Abstract
Convergent evidences have revealed that schizophrenia is associated with brain dysconnectivity, which leads to abnormal network organization. However, discrepancies were apparent between the structural connectivity (SC) and functional connectivity (FC) studies, and the relationship between structural and functional deficits in schizophrenia remains largely unknown. In this study, resting‐state functional magnetic resonance imaging and structural diffusion tensor imaging were performed in 20 patients with schizophrenia and 20 matched healthy volunteers (patients/controls = 19/17 after head motion rejection). Functional and structural brain networks were obtained for each participant. Graph theoretical approaches were employed to parcellate the FC networks into functional modules. The relationships between the entries of SC and FC were estimated within each module to identify group differences and their correlations with clinical symptoms. Although five common functional modules (including the default mode, occipital, subcortical, frontoparietal, and central modules) were identified in both groups, the patients showed a significantly reduced modularity in comparison with healthy participants. Furthermore, we found that schizophrenia‐related aberrations of SC–FC coupling exhibited complex patterns among modules. Compared with controls, patients showed an increased SC–FC coupling in the default mode and the central modules. Moreover, significant SC–FC decoupling was demonstrated in the occipital and the subcortical modules, which was associated with longer duration of illness and more severe clinical manifestations of schizophrenia. Taken together, these findings demonstrated that altered module‐dependent SC–FC coupling may underlie abnormal brain function and clinical symptoms observed in schizophrenia and highlighted the potential for using new multimodal neuroimaging biomarkers for diagnosis and severity evaluation of schizophrenia. Hum Brain Mapp 38:2008–2025, 2017. © 2017 Wiley Periodicals, Inc.
Keywords: resting‐state fMRI, diffusion tensor imaging (DTI), structural–functional coupling, modular architecture, schizophrenia
INTRODUCTION
A recent conceptualization suggests that the human brain forms a large‐scale complex network of interconnected regions within the human connectome, which provides the anatomical substrate for neural communication, functional processing, and information integration in the brain [Sporns, 2011]. Although it is commonly assumed that functional brain connectivity (assessed using functional MRI) reflects the underlying structural brain connectivity (assessed using diffusion tractography), the nature of the structure‐function relationship, particularly how the structure of the neural connectional network underlies cognitive functions, remains one of the most challenging questions in neuroscience [Honey et al., 2009; Liao et al., 2015; Skudlarski et al., 2008; Wang et al., 2015]. More importantly, studies on the progression of this relationship under various brain disorders remain in its infancy [Damoiseaux and Greicius, 2009].
Schizophrenia is a severe psychiatric disorder with a myriad of clinical manifestations. While the precise neural substrates underpinning the clinical manifestations of schizophrenia are far from understood, the disorder is thought to be related to dysconnectivity among brain regions [Friston, 1998; Pettersson‐Yeo et al., 2011; Stephan et al., 2006, 2009]. Lately, studies of brain structural/functional networks in schizophrenia have revealed convergent trend of wide‐spread dysconnectivity [Canu et al., 2015; Ellison‐Wright and Bullmore, 2009; Fornito et al., 2012; Van den Heuvel and Fornito, 2014], which leads to abnormal network organization in patients, including increase of minimum path length [Zalesky et al., 2011; Zhang et al., 2012], loss of hubs (highly interconnected brain regions) mainly in frontal regions [Rubinov and Bullmore, 2013], and an abnormal rich club organization (highly interconnected hubs) [Van den Heuvel et al., 2013]. It is noteworthy that most of the reported aberrations in brain network organization were revealed using single neuroimaging modality, therefore providing limited insights into the understanding of schizophrenia [Skudlarski et al., 2010]. Furthermore, discrepancies were also apparent between the structural and functional network topology [Fornito et al., 2012; Van den Heuvel and Fornito, 2014], reiterating the potentially complex relationship between structural and functional brain network alterations in schizophrenia.
Most recently, the emerging field of combining both structural and functional connectivity has provided some of the first quantitative and comprehensive insights into schizophrenia‐related alterations in brain connectivity [Camchong et al., 2011; Cocchi et al., 2014; Skudlarski et al., 2010; Van den Heuvel et al., 2013]. For instance, Skudlarski et al., showed that patients with schizophrenia exhibited nearly uniform decrease of structural connectivity (SC) with complex functional connectivity (FC) alterations, leading to a decoupling interaction between structural and functional connectivity which predominantly resided in the posterior cingulate cortex, the task‐positive network and the default mode network (DMN) [Skudlarski et al., 2010]. Similar findings of reduced regional SC–FC coupling in schizophrenia were also observed by Cocchi et al. [2014]. Meanwhile, an increased coupling between structural and functional connectivity, aggregated over the whole brain, was revealed [Van den Heuvel et al., 2013]. These studies have focused on microscopic (regional pair) [Cocchi et al., 2014; Skudlarski et al., 2010] or macroscopic (whole‐brain) [Van den Heuvel et al., 2013] levels of SC–FC coupling, however, the aberrations of SC–FC coupling in schizophrenia at intermediate level, which could be assessed in modular architecture, has yet been explored.
Modularity is a fundamental concept in systems neuroscience, referring to an optimal partition of a brain network into smaller functional communities with dense connectivity within modules and sparse connectivity between modules [Bullmore and Sporns, 2009; Newman and Girvan, 2004]. The identification of non‐random modular architecture in brain networks has made a great impact on our understanding of the topological organization of brain networks as it contributes to various aspects of the intrinsic functional organization of the human brain such as the balance of functional segregation and integration whilst conserving wiring length (efficient local information processing within modules and rapid information communication among different modules), high resilience to network node or edge failure, and adaptability to multiple or distinct selection criteria over time (for a review, see [Meunier et al., 2010]). Detection and characterization of modular structure in brain system can help us to identify groups of associated components that perform specific biological functions [He et al., 2009]. Graph theoretical analysis has consistently revealed modular architecture in brain networks of healthy individuals, which broadly agree with several well‐defined functional subdivisions, such as the auditory, visual, and somato‐sensory‐motor systems [He et al., 2009; Kim et al., 2014; Meunier et al., 2010]. In the context of the recent focus on the developmental phenotypes of neuropsychiatric disease [Giedd et al., 2009; Gogtay et al., 2008], investigating the measurements that is theoretically linked to network development, such as modularity, would provide sensitive markers of abnormal brain development in schizophrenia [Alexander‐Bloch et al., 2010]. Of note, dysmodularity in schizophrenia has already been proposed as a neuropsychological theory [David, 1994], implying the breakdown of information encapsulation between brain subsystems that are specialized to carry out different tasks. However, the topological modularity of brain functional networks in schizophrenia is only beginning to be revealed [Alexander‐Bloch et al., 2010, 2012; Yu et al., 2011].
Given the paucity of schizophrenia‐related multimodal neuroimaging brain network research [Camchong et al., 2011; Cocchi et al., 2014; Skudlarski et al., 2010; Van den Heuvel et al., 2013] and the widely observed cognitive deficits in attention, working memory, visual processing, and language in schizophrenia [Javitt, 2009; Minzenberg et al., 2009], we believe that examining the characteristics of aberrant SC–FC coupling at the intermediate functional modular level may lead to a better understanding of the underlying nature of schizophrenia‐related alterations in brain connectivity, and potentially help to elucidate the etiology of the disorder. In this exploratory study, we employed connectomic techniques on resting‐state functional as well as diffusion imaging data in a sample of well characterized patients with schizophrenia and a carefully matched group of healthy participants. A graph theoretical analysis framework was utilized to investigate the functional modular architecture in schizophrenia. Based upon consistent observations of the modular architecture in healthy participants [Meunier et al., 2010], we hypothesized that patients with schizophrenia would also demonstrate modular architecture in the functional brain network. This functional module analysis was followed by an assessment of module‐dependent SC–FC coupling in schizophrenia. Based upon the wide‐spread inhomogeneous alterations of SC and FC across the whole brain [Pettersson‐Yeo et al., 2011], we further hypothesized that schizophrenia‐related abnormal SC–FC coupling would exhibit complex patterns among modules. Specifically, we expect that increased SC–FC coupling in patients with schizophrenia compared with controls may be indicative of less dynamic brain function. Conversely, weaker coupling may indicate a relaxation of the normal constraints imposed by anatomical interactions on brain function.
METHODS AND MATERIALS
Subjects
The study sample consisted of 20 patients with schizophrenia (male/female = 10/10, age = 38.4 ± 9.6 years, range: 24–56 years) recruited at the Institute of Mental Health (IMH), Singapore, and 20 normal subjects (male/female = 10/10, age = 37.8 ± 8.8 years, range: 28–59 years) recruited from the local community through advertisements. For all participants, presence and absence of psychopathology was established by a board‐certificated psychiatrist (K.S.) using information obtained from the clinical history, mental status examination, existing medical records, interviews with significant others as well as the administration of the Structural Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM‐IV). Patients were eligible for the study if they met DSM‐IV criteria for schizophrenia. The age of onset of first psychosis was recorded and the total duration of illness was calculated. The type and daily dose of antipsychotic therapy was also recorded and converted to a chlorpromazine equivalent according to [Woods, 2003].
Participants were excluded if they had any of the following characteristics: (1) a history of any significant neurological illness such as brain trauma, epilepsy, or cerebral vascular accident and (2) met DSM‐IV criteria for alcohol or other substance abuse. Normal subjects were additionally screened for family history of mental illness: subjects with a first degree relative suffering from mental illness would be excluded in this study. The psychopathology and symptom severity was assessed using the positive and negative syndrome scale (PANSS) [Kay et al., 1987]; while the general level of functioning were evaluated with the global assessment scale [Endicott et al., 1976]. All participants provided written informed consent as approved by the Institutional Review Boards of the IMH, Singapore. This study was carried out in compliance with the Declaration of Helsinki. At the time of scanning, all participants were regularly receiving antipsychotic medication and had no changes to their medications for the last 4 weeks.
Data Acquisition
Images were acquired on a 3‐Tesla Philips Achieva scanner (Philips, The Netherlands) with an 8‐channel SENSE head‐coil at National Neuroscience Institute, Singapore. Subjects were instructed to keep still and remain as motionless as possible before the scanning.
