Abstract
Multiple sclerosis (MS) is a demyelinating disease that results in a broad array of symptoms, including impaired motor performance. How such demyelination of fibers affects the inherent neurophysiological activity in motor circuits, however, remains largely unknown. Potentially, the movement errors associated with MS may be due to imperfections in the internal model used to make predictions of the motor output that will meet the task demands. Prior magnetoencephalographic (MEG) and electroencephalographic brain imaging experiments have established that the beta (15‐30 Hz) oscillatory activity in the sensorimotor cortices is related to the control of movement. Specifically, it has been suggested that the strength of the post‐movement beta rebound may indicate the certainty of the internal model. In this study, we used MEG to evaluate the neural oscillatory activity in the sensorimotor cortices of individuals with MS and healthy individuals during a goal‐directed isometric knee force task. Our results showed no difference between the individuals with MS and healthy individuals in the beta activity during the planning and execution stages of movement. However, we did find that individuals with MS exhibited a weaker post‐movement beta rebound in the pre/postcentral gyri relative to healthy controls. Additionally, we found that the behavioral performance of individuals with MS was aberrant, and related to the strength of the post‐movement beta rebound. These results suggest that the internal model may be faulty in individuals with MS. Hum Brain Mapp 38:4009–4018, 2017. © 2017 Wiley Periodicals, Inc.
Keywords: magnetoencephalography, lower extremity, beta frequency, PMBR
INTRODUCTION
Multiple sclerosis (MS) is a demyelinating disease that impacts the function of the central nervous system, and often results in impaired muscular performance. Previously, we have shown that individuals with MS have greater errors when attempting to control the precision of the lower extremity force production [Arpin et al., 2016; Davies et al., 2015]. While these results are insightful, the neurophysiological abnormalities that may be responsible for the reduced muscular force control remains unknown. Potentially, the errors in the precision of the force production may partly be a result of imperfections in the internal model that is used to make accurate predictions of the motor output that will meet the task demands.
Prior research has established that the brain maintains and updates an internal model that is used to predict the muscular synergies necessary to achieve a motor goal [Kording et al., 2004; Shadmer, 2004; Wolpert, 2007]. This internal model is used to formulate a motor plan based on sensory feedback and knowledge of results from prior attempts to achieve the motor goal. The motor plan is then transformed into a motor command, which contains the predicted muscular synergies required to achieve the motor goal. Once the motor command is executed, the sensory feedback that occurs can then be compared with the sensory feedback expected by the internal model. Any mismatch between the actual and expected sensory feedback can be used to make corrections to the movement trajectory [Kording et al., 2004; Shadmer, 2004; Wolpert, 2007]. A breakdown in any of these processes may contribute to the errors observed in the precision of the force production of individuals with MS. However, determining where that breakdown may occur (i.e., motor planning, execution, or feedback stage) is inherently difficult due to the speed at which each of these processes occurs.
Advances in neuroimaging and neurophysiological techniques have allowed the neural oscillatory activity serving motor control to be noninvasively imaged in human participants, and such studies have suggested that distinct neural oscillations correspond to the planning and execution aspects of movement. Electroencephalography (EEG) and magnetoencephalography (MEG) are currently the only brain imaging techniques with sufficient temporal resolution to assess these neural oscillations. Numerous EEG and MEG experiments have shown that prior to the onset of movement, cortical oscillatory activity across the sensorimotor cortices decreases in the beta frequency range (15–30 Hz) [Cheyne et al., 2006; Jurkiewicz et al., 2006; Pfurtscheller and Lopes da Silva, 1999]. This decrease in the amount of power found in the beta band, commonly termed beta desynchronization, is thought to reflect task‐related changes in the firing rate of local populations of neurons, as they begin to prepare for the specific demands of the pending movement. The consensus is that this beta event‐related desynchronization (ERD) is related to the formulation of the motor plan, because it occurs well before the onset of movement [Alegre et al., 2003], occurs earlier for easier motor tasks [Kaiser et al., 2001], and because the amplitude of the response is influenced by the certainty of the movement pattern to be performed [Tzagarakis et al., 2010]. Additionally, upon completion of a movement, there is a robust beta frequency event‐related synchronization, which is referred to as the post‐movement beta rebound (PMBR) [Gaetz et al., 2011, 2010; Tzagarakis et al., 2010; Wilson et al., 2014, 2010, 2011]. Traditionally, this PMBR was believed to represent the active inhibition of neuronal networks after movement termination [Neuper and Pfurtscheller, 2001; Salmelin et al., 1995; Solis‐Escalante et al., 2012] and/or afferent feedback to the motor cortices [Cassim et al., 2001; Houdayer et al., 2006; Parkes et al., 2006]. However, recent experimental work has shown that changes in the PMBR may reflect the certainty of the feedforward motor actions that were executed based on the internal model [Tan et al., 2016].
