE. However, little is known regarding the neural mechanisms underlying the SRE in a social context such as a person‐to‐person relationship.
In the present study, using an event‐related fMRI method, we investigated the neural mechanisms related to self‐referential processing during the encoding of face memories in a social context modulated by imagining the person‐to‐person relationship. On the basis of previous studies, we made three predictions. First, memories of unfamiliar faces encoded by self‐referential processing in imagining participants' own future friendships would be enhanced in subsequent retrieval compared to those encoded by imagining future friendship with others. Second, the CMS regions including the mPFC would show greater activation during the encoding of unfamiliar faces imagined a future friendship with the participants themselves, than those imagined a future relationship with familiar or unfamiliar others. Third, functional connectivity between the CMS regions including the mPFC, which reflects self‐referential processing, and the MTL, which reflects successful encoding [Paller and Wagner, 2002], would contribute to the SRE in the social context of a person‐to‐person relationship.
MATERIALS AND METHODS
Participants
In the present study, we recruited 12 pairs of personal friends of the same sex (12 females and 12 males). They were graduate or undergraduate students at Kyoto University or Osaka University and were paid for their participation in our experiment. All participants were healthy, right‐handed, native Japanese‐speaking individuals with no history of neurological or psychiatric disease. To make a cue stimulus used in the encoding task, photos of individual faces with neutral expressions were taken before the experiment. The data from five participants were excluded from behavioral and fMRI analyses because neuroimaging data were not acquired for four participants due to device malfunction and one showed significantly higher false alarms (63.5%) than the other participants (mean = 19.8%, Smirnov–Grubbs test, P < 0.01). Thus, we analyzed behavioral and fMRI data from a total of 19 participants (9 females and 10 males, mean age = 22.5 years, SD = 1.6). All participants gave informed consent to the protocol that had been approved by the Institutional Review Board (IRB) of the Graduate School of Human and Environmental Studies, Kyoto University (24‐H‐9), and the Graduate School of Medicine, Kyoto University (C644).
Stimuli
For the target and distracter stimuli, 288 pictures of unfamiliar faces with emotionally neutral expressions (144 females and 144 males) were prepared. In these images, the eyes were directed to the front, and the ages of those shown ranged from thirties to fifties. Half of them were chosen from the in‐house face database, and the other half were collected from material on web sites. The in‐house face database was made by the voluntary participation of pedestrians in the downtown area of Kyoto city, and the faces from web sites mainly included the local council members in Japanese cities or prefectures other than Kyoto city and the neighborhood areas. We confirmed that all faces used in our experiment were unfamiliar for participants. For cue stimuli to instruct the experimental condition, face photos of participants and their friends who were recruited together in the experiment were employed. In addition, photos of one male and one female face, which were in the same generation as the participants (twenties) and unfamiliar to all participants, were used as cue stimuli. All stimuli of faces were automatically corrected in tone, contrast, and level using image‐processing software (Adobe Photoshop CS 5.1) and were then converted into gray‐scale images with a resolution of 96 dpi and a dimension of approximately 256 × 296 pixels. The 288 unfamiliar faces of the target and distracter stimuli were divided into four lists, among which age and sex of stimuli were equalized. Three of the four lists were applied to three encoding conditions of Self, Friend, and Other (see below), and another list was used for the distracter stimuli in the retrieval phase. These four lists of face photos were counterbalanced across the participants.
Experimental Procedures
All pairs of participants performed both encoding and retrieval tasks, and each participant of the pair was examined individually in the fMRI scanning session. fMRI scanning was applied to both the encoding and retrieval phases. fMRI experiments during both encoding and retrieval were designed by the event‐related fMRI method. Before fMRI scanning, participants were fully trained using the encoding and retrieval practice tasks on a Windows PC outside of the fMRI, and the experimenters confirmed that the participants comprehended the experimental procedures completely. However, this manuscript focused only on encoding‐related activation, because the aim of this study was to identify the effect of encoding operations of self‐referential process on behavioral responses and encoding‐related activation.
An example of encoding trials is illustrated in Figure 1. During a block of encoding, participants were presented with face pairs one by one: the face on the left side was used as a cue to instruct the encoding condition and the face on the right side was presented as a target to be remembered. Participants were instructed to subjectively rate how appropriate the target face is to the cued face in forming a potential friendship, and were told that their memory for the target faces would be tested in the later retrieval task. Responses for the ratings were recorded by pressing one of four buttons (DI, definitely inappropriate; PI, probably inappropriate; PA, probably appropriate; DA, definitely appropriate). Cues on the left side included faces of the participant, the participant's friend who was recruited to this experiment with the participant, and unfamiliar people of the same sex and generation as the participant, and these faces indicated the encoding condition of Self, Friend, and Other, respectively.
Figure 1.

Examples of the encoding and retrieval trials. During encoding, participants were required to rate how appropriate a friendship would be between a cued face on the left side, which indicated one of three encoding conditions (Self, Friend, or Other), and a target face on the right side, which was remembered. The rating scale was as follows: DI, definitely inappropriate; PI, probably inappropriate; PA, probably appropriate; DA, definitely appropriate. During retrieval, participants were presented with previously learned (target) and new (distracter) faces one by one and were required to recognize whether the faces were target or distracter faces using four response options (DO, definitely old; PO, probably old; PN, probably new; DN, definitely new). The example pictures shown in this figure were not included in the stimulus sets of our experiment, and all verbal labels were actually presented in Japanese. English labels are used here for illustration purposes only.
In the Self condition, participants were presented with their own face as a cue on the left side and were required to rate how appropriate a friendship would be between the target face on the right side and the participants' own face. In the Friend condition, participants were presented with a cued face of their friend who participated in the experiment and were required to rate how appropriate a friendship would be between the target face and the participant's friend. In the Other condition, participants were presented with a cued face of an unfamiliar person on the left side and were required to rate the appropriateness of a potential friendship between the cued and target faces. During encoding, each pair of cued and target faces was presented on the screen for 5.5 s, and a visual fixation was then presented as an interstimulus interval (ISI) for variable durations (0.5–6.5 s). In each block of encoding, we prepared 72 trials including 24 trials for each encoding condition, and these trials were presented in a random order across participants.
