Abstract
Language network reorganization in aphasia may depend on the degree of damage in critical language areas, making it difficult to determine how reorganization impacts performance. Prior studies on remapping of function in aphasia have not accounted for the location of the lesion relative to critical language areas. They rectified this problem by using a multimodal approach, combining multivariate lesion‐symptom mapping and fMRI in chronic aphasia to understand the independent contributions to naming performance of the lesion and the activity in both hemispheres. Activity was examined during two stages of naming: covert retrieval, and overt articulation. Regions of interest were drawn based on over‐ and under‐activation, and in areas where activity had a bivariate relationship with naming. Regressions then tested whether activation of these regions predicted naming ability, while controlling for lesion size and damage in critical left hemisphere naming areas, as determined by lesion‐symptom mapping. Engagement of the right superior temporal sulcus (STS) and disengagement of the left dorsal pars opercularis (dPOp) during overt naming was associated with better than predicted naming performance. Lesions in the left STS prevented right STS engagement and resulted in persistent left dPOp activation. In summary, changes in activity during overt articulation independently relate to naming outcomes, controlling for stroke severity. Successful remapping relates to network disruptions that depend on the location of the lesion in the left hemisphere. Hum Brain Mapp 38:2051–2066, 2017. © 2017 Wiley Periodicals, Inc.
Keywords: aphasia, stroke, fMRI, naming, language production
INTRODUCTION
One‐third of stroke survivors suffer from loss of language ability, and there is much variability in recovery. By some estimates, two thirds of these individuals never fully recover language ability, and our understanding of which variables contribute to recovery from aphasia is poor [Gottesman and Hillis, 2010; Pedersen et al., 1995].
A well‐established set of findings in the neuroimaging and aphasia literature is that activation in preserved left hemisphere tissue is associated with better performance on language tasks [Anglade et al., 2014; Heiss et al., 2003; Meinzer and Breitenstein, 2008]. Studies have confirmed this finding by showing that recovery is related to increased activation of the left hemisphere at rest [Karbe et al., 1995], during sentence comprehension [Saur et al., 2006] and while performing semantic matching tasks [Winhuisen et al., 2005, 2007]. Activation of the left hemisphere during an overt naming task has been positively correlated with naming abilities in people with aphasia [Fridriksson et al., 2010]. Changing left hemisphere activation following treatment has also been associated with responsiveness to naming treatment [Fridriksson et al., 2012]. There is thus ample evidence that activation in the left hemisphere in general relates to good outcomes in aphasia.
The role of the right hemisphere, however, is still difficult to understand. It is clear that after a left hemisphere stroke causing aphasia, activity in the right hemisphere often increases in areas that mirror the left hemisphere language network [Turkeltaub et al., 2011]. In some studies, right hemisphere activity appears particularly robust in areas directly opposite the lesion [Blank et al., 2003; Turkeltaub et al., 2011], but this increase in activity is not always correlated with performance [Meltzer et al., 2013]. This pattern is sometimes explained as a consequence of released interhemispheric inhibition. This hypothesis states that, in healthy people, there is cross‐hemispheric inhibition between language areas, similar to what has been demonstrated in motor areas [Duque et al., 2005; Murase et al., 2004]. A stroke in the left hemisphere disrupts this balance, leading to overactivation of right hemispheric language areas homotopic to the lesion. In the context of the interhemispheric inhibition theory, the overactivated right hemisphere is thought to maladaptively inhibit perilesional left hemisphere areas, resulting in worse outcomes [Hamilton et al., 2011]. An alternative view is that right hemisphere processors homotopic to the nodes damaged by the stroke are incorporated into the surviving language network and serve to compensate for the damaged left hemisphere language nodes [Turkeltaub et al., 2011], albeit less efficiently than native left hemisphere language areas [Heiss et al., 1999].
In support of a compensatory role for the right hemisphere, neuropsychological case studies have identified people who had mostly recovered from aphasia following left hemisphere stroke, who then re‐develop aphasia after a later right hemisphere disruption [Basso et al., 1989; Kinsbourne, 1971; Turkeltaub et al., 2012; Yarnell et al., 1979]. These studies suggest that the right hemisphere can, to some degree, take over the language functions that the left hemisphere can no longer perform. Alternatively, transcranial magnetic stimulation studies show that inhibiting right hemisphere regions, particularly the right inferior frontal gyrus pars triangularis (PTr), improves fluency, naming and many other language measures in people with left hemisphere stroke [Barwood et al., 2011; Hamilton et al., 2011; Martin et al., 2009; Naeser et al., 2005; Ren et al., 2014; Winhuisen et al., 2005].
Neuroimaging studies have also produced inconsistent results about the right hemisphere in aphasia. Longitudinal studies show that early engagement of the right hemisphere during the acute phase promotes recovery, but that disengagement of the right hemisphere at later, chronic stages is associated with improved recovery [Breier et al., 2009; Fernandez et al., 2004; Kurland et al., 2008; Saur et al., 2006]. Several studies have looked at activation patterns over time in the right hemisphere specifically in response to overt naming or name retrieval. Fernandez et al. [2004] found that right hemisphere over‐activation during a picture‐word rhyme judgment task was related to poor recovery. Others have also found that over‐reliance on the right hemisphere during overt picture naming is related to poor naming performance, and that right hemisphere activation is greater during errors than successful naming [Cao et al., 1999; Postman‐Caucheteux et al., 2010].
However, it remains unclear whether greater right hemisphere activation is related directly to outcomes, or whether it is instead associated with greater damage to the critical left hemisphere regions for naming. Recent anatomical studies show that, although left hemisphere damage in specific sites such as the arcuate fasciculus provides strong predictive power for language outcomes [Marchina et al., 2011; Wang et al., 2013], the models with the most predictive power take into account structural integrity in both the left and right hemispheres [Forkel et al., 2014; Pani et al., 2016; Xing et al., 2016]. Studying lesion distribution in the left hemisphere appears to be critical to understanding the role of the right hemisphere in recovery.
Prior functional MRI studies have used group‐level quantification of lesion distribution to estimate average levels of damage, and to determine the borders of what can be called peri‐lesional tissue [Fridriksson et al., 2010; Kielar et al., 2016]. However, prior fMRI studies of aphasia recovery have not accounted for the degree to which critical areas, as defined by the deficit, are damaged by the stroke. The degree to which critical areas are spared or damaged may influence the degree to which right hemisphere remapping is beneficial or inhibitory to recovery. It is possible that right hemisphere over‐activation only occurs in the most severe cases, when the left hemisphere is devastated and unable to recover function [Heiss et al., 2003] in which case activation is interacting with lesion severity. A person with aphasia following a lesion that completely destroys a key naming region, such as Broca's area, may activate the right hemisphere simply because there is no healthy left hemisphere tissue remaining. If this is true, then the right hemisphere activity correlating with poor performance may actually be spurious, in that both measures are directly impacted by the size and location of the lesion itself. Similarly, the association of left hemisphere activity with good outcomes may simply reflect the fact that less severe strokes cause milder aphasia and also spare more left hemisphere cortex.
These relationships lead to the impression that the brain activity pattern determines the ability to recover, when in fact the performance simply reflects the severity of the stroke, which also impacts the pattern of activity in the intact portions of the brain.
To understand the role that brain activity actually contributes to performance and recovery, it is necessary to first understand the direct impact of the stroke itself on performance, and then to ask whether brain activity relates to deviations, either positive or negative, from that expected performance.
Processes of Naming
In this experiment, we examine two distinct stages of naming: lexical‐phonological retrieval and post‐lexical output [Goldrick and Rapp, 2007]. Lexical‐retrieval processes involve accessing concept knowledge and mapping phonological representations, stored in long‐term memory. Phonological retrieval is often ascribed to a ventral stream of processing, in which phonological representations in the posterior superior temporal lobe map onto semantic and conceptual information in the angular gyrus and anterior temporal lobes [Hickok and Poeppel, 2007].
Post‐lexical output is the production stage, in which phonological representations are mapped to motor representations and speech occurs. Some dual‐stream models assign this stage to the dorsal stream, in that the phonological representations in the superior temporal gyrus (STG) are mapped to motor sequence representations in the temporoparietal junction and posterior inferior frontal lobes [Hickok and Poeppel, 2007].