Pre‐scan training and practice were performed to achieve increased patient cooperation. During the whole data acquisition period, subjects lay supine with the head snugly fixed by foam pads provided by the scanner manufacturer to further minimize the head motion. The following sequences were obtained in a single session without altering position: (a) one structural T1 MRI; (b) one resting‐state fMRI; and (c) two volumes of diffusion encoded images. Of note, during the resting‐state fMRI scanning, all participants were instructed to stay awake, keep their gaze on a central fixation cross at all times and not to think systematically. No participants fell asleep, which was confirmed by self‐reports on their feedback in the scanner after the scanning.
Structural images for co‐registration, normalization and cortical parcellation were acquired using a high‐resolution T1‐weighted magnetization prepared rapid gradient‐recalled sequence (repetition time [TR] = 7.2 ms; echo time [TE] = 3.3 ms; slice number = 180; thickness = 0.9 mm; field of view [FOV] = 230 × 230 mm2; acquisition matrix = 256 × 256; in‐plane resolution = 0.9 × 0.9 mm2) in the direction of the anterior‐posterior commissures. Resting‐state fMRI data were collected during one run of 240 images at TR/TE of 2,000/35 ms, flip angle 90°, 36 slices, 3‐mm slice thickness; FOV 230 × 230 mm2; 64 × 64 matrix, 3.5 × 3.5 mm2 in‐plane resolution. A single‐shot echo‐planar sequence (TR/TE = 4,323/60 ms, flip angle = 90°; b‐factor = 800 s/mm2; 1 baseline image with b0 = 0 s/mm2) from 32 separate nonparallel directions was utilized to obtain diffusion encoded images (each volumes containing 45 slices, 3.0 mm with no gap; FOV = 230 × 230 mm2; acquisition matrix = 112 × 109, reconstructed to 256 × 256). For each participant, the diffusion sequences were scanned twice to improve the signal‐to‐noise ratio.
Functional Data Preprocessing and Network Construction
Functional imaging data preprocessing was performed using the SPM12 package (http://www.fil.ion.ucl.ac.uk/spm/software/spm12/; Wellcome Trust Center for Neuroimaging, University College London), resting‐state fMRI data analysis toolkit [Song et al., 2011], and DPARSF (data processing assistant for resting‐state fMRI) [Yan and Zang, 2010], running under Matlab 2012a (Mathworks, USA). After removing the first 10 volumes to allow for T1 equilibrations effects and subjects' adaption to the environment, the remaining functional volumes were corrected for time offsets between slices. Then the time series of images were realigned to the first volume (the original 11th volume) to compensate the inter‐scan head motion artifacts using a six‐parameter rigid‐body transform. This realigning step also provided a record for inter‐scan head motions. The individual anatomical T1‐weighted images were coregistered to functional images after motion correction using a linear transformation and were segmented into gray matter, white matter, and cerebrospinal fluid (CSF) tissue maps through a unified segmentation approach [Ashburner and Friston, 2005]. To further reduce the variance estimates, nuisance signal corrections were also performed on the 24 head‐motion profiles (Friston 24‐parameter model) [Friston et al., 1996], white matter, CSF signals, and global signals [Yan and Zang, 2010]. Subsequently, all corrected functional data were normalized to the Montreal Neurological Institute (MNI) template and further resampled to 3 × 3 × 3 mm3 isotropic resolution and spatially smoothed by convolution with a Gaussian kernel (FWHM = 4 mm). Previous studies showed that correlated endogenous dynamics in resting‐state functional data are particularly salient in frequencies below 0.1 Hz [Lowe et al., 1998]. Therefore, in this study, the temporal waveform of each voxel was band‐passed into 0.01–0.1 Hz to reduce the effect of very low frequency drift and the high frequency physiological noise.
It is known that head motion can introduce substantial changes in the time courses of resting state functional connectivity [Power et al., 2012; Van Dijk et al., 2012; Yan et al., 2013a]. Hence, two strategies were adopted in the current study to control for head motion. First, to account for the transient excessive movement, subjects were discarded if their head moved more than 2 mm of translation or 2° of rotation in any direction. No subject was rejected at this step. Additionally, we addressed the residual effects of head motion in group analysis through frame‐wise displacement (FD) derived with Jenkinson's relative root mean square algorithm as nuisance covariate [Jenkinson et al., 2002]. Those subjects with mean FD (Jenkinson) greater than 2 × S.D. above the group mean motion were excluded from the following graph theoretical analysis (thresholdNC = 0.175, thresholdSCZ = 0.193) [Yan et al., 2013b]. Four subjects were excluded at this step, leading to a final subject number of 36 (NC/SCZ = 17/19). Head motion between both groups was not significantly different (t 34 = −0.923, P = 0.362). The detailed demographic and clinical characteristics of the included subjects were presented in Table 1.
Table 1.
Demographic and clinical characteristics of the samplesa
| Characteristics |
Patients with schizophrenia (n = 19) |
Normal controls (n = 17) |
Statistical analysis | |
|---|---|---|---|---|
| Age (years) | 24–56 (38.8 ± 9.7) | 29–59 (37.4 ± 8.4) | t 38 = 0.469 | P = 0.642 |
| Gender: Male/Female | 9/10 | 9/8 | χ 1 2 = 0.111 | P = 0.738 |
| Handedness: Right/Left | 18/1 | 17/0 | χ 1 2 = 0.920 | P = 0.337 |
| Ethnicb: C/M/I/O | 14/3/1/1 | 14/2/1/0 | χ 3 2 = 1.092 | P = 0.779 |
| Educationc (years) | 6–16 (11.3 ± 3.2) | 10–19 (15.1 ± 2.2) | t 37 = −4.078 | P = 2.59 e−4 |
| WRAT3 scored | 29–57 (49.0 ± 6.0) | 39–54 (49.0 ± 4.0) | t 38 = 0.283 | P = 0.779 |
| Age of onset (years) | 14–47 (26.4 ± 8.5) | – | – | – |
| Duration of illness (years) | 1–30 (11.1 ± 8.5) | – | – | – |
| Antipsychotic medication | ||||
| Medication dosee (mg/day) | 50–650 (254.7 ± 160.5) | – | – | – |
| typical/atypical/mixed | 2/10/7 | – | – | – |
| PANSS symptomse | ||||
| Positive symptoms | 7–14 (9.8 ± 2.8) | – | – | – |
| Negative symptoms | 7–26 (10.6 ± 5.1) | – | – | – |
| General symptoms | 16–28 (19.8 ± 3.5) | – | – | – |
| Overall symptoms | 30–53 (40.2 ± 7.1) | – | – | – |
Unless otherwise indicated, data are expressed as a range of minimum–maximum (mean ± SD).
Ethnic: C = Chinese, M = Malay, I = Indian, O = Other.
Data was missing for one normal control.
WRAT3: wide range achievement test (3rd edition) was estimated for the IQ evaluation.
The antipsychotic medication dosage was converted to daily chlorpromazine milligram equivalents according to Woods [2003].
The positive and negative syndrome scale (PANSS) [Kay et al., 1987] was used to assess the psychopathology and symptom severity.
To construct the functional connectivity matrix, we employed a previously validated automatically labeled template (i.e., automated anatomical labeling [AAL]) to parcellate the brain into 90 regions of interest (Fig. 1A, and Supporting Information Table I) [Tzourio‐Mazoyer et al., 2002]. The cerebellum was excluded from analysis due to inconsistent sampling across participants during data acquisition. The representative time series of each ROI was obtained by averaging the time series of each voxel within that region (Fig. 1B). Then the averaged regional time series of all pairs of regions were correlated, taken as the level of functional coupling and the correlation coefficient was included in the functional connectivity matrix. A Fisher's‐Z transformation was further applied to the correlation matrices to improve the normality of the correlation coefficients. To de‐noise spurious interregional correlations, we performed univariate tests for each elements of the functional connectivity matrices for both groups and retained those with corresponding p‐values that passed a statistical threshold (P < 0.01, false discovery rate (FDR)‐corrected) [Wang et al., 2013]. Given the ongoing debate of the physiological meaning of negative correlations [Anderson et al., 2011; Chang and Glover, 2009], the elements of significantly negative correlations were set to zero.
Figure 1.
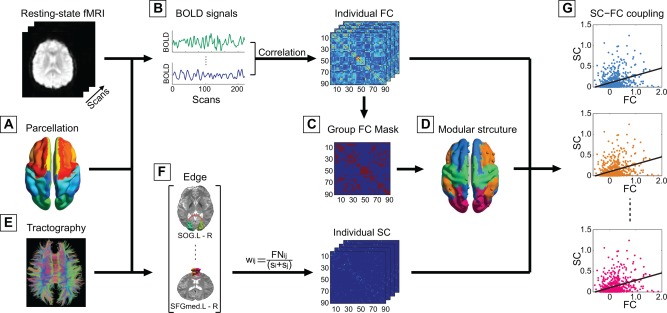
The flowchart of the analytical steps. (A) Automated anatomical labeling template was used to parcellate the brain into 90 distinct brain regions (the names and abbreviations of the brain regions, see Supporting Information Table SI), which also served as nodes of the individual brain networks. (B) Functional connectivity between pairs of nodes was computed as their level of correlation between the averaged BOLD time series within each region, resulting in the individual functional connectivity matrix (FC). (C) A representative functional brain network was generated for each group through averaging the individual connectivity matrix where only significantly (P < 0.01, FDR‐corrected) positive connections were retained. (D) A modified greedy optimization algorithm was adopted for community detection. (E) Streamline tractography was applied to the diffusion tensor imaging (DTI) data to reconstruct cortico‐cortical white matter pathways. (F) Edge weight of the structural connectivity (SC) was estimated by streamline density to compensate for the effect of different size of the ROIs. (G) The level of coupling between FC and SC was examined in each of the obtained module through computing the levels of correlation between the weights of FC and SC. The results were referred to as the level of SC–FC coupling, which would be statistically compared between both groups using permutation testing. [Color figure can be viewed at http://wileyonlinelibrary.com]
Structural Data Preprocessing and Network Construction
Structural data preprocessing and brain anatomical network construction were conducted using FMRIB Software Library (FSL, 5.0) [Smith et al., 2004], diffusion toolkit [Wang et al., 2007], PANDA (a pipeline tool for diffusion MRI analysis) [Cui et al., 2013] and has been described in detail previously [Sun et al., 2015, 2016]. Briefly, the two diffusion weighted images (DWIs) were realigned and corrected for simple head motion and eddy current distortions [Jenkinson et al., 2002]. The six independent components of the diffusion tensor were estimated, from which fractional anisotropy (FA, a measure of the directional coherence for white matter tracts) was calculated. A widely used deterministic streamline tracking algorithm was subsequently performed to obtain the whole‐brain tractography (Fig. 1E) [Mori et al., 1999]. The tracking procedure started from the deep white matter regions and terminated at a voxel with an FA < 0.15 or reached a voxel with turning angle greater than 45°. In order to enable estimation and direct comparison of multimodal coupling between the functional and structural brain networks, we employed the same AAL template to segment the entire cerebral cortex of each subject into 90 regions. Specifically, a linear transformation was applied within each subject's DTI image correlated with T1‐weighted image to coregister them to the b0 image with DTI space followed by applying a nonlinear transformation to map to the MNI template. Then, the subject‐specific AAL mask was weaved from the MNI space to the DTI native space with the corresponding inverse transformation, such that separate labeling values were maintained via nearest‐neighbor interpolation [Cui et al., 2013; Gong et al., 2009a]. Then the structural connectivity network was created through combining the collection of reconstructed fiber tracts with the individual parcellation scheme. Two ROIs were considered to be connected if the two end points of the reconstructed fiber bundles were located between them. Here edge weight of the structural connectivity network was computed as the streamline density (computed as the number of streamlines normalized by the volumes of the two interconnected ROIs at individual native space) to account for different size of the ROIs (Fig. 1F) [Buchanan et al., 2014]. To reduce the influence of pseudoconnections due to possible noise effect on the whole‐brain tractography, a predefined threshold (>3 fibers) was chosen: only pairs of regions with more than 3 fibers were considered connected [Shu et al., 2011]. Moreover, as mentioned in some previous studies [Gong et al., 2009a; Wang et al., 2012], fiber bundles may be chosen erroneously if too many white matter voxels were contained in an AAL template that are not truly adjacent to the cortex. To address this issue and meanwhile reduce the possibility of excluding the fiber tracts that actually belong to the specific AAL region, we employed a simple method introduced by Gong and colleagues [Gong et al., 2009a], that is, remove white matter voxels in the raw AAL template if no cortical voxels existed within their 2 mm cubic neighborhood.
