Abstract
Altered serotonergic neurotransmission has been found to cause impulsive and aggressive behavior, as well as increased motor activity, all exemplifying key symptoms of ADHD. The main objectives of this positron emission tomography (PET) study were to investigate the serotonin transporter binding potential (SERT BPND) in patients with ADHD and to assess associations of SERT BPND between the brain regions. 25 medication‐free patients with ADHD (age ± SD; 32.39 ± 10.15; 10 females) without any psychiatric comorbidity and 25 age and sex matched healthy control subjects (33.74 ± 10.20) were measured once with PET and the highly selective and specific radioligand [11C]DASB. SERT BPND maps in nine a priori defined ROIs exhibiting high SERT binding were compared between groups by means of a linear mixed model. Finally, adopted from structural and functional connectivity analyses, we performed correlational analyses using regional SERT binding potentials to examine molecular interregional associations between all selected ROIs. We observed significant differences in the interregional correlations between the precuneus and the hippocampus in patients with ADHD compared to healthy controls, using SERT BPND of the investigated ROIs (P < 0.05; Bonferroni corrected). When correlating SERT BPND and age in the ADHD and the healthy control group, we confirmed an age‐related decline in brain SERT binding in the thalamus and insula (R 2 = 0.284, R 2 = 0.167, Ps < 0.05; Bonferroni corrected). The results show significantly different interregional molecular associations of the SERT expression for the precuneus with hippocampus in patients with ADHD, indicating presumably altered functional coupling. Altered interregional coupling between brain regions might be a sensitive approach to demonstrate functional and molecular alterations in psychiatric conditions. Hum Brain Mapp 38:792–802, 2017. © 2016 Wiley Periodicals, Inc.
Keywords: neuroimaging, ADHD, positron emission tomography, PET, serotonin, SERT, interregional molecular associations
Abbreviations
- AAL
Automated anatomical labelling
- ADHD
Attention deficit hyperactivity disorder
- HC
Healthy control subects
- PET
Positron emission tomography
- SERT
Serotonin Transporter
- SPECT
Single photon emission tomography
INTRODUCTION
Attention deficit hyperactivity disorder (ADHD) is characterized by inappropriate inattention, hyperactivity, impulsive behaviour and emotional dysregulation [American Psychiatric Association, 2013; Rosler et al., 2010], as well as by a certain constellation of deficits in executive functions. ADHD is considered to be the most prevalent neurodevelopmental disorder, prevalance rates are estimated to range between 8% and 12% in childhood [Biederman and Faraone, 2005]. In about 30% of children diagnosed with ADHD [Barbaresi et al., 2013], especially inattentive symptoms persist into adulthood.
Frequently prescribed stimulant and non‐stimulant psychopharmacological treatment for patients with ADHD are suggested to unfold efficacy through modulation of dopaminergic (DA) and norepinephrinergic neurotransmission in cortical and subcortical brain circuits and improvement of neurocognitive deficits [Castells et al., 2011; Chamberlain et al., 2009; Retz et al., 2011]. Although the serotoninergic system is not a direct target for ADHD medication, evidence from pharmacological, genetic and animal studies suggest an involvement of the serotonergic neurotransmission in the neurobiological mechanisms of ADHD [for review see (Banerjee and Nandagopal, 2015)].
Although methylphenidate does not inhibit the serotonin transporter, amphetamines enhance serotonergic release [Bymaster et al., 2002; Kuczenski and Segal, 1997]. A recently published positron emission tomography (PET) animal study found that atomoxetine applied at clinical dosage blocks the norepinephrine transporter as well as the serotonin transporter (SERT) [Ding et al., 2014] and atomoxetine has been shown to significantly alleviate symptoms in adult ADHD patients [Adler et al., 2009]. This has led some researchers to suggest that serotonergic transmission might also be of relevant to ADHD treatment and neuropathology [Gainetdinov et al., 1999].
Several lines of evidence suggest that serotonin is involved in impulsive behaviour and extensive motor activity [Dalley and Roiser, 2012; Winstanley et al., 2006]. Serotonergic neurons in the medial and dorsal raphe project into the striatum, ventral tegmental area and nucleus accumbens as well as into the amygdala, hippocampus and the frontal cortex [Muller and Jacobs, 2009]. Serotonin regulates dopaminergic neurotransmission via projections to the dopaminergic neurons in the midbrain and neuronal interactions between these neurotransmitters are found to profoundly modulate impulsive behaviour [Oades, 2008; Wood and Wren, 2008]. Furthermore, a deficit to withhold attention for an adequate time, related to a specific context, can lead to emotional dysregulation, a symptom of ADHD that affects patients markedly throughout lifetime. Brain regions implicated in emotional dysregulation comprise the striatum, amygdala and the medial prefrontal cortex, regions that are strongly modulated by serotonergic neurotransmission [Shaw et al., 2014].
Neuroimaging studies have been demonstrating that patients with ADHD display altered neural activation for inhibition and attention in frontal, parietal and thalamic brain regions as well as in the basal ganglia [Aron and Poldrack, 2005; Hart et al., 2013]. In comparison to healthy control subjects (HC), the administration of fluoxetine, a selective serotonin reuptake inhibitor, prior to functional magnetic resonance imaging (fMRI) measurements, has been shown to normalize neuronal activation during a stop signal task measuring motor inhibition in the orbitofrontal cortex and in the basal ganglia in 18 patients with ADHD [Chantiluke et al., 2015]. Fluoxetine, as well as its metabolite norfluoxetine, also binds to the norepinephrine transporter, although to a far lesser extent [Wong et al., 1993]. In addition, Fluoxetine has been found to be effective to improve attention and alleviate hyperactivity in children with ADHD and non‐bipolar comorbid mood‐disorders [Barrickman et al., 1991; Quintana et al., 2007].
With a remarkable heritability estimated to be 77% [Faraone et al., 2005], ADHD exemplifies a spectrum disorder with behavioural and personality traits, which underlie a combination and an interaction of genetic and environmental factors [Fliers et al., 2012]. The gene encoding the serotonin transporter (SERT; SLC6A4) as well as the genes encoding certain serotonergic receptors comprise various single nucleotide polymorphisms that have been examined in ADHD and other neuropsychiatric disorders and were found to be influencing the susceptibility to ADHD [Faraone and Khan, 2006; van der Meer et al., 2014]. Thus, the SERT gene is alleged to play a main role in ADHD pathogenesis.
Studies applying PET or single photon emission tomography (SPECT) in adult patients with ADHD have explored glucose, blood flow metabolismand [for review see (Zimmer, 2009)] and especially the dopaminergic and noradrenergic neurotransmitter systems. Dysfunctional dopaminergic signaling, including investigations on the dopamine transporter [Fusar‐Poli et al., 2012] and dopamine receptors [del Campo et al., 2013; Volkow et al., 2009], has been identified in different brain regions, though results remain inconsistent. In a recently published PET study, we found no difference in norepinephrine transporter in subcortical regions between patients with ADHD and HC [Vanicek et al., 2014]. A PET study has investigated serotonin transporter binding potential (SERT BPND) in patients with ADHD [Karlsson et al., 2013], using [11C]MADAM, which is a frequently used tracer for estimating brain SERT levels. The results depict no differences compared to HC. However the findings are preliminary, since the sample size is too small to exemplify reasonable size for power analysis and the tracer. As mentioned above, evidence from behavioral, neuroimaging and genetic studies suggest an involvement of the serotonergic system in ADHD. The SERT terminates serotonin from the synaptic cleft, therefore withholding a pivotal role in the regulation of serotonergic signaling. The SERT BPND has been investigated in the past with [11C]DASB and PET in various neuropsychiatric disorders [Spies et al., 2015].