Approach of This Study
The experiment described here is a cross‐sectional study of naming in chronic aphasia, and advances the current literature in three vital ways. The first way is by separating out two major stages of naming during the fMRI task. In our study, we operationalized the retrieval stage through covert naming, by asking participants to retrieve the picture name silently. Then after a short period, the motor output phase was activated as the participants produced the name overtly. In this design, we can look at the separate fMRI effects of retrieval and production in naming, which, to our knowledge, has not previously been done in people with aphasia.
The second way that this study is unique is that it investigates the degree to which activation in intact sites explains naming performance, beyond what we can quantify about the lesion itself. We tested the relationship between activity and naming performance, controlling for both lesion size and the amount of damage in critical left hemisphere naming areas. In this study, we use multivariate lesion symptom mapping to identify critical left hemisphere language areas, and quantify damage in these regions in each individual. This allows us to estimate expected language deficits in each individual, based on the site and size of their lesion. We then test the degree to which individual differences in left and right hemisphere activity can account for deviations from predicted naming performance.
Finally, we examine interactions between lesion distribution and activity to elucidate the mechanisms by which structural damage induces changes, both adaptive and maladaptive, in remaining brain networks after stroke.
MATERIALS AND METHODS
Participants
Forty‐six chronic left‐hemisphere stroke survivors with a history of aphasia were recruited. All participants in the aphasia group were native English speakers, and testing occurred at least 6 months after the stroke. Participants were screened based on ability to follow testing instructions and had no history of other significant neurological illness. Fourteen participants were then excluded based on fMRI task performance (see below), resulting in a final sample of 32 participants in the aphasia group (Table 1 and Supporting Information). On average, the participants removed due to low accuracy had larger lesions (mean = 38,439 voxels, range = 2,651–110,222 voxels) than the participants included (mean = 28,582 voxels, range = 402–110,213), but the ranges overlapped significantly, and the final group included participants with both small and large lesions.
Table 1.
Mean demographic information for participants in the aphasia group and the control group
| Aphasia group (N = 31) | Control group (N = 25) | |
|---|---|---|
| Age (years) | 58.78 (8.61) | 59.88 (14.55) |
| Gender (M/F) | 19/12 | 10/15 |
| Education (years) | 16.23 (3.03) | 16.46 (2.50) |
| Handedness (right/left/ambidextrous) | 26/3/2 | 22/2/1 |
| Time since stroke (months) | 40.88 (36.12) | – |
| Lesion size (1 mm3 voxels) | 27484 (22896) | – |
| WAB Aphasia Quotient | 77.66 (20.98) | – |
| WAB Naming/Word‐Finding | 7.47 (2.40) | – |
| WAB Auditory–Verbal Comprehension | 8.33 (1.68) | – |
| WAB Repetition | 7.30 (2.41) | – |
| WAB Spontaneous Speech | 14.71 (5.13) | – |
Standard deviation shown in parentheses. More details on the participants in the aphasia group can be found in the Supporting Information.
Twenty‐five healthy control subjects, with no neurologic or psychiatric disorders were also tested. Participants in the control group were matched to the aphasia group on age (Table 1). Sex was not matched between the two groups (the aphasia group had more males, the control group had more females). Sex was included as a covariate in all regressions in this study, and was not found to have any effect in any of our analyses.
The study was approved by the Georgetown University Institutional Review Board, and written informed consent was obtained from all study participants prior to enrollment in the study.
Experimental Design
Visual stimuli were 54 line drawings, with 92%–100% name agreement based on norming in 55 older controls, representing one‐, two‐, and three‐syllable words. To reduce individual differences in in‐scanner performance, participants were presented with one of two 32‐items sets during scanning based on the severity of their deficits. Five participants whose naming and repetition deficits were severe in pre‐MRI testing were given 32 one‐ and two‐syllable items, while all other participants, including controls were given 32 two‐ and three‐syllable items during scanning. The one‐syllable words also had overall higher frequency than the three‐syllable words.
The fMRI task followed a slow jittered event related design. The trials were presented in a pseudo‐randomized order, with three stages for each trial. First, a single line drawing appeared centered on the screen, surrounded by a red border. This image remained on the screen for 7,500–9,000 ms, during which time the participant named the object in the image silently. Then, the border around the image changed from red to green and remained on screen for 5,500 ms. Both covert and overt naming trial durations were the same length regardless of whether participants received the one‐ and two‐syllable items, or the two‐ and three‐syllable items. During this time, the participant was asked to produce the name of the object aloud. Finally, the line drawing and the surrounding box disappeared and the participant fixated on a crosshair for 14,000 ms. A similar experimental design has been used previously to identify activity specific to covert and overt naming in healthy young adults [Kemeny et al., 2006]. Our design differed in that a slow event related design was chosen to allow for wash out of the hemodynamic response, which may be slower in stroke survivors [Bonakdarpour et al., 2007], and to provide adequate time for even severe participants to make a response. Images were presented using E‐Prime software (Psychology Software Tools Inc., Pittsburg, PA), and responses were recorded using a MRI safe microphone (Opto‐acoustics, FOMRI‐III). Before the scan, participants practiced the task on images not included in the fMRI task.
If a participant produced the correct name at any point during the overt naming period, the item was counted as correct. Incorrect trials were removed from analysis at all three stages: covert naming, overt naming and fixation. Participants who correctly named fewer than 20% of the trials were removed from all further analysis.
Naming ability was tested using a 60‐item version of the Philadelphia Naming Test [PNT; Roach et al., 1996], made up of items independent of those used in the scanning task. Testing took place within one week of the MRI scan. We counted the total number of items on the PNT that were named correctly on the first attempt.
Scanning Parameters
MRI data were collected on a 3.0 T Siemens Trio Scanner at the Georgetown University Medical Center. A high resolution T1‐weighted MPRAGE was collected with the following parameters: repetition time = 1,900 ms, echo time = 2.56 ms, flip angle = 9°, 160 contiguous 1‐mm slices, field of view = 250 × 250 mm, matrix size = 246 × 256, voxel size = 1 × 1 × 1 mm.
Functional T2*‐weighted images were acquired using a gradient‐echo echo‐planar pulse sequence, with the following parameters: repetition time = 2,000 ms, echo time = 30 ms, flip angle = 90°, 38 contiguous 3.2 mm slices, field of view = 250 × 250 mm, voxel size = 3.2 × 3.2 × 3.2 mm. The functional scan lasted for approximately 15 minutes, including an opening and closing screen and 32 total trials.
Lesion Symptom Mapping and Identification of Critical Naming Areas
Lesion masks were created by manually tracing stroke damage on the T1‐weighted images, in native space, in MRIcron [Rorden and Brett, 2000]. All lesion masks were checked by two board certified neurologists (S.X. and P.E.T.). Lesions were then warped to a template in MNI space created from MRIs of older adults, provided in the MRIcron Clinical Toolbox [Rorden et al., 2012; http://www.mccauslandcenter.sc.edu/CRNL/clinical-toolbox]. The warping was carried out by applying deformation fields derived from a 12‐parameter affine linear and non‐linear warping transformations, using the VBM8 toolbox in Statistical Parametric Mapping Software (SPM8; http://www.fil.ion.ucl.ac.uk/spm). A lesion overlap map is shown in Figure 1.
Figure 1.
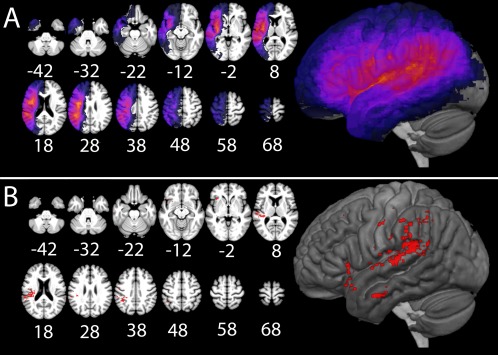
(A) Lesion overlap map revealing where the greatest number of lesions occurred in our sample. (B) Results of the SVR‐LSM map showing the critical areas for naming, P < 0.005, in red. [Color figure can be viewed at http://wileyonlinelibrary.com]
Support Vector Regression‐based Lesion Symptom Mapping (SVR‐LSM), a multivariate lesion‐symptom mapping approach [Zhang et al., 2014], was used to define critical left hemisphere areas in which damage relates to naming impairment, as measured by the overall accuracy on the PNT. These results were used to quantify individual differences in left hemisphere stroke locations that related to behavioral scores, which is vital to determine if changes in functional cortical activity contribute additional variance to scores. SVR‐LSM uses a machine leaning‐based multivariate support vector regression algorithm to find lesion‐symptom relationships [Zhang et al., 2014]. SVR‐LSM analysis was carried out in Matlab 2013b. Only voxels damaged in at least 20% of participants in the aphasia group were included in the analysis. Probabilistic maps created using 10,000 permutations of the behavioral scores were thresholded at P < 0.005 to define the critical areas in the left hemisphere in which damage causes deficits to naming. The Proportion of Critical Area Damaged (PCAD) was then calculated for each participant in the aphasia group. Although lesion size was controlled when determining the critical area, the PCAD was still highly correlated with lesion size, r = 0.79, t(30) =7.14, P < 0.001.
fMRI Preprocessing and Whole Brain Analysis
fMRI data were preprocessed and analyzed using FSL 5.0.6 [Jenkinson et al., 2012]. Preprocessing included application of a high pass temporal filter, standard correction for head motion using MCFLIRT, interleaved slice timing correction, intensity normalization across volumes, and spatial smoothing to 5 mm FWHM. Lesions were also masked out of the functional data for each individual at this level. Registration and normalization to the older adult MNI template brain was carried out using deformation fields derived from the SVR‐LSM analysis above. Normalization accuracy was confirmed through visual inspection of all participants after warping.