Modularity
Modularity is one of the most fundamental and intriguing properties and has been revealed in many biological networks [Bullmore and Sporns, 2009; Hartwell et al., 1999]. In a network with modular structure, nodes in the same module are densely connected to each other, while nodes in different modules are sparsely connected [Newman and Girvan, 2004; Radicchi et al., 2004]. Modularity reflects how well the network can be delineated into communities and modular analysis aims at finding a specific partition that yields the maximum modularity.
Specifically, the modularity value QW of a weighted network G with N nodes for a given partition is defined as the proportion of G's edges that fall within modules, subtracted by the proportion that would be expected due to random chance alone [Newman and Girvan, 2004]:
| (1) |
where lW is the sum of all weights in the network, computed as , is the strength of node i, computed as , mi is the module containing node i, and is 1 if node i and j are in the same module and 0 otherwise (ensure that only intra‐modular edges are added to the sum). QW values range from 0 to 1 with a strongly modular network having QW value close to 1 and a network without modular organization having QW value close to 0. It is generally accepted that maximum values of QW ≥ 0.3 are indicative of non‐random community structure [Newman and Girvan, 2004]. Modular analysis is to find a specific module partition that yields the maximum modularity. Here, a widely employed modified greedy optimization algorithm was adopted to detect the modular partition of the group representative brain functional network [Chen et al., 2011]. The advantage of this algorithm is that it takes into account of the heterogeneity of module size normally observed in real networks [Danon et al., 2006].
In previous studies, researchers showed that employing a high sparsity threshold (including more connections) would generate graphs with low modularity equivalent to a random graph, while utilizing a low sparsity threshold (including fewer connections) would lead to disconnected graphs where some regions were isolated [Meunier et al., 2009]. Because there is no definitive way to select a single threshold, therefore in the current study, modularity of functional brain network for each participant was initially estimated over a sparsity (sparsity = number of actual edges/number of all possible edges) range of 5 – 20% (Supporting Information Fig. S2) to focus on fully‐connected but non‐random aspects of brain network organization. In order to reduce the dependency of any significant differences in the modularity (QW) on the arbitrary choice of a single threshold, an integrated network metric was estimated for the modularity over the predefined sparsity range [Achard and Bullmore, 2007; He et al., 2009]. Mathematically, the integrated modularity corresponds to the areas under the curve (AUC). Subsequently, statistical analysis was conducted on the integrated values to inspect the presence of between‐group difference (Supporting Information Fig. S3).
Modular Structure and Regional Role Assignment
In order to quantitatively assess the schizophrenia‐related disruption of modular topology and ensure the comparability needed to evaluate the between‐group differences of the structure‐function coupling, we performed modular analysis on the group‐level functional brain network, which was constructed through averaging the obtained functional connectivity network within each group [Meunier et al., 2009; Shin et al., 2014]. Hence, more fine‐grained analysis of the community structure was subsequently performed at a specific sparsity threshold 10% in the current study, where we could capture the network backbone underlying the modular organization of the most sparse network and at the same time maintain the connectedness (the ability for each node to reach other nodes in the network) for both groups (more than half of the participants within each group are fully connected) (Supporting Information Fig. S2). Once a modular structure has been obtained, we could further distinguish the roles of nodes in terms of their intra‐ and inter‐module connectivity patterns. Here we adopted two additional network metrics, i.e., nodal efficiency ( ) [Achard and Bullmore, 2007] and participation coefficient (PC(i)) [Guimera et al., 2005; Guimera and Nunes Amaral, 2005].
Nodal efficiency was originally proposed by [Achard and Bullmore, 2007] as a measure that quantify the importance of each node for the communication within the network [Gong et al., 2009b]. In the current study, this measure was employed within each module to assess the intra‐module connectivity. For a given network GM with NM nodes, is defined as the inverse of the harmonic mean of the shortest path length between this node and all other nodes [Achard and Bullmore, 2007]:
| (2) |
where Lij is the path length between node i and j and is defined as the reciprocal of the edge weight (Lij = 1/wij), is the shortest path length between node i and j. Of note, is estimated within the module where node i belongs to. A node with high indicates great interconnectivity with other regions within the module. Region i was considered as a hub if was at least 1 standard deviation higher than the average of the metrics within the module ( > mean + S.D.).
The participation coefficient (PC(i)) measures the inter‐module connectivity of a node i and is defined as [Guimera et al., 2005; Guimera and Nunes Amaral, 2005]:
| (3) |
where ki is the total number of edges linking to node i, kim is the number of edges linking node i to other nodes in the module m, and M is the total number of modules. PC(i) ranges from 0 to 1, that is, if node i has a homogeneous connection distribution with all the modules PC(i) will be close to 1; if node i is linked exclusively to other nodes in its own module, PC(i) is 0. In terms of PC(i), node i would be characterized as a connector node if PC(i) > 0.25 and node i with PC(i) ≤0.25 is classified as a provincial node.
Based upon these two network metrics, we could assign roles to the brain regions according to the following criteria: (1) connector hub with > mean + S.D. and PC(i) > 0.25; (2) connector node with ≤imean + S.D. and PC(i) > 0.25; (3) provincial hub with > mean + S.D. and PC(i) ≤0.25; and (4) provincial node with ≤imean + S.D. and PC(i) ≤0.25.
Modular Characteristics
Based upon the topological roles of brain regions within each module, we further investigate the modular characteristics differences in the brain networks between both groups. The modular characteristics could be defined in terms of the proportion of connector nodes within the module and the number of links that connect it to each other module in the network. Specifically, in the group representative network, connector coefficient of each module was calculated as the ratio between the number of connector nodes and the number of all nodes within the module. A module could be defined as connector module if it has a high connector coefficient (connector coefficient > 0.6) and a high ratio of intermodule connections (> 1/number of modules) [Guimera et al., 2005; Guimera and Nunes Amaral, 2005; Meunier et al., 2009].
Intra‐modular connectivity strength of a particular module is another measurement to assess the significance of the module within the whole network [Shin et al., 2014] and it is calculated as the mean of all edge weights within the module. Here, the intra‐module connectivity strength was estimated for each participant and group differences were statistically assessed using the non‐parametric permutation test (10,000 permutations).
SC–FC Coupling
Once the modular structure has been obtained, a correlation analysis was performed within each module between the strength of the structural connections and their functional counterparts. In one recent review, Damoiseaux and Greicius showed that the strength of resting‐state functional connectivity is positively correlated with structural connectivity strength; more importantly, functional connectivity is also observed between regions where there is little or no direct structural connectivity [Damoiseaux and Greicius, 2009]. Therefore in the current study, the entries of the functional connections within each module were selected and correlated with their counterparts in the structural connectivity matrices for each participant (regardless of whether structural connections are present or not), resulting in a single SC–FC coupling metric for each module of each participant (Fig. 1G) [Honey et al., 2009; Van den Heuvel et al., 2013]. In addition, to validate the reproducibility of the module‐dependent abnormal SC–FC coupling in schizophrenia, we performed additional SC–FC coupling estimation between the nonzero entries of the structural connections (i.e., only considering the node pairs where structural connections were present) and their functional counterparts within each module.
The SC strengths produced by the streamline density were exponentially distributed and spanned several orders of magnitude. To control the distribution of SC strengths, we performed additional SC–FC coupling analysis via rescaling the SC weights to a Gaussian distribution. Specifically, for a given structural connectivity network GSC with X edges , we generated X random samples from a Gaussian distribution and replaced the original edge weight with the randomly sampled value according to the weight rank [Honey et al., 2009; Van den Heuvel et al., 2013]. This produced a set of X resampled data values distributed according to a standard Gaussian, which we rescaled to a mean of 0.5 and a standard deviation of 0.1 dimensionless units [Honey et al., 2009]. In the current study, the empirical results (without rescaling SC) were presented as the main observation. We found that rescaling did not change the nature of our findings (Supporting Information Results and Supporting Information Fig. S4).
High‐Resolution Brain Network
Recent studies have continuously suggested that different parcellation schemes might lead to different properties of brain networks [De Reus and van den Heuvel, 2013]. Several recent studies have suggested the use of higher‐resolution networks, of up to 1,000 smaller ROIs, instead of using a coarse parcellation scheme (Supporting Information Fig. S1A) [Hagmann et al., 2008; Zalesky et al., 2010]. Therefore, in the current work, a high‐resolution parcellation scheme was adopted to further assess the abnormal SC–FC coupling of modular structure in schizophrenia. Specifically, the high‐resolution parcellation scheme was obtained through subdividing the coarse AAL template into 1024 ROIs with equal size (Supporting Information Fig. S1B) according to the approach introduced in Zalesky et al. [2010]. The network construction procedures and SC–FC coupling estimations were repeated for high‐resolution parcellation scales.