While the central role of beta neural oscillatory activity in motor performance is well appreciated, there has been limited effort to use this knowledge to more precisely characterize the motor deficits seen in individuals with MS. Of note, one previous EEG study assessed beta neural oscillations in patients with MS during a self‐paced hand movement task [Leocani et al., 2001], but this study was limited to scalp‐level analyzes and movement of the upper extremities. Additionally, a recent MEG study examined motor‐related oscillations in patients with MS [Barratt et al., 2017], but this study also focused on the upper extremities and no studies to date have examined oscillatory activity during a lower extremity motor task in patients with MS. Therefore, the purpose of this study was (1) to determine if beta oscillatory activity is altered in individuals with MS compared to healthy controls when completing a knee extension target matching task, and (2) to identify if there is a relationship between beta oscillatory activity and the precision of the knee joint muscular force production.
METHODS
Subjects
Fifteen individuals with relapsing‐remitting or secondary progressive MS (Age = 56.1 ± 6.5 years; Female = 12) and fifteen healthy age and sex matched individuals (Age = 55.1 ± 6.9 years; Female = 12) participated in this study. The individuals with MS had an average Kurtzke Expanded Disability Status Scale of 5.5 ± 0.7, which indicated that on average they could walk independently for at least 100 m. At the time of data collection, none of the patients had a relapse or a change in medication for at least 3 months. All testing was done at the University of Nebraska Medical Center. The Institutional Review Board at the University of Nebraska Medical Center reviewed and approved the protocol for this investigation. Additionally, all participants provided informed consent prior to participation in this study.
Experimental Paradigm
The participants were seated upright in a magnetically‐silent chair. The experimental paradigm consisted of an isometric knee extension target matching task. The participants used their most affected leg (nondominant for the healthy comparison group) to match target forces that varied randomly between 5 and 30% of the participant's maximum isometric knee extension force. The target force was visually displayed as a box on a back‐projection screen that was ∼1 meter in front of the participant at eye level, and the force generated by the participant was shown as a smaller box (beneath the larger box) that moved vertically based on the isometric force generated (Fig. 1A). Each participant performed 120 target matching trials. Each trial lasted 5.0 s and was followed by a 5.0 s rest period. If the participant successfully matched the target, the trial ended early and any additional time was added to the 5.0 s of rest. A successful match occurred when the box representing the participant's isometric force was inside the target box for 0.3 s, and this was followed by the presentation of a fixation cross until the start of the next trial.
Figure 1.
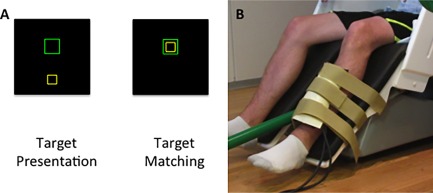
(A) Depiction of the target matching task. The isometric knee extension force generated by the participant animates the yellow box to ascend vertically to match the green target box. Each trial lasted 5.0 s and was followed by a 5.0 s rest period. If the participant successfully matched the target, the trial ended early and any additional time was added to the 5.0 s of rest. A successful match occurred when the box representing the participant's isometric force was inside the target box for 0.3 s. (B) Depiction of the custom‐built pneumatic force transducer that was positioned just proximal to the lateral malleolus of the participant. [Color figure can be viewed at http://wileyonlinelibrary.com]
A custom‐built magnetically‐silent force transducer was used to measure the isometric knee extension forces generated by the participants (Fig. 1B). This device consisted of a 20 x 10 cm airbladder that was inflated to 317 kPa, and fixed to the anterior portion of the lower leg just proximal to the lateral malleoli (ankle). A thermoplastic shell encased the outer portion of the airbladder and was secured to the chair with ridged strappings. Changes in the pressure of the airbag as the participant generated an isometric contraction were quantified by an air pressure sensor (Phidgets Inc., Calgary, Alberta, CA), and were subsequently converted into units of force. The force data was concurrently collected with the MEG data at 1 kHz. For each trial, the reaction time, amount of overshoot, average rate of force development to the target, time to initially reach the target, and the time to successfully match the target were computed offline. Separate t‐tests at the 0.05 alpha level were used to determine if there were differences in the behavioral variables of the respective groups.
MEG Data Acquisition and Coregistration
All MEG recordings were conducted in a one‐layer magnetically shielded room (ETS‐Lindgren Oy, Eura, Finland) with active shielding engaged for advanced environmental noise compensation. During data acquisition, participants were monitored via real‐time audio‐video feeds from inside the shielded room. Neuromagnetic responses were acquired with a bandwidth of 0.1–330 Hz and were sampled continuously at 1 kHz using an Elekta Neuromag system (Helsinki, Finland) with 306 MEG sensors, including 204 planar gradiometers and 102 magnetometers. With the use of the MaxFilter software (Elekta), each MEG dataset was individually corrected for head motion during task performance, and subjected to noise reduction using the signal space separation method with a temporal extension [Taulu and Simola, 2006]. Note that mean head movement was less than 5 mm across the whole recording in both groups (MS: 0.47 cm; Controls: 0.31 cm), and was corrected to the starting head position prior to MEG data pre‐processing.