For each condition in each trial, a canonical double‐gamma hemodynamic response function was constructed for the duration of the event. For covert naming, this event covered the duration that the image appeared within the red box (7,500–9,000 ms), and for overt naming this included the entire duration that the image was in the green box (5,500 ms). Motion parameters were then included as covariates in the model. Both covert naming and overt naming conditions were contrasted against baseline. At the second level, a gray matter mask derived from the older‐adult template was applied to all contrasts. Two sets of whole‐brain voxelwise analyses were performed.
Between‐group contrasts and relationship to naming ability
Group contrasts were carried out, examining activation to both covert naming and overt naming relative to baseline, allowing us to look for regions over‐activated or under‐activated by people in the aphasia group compared with the control group. Areas deactivated by both groups (based on median task activation) were excluded from the group‐difference maps. Group different maps were thresholded at z = 2.3, cluster‐corrected to P < 0.05.
To test if over‐ or under‐activation by people with aphasia related to naming ability, regions of interest (ROIs) were created using the aphasia > control and control > aphasia maps, for both the covert > fixation and overt > fixation contrasts. Clusters were drawn at thresholds that allowed for successful differentiation of critical peaks. For covert > fixation between group contrasts, the ROIs were selected at a threshold of z = 3.1 (P < 0.001 uncorrected). However, when the overt naming maps were set to that threshold, several peaks bled together into large clusters extended across multiple lobes. For this reason, the overt naming between‐group contrasts were set to a threshold of z = 3.5 for ROI selection. Clusters were selected as ROIs if they survived this threshold and were larger than 500 mm3. Clusters were excluded if they appeared in the brainstem or primary visual cortex. Clusters were also excluded if they were not significantly active within group, meaning that clusters identified in the control > aphasia contrast were only included if the median z‐value for the control group was greater than 2.3. All thresholding for ROIs was done a priori to the regressions.
Mean contrast parameter estimates were extracted from each ROI for each participant in the aphasia group, based on the task contrast used to select it. In other words, activation in the covert > fixation contrast was extracted for ROIs drawn in the covert activation maps. Then we tested whether activation of each ROI during a naming task could predict naming performance as measured by the 60‐item PNT, outside of the scanner. We first performed bivariate correlations between the mean activity in each ROI and PNT scores. For ROIs with significant correlations after Bonferroni correction, we then tested if these relationships were independent of lesion and demographic factors using linear regressions in R 3.1.0 [R Development Core Team, 2010].
First, we constructed a linear regression, in which number of items named correctly in the PNT was the dependent variable. In the first level regression, age, sex, education, handedness, time since stroke, lesion size, and PCAD were included as predictors. Then, for each ROI, a second regression was created in which the contrast parameter estimate was added as the independent variable. The change between the two regressions for each ROI determined whether activation of that ROI during naming actually predicted naming performance, beyond the prediction based on the nature of the stroke.
Voxelwise relationship between activity and naming ability
To directly examine relationships between activity and naming performance irrespective of the group‐level activity, we next carried out a voxelwise bivariate correlation between activity in each voxel and PNT scores in the aphasia group (voxelwise z > 2.3, cluster‐corrected P < 0.05). To examine the nature of significant relationships in more detail, ROIs were identified at a more restrictive cluster extent threshold to match the ROI analysis above (z > 2.3, k > 500 mm3). For each ROI, we then determined whether the relationship between activity and naming ability could be accounted for by other factors. Activity in response to the task was extracted from each individual, and a regression was carried out with activity as the primary predictor, with age, education, sex, handedness, PCAD, and lesion size included as covariates. For any ROI in which activity was significantly related to performance, when controlling for all covariates, we then tested whether the aphasia group as a whole over‐ or under‐activated the ROI relative to the age‐matched control group.
Differences in Activity During Successful Versus Unsuccessful Naming
Finally, we carried out a whole volume analysis within the aphasia group, to look for activity related to successful versus unsuccessful naming. Accuracy for each trial was determined based on responses made during the overt naming period of the task. For this analysis, we only included participants who scored between 20% and 80% accuracy in the scanner (so that there would be enough trials in both the incorrect and correct conditions), which resulted in a sub‐sample of 24 participants with aphasia. We then carried out a correct trials versus incorrectly named trials contrast, using functional data collected during both the covert naming period and overt naming periods.
RESULTS
Behavioral Results
No participants in the control group named more than two items incorrectly, with a mean accuracy of 0.99. For the aphasia group, overall mean accuracy was 0.74 (SD 0.27).
On the PNT, the participants in the aphasia group named an average of 41.6/60 items correctly (SD 17.8, Range 1–59/60). Individual participant performance on the PNT and other common aphasia tests can be found in the Supporting Information.
Neuroimaging Results
Critical areas for naming
First, we used SVR‐LSM to identify critical left hemisphere areas for naming as measured by the PNT (Fig. 1). The peak area in which lesion status was significantly related to impairment on the PNT was in the posterior STG. Other significant areas included angular gyrus, intraparietal sulcus (IPS), and parts of the PTr.
We then calculated the proportion of this map that was lesioned in each individual in the aphasia group. The resulting PCAD score ranged from 0 to 0.995 across the participants. PCAD significantly correlated with PNT scores, R = −0.73, t(30) = −5.92, P < 0.001.
It is worth noting that this approach likely leads to an overestimation of the predictive power of the PCAD measure, because the critical areas were determined using the same participants that were then entered into the correlation. Ultimately, our inclusion of PCAD, measured in this way, in later regressions is a conservative decision. By including a PCAD calculated on the same participants as the dependent variables (PNT accuracy), we underestimate the amount of variance remaining that can be accounted for by activity in the ROI, and thus reduce the chance of type 1 error.
Naming activity in aphasia and control groups
In order to identify areas activated during covert and overt naming in the two groups, and areas in which naming activity differed between the groups, we first performed whole brain single‐group analyses, and between group contrasts.
Covert naming
Both patients and controls showed widespread overlapping activation (Fig. 2A,B) during covert naming compared with fixation in the bilateral occipital lobe, bilateral pars opercularis (POp) and PTr, bilateral supplementary motor area (SMA), bilateral IPS, left middle frontal gyrus, and right superior temporal pole (TP). Participants in the control group, but not aphasia group, also showed activation in the left superior TP and left posterior hippocampus. Both groups showed activation in left and right basal ganglia as well, but the activation was larger for the aphasia group.
Figure 2.
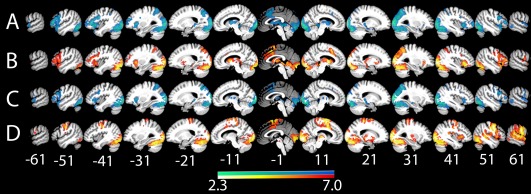
Within‐group fMRI contrasts. (A) Covert naming > fixation in controls, (B) covert naming > fixation in the aphasia group, (C) overt naming > fixation in controls, (D) overt naming > fixation in the aphasia group. All results are thresholded at P < 0.05, cluster corrected. [Color figure can be viewed at http://wileyonlinelibrary.com]
We then examined the between group contrasts (Fig. 3A). At a threshold of z = 3.1, cluster corrected to P < 0.05 for whole brain analysis, the control group showed greater activation than the aphasia group only in the left occipital lobe, specifically the calcarine fissure and lingual gyrus. The aphasia group showed greater activation than the control group in the right precuneus.