Statistical Analysis
Here, a nonparametric permutation test [Nichols and Holmes, 2002] was used to investigate the differences of the modularity and SC–FC coupling of modular structure between the healthy volunteers and the patients with schizophrenia. Briefly, for SC–FC coupling of each module, the between‐group difference of the mean values was initially calculated. An empirical null distribution of the difference was then obtained by randomly assigning all of the values into two groups (with group sizes intact) for 10,000 times and computing the mean differences between the two randomized groups in each iteration. Threshold level for establishing the significant difference was set at the 95% point of the empirical distribution in a two‐tailed test of whether the observed differences could occur by chance. To address the problem of multiple comparisons, effects were also tested on whether they survived a Bonferroni correction. All statistical analyses were performed using the SPSS 17 software.
Relationship between the SC–FC coupling scores and clinical variables were also explored in the patient group. Specifically, multiple linear regressions were employed and implemented in the statistical package, R (http://www.r-project.org/) in the current study, with the covariates of age, gender, and age‐by‐gender interaction. Because these analyses were exploratory in nature, correction for multiple comparisons was not applied, and an uncorrected P‐value of 0.05 was considered for establishment of a significant relationship.
RESULTS
Altered Modular Topology of Functional Network in Schizophrenia
For both groups, maximum modularity declined monotonically as a function of increasing sparsity where maximum modularity was revealed for the most sparse yet fully connected networks considered (Supporting Information Fig. S2). Of note, a community structure would be considered as nonrandom community if its modularity fulfills QW ≥ 0.3 [Newman and Girvan, 2004]. In the current work, the functional brain networks in both groups consistently exhibited a modular community structure (QW > 0.4 over the predefined sparsity band) (Supporting Information Fig. S3). Compared with healthy controls, significant reduction of the integrated maximum modularity over the entire sparsity range (corresponding to the areas under the metric curve, AUC(QW)) was revealed (P = 0.041, 10,000 permutations) (Supporting Information Fig. S3A).
More fine‐grained analysis was subsequently performed on the community structure arising in both groups at a sparse connection density (i.e., 10%), where we were able to capture the network backbone underlying the modular organization of the most sparse network, yet maintain the connectedness for both groups (Supporting Information Fig. S2A). The healthy functional brain network comprised five connected modules, designated as the default mode module (Module I, including 24 regions), the occipital module (Module II, 16 regions), the subcortical module (Module III, 16 regions), the frontoparietal module (Module IV, 14 regions), and the central module (Module V, 20 regions). The same five modules were revealed in the patient group with minor differences in relative size and topological role profile of the brain regions (Fig. 2, Supporting Information Table III and Supporting Information results). Interestingly, Module IV was identified as connector module for both groups as it exhibited both great connector efficient (>0.6) and high intermodule connections (>1/(number of modules)) (Table 2, Supporting Information results).
Figure 2.
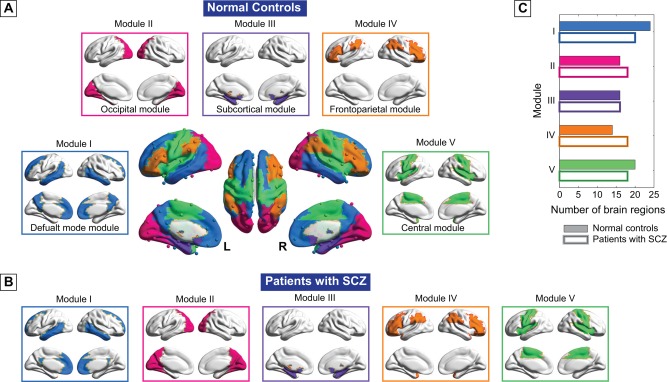
Modular structure of group representative brain functional network for both (A) normal controls and (B) patients with schizophrenia at sparsity level of 10%. The number of brain regions within each module was presented in (C). Five functionally interconnected modules were identified in both groups, including the default‐mode module (Module I), the occipital module (Module II), the subcortical module (Module III), the frontoparietal module (Module IV), and the central module (Module V). [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 2.
The topological roles of the modules in normal controls and patients with schizophrenia
| Normal controls | Patients with schizophrenia | |||||
|---|---|---|---|---|---|---|
| Module | Number of regions | Connectors | Intermodule | Number of regions | Connectors | Intermodule |
| I | 24 | 7 (0.292) | 26 (0.228) | 20 | 10 (0.500) | 28 (0.241) |
| II | 16 | 3 (0.188) | 9 (0.079) | 18 | 5 (0.278) | 12 (0.103) |
| III | 16 | 7 (0.438) | 16 (0.140) | 16 | 4 (0.250) | 14 (0.121) |
| IV | 14 | 11 (0.786) | 36 (0.316) | 18 | 13 (0.722) | 37 (0.319) |
| V | 20 | 7 (0.350) | 27 (0.237) | 18 | 5 (0.278) | 25 (0.216) |
| Total | 90 | 35 | 57 | 90 | 37 | 58 |
Here, the community structure was estimated at a specific sparsity of 10%. Values in the “Number of regions” column are the region number within each module, corresponding to Figure 2(C). The “Connectors” columns represent the number of connector nodes in each module and its ratio with respect to the total number of brain regions within the module; the values in the “Intermodule” column indicate the numbers of intermodule connections and its ratio to the total number of intermodule connections in all modules. Modules with both great connector coefficient (>0.6) and high intermodule connections (>1/(number of modules)) were considered as connector‐module [Wu et al., 2012].
In addition, significant group effects were revealed in the intra‐modular functional connectivity strength for three modules. Specifically, compared with healthy controls, a significant hyper‐connectivity (P = 0.003*, 10,000 permutations, * indicates statistical results that survived Bonferroni correction) was found in the occipital module, whereas the intra‐modular connectivity was significantly reduced in the subcortical module (P = 0.006*, 10,000 permutations) and the frontoparietal module (P = 0.001*, 10,000 permutations) in patients. No statistically significant difference was observed in the intra‐modular structural connectivity strength between both groups (P > 0.05, 10,000 permutations) (Supporting Information Fig. S5).
Abnormal SC–FC Coupling in Schizophrenia
The SC–FC coupling within each module exhibited different patterns between both groups (Fig. 3). Specifically, the SC–FC coupling was found to be significantly increased in the default mode module (NC vs. SCZ: 0.336 ± 0.014 vs. 0.370 ± 0.011 (mean ± standard error), P = 0.032, 10,000 permutations) and the central module (NC vs. SCZ: 0.224 ± 0.010 vs. 0.281 ± 0.012, P < 0.001*, 10,000 permutations) in patients relative to controls. In addition, a significant decline of SC–FC coupling was observed in the occipital module (NC vs. SCZ: 0.420 ± 0.017 vs. 0.365 ± 0.015, P = 0.012, 10,000 permutations) and the subcortical module (NC vs. SCZ: 0.243 ± 0.019 vs. 0.176 ± 0.017, P = 0.007*, 10,000 permutations) for patients with schizophrenia. Additional analyses were performed to account for the potential confounding effects of age, gender, years of educations, and medication dosage on the observed between‐group differences of SC–FC coupling. These confounds were included as linear regressors and the differences of the residuals between both groups were statistically assessed using permutation test. Results indicated that the reported SC–FC coupling differences were not explained by variance related to any of these factors. Moreover, in our validation analysis of SC–FC coupling with only present structural connections, we found the same module‐dependent disruption of SC–FC coupling in patients with schizophrenia (Supporting Information Results and Supporting Information Fig. S6).
Figure 3.
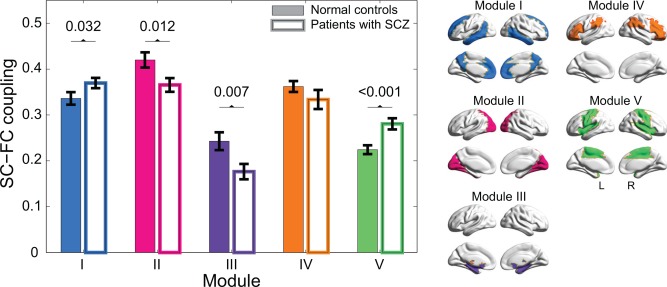
SC–FC coupling in each module of normal controls (color‐filled bar) and patients with schizophrenia (unfilled bar). Bars represent mean ± standard error of mean. Each horizontal line and the associated number represent the p‐value of a permutation test on the SC–FC coupling parameters. Compared with normal controls, significantly higher SC–FC couplings were observed in Module I (P = 0.032, 10,000 permutations) and V (P < 0.001*, 10,000 permutations, * indicates statistical results that survived Bonferroni correction), whereas a significantly lower SC–FC coupling was revealed in Module II (P = 0.012, 10,000 permutations) and III (P = 0.007*, 10,000 permutations) for patients with schizophrenia. The modular structure was also presented in the right panel for ease of interpretation. [Color figure can be viewed at http://wileyonlinelibrary.com]
Relationship Between SC–FC Coupling and Clinical Features
Within the schizophrenia group, the SC–FC coupling scores of the occipital module correlated significantly with the illness duration (SC–FC (MII), r = −0.629, P = 0.009) and PANSS overall symptoms (r = −0.548, P = 0.028). That is the lower SC–FC coupling scores in the occipital module, the longer illness duration and more severe overall psychopathology. In addition, the SC–FC coupling scores of the subcortical module also exhibited a significantly negative correlation with the PANSS overall symptoms (SC–FC (MIII), r = −0.509, P = 0.044). A trend of significantly negative association between the SC–FC coupling scores of the subcortical module and illness duration was also revealed (r = −0.488, P = 0.055) (Table 3).
Table 3.