Four coils were affixed to the head of each participant and were used for continuous head localization during the MEG experiment. Before the experiment, the location of these coils, three fiducial points, and the scalp surface were digitized to determine their three‐dimensional position (Fastrak 3SF0002, Polhemus Navigator Sciences, Colchester, VT, USA). Once the participant was positioned for MEG recording, an electric current with a unique frequency label (e.g., 322 Hz) was fed to each of the four coils. This induced a measurable magnetic field and allowed each coil to be localized in reference to the sensors throughout the recording session. Since the coil locations were also known in head coordinates, all MEG measurements could be transformed into a common coordinate system. With this coordinate system (including the scalp surface points), each participant's MEG data was coregistered with structural T1‐weighted MRI data using three external landmarks (i.e., fiducials) and the digitized scalp surface points prior to source space analyzes. Structural MRI data were aligned parallel to the anterior and posterior commissures and transformed into the Talairach coordinate system [Talairach and Tournoux, 1998] using the volumetric subspace warping method implemented in BrainVoyager QX version 2.2 (Brain Innovations, The Netherlands).
MEG Pre‐Processing
Artifact rejection was based on a fixed threshold method, supplemented with visual inspection. In brevity, this fixed threshold methodology was applied to each participant's data individually, and consisted of rejecting epochs containing signal amplitudes and/or gradients that exceeded a given threshold on any planar gradiometer. Two participants with MS and two controls were excluded from data analysis due to excessive MEG artifacts, which resulted in a large number of rejected epochs. To assess the beta ERD, 10.0 s epochs were defined from −3.0 to +7.0 s, with the onset of movement defined as time 0.0 s and the baseline defined as −1.6 to −1.2 s. To assess the PMBR, 10.0 s epochs were defined from −4.8 to +5.2 s, with the offset of movement defined as time 0.0 s and the baseline defined as −4.6 to −4.2 s. Note that the baseline and epoch definitions were adjusted to accommodate the temporal location of movement, which naturally varied due to the different definition of time zero (i.e., movement‐onset versus movement‐offset) across the two analysis types (i.e., ERD and PMBR). Artifact‐free epochs for each sensor were transformed into the time‐frequency domain using complex demodulation (resolution: 2.0 Hz, 25 ms) and averaged over the respective trials to generate plots of the mean spectral density. These data were then normalized by dividing the power value of each time‐frequency bin by the mean power during the respective baseline periods.
Sensor Level Statistics
We determined the precise time‐frequency bins of interest for both the beta ERD and the PMBR by conducting separate statistical analyzes of the spectrograms corresponding to a subset of planar gradiometers (106 sensors) centered on the midline near the sensorimotor cortices. Each time‐frequency bin in the spectrogram was initially evaluated using a mass univariate approach based on the general linear model. Briefly, we conducted one sample t‐tests on each time‐frequency bin across all participants, and the output spectrograms of t‐values (one per sensor) were thresholded at P < 0.05. Next, the time‐frequency bins that survived this threshold were clustered with temporally and/or spectrally neighboring bins that were also above the threshold, and a cluster value was derived by summing all of the t‐values of all data points in the cluster. Nonparametric permutation testing was then used to derive a distribution of cluster values, and the significance level of the observed clusters was tested directly using this distribution [Ernst, 2004; Maris and Oostenveld, 2007]. Note that we have followed a similar statistical method in several recent publications [Heinrichs‐Graham et al., 2014, 2015, 2016a, 2016b]. For each comparison, 10,000 permutations were computed to build a distribution of cluster values. Based on this analysis, the time‐frequency windows that contained significant oscillatory events across all participants were identified for the beamforming analyzes.
MEG Source Imaging and Virtual Sensor Extraction
A minimum variance vector beamforming algorithm was used to calculate the source power across the entire brain volume [Gross et al., 2001; van Veen et al., 1997]. The single images were derived from the cross spectral densities of all combinations of the 204 MEG planer gradiometers within the time‐frequency ranges of interest, and the solution of the forward problem for each location on a grid specified by input voxel space. Following convention, the source power in these images were normalized per participant using a separately averaged pre‐stimulus noise period of equal duration and bandwidth [Hillebrand et al., 2005]. Thus, the normalized power per voxel was computed for the time‐frequency ranges of interest over the entire brain volume per participant at 4.0 × 4.0 × 4.0 mm resolution. Each participant's functional images were transformed into a standardized space using the transform previously applied to the structural MRI volume [Talairach and Tournoux 1998]. The MEG pre‐processing and imaging was performed using the BESA software (BESA version 6.0), and MEG‐MRI coregistration was performed using the BrainVoyager QX (Version 2.2) software.