In the last decades neuroimaging investigations have begun to change the conceptual focus from activation paradigms towards connectivity analysis, from univariate, where activation in cue‐related regions is explored, to multivariate analysis, where correlations of activation across brain regions are evaluated [Bullmore, 2012]. To disclose a possible involvement of a specific neurotransmitter system in neuropsychiatric disorders, PET imaging has predominantly been used to observe regional availability of a particular transporter or receptor in a specific brain region. Though, through performing interregional correlation analyses, PET imaging has also been applied to explore brain connectivity in HC, major depressive disorder, autism and obsessive‐compulsive disorder, Alzheimer's disease and epilepsy [Baldinger et al., 2014; Horwitz et al., 1984; Lee et al., 2008; Morbelli et al., 2013; Vanicek et al., 2016].
The serotonergic system represents one of the chief modulatory neurotransmitter systems in the human brain, where neurons from the raphe nuclei innervate nearly all cortical regions and several subcortical structures. Therefore, serotonin is associated with almost all emotional and cognitive functions. Since the SERT expression is modified via available and released serotonin [Benmansour et al., 2002], investigations on the relation of SERT expression between different brain regions may exemplify a valuable method to understand the function on a more global level of this neurotransmitter system. Studies from our group showed that molecular associations of the serotonergic neurotransmitter system (serotonin‐1A receptor and SERT) differed between depressive patients and HC [Baldinger et al., 2014; Hahn et al., 2014; Lanzenberger et al., 2012], implicating that interregional molecular correlation analyses is a promising method to generate more insight to the complexity of neurotransmitter systems and their role in neuronal pathophysiology.
Therefore, we applied [11C]DASB and PET to assess SERT BPND in SERT rich regions to observe differences in SERT availability between adult patients with ADHD and HC. Furthermore, we performed a correlational analysis, to examine interregional association of SERT binding as an index for interregional molecular balance of serotonergic neurotransmission. We hypothesized that SERT BPND and interregional molecular associations of SERT availability across brain areas will reflect a characteristic pattern that differs between patients with ADHD and HC.
METHODS
Subjects
Twenty‐five adult patients with ADHD (age ± SD; 32.39 ± 10.15; 10 females) and 25 age and sex matched HC (aged 33.74 ± 10.20) were recruited through the ADHD outpatient clinic at the Department of Psychiatry and Psychotherapy, Medical University of Vienna and from the local community via advertisement. Patients were free from psychopharmacologic treatment for at least six months prior to the screening visit while HC were naïve to all psychopharmacologic treatment. Four patients used methylphenidate in the past, one patient atomoxetine and one antidepressant medication. Written informed consent was obtained from all participants after detailed explanation of the study protocol and subjects received financial reimbursement for their participation. This study was approved by the Ethics Committee of the Medical University of Vienna and the General Hospital of Vienna (EK 552/2010).
Medical Examination and Clinical Exploration
Subjects underwent standard medical examination including a general physical and neurological status, electrocardiography and routine laboratory tests at the screening‐ and final visit in order to ensure physical health. Female participants underwent a urine‐pregnancy test at the screening visit and prior to PET measurement. A multidrug‐urine test was performed at the screening visit in order to exclude current substance abuse. Participants were interviewed by experienced psychiatrists using Conners' Adult ADHD Diagnostic Interview for DSM IV (CAADID, Conners 1999) to evaluate current and childhood attentional and hyperactivity/impulsivity symptoms and to attest ADHD diagnosis. (ADHD: impulsive symptoms: 20.05± 4.34 hyperactive symptoms: 20.05 ± 4.42; HC: impulsive symptoms: 0.55± 0.92 hyperactive symptoms: 0.35 ± 0.79). For five patients hyperactivity/impulsivity symptoms were not recorded, thus we excluded these patients and their matched HC from this analysis. Structured Clinical Interview for DSM IV Axis I and Axis II disorders (SCID‐I, SCID‐II) was performed to exclude comorbid psychiatric disorders. Smoking status was recorded and subjects were subdivided into groups best describing their smoking status according to quantity of consumption (non‐smokers, five cigarettes/week, five cigarettes/day, five to ten cigarettes/day, ten cigarettes/day, ten to 15 cigarettes/day, 15 cigarettes/day and 20 cigarettes/day; ranks 1‐8, respectively). ADHD patients did not significantly differ in smoking status compared to HC (Mann‐Whitney U = 161.5, Z=−1.25, P = 0.30). Subjects with PET‐ or MRI‐incompatible implants or in pregnancy or breastfeeding were also excluded.
Data Acquisition
All PET scans were carried out at the Dept of Biomedical Imaging and Image‐guided Therapy, Division of Nuclear Medicine, Medical University of Vienna using a full‐ring scanner (General Electric Medical Systems, Milwaukee, WI, USA) in 3D acquisition mode. We applied [11C]DASB [Haeusler et al., 2009], which is currently among the most suitable PET tracers for in vivo SERT quantification as reported previously in detail [Lanzenberger et al., 2012]. A 5 min transmission scan using retractable 68Ge rod sources for tissue attenuation correction was performed prior to the emission scan. Data acquisition started with a bolus i.v. injection. Brain radioactivity was measured in a series of 50 consecutive time frames (12 × 5 s, 6 × 10 s, 3 × 20 s, 6 × 30 s, 4 × 1 min, 5 × 2 min, 14 × 5 min) with a total measurement time of 90 min after bolus. Acquired data were reconstructed in volumes consisting of 35 transaxial sections (128 × 128 matrix) using an iterative filtered back‐projection algorithm (FORE‐ITER) with a spatial resolution of 4.36 mm full‐width at half maximum 1 cm next to the center of the field of view. For coregistration, magnetic resonance (MR) images were acquired from all participants on a 3 Tesla (T) Philips scanner (Achieva) using a 3D T1 FFE weighted sequence, yielding 0.88 mm slice thickness and inplane resolution of 0.8 × 0.8 mm.
Data Quantification
Each time frame of the dynamic PET scan was realigned to the mean of frames with no head motion, identified by visual inspection. Subsequently, each summed image (PET integral image from realigned data) was coregistered (rigid body transformation) to each subject's MRI using a normalized mutual information algorithm implemented in SPM12 (Wellcome Trust Centre for Neuroimaging, London, UK; http://www.fil.ion.ucl.ac.uk/spm/). Individual MRIs were spatially normalized to the T1‐weighted MRI template provided in SPM. Resulting transformation matrices were applied to the coregistered PET images, warping them into MNI standard space. Parametric images of BPND [Innis et al., 2007] values were calculated using the multilinear reference tissue model with two parameters (MRTM2) implemented in PMOD image analysis software, version 3.509 (PMOD Technologies Ltd., Zurich, Switzerland; http://www.pmod.com). Thalamus was used as the receptor‐rich region and cerebellar grey matter as the reference region because it contains negligible availability of SERT and has been demonstrated to represent the optimal reference region for [11C]DASB [Parsey et al., 2006].