Partial correlation coefficients between the SC–FC coupling scores and clinical characteristics of patients with schizophrenia at low resolution
| SC–FC coupling | Partial correlation coefficients (P‐value) | ||||
|---|---|---|---|---|---|
| Duration | PANSS positive | PANSS negative | PANSS general | PANSS overall | |
| MI | 0.089 (0.744) | 0.049 (0.856) | −0.146 (0.588) | 0.440 (0.088) | 0.328 (0.214) |
| MII | −0.629 (0.009) | −0.153 (0.571) | −0.433 (0.094) | −0.460 (0.073) | −0.548 (0.028) |
| MIII | −0.488 (0.055) | −0.019 (0.944) | −0.458 (0.074) | −0.451 (0.079) | −0.509 (0.044) |
| MIV | 0.089 (0.744) | 0.434 (0.092) | 0.098 (0.718) | 0.091 (0.739) | 0.145 (0.592) |
| MV | −0.030 (0.912) | 0.162 (0.549) | −0.282 (0.290) | 0.221 (0.411) | −0.002 (0.994) |
Note: the partial correlation coefficients were estimated via multiple linear regressions with age, gender, and age‐by‐gender interaction as covariates. Significant correlations (P < 0.05, uncorrected) were indicated by the bold text. MI–MV indicate Module I to Module V (see Fig. 2).
PANSS: positive and negative syndrome scale.
Results of High‐Resolution Network
Similar to the patterns observed in the low‐resolution brain network, the maximum modularity obtained in the high‐resolution network analysis declined as a function of increasing of sparsity (Supporting Information Fig. S3). Significant group difference (P = 0.031, 10,000 permutations) of the integrated modularity (AUC(QW)) was also revealed.
Again, the same sparsity (i.e., 10%) was employed for the high‐resolution brain network to perform more fine‐grained analysis on the community structure. The healthy brain network comprised 6 connected modules, where most of them (4 of 6) were also identified in the low‐resolution network analysis, including the default mode module (Module I), the occipital module (Module II), the subcortical module (Module III), and the frontoparietal module (Module IV). The central module revealed in the low‐resolution network was separated into two modules, designated as the central module (Module V) and the cingulo‐opercular module (Module VI) (Supporting Information Results and Supporting Information Fig. S7). The same 6 modules were revealed in the patient group. Interestingly, the frontoparietal module and the cingulo‐opercular module were identified as connector module in normal controls, whereas only the frontoparietal module exhibited properties for connector module in patients group (Supporting Information Table III). Additional statistical analysis of SC–FC coupling revealed significantly higher SC–FC scores in the default mode module (NC vs. SCZ: 0.124 ± 0.006 vs. 0.167 ± 0.006 (mean ± standard error), P < 0.001*, 10,000 permutations) and the cingulo‐opercular module (NC vs. SCZ: 0.162 ± 0.008 vs. 0.195 ± 0.004, P < 0.001*, 10,000 permutations) in patients with schizophrenia, while significantly reduced SC–FC coupling was discovered in the occipital module (NC vs. SCZ: 0.213 ± 0.009 vs. 0.204 ± 0.005, P = 0.037, 10,000 permutations) (Supporting Information Fig. S8). These observations were comparable with the results of the low‐resolution analyses.
DISCUSSION
In this study, using multimodal neuroimaging techniques, we examined the altered structural–functional coupling of brain networks at the functional modular level in patients with schizophrenia. The significant findings are as follows: first, although the optimal modular architecture was preserved, a significantly reduced modularity was revealed in functional brain networks of patients with schizophrenia; second, SC–FC coupling exhibited module‐dependent aberrations between both groups, attributing to complex alterations of intra‐modular functional connectivity in schizophrenia; third, the aberration of SC–FC coupling was correlated with the clinical features of schizophrenia. These findings are discussed in greater detail below.
Although modularity has been widely considered as one of the main organization principles of complex biological networks, it remains controversial in the brain networks, with arguments concerning the existence and emergence of the modular organization [Bullinaria, 2007]. Our findings provided further evidence to support the presence of the modular structure in the spontaneous human brain functional networks. Specifically, we identified five cohesive modules in both groups, which correspond to several well‐known functional subsystems such as default mode, visual, subcortical, frontoparietal, and motor/sensorimotor systems. Despite methodological variations, the number and organization of modules resembled what was found in previous fMRI studies of healthy participants [Bertolero et al., 2015; He et al., 2009; Meunier et al., 2009]. Among these five modules, the frontoparietal module was further classified as a connector module for having a high proportion of connector nodes and intermodular connections. Most recently, Bertolero and colleagues employed a resting‐state brain network model and investigated the modular functional architecture of the human brain by analyzing activity at different types of nodes in the network across 9,208 experiments of 77 cognitive tasks [Bertolero et al., 2015]. Like in the present study, connector nodes were shown to mainly reside in the inferior frontal and parietal areas. Conceptually, connector nodes are thought to have access to information across or coordinate connectivity between different modules; connector module is therefore implicated in a diverse range of tasks [Yeo et al., 2015]. Using task‐state connectivity methods, Cole et al., showed that the frontoparietal brain network was composed of brain regions that flexibly and rapidly altered their functional connectivity with other functionally specialized neural networks [Cole et al., 2013]. Therefore, our findings provide further neuroimaging evidence to support the notion that the frontoparietal module might play a critical role in coordinating activity across the brain network as a whole and in mediating interactions among other modules.
The first resting‐state fMRI study investigating schizophrenia‐related modularity alterations in functional brain networks was conducted by Alexander‐Bloch et al. [2010]. Using three different decomposition algorithms (including the one employed in the current study), they discovered reduced modularity in patients with childhood‐onset schizophrenia, suggesting a loss of intra‐ relative to inter‐modular connectivity. Similar observation of schizophrenia‐related modularity decline was also evidenced in their recent study using a new community partition method that is independent of anatomy or function [Alexander‐Bloch et al., 2012]. In line with these findings, significantly reduced functional modularity in the patient group with preserved modular architecture was also revealed in the present work. Contemporary theories suggested that the complex clinical presentations of schizophrenia are related to dysconnectivity between distinct brain regions and emphasized the role of the brain's integrative process—the substrate of which is connectivity—in the pathophysiology of schizophrenia [Bullmore et al., 1997; Friston, 1998; Stephan et al., 2006]. Such a concept can be alternatively described as an aberration in the efficiency of information exchange between separate neural networks/modules. Along this line, our observations of significantly reduced modularity provide further evidence for the notion of schizophrenia as a disconnection syndrome. However, our findings of relatively preserved modular architecture are unlike one recent study, which revealed more and smaller modules in patients with schizophrenia [Yu et al., 2011]. The discrepancies could stem from the following two aspects. Firstly, different functional brain network construction methods were employed, that is, functional connectivity was estimated through computing the correlations between spatially decomposed functional independent components in [Yu et al., 2011], whereas functional association was obtained using a parcellation scheme in the present study. Secondly, patients with less severe symptoms were employed in the present work in comparison with those participated in [Yu et al., 2011]. Given the paucity of research in schizophrenia‐related disrupted functional modular organization, further studies may help to reconcile such inconsistencies.
Another main observation is module‐dependent altered SC–FC coupling in schizophrenia, attributing to the complex intra‐modular functional dysconnectivity with preserved structural connectivity in the patient group. Our findings may thus converge on the notion of complex relationship between SC and FC in schizophrenia [Cocchi et al., 2014; Skudlarski et al., 2010; Van den Heuvel et al., 2013]. Specifically, an increased coupling between SC and FC in patients with schizophrenia was revealed in the default mode module (Module I) and the central module (Module V), suggesting that the functional interactions within these modules are more directly related to the underlying anatomical connectivity in schizophrenia. Of note, we did not find significantly aberrant intra‐modular functional connectivity strength within both modules in schizophrenia. While there is evidence of altered functional connectivity in the DMN and the cingulo‐opercular network (part of central module) in schizophrenia, these prior studies have typically focused on specific interregional connections and findings of these studies have been inconsistent [Greicius, 2008; Repovs et al., 2011; Tu et al., 2012; Whitfield‐Gabrieli and Ford, 2012; Whitfield‐Gabrieli et al., 2009]. Given the important role of the DMN and the cingulo‐opercular network in various cognitive tasks [Weissman et al., 2006; Whitfield‐Gabrieli et al., 2009], we speculate that the increased SC–FC coupling may be indicative of more stringent and less dynamic brain function [Van den Heuvel et al., 2013] and may represent less efficient modulation of the DMN and cingulo‐opercular network activation in patients with schizophrenia, leading to the observed cognitive deficits in attention, working memory, and language.
Furthermore, significantly reduced SC–FC coupling was shown in the occipital module (Module II) and the subcortical module (Module III) in the patient group. We found statistically lower intra‐modular connectivity strength in the subcortical module in schizophrenia. Pathology of subcortical regions (including hippocampus and thalamus) has been repeatedly implicated as robust findings in the pathogenesis of schizophrenia and relationship with various clinical manifestations [Ho et al., 2017; Nelson et al., 1998; Pergola et al., 2015; Qiu et al., 2010]. Most recently, in one volumetric study of subcortical brain aberrations in 2,028 patients with schizophrenia, Van Erp et al., revealed significantly reduced volumes of subcortical regions in patients group [Van Erp et al., 2016], thus resembling the observations in the present study. Of note, we observed abnormally high intra‐modular functional connectivity strength in the occipital module in patients with schizophrenia. This finding is consistent with one recent study where schizophrenia‐related higher degree centrality of functional connectivity was revealed in the visual processing regions [Palaniyappan and Liddle, 2014]. Using a direct three‐dimensional counting method, Selemon and colleagues also found increased neuronal density in the occipital area in the schizophrenia brain [Selemon et al., 1995]. We speculate that the functional hyper‐connectivity and decoupled SC–FC association in the occipital module may indicate impairment of early cortical processing in schizophrenia involving the visual cortex [Butler and Javitt, 2005; Butler et al., 2008; Ford, 1999; Seymour et al., 2013]. Importantly, the significant decoupling in the occipital module and the subcortical module was associated with increased illness duration and greater severity of psychopathology of schizophrenia. The findings suggest that the SC–FC coupling in the occipital module and the subcortical module may be related to the severity and progression of the long‐term impairment in patients.