Figure 3.
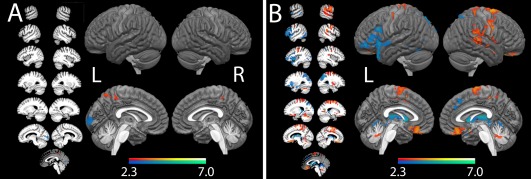
Between‐group fMRI contrasts. (A) Covert naming and (B) overt naming. Red‐orange represents regions the aphasia group over‐activated relative to the control group, while blue represents regions the aphasia group under‐activated relative to controls. All results are shown at P < 0.05, cluster corrected. [Color figure can be viewed at http://wileyonlinelibrary.com]
Overt naming
For overt naming versus fixation (Fig. 2C,D), both groups significantly activated large portions of bilateral visual cortex. The group map for the controls appeared very similar to the control > aphasia contrast described above. However, the group map for the aphasia group revealed additional activations in right ventral postcentral gyrus, right superior temporal sulcus (STS), and STG, and right Heschl's gyrus. Subcortical activation for the aphasia group was also identified in the right globus pallidus.
At a threshold of z = 3.1, cluster corrected to P < 0.05 for whole brain analysis, controls showed greater activation than the aphasia group in many regions of the left hemisphere language network. This included the left dorsal pars opercularis (dPOp), PTr and pars orbitalis, left premotor cortex, the left insula, left preSMA, left STG into the TP, and left parahippocampus. The control group also showed greater activation in the bilateral IPS.
In contrast, the aphasia group showed greater activation than controls in response to overt naming mostly in right hemisphere regions (Fig. 3B). The aphasia group over‐activated the right central sulcus, along the right middle temporal gyrus, the right anterior supramarginal gyrus and in a small cluster in the right insula. Participants in the aphasia group also over‐activated bilateral medial orbitofrontal cortex and several clusters in the bilateral medial SMA and left precuneus.
Relationship between brain activity and naming ability
The next set of analyses aimed to identify relationships between brain activity and naming ability, and to determine if these relationships were independent of other individual differences between people with aphasia.
Regressions of lesion and demographic factors with naming ability
Before activation levels were examined, we constructed the first step of a hierarchical regression using the data from the aphasia group. The dependent variable was number of correct trials in the PNT, and the predictors were PCAD, lesion volume, time since stroke, age, sex, education and handedness. As predicted, PCAD was the strongest predictor of PNT performance, t(24) = −3.76, P < 0.001. Time since stroke was also a significant predictor, t(24) = 2.13, P < 0.05. No other variables significantly predicted PNT performance.
Relationship of over‐ and under‐activation in aphasia to naming ability
The between‐group contrasts above demonstrate that people with aphasia over‐activate some areas and under‐activate others relative to control subjects. To test if these alterations in activity relate to naming ability, we extracted ROIs from the between‐group contrasts above and examined relationships between activity in these ROIs and PNT scores. We first tested for bivariate relationships using correlations. For ROIs with significant correlations, we then determined if these relationships were independent of other factors by adding activity in the ROI as an additional predictor to the regression analysis above.
The covert naming between‐group contrasts produced no ROIs that met the threshold requirement for further analysis.
A total of 11 ROIs survived from the overt naming map, four from the control > aphasia map, and seven from the aphasia > control map (Table 2, Fig. 4B–E).
Table 2.
Description of overt naming ROIs and results of overt naming regressions
| ROI | Group contrast | Size | Peak | x | y | z | r | t |
|---|---|---|---|---|---|---|---|---|
| right IPS | Control > Aphasia | 798 | 4.74 | 25 | −64 | 44 | 0.09 | |
| left IPS | Control > Aphasia | 1750 | 4.47 | 23 | −68 | 34 | −0.02 | |
| left PTr | Control > Aphasia | 798 | 4.00 | −49 | 34 | 6 | −0.31 | |
| left dPOp | Control > Aphasia | 698 | 4.75 | −49 | 20 | 26 | −0.66** | −2.91** |
| right superior motor cortex | Aphasia > Control | 6382 | 4.72 | 23 | −1 | 66 | 0.46 | |
| right ventral motor cortex | Aphasia > Control | 2334 | 4.52 | 55 | 0 | 34 | 0.68** | 1.88 |
| right supramarginal sulcus | Aphasia > Control | 1683 | 4.47 | 50 | −37 | 17 | 0.44 | |
| left medial SMA | Aphasia > Control | 894 | 4.45 | −11 | −32 | 50 | 0.34 | |
| right marginal sulcus | Aphasia > Control | 466 | 3.99 | 16 | −37 | 64 | 0.41 | |
| left dorsal motor cortex | Aphasia > Control | 452 | 4.21 | −32 | −16 | 40 | 0.24 | |
| right STS | Aphasia > Control | 292 | 4.46 | 46 | −39 | −3 | 0.63** | 2.80* |
ROI size is measured in mm3, and coordinates are in MNI space. Peak refers to the z‐value at the peak of the cluster, r refers to Pearson's r from the bivariate correlation with PNT. Only ROIs with significant correlations were then entered into the regression. The t column shows the relationship between activity and PNT, controlling for all other factors.
*P < 0.05; **P < 0.01.
Figure 4.
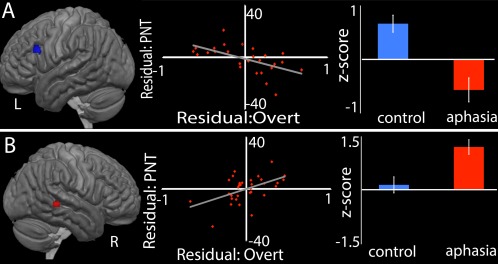
Regions in which activity predicts naming ability, controlling for lesion size, PCAD, and other variables. Each section presents the ROI where activity significantly predicts naming performance, the residual plot showing predicted PNT against residual activation, and a bar graph showing raw activation for the control and aphasia groups. The ROIs where activity significantly predicted performance are (A) the left dPOp and (B) the right STS. [Color figure can be viewed at http://wileyonlinelibrary.com]
From the control > aphasia map, activation during overt naming in the left dPOp had negative correlation with PNT scores (P < 0.05, Bonferroni‐corrected for 11 ROIs). This negative relationship with PNT performance remained significant in the regression analysis (t(23) = −2.91, P < 0.01), demonstrating that the relationship was independent of lesion and demographic factors.
From the aphasia> control map, overt naming activity in two ROIs, the right STS and right ventral motor cortex, had positive correlations with PNT scores (P < 0.05, Bonferroni‐corrected for 11 ROIs). In the regression analyses, only the positive relationship between right STS activity and PNT remained significant (t(23) = 2.80, P = 0.01).
Voxelwise relationships between activity and naming ability
The above analysis was only sensitive to areas in which activity differed between people with aphasia and controls at the group level and so may have missed areas important for naming without group‐level differences. Thus, we next performed a complementary analysis to directly examine relationships between activity and naming ability, irrespective of the group‐level differences. We performed a voxelwise bivariate correlation between activity during the task and naming ability outside of the scanner, and then extracted ROIs from significant clusters meeting correction for multiple comparisons to test if these relationships were independent of lesion and demographic factors in regression analyses (Table 3, Fig. 5).
Table 3.
Description of ROIs defined by voxelwise correlation between activity and PNT scores
| ROI | Task contrast | Relationship with PNT | size | peak | x | y | z | t‐value |
|---|---|---|---|---|---|---|---|---|
| Left SMA | Covert > fixation | Negative | 4842 | −4.15 | −14 | −6 | 66 | −0.81 |
| Right dorsal central sulcus | Covert > fixation | Negative | 632 | −3.15 | 28 | −14 | 64 | −1.03 |
| Right medial SMA | Covert > fixation | Negative | 556 | −3.26 | 12 | 12 | 62 | −0.29 |
| Left supramarginal gyrus | Covert > fixation | Negative | 519 | −3.33 | −40 | −38 | 50 | −1.48 |
| Left temporal pole | Covert > fixation | Positive | 1672 | 4.27 | −50 | 6 | −2 | 2.64* |
| Left MFG | Overt > fixation | Negative | 6422 | −3.93 | −48 | 28 | 26 | −1.98 |
| Left frontal pole | Overt > fixation | Negative | 1423 | −3.33 | −46 | 44 | 0 | −2.50* |
| Left angular gyrus | Overt > fixation | Negative | 622 | −3.36 | −48 | −68 | 46 | 0.54 |
| Right post‐central sulcus | Overt > fixation | Positive | 968 | 3.51 | 52 | −8 | 26 | 2.83* |
| Left posterior STG | Overt > fixation | Positive | 643 | 3.37 | −50 | −48 | 18 | 2.05 |
| Right visual cortex | Overt > fixation | Positive | 621 | 3.40 | 18 | −90 | −18 | 1.00 |
| Right posterior STS | Overt > fixation | Positive | 569 | 3.53 | 58 | −28 | −4 | 2.65* |
* P < 0.05.