Regions of Interests (ROIs)
Selected ROIs included SERT rich brain regions, based on previous PET, in vivo, human brain studies [Savli et al., 2012], including the anterior cingulate cortex, amygdala, dorsal raphe nuclei as well as the hippocampus, insula, precuneus, posterior cingulate cortex, striatum and thalamus. Binding potential values were extracted from an automated anatomical labelling (AAL)‐based atlas [Savli et al., 2012], including manually delineated ROIs for the dorsal and medial raphe nucleus.
Statistical Analysis
Data was analysed using linear mixed models for the outcome measure SERT BPND with group, sex, and ROI as fixed factors, with ROI as repeated factor, and subjects and matched participant pairs as random factors. Fixed effects were included in the model in a multifactorial approach whereas interaction effects were dropped in case of non‐significance. In case of significant interactions or main effects, post‐hoc pairwise comparisons were computed and Bonferroni corrected for multiple comparisons. In a second exploratory approach to examine the effects of age and smoking status, a mixed model was calculated using a stepwise procedure with backward elimination, i.e., starting with all candidate variables (including subject groups and ROI) followed by a stepwise deletion of interactions and variables with largest P‐values. Finally, mixed models using the same procedure were applied to investigate the effects of clinical variables CAARS‐inattentiveness and CAARS‐hyperactivity/impulsivity. According to Akaike's information criterion [Akaike, 1974], repeated measurements were modelled using the diagonal structure. SPSS version 19.0 for Windows was used for statistical computations. The two‐tailed significance level was set at 0.05.
Interregional molecular association matrices were calculated between each ROI pair using Spearman's rank correlation coefficient (Δρ) for each group separately. For the assessment of statistically significant differences (P < 0.05) in balance between patients with ADHD and HC, correlation matrices were transformed using Fisher's r‐to‐z‐transformation and a 10,000 fold permutation test was performed. Results were Bonferroni correction for multiple comparisons.
RESULTS
Linear mixed models analysis revealed a main effect of ROI (F 800.22 = 72.08, P < 0.001) and of subject group (F 29.35 = 261.37, P < 0.001; Table 1; Fig. 1), but no main effects for sex (F 1.21 = 21.26, P = 0.1) and no interaction effects (all P > 0.1). Post‐hoc pairwise comparisons revealed significant attenuated SERT BPND in patients with ADHD compared to HC in the striatum (P = 0.029; uncorrected) as well as trend in the anterior cingulate cortex and insula (P = 0.066 and P = 0.085; uncorrected). After applying Bonferroni correction for multiple comparisons, we were not able to detect any significant differences (Table 1).
Figure 1.
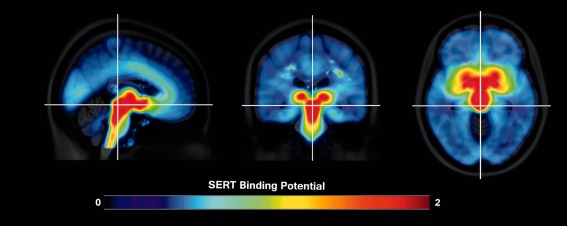
Average [11C]DASB distribution in 25 patients with ADHD normalized to MNI T1 template. Highest SERT BPND is found in the dorsal raphe nuclei ROIs. The color table represents binding potential at each voxel, blue indicates lowest and red highest SERT BPND. Crosshair is set on the dorsal raphe nuclei in MNI space. [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 1.
SERT BPND by region of interest
| Region of interest | HC | ADHD | p‐value |
|---|---|---|---|
| Anterior cingulate cortex | 0.318 ±0.071 | 0.278 ±0.076 | 0.066 |
| Amygdala | 1.025 ±0.158 | 0.958 ±0.181 | 0.183 |
| Dorsal raphe nucleus | 3.522 ±0.653 | 3.463 ±0.526 | 0.697 |
| Hippocampus | 0.605 ±0.127 | 0.563 ±0.118 | 0.194 |
| Insula | 0.558 ±0.103 | 0.518 ±0.101 | 0.085 |
| Precuneus | 0.240 ±0.052 | 0.198 ±0.070 | 0.723 |
| Posterior cingulate cortex | 0.246 ±0.078 | 0.228 ±0.085 | 0.433 |
| Striatum | 1.748 ±0.248 | 1.603 ±0.209 | 0.029* |
| Thalamus | 1.880 ±0.266 | 1.772 ±0.256 | 0.096 |
Mean SERT BPND and standard deviations are listed from automated AAL, including manually delineated ROI dorsal raphe nuclei for patients with ADHD and HC.
*Marks significant differences between patients with ADHD and HC, though, after Bonferroni correction for multiple comparisons, differences are not significant different.
AAL: anatomical labelling atlas; ADHD: attention deficit/hyperactivity disorder; HC: healthy control subjects; SERT BPND: serotonin transporter binding potential; ROIs: regions of interest.
When investigating the potential effects of age, mixed models analysis for ROI SERT BPND based on AAL atlas revealed an interaction effect between ROI and age (F 3.53 = 69.79, P < 0.002), in addition to a main effect of ROI main effect of age (F 7.15 = 21.26, P = 0.014). Post‐hoc correlation analyses between regional SERT BPND and age revealed negative correlations in the thalamus and insula (R 2 = 0.284, R 2 = 0.167, Ps < 0.05; Bonferroni corrected; Fig. 2). Furthermore, negative correlations in the anterior cingulate cortex (R 2 = 0.128), posterior cingulate cortex (R 2 = 0.119) and the precuneus (R 2 = 0.129) were detected, however not significant after Bonferroni correction. These correlations did not differ between HC and ADHD patients. Smoking status had no effect on SERT BPND nor did they lead to any significant interactions. Additionally, no main or interaction effects were observed for clinical variables (CAARS‐Inattentiveness, CAARS‐Hyperactivity/Impulsivity) and SERT BPND.
Figure 2.
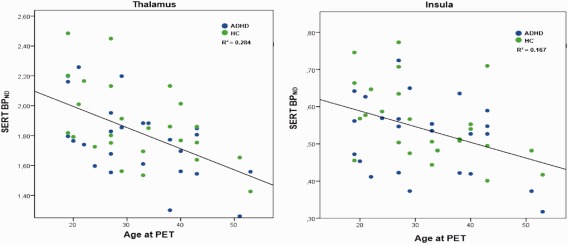
Negative correlation of SERT BPND and age in the thalamus and insula in both patients with ADHD and HC. Scatterplots showing a significant negative correlation between SERT BPND and age in the thalamus (R 2 = 0.284) and insula (R 2 = 0.167). ROIs were extracted from automated AAL. Age is given in years, significance level was set to P < 0.05 and results were Bonferroni corrected for multiple comparisons. AAL: anatomical labelling atlas; ADHD: attention deficit/hyperactivity disorder, SERT BPND: serotonin transporter binding potential, ROIs: regions of interest. [Color figure can be viewed at http://wileyonlinelibrary.com]
When comparing interregional SERT BPND correlations between patients with ADHD and HC, we found a significant difference in the correlation of precuneus with amygdala, hippocampus, insula, DRN and ACC, of the hippocampus with insula and ACC as well as of the PCC and the ACC. Only the differences in interregional molecular correlations of precuneus with hippocampus survived Bonferroni correction for multiple comparisons (P = 0.0324; see Table 2, Figs. 3 and 4).