It is noteworthy to mention that in their study of schizophrenia‐related disruption of structure‐function coupling, Cocchi and colleagues revealed a general trend of reduction in both functional and structural connectivity in a subnetwork that encompassed the frontal, thalamic and striatal regions, which led to a reduced structure‐function coupling in schizophrenia [Cocchi et al., 2014]. Similar deficits in fronto‐striatal coupling have been consistently found in schizophrenia [Fornito et al., 2013; Fusar‐Poli et al., 2010]. However, our additional analysis did not reveal significant between‐group differences in the inter‐modular connectivity strength between the subcortical module and fronto‐parietal module. The discrepancies could stem from the different analytical frameworks employed in the current study and previous work. According to [Cocchi et al., 2014], a whole brain‐wide bivariate statistical comparison and network‐based statistic were initially employed to identify subnetworks with significant between‐group differences in functional connectivity. The obtained subnetworks were then used as the template for selective structural connectivity construction and structure‐function coupling estimation [Cocchi et al., 2014]. Given the primary focus of the current study was to investigate the schizophrenia‐related abnormal SC–FC coupling within specific modules, modular architecture was employed to parcellate the brain into functional modules that were utilized as the template for estimating SC–FC coupling and calculating inter‐module connectivity strength. Modular analyses may not be sensitive to reveal altered fronto‐striatal coupling that is detectable with bivariate statistics. Further studies may help to reconcile such apparent inconsistencies.
Some issues should be taken into account when interpreting our results. Firstly, previous neuroimaging studies of schizophrenia have reported pharmacological changes in localized brain regions and connections [Ahmed et al., 2015; Ho et al., 2011]. However, evidences pertaining to the association between network measurements and medication dosages are not entirely consistent [Liu et al., 2008; Rubinov et al., 2009]. In fact, Rubinov et al., suggested that medication is unlikely to be a confounding factor and may, on the contrary, exert a normalizing influence [Rubinov et al., 2009]. Reports of connectivity disturbances in patients' unaffected siblings [Liu et al., 2012; Repovs et al., 2011] further suggest that at least some network connectivity abnormalities may reflect an inherited susceptibility to the disease. These studies therefore lead us to believe that the observed between‐group differences may reflect the intrinsic disease process rather than the effects of direct pharmacological treatment. Secondly, the widely used AAL template was used for the network construction to maximize the number of existing studies with which our results could be directly compared without the need to determine a template‐to‐template mapping between discordant regional definitions. However, regions on the template differ in size, which may introduce a confounding effect on the link weight among the network nodes [Wang et al., 2012]. Furthermore, it has been previously demonstrated that a large anatomical region might have heterogeneous resting‐state fMRI signals [Smith et al., 2011]. To address these issues, we further performed a high‐resolution parcellation scheme (with equal size ROIs) for network construction [Zalesky et al., 2010] and employed a streamline density approach to account for different sizes of the ROIs [Buchanan et al., 2014]. The network analysis of modular structure and the following SC–FC coupling investigations yielded comparable results between both parcellation schemes. Nonetheless, there were some discrepancies which may result from differences in the graph properties under the different node scales [De Reus and van den Heuvel, 2013; Zalesky et al., 2010]. The choice of the most accurate representation of the underlying neurobiological connectivity [Jones et al., 2013] and the optimal template resolution for patient studies remains an open question. New advances in functional parcellation approaches [Power et al., 2011; Yeo et al., 2011] and edge weighting methods, examining disrupted structural–functional coupling across different resolutions, are therefore of interest. Thirdly, a computational inexpensive deterministic tractography method was adopted for structural network construction [Mori et al., 1999]. Due to limitations in the model that is used to infer fiber bundle orientation, DTI has difficulty in detecting crossing fiber bundles, which may hinder the tracking algorithm from correctly tracing fiber streamlines [Jones et al., 2013]. This may in turn result in an underrepresentation of the number of connections of the structural network (false negatives) with reduced connectome sensitivity. We assessed the credibility of our tracking results in Supporting Information materials by showing 7 well‐known WM fiber bundles (including 2 short WM tracts and 5 major tracts) from 3 randomly selected subjects (Supporting Information Fig. S9) and found that the reconstructed fiber bundles were faithful to the human WM anatomy from previous studies [Gong et al., 2009a; Li et al., 2009]. Although probabilistic tractography method is advantageous in overcoming the fiber crossing problem [Behrens et al., 2007], recent research has indicated that such method would yield dense connectomes with increased false positive connections and reduced specificity of connectome constructions [Zalesky et al., 2016]. According to [Zalesky et al., 2016], connectome specificity is at least twice as important as connectome sensitivity with respect to analysis of the topological properties of brain networks. In this study, our credible tracking results suggested that a single tensor model and a deterministic tracking method might be the optimal choice. Nonetheless, further studies with cautious application of advanced probabilistic fiber tracking methods and crossing‐fiber models to high‐quality data is recommended to confirm our observations. Fourthly, although global signal regression (GSR) is among the most commonly used techniques in resting‐state fMRI filed, there is still an ongoing controversy as to the removal of the global brain signal since it could lead to ambiguous interpretations of the biological mechanisms of correlations [Fox et al., 2009; Murphy et al., 2009; Power et al., 2014; Saad et al., 2012]. Specifically, criticisms center around the potential for GSR to artifactually generate negative correlations [Murphy et al., 2009], which might alter the interregional correlation patterns across participants [Fox et al., 2009; Saad et al., 2012]. However, the findings of the influence of GSR on group comparisons are not entirely consistent [Gotts et al., 2013; Tyszka et al., 2014]. In one recent work, Power and colleagues demonstrated empirical evidence that global signal regression is highly effective in removing artifactual variance in resting‐state functional connectivity data and suggested that the empirical benefits outweigh the theoretical costs of GSR, that is, motion‐related group differences are 1–2 order of magnitude less with than without GSR [Power et al., 2014]. Moreover, to test the influence of GSR on our reported aberrations of module‐dependent SC–FC coupling, an additional analysis was performed, in which GSR was left out. Similar to the main findings, the same module‐dependent SC–FC disruptions were found in patients (Supporting Information Fig. S10). Finally, as our study is one of the first exploratory investigations of abnormal structure‐function coupling at modular level in schizophrenia, an un‐corrected P‐value of 0.05 was employed for establishing the significance and presenting the results. It is possible that some of the results may have occurred by chance and some caution is needed when interpreting these results. In this work, we mainly focused on the interpretation of the patterns of the findings and highlighted those that survived correction for multiple comparisons for reader's interpretation. Considering the small number of subjects used in this study, future studies using a larger independent study sample and hypothesis‐driven study design are expected.
In summary, using multimodal brain connectome, we investigated the altered structural–functional coupling at functional modular level in schizophrenia. Although patients exhibited the optimal modular architecture in functional brain networks, module‐dependent aberrations of SC–FC coupling were revealed in schizophrenia, which were correlated with clinical features of the illness. These findings provide evidence to support the notion that altered SC–FC coupling may underlie abnormal brain function and clinical symptoms observed in schizophrenia [Cocchi et al., 2014; Skudlarski et al., 2010; Van den Heuvel et al., 2013]. When replicated in larger samples, our findings will potentially lead to new neural biomarkers for diagnosis and severity evaluation of schizophrenia as well as understanding the pathophysiologic mechanisms of the disease.
Supporting information
Supporting Information
The authors declared no potential conflict of interests with respect to the authorship and publication of this article.
REFERENCES
- Achard S, Bullmore E (2007): Efficiency and cost of economical brain functional networks. PLoS Comput. Biol 3:e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed M, Cannon DM, Scanlon C, Holleran L, Schmidt H, McFarland J, Langan C, McCarthy P, Barker GJ, Hallahan B, McDonald C (2015): Progressive brain atrophy and cortical thinning in schizophrenia after commencing clozapine treatment. Neuropsychopharmacology 40:2409–2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander‐Bloch AF, Gogtay N, Meunier D, Birn R, Clasen L, Lalonde F, Lenroot R, Giedd J, Bullmore ET (2010): Disrupted modularity and local connectivity of brain functional networks in childhood‐onset schizophrenia. Front Syst Neurosci 4:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander‐Bloch A, Lambiotte R, Roberts B, Giedd J, Gogtay N, Bullmore E (2012): The discovery of population differences in network community structure: new methods and applications to brain functional networks in schizophrenia. NeuroImage 59:3889–3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JS, Druzgal TJ, Lopez‐Larson M, Jeong EK, Desai K, Yurgelun‐Todd D (2011): Network anticorrelations, global regression, and phase‐shifted soft tissue correction. Hum Brain Mapp 32:919–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ (2005): Unified segmentation. NeuroImage 26:839–851. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW (2007): Probabilistic diffusion tractography with multiple fibre orientations: What can we gain?. NeuroImage 34:144–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolero MA, Yeo BT, D'Esposito M (2015): The modular and integrative functional architecture of the human brain. Proc Natl Acad Sci U S A 112:E6798–E6807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan CR, Pernet CR, Gorgolewski KJ, Storkey AJ, Bastin ME (2014): Test‐retest reliability of structural brain networks from diffusion MRI. NeuroImage 86:231–243. [DOI] [PubMed] [Google Scholar]