Once all functional images were in the same anatomical space, the ERD and PMBR beamformer images were averaged across all participants separately to identify the peak responses. We then extracted virtual sensors corresponding to the peak voxel of each response. The virtual sensors were created by applying the sensor weighting matrix derived through the forward computation to the preprocessed signal vector, which resulted in a time series with the same temporal resolution as the original MEG recording [Cheyne et al., 2006; Heinrichs‐Graham and Wilson, 2016a,b]. Once the virtual sensors were extracted, they were transformed into the time‐frequency domain and the power, relative to baseline, was averaged across the frequency window of interest per unit time for each individual to derive the temporal evolution of the key oscillatory responses. Statistical analysis of these voxel time series was then performed using a two‐stage approach similar to that used for the sensor‐level analyzes, except that two sample t‐tests (not one‐sample) were conducted on each data point in the time series. The output was thresholded at P < 0.05 and we controlled for Type 1 error by conducting nonparametric permutation testing using the same cluster t‐value and 10,000 permutations approach that was used in the sensor‐level analyzes [Ernst, 2004; Maris and Oostenveld, 2007]. Finally, we averaged the power across the time windows of significant difference for each individual to derive the strength of the event‐related neural activity (see below), and these data were entered into Pearson product moment correlations to determine if there was a correlation between event‐related neural activity and the respective behavioral variables.
RESULTS
Demographic and Behavioral Data
As noted in the MEG pre‐processing section, two participants with MS and two controls were excluded due to excessive MEG artifacts. Following these exclusions, the two groups did not statistically differ on age or sex (MS: age = 57.1 ± 6.3 years, females = 11; Controls: age = 55.1 ± 6.9, females = 12). However, significant differences were found between the two groups for all behavioral measures (Fig. 2). Individuals with MS had a longer reaction time (MS = 0.49 ± 0.16 s, Controls = 0.36 ± 0.06 s, P = 0.01), greater amount of overshoot (MS = 7.43 ± 2.69%, Controls = 4.58 ± 1.59%, P = 0.003), slower average rate of force development to the target (MS = 40.0 ± 19.7 N/s, Controls = 60.7 ± 22.0 N/s, P = 0.02), longer time to initially reach the target (MS = 1.20 ± 0.36 s, Controls = 0.91 ± 0.23 s, P = 0.02), and longer time to match the target (MS = 2.72 ± 0.47 s, Controls = 2.01 ± 0.23 s, P = 0.00005). Altogether the results indicate that the precision of the isometric knee force production was reduced for the individuals with MS.
Figure 2.
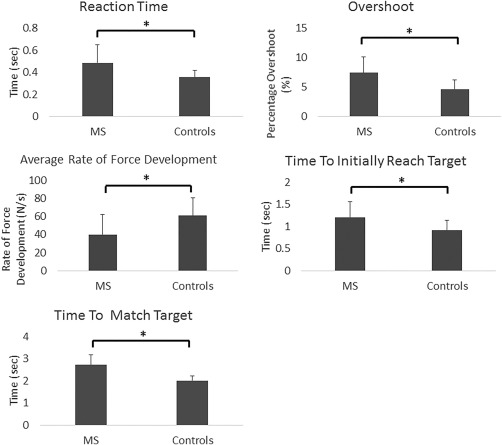
Group averages (mean ± SD) for reaction time, amount of overshoot, average rate of force development to the target, time to initially reach the target, and time to match the target. * p < .05.
Sensor Level Analysis
Grand averages of the peak sensor, which was located near the leg area of the sensorimotor cortices, showed a more robust beta ERD when time‐locked to movement onset (Fig. 3A), and a stronger PMBR when time‐locked to movement offset (Fig. 3B). Based on our statistical analysis, we found a significant beta ERD in the 14–30 Hz frequency range from approximately −0.6 to 2.45 s (P < 0.00001; cluster‐corrected). Additionally, a significant PMBR was found ranging from 14 to 30 Hz from approximately 1.275 to 4.075 s (P < 0.00001; cluster‐corrected). To image these neural responses, we focused on the 400 ms time‐frequency windows corresponding to the maximum beta ERD and PMBR respectively, determined by the grand average of the peak sensor for movement onset and offset (i.e., Beta ERD = 16–26 Hz, −0.4 to 0 s; PMBR = 16–26 Hz, 1.9 to 2.3 s).
Figure 3.