Table 2.
Significant differences in interregional molecular correlations of the SERT BPND
| Region of interest | Δρ | P‐value | P‐value Bonferroni corrected |
|---|---|---|---|
| Precuneus—amygdala | 0.3846 | 0.0206a | 0.7416 |
| Precuneus—hippocampus | 0.74308 | 0.0009a | 0.0324b |
| Precuneus—insula | 0.52231 | 0.0072a | 0.2592 |
| Precuneus—dorsal raphe nucleus | 0.68 | 0.0134a | 0.4824 |
| Precuneus—anterior cingulate cortex | 0.50923 | 0.0077a | 0.2772 |
| Hippocampus—posterior cingulate cortex | 0.42 | 0.0218a | 0.7848 |
| Hippocampus—insula | 0.30385 | 0.0243a | 0.8748 |
| Anterior cingulate cortex—posterior cingulate cortex | 0.42769 | 0.0259a | 0.9324 |
We observed significant stronger interregional associations of SERT BPND between the listed ROIs in patients with ADHD and healthy control subjects (Spearman's delta rho; P < 0.05; corrected for multiple comparisons).
Marks significant differences between patients with ADHD and HC.
Marks significant differences between patients with ADHD and HC after Bonferroni correction for multiple comparisons.
ADHD: attention deficit/hyperactivity disorder; HC: healthy control subjects; SERT BPND: serotonin transporter binding potential; ROIs: regions of interest.
Figure 3.
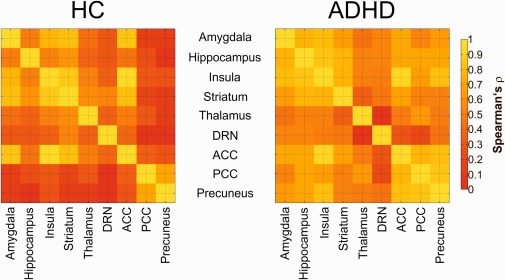
Molecular interregional molecular correlations of patients with ADHD and HC. Left map shows the correlation (Spearman's ρ) of SERT BPND in HC indicating interregional differences in functional coupling. Right map denote the condition in patients with ADHD. The color table represents the strength of interregional associations, red indicates lowest and yellow highest interregional associations. ADHD: attention deficit/hyperactivity disorder, SERT BPND: serotonin transporter binding potential, ROIs: regions of interest. HC: healthy controls. [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 4.
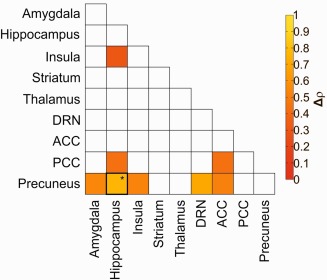
Difference in the interregional SERT balance between patients with ADHD and HC subjects. Color marked squares demonstrate significant differences in interregional SERT coupling in patients with ADHD compared to HC subjects (P < 0.05). After Bonferroni correction for multiple comparisons, we found significant differences in interregional correlations of precuneus with hippocampus (P = 0.0324), marked with *. The color table represents the difference in interregional associations (in Spearman's delta rho), red indicates lowest and yellow highest interregional associations. ADHD: attention deficit/hyperactivity disorder, SERT: serotonin transporter, ROIs: regions of interest. [Color figure can be viewed at http://wileyonlinelibrary.com]
DISCUSSION
In this cross‐sectional PET study we aimed to investigate SERT availability in adult, medication free patients with ADHD. When comparing groups, we observed lower SERT availability for all ROIs pooled together in patients with ADHD compared HC. For separate brain regions and after correction for multiple comparisons, results show no significant differences in SERT BPND between patients with ADHD and HC. When comparing interregional SERT BPND correlations between groups, we found a significant increase for interregional SERT BPND correlations of the precuneus with hippocampus. In addition, we observed a negative correlation for SERT BPND and age for patients and HC in the thalamus and the insula.
Previously published PET and SPECT imaging studies found no changes in SERT availability between patients with ADHD and HC [Hesse et al., 2009; Karlsson et al., 2013]. Though, findings are preliminary and should be interpreted with caution, since Karlsson et al. investigated SERT BPND in eight patients with ADHD, a sample size insufficient in power to detect putative differences [Karlsson et al., 2013]. Another study observed SERT availability with [123I]FP‐CIT, a SPECT radiotracer showing only moderate specificity to the SERT in subcortical regions [Hesse et al., 2009] and demonstrated no alteration between patients and HC. We found attenuated SERT binding in patients with ADHD at uncorrected P‐value in the striatum, a region that has been found to exhibit ADHD specific morphological and functional alterations [Plichta et al., 2009; Qiu et al., 2009]. Elevated SERT availability has been shown to be correlated with cognitive performance in the caudate as well as in other brain regions in HC [Madsen et al., 2011] whereas a negative association has been found between SERT binding and impulsive behavior in suicide attempters [Ryding et al., 2006]. Our finding may suggest a contribution of the SERT to the pathophysiology in ADHD, which may be key for impulsive symptoms. Nevertheless, and in line with previous SERT imaging in ADHD, we found no differences in SERT BPND after correction for multiple testing in patients with ADHD in comparison to HC.
The findings further demonstrate a decrease of SERT BPND with increasing age in the thalamus, insula, precuneus and anterior and posterior cingulate cortex in patients and HC. This validates previous SERT investigations [Hesse et al., 2003; Yamamoto et al., 2002] as well as PET studies observing the noradrenergic transmitter system [Ding et al., 2014; Vanicek et al., 2014], which demonstrated a negative correlation of monoaminergic transporters with age in HC as well as in patients with ADHD.
In addition to the comparison of SERT BPND between groups, we performed an interregional molecular correlational analysis to evaluate associations of SERT BPND between the selected ROIs. Our approach is similar to MRI studies, where structural and functional connectivity analyses aim to capture the complexity of large‐scale brain networks and findings show widespread and distinct alterations in connectivity in ADHD [Matthews et al., 2014]. These methods were adapted to PET imaging in order to explore if interregional correlations of SERT mirror morphological correlates in the pathophysiology of ADHD. The assumption that regional up‐ or down‐regulation of a single protein, such as the SERT, might be sufficient to differentiate healthy from disordered brains may seem over‐simplifying. Therefore, this approach might allow for an even more precise understanding of inherent specificities of the serotonergic system rather than simply comparing transporter binding in various regions between subject groups.