- Bullinaria JA (2007): Understanding the emergence of modularity in neural systems. Cogn Sci 31:673–695. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Sporns O (2009): Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci 10:186–198. [DOI] [PubMed] [Google Scholar]
- Bullmore ET, Frangou S, Murray RM (1997): The dysplastic net hypothesis: an integration of developmental and dysconnectivity theories of schizophrenia. Schizophr Res 28:143–156. [DOI] [PubMed] [Google Scholar]
- Butler PD, Javitt DC (2005): Early‐stage visual processing deficits in schizophrenia. Curr Opin Psychiatry 18:151–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler PD, Silverstein SM, Dakin SC (2008): Visual perception and its impairment in schizophrenia. Biol Psychiatry 64:40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camchong J, MacDonald AW, 3rd , Bell C, Mueller BA, Lim KO (2011): Altered functional and anatomical connectivity in schizophrenia. Schizophr Bull 37:640–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canu E, Agosta F, Filippi M (2015): A selective review of structural connectivity abnormalities of schizophrenic patients at different stages of the disease. Schizophr Res 161:19–28. [DOI] [PubMed] [Google Scholar]
- Chang C, Glover GH (2009): Effects of model‐based physiological noise correction on default mode network anti‐correlations and correlations. NeuroImage 47:1448–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZJ, He Y, Rosa‐Neto P, Gong G, Evans AC (2011): Age‐related alterations in the modular organization of structural cortical network by using cortical thickness from MRI. NeuroImage 56:235–245. [DOI] [PubMed] [Google Scholar]
- Cocchi L, Harding IH, Lord A, Pantelis C, Yucel M, Zalesky A (2014): Disruption of structure‐function coupling in the schizophrenia connectome. NeuroImage Clin 4:779–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Reynolds JR, Power JD, Repovs G, Anticevic A, Braver TS (2013): Multi‐task connectivity reveals flexible hubs for adaptive task control. Nat Neurosci 16:1348–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Z, Zhong S, Xu P, He Y, Gong G (2013): PANDA: a pipeline toolbox for analyzing brain diffusion images. Front Hum Neurosci 7:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Greicius MD (2009): Greater than the sum of its parts: a review of studies combining structural connectivity and resting‐state functional connectivity. Brain Struct Funct 213:525–533. [DOI] [PubMed] [Google Scholar]
- Danon L, Diaz‐Guilera A, Arenas A (2006): The effect of size heterogeneity on community identification in complex networks. J Stat Mech‐Theory E 68:P11010. [Google Scholar]
- David AS (1994): Dysmodularity: a neurocognitive model for schizophrenia. Schizophr Bull 20:249–255. [DOI] [PubMed] [Google Scholar]
- De Reus MA, van den Heuvel MP (2013): The parcellation‐based connectome: limitations and extensions. NeuroImage 80:397–404. [DOI] [PubMed] [Google Scholar]
- Ellison‐Wright I, Bullmore E (2009): Meta‐analysis of diffusion tensor imaging studies in schizophrenia. Schizophr Res 108:3–10. [DOI] [PubMed] [Google Scholar]
- Endicott J, Spitzer RL, Fleiss JL, Cohen J (1976): The global assessment scale. a procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry 33:766–771. [DOI] [PubMed] [Google Scholar]
- Ford JM (1999): Schizophrenia: the broken P300 and beyond. Psychophysiology 36:667–682. [PubMed] [Google Scholar]
- Fornito A, Zalesky A, Pantelis C, Bullmore ET (2012): Schizophrenia, neuroimaging and connectomics. NeuroImage 62:2296–2314. [DOI] [PubMed] [Google Scholar]
- Fornito A, Harrison BJ, Goodby E, Dean A, Ooi C, Nathan PJ, Lennox BR, Jones PB, Suckling J, Bullmore ET (2013): Functional dysconnectivity of corticostriatal circuitry as a risk phenotype for psychosis. JAMA Psychiatry 70:1143–1151. [DOI] [PubMed] [Google Scholar]
- Fox MD, Zhang D, Snyder AZ, Raichle ME (2009): The global signal and observed anticorrelated resting state brain networks. J Neurophysiol 101:3270–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ (1998): The disconnection hypothesis. Schizophr Res 30:115–125. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R (1996): Movement‐related effects in fMRI time‐series. Magn Reson Med 35:346–355. [DOI] [PubMed] [Google Scholar]
- Fusar‐Poli P, Howes OD, Allen P, Broome M, Valli I, Asselin MC, Grasby PM, McGuire PK (2010): Abnormal frontostriatal interactions in people with prodromal signs of psychosis: a multimodal imaging study. Arch Gen Psychiatry 67:683–691. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Lalonde FM, Celano MJ, White SL, Wallace GL, Lee NR, Lenroot RK (2009): Anatomical brain magnetic resonance imaging of typically developing children and adolescents. J Am Acad Child Adolesc Psychiatry 48:465–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Lu A, Leow AD, Klunder AD, Lee AD, Chavez A, Greenstein D, Giedd JN, Toga AW, Rapoport JL, Thompson PM (2008): Three‐dimensional brain growth abnormalities in childhood‐onset schizophrenia visualized by using tensor‐based morphometry. Proc Natl Acad Sci U S A 105:15979–15984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong G, He Y, Concha L, Lebel C, Gross DW, Evans AC, Beaulieu C (2009a): Mapping anatomical connectivity patterns of human cerebral cortex using in vivo diffusion tensor imaging tractography. Cereb Cortex 19:524–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong G, Rosa‐Neto P, Carbonell F, Chen ZJ, He Y, Evans AC (2009b): Age‐ and gender‐related differences in the cortical anatomical network. J Neurosci 29:15684–15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotts SJ, Saad ZS, Jo HJ, Wallace GL, Cox RW, Martin A (2013): The perils of global signal regression for group comparisons: a case study of Autism Spectrum Disorders. Front Hum Neurosci 7:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M (2008): Resting‐state functional connectivity in neuropsychiatric disorders. Curr Opin Neurol 21:424–430. [DOI] [PubMed] [Google Scholar]
- Guimera R, Nunes Amaral LA (2005): Functional cartography of complex metabolic networks. Nature 433:895–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimera R, Mossa S, Turtschi A, Amaral LA (2005): The worldwide air transportation network: Anomalous centrality, community structure, and cities' global roles. Proc Natl Acad Sci U S A 102:7794–7799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, Sporns O (2008): Mapping the structural core of human cerebral cortex. PLoS Biol 6:e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell LH, Hopfield JJ, Leibler S, Murray AW (1999): From molecular to modular cell biology. Nature 402:C47–C52. [DOI] [PubMed] [Google Scholar]
- He Y, Wang J, Wang L, Chen ZJ, Yan C, Yang H, Tang H, Zhu C, Gong Q, Zang Y, Evans AC (2009): Uncovering intrinsic modular organization of spontaneous brain activity in humans. PLoS One 4:e5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho BC, Andreasen NC, Ziebell S, Pierson R, Magnotta V (2011): Long‐term antipsychotic treatment and brain volumes: a longitudinal study of first‐episode schizophrenia. Arch Gen Psychiatry 68:128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho NF, Iglesias JE, Sum MY, Kuswanto CN, Sitoh YY, De Souza J, Hong Z, Fischl B, Roffman JL, Zhou J, Sim K, Holt DJ (2017): Progression from selective to general involvement of hippocampal subfields in schizophrenia. Mol Psychiatry. 22:142–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey CJ, Sporns O, Cammoun L, Gigandet X, Thiran JP, Meuli R, Hagmann P (2009): Predicting human resting‐state functional connectivity from structural connectivity. Proc Natl Acad Sci U S A 106:2035–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC (2009): When doors of perception close: bottom‐up models of disrupted cognition in schizophrenia. Annu Rev Clin Psychol 5:249–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S (2002): Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage 17:825–841. [DOI] [PubMed] [Google Scholar]
- Jones DK, Knosche TR, Turner R (2013): White matter integrity, fiber count, and other fallacies: the do's and don'ts of diffusion MRI. NeuroImage 73:239–254. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA (1987): The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 13:261–276. [DOI] [PubMed] [Google Scholar]
- Kim DJ, Kent JS, Bolbecker AR, Sporns O, Cheng H, Newman SD, Puce A, O'Donnell BF, Hetrick WP (2014): Disrupted modular architecture of cerebellum in schizophrenia: a graph theoretic analysis. Schizophr Bull 40:1216–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Liu Y, Li J, Qin W, Li K, Yu C, Jiang T (2009): Brain anatomical network and intelligence. PLoS Comput Biol 5:e1000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao X, Yuan L, Zhao T, Dai Z, Shu N, Xia M, Yang Y, Evans A, He Y (2015): Spontaneous functional network dynamics and associated structural substrates in the human brain. Front Hum Neurosci 9:478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Liang M, Zhou Y, He Y, Hao Y, Song M, Yu C, Liu H, Liu Z, Jiang T (2008): Disrupted small‐world networks in schizophrenia. Brain 131:945–961. [DOI] [PubMed] [Google Scholar]
- Liu H, Kaneko Y, Ouyang X, Li L, Hao Y, Chen EY, Jiang T, Zhou Y, Liu Z (2012): Schizophrenic patients and their unaffected siblings share increased resting‐state connectivity in the task‐negative network but not its anticorrelated task‐positive network. Schizophr Bull 38:285–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe MJ, Mock BJ, Sorenson JA (1998): Functional connectivity in single and multislice echoplanar imaging using resting‐state fluctuations. NeuroImage 7:119–132. [DOI] [PubMed] [Google Scholar]
- Meunier D, Achard S, Morcom A, Bullmore E (2009): Age‐related changes in modular organization of human brain functional networks. NeuroImage 44:715–723. [DOI] [PubMed] [Google Scholar]
- Meunier D, Lambiotte R, Bullmore ET (2010): Modular and hierarchically modular organization of brain networks. Front Neurosci 4:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC (2009): Meta‐analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry 66:811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Crain BJ, Chacko VP, van Zijl PC (1999): Three‐dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol 45:265–269. [DOI] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA (2009): The impact of global signal regression on resting state correlations: are anti‐correlated networks introduced?. NeuroImage 44:893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson MD, Saykin AJ, Flashman LA, Riordan HJ (1998): Hippocampal volume reduction in schizophrenia as assessed by magnetic resonance imaging: a meta‐analytic study. Arch Gen Psychiatry 55:433–440. [DOI] [PubMed] [Google Scholar]
- Newman ME, Girvan M (2004): Finding and evaluating community structure in networks. Phys Rev E 69:026113. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP (2002): Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp 15:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palaniyappan L, Liddle PF (2014): Diagnostic discontinuity in psychosis: a combined study of cortical gyrification and functional connectivity. Schizophr Bull 40:675–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pergola G, Selvaggi P, Trizio S, Bertolino A, Blasi G (2015): The role of the thalamus in schizophrenia from a neuroimaging perspective. Neurosci Biobehav Rev 54:57–75. [DOI] [PubMed] [Google Scholar]