Figure 5.
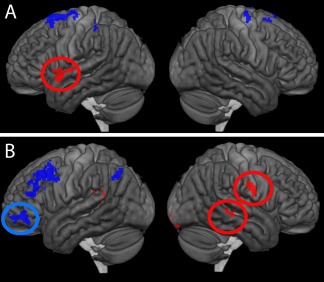
Regions of interest defined based on voxelwise bivariate correlations between activity and PNT during (A) covert naming and (B) overt naming. Red indicates a positive correlation, while blue indicates a negative correlation. Not shown is the right medial SMA ROI. Circles indicate ROIs were the relationship remained significant in a regression with all other factors were controlled. [Color figure can be viewed at http://wileyonlinelibrary.com]
Five significant clusters were identified in the covert naming > fixation contrast, four in which activity was negatively related to PNT, and one (the left TP) in which greater activity was related to better PNT scores. However, only the left TP had a significant relationship with PNT when all other variables, including PCAD, were controlled, t(14) = 2.73, P < 0.05. Activity did not differ between people with aphasia (mean z = 1.07) and controls (mean z = 1.03) in this ROI during covert naming.
Seven clusters were identified in the overt naming > fixation contrast, three in which activity was negatively related to PNT and four in which activity had a positive relationship with PNT. Of the ROIs with negative relationships between activity and PNT, only activity in the left frontal pole was significantly related to PNT after controlling for all other variables, t(19) = −2.94, P < 0.01. The activity in the aphasia group (mean z = −1.00) did not differ significantly from the control group (mean z = 0.26).
Of the four ROIs in which overt naming activity had a positive relationship with naming ability, two were still significant when all other variables were controlled: the right post‐central gyrus (t(23) = 2.83, P < 0.01) and the right STS, just anterior to the ROI identified from the between‐group contrasts (t(23) = 2.65, P = 0.014). For both of these ROIs, t‐tests revealed that the aphasia group showed significantly more activity than the control group during overt naming (post‐central gyrus, aphasia mean z = 3.06, control mean z = 1.13, P < 0.01; STS, aphasia mean z = 1.70, control group mean z = 0.25, P < 0.01).
Activity related to successful versus unsuccessful naming
Finally, because some studies have addressed the relationship between brain activity and naming ability by examining differences between correct and incorrect naming attempts, we tested for such differences in a whole‐brain analysis. At a relatively lenient threshold of z = 2.3, cluster corrected to P < 0.05, we found no regions where activity consistently differed during successful naming versus unsuccessful naming in participants with aphasia.
Relationships between activation and lesion site
To better understand the mechanisms of recruitment and relationship to naming performance, we examined two notable regions further: the left dPOp and the right STS from the between‐group analysis above. These areas are well known to be part of the language network for both people with aphasia and controls [Turkeltaub et al., 2011] and are part of the dorsal articulation network [Hickok and Poeppel 2004, 2007]. Furthermore, the negative relationship between activation of the preserved left POp and performance is counter to most literature in the field.
Since the activation in these areas had opposite relationships with PNT, we first examined the direct relationship between activation of the left dPOp and the right STS, and found a significant negative relationship, r = −0.74, t(30) = −6.05, P < 0.001, suggesting either that these areas interact directly or that their activity levels relate oppositely to a common underlying factor.
Next we examined whether activation of these areas related to lesion location. To accomplish this, we performed two SVR‐LSM analyses using residual activation of the right STS and the left dPOp as the dependent variables (Fig. 6). For the left dPOp analysis, we excluded six participants with strokes involving the left dPOp. The results were examined at P < 0.01, with a minimum cluster size of 500 voxels.
Figure 6.
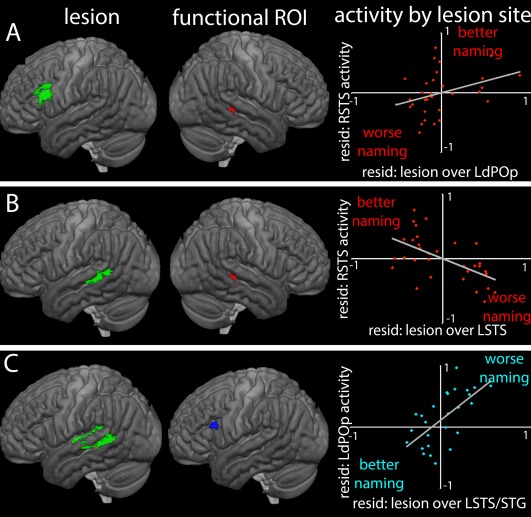
SVR‐LSM maps (left) show the lesion locations associated with activity in functional ROIs (middle). The graphs on the right show the relationships between lesions at these sites, activity in the corresponding ROIs, and naming performance relative to the model prediction. The aphasia group, as a whole, overactivated the right STS (red) and underactivated the left dPOp (blue). (A) Lesion locations associated with greater activity in the right STS; (B) Lesion locations associated with less activity in right STS, that is, activity similar to controls; (C) Lesions associated with greater activity in left dPOp, that is, activity similar to controls. [Color figure can be viewed at http://wileyonlinelibrary.com]
In the right STS, which was over‐activated in the aphasia group as a whole, greater activation was associated with lesions precisely overlapping with the left dPOp ROI in which lower fMRI activity was associated with better performance. That is to say, in people with lesions of the left dPOp, there was greater activation of the right STS, which was associated with better‐than‐predicted naming outcomes; in people without lesions in the left dPOp, decreased activation of this area was also related to greater activation of the right STS as shown by the direct correlation above, and better‐than‐predicted naming outcomes. The SVR‐LSM analysis further showed that low activation of right STS (i.e., no over‐activation compared with controls) was associated with lesions in the left STS, directly contralateral to the right STS ROI, and just ventral to the STG region identified as critical to PNT performance in the first SVR‐LSM analysis.
In the left dPOp, which was under‐activated in the aphasia group as a whole, greater activity (i.e., closer to normal levels in controls) related to lesions in left STG, left medial white matter below the precuneus, and most notably, the same region in the left STS that was related to low levels of activation of the right STS. There were no areas where lesions significantly predicted low levels of left dPOp activation.
The relationships between activation, lesion site and naming performance is depicted in Figure 6. In sum, these findings suggest that when critical left hemisphere naming areas are damaged, the left dPOp is disengaged and, especially when the left dPOp is damaged, the right STS is recruited. Both of these changes in activation are associated with better‐than‐predicted naming outcomes. However, when left STS is damaged, these compensatory changes fail to occur.
DISCUSSION
Three major findings emerged from this study. The first notable finding is that the classic pattern of right hemisphere over‐activation during naming in people with aphasia [Turkeltaub et al., 2011] relates primarily to post‐lexical production rather than lexical retrieval. Second, activation in spared brain areas relates to naming outcomes, independent of the direct effects of the stroke, suggesting that activation of some spared areas is not simply a correlate of stroke damage, but independently contributes to outcomes. Finally, interactions between activation of spared areas and lesion distributions suggest complex network dynamics at play in the brain's response to stroke.
Right Hemisphere Activation Relates to Better Performance and Depends on the Task
The right hemisphere, especially in the inferior frontal lobes, is a target for inhibition through noninvasive brain stimulation studies in chronic aphasia populations, and these studies have repeatedly been shown to improve picture naming scores [Barwood et al., 2011; Martin et al., 2009; Naeser et al., 2005; Turkeltaub et al., 2012; Winhuisen et al., 2005]. Based on these findings and some functional imaging results, a hypothesis has gained traction that engagement of the right hemisphere becomes less helpful and more detrimental to recovery as more time passes after the stroke, especially in people with small to moderate sized strokes [Anglade et al., 2014].