Using this interregional molecular correlational analysis we found significant interregional differences of SERT BPND correlations for the precuneus and the hippocampus. The serotonergic system projects from raphe nuclei to the precuneus and the hippocampus, therefore modulating regional and network specific function of these brain regions. The precuneus is part of the posterior components of the default mode network, a network that is activated during no‐goal directed processes, which has been shown to be dysfunctional in ADHD [Castellanos et al., 2008]. Using resting‐state fMRI, altered functional connectivity between the precuneus and other brain regions, specifically the ventromedial prefrontal cortex, a region which is highly innervated and modulated by serotonin action, has been demonstrated. In addition, the precuneus has also been found to be involved in timing functions, exhibiting increased activation patterns in patients with ADHD relative to HC using fMRI [Hart et al., 2012]. Timing deficits have been observed in patients with ADHD and linked to impulsiveness [Rubia et al., 2009].
The hippocampus is associated with learning and memory and is implicated in encoding novel stimuli, processing spatial information as well as in attention [Goldfarb et al., 2016; Jarrard, 1995; Kaplan et al., 2014; Van Petten, 2004]. Being a component of the limbic region, the hippocampus is highly modulated by serotonergic neurotransmitter system, receiving projections from midbrain serotonergic cells [Hensler, 2006]. During a decision‐making task, measured with [15O]H2O and PET, regional blood flow has been found to be reduced in the hippocampus in patients with ADHD [Ernst et al., 2003]. The altered activation in the hippocampus as well as in other regions in ADHD is interpreted as a diminished involvement of brain regions associated with complex cognitive‐emotional functions. A MRI study found reduced hippocampal volume and connectivity of the hippocampus with the prefrontal cortex, whereas structural findings were associated with depressive symptoms [Posner et al., 2014]. Though other structural MRI investigations have showed inconclusive data in ADHD, depicting higher or no differences in hippocampal volume between patients with ADHD and HC [Castellanos et al., 1996; Plessen et al., 2006].
Imaging and behavioral studies have demonstrated that serotonergic neurotransmission affect impulsive behavior [Dalley and Roiser, 2012], motor planning and sensory perception [Biskup et al., 2016] and modulates the default mode network [Hahn et al., 2010]. Recently, it has been found that, compared to HC, patients with ADHD show elevated functional connectivity of the default mode network and attenuated functional connectivity in a state of diminished brain serotonin levels, evoked through acute tryptophan depletion [Biskup et al., 2016]. We found a higher molecular correlation of the SERT between the precuneus and the hippocampus in patients with ADHD and in general lower correlations in HC, which may reflect higher impulsivity in patients and might be explained by a more diverse, region specific modulated serotonergic system in HC and by more rigid and less variable serotonergic signaling in ADHD.
This PET study has limitations that compromise the interpretation of its results. Regarding group differences in regional SERT binding a main effect was observed, but only trends for significant differences were obtained in separate brain regions. Although the sample size of this study is common for investigations with PET [Kranz et al., 2015; Volkow et al., 2007], it is still possible that more subjects are required to identify more subtle differences. On the other hand, the significance in the main effect but not for single ROIs might be driven by a more reliable variance estimate for the former one. Next to the thalamus and the insula, we found an association between age and SERT binding in the anterior cingulate cortex, posterior cingulate cortex and the precuneus, though not significant after applying Bonferroni correction. Previous PET studies found a decline in SERT with age in the raphe nuclei, though, we did not observe an age‐related decline of SERT in the dorsal raphe nuclei. The dorsal raphe nuclei is relatively small structures in the midbrain where signal to noise ratio is rather low. Therefore, it is possible that there is an age‐related decline in SERT in this region, although we did not detect an association. In addition, no blood sampling was carried out in this study. This impedes the evaluation of potential differences in the cerebellum, which was however suggested to represent an optimal reference region [Parsey et al., 2006].
CONCLUSION
In conclusion, we observed altered interregional SERT BPND correlation of the precuneus and the hippocampus in patients with ADHD, underlining the involvement of these brain areas in the pathophysiology of ADHD. On the other hand, SERT binding does not differ after applying correction for multiple comparisons on a regional level between patients with ADHD and HC. Given the fact that the SERT expression is modulated by regional serotonergic release, our results are compatible with alterations of interregional coupling within the serotonergic system in ADHD.
DISCLOSURE OF BIOMEDICAL FINANCIAL INTERESTS AND POTENTIAL CONFLICTS OF INTEREST
All authors declare no competing financial interests in relation to the work described. Without any relevance to thisƒ work, M Hacker has received conference speaker honoraria from Covidian, GE Healthcare, IBA and Endocyte and consults advisory boards of Endocyte. S Kasper declares that he has received grant/research support from Austrian National Bank (OENB), Eli Lilly, Lundbeck A/S, Bristol‐Myers Squibb, Fonds für wissenschaftliche Förderung (FWF), Servier, Sepracor, GlaxoSmithKline, Organon, Dr. Willmar Schwabe GmbH & Co. KG and has served as a consultant or on advisory boards for AOP Pharma, AstraZeneca, Austrian Sick Found, Austrian National Bank (OENB), Bristol‐Myers Squibb, German Research Foundation (DFG), Generali Insurance Company, GlaxoSmithKline, Eli Lily, Lundbeck A/S, Pfizer, Organon, Sepracor, Janssen, and Novartis, and has served on speakers' bureaus for AOP Pharma, AstraZeneca, Eli Lilly, Lundbeck A/S, Neuraxpharm, Servier, Sepracor and Janssen. GS Kranz received travel grants from Roche and AOP Orphan. A Kutzelnigg has received travel grants from Eli Lilly and Company, Affiris AG, Novartis Pharmaceuticals Corporation, and AstraZeneca, payment for lectures including service on speakers' bureaus from Eli Lilly and Company, Novartis Pharmaceuticals Corporation, AstraZeneca and Affiris AG and has served as a consultant and on advisory boards for the Austrian Federal Ministry of Health, Eli Lilly and Company, Biogen‐Idec and Medice Arzneimittel GmbH. R. Lanzenberger received travel grants and/or conference speaker honoraria from AstraZeneca, Lundbeck A/S, Dr. Willmar Schwabe GmbH, AOP Orphan Pharmaceuticals AG, Janssen, and Roche Austria GmbH. T. Vanicek received travel grants and compensation for workshop participation from Pfizer and Eli Lilly. W Wadsak has received research support from Rotem GmbH, ABX, Iason, Advion and Raytest Austria and has served as a consultant/trainer for Bayer and THP. The authors A Hahn, A Höflich, Gregory M. James, A Kautzky, M Mitterhauser, Cecile Philippe, Helen L. Sigurdardottir, T Traub‐Weidinger report no financial relationships with commercial interests. Funding/Support: This research was supported by a grant from the Austrian National Bank (OeNB), FONDS/Jubiläumsfonds (Project Nr. 13675) awarded to M Mitterhauser.
ACKNOWLEDGMENTS
The authors thank M Stamenkovic, C Klier, B Hackenberg, A Konstantinidis, P Baldinger, D Meshkat, J Losak, C Kraus and R Gößler for medical support. Further they thank the PET team, especially G Karanikas, L Nics, D Häusler, C Rami‐Mark for the technical support. For administrative support, we thank G Gryglewski, M Cotton, J Unterholzer, and MG Godbersen.