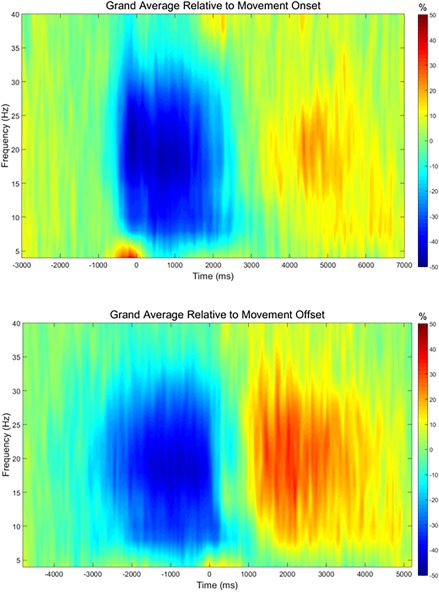
Grand averaged time‐frequency plots of the peak sensor, which was located near the leg area of the sensorimotor strip. The same sensor was used in all participants for both the ERD and PMBR analyzes. The analysis was time‐locked to movement onset (top) to assess the beta ERD, and to movement offset (bottom) to assess the PMBR. For the beta ERD analysis (top), movement onset was defined as time 0.0 s and the baseline was defined as −1.6 to −1.2 s. A strong pre‐ and peri‐movement ERD (blue) can be seen from approximately −0.6 to 2.2 s. This response was strongest in the beta range, but also stretched into the alpha range. For the PMBR analysis (bottom), movement offset was defined as time 0.0 s and the baseline was defined as −4.6 to −4.2 s. A strong PMBR (red) could be seen from approximately 1.2 to 4.0 s. As with the ERD, the response was strongest in the beta range, but also stretched down into the alpha band. [Color figure can be viewed at http://wileyonlinelibrary.com]
Beamformer and Peak Voxel Analysis
Beamformer images corresponding to the maximum beta ERD (16–26 Hz, −0.4 to 0.0 s) and PMBR (16–26 Hz, 1.9 to 2.3 s) were computed in each participant and averaged across both groups. The peak voxel time series (i.e., virtual sensor) were then extracted in each participant individually, and examined statistically for group differences as a function of time. No significant between‐group differences were found for the beta ERD during the planning or execution period of the virtual sensor time course (P = 0.73; cluster‐corrected). However, group differences were found in the virtual sensor time course of the PMBR, and these reflected significantly weaker PMBR activity in the individuals with MS from 1.25 to 1.65 s (P = 0.049; cluster‐corrected; Fig. 4). A weaker PMBR response was also seen from 1.675 to 2.05 s in the individuals with MS, but this difference did not survive correction for multiple comparisons (P = 0.070; cluster‐corrected).
Figure 4.
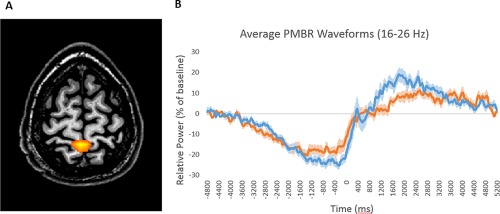
(A) Grand averaged beamformer image of the post‐movement beta rebound (PMBR; 16–26 Hz, 1.9–2.3 s) from all participants indicated that the response was generated by the leg area of the pre/postcentral gyri. (B) Group averaged time series of the beta activity (16–26 Hz) extracted from the peak voxel. Time is shown on the x‐axis, with movement offset occurring at 0.0 s, while relative power (expressed as a percentage from baseline) is shown on the y‐axis. The PMBR was significantly stronger in healthy controls (blue line) relative to patients with MS (orange line) from 1.25 to 1.65 s. The shaded area around each line denotes the standard error of the mean (SEM). [Color figure can be viewed at http://wileyonlinelibrary.com]
Lastly, we also found moderate negative correlations between the strength of the PMBR (averaged from 1.25 to 1.65 s) and the time to successfully match the target (r = −0.54, P = 0.004), and time to initially reach the target (r = −0.42, P = 0.03). We also found a moderate positive correlation between the strength of the PMBR and the rate of force development (r = 0.40, P = 0.05). No significant correlations were found between the strength of the PMBR and the amount of overshoot (P = 0.32), or reaction time (P = 0.07).
DISCUSSION
The purpose of this study was to evaluate neural oscillatory activity in the sensorimotor cortices of individuals with MS and healthy individuals during a goal‐directed knee extension task. Our primary finding was that individuals with MS exhibited a weaker PMBR in the precentral and postcentral gyri relative to healthy individuals. Our results also demonstrated that the precision of the isometric knee force production was reduced in individuals with MS, and that the strength of the PMBR was correlated with performance of the isometric knee force task.
Our MEG results showed no differences between individuals with MS and healthy individuals in the pre‐ and peri‐movement beta ERD. This finding was contrary to our prediction, as motor planning deficits have previously been reported in individuals with MS [Ternes et al., 2014], and multiple studies have connected the beta ERD response to motor planning [e.g., Alegre et al., 2003; Heinrichs‐Graham and Wilson, 2015; Kaiser et al., 2001; Tzagarakis et al., 2010]. Prior EEG work also found that the latency and amplitude of the beta ERD did not differ between healthy individuals and nonfatigued individuals with MS (classified by the Fatigue Severity Scale) [Leocani et al., 2001]. However, this study did find increased beta ERD in fatigued individuals with MS compared to nonfatigued individuals and healthy individuals [Leocani et al., 2001]. This may suggest that fatigue is related to the beta ERD response in individuals with MS. Given these somewhat conflicting reports, additional studies are warranted to further identify the interrelationships between motor planning deficits in patients with MS, beta ERD responses, and the severity of fatigue symptoms.