Immediately after the encoding block, participants performed the retrieval task. An example of retrieval trials is illustrated in Figure 1. During a retrieval block, participants were randomly presented with 96 faces individually, including 72 target and 24 distracter faces, and were required to judge whether the faces had been learned in the encoding block just before. “Old” or “New” responses were recorded by two levels of confidence, and hence, four response options were given: “definitely old (DO),” “probably old (PO),” “probably new (PN),” and “definitely new (DN),” one of which was chosen by pressing one of four buttons. Each face was presented for 3.5 s, and a visual fixation was then shown as an ISI for variable durations (0.5–6.5 s). The procedures for the encoding and retrieval blocks were alternatively repeated three times with different stimulus sets. Thus, participants performed the encoding and retrieval tasks for 216 target and 72 distracter faces.
After finishing all blocks of the encoding and retrieval tasks, participants subjectively rated how close the personal relationship was between themselves and their friend who participated in the experiment using a visual analogue scale (VAS) of 10 cm in length (from 0 cm: self to 10 cm: unfamiliar other). The rationale of this rating task was to confirm that participants mentally positioned their friends between themselves and unfamiliar others in personal relationship. The results showed that the mean value of this closeness rating was 2.3 cm (SD = 1.3). Thus, we confirmed that participants appropriately positioned their friends between themselves and unfamiliar others in their mental distance of personal relationship. The results of this rating were not disclosed to the friend of the participant.
MRI Acquisition
All MRI data were acquired using a Siemens MAGNETOM Trio 3T scanner at the Human Brain Research Center, Kyoto University. The stimulus presentation and recording of behavioral responses were controlled by MATLAB programs (http://www.mathworks.com) on a Windows PC. All stimuli were visually presented on an MRI‐compatible display (Nordic Neuro Lab, Inc., Bergen, Norway), and the participants viewed the stimuli through a mirror attached to the head coil of the scanner. Behavioral responses were recorded using an eight‐button optic fiber response device (Current designs, Inc., Philadelphia, PA, USA), which was composed of two response boxes with four buttons each. In the present study, only one four‐button response box was assigned to the right hand to record four response options in both the encoding and retrieval blocks. The scanner noise was reduced with earplugs, and head motion was minimized by foam pads.
During MRI scanning, T1‐weighted sagittal localizer scanning was acquired first. Second, gradient‐echo echo‐planar functional images (EPIs), which are sensitive to blood‐oxygenation‐level dependent (BOLD) contrasts, were acquired for functional scanning during encoding and retrieval (TR = 2 s, TE = 25 ms, flip angle = 70°, FOV = 24.3 × 24.3 cm, matrix size = 64 × 64, slice thickness/gap = 3.8/0 mm, 34 horizontal slices). Finally, high‐resolution T1‐weighted structural images were collected (MPRAGE: TR = 2 s, TE = 4.38 ms, FOV = 17.6 × 19.2 cm, matrix size = 176 × 192, slice thickness/gap = 1.0 mm/0, 160 horizontal slices).
fMRI Data Analysis
For the analysis of the fMRI data, we employed Statistical Parametric Mapping 8 (SPM 8: Wellcome Department of Cognitive Neurology, London, UK) implemented in MATLAB. All functional images were corrected for slice‐timing and image movement related to head motion and magnetic field drift. These corrected images were spatially normalized into the Montreal Neurological Institute (MNI) template (resampled resolution: 3.8 × 3.8 × 3.8 mm) and then spatially smoothed using a Gaussian Kernel of FWHM 8 mm.
After preprocessing, all fMRI images were statistically analyzed at two levels: the individual level of each participant and the group level of multiple participants. In the individual‐level (fixed‐effect) analyses, trial‐related activation was modeled by convolving vectors of onset with a canonical hemodynamic response function (HRF) in the context of the general linear model (GLM). The timing of onsets was set with the responses to the target stimuli. Confounding variables of image movement induced by head motion and magnetic field drift were also included in this model. All encoding trials were divided into two categories, subsequently remembered (Hit) and subsequently forgotten (Miss) trials [Paller and Wagner, 2002]. The Hit trials included encoding trials showing subsequent hits with DO and PO responses, and the Miss trials included encoding trials showing subsequent misses with DN and PN responses. In addition, the Hit and Miss trials were subdivided by the encoding condition of Self, Friend, or Other. The rationale of collapsing two levels of confidence in categorization of the Hit and Miss trials was that four participants showed only eight trials or less of hits with high confidence in one encoding condition. Thus, trial‐related activation during encoding was modeled with six experimental conditions determined by two factors, encoding condition and subsequent memory (Self‐Hit, Self‐Miss, Friend‐Hit, Friend‐Miss, Other‐Hit, and Other‐Miss), and with one no‐response for the encoding and/or subsequent retrieval tasks. In the individual data statistics, significant activation in each condition was defined by comparing encoding‐related activation in each of the six experimental conditions with baseline activation while viewing a visual fixation. Contrasts reflecting the activation of these regions identified in each condition yielded a t‐statistic in each voxel.
In the group‐level (random‐effect) analyses, the six contrasts identified in the individual‐level analyses were analyzed by a two‐way repeated‐measure ANOVA with the factors of encoding condition (Self, Friend, and Other) and subsequent memory (Hit and Miss). The ANOVA model was conducted by a flexible factorial design in SPM 8, and a subject factor was included in the model. In this ANOVA, three types of statistical analyses were performed. First, to identify regions related to the self‐referential processes during encoding, an F‐contrast reflecting a main effect of encoding condition was inclusively masked by two t‐contrasts of Self versus Friend and Self versus Other (P < 0.05). This procedure yielded an activation map reflecting a significant main effect of encoding condition and showing greater activation in the Self condition than in the Friend and Other conditions. Second, successful encoding activation was identified in an F‐contrast reflecting a main effect of subsequent memory masked inclusively by a t‐contrast of Hit versus Miss (P < 0.05). This procedure yielded an activation map reflecting a significant main effect of subsequent memory and showing greater activation in Hit than in Miss. Finally, to find significant activation related to the successful encoding of self‐related faces, we analyzed an F‐contrast reflecting an interaction between the factors of encoding condition and subsequent memory. In these analyses, the height threshold at the voxel level was corrected for whole‐brain multiple comparisons (FWE, P < 0.05). In regions fulfilling the voxel‐level threshold, regions with a cluster size of 2 or more successive voxels were considered as significant activation in the present study. In addition, the hypothesis‐driven small volume correction (SVC) [Worsley et al., 1996] was applied to regions of interest (ROIs) in the mPFC and MTL (corrected by FWE, P < 0.05 at the voxel level). The mPFC ROI for the self‐referential process during encoding was defined by methods in a previous study [Fuster, 2001], in which this ROI was formed by combining multiple ROIs of the bilateral orbital medial frontal gyri, superior medial frontal gyri, and anterior cingulate gyri obtained from the AAL ROI package [Tzourio‐Mazoyer et al., 2002]. The MTL ROI for successful encoding included the bilateral hippocampi and parahippocampal gyri obtained from the AAL ROI package.