- Pettersson‐Yeo W, Allen P, Benetti S, McGuire P, Mechelli A (2011): Dysconnectivity in schizophrenia: where are we now?. Neurosci Biobehav Rev 35:1110–1124. [DOI] [PubMed] [Google Scholar]
- Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, Vogel AC, Laumann TO, Miezin FM, Schlaggar BL, Petersen SE (2011): Functional network organization of the human brain. Neuron 72:665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE (2012): Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage 59:2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE (2014): Methods to detect, characterize, and remove motion artifact in resting state fMRI. NeuroImage 84:320–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu A, Tuan TA, Woon PS, Abdul‐Rahman MF, Graham S, Sim K (2010): Hippocampal‐cortical structural connectivity disruptions in schizophrenia: an integrated perspective from hippocampal shape, cortical thickness, and integrity of white matter bundles. NeuroImage 52:1181–1189. [DOI] [PubMed] [Google Scholar]
- Radicchi F, Castellano C, Cecconi F, Loreto V, Parisi D (2004): Defining and identifying communities in networks. Proc Natl Acad Sci U S A 101:2658–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repovs G, Csernansky JG, Barch DM (2011): Brain network connectivity in individuals with schizophrenia and their siblings. Biol Psychiatry 69:967–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M, Bullmore E (2013): Schizophrenia and abnormal brain network hubs. Dialogues Clin Neurosci 15:339–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M, Knock SA, Stam CJ, Micheloyannis S, Harris AW, Williams LM, Breakspear M (2009): Small‐world properties of nonlinear brain activity in schizophrenia. Hum Brain Mapp 30:403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad ZS, Gotts SJ, Murphy K, Chen G, Jo HJ, Martin A, Cox RW (2012): Trouble at rest: how correlation patterns and group differences become distorted after global signal regression. Brain Connect 2:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemon LD, Rajkowska G, Goldman‐Rakic PS (1995): Abnormally high neuronal density in the schizophrenic cortex. A morphometric analysis of prefrontal area 9 and occipital area 17. Arch Gen Psychiatry 52:805–820. [DOI] [PubMed] [Google Scholar]
- Seymour K, Stein T, Sanders LL, Guggenmos M, Theophil I, Sterzer P (2013): Altered contextual modulation of primary visual cortex responses in schizophrenia. Neuropsychopharmacology 38:2607–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin DJ, Jung WH, He Y, Wang J, Shim G, Byun MS, Jang JH, Kim SN, Lee TY, Park HY, Kwon JS (2014): The effects of pharmacological treatment on functional brain connectome in obsessive‐compulsive disorder. Biol Psychiatry 75:606–614. [DOI] [PubMed] [Google Scholar]
- Shu N, Liu Y, Li K, Duan Y, Wang J, Yu C, Dong H, Ye J, He Y (2011): Diffusion tensor tractography reveals disrupted topological efficiency in white matter structural networks in multiple sclerosis. Cereb Cortex 21:2565–2577. [DOI] [PubMed] [Google Scholar]
- Skudlarski P, Jagannathan K, Calhoun VD, Hampson M, Skudlarska BA, Pearlson G (2008): Measuring brain connectivity: diffusion tensor imaging validates resting state temporal correlations. NeuroImage 43:554–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skudlarski P, Jagannathan K, Anderson K, Stevens MC, Calhoun VD, Skudlarska BA, Pearlson G (2010): Brain connectivity is not only lower but different in schizophrenia: a combined anatomical and functional approach. Biol Psychiatry 68:61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen‐Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, Stefano ND, Brady JM, Matthews PM (2004): Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 23 Suppl 1:S208–S219. [DOI] [PubMed] [Google Scholar]
- Smith SM, Miller KL, Salimi‐Khorshidi G, Webster M, Beckmann CF, Nichols TE, Ramsey JD, Woolrich MW (2011): Network modelling methods for FMRI. NeuroImage 54:875–891. [DOI] [PubMed] [Google Scholar]
- Song XW, Dong ZY, Long XY, Li SF, Zuo XN, Zhu CZ, He Y, Yan CG, Zang YF (2011): REST: a toolkit for resting‐state functional magnetic resonance imaging data processing. PLoS One 6:e25031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O (2011): The human connectome: a complex network. Ann N Y Acad Sci 1224:109–125. [DOI] [PubMed] [Google Scholar]
- Stephan KE, Baldeweg T, Friston KJ (2006): Synaptic plasticity and dysconnection in schizophrenia. Biol Psychiatry 59:929–939. [DOI] [PubMed] [Google Scholar]
- Stephan KE, Friston KJ, Frith CD (2009): Dysconnection in schizophrenia: from abnormal synaptic plasticity to failures of self‐monitoring. Schizophr Bull 35:509–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Chen Y, Collinson SL, Bezerianos A, Sim K (2015): Reduced hemispheric asymmetry of brain anatomical networks is linked to schizophrenia: a connectome study. Cereb Cortex DOI: 10.1093/cercor/bhv255 [DOI] [PubMed] [Google Scholar]
- Sun Y, Chen Y, Lee R, Bezerianos A, Collinson SL, Sim K (2016): Disruption of brain anatomical networks in schizophrenia: A longitudinal, diffusion tensor imaging based study. Schizophr Res 171:149–157. [DOI] [PubMed] [Google Scholar]
- Tu PC, Hsieh JC, Li CT, Bai YM, Su TP (2012): Cortico‐striatal disconnection within the cingulo‐opercular network in schizophrenia revealed by intrinsic functional connectivity analysis: a resting fMRI study. NeuroImage 59:238–247. [DOI] [PubMed] [Google Scholar]
- Tyszka JM, Kennedy DP, Paul LK, Adolphs R (2014): Largely typical patterns of resting‐state functional connectivity in high‐functioning adults with autism. Cereb Cortex 24:1894–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. NeuroImage 15:273–289. [DOI] [PubMed] [Google Scholar]
- Van den Heuvel MP, Fornito A (2014): Brain networks in schizophrenia. Neuropsychol Rev 24:32–48. [DOI] [PubMed] [Google Scholar]
- Van den Heuvel MP, Sporns O, Collin G, Scheewe T, Mandl RC, Cahn W, Goni J, Hulshoff Pol HE, Kahn RS (2013): Abnormal rich club organization and functional brain dynamics in schizophrenia. JAMA Psychiatry 70:783–792. [DOI] [PubMed] [Google Scholar]
- Van Dijk KR, Sabuncu MR, Buckner RL (2012): The influence of head motion on intrinsic functional connectivity MRI. NeuroImage 59:431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Erp TG, Hibar DP, Rasmussen JM, Glahn DC, Pearlson GD, Andreassen OA, Agartz I, Westlye LT, Haukvik UK, Dale AM, Melle I, Hartberg CB, Gruber O, Kraemer B, Zilles D, Donohoe G, Kelly S, McDonald C, Morris DW, Cannon DM, Corvin A, Machielsen MWJ, Koenders L, de Hann L, Veltman DJ, Satterthwaite TD, Wolf H, Gur RC, Gur RE, Potkin SG, Mathalon DH, Mueller BA, Preda A, Macciardi F, Ehrlich S, Walton E, Hass J, Calhoun VD, Bockholt HJ, Sponheim SR, Shoemaker JM, van Haren NEM, Pol HEH, Ophoff RA, Kahn RS, Roiz‐Santianez R, Crespo‐Facorro B, Wang L, Alpert KI, Jonsson EG, Dimitrova R, Bois C, Whalley HC, McIntosh AM, Lawrie SM, Hashimoto R, Thompson PM, Turner JA, for the ENIGMA Schizophrenia Working Group (2016): Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol Psychiatry 21:547–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Benner T, Sorensen A, Wedeen V (2007): Diffusion toolkit: a software package for diffusion imaging data processing and tractography; Proc Intl Soc Mag Reson Med 15, Berlin, Germany, p 3720.
- Wang QF, Su TP, Zhou Y, Chou KH, Chen IY, Jiang TZ, Lin CP (2012): Anatomical insights into disrupted small‐world networks in schizophrenia. NeuroImage 59:1085–1093. [DOI] [PubMed] [Google Scholar]
- Wang J, Zuo X, Dai Z, Xia M, Zhao Z, Zhao X, Jia J, Han Y, He Y (2013): Disrupted functional brain connectome in individuals at risk for Alzheimer's disease. Biol Psychiatry 73:472–481. [DOI] [PubMed] [Google Scholar]
- Wang Z, Dai Z, Gong G, Zhou C, He Y (2015): Understanding structural‐functional relationships in the human brain: a large‐scale network perspective. Neuroscientist 21:290–305. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Roberts KC, Visscher KM, Woldorff MG (2006): The neural bases of momentary lapses in attention. Nat Neurosci 9:971–978. [DOI] [PubMed] [Google Scholar]
- Whitfield‐Gabrieli S, Ford JM (2012): Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol 8:49–76. [DOI] [PubMed] [Google Scholar]
- Whitfield‐Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, Shenton ME, Green AI, Nieto‐Castanon A, LaViolette P, Wojcik J, Gabrieli JDE, Seidman LJ, Raichle ME (2009): Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first‐degree relatives of persons with schizophrenia. Proc Natl Acad Sci U S A 106:1279–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SW (2003): Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry 64:663–667. [DOI] [PubMed] [Google Scholar]
- Wu K, Taki Y, Sato K, Kinomura S, Goto R, Okada K, Kawashima R, He Y, Evans AC, Fukuda H (2012): Age‐related changes in topological organization of structural brain networks in healthy individuals. Hum Brain Mapp 33:552–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan CG, Zang YF (2010): DPARSF: A MATLAB toolbox for “Pipeline” data analysis of resting‐state fMRI. Front Syst Neurosci 4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan CG, Cheung B, Kelly C, Colcombe S, Craddock RC, D, Martino A, Li Q, Zuo XN, Castellanos FX, Milham MP (2013a): A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. NeuroImage 76:183–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan CG, Craddock RC, Zuo XN, Zang YF, Milham MP (2013b): Standardizing the intrinsic brain: towards robust measurement of inter‐individual variation in 1000 functional connectomes. NeuroImage 80:246–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Eickhoff SB, Yaakub SN, Fox PT, Buckner RL, Asplund CL, Chee MW (2015): Functional specialization and flexibility in human association cortex. Cereb Cortex 5:3654–3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zollei L, Polimeni JR, Fischl B, Liu H, Buckner RL (2011): The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol 106:1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Plis SM, Erhardt EB, Allen EA, Sui J, Kiehl KA, Pearlson G, Calhoun VD (2011): Modular organization of functional network connectivity in healthy controls and patients with schizophrenia during the resting state. Front Syst Neurosci 5:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalesky A, Fornito A, Harding IH, Cocchi L, Yucel M, Pantelis C, Bullmore ET (2010): Whole‐brain anatomical networks: does the choice of nodes matter?. NeuroImage 50:970–983. [DOI] [PubMed] [Google Scholar]
- Zalesky A, Fornito A, Seal ML, Cocchi L, Westin CF, Bullmore ET, Egan GF, Pantelis C (2011): Disrupted axonal fiber connectivity in schizophrenia. Biol Psychiatry 69:80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalesky A, Fornito A, Cocchi L, Gollo LL, van den Heuvel MP, Breakspear M (2016): Connectome sensitivity or specificity: which is more important? NeuroImage 142:407–420. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Lin L, Lin CP, Zhou Y, Chou KH, Lo CY, Su TP, Jiang T (2012): Abnormal topological organization of structural brain networks in schizophrenia. Schizophr Res 141:109–118. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