In our study, individuals with MS exhibited a weaker PMBR in the pre‐ and post‐central gyri relative to healthy individuals. Similar findings were previously reported in an EEG study of self‐paced movements of the hand in individuals with MS [Leocani et al., 2001], and abnormal PMBR responses were also detected in a recent MEG study of visuomotor performance involving the hands of patients with MS [Barratt et al., 2017]. Together, these results provide mounting evidence that the PBMR response is disturbed in individuals with MS. Recent work indicates that the amplitude of the PMBR may be related to the uncertainty in the feedforward estimations of the internal model [Tan et al., 2016]. Since a stronger PMBR appears to be related to improved certainty of the internal model, we speculate that the internal model may be faulty in individuals with MS. Prior work appears to agree with this hypothesis. Using a multisensory model of sensory feedback control, Heenan et al. [2014] found that there appears to be a mismatch between the predicted and actual arm dynamics exhibited by individuals with MS during a reaching task. Furthermore, they suggest that the muscular control problems seen in individuals with MS may be due to an inability to adapt the internal estimate of movement duration to account for increases in the visual processing time. Taken together, this suggests that the internal model may become corrupt overtime due to demyelination in the cortical and spinal tracts that are necessary for relaying sensory feedback and properly updating the internal model. Alternatively, it has also been hypothesized that the PMBR may represent afferent feedback to the motor cortices [Cassim et al., 2001; Houdayer et al., 2006; Parkes et al., 2006]. This hypothesis may be linked to the hypothesis that the PMBR is related to the certainty of the internal model, as sensory feedback is crucial to maintaining and updating the internal model. Regardless of this connection, the idea that a reduced PMBR may indicate a disruption in afferent feedback could explain the impaired behavioral results of the individuals with MS, as afferent feedback is necessary to make corrections to the movement trajectory [Kording et al., 2004; Shadmer, 2004; Wolpert, 2007]. Additionally, the PMBR has also been suggested to represent the active inhibition of neuronal networks after movement termination [Neuper and Pfurtscheller, 2001; Salmelin et al., 1995; Solis‐Escalante et al., 2012; Heinrichs‐Graham et al., 2017]. This hypothesis may also account for the reduced behavioral performance of our individuals with MS, as a reduced PMBR may impair the inhibition of the motor cortex, allowing for unwanted movements to occur.
Our behavioral results show that individuals with MS have impairments in the precision of the lower extremity force production, which is consistent with our previous work [Arpin et al., 2016; Davies et al., 2015]. Specifically, we found that individuals with MS had slower reaction times and a greater amount of overshoot of the presented targets. These impairments in behavioral performance may suggest motor planning deficits. However, no differences were seen in the pre‐movement beta ERD, suggesting that motor planning was intact in these individuals. We propose that this apparent contradiction could be due to a number of factors. While motor planning may be intact, the demyelination of the cortical and spinal tracts may cause a delay in the signal from the cortex to the muscle [Conte et al., 2009]. Alternatively, it is possible that the difference in reaction time is due to increased processing time required by individuals with MS to perform the appropriate sensorimotor transformations, as these fiber tracts may be damaged [Bonzano et al., 2009; Bonfiglio et al., 2006]. This may be the best explanation, as there does not appear to be a difference in the latency of the pre‐movement beta ERD, indicating the delayed reaction time is occurring prior to the formulation and execution of the motor plan. Finally, although the beta ERD appears similar, the motor plan is likely corrupt since the overshoot is substantially greater, indicating heightened errors in the motor output. This increase in the amount of overshoot may also indicate deficits in the ability to properly estimate the amount of force required to reach the target, further suggesting that the internal model may be corrupt in individuals with MS. Again, this overall pattern of findings suggests that future work is needed to identify the precise relationship between motor planning and beta ERD activity in MS.
Lastly, we found correlations between the strength of the PMBR and the time to successfully match the target, time to initially reach the target, and rate of force development. These correlations suggest that a stronger PMBR is partially related to improved performance on the goal‐directed knee force task. Moreover, these correlations imply that the strength of the PMBR is related to the certainty of the internal model. Specifically, time to match the target may indicate the integrity of the internal model by representing a measure of the ongoing comparisons that occur between the internal model and the current motor outcome [Kording et al., 2004; Shadmer, 2004; Wolpert, 2007]. Likewise, we speculate that the time to initially reach the target and the rate of force development difference might represent a delay in the sensorimotor transformation, which could impact the ability to maintain and update the internal model. An alternative view is that our results reflect differences in the movement kinematics between individuals with MS and healthy individuals. Prior work in healthy adults has shown that the strength of the PMBR increases with higher force output and a greater rate of force development [Fry et al., 2016]. Thus, the differences we observed in the PMBR, as well as the correlations we found, may be due to the reduced rate of force development in the individuals with MS. However, such differences in the rate of force development could be due to an inability to properly update the internal model in the group with MS, which may impair their ability to increase the rate of force development.
CONCLUSIONS
Our results show that individuals with MS have impairments in the precision of the lower extremity force production, as well as reduced cortical oscillatory activity following movement termination. Since a stronger PMBR has been previously linked to improved certainty of the internal model, we speculate that the internal model is faulty in individuals with MS. Potentially, the internal model may become corrupt over time due to the demyelination in the cortical and spinal tracts that are necessary for relaying sensory feedback and properly updating the internal model. We suggest that degradation in the PBMR deserves further attention because it may result in a novel biomarker that can be used to assess the efficacy of the current treatment protocols that are being used in MS.