Our results revealed that levels of activation in some right hemisphere areas during the articulatory output phase was associated with better naming performance. The group with aphasia over‐activated several right hemisphere areas compared with controls during overt naming, and in some of these areas greater activity related to better outcomes. In the voxelwise analysis, we did identify two right hemisphere areas where activity was negatively correlated to PNT scores, but this effect disappeared when other variables were controlled. Given our control variables, these results show that participants with aphasia who had higher levels of right hemisphere activation in certain areas performed better than expected, given the severity of the lesion and the degree to which critical naming sites were damaged. Interpreted broadly, this finding could be seen to contradict the noninvasive brain stimulation literature in aphasia, which has suggested that right hemisphere inhibition improves performance. However, the neuronal effects of stimulation are not entirely clear. For example, direct current stimulation used to inhibit cortical excitability appears to have a nonlinear effect, and high doses of stimulation can have a reverse effect on local tissue [Batsikadze et al., 2013]. Furthermore, the large scale reorganization of cortical networks resulting from noninvasive brain stimulation treatment [Dayan et al., 2013] is still poorly understood. For example, it remains unclear whether stimulation protocols known to cause short‐term inhibition also cause longer lasting local inhibition associated with the lasting behavioral effects. In addition, it is unclear if local effects or downstream effects in other regions, both inhibitory and excitatory, are most important for recovery [see Turkeltaub, 2015 for review).
It should also be noted that, with only a few exceptions [e.g., You et al., 2011] the positive effects of right hemisphere inhibition have been observed specifically in the right prefrontal cortex, not the areas we identified here. Ideas about the maladaptive role of the right hemisphere primarily derive from inhibitory brain stimulation studies of right inferior frontal gyrus (IFG) and imaging findings in the same area. Although involvement of the entire right hemisphere is sometimes viewed as maladaptive in aphasia recovery, we have previously noted that brain stimulation and imaging studies suggest that the right PTr specifically may impair naming, while the rest of the right hemisphere compensates for deficits [Turkeltaub et al., 2011, 2012; Turkeltaub, 2015]. Here, we found no relationship between right IFG activation and naming performance. Thus, although our findings do not support the hypothesis that right PTr activity is maladaptive in aphasia recovery, they cannot clearly be said to conflict with this hypothesis either.
The positive relationships we observed here between right hemisphere over‐activation and naming outcomes elsewhere in the right hemisphere support prior evidence of right hemisphere compensation outside the PTr [Allendorfer et al., 2012; Elkana et al., 2013; Gold and Kertesz, 2000; Kinsbourne, 1971; Teki et al., 2013; Yarnell et al., 1979]. Our findings in the right STS were particularly robust, appearing in both the between‐group analysis and the voxelwise correlations with PNT. Activation in the right posterior STS has been associated with semantic retrieval in people with aphasia [Gold and Kertesz, 2000], articulation and auditory processing [Allendorfer et al., 2012], and phoneme processing [Teki et al., 2013]. Interestingly, the beneficial over‐activation observed here was restricted to the overt, articulatory output stage. The results in the right posterior STS are also consistent with a recent voxel‐based morphometry study, in which we found that hypertrophy in the same right hemisphere region was associated with better performance on speech production tasks [Xing et al., 2016], and a recent MEG study also found that recruitment of right temporoparietal areas was related to improved recovery on a semantic task [Kielar et al., 2016]. Recent transcranial magnetic stimulation studies in healthy adults found that disruption of a neighboring region, the right supramarginal gyrus, interefered with word production [Sollmann et al., 2014] and inhibited phonological decision making, but not semantic decision making, the latter of which would be better captured in the covert naming phase [Cogan et al., 2014; Hartwigsen et al., 2010]. These findings suggest that posterior regions in the right hemisphere language network may already be involved in production, but not necessarily retrieval, in healthy individuals, and are therefore better candidates for plastic reorganization of production processes following left hemisphere stroke. These findings have significant implications for brain stimulation treatments for naming.
Interestingly, we were unable to find any regions where activity was consistently different across our group of people with aphasia during correctly named trials versus incorrect trials. This suggests that right hemisphere activity associated with better performance is more closely related to overall impairment, rather than the momentary demands of the specific item to be named. At first glance, it appears that our results differ from Postman‐Caucheteux et al. [2010], who found robust right hemisphere activation during incorrect picture naming, significantly greater than during correct naming in all three of their participants with nonfluent aphasia. However, the precise location of the right hemisphere activity was not consistent across all three participants. In fact, what is most striking about their finding is that the right hemisphere response during incorrect trials is in the right hemisphere homologue of each individual participant's lesion. Perhaps a more detailed subject‐by‐subject analysis would reveal differences between correct and incorrect trials at the single subject level in our study. The null results in our larger sample of more varied aphasia, further supports the need to account for lesion size and location when examining functional task responses in aphasia. Depending on the specific research question, the analysis approach may control for these differences as we did in our main analyses here, or alternatively look for relationships between lesion location and activity to better understand the nature of the activity, as in the secondary analyses discussed below.
Relationship between Lesion Site and Activation of Left and Right Language Areas
Our results suggest that the degree to which individuals are able to successfully recruit right hemisphere language homologues depends on the location of the lesions in the left hemisphere. Overall, when participants with aphasia were able to over‐activate the right STS, they performed better on naming tasks—even better than predicted by models that controlled for the degree to which critical naming areas were damaged. However, not all people with aphasia were able to successfully recruit the right STS. Participants with left STS lesions failed to over‐activate the right STS compared with controls. This finding stands in stark contrast to ideas that right hemisphere nodes are recruited either due to a release of interhemispheric inhibition, or to replace their damaged left hemisphere counterparts. Both hypotheses predict that lesions of the left STS should cause increased right STS activity, whereas we found that these lesions relate to a failure to recruit the right STS. The failure to recruit the right STS when the left STS is damaged may relate to disruption of cross‐hemispheric fibers required for communication between homotopic areas of the two hemispheres. When the left STS is damaged, the right STS lacks a critical input necessary to modify activity. The right STS may thus serve to compensate for network‐level dysfunction of circuits involving the left STS, but cannot be recruited for this purpose when the route of communication from the left STS is directly damaged.
This hypothesis is supported by the finding that people with lesions in the left dPOp, an area strongly connected to the left STS [Kelly et al., 2010; Xiang et al., 2010], over‐activated portions of the right STS, to their own benefit. Additionally, in people without damage in the left dPOp, under‐activation in this area was also associated with greater activation of the right STS, which again was related to better naming performance. The relationships observed here between both lesion status and activation of the dPOp, left STS, and right STS align with dual stream models of speech perception and production [Hickok and Poeppel 2004, 2007]. These models propose a bi‐directional stream of processing that connects the bilateral posterior STS and left posterior IFG, including the dPOp. The bilateral STS is proposed to store a sound‐based representation of speech, because it is active during passive speech perception [Schlosser et al., 1998] and during silent word rehearsal [Buchsbaum et al., 2001]. The sensory representations in this region are assumed to be activated during production as well as perception [Hickok, 2012]. The STS communicates with posterior IFG, where articulatory/motor codes are matched to the sound‐based representations. The interaction of these regions specifically during the matching of phonological codes to motor codes may explain why these regions were particularly important during the overt naming stage here. Damage in the left STS may render activity in the posterior IFG (dPOp) ineffective since the phonological codes are not retrieved correctly, resulting in worse naming outcomes when activity in this area is retained. Conversely, damage in the left dPOp may disrupt feedback to the left STS, thus eliciting increased recruitment of the right STS to aid in retrieving phonological codes.
The negative relationship between left dPOp activation and naming performance is seemingly contradictory to some of the previous literature, in which others have found a positive relationship between activation of preserved left frontal regions and naming [Fridriksson et al., 2010; Berthier and Pulvermüller, 2011; Hodgson et al., 2014]. This finding has not been entirely consistent however, as others have found that lexical therapy resulted in decreased activity in the left IFG that also related to better outcomes in naming [Abel et al., 2015], which was interpreted as being driven by ease of processing following treatment. Meier et al. [2016] recently reported that greater inhibitory pressure between the left middle temporal gyrus (MTG) and left IFG correlated with better naming in the scanner, and this inhibitory connectivity was associated with spared MTG. Furthermore, two studies have found that (inhibitory) cathodal direct current stimulation of left perilesional tissue led to better outcomes in aphasia than (excitatory) anodal stimulation [Monti et al., 2008; Shah‐Basak et al., 2015]. In some cases, disengagement of left frontal areas in concert with engagement of alternate language processors elsewhere, such as the right STS, may result in improved outcomes overall. Our results suggest that the degree to which recovering or enhancing function in left hemisphere sites is helpful depends at least partially on lesion location.