To investigate regions showing significant functional connectivity with the bilateral hippocampi related to successful encoding, generalized psychophysiological interactions (gPPI) [McLaren et al., 2012] were analyzed between the activation in seed regions of the bilateral hippocampi and in other regions. Before performing the gPPI analyses, three encoding runs were collapsed into one run, and trial‐related activation during encoding was remodeled by convolving onset vectors of the same timing with the original GLM. This new one‐run GLM produced in each participant included six experimental conditions (Self‐Hit, Self‐Miss, Friend‐Hit, Friend‐Miss, Other‐Hit, and Other‐Miss) and one no‐response condition with six variables of confounding factors (head motion and magnetic field drift). In this model, each of the left and right hippocampal seeds was defined as a volume‐of‐interest (VOI) sphere with a 6 mm radius around the peak voxels, which corresponded to coordinates of the bilateral hippocampi reflecting successful encoding in the ANOVA analysis (left: x = −25, y = −6, z = −20; right: x = 21, y = −9, z = −16). Each VOI of the left and right hippocampi in individual participants was explored in the ipsilateral MTL (hippocampus and parahippocampal gyrus) ROI obtained from the AAL ROI package.
In the present study, we used the gPPI toolbox (http://www.nitrc.org/projects/gppi) to analyze patterns of functional connectivity. This toolbox produces a design matrix with three columns of condition‐related onsets with canonical HRF, BOLD signals deconvolved from the seed region and PPI regressors at the individual level. Thus, the GLM of this analysis included PPI and condition regressors of the six experimental conditions (Self‐Hit, Self‐Miss, Friend‐Hit, Friend‐Miss, Other‐Hit, and Other‐Miss) and one no‐response condition, and BOLD signals in a seed VOI of the left or right hippocampus. In addition, six motion‐related regressors were also included in this GLM. The gPPI toolbox estimated the model parameters and computed the linear contrasts in the GLMs, which were created by each seed of the left and right hippocampus. Regions showing a significant effect in contrasts of the PPI regressor were considered to be functionally connected with the hippocampal seeds on the statistical threshold. In the gPPI analysis for the right hippocampal seed, data from one participant were excluded because the right hippocampal VOI used as the seed region in this participant was not appropriately defined using the criteria for the VOI definition mentioned above. Thus, the PPI regressor contrasts reflecting functional connectivity with the right hippocampal seed were acquired from a total of 18 participants, and the PPI contrasts related to the left hippocampal seed were identified in all 19 participants. These PPI regressor contrasts were applied to the group‐level (random‐effect) analyses.
In the individual‐level (fixed‐effect) analysis of the gPPI, brain regions showing functional connectivity with each of the left and right hippocampal seeds during successful encoding were explored in two PPI regressor contrasts of Self‐Hit and Other‐Hit. The rationale of comparing PPI regressors only between Self‐Hit and Other‐Hit was that a significant difference of hit rates for faces was found between the Self and Other conditions, but not between the Self and Friend conditions in behavioral results (see below). In the group‐level (random‐effect) analysis, regions reflecting significant functional connectivity with the left or right hippocampal seed in each condition of Self‐Hit and Other‐Hit were analyzed by one‐sample t tests for PPI regressor contrasts identified in the individual‐level analysis. In addition, to identify regions showing significant functional connectivity with the hippocampal seeds in the Self‐Hit but not in the Other‐Hit conditions, regions identified in a one‐sample t test for PPI regressor contrasts of the Self‐Hit condition were exclusively masked by regions identified in the one‐sample t test for PPI regressor contrasts of the Other‐Hit condition (P < 0.05). This analysis was carried out in each seed of the left and right hippocampi. In the gPPI analysis, the height threshold at the voxel level was corrected for whole‐brain multiple comparisons (FWE, P < 0.05). In regions fulfilling the voxel‐level threshold, regions with a cluster size of two or more successive voxels were considered as significant functional connectivity in the present study. Anatomical sites showing significant activation and functional connectivity were primarily defined by the SPM Anatomy Toolbox [Eickhoff et al., 2006, 2007, 2005].
RESULTS
Behavioral Data
Confirming our first prediction, faces encoded by the self‐referential process in a social context were recognized more accurately than those encoded by the other‐referential process in a social context. Table 1 summarizes the hit rates, false alarm rates, and response times (RTs) in each encoding condition. As shown in Figure 2, a one‐way repeated‐measure ANOVA for hit rates showed a significant effect of encoding condition [F(2,36) = 3.56, P < 0.05, η 2 = 0.17], in which the hit rates of faces encoded in the Self condition were significantly higher than those in the Other condition [multiple comparisons by the Ryan's method: t(36) = 2.67, P < 0.05, r = 0.41]. However, there was no significant difference of hit rates between the Self and Friend conditions [multiple comparisons by the Ryan's method: t(36) = 1.28, n.s., r = 0.21], and between the Friend and Other conditions [multiple comparisons by the Ryan's method: t(36) = 1.38, n.s., r = 0.23]. In addition, mean d‐primes were 1.16 (SD = 0.42), which was computed by hit rates (%) for all target faces (mean = 58.7, SD = 14.2) and false alarm rates (%) for distracter faces (mean = 19.8, SD = 9.4). Thus, the retrieval performance would not be affected by response biases toward false positive recognition. RT data (ms) during encoding were analyzed by a two‐way repeated‐measure ANOVA with the factors of encoding condition (Self, Friend, Other) and subsequent memory (Hit, Miss). This analysis demonstrated a significant main effect of encoding condition [F(2,36) = 6.65, P < 0.01, η p 2 = 0.27], in which a post hoc test (multiple comparisons by the Ryan's method) showed significantly shorter RTs in the Self condition than in the Other condition [t(36) = 3.63, P < 0.05, r = 0.52]. However, the post hoc tests did not show a significant difference of RTs between the Self and Friend conditions [t(36) = 2.09, n.s., r = 0.33], and between the Friend and Other conditions [t(36) = 1.54, n.s., r = 0.25]. A main effect of subsequent memory [F(1,18) = 0.59, P = 0.45, η p 2 = 0.03] and the interaction between the factors of encoding condition and subsequent memory [F(2,36) = 0.24, P = 0.79, η p 2 = 0.01] were not significant. RT data (ms) during retrieval were also analyzed by a two‐way repeated‐measure ANOVA with the factors of encoding condition (Self, Friend, and Other) and memory (Hit and Miss). In this analysis, we found a significant main effect of memory [F(1,18) = 14.82, P < 0.01, η p 2 = 0.45], in which the RTs in the Hit condition were significantly shorter than those in the Miss condition. However, a main effect of encoding condition [F(2,36) = 1.81, P = 0.18, η p 2 = 0.09] and the interaction between the two factors [F(2,36) = 1.19, P = 0.32, η p 2 = 0.06] were not significant.