REFERENCES
- Alegre M, Gurtubay IG, Labarga A, Iriarte J, Malanda A, Artieda J (2003): Alpha and beta oscillatory changes during stimulus‐induced movement paradigms: effect of stimulus predictability. Neuroreport 14:381–385. [DOI] [PubMed] [Google Scholar]
- Arpin DJ, Davies BL, Kurz MJ (2016): Multiple sclerosis influences the precision of the ankle plantarflexon muscular force production. Gait Posture 45:170–174. [DOI] [PubMed] [Google Scholar]
- Barratt EL, Tewarie PK, Clarke MA, Hall EL, Gowland PA, Morris PG, Francis ST, Evangelou N, Brookes MJ (2017): Abnormal task driven neural oscillations in multiple sclerosis: A visuomotor MEG study. Hum Brain Mapp 38:2441‐2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfiglio L, Rossi B, Sartucci F (2006): Prolonged intracortical delay of long‐latency reflexes: electrophysiological evidence for a cortical dysfunction in multiple sclerosis. Brain Res Bull 69:606–613. [DOI] [PubMed] [Google Scholar]
- Bonzano L, Pardini M, Mancardi GL, Pizzorno M, Roccatagliata L (2009): Structural connectivity influences brain activation during during PVSAT in Multiple Sclerosis. Neuroimage 44:9–15. [DOI] [PubMed] [Google Scholar]
- Cassim F, Monaca C, Szurhaj W, Bourriez JL, Defebvre L, Derambure P, Guieu JD (2001): Does post‐movement beta synchronization reflect an idling motor cortex? Neuroreport 12:3859–3863. [DOI] [PubMed] [Google Scholar]
- Cheyne D, Bakhtazad L, Gaetz W (2006): Spatiotemporal mapping of cortical activity accompanying voluntary movements using an event‐related beamforming approach. Hum Brain Mapp 27:213–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte A, Lenzi D, Frasca V, Gilio F, Giacomelli E, Gabriele M, Bettolo CM, Iacovelli E, Pantano P, Pozzilli C, Inghilleri M (2009): Intracortical excitability in patients with relapsing–remitting and secondary progressive multiple sclerosis. J Neurol 256:933–938. [DOI] [PubMed] [Google Scholar]
- Davies BL, Arpin DJ, Volkman KG, Corr B, Reelfs H, Harbourne RT, Healey K, Zabad R, Kurz MJ (2015): Neurorehabilitation strategies focusing on ankle control improve mobility and posture in persons with multiple sclerosis. J Neurol Phys Ther 39:225–232. [DOI] [PubMed] [Google Scholar]
- Ernst MD (2004): Permutation methods: A basis for exact inference. Stat Sci 19:676–685. [Google Scholar]
- Fry A, Mullinger KJ, O'Neill GC, Barratt EL, Morris PG, Bauer M, Folland JP, Brookes MJ (2016): Modulation of post‐movement beta rebound by contraction force and rate of force development. Hum Brain Map 37:2493–2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaetz W, Edgar JC, Wang DJ, Roberts TP (2011): Relating MEG measured motor cortical oscillations to resting gamma‐aminobutyric acid (GABA) concentration. Neuroimage 55:616–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaetz W, Macdonald M, Cheyne D, Snead OC (2010): Neuromagnetic imaging of movement‐related cortical oscillations in children and adults: age predicts post‐movement beta rebound. Neuroimage 51:792–807. [DOI] [PubMed] [Google Scholar]
- Gross J, Kujala J, Hamalainen M, Timmermann L, Schnitzler A, Salmelin R (2001): Dynamic imaging of coherent sources: Studying neural interactions in the human brain. Proc Nati Acad Sci U S A 98:694–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heenan M, Scheidt RA, Woo D, Beardsley SA (2014): Intention tremor and deficits of sensory feedback control in multiple sclerosis: A pilot study. J Neruoeng Rehabil 11:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs‐Graham E, Kurz MJ, Gehringer JE, Wilson TW (2017): The functional role of post‐movement beta oscillations in motor termination. Brain Struct Funct (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs‐Graham E, Wilson TW (2015): Coding complexity in the human motor circuit. Hum Brain Mapp 36:5155–5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs‐Graham E, Wilson TW (2016a): Is an absolute level of cortical beta suppression required for proper movement? Magnetoencephalographic evidence from healthy aging. Neuroimage 134:514–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs‐Graham E, Arpin DJ, Wilson TW (2016b): Cue‐related temporal factors modulate movement‐related beta oscillatory activity in the human motor circuit. J Cogn Neurosci 28:1039–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs‐Graham E, Wilson TW, Santamaria PM, Heithoff SK, Torres‐Russotto D, Hutter‐Saunders JA, Estes KA, Meza JL, Mosley RL, Gendelman HE (2014): Neuromagnetic evidence of abnormal movement‐related beta desynchronization in Parkinson's disease. Cereb Cortex 24:2669–2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillebrand A, Singh KD, Holliday IE, Furlong PL, Barnes GR (2005): A new approach to neuroimaging with magnetoencephalography. Hum Brain Mapp 25:199–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houdayer E, Labyt E, Cassim F, Bourriez JL, Derambure P (2006): Relationship between event‐related beta synchronization and afferent inputs: analysis of finger movement and peripheral nerve stimulations. Clin Neurophysiol 117:628–636. [DOI] [PubMed] [Google Scholar]