REFERENCES
- Adler L, Wilens T, Zhang S, Durell T, Walker D, Schuh L, Jin L, Feldman P, Trzepacz P (2009): Retrospective safety analysis of atomoxetine in adult ADHD patients with or without comorbid alcohol abuse and dependence. Am J Addict/Am Acad Psychiatr Alcohol Addict 18:393–401. [DOI] [PubMed] [Google Scholar]
- Akaike H (1974): A new look at the statistical model identification. System identification and time‐series analysis. IEEE Trans Automatic Control AC‐1:716–723. [Google Scholar]
- Aron AR, Poldrack RA (2005): The cognitive neuroscience of response inhibition: Relevance for genetic research in attention‐deficit/hyperactivity disorder. Biol Psychiatry 57:1285–1292. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013) Diagnostic and Statistical Manual of Mental Health Disorders: DSM‐5, 5th ed Washington, DC: American Psychiatric Publishing. [Google Scholar]
- Baldinger P, Kranz GS, Haeusler D, Savli M, Spies M, Philippe C, Hahn A, Hoflich A, Wadsak W, Mitterhauser M, Lanzenberger R, Kasper S (2014): Regional differences in SERT occupancy after acute and prolonged SSRI intake investigated by brain PET. Neuroimage 88:252–262. [DOI] [PubMed] [Google Scholar]
- Banerjee E, Nandagopal K (2015): Does serotonin deficit mediate susceptibility to ADHD?. Neurochem Int 82:52–68. [DOI] [PubMed] [Google Scholar]
- Barbaresi WJ, Colligan RC, Weaver AL, Voigt RG, Killian JM, Katusic SK (2013): Mortality, ADHD, and psychosocial adversity in adults with childhood ADHD: A prospective study. Pediatrics 131:637–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrickman L, Noyes R, Kuperman S, Schumacher E, Verda M (1991): Treatment of ADHD with fluoxetine: A preliminary trial. J Am Acad Child Adolesc Psychiatry 30:762–767. [PubMed] [Google Scholar]
- Benmansour S, Owens WA, Cecchi M, Morilak DA, Frazer A (2002): Serotonin clearance in vivo is altered to a greater extent by antidepressant‐induced downregulation of the serotonin transporter than by acute blockade of this transporter. J Neurosci 22:6766–6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J, Faraone SV (2005): Attention‐deficit hyperactivity disorder. Lancet 366:237–248. [DOI] [PubMed] [Google Scholar]
- Biskup CS, Helmbold K, Baurmann D, Klasen M, Gaber TJ, Bubenzer‐Busch S, Konigschulte W, Fink GR, Zepf FD (2016): Resting state default mode network connectivity in children and adolescents with ADHD after acute tryptophan depletion. Acta Psychiatr Scand 134:161–171. [DOI] [PubMed] [Google Scholar]
- Bullmore E (2012): The future of functional MRI in clinical medicine. Neuroimage 62:1267–1271. [DOI] [PubMed] [Google Scholar]
- Bymaster FP, Katner JS, Nelson DL, Hemrick‐Luecke SK, Threlkeld PG, Heiligenstein JH, Morin SM, Gehlert DR, Perry KW (2002): Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: A potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology 27:699–711. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Giedd JN, Marsh WL, Hamburger SD, Vaituzis AC, Dickstein DP, Sarfatti SE, Vauss YC, Snell JW, Lange N, Kaysen D, Krain AL, Ritchie GF, Rajapakse JC, Rapoport JL (1996): Quantitative brain magnetic resonance imaging in attention‐deficit hyperactivity disorder. Arch Gen Psychiatry 53:607–616. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Margulies DS, Kelly C, Uddin LQ, Ghaffari M, Kirsch A, Shaw D, Shehzad Z, Di Martino A, Biswal B, Sonuga‐Barke EJ, Rotrosen J, Adler LA, Milham MP (2008): Cingulate‐precuneus interactions: A new locus of dysfunction in adult attention‐deficit/hyperactivity disorder. Biol Psychiatry 63:332–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castells X, Ramos‐Quiroga JA, Rigau D, Bosch R, Nogueira M, Vidal X, Casas M (2011): Efficacy of methylphenidate for adults with attention‐deficit hyperactivity disorder: A meta‐regression analysis. CNS Drugs 25:157–169. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Hampshire A, Muller U, Rubia K, Del Campo N, Craig K, Regenthal R, Suckling J, Roiser JP, Grant JE, Bullmore ET, Robbins TW, Sahakian BJ (2009): Atomoxetine modulates right inferior frontal activation during inhibitory control: A pharmacological functional magnetic resonance imaging study. Biol Psychiatry 65:550–555. [DOI] [PubMed] [Google Scholar]
- Chantiluke K, Barrett N, Giampietro V, Santosh P, Brammer M, Simmons A, Murphy DG, Rubia K (2015): Inverse fluoxetine effects on inhibitory brain activation in non‐comorbid boys with ADHD and with ASD. Psychopharmacology (Berl) 232:2071–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Roiser JP (2012): Dopamine, serotonin and impulsivity. Neuroscience 215:42–58. [DOI] [PubMed] [Google Scholar]
- del Campo N, Fryer TD, Hong YT, Smith R, Brichard L, Acosta‐Cabronero J, Chamberlain SR, Tait R, Izquierdo D, Regenthal R, Dowson J, Suckling J, Baron JC, Aigbirhio FI, Robbins TW, Sahakian BJ, Muller U (2013): A positron emission tomography study of nigro‐striatal dopaminergic mechanisms underlying attention: Implications for ADHD and its treatment. Brain 136:3252–3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding YS, Naganawa M, Gallezot JD, Nabulsi N, Lin SF, Ropchan J, Weinzimmer D, McCarthy TJ, Carson RE, Huang Y, Laruelle M (2014): Clinical doses of atomoxetine significantly occupy both norepinephrine and serotonin transports: Implications on treatment of depression and ADHD. Neuroimage 86:164–171. [DOI] [PubMed] [Google Scholar]
- Ernst M, Kimes AS, London ED, Matochik JA, Eldreth D, Tata S, Contoreggi C, Leff M, Bolla K (2003): Neural substrates of decision making in adults with attention deficit hyperactivity disorder. Am J Psychiatry 160:1061–1070. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Khan SA (2006): Candidate gene studies of attention‐deficit/hyperactivity disorder. J Clin Psychiatry 67 Suppl 8:13–20. [PubMed] [Google Scholar]
- Faraone SV, Perlis RH, Doyle AE, Smoller JW, Goralnick JJ, Holmgren MA, Sklar P (2005): Molecular genetics of attention‐deficit/hyperactivity disorder. Biol Psychiatry 57:1313–1323. [DOI] [PubMed] [Google Scholar]
- Fliers EA, Vasquez AA, Poelmans G, Rommelse N, Altink M, Buschgens C, Asherson P, Banaschewski T, Ebstein R, Gill M, Miranda A, Mulas F, Oades RD, Roeyers H, Rothenberger A, Sergeant J, Sonuga‐Barke E, Steinhausen HC, Faraone SV, Buitelaar JK, Franke B (2012): Genome‐wide association study of motor coordination problems in ADHD identifies genes for brain and muscle function. World J Biol Psychiatry 13:211–222. [DOI] [PubMed] [Google Scholar]