Table 1.
Behavioral results
| Condition | Self (SD) | Friend (SD) | Other (SD) | Distracter (SD) |
|---|---|---|---|---|
| Proportion (%) of recognition responses | ||||
| Hit | 61.0 (15.9) | 58.8 (13.5) | 56.4 (15.0) | – |
| False alarm | – | – | – | 19.8 (9.4) |
| RT (ms) during encoding | ||||
| Subsequent hit | 2,173.1 (469.0) | 2,242.9 (577.6) | 2,292.4 (584.8) | – |
| Subsequent miss | 2,127.1 (571.0) | 2,217.0 (634.2) | 2,285.1 (592.8) | – |
| RT (ms) during retrieval | ||||
| Hit | 1,653.5 (374.0) | 1,664.6 (334.2) | 1,727.4 (368.7) | – |
| Miss | 1,821.5 (359.1) | 1,817.5 (357.8) | 1,825.8 (409.2) | – |
| Correct rejection | – | – | – | 1,737.0 (360.5) |
| False alarm | – | – | – | 1,866.6 (445.1) |
RT, response time; SD, standard deviation.
Figure 2.
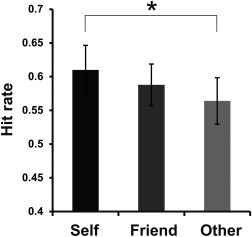
Retrieval accuracy for faces encoded in the three encoding conditions of Self, Friend, and Other. Hit rates [hits/(hits + misses)] were calculated for each participant. Error bars represent standard error (SE). *P < 0.05.
fMRI Data
Confirming our second prediction, activation in the mPFC was associated with the self‐referential process during encoding, and activation in the hippocampus reflected the successful encoding process. As shown in Figure 3, a two‐way repeated‐measure ANOVA with the factors of encoding condition (Self, Friend, and Other) and subsequent memory (Hit and Miss) demonstrated that the left mPFC showed a significant main effect of encoding condition and that the activation was significantly greater in the Self condition than in the Friend and Other conditions. The same pattern of activation was identified in the right inferior temporal gyrus. In this ANOVA, we found regions reflecting a significant main effect of subsequent memory, in which activation in the bilateral hippocampi and right inferior temporal gyrus was significantly greater in the Hit condition than in the Miss condition. However, there was no significant interaction between encoding condition and subsequent memory in the activation of any regions. Detailed findings from this ANOVA are summarized in Table 2.
Figure 3.
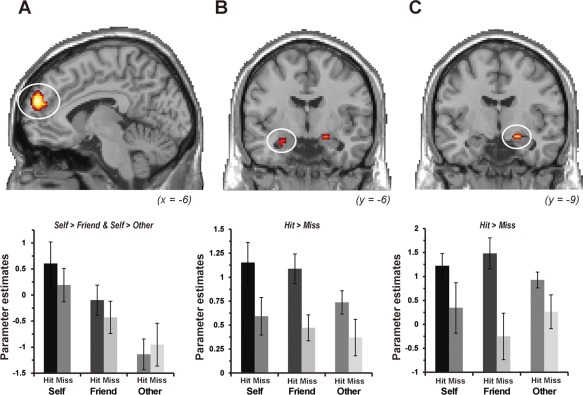
Regions showing significant main effects of encoding condition and subsequent memory. (A) The left mPFC (x = −6, y = 55, z = 22) showed greater activation in the Self condition than in the Friend and Other conditions during encoding. (B) The left hippocampus (x = −25, y = −6, z = −20) and (C) the right hippocampus (x = 21, y = −9, z = −16) showed greater activation in the trials with subsequent hits than in the trials with subsequent misses. Parameter estimates on graphs were extracted from peak voxels within activation clusters in each region. Error bars represent standard error (SE).
Table 2.
Regions showing significant activation
| Coordinates | ||||||
|---|---|---|---|---|---|---|
| Regions | L/R | BA | x | y | z | Z value |
| Main effect of encoding condition (masked by Self > Friend and Self > Other) | ||||||
| ROI analysis | ||||||
| Medial prefrontal cortex | L | 10 | −6 | 55 | 22 | 4.60 |
| Whole‐brain analysis | ||||||
| Medial prefrontal cortex | L | 10 | −6 | 55 | 22 | 4.60 |
| Inferior temporal gyrus | R | 37 | 51 | −59 | −8 | 4.82 |
| Main effect of subsequent memory (masked by Hit > Miss) | ||||||
| ROI analysis | ||||||
| Hippocampus | L | −25 | −6 | −20 | 3.74 | |
| Hippocampus | R | 21 | −9 | −16 | 3.64 | |
| Whole‐brain analysis | ||||||
| Inferior temporal gyrus | R | 37 | 51 | −59 | −8 | 4.78 |
| Interaction between encoding condition and subsequent memory | ||||||
| No significant activation | ||||||
L, left; R, right; BA, Brodmann area; SVC, small volume correction; ROI, region of interest.