- Jurkiewicz MT, Gaetz WC, Bostan AC, Cheyne D (2006): Post‐movement beta rebound is generated in motor cortex: Evidence from neuromagnetic recordings. Neuroimage 32:1281–1289. [DOI] [PubMed] [Google Scholar]
- Kaiser J, Birbaumer N, Lutzenberger W (2001): Event‐related beta desynchroniztion indicates timing of response selection in a delayed‐response paradigm in humans. Neurosci Lett 312:149–152. [DOI] [PubMed] [Google Scholar]
- Kording K, Ku S, Wolpert DM (2004): Bayesian integration in force estimation. J Neurophysiol 92:3161–3165. [DOI] [PubMed] [Google Scholar]
- Leocani L, Colombo B, Magnani G, Martinelli‐Boneschi F, Cursi M, Rossi P, Martinelli V, Comi G (2001): Fatigue in multiple sclerosis is associated with abnormal cortical activation to voluntary movement–EEG evidence. Neuroimage 13:1186–1192. [DOI] [PubMed] [Google Scholar]
- Maris E, Oostenveld R (2007): Nonparametric statistical testing of EEG‐ and MEG‐data. J Neurosci Methods 164:177–190. [DOI] [PubMed] [Google Scholar]
- Neuper C, Pfurtscheller G (2001): Evidence for distinct beta resonance frequencies in human EEG related to specific sensorimotor cortical areas. Clin Neurophysiol 112:2084–2097. [DOI] [PubMed] [Google Scholar]
- Parkes LM, Bastiaansen MC, Norris DG (2006): Combining EEG and fMRI to investigate the post‐movement beta rebound. Neuroimage 29:685–696. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Lopes da Silva FH (1999): Event‐related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol 110:1842–1857. [DOI] [PubMed] [Google Scholar]
- Salmelin R, Hamalainen M, Kajola M, Hari R (1995): Functional segregation of movement‐related rhythmic activity in the human brain. Neuroimage 2:237–243. [DOI] [PubMed] [Google Scholar]
- Shadmer R (2004): Generalization as a behavioral window to the neural mechanisms of learning internal models. Hum Mov Sci 23:543–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solis‐Escalante T, Muller‐Putz GR, Pfurtscheller G, Neuper C (2012): Cue‐induced beta rebound during withholding of overt and covert foot movement. Clin Neurophysiol 123:1182–1190. [DOI] [PubMed] [Google Scholar]
- Talairach G, Tournoux P. (1998): Co‐planar Stereotaxic Atlas of the Human Brain. New York, NY: Thieme. [Google Scholar]
- Tan H, Wade C, Brown P (2016): Post‐movement beta activity in sensorimotor cortex indexes confidence in the estimations from internal models. J Neurosci 36:1516–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taulu S, Simola J (2006): Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Phys Med Biol 51:1759–1768. [DOI] [PubMed] [Google Scholar]
- Ternes AM, Fielding J, Corben LA, White OB, Bradshaw JL, Hocking DR, Georgiou‐Karistianis N (2014): Movement planning and online control in multiple sclerosis: assessment using a Fitts law reciprocal aiming task. Cogn Behav Neurol 27:139–147. [DOI] [PubMed] [Google Scholar]
- Tzagarakis C, Ince NF, Leuthold AC, Pellizzer G (2010): Beta‐band activity during motor planning reflects response uncertainty. J Neurosci 30:11270–11277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Veen BD, van Drongelen W, Yuchtman M, Suzuki A (1997): Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. IEEE Trans Biomed Eng 44:867–880. [DOI] [PubMed] [Google Scholar]
- Wilson TW, Heinrichs‐Graham E, Becker KM (2014): Circadian modulation of motor‐related beta oscillatory responses. Neuroimage 102:531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TW, Slason E, Asherin R, Kronberg E, Reite ML, Teale PD, Rojas DC (2010): An extended motor network generates beta and gamma oscillatory perturbations during development. Brain Cogn 73:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TW, Slason E, Asherin R, Kronberg E, Reite ML, Teale PD, Rojas DC (2011): Abnormal gamma and beta MEG activity during finger movements in early‐onset psychosis. Dev Neuropsychol 36:596–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpert DM (2007): Probabilistic models in human sensorimotor control. Hum Mov Sci 26:511–524. [DOI] [PMC free article] [PubMed] [Google Scholar]