- Fusar‐Poli P, Rubia K, Rossi G, Sartori G, Balottin U (2012): Striatal dopamine transporter alterations in ADHD: Pathophysiology or adaptation to psychostimulants? A meta‐analysis. Am J Psychiatry 169:264–272. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Wetsel WC, Jones SR, Levin ED, Jaber M, Caron MG (1999): Role of serotonin in the paradoxical calming effect of psychostimulants on hyperactivity. Science 283:397–401. [DOI] [PubMed] [Google Scholar]
- Goldfarb EV, Chun MM, Phelps EA (2016): Memory‐Guided Attention: Independent Contributions of the Hippocampus and Striatum. Neuron 89:317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeusler D, Mien LK, Nics L, Ungersboeck J, Philippe C, Lanzenberger RR, Kletter K, Dudczak R, Mitterhauser M, Wadsak W (2009): Simple and rapid preparation of [11C]DASB with high quality and reliability for routine applications. Appl Radiat Isot 67:1654–1660. [DOI] [PubMed] [Google Scholar]
- Hahn A, Haeusler D, Kraus C, Hoflich AS, Kranz GS, Baldinger P, Savli M, Mitterhauser M, Wadsak W, Karanikas G, Kasper S, Lanzenberger R (2014): Attenuated serotonin transporter association between dorsal raphe and ventral striatum in major depression. Hum Brain Mapp 35:3857–3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn A, Lanzenberger R, Wadsak W, Spindelegger C, Moser U, Mien LK, Mitterhauser M, Kasper S (2010): Escitalopram enhances the association of serotonin‐1A autoreceptors to heteroreceptors in anxiety disorders. J Neurosci 30:14482–14489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart H, Radua J, Mataix‐Cols D, Rubia K (2012): Meta‐analysis of fMRI studies of timing in attention‐deficit hyperactivity disorder (ADHD). Neurosci Biobehav Rev 36:2248–2256. [DOI] [PubMed] [Google Scholar]
- Hart H, Radua J, Nakao T, Mataix‐Cols D, Rubia K (2013): Meta‐analysis of functional magnetic resonance imaging studies of inhibition and attention in attention‐deficit/hyperactivity disorder: Exploring task‐specific, stimulant medication, and age effects. JAMA Psychiatry 70:185–198. [DOI] [PubMed] [Google Scholar]
- Hensler JG (2006): Serotonergic modulation of the limbic system. Neurosci Biobehav Rev 30:203–214. [DOI] [PubMed] [Google Scholar]
- Hesse S, Barthel H, Murai T, Muller U, Muller D, Seese A, Kluge R, Sabri O (2003): Is correction for age necessary in neuroimaging studies of the central serotonin transporter? Eur J Nucl Med Mol Imaging 30:427–430. [DOI] [PubMed] [Google Scholar]
- Hesse S, Ballaschke O, Barthel H, Sabri O (2009): Dopamine transporter imaging in adult patients with attention‐deficit/hyperactivity disorder. Psychiatry Res 171:120–128. [DOI] [PubMed] [Google Scholar]
- Horwitz B, Duara R, Rapoport SI (1984): Intercorrelations of glucose metabolic rates between brain regions: Application to healthy males in a state of reduced sensory input. J Cereb Blood Flow Metab 4:484–499. [DOI] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, Holden J, Houle S, Huang SC, Ichise M, Iida H, Ito H, Kimura Y, Koeppe RA, Knudsen GM, Knuuti J, Lammertsma AA, Laruelle M, Logan J, Maguire RP, Mintun MA, Morris ED, Parsey R, Price JC, Slifstein M, Sossi V, Suhara T, Votaw JR, Wong DF, Carson RE (2007): Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab 27:1533–1539. [DOI] [PubMed] [Google Scholar]
- Jarrard LE (1995): What does the hippocampus really do? Behav Brain Res 71:1–10. [DOI] [PubMed] [Google Scholar]
- Kaplan R, Horner AJ, Bandettini PA, Doeller CF, Burgess N (2014): Human hippocampal processing of environmental novelty during spatial navigation. Hippocampus 24:740–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson L, Tuominen L, Huotarinen A, Leppamaki S, Sihvola E, Helin S, Sipila M, Tani P, Hirvonen J, Hietala J, Karlsson H (2013): Serotonin transporter in attention‐deficit hyperactivity disorder–preliminary results from a positron emission tomography study. Psychiatry Res 212:164–165. [DOI] [PubMed] [Google Scholar]
- Kranz GS, Wadsak W, Kaufmann U, Savli M, Baldinger P, Gryglewski G, Haeusler D, Spies M, Mitterhauser M, Kasper S, Lanzenberger R (2015): High‐dose testosterone treatment increases serotonin transporter binding in transgender people. Biol Psychiatry 78:525–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS (1997): Effects of methylphenidate on extracellular dopamine, serotonin, and norepinephrine: Comparison with amphetamine. J Neurochem 68:2032–2037. [DOI] [PubMed] [Google Scholar]
- Lanzenberger R, Kranz GS, Haeusler D, Akimova E, Savli M, Hahn A, Mitterhauser M, Spindelegger C, Philippe C, Fink M, Wadsak W, Karanikas G, Kasper S (2012): Prediction of SSRI treatment response in major depression based on serotonin transporter interplay between median raphe nucleus and projection areas. Neuroimage 63:874–881. [DOI] [PubMed] [Google Scholar]
- Lee DS, Kang H, Kim H, Park H, Oh JS, Lee JS, Lee MC (2008): Metabolic connectivity by interregional correlation analysis using statistical parametric mapping (SPM) and FDG brain PET; methodological development and patterns of metabolic connectivity in adults. Eur J Nucl Med Mol Imaging 35:1681–1691. [DOI] [PubMed] [Google Scholar]
- Madsen K, Erritzoe D, Mortensen EL, Gade A, Madsen J, Baare W, Knudsen GM, Hasselbalch SG (2011): Cognitive function is related to fronto‐striatal serotonin transporter levels–a brain PET study in young healthy subjects. Psychopharmacology (Berl) 213:573–581. [DOI] [PubMed] [Google Scholar]
- Matthews M, Nigg JT, Fair DA (2014): Attention deficit hyperactivity disorder. Curr Top Behav Neurosci 16:235–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morbelli S, Perneczky R, Drzezga A, Frisoni GB, Caroli A, van Berckel BN, Ossenkoppele R, Guedj E, Didic M, Brugnolo A, Naseri M, Sambuceti G, Pagani M, Nobili F (2013): Metabolic networks underlying cognitive reserve in prodromal Alzheimer disease: A European Alzheimer disease consortium project. J Nucl Med 54:894–902. [DOI] [PubMed] [Google Scholar]
- Muller CP, Jacobs BL (2009): Handbook of the Behavioral Neurobiology of Serotonin, 1st Edition. Elsevier, London, England. [Google Scholar]