Confirming our third prediction, activation in the right hippocampal seed region was functionally connected with activation in the mPFC and precuneus within the CMS regions, and the functional connectivity was identified in the successful encoding of self‐related faces but not of other‐related faces. The gPPI analysis to investigate functional connectivity with the right hippocampus used as a seed region demonstrated that significant functional connectivity during the successful encoding of self‐related faces was found in the right mPFC, bilateral precuneus, left cuneus, and left calcarine sulcus within the CMS regions, and the left superior parietal lobule and left middle occipital gyrus in a PPI regressor contrast of Self‐Hit masked exclusively with a PPI regressor contrast of Other‐Hit. The right mPFC and precuneus reflecting significant functional connectivity with the right hippocampal seed are shown in Figure 4. The gPPI analysis for the left hippocampus used as a seed region showed no significant functional connectivity in any regions during the successful encoding of self‐related faces. The left–right difference of functional connectivity in the hippocampus might reflect that faces as a pictorial stimulus were employed to identify the SRE‐related activations in the present study [Persson and Soderlund, 2015]. Detailed results from the gPPI analysis are summarized in Table 3.
Figure 4.
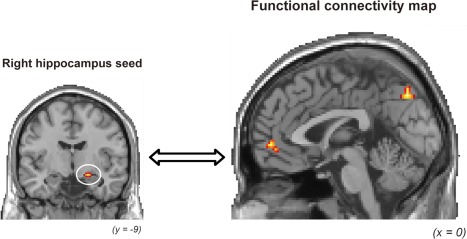
Regions showing significant functional connectivity with activation in the right hippocampal seed during successful encoding in the Self condition. The cortical midline structures (CMSs) including the mPFC and precuneus showed significant functional connectivity with the right hippocampus.
Table 3.
Regions showing significant functional connectivity with hippocampal seeds during the successful encoding of self‐related faces
| Coordinates | ||||||
|---|---|---|---|---|---|---|
| Regions | L/R | BA | x | y | z | Z value |
| Functional connectivity with the left hippocampus | ||||||
| No region showing significant functional connectivity | ||||||
| Functional connectivity with the right hippocampus | ||||||
| Medial prefrontal cortex | R | 10 | 2 | 51 | −4 | 5.20 |
| Superior parietal lobule | L | 2 | −36 | −47 | 60 | 4.92 |
| Precuneus | L | 7 | −2 | −74 | 37 | 5.34 |
| Precuneus | R | 7 | 17 | −51 | 37 | 4.92 |
| Middle occipital gyrus | L | 19 | −40 | −74 | 37 | 4.88 |
| Cuneus | L | 18 | −6 | −89 | 22 | 4.89 |
| Calcarine sulcus | L | 17 | −2 | −82 | 7 | 4.91 |
L, left; R, right; BA, Brodmann area.
DISCUSSION
Three major findings emerged from the present study. First, target faces encoded with the participants' own face were remembered more accurately than those encoded with unfamiliar faces. Second, the mPFC showed greater activation during the encoding of target faces paired with the participants' own face than the encoding of target faces paired with each face of familiar and unfamiliar others. Third, functional connectivity between the hippocampal seed region and the CMS regions, including the mPFC and precuneus, was significant in the successful encoding of faces paired with the participants' own face. These findings suggest that memories encoded by the self‐referential process in a social context could be enhanced in subsequent retrieval, and the memory enhancement could be associated with functional connectivity between the CMS regions related to the self‐referential process and the hippocampus related to the successful encoding. These findings are discussed in separate sections below.
SRE on Face Memories in Social Context
The first main finding of this study was that memories for target faces encoded with the participants' own face were significantly better than those faces presented with strangers' face (Fig. 2). This finding suggests that memories encoded by the self‐referential process could be enhanced compared to memories encoded by the other‐referential process, and the memory enhancement by the self‐referential process, or the SRE, could be active even in a social context such as personal relationships.
The present finding, which is the first to demonstrate the SRE on memories in a social context, was consistent with previous findings in which items related to the self were remembered more accurately than items related to others or items processed semantically [Rogers et al., 1977; Symons and Johnson, 1997]. For example, one of the traditional psychological studies investigating the SRE reported that memories for adjectives encoded by judging whether the adjectives appropriately described personalities of selves were significantly better than those encoded by the perceptual or semantic judgments of the adjectives [Rogers et al., 1977]. There is evidence from a meta‐analysis showing that the SRE have been frequently observed by the traditional paradigm in which words describing personality traits are used for memory items [Symons and Johnson, 1997]. In the present study, we identified a significant SRE on face memories by an encoding operation in which participants were asked to imagine a possible friendship between themselves and unfamiliar faces. Thus, this finding extends previous findings by showing that memory enhancement by an encoding operation with a self‐referential process was significant in a social context such as judging the possibility of a personal relationship, and in non‐social laboratory‐based experiments such as adjective judgments.
Regions Associated With the Self‐Referential Process
The second main finding of this study was that the mPFC showed greater activation during the encoding of target faces paired with a participants' own face (Self) than during the encoding of target faces paired with a friend's (Friend) or stranger's face (Other). The finding suggests that the mPFC region could contribute to face encoding by the self‐referential process in a social context such as a person‐to‐person relationship.
The present finding of mPFC activation associated with the self‐referential process during encoding is consistent with previous findings [Gusnard et al., 2001; Gutchess et al., 2007a; Leshikar and Duarte, 2012]. For example, one fMRI study reported that the mPFC and mid‐cingulate cortex showed significant activation during encoding by the self‐referential process, which was induced by judging whether adjectives appropriately described personality traits of themselves, and activation of these regions was identified in both young and older adults [Gutchess et al., 2007a]. In another fMRI study, memories for objects were significantly enhanced by the self‐referential encoding of object–scene pairs, and significant activation during the self‐referential encoding was identified in the mPFC [Leshikar and Duarte, 2012]. A previous review study proposed the theory that the mPFC could represent the “event simulators” (elators) which give rise to social event knowledge [Krueger et al., 2009]. Thus, the present findings extend previous findings by showing that mPFC activation was found even during the self‐referential encoding modulated by the processing of a social context such as social relationships with others.