CONCLUSIONS
In this study, we replicated some findings in the naming and aphasia literature and extended them by showing that activation of the right hemisphere depends on the stage of the picture naming process. By controlling for the proportion of critical areas damaged as well as total lesion size and other factors, we were able to identify right hemisphere regions where activation was related not just to raw performance, but to better than predicted performance given all that we know about the individuals' lesions. Furthermore, we identified a left hemisphere region, the dPOp, in which retained activity is maladaptive and disengagement produces better than predicted naming outcomes. In both cases, the compensatory changes in brain activity relate to particular lesion distributions suggesting network level disruption triggering distant effects that impact recovery. These findings have significant implications for understanding mechanisms of plasticity in language networks that may ultimately lead to more effective treatments for aphasia.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
We thank Katherine Spiegel, Mackenzie Fama, Laura Hussey, Lauren Taylor, Jessica Friedman, Molly Stamp and Thomas Mitchell for contributing to data collection and organization. The authors have no conflicts of interest to declare.
Contributor Information
Laura M. Skipper‐Kallal, Email: laurakallal.research@gmail.com.
Peter E. Turkeltaub, Email: peter.turkeltaub@georgetown.edu.
REFERENCES
- Abel S, Weiller C, Huber W, Willmes K, Specht K (2015): Therapy‐induced brain reorganization patterns in aphasia. Brain 138:1097–1112. [DOI] [PubMed] [Google Scholar]
- Allendorfer JB, Kissela BM, Holland SK, Szaflarski JP (2012): Different patterns of language activation in post‐stroke aphasia are detected by overt and covert versions of the verb generation task. Med Sci Monitor 18:CR135–CR137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anglade C, Thiel A, Ansaldo AI (2014): The complementary role of the cerebral hemispheres in recovery from aphasia after stroke: A critical review of the literature. Brain Injury 28:138–145. [DOI] [PubMed] [Google Scholar]
- Barwood CH, Murdoch BE, Whelen BM, Lloyd D, Riek S, O'Sullivan JD, Coulthard A, Wong A (2011): Improved language performance subsequent to low‐frequency rTMS in patients with chronic non‐fluent aphasia psot‐stroke. Eur J Neurol 18:935–943. [DOI] [PubMed] [Google Scholar]
- Barwood CHS, Murdoch BE, Whelan B‐M, Lloyd D, Riek S, O'Sullivan JD, et al. (2010): Improved language performance subsequent to low‐frequency rTMS in patients with chronic non‐fluent aphasia post‐stroke. Eur J Neurol 18:935–943. [DOI] [PubMed] [Google Scholar]
- Basso A, Gardelli M, Grassi MP, Mariotti M (1989): The role of the right hemisphere in recovery from aphasia: Two case studies. Cortex 25:555–566. [DOI] [PubMed] [Google Scholar]
- Batsikadze G, Moliadze V, Paulus W, Kuo M‐F, Nitsche MA (2013): Partially non‐linear stimulation intensity‐dependent effects of direct current stimulation on motor cortex excitability in humans. J Physiol 591:1987–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthier M, Pulvermüller F (2011): Neuroscience insights improve neurorehabilitation of poststroke aphasia. Nat Rev Neurol 7:86–97. [DOI] [PubMed] [Google Scholar]
- Blank SC, Bird H, Turkheimer F, Wise RJS (2003): Speech production after stroke: The role of the right pars opercularis. Ann Neurol 54:310–320. [DOI] [PubMed] [Google Scholar]
- Bonakdarpour B, Parris TB, Thompson CK (2007): Hemodynamic response function in patients with stroke‐induced aphasia: Implications for fMRI data analysis. NeuroImage 36:322–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breier JI, Juranek J, Maher LM, Schmadeke S, Men D, Papanicolaou AC (2009): Behavioral and neurphysiologic response to therapy for chronic aphasia. Arch Phys Med Rehabil 90:2026–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchsbaum BR, Hickok G, Humphries C (2001): Role of the left posterior superior temporal gyrus in phonological processing for speech perception and production. Cogn Sci 25:663–678. [Google Scholar]
- Cao Y, Vikingstad EM, George KP, Johnson AF, Welch KMA (1999): Corticial language activation in stroke patients recovering from aphasia with functional MRI. Stroke 30:2331–2340. [DOI] [PubMed] [Google Scholar]
- Dayan E, Censor N, Buch ER, Sandrini M, Cohen LG (2013): Noninvasive brain stimulation: From physiology to network dynamics and back. Nat Neurosci 16:838–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J, Hummel F, Celnik P, Murase N, Mazzocchio R, Cohen LG (2005): Transcallosal inhibition in chronic subcortical stroke. NeuroImage 28:940–946. [DOI] [PubMed] [Google Scholar]
- Elkana O, Frost R, Kramer U, Ben‐Bashat D, Schweiger A (2013): Cerebral language reorganization in the chronic stage of recovery: A longitudinal fMRI study. Cortex 49:71–81. [DOI] [PubMed] [Google Scholar]
- Fernandez B, Cardebat D, Demonet J‐F, Joseph PA, Mazaux J‐M, Barat M, et al. (2004): Functional MRI follow‐up study of language processes in healthy subjects and during recovery in a case of aphasia. Stroke 35:2171–2176. [DOI] [PubMed] [Google Scholar]
- Forkel SJ, Thiebaut de Schotten M, Dell'Acqua F, Kalra L, Murphy DG, Williams SC, Catani M (2014): Anatomical predictors of aphasia recovery: A tractography study of bilateral perisylvian language networks. Brain 137:2027–2039. [DOI] [PubMed] [Google Scholar]
- Fridriksson J, Bonilha L, Baker JM, Moser D, Rorden C (2010): Activity in preserved left hemisphere regions predicts anomia and severity in aphasia. Cerebr Cortex 20:1013–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridriksson J, Richardson JD, Fillmore P, Cai B (2012): Left hemisphere plasticity in aphasia recovery. NeuroImage 60:854–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold BT, Kertesz A (2000): Right hemisphere semantic processing of visual words in an aphasic patient: An fMRI study. Brain Lang 73:456–465. [DOI] [PubMed] [Google Scholar]
- Goldrick M, Rapp B (2007): Lexical and post‐lexical phonological representations in spoken production. Cognition 102:219–260. [DOI] [PubMed] [Google Scholar]
- Gottesman R, Hillis A (2010): Predictors and assessment of cognitive dysfunction resulting from ischaemic stroke. Lancet Neurol 9:895–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton RH, Chrysikou EG, Coslett B (2011): Mechanisms of aphasia recovery after stroke and the role of noninvasive brain stimulation. Brain Lang 118:40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwigsen G, Baumgaertner A, Price CJ, Koehnke M, Ulmer S, Siebner HR (2010): Phonological decisions require both the left and right supramarginal gyri. pnas 107:16494–16499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiss W‐D, Kessler J, Thiel A, Ghaemi M, Karbe H (1999): Differential capacity of left and right hemispheric areas for compensation of poststroke aphasia. Ann Neurol 45:430–438. [DOI] [PubMed] [Google Scholar]
- Heiss W‐D, Thiel A, Kessler J, Herholz K (2003): Disturbance and recovery of language function: Correlates in PET activation studies. NeuroImage 20:S42–S49. [DOI] [PubMed] [Google Scholar]
- Hickok G (2012): Computational neuroanatomy of speech production. Nat Rev Neurosci 13:135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G, Poeppel D (2004): Dorsal and ventral streams: A framework for understanding aspects of the functional anatomy of language. Cognition 92:67–99. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D (2007): The cortical organization of speech processing. Nat Rev Neurosci 8:393–402. [DOI] [PubMed] [Google Scholar]
- Hodgson JC, Benattayallah A, Hodgson TL (2014): The role of the dominant versus non‐dominant hemisphere: An fMRI study of aphasia recovery following stroke. Aphasiology 28:1426–1477. [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM (2012): FSL. NeuroImage 62:782–790. [DOI] [PubMed] [Google Scholar]
- Karbe H, Kessler J, Herholz K, Fink GR, Heiss W‐D (1995): Long‐term prognosis of poststroke aphasia studied with positron emission tomography. Arch Neurol 52:186–190. [DOI] [PubMed] [Google Scholar]