- Oades RD (2008): Dopamine‐serotonin interactions in attention‐deficit hyperactivity disorder (ADHD). Prog Brain Res 172:543–565. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Ojha A, Ogden RT, Erlandsson K, Kumar D, Landgrebe M, Van Heertum R, Mann JJ (2006): Metabolite considerations in the in vivo quantification of serotonin transporters using 11C‐DASB and PET in humans. J Nucl Med 47:1796–1802. [PubMed] [Google Scholar]
- Plessen KJ, Bansal R, Zhu H, Whiteman R, Amat J, Quackenbush GA, Martin L, Durkin K, Blair C, Royal J, Hugdahl K, Peterson BS (2006): Hippocampus and amygdala morphology in attention‐deficit/hyperactivity disorder. Arch Gen Psychiatry 63:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plichta MM, Vasic N, Wolf RC, Lesch KP, Brummer D, Jacob C, Fallgatter AJ, Gron G (2009): Neural hyporesponsiveness and hyperresponsiveness during immediate and delayed reward processing in adult attention‐deficit/hyperactivity disorder. Biol Psychiatry 65:7–14. [DOI] [PubMed] [Google Scholar]
- Posner J, Siciliano F, Wang Z, Liu J, Sonuga‐Barke E, Greenhill L (2014): A multimodal MRI study of the hippocampus in medication‐naive children with ADHD: What connects ADHD and depression?. Psychiatry Res 224:112–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu A, Crocetti D, Adler M, Mahone EM, Denckla MB, Miller MI, Mostofsky SH (2009): Basal ganglia volume and shape in children with attention deficit hyperactivity disorder. Am J Psychiatry 166:74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana H, Butterbaugh GJ, Purnell W, Layman AK (2007): Fluoxetine monotherapy in attention‐deficit/hyperactivity disorder and comorbid non‐bipolar mood disorders in children and adolescents. Child Psychiatry Hum Dev 37:241–253. [DOI] [PubMed] [Google Scholar]
- Retz W, Retz‐Junginger P, Thome J, Rosler M (2011): Pharmacological treatment of adult ADHD in Europe. World J Biol Psychiatry 12 Suppl 1:89–94. [DOI] [PubMed] [Google Scholar]
- Rosler M, Casas M, Konofal E, Buitelaar J (2010): Attention deficit hyperactivity disorder in adults. World J Biol Psychiatry 11:684–698. [DOI] [PubMed] [Google Scholar]
- Rubia K, Halari R, Christakou A, Taylor E (2009): Impulsiveness as a timing disturbance: Neurocognitive abnormalities in attention‐deficit hyperactivity disorder during temporal processes and normalization with methylphenidate. Philos Trans R Soc Lond B Biol Sci 364:1919–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryding E, Ahnlide JA, Lindstrom M, Rosen I, Traskman‐Bendz L (2006): Regional brain serotonin and dopamine transporter binding capacity in suicide attempters relate to impulsiveness and mental energy. Psychiatry Res 148:195–203. [DOI] [PubMed] [Google Scholar]
- Savli M, Bauer A, Mitterhauser M, Ding YS, Hahn A, Kroll T, Neumeister A, Haeusler D, Ungersboeck J, Henry S, Isfahani SA, Rattay F, Wadsak W, Kasper S, Lanzenberger R (2012): Normative database of the serotonergic system in healthy subjects using multi‐tracer PET. Neuroimage 63:447–459. [DOI] [PubMed] [Google Scholar]
- Shaw P, Stringaris A, Nigg J, Leibenluft E (2014): Emotion dysregulation in attention deficit hyperactivity disorder. Am J Psychiatry 171:276–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spies M, Knudsen GM, Lanzenberger R, Kasper S (2015): The serotonin transporter in psychiatric disorders: Insights from PET imaging. Lancet Psychiatry 2:743–755. [DOI] [PubMed] [Google Scholar]
- van der Meer D, Hartman CA, Richards J, Bralten JB, Franke B, Oosterlaan J, Heslenfeld DJ, Faraone SV, Buitelaar JK, Hoekstra PJ (2014): The serotonin transporter gene polymorphism 5‐HTTLPR moderates the effects of stress on attention‐deficit/hyperactivity disorder. J Child Psychol Psychiatry 55:1363–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Petten C (2004): Relationship between hippocampal volume and memory ability in healthy individuals across the lifespan: Review and meta‐analysis. Neuropsychologia 42:1394–1413. [DOI] [PubMed] [Google Scholar]
- Vanicek T, Spies M, Rami‐Mark C, Savli M, Hoflich A, Kranz GS, Hahn A, Kutzelnigg A, Traub‐Weidinger T, Mitterhauser M, Wadsak W, Hacker M, Volkow ND, Kasper S, Lanzenberger R (2014): The norepinephrine transporter in attention‐deficit/hyperactivity disorder investigated with positron emission tomography. JAMA Psychiatry 71:1340–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanicek T, Hahn A, Traub‐Weidinger T, Hilger E, Spies M, Wadsak W, Lanzenberger R, Pataraia E, Asenbaum‐Nan S (2016): Insights into Intrinsic Brain Networks based on Graph Theory and PET in right‐ compared to left‐sided Temporal Lobe Epilepsy. Sci Rep 6:28513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Newcorn J, Fowler JS, Telang F, Solanto MV, Logan J, Wong C, Ma Y, Swanson JM, Schulz K, Pradhan K (2007): Brain dopamine transporter levels in treatment and drug naive adults with ADHD. Neuroimage 34:1182–1190. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Kollins SH, Wigal TL, Newcorn JH, Telang F, Fowler JS, Zhu W, Logan J, Ma Y, Pradhan K, Wong C, Swanson JM (2009): Evaluating dopamine reward pathway in ADHD: Clinical implications. JAMA 302:1084–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Dalley JW, Cardinal RN, Robbins TW (2006): Double dissociation between serotonergic and dopaminergic modulation of medial prefrontal and orbitofrontal cortex during a test of impulsive choice. Cereb Cortex 16:106–114. [DOI] [PubMed] [Google Scholar]
- Wong DT, Bymaster FP, Reid LR, Mayle DA, Krushinski JH, Robertson DW (1993): Norfluoxetine enantiomers as inhibitors of serotonin uptake in rat brain. Neuropsychopharmacology 8:337–344. [DOI] [PubMed] [Google Scholar]
- Wood MD, Wren PB (2008): Serotonin‐dopamine interactions: Implications for the design of novel therapeutic agents for psychiatric disorders. Prog Brain Res 172:213–230. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Suhara T, Okubo Y, Ichimiya T, Sudo Y, Inoue M, Takano A, Yasuno F, Yoshikawa K, Tanada S (2002): Age‐related decline of serotonin transporters in living human brain of healthy males. Life Sci 71:751. [DOI] [PubMed] [Google Scholar]
- Zimmer L (2009): Positron emission tomography neuroimaging for a better understanding of the biology of ADHD. Neuropharmacology 57:601. [DOI] [PubMed] [Google Scholar]