In the present study, activation related to the self‐referential process during encoding was identified in the right inferior temporal gyrus. In addition, activation in this region was also found in a contrast of subsequently remembered trials versus subsequently forgotten trials. The right inferior temporal activation could be associated with previous findings, in which this activation has been consistently observed in the successful encoding of faces [Prince et al., 2009; Tsukiura and Cabeza, 2011]. For example, one fMRI study reported that the right inferior temporal gyrus showed linearly increasing activation associated with the subjective rating scores of facial attractiveness during the encoding of faces [Tsukiura and Cabeza, 2011]. Another fMRI study demonstrated that activation in the right fusiform gyrus reflected a significant interaction between photo type and subsequent memory during the encoding of faces and scenes, and the activation was significantly associated with the successful encoding and retrieval of faces but not of scenes [Prince et al., 2009]. Taken together with the present findings, activation in the right inferior temporal gyrus—including the fusiform gyrus—could reflect the successful encoding of face‐related source memories by the enhanced vividness of faces, which is induced by face‐based socioemotional signals from the self‐referential process in a social context.
Functional Connectivity Between the Hippocampus and Cortical Midline Structures
The third main finding of this study was that activation in the right hippocampus used as a seed region was functionally connected with activation in the CMS regions, including the mPFC and precuneus, during successful encoding with a self‐referential process. The finding suggests that the interacting mechanisms between the memory‐related hippocampus and the CMS regions related to the self‐referential process could contribute to the SRE modulated by social context.
The present finding, which is the first to show significant functional connectivity between the CMS regions and hippocampus during encoding with a self‐referential process in a social context, was consistent with previous studies which demonstrated that functional connectivity between the mPFC and posterior cingulate gyrus (PCC) and between the hippocampus and PCC was important in the laboratory‐based SRE [Morel et al., 2014]. In this fMRI study, the SRE on memories was observed when participants judged whether adjective words appropriately described the personality traits of themselves compared to when participants judged whether the words were emotionally positive. Thus, the present finding suggests that the interaction between the CMS regions, including the mPFC and precuneus, which are related to the self‐referential process, and the hippocampus related to the memory process could contribute to the SRE in a social context such as judging social relationships with other people as well as to the SRE in a nonsocial context such as judging adjectives.
The importance of functional connectivity between the CMS regions and hippocampus in the social context‐dependent SRE is well supported by previous findings from anatomical studies in experimental animals and resting‐state fMRI studies in human participants. For example, anatomical connectivity between the hippocampus and mPFC was identified in rhesus monkeys [Barbas and Blatt, 1995]. In addition, a review article of the resting‐state fMRI studies implied that the default mode network (DMN) including the precuneus/PCC, mPFC, inferior parietal lobe, medial temporal lobe, and lateral temporal cortex could be divided into two subsystems, which include the self‐referential process and the retrieval of episodic memory [Buckner et al., 2008]. In other words, a subsystem of the anterior domain of the DMN including the mPFC could be involved in the processing of self‐related information, whereas another subsystem of the posterior domain of the DMN including the medial temporal lobe could be involved in the reconstruction and retrieval of episodic memory. These two subsystems could be integrated in the PCC region. Thus, the present finding of functional connectivity between the CMS regions and hippocampus during encoding with a self‐referential process in a social context could reflect the interaction between an anterior portion of the DMN related to the self‐referential process and a posterior portion of the DMN related to the episodic encoding and retrieval.
The present findings, in which the social context‐dependent SRE was involved in functional connectivity between the CMS regions and hippocampus, could be explained by the framework of a schema effect on memory processes. Information that is congruent with existing knowledge (schema) is better remembered than less congruent information [van Kesteren et al., 2012]. One neuropsychological study reported that amnesic patients with relatively intact semantic systems showed a beneficial effect of schema on memory functions, whereas the schema effect on memory was not found in patients with compromised semantic systems [Kan et al., 2009]. In addition, there is neuroscientific evidence that the efficiency of learning by a schema is supported by the MTL–mPFC interactions [Tse et al., 2011; van Kesteren et al., 2012]. Given that in the Self condition of this study, participants were required to rate the appropriateness of potential friendship between themselves and unfamiliar others, the memory advantage by the SRE in a social context could be induced by accessing schemas of pre‐existing knowledge about the social relationship in autobiographical memories. The schema effect on memories encoded by the self‐referential process in a social context could be associated with functional connectivity between the CMS regions and hippocampus.
Limitations
Although the present findings showed the contribution of CMS–hippocampus interaction to the SRE in a social context, there are a few potential limitations in this study. The first potential limitation is whether the social context‐dependent SRE is induced even in the absence of an explicit task demand to engage in the self‐referential process. Previous studies have reported that self‐sensitive brain regions including the CMS regions are recruited in implicit responses to the self‐related stimuli [Moran et al., 2009; Rameson et al., 2010]. In the Self condition of this study, participants were presented with face pairs, which included their own face as a cue on the left side and target faces on the right side, and were required to rate how appropriate a future friendship would be between the face pairs. Given that explicit psychological processes such as the self–other discrimination or imagination of social relationship between the self and other faces were occurred in this condition, our experimental design might not be enough to decide that the CMS–hippocampus interaction reflected only the social context‐dependent SRE. Further analyses would be required to clarify whether the SRE in a social context is also observed in the implicit task and the explicit task.
The second potential limitation is that the perceived characteristics of facial stimuli such as facial attractiveness was not measured and not statistically controlled across four lists of faces. One fMRI study reported that attractive faces were remembered more accurately than neutral or unattractive faces, and that the beneficial effect of facial attractiveness was involved in an interaction between activation in the attractiveness‐related orbitofrontal cortex and memory‐related hippocampus [Tsukiura and Cabeza, 2011]. Thus, it is ideal to equalize the perceived characteristics of faces in investigating the neural mechanisms related to face memories. However, facial stimuli employed in the present study were divided into four lists, among which age and sex of stimuli as perceived characteristics were equalized, and the lists of facial stimuli were counterbalanced across the participants. The experimental manipulations in constructing the lists could be helpful to minimize the possible artifacts conveyed from the perceived characteristics of faces.
The third potential limitation is that the mPFC region identified in the activation analysis was located in a relatively dorsal part of this region, whereas the mPFC region in the functional connectivity analysis was found in a relatively ventral part of this region. A previous study of the meta‐analysis demonstrated that a dorsal part of the mPFC was functionally connected with the inferior frontal gyrus, temporoparietal junction, and middle temporal gyrus, whereas a ventral part of the mPFC was functionally connected with the nucleus accumbens, hippocampus, posterior cingulate cortex, and retrosplenial cortex [Bzdok et al., 2013]. Taken together with the present findings, activation in a relatively dorsal part of the mPFC might contribute to the top–down processing such as the self‐referential process or mentalizing process, and functional connectivity between a relatively ventral part of the mPFC and hippocampus might be involved in the encoding of self‐related memories. The coordination between these functions related to each part of the mPFC might contribute to the SRE in a social context. Further investigations would be required to find how the social context‐dependent SRE is modulated by the interacting mechanism between these separate regions.