- Kelly C, Uddin LQ, Shehzard Z, Margulies DS, Castellanos FX, Milham MP, et al. (2010): Broca's region: Linking human brain functional connectivity data and non‐human primate tracing anatomy studies. Eur J Neurosci 32:383–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemeny S, Xu J, Park GH, Hosey LA, Wettig CM, Braun AR (2006): Temporal dissociation of early lexical access and articulation using a delayed naming task – an fMRI study. Cerebr Cortex 16:587–595. [DOI] [PubMed] [Google Scholar]
- Kielar A, Deschamps T, Jokel R, Meltzer JA (2016): Functional reorganization of language networks for semantics and syntax in chronic stroke: Evidence from MEG. Hum Brain Mapp 37:2869–2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsbourne M (1971): The minor cerebral hemisphere as a source of aphasic speech. Arch Neurol 25:302–306. [DOI] [PubMed] [Google Scholar]
- Kurland J, Cortes CR, Wilke M, Sperling AJ, Lott SN, Tagamets MA, et al. (2008): Neural mechanisms underlying learning following semantic mediation treatment in a case of phonological alexia. Brain Imaging Behav 2:147–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchina S, Zhu LL, Norton A, Zipse L, Wan CY, Schlaug G (2011): Impairment of speech production predicted by lesion load of the left arcuate fasciculus. Stroke 42:2251–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PI, Naeser MA, Ho M, Treglia E, Kaplan E, Baker E (2009): Magnetic stimulation in the treatment of aphasia. Curr Neurol Neurosci Rep 9:451–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier EL, Kapse KJ, Kiran S (2016): The relationship between frontotemporal effective connectivity during picture naming, behavior, and preserved cortical tissue in chronic aphasia. Front Hum Neurosci 10:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinzer M, Breitenstein C (2008): Functional imaging studies of treatment‐induced recovery in chronic aphasia. Aphasiology 22:1251–1268. [Google Scholar]
- Meltzer JA, Wagage S, Ryder J, Solomon B, Braun AR (2013): Adaptive significance of right hemisphere activation in aphasic language comprehension. Neuropsychologia 51:1248–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti A, Cogiamanian F, Marceglia S, Ferrucci R, Mameli F, Mrakic‐Sposta S, et al. (2008): Improved naming after transcranial direct current stimulation in aphasia. J Neurol Neurosurg Psychiatry 79:451–453. [DOI] [PubMed] [Google Scholar]
- Murase N, Duque J, Mazzocchio R, Cohen LG (2004): Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol 55:400–409. [DOI] [PubMed] [Google Scholar]
- Naeser MA, Martin PI, Nicholas M, Baker EH, Seekins H, Kobayashi M, et al. (2005): Improved picture naming in chronic aphasia after TMS to part of right Broca's area: An open‐protocol study. Brain Lang 93:95–105. [DOI] [PubMed] [Google Scholar]
- Pani E, Zheng X, Wang J, Norton A, Schlaug G (2016): Right hemisphere structures predict poststroke speech fluency. Neurology 86:1574–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen P, JØrgensen H, Nakayama H, Raaschou HO, Olsen TH (1995): Aphasia in acute stroke: Incidence, determinants and recovery. Ann Neurol 38:659–666. [DOI] [PubMed] [Google Scholar]
- Postman‐Caucheteux WA, Birn RM, Pursley RH, Butman JA, Solomon JM, Picchioni D, et al. (2010): Single‐trial fMRI shows contralesional activity linked to overt naming errors in chronic aphasia patients. J Cogn Neurosci 22:1299–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team . (2010): R: A Language and Environment for Statistical Computing. Vienna, Austria: The R Foundation for Statistical Computing. [Google Scholar]
- Ren C‐L, Zhang G‐F, Xia N, Jin C‐H, Zhang X‐H, Hao J‐F, et al. (2014): Effect of low‐frequency rTMS on aphasia in stroke patients: A meta‐analysis of randomized controlled trials. PLoS One 9:e102557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach A, Schwartz MF, Martin N, Grewal RS, Brecher A (1996): The Philadelphia naming test: Scoring and rationale. Clin Aphasiol 24:121–133. [Google Scholar]
- Rorden C, Brett M (2000): Stereotaxic display of brain lesions. Behav Neurol 12:191–200. [DOI] [PubMed] [Google Scholar]
- Rorden C, Bonilha L, Fridriksson J, Bender B, Karnath H‐O (2012): Age‐specific CT and MRI templates for spatial normalization. NeuroImage 61:957–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saur D, Lange R, Baumgaertner A, Schraknepper V, Willmes K, Rijntjes M, et al. (2006): Dynamics of language reorganization after stroke. Brain 129:1371–1384. [DOI] [PubMed] [Google Scholar]
- Schlosser MJ, Aoyagi N, Fulbright RK, Gore JC, McCarthey G (1998): Functional MRI studies of auditory comprehension. Hum Brain Mapp 6:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah‐Basak PP, Norise C, Garcia G, Torres J, Faseyitan O, Hamilton RH (2015): Individualized treatment with transcranial direct current stimulation in patients with chronic non‐fluent aphasia due to stroke. Front Hum Neurosci 9:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollmann N, Tanigawa N, Ringel F, Zimmer C, Meyer B, Krieg SM (2014): Language and its right‐hemispheric distribution in healthy brains: An investigation by repetitive transcranial magnetic stimulation. NeuroImage 102:776–788. [DOI] [PubMed] [Google Scholar]
- Teki S, Barnes GR, Penny WD, Iverson P, Woodhead ZVJ, Griffiths TD, et al. (2013): The right hemisphere supports but does not replace left hemisphere auditory function in patients with persisting aphasia. Brain 136:1901–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub PE (2015): Brain stimulation and the role of the right hemisphere in aphasia recovery. Curr Neurol Neurosci Rep 15:1–9. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Messing S, Norise C, Hamilton RH (2011): Are networks for residual language function and recovery consistent across aphasic patients? Neurology 76:1726–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub PE, Coslett HB, Thomas AL, Faseyitan O, Benson J, Norise C, et al. (2012): The right hemisphere is not unitary in its role in aphasia recovery. Cortex 48:1179–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker GM, Hickok G (2015): Bridging computational approaches to speech production: The semantic‐lexical‐auditory‐motor model (SLAM). Psychon Bull Rev 23:339–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Marchina S, Norton AC, Wan CY, Schlaug G (2013): Predicting speech fluency and naming abilities in aphasic patients. Front Hum Neurosci 7:831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winhuisen L, Thiel A, Schumacher B, Kessler J, Rudolf J, Haupt WF, et al. (2005): Role of the contralateral inferior frontal gyrus in recovery of language function in poststroke aphasia: A combined repetitive transcranial magnetic stimulation and positron emission tomography study. Stroke 36:1759–1763. [DOI] [PubMed] [Google Scholar]
- Winhuisen L, Thiel A, Schumacher B, Kessler J, Rudolf J, Haupt WF, et al. (2007): The right inferior frontal gyrus and poststroke aphasia: A follow‐up investigation. Stroke 38:1286–1292. [DOI] [PubMed] [Google Scholar]
- Xiang H‐D, Fonteijn HM, Norris DG, Hagoort P (2010): Topographical functional connectivity pattern in the perisylvian language networks. Cerebr Cortex 20:549–560. [DOI] [PubMed] [Google Scholar]
- Xing S, Lacey E, Skipper‐Kallal LM, Jiang X, Harris‐Love ML, Zeng J, et al. (2016): Right hemisphere grey matter structure and language outcomes in chronic left hemisphere stroke. Brain 139:227–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarnell P, Monroe P, Sobel L (1979): Aphasia outcome in stroke: A clinical neuroradiological correlation. Stroke 7:516–523. [DOI] [PubMed] [Google Scholar]
- You DS, Kim D‐Y, Chun MH, Jung SE, Park SJ (2011): Cathodal transcranial direct current stimulation of the right Wernicke's area improves comprehension in subacute stroke patients. Brain Lang 119:1–5. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Kimberg DY, Coslett HB, Schwartz MF, Wang Z (2014): Support vector regression based multivariate lesion‐symptom mapping. Hum Brain Mapp 35:5599–5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