CONCLUSIONS
In the present study, we investigated the neural mechanisms underlying the SRE on memory of faces in a social context modulated by the social relationship with others. Three major findings emerged from this study. First, target faces encoded by imagining a social relationship with oneself were remembered more accurately than those target faces encoded by imagining a social relationship with a stranger. Second, the mPFC showed greater encoding‐related activation when the potential friendship was imagined between the participants' own face and the target faces than when the potential friendship was imagined between their friend's face and the target faces or between the stranger's face and the target faces. Third, functional connectivity between the hippocampal seed region and the CMS regions—including the mPFC and precuneus—was significant in the successful encoding of the target faces paired with the participants' own face but not in the successful encoding of the target faces paired with the stranger's face. These findings suggest that the SRE could be effective even in a social context modulated by social relationship, and the SRE in a social context could be involved in functional connectivity between the CMS regions related to the self‐referential process and the hippocampus related to the successful encoding process.
ACKNOWLEDGMENTS
The fMRI experiment in this study was conducted using the MRI scanner and related facilities at Human Brain Research Center, Kyoto University.
REFERENCES
- Barbas H, Blatt GJ (1995): Topographically specific hippocampal projections target functionally distinct prefrontal areas in the rhesus monkey. Hippocampus 5:511–533. [DOI] [PubMed] [Google Scholar]
- Bower GH, Gilligan SG (1979): Remembering information related to ones‐self. J Res Pers 13:420–432. [Google Scholar]
- Buckner RL, Andrews‐Hanna JR, Schacter DL (2008): The brain's default network: Anatomy, function, and relevance to disease. Ann N Y Acad Sci 1124:1–38. [DOI] [PubMed] [Google Scholar]
- Bzdok D, Langner R, Schilbach L, Engemann DA, Laird AR, Fox PT, Eickhoff SB (2013): Segregation of the human medial prefrontal cortex in social cognition. Front Hum Neurosci 7:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Prince SE, Daselaar SM, Greenberg DL, Budde M, Dolcos F, LaBar KS, Rubin DC (2004): Brain activity during episodic retrieval of autobiographical and laboratory events: An fMRI study using a novel photo paradigm. J Cogn Neurosci 16:1583–1594. [DOI] [PubMed] [Google Scholar]
- Craik FIM, Moroz TM, Moscovitch M, Stuss DT, Winocur G, Tulving E, Kapur S (1999): In search of the self: A positron emission tomography study. Psychol Sci 10:26–34. [Google Scholar]
- Cunningham SJ, Brebner JL, Quinn F, Turk DJ (2014): The self‐reference effect on memory in early childhood. Child Dev 85:808–823. [DOI] [PubMed] [Google Scholar]
- Cunningham SJ, Turk DJ, Macdonald LM, Neil Macrae C (2008): Yours or mine? Ownership and memory. Conscious Cogn 17:312–318. [DOI] [PubMed] [Google Scholar]
- Cunningham SJ, Vergunst F, Macrae CN, Turk DJ (2013): Exploring early self‐referential memory effects through ownership. Br J Dev Psychol 31:289–301. [DOI] [PubMed] [Google Scholar]
- D'Argembeau A, Comblain C, Van der Linden M (2005): Affective valence and the self‐reference effect: Influence of retrieval conditions. Br J Psychol 96:457–466. [DOI] [PubMed] [Google Scholar]
- Davachi L (2006): Item, context and relational episodic encoding in humans. Curr Opin Neurobiol 16:693–700. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Heim S, Zilles K, Amunts K (2006): Testing anatomically specified hypotheses in functional imaging using cytoarchitectonic maps. Neuroimage 32:570–582. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Paus T, Caspers S, Grosbras MH, Evans AC, Zilles K, Amunts K (2007): Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. Neuroimage 36:511–521. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K (2005): A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage 25:1325–1335. [DOI] [PubMed] [Google Scholar]
- Feyers D, Collette F, D'Argembeau A, Majerus S, Salmon E (2010): Neural networks involved in self‐judgement in young and elderly adults. Neuroimage 53:341–347. [DOI] [PubMed] [Google Scholar]
- Fuster JM (2001): The prefrontal cortex–An update: Time is of the essence. Neuron 30:319–333. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME (2001): Medial prefrontal cortex and self‐referential mental activity: Relation to a default mode of brain function. Proc Natl Acad Sci U S A 98:4259–4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutchess AH, Kensinger EA, Schacter DL (2007a): Aging, self‐referencing, and medial prefrontal cortex. Soc Neurosci 2:117–133. [DOI] [PubMed] [Google Scholar]
- Gutchess AH, Kensinger EA, Yoon C, Schacter DL (2007b): Ageing and the self‐reference effect in memory. Memory 15:822–837. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Wyland CL, Macrae CN, Demos KE, Denny BT, Kelley WM (2006): Medial prefrontal activity differentiates self from close others. Soc Cogn Affect Neurosci 1:18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, Baxter LC, Wilder LS, Pipe JG, Heiserman JE, Prigatano GP (2002): Neural correlates of self‐reflection. Brain 125:1808–1814. [DOI] [PubMed] [Google Scholar]
- Kan IP, Alexander MP, Verfaellie M (2009): Contribution of prior semantic knowledge to new episodic learning in amnesia. J Cogn Neurosci 21:938–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF (2002): Finding the self? An event‐related fMRI study. J Cogn Neurosci 14:785–794. [DOI] [PubMed] [Google Scholar]
- Kesebir S, Oishi S (2010): A spontaneous self‐reference effect in memory: Why some birthdays are harder to remember than others. Psychol Sci 21:1525–1531. [DOI] [PubMed] [Google Scholar]
- Kim H (2012): A dual‐subsystem model of the brain's default network: Self‐referential processing, memory retrieval processes, and autobiographical memory retrieval. Neuroimage 61:966–977. [DOI] [PubMed] [Google Scholar]
- Krueger F, Barbey AK, Grafman J (2009): The medial prefrontal cortex mediates social event knowledge. Trends Cogn Sci 13:103–109. [DOI] [PubMed] [Google Scholar]
- Kuiper NA, Rogers TB (1979): Encoding of personal information: Self‐other differences. J Pers Soc Psychol 37:499–514. [DOI] [PubMed] [Google Scholar]
- Leshikar ED, Duarte A (2012): Medial prefrontal cortex supports source memory accuracy for self‐referenced items. Soc Neurosci 7:126–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshikar ED, Park JM, Gutchess AH (2015): Similarity to the self affects memory for impressions of others in younger and older adults. J Gerontol B Psychol Sci Soc Sci 70:737–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou HC, Luber B, Crupain M, Keenan JP, Nowak M, Kjaer TW, Sackeim HA, Lisanby SH (2004): Parietal cortex and representation of the mental self. Proc Natl Acad Sci U S A 101:6827–6832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mano Y, Sugiura M, Tsukiura T, Chiao JY, Yomogida Y, Jeong H, Sekiguchi A, Kawashima R (2011): The representation of social interaction in episodic memory: A functional MRI study. Neuroimage 57:1234–1242. [DOI] [PubMed] [Google Scholar]
- Martinelli P, Sperduti M, Piolino P (2013): Neural substrates of the self‐memory system: New insights from a meta‐analysis. Hum Brain Mapp 34:1515–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren DG, Ries ML, Xu G, Johnson SC (2012): A generalized form of context‐dependent psychophysiological interactions (gPPI): A comparison to standard approaches. Neuroimage 61:1277–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millward C, Powell S, Messer D, Jordan R (2000): Recall for self and other in autism: Children's memory for events experienced by themselves and their peers. J Autism Dev Disord 30:15–28. [DOI] [PubMed] [Google Scholar]
- Moran JM, Heatherton TF, Kelley WM (2009): Modulation of cortical midline structures by implicit and explicit self‐relevance evaluation. Soc Neurosci 4:197–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel N, Villain N, Rauchs G, Gaubert M, Piolino P, Landeau B, Mezenge F, Desgranges B, Eustache F, Chetelat G (2014): Brain activity and functional coupling changes associated with self‐reference effect during both encoding and retrieval. PLoS One 9:e90488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J (2006): Self‐referential processing in our brain–A meta‐analysis of imaging studies on the self. Neuroimage 31:440–457. [DOI] [PubMed] [Google Scholar]
- Paller KA, Wagner AD (2002): Observing the transformation of experience into memory. Trends Cogn Sci 6:93–102. [DOI] [PubMed] [Google Scholar]
- Persson J, Soderlund H (2015): Hippocampal hemispheric and long‐axis differentiation of stimulus content during episodic memory encoding and retrieval: An activation likelihood estimation meta‐analysis. Hippocampus 25:1614–1631. [DOI] [PubMed] [Google Scholar]
- Prince SE, Dennis NA, Cabeza R (2009): Encoding and retrieving faces and places: Distinguishing process‐ and stimulus‐specific differences in brain activity. Neuropsychologia 47:2282–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullyblank J, Bisanz J, Scott C, Champion MA (1985): Developmental invariance in the effects of functional self‐knowledge on memory. Child Dev 56:1447–1454. [Google Scholar]
- Rameson LT, Satpute AB, Lieberman MD (2010): The neural correlates of implicit and explicit self‐relevant processing. Neuroimage 50:701–708. [DOI] [PubMed] [Google Scholar]
- Rogers TB, Kuiper NA, Kirker WS (1977): Self‐reference and the encoding of personal information. J Pers Soc Psychol 35:677–688. [DOI] [PubMed] [Google Scholar]
- Saxe R, Moran JM, Scholz J, Gabrieli J (2006): Overlapping and non‐overlapping brain regions for theory of mind and self reflection in individual subjects. Soc Cogn Affect Neurosci 1:229–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serbun SJ, Shih JY, Gutchess AH (2011): Memory for details with self‐referencing. Memory 19:1004–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui J, Zhu Y (2005): Five‐year‐olds can show the self‐reference advantage. Int J Behav Dev 29:382–387. [Google Scholar]
- Symons CS, Johnson BT (1997): The self‐reference effect in memory: A meta‐analysis. Psychol Bull 121:371–394. [DOI] [PubMed] [Google Scholar]
- Toichi M, Kamio Y, Okada T, Sakihama M, Youngstrom EA, Findling RL, Yamamoto K (2002): A lack of self‐consciousness in autism. Am J Psychiatry 159:1422–1424. [DOI] [PubMed] [Google Scholar]
- Tse D, Takeuchi T, Kakeyama M, Kajii Y, Okuno H, Tohyama C, Bito H, Morris RG (2011): Schema‐dependent gene activation and memory encoding in neocortex. Science 333:891–895. [DOI] [PubMed] [Google Scholar]
- Tsukiura T, Cabeza R (2011): Remembering beauty: Roles of orbitofrontal and hippocampal regions in successful memory encoding of attractive faces. Neuroimage 54:653–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk DJ, Brady‐van den Bos M, Collard P, Gillespie‐Smith K, Conway MA, Cunningham SJ (2013): Divided attention selectively impairs memory for self‐relevant information. Mem Cognit 41:503–510. [DOI] [PubMed] [Google Scholar]
- Turk DJ, Cunningham SJ, Macrae CN (2008): Self‐memory biases in explicit and incidental encoding of trait adjectives. Conscious Cogn 17:1040–1045. [DOI] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. Neuroimage 15:273–289. [DOI] [PubMed] [Google Scholar]
- van Kesteren MT, Ruiter DJ, Fernandez G, Henson RN (2012): How schema and novelty augment memory formation. Trends Neurosci 35:211–219. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC (1996): A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp 4:58–73. [DOI] [PubMed] [Google Scholar]
- Yang L, Truong L, Fuss S, Bislimovic S (2012): The effects of ageing and divided attention on the self‐reference effect in emotional memory: Spontaneous or effortful mnemonic benefits? Memory 20:596–607. [DOI] [PubMed] [Google Scholar]
- Zhang T, Zhu Y, Wu Y (2014): Losing oneself upon placement in another's position: The influence of perspective on self‐referential processing. Conscious Cogn 27:53–61. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Zhang L, Fan J, Han SH (2007): Neural basis of cultural influence on self‐representation. Neuroimage 34:1310–1316. [DOI] [PubMed] [Google Scholar]


